

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 5-hydroxytryptamine receptor 1A (Rattus norvegicus (rat)) | BDBM50020178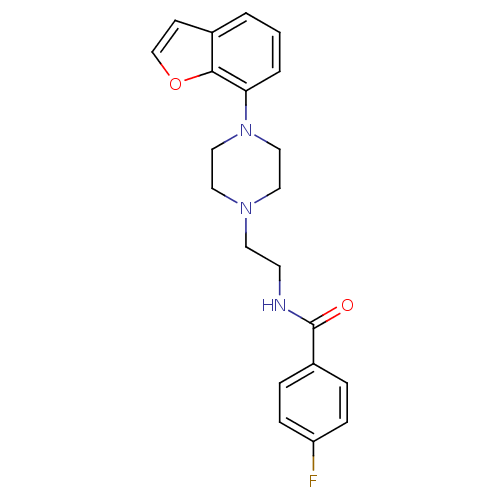 (CHEMBL294633 | CHEMBL555633 | N-[2-(4-Benzofuran-7...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 0.150 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Solvay Pharma Curated by ChEMBL | Assay Description Binding affinity was evaluated by determining in vitro displacement of [3H]-8-OH-DPAT from the central 5-hydroxytryptamine 1A receptor recognition si... | J Med Chem 40: 300-12 (1997) Article DOI: 10.1021/jm960496o BindingDB Entry DOI: 10.7270/Q27W6B96 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1A (Rattus norvegicus (rat)) | BDBM50039824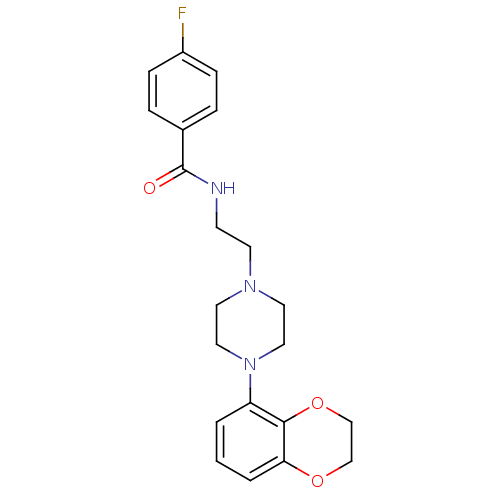 (CHEMBL81728 | N-{2-[4-(2,3-Dihydro-benzo[1,4]dioxi...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Solvay Pharma Curated by ChEMBL | Assay Description Binding affinity was evaluated by determining in vitro displacement of [3H]-8-OH-DPAT from the central 5-hydroxytryptamine 1A receptor recognition si... | J Med Chem 40: 300-12 (1997) Article DOI: 10.1021/jm960496o BindingDB Entry DOI: 10.7270/Q27W6B96 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1A (Rattus norvegicus (rat)) | BDBM50056040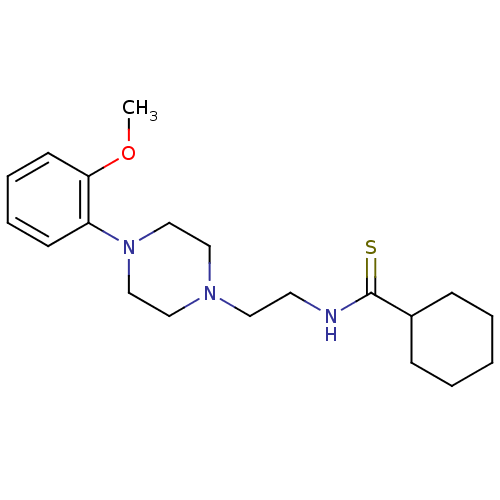 (CHEMBL107141 | Cyclohexanecarbothioic acid {2-[4-(...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | 0.320 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Solvay Pharma Curated by ChEMBL | Assay Description Binding affinity was evaluated by determining in vitro displacement of [3H]-8-OH-DPAT from the central 5-hydroxytryptamine 1A receptor recognition si... | J Med Chem 40: 300-12 (1997) Article DOI: 10.1021/jm960496o BindingDB Entry DOI: 10.7270/Q27W6B96 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Homo sapiens (Human)) | BDBM50150945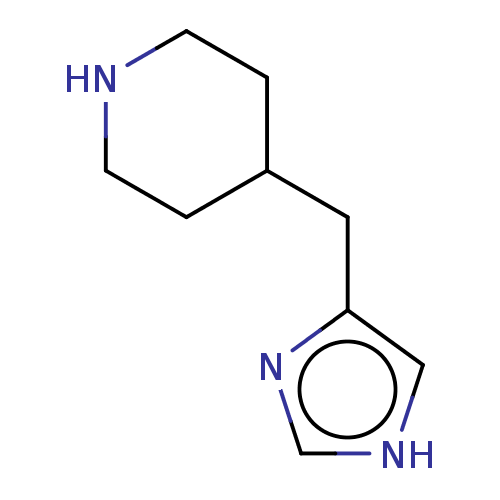 (CHEBI:81390 | Immepip) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase KEGG PC cid PC sid PDB UniChem Similars | Article PubMed | 0.479 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vrije Universiteit Curated by ChEMBL | Assay Description Inhibitory activity measured by [3H]- N alpha- methyl-histamine binding to membranes of SK-N-MC cells expressing the human Histamine H3 receptor | J Med Chem 46: 5445-57 (2003) Article DOI: 10.1021/jm030905y BindingDB Entry DOI: 10.7270/Q2FN18X0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1A (Rattus norvegicus (rat)) | BDBM50056034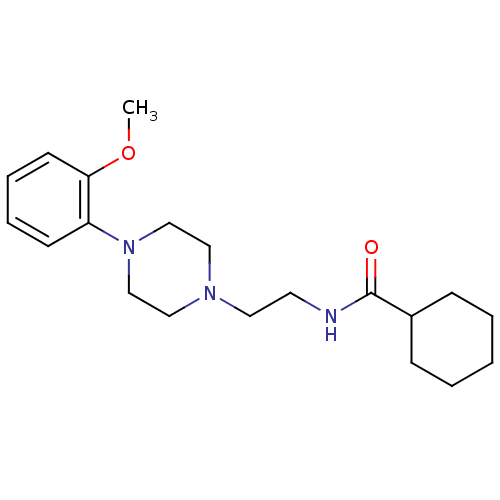 (CHEMBL108229 | Cyclohexanecarboxylic acid {2-[4-(2...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.800 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Solvay Pharma Curated by ChEMBL | Assay Description Binding affinity was evaluated by determining in vitro displacement of [3H]-8-OH-DPAT from the central 5-hydroxytryptamine 1A receptor recognition si... | J Med Chem 40: 300-12 (1997) Article DOI: 10.1021/jm960496o BindingDB Entry DOI: 10.7270/Q27W6B96 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1A (Rattus norvegicus (rat)) | BDBM50019959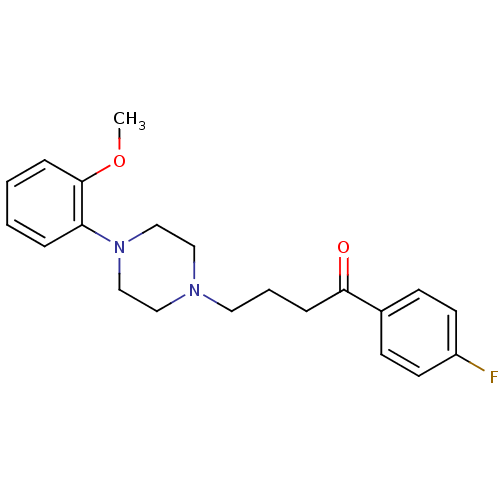 (1-(4-Fluoro-phenyl)-4-[4-(2-methoxy-phenyl)-pipera...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Solvay Pharma Curated by ChEMBL | Assay Description Binding affinity was evaluated by determining in vitro displacement of [3H]-8-OH-DPAT from the central 5-hydroxytryptamine 1A receptor recognition si... | J Med Chem 40: 300-12 (1997) Article DOI: 10.1021/jm960496o BindingDB Entry DOI: 10.7270/Q27W6B96 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1A (Rattus norvegicus (rat)) | BDBM50056029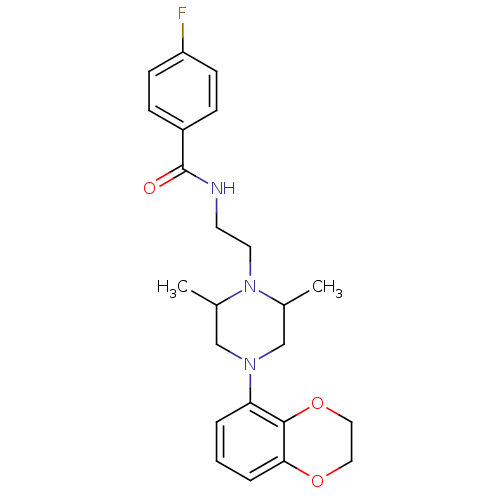 (CHEMBL316960 | N-{2-[4-(2,3-Dihydro-benzo[1,4]diox...