 Found 511 hits with Last Name = 'spalding' and Initial = 'ta'
Found 511 hits with Last Name = 'spalding' and Initial = 'ta' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
D(2) dopamine receptor
(Homo sapiens (Human)) | BDBM21398

(4-[4-(4-Chloro-phenyl)-4-hydroxy-piperidin-1-yl]-1...)Show SMILES OC1(CCN(CCCC(=O)c2ccc(F)cc2)CC1)c1ccc(Cl)cc1 Show InChI InChI=1S/C21H23ClFNO2/c22-18-7-5-17(6-8-18)21(26)11-14-24(15-12-21)13-1-2-20(25)16-3-9-19(23)10-4-16/h3-10,26H,1-2,11-15H2 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
Article
PubMed
| 0.700 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
ACADIA Pharmaceuticals, Inc.,
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 310: 943-51 (2004)
Article DOI: 10.1124/jpet.104.066688
BindingDB Entry DOI: 10.7270/Q28S4NH4 |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 2A
(Homo sapiens (Human)) | BDBM22869

(6-chloro-10-(4-methylpiperazin-1-yl)-2,9-diazatric...)Show SMILES CN1CCN(CC1)C1=c2ccccc2=Nc2ccc(Cl)cc2N1 |c:8,15| Show InChI InChI=1S/C18H19ClN4/c1-22-8-10-23(11-9-22)18-14-4-2-3-5-15(14)20-16-7-6-13(19)12-17(16)21-18/h2-7,12,21H,8-11H2,1H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
ACADIA Pharmaceuticals, Inc.,
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 310: 943-51 (2004)
Article DOI: 10.1124/jpet.104.066688
BindingDB Entry DOI: 10.7270/Q28S4NH4 |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 2C
(Homo sapiens (Human)) | BDBM22869

(6-chloro-10-(4-methylpiperazin-1-yl)-2,9-diazatric...)Show SMILES CN1CCN(CC1)C1=c2ccccc2=Nc2ccc(Cl)cc2N1 |c:8,15| Show InChI InChI=1S/C18H19ClN4/c1-22-8-10-23(11-9-22)18-14-4-2-3-5-15(14)20-16-7-6-13(19)12-17(16)21-18/h2-7,12,21H,8-11H2,1H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 1.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
ACADIA Pharmaceuticals, Inc.,
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 310: 943-51 (2004)
Article DOI: 10.1124/jpet.104.066688
BindingDB Entry DOI: 10.7270/Q28S4NH4 |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 2C
(Homo sapiens (Human)) | BDBM86496
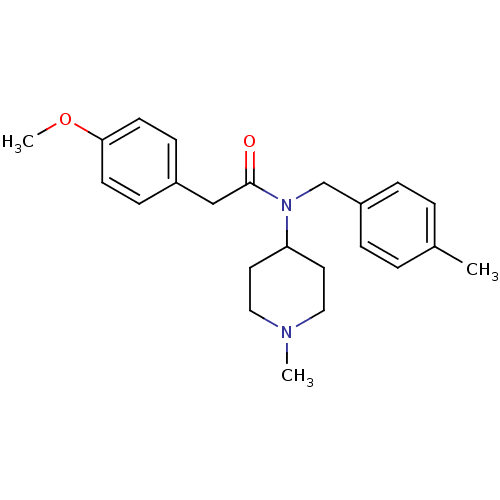
(AC-90179 | N-(4-Methylbenzyl)-N-(1-methyl-4-piperi...)Show SMILES COc1ccc(CC(=O)N(Cc2ccc(C)cc2)C2CCN(C)CC2)cc1 Show InChI InChI=1S/C23H30N2O2/c1-18-4-6-20(7-5-18)17-25(21-12-14-24(2)15-13-21)23(26)16-19-8-10-22(27-3)11-9-19/h4-11,21H,12-17H2,1-3H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 2.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
ACADIA Pharmaceuticals, Inc.,
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 310: 943-51 (2004)
Article DOI: 10.1124/jpet.104.066688
BindingDB Entry DOI: 10.7270/Q28S4NH4 |
More data for this
Ligand-Target Pair | |
D(2) dopamine receptor
(Homo sapiens (Human)) | BDBM22869

(6-chloro-10-(4-methylpiperazin-1-yl)-2,9-diazatric...)Show SMILES CN1CCN(CC1)C1=c2ccccc2=Nc2ccc(Cl)cc2N1 |c:8,15| Show InChI InChI=1S/C18H19ClN4/c1-22-8-10-23(11-9-22)18-14-4-2-3-5-15(14)20-16-7-6-13(19)12-17(16)21-18/h2-7,12,21H,8-11H2,1H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 49.9 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
ACADIA Pharmaceuticals, Inc.,
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 310: 943-51 (2004)
Article DOI: 10.1124/jpet.104.066688
BindingDB Entry DOI: 10.7270/Q28S4NH4 |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 2A
(Homo sapiens (Human)) | BDBM21398

(4-[4-(4-Chloro-phenyl)-4-hydroxy-piperidin-1-yl]-1...)Show SMILES OC1(CCN(CCCC(=O)c2ccc(F)cc2)CC1)c1ccc(Cl)cc1 Show InChI InChI=1S/C21H23ClFNO2/c22-18-7-5-17(6-8-18)21(26)11-14-24(15-12-21)13-1-2-20(25)16-3-9-19(23)10-4-16/h3-10,26H,1-2,11-15H2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 70.2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
ACADIA Pharmaceuticals, Inc.,
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 310: 943-51 (2004)
Article DOI: 10.1124/jpet.104.066688
BindingDB Entry DOI: 10.7270/Q28S4NH4 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase Lck
(Homo sapiens (Human)) | BDBM50311926
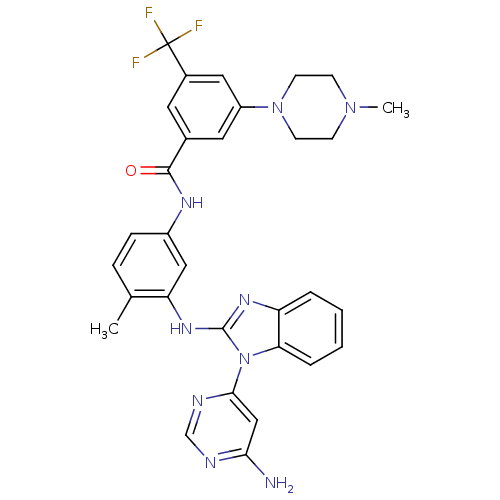
(CHEMBL1076436 | N-(3-(1-(6-aminopyrimidin-4-yl)-1H...)Show SMILES CN1CCN(CC1)c1cc(cc(c1)C(F)(F)F)C(=O)Nc1ccc(C)c(Nc2nc3ccccc3n2-c2cc(N)ncn2)c1 Show InChI InChI=1S/C31H30F3N9O/c1-19-7-8-22(38-29(44)20-13-21(31(32,33)34)15-23(14-20)42-11-9-41(2)10-12-42)16-25(19)40-30-39-24-5-3-4-6-26(24)43(30)28-17-27(35)36-18-37-28/h3-8,13-18H,9-12H2,1-2H3,(H,38,44)(H,39,40)(H2,35,36,37) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Genomics Institute of the Novartis Research Foundation (GNF)
Curated by ChEMBL
| Assay Description
Inhibition of Tel-fused LCK expressed in mouse BAF3 cells |
Bioorg Med Chem Lett 19: 6691-5 (2009)
Article DOI: 10.1016/j.bmcl.2009.09.123
BindingDB Entry DOI: 10.7270/Q2Q52PRR |
More data for this
Ligand-Target Pair | |
Vascular endothelial growth factor receptor 2
(Homo sapiens (Human)) | BDBM50311921

(CHEMBL1086731 | N-(3-(1-(6-aminopyrimidin-4-yl)-1H...)Show SMILES Cc1ccc(NC(=O)c2ccc(s2)C(C)(C)C)cc1Nc1nc2ccccc2n1-c1cc(N)ncn1 Show InChI InChI=1S/C27H27N7OS/c1-16-9-10-17(31-25(35)21-11-12-22(36-21)27(2,3)4)13-19(16)33-26-32-18-7-5-6-8-20(18)34(26)24-14-23(28)29-15-30-24/h5-15H,1-4H3,(H,31,35)(H,32,33)(H2,28,29,30) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
| Article
PubMed
| n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
Genomics Institute of the Novartis Research Foundation (GNF)
Curated by ChEMBL
| Assay Description
Inhibition of Tel-fused KDR expressed in mouse BAF3 cells |
Bioorg Med Chem Lett 19: 6691-5 (2009)
Article DOI: 10.1016/j.bmcl.2009.09.123
BindingDB Entry DOI: 10.7270/Q2Q52PRR |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase Lck
(Homo sapiens (Human)) | BDBM50311921

