 Found 6774 hits with Last Name = 'spencer' and Initial = 'j'
Found 6774 hits with Last Name = 'spencer' and Initial = 'j' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Coagulation factor VII/Tissue factor
(Homo sapiens (Human)) | BDBM50103651

(2-(3'-Amino-5-chloro-2-hydroxy-biphenyl-3-yl)-1H-b...)Show SMILES NC(=N)c1ccc2nc([nH]c2c1)-c1cc(Cl)cc(-c2cccc(N)c2)c1O Show InChI InChI=1S/C20H16ClN5O/c21-12-8-14(10-2-1-3-13(22)6-10)18(27)15(9-12)20-25-16-5-4-11(19(23)24)7-17(16)26-20/h1-9,27H,22H2,(H3,23,24)(H,25,26) | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 0.0780 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Axys Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
Binding affinity for factor VIIa/TF |
Bioorg Med Chem Lett 11: 2253-6 (2001)
BindingDB Entry DOI: 10.7270/Q2XG9QD9 |
More data for this
Ligand-Target Pair | |
Gastrin/cholecystokinin type B receptor
(Homo sapiens (Human)) | BDBM50411339
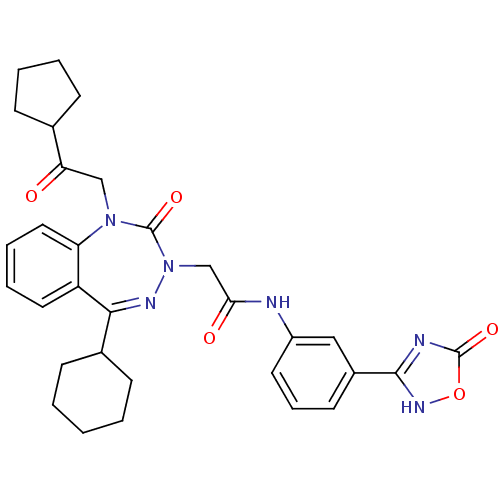
(CHEMBL227276)Show SMILES O=C(CN1N=C(C2CCCCC2)c2ccccc2N(CC(=O)C2CCCC2)C1=O)Nc1cccc(c1)-c1nc(=O)o[nH]1 |t:4| Show InChI InChI=1S/C31H34N6O5/c38-26(20-9-4-5-10-20)18-36-25-16-7-6-15-24(25)28(21-11-2-1-3-12-21)34-37(31(36)41)19-27(39)32-23-14-8-13-22(17-23)29-33-30(40)42-35-29/h6-8,13-17,20-21H,1-5,9-12,18-19H2,(H,32,39)(H,33,35,40) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.110 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
James Black Foundation
Curated by ChEMBL
| Assay Description
Displacement of [3H]BH-CCK-8S from human recombinant CCK2 receptor expressed in NIH3T3 cells |
J Med Chem 50: 3101-12 (2007)
Checked by Author
Article DOI: 10.1021/jm070139l
BindingDB Entry DOI: 10.7270/Q2QJ7JHN |
More data for this
Ligand-Target Pair | |
Gastrin/cholecystokinin type B receptor
(Homo sapiens (Human)) | BDBM50056102
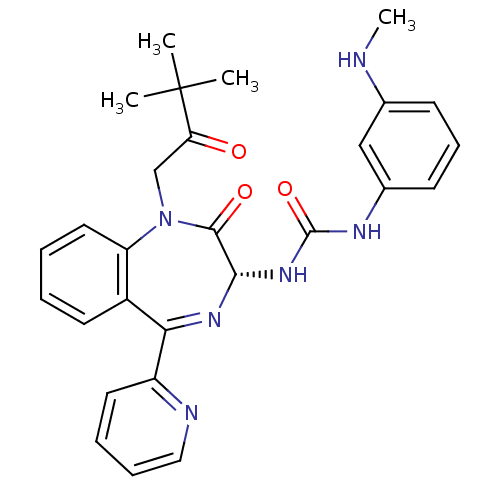
((R)-1-(1-(3,3-dimethyl-2-oxobutyl)-2-oxo-5-(pyridi...)Show SMILES CNc1cccc(NC(=O)N[C@@H]2N=C(c3ccccn3)c3ccccc3N(CC(=O)C(C)(C)C)C2=O)c1 |t:12| Show InChI InChI=1S/C28H30N6O3/c1-28(2,3)23(35)17-34-22-14-6-5-12-20(22)24(21-13-7-8-15-30-21)32-25(26(34)36)33-27(37)31-19-11-9-10-18(16-19)29-4/h5-16,25,29H,17H2,1-4H3,(H2,31,33,37)/t25-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.140 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
James Black Foundation
Curated by ChEMBL
| Assay Description
Displacement of [3H]BH-CCK-8S from human recombinant CCK2 receptor expressed in NIH3T3 cells |
J Med Chem 50: 3101-12 (2007)
Checked by Author
Article DOI: 10.1021/jm070139l
BindingDB Entry DOI: 10.7270/Q2QJ7JHN |
More data for this
Ligand-Target Pair | |
Fatty-acid amide hydrolase 1 [30-579]
(Rattus norvegicus (rat)) | BDBM50161518

(1-(oxazolo[4,5-b]pyridin-2-yl)-6-phenylhexan-1-one...)Show InChI InChI=1S/C18H18N2O2/c21-15(18-20-17-16(22-18)12-7-13-19-17)11-6-2-5-10-14-8-3-1-4-9-14/h1,3-4,7-9,12-13H,2,5-6,10-11H2 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ de Lille
Curated by ChEMBL
| Assay Description
Inhibition of rat FAAH assessed as reduction in [14C]oleamide conversion to oleic acid by Lineweaver-Burk plot analysis |
J Med Chem 60: 4-46 (2017)
Article DOI: 10.1021/acs.jmedchem.6b00538
BindingDB Entry DOI: 10.7270/Q2348NZC |
More data for this
Ligand-Target Pair | |
Gastrin/cholecystokinin type B receptor
(Homo sapiens (Human)) | BDBM50411341
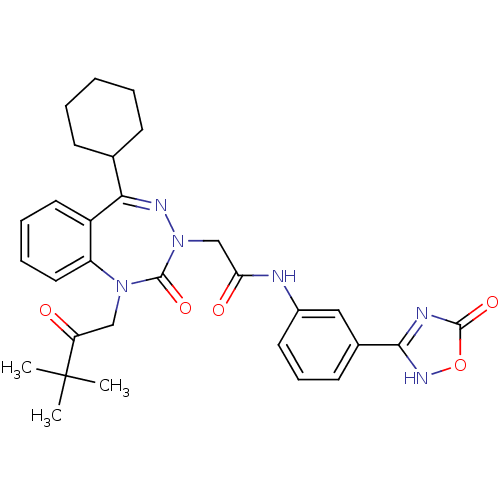
(CHEMBL226583)Show SMILES CC(C)(C)C(=O)CN1c2ccccc2C(=NN(CC(=O)Nc2cccc(c2)-c2nc(=O)o[nH]2)C1=O)C1CCCCC1 |c:15| Show InChI InChI=1S/C30H34N6O5/c1-30(2,3)24(37)17-35-23-15-8-7-14-22(23)26(19-10-5-4-6-11-19)33-36(29(35)40)18-25(38)31-21-13-9-12-20(16-21)27-32-28(39)41-34-27/h7-9,12-16,19H,4-6,10-11,17-18H2,1-3H3,(H,31,38)(H,32,34,39) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
James Black Foundation
Curated by ChEMBL
| Assay Description
Displacement of [3H]BH-CCK-8S from human recombinant CCK2 receptor expressed in NIH3T3 cells |
J Med Chem 50: 3101-12 (2007)
Checked by Author
Article DOI: 10.1021/jm070139l
BindingDB Entry DOI: 10.7270/Q2QJ7JHN |
More data for this
Ligand-Target Pair | |
Gastrin/cholecystokinin type B receptor
(Homo sapiens (Human)) | BDBM50002875
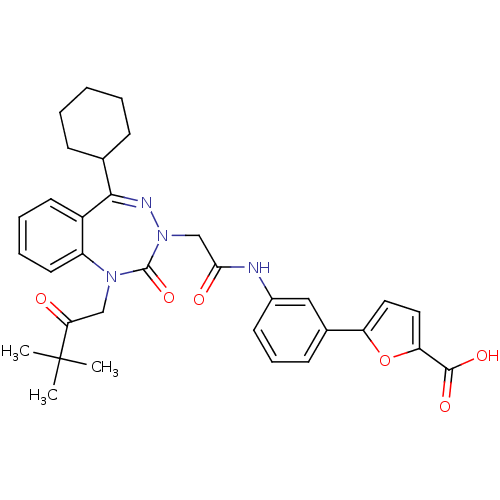
(CHEMBL388144)Show SMILES CC(C)(C)C(=O)CN1c2ccccc2C(=NN(CC(=O)Nc2cccc(c2)-c2ccc(o2)C(O)=O)C1=O)C1CCCCC1 |c:15| Show InChI InChI=1S/C33H36N4O6/c1-33(2,3)28(38)19-36-25-15-8-7-14-24(25)30(21-10-5-4-6-11-21)35-37(32(36)42)20-29(39)34-23-13-9-12-22(18-23)26-16-17-27(43-26)31(40)41/h7-9,12-18,21H,4-6,10-11,19-20H2,1-3H3,(H,34,39)(H,40,41) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.25 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
James Black Foundation
Curated by ChEMBL
| Assay Description
Displacement of [3H]BH-CCK-8S from human recombinant CCK2 receptor expressed in NIH3T3 cells |
J Med Chem 50: 3101-12 (2007)
Checked by Author
Article DOI: 10.1021/jm070139l
BindingDB Entry DOI: 10.7270/Q2QJ7JHN |
More data for this
Ligand-Target Pair | |
Fatty-acid amide hydrolase 1 [30-579]
(Rattus norvegicus (rat)) | BDBM23316
