

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Acetyl-CoA carboxylase 2 (Homo sapiens (Human)) | BDBM50439646 (CHEMBL2419600 | US8993586, 110) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.70 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Inhibition of human ACC2 using acetyl-CoA as substrate assessed as [14C]malonyl-CoA synthesis preincubated for 10 mins prior to substrate addition me... | J Med Chem 56: 7110-9 (2013) Article DOI: 10.1021/jm401033t BindingDB Entry DOI: 10.7270/Q2JW8G9D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetyl-CoA carboxylase 1 (Homo sapiens (Human)) | BDBM50439646 (CHEMBL2419600 | US8993586, 110) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.80 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Inhibition of human ACC1 using acetyl-CoA as substrate assessed as [14C]malonyl-CoA synthesis preincubated for 10 mins prior to substrate addition me... | J Med Chem 56: 7110-9 (2013) Article DOI: 10.1021/jm401033t BindingDB Entry DOI: 10.7270/Q2JW8G9D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Free fatty acid receptor 1 (Homo sapiens (Human)) | BDBM50277775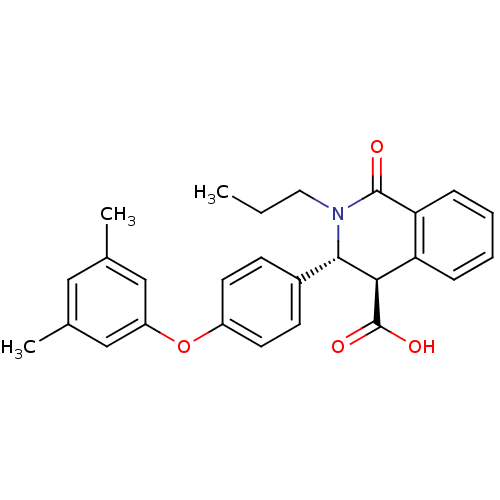 ((3R,4R)-3-(4-(3,5-dimethylphenoxy)phenyl)-1-oxo-2-...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global R&D Curated by ChEMBL | Assay Description Antagonist activity at human GPR40 expressed HEK293 cells assessed as effect on intracellular calcium concentration by FLIPR assay | Bioorg Med Chem Lett 19: 2400-3 (2009) Article DOI: 10.1016/j.bmcl.2009.03.082 BindingDB Entry DOI: 10.7270/Q23N238P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetyl-CoA carboxylase 2 (Homo sapiens (Human)) | BDBM50439642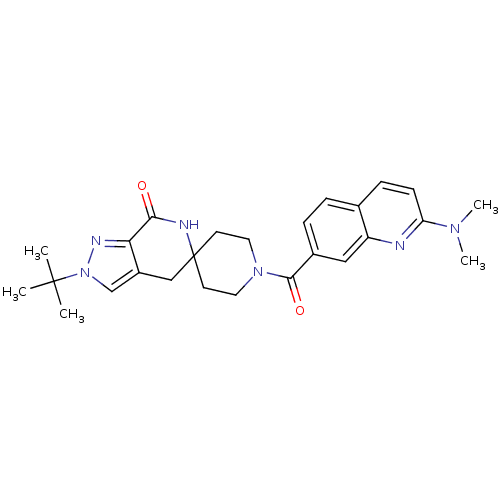 (CHEMBL2419589 | US8993586, 105) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Inhibition of human ACC2 using acetyl-CoA as substrate assessed as [14C]malonyl-CoA synthesis preincubated for 10 mins prior to substrate addition me... | J Med Chem 56: 7110-9 (2013) Article DOI: 10.1021/jm401033t BindingDB Entry DOI: 10.7270/Q2JW8G9D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetyl-CoA carboxylase 2 (Homo sapiens (Human)) | BDBM50439644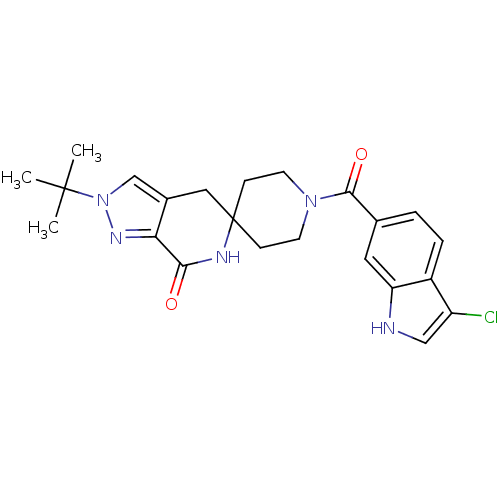 (CHEMBL2419593 | US8993586, 86) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Inhibition of human ACC2 using acetyl-CoA as substrate assessed as [14C]malonyl-CoA synthesis preincubated for 10 mins prior to substrate addition me... | J Med Chem 56: 7110-9 (2013) Article DOI: 10.1021/jm401033t BindingDB Entry DOI: 10.7270/Q2JW8G9D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Free fatty acid receptor 1 (Homo sapiens (Human)) | BDBM50277776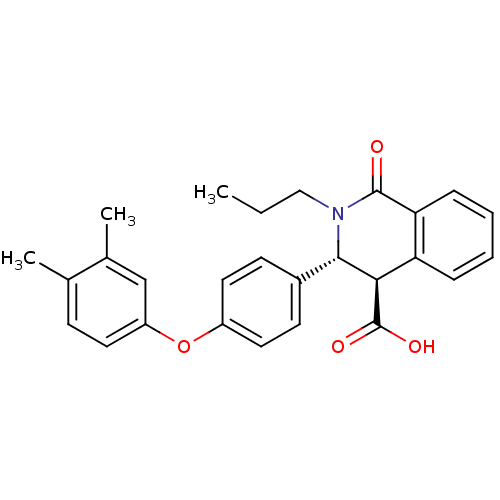 ((3R,4R)-3-(4-(3,4-dimethylphenoxy)phenyl)-1-oxo-2-...