

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Neurotensin receptor type 2 (Homo sapiens (Human)) | BDBM50553771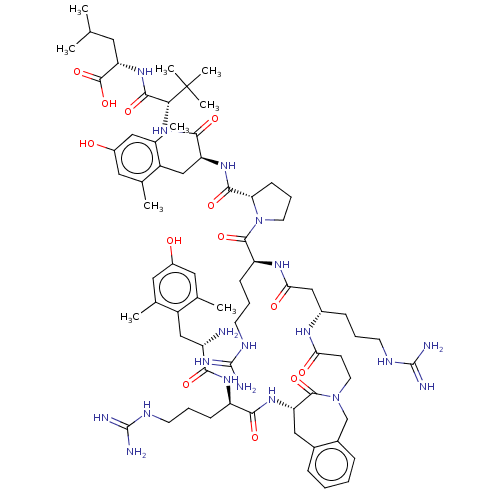 (CHEMBL4776986) | Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.00300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Displacement of [125I]-neurotensin from human recombinant NTS2 stably expressed in human 1321N1 cell membranes incubated for 60 mins by gamma counter... | Citation and Details Article DOI: 10.1021/acs.jmedchem.0c01376 BindingDB Entry DOI: 10.7270/Q2H41W2P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Homo sapiens (Human)) | BDBM50240938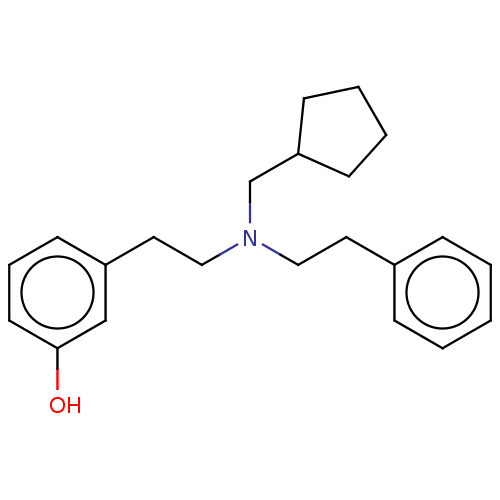 (CHEMBL4071862) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.0170 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Innsbruck Curated by ChEMBL | Assay Description Displacement of [3H]U69,593 from human kappa opioid receptor expressed in CHO cell membranes after 60 mins by liquid scintillation counting method | J Med Chem 60: 7579-7590 (2017) Article DOI: 10.1021/acs.jmedchem.7b00981 BindingDB Entry DOI: 10.7270/Q21C202C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Rattus norvegicus (rat)) | BDBM50127165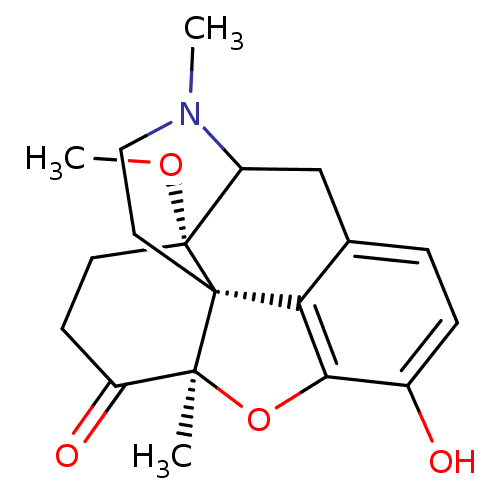 (10-hydroxy-17-methoxy-4,13-dimethyl-(13R,17S)-12-o...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 0.0230 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Innsbruck Curated by ChEMBL | Assay Description In vitro binding affinity at opioid receptor mu 1 was determined in C6 rat glioma cells using [3H]DAMGO | J Med Chem 46: 1758-63 (2003) Article DOI: 10.1021/jm021118o BindingDB Entry DOI: 10.7270/Q2V125JQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neurotensin receptor type 2 (Homo sapiens (Human)) | BDBM50553769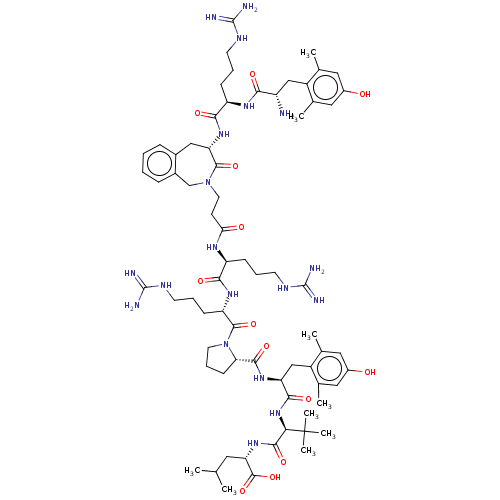 (CHEMBL4798887) | Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.0300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Displacement of [125I]-neurotensin from human recombinant NTS2 stably expressed in human 1321N1 cell membranes incubated for 60 mins by gamma counter... | Citation and Details Article DOI: 10.1021/acs.jmedchem.0c01376 BindingDB Entry DOI: 10.7270/Q2H41W2P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Homo sapiens (Human)) | BDBM50469380 (CHEMBL4276875) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.0370 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vrije Universiteit Brussel Curated by ChEMBL | Assay Description Displacement of [3H]DAMGO from human MOR expressed in CHO cell membranes after 60 mins by liquid scintillation counting | J Med Chem 61: 9784-9789 (2018) Article DOI: 10.1021/acs.jmedchem.8b01282 BindingDB Entry DOI: 10.7270/Q2F76G8Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Homo sapiens (Human)) | BDBM50240954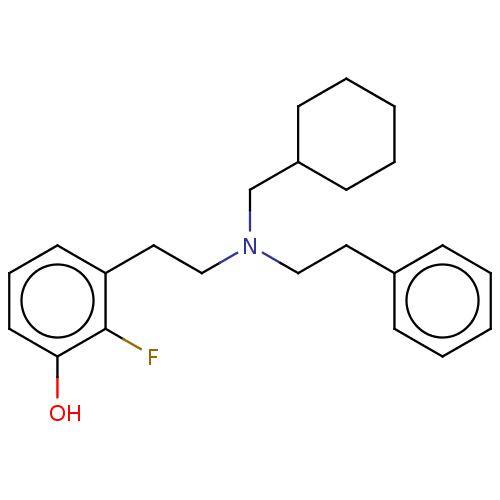 (CHEMBL4101576) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.0400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Innsbruck Curated by ChEMBL | Assay Description Displacement of [3H]U69,593 from human kappa opioid receptor expressed in CHO cell membranes after 60 mins by liquid scintillation counting method | J Med Chem 60: 7579-7590 (2017) Article DOI: 10.1021/acs.jmedchem.7b00981 