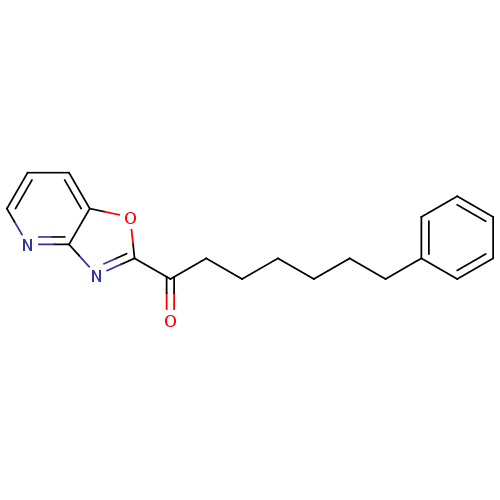
(7-phenyl-1-{pyrido[2,3-d][1,3]oxazol-2-yl}heptan-1...)Show InChI InChI=1S/C19H20N2O2/c22-16(19-21-18-17(23-19)13-8-14-20-18)12-7-2-1-4-9-15-10-5-3-6-11-15/h3,5-6,8,10-11,13-14H,1-2,4,7,9,12H2 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.280 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ de Lille
Curated by ChEMBL
| Assay Description
Inhibition of rat FAAH assessed as reduction in [14C]oleamide conversion to oleic acid by Lineweaver-Burk plot analysis |
J Med Chem 60: 4-46 (2017)
Article DOI: 10.1021/acs.jmedchem.6b00538
BindingDB Entry DOI: 10.7270/Q2348NZC |
More data for this
Ligand-Target Pair | |
Gastrin/cholecystokinin type B receptor
(Homo sapiens (Human)) | BDBM50411340
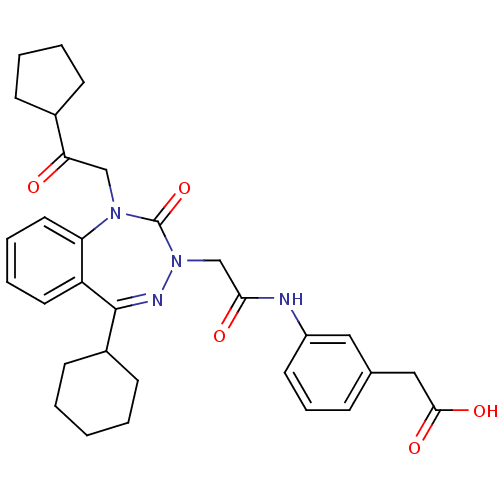
(CHEMBL387948)Show SMILES OC(=O)Cc1cccc(NC(=O)CN2N=C(C3CCCCC3)c3ccccc3N(CC(=O)C3CCCC3)C2=O)c1 |t:14| Show InChI InChI=1S/C31H36N4O5/c36-27(22-10-4-5-11-22)19-34-26-16-7-6-15-25(26)30(23-12-2-1-3-13-23)33-35(31(34)40)20-28(37)32-24-14-8-9-21(17-24)18-29(38)39/h6-9,14-17,22-23H,1-5,10-13,18-20H2,(H,32,37)(H,38,39) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.280 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
James Black Foundation
Curated by ChEMBL
| Assay Description
Displacement of [3H]BH-CCK-8S from human recombinant CCK2 receptor expressed in NIH3T3 cells |
J Med Chem 50: 3101-12 (2007)
Checked by Author
Article DOI: 10.1021/jm070139l
BindingDB Entry DOI: 10.7270/Q2QJ7JHN |
More data for this
Ligand-Target Pair | |
Fatty-acid amide hydrolase 1 [30-579]
(Rattus norvegicus (rat)) | BDBM50275119
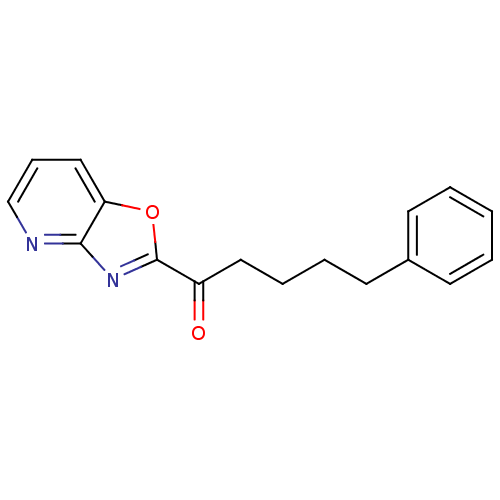
(1-(oxazolo[4,5-b]pyridin-2-yl)-5-phenylpentan-1-on...)Show InChI InChI=1S/C17H16N2O2/c20-14(10-5-4-9-13-7-2-1-3-8-13)17-19-16-15(21-17)11-6-12-18-16/h1-3,6-8,11-12H,4-5,9-10H2 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ de Lille
Curated by ChEMBL
| Assay Description
Inhibition of rat FAAH assessed as reduction in [14C]oleamide conversion to oleic acid by Lineweaver-Burk plot analysis |
J Med Chem 60: 4-46 (2017)
Article DOI: 10.1021/acs.jmedchem.6b00538
BindingDB Entry DOI: 10.7270/Q2348NZC |
More data for this
Ligand-Target Pair | |
Gastrin/cholecystokinin type B receptor
(Homo sapiens (Human)) | BDBM50411344
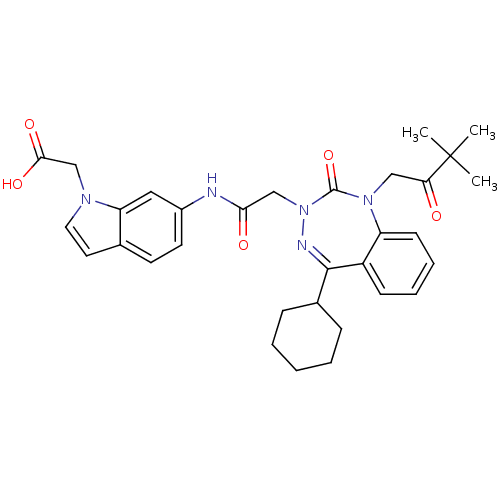
(CHEMBL389711)Show SMILES CC(C)(C)C(=O)CN1c2ccccc2C(=NN(CC(=O)Nc2ccc3ccn(CC(O)=O)c3c2)C1=O)C1CCCCC1 |c:15| Show InChI InChI=1S/C32H37N5O5/c1-32(2,3)27(38)18-36-25-12-8-7-11-24(25)30(22-9-5-4-6-10-22)34-37(31(36)42)19-28(39)33-23-14-13-21-15-16-35(20-29(40)41)26(21)17-23/h7-8,11-17,22H,4-6,9-10,18-20H2,1-3H3,(H,33,39)(H,40,41) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
James Black Foundation
Curated by ChEMBL
| Assay Description
Displacement of [3H]BH-CCK-8S from human recombinant CCK2 receptor expressed in NIH3T3 cells |
J Med Chem 50: 3101-12 (2007)
Checked by Author
Article DOI: 10.1021/jm070139l
BindingDB Entry DOI: 10.7270/Q2QJ7JHN |
More data for this
Ligand-Target Pair | |
Gastrin/cholecystokinin type B receptor
(Homo sapiens (Human)) | BDBM50411342
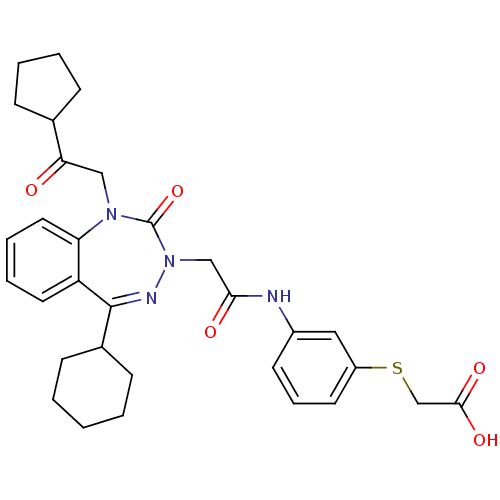
(CHEMBL227333)Show SMILES OC(=O)CSc1cccc(NC(=O)CN2N=C(C3CCCCC3)c3ccccc3N(CC(=O)C3CCCC3)C2=O)c1 |t:15| Show InChI InChI=1S/C31H36N4O5S/c36-27(21-9-4-5-10-21)18-34-26-16-7-6-15-25(26)30(22-11-2-1-3-12-22)33-35(31(34)40)19-28(37)32-23-13-8-14-24(17-23)41-20-29(38)39/h6-8,13-17,21-22H,1-5,9-12,18-20H2,(H,32,37)(H,38,39) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.330 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
James Black Foundation
Curated by ChEMBL
| Assay Description
Displacement of [3H]BH-CCK-8S from human recombinant CCK2 receptor expressed in NIH3T3 cells |
J Med Chem 50: 3101-12 (2007)
Checked by Author
Article DOI: 10.1021/jm070139l
BindingDB Entry DOI: 10.7270/Q2QJ7JHN |
More data for this
Ligand-Target Pair | |
Gastrin/cholecystokinin type B receptor
(Homo sapiens (Human)) | BDBM50411336

(CHEMBL227330)Show SMILES OC(=O)CCc1cccc(NC(=O)CN2N=C(C3CCCCC3)c3ccccc3N(CC(=O)C3CCCC3)C2=O)c1 |t:15| Show InChI InChI=1S/C32H38N4O5/c37-28(23-10-4-5-11-23)20-35-27-16-7-6-15-26(27)31(24-12-2-1-3-13-24)34-36(32(35)41)21-29(38)33-25-14-8-9-22(19-25)17-18-30(39)40/h6-9,14-16,19,23-24H,1-5,10-13,17-18,20-21H2,(H,33,38)(H,39,40) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.340 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
James Black Foundation
Curated by ChEMBL
| Assay Description
Displacement of [3H]BH-CCK-8S from human recombinant CCK2 receptor expressed in NIH3T3 cells |
J Med Chem 50: 3101-12 (2007)
Checked by Author
Article DOI: 10.1021/jm070139l
BindingDB Entry DOI: 10.7270/Q2QJ7JHN |
More data for this
Ligand-Target Pair | |
Gastrin/cholecystokinin type B receptor
(Homo sapiens (Human)) | BDBM50002880
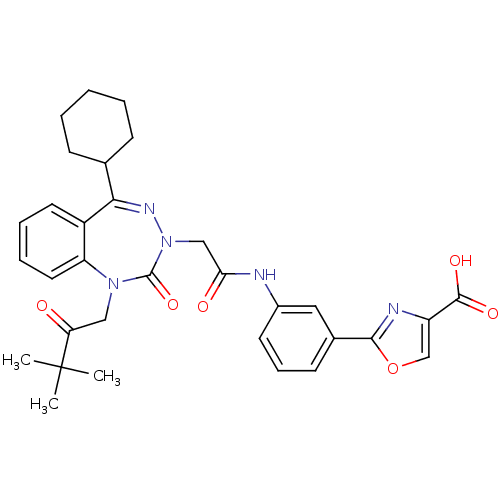
(CHEMBL437736)Show SMILES CC(C)(C)C(=O)CN1c2ccccc2C(=NN(CC(=O)Nc2cccc(c2)-c2nc(co2)C(O)=O)C1=O)C1CCCCC1 |c:15| Show InChI InChI=1S/C32H35N5O6/c1-32(2,3)26(38)17-36-25-15-8-7-14-23(25)28(20-10-5-4-6-11-20)35-37(31(36)42)18-27(39)33-22-13-9-12-21(16-22)29-34-24(19-43-29)30(40)41/h7-9,12-16,19-20H,4-6,10-11,17-18H2,1-3H3,(H,33,39)(H,40,41) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| 0.360 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