(CHEMBL1086731 | N-(3-(1-(6-aminopyrimidin-4-yl)-1H...)Show SMILES Cc1ccc(NC(=O)c2ccc(s2)C(C)(C)C)cc1Nc1nc2ccccc2n1-c1cc(N)ncn1 Show InChI InChI=1S/C27H27N7OS/c1-16-9-10-17(31-25(35)21-11-12-22(36-21)27(2,3)4)13-19(16)33-26-32-18-7-5-6-8-20(18)34(26)24-14-23(28)29-15-30-24/h5-15H,1-4H3,(H,31,35)(H,32,33)(H2,28,29,30) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
| Article
PubMed
| n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
Genomics Institute of the Novartis Research Foundation (GNF)
Curated by ChEMBL
| Assay Description
Inhibition of Tel-fused LCK expressed in mouse BAF3 cells |
Bioorg Med Chem Lett 19: 6691-5 (2009)
Article DOI: 10.1016/j.bmcl.2009.09.123
BindingDB Entry DOI: 10.7270/Q2Q52PRR |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase Lck
(Homo sapiens (Human)) | BDBM50311927
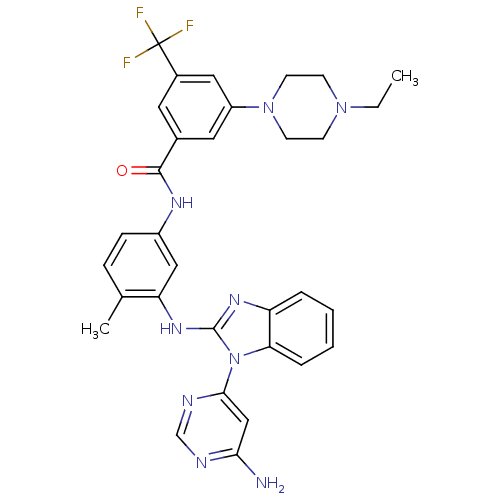
(CHEMBL1076437 | N-(3-(1-(6-aminopyrimidin-4-yl)-1H...)Show SMILES CCN1CCN(CC1)c1cc(cc(c1)C(F)(F)F)C(=O)Nc1ccc(C)c(Nc2nc3ccccc3n2-c2cc(N)ncn2)c1 Show InChI InChI=1S/C32H32F3N9O/c1-3-42-10-12-43(13-11-42)24-15-21(14-22(16-24)32(33,34)35)30(45)39-23-9-8-20(2)26(17-23)41-31-40-25-6-4-5-7-27(25)44(31)29-18-28(36)37-19-38-29/h4-9,14-19H,3,10-13H2,1-2H3,(H,39,45)(H,40,41)(H2,36,37,38) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
Genomics Institute of the Novartis Research Foundation (GNF)
Curated by ChEMBL
| Assay Description
Inhibition of Tel-fused LCK expressed in mouse BAF3 cells |
Bioorg Med Chem Lett 19: 6691-5 (2009)
Article DOI: 10.1016/j.bmcl.2009.09.123
BindingDB Entry DOI: 10.7270/Q2Q52PRR |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase Lck
(Homo sapiens (Human)) | BDBM50311924
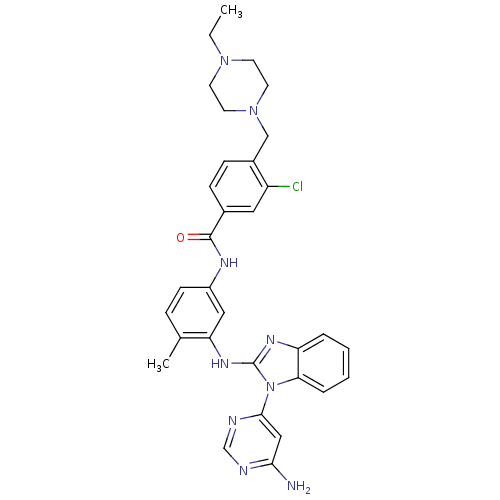
(CHEMBL1076433 | N-(3-(1-(6-aminopyrimidin-4-yl)-1H...)Show SMILES CCN1CCN(Cc2ccc(cc2Cl)C(=O)Nc2ccc(C)c(Nc3nc4ccccc4n3-c3cc(N)ncn3)c2)CC1 Show InChI InChI=1S/C32H34ClN9O/c1-3-40-12-14-41(15-13-40)19-23-10-9-22(16-25(23)33)31(43)37-24-11-8-21(2)27(17-24)39-32-38-26-6-4-5-7-28(26)42(32)30-18-29(34)35-20-36-30/h4-11,16-18,20H,3,12-15,19H2,1-2H3,(H,37,43)(H,38,39)(H2,34,35,36) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
Genomics Institute of the Novartis Research Foundation (GNF)
Curated by ChEMBL
| Assay Description
Inhibition of LCK |
Bioorg Med Chem Lett 19: 6691-5 (2009)
Article DOI: 10.1016/j.bmcl.2009.09.123
BindingDB Entry DOI: 10.7270/Q2Q52PRR |
More data for this
Ligand-Target Pair | |
Insulin receptor
(Homo sapiens (Human)) | BDBM50311921

(CHEMBL1086731 | N-(3-(1-(6-aminopyrimidin-4-yl)-1H...)Show SMILES Cc1ccc(NC(=O)c2ccc(s2)C(C)(C)C)cc1Nc1nc2ccccc2n1-c1cc(N)ncn1 Show InChI InChI=1S/C27H27N7OS/c1-16-9-10-17(31-25(35)21-11-12-22(36-21)27(2,3)4)13-19(16)33-26-32-18-7-5-6-8-20(18)34(26)24-14-23(28)29-15-30-24/h5-15H,1-4H3,(H,31,35)(H,32,33)(H2,28,29,30) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
| Article
PubMed
| n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
Genomics Institute of the Novartis Research Foundation (GNF)
Curated by ChEMBL
| Assay Description
Inhibition of Tel-fused InsR expressed in mouse BAF3 cells |
Bioorg Med Chem Lett 19: 6691-5 (2009)
Article DOI: 10.1016/j.bmcl.2009.09.123
BindingDB Entry DOI: 10.7270/Q2Q52PRR |
More data for this
Ligand-Target Pair | |
Proto-oncogene tyrosine-protein kinase Src
(Homo sapiens (Human)) | BDBM50311921

(CHEMBL1086731 | N-(3-(1-(6-aminopyrimidin-4-yl)-1H...)Show SMILES Cc1ccc(NC(=O)c2ccc(s2)C(C)(C)C)cc1Nc1nc2ccccc2n1-c1cc(N)ncn1 Show InChI InChI=1S/C27H27N7OS/c1-16-9-10-17(31-25(35)21-11-12-22(36-21)27(2,3)4)13-19(16)33-26-32-18-7-5-6-8-20(18)34(26)24-14-23(28)29-15-30-24/h5-15H,1-4H3,(H,31,35)(H,32,33)(H2,28,29,30) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
| Article
PubMed
| n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
Genomics Institute of the Novartis Research Foundation (GNF)
Curated by ChEMBL
| Assay Description
Inhibition of Tel-fused SRC expressed in mouse BAF3 cells |
Bioorg Med Chem Lett 19: 6691-5 (2009)
Article DOI: 10.1016/j.bmcl.2009.09.123
BindingDB Entry DOI: 10.7270/Q2Q52PRR |
More data for this
Ligand-Target Pair | |
Vascular endothelial growth factor receptor 2
(Homo sapiens (Human)) | BDBM50311927

(CHEMBL1076437 | N-(3-(1-(6-aminopyrimidin-4-yl)-1H...)Show SMILES CCN1CCN(CC1)c1cc(cc(c1)C(F)(F)F)C(=O)Nc1ccc(C)c(Nc2nc3ccccc3n2-c2cc(N)ncn2)c1 Show InChI InChI=1S/C32H32F3N9O/c1-3-42-10-12-43(13-11-42)24-15-21(14-22(16-24)32(33,34)35)30(45)39-23-9-8-20(2)26(17-23)41-31-40-25-6-4-5-7-27(25)44(31)29-18-28(36)37-19-38-29/h4-9,14-19H,3,10-13H2,1-2H3,(H,39,45)(H,40,41)(H2,36,37,38) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
Genomics Institute of the Novartis Research Foundation (GNF)
Curated by ChEMBL
| Assay Description
Inhibition of Tel-fused KDR expressed in mouse BAF3 cells |
Bioorg Med Chem Lett 19: 6691-5 (2009)
Article DOI: 10.1016/j.bmcl.2009.09.123
BindingDB Entry DOI: 10.7270/Q2Q52PRR |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase Lck
(Homo sapiens (Human)) | BDBM50311924