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global R&D Curated by ChEMBL | Assay Description Antagonist activity at human GPR40 expressed HEK293 cells assessed as effect on intracellular calcium concentration by FLIPR assay | Bioorg Med Chem Lett 19: 2400-3 (2009) Article DOI: 10.1016/j.bmcl.2009.03.082 BindingDB Entry DOI: 10.7270/Q23N238P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetyl-CoA carboxylase 2 (Homo sapiens (Human)) | BDBM50439634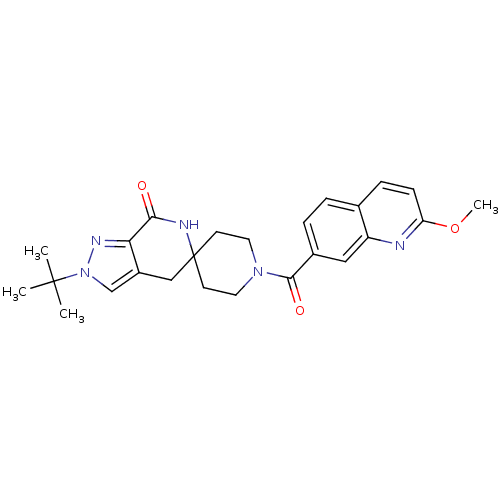 (CHEMBL2419596 | US8993586, 71) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Inhibition of human ACC2 using acetyl-CoA as substrate assessed as [14C]malonyl-CoA synthesis preincubated for 10 mins prior to substrate addition me... | J Med Chem 56: 7110-9 (2013) Article DOI: 10.1021/jm401033t BindingDB Entry DOI: 10.7270/Q2JW8G9D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Free fatty acid receptor 1 (Homo sapiens (Human)) | BDBM50277814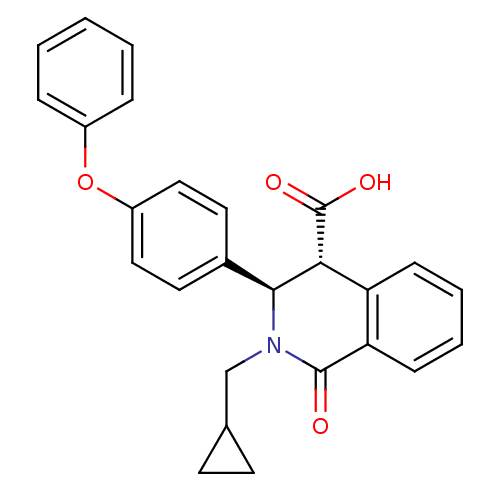 ((3R,4R)-2-(cyclopropylmethyl)-1-oxo-3-(4-phenoxyph...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global R&D Curated by ChEMBL | Assay Description Antagonist activity at human GPR40 expressed HEK293 cells assessed as effect on intracellular calcium concentration by FLIPR assay | Bioorg Med Chem Lett 19: 2400-3 (2009) Article DOI: 10.1016/j.bmcl.2009.03.082 BindingDB Entry DOI: 10.7270/Q23N238P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetyl-CoA carboxylase 2 (Homo sapiens (Human)) | BDBM50439641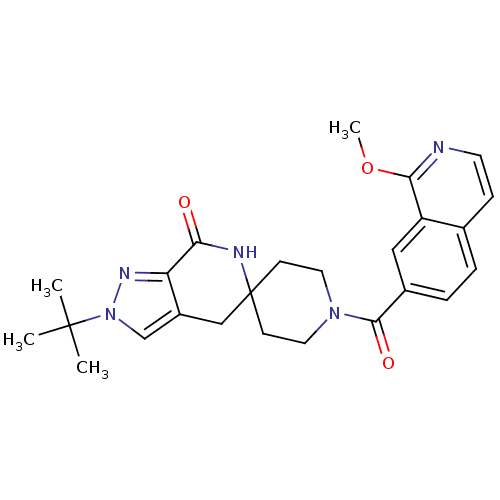 (CHEMBL2419597 | US8993586, 55) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 4.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Inhibition of human ACC2 using acetyl-CoA as substrate assessed as [14C]malonyl-CoA synthesis preincubated for 10 mins prior to substrate addition me... | J Med Chem 56: 7110-9 (2013) Article DOI: 10.1021/jm401033t BindingDB Entry DOI: 10.7270/Q2JW8G9D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetyl-CoA carboxylase 2 (Homo sapiens (Human)) | BDBM50439643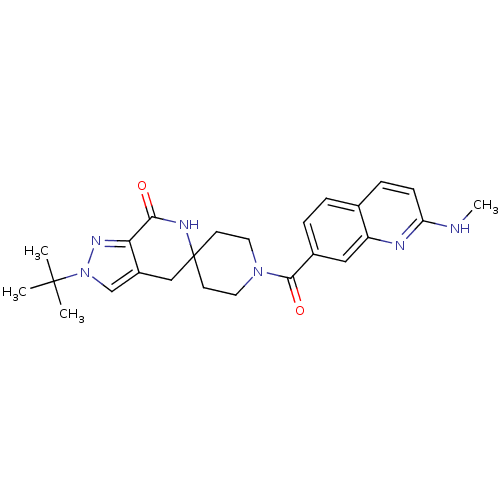 (CHEMBL2419598 | US8993586, 76) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 4.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Inhibition of human ACC2 using acetyl-CoA as substrate assessed as [14C]malonyl-CoA synthesis preincubated for 10 mins prior to substrate addition me... | J Med Chem 56: 7110-9 (2013) Article DOI: 10.1021/jm401033t BindingDB Entry DOI: 10.7270/Q2JW8G9D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetyl-CoA carboxylase 2 (Homo sapiens (Human)) | BDBM50439645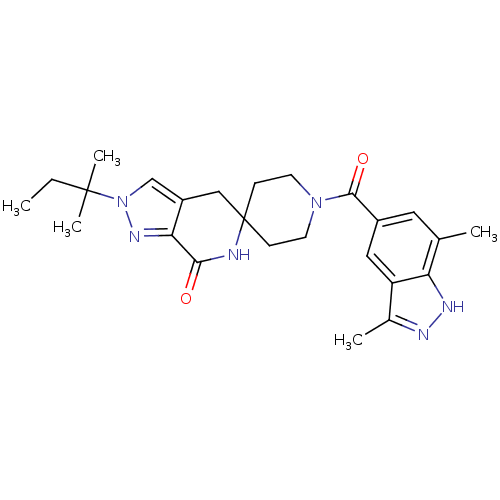 (CHEMBL2419607) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 4.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Inhibition of human ACC2 using acetyl-CoA as substrate assessed as [14C]malonyl-CoA synthesis preincubated for 10 mins prior to substrate addition me... | J Med Chem 56: 7110-9 (2013) Article DOI: 10.1021/jm401033t BindingDB Entry DOI: 10.7270/Q2JW8G9D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetyl-CoA carboxylase 1 (Homo sapiens (Human)) | BDBM50439642 (CHEMBL2419589 | US8993586, 105) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Inhibition of human ACC1 using acetyl-CoA as substrate assessed as [14C]malonyl-CoA synthesis preincubated for 10 mins prior to substrate addition me... | J Med Chem 56: 7110-9 (2013) Article DOI: 10.1021/jm401033t BindingDB Entry DOI: 10.7270/Q2JW8G9D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetyl-CoA carboxylase 2 (Homo sapiens (Human)) | BDBM50439635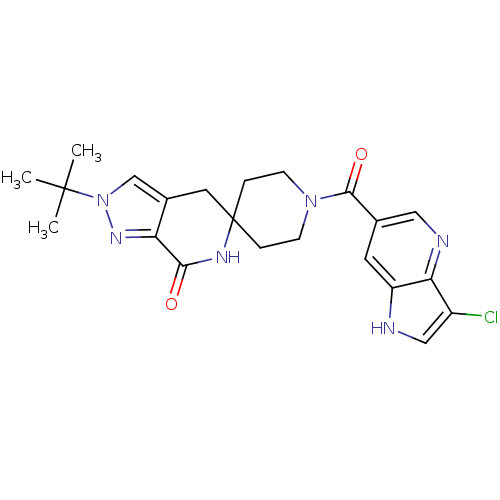 (CHEMBL2419594 | US8993586, 88) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Inhibition