James Black Foundation
Curated by ChEMBL
| Assay Description
Displacement of [3H]BH-CCK-8S from human recombinant CCK2 receptor expressed in NIH3T3 cells |
J Med Chem 50: 3101-12 (2007)
Checked by Author
Article DOI: 10.1021/jm070139l
BindingDB Entry DOI: 10.7270/Q2QJ7JHN |
More data for this
Ligand-Target Pair | |
Fatty-acid amide hydrolase 1 [30-579]
(Rattus norvegicus (rat)) | BDBM50161520
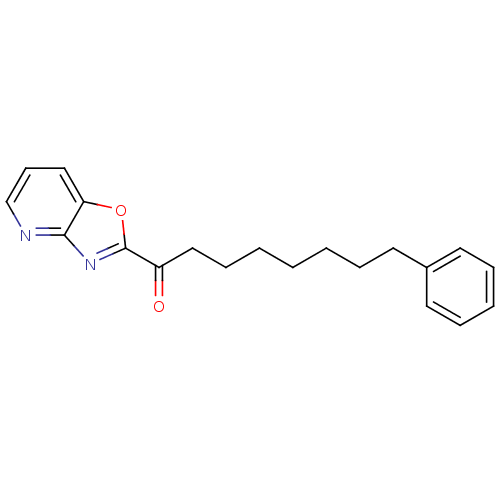
(1-(oxazolo[4,5-b]pyridin-2-yl)-8-phenyloctan-1-one...)Show InChI InChI=1S/C20H22N2O2/c23-17(20-22-19-18(24-20)14-9-15-21-19)13-8-3-1-2-5-10-16-11-6-4-7-12-16/h4,6-7,9,11-12,14-15H,1-3,5,8,10,13H2 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.390 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ de Lille
Curated by ChEMBL
| Assay Description
Inhibition of rat FAAH assessed as reduction in [14C]oleamide conversion to oleic acid by Lineweaver-Burk plot analysis |
J Med Chem 60: 4-46 (2017)
Article DOI: 10.1021/acs.jmedchem.6b00538
BindingDB Entry DOI: 10.7270/Q2348NZC |
More data for this
Ligand-Target Pair | |
Urokinase-type plasminogen activator
(Homo sapiens (Human)) | BDBM50103651

(2-(3'-Amino-5-chloro-2-hydroxy-biphenyl-3-yl)-1H-b...)Show SMILES NC(=N)c1ccc2nc([nH]c2c1)-c1cc(Cl)cc(-c2cccc(N)c2)c1O Show InChI InChI=1S/C20H16ClN5O/c21-12-8-14(10-2-1-3-13(22)6-10)18(27)15(9-12)20-25-16-5-4-11(19(23)24)7-17(16)26-20/h1-9,27H,22H2,(H3,23,24)(H,25,26) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 0.460 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Axys Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
Binding affinity of the compound towards urokinase-type plasminogen activator (microPa) |
Bioorg Med Chem Lett 11: 2253-6 (2001)
BindingDB Entry DOI: 10.7270/Q2XG9QD9 |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50103651

(2-(3'-Amino-5-chloro-2-hydroxy-biphenyl-3-yl)-1H-b...)Show SMILES NC(=N)c1ccc2nc([nH]c2c1)-c1cc(Cl)cc(-c2cccc(N)c2)c1O Show InChI InChI=1S/C20H16ClN5O/c21-12-8-14(10-2-1-3-13(22)6-10)18(27)15(9-12)20-25-16-5-4-11(19(23)24)7-17(16)26-20/h1-9,27H,22H2,(H3,23,24)(H,25,26) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 0.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Axys Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
Binding affinity of the compound towards Coagulation factor X |
Bioorg Med Chem Lett 11: 2253-6 (2001)
BindingDB Entry DOI: 10.7270/Q2XG9QD9 |
More data for this
Ligand-Target Pair | |
Fatty-acid amide hydrolase 1 [30-579]
(Rattus norvegicus (rat)) | BDBM50161525
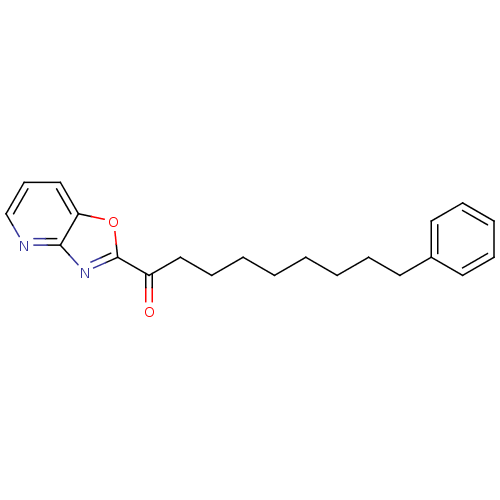
(1-(oxazolo[4,5-b]pyridin-2-yl)-9-phenylnonan-1-one...)Show InChI InChI=1S/C21H24N2O2/c24-18(21-23-20-19(25-21)15-10-16-22-20)14-9-4-2-1-3-6-11-17-12-7-5-8-13-17/h5,7-8,10,12-13,15-16H,1-4,6,9,11,14H2 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.520 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ de Lille
Curated by ChEMBL
| Assay Description
Inhibition of rat FAAH assessed as reduction in [14C]oleamide conversion to oleic acid by Lineweaver-Burk plot analysis |
J Med Chem 60: 4-46 (2017)
Article DOI: 10.1021/acs.jmedchem.6b00538
BindingDB Entry DOI: 10.7270/Q2348NZC |
More data for this
Ligand-Target Pair | |
Fatty-acid amide hydrolase 1 [30-579]
(Rattus norvegicus (rat)) | BDBM50350547
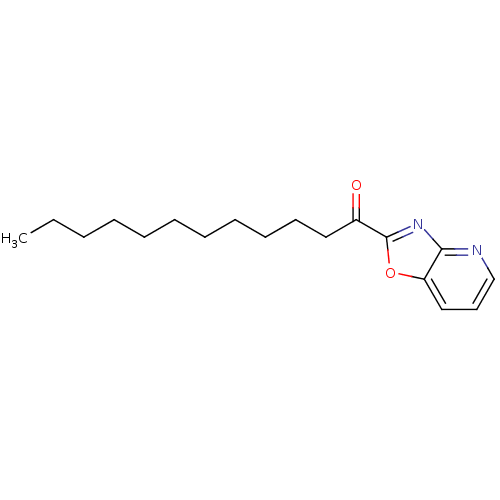
(CHEMBL257307)Show InChI InChI=1S/C18H26N2O2/c1-2-3-4-5-6-7-8-9-10-12-15(21)18-20-17-16(22-18)13-11-14-19-17/h11,13-14H,2-10,12H2,1H3 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.570 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ de Lille
Curated by ChEMBL
| Assay Description
Inhibition of rat FAAH assessed as reduction in [14C]oleamide conversion to oleic acid by Lineweaver-Burk plot analysis |
J Med Chem 60: 4-46 (2017)
Article DOI: 10.1021/acs.jmedchem.6b00538
BindingDB Entry DOI: 10.7270/Q2348NZC |
More data for this
Ligand-Target Pair | |
Gastrin/cholecystokinin type B receptor
(Homo sapiens (Human)) | BDBM50411345
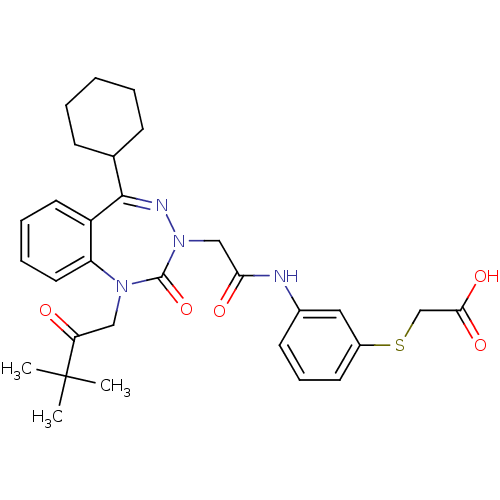
(CHEMBL389639)Show SMILES CC(C)(C)C(=O)CN1c2ccccc2C(=NN(CC(=O)Nc2cccc(SCC(O)=O)c2)C1=O)C1CCCCC1 |c:15| Show InChI InChI=1S/C30H36N4O5S/c1-30(2,3)25(35)17-33-24-15-8-7-14-23(24)28(20-10-5-4-6-11-20)32-34(29(33)39)18-26(36)31-21-12-9-13-22(16-21)40-19-27(37)38/h7-9,12-16,20H,4-6,10-11,17-19H2,1-3H3,(H,31,36)(H,37,38) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.650 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
James Black Foundation
Curated by ChEMBL
| Assay Description
Displacement of [3H]BH-CCK-8S from human recombinant CCK2 receptor expressed in NIH3T3 cells |
J Med Chem 50: 3101-12 (2007)
Checked by Author
Article DOI: 10.1021/jm070139l
BindingDB Entry DOI: 10.7270/Q2QJ7JHN |
More data for this
Ligand-Target Pair | |
Gastrin/cholecystokinin type B receptor
(Homo sapiens (Human)) | BDBM50002878