(CHEMBL1076433 | N-(3-(1-(6-aminopyrimidin-4-yl)-1H...)Show SMILES CCN1CCN(Cc2ccc(cc2Cl)C(=O)Nc2ccc(C)c(Nc3nc4ccccc4n3-c3cc(N)ncn3)c2)CC1 Show InChI InChI=1S/C32H34ClN9O/c1-3-40-12-14-41(15-13-40)19-23-10-9-22(16-25(23)33)31(43)37-24-11-8-21(2)27(17-24)39-32-38-26-6-4-5-7-28(26)42(32)30-18-29(34)35-20-36-30/h4-11,16-18,20H,3,12-15,19H2,1-2H3,(H,37,43)(H,38,39)(H2,34,35,36) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
Genomics Institute of the Novartis Research Foundation (GNF)
Curated by ChEMBL
| Assay Description
Inhibition of Tel-fused LCK expressed in mouse BAF3 cells |
Bioorg Med Chem Lett 19: 6691-5 (2009)
Article DOI: 10.1016/j.bmcl.2009.09.123
BindingDB Entry DOI: 10.7270/Q2Q52PRR |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase Lyn
(Homo sapiens (Human)) | BDBM50311920
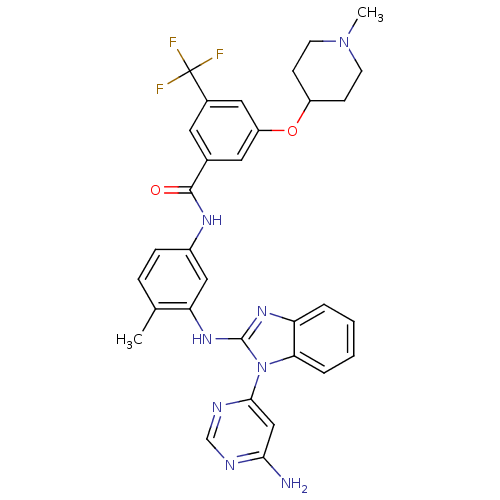
(CHEMBL1076432 | N-(3-(1-(6-aminopyrimidin-4-yl)-1H...)Show SMILES CN1CCC(CC1)Oc1cc(cc(c1)C(F)(F)F)C(=O)Nc1ccc(C)c(Nc2nc3ccccc3n2-c2cc(N)ncn2)c1 Show InChI InChI=1S/C32H31F3N8O2/c1-19-7-8-22(16-26(19)41-31-40-25-5-3-4-6-27(25)43(31)29-17-28(36)37-18-38-29)39-30(44)20-13-21(32(33,34)35)15-24(14-20)45-23-9-11-42(2)12-10-23/h3-8,13-18,23H,9-12H2,1-2H3,(H,39,44)(H,40,41)(H2,36,37,38) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
Genomics Institute of the Novartis Research Foundation (GNF)
Curated by ChEMBL
| Assay Description
Inhibition of Tel-fused LYN expressed in mouse BAF3 cells |
Bioorg Med Chem Lett 19: 6691-5 (2009)
Article DOI: 10.1016/j.bmcl.2009.09.123
BindingDB Entry DOI: 10.7270/Q2Q52PRR |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase Lyn
(Homo sapiens (Human)) | BDBM50311921

(CHEMBL1086731 | N-(3-(1-(6-aminopyrimidin-4-yl)-1H...)Show SMILES Cc1ccc(NC(=O)c2ccc(s2)C(C)(C)C)cc1Nc1nc2ccccc2n1-c1cc(N)ncn1 Show InChI InChI=1S/C27H27N7OS/c1-16-9-10-17(31-25(35)21-11-12-22(36-21)27(2,3)4)13-19(16)33-26-32-18-7-5-6-8-20(18)34(26)24-14-23(28)29-15-30-24/h5-15H,1-4H3,(H,31,35)(H,32,33)(H2,28,29,30) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
| Article
PubMed
| n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
Genomics Institute of the Novartis Research Foundation (GNF)
Curated by ChEMBL
| Assay Description
Inhibition of Tel-fused LYN expressed in mouse BAF3 cells |
Bioorg Med Chem Lett 19: 6691-5 (2009)
Article DOI: 10.1016/j.bmcl.2009.09.123
BindingDB Entry DOI: 10.7270/Q2Q52PRR |
More data for this
Ligand-Target Pair | |
Proto-oncogene tyrosine-protein kinase Src
(Homo sapiens (Human)) | BDBM50263515
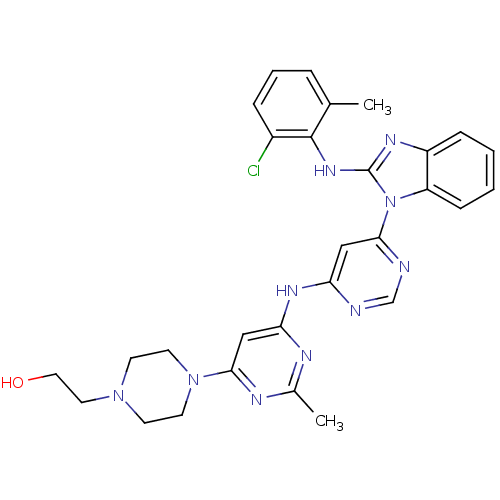
(2-(4-(6-(6-(2-(2-chloro-6-methylphenylamino)-1H-be...)Show SMILES Cc1nc(Nc2cc(ncn2)-n2c(Nc3c(C)cccc3Cl)nc3ccccc23)cc(n1)N1CCN(CCO)CC1 Show InChI InChI=1S/C29H31ClN10O/c1-19-6-5-7-21(30)28(19)37-29-35-22-8-3-4-9-23(22)40(29)26-16-24(31-18-32-26)36-25-17-27(34-20(2)33-25)39-12-10-38(11-13-39)14-15-41/h3-9,16-18,41H,10-15H2,1-2H3,(H,35,37)(H,31,32,33,34,36) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
Genomics Institute of the Novartis Research Foundation (GNF)
Curated by ChEMBL
| Assay Description
Inhibition of TEL fused Src-mediated proliferation of TEL-Src transformed mouse BA/F3 cells after 48 hrs by bright-glo luciferase assay |
Bioorg Med Chem Lett 18: 5618-21 (2008)
Article DOI: 10.1016/j.bmcl.2008.08.104
BindingDB Entry DOI: 10.7270/Q2DN44WQ |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase Lck
(Homo sapiens (Human)) | BDBM50311931
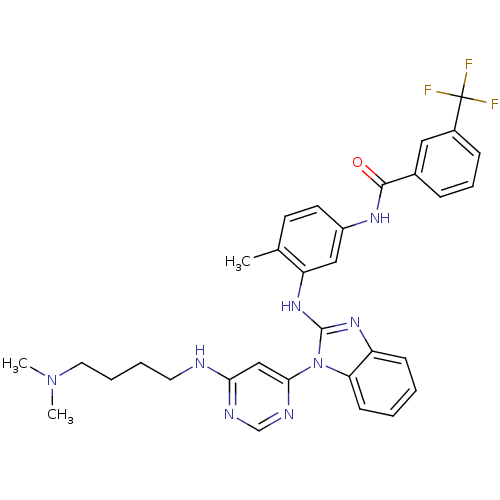
(CHEMBL1076469 | N-(3-(1-(6-(4-(dimethylamino)butyl...)Show SMILES CN(C)CCCCNc1cc(ncn1)-n1c(Nc2cc(NC(=O)c3cccc(c3)C(F)(F)F)ccc2C)nc2ccccc12 Show InChI InChI=1S/C32H33F3N8O/c1-21-13-14-24(39-30(44)22-9-8-10-23(17-22)32(33,34)35)18-26(21)41-31-40-25-11-4-5-12-27(25)43(31)29-19-28(37-20-38-29)36-15-6-7-16-42(2)3/h4-5,8-14,17-20H,6-7,15-16H2,1-3H3,(H,39,44)(H,40,41)(H,36,37,38) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
Genomics Institute of the Novartis Research Foundation (GNF)
Curated by ChEMBL
| Assay Description
Inhibition of LCK |
Bioorg Med Chem Lett 19: 6691-5 (2009)
Article DOI: 10.1016/j.bmcl.2009.09.123
BindingDB Entry DOI: 10.7270/Q2Q52PRR |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase Lyn
(Homo sapiens (Human)) | BDBM50311926

