 Found 3957 hits with Last Name = 'srivastava' and Initial = 'a'
Found 3957 hits with Last Name = 'srivastava' and Initial = 'a' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Serine/threonine-protein kinase PknB
(Mycobacterium tuberculosis) | BDBM50035670

(CHEMBL3338456)Show InChI InChI=1S/C9H9ClFN3O2/c10-6-3-5(1-2-7(6)11)13-8(15)4-9(16)14-12/h1-3H,4,12H2,(H,13,15)(H,14,16) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 3.53E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
CSIR-Central Drug Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of Mycobacterium tuberculosis PknB |
Bioorg Med Chem Lett 24: 5181-4 (2014)
Article DOI: 10.1016/j.bmcl.2014.09.080
BindingDB Entry DOI: 10.7270/Q29G5PDS |
More data for this
Ligand-Target Pair | |
Falcipain 2
(Plasmodium falciparum) | BDBM50261135
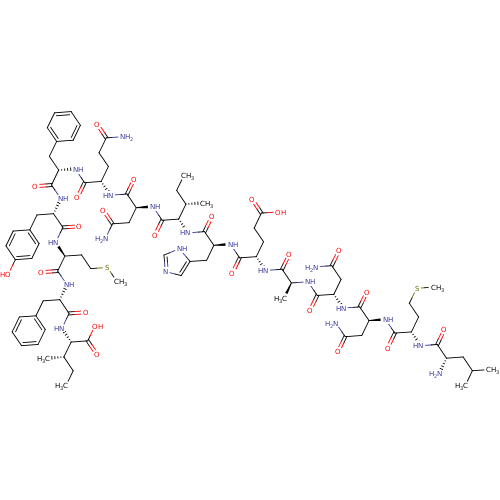
(CHEMBL525938 | LMNNAEHINQFYMFI)Show SMILES CC[C@H](C)[C@H](NC(=O)[C@H](Cc1ccccc1)NC(=O)[C@H](CCSC)NC(=O)[C@H](Cc1ccc(O)cc1)NC(=O)[C@H](Cc1ccccc1)NC(=O)[C@H](CCC(N)=O)NC(=O)[C@H](CC(N)=O)NC(=O)[C@@H](NC(=O)[C@H](Cc1cnc[nH]1)NC(=O)[C@H](CCC(O)=O)NC(=O)[C@H](C)NC(=O)[C@H](CC(N)=O)NC(=O)[C@H](CC(N)=O)NC(=O)[C@H](CCSC)NC(=O)[C@@H](N)CC(C)C)[C@@H](C)CC)C(O)=O |r| Show InChI InChI=1S/C86H125N21O23S2/c1-10-45(5)70(106-84(127)61(38-51-42-92-43-93-51)102-75(118)55(27-29-69(113)114)95-72(115)47(7)94-78(121)62(39-66(89)110)104-82(125)63(40-67(90)111)103-76(119)56(30-32-131-8)96-73(116)53(87)34-44(3)4)85(128)105-64(41-68(91)112)81(124)97-54(26-28-65(88)109)74(117)99-58(35-48-18-14-12-15-19-48)80(123)101-59(37-50-22-24-52(108)25-23-50)79(122)98-57(31-33-132-9)77(120)100-60(36-49-20-16-13-17-21-49)83(126)107-71(86(129)130)46(6)11-2/h12-25,42-47,53-64,70-71,108H,10-11,26-41,87H2,1-9H3,(H2,88,109)(H2,89,110)(H2,90,111)(H2,91,112)(H,92,93)(H,94,121)(H,95,115)(H,96,116)(H,97,124)(H,98,122)(H,99,117)(H,100,120)(H,101,123)(H,102,118)(H,103,119)(H,104,125)(H,105,128)(H,106,127)(H,107,126)(H,113,114)(H,129,130)/t45-,46-,47-,53-,54-,55-,56-,57-,58-,59-,60-,61-,62-,63-,64-,70-,71-/m0/s1 | PDB
MMDB
UniProtKB/TrEMBL
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 3.80E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
International Center for Genetic Engineering and Biotechnology
Curated by ChEMBL
| Assay Description
Inhibition of Plasmodium falciparum recombinant falcipain-2 |
J Med Chem 51: 3116-23 (2008)
Article DOI: 10.1021/jm070735f
BindingDB Entry DOI: 10.7270/Q20G3JZR |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase PknB
(Mycobacterium tuberculosis) | BDBM50035656

(CHEMBL1986389)Show InChI InChI=1S/C10H11N3O4/c11-13-9(15)5-8(14)12-7-3-1-6(2-4-7)10(16)17/h1-4H,5,11H2,(H,12,14)(H,13,15)(H,16,17) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 3.86E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
CSIR-Central Drug Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of Mycobacterium tuberculosis PknB |
Bioorg Med Chem Lett 24: 5181-4 (2014)
Article DOI: 10.1016/j.bmcl.2014.09.080
BindingDB Entry DOI: 10.7270/Q29G5PDS |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase PknB
(Mycobacterium tuberculosis) | BDBM50035662

(CHEMBL3338464)Show InChI InChI=1S/C11H15N3O4/c1-17-7-3-4-8(9(5-7)18-2)13-10(15)6-11(16)14-12/h3-5H,6,12H2,1-2H3,(H,13,15)(H,14,16) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 5.25E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
CSIR-Central Drug Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of Mycobacterium tuberculosis PknB |
Bioorg Med Chem Lett 24: 5181-4 (2014)
Article DOI: 10.1016/j.bmcl.2014.09.080
BindingDB Entry DOI: 10.7270/Q29G5PDS |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase PknB
(Mycobacterium tuberculosis) | BDBM50035666
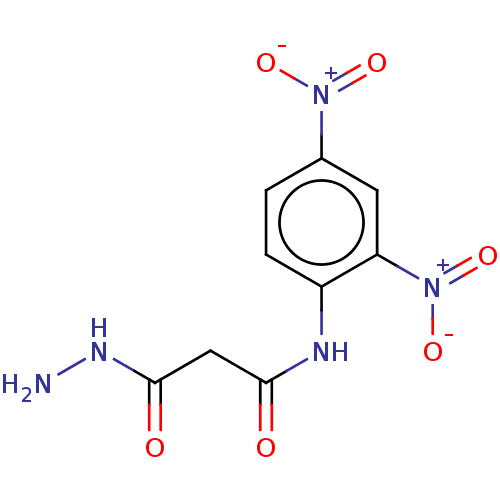
(CHEMBL3338460)Show SMILES NNC(=O)CC(=O)Nc1ccc(cc1[N+]([O-])=O)[N+]([O-])=O Show InChI InChI=1S/C9H9N5O6/c10-12-9(16)4-8(15)11-6-2-1-5(13(17)18)3-7(6)14(19)20/h1-3H,4,10H2,(H,11,15)(H,12,16) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 6.38E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
CSIR-Central Drug Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of Mycobacterium tuberculosis PknB |
Bioorg Med Chem Lett 24: 5181-4 (2014)
Article DOI: 10.1016/j.bmcl.2014.09.080
BindingDB Entry DOI: 10.7270/Q29G5PDS |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase PknB
(Mycobacterium tuberculosis) | BDBM50035663

