 Found 3737 hits with Last Name = 'sprengeler' and Initial = 'pa'
Found 3737 hits with Last Name = 'sprengeler' and Initial = 'pa' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Coagulation factor VII/Tissue factor
(Homo sapiens (Human)) | BDBM50103651

(2-(3'-Amino-5-chloro-2-hydroxy-biphenyl-3-yl)-1H-b...)Show SMILES NC(=N)c1ccc2nc([nH]c2c1)-c1cc(Cl)cc(-c2cccc(N)c2)c1O Show InChI InChI=1S/C20H16ClN5O/c21-12-8-14(10-2-1-3-13(22)6-10)18(27)15(9-12)20-25-16-5-4-11(19(23)24)7-17(16)26-20/h1-9,27H,22H2,(H3,23,24)(H,25,26) | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 0.0780 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Axys Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
Binding affinity for factor VIIa/TF |
Bioorg Med Chem Lett 11: 2253-6 (2001)
BindingDB Entry DOI: 10.7270/Q2XG9QD9 |
More data for this
Ligand-Target Pair | |
Urokinase-type plasminogen activator
(Homo sapiens (Human)) | BDBM50103651

(2-(3'-Amino-5-chloro-2-hydroxy-biphenyl-3-yl)-1H-b...)Show SMILES NC(=N)c1ccc2nc([nH]c2c1)-c1cc(Cl)cc(-c2cccc(N)c2)c1O Show InChI InChI=1S/C20H16ClN5O/c21-12-8-14(10-2-1-3-13(22)6-10)18(27)15(9-12)20-25-16-5-4-11(19(23)24)7-17(16)26-20/h1-9,27H,22H2,(H3,23,24)(H,25,26) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 0.460 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Axys Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
Binding affinity of the compound towards urokinase-type plasminogen activator (microPa) |
Bioorg Med Chem Lett 11: 2253-6 (2001)
BindingDB Entry DOI: 10.7270/Q2XG9QD9 |
More data for this
Ligand-Target Pair | |
Plasma kallikrein
(Homo sapiens (Human)) | BDBM50180517
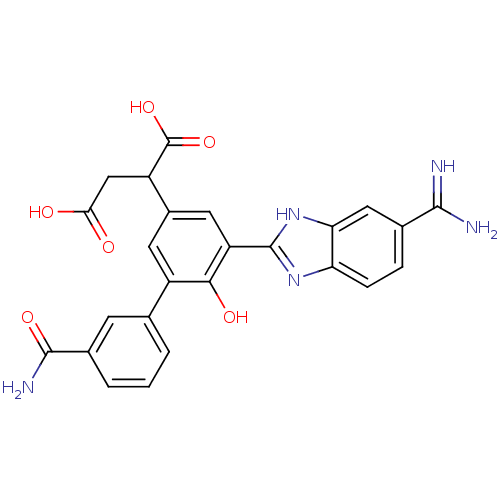
(2-[5-(5-carbamimidoyl-1H-benzoimidazol-2-yl)-3'-ca...)Show SMILES NC(=N)c1ccc2nc([nH]c2c1)-c1cc(cc(-c2cccc(c2)C(N)=O)c1O)C(CC(O)=O)C(O)=O Show InChI InChI=1S/C25H21N5O6/c26-22(27)12-4-5-18-19(9-12)30-24(29-18)17-8-14(16(25(35)36)10-20(31)32)7-15(21(17)33)11-2-1-3-13(6-11)23(28)34/h1-9,16,33H,10H2,(H3,26,27)(H2,28,34)(H,29,30)(H,31,32)(H,35,36) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Celera Genomics
Curated by ChEMBL
| Assay Description
Binding affinity to plasma kallikrein |
Bioorg Med Chem Lett 16: 2034-6 (2006)
Article DOI: 10.1016/j.bmcl.2005.12.060
BindingDB Entry DOI: 10.7270/Q2P55N3N |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50101882
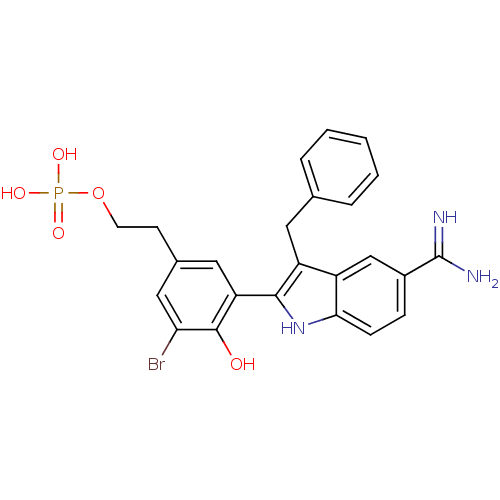
(CHEMBL53829 | Phosphoric acid mono-{2-[3-(3-benzyl...)Show SMILES NC(=N)c1ccc2[nH]c(c(Cc3ccccc3)c2c1)-c1cc(CCOP(O)(O)=O)cc(Br)c1O Show InChI InChI=1S/C24H23BrN3O5P/c25-20-12-15(8-9-33-34(30,31)32)11-19(23(20)29)22-18(10-14-4-2-1-3-5-14)17-13-16(24(26)27)6-7-21(17)28-22/h1-7,11-13,28-29H,8-10H2,(H3,26,27)(H2,30,31,32) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 0.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Axys Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
Inhibitory activity against Coagulation factor X in human plasma |
Bioorg Med Chem Lett 11: 1797-800 (2001)
BindingDB Entry DOI: 10.7270/Q20K27TW |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50101871

(3-[3-(3-Benzyl-5-carbamimidoyl-1H-indol-2-yl)-5-br...)Show SMILES NC(=N)c1ccc2[nH]c(c(Cc3ccccc3)c2c1)-c1cc(CCC(O)=O)cc(Br)c1O Show InChI InChI=1S/C25H22BrN3O3/c26-20-12-15(6-9-22(30)31)11-19(24(20)32)23-18(10-14-4-2-1-3-5-14)17-13-16(25(27)28)7-8-21(17)29-23/h1-5,7-8,11-13,29,32H,6,9-10H2,(H3,27,28)(H,30,31) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 0.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Axys Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
Inhibitory activity against Coagulation factor X in human plasma |
Bioorg Med Chem Lett 11: 1797-800 (2001)
BindingDB Entry DOI: 10.7270/Q20K27TW |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50103651