(CHEMBL1076436 | N-(3-(1-(6-aminopyrimidin-4-yl)-1H...)Show SMILES CN1CCN(CC1)c1cc(cc(c1)C(F)(F)F)C(=O)Nc1ccc(C)c(Nc2nc3ccccc3n2-c2cc(N)ncn2)c1 Show InChI InChI=1S/C31H30F3N9O/c1-19-7-8-22(38-29(44)20-13-21(31(32,33)34)15-23(14-20)42-11-9-41(2)10-12-42)16-25(19)40-30-39-24-5-3-4-6-26(24)43(30)28-17-27(35)36-18-37-28/h3-8,13-18H,9-12H2,1-2H3,(H,38,44)(H,39,40)(H2,35,36,37) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
Genomics Institute of the Novartis Research Foundation (GNF)
Curated by ChEMBL
| Assay Description
Inhibition of Tel-fused LYN expressed in mouse BAF3 cells |
Bioorg Med Chem Lett 19: 6691-5 (2009)
Article DOI: 10.1016/j.bmcl.2009.09.123
BindingDB Entry DOI: 10.7270/Q2Q52PRR |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase Lck
(Homo sapiens (Human)) | BDBM50311893
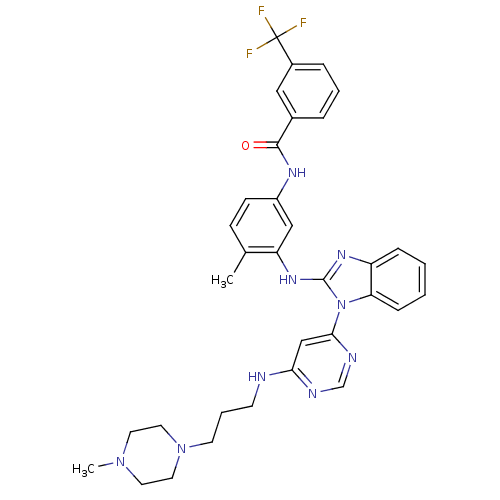
(CHEMBL1076478 | N-(4-methyl-3-(1-(6-(3-(4-methylpi...)Show SMILES CN1CCN(CCCNc2cc(ncn2)-n2c(Nc3cc(NC(=O)c4cccc(c4)C(F)(F)F)ccc3C)nc3ccccc23)CC1 Show InChI InChI=1S/C34H36F3N9O/c1-23-11-12-26(41-32(47)24-7-5-8-25(19-24)34(35,36)37)20-28(23)43-33-42-27-9-3-4-10-29(27)46(33)31-21-30(39-22-40-31)38-13-6-14-45-17-15-44(2)16-18-45/h3-5,7-12,19-22H,6,13-18H2,1-2H3,(H,41,47)(H,42,43)(H,38,39,40) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
Genomics Institute of the Novartis Research Foundation (GNF)
Curated by ChEMBL
| Assay Description
Inhibition of LCK |
Bioorg Med Chem Lett 19: 6691-5 (2009)
Article DOI: 10.1016/j.bmcl.2009.09.123
BindingDB Entry DOI: 10.7270/Q2Q52PRR |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase Lck
(Homo sapiens (Human)) | BDBM50311917
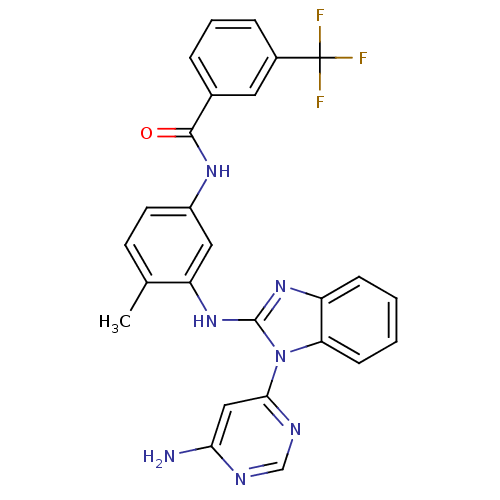
(CHEMBL1079945 | N-(3-(1-(6-aminopyrimidin-4-yl)-1H...)Show SMILES Cc1ccc(NC(=O)c2cccc(c2)C(F)(F)F)cc1Nc1nc2ccccc2n1-c1cc(N)ncn1 Show InChI InChI=1S/C26H20F3N7O/c1-15-9-10-18(33-24(37)16-5-4-6-17(11-16)26(27,28)29)12-20(15)35-25-34-19-7-2-3-8-21(19)36(25)23-13-22(30)31-14-32-23/h2-14H,1H3,(H,33,37)(H,34,35)(H2,30,31,32) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 7 | n/a | n/a | n/a | n/a | n/a | n/a |
Genomics Institute of the Novartis Research Foundation (GNF)
Curated by ChEMBL
| Assay Description
Inhibition of Tel-fused LCK expressed in mouse BAF3 cells |
Bioorg Med Chem Lett 19: 6691-5 (2009)
Article DOI: 10.1016/j.bmcl.2009.09.123
BindingDB Entry DOI: 10.7270/Q2Q52PRR |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase Lck
(Homo sapiens (Human)) | BDBM50311940
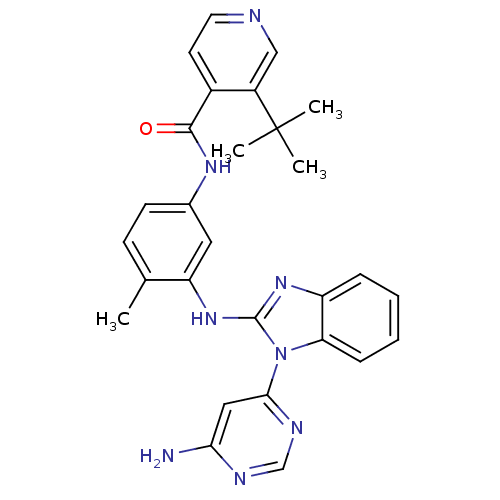
(CHEMBL1080475 | N-(3-(1-(6-aminopyrimidin-4-yl)-1H...)Show SMILES Cc1ccc(NC(=O)c2ccncc2C(C)(C)C)cc1Nc1nc2ccccc2n1-c1cc(N)ncn1 Show InChI InChI=1S/C28H28N8O/c1-17-9-10-18(33-26(37)19-11-12-30-15-20(19)28(2,3)4)13-22(17)35-27-34-21-7-5-6-8-23(21)36(27)25-14-24(29)31-16-32-25/h5-16H,1-4H3,(H,33,37)(H,34,35)(H2,29,31,32) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 7 | n/a | n/a | n/a | n/a | n/a | n/a |
Genomics Institute of the Novartis Research Foundation (GNF)
Curated by ChEMBL
| Assay Description
Inhibition of Tel-fused LCK expressed in mouse BAF3 cells |
Bioorg Med Chem Lett 19: 6691-5 (2009)
Article DOI: 10.1016/j.bmcl.2009.09.123
BindingDB Entry DOI: 10.7270/Q2Q52PRR |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase Lck
(Homo sapiens (Human)) | BDBM50311920

(CHEMBL1076432 | N-(3-(1-(6-aminopyrimidin-4-yl)-1H...)Show SMILES CN1CCC(CC1)Oc1cc(cc(c1)C(F)(F)F)C(=O)Nc1ccc(C)c(Nc2nc3ccccc3n2-c2cc(N)ncn2)c1 Show InChI InChI=1S/C32H31F3N8O2/c1-19-7-8-22(16-26(19)41-31-40-25-5-3-4-6-27(25)43(31)29-17-28(36)37-18-38-29)39-30(44)20-13-21(32(33,34)35)15-24(14-20)45-23-9-11-42(2)12-10-23/h3-8,13-18,23H,9-12H2,1-2H3,(H,39,44)(H,40,41)(H2,36,37,38) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 7 | n/a | n/a | n/a | n/a | n/a | n/a |
Genomics Institute of the Novartis Research Foundation (GNF)
Curated by ChEMBL
| Assay Description
Inhibition of Tel-fused LCK expressed in mouse BAF3 cells |
Bioorg Med Chem Lett 19: 6691-5 (2009)
Article DOI: 10.1016/j.bmcl.2009.09.123
BindingDB Entry DOI: 10.7270/Q2Q52PRR |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase Lck
(Homo sapiens (Human)) | BDBM50311897
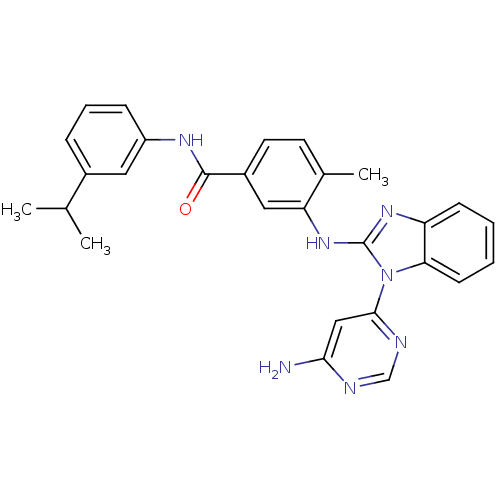
(3-(1-(6-aminopyrimidin-4-yl)-1H-benzo[d]imidazol-2...)Show SMILES CC(C)c1cccc(NC(=O)c2ccc(C)c(Nc3nc4ccccc4n3-c3cc(N)ncn3)c2)c1 Show InChI InChI=1S/C28H27N7O/c1-17(2)19-7-6-8-21(13-19)32-27(36)20-12-11-18(3)23(14-20)34-28-33-22-9-4-5-10-24(22)35(28)26-15-25(29)30-16-31-26/h4-17H,1-3H3,(H,32,36)(H,33,34)(H2,29,30,31) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 7 | n/a | n/a | n/a | n/a | n/a | n/a |
Genomics Institute of the Novartis Research Foundation (GNF)
Curated by ChEMBL
| Assay Description
Inhibition of Tel-fused LCK expressed in mouse BAF3 cells |
Bioorg Med Chem Lett 19: 6691-5 (2009)
Article DOI: 10.1016/j.bmcl.2009.09.123
BindingDB Entry DOI: 10.7270/Q2Q52PRR |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase Lyn
(Homo sapiens (Human)) | BDBM50311940

