

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Thymidylate synthase (Homo sapiens (Human)) | BDBM50028107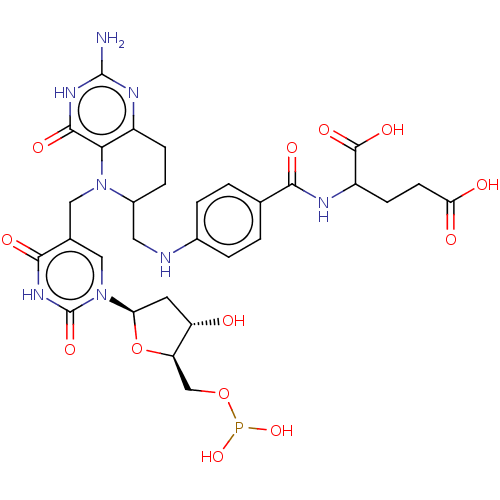 (2-{4-[(2-Amino-5-{1-[5-(dihydroxy-phosphanyloxymet...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 58 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibitory activity against human thymidylate synthase with variable concentration of dUMP and fixed N5,10-CH2-H4PteGlu (200 uM) | J Med Chem 27: 1710-7 (1985) BindingDB Entry DOI: 10.7270/Q2N87BBJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| DNA-(apurinic or apyrimidinic site) endonuclease (Homo sapiens (Human)) | BDBM318446 (NSC332395 | US9624235, Compound 4) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 120 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Pittsburgh—Of the Commonwealth System of Higher Education US Patent | Assay Description The initial screen was designed to look at the effect of potential inhibitors on APE1 repair kinetics. The approach can be used as a high throughput ... | US Patent US9624235 (2017) BindingDB Entry DOI: 10.7270/Q2SQ92GH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| DNA-(apurinic or apyrimidinic site) endonuclease (Homo sapiens (Human)) | BDBM92732 (NSC332395, 4) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 120 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Pittsburgh | Assay Description A fluorescenc-based molecular beacon assay to measure APE-1 protein activity and inhibition was performed as described. | Biochemistry 51: 6246-59 (2012) Article DOI: 10.1021/bi300490r BindingDB Entry DOI: 10.7270/Q2D7991B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| DNA-(apurinic or apyrimidinic site) endonuclease (Homo sapiens (Human)) | BDBM318443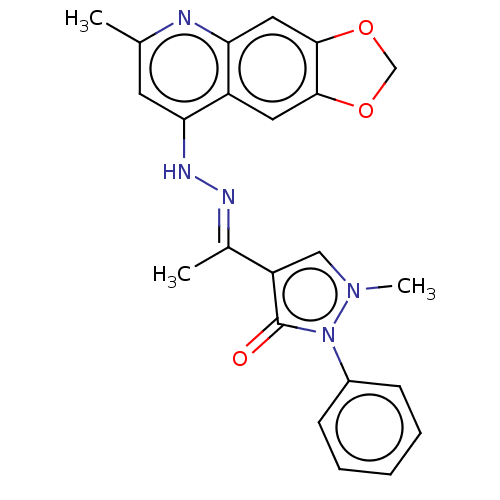 (NSC332398 | US9624235, Compound 1) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 180 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Pittsburgh—Of the Commonwealth System of Higher Education US Patent | Assay Description The initial screen was designed to look at the effect of potential inhibitors on APE1 repair kinetics. The approach can be used as a high throughput ... | US Patent US9624235 (2017) BindingDB Entry DOI: 10.7270/Q2SQ92GH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| DNA-(apurinic or apyrimidinic site) endonuclease (Homo sapiens (Human)) | BDBM92729 (NSC332398, 1) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 180 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Pittsburgh | Assay Description A fluorescenc-based molecular beacon assay to measure APE-1 protein activity and inhibition was performed as described. | Biochemistry 51: 6246-59 (2012) Article DOI: 10.1021/bi300490r BindingDB Entry DOI: 10.7270/Q2D7991B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| DNA-(apurinic or apyrimidinic site) endonuclease (Homo sapiens (Human)) | BDBM318445 (NSC332389 | US9624235, Compound 3) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 190 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Pittsburgh—Of the Commonwealth System of Higher Education US Patent | Assay Description The initial screen was designed to look at the effect of potential inhibitors on APE1 repair kinetics. The approach can be used as a high throughput ... | US Patent US9624235 (2017) BindingDB Entry DOI: 10.7270/Q2SQ92GH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| DNA-(apurinic or apyrimidinic site) endonuclease (Homo sapiens (Human)) | BDBM92731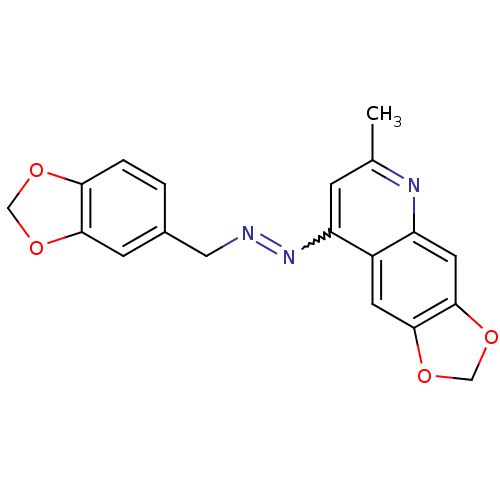 (NSC332389, 3) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 190 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Pittsburgh | Assay Description A fluorescenc-based molecular beacon assay to measure APE-1 protein activity and inhibition was performed as described. | Biochemistry 51: 6246-59 (2012) Article DOI: 10.1021/bi300490r BindingDB Entry DOI: 10.7270/Q2D7991B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| DNA-(apurinic or apyrimidinic site) endonuclease (Homo sapiens (Human)) | BDBM318444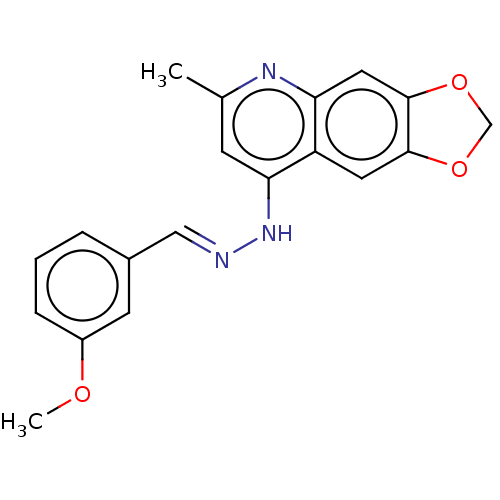 (NSC332384 | US9624235, Compound 2) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 220 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Pittsburgh—Of the Commonwealth System of Higher Education US Patent | Assay Description The initial screen was designed to look at the effect of potential inhibitors on APE1 repair kinetics. The approach can be used as a high throughput ... | US Patent US9624235 (2017) BindingDB Entry DOI: 10.7270/Q2SQ92GH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| DNA-(apurinic or apyrimidinic site) endonuclease (Homo sapiens (Human)) | BDBM92730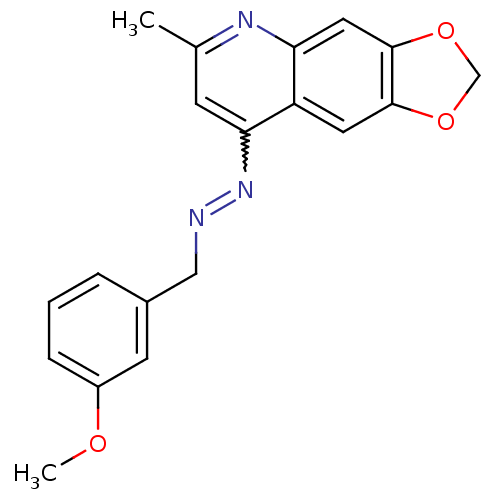 (NSC332384, 2) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 220 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Pittsburgh | Assay Description A fluorescenc-based molecular beacon assay to measure APE-1 protein activity and inhibition was performed as described. | Biochemistry 51: 6246-59 (2012) Article DOI: 10.1021/bi300490r BindingDB Entry DOI: 10.7270/Q2D7991B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Thymidylate synthase (Homo sapiens (Human)) | BDBM50028107 (2-{4-[(2-Amino-5-{1-[5-(dihydroxy-phosphanyloxymet...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 250 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibitory activity against human thymidylate synthase with variable concentration of dUMP and fixed N5,10-CH2-H4PteGlu (200 uM) | J Med Chem 27: 1710-7 (1985) BindingDB Entry DOI: 10.7270/Q2N87BBJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| DNA-(apurinic or apyrimidinic site) endonuclease (Homo sapiens (Human)) | BDBM318448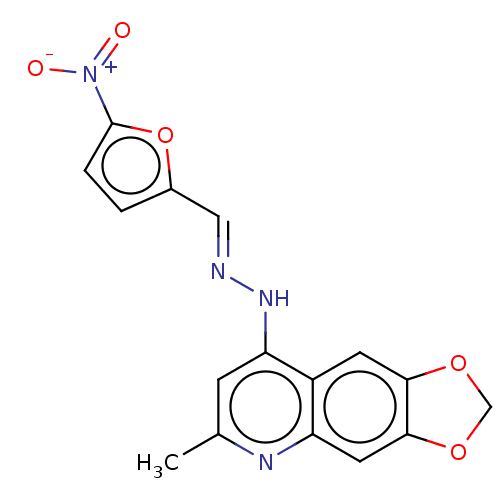 (NSC332397 | US9624235, Compound 6) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 640 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Pittsburgh—Of the Commonwealth System of Higher Education US Patent | Assay Description The initial screen was designed to look at the effect of potential inhibitors on APE1 repair kinetics. The approach can be used as a high throughput ... | US Patent US9624235 (2017) BindingDB Entry DOI: 10.7270/Q2SQ92GH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| DNA-(apurinic or apyrimidinic site) endonuclease (Homo sapiens (Human)) | BDBM318447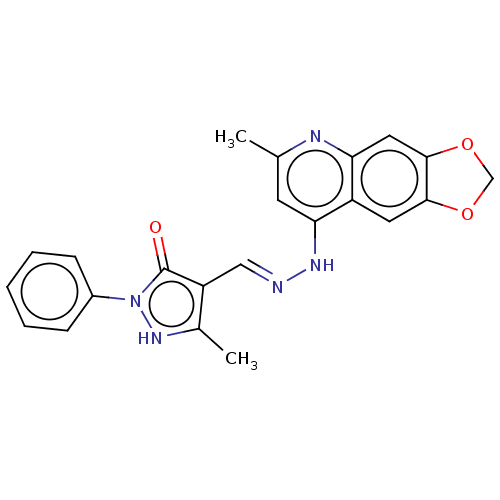 (NSC332396 | US9624235, Compound 5) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 680 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Pittsburgh—Of the Commonwealth System of Higher Education US Patent | Assay Description The initial screen was designed to look at the effect of potential inhibitors on APE1 repair kinetics. The approach can be used as a high throughput ... | US Patent US9624235 (2017) BindingDB Entry DOI: 10.7270/Q2SQ92GH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| DNA-(apurinic or apyrimidinic site) endonuclease (Homo sapiens (Human)) | BDBM92733 (NSC332396, 5) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 680 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Pittsburgh | Assay Description A fluorescenc-based molecular beacon assay to measure APE-1 protein activity and inhibition was performed as described. | Biochemistry 51: 6246-59 (2012) Article DOI: 10.1021/bi300490r BindingDB Entry DOI: 10.7270/Q2D7991B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Thymidylate synthase (Homo sapiens (Human)) | BDBM50028108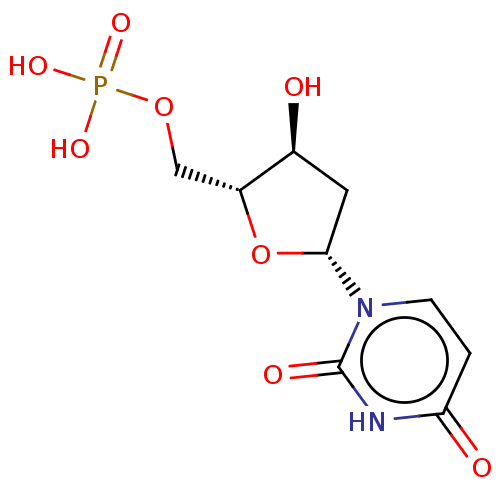 (2'-Deoxyuridinemonophosphate | DEOXYURIDINE MONOPH...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase DrugBank KEGG PC cid PC sid PDB UniChem Similars | PubMed | 3.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibitory activity against human thymidylate synthase with variable concentration of dUMP and fixed N5,10-CH2-H4PteGlu (200 uM) | J Med Chem 27: 1710-7 (1985) BindingDB Entry DOI: 10.7270/Q2N87BBJ | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (docked) | ||||||||||||
| DNA-(apurinic or apyrimidinic site) endonuclease (Homo sapiens (Human)) | BDBM92740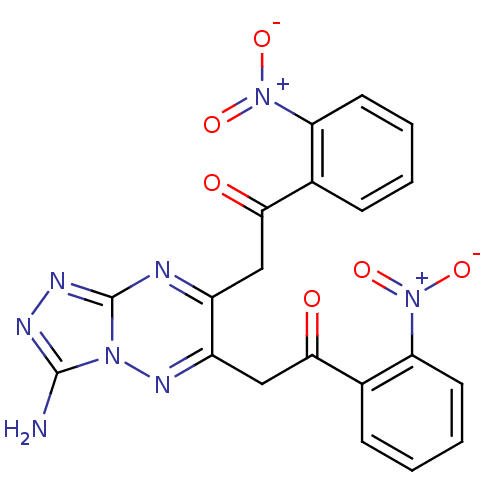 (NSC614430, 13) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 4.90E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Pittsburgh | Assay Description A fluorescenc-based molecular beacon assay to measure APE-1 protein activity and inhibition was performed as described. | Biochemistry 51: 6246-59 (2012) Article DOI: 10.1021/bi300490r BindingDB Entry DOI: 10.7270/Q2D7991B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| DNA-(apurinic or apyrimidinic site) endonuclease (Homo sapiens (Human)) | BDBM318454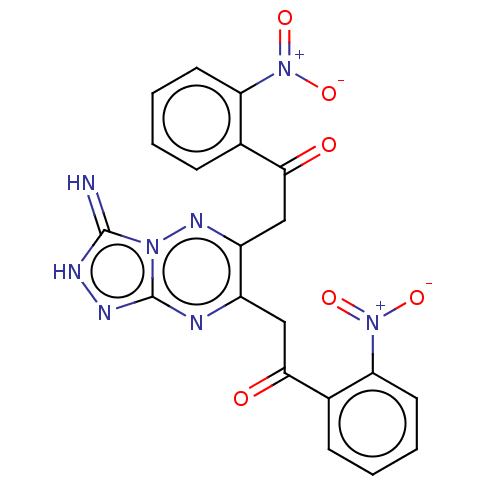 (NSC614430 | US9624235, Compound 13) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 4.90E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Pittsburgh—Of the Commonwealth System of Higher Education US Patent | Assay Description The initial screen was designed to look at the effect of potential inhibitors on APE1 repair kinetics. The approach can be used as a high throughput ... | US Patent US9624235 (2017) BindingDB Entry DOI: 10.7270/Q2SQ92GH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| DNA-(apurinic or apyrimidinic site) endonuclease (Homo sapiens (Human)) | BDBM92738 (NSC131534, 11) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 6.70E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Pittsburgh | Assay Description A fluorescenc-based molecular beacon assay to measure APE-1 protein activity and inhibition was performed as described. | Biochemistry 51: 6246-59 (2012) Article DOI: 10.1021/bi300490r BindingDB Entry DOI: 10.7270/Q2D7991B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| DNA-(apurinic or apyrimidinic site) endonuclease (Homo sapiens (Human)) | BDBM318452 (NSC131534 | US9624235, Compound 11) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 6.70E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Pittsburgh—Of the Commonwealth System of Higher Education US Patent | Assay Description The initial screen was designed to look at the effect of potential inhibitors on APE1 repair kinetics. The approach can be used as a high throughput ... | US Patent US9624235 (2017) BindingDB Entry DOI: 10.7270/Q2SQ92GH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| DNA-(apurinic or apyrimidinic site) endonuclease (Homo sapiens (Human)) | BDBM318449 (NSC300598 | US9624235, Compound 7) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 1.09E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Pittsburgh—Of the Commonwealth System of Higher Education US Patent | Assay Description The initial screen was designed to look at the effect of potential inhibitors on APE1 repair kinetics. The approach can be used as a high throughput ... | US Patent US9624235 (2017) BindingDB Entry DOI: 10.7270/Q2SQ92GH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| DNA-(apurinic or apyrimidinic site) endonuclease (Homo sapiens (Human)) | BDBM92735 (NSC300598, 7) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 1.09E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Pittsburgh | Assay Description A fluorescenc-based molecular beacon assay to measure APE-1 protein activity and inhibition was performed as described. | Biochemistry 51: 6246-59 (2012) Article DOI: 10.1021/bi300490r BindingDB Entry DOI: 10.7270/Q2D7991B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| DNA-(apurinic or apyrimidinic site) endonuclease (Homo sapiens (Human)) | BDBM318450 (NSC89640 | US9624235, Compound 9) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 1.30E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Pittsburgh—Of the Commonwealth System of Higher Education US Patent | Assay Description The initial screen was designed to look at the effect of potential inhibitors on APE1 repair kinetics. The approach can be used as a high throughput ... | US Patent US9624235 (2017) BindingDB Entry DOI: 10.7270/Q2SQ92GH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| DNA-(apurinic or apyrimidinic site) endonuclease (Homo sapiens (Human)) | BDBM92736 (NSC89640, 9) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase PC cid PC sid UniChem | Article PubMed | 1.30E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Pittsburgh | Assay Description A fluorescenc-based molecular beacon assay to measure APE-1 protein activity and inhibition was performed as described. | Biochemistry 51: 6246-59 (2012) Article DOI: 10.1021/bi300490r BindingDB Entry DOI: 10.7270/Q2D7991B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| DNA-(apurinic or apyrimidinic site) endonuclease (Homo sapiens (Human)) | BDBM92737 (NSC107215, 10) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 1.70E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Pittsburgh | Assay Description A fluorescenc-based molecular beacon assay to measure APE-1 protein activity and inhibition was performed as described. | Biochemistry 51: 6246-59 (2012) Article DOI: 10.1021/bi300490r BindingDB Entry DOI: 10.7270/Q2D7991B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| DNA-(apurinic or apyrimidinic site) endonuclease (Homo sapiens (Human)) | BDBM318451 (NSC107215 | US9624235, Compound 10) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 1.70E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Pittsburgh—Of the Commonwealth System of Higher Education US Patent | Assay Description The initial screen was designed to look at the effect of potential inhibitors on APE1 repair kinetics. The approach can be used as a high throughput ... | US Patent US9624235 (2017) BindingDB Entry DOI: 10.7270/Q2SQ92GH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| DNA-(apurinic or apyrimidinic site) endonuclease (Homo sapiens (Human)) | BDBM92739 (NSC375491, 12 | US9624235, Compound 12) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 3.20E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Pittsburgh | Assay Description A fluorescenc-based molecular beacon assay to measure APE-1 protein activity and inhibition was performed as described. | Biochemistry 51: 6246-59 (2012) Article DOI: 10.1021/bi300490r BindingDB Entry DOI: 10.7270/Q2D7991B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| DNA-(apurinic or apyrimidinic site) endonuclease (Homo sapiens (Human)) | BDBM92739 (NSC375491, 12 | US9624235, Compound 12) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 3.20E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Pittsburgh—Of the Commonwealth System of Higher Education US Patent | Assay Description The initial screen was designed to look at the effect of potential inhibitors on APE1 repair kinetics. The approach can be used as a high throughput ... | US Patent US9624235 (2017) BindingDB Entry DOI: 10.7270/Q2SQ92GH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| DNA-(apurinic or apyrimidinic site) endonuclease (Homo sapiens (Human)) | BDBM92741 (NSC402686, 14) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 3.70E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Pittsburgh | Assay Description A fluorescenc-based molecular beacon assay to measure APE-1 protein activity and inhibition was performed as described. | Biochemistry 51: 6246-59 (2012) Article DOI: 10.1021/bi300490r BindingDB Entry DOI: 10.7270/Q2D7991B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| DNA-(apurinic or apyrimidinic site) endonuclease (Homo sapiens (Human)) | BDBM318455 (NSC402686 | US9624235, Compound 14) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase PC cid PC sid UniChem | US Patent | 3.70E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Pittsburgh—Of the Commonwealth System of Higher Education US Patent | Assay Description The initial screen was designed to look at the effect of potential inhibitors on APE1 repair kinetics. The approach can be used as a high throughput ... | US Patent US9624235 (2017) BindingDB Entry DOI: 10.7270/Q2SQ92GH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Thymidylate synthase (Homo sapiens (Human)) | BDBM50028109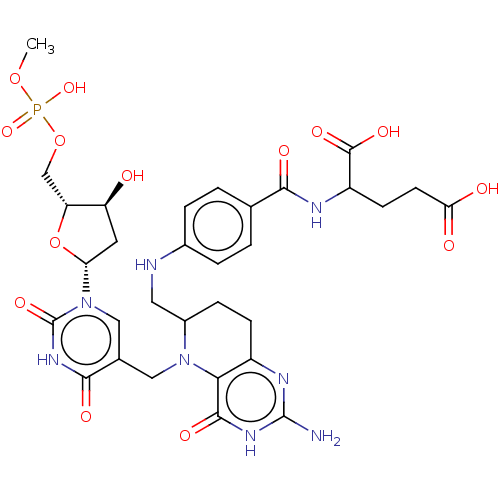 (2-{4-[(2-Amino-5-{1-[4-hydroxy-5-(hydroxy-methoxy-...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 1.20E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibitory activity against human thymidylate synthase with variable concentration of dUMP and fixed N5,10-CH2-H4PteGlu (200 uM) | J Med Chem 27: 1710-7 (1985) BindingDB Entry DOI: 10.7270/Q2N87BBJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Thymidylate synthase (Homo sapiens (Human)) | BDBM50028106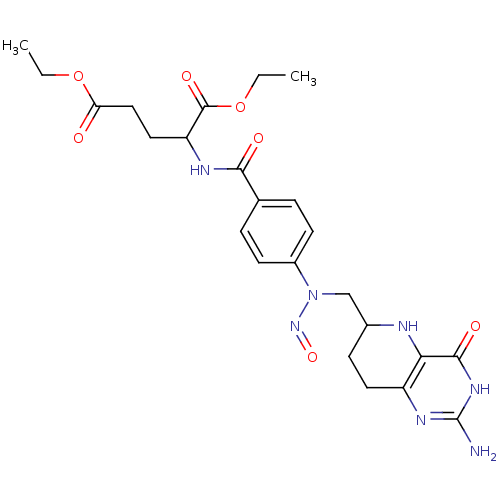 (CHEMBL46945 | diethyl 2-{4-[2-amino-4-oxo-3,4,5,6,...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 1.50E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibitory activity against human thymidylate synthase with variable concentration of N5, 10-CH2-H4PteGlu and fixed dUMP (100 uM) | J Med Chem 27: 1710-7 (1985) BindingDB Entry DOI: 10.7270/Q2N87BBJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Thymidylate synthase (Homo sapiens (Human)) | BDBM50028110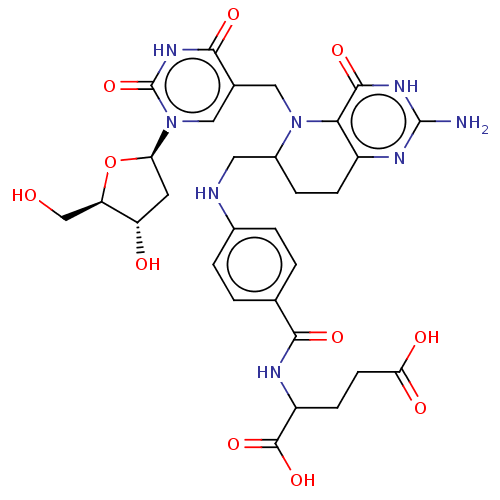 (2-[4-({2-Amino-5-[1-(4-hydroxy-5-hydroxymethyl-tet...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 1.50E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibitory activity against human thymidylate synthase with variable concentration of dUMP and fixed N5,10-CH2-H4PteGlu (200 uM) | J Med Chem 27: 1710-7 (1985) BindingDB Entry DOI: 10.7270/Q2N87BBJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Thymidylate synthase (Homo sapiens (Human)) | BDBM50028106 (CHEMBL46945 | diethyl 2-{4-[2-amino-4-oxo-3,4,5,6,...