(2-(3'-Amino-5-chloro-2-hydroxy-biphenyl-3-yl)-1H-b...)Show SMILES NC(=N)c1ccc2nc([nH]c2c1)-c1cc(Cl)cc(-c2cccc(N)c2)c1O Show InChI InChI=1S/C20H16ClN5O/c21-12-8-14(10-2-1-3-13(22)6-10)18(27)15(9-12)20-25-16-5-4-11(19(23)24)7-17(16)26-20/h1-9,27H,22H2,(H3,23,24)(H,25,26) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 0.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Axys Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
Binding affinity of the compound towards Coagulation factor X |
Bioorg Med Chem Lett 11: 2253-6 (2001)
BindingDB Entry DOI: 10.7270/Q2XG9QD9 |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50101881
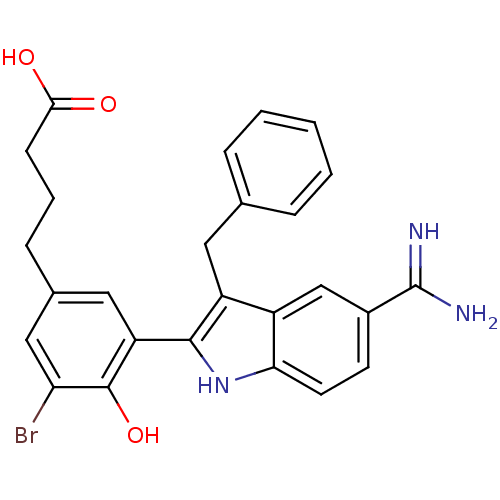
(4-[3-(3-Benzyl-5-carbamimidoyl-1H-indol-2-yl)-5-br...)Show SMILES NC(=N)c1ccc2[nH]c(c(Cc3ccccc3)c2c1)-c1cc(CCCC(O)=O)cc(Br)c1O Show InChI InChI=1S/C26H24BrN3O3/c27-21-13-16(7-4-8-23(31)32)12-20(25(21)33)24-19(11-15-5-2-1-3-6-15)18-14-17(26(28)29)9-10-22(18)30-24/h1-3,5-6,9-10,12-14,30,33H,4,7-8,11H2,(H3,28,29)(H,31,32) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 0.700 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Axys Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
Inhibitory activity against Coagulation factor X in human plasma |
Bioorg Med Chem Lett 11: 1797-800 (2001)
BindingDB Entry DOI: 10.7270/Q20K27TW |
More data for this
Ligand-Target Pair | |
Coagulation factor VII/Tissue factor
(Homo sapiens (Human)) | BDBM50181921
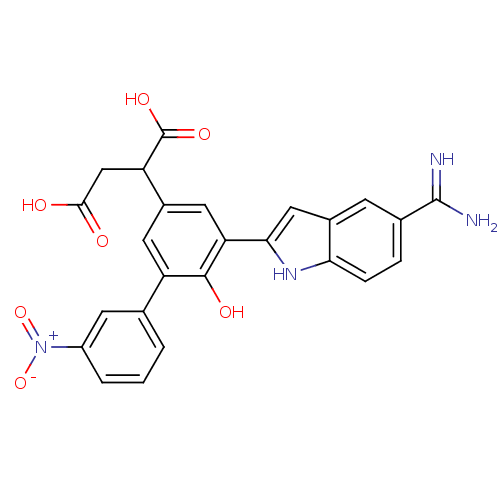
(2-[5-(5-carbamimidoyl-1H-indol-2-yl)-6-hydroxy-3'-...)Show SMILES NC(=N)c1ccc2[nH]c(cc2c1)-c1cc(cc(c1O)-c1cccc(c1)[N+]([O-])=O)C(CC(O)=O)C(O)=O Show InChI InChI=1S/C25H20N4O7/c26-24(27)13-4-5-20-15(6-13)10-21(28-20)19-9-14(18(25(33)34)11-22(30)31)8-17(23(19)32)12-2-1-3-16(7-12)29(35)36/h1-10,18,28,32H,11H2,(H3,26,27)(H,30,31)(H,33,34) | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Celera Genomics
Curated by ChEMBL
| Assay Description
Binding affinity to factor7a/TF complex |
Bioorg Med Chem Lett 16: 2243-6 (2006)
Article DOI: 10.1016/j.bmcl.2006.01.037
BindingDB Entry DOI: 10.7270/Q2GB23NX |
More data for this
Ligand-Target Pair | |
Coagulation factor VII
(Homo sapiens (Human)) | BDBM50180400
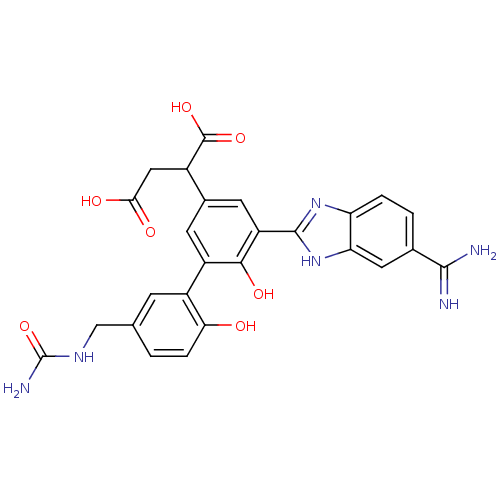
(2-[5-(5-carbamimidoyl-1H-benzoimidazol-2-yl)-6,2'-...)Show SMILES NC(=O)NCc1ccc(O)c(c1)-c1cc(cc(-c2nc3ccc(cc3[nH]2)C(N)=N)c1O)C(CC(O)=O)C(O)=O Show InChI InChI=1S/C26H24N6O7/c27-23(28)12-2-3-18-19(8-12)32-24(31-18)17-7-13(14(25(37)38)9-21(34)35)6-16(22(17)36)15-5-11(1-4-20(15)33)10-30-26(29)39/h1-8,14,33,36H,9-10H2,(H3,27,28)(H,31,32)(H,34,35)(H,37,38)(H3,29,30,39) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Celera
Curated by ChEMBL
| Assay Description
Binding affinity to f7a |
Bioorg Med Chem Lett 16: 3829-32 (2006)
Article DOI: 10.1016/j.bmcl.2006.04.018
BindingDB Entry DOI: 10.7270/Q2DN44NK |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Serine protease 1
(Bos taurus (bovine)) | BDBM14338
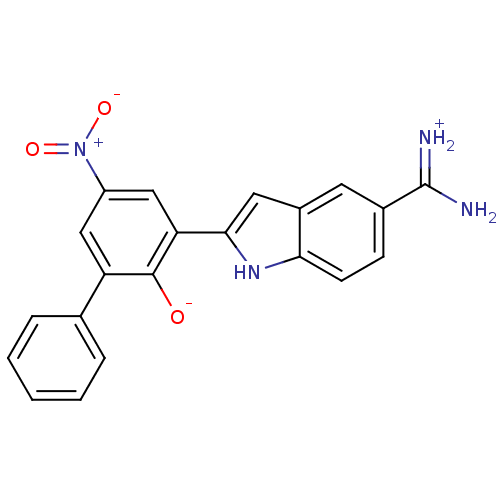
(2-{5-[amino(iminiumyl)methyl]-1H-indol-2-yl}-4-nit...)Show SMILES NC(=[NH2+])c1ccc2[nH]c(cc2c1)-c1cc(cc(-c2ccccc2)c1[O-])[N+]([O-])=O Show InChI InChI=1S/C21H16N4O3/c22-21(23)13-6-7-18-14(8-13)9-19(24-18)17-11-15(25(27)28)10-16(20(17)26)12-4-2-1-3-5-12/h1-11,24,26H,(H3,22,23) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Celera
| Assay Description
Each enzyme was assayed with a set of different concentrations of each inhibitor. After addition of the appropriate substrate, the rate of hydrolysis... |
J Mol Biol 329: 93-120 (2003)
Article DOI: 10.1016/s0022-2836(03)00399-1
BindingDB Entry DOI: 10.7270/Q2R78CGQ |
More data for this
Ligand-Target Pair | |
Coagulation factor VII/Tissue factor
(Homo sapiens (Human)) | BDBM50189939
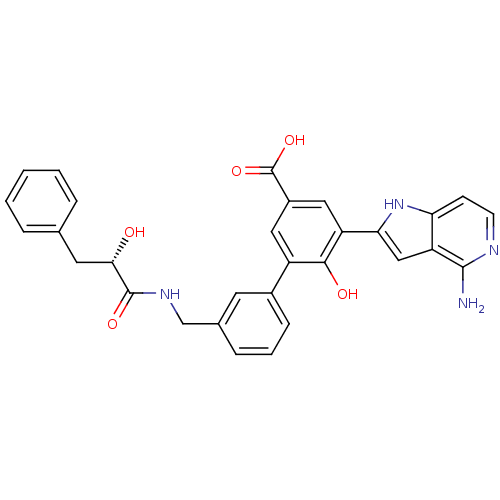
(5-(4-amino-1H-pyrrolo[3,2-c]pyridin-2-yl)-6-hydrox...)Show SMILES Nc1nccc2[nH]c(cc12)-c1cc(cc(-c2cccc(CNC(=O)[C@@H](O)Cc3ccccc3)c2)c1O)C(O)=O Show InChI InChI=1S/C30H26N4O5/c31-28-23-15-25(34-24(23)9-10-32-28)22-14-20(30(38)39)13-21(27(22)36)19-8-4-7-18(11-19)16-33-29(37)26(35)12-17-5-2-1-3-6-17/h1-11,13-15,26,34-36H,12,16H2,(H2,31,32)(H,33,37)(H,38,39)/t26-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Celera Genomics
Curated by ChEMBL
| Assay Description
Binding affinity to f7a/TF complex |
Bioorg Med Chem Lett 16: 4567-70 (2006)
Article DOI: 10.1016/j.bmcl.2006.06.016
BindingDB Entry DOI: 10.7270/Q2NG4Q67 |
More data for this
Ligand-Target Pair | |
Dimer of Tryptase beta-2
(Homo sapiens (Human)) | BDBM50187167
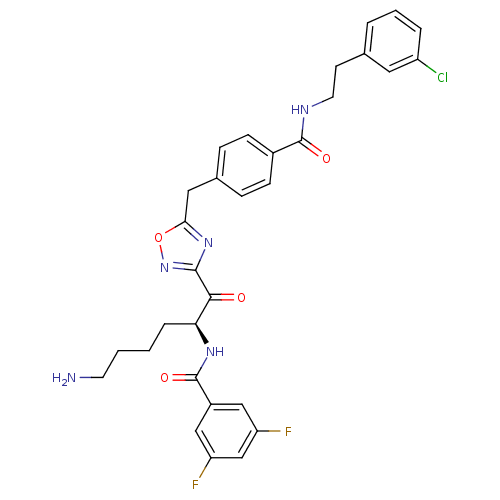
(CHEMBL211357 | N-[(S)-5-amino-1-(5-{4-[2-(3-chloro...)Show SMILES NCCCC[C@H](NC(=O)c1cc(F)cc(F)c1)C(=O)c1noc(Cc2ccc(cc2)C(=O)NCCc2cccc(Cl)c2)n1 Show InChI InChI=1S/C31H30ClF2N5O4/c32-23-5-3-4-19(14-23)11-13-36-30(41)21-9-7-20(8-10-21)15-27-38-29(39-43-27)28(40)26(6-1-2-12-35)37-31(42)22-16-24(33)18-25(34)17-22/h3-5,7-10,14,16-18,26H,1-2,6,11-13,15,35H2,(H,36,41)(H,37,42)/t26-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Celera
Curated by ChEMBL
| Assay Description
Inhibition of human beta tryptase |
Bioorg Med Chem Lett 16: 4036-40 (2006)
Article DOI: 10.1016/j.bmcl.2006.05.009
BindingDB Entry DOI: 10.7270/Q20864ZV |
More data for this
Ligand-Target Pair | |
Dimer of Tryptase beta-2
(Homo sapiens (Human)) | BDBM50187166
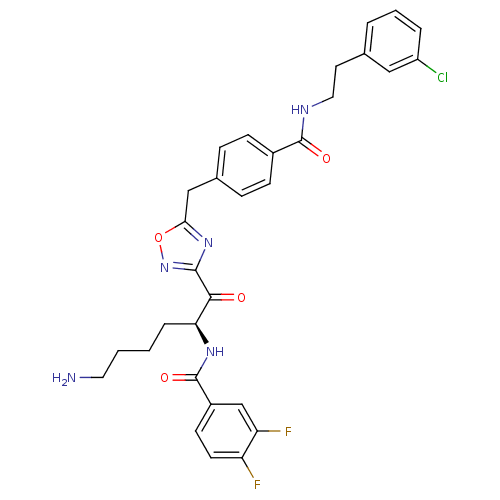
(CHEMBL380293 | N-[(S)-5-amino-1-(5-{4-[2-(3-chloro...)Show SMILES NCCCC[C@H](NC(=O)c1ccc(F)c(F)c1)C(=O)c1noc(Cc2ccc(cc2)C(=O)NCCc2cccc(Cl)c2)n1 Show InChI InChI=1S/C31H30ClF2N5O4/c32-23-5-3-4-19(16-23)13-15-36-30(41)21-9-7-20(8-10-21)17-27-38-29(39-43-27)28(40)26(6-1-2-14-35)37-31(42)22-11-12-24(33)25(34)18-22/h3-5,7-12,16,18,26H,1-2,6,13-15,17,35H2,(H,36,41)(H,37,42)/t26-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Celera
Curated by ChEMBL
| Assay Description
Inhibition of human beta tryptase |
Bioorg Med Chem Lett 16: 4036-40 (2006)
Article DOI: 10.1016/j.bmcl.2006.05.009
BindingDB Entry DOI: 10.7270/Q20864ZV |
More data for this
Ligand-Target Pair | |
Dimer of Tryptase beta-2
(Homo sapiens (Human)) | BDBM50187177
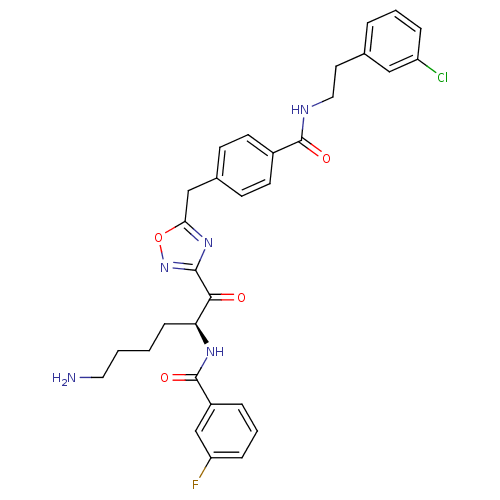
(CHEMBL212774 | N-[(2S)-6-amino-1-{5-[(4-{[2-(3-chl...)Show SMILES NCCCC[C@H](NC(=O)c1cccc(F)c1)C(=O)c1noc(Cc2ccc(cc2)C(=O)NCCc2cccc(Cl)c2)n1 Show InChI InChI=1S/C31H31ClFN5O4/c32-24-7-3-5-20(17-24)14-16-35-30(40)22-12-10-21(11-13-22)18-27-37-29(38-42-27)28(39)26(9-1-2-15-34)36-31(41)23-6-4-8-25(33)19-23/h3-8,10-13,17,19,26H,1-2,9,14-16,18,34H2,(H,35,40)(H,36,41)/t26-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Celera
Curated by ChEMBL
| Assay Description
Inhibition of human beta tryptase |
Bioorg Med Chem Lett 16: 4036-40 (2006)
Article DOI: 10.1016/j.bmcl.2006.05.009
BindingDB Entry DOI: 10.7270/Q20864ZV |
More data for this
Ligand-Target Pair | |
Dimer of Tryptase beta-2
(Homo sapiens (Human)) | BDBM50187160