(CHEMBL1080475 | N-(3-(1-(6-aminopyrimidin-4-yl)-1H...)Show SMILES Cc1ccc(NC(=O)c2ccncc2C(C)(C)C)cc1Nc1nc2ccccc2n1-c1cc(N)ncn1 Show InChI InChI=1S/C28H28N8O/c1-17-9-10-18(33-26(37)19-11-12-30-15-20(19)28(2,3)4)13-22(17)35-27-34-21-7-5-6-8-23(21)36(27)25-14-24(29)31-16-32-25/h5-16H,1-4H3,(H,33,37)(H,34,35)(H2,29,31,32) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 7 | n/a | n/a | n/a | n/a | n/a | n/a |
Genomics Institute of the Novartis Research Foundation (GNF)
Curated by ChEMBL
| Assay Description
Inhibition of Tel-fused LYN expressed in mouse BAF3 cells |
Bioorg Med Chem Lett 19: 6691-5 (2009)
Article DOI: 10.1016/j.bmcl.2009.09.123
BindingDB Entry DOI: 10.7270/Q2Q52PRR |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase Lck
(Homo sapiens (Human)) | BDBM50263515

(2-(4-(6-(6-(2-(2-chloro-6-methylphenylamino)-1H-be...)Show SMILES Cc1nc(Nc2cc(ncn2)-n2c(Nc3c(C)cccc3Cl)nc3ccccc23)cc(n1)N1CCN(CCO)CC1 Show InChI InChI=1S/C29H31ClN10O/c1-19-6-5-7-21(30)28(19)37-29-35-22-8-3-4-9-23(22)40(29)26-16-24(31-18-32-26)36-25-17-27(34-20(2)33-25)39-12-10-38(11-13-39)14-15-41/h3-9,16-18,41H,10-15H2,1-2H3,(H,35,37)(H,31,32,33,34,36) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 7 | n/a | n/a | n/a | n/a | n/a | n/a |
Genomics Institute of the Novartis Research Foundation (GNF)
Curated by ChEMBL
| Assay Description
Inhibition of TEL fused Lck (unknown origin)-mediated proliferation of TEL-Lck transformed mouse BA/F3 cells after 48 hrs by bright-glo luciferase as... |
Bioorg Med Chem Lett 18: 5618-21 (2008)
Article DOI: 10.1016/j.bmcl.2008.08.104
BindingDB Entry DOI: 10.7270/Q2DN44WQ |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase Lck
(Homo sapiens (Human)) | BDBM50311925
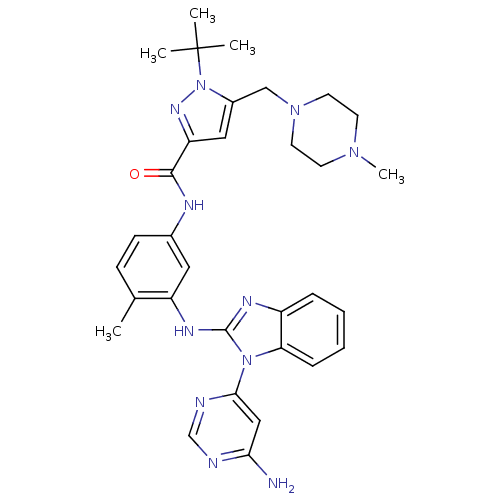
(CHEMBL1075628 | N-(3-(1-(6-aminopyrimidin-4-yl)-1H...)Show SMILES CN1CCN(Cc2cc(nn2C(C)(C)C)C(=O)Nc2ccc(C)c(Nc3nc4ccccc4n3-c3cc(N)ncn3)c2)CC1 Show InChI InChI=1S/C32H39N11O/c1-21-10-11-22(36-30(44)26-17-23(43(39-26)32(2,3)4)19-41-14-12-40(5)13-15-41)16-25(21)38-31-37-24-8-6-7-9-27(24)42(31)29-18-28(33)34-20-35-29/h6-11,16-18,20H,12-15,19H2,1-5H3,(H,36,44)(H,37,38)(H2,33,34,35) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 8 | n/a | n/a | n/a | n/a | n/a | n/a |
Genomics Institute of the Novartis Research Foundation (GNF)
Curated by ChEMBL
| Assay Description
Inhibition of LCK |
Bioorg Med Chem Lett 19: 6691-5 (2009)
Article DOI: 10.1016/j.bmcl.2009.09.123
BindingDB Entry DOI: 10.7270/Q2Q52PRR |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase HCK
(Homo sapiens (Human)) | BDBM50311924

(CHEMBL1076433 | N-(3-(1-(6-aminopyrimidin-4-yl)-1H...)Show SMILES CCN1CCN(Cc2ccc(cc2Cl)C(=O)Nc2ccc(C)c(Nc3nc4ccccc4n3-c3cc(N)ncn3)c2)CC1 Show InChI InChI=1S/C32H34ClN9O/c1-3-40-12-14-41(15-13-40)19-23-10-9-22(16-25(23)33)31(43)37-24-11-8-21(2)27(17-24)39-32-38-26-6-4-5-7-28(26)42(32)30-18-29(34)35-20-36-30/h4-11,16-18,20H,3,12-15,19H2,1-2H3,(H,37,43)(H,38,39)(H2,34,35,36) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 9 | n/a | n/a | n/a | n/a | n/a | n/a |
Genomics Institute of the Novartis Research Foundation (GNF)
Curated by ChEMBL
| Assay Description
Inhibition of HCK |
Bioorg Med Chem Lett 19: 6691-5 (2009)
Article DOI: 10.1016/j.bmcl.2009.09.123
BindingDB Entry DOI: 10.7270/Q2Q52PRR |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase Lyn
(Homo sapiens (Human)) | BDBM50311927

(CHEMBL1076437 | N-(3-(1-(6-aminopyrimidin-4-yl)-1H...)Show SMILES CCN1CCN(CC1)c1cc(cc(c1)C(F)(F)F)C(=O)Nc1ccc(C)c(Nc2nc3ccccc3n2-c2cc(N)ncn2)c1 Show InChI InChI=1S/C32H32F3N9O/c1-3-42-10-12-43(13-11-42)24-15-21(14-22(16-24)32(33,34)35)30(45)39-23-9-8-20(2)26(17-23)41-31-40-25-6-4-5-7-27(25)44(31)29-18-28(36)37-19-38-29/h4-9,14-19H,3,10-13H2,1-2H3,(H,39,45)(H,40,41)(H2,36,37,38) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 9 | n/a | n/a | n/a | n/a | n/a | n/a |
Genomics Institute of the Novartis Research Foundation (GNF)
Curated by ChEMBL
| Assay Description
Inhibition of Tel-fused LYN expressed in mouse BAF3 cells |
Bioorg Med Chem Lett 19: 6691-5 (2009)
Article DOI: 10.1016/j.bmcl.2009.09.123
BindingDB Entry DOI: 10.7270/Q2Q52PRR |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase Lyn
(Homo sapiens (Human)) | BDBM50311924

(CHEMBL1076433 | N-(3-(1-(6-aminopyrimidin-4-yl)-1H...)Show SMILES CCN1CCN(Cc2ccc(cc2Cl)C(=O)Nc2ccc(C)c(Nc3nc4ccccc4n3-c3cc(N)ncn3)c2)CC1 Show InChI InChI=1S/C32H34ClN9O/c1-3-40-12-14-41(15-13-40)19-23-10-9-22(16-25(23)33)31(43)37-24-11-8-21(2)27(17-24)39-32-38-26-6-4-5-7-28(26)42(32)30-18-29(34)35-20-36-30/h4-11,16-18,20H,3,12-15,19H2,1-2H3,(H,37,43)(H,38,39)(H2,34,35,36) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
Genomics Institute of the Novartis Research Foundation (GNF)
Curated by ChEMBL
| Assay Description
Inhibition of Tel-fused LYN expressed in mouse BAF3 cells |
Bioorg Med Chem Lett 19: 6691-5 (2009)
Article DOI: 10.1016/j.bmcl.2009.09.123
BindingDB Entry DOI: 10.7270/Q2Q52PRR |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase Lck
(Homo sapiens (Human)) | BDBM50311940

(CHEMBL1080475 | N-(3-(1-(6-aminopyrimidin-4-yl)-1H...)Show SMILES Cc1ccc(NC(=O)c2ccncc2C(C)(C)C)cc1Nc1nc2ccccc2n1-c1cc(N)ncn1 Show InChI InChI=1S/C28H28N8O/c1-17-9-10-18(33-26(37)19-11-12-30-15-20(19)28(2,3)4)13-22(17)35-27-34-21-7-5-6-8-23(21)36(27)25-14-24(29)31-16-32-25/h5-16H,1-4H3,(H,33,37)(H,34,35)(H2,29,31,32) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
Genomics Institute of the Novartis Research Foundation (GNF)
Curated by ChEMBL
| Assay Description
Inhibition of LCK |
Bioorg Med Chem Lett 19: 6691-5 (2009)
Article DOI: 10.1016/j.bmcl.2009.09.123
BindingDB Entry DOI: 10.7270/Q2Q52PRR |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase HCK
(Homo sapiens (Human)) | BDBM50311925