BindingDB Entry DOI: 10.7270/Q21C202C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Homo sapiens (Human)) | BDBM50250450 (CHEMBL4103328) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.0480 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Innsbruck Curated by ChEMBL | Assay Description Displacement of [3H]-DAMGO from recombinant human MOR expressed in CHO cell membranes after 60 mins by liquid scintillation counting | J Med Chem 60: 9407-9412 (2017) Article DOI: 10.1021/acs.jmedchem.7b01363 BindingDB Entry DOI: 10.7270/Q2PZ5C72 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Homo sapiens (Human)) | BDBM50240970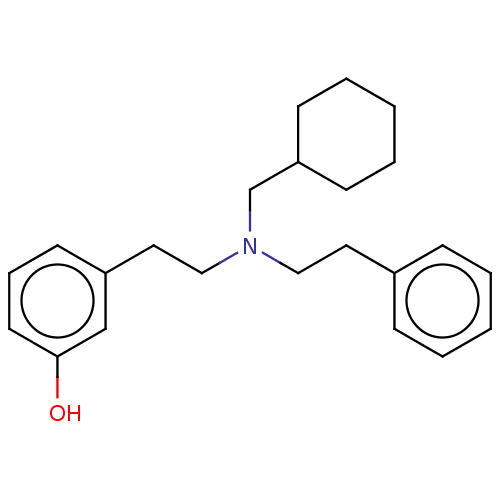 (CHEMBL4092640) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.0610 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Innsbruck Curated by ChEMBL | Assay Description Displacement of [3H]U69,593 from human kappa opioid receptor expressed in CHO cell membranes after 60 mins by liquid scintillation counting method | J Med Chem 60: 7579-7590 (2017) Article DOI: 10.1021/acs.jmedchem.7b00981 BindingDB Entry DOI: 10.7270/Q21C202C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Delta-type opioid receptor (Homo sapiens (Human)) | BDBM21864 ((21R)-22-(cyclopropylmethyl)-14-oxa-11,22-diazahep...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase PC cid PC sid UniChem Similars | PubMed | 0.0620 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Innsbruck Curated by ChEMBL | Assay Description The ability of compound to inhibit [35S]GTP-delta-S binding in guinea pig caudate stimulated by SNC80 (Opioid receptor delta 1) antagonist | J Med Chem 45: 5378-83 (2002) BindingDB Entry DOI: 10.7270/Q2R21244 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Homo sapiens (Human)) | BDBM50240967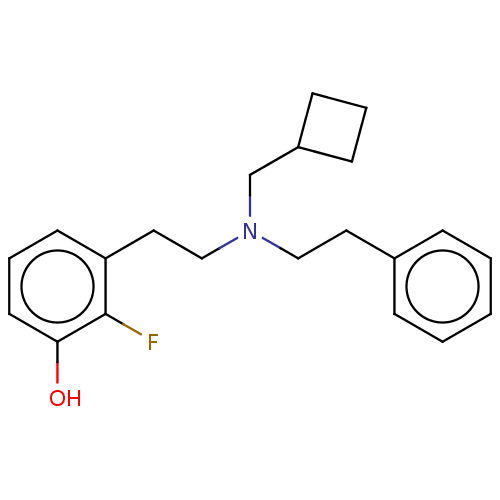 (CHEMBL4090181) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.0720 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Innsbruck Curated by ChEMBL | Assay Description Displacement of [3H]U69,593 from human kappa opioid receptor expressed in CHO cell membranes after 60 mins by liquid scintillation counting method | J Med Chem 60: 7579-7590 (2017) Article DOI: 10.1021/acs.jmedchem.7b00981 BindingDB Entry DOI: 10.7270/Q21C202C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Homo sapiens (Human)) | BDBM50127167 (10-hydroxy-17-(3-phenylpropoxy)-4-tetrahydro-2-fur...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | 0.0800 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Innsbruck Curated by ChEMBL | Assay Description In vitro binding affinity at opioid receptor kappa 1 was determined in human CHO cells using [3H]U-69593 | J Med Chem 46: 1758-63 (2003) Article DOI: 10.1021/jm021118o BindingDB Entry DOI: 10.7270/Q2V125JQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neurotensin receptor type 2 (Homo sapiens (Human)) | BDBM50553767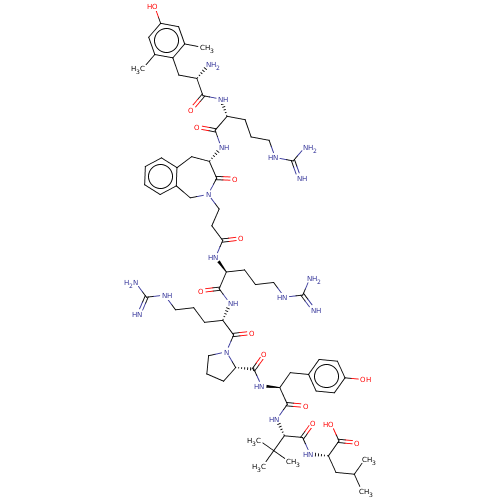 (CHEMBL4789659) | Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.0900 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Displacement of [125I]-neurotensin from human recombinant NTS2 stably expressed in human 1321N1 cell membranes incubated for 60 mins by gamma counter... | Citation and Details Article DOI: 10.1021/acs.jmedchem.0c01376 BindingDB Entry DOI: 10.7270/Q2H41W2P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Rattus norvegicus (rat)) | BDBM50127162 (10-hydroxy-17-(3-phenylpropoxy)-4-propyl-(13R,17S)...