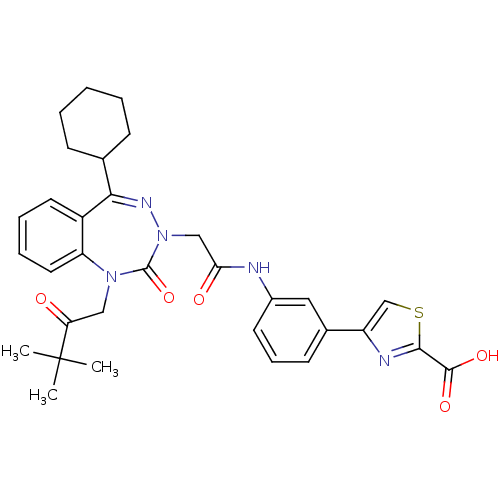
(CHEMBL227275)Show SMILES CC(C)(C)C(=O)CN1c2ccccc2C(=NN(CC(=O)Nc2cccc(c2)-c2csc(n2)C(O)=O)C1=O)C1CCCCC1 |c:15| Show InChI InChI=1S/C32H35N5O5S/c1-32(2,3)26(38)17-36-25-15-8-7-14-23(25)28(20-10-5-4-6-11-20)35-37(31(36)42)18-27(39)33-22-13-9-12-21(16-22)24-19-43-29(34-24)30(40)41/h7-9,12-16,19-20H,4-6,10-11,17-18H2,1-3H3,(H,33,39)(H,40,41) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| 0.660 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
James Black Foundation
Curated by ChEMBL
| Assay Description
Displacement of [3H]BH-CCK-8S from human recombinant CCK2 receptor expressed in NIH3T3 cells |
J Med Chem 50: 3101-12 (2007)
Checked by Author
Article DOI: 10.1021/jm070139l
BindingDB Entry DOI: 10.7270/Q2QJ7JHN |
More data for this
Ligand-Target Pair | |
Fatty-acid amide hydrolase 1 [30-579]
(Rattus norvegicus (rat)) | BDBM50350545
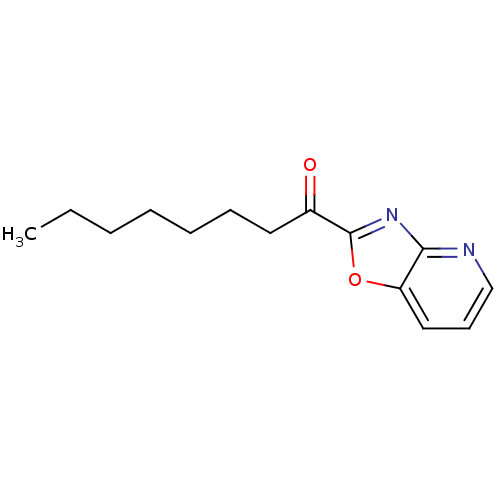
(CHEMBL257305)Show InChI InChI=1S/C14H18N2O2/c1-2-3-4-5-6-8-11(17)14-16-13-12(18-14)9-7-10-15-13/h7,9-10H,2-6,8H2,1H3 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.690 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ de Lille
Curated by ChEMBL
| Assay Description
Inhibition of rat FAAH assessed as reduction in [14C]oleamide conversion to oleic acid by Lineweaver-Burk plot analysis |
J Med Chem 60: 4-46 (2017)
Article DOI: 10.1021/acs.jmedchem.6b00538
BindingDB Entry DOI: 10.7270/Q2348NZC |
More data for this
Ligand-Target Pair | |
Gastrin/cholecystokinin type B receptor
(Homo sapiens (Human)) | BDBM50002876
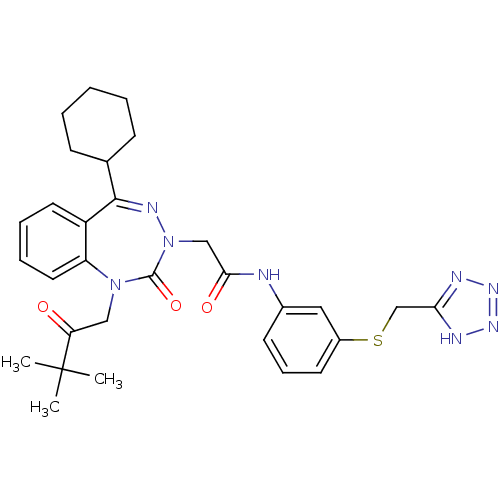
(CHEMBL435143)Show SMILES CC(C)(C)C(=O)CN1c2ccccc2C(=NN(CC(=O)Nc2cccc(SCc3nn[nH]n3)c2)C1=O)C1CCCCC1 |c:15| Show InChI InChI=1S/C30H36N8O3S/c1-30(2,3)25(39)17-37-24-15-8-7-14-23(24)28(20-10-5-4-6-11-20)34-38(29(37)41)18-27(40)31-21-12-9-13-22(16-21)42-19-26-32-35-36-33-26/h7-9,12-16,20H,4-6,10-11,17-19H2,1-3H3,(H,31,40)(H,32,33,35,36) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.710 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
James Black Foundation
Curated by ChEMBL
| Assay Description
Displacement of [3H]BH-CCK-8S from human recombinant CCK2 receptor expressed in NIH3T3 cells |
J Med Chem 50: 3101-12 (2007)
Checked by Author
Article DOI: 10.1021/jm070139l
BindingDB Entry DOI: 10.7270/Q2QJ7JHN |
More data for this
Ligand-Target Pair | |
Fatty-acid amide hydrolase 1 [30-579]
(Rattus norvegicus (rat)) | BDBM50350546
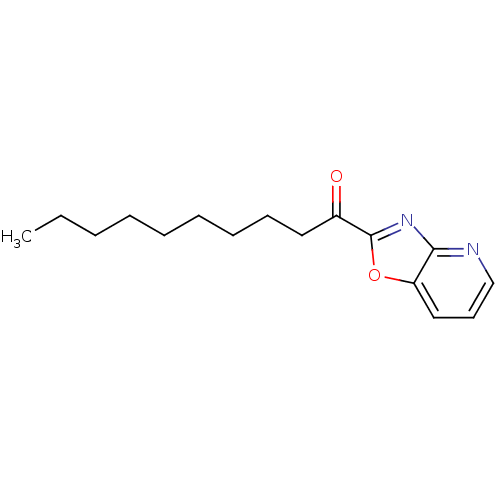
(CHEMBL1812708)Show InChI InChI=1S/C16H22N2O2/c1-2-3-4-5-6-7-8-10-13(19)16-18-15-14(20-16)11-9-12-17-15/h9,11-12H,2-8,10H2,1H3 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.75 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ de Lille
Curated by ChEMBL
| Assay Description
Inhibition of rat FAAH assessed as reduction in [14C]oleamide conversion to oleic acid by Lineweaver-Burk plot analysis |
J Med Chem 60: 4-46 (2017)
Article DOI: 10.1021/acs.jmedchem.6b00538
BindingDB Entry DOI: 10.7270/Q2348NZC |
More data for this
Ligand-Target Pair | |
Gastrin/cholecystokinin type B receptor
(Homo sapiens (Human)) | BDBM50002889
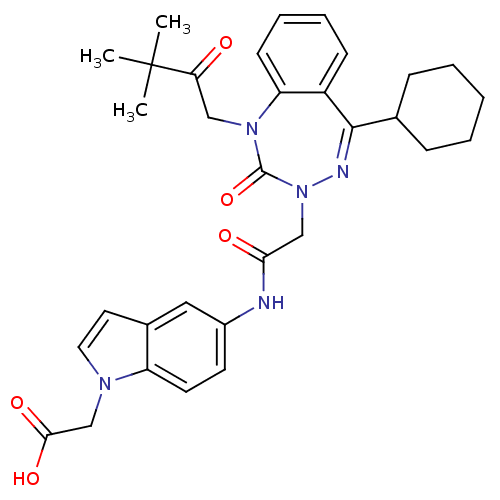
(CHEMBL438843)Show SMILES CC(C)(C)C(=O)CN1c2ccccc2C(=NN(CC(=O)Nc2ccc3n(CC(O)=O)ccc3c2)C1=O)C1CCCCC1 |c:15| Show InChI InChI=1S/C32H37N5O5/c1-32(2,3)27(38)18-36-26-12-8-7-11-24(26)30(21-9-5-4-6-10-21)34-37(31(36)42)19-28(39)33-23-13-14-25-22(17-23)15-16-35(25)20-29(40)41/h7-8,11-17,21H,4-6,9-10,18-20H2,1-3H3,(H,33,39)(H,40,41) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.780 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
James Black Foundation
Curated by ChEMBL
| Assay Description
Displacement of [3H]BH-CCK-8S from human recombinant CCK2 receptor expressed in NIH3T3 cells |
J Med Chem 50: 3101-12 (2007)
Checked by Author
Article DOI: 10.1021/jm070139l
BindingDB Entry DOI: 10.7270/Q2QJ7JHN |
More data for this
Ligand-Target Pair | |
Gastrin/cholecystokinin type B receptor
(Homo sapiens (Human)) | BDBM50002902
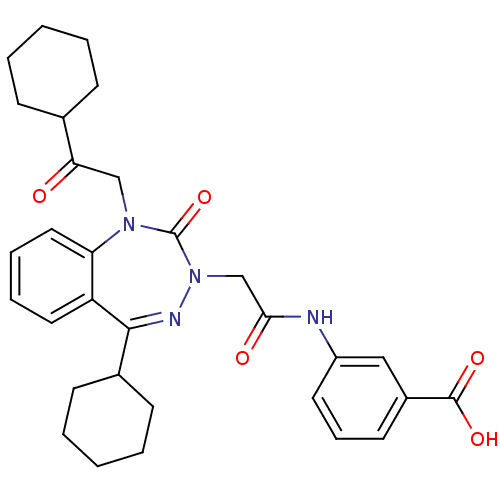
(CHEMBL226622)Show SMILES OC(=O)c1cccc(NC(=O)CN2N=C(C3CCCCC3)c3ccccc3N(CC(=O)C3CCCCC3)C2=O)c1 |t:13| Show InChI InChI=1S/C31H36N4O5/c36-27(21-10-3-1-4-11-21)19-34-26-17-8-7-16-25(26)29(22-12-5-2-6-13-22)33-35(31(34)40)20-28(37)32-24-15-9-14-23(18-24)30(38)39/h7-9,14-18,21-22H,1-6,10-13,19-20H2,(H,32,37)(H,38,39) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.790 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
James Black Foundation
Curated by ChEMBL
| Assay Description
Displacement of [3H]BH-CCK-8S from human recombinant CCK2 receptor expressed in NIH3T3 cells |
J Med Chem 50: 3101-12 (2007)
Checked by Author
Article DOI: 10.1021/jm070139l
BindingDB Entry DOI: 10.7270/Q2QJ7JHN |
More data for this
Ligand-Target Pair | |
Gastrin/cholecystokinin type B receptor
(Homo sapiens (Human)) | BDBM50002888
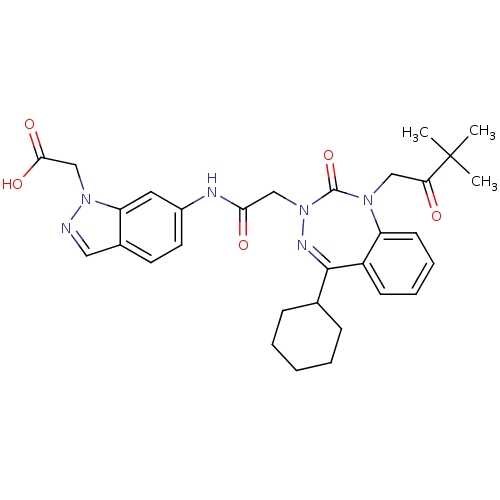