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Solvay Pharma Curated by ChEMBL | Assay Description Binding affinity was evaluated by determining in vitro displacement of [3H]-8-OH-DPAT from the central 5-hydroxytryptamine 1A receptor recognition si... | J Med Chem 40: 300-12 (1997) Article DOI: 10.1021/jm960496o BindingDB Entry DOI: 10.7270/Q27W6B96 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1A (Rattus norvegicus (rat)) | BDBM50019960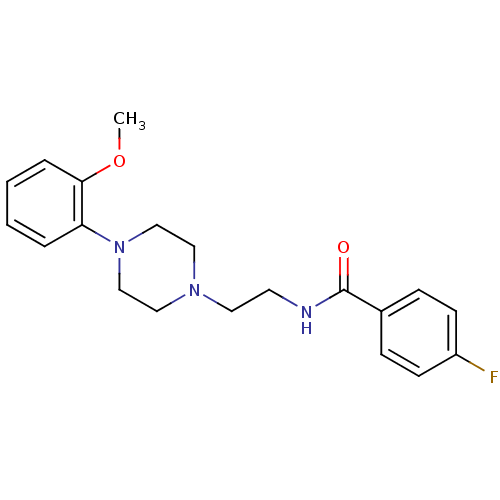 (4-Fluoro-N-{2-[4-(2-methoxy-phenyl)-piperazin-1-yl...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Solvay Pharma Curated by ChEMBL | Assay Description Binding affinity was evaluated by determining in vitro displacement of [3H]-8-OH-DPAT from the central 5-hydroxytryptamine 1A receptor recognition si... | J Med Chem 40: 300-12 (1997) Article DOI: 10.1021/jm960496o BindingDB Entry DOI: 10.7270/Q27W6B96 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A3 (Rattus norvegicus) | BDBM50118812 ((2S,3S,4R,5R)-3,4-Dihydroxy-5-[6-(3-iodo-benzylami...) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE PC cid PC sid PDB UniChem Patents Similars | Article PubMed | 1.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Leiden University Curated by ChEMBL | Assay Description Binding affinity for rat adenosine A3 receptor | J Med Chem 42: 1384-92 (1999) Article DOI: 10.1021/jm9804984 BindingDB Entry DOI: 10.7270/Q2X34Z4X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1A (Rattus norvegicus (rat)) | BDBM50056026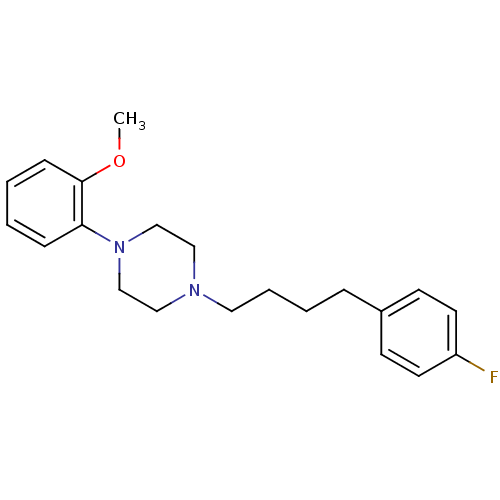 (1-[4-(4-Fluoro-phenyl)-butyl]-4-(2-methoxy-phenyl)...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Solvay Pharma Curated by ChEMBL | Assay Description Binding affinity was evaluated by determining in vitro displacement of [3H]-8-OH-DPAT from the central 5-hydroxytryptamine 1A receptor recognition si... | J Med Chem 40: 300-12 (1997) Article DOI: 10.1021/jm960496o BindingDB Entry DOI: 10.7270/Q27W6B96 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A3 (Homo sapiens (Human)) | BDBM50118812 ((2S,3S,4R,5R)-3,4-Dihydroxy-5-[6-(3-iodo-benzylami...) | Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE PC cid PC sid PDB UniChem Patents Similars | Article PubMed | 1.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Leiden University Curated by ChEMBL | Assay Description Displacement of [125I]AB-MECA from membranes of HEK 293 cells expressing human Adenosine A3 receptor | J Med Chem 42: 1384-92 (1999) Article DOI: 10.1021/jm9804984 BindingDB Entry DOI: 10.7270/Q2X34Z4X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1A (Rattus norvegicus (rat)) | BDBM50056024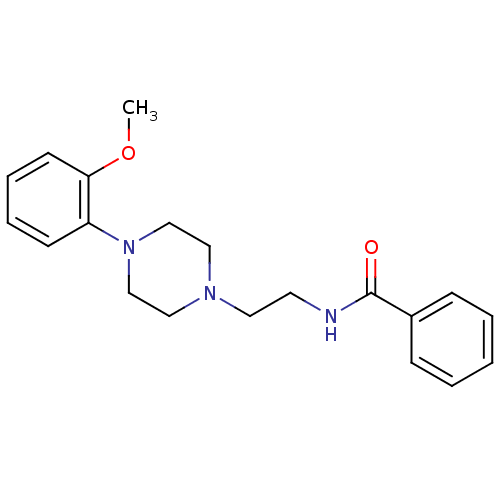 (CHEMBL106854 | N-{2-[4-(2-Methoxy-phenyl)-piperazi...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 1.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Solvay Pharma Curated by ChEMBL | Assay Description Binding affinity was evaluated by determining in vitro displacement of [3H]-8-OH-DPAT from the central 5-hydroxytryptamine 1A receptor recognition si... | J Med Chem 40: 300-12 (1997) Article DOI: 10.1021/jm960496o BindingDB Entry DOI: 10.7270/Q27W6B96 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Homo sapiens (Human)) | BDBM50474424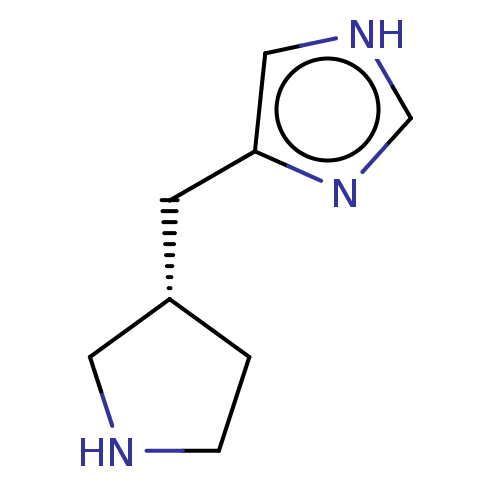 (CHEMBL153051) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase PC cid PC sid UniChem | Article PubMed | 1.