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 1.50E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibitory activity against human thymidylate synthase with variable concentration of N5, 10-CH2-H4PteGlu and fixed dUMP (100 uM) | J Med Chem 27: 1710-7 (1985) BindingDB Entry DOI: 10.7270/Q2N87BBJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Thymidylate synthase (Homo sapiens (Human)) | BDBM50028110 (2-[4-({2-Amino-5-[1-(4-hydroxy-5-hydroxymethyl-tet...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 2.40E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibitory activity against human thymidylate synthase with variable concentration of dUMP and fixed N5,10-CH2-H4PteGlu (200 uM) | J Med Chem 27: 1710-7 (1985) BindingDB Entry DOI: 10.7270/Q2N87BBJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Thymidylate synthase (Homo sapiens (Human)) | BDBM50028109 (2-{4-[(2-Amino-5-{1-[4-hydroxy-5-(hydroxy-methoxy-...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 7.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibitory activity against human thymidylate synthase with variable concentration of dUMP and fixed N5,10-CH2-H4PteGlu (200 uM) | J Med Chem 27: 1710-7 (1985) BindingDB Entry DOI: 10.7270/Q2N87BBJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neurotensin receptor type 1 (Homo sapiens (Human)) | BDBM50130880 (CHEMBL407196 | NT(1-13) | neurotensin | pGlu-Leu-T...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a |
Washington University School of Medicine Curated by ChEMBL | Assay Description Binding affinity towards neurotensin receptor in membranes prepared from HT-29 cell line, relative to [111In]-labeled neurotensin peptide | J Med Chem 46: 3403-11 (2003) Article DOI: 10.1021/jm030081k BindingDB Entry DOI: 10.7270/Q2222VHR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neurotensin receptor type 1 (Homo sapiens (Human)) | BDBM50130895 (CHEMBL408127 | DTPA-Cha(tr-4-Mam)-Pro-Arg-Gly(PipA...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a |
Washington University School of Medicine Curated by ChEMBL | Assay Description Binding affinity towards neurotensin receptor in membranes prepared from HT-29 cell line, relative to [111In]-labeled neurotensin peptide | J Med Chem 46: 3403-11 (2003) Article DOI: 10.1021/jm030081k BindingDB Entry DOI: 10.7270/Q2222VHR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neurotensin receptor type 1 (Homo sapiens (Human)) | BDBM50130898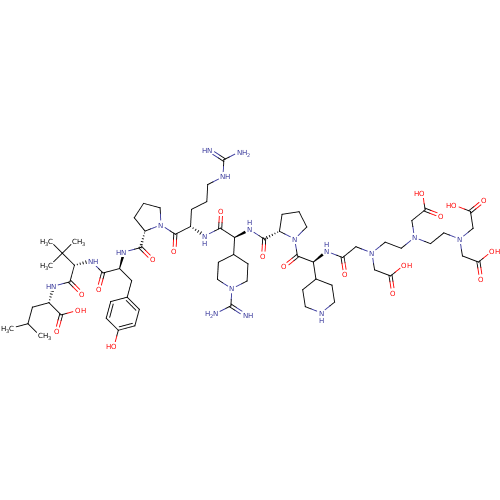 (CHEMBL408381 | DTPA-Gly(Pip)-Pro-Arg-Gly(PipAm)-Ar...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a |
Washington University School of Medicine Curated by ChEMBL | Assay Description Binding affinity towards neurotensin receptor in membranes prepared from HT-29 cell line, relative to [111In]-labeled neurotensin peptide | J Med Chem 46: 3403-11 (2003) Article DOI: 10.1021/jm030081k BindingDB Entry DOI: 10.7270/Q2222VHR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neurotensin receptor type 1 (Homo sapiens (Human)) | BDBM50130885 (CHEMBL412699 | DTPA-DLys-Pro-Arg-Phe(4-Gu)-Pro-Tyr...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a |