(CHEMBL389713)Show SMILES CC(C)(C)C(=O)CN1c2ccccc2C(=NN(CC(=O)Nc2ccc3cnn(CC(O)=O)c3c2)C1=O)C1CCCCC1 |c:15| Show InChI InChI=1S/C31H36N6O5/c1-31(2,3)26(38)17-35-24-12-8-7-11-23(24)29(20-9-5-4-6-10-20)34-37(30(35)42)18-27(39)33-22-14-13-21-16-32-36(19-28(40)41)25(21)15-22/h7-8,11-16,20H,4-6,9-10,17-19H2,1-3H3,(H,33,39)(H,40,41) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.830 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
James Black Foundation
Curated by ChEMBL
| Assay Description
Displacement of [3H]BH-CCK-8S from human recombinant CCK2 receptor expressed in NIH3T3 cells |
J Med Chem 50: 3101-12 (2007)
Checked by Author
Article DOI: 10.1021/jm070139l
BindingDB Entry DOI: 10.7270/Q2QJ7JHN |
More data for this
Ligand-Target Pair | |
Gastrin/cholecystokinin type B receptor
(Homo sapiens (Human)) | BDBM50002872
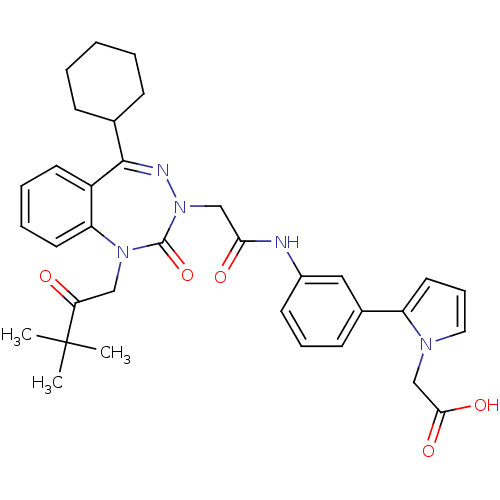
(CHEMBL437930)Show SMILES CC(C)(C)C(=O)CN1c2ccccc2C(=NN(CC(=O)Nc2cccc(c2)-c2cccn2CC(O)=O)C1=O)C1CCCCC1 |c:15| Show InChI InChI=1S/C34H39N5O5/c1-34(2,3)29(40)20-38-28-16-8-7-15-26(28)32(23-11-5-4-6-12-23)36-39(33(38)44)21-30(41)35-25-14-9-13-24(19-25)27-17-10-18-37(27)22-31(42)43/h7-10,13-19,23H,4-6,11-12,20-22H2,1-3H3,(H,35,41)(H,42,43) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.910 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
James Black Foundation
Curated by ChEMBL
| Assay Description
Displacement of [3H]BH-CCK-8S from human recombinant CCK2 receptor expressed in NIH3T3 cells |
J Med Chem 50: 3101-12 (2007)
Checked by Author
Article DOI: 10.1021/jm070139l
BindingDB Entry DOI: 10.7270/Q2QJ7JHN |
More data for this
Ligand-Target Pair | |
Parathyroid hormone/parathyroid hormone-related peptide receptor
(Homo sapiens (Human)) | BDBM50002926
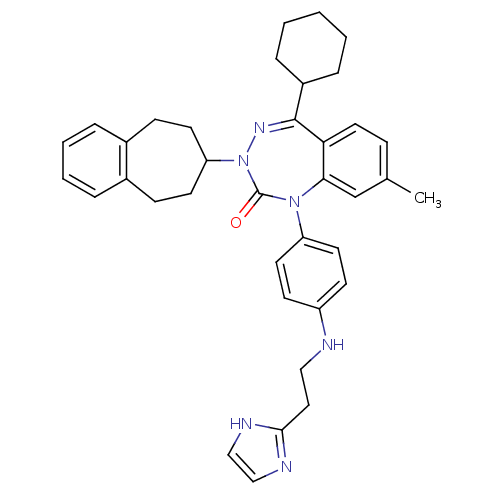
(CHEMBL244325)Show SMILES Cc1ccc2c(c1)N(c1ccc(NCCc3ncc[nH]3)cc1)C(=O)N(N=C2C1CCCCC1)C1CCc2ccccc2CC1 |c:28| Show InChI InChI=1S/C37H42N6O/c1-26-11-20-33-34(25-26)42(31-18-14-30(15-19-31)38-22-21-35-39-23-24-40-35)37(44)43(41-36(33)29-9-3-2-4-10-29)32-16-12-27-7-5-6-8-28(27)13-17-32/h5-8,11,14-15,18-20,23-25,29,32,38H,2-4,9-10,12-13,16-17,21-22H2,1H3,(H,39,40) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.910 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
James Black Foundation
Curated by ChEMBL
| Assay Description
Displacement of [125I]-[Nle,8,18 Tyr34]-hPTH(1-34) from human recombinant PTH1R expressed in HEK293 cells |
J Med Chem 50: 4789-92 (2007)
Checked by Author
Article DOI: 10.1021/jm0707626
BindingDB Entry DOI: 10.7270/Q2Z039C3 |
More data for this
Ligand-Target Pair | |
Gastrin/cholecystokinin type B receptor
(Homo sapiens (Human)) | BDBM50411348
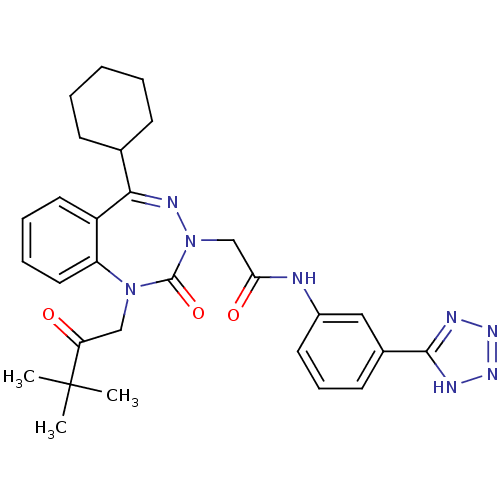
(CHEMBL226533)Show SMILES CC(C)(C)C(=O)CN1c2ccccc2C(=NN(CC(=O)Nc2cccc(c2)-c2nnn[nH]2)C1=O)C1CCCCC1 |c:15| Show InChI InChI=1S/C29H34N8O3/c1-29(2,3)24(38)17-36-23-15-8-7-14-22(23)26(19-10-5-4-6-11-19)33-37(28(36)40)18-25(39)30-21-13-9-12-20(16-21)27-31-34-35-32-27/h7-9,12-16,19H,4-6,10-11,17-18H2,1-3H3,(H,30,39)(H,31,32,34,35) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
James Black Foundation
Curated by ChEMBL
| Assay Description
Displacement of [3H]BH-CCK-8S from human recombinant CCK2 receptor expressed in NIH3T3 cells |
J Med Chem 50: 3101-12 (2007)
Checked by Author
Article DOI: 10.1021/jm070139l
BindingDB Entry DOI: 10.7270/Q2QJ7JHN |
More data for this
Ligand-Target Pair | |
Gastrin/cholecystokinin type B receptor
(Homo sapiens (Human)) | BDBM50411347
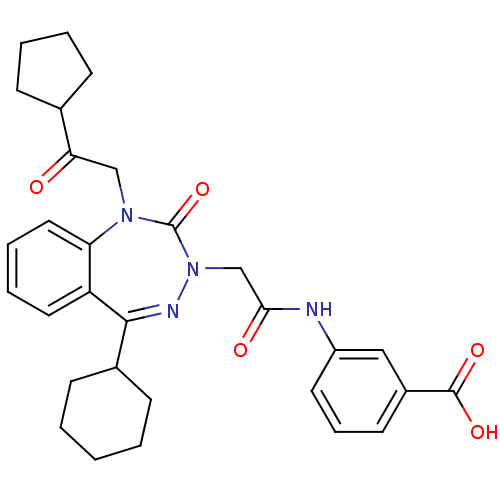
(CHEMBL226620)Show SMILES OC(=O)c1cccc(NC(=O)CN2N=C(C3CCCCC3)c3ccccc3N(CC(=O)C3CCCC3)C2=O)c1 |t:13| Show InChI InChI=1S/C30H34N4O5/c35-26(20-9-4-5-10-20)18-33-25-16-7-6-15-24(25)28(21-11-2-1-3-12-21)32-34(30(33)39)19-27(36)31-23-14-8-13-22(17-23)29(37)38/h6-8,13-17,20-21H,1-5,9-12,18-19H2,(H,31,36)(H,37,38) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
James Black Foundation
Curated by ChEMBL
| Assay Description
Displacement of [3H]BH-CCK-8S from human recombinant CCK2 receptor expressed in NIH3T3 cells |
J Med Chem 50: 3101-12 (2007)
Checked by Author
Article DOI: 10.1021/jm070139l
BindingDB Entry DOI: 10.7270/Q2QJ7JHN |
More data for this
Ligand-Target Pair | |
Gastrin/cholecystokinin type B receptor
(Homo sapiens (Human)) | BDBM50002895
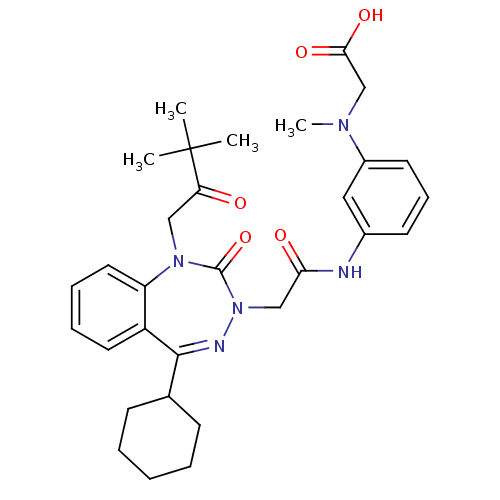
(CHEMBL226726)Show SMILES CN(CC(O)=O)c1cccc(NC(=O)CN2N=C(C3CCCCC3)c3ccccc3N(CC(=O)C(C)(C)C)C2=O)c1 |t:16| Show InChI InChI=1S/C31H39N5O5/c1-31(2,3)26(37)18-35-25-16-9-8-15-24(25)29(21-11-6-5-7-12-21)33-36(30(35)41)19-27(38)32-22-13-10-14-23(17-22)34(4)20-28(39)40/h8-10,13-17,21H,5-7,11-12,18-20H2,1-4H3,(H,32,38)(H,39,40) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 1.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
James Black Foundation
Curated by ChEMBL
| Assay Description
Displacement of [3H]BH-CCK-8S from human recombinant CCK2 receptor expressed in NIH3T3 cells |
J Med Chem 50: 3101-12 (2007)
Checked by Author
Article DOI: 10.1021/jm070139l
BindingDB Entry DOI: 10.7270/Q2QJ7JHN |
More data for this
Ligand-Target Pair | |
Gastrin/cholecystokinin type B receptor
(Homo sapiens (Human)) | BDBM50002898
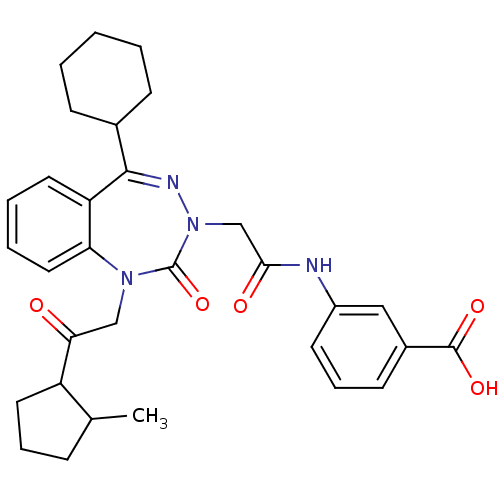