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vrije Universiteit Curated by ChEMBL | Assay Description Inhibitory activity measured by [3H]- N alpha- methyl-histamine binding to membranes of SK-N-MC cells expressing the human Histamine H3 receptor | J Med Chem 46: 5445-57 (2003) Article DOI: 10.1021/jm030905y BindingDB Entry DOI: 10.7270/Q2FN18X0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1A (Rattus norvegicus (rat)) | BDBM50056027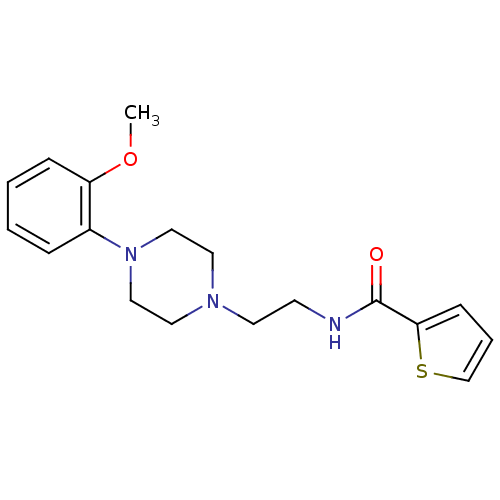 (CHEMBL419910 | Thiophene-2-carboxylic acid {2-[4-(...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Solvay Pharma Curated by ChEMBL | Assay Description Binding affinity was evaluated by determining in vitro displacement of [3H]-8-OH-DPAT from the central 5-hydroxytryptamine 1A receptor recognition si... | J Med Chem 40: 300-12 (1997) Article DOI: 10.1021/jm960496o BindingDB Entry DOI: 10.7270/Q27W6B96 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1A (Rattus norvegicus (rat)) | BDBM50454759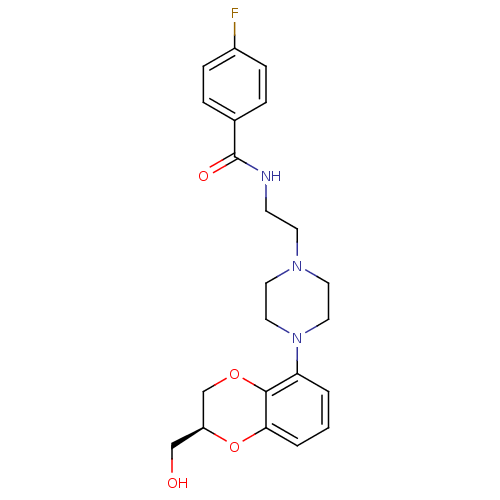 (Flesinoxan) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase PC cid PC sid UniChem Similars | Article PubMed | 1.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Solvay Pharma Curated by ChEMBL | Assay Description Binding affinity was evaluated by determining in vitro displacement of [3H]-8-OH-DPAT from the central 5-hydroxytryptamine 1A receptor recognition si... | J Med Chem 40: 300-12 (1997) Article DOI: 10.1021/jm960496o BindingDB Entry DOI: 10.7270/Q27W6B96 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1A (Rattus norvegicus (rat)) | BDBM50056023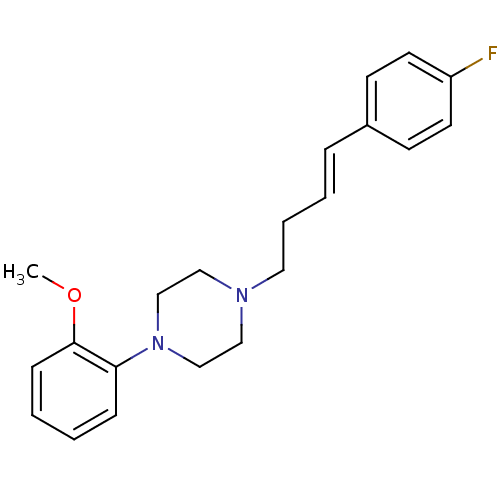 (1-[(E)-4-(4-Fluoro-phenyl)-but-3-enyl]-4-(2-methox...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | 1.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Solvay Pharma Curated by ChEMBL | Assay Description Binding affinity was evaluated by determining in vitro displacement of [3H]-8-OH-DPAT from the central 5-hydroxytryptamine 1A receptor recognition si... | J Med Chem 40: 300-12 (1997) Article DOI: 10.1021/jm960496o BindingDB Entry DOI: 10.7270/Q27W6B96 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1A (Rattus norvegicus (rat)) | BDBM50056039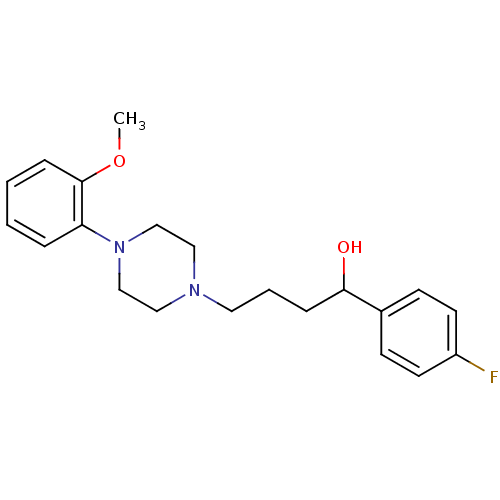 (1-(4-Fluoro-phenyl)-4-[4-(2-methoxy-phenyl)-pipera...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 2.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Solvay Pharma Curated by ChEMBL | Assay Description Binding affinity was evaluated by determining in vitro displacement of [3H]-8-OH-DPAT from the central 5-hydroxytryptamine 1A receptor recognition si... | J Med Chem 40: 300-12 (1997) Article DOI: 10.1021/jm960496o BindingDB Entry DOI: 10.7270/Q27W6B96 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(2) dopamine receptor (Rattus norvegicus (rat)) | BDBM50019959 (1-(4-Fluoro-phenyl)-4-[4-(2-methoxy-phenyl)-pipera...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | 3.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Solvay Pharma Curated by ChEMBL | Assay Description In vitro displacement of [3H]-spiperone from Dopamine receptor D2 binding site in rat striatum. | J Med Chem 40: 300-12 (1997) Article DOI: 10.1021/jm960496o BindingDB Entry DOI: 10.7270/Q27W6B96 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(2) dopamine receptor (Rattus norvegicus (rat)) | BDBM50056038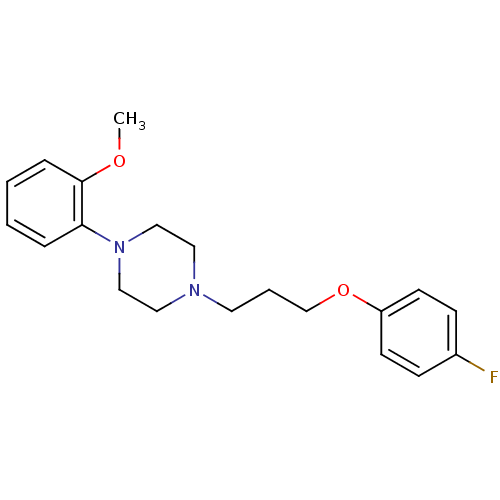 (1-[3-(4-Fluoro-phenoxy)-propyl]-4-(2-methoxy-pheny...