of human ACC2 using acetyl-CoA as substrate assessed as [14C]malonyl-CoA synthesis preincubated for 10 mins prior to substrate addition me... | J Med Chem 56: 7110-9 (2013) Article DOI: 10.1021/jm401033t BindingDB Entry DOI: 10.7270/Q2JW8G9D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetyl-CoA carboxylase 1 (Homo sapiens (Human)) | BDBM50439643 (CHEMBL2419598 | US8993586, 76) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Inhibition of human ACC1 using acetyl-CoA as substrate assessed as [14C]malonyl-CoA synthesis preincubated for 10 mins prior to substrate addition me... | J Med Chem 56: 7110-9 (2013) Article DOI: 10.1021/jm401033t BindingDB Entry DOI: 10.7270/Q2JW8G9D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetyl-CoA carboxylase 2 (Homo sapiens (Human)) | BDBM50439633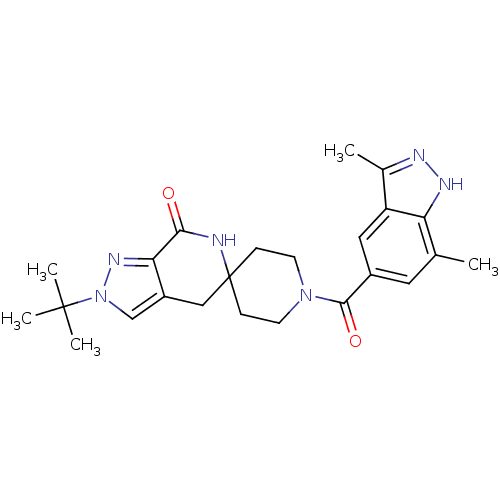 (CHEMBL2419604) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Inhibition of human ACC2 using acetyl-CoA as substrate assessed as [14C]malonyl-CoA synthesis preincubated for 10 mins prior to substrate addition me... | J Med Chem 56: 7110-9 (2013) Article DOI: 10.1021/jm401033t BindingDB Entry DOI: 10.7270/Q2JW8G9D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetyl-CoA carboxylase 2 (Homo sapiens (Human)) | BDBM50439638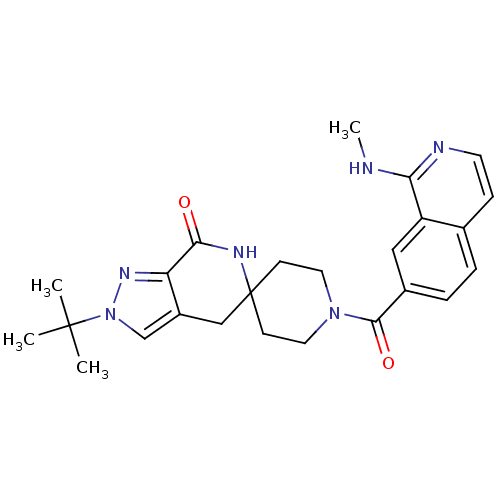 (CHEMBL2419599 | US8993586, 82) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Inhibition of human ACC2 using acetyl-CoA as substrate assessed as [14C]malonyl-CoA synthesis preincubated for 10 mins prior to substrate addition me... | J Med Chem 56: 7110-9 (2013) Article DOI: 10.1021/jm401033t BindingDB Entry DOI: 10.7270/Q2JW8G9D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protein-tyrosine kinase 2-beta (Homo sapiens (Human)) | BDBM50402996 (CHEMBL2207440) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 6.40 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Inhibition of human recombinant C-terminal His-tagged PYK2 using ATP as substrate incubated for 1 hr prior to substrate addition | Bioorg Med Chem Lett 22: 7523-9 (2012) Article DOI: 10.1016/j.bmcl.2012.10.039 BindingDB Entry DOI: 10.7270/Q2319X2C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetyl-CoA carboxylase 2 (Homo sapiens (Human)) | BDBM50439639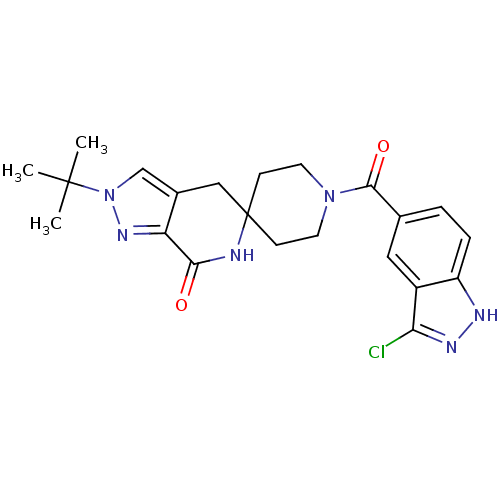 (CHEMBL2419591 | US8993586, 64) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 6.70 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Inhibition of human ACC2 using acetyl-CoA as substrate assessed as [14C]malonyl-CoA synthesis preincubated for 10 mins prior to substrate addition me... | J Med Chem 56: 7110-9 (2013) Article DOI: 10.1021/jm401033t BindingDB Entry DOI: 10.7270/Q2JW8G9D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetyl-CoA carboxylase 2 (Homo sapiens (Human)) | BDBM50439636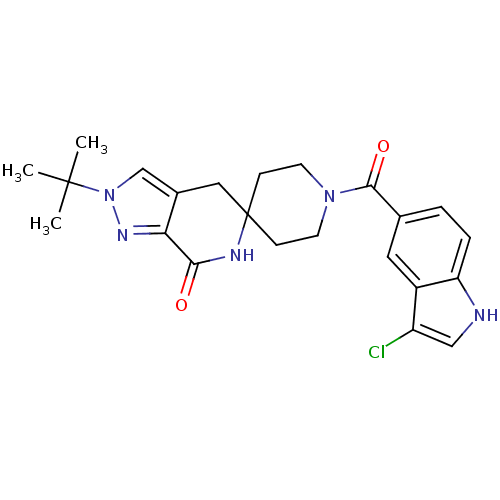 (CHEMBL2419592 | US8993586, 85) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 6.80 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Inhibition of human ACC2 using acetyl-CoA as substrate assessed as [14C]malonyl-CoA synthesis preincubated for 10 mins prior to substrate addition me... | J Med Chem 56: 7110-9 (2013) Article DOI: 10.1021/jm401033t