(CHEMBL388068)Show SMILES CC1CCCC1C(=O)CN1c2ccccc2C(=NN(CC(=O)Nc2cccc(c2)C(O)=O)C1=O)C1CCCCC1 |c:18| Show InChI InChI=1S/C31H36N4O5/c1-20-9-7-15-24(20)27(36)18-34-26-16-6-5-14-25(26)29(21-10-3-2-4-11-21)33-35(31(34)40)19-28(37)32-23-13-8-12-22(17-23)30(38)39/h5-6,8,12-14,16-17,20-21,24H,2-4,7,9-11,15,18-19H2,1H3,(H,32,37)(H,38,39) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
James Black Foundation
Curated by ChEMBL
| Assay Description
Displacement of [3H]BH-CCK-8S from human recombinant CCK2 receptor expressed in NIH3T3 cells |
J Med Chem 50: 3101-12 (2007)
Checked by Author
Article DOI: 10.1021/jm070139l
BindingDB Entry DOI: 10.7270/Q2QJ7JHN |
More data for this
Ligand-Target Pair | |
Serine protease 1
(Bos taurus (bovine)) | BDBM14338
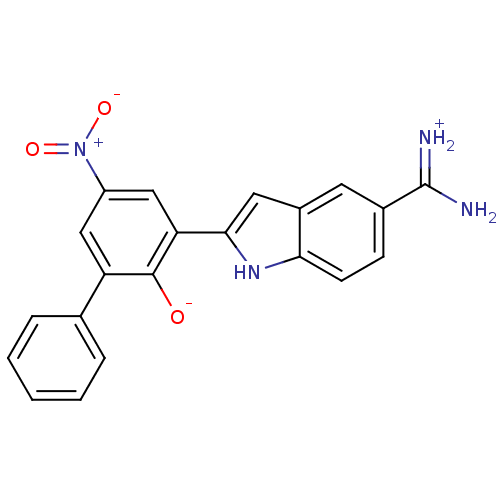
(2-{5-[amino(iminiumyl)methyl]-1H-indol-2-yl}-4-nit...)Show SMILES NC(=[NH2+])c1ccc2[nH]c(cc2c1)-c1cc(cc(-c2ccccc2)c1[O-])[N+]([O-])=O Show InChI InChI=1S/C21H16N4O3/c22-21(23)13-6-7-18-14(8-13)9-19(24-18)17-11-15(25(27)28)10-16(20(17)26)12-4-2-1-3-5-12/h1-11,24,26H,(H3,22,23) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Celera
| Assay Description
Each enzyme was assayed with a set of different concentrations of each inhibitor. After addition of the appropriate substrate, the rate of hydrolysis... |
J Mol Biol 329: 93-120 (2003)
Article DOI: 10.1016/s0022-2836(03)00399-1
BindingDB Entry DOI: 10.7270/Q2R78CGQ |
More data for this
Ligand-Target Pair | |
Parathyroid hormone/parathyroid hormone-related peptide receptor
(Homo sapiens (Human)) | BDBM50002913
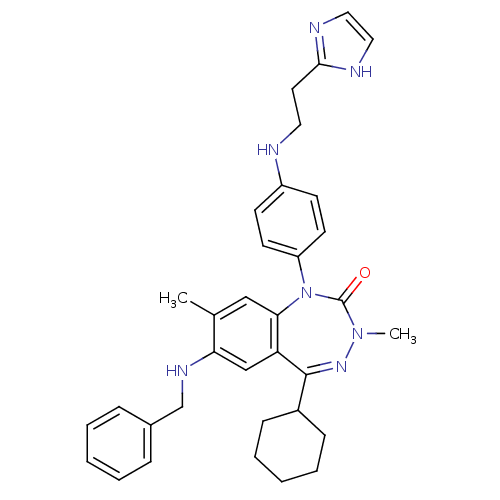
(CHEMBL389783)Show SMILES CN1N=C(C2CCCCC2)c2cc(NCc3ccccc3)c(C)cc2N(c2ccc(NCCc3ncc[nH]3)cc2)C1=O |t:2| Show InChI InChI=1S/C34H39N7O/c1-24-21-31-29(22-30(24)38-23-25-9-5-3-6-10-25)33(26-11-7-4-8-12-26)39-40(2)34(42)41(31)28-15-13-27(14-16-28)35-18-17-32-36-19-20-37-32/h3,5-6,9-10,13-16,19-22,26,35,38H,4,7-8,11-12,17-18,23H2,1-2H3,(H,36,37) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 1.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
James Black Foundation
Curated by ChEMBL
| Assay Description
Displacement of [125I]-[Nle,8,18 Tyr34]-hPTH(1-34) from human recombinant PTH1R expressed in HEK293 cells |
J Med Chem 50: 4789-92 (2007)
Checked by Author
Article DOI: 10.1021/jm0707626
BindingDB Entry DOI: 10.7270/Q2Z039C3 |
More data for this
Ligand-Target Pair | |
Dimer of Tryptase beta-2
(Homo sapiens (Human)) | BDBM50187167
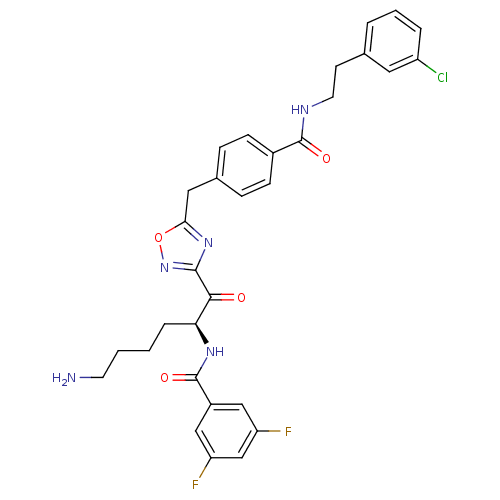
(CHEMBL211357 | N-[(S)-5-amino-1-(5-{4-[2-(3-chloro...)Show SMILES NCCCC[C@H](NC(=O)c1cc(F)cc(F)c1)C(=O)c1noc(Cc2ccc(cc2)C(=O)NCCc2cccc(Cl)c2)n1 Show InChI InChI=1S/C31H30ClF2N5O4/c32-23-5-3-4-19(14-23)11-13-36-30(41)21-9-7-20(8-10-21)15-27-38-29(39-43-27)28(40)26(6-1-2-12-35)37-31(42)22-16-24(33)18-25(34)17-22/h3-5,7-10,14,16-18,26H,1-2,6,11-13,15,35H2,(H,36,41)(H,37,42)/t26-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Celera
Curated by ChEMBL
| Assay Description
Inhibition of human beta tryptase |
Bioorg Med Chem Lett 16: 4036-40 (2006)
Article DOI: 10.1016/j.bmcl.2006.05.009
BindingDB Entry DOI: 10.7270/Q20864ZV |
More data for this
Ligand-Target Pair | |
Gastrin/cholecystokinin type B receptor
(Homo sapiens (Human)) | BDBM50002877
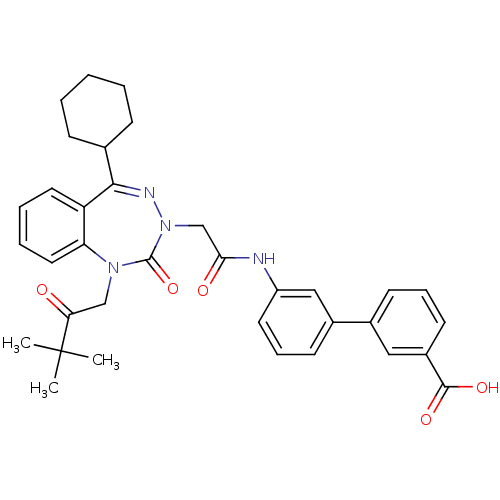
(CHEMBL388143)Show SMILES CC(C)(C)C(=O)CN1c2ccccc2C(=NN(CC(=O)Nc2cccc(c2)-c2cccc(c2)C(O)=O)C1=O)C1CCCCC1 |c:15| Show InChI InChI=1S/C35H38N4O5/c1-35(2,3)30(40)21-38-29-18-8-7-17-28(29)32(23-11-5-4-6-12-23)37-39(34(38)44)22-31(41)36-27-16-10-14-25(20-27)24-13-9-15-26(19-24)33(42)43/h7-10,13-20,23H,4-6,11-12,21-22H2,1-3H3,(H,36,41)(H,42,43) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 1.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
James Black Foundation
Curated by ChEMBL
| Assay Description
Displacement of [3H]BH-CCK-8S from human recombinant CCK2 receptor expressed in NIH3T3 cells |
J Med Chem 50: 3101-12 (2007)
Checked by Author
Article DOI: 10.1021/jm070139l
BindingDB Entry DOI: 10.7270/Q2QJ7JHN |
More data for this
Ligand-Target Pair | |
Dimer of Tryptase beta-2
(Homo sapiens (Human)) | BDBM50187166
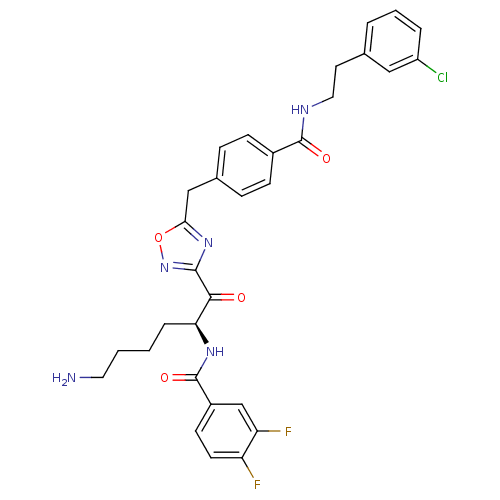
(CHEMBL380293 | N-[(S)-5-amino-1-(5-{4-[2-(3-chloro...)Show SMILES NCCCC[C@H](NC(=O)c1ccc(F)c(F)c1)C(=O)c1noc(Cc2ccc(cc2)C(=O)NCCc2cccc(Cl)c2)n1 Show InChI InChI=1S/C31H30ClF2N5O4/c32-23-5-3-4-19(16-23)13-15-36-30(41)21-9-7-20(8-10-21)17-27-38-29(39-43-27)28(40)26(6-1-2-14-35)37-31(42)22-11-12-24(33)25(34)18-22/h3-5,7-12,16,18,26H,1-2,6,13-15,17,35H2,(H,36,41)(H,37,42)/t26-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Celera
Curated by ChEMBL
| Assay Description
Inhibition of human beta tryptase |
Bioorg Med Chem Lett 16: 4036-40 (2006)
Article DOI: 10.1016/j.bmcl.2006.05.009
BindingDB Entry DOI: 10.7270/Q20864ZV |
More data for this
Ligand-Target Pair | |
Dimer of Tryptase beta-2
(Homo sapiens (Human)) | BDBM50187177
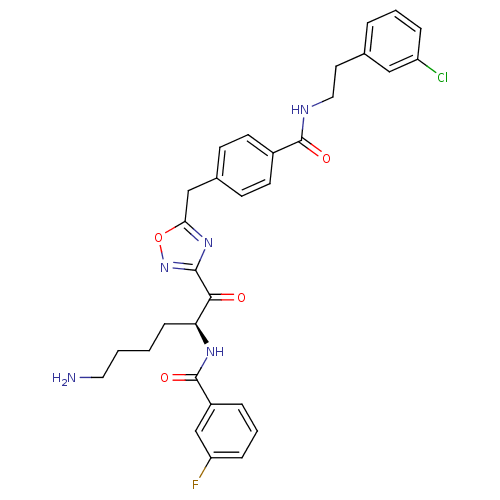