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 3.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Solvay Pharma Curated by ChEMBL | Assay Description In vitro displacement of [3H]-spiperone from Dopamine receptor D2 binding site in rat striatum. | J Med Chem 40: 300-12 (1997) Article DOI: 10.1021/jm960496o BindingDB Entry DOI: 10.7270/Q27W6B96 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(2) dopamine receptor (Rattus norvegicus (rat)) | BDBM50056026 (1-[4-(4-Fluoro-phenyl)-butyl]-4-(2-methoxy-phenyl)...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 3.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Solvay Pharma Curated by ChEMBL | Assay Description In vitro displacement of [3H]-spiperone from Dopamine receptor D2 binding site in rat striatum. | J Med Chem 40: 300-12 (1997) Article DOI: 10.1021/jm960496o BindingDB Entry DOI: 10.7270/Q27W6B96 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1A (Rattus norvegicus (rat)) | BDBM50056038 (1-[3-(4-Fluoro-phenoxy)-propyl]-4-(2-methoxy-pheny...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 3.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Solvay Pharma Curated by ChEMBL | Assay Description Binding affinity was evaluated by determining in vitro displacement of [3H]-8-OH-DPAT from the central 5-hydroxytryptamine 1A receptor recognition si... | J Med Chem 40: 300-12 (1997) Article DOI: 10.1021/jm960496o BindingDB Entry DOI: 10.7270/Q27W6B96 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(2) dopamine receptor (Rattus norvegicus (rat)) | BDBM50056039 (1-(4-Fluoro-phenyl)-4-[4-(2-methoxy-phenyl)-pipera...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 3.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Solvay Pharma Curated by ChEMBL | Assay Description In vitro displacement of [3H]-spiperone from Dopamine receptor D2 binding site in rat striatum. | J Med Chem 40: 300-12 (1997) Article DOI: 10.1021/jm960496o BindingDB Entry DOI: 10.7270/Q27W6B96 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Homo sapiens (Human)) | BDBM50474427 (CHEMBL153061) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase PC cid PC sid UniChem | Article PubMed | 3.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vrije Universiteit Curated by ChEMBL | Assay Description Inhibitory activity measured by [3H]- N alpha- methyl-histamine binding to membranes of SK-N-MC cells expressing the human Histamine H3 receptor | J Med Chem 46: 5445-57 (2003) Article DOI: 10.1021/jm030905y BindingDB Entry DOI: 10.7270/Q2FN18X0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A1 (Homo sapiens (Human)) | BDBM50118812 ((2S,3S,4R,5R)-3,4-Dihydroxy-5-[6-(3-iodo-benzylami...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE PC cid PC sid PDB UniChem Patents Similars | Article PubMed | 3.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Leiden University Curated by ChEMBL | Assay Description Binding affinity for human adenosine A1 receptor | J Med Chem 42: 1384-92 (1999) Article DOI: 10.1021/jm9804984 BindingDB Entry DOI: 10.7270/Q2X34Z4X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Homo sapiens (Human)) | BDBM50474428 (CHEMBL152936) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vrije Universiteit Curated by ChEMBL | Assay Description Inhibitory activity measured by [3H]- N alpha- methyl-histamine binding to membranes of SK-N-MC cells expressing the human Histamine H3 receptor | J Med Chem 46: 5445-57 (2003) Article DOI: 10.1021/jm030905y BindingDB Entry DOI: 10.7270/Q2FN18X0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(2) dopamine receptor (Rattus norvegicus (rat)) | BDBM50056023 (1-[(E)-4-(4-Fluoro-phenyl)-but-3-enyl]-4-(2-methox...