(CHEMBL1075628 | N-(3-(1-(6-aminopyrimidin-4-yl)-1H...)Show SMILES CN1CCN(Cc2cc(nn2C(C)(C)C)C(=O)Nc2ccc(C)c(Nc3nc4ccccc4n3-c3cc(N)ncn3)c2)CC1 Show InChI InChI=1S/C32H39N11O/c1-21-10-11-22(36-30(44)26-17-23(43(39-26)32(2,3)4)19-41-14-12-40(5)13-15-41)16-25(21)38-31-37-24-8-6-7-9-27(24)42(31)29-18-28(33)34-20-35-29/h6-11,16-18,20H,12-15,19H2,1-5H3,(H,36,44)(H,37,38)(H2,33,34,35) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 11 | n/a | n/a | n/a | n/a | n/a | n/a |
Genomics Institute of the Novartis Research Foundation (GNF)
Curated by ChEMBL
| Assay Description
Inhibition of HCK |
Bioorg Med Chem Lett 19: 6691-5 (2009)
Article DOI: 10.1016/j.bmcl.2009.09.123
BindingDB Entry DOI: 10.7270/Q2Q52PRR |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase Lck
(Homo sapiens (Human)) | BDBM50311911

(4-methyl-3-(1-(6-(2-(4-methylpiperazin-1-yl)ethyla...)Show SMILES CN1CCN(CCNc2cc(ncn2)-n2c(Nc3cc(ccc3C)C(=O)Nc3cccc(c3)C(F)(F)F)nc3ccccc23)CC1 Show InChI InChI=1S/C33H34F3N9O/c1-22-10-11-23(31(46)40-25-7-5-6-24(19-25)33(34,35)36)18-27(22)42-32-41-26-8-3-4-9-28(26)45(32)30-20-29(38-21-39-30)37-12-13-44-16-14-43(2)15-17-44/h3-11,18-21H,12-17H2,1-2H3,(H,40,46)(H,41,42)(H,37,38,39) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 11 | n/a | n/a | n/a | n/a | n/a | n/a |
Genomics Institute of the Novartis Research Foundation (GNF)
Curated by ChEMBL
| Assay Description
Inhibition of LCK |
Bioorg Med Chem Lett 19: 6691-5 (2009)
Article DOI: 10.1016/j.bmcl.2009.09.123
BindingDB Entry DOI: 10.7270/Q2Q52PRR |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase Lck
(Homo sapiens (Human)) | BDBM50311917

(CHEMBL1079945 | N-(3-(1-(6-aminopyrimidin-4-yl)-1H...)Show SMILES Cc1ccc(NC(=O)c2cccc(c2)C(F)(F)F)cc1Nc1nc2ccccc2n1-c1cc(N)ncn1 Show InChI InChI=1S/C26H20F3N7O/c1-15-9-10-18(33-24(37)16-5-4-6-17(11-16)26(27,28)29)12-20(15)35-25-34-19-7-2-3-8-21(19)36(25)23-13-22(30)31-14-32-23/h2-14H,1H3,(H,33,37)(H,34,35)(H2,30,31,32) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 11 | n/a | n/a | n/a | n/a | n/a | n/a |
Genomics Institute of the Novartis Research Foundation (GNF)
Curated by ChEMBL
| Assay Description
Inhibition of LCK |
Bioorg Med Chem Lett 19: 6691-5 (2009)
Article DOI: 10.1016/j.bmcl.2009.09.123
BindingDB Entry DOI: 10.7270/Q2Q52PRR |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase Lck
(Homo sapiens (Human)) | BDBM50311926

(CHEMBL1076436 | N-(3-(1-(6-aminopyrimidin-4-yl)-1H...)Show SMILES CN1CCN(CC1)c1cc(cc(c1)C(F)(F)F)C(=O)Nc1ccc(C)c(Nc2nc3ccccc3n2-c2cc(N)ncn2)c1 Show InChI InChI=1S/C31H30F3N9O/c1-19-7-8-22(38-29(44)20-13-21(31(32,33)34)15-23(14-20)42-11-9-41(2)10-12-42)16-25(19)40-30-39-24-5-3-4-6-26(24)43(30)28-17-27(35)36-18-37-28/h3-8,13-18H,9-12H2,1-2H3,(H,38,44)(H,39,40)(H2,35,36,37) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 11 | n/a | n/a | n/a | n/a | n/a | n/a |
Genomics Institute of the Novartis Research Foundation (GNF)
Curated by ChEMBL
| Assay Description
Inhibition of LCK |
Bioorg Med Chem Lett 19: 6691-5 (2009)
Article DOI: 10.1016/j.bmcl.2009.09.123
BindingDB Entry DOI: 10.7270/Q2Q52PRR |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase Lck
(Homo sapiens (Human)) | BDBM50311927

(CHEMBL1076437 | N-(3-(1-(6-aminopyrimidin-4-yl)-1H...)Show SMILES CCN1CCN(CC1)c1cc(cc(c1)C(F)(F)F)C(=O)Nc1ccc(C)c(Nc2nc3ccccc3n2-c2cc(N)ncn2)c1 Show InChI InChI=1S/C32H32F3N9O/c1-3-42-10-12-43(13-11-42)24-15-21(14-22(16-24)32(33,34)35)30(45)39-23-9-8-20(2)26(17-23)41-31-40-25-6-4-5-7-27(25)44(31)29-18-28(36)37-19-38-29/h4-9,14-19H,3,10-13H2,1-2H3,(H,39,45)(H,40,41)(H2,36,37,38) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 11 | n/a | n/a | n/a | n/a | n/a | n/a |
Genomics Institute of the Novartis Research Foundation (GNF)
Curated by ChEMBL
| Assay Description
Inhibition of LCK |
Bioorg Med Chem Lett 19: 6691-5 (2009)
Article DOI: 10.1016/j.bmcl.2009.09.123
BindingDB Entry DOI: 10.7270/Q2Q52PRR |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase Lck
(Homo sapiens (Human)) | BDBM50311915
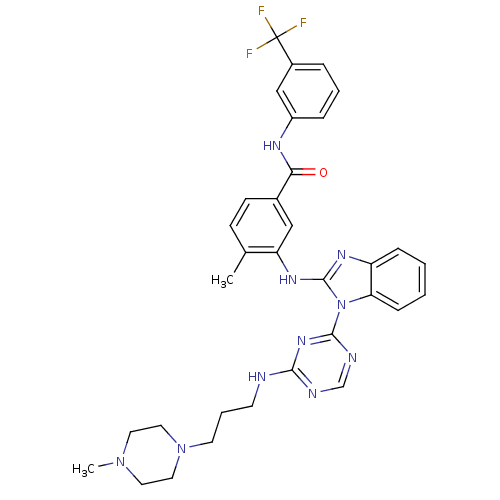
(4-methyl-3-(1-(4-(3-(4-methylpiperazin-1-yl)propyl...)Show SMILES CN1CCN(CCCNc2ncnc(n2)-n2c(Nc3cc(ccc3C)C(=O)Nc3cccc(c3)C(F)(F)F)nc3ccccc23)CC1 Show InChI InChI=1S/C33H35F3N10O/c1-22-11-12-23(29(47)40-25-8-5-7-24(20-25)33(34,35)36)19-27(22)42-32-41-26-9-3-4-10-28(26)46(32)31-39-21-38-30(43-31)37-13-6-14-45-17-15-44(2)16-18-45/h3-5,7-12,19-21H,6,13-18H2,1-2H3,(H,40,47)(H,41,42)(H,37,38,39,43) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 12 | n/a | n/a | n/a | n/a | n/a | n/a |
Genomics Institute of the Novartis Research Foundation (GNF)
Curated by ChEMBL
| Assay Description
Inhibition of LCK |
Bioorg Med Chem Lett 19: 6691-5 (2009)
Article DOI: 10.1016/j.bmcl.2009.09.123
BindingDB Entry DOI: 10.7270/Q2Q52PRR |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase Lck
(Homo sapiens (Human)) | BDBM50311932
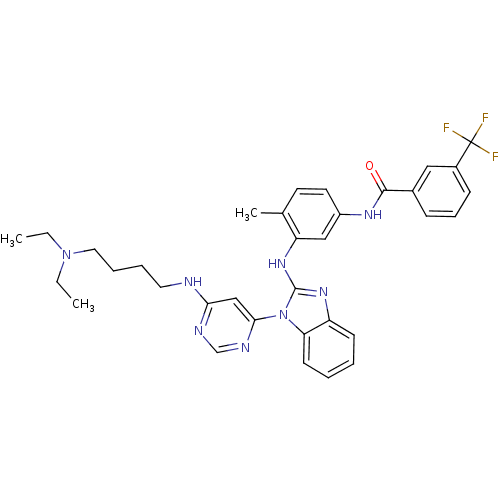
(CHEMBL1076470 | N-(3-(1-(6-(4-(diethylamino)butyla...)Show SMILES CCN(CC)CCCCNc1cc(ncn1)-n1c(Nc2cc(NC(=O)c3cccc(c3)C(F)(F)F)ccc2C)nc2ccccc12 Show InChI InChI=1S/C34H37F3N8O/c1-4-44(5-2)18-9-8-17-38-30-21-31(40-22-39-30)45-29-14-7-6-13-27(29)42-33(45)43-28-20-26(16-15-23(28)3)41-32(46)24-11-10-12-25(19-24)34(35,36)37/h6-7,10-16,19-22H,4-5,8-9,17-18H2,1-3H3,(H,41,46)(H,42,43)(H,38,39,40) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 12 | n/a | n/a | n/a | n/a | n/a | n/a |
Genomics Institute of the Novartis Research Foundation (GNF)
Curated by ChEMBL
| Assay Description
Inhibition of LCK |
Bioorg Med Chem Lett 19: 6691-5 (2009)
Article DOI: 10.1016/j.bmcl.2009.09.123
BindingDB Entry DOI: 10.7270/Q2Q52PRR |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase Lck
(Homo sapiens (Human)) | BDBM50263516
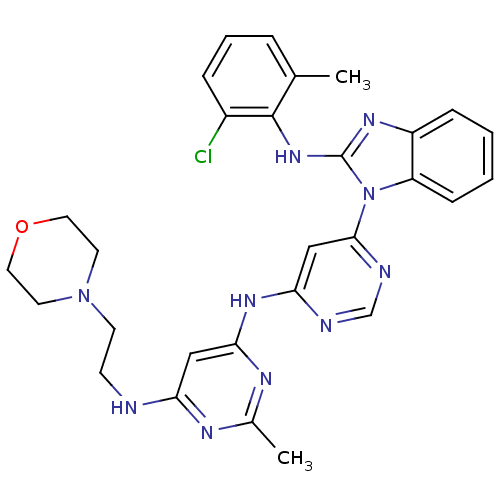
(CHEMBL450092 | N4-(6-(2-(2-chloro-6-methylphenylam...)Show SMILES Cc1nc(NCCN2CCOCC2)cc(Nc2cc(ncn2)-n2c(Nc3c(C)cccc3Cl)nc3ccccc23)n1 Show InChI InChI=1S/C29H31ClN10O/c1-19-6-5-7-21(30)28(19)38-29-36-22-8-3-4-9-23(22)40(29)27-17-25(32-18-33-27)37-26-16-24(34-20(2)35-26)31-10-11-39-12-14-41-15-13-39/h3-9,16-18H,10-15H2,1-2H3,(H,36,38)(H2,31,32,33,34,35,37) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 13 | n/a | n/a | n/a | n/a | n/a | n/a |
Genomics Institute of the Novartis Research Foundation (GNF)
Curated by ChEMBL
| Assay Description
Inhibition of TEL fused Lck (unknown origin)-mediated proliferation of TEL-Lck transformed mouse BA/F3 cells after 48 hrs by bright-glo luciferase as... |
Bioorg Med Chem Lett 18: 5618-21 (2008)
Article DOI: 10.1016/j.bmcl.2008.08.104
BindingDB Entry DOI: 10.7270/Q2DN44WQ |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase Lck
(Homo sapiens (Human)) | BDBM50263516