(CHEMBL213928 | N-[(2S)-6-amino-1-{5-[(4-{[2-(3-chl...)Show SMILES NCCCC[C@H](NC(=O)c1ccc(F)cc1)C(=O)c1noc(Cc2ccc(cc2)C(=O)NCCc2cccc(Cl)c2)n1 Show InChI InChI=1S/C31H31ClFN5O4/c32-24-5-3-4-20(18-24)15-17-35-30(40)22-9-7-21(8-10-22)19-27-37-29(38-42-27)28(39)26(6-1-2-16-34)36-31(41)23-11-13-25(33)14-12-23/h3-5,7-14,18,26H,1-2,6,15-17,19,34H2,(H,35,40)(H,36,41)/t26-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Celera
Curated by ChEMBL
| Assay Description
Inhibition of human beta tryptase |
Bioorg Med Chem Lett 16: 4036-40 (2006)
Article DOI: 10.1016/j.bmcl.2006.05.009
BindingDB Entry DOI: 10.7270/Q20864ZV |
More data for this
Ligand-Target Pair | |
Dimer of Tryptase beta-2
(Homo sapiens (Human)) | BDBM50187163
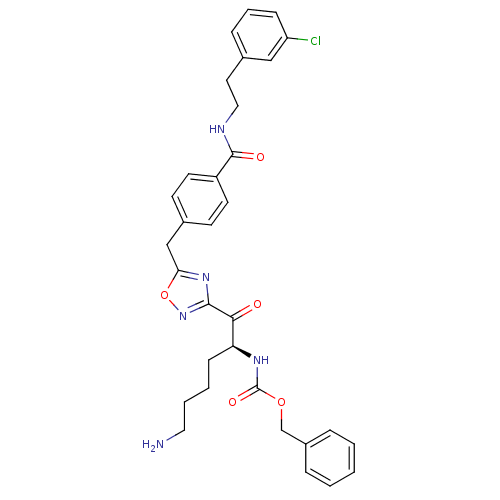
((S)-benzyl 1-(5-(4-((3-chlorophenethyl)carbamoyl)b...)Show SMILES NCCCC[C@H](NC(=O)OCc1ccccc1)C(=O)c1noc(Cc2ccc(cc2)C(=O)NCCc2cccc(Cl)c2)n1 Show InChI InChI=1S/C32H34ClN5O5/c33-26-10-6-9-22(19-26)16-18-35-31(40)25-14-12-23(13-15-25)20-28-37-30(38-43-28)29(39)27(11-4-5-17-34)36-32(41)42-21-24-7-2-1-3-8-24/h1-3,6-10,12-15,19,27H,4-5,11,16-18,20-21,34H2,(H,35,40)(H,36,41)/t27-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Celera
Curated by ChEMBL
| Assay Description
Inhibition of human beta tryptase |
Bioorg Med Chem Lett 16: 4036-40 (2006)
Article DOI: 10.1016/j.bmcl.2006.05.009
BindingDB Entry DOI: 10.7270/Q20864ZV |
More data for this
Ligand-Target Pair | |
Dimer of Tryptase beta-2
(Homo sapiens (Human)) | BDBM50187168
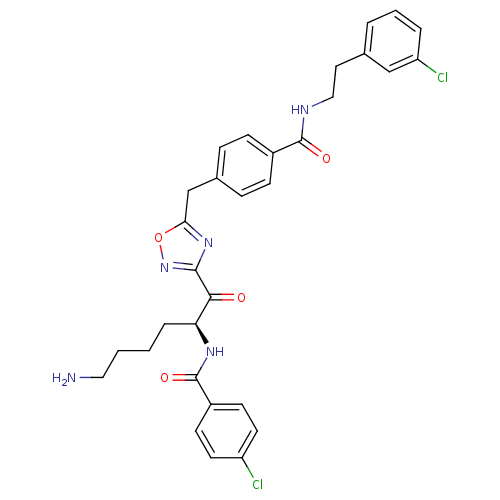
(CHEMBL377656 | N-[(2S)-6-amino-1-{5-[(4-{[2-(3-chl...)Show SMILES NCCCC[C@H](NC(=O)c1ccc(Cl)cc1)C(=O)c1noc(Cc2ccc(cc2)C(=O)NCCc2cccc(Cl)c2)n1 Show InChI InChI=1S/C31H31Cl2N5O4/c32-24-13-11-23(12-14-24)31(41)36-26(6-1-2-16-34)28(39)29-37-27(42-38-29)19-21-7-9-22(10-8-21)30(40)35-17-15-20-4-3-5-25(33)18-20/h3-5,7-14,18,26H,1-2,6,15-17,19,34H2,(H,35,40)(H,36,41)/t26-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Celera
Curated by ChEMBL
| Assay Description
Inhibition of human beta tryptase |
Bioorg Med Chem Lett 16: 4036-40 (2006)
Article DOI: 10.1016/j.bmcl.2006.05.009
BindingDB Entry DOI: 10.7270/Q20864ZV |
More data for this
Ligand-Target Pair | |
Dimer of Tryptase beta-2
(Homo sapiens (Human)) | BDBM50187176
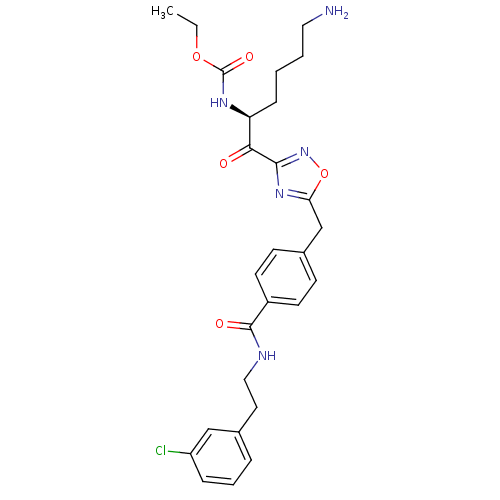
((S)-ethyl 1-(5-(4-((3-chlorophenethyl)carbamoyl)be...)Show SMILES CCOC(=O)N[C@@H](CCCCN)C(=O)c1noc(Cc2ccc(cc2)C(=O)NCCc2cccc(Cl)c2)n1 Show InChI InChI=1S/C27H32ClN5O5/c1-2-37-27(36)31-22(8-3-4-14-29)24(34)25-32-23(38-33-25)17-19-9-11-20(12-10-19)26(35)30-15-13-18-6-5-7-21(28)16-18/h5-7,9-12,16,22H,2-4,8,13-15,17,29H2,1H3,(H,30,35)(H,31,36)/t22-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Celera
Curated by ChEMBL
| Assay Description
Inhibition of human beta tryptase |
Bioorg Med Chem Lett 16: 4036-40 (2006)
Article DOI: 10.1016/j.bmcl.2006.05.009
BindingDB Entry DOI: 10.7270/Q20864ZV |
More data for this
Ligand-Target Pair | |
Dimer of Tryptase beta-2
(Homo sapiens (Human)) | BDBM50187162
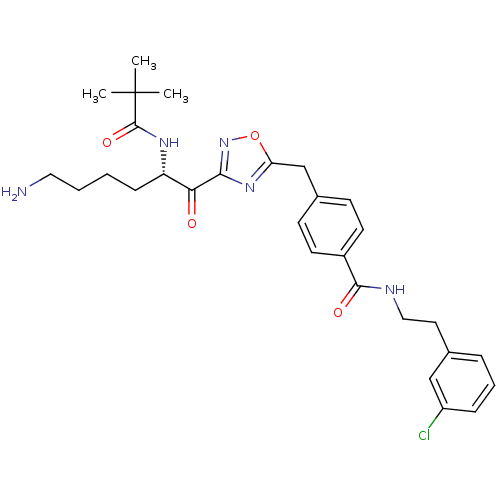
((S)-N-(3-chlorophenethyl)-4-((3-(6-amino-2-pivalam...)Show SMILES CC(C)(C)C(=O)N[C@@H](CCCCN)C(=O)c1noc(Cc2ccc(cc2)C(=O)NCCc2cccc(Cl)c2)n1 Show InChI InChI=1S/C29H36ClN5O4/c1-29(2,3)28(38)33-23(9-4-5-15-31)25(36)26-34-24(39-35-26)18-20-10-12-21(13-11-20)27(37)32-16-14-19-7-6-8-22(30)17-19/h6-8,10-13,17,23H,4-5,9,14-16,18,31H2,1-3H3,(H,32,37)(H,33,38)/t23-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Celera
Curated by ChEMBL
| Assay Description
Inhibition of human beta tryptase |
Bioorg Med Chem Lett 16: 4036-40 (2006)
Article DOI: 10.1016/j.bmcl.2006.05.009
BindingDB Entry DOI: 10.7270/Q20864ZV |
More data for this
Ligand-Target Pair | |
Coagulation factor VII/Tissue factor
(Homo sapiens (Human)) | BDBM50181916
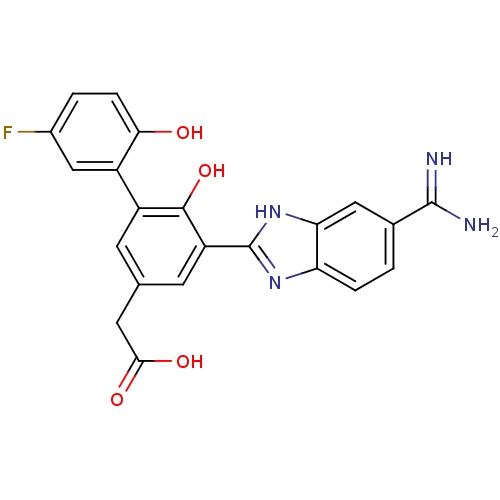
(CHEMBL206168 | [5-(5-carbamimidoyl-1H-benzoimidazo...)Show SMILES NC(=N)c1ccc2nc([nH]c2c1)-c1cc(CC(O)=O)cc(c1O)-c1cc(F)ccc1O Show InChI InChI=1S/C22H17FN4O4/c23-12-2-4-18(28)13(9-12)14-5-10(7-19(29)30)6-15(20(14)31)22-26-16-3-1-11(21(24)25)8-17(16)27-22/h1-6,8-9,28,31H,7H2,(H3,24,25)(H,26,27)(H,29,30) | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Celera Genomics
Curated by ChEMBL
| Assay Description
Binding affinity to factor7a/TF complex |
Bioorg Med Chem Lett 16: 2243-6 (2006)
Article DOI: 10.1016/j.bmcl.2006.01.037
BindingDB Entry DOI: 10.7270/Q2GB23NX |
More data for this
Ligand-Target Pair | |
Coagulation factor VII/Tissue factor
(Homo sapiens (Human)) | BDBM50180400