BindingDB Entry DOI: 10.7270/Q2JW8G9D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetyl-CoA carboxylase 1 (Homo sapiens (Human)) | BDBM50439644 (CHEMBL2419593 | US8993586, 86) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 7 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Inhibition of human ACC1 using acetyl-CoA as substrate assessed as [14C]malonyl-CoA synthesis preincubated for 10 mins prior to substrate addition me... | J Med Chem 56: 7110-9 (2013) Article DOI: 10.1021/jm401033t BindingDB Entry DOI: 10.7270/Q2JW8G9D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetyl-CoA carboxylase 2 (Homo sapiens (Human)) | BDBM50439637 (CHEMBL2419601) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 9.80 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Inhibition of human recombinant ACC2 after 1 hr by transcreener assay | J Med Chem 57: 10512-26 (2014) Article DOI: 10.1021/jm5016022 BindingDB Entry DOI: 10.7270/Q2SN0BJM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Free fatty acid receptor 1 (Homo sapiens (Human)) | BDBM50277853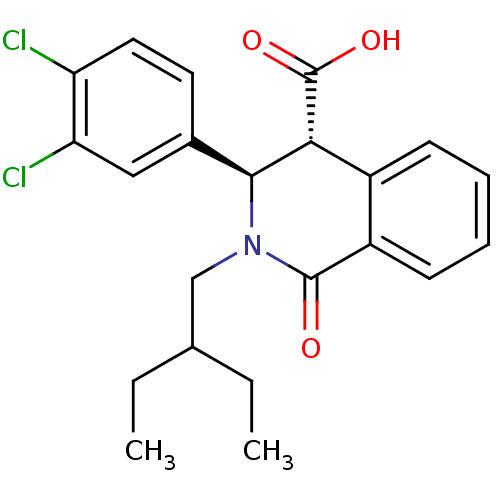 ((3R,4R)-3-(3,4-dichlorophenyl)-2-(2-ethylbutyl)-1-...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global R&D Curated by ChEMBL | Assay Description Antagonist activity at human GPR40 expressed HEK293 cells assessed as effect on intracellular calcium concentration by FLIPR assay | Bioorg Med Chem Lett 19: 2400-3 (2009) Article DOI: 10.1016/j.bmcl.2009.03.082 BindingDB Entry DOI: 10.7270/Q23N238P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetyl-CoA carboxylase 2 (Homo sapiens (Human)) | BDBM50439637 (CHEMBL2419601) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Inhibition of human ACC2 using acetyl-CoA as substrate assessed as [14C]malonyl-CoA synthesis preincubated for 10 mins prior to substrate addition me... | J Med Chem 56: 7110-9 (2013) Article DOI: 10.1021/jm401033t BindingDB Entry DOI: 10.7270/Q2JW8G9D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Free fatty acid receptor 1 (Homo sapiens (Human)) | BDBM50277728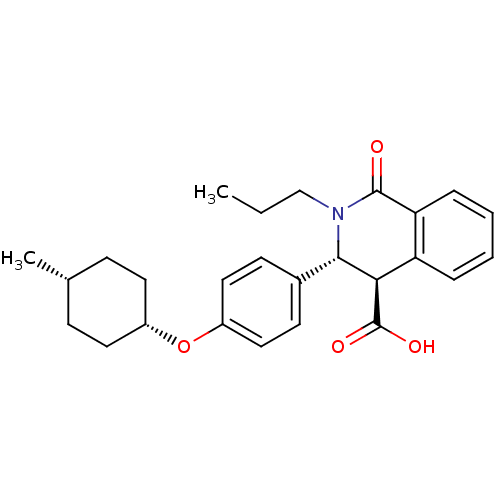 ((3R,4R)-3-(4-((cis)-4-methylcyclohexyloxy)phenyl)-...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global R&D Curated by ChEMBL | Assay Description Antagonist activity at human GPR40 expressed HEK293 cells assessed as effect on intracellular calcium concentration by FLIPR assay | Bioorg Med Chem Lett 19: 2400-3 (2009) Article DOI: 10.1016/j.bmcl.2009.03.082 BindingDB Entry DOI: 10.7270/Q23N238P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetyl-CoA carboxylase 2 (Homo sapiens (Human)) | BDBM50439626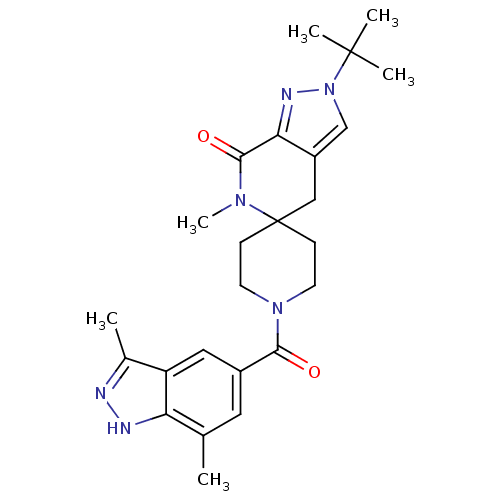 (CHEMBL2419610) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Inhibition of human ACC2 using acetyl-CoA as substrate assessed as [14C]malonyl-CoA synthesis preincubated for 10 mins prior to substrate addition me... | J Med Chem 56: 7110-9 (2013) Article DOI: 10.1021/jm401033t BindingDB Entry DOI: 10.7270/Q2JW8G9D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetyl-CoA carboxylase 1 (Homo sapiens (Human)) | BDBM50439634 (CHEMBL2419596 | US8993586, 71) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Inhibition of human ACC1 using acetyl-CoA as substrate assessed as [14C]malonyl-CoA synthesis preincubated for 10 mins prior to substrate addition me... | J Med Chem 56: 7110-9 (2013) Article DOI: 10.1021/jm401033t BindingDB Entry DOI: 10.7270/Q2JW8G9D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Free fatty acid receptor 1 (Homo sapiens (Human)) | BDBM50277887 ((3R,4R)-3-(benzo[d]thiazol-2-yl)-2-(2-ethylbutyl)-...