(CHEMBL3338463)Show InChI InChI=1S/C11H15N3O4/c1-17-7-3-4-9(18-2)8(5-7)13-10(15)6-11(16)14-12/h3-5H,6,12H2,1-2H3,(H,13,15)(H,14,16) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 7.01E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
CSIR-Central Drug Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of Mycobacterium tuberculosis PknB |
Bioorg Med Chem Lett 24: 5181-4 (2014)
Article DOI: 10.1016/j.bmcl.2014.09.080
BindingDB Entry DOI: 10.7270/Q29G5PDS |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase PknB
(Mycobacterium tuberculosis) | BDBM50035657

(CHEMBL3338469)Show InChI InChI=1S/C10H11N3O4/c11-13-9(15)5-8(14)12-7-4-2-1-3-6(7)10(16)17/h1-4H,5,11H2,(H,12,14)(H,13,15)(H,16,17) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 7.32E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
CSIR-Central Drug Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of Mycobacterium tuberculosis PknB |
Bioorg Med Chem Lett 24: 5181-4 (2014)
Article DOI: 10.1016/j.bmcl.2014.09.080
BindingDB Entry DOI: 10.7270/Q29G5PDS |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase PknB
(Mycobacterium tuberculosis) | BDBM50035658
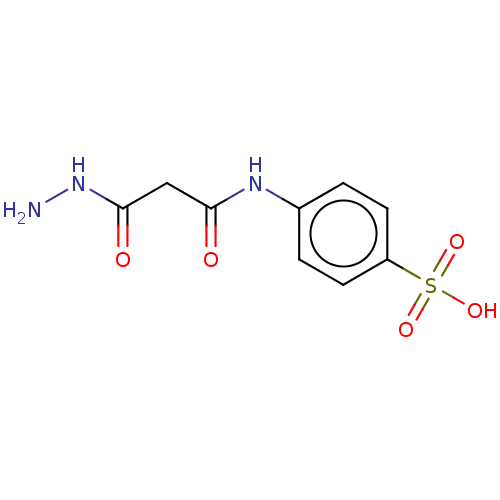
(CHEMBL3338468)Show InChI InChI=1S/C9H11N3O5S/c10-12-9(14)5-8(13)11-6-1-3-7(4-2-6)18(15,16)17/h1-4H,5,10H2,(H,11,13)(H,12,14)(H,15,16,17) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
| Article
PubMed
| 7.59E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
CSIR-Central Drug Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of Mycobacterium tuberculosis PknB |
Bioorg Med Chem Lett 24: 5181-4 (2014)
Article DOI: 10.1016/j.bmcl.2014.09.080
BindingDB Entry DOI: 10.7270/Q29G5PDS |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase PknB
(Mycobacterium tuberculosis) | BDBM50035665
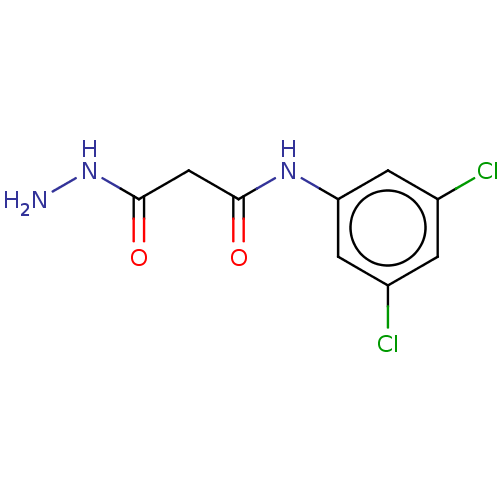
(CHEMBL3338461)Show InChI InChI=1S/C9H9Cl2N3O2/c10-5-1-6(11)3-7(2-5)13-8(15)4-9(16)14-12/h1-3H,4,12H2,(H,13,15)(H,14,16) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 7.68E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
CSIR-Central Drug Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of Mycobacterium tuberculosis PknB |
Bioorg Med Chem Lett 24: 5181-4 (2014)
Article DOI: 10.1016/j.bmcl.2014.09.080
BindingDB Entry DOI: 10.7270/Q29G5PDS |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase PknB
(Mycobacterium tuberculosis) | BDBM50035659

(CHEMBL3338467)Show InChI InChI=1S/C12H17N3O2/c1-2-3-9-4-6-10(7-5-9)14-11(16)8-12(17)15-13/h4-7H,2-3,8,13H2,1H3,(H,14,16)(H,15,17) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 9.04E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
CSIR-Central Drug Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of Mycobacterium tuberculosis PknB |
Bioorg Med Chem Lett 24: 5181-4 (2014)
Article DOI: 10.1016/j.bmcl.2014.09.080
BindingDB Entry DOI: 10.7270/Q29G5PDS |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase PknB
(Mycobacterium tuberculosis) | BDBM50035664

(CHEMBL3338462)Show InChI InChI=1S/C9H9Cl2N3O2/c10-6-2-1-5(3-7(6)11)13-8(15)4-9(16)14-12/h1-3H,4,12H2,(H,13,15)(H,14,16) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 9.21E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
CSIR-Central Drug Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of Mycobacterium tuberculosis PknB |
Bioorg Med Chem Lett 24: 5181-4 (2014)
Article DOI: 10.1016/j.bmcl.2014.09.080
BindingDB Entry DOI: 10.7270/Q29G5PDS |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase PknB
(Mycobacterium tuberculosis) | BDBM50035667

(CHEMBL3338459)Show InChI InChI=1S/C9H10N4O4/c10-12-9(15)5-8(14)11-6-1-3-7(4-2-6)13(16)17/h1-4H,5,10H2,(H,11,14)(H,12,15) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
| Article
PubMed
| 1.08E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
CSIR-Central Drug Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of Mycobacterium tuberculosis PknB |
Bioorg Med Chem Lett 24: 5181-4 (2014)
Article DOI: 10.1016/j.bmcl.2014.09.080
BindingDB Entry DOI: 10.7270/Q29G5PDS |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase PknB
(Mycobacterium tuberculosis) | BDBM50035668

(CHEMBL3338458)Show InChI InChI=1S/C9H10N4O4/c10-12-9(15)5-8(14)11-6-2-1-3-7(4-6)13(16)17/h1-4H,5,10H2,(H,11,14)(H,12,15) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 1.25E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
CSIR-Central Drug Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of Mycobacterium tuberculosis PknB |
Bioorg Med Chem Lett 24: 5181-4 (2014)
Article DOI: 10.1016/j.bmcl.2014.09.080
BindingDB Entry DOI: 10.7270/Q29G5PDS |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase PknB
(Mycobacterium tuberculosis) | BDBM50035661
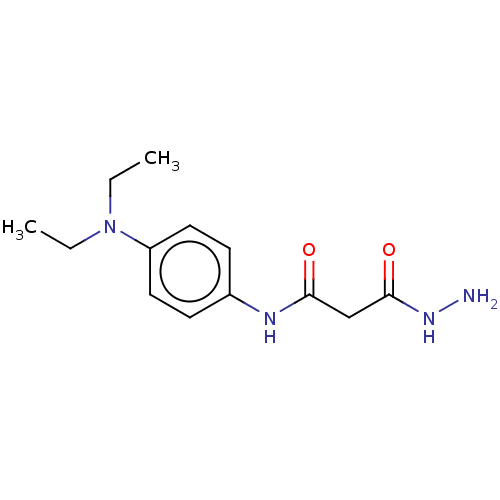
(CHEMBL3338465)Show InChI InChI=1S/C13H20N4O2/c1-3-17(4-2)11-7-5-10(6-8-11)15-12(18)9-13(19)16-14/h5-8H,3-4,9,14H2,1-2H3,(H,15,18)(H,16,19) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.31E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
CSIR-Central Drug Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of Mycobacterium tuberculosis PknB |
Bioorg Med Chem Lett 24: 5181-4 (2014)
Article DOI: 10.1016/j.bmcl.2014.09.080
BindingDB Entry DOI: 10.7270/Q29G5PDS |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase PknB
(Mycobacterium tuberculosis) | BDBM50035655