(2-[5-(5-carbamimidoyl-1H-benzoimidazol-2-yl)-6,2'-...)Show SMILES NC(=O)NCc1ccc(O)c(c1)-c1cc(cc(-c2nc3ccc(cc3[nH]2)C(N)=N)c1O)C(CC(O)=O)C(O)=O Show InChI InChI=1S/C26H24N6O7/c27-23(28)12-2-3-18-19(8-12)32-24(31-18)17-7-13(14(25(37)38)9-21(34)35)6-16(22(17)36)15-5-11(1-4-20(15)33)10-30-26(29)39/h1-8,14,33,36H,9-10H2,(H3,27,28)(H,31,32)(H,34,35)(H,37,38)(H3,29,30,39) | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Celera Genomics
Curated by ChEMBL
| Assay Description
Binding affinity to F7a/TF complex |
Bioorg Med Chem Lett 16: 2037-41 (2006)
Article DOI: 10.1016/j.bmcl.2005.12.059
BindingDB Entry DOI: 10.7270/Q2639PBP |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50101883
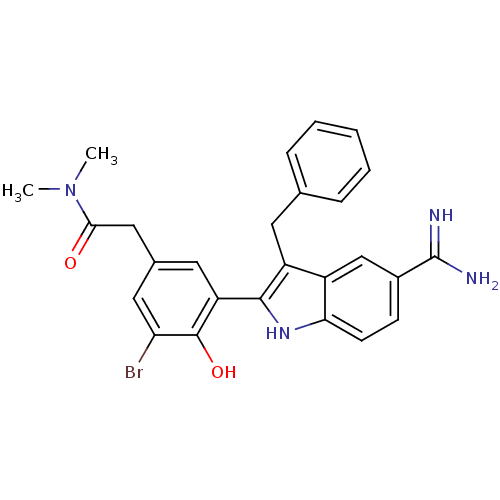
(2-[3-(3-Benzyl-5-carbamimidoyl-1H-indol-2-yl)-5-br...)Show SMILES CN(C)C(=O)Cc1cc(Br)c(O)c(c1)-c1[nH]c2ccc(cc2c1Cc1ccccc1)C(N)=N Show InChI InChI=1S/C26H25BrN4O2/c1-31(2)23(32)13-16-11-20(25(33)21(27)12-16)24-19(10-15-6-4-3-5-7-15)18-14-17(26(28)29)8-9-22(18)30-24/h3-9,11-12,14,30,33H,10,13H2,1-2H3,(H3,28,29) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Axys Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
Inhibitory activity against Coagulation factor X in human plasma |
Bioorg Med Chem Lett 11: 1797-800 (2001)
BindingDB Entry DOI: 10.7270/Q20K27TW |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50101885
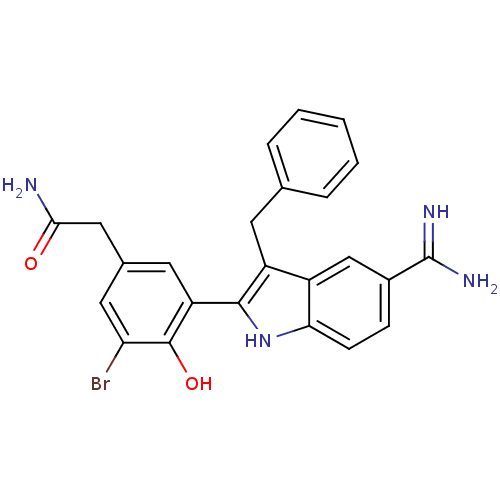
(2-[3-(3-Benzyl-5-carbamimidoyl-1H-indol-2-yl)-5-br...)Show SMILES NC(=O)Cc1cc(Br)c(O)c(c1)-c1[nH]c2ccc(cc2c1Cc1ccccc1)C(N)=N Show InChI InChI=1S/C24H21BrN4O2/c25-19-10-14(11-21(26)30)9-18(23(19)31)22-17(8-13-4-2-1-3-5-13)16-12-15(24(27)28)6-7-20(16)29-22/h1-7,9-10,12,29,31H,8,11H2,(H2,26,30)(H3,27,28) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Axys Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
Inhibitory activity against Coagulation factor X in human plasma |
Bioorg Med Chem Lett 11: 1797-800 (2001)
BindingDB Entry DOI: 10.7270/Q20K27TW |
More data for this
Ligand-Target Pair | |
Dimer of Tryptase beta-2
(Homo sapiens (Human)) | BDBM50187182