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global R&D Curated by ChEMBL | Assay Description Antagonist activity at human GPR40 expressed HEK293 cells assessed as effect on intracellular calcium concentration by FLIPR assay | Bioorg Med Chem Lett 19: 2400-3 (2009) Article DOI: 10.1016/j.bmcl.2009.03.082 BindingDB Entry DOI: 10.7270/Q23N238P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetyl-CoA carboxylase 1 (Homo sapiens (Human)) | BDBM50439641 (CHEMBL2419597 | US8993586, 55) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 11 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Inhibition of human ACC1 using acetyl-CoA as substrate assessed as [14C]malonyl-CoA synthesis preincubated for 10 mins prior to substrate addition me... | J Med Chem 56: 7110-9 (2013) Article DOI: 10.1021/jm401033t BindingDB Entry DOI: 10.7270/Q2JW8G9D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetyl-CoA carboxylase 1 (Homo sapiens (Human)) | BDBM50439635 (CHEMBL2419594 | US8993586, 88) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 11 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Inhibition of human ACC1 using acetyl-CoA as substrate assessed as [14C]malonyl-CoA synthesis preincubated for 10 mins prior to substrate addition me... | J Med Chem 56: 7110-9 (2013) Article DOI: 10.1021/jm401033t BindingDB Entry DOI: 10.7270/Q2JW8G9D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Free fatty acid receptor 1 (Homo sapiens (Human)) | BDBM50277729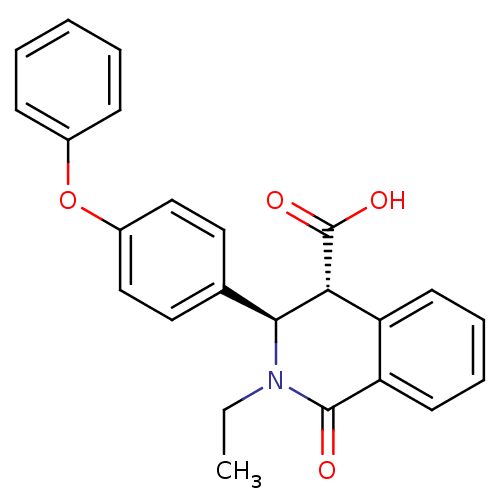 ((3R,4R)-2-ethyl-1-oxo-3-(4-phenoxyphenyl)-1,2,3,4-...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 12 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global R&D Curated by ChEMBL | Assay Description Antagonist activity at human GPR40 expressed HEK293 cells assessed as effect on intracellular calcium concentration by FLIPR assay | Bioorg Med Chem Lett 19: 2400-3 (2009) Article DOI: 10.1016/j.bmcl.2009.03.082 BindingDB Entry DOI: 10.7270/Q23N238P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Free fatty acid receptor 1 (Homo sapiens (Human)) | BDBM50277852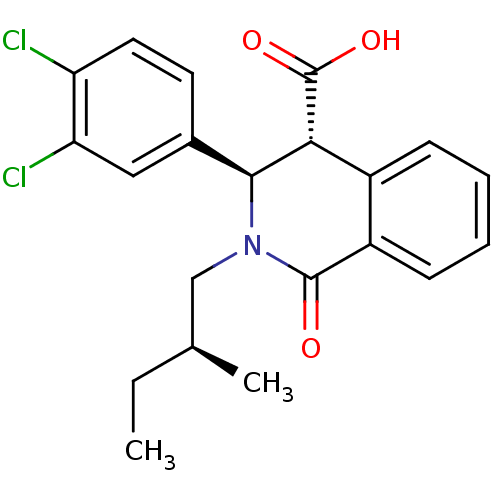 ((3R,4R)-3-(3,4-dichlorophenyl)-2-((S)-2-methylbuty...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 13 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global R&D Curated by ChEMBL | Assay Description Antagonist activity at human GPR40 expressed HEK293 cells assessed as effect on intracellular calcium concentration by FLIPR assay | Bioorg Med Chem Lett 19: 2400-3 (2009) Article DOI: 10.1016/j.bmcl.2009.03.082 BindingDB Entry DOI: 10.7270/Q23N238P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetyl-CoA carboxylase 1 (Homo sapiens (Human)) | BDBM50439636 (CHEMBL2419592 | US8993586, 85) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 13 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Inhibition