(CHEMBL450092 | N4-(6-(2-(2-chloro-6-methylphenylam...)Show SMILES Cc1nc(NCCN2CCOCC2)cc(Nc2cc(ncn2)-n2c(Nc3c(C)cccc3Cl)nc3ccccc23)n1 Show InChI InChI=1S/C29H31ClN10O/c1-19-6-5-7-21(30)28(19)38-29-36-22-8-3-4-9-23(22)40(29)27-17-25(32-18-33-27)37-26-16-24(34-20(2)35-26)31-10-11-39-12-14-41-15-13-39/h3-9,16-18H,10-15H2,1-2H3,(H,36,38)(H2,31,32,33,34,35,37) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 13 | n/a | n/a | n/a | n/a | n/a | n/a |
Genomics Institute of the Novartis Research Foundation (GNF)
Curated by ChEMBL
| Assay Description
Inhibition of TEL fused Lck (unknown origin)-mediated proliferation of TEL-Lck transformed mouse BA/F3 cells after 48 hrs by bright-glo luciferase as... |
Bioorg Med Chem Lett 18: 5618-21 (2008)
Article DOI: 10.1016/j.bmcl.2008.08.104
BindingDB Entry DOI: 10.7270/Q2DN44WQ |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase Lck
(Homo sapiens (Human)) | BDBM50311944
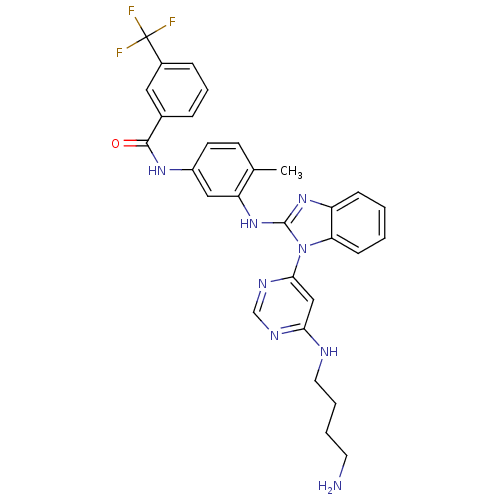
(CHEMBL1076466 | N-(3-(1-(6-(4-aminobutylamino)pyri...)Show SMILES Cc1ccc(NC(=O)c2cccc(c2)C(F)(F)F)cc1Nc1nc2ccccc2n1-c1cc(NCCCCN)ncn1 Show InChI InChI=1S/C30H29F3N8O/c1-19-11-12-22(38-28(42)20-7-6-8-21(15-20)30(31,32)33)16-24(19)40-29-39-23-9-2-3-10-25(23)41(29)27-17-26(36-18-37-27)35-14-5-4-13-34/h2-3,6-12,15-18H,4-5,13-14,34H2,1H3,(H,38,42)(H,39,40)(H,35,36,37) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 13 | n/a | n/a | n/a | n/a | n/a | n/a |
Genomics Institute of the Novartis Research Foundation (GNF)
Curated by ChEMBL
| Assay Description
Inhibition of LCK |
Bioorg Med Chem Lett 19: 6691-5 (2009)
Article DOI: 10.1016/j.bmcl.2009.09.123
BindingDB Entry DOI: 10.7270/Q2Q52PRR |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase Lyn
(Homo sapiens (Human)) | BDBM50311917

(CHEMBL1079945 | N-(3-(1-(6-aminopyrimidin-4-yl)-1H...)Show SMILES Cc1ccc(NC(=O)c2cccc(c2)C(F)(F)F)cc1Nc1nc2ccccc2n1-c1cc(N)ncn1 Show InChI InChI=1S/C26H20F3N7O/c1-15-9-10-18(33-24(37)16-5-4-6-17(11-16)26(27,28)29)12-20(15)35-25-34-19-7-2-3-8-21(19)36(25)23-13-22(30)31-14-32-23/h2-14H,1H3,(H,33,37)(H,34,35)(H2,30,31,32) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 14 | n/a | n/a | n/a | n/a | n/a | n/a |
Genomics Institute of the Novartis Research Foundation (GNF)
Curated by ChEMBL
| Assay Description
Inhibition of Tel-fused LYN expressed in mouse BAF3 cells |
Bioorg Med Chem Lett 19: 6691-5 (2009)
Article DOI: 10.1016/j.bmcl.2009.09.123
BindingDB Entry DOI: 10.7270/Q2Q52PRR |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase Lck
(Homo sapiens (Human)) | BDBM50311889
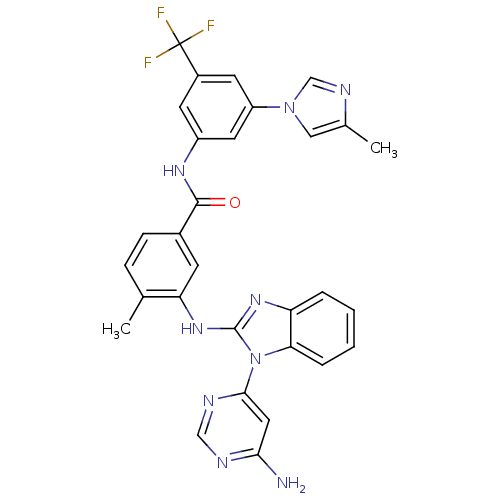
(3-(1-(6-aminopyrimidin-4-yl)-1H-benzo[d]imidazol-2...)Show SMILES Cc1cn(cn1)-c1cc(NC(=O)c2ccc(C)c(Nc3nc4ccccc4n3-c3cc(N)ncn3)c2)cc(c1)C(F)(F)F Show InChI InChI=1S/C30H24F3N9O/c1-17-7-8-19(28(43)38-21-10-20(30(31,32)33)11-22(12-21)41-14-18(2)37-16-41)9-24(17)40-29-39-23-5-3-4-6-25(23)42(29)27-13-26(34)35-15-36-27/h3-16H,1-2H3,(H,38,43)(H,39,40)(H2,34,35,36) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 14 | n/a | n/a | n/a | n/a | n/a | n/a |
Genomics Institute of the Novartis Research Foundation (GNF)
Curated by ChEMBL
| Assay Description
Inhibition of Tel-fused LCK expressed in mouse BAF3 cells |
Bioorg Med Chem Lett 19: 6691-5 (2009)
Article DOI: 10.1016/j.bmcl.2009.09.123
BindingDB Entry DOI: 10.7270/Q2Q52PRR |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase Lck
(Homo sapiens (Human)) | BDBM50263513