(CHEMBL3338470)Show InChI InChI=1S/C8H11N3O3/c9-11-8(13)4-7(12)10-5-6-2-1-3-14-6/h1-3H,4-5,9H2,(H,10,12)(H,11,13) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.85E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
CSIR-Central Drug Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of Mycobacterium tuberculosis PknB |
Bioorg Med Chem Lett 24: 5181-4 (2014)
Article DOI: 10.1016/j.bmcl.2014.09.080
BindingDB Entry DOI: 10.7270/Q29G5PDS |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase PknB
(Mycobacterium tuberculosis) | BDBM50035669
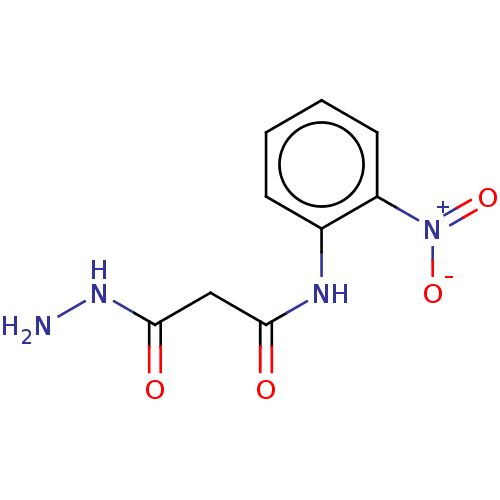
(CHEMBL3338457)Show InChI InChI=1S/C9H10N4O4/c10-12-9(15)5-8(14)11-6-3-1-2-4-7(6)13(16)17/h1-4H,5,10H2,(H,11,14)(H,12,15) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 2.29E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
CSIR-Central Drug Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of Mycobacterium tuberculosis PknB |
Bioorg Med Chem Lett 24: 5181-4 (2014)
Article DOI: 10.1016/j.bmcl.2014.09.080
BindingDB Entry DOI: 10.7270/Q29G5PDS |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 1
(Homo sapiens (Human)) | BDBM93118

(PTP1B Inhibitor, 19)Show SMILES CC(=O)Oc1ccc(C2=NOC(C2)c2ccccc2)c(OC(C)=O)c1 |t:8| Show InChI InChI=1S/C19H17NO5/c1-12(21)23-15-8-9-16(19(10-15)24-13(2)22)17-11-18(25-20-17)14-6-4-3-5-7-14/h3-10,18H,11H2,1-2H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | 2.50E+4 | n/a | 6.27E+4 | n/a | n/a | n/a | n/a | 7.0 | n/a |
Central Drug Research Institute,
| Assay Description
The effect of the test compounds on PTP1B was studied by preincubating the test compound with enzyme in the reaction system for 10 min and determinin... |
Medicinal Chemistry Research 17: 123-136 (2008)
BindingDB Entry DOI: 10.7270/Q2JQ0ZMG |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 1
(Homo sapiens (Human)) | BDBM50266158
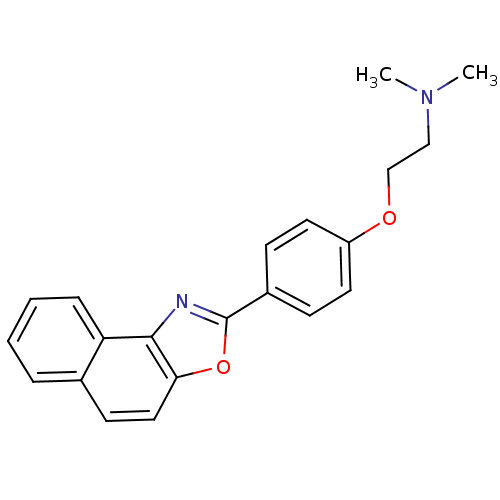
(CHEMBL510225 | Dimethyl-[2-(4-naphtho[1,2-d]oxazol...)Show InChI InChI=1S/C21H20N2O2/c1-23(2)13-14-24-17-10-7-16(8-11-17)21-22-20-18-6-4-3-5-15(18)9-12-19(20)25-21/h3-12H,13-14H2,1-2H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 2.51E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Central Drug Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of of PTP1B (unknown origin) assessed as residual enzyme activity |
Eur J Med Chem 44: 109-16 (2008)
Article DOI: 10.1016/j.ejmech.2008.03.009
BindingDB Entry DOI: 10.7270/Q2FN1602 |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase PknB
(Mycobacterium tuberculosis) | BDBM50035660
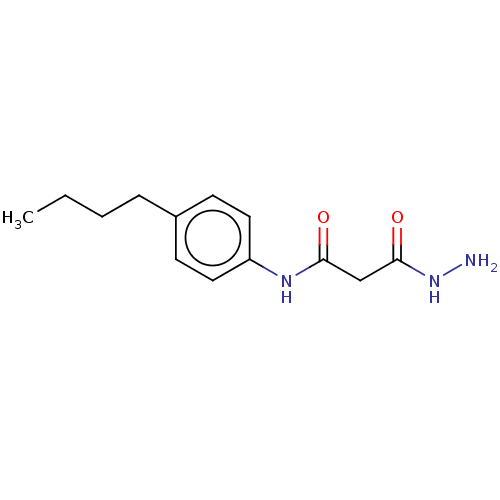
(CHEMBL3338466)Show InChI InChI=1S/C13H19N3O2/c1-2-3-4-10-5-7-11(8-6-10)15-12(17)9-13(18)16-14/h5-8H,2-4,9,14H2,1H3,(H,15,17)(H,16,18) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 2.65E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
CSIR-Central Drug Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of Mycobacterium tuberculosis PknB |
Bioorg Med Chem Lett 24: 5181-4 (2014)
Article DOI: 10.1016/j.bmcl.2014.09.080
BindingDB Entry DOI: 10.7270/Q29G5PDS |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 1
(Homo sapiens (Human)) | BDBM50266161
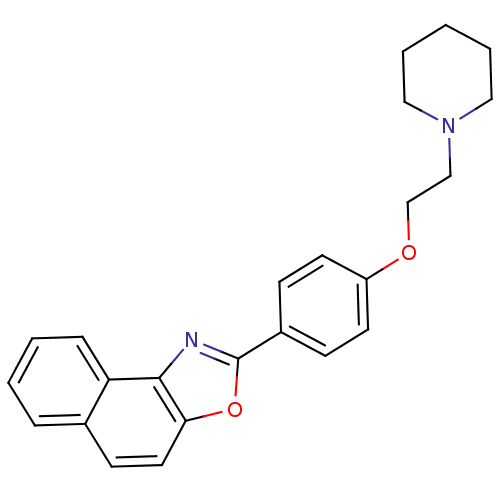
(2-[4-(2-Piperidine-1-yl-ethoxy)-phenyl]-naphtho[1,...)Show SMILES C(CN1CCCCC1)Oc1ccc(cc1)-c1nc2c(ccc3ccccc23)o1 Show InChI InChI=1S/C24H24N2O2/c1-4-14-26(15-5-1)16-17-27-20-11-8-19(9-12-20)24-25-23-21-7-3-2-6-18(21)10-13-22(23)28-24/h2-3,6-13H,1,4-5,14-17H2 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 2.71E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Central Drug Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of of PTP1B (unknown origin) assessed as residual enzyme activity in presence of Triton X-100 |
Eur J Med Chem 44: 109-16 (2008)
Article DOI: 10.1016/j.ejmech.2008.03.009
BindingDB Entry DOI: 10.7270/Q2FN1602 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 1
(Homo sapiens (Human)) | BDBM50198352
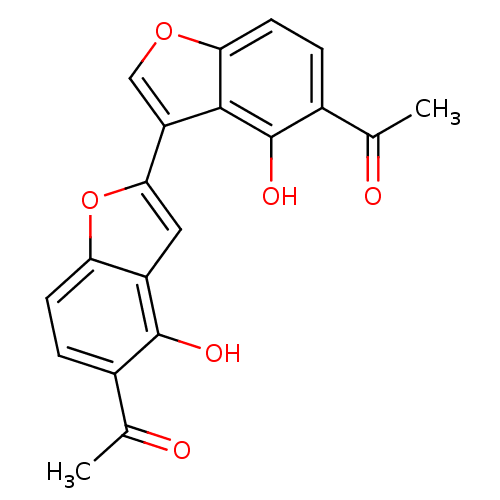
(1-(5-acetyl-6,6'-dihydroxy-2',3'-dihydro-[2,3']-bi...)Show SMILES CC(=O)c1ccc2oc(cc2c1O)-c1coc2ccc(C(C)=O)c(O)c12 Show InChI InChI=1S/C20H14O6/c1-9(21)11-3-5-15-13(19(11)23)7-17(26-15)14-8-25-16-6-4-12(10(2)22)20(24)18(14)16/h3-8,23-24H,1-2H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 2.75E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Central Drug Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of PTP1B in presence of 0.01% Triton X-100 |
Bioorg Med Chem 15: 727-34 (2006)
Article DOI: 10.1016/j.bmc.2006.10.053
BindingDB Entry DOI: 10.7270/Q2CR5T1W |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 1
(Homo sapiens (Human)) | BDBM50182133
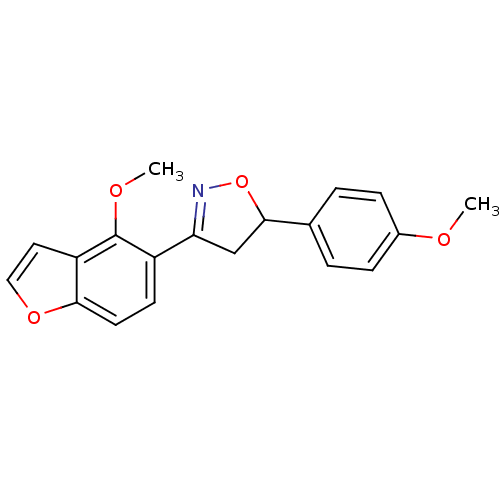
(3-(4-methoxybenzofuran-5-yl)-5-(4-methoxyphenyl)-4...)Show SMILES COc1ccc(cc1)C1CC(=NO1)c1ccc2occc2c1OC |c:11| Show InChI InChI=1S/C19H17NO4/c1-21-13-5-3-12(4-6-13)18-11-16(20-24-18)14-7-8-17-15(9-10-23-17)19(14)22-2/h3-10,18H,11H2,1-2H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 2.75E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Central Drug Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of PTP1B |
Bioorg Med Chem Lett 16: 2139-43 (2006)
Article DOI: 10.1016/j.bmcl.2006.01.062
BindingDB Entry DOI: 10.7270/Q29K49ST |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 1
(Homo sapiens (Human)) | BDBM50266161