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | 4.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Solvay Pharma Curated by ChEMBL | Assay Description In vitro displacement of [3H]-spiperone from Dopamine receptor D2 binding site in rat striatum. | J Med Chem 40: 300-12 (1997) Article DOI: 10.1021/jm960496o BindingDB Entry DOI: 10.7270/Q27W6B96 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Homo sapiens (Human)) | BDBM50170164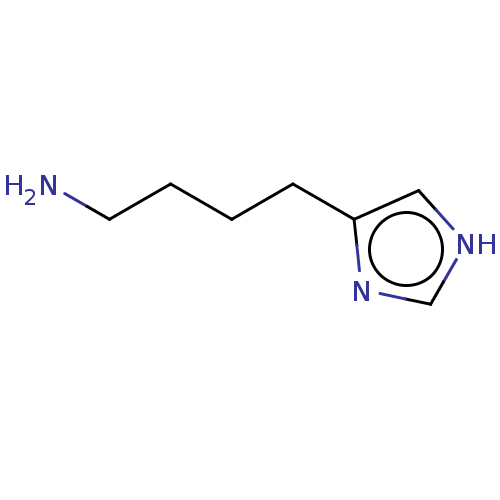 (Imbutamine) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase PC cid PC sid UniChem Patents | Article PubMed | 4.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vrije Universiteit Curated by ChEMBL | Assay Description Inhibitory activity measured by [3H]- N alpha- methyl-histamine binding to membranes of SK-N-MC cells expressing the human Histamine H3 receptor | J Med Chem 46: 5445-57 (2003) Article DOI: 10.1021/jm030905y BindingDB Entry DOI: 10.7270/Q2FN18X0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Homo sapiens (Human)) | BDBM50215536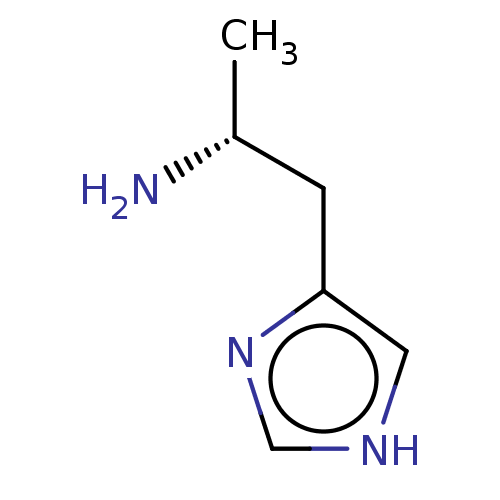 ((R)-Alpha-Methylhistamine | CHEBI:73337 | CHEMBL26...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | Article PubMed | 4.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vrije Universiteit Curated by ChEMBL | Assay Description Inhibitory activity measured by [3H]- N alpha- methyl-histamine binding to membranes of SK-N-MC cells expressing the human Histamine H3 receptor | J Med Chem 46: 5445-57 (2003) Article DOI: 10.1021/jm030905y BindingDB Entry DOI: 10.7270/Q2FN18X0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Homo sapiens (Human)) | BDBM50409817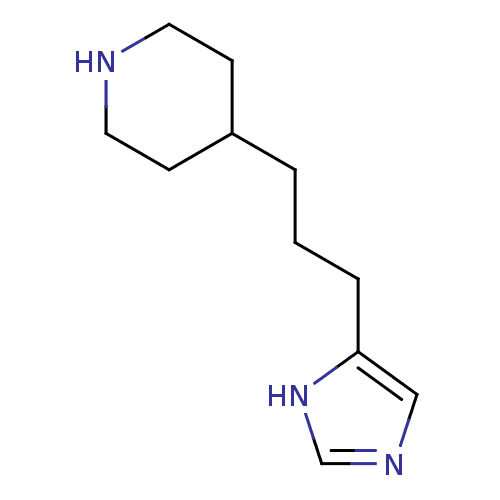 (CHEMBL78498 | VUF-5681) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | Article PubMed | 4.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vrije Universiteit Curated by ChEMBL | Assay Description Inhibitory activity measured by [3H]- N alpha- methyl-histamine binding to membranes of SK-N-MC cells expressing the human Histamine H3 receptor | J Med Chem 46: 5445-57 (2003) Article DOI: 10.1021/jm030905y BindingDB Entry DOI: 10.7270/Q2FN18X0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1A (Rattus norvegicus (rat)) | BDBM50056025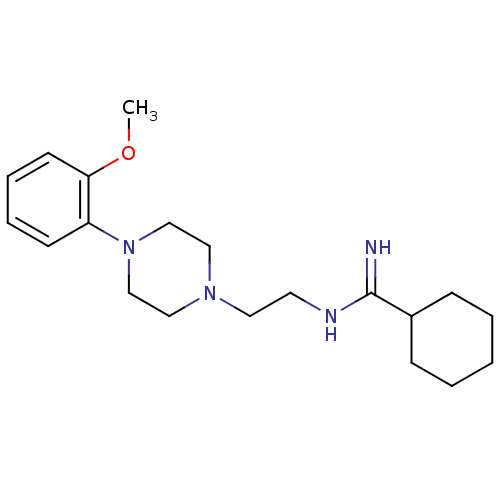 (CHEMBL107903 | N-{2-[4-(2-Methoxy-phenyl)-piperazi...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | 4.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Solvay Pharma Curated by ChEMBL | Assay Description Binding affinity was evaluated by determining in vitro displacement of [3H]-8-OH-DPAT from the central 5-hydroxytryptamine 1A receptor recognition si... | J Med Chem 40: 300-12 (1997) Article DOI: 10.1021/jm960496o BindingDB Entry DOI: 10.7270/Q27W6B96 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1A (Rattus norvegicus (rat)) | BDBM50056037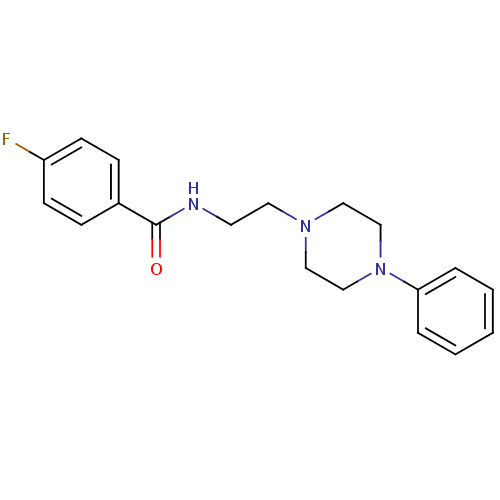 (4-Fluoro-N-[2-(4-phenyl-piperazin-1-yl)-ethyl]-ben...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 4.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Solvay Pharma Curated by ChEMBL | Assay Description Binding affinity was evaluated by determining in vitro displacement of [3H]-8-OH-DPAT from the central 5-hydroxytryptamine 1A receptor recognition si... | J Med Chem 40: 300-12 (1997) Article DOI: 10.1021/jm960496o BindingDB Entry DOI: 10.7270/Q27W6B96 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(2) dopamine receptor (Rattus norvegicus (rat)) | BDBM50019960 (4-Fluoro-N-{2-[4-(2-methoxy-phenyl)-piperazin-1-yl...