of human ACC1 using acetyl-CoA as substrate assessed as [14C]malonyl-CoA synthesis preincubated for 10 mins prior to substrate addition me... | J Med Chem 56: 7110-9 (2013) Article DOI: 10.1021/jm401033t BindingDB Entry DOI: 10.7270/Q2JW8G9D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protein-tyrosine kinase 2-beta (Homo sapiens (Human)) | BDBM50402999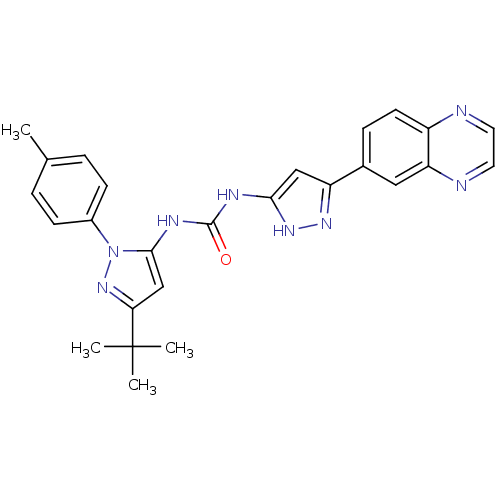 (CHEMBL2207441) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 13 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Inhibition of human recombinant C-terminal His-tagged PYK2 using ATP as substrate incubated for 1 hr prior to substrate addition | Bioorg Med Chem Lett 22: 7523-9 (2012) Article DOI: 10.1016/j.bmcl.2012.10.039 BindingDB Entry DOI: 10.7270/Q2319X2C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetyl-CoA carboxylase 2 (Homo sapiens (Human)) | BDBM50439640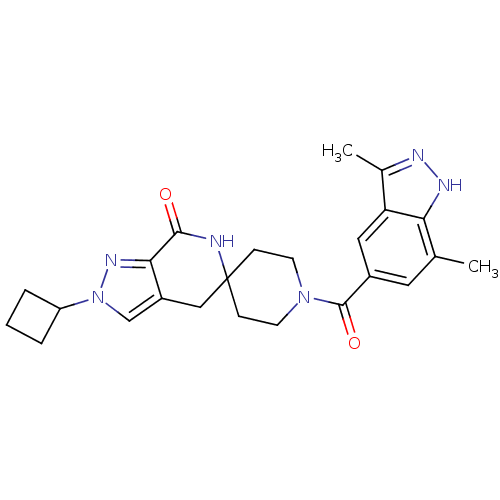 (CHEMBL2419606 | US8993586, 57) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 16 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Inhibition of human ACC2 using acetyl-CoA as substrate assessed as [14C]malonyl-CoA synthesis preincubated for 10 mins prior to substrate addition me... | J Med Chem 56: 7110-9 (2013) Article DOI: 10.1021/jm401033t BindingDB Entry DOI: 10.7270/Q2JW8G9D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetyl-CoA carboxylase 1 (Rattus norvegicus (Rat)) | BDBM50127711 (CHEMBL3629719) | PDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid PDB UniChem Patents | Article PubMed | n/a | n/a | 16 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Curated by ChEMBL | Assay Description Inhibition of rat liver ACC1 preincubated for 10 mins followed by acetyl-CoA/KHCO3/[14C]-NaHCO3/ATP addition measured after 20 mins by liquid scintil... | Bioorg Med Chem Lett 25: 5352-6 (2015) Article DOI: 10.1016/j.bmcl.2015.09.035 BindingDB Entry DOI: 10.7270/Q2W37Z4R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetyl-CoA carboxylase 1 (Homo sapiens (Human)) | BDBM50439639 (CHEMBL2419591 | US8993586, 64) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 17 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Inhibition of human ACC1 using acetyl-CoA as substrate assessed as [14C]malonyl-CoA synthesis preincubated for 10 mins prior to substrate addition me... | J Med Chem 56: 7110-9 (2013) Article DOI: 10.1021/jm401033t BindingDB Entry DOI: 10.7270/Q2JW8G9D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetyl-CoA carboxylase 2 (Homo sapiens (Human)) | BDBM50034496 (CHEMBL3359261) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 17 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Inhibition of human recombinant ACC2 after 1 hr by transcreener assay | J Med Chem 57: 10512-26 (2014) Article DOI: 10.1021/jm5016022 BindingDB Entry DOI: 10.7270/Q2SN0BJM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetyl-CoA carboxylase 1 (Homo sapiens (Human)) | BDBM50439638 (CHEMBL2419599 | US8993586, 82) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 18 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Inhibition of human ACC1 using acetyl-CoA as substrate assessed as [14C]malonyl-CoA synthesis preincubated for 10 mins prior