(2-[4-(2-Piperidine-1-yl-ethoxy)-phenyl]-naphtho[1,...)Show SMILES C(CN1CCCCC1)Oc1ccc(cc1)-c1nc2c(ccc3ccccc23)o1 Show InChI InChI=1S/C24H24N2O2/c1-4-14-26(15-5-1)16-17-27-20-11-8-19(9-12-20)24-25-23-21-7-3-2-6-18(21)10-13-22(23)28-24/h2-3,6-13H,1,4-5,14-17H2 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 2.75E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Central Drug Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of of PTP1B (unknown origin) assessed as residual enzyme activity |
Eur J Med Chem 44: 109-16 (2008)
Article DOI: 10.1016/j.ejmech.2008.03.009
BindingDB Entry DOI: 10.7270/Q2FN1602 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 1
(Homo sapiens (Human)) | BDBM50266158

(CHEMBL510225 | Dimethyl-[2-(4-naphtho[1,2-d]oxazol...)Show InChI InChI=1S/C21H20N2O2/c1-23(2)13-14-24-17-10-7-16(8-11-17)21-22-20-18-6-4-3-5-15(18)9-12-19(20)25-21/h3-12H,13-14H2,1-2H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 2.87E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Central Drug Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of of PTP1B (unknown origin) assessed as residual enzyme activity in presence of Triton X-100 |
Eur J Med Chem 44: 109-16 (2008)
Article DOI: 10.1016/j.ejmech.2008.03.009
BindingDB Entry DOI: 10.7270/Q2FN1602 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 1
(Homo sapiens (Human)) | BDBM93109