Washington University School of Medicine Curated by ChEMBL | Assay Description Binding affinity towards neurotensin receptor in membranes prepared from HT-29 cell line, relative to [111In]-labeled neurotensin peptide | J Med Chem 46: 3403-11 (2003) Article DOI: 10.1021/jm030081k BindingDB Entry DOI: 10.7270/Q2222VHR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neurotensin receptor type 1 (Homo sapiens (Human)) | BDBM50130899 (CHEMBL408638 | DTPA-DLys-Pro-Arg-Gly(PipAm)-Arg-Pr...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a |
Washington University School of Medicine Curated by ChEMBL | Assay Description Binding affinity towards neurotensin receptor in membranes prepared from HT-29 cell line, relative to [111In]-labeled neurotensin peptide | J Med Chem 46: 3403-11 (2003) Article DOI: 10.1021/jm030081k BindingDB Entry DOI: 10.7270/Q2222VHR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neurotensin receptor type 1 (Homo sapiens (Human)) | BDBM50130876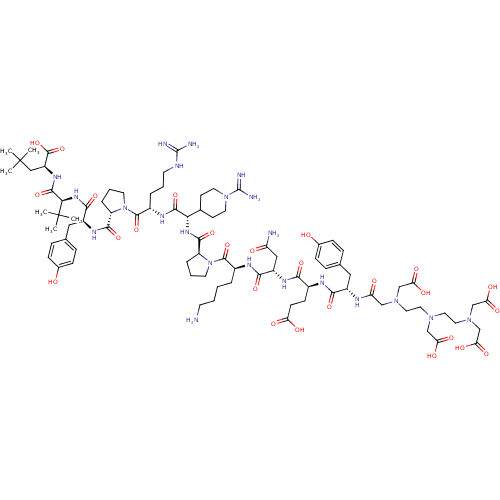 (CHEMBL404942 | DTPA-DTyr-Glu-Asn-Lys-Pro-Gly(PipAm...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a |
Washington University School of Medicine Curated by ChEMBL | Assay Description Binding affinity towards neurotensin receptor in membranes prepared from HT-29 cell line, relative to [111In]-labeled neurotensin peptide | J Med Chem 46: 3403-11 (2003) Article DOI: 10.1021/jm030081k BindingDB Entry DOI: 10.7270/Q2222VHR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neurotensin receptor type 1 (Homo sapiens (Human)) | BDBM50130873 (CHEMBL409477 | DTPA-Arg-Arg-Pro-Tur-Ile-Leu-OH) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a |
Washington University School of Medicine Curated by ChEMBL | Assay Description Binding affinity towards neurotensin receptor in membranes prepared from HT-29 cell line, relative to [111In]-labeled neurotensin peptide | J Med Chem 46: 3403-11 (2003) Article DOI: 10.1021/jm030081k BindingDB Entry DOI: 10.7270/Q2222VHR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neurotensin receptor type 1 (Homo sapiens (Human)) | BDBM50130882 (CHEMBL406127 | DTPA-DLys-Pro-Arg-Gly(PipAm)-Arg-Pr...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a |
Washington University School of Medicine Curated by ChEMBL | Assay Description Binding affinity towards neurotensin receptor in membranes prepared from HT-29 cell line, relative to [111In]-labeled neurotensin peptide | J Med Chem 46: 3403-11 (2003) Article DOI: 10.1021/jm030081k BindingDB Entry DOI: 10.7270/Q2222VHR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neurotensin receptor type 1 (Homo sapiens (Human)) | BDBM50370236 (CHEMBL1790850) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Washington University School of Medicine Curated by ChEMBL | Assay Description Binding affinity towards neurotensin receptor in membranes prepared from HT-29 cell line, relative to [111In]-labeled neurotensin peptide | J Med Chem 46: 3403-11 (2003) Article DOI: 10.1021/jm030081k BindingDB Entry DOI: 10.7270/Q2222VHR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neurotensin receptor type 1 (Homo sapiens (Human)) | BDBM50130886 (CHEMBL437288 | alpha-FITC-Gly(Pip)-Pro-Gly(PipAm)-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Washington University School of Medicine Curated by ChEMBL | Assay Description Binding affinity towards neurotensin receptor in membranes prepared from HT-29 cell line, relative to [111In]-labeled neurotensin peptide | J Med Chem 46: 3403-11 (2003) Article DOI: 10.1021/jm030081k BindingDB Entry DOI: 10.7270/Q2222VHR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neurotensin receptor type 1 (Homo sapiens (Human)) | BDBM50130875 (CHEMBL386387 | DTPA-DTyr-Glu-Asn-Lys-Pro-Gly(PipAm...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.600 | n/a | n/a | n/a | n/a | n/a | n/a |