to substrate addition me... | J Med Chem 56: 7110-9 (2013) Article DOI: 10.1021/jm401033t BindingDB Entry DOI: 10.7270/Q2JW8G9D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetyl-CoA carboxylase 1 (Homo sapiens (Human)) | BDBM50439637 (CHEMBL2419601) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 19 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Inhibition of human ACC1 using acetyl-CoA as substrate assessed as [14C]malonyl-CoA synthesis preincubated for 10 mins prior to substrate addition me... | J Med Chem 56: 7110-9 (2013) Article DOI: 10.1021/jm401033t BindingDB Entry DOI: 10.7270/Q2JW8G9D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetyl-CoA carboxylase 2 (Homo sapiens (Human)) | BDBM50439630 (CHEMBL2419590 | US8993586, 80) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 19 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Inhibition of human ACC2 using acetyl-CoA as substrate assessed as [14C]malonyl-CoA synthesis preincubated for 10 mins prior to substrate addition me... | J Med Chem 56: 7110-9 (2013) Article DOI: 10.1021/jm401033t BindingDB Entry DOI: 10.7270/Q2JW8G9D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Free fatty acid receptor 1 (Homo sapiens (Human)) | BDBM50277888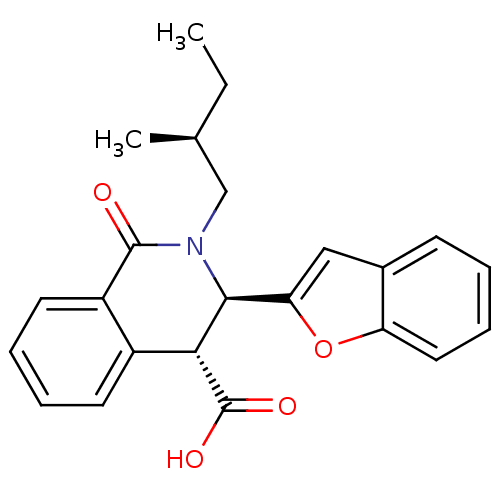 ((3R,4R)-3-(benzofuran-2-yl)-2-((S)-2-methylbutyl)-...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 20 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global R&D Curated by ChEMBL | Assay Description Antagonist activity at human GPR40 expressed HEK293 cells assessed as effect on intracellular calcium concentration by FLIPR assay | Bioorg Med Chem Lett 19: 2400-3 (2009) Article DOI: 10.1016/j.bmcl.2009.03.082 BindingDB Entry DOI: 10.7270/Q23N238P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetyl-CoA carboxylase 2 (Homo sapiens (Human)) | BDBM50034494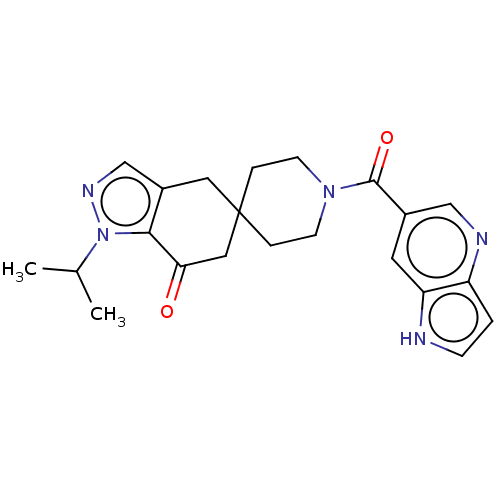 (CHEMBL3359264) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 23 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Inhibition of human recombinant ACC2 after 1 hr by transcreener assay | J Med Chem 57: 10512-26 (2014) Article DOI: 10.1021/jm5016022 BindingDB Entry DOI: 10.7270/Q2SN0BJM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetyl-CoA carboxylase 1 (Homo sapiens (Human)) | BDBM50439645 (CHEMBL2419607) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 23 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Inhibition of human ACC1 using acetyl-CoA as substrate assessed as [14C]malonyl-CoA synthesis preincubated for 10 mins prior to substrate addition me... | J Med Chem 56: 7110-9 (2013) Article DOI: 10.1021/jm401033t BindingDB Entry DOI: 10.7270/Q2JW8G9D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetyl-CoA carboxylase 1 (Rattus norvegicus (Rat)) | BDBM50034371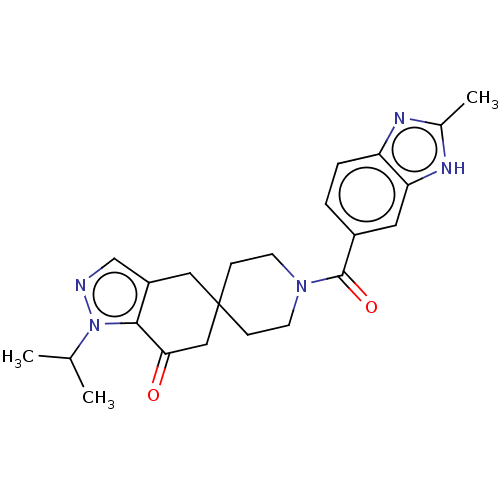 (CHEMBL3359265) | PDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 24 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Inhibition of rat recombinant ACC1 assessed as incorporation of [14C]bicarbonate into [14C]malonyl-CoA by radiometric assay | J Med Chem 57: 10512-26 (2014) Article DOI: 10.1021/jm5016022 BindingDB Entry DOI: 10.7270/Q2SN0BJM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protein-tyrosine