((S)-N-(1-(5-(4-((3-chlorophenethyl)carbamoyl)benzy...)Show SMILES NCCCC[C@H](NC(=O)c1ccc2OCOc2c1)C(=O)c1noc(Cc2ccc(cc2)C(=O)NCCc2cccc(Cl)c2)n1 Show InChI InChI=1S/C32H32ClN5O6/c33-24-5-3-4-20(16-24)13-15-35-31(40)22-9-7-21(8-10-22)17-28-37-30(38-44-28)29(39)25(6-1-2-14-34)36-32(41)23-11-12-26-27(18-23)43-19-42-26/h3-5,7-12,16,18,25H,1-2,6,13-15,17,19,34H2,(H,35,40)(H,36,41)/t25-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 2.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Celera
Curated by ChEMBL
| Assay Description
Inhibition of human beta tryptase |
Bioorg Med Chem Lett 16: 4036-40 (2006)
Article DOI: 10.1016/j.bmcl.2006.05.009
BindingDB Entry DOI: 10.7270/Q20864ZV |
More data for this
Ligand-Target Pair | |
Urokinase-type plasminogen activator
(Homo sapiens (Human)) | BDBM14352
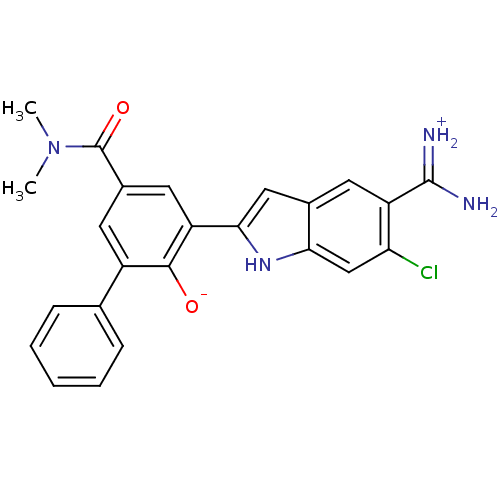
(2-{5-[amino(iminiumyl)methyl]-6-chloro-1H-indol-2-...)Show SMILES CN(C)C(=O)c1cc(-c2cc3cc(C(N)=[NH2+])c(Cl)cc3[nH]2)c([O-])c(c1)-c1ccccc1 Show InChI InChI=1S/C24H21ClN4O2/c1-29(2)24(31)15-9-16(13-6-4-3-5-7-13)22(30)18(10-15)21-11-14-8-17(23(26)27)19(25)12-20(14)28-21/h3-12,28,30H,1-2H3,(H3,26,27) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 2.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Celera
| Assay Description
Each enzyme was assayed with a set of different concentrations of each inhibitor. After addition of the appropriate substrate, the rate of hydrolysis... |
J Mol Biol 329: 93-120 (2003)
Article DOI: 10.1016/s0022-2836(03)00399-1
BindingDB Entry DOI: 10.7270/Q2R78CGQ |
More data for this
Ligand-Target Pair | |
Dimer of Tryptase beta-2
(Homo sapiens (Human)) | BDBM50187164
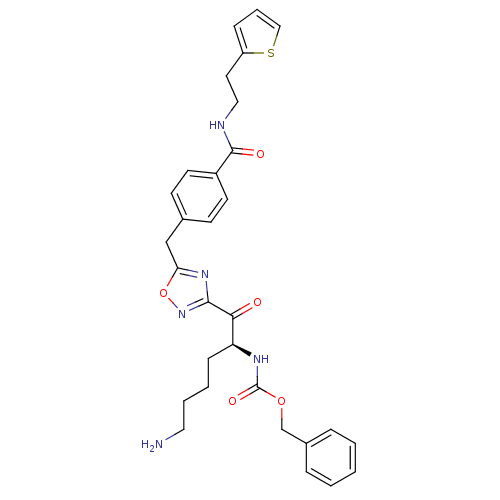
((S)-benzyl 1-(5-(4-((2-(thiophen-2-yl)ethyl)carbam...)Show SMILES NCCCC[C@H](NC(=O)OCc1ccccc1)C(=O)c1noc(Cc2ccc(cc2)C(=O)NCCc2cccs2)n1 Show InChI InChI=1S/C30H33N5O5S/c31-16-5-4-10-25(33-30(38)39-20-22-7-2-1-3-8-22)27(36)28-34-26(40-35-28)19-21-11-13-23(14-12-21)29(37)32-17-15-24-9-6-18-41-24/h1-3,6-9,11-14,18,25H,4-5,10,15-17,19-20,31H2,(H,32,37)(H,33,38)/t25-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 2.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Celera
Curated by ChEMBL
| Assay Description
Inhibition of human beta tryptase |
Bioorg Med Chem Lett 16: 4036-40 (2006)
Article DOI: 10.1016/j.bmcl.2006.05.009
BindingDB Entry DOI: 10.7270/Q20864ZV |
More data for this
Ligand-Target Pair | |
Coagulation factor VII/Tissue factor
(Homo sapiens (Human)) | BDBM50189938
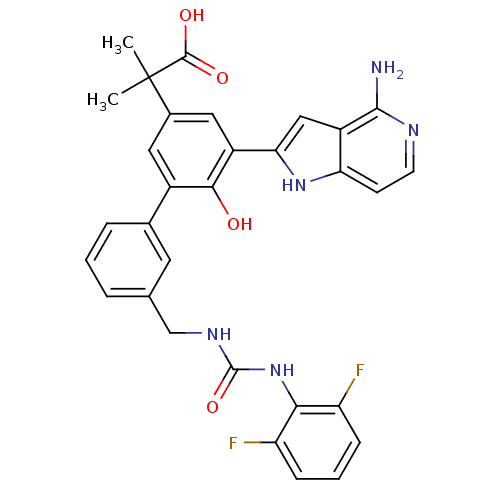
(2-{5-(4-amino-1H-pyrrolo[3,2-c]pyridin-2-yl)-3'-[3...)Show SMILES CC(C)(C(O)=O)c1cc(-c2cc3c(N)nccc3[nH]2)c(O)c(c1)-c1cccc(CNC(=O)Nc2c(F)cccc2F)c1 Show InChI InChI=1S/C31H27F2N5O4/c1-31(2,29(40)41)18-12-19(27(39)20(13-18)25-14-21-24(37-25)9-10-35-28(21)34)17-6-3-5-16(11-17)15-36-30(42)38-26-22(32)7-4-8-23(26)33/h3-14,37,39H,15H2,1-2H3,(H2,34,35)(H,40,41)(H2,36,38,42) | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 2.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Celera Genomics
Curated by ChEMBL
| Assay Description
Binding affinity to f7a/TF complex |
Bioorg Med Chem Lett 16: 4567-70 (2006)
Article DOI: 10.1016/j.bmcl.2006.06.016
BindingDB Entry DOI: 10.7270/Q2NG4Q67 |
More data for this
Ligand-Target Pair | |
Urokinase-type plasminogen activator
(Homo sapiens (Human)) | BDBM14351

(2-{5-[amino(iminiumyl)methyl]-6-chloro-1H-indol-2-...)Show SMILES COc1cc(-c2cc3cc(C(N)=[NH2+])c(Cl)cc3[nH]2)c([O-])c(c1)-c1ccccc1 Show InChI InChI=1S/C22H18ClN3O2/c1-28-14-9-15(12-5-3-2-4-6-12)21(27)17(10-14)20-8-13-7-16(22(24)25)18(23)11-19(13)26-20/h2-11,26-27H,1H3,(H3,24,25) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 2.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Celera
| Assay Description
Each enzyme was assayed with a set of different concentrations of each inhibitor. After addition of the appropriate substrate, the rate of hydrolysis... |
J Mol Biol 329: 93-120 (2003)
Article DOI: 10.1016/s0022-2836(03)00399-1
BindingDB Entry DOI: 10.7270/Q2R78CGQ |
More data for this
Ligand-Target Pair | |
Dimer of Tryptase beta-2
(Homo sapiens (Human)) | BDBM14312
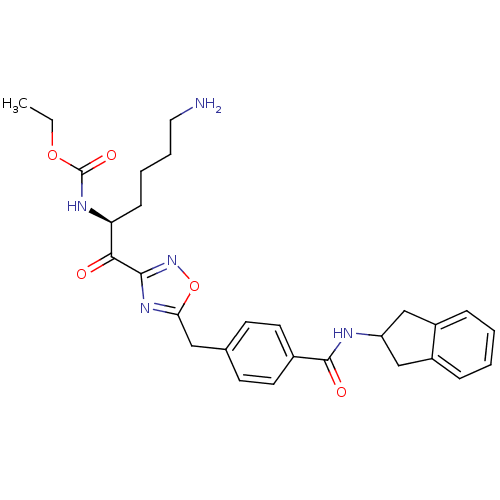
(CHEMBL214368 | CRA23 | ethyl N-[(2S)-6-amino-1-(5-...)Show SMILES CCOC(=O)N[C@@H](CCCCN)C(=O)c1noc(Cc2ccc(cc2)C(=O)NC2Cc3ccccc3C2)n1 |r| Show InChI InChI=1S/C28H33N5O5/c1-2-37-28(36)31-23(9-5-6-14-29)25(34)26-32-24(38-33-26)15-18-10-12-19(13-11-18)27(35)30-22-16-20-7-3-4-8-21(20)17-22/h3-4,7-8,10-13,22-23H,2,5-6,9,14-17,29H2,1H3,(H,30,35)(H,31,36)/t23-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| 2.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Celera
Curated by ChEMBL
| Assay Description
Inhibition of human beta tryptase |
Bioorg Med Chem Lett 16: 4036-40 (2006)
Article DOI: 10.1016/j.bmcl.2006.05.009
BindingDB Entry DOI: 10.7270/Q20864ZV |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Dimer of Tryptase beta-2
(Homo sapiens (Human)) | BDBM50187169
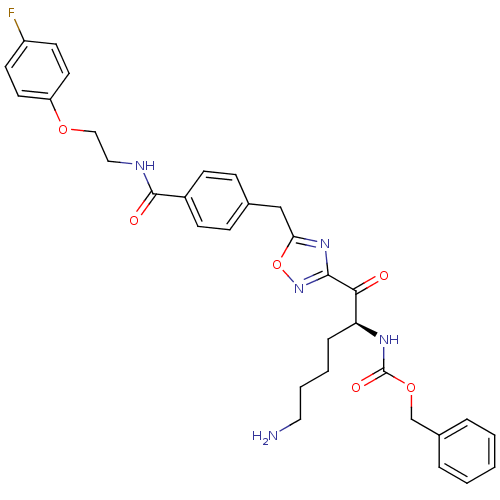
((S)-benzyl 1-(5-(4-((2-(4-fluorophenoxy)ethyl)carb...)Show SMILES NCCCC[C@H](NC(=O)OCc1ccccc1)C(=O)c1noc(Cc2ccc(cc2)C(=O)NCCOc2ccc(F)cc2)n1 Show InChI InChI=1S/C32H34FN5O6/c33-25-13-15-26(16-14-25)42-19-18-35-31(40)24-11-9-22(10-12-24)20-28-37-30(38-44-28)29(39)27(8-4-5-17-34)36-32(41)43-21-23-6-2-1-3-7-23/h1-3,6-7,9-16,27H,4-5,8,17-21,34H2,(H,35,40)(H,36,41)/t27-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 2.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Celera
Curated by ChEMBL
| Assay Description
Inhibition of human beta tryptase |
Bioorg Med Chem Lett 16: 4036-40 (2006)
Article DOI: 10.1016/j.bmcl.2006.05.009
BindingDB Entry DOI: 10.7270/Q20864ZV |
More data for this
Ligand-Target Pair | |
Coagulation factor VII/Tissue factor
(Homo sapiens (Human)) | BDBM50103655
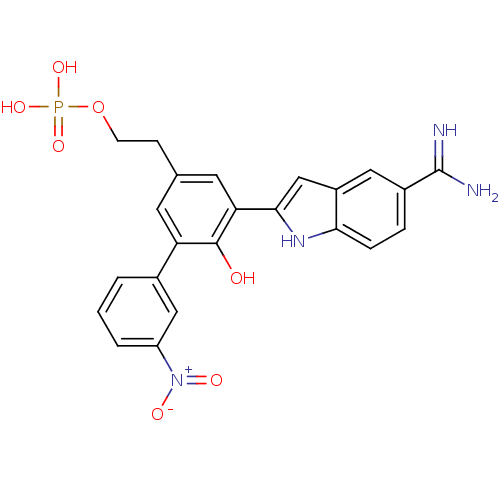
(CHEMBL72231 | Phosphoric acid mono-{2-[5-(5-carbam...)Show SMILES NC(=N)c1ccc2[nH]c(cc2c1)-c1cc(CCO[P+](O)(O)[O-])cc(c1O)-c1cccc(c1)[N+]([O-])=O Show InChI InChI=1S/C23H21N4O7P/c24-23(25)15-4-5-20-16(10-15)12-21(26-20)19-9-13(6-7-34-35(31,32)33)8-18(22(19)28)14-2-1-3-17(11-14)27(29)30/h1-5,8-12,26,28H,6-7H2,(H3,24,25)(H2,31,32,33) | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Axys Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
Binding affinity for factor VIIa/TF |
Bioorg Med Chem Lett 11: 2253-6 (2001)
BindingDB Entry DOI: 10.7270/Q2XG9QD9 |
More data for this
Ligand-Target Pair | |
Plasma kallikrein
(Homo sapiens (Human)) | BDBM14921
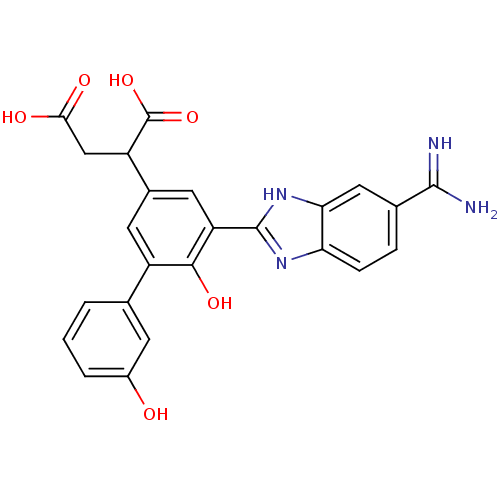
(2-[3-(5-carbamimidoyl-1H-1,3-benzodiazol-2-yl)-4-h...)Show SMILES NC(=N)c1ccc2nc([nH]c2c1)-c1cc(cc(-c2cccc(O)c2)c1O)C(CC(O)=O)C(O)=O Show InChI InChI=1S/C24H20N4O6/c25-22(26)12-4-5-18-19(9-12)28-23(27-18)17-8-13(16(24(33)34)10-20(30)31)7-15(21(17)32)11-2-1-3-14(29)6-11/h1-9,16,29,32H,10H2,(H3,25,26)(H,27,28)(H,30,31)(H,33,34) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Celera Genomics
Curated by ChEMBL
| Assay Description
Binding affinity to plasma kallikrein |
Bioorg Med Chem Lett 16: 2034-6 (2006)
Article DOI: 10.1016/j.bmcl.2005.12.060
BindingDB Entry DOI: 10.7270/Q2P55N3N |
More data for this
Ligand-Target Pair | |
Plasma kallikrein
(Homo sapiens (Human)) | BDBM14917
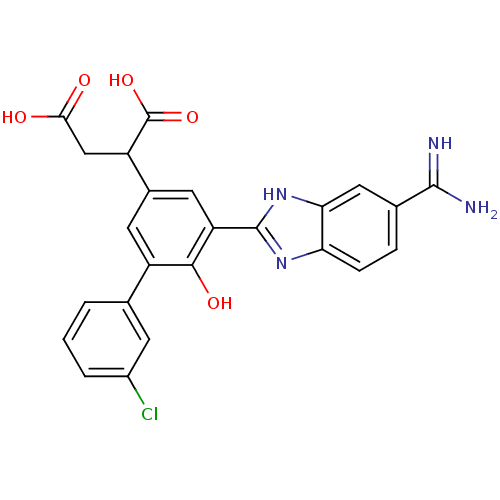
(2-[3-(5-carbamimidoyl-1H-1,3-benzodiazol-2-yl)-5-(...)Show SMILES NC(=N)c1ccc2nc([nH]c2c1)-c1cc(cc(-c2cccc(Cl)c2)c1O)C(CC(O)=O)C(O)=O Show InChI InChI=1S/C24H19ClN4O5/c25-14-3-1-2-11(6-14)15-7-13(16(24(33)34)10-20(30)31)8-17(21(15)32)23-28-18-5-4-12(22(26)27)9-19(18)29-23/h1-9,16,32H,10H2,(H3,26,27)(H,28,29)(H,30,31)(H,33,34) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Celera Genomics
Curated by ChEMBL
| Assay Description
Binding affinity to plasma kallikrein |
Bioorg Med Chem Lett 16: 2034-6 (2006)
Article DOI: 10.1016/j.bmcl.2005.12.060
BindingDB Entry DOI: 10.7270/Q2P55N3N |
More data for this
Ligand-Target Pair | |
Coagulation factor VII/Tissue factor
(Homo sapiens (Human)) | BDBM13778