(PTP1B Inhibitor, 5)Show SMILES COc1c(ccc2OC(C)(C)C=Cc12)C1=NOC(C1)c1ccccc1 |c:11,t:16| Show InChI InChI=1S/C21H21NO3/c1-21(2)12-11-16-18(24-21)10-9-15(20(16)23-3)17-13-19(25-22-17)14-7-5-4-6-8-14/h4-12,19H,13H2,1-3H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| | 3.00E+4 | n/a | 7.00E+4 | n/a | n/a | n/a | n/a | 7.0 | n/a |
Central Drug Research Institute,
| Assay Description
The effect of the test compounds on PTP1B was studied by preincubating the test compound with enzyme in the reaction system for 10 min and determinin... |
Medicinal Chemistry Research 17: 123-136 (2008)
BindingDB Entry DOI: 10.7270/Q2JQ0ZMG |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 1
(Homo sapiens (Human)) | BDBM50198349
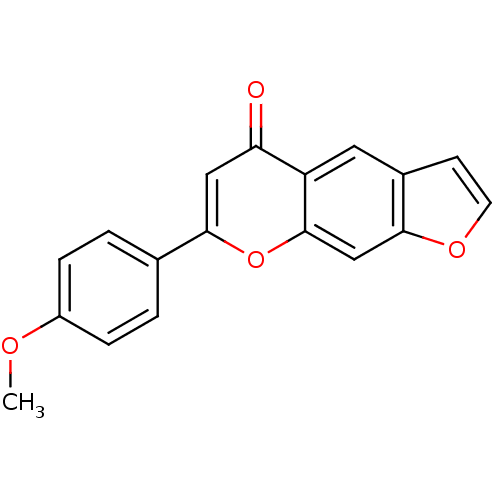
(7-(4-methoxy-phenyl)-furo[3,2-g]chromen-5-one | CH...)Show InChI InChI=1S/C18H12O4/c1-20-13-4-2-11(3-5-13)17-9-15(19)14-8-12-6-7-21-16(12)10-18(14)22-17/h2-10H,1H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 3.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Central Drug Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of PTP1B |
Bioorg Med Chem 15: 727-34 (2006)
Article DOI: 10.1016/j.bmc.2006.10.053
BindingDB Entry DOI: 10.7270/Q2CR5T1W |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 1
(Homo sapiens (Human)) | BDBM50182134
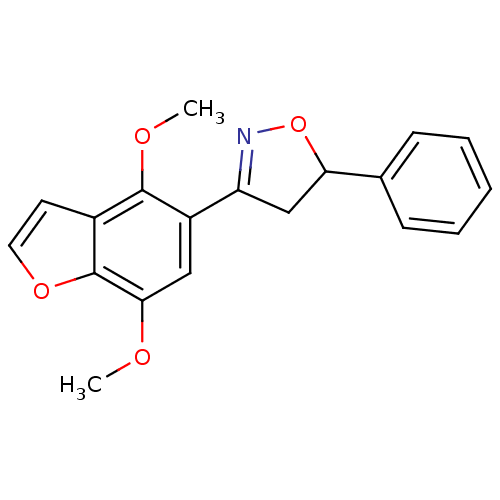
(3-(4,7-dimethoxybenzofuran-5-yl)-5-phenyl-4,5-dihy...)Show SMILES COc1cc(C2=NOC(C2)c2ccccc2)c(OC)c2ccoc12 |t:5| Show InChI InChI=1S/C19H17NO4/c1-21-17-10-14(18(22-2)13-8-9-23-19(13)17)15-11-16(24-20-15)12-6-4-3-5-7-12/h3-10,16H,11H2,1-2H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 3.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Central Drug Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of PTP1B |
Bioorg Med Chem Lett 16: 2139-43 (2006)
Article DOI: 10.1016/j.bmcl.2006.01.062
BindingDB Entry DOI: 10.7270/Q29K49ST |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 1
(Homo sapiens (Human)) | BDBM50182131
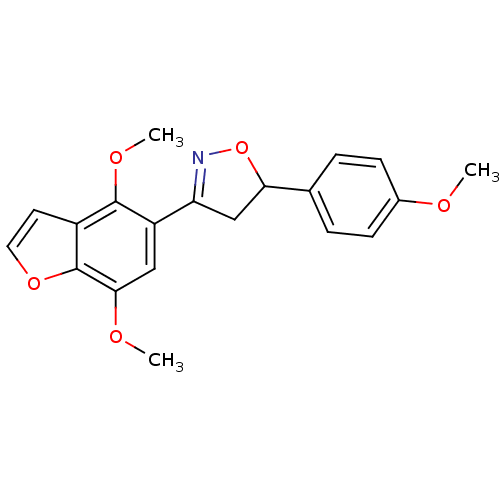
(3-(4,7-dimethoxybenzofuran-5-yl)-5-(4-methoxypheny...)Show SMILES COc1ccc(cc1)C1CC(=NO1)c1cc(OC)c2occc2c1OC |c:11| Show InChI InChI=1S/C20H19NO5/c1-22-13-6-4-12(5-7-13)17-11-16(21-26-17)15-10-18(23-2)20-14(8-9-25-20)19(15)24-3/h4-10,17H,11H2,1-3H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 3.20E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Central Drug Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of PTP1B |
Bioorg Med Chem Lett 16: 2139-43 (2006)
Article DOI: 10.1016/j.bmcl.2006.01.062
BindingDB Entry DOI: 10.7270/Q29K49ST |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 1
(Homo sapiens (Human)) | BDBM93111
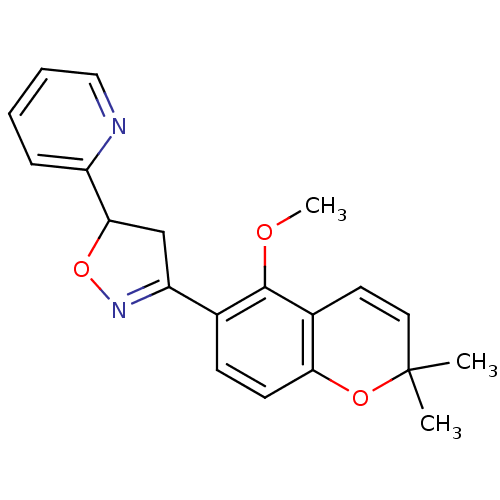
(PTP1B Inhibitor, 7)Show SMILES COc1c(ccc2OC(C)(C)C=Cc12)C1=NOC(C1)c1ccccn1 |c:11,t:16| Show InChI InChI=1S/C20H20N2O3/c1-20(2)10-9-14-17(24-20)8-7-13(19(14)23-3)16-12-18(25-22-16)15-6-4-5-11-21-15/h4-11,18H,12H2,1-3H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | 3.40E+4 | n/a | 8.70E+4 | n/a | n/a | n/a | n/a | 7.0 | n/a |
Central Drug Research Institute,
| Assay Description
The effect of the test compounds on PTP1B was studied by preincubating the test compound with enzyme in the reaction system for 10 min and determinin... |
Medicinal Chemistry Research 17: 123-136 (2008)
BindingDB Entry DOI: 10.7270/Q2JQ0ZMG |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 1
(Homo sapiens (Human)) | BDBM50266231

(CHEMBL510460 | Dimethyl-[3-(4-naphtho[1,2-d]oxazol...)Show InChI InChI=1S/C22H22N2O2/c1-24(2)14-5-15-25-18-11-8-17(9-12-18)22-23-21-19-7-4-3-6-16(19)10-13-20(21)26-22/h3-4,6-13H,5,14-15H2,1-2H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 3.41E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Central Drug Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of of PTP1B (unknown origin) assessed as residual enzyme activity |
Eur J Med Chem 44: 109-16 (2008)
Article DOI: 10.1016/j.ejmech.2008.03.009
BindingDB Entry DOI: 10.7270/Q2FN1602 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 1
(Homo sapiens (Human)) | BDBM50266160

(2-[4-(2-Pyrrolidin-1-yl-ethoxy)-phenyl]-naphtho[1,...)Show SMILES C(CN1CCCC1)Oc1ccc(cc1)-c1nc2c(ccc3ccccc23)o1 Show InChI InChI=1S/C23H22N2O2/c1-2-6-20-17(5-1)9-12-21-22(20)24-23(27-21)18-7-10-19(11-8-18)26-16-15-25-13-3-4-14-25/h1-2,5-12H,3-4,13-16H2 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 3.42E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Central Drug Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of of PTP1B (unknown origin) assessed as residual enzyme activity |
Eur J Med Chem 44: 109-16 (2008)
Article DOI: 10.1016/j.ejmech.2008.03.009
BindingDB Entry DOI: 10.7270/Q2FN1602 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 1
(Homo sapiens (Human)) | BDBM50266159
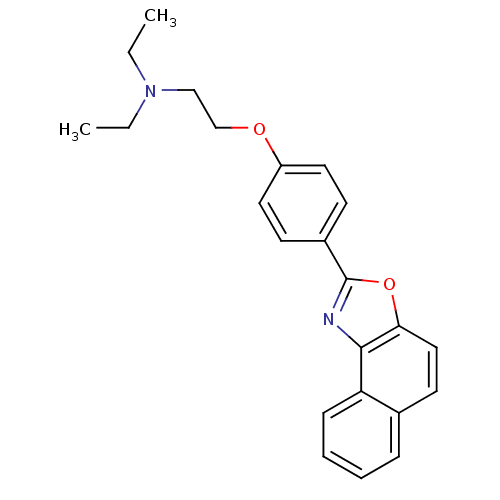
(CHEMBL506882 | Diethyl-[2-(4-naphtho[1,2-d]oxazol-...)Show InChI InChI=1S/C23H24N2O2/c1-3-25(4-2)15-16-26-19-12-9-18(10-13-19)23-24-22-20-8-6-5-7-17(20)11-14-21(22)27-23/h5-14H,3-4,15-16H2,1-2H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 3.50E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Central Drug Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of of PTP1B (unknown origin) assessed as residual enzyme activity |
Eur J Med Chem 44: 109-16 (2008)
Article DOI: 10.1016/j.ejmech.2008.03.009
BindingDB Entry DOI: 10.7270/Q2FN1602 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 1
(Homo sapiens (Human)) | BDBM50198352