Washington University School of Medicine Curated by ChEMBL | Assay Description Binding affinity towards neurotensin receptor in membranes prepared from HT-29 cell line, relative to [111In]-labeled neurotensin peptide | J Med Chem 46: 3403-11 (2003) Article DOI: 10.1021/jm030081k BindingDB Entry DOI: 10.7270/Q2222VHR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neurotensin receptor type 1 (Homo sapiens (Human)) | BDBM50130883 (CHEMBL406456 | epsilon-FITC-DLys-Pro-Gly(PipAm)-Ar...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.600 | n/a | n/a | n/a | n/a | n/a | n/a |
Washington University School of Medicine Curated by ChEMBL | Assay Description Binding affinity towards neurotensin receptor in membranes prepared from HT-29 cell line, relative to [111In]-labeled neurotensin peptide | J Med Chem 46: 3403-11 (2003) Article DOI: 10.1021/jm030081k BindingDB Entry DOI: 10.7270/Q2222VHR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neurotensin receptor type 1 (Homo sapiens (Human)) | BDBM50130888 (CHEMBL408534 | alpha-FITC-DLys-Pro-Gly(PipAm)-Arg-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Washington University School of Medicine Curated by ChEMBL | Assay Description Binding affinity towards neurotensin receptor in membranes prepared from HT-29 cell line, relative to [111In]-labeled neurotensin peptide | J Med Chem 46: 3403-11 (2003) Article DOI: 10.1021/jm030081k BindingDB Entry DOI: 10.7270/Q2222VHR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neurotensin receptor type 1 (Homo sapiens (Human)) | BDBM50130896 (CHEMBL437707 | DTPA-DTyr-Glu-Asn-Lys-Pro-Gly(PipAm...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Washington University School of Medicine Curated by ChEMBL | Assay Description Binding affinity towards neurotensin receptor in membranes prepared from HT-29 cell line, relative to [111In]-labeled neurotensin peptide | J Med Chem 46: 3403-11 (2003) Article DOI: 10.1021/jm030081k BindingDB Entry DOI: 10.7270/Q2222VHR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neurotensin receptor type 1 (Homo sapiens (Human)) | BDBM50130878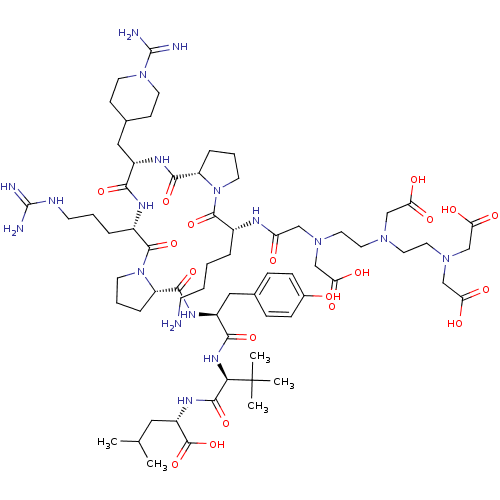 (CHEMBL424896 | DTPA-DLys-Pro-Arg-Ala(PipAm)-Arg-Pr...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Washington University School of Medicine Curated by ChEMBL | Assay Description Binding affinity towards neurotensin receptor in membranes prepared from HT-29 cell line, relative to [111In]-labeled neurotensin peptide | J Med Chem 46: 3403-11 (2003) Article DOI: 10.1021/jm030081k BindingDB Entry DOI: 10.7270/Q2222VHR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neurotensin receptor type 1 (Homo sapiens (Human)) | BDBM50130893 (CHEMBL439694 | alpha,epsilon-Bis(FITC)-DLys-Pro-Gl...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3.40 | n/a | n/a | n/a | n/a | n/a | n/a |
Washington University School of Medicine Curated by ChEMBL | Assay Description Binding affinity towards neurotensin receptor in membranes prepared from HT-29 cell line, relative to [111In]-labeled neurotensin peptide | J Med Chem 46: 3403-11 (2003) Article DOI: 10.1021/jm030081k BindingDB Entry DOI: 10.7270/Q2222VHR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 75 total ) | Next | Last >> |