(2-[3-(5-carbamimidoyl-1H-indol-2-yl)-4-hydroxy-5-(...)Show SMILES NC(=N)c1ccc2[nH]c(cc2c1)-c1cc(CC(O)=O)cc(c1O)-c1cccc(c1)[N+]([O-])=O Show InChI InChI=1S/C23H18N4O5/c24-23(25)14-4-5-19-15(9-14)11-20(26-19)18-7-12(8-21(28)29)6-17(22(18)30)13-2-1-3-16(10-13)27(31)32/h1-7,9-11,26,30H,8H2,(H3,24,25)(H,28,29) | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Celera Genomics
Curated by ChEMBL
| Assay Description
Binding affinity to factor7a/TF complex |
Bioorg Med Chem Lett 16: 2243-6 (2006)
Article DOI: 10.1016/j.bmcl.2006.01.037
BindingDB Entry DOI: 10.7270/Q2GB23NX |
More data for this
Ligand-Target Pair | |
Dimer of Tryptase beta-2
(Homo sapiens (Human)) | BDBM50187179
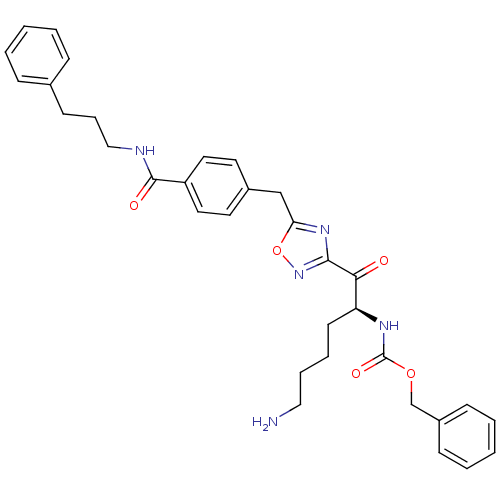
((S)-benzyl 1-(5-(4-((3-phenylpropyl)carbamoyl)benz...)Show SMILES NCCCC[C@H](NC(=O)OCc1ccccc1)C(=O)c1noc(Cc2ccc(cc2)C(=O)NCCCc2ccccc2)n1 Show InChI InChI=1S/C33H37N5O5/c34-20-8-7-15-28(36-33(41)42-23-26-12-5-2-6-13-26)30(39)31-37-29(43-38-31)22-25-16-18-27(19-17-25)32(40)35-21-9-14-24-10-3-1-4-11-24/h1-6,10-13,16-19,28H,7-9,14-15,20-23,34H2,(H,35,40)(H,36,41)/t28-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Celera
Curated by ChEMBL
| Assay Description
Inhibition of human beta tryptase |
Bioorg Med Chem Lett 16: 4036-40 (2006)
Article DOI: 10.1016/j.bmcl.2006.05.009
BindingDB Entry DOI: 10.7270/Q20864ZV |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50101880
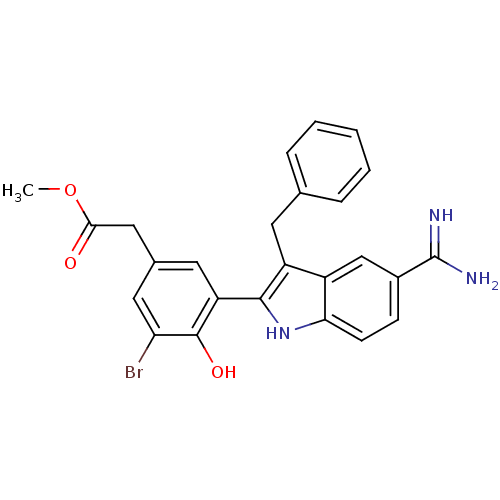
(CHEMBL416127 | [3-(3-Benzyl-5-carbamimidoyl-1H-ind...)Show SMILES COC(=O)Cc1cc(Br)c(O)c(c1)-c1[nH]c2ccc(cc2c1Cc1ccccc1)C(N)=N Show InChI InChI=1S/C25H22BrN3O3/c1-32-22(30)12-15-10-19(24(31)20(26)11-15)23-18(9-14-5-3-2-4-6-14)17-13-16(25(27)28)7-8-21(17)29-23/h2-8,10-11,13,29,31H,9,12H2,1H3,(H3,27,28) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Axys Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
Inhibitory activity against Coagulation factor X in human plasma |
Bioorg Med Chem Lett 11: 1797-800 (2001)
BindingDB Entry DOI: 10.7270/Q20K27TW |
More data for this
Ligand-Target Pair | |
Coagulation factor VII
(Homo sapiens (Human)) | BDBM50186211

(CHEMBL211482 | N-[3'-(5-carbamimidoyl-1H-indol-2-y...)Show SMILES NC(=N)c1ccc2[nH]c(cc2c1)-c1cccc(c1O)-c1cc(CNC(=O)CO)ccc1O Show InChI InChI=1S/C24H22N4O4/c25-24(26)14-5-6-19-15(9-14)10-20(28-19)17-3-1-2-16(23(17)32)18-8-13(4-7-21(18)30)11-27-22(31)12-29/h1-10,28-30,32H,11-12H2,(H3,25,26)(H,27,31) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Celera
Curated by ChEMBL
| Assay Description
Binding affinity to f7a |
Bioorg Med Chem Lett 16: 3829-32 (2006)
Article DOI: 10.1016/j.bmcl.2006.04.018
BindingDB Entry DOI: 10.7270/Q2DN44NK |
More data for this
Ligand-Target Pair | |
Coagulation factor VII/Tissue factor
(Homo sapiens (Human)) | BDBM13778