(CHEMBL212774 | N-[(2S)-6-amino-1-{5-[(4-{[2-(3-chl...)Show SMILES NCCCC[C@H](NC(=O)c1cccc(F)c1)C(=O)c1noc(Cc2ccc(cc2)C(=O)NCCc2cccc(Cl)c2)n1 Show InChI InChI=1S/C31H31ClFN5O4/c32-24-7-3-5-20(17-24)14-16-35-30(40)22-12-10-21(11-13-22)18-27-37-29(38-42-27)28(39)26(9-1-2-15-34)36-31(41)23-6-4-8-25(33)19-23/h3-8,10-13,17,19,26H,1-2,9,14-16,18,34H2,(H,35,40)(H,36,41)/t26-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Celera
Curated by ChEMBL
| Assay Description
Inhibition of human beta tryptase |
Bioorg Med Chem Lett 16: 4036-40 (2006)
Article DOI: 10.1016/j.bmcl.2006.05.009
BindingDB Entry DOI: 10.7270/Q20864ZV |
More data for this
Ligand-Target Pair | |
Dimer of Tryptase beta-2
(Homo sapiens (Human)) | BDBM50187163
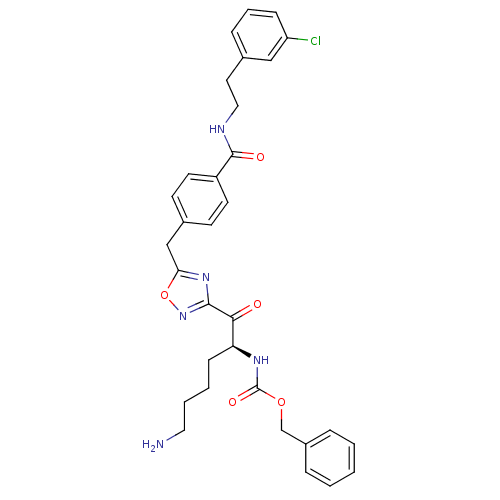
((S)-benzyl 1-(5-(4-((3-chlorophenethyl)carbamoyl)b...)Show SMILES NCCCC[C@H](NC(=O)OCc1ccccc1)C(=O)c1noc(Cc2ccc(cc2)C(=O)NCCc2cccc(Cl)c2)n1 Show InChI InChI=1S/C32H34ClN5O5/c33-26-10-6-9-22(19-26)16-18-35-31(40)25-14-12-23(13-15-25)20-28-37-30(38-43-28)29(39)27(11-4-5-17-34)36-32(41)42-21-24-7-2-1-3-8-24/h1-3,6-10,12-15,19,27H,4-5,11,16-18,20-21,34H2,(H,35,40)(H,36,41)/t27-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Celera
Curated by ChEMBL
| Assay Description
Inhibition of human beta tryptase |
Bioorg Med Chem Lett 16: 4036-40 (2006)
Article DOI: 10.1016/j.bmcl.2006.05.009
BindingDB Entry DOI: 10.7270/Q20864ZV |
More data for this
Ligand-Target Pair | |
Parathyroid hormone/parathyroid hormone-related peptide receptor
(Homo sapiens (Human)) | BDBM50002915
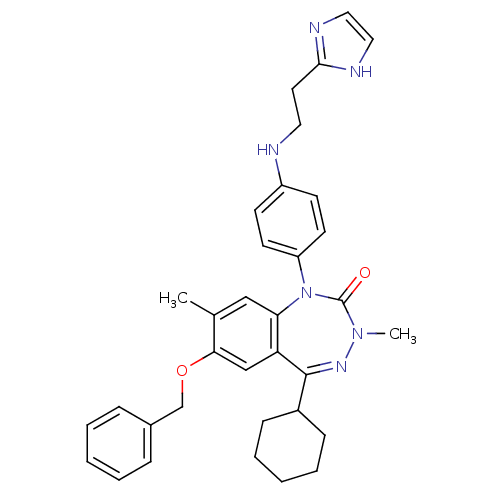
(CHEMBL389787)Show SMILES CN1N=C(C2CCCCC2)c2cc(OCc3ccccc3)c(C)cc2N(c2ccc(NCCc3ncc[nH]3)cc2)C1=O |t:2| Show InChI InChI=1S/C34H38N6O2/c1-24-21-30-29(22-31(24)42-23-25-9-5-3-6-10-25)33(26-11-7-4-8-12-26)38-39(2)34(41)40(30)28-15-13-27(14-16-28)35-18-17-32-36-19-20-37-32/h3,5-6,9-10,13-16,19-22,26,35H,4,7-8,11-12,17-18,23H2,1-2H3,(H,36,37) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
James Black Foundation
Curated by ChEMBL
| Assay Description
Displacement of [125I]-[Nle,8,18 Tyr34]-hPTH(1-34) from human recombinant PTH1R expressed in HEK293 cells |
J Med Chem 50: 4789-92 (2007)
Checked by Author
Article DOI: 10.1021/jm0707626
BindingDB Entry DOI: 10.7270/Q2Z039C3 |
More data for this
Ligand-Target Pair | |
Dimer of Tryptase beta-2
(Homo sapiens (Human)) | BDBM50187160

(CHEMBL213928 | N-[(2S)-6-amino-1-{5-[(4-{[2-(3-chl...)Show SMILES NCCCC[C@H](NC(=O)c1ccc(F)cc1)C(=O)c1noc(Cc2ccc(cc2)C(=O)NCCc2cccc(Cl)c2)n1 Show InChI InChI=1S/C31H31ClFN5O4/c32-24-5-3-4-20(18-24)15-17-35-30(40)22-9-7-21(8-10-22)19-27-37-29(38-42-27)28(39)26(6-1-2-16-34)36-31(41)23-11-13-25(33)14-12-23/h3-5,7-14,18,26H,1-2,6,15-17,19,34H2,(H,35,40)(H,36,41)/t26-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Celera
Curated by ChEMBL
| Assay Description
Inhibition of human beta tryptase |
Bioorg Med Chem Lett 16: 4036-40 (2006)
Article DOI: 10.1016/j.bmcl.2006.05.009
BindingDB Entry DOI: 10.7270/Q20864ZV |
More data for this
Ligand-Target Pair | |
Dimer of Tryptase beta-2
(Homo sapiens (Human)) | BDBM50187168
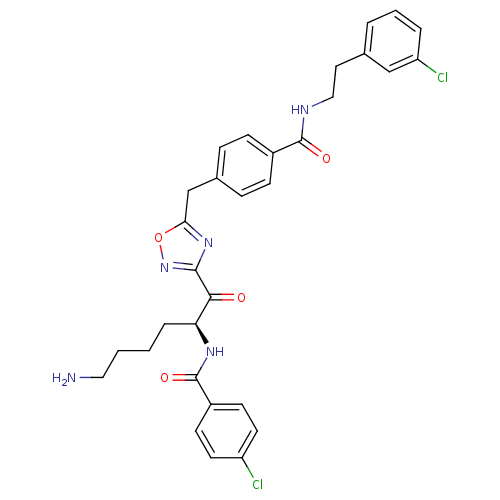
(CHEMBL377656 | N-[(2S)-6-amino-1-{5-[(4-{[2-(3-chl...)Show SMILES NCCCC[C@H](NC(=O)c1ccc(Cl)cc1)C(=O)c1noc(Cc2ccc(cc2)C(=O)NCCc2cccc(Cl)c2)n1 Show InChI InChI=1S/C31H31Cl2N5O4/c32-24-13-11-23(12-14-24)31(41)36-26(6-1-2-16-34)28(39)29-37-27(42-38-29)19-21-7-9-22(10-8-21)30(40)35-17-15-20-4-3-5-25(33)18-20/h3-5,7-14,18,26H,1-2,6,15-17,19,34H2,(H,35,40)(H,36,41)/t26-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Celera
Curated by ChEMBL
| Assay Description
Inhibition of human beta tryptase |
Bioorg Med Chem Lett 16: 4036-40 (2006)
Article DOI: 10.1016/j.bmcl.2006.05.009
BindingDB Entry DOI: 10.7270/Q20864ZV |
More data for this
Ligand-Target Pair | |
Dimer of Tryptase beta-2
(Homo sapiens (Human)) | BDBM50187176
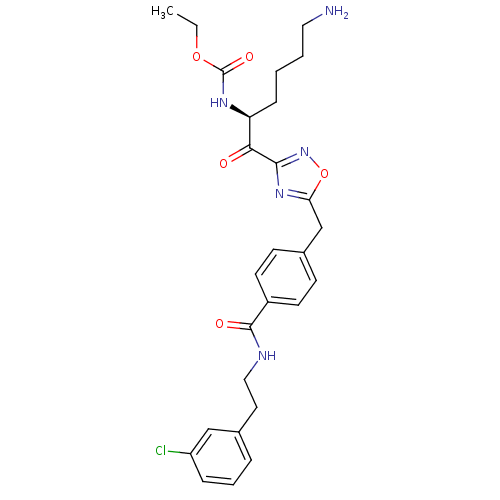
((S)-ethyl 1-(5-(4-((3-chlorophenethyl)carbamoyl)be...)Show SMILES CCOC(=O)N[C@@H](CCCCN)C(=O)c1noc(Cc2ccc(cc2)C(=O)NCCc2cccc(Cl)c2)n1 Show InChI InChI=1S/C27H32ClN5O5/c1-2-37-27(36)31-22(8-3-4-14-29)24(34)25-32-23(38-33-25)17-19-9-11-20(12-10-19)26(35)30-15-13-18-6-5-7-21(28)16-18/h5-7,9-12,16,22H,2-4,8,13-15,17,29H2,1H3,(H,30,35)(H,31,36)/t22-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Celera
Curated by ChEMBL
| Assay Description
Inhibition of human beta tryptase |
Bioorg Med Chem Lett 16: 4036-40 (2006)
Article DOI: 10.1016/j.bmcl.2006.05.009
BindingDB Entry DOI: 10.7270/Q20864ZV |
More data for this
Ligand-Target Pair | |
Gastrin/cholecystokinin type B receptor
(Homo sapiens (Human)) | BDBM50411343
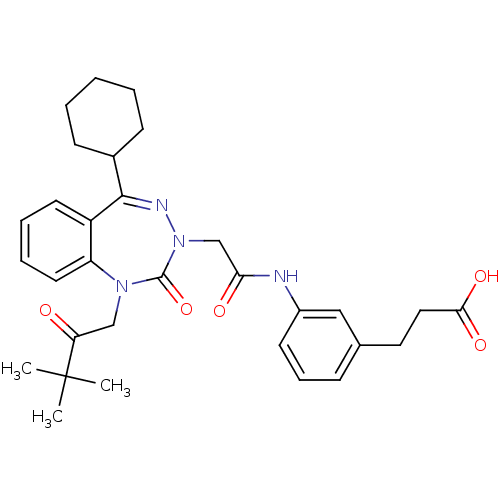
(CHEMBL226675)Show SMILES CC(C)(C)C(=O)CN1c2ccccc2C(=NN(CC(=O)Nc2cccc(CCC(O)=O)c2)C1=O)C1CCCCC1 |c:15| Show InChI InChI=1S/C31H38N4O5/c1-31(2,3)26(36)19-34-25-15-8-7-14-24(25)29(22-11-5-4-6-12-22)33-35(30(34)40)20-27(37)32-23-13-9-10-21(18-23)16-17-28(38)39/h7-10,13-15,18,22H,4-6,11-12,16-17,19-20H2,1-3H3,(H,32,37)(H,38,39) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
James Black Foundation
Curated by ChEMBL
| Assay Description
Displacement of [3H]BH-CCK-8S from human recombinant CCK2 receptor expressed in NIH3T3 cells |