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | 0.0900 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Innsbruck Curated by ChEMBL | Assay Description In vitro binding affinity at opioid receptor mu 1 was determined in C6 rat glioma cells using [3H]DAMGO | J Med Chem 46: 1758-63 (2003) Article DOI: 10.1021/jm021118o BindingDB Entry DOI: 10.7270/Q2V125JQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Delta-type opioid receptor (Rattus norvegicus (rat)) | BDBM50127167 (10-hydroxy-17-(3-phenylpropoxy)-4-tetrahydro-2-fur...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | 0.0900 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Innsbruck Curated by ChEMBL | Assay Description In vitro binding affinity at opioid receptor delta 1 was determined in C6 rat glioma cells using [3H]Ile5,6 deltorphin II | J Med Chem 46: 1758-63 (2003) Article DOI: 10.1021/jm021118o BindingDB Entry DOI: 10.7270/Q2V125JQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Rattus norvegicus (rat)) | BDBM50326662 (10-hydroxy-17-methoxy-4-methyl-(13R,17S)-12-oxa-4-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 0.100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Displacement of [3H]DAMGO from mu opioid receptor in rat brain membranes incubated for 45 mins by liquid scintillation counting method | Citation and Details Article DOI: 10.1021/acs.jmedchem.8b01327 BindingDB Entry DOI: 10.7270/Q2RB7870 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Rattus norvegicus (rat)) | BDBM86446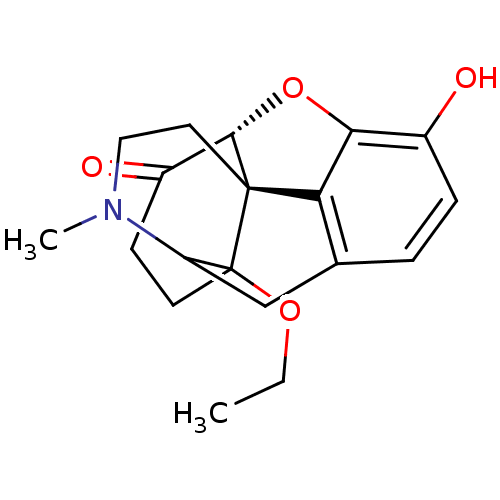 (14-O-methyloxymorphone) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 0.100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Innsbruck Curated by PDSP Ki Database | Eur J Pharmacol 483: 301-8 (2004) Article DOI: 10.1016/j.ejphar.2003.10.049 BindingDB Entry DOI: 10.7270/Q2TQ603X | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Rattus norvegicus (rat)) | BDBM50326663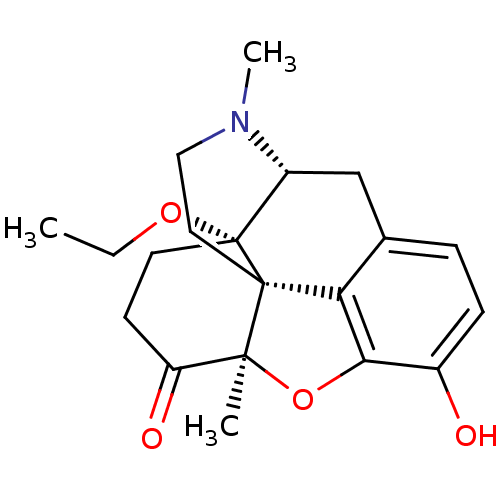 (CHEMBL1255022) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Innsbruck and Center for Molecular Biosciences Innsbruck-CMBI Curated by ChEMBL | Assay Description Displacement of [3H]DAMGO from mu opioid receptor in rat brain membrane | Bioorg Med Chem 18: 5071-80 (2010) Article DOI: 10.1016/j.bmc.2010.05.071 BindingDB Entry DOI: 10.7270/Q2XP75XQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Rattus norvegicus (rat)) | BDBM50326662 (10-hydroxy-17-methoxy-4-methyl-(13R,17S)-12-oxa-4-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 0.100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University La Sapienza Curated by ChEMBL | Assay Description Inhibition of [3H]DAMGO binding to opioid receptor mu from rat brain membranes | J Med Chem 48: 3372-8 (2005) Article DOI: 10.1021/jm040894o BindingDB Entry DOI: 10.7270/Q2X92C3R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Homo sapiens (Human)) | BDBM50127163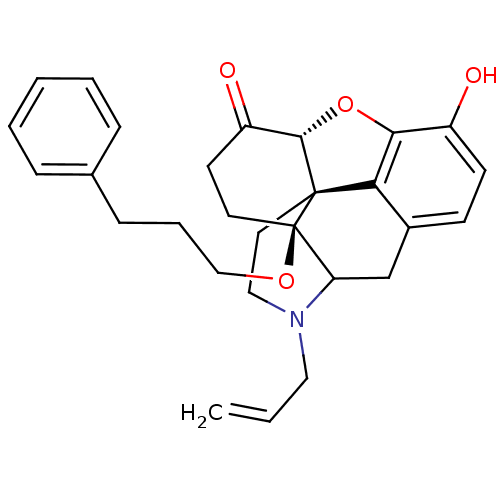 (4-allyl-10-hydroxy-17-(3-phenylpropoxy)-(13R,17S)-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | 0.110 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Innsbruck Curated by ChEMBL | Assay Description In vitro binding affinity at opioid receptor kappa 1 was determined in human CHO cells using [3H]U-69593 | J Med Chem 46: 1758-63 (2003) Article DOI: 10.1021/jm021118o BindingDB Entry DOI: 10.7270/Q2V125JQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neurotensin receptor type 2 (Homo sapiens (Human)) | BDBM50553779 (CHEMBL4742999) | Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.110 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Displacement of [125I]-neurotensin from human recombinant NTS2 stably expressed in human 1321N1 cell membranes incubated for 60 mins by gamma counter... | Citation and Details Article DOI: 10.1021/acs.jmedchem.0c01376 BindingDB Entry DOI: 10.7270/Q2H41W2P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Homo sapiens (Human)) | BDBM50240955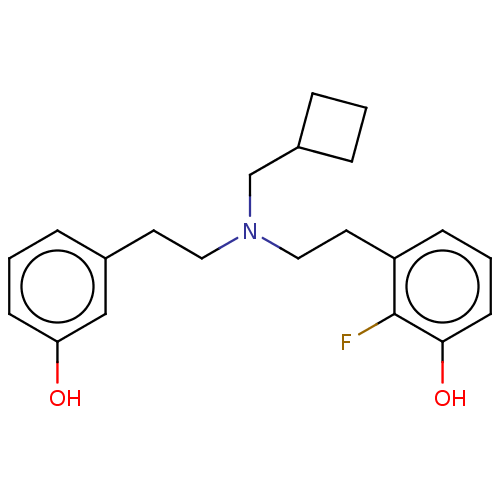 (CHEMBL4083571) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL MMDB PC cid PC sid UniChem Patents Similars | Article PubMed | 0.120 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Innsbruck Curated by ChEMBL | Assay Description Displacement of [3H]U69,593 from human kappa opioid receptor expressed in CHO cell membranes after 60 mins by liquid scintillation counting method | J Med Chem 60: 7579-7590 (2017) Article DOI: 10.1021/acs.jmedchem.7b00981 BindingDB Entry DOI: 10.7270/Q21C202C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Rattus norvegicus (rat)) | BDBM50166216 (17-benzyloxy-10-hydroxy-4-methyl-(13R,17S)-12-oxa-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 0.120 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University La Sapienza Curated by ChEMBL | Assay Description Inhibition of [3H]DAMGO binding to opioid receptor mu from rat brain membranes | J Med Chem 48: 3372-8 (2005) Article DOI: 10.1021/jm040894o BindingDB Entry DOI: 10.7270/Q2X92C3R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Rattus norvegicus (rat)) | BDBM50133226 (15-methoxy-3-methyl-19-(1-methylbutyl)-13-oxa-3-az...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.130 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Innsbruck Curated by ChEMBL | Assay Description Binding affinity against Opioid receptor kappa 1 isolated from rat brain membarane was determined using [3H]U-69593 as radioligand | J Med Chem 46: 4182-7 (2003) Article DOI: 10.1021/jm030878b BindingDB Entry DOI: 10.7270/Q22V2GVM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Homo sapiens (Human)) | BDBM50240958 (CHEMBL4065350) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | 0.140 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Innsbruck Curated by ChEMBL | Assay Description Displacement of [3H]U69,593 from human kappa opioid receptor expressed in CHO cell membranes after 60 mins by liquid scintillation counting method | J Med Chem 60: 7579-7590 (2017) Article DOI: 10.1021/acs.jmedchem.7b00981 BindingDB Entry DOI: 10.7270/Q21C202C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Homo sapiens (Human)) | BDBM50070386 (CHEMBL3408737) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.140 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Displacement of [3H]-DAMGO from recombinant human mu opioid receptor expressed in CHO cell membrane incubated for 60 mins by liquid scintillation cou... | Citation and Details Article DOI: 10.1021/acs.jmedchem.0c01376 BindingDB Entry DOI: 10.7270/Q2H41W2P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Delta-type opioid receptor (Rattus norvegicus (rat)) | BDBM50133224 (10-hydroxy-4,13-dimethyl-17-(3-phenylpropoxy)-12-o...