(2-[3-(5-carbamimidoyl-1H-indol-2-yl)-4-hydroxy-5-(...)Show SMILES NC(=N)c1ccc2[nH]c(cc2c1)-c1cc(CC(O)=O)cc(c1O)-c1cccc(c1)[N+]([O-])=O Show InChI InChI=1S/C23H18N4O5/c24-23(25)14-4-5-19-15(9-14)11-20(26-19)18-7-12(8-21(28)29)6-17(22(18)30)13-2-1-3-16(10-13)27(31)32/h1-7,9-11,26,30H,8H2,(H3,24,25)(H,28,29) | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Axys Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
Binding affinity for factor VIIa/TF |
Bioorg Med Chem Lett 11: 2253-6 (2001)
BindingDB Entry DOI: 10.7270/Q2XG9QD9 |
More data for this
Ligand-Target Pair | |
Tryptase beta-2
(Homo sapiens (Human)) | BDBM14312

(CHEMBL214368 | CRA23 | ethyl N-[(2S)-6-amino-1-(5-...)Show SMILES CCOC(=O)N[C@@H](CCCCN)C(=O)c1noc(Cc2ccc(cc2)C(=O)NC2Cc3ccccc3C2)n1 |r| Show InChI InChI=1S/C28H33N5O5/c1-2-37-28(36)31-23(9-5-6-14-29)25(34)26-32-24(38-33-26)15-18-10-12-19(13-11-18)27(35)30-22-16-20-7-3-4-8-21(20)17-22/h3-4,7-8,10-13,22-23H,2,5-6,9,14-17,29H2,1H3,(H,30,35)(H,31,36)/t23-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| 3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Celera
| Assay Description
Each enzyme was assayed with a set of different concentrations of each inhibitor. After addition of the appropriate substrate, the rate of hydrolysis... |
Biochemistry 45: 5964-73 (2006)
Article DOI: 10.1021/bi060173m
BindingDB Entry DOI: 10.7270/Q2W09450 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50101879
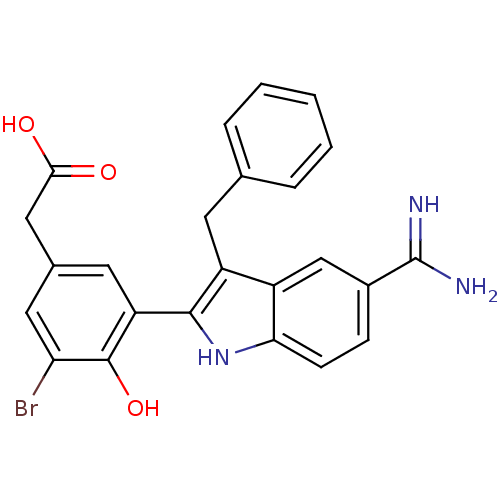
(CHEMBL50924 | [3-(3-Benzyl-5-carbamimidoyl-1H-indo...)Show SMILES NC(=N)c1ccc2[nH]c(c(Cc3ccccc3)c2c1)-c1cc(CC(O)=O)cc(Br)c1O Show InChI InChI=1S/C24H20BrN3O3/c25-19-10-14(11-21(29)30)9-18(23(19)31)22-17(8-13-4-2-1-3-5-13)16-12-15(24(26)27)6-7-20(16)28-22/h1-7,9-10,12,28,31H,8,11H2,(H3,26,27)(H,29,30) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Axys Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
Inhibitory activity against Coagulation factor X in human plasma |
Bioorg Med Chem Lett 11: 1797-800 (2001)
BindingDB Entry DOI: 10.7270/Q20K27TW |
More data for this
Ligand-Target Pair | |
Dimer of Tryptase beta-2
(Homo sapiens (Human)) | BDBM50187154
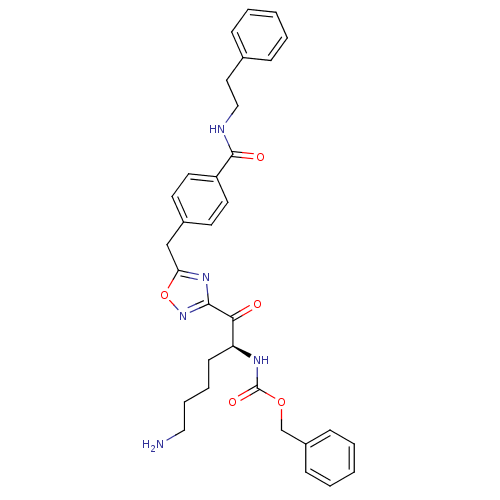
((S)-benzyl 1-(5-(4-(phenethylcarbamoyl)benzyl)-1,2...)Show SMILES NCCCC[C@H](NC(=O)OCc1ccccc1)C(=O)c1noc(Cc2ccc(cc2)C(=O)NCCc2ccccc2)n1 Show InChI InChI=1S/C32H35N5O5/c33-19-8-7-13-27(35-32(40)41-22-25-11-5-2-6-12-25)29(38)30-36-28(42-37-30)21-24-14-16-26(17-15-24)31(39)34-20-18-23-9-3-1-4-10-23/h1-6,9-12,14-17,27H,7-8,13,18-22,33H2,(H,34,39)(H,35,40)/t27-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 3.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Celera
Curated by ChEMBL
| Assay Description
Inhibition of human beta tryptase |
Bioorg Med Chem Lett 16: 4036-40 (2006)
Article DOI: 10.1016/j.bmcl.2006.05.009
BindingDB Entry DOI: 10.7270/Q20864ZV |
More data for this
Ligand-Target Pair | |
Plasminogen
(Homo sapiens (Human)) | BDBM50103651

(2-(3'-Amino-5-chloro-2-hydroxy-biphenyl-3-yl)-1H-b...)Show SMILES NC(=N)c1ccc2nc([nH]c2c1)-c1cc(Cl)cc(-c2cccc(N)c2)c1O Show InChI InChI=1S/C20H16ClN5O/c21-12-8-14(10-2-1-3-13(22)6-10)18(27)15(9-12)20-25-16-5-4-11(19(23)24)7-17(16)26-20/h1-9,27H,22H2,(H3,23,24)(H,25,26) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 3.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Axys Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
Binding affinity of the compound towards Plasmin |
Bioorg Med Chem Lett 11: 2253-6 (2001)
BindingDB Entry DOI: 10.7270/Q2XG9QD9 |
More data for this
Ligand-Target Pair | |
Urokinase-type plasminogen activator
(Homo sapiens (Human)) | BDBM14354
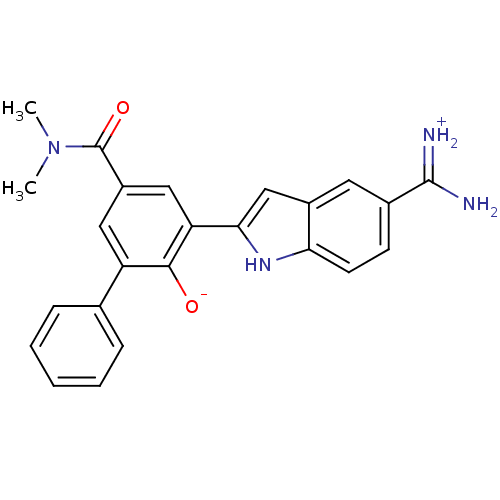
(2-{5-[amino(iminiumyl)methyl]-1H-indol-2-yl}-4-(di...)Show SMILES CN(C)C(=O)c1cc(-c2cc3cc(ccc3[nH]2)C(N)=[NH2+])c([O-])c(c1)-c1ccccc1 Show InChI InChI=1S/C24H22N4O2/c1-28(2)24(30)17-11-18(14-6-4-3-5-7-14)22(29)19(12-17)21-13-16-10-15(23(25)26)8-9-20(16)27-21/h3-13,27,29H,1-2H3,(H3,25,26) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 3.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Celera
| Assay Description
Each enzyme was assayed with a set of different concentrations of each inhibitor. After addition of the appropriate substrate, the rate of hydrolysis... |
J Mol Biol 329: 93-120 (2003)
Article DOI: 10.1016/s0022-2836(03)00399-1
BindingDB Entry DOI: 10.7270/Q2R78CGQ |
More data for this
Ligand-Target Pair | |
Dimer of Tryptase beta-2
(Homo sapiens (Human)) | BDBM50187157