kinase 2-beta (Homo sapiens (Human)) | BDBM50403004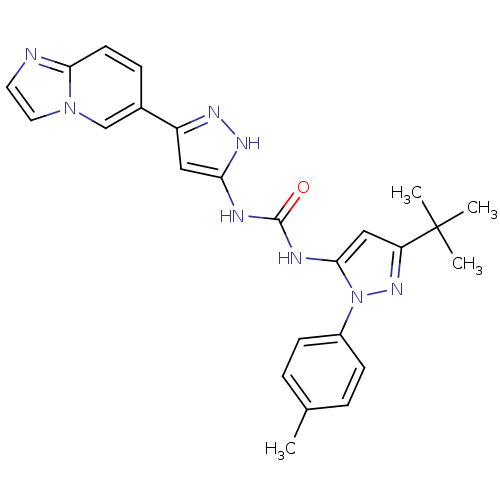 (CHEMBL2207439) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 25 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Inhibition of human recombinant C-terminal His-tagged PYK2 using ATP as substrate incubated for 1 hr prior to substrate addition | Bioorg Med Chem Lett 22: 7523-9 (2012) Article DOI: 10.1016/j.bmcl.2012.10.039 BindingDB Entry DOI: 10.7270/Q2319X2C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetyl-CoA carboxylase 1 (Rattus norvegicus (Rat)) | BDBM50127710 (CHEMBL3629720) | PDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents | Article PubMed | n/a | n/a | 26 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Curated by ChEMBL | Assay Description Inhibition of rat liver ACC1 preincubated for 10 mins followed by acetyl-CoA/KHCO3/[14C]-NaHCO3/ATP addition measured after 20 mins by liquid scintil... | Bioorg Med Chem Lett 25: 5352-6 (2015) Article DOI: 10.1016/j.bmcl.2015.09.035 BindingDB Entry DOI: 10.7270/Q2W37Z4R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetyl-CoA carboxylase 1 (Homo sapiens (Human)) | BDBM50034371 (CHEMBL3359265) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 27 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Inhibition of human recombinant ACC1 assessed as incorporation of [14C]bicarbonate into [14C]malonyl-CoA by radiometric assay | J Med Chem 57: 10512-26 (2014) Article DOI: 10.1021/jm5016022 BindingDB Entry DOI: 10.7270/Q2SN0BJM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetyl-CoA carboxylase 1 (Homo sapiens (Human)) | BDBM50439633 (CHEMBL2419604) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 28 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Inhibition of human ACC1 using acetyl-CoA as substrate assessed as [14C]malonyl-CoA synthesis preincubated for 10 mins prior to substrate addition me... | J Med Chem 56: 7110-9 (2013) Article DOI: 10.1021/jm401033t BindingDB Entry DOI: 10.7270/Q2JW8G9D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetyl-CoA carboxylase 2 (Homo sapiens (Human)) | BDBM50439632 (CHEMBL2419605) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 29 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Inhibition of human ACC2 using acetyl-CoA as substrate assessed as [14C]malonyl-CoA synthesis preincubated for 10 mins prior to substrate addition me... | J Med Chem 56: 7110-9 (2013) Article DOI: 10.1021/jm401033t BindingDB Entry DOI: 10.7270/Q2JW8G9D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Free fatty acid receptor 1 (Homo sapiens (Human)) | BDBM50277777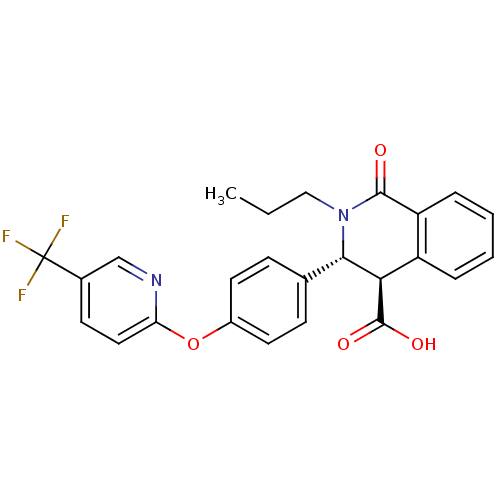 ((3R,4R)-1-oxo-2-propyl-3-(4-(5-(trifluoromethyl)py...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 29 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global R&D Curated by ChEMBL | Assay Description Antagonist activity at human GPR40 expressed HEK293 cells assessed as effect on intracellular calcium concentration by FLIPR assay | Bioorg Med Chem Lett 19: 2400-3 (2009) Article DOI: 10.1016/j.bmcl.2009.03.082 BindingDB Entry DOI: 10.7270/Q23N238P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 172 total ) | Next | Last >> |