(1-(5-acetyl-6,6'-dihydroxy-2',3'-dihydro-[2,3']-bi...)Show SMILES CC(=O)c1ccc2oc(cc2c1O)-c1coc2ccc(C(C)=O)c(O)c12 Show InChI InChI=1S/C20H14O6/c1-9(21)11-3-5-15-13(19(11)23)7-17(26-15)14-8-25-16-6-4-12(10(2)22)20(24)18(14)16/h3-8,23-24H,1-2H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 3.50E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Central Drug Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of PTP1B |
Bioorg Med Chem 15: 727-34 (2006)
Article DOI: 10.1016/j.bmc.2006.10.053
BindingDB Entry DOI: 10.7270/Q2CR5T1W |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 1
(Homo sapiens (Human)) | BDBM50198349

(7-(4-methoxy-phenyl)-furo[3,2-g]chromen-5-one | CH...)Show InChI InChI=1S/C18H12O4/c1-20-13-4-2-11(3-5-13)17-9-15(19)14-8-12-6-7-21-16(12)10-18(14)22-17/h2-10H,1H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 3.60E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Central Drug Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of PTP1B in presence of 0.01% Triton X-100 |
Bioorg Med Chem 15: 727-34 (2006)
Article DOI: 10.1016/j.bmc.2006.10.053
BindingDB Entry DOI: 10.7270/Q2CR5T1W |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 1
(Homo sapiens (Human)) | BDBM50266231

(CHEMBL510460 | Dimethyl-[3-(4-naphtho[1,2-d]oxazol...)Show InChI InChI=1S/C22H22N2O2/c1-24(2)14-5-15-25-18-11-8-17(9-12-18)22-23-21-19-7-4-3-6-16(19)10-13-20(21)26-22/h3-4,6-13H,5,14-15H2,1-2H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 3.63E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Central Drug Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of of PTP1B (unknown origin) assessed as residual enzyme activity in presence of Triton X-100 |
Eur J Med Chem 44: 109-16 (2008)
Article DOI: 10.1016/j.ejmech.2008.03.009
BindingDB Entry DOI: 10.7270/Q2FN1602 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 1
(Homo sapiens (Human)) | BDBM50266160

(2-[4-(2-Pyrrolidin-1-yl-ethoxy)-phenyl]-naphtho[1,...)Show SMILES C(CN1CCCC1)Oc1ccc(cc1)-c1nc2c(ccc3ccccc23)o1 Show InChI InChI=1S/C23H22N2O2/c1-2-6-20-17(5-1)9-12-21-22(20)24-23(27-21)18-7-10-19(11-8-18)26-16-15-25-13-3-4-14-25/h1-2,5-12H,3-4,13-16H2 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 3.70E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Central Drug Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of of PTP1B (unknown origin) assessed as residual enzyme activity in presence of Triton X-100 |
Eur J Med Chem 44: 109-16 (2008)
Article DOI: 10.1016/j.ejmech.2008.03.009
BindingDB Entry DOI: 10.7270/Q2FN1602 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 1
(Homo sapiens (Human)) | BDBM50198350
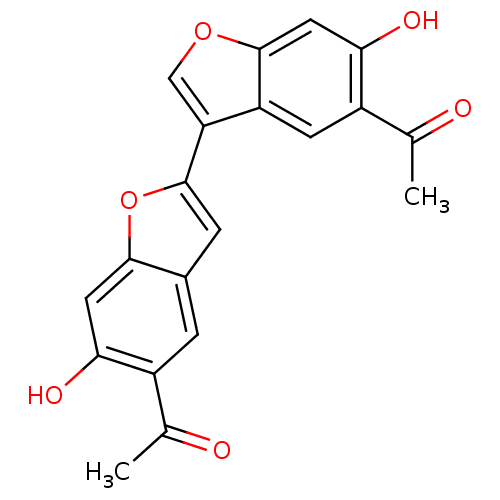
(1-(5-acetyl-4,4'-dihydroxy-2',3'-dihydro-[2,3']bib...)Show SMILES CC(=O)c1cc2cc(oc2cc1O)-c1coc2cc(O)c(cc12)C(C)=O Show InChI InChI=1S/C20H14O6/c1-9(21)12-3-11-4-20(26-18(11)6-16(12)23)15-8-25-19-7-17(24)13(10(2)22)5-14(15)19/h3-8,23-24H,1-2H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 3.70E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Central Drug Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of PTP1B |
Bioorg Med Chem 15: 727-34 (2006)
Article DOI: 10.1016/j.bmc.2006.10.053
BindingDB Entry DOI: 10.7270/Q2CR5T1W |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 1
(Homo sapiens (Human)) | BDBM50266232
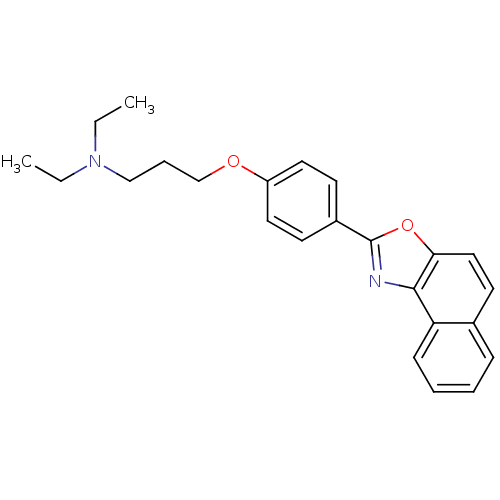
(CHEMBL458009 | Diethyl-[3-(4-naphtho[1,2-d]oxazol-...)Show SMILES CCN(CC)CCCOc1ccc(cc1)-c1nc2c(ccc3ccccc23)o1 Show InChI InChI=1S/C24H26N2O2/c1-3-26(4-2)16-7-17-27-20-13-10-19(11-14-20)24-25-23-21-9-6-5-8-18(21)12-15-22(23)28-24/h5-6,8-15H,3-4,7,16-17H2,1-2H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 4.05E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Central Drug Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of of PTP1B (unknown origin) assessed as residual enzyme activity |
Eur J Med Chem 44: 109-16 (2008)
Article DOI: 10.1016/j.ejmech.2008.03.009
BindingDB Entry DOI: 10.7270/Q2FN1602 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 1
(Homo sapiens (Human)) | BDBM50266162
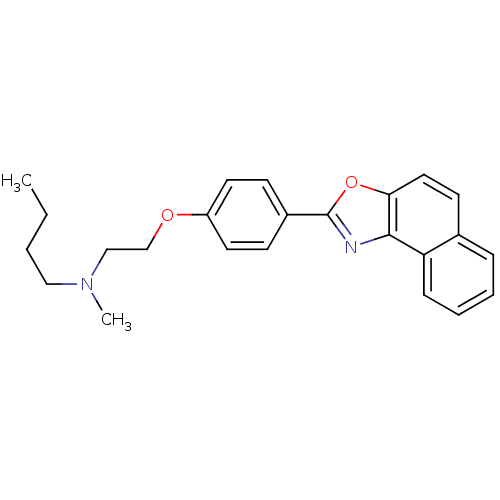
(Butyl-methyl-[2-(4-naphtho[1,2-d]oxazol-2-yl-pheno...)Show SMILES CCCCN(C)CCOc1ccc(cc1)-c1nc2c(ccc3ccccc23)o1 Show InChI InChI=1S/C24H26N2O2/c1-3-4-15-26(2)16-17-27-20-12-9-19(10-13-20)24-25-23-21-8-6-5-7-18(21)11-14-22(23)28-24/h5-14H,3-4,15-17H2,1-2H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 4.10E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Central Drug Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of of PTP1B (unknown origin) assessed as residual enzyme activity in presence of Triton X-100 |
Eur J Med Chem 44: 109-16 (2008)
Article DOI: 10.1016/j.ejmech.2008.03.009
BindingDB Entry DOI: 10.7270/Q2FN1602 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 1
(Homo sapiens (Human)) | BDBM50266232