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.140 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Innsbruck Curated by ChEMBL | Assay Description Binding affinity against Opioid receptor delta 1 isolated from rat brain membarane was determined using [3H][Ile]-deltorphin as radioligand | J Med Chem 46: 4182-7 (2003) Article DOI: 10.1021/jm030878b BindingDB Entry DOI: 10.7270/Q22V2GVM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Homo sapiens (Human)) | BDBM85731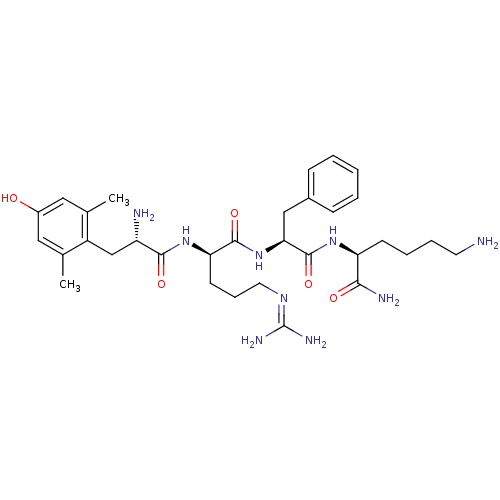 ([Dmt1]DALDA) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 0.143 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vrije Universiteit Brussel Curated by ChEMBL | Assay Description Binding affinity to mu opioid receptor | J Med Chem 55: 9549-61 (2012) Article DOI: 10.1021/jm3008079 BindingDB Entry DOI: 10.7270/Q2TM7C82 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Rattus norvegicus (rat)) | BDBM85731 ([Dmt1]DALDA) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 0.143 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vrije Universiteit Brussel Curated by ChEMBL | Assay Description Displacement of [3H]DAMGO from mu opioid receptor in rat brain membranes after 2 hrs | J Med Chem 55: 9549-61 (2012) Article DOI: 10.1021/jm3008079 BindingDB Entry DOI: 10.7270/Q2TM7C82 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Rattus norvegicus (rat)) | BDBM50346330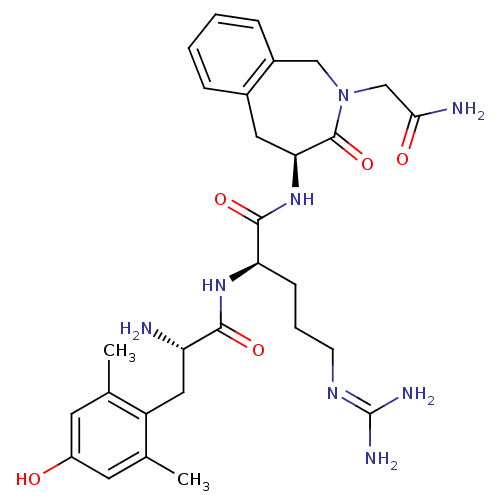 ((R)-N-((S)-2-(2-amino-2-oxoethyl)-3-oxo-2,3,4,5-te...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.150 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vrije Universiteit Brussel Curated by ChEMBL | Assay Description Displacement of [3H]DAMGO from mu opioid receptor in rat brain membranes after 2 hrs | J Med Chem 55: 9549-61 (2012) Article DOI: 10.1021/jm3008079 BindingDB Entry DOI: 10.7270/Q2TM7C82 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Rattus norvegicus (rat)) | BDBM86261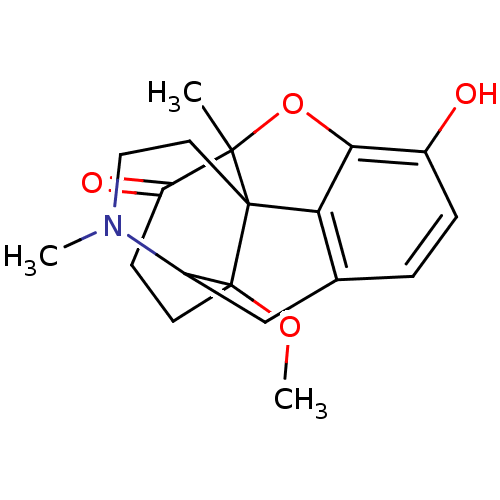 (14-methoxymetopon | CAS_131575-03-6 | NSC_125489) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 0.150 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Innsbruck Curated by PDSP Ki Database | Eur J Neurosci 18: 290-5 (2003) Article DOI: 10.1046/j.1460-9568.2003.02744.x BindingDB Entry DOI: 10.7270/Q2P55M37 | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Rattus norvegicus (rat)) | BDBM50127165 (10-hydroxy-17-methoxy-4,13-dimethyl-(13R,17S)-12-o...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 0.150 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University La Sapienza Curated by ChEMBL | Assay Description Inhibition of [3H]DAMGO binding to opioid receptor mu from rat brain membranes | J Med Chem 48: 3372-8 (2005) Article DOI: 10.1021/jm040894o BindingDB Entry DOI: 10.7270/Q2X92C3R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Rattus norvegicus (rat)) | BDBM50133227 (10,17-dimethoxy-4,13-dimethyl-12-oxa-4-azapentacyc...