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Solvay Pharma Curated by ChEMBL | Assay Description In vitro displacement of [3H]-spiperone from Dopamine receptor D2 binding site in rat striatum. | J Med Chem 40: 300-12 (1997) Article DOI: 10.1021/jm960496o BindingDB Entry DOI: 10.7270/Q27W6B96 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Homo sapiens (Human)) | BDBM50474426 (Impentamine) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase PC cid PC sid UniChem | Article PubMed | 5.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vrije Universiteit Curated by ChEMBL | Assay Description Inhibitory activity measured by [3H]- N alpha- methyl-histamine binding to membranes of SK-N-MC cells expressing the human Histamine H3 receptor | J Med Chem 46: 5445-57 (2003) Article DOI: 10.1021/jm030905y BindingDB Entry DOI: 10.7270/Q2FN18X0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Homo sapiens (Human)) | BDBM50474433 (CHEMBL440730) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase PC cid PC sid UniChem | Article PubMed | 5.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vrije Universiteit Curated by ChEMBL | Assay Description Inhibitory activity measured by [3H]- N alpha- methyl-histamine binding to membranes of SK-N-MC cells expressing the human Histamine H3 receptor | J Med Chem 46: 5445-57 (2003) Article DOI: 10.1021/jm030905y BindingDB Entry DOI: 10.7270/Q2FN18X0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(2) dopamine receptor (Rattus norvegicus (rat)) | BDBM50020178 (CHEMBL294633 | CHEMBL555633 | N-[2-(4-Benzofuran-7...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 5.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Solvay Pharma Curated by ChEMBL | Assay Description In vitro displacement of [3H]-spiperone from Dopamine receptor D2 binding site in rat striatum. | J Med Chem 40: 300-12 (1997) Article DOI: 10.1021/jm960496o BindingDB Entry DOI: 10.7270/Q27W6B96 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A1 (Rattus norvegicus (rat)) | BDBM25400 ((2R,3R,4S,5R)-2-[6-(cyclopentylamino)-9H-purin-9-y...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL MCE MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | 5.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Leiden/Amsterdam Center for Drug Research Curated by ChEMBL | Assay Description Displacement of [3H]DPCPX (without GTP) from Adenosine A1 receptor of rat cortical membrane | J Med Chem 39: 1463-71 (1996) Article DOI: 10.1021/jm950267m BindingDB Entry DOI: 10.7270/Q2WH2QNT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Homo sapiens (Human)) | BDBM50474425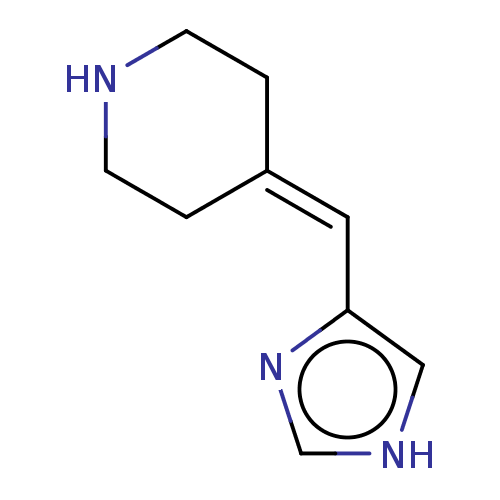 (CHEMBL356666) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 5.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vrije Universiteit Curated by ChEMBL | Assay Description Inhibitory activity measured by [3H]- N alpha- methyl-histamine binding to membranes of SK-N-MC cells expressing the human Histamine H3 receptor | J Med Chem 46: 5445-57 (2003) Article DOI: 10.1021/jm030905y BindingDB Entry DOI: 10.7270/Q2FN18X0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(2) dopamine receptor (Rattus norvegicus (rat)) | BDBM50056040 (CHEMBL107141 | Cyclohexanecarbothioic acid {2-[4-(...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | 6.