(CHEMBL458009 | Diethyl-[3-(4-naphtho[1,2-d]oxazol-...)Show SMILES CCN(CC)CCCOc1ccc(cc1)-c1nc2c(ccc3ccccc23)o1 Show InChI InChI=1S/C24H26N2O2/c1-3-26(4-2)16-7-17-27-20-13-10-19(11-14-20)24-25-23-21-9-6-5-8-18(21)12-15-22(23)28-24/h5-6,8-15H,3-4,7,16-17H2,1-2H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 4.10E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Central Drug Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of of PTP1B (unknown origin) assessed as residual enzyme activity in presence of Triton X-100 |
Eur J Med Chem 44: 109-16 (2008)
Article DOI: 10.1016/j.ejmech.2008.03.009
BindingDB Entry DOI: 10.7270/Q2FN1602 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 1
(Homo sapiens (Human)) | BDBM50266162

(Butyl-methyl-[2-(4-naphtho[1,2-d]oxazol-2-yl-pheno...)Show SMILES CCCCN(C)CCOc1ccc(cc1)-c1nc2c(ccc3ccccc23)o1 Show InChI InChI=1S/C24H26N2O2/c1-3-4-15-26(2)16-17-27-20-12-9-19(10-13-20)24-25-23-21-8-6-5-7-18(21)11-14-22(23)28-24/h5-14H,3-4,15-17H2,1-2H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 4.44E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Central Drug Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of of PTP1B (unknown origin) assessed as residual enzyme activity |
Eur J Med Chem 44: 109-16 (2008)
Article DOI: 10.1016/j.ejmech.2008.03.009
BindingDB Entry DOI: 10.7270/Q2FN1602 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 1
(Homo sapiens (Human)) | BDBM50182135