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.150 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Innsbruck Curated by ChEMBL | Assay Description Binding affinity against Opioid receptor mu 1 isolated from rat brain membarane was determined using [3H]DAMGO as radioligand | J Med Chem 46: 4182-7 (2003) Article DOI: 10.1021/jm030878b BindingDB Entry DOI: 10.7270/Q22V2GVM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Rattus norvegicus (rat)) | BDBM86261 (14-methoxymetopon | CAS_131575-03-6 | NSC_125489) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 0.150 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Innsbruck Curated by PDSP Ki Database | Eur J Pharmacol 483: 301-8 (2004) Article DOI: 10.1016/j.ejphar.2003.10.049 BindingDB Entry DOI: 10.7270/Q2TQ603X | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neurotensin receptor type 2 (Homo sapiens (Human)) | BDBM50553770 (CHEMBL4758754) | Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.160 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Displacement of [125I]-neurotensin from human recombinant NTS2 stably expressed in human 1321N1 cell membranes incubated for 60 mins by gamma counter... | Citation and Details Article DOI: 10.1021/acs.jmedchem.0c01376 BindingDB Entry DOI: 10.7270/Q2H41W2P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Rattus norvegicus (rat)) | BDBM50166212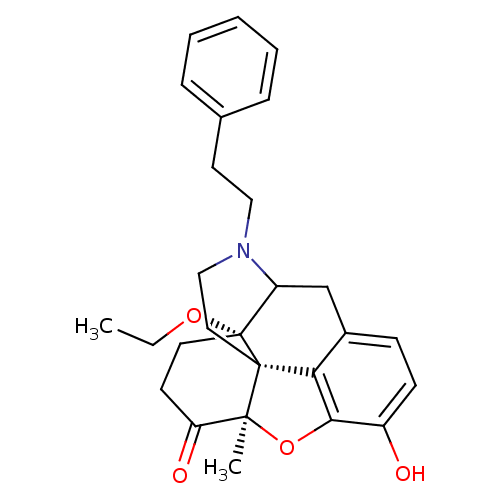 (17-ethoxy-10-hydroxy-13-methyl-4-phenethyl-(13R,17...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 0.160 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University La Sapienza Curated by ChEMBL | Assay Description Inhibition of [3H]DAMGO binding to opioid receptor mu from rat brain membranes | J Med Chem 48: 3372-8 (2005) Article DOI: 10.1021/jm040894o BindingDB Entry DOI: 10.7270/Q2X92C3R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Rattus norvegicus (rat)) | BDBM50166214 (10-hydroxy-4-methyl-17-(1-naphthylmethoxy)-(13R,17...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 0.180 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University La Sapienza Curated by ChEMBL | Assay Description Inhibition of [3H]DAMGO binding to opioid receptor mu from rat brain membranes | J Med Chem 48: 3372-8 (2005) Article DOI: 10.1021/jm040894o BindingDB Entry DOI: 10.7270/Q2X92C3R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Rattus norvegicus (rat)) | BDBM50166215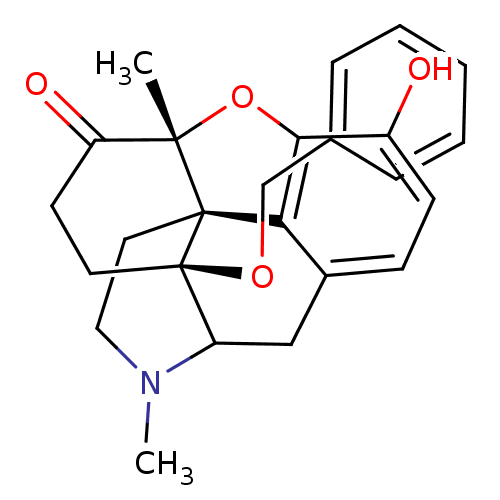 (17-benzyloxy-10-hydroxy-4,13-dimethyl-(13R,17S)-12...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 0.180 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University La Sapienza Curated by ChEMBL | Assay Description Inhibition of [3H]DAMGO binding to opioid receptor mu from rat brain membranes | J Med Chem 48: 3372-8 (2005) Article DOI: 10.1021/jm040894o BindingDB Entry DOI: 10.7270/Q2X92C3R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Rattus norvegicus (rat)) | BDBM50367123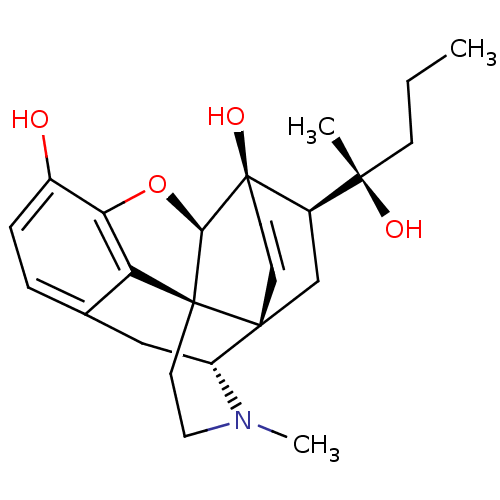 (ETORPHINE | M99) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 