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Solvay Pharma Curated by ChEMBL | Assay Description In vitro displacement of [3H]-spiperone from Dopamine receptor D2 binding site in rat striatum. | J Med Chem 40: 300-12 (1997) Article DOI: 10.1021/jm960496o BindingDB Entry DOI: 10.7270/Q27W6B96 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Homo sapiens (Human)) | BDBM50121205 (CHEBI:18295 | Histamine) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE KEGG PC cid PC sid PDB UniChem Patents | Article PubMed | 9.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vrije Universiteit Curated by ChEMBL | Assay Description Inhibitory activity measured by [3H]- N alpha- methyl-histamine binding to membranes of SK-N-MC cells expressing the human Histamine H3 receptor | J Med Chem 46: 5445-57 (2003) Article DOI: 10.1021/jm030905y BindingDB Entry DOI: 10.7270/Q2FN18X0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Homo sapiens (Human)) | BDBM50127605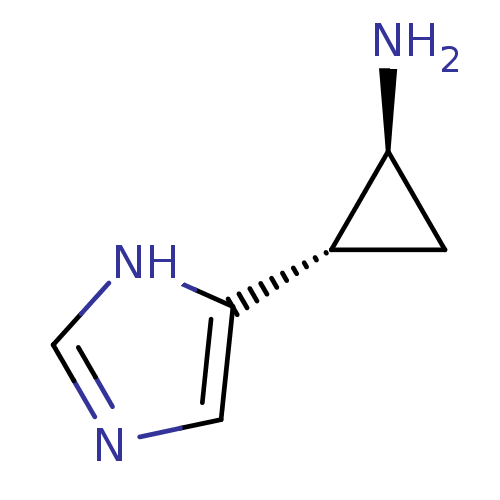 ((1S,2S)-2-(1H-Imidazol-4-yl)-cyclopropylamine | (1...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 9.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vrije Universiteit Curated by ChEMBL | Assay Description Inhibitory activity measured by [3H]- N alpha- methyl-histamine binding to membranes of SK-N-MC cells expressing the human Histamine H3 receptor | J Med Chem 46: 5445-57 (2003) Article DOI: 10.1021/jm030905y BindingDB Entry DOI: 10.7270/Q2FN18X0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Homo sapiens (Human)) | BDBM50407522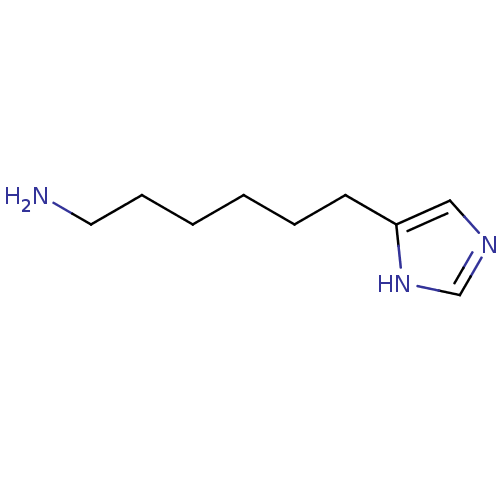 (CHEMBL554031) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vrije Universiteit Curated by ChEMBL | Assay Description Inhibitory activity measured by [3H]- N alpha- methyl-histamine binding to membranes of SK-N-MC cells expressing the human Histamine H3 receptor | J Med Chem 46: 5445-57 (2003) Article DOI: 10.1021/jm030905y BindingDB Entry DOI: 10.7270/Q2FN18X0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(2) dopamine receptor (Rattus norvegicus (rat)) | BDBM50056030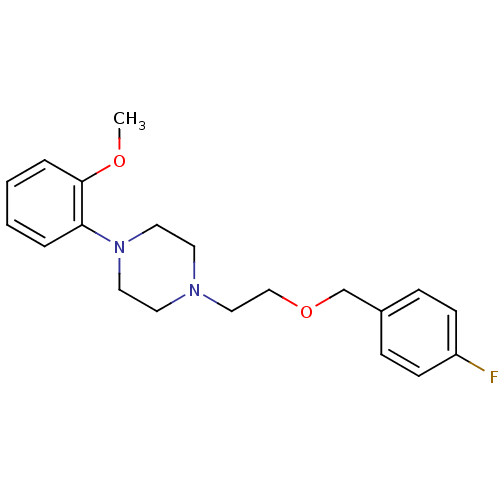 (1-[2-(4-Fluoro-benzyloxy)-ethyl]-4-(2-methoxy-phen...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | 10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Solvay Pharma Curated by ChEMBL | Assay Description In vitro displacement of [3H]-spiperone from Dopamine receptor D2 binding site in rat striatum. | J Med Chem 40: 300-12 (1997) Article DOI: 10.1021/jm960496o BindingDB Entry DOI: 10.7270/Q27W6B96 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A3 (Homo sapiens (Human)) | BDBM21220 ((2S,3S,4R,5R)-5-(6-amino-9H-purin-9-yl)-N-ethyl-3,...) | Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE MMDB PC cid PC sid PDB UniChem Similars | Article PubMed | 11 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Leiden University Curated by ChEMBL | Assay Description Ability to displace radioligand [125I]AB-MECA from membrane of HEK 293 cells stably transfected with human adenosine A3 receptor cDNA | J Med Chem 42: 1384-92 (1999) Article DOI: 10.1021/jm9804984 BindingDB Entry DOI: 10.7270/Q2X34Z4X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(2) dopamine receptor (Rattus norvegicus (rat)) | BDBM50056024 (CHEMBL106854 | N-{2-[4-(2-Methoxy-phenyl)-piperazi...