J Med Chem 50: 3101-12 (2007)
Checked by Author
Article DOI: 10.1021/jm070139l
BindingDB Entry DOI: 10.7270/Q2QJ7JHN |
More data for this
Ligand-Target Pair | |
Gastrin/cholecystokinin type B receptor
(Homo sapiens (Human)) | BDBM50411338
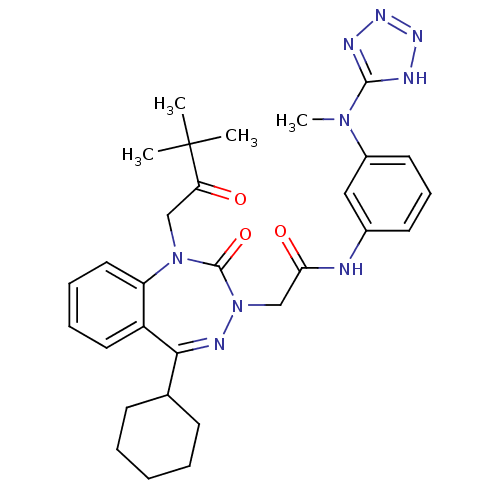
(CHEMBL227254)Show SMILES CN(c1nnn[nH]1)c1cccc(NC(=O)CN2N=C(C3CCCCC3)c3ccccc3N(CC(=O)C(C)(C)C)C2=O)c1 |t:18| Show InChI InChI=1S/C30H37N9O3/c1-30(2,3)25(40)18-38-24-16-9-8-15-23(24)27(20-11-6-5-7-12-20)34-39(29(38)42)19-26(41)31-21-13-10-14-22(17-21)37(4)28-32-35-36-33-28/h8-10,13-17,20H,5-7,11-12,18-19H2,1-4H3,(H,31,41)(H,32,33,35,36) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
James Black Foundation
Curated by ChEMBL
| Assay Description
Displacement of [3H]BH-CCK-8S from human recombinant CCK2 receptor expressed in NIH3T3 cells |
J Med Chem 50: 3101-12 (2007)
Checked by Author
Article DOI: 10.1021/jm070139l
BindingDB Entry DOI: 10.7270/Q2QJ7JHN |
More data for this
Ligand-Target Pair | |
Dimer of Tryptase beta-2
(Homo sapiens (Human)) | BDBM50187162
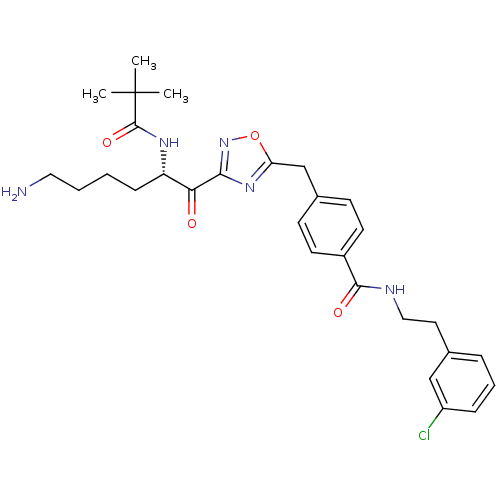
((S)-N-(3-chlorophenethyl)-4-((3-(6-amino-2-pivalam...)Show SMILES CC(C)(C)C(=O)N[C@@H](CCCCN)C(=O)c1noc(Cc2ccc(cc2)C(=O)NCCc2cccc(Cl)c2)n1 Show InChI InChI=1S/C29H36ClN5O4/c1-29(2,3)28(38)33-23(9-4-5-15-31)25(36)26-34-24(39-35-26)18-20-10-12-21(13-11-20)27(37)32-16-14-19-7-6-8-22(30)17-19/h6-8,10-13,17,23H,4-5,9,14-16,18,31H2,1-3H3,(H,32,37)(H,33,38)/t23-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Celera
Curated by ChEMBL
| Assay Description
Inhibition of human beta tryptase |
Bioorg Med Chem Lett 16: 4036-40 (2006)
Article DOI: 10.1016/j.bmcl.2006.05.009
BindingDB Entry DOI: 10.7270/Q20864ZV |
More data for this
Ligand-Target Pair | |
Gastrin/cholecystokinin type B receptor
(Homo sapiens (Human)) | BDBM50411346
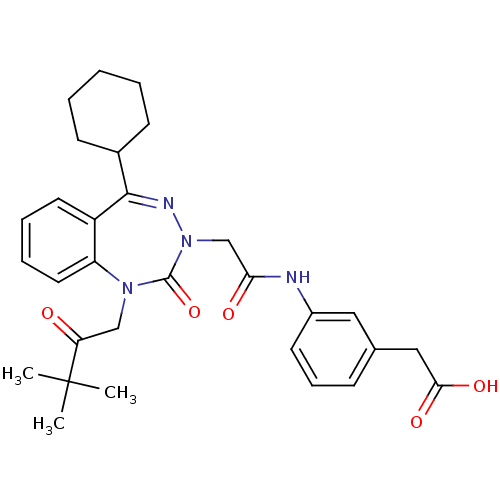
(CHEMBL226673)Show SMILES CC(C)(C)C(=O)CN1c2ccccc2C(=NN(CC(=O)Nc2cccc(CC(O)=O)c2)C1=O)C1CCCCC1 |c:15| Show InChI InChI=1S/C30H36N4O5/c1-30(2,3)25(35)18-33-24-15-8-7-14-23(24)28(21-11-5-4-6-12-21)32-34(29(33)39)19-26(36)31-22-13-9-10-20(16-22)17-27(37)38/h7-10,13-16,21H,4-6,11-12,17-19H2,1-3H3,(H,31,36)(H,37,38) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 2.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
James Black Foundation
Curated by ChEMBL
| Assay Description
Displacement of [3H]BH-CCK-8S from human recombinant CCK2 receptor expressed in NIH3T3 cells |
J Med Chem 50: 3101-12 (2007)
Checked by Author
Article DOI: 10.1021/jm070139l
BindingDB Entry DOI: 10.7270/Q2QJ7JHN |
More data for this
Ligand-Target Pair | |
Dimer of Tryptase beta-2
(Homo sapiens (Human)) | BDBM50187182

((S)-N-(1-(5-(4-((3-chlorophenethyl)carbamoyl)benzy...)Show SMILES NCCCC[C@H](NC(=O)c1ccc2OCOc2c1)C(=O)c1noc(Cc2ccc(cc2)C(=O)NCCc2cccc(Cl)c2)n1 Show InChI InChI=1S/C32H32ClN5O6/c33-24-5-3-4-20(16-24)13-15-35-31(40)22-9-7-21(8-10-22)17-28-37-30(38-44-28)29(39)25(6-1-2-14-34)36-32(41)23-11-12-26-27(18-23)43-19-42-26/h3-5,7-12,16,18,25H,1-2,6,13-15,17,19,34H2,(H,35,40)(H,36,41)/t25-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 2.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Celera
Curated by ChEMBL
| Assay Description
Inhibition of human beta tryptase |
Bioorg Med Chem Lett 16: 4036-40 (2006)
Article DOI: 10.1016/j.bmcl.2006.05.009
BindingDB Entry DOI: 10.7270/Q20864ZV |
More data for this
Ligand-Target Pair | |
Urokinase-type plasminogen activator
(Homo sapiens (Human)) | BDBM14352
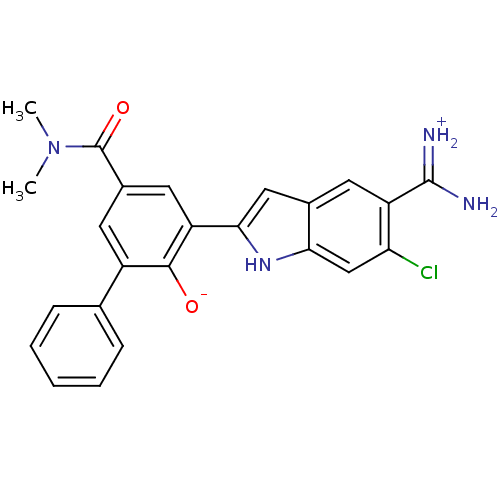
(2-{5-[amino(iminiumyl)methyl]-6-chloro-1H-indol-2-...)Show SMILES CN(C)C(=O)c1cc(-c2cc3cc(C(N)=[NH2+])c(Cl)cc3[nH]2)c([O-])c(c1)-c1ccccc1 Show InChI InChI=1S/C24H21ClN4O2/c1-29(2)24(31)15-9-16(13-6-4-3-5-7-13)22(30)18(10-15)21-11-14-8-17(23(26)27)19(25)12-20(14)28-21/h3-12,28,30H,1-2H3,(H3,26,27) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 2.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Celera
| Assay Description
Each enzyme was assayed with a set of different concentrations of each inhibitor. After addition of the appropriate substrate, the rate of hydrolysis... |
J Mol Biol 329: 93-120 (2003)
Article DOI: 10.1016/s0022-2836(03)00399-1
BindingDB Entry DOI: 10.7270/Q2R78CGQ |
More data for this
Ligand-Target Pair | |
Fatty-acid amide hydrolase 1 [30-579]
(Rattus norvegicus (rat)) | BDBM50100865
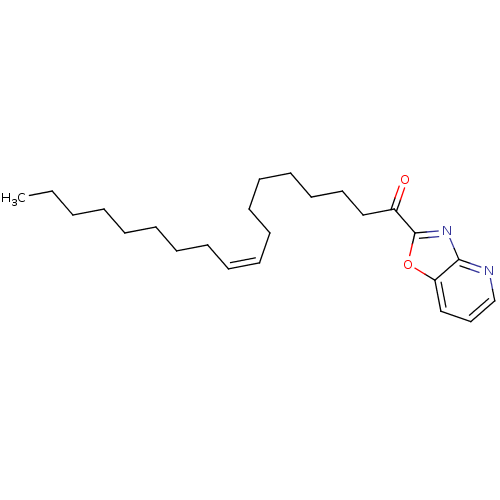
((Z)-1-(oxazolo[4,5-b]pyridin-2-yl)octadec-9-en-1-o...)Show InChI InChI=1S/C24H36N2O2/c1-2-3-4-5-6-7-8-9-10-11-12-13-14-15-16-18-21(27)24-26-23-22(28-24)19-17-20-25-23/h9-10,17,19-20H,2-8,11-16,18H2,1H3/b10-9- | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 2.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ de Lille
Curated by ChEMBL
| Assay Description
Inhibition of rat FAAH assessed as reduction in [14C]oleamide conversion to oleic acid by Lineweaver-Burk plot analysis |
J Med Chem 60: 4-46 (2017)
Article DOI: 10.1021/acs.jmedchem.6b00538
BindingDB Entry DOI: 10.7270/Q2348NZC |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data