0.180 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Innsbruck Curated by ChEMBL | Assay Description Binding affinity against Opioid receptor kappa 1 isolated from rat brain membarane was determined using [3H]U-69593 as radioligand | J Med Chem 46: 4182-7 (2003) Article DOI: 10.1021/jm030878b BindingDB Entry DOI: 10.7270/Q22V2GVM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Rattus norvegicus (rat)) | BDBM50133226 (15-methoxy-3-methyl-19-(1-methylbutyl)-13-oxa-3-az...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.190 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Innsbruck Curated by ChEMBL | Assay Description Binding affinity against Opioid receptor mu 1 isolated from rat brain membarane was determined using [3H]DAMGO as radioligand | J Med Chem 46: 4182-7 (2003) Article DOI: 10.1021/jm030878b BindingDB Entry DOI: 10.7270/Q22V2GVM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Rattus norvegicus (rat)) | BDBM50548314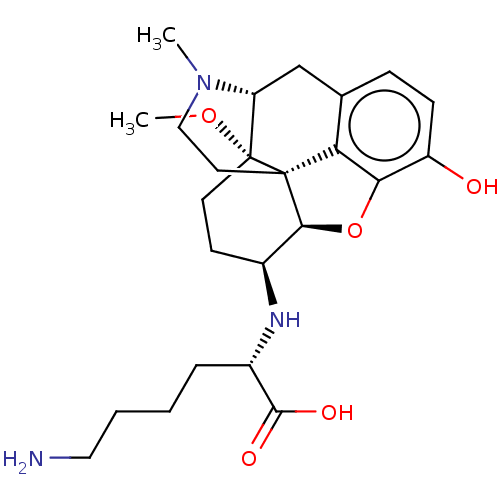 (CHEMBL4793888) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.190 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Displacement of [3H]DAMGO from mu opioid receptor in rat brain membranes incubated for 45 mins by liquid scintillation counting method | Citation and Details Article DOI: 10.1021/acs.jmedchem.8b01327 BindingDB Entry DOI: 10.7270/Q2RB7870 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Rattus norvegicus (rat)) | BDBM50127167 (10-hydroxy-17-(3-phenylpropoxy)-4-tetrahydro-2-fur...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Innsbruck Curated by ChEMBL | Assay Description In vitro binding affinity at opioid receptor mu 1 was determined in C6 rat glioma cells using [3H]DAMGO | J Med Chem 46: 1758-63 (2003) Article DOI: 10.1021/jm021118o BindingDB Entry DOI: 10.7270/Q2V125JQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Rattus norvegicus (rat)) | BDBM50127163 (4-allyl-10-hydroxy-17-(3-phenylpropoxy)-(13R,17S)-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Innsbruck Curated by ChEMBL | Assay Description In vitro binding affinity at opioid receptor mu 1 was determined in C6 rat glioma cells using [3H]DAMGO | J Med Chem 46: 1758-63 (2003) Article DOI: 10.1021/jm021118o BindingDB Entry DOI: 10.7270/Q2V125JQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Homo sapiens (Human)) | BDBM50000296 (CHEMBL441765 | CHEMBL482811 | U-50488H | US1149237...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL KEGG PC cid PC sid UniChem Patents Similars | Article PubMed | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Innsbruck Curated by ChEMBL | Assay Description Displacement of [3H]U69,593 from human kappa opioid receptor expressed in CHO cell membranes incubated for 60 mins by liquid scintillation counting | J Med Chem 55: 10302-6 (2012) Article DOI: 10.1021/jm301258w BindingDB Entry DOI: 10.7270/Q24X58ZX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Rattus norvegicus (rat)) | BDBM50133224 (10-hydroxy-4,13-dimethyl-17-(3-phenylpropoxy)-12-o...