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 13 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Solvay Pharma Curated by ChEMBL | Assay Description In vitro displacement of [3H]-spiperone from Dopamine receptor D2 binding site in rat striatum. | J Med Chem 40: 300-12 (1997) Article DOI: 10.1021/jm960496o BindingDB Entry DOI: 10.7270/Q27W6B96 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1A (Rattus norvegicus (rat)) | BDBM50035042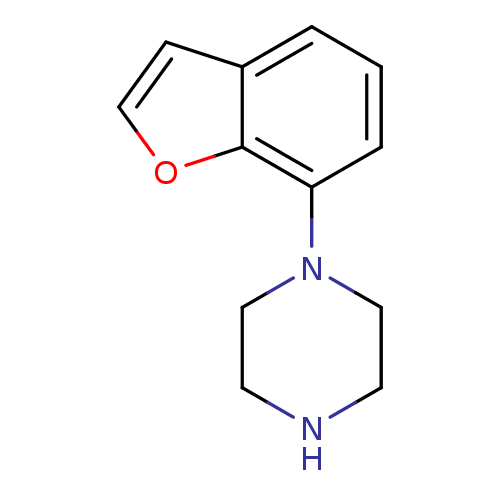 (1-Benzofuran-7-yl-piperazine | CHEMBL61032) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 13 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Solvay Pharma Curated by ChEMBL | Assay Description Binding affinity was evaluated by determining in vitro displacement of [3H]-8-OH-DPAT from the central 5-hydroxytryptamine 1A receptor recognition si... | J Med Chem 40: 300-12 (1997) Article DOI: 10.1021/jm960496o BindingDB Entry DOI: 10.7270/Q27W6B96 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A1 (Homo sapiens (Human)) | BDBM21220 ((2S,3S,4R,5R)-5-(6-amino-9H-purin-9-yl)-N-ethyl-3,...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE MMDB PC cid PC sid PDB UniChem Similars | Article PubMed | 14 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Leiden University Curated by ChEMBL | Assay Description Binding affinity for human adenosine A1 receptor | J Med Chem 42: 1384-92 (1999) Article DOI: 10.1021/jm9804984 BindingDB Entry DOI: 10.7270/Q2X34Z4X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(2) dopamine receptor (Rattus norvegicus (rat)) | BDBM50039824 (CHEMBL81728 | N-{2-[4-(2,3-Dihydro-benzo[1,4]dioxi...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 14 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Solvay Pharma Curated by ChEMBL | Assay Description In vitro displacement of [3H]-spiperone from Dopamine receptor D2 binding site in rat striatum. | J Med Chem 40: 300-12 (1997) Article DOI: 10.1021/jm960496o BindingDB Entry DOI: 10.7270/Q27W6B96 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H4 receptor (Homo sapiens (Human)) | BDBM50121205 (CHEBI:18295 | Histamine) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE KEGG PC cid PC sid PDB UniChem Patents | PDB Article PubMed | 14 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vrije Universiteit Curated by ChEMBL | Assay Description Inhibition of [3H]-histamine binding to membranes of SK-N-MC cells expressing the human Histamine H4 receptor | J Med Chem 46: 5445-57 (2003) Article DOI: 10.1021/jm030905y BindingDB Entry DOI: 10.7270/Q2FN18X0 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Histamine H4 receptor (Homo sapiens (Human)) | BDBM50170164 (Imbutamine) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase PC cid PC sid UniChem Patents | Article PubMed | 14.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vrije Universiteit Curated by ChEMBL | Assay Description Inhibitory activity measured by [3H]- N alpha- methyl-histamine binding to membranes of SK-N-MC cells expressing the human Histamine H4 receptor | J Med Chem 46: 5445-57 (2003) Article DOI: 10.1021/jm030905y BindingDB Entry DOI: 10.7270/Q2FN18X0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A2a (Rattus norvegicus (rat)) | BDBM35804 ((CGS21680) 3-(4-{2-[6-Amino-9-(5-ethylcarbamoylmet...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | 15 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Leiden University Curated by ChEMBL | Assay Description Ability to displace radioligand [3H]- CGS 21680 from adenosine A2A receptor on rat striatal membrane | J Med Chem 42: 1384-92 (1999) Article DOI: 10.1021/jm9804984 BindingDB Entry DOI: 10.7270/Q2X34Z4X | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Displayed 1 to 50 (of 215 total ) | Next | Last >> |