((S)-benzyl 1-(5-(4-((3,5-difluorophenethyl)carbamo...)Show SMILES NCCCC[C@H](NC(=O)OCc1ccccc1)C(=O)c1noc(Cc2ccc(cc2)C(=O)NCCc2cc(F)cc(F)c2)n1 Show InChI InChI=1S/C32H33F2N5O5/c33-25-16-23(17-26(34)19-25)13-15-36-31(41)24-11-9-21(10-12-24)18-28-38-30(39-44-28)29(40)27(8-4-5-14-35)37-32(42)43-20-22-6-2-1-3-7-22/h1-3,6-7,9-12,16-17,19,27H,4-5,8,13-15,18,20,35H2,(H,36,41)(H,37,42)/t27-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 3.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Celera
Curated by ChEMBL
| Assay Description
Inhibition of human beta tryptase |
Bioorg Med Chem Lett 16: 4036-40 (2006)
Article DOI: 10.1016/j.bmcl.2006.05.009
BindingDB Entry DOI: 10.7270/Q20864ZV |
More data for this
Ligand-Target Pair | |
Dimer of Tryptase beta-2
(Homo sapiens (Human)) | BDBM50187165
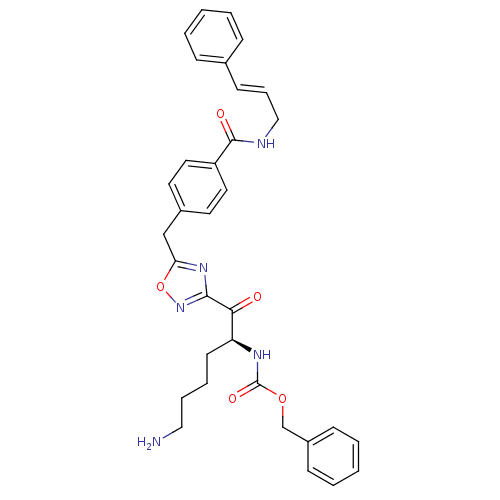
((S)-benzyl 1-(5-(4-(cinnamylcarbamoyl)benzyl)-1,2,...)Show SMILES NCCCC[C@H](NC(=O)OCc1ccccc1)C(=O)c1noc(Cc2ccc(cc2)C(=O)NC\C=C\c2ccccc2)n1 Show InChI InChI=1S/C33H35N5O5/c34-20-8-7-15-28(36-33(41)42-23-26-12-5-2-6-13-26)30(39)31-37-29(43-38-31)22-25-16-18-27(19-17-25)32(40)35-21-9-14-24-10-3-1-4-11-24/h1-6,9-14,16-19,28H,7-8,15,20-23,34H2,(H,35,40)(H,36,41)/b14-9+/t28-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 3.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Celera
Curated by ChEMBL
| Assay Description
Inhibition of human beta tryptase |
Bioorg Med Chem Lett 16: 4036-40 (2006)
Article DOI: 10.1016/j.bmcl.2006.05.009
BindingDB Entry DOI: 10.7270/Q20864ZV |
More data for this
Ligand-Target Pair | |
Dimer of Tryptase beta-2
(Homo sapiens (Human)) | BDBM50187158
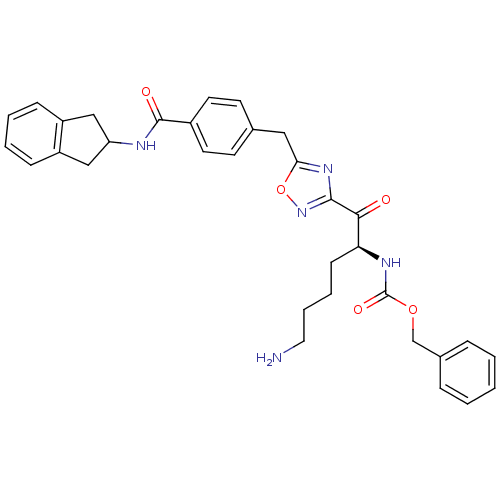
((S)-benzyl 1-(5-(4-((2,3-dihydro-1H-inden-2-yl)car...)Show SMILES NCCCC[C@H](NC(=O)OCc1ccccc1)C(=O)c1noc(Cc2ccc(cc2)C(=O)NC2Cc3ccccc3C2)n1 Show InChI InChI=1S/C33H35N5O5/c34-17-7-6-12-28(36-33(41)42-21-23-8-2-1-3-9-23)30(39)31-37-29(43-38-31)18-22-13-15-24(16-14-22)32(40)35-27-19-25-10-4-5-11-26(25)20-27/h1-5,8-11,13-16,27-28H,6-7,12,17-21,34H2,(H,35,40)(H,36,41)/t28-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 3.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Celera
Curated by ChEMBL
| Assay Description
Inhibition of human beta tryptase |
Bioorg Med Chem Lett 16: 4036-40 (2006)
Article DOI: 10.1016/j.bmcl.2006.05.009
BindingDB Entry DOI: 10.7270/Q20864ZV |
More data for this
Ligand-Target Pair | |
Coagulation factor VII
(Homo sapiens (Human)) | BDBM13778

(2-[3-(5-carbamimidoyl-1H-indol-2-yl)-4-hydroxy-5-(...)Show SMILES NC(=N)c1ccc2[nH]c(cc2c1)-c1cc(CC(O)=O)cc(c1O)-c1cccc(c1)[N+]([O-])=O Show InChI InChI=1S/C23H18N4O5/c24-23(25)14-4-5-19-15(9-14)11-20(26-19)18-7-12(8-21(28)29)6-17(22(18)30)13-2-1-3-16(10-13)27(31)32/h1-7,9-11,26,30H,8H2,(H3,24,25)(H,28,29) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 4 | -47.5 | n/a | n/a | n/a | n/a | n/a | 7.4 | 22 |
Celera
| Assay Description
The enzyme reactions were initiated by the addition of substrate, and the color developed from the release of p-nitroanilide from each chromogenic su... |
Bioorg Med Chem Lett 16: 2270-3 (2006)
Article DOI: 10.1016/j.bmcl.2006.01.017
BindingDB Entry DOI: 10.7270/Q2RF5S8X |
More data for this
Ligand-Target Pair | |
Coagulation factor VII
(Homo sapiens (Human)) | BDBM14898
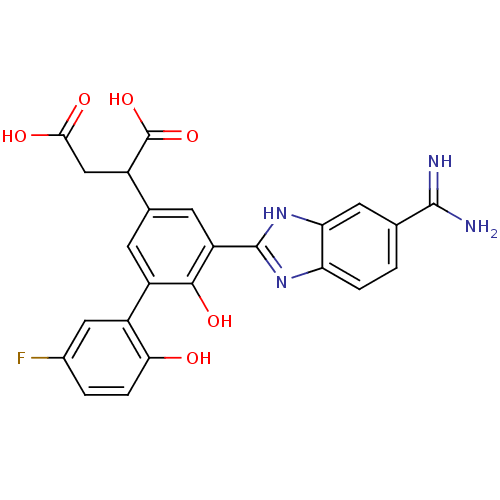
(2-[3-(5-carbamimidoyl-1H-1,3-benzodiazol-2-yl)-5-(...)Show SMILES NC(=N)c1ccc2nc([nH]c2c1)-c1cc(cc(c1O)-c1cc(F)ccc1O)C(CC(O)=O)C(O)=O Show InChI InChI=1S/C24H19FN4O6/c25-12-2-4-19(30)14(8-12)15-5-11(13(24(34)35)9-20(31)32)6-16(21(15)33)23-28-17-3-1-10(22(26)27)7-18(17)29-23/h1-8,13,30,33H,9H2,(H3,26,27)(H,28,29)(H,31,32)(H,34,35) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 4 | -47.5 | n/a | n/a | n/a | n/a | n/a | 7.4 | 22 |
Celera
| Assay Description
The enzyme reactions were initiated by the addition of substrate, and the color developed from the release of p-nitroanilide from each chromogenic su... |
Bioorg Med Chem Lett 16: 1596-600 (2006)
Article DOI: 10.1016/j.bmcl.2005.12.040
BindingDB Entry DOI: 10.7270/Q2VX0DSZ |
More data for this
Ligand-Target Pair | |
Tryptase beta-2
(Homo sapiens (Human)) | BDBM14311
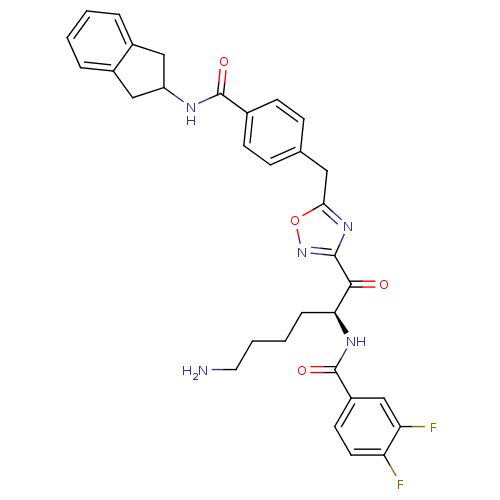
(CRA22 | N-[(2S)-6-amino-1-(5-{[4-(2,3-dihydro-1H-i...)Show SMILES NCCCC[C@H](NC(=O)c1ccc(F)c(F)c1)C(=O)c1noc(Cc2ccc(cc2)C(=O)NC2Cc3ccccc3C2)n1 |r| Show InChI InChI=1S/C32H31F2N5O4/c33-25-13-12-23(18-26(25)34)32(42)37-27(7-3-4-14-35)29(40)30-38-28(43-39-30)15-19-8-10-20(11-9-19)31(41)36-24-16-21-5-1-2-6-22(21)17-24/h1-2,5-6,8-13,18,24,27H,3-4,7,14-17,35H2,(H,36,41)(H,37,42)/t27-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Celera
| Assay Description
Each enzyme was assayed with a set of different concentrations of each inhibitor. After addition of the appropriate substrate, the rate of hydrolysis... |
Biochemistry 45: 5964-73 (2006)
Article DOI: 10.1021/bi060173m
BindingDB Entry DOI: 10.7270/Q2W09450 |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50101870
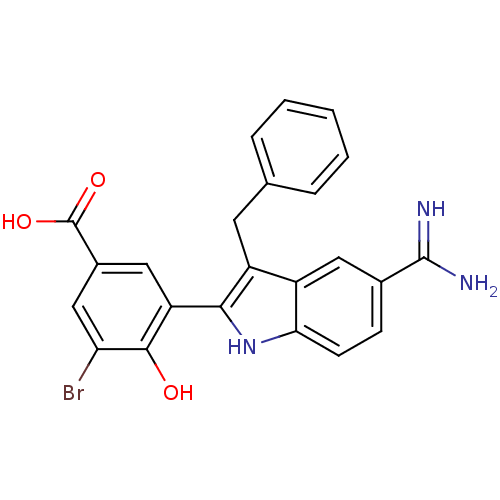
(3-(3-Benzyl-5-carbamimidoyl-1H-indol-2-yl)-5-bromo...)Show SMILES NC(=N)c1ccc2[nH]c(c(Cc3ccccc3)c2c1)-c1cc(cc(Br)c1O)C(O)=O Show InChI InChI=1S/C23H18BrN3O3/c24-18-11-14(23(29)30)10-17(21(18)28)20-16(8-12-4-2-1-3-5-12)15-9-13(22(25)26)6-7-19(15)27-20/h1-7,9-11,27-28H,8H2,(H3,25,26)(H,29,30) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Axys Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
Inhibitory activity against Coagulation factor X in human plasma |
Bioorg Med Chem Lett 11: 1797-800 (2001)
BindingDB Entry DOI: 10.7270/Q20K27TW |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data