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Innsbruck Curated by ChEMBL | Assay Description Binding affinity against Opioid receptor mu 1 isolated from rat brain membarane was determined using [3H]DAMGO as radioligand | J Med Chem 46: 4182-7 (2003) Article DOI: 10.1021/jm030878b BindingDB Entry DOI: 10.7270/Q22V2GVM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Rattus norvegicus (rat)) | BDBM50336600 (CHEMBL3216936 | [[17-Cyclopropylmethyl-4,5alpha-ep...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Innsbruck Curated by ChEMBL | Assay Description Displacement of [3H]DAMGO from mu opioid receptor in Sprague-Dawley rat brain membrane by liquid scintillation counting | J Med Chem 54: 980-8 (2011) Article DOI: 10.1021/jm101211p BindingDB Entry DOI: 10.7270/Q2MP53KV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Delta-type opioid receptor (Rattus norvegicus (rat)) | BDBM50133226 (15-methoxy-3-methyl-19-(1-methylbutyl)-13-oxa-3-az...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Innsbruck Curated by ChEMBL | Assay Description Binding affinity against Opioid receptor delta 1 isolated from rat brain membarane was determined using [3H][Ile]-deltorphin as radioligand | J Med Chem 46: 4182-7 (2003) Article DOI: 10.1021/jm030878b BindingDB Entry DOI: 10.7270/Q22V2GVM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Rattus norvegicus (rat)) | BDBM50336591 ((1R,5R,13R,17S)-10-hydroxy-4,13-dimethyl-17-(3-phe...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Innsbruck Curated by ChEMBL | Assay Description Displacement of [3H]DAMGO from mu opioid receptor in Sprague-Dawley rat brain membrane by liquid scintillation counting | J Med Chem 54: 980-8 (2011) Article DOI: 10.1021/jm101211p BindingDB Entry DOI: 10.7270/Q2MP53KV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Rattus norvegicus (rat)) | BDBM50399643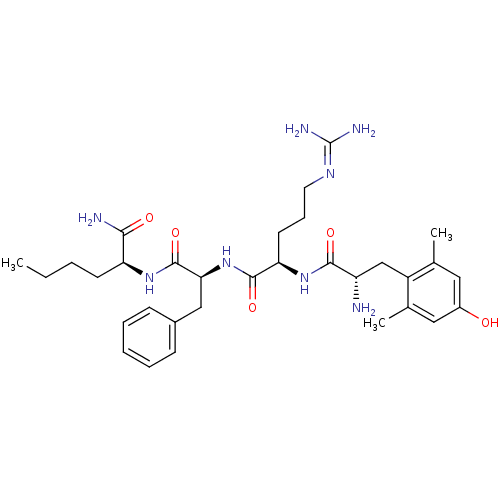 (CHEMBL2181201) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.230 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vrije Universiteit Brussel Curated by ChEMBL | Assay Description Displacement of [3H]DAMGO from mu opioid receptor in rat brain membranes after 2 hrs | J Med Chem 55: 9549-61 (2012) Article DOI: 10.1021/jm3008079 BindingDB Entry DOI: 10.7270/Q2TM7C82 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Homo sapiens (Human)) | BDBM50250449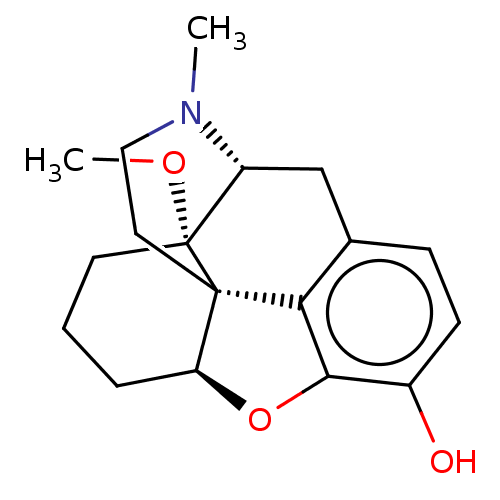 (CHEMBL4065490) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 0.240 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Innsbruck Curated by ChEMBL | Assay Description Displacement of [3H]-DAMGO from recombinant human MOR expressed in CHO cell membranes after 60 mins by liquid scintillation counting | J Med Chem 60: 9407-9412 (2017) Article DOI: 10.1021/acs.jmedchem.7b01363 BindingDB Entry DOI: 10.7270/Q2PZ5C72 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Rattus norvegicus (rat)) | BDBM50127170 (4-cyclobutylmethyl-10-hydroxy-17-(3-phenylpropoxy)...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | 0.25 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Innsbruck Curated by ChEMBL | Assay Description In vitro binding affinity at opioid receptor mu 1 was determined in C6 rat glioma cells using [3H]DAMGO | J Med Chem 46: 1758-63 (2003) Article DOI: 10.1021/jm021118o BindingDB Entry DOI: 10.7270/Q2V125JQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 1063 total ) | Next | Last >> |