(3-(4-methoxybenzofuran-5-yl)-5-phenyl-4,5-dihydroi...)Show InChI InChI=1S/C18H15NO3/c1-20-18-13(7-8-16-14(18)9-10-21-16)15-11-17(22-19-15)12-5-3-2-4-6-12/h2-10,17H,11H2,1H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 4.50E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Central Drug Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of PTP1B |
Bioorg Med Chem Lett 16: 2139-43 (2006)
Article DOI: 10.1016/j.bmcl.2006.01.062
BindingDB Entry DOI: 10.7270/Q29K49ST |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 1
(Homo sapiens (Human)) | BDBM50198348
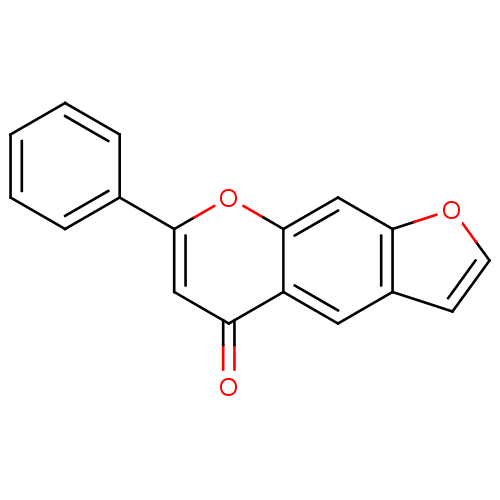
(7-phenyl-furo[3,2-g]chromen-5-one | CHEMBL388814)Show InChI InChI=1S/C17H10O3/c18-14-9-16(11-4-2-1-3-5-11)20-17-10-15-12(6-7-19-15)8-13(14)17/h1-10H | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 4.50E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Central Drug Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of PTP1B in presence of 0.01% Triton X-100 |
Bioorg Med Chem 15: 727-34 (2006)
Article DOI: 10.1016/j.bmc.2006.10.053
BindingDB Entry DOI: 10.7270/Q2CR5T1W |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 1
(Homo sapiens (Human)) | BDBM93113
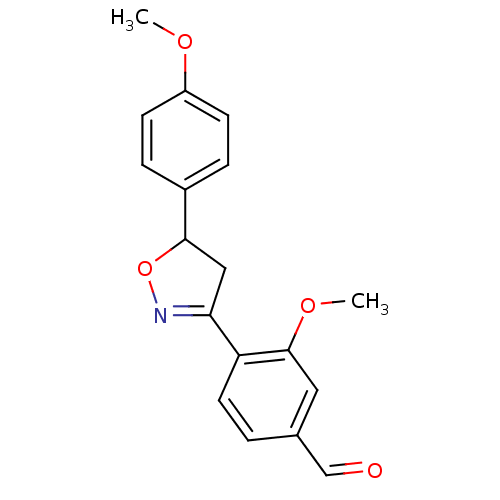
(PTP1B Inhibitor, 11)Show SMILES COc1ccc(cc1)C1CC(=NO1)c1ccc(C=O)cc1OC |c:11| Show InChI InChI=1S/C18H17NO4/c1-21-14-6-4-13(5-7-14)17-10-16(19-23-17)15-8-3-12(11-20)9-18(15)22-2/h3-9,11,17H,10H2,1-2H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| | 4.80E+4 | n/a | 6.70E+4 | n/a | n/a | n/a | n/a | 7.0 | n/a |
Central Drug Research Institute,
| Assay Description
The effect of the test compounds on PTP1B was studied by preincubating the test compound with enzyme in the reaction system for 10 min and determinin... |
Medicinal Chemistry Research 17: 123-136 (2008)
BindingDB Entry DOI: 10.7270/Q2JQ0ZMG |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 1
(Homo sapiens (Human)) | BDBM93112
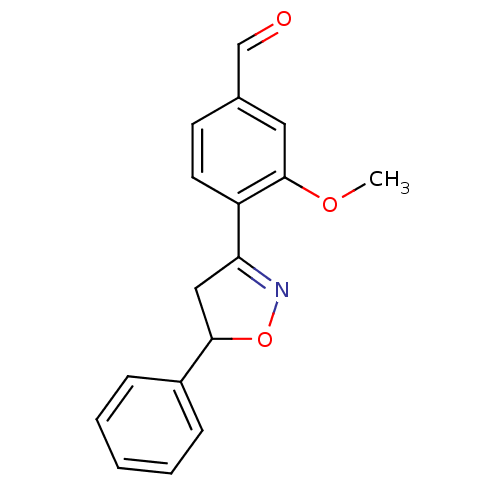
(PTP1B Inhibitor, 10)Show InChI InChI=1S/C17H15NO3/c1-20-17-9-12(11-19)7-8-14(17)15-10-16(21-18-15)13-5-3-2-4-6-13/h2-9,11,16H,10H2,1H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| | 4.80E+4 | n/a | 6.90E+4 | n/a | n/a | n/a | n/a | 7.0 | n/a |
Central Drug Research Institute,
| Assay Description
The effect of the test compounds on PTP1B was studied by preincubating the test compound with enzyme in the reaction system for 10 min and determinin... |
Medicinal Chemistry Research 17: 123-136 (2008)
BindingDB Entry DOI: 10.7270/Q2JQ0ZMG |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 1
(Homo sapiens (Human)) | BDBM50487364
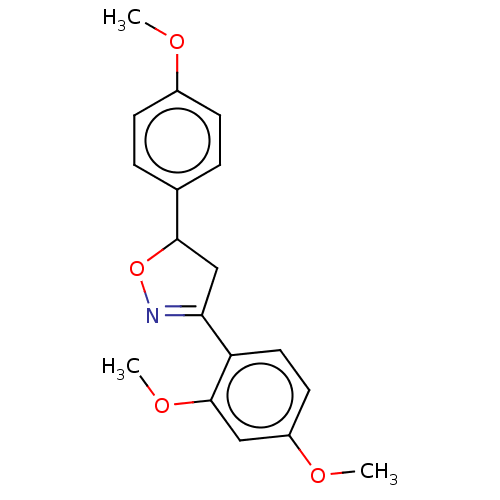
(CHEMBL2259741)Show SMILES COc1ccc(cc1)C1CC(=NO1)c1ccc(OC)cc1OC |c:11| Show InChI InChI=1S/C18H19NO4/c1-20-13-6-4-12(5-7-13)17-11-16(19-23-17)15-9-8-14(21-2)10-18(15)22-3/h4-10,17H,11H2,1-3H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | 4.80E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Central Drug Research Institute,
| Assay Description
The effect of the test compounds on PTP1B was studied by preincubating the test compound with enzyme in the reaction system for 10 min and determinin... |
Medicinal Chemistry Research 17: 123-136 (2008)
BindingDB Entry DOI: 10.7270/Q2JQ0ZMG |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 1
(Homo sapiens (Human)) | BDBM50487368
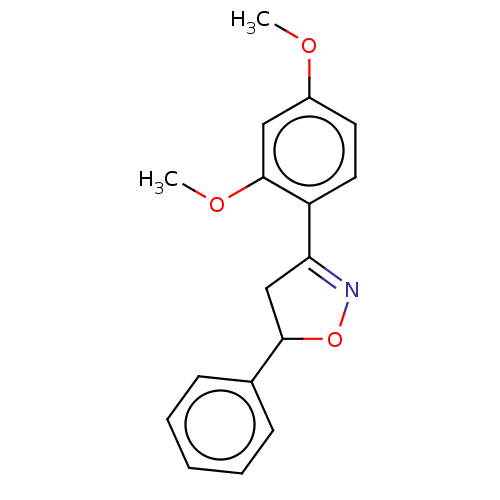
(CHEMBL2259740)Show InChI InChI=1S/C17H17NO3/c1-19-13-8-9-14(17(10-13)20-2)15-11-16(21-18-15)12-6-4-3-5-7-12/h3-10,16H,11H2,1-2H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | 4.80E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Central Drug Research Institute,
| Assay Description
The effect of the test compounds on PTP1B was studied by preincubating the test compound with enzyme in the reaction system for 10 min and determinin... |
Medicinal Chemistry Research 17: 123-136 (2008)
BindingDB Entry DOI: 10.7270/Q2JQ0ZMG |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 1
(Homo sapiens (Human)) | BDBM50198351
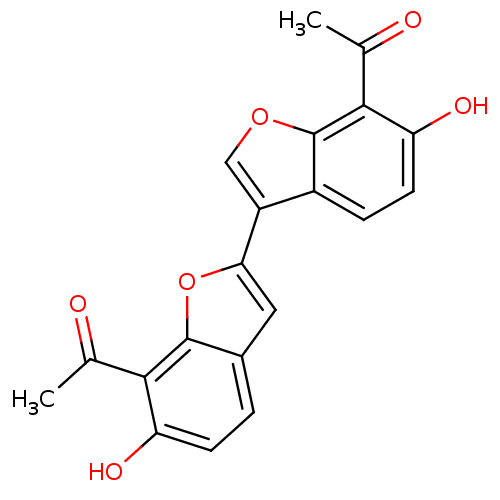
(1-(7'-acetyl-6,6'-dihydroxy-2',3'-dihydro-[2,3']bi...)Show SMILES CC(=O)c1c(O)ccc2cc(oc12)-c1coc2c(C(C)=O)c(O)ccc12 Show InChI InChI=1S/C20H14O6/c1-9(21)17-14(23)5-3-11-7-16(26-19(11)17)13-8-25-20-12(13)4-6-15(24)18(20)10(2)22/h3-8,23-24H,1-2H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 5.40E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Central Drug Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of PTP1B in presence of 0.01% Triton X-100 |
Bioorg Med Chem 15: 727-34 (2006)
Article DOI: 10.1016/j.bmc.2006.10.053
BindingDB Entry DOI: 10.7270/Q2CR5T1W |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 1
(Homo sapiens (Human)) | BDBM93110
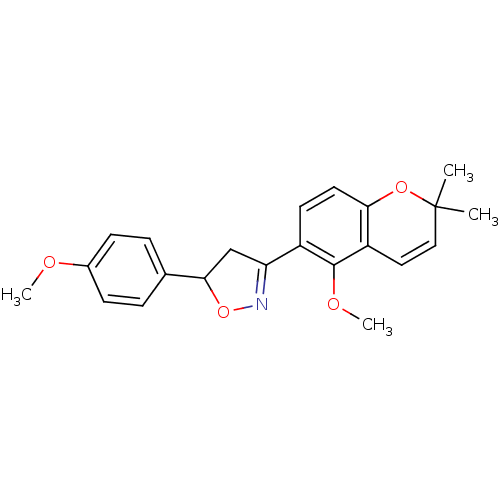
(PTP1B Inhibitor, 6)Show SMILES COc1ccc(cc1)C1CC(=NO1)c1ccc2OC(C)(C)C=Cc2c1OC |c:11,23| Show InChI InChI=1S/C22H23NO4/c1-22(2)12-11-17-19(26-22)10-9-16(21(17)25-4)18-13-20(27-23-18)14-5-7-15(24-3)8-6-14/h5-12,20H,13H2,1-4H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| | 5.60E+4 | n/a | 9.10E+4 | n/a | n/a | n/a | n/a | 7.0 | n/a |
Central Drug Research Institute,
| Assay Description
The effect of the test compounds on PTP1B was studied by preincubating the test compound with enzyme in the reaction system for 10 min and determinin... |
Medicinal Chemistry Research 17: 123-136 (2008)
BindingDB Entry DOI: 10.7270/Q2JQ0ZMG |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 1
(Homo sapiens (Human)) | BDBM50198348

(7-phenyl-furo[3,2-g]chromen-5-one | CHEMBL388814)Show InChI InChI=1S/C17H10O3/c18-14-9-16(11-4-2-1-3-5-11)20-17-10-15-12(6-7-19-15)8-13(14)17/h1-10H | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 5.70E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Central Drug Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of PTP1B |
Bioorg Med Chem 15: 727-34 (2006)
Article DOI: 10.1016/j.bmc.2006.10.053
BindingDB Entry DOI: 10.7270/Q2CR5T1W |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data