(CHEMBL477172 | N-(2-chloro-6-methylphenyl)-1-(6-(4...)Show SMILES Cc1cccc(Cl)c1Nc1nc2ccccc2n1-c1cc(Nc2cc(CN3CCOCC3)ccn2)ncn1 Show InChI InChI=1S/C28H27ClN8O/c1-19-5-4-6-21(29)27(19)35-28-33-22-7-2-3-8-23(22)37(28)26-16-25(31-18-32-26)34-24-15-20(9-10-30-24)17-36-11-13-38-14-12-36/h2-10,15-16,18H,11-14,17H2,1H3,(H,33,35)(H,30,31,32,34) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 14 | n/a | n/a | n/a | n/a | n/a | n/a |
Genomics Institute of the Novartis Research Foundation (GNF)
Curated by ChEMBL
| Assay Description
Inhibition of Lck by lance assay |
Bioorg Med Chem Lett 18: 5618-21 (2008)
Article DOI: 10.1016/j.bmcl.2008.08.104
BindingDB Entry DOI: 10.7270/Q2DN44WQ |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase Lyn
(Homo sapiens (Human)) | BDBM50311897

(3-(1-(6-aminopyrimidin-4-yl)-1H-benzo[d]imidazol-2...)Show SMILES CC(C)c1cccc(NC(=O)c2ccc(C)c(Nc3nc4ccccc4n3-c3cc(N)ncn3)c2)c1 Show InChI InChI=1S/C28H27N7O/c1-17(2)19-7-6-8-21(13-19)32-27(36)20-12-11-18(3)23(14-20)34-28-33-22-9-4-5-10-24(22)35(28)26-15-25(29)30-16-31-26/h4-17H,1-3H3,(H,32,36)(H,33,34)(H2,29,30,31) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 15 | n/a | n/a | n/a | n/a | n/a | n/a |
Genomics Institute of the Novartis Research Foundation (GNF)
Curated by ChEMBL
| Assay Description
Inhibition of Tel-fused LYN expressed in mouse BAF3 cells |
Bioorg Med Chem Lett 19: 6691-5 (2009)
Article DOI: 10.1016/j.bmcl.2009.09.123
BindingDB Entry DOI: 10.7270/Q2Q52PRR |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase Lyn
(Homo sapiens (Human)) | BDBM50311901
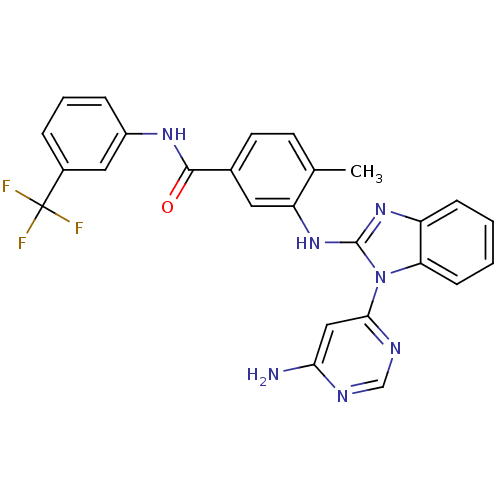
(3-(1-(6-aminopyrimidin-4-yl)-1H-benzo[d]imidazol-2...)Show SMILES Cc1ccc(cc1Nc1nc2ccccc2n1-c1cc(N)ncn1)C(=O)Nc1cccc(c1)C(F)(F)F Show InChI InChI=1S/C26H20F3N7O/c1-15-9-10-16(24(37)33-18-6-4-5-17(12-18)26(27,28)29)11-20(15)35-25-34-19-7-2-3-8-21(19)36(25)23-13-22(30)31-14-32-23/h2-14H,1H3,(H,33,37)(H,34,35)(H2,30,31,32) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 15 | n/a | n/a | n/a | n/a | n/a | n/a |
Genomics Institute of the Novartis Research Foundation (GNF)
Curated by ChEMBL
| Assay Description
Inhibition of Tel-fused LYN expressed in mouse BAF3 cells |
Bioorg Med Chem Lett 19: 6691-5 (2009)
Article DOI: 10.1016/j.bmcl.2009.09.123
BindingDB Entry DOI: 10.7270/Q2Q52PRR |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase Lck
(Homo sapiens (Human)) | BDBM50311909
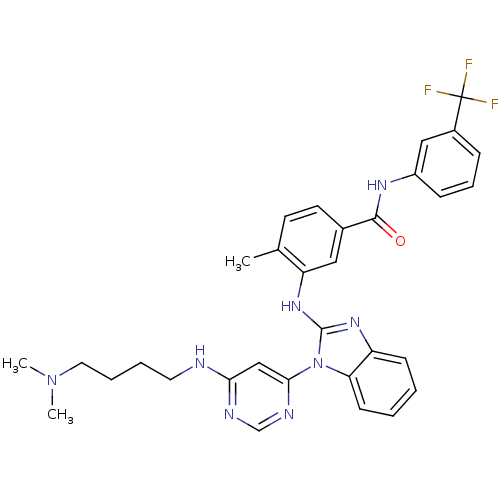
(3-(1-(6-(4-(dimethylamino)butylamino)pyrimidin-4-y...)Show SMILES CN(C)CCCCNc1cc(ncn1)-n1c(Nc2cc(ccc2C)C(=O)Nc2cccc(c2)C(F)(F)F)nc2ccccc12 Show InChI InChI=1S/C32H33F3N8O/c1-21-13-14-22(30(44)39-24-10-8-9-23(18-24)32(33,34)35)17-26(21)41-31-40-25-11-4-5-12-27(25)43(31)29-19-28(37-20-38-29)36-15-6-7-16-42(2)3/h4-5,8-14,17-20H,6-7,15-16H2,1-3H3,(H,39,44)(H,40,41)(H,36,37,38) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 15 | n/a | n/a | n/a | n/a | n/a | n/a |
Genomics Institute of the Novartis Research Foundation (GNF)
Curated by ChEMBL
| Assay Description
Inhibition of Tel-fused LCK expressed in mouse BAF3 cells |
Bioorg Med Chem Lett 19: 6691-5 (2009)
Article DOI: 10.1016/j.bmcl.2009.09.123
BindingDB Entry DOI: 10.7270/Q2Q52PRR |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase HCK
(Homo sapiens (Human)) | BDBM50311927

(CHEMBL1076437 | N-(3-(1-(6-aminopyrimidin-4-yl)-1H...)Show SMILES CCN1CCN(CC1)c1cc(cc(c1)C(F)(F)F)C(=O)Nc1ccc(C)c(Nc2nc3ccccc3n2-c2cc(N)ncn2)c1 Show InChI InChI=1S/C32H32F3N9O/c1-3-42-10-12-43(13-11-42)24-15-21(14-22(16-24)32(33,34)35)30(45)39-23-9-8-20(2)26(17-23)41-31-40-25-6-4-5-7-27(25)44(31)29-18-28(36)37-19-38-29/h4-9,14-19H,3,10-13H2,1-2H3,(H,39,45)(H,40,41)(H2,36,37,38) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 16 | n/a | n/a | n/a | n/a | n/a | n/a |
Genomics Institute of the Novartis Research Foundation (GNF)
Curated by ChEMBL
| Assay Description
Inhibition of HCK |
Bioorg Med Chem Lett 19: 6691-5 (2009)
Article DOI: 10.1016/j.bmcl.2009.09.123
BindingDB Entry DOI: 10.7270/Q2Q52PRR |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase Lyn
(Homo sapiens (Human)) | BDBM50311898
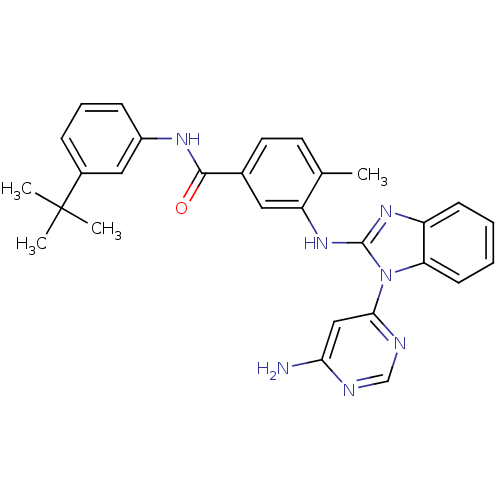
(3-(1-(6-aminopyrimidin-4-yl)-1H-benzo[d]imidazol-2...)Show SMILES Cc1ccc(cc1Nc1nc2ccccc2n1-c1cc(N)ncn1)C(=O)Nc1cccc(c1)C(C)(C)C Show InChI InChI=1S/C29H29N7O/c1-18-12-13-19(27(37)33-21-9-7-8-20(15-21)29(2,3)4)14-23(18)35-28-34-22-10-5-6-11-24(22)36(28)26-16-25(30)31-17-32-26/h5-17H,1-4H3,(H,33,37)(H,34,35)(H2,30,31,32) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 16 | n/a | n/a | n/a | n/a | n/a | n/a |
Genomics Institute of the Novartis Research Foundation (GNF)
Curated by ChEMBL
| Assay Description
Inhibition of Tel-fused LYN expressed in mouse BAF3 cells |
Bioorg Med Chem Lett 19: 6691-5 (2009)
Article DOI: 10.1016/j.bmcl.2009.09.123
BindingDB Entry DOI: 10.7270/Q2Q52PRR |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data