

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Prothrombin (Homo sapiens (Human)) | BDBM50096629 (3-(4-Amino-cyclohexyl)-2-oxo-3-[((6S,8aS)-4-oxo-2-...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.0900 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
BioChem Pharma Inc. Curated by ChEMBL | Assay Description In vitro activity of the compound against human alpha thrombin was determined | Bioorg Med Chem Lett 11: 287-90 (2001) BindingDB Entry DOI: 10.7270/Q2P55MS6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM50072528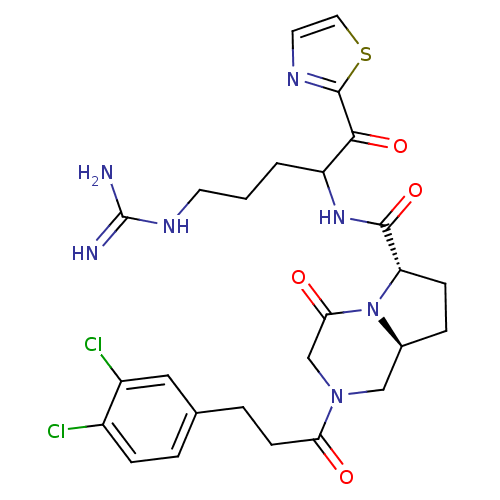 ((6S,8aS)-2-[3-(3,4-Dichloro-phenyl)-propionyl]-4-o...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.106 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
BioChem Therapeutic Inc. Curated by ChEMBL | Assay Description Binding affinity of the compound against thrombin | Bioorg Med Chem Lett 8: 3193-8 (1999) BindingDB Entry DOI: 10.7270/Q2HT2NGF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM50096623 (3-(4-Amino-cyclohexyl)-2-oxo-3-[(4-oxo-2-phenylmet...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.130 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
BioChem Pharma Inc. Curated by ChEMBL | Assay Description In vitro activity of the compound against human alpha thrombin was determined | Bioorg Med Chem Lett 11: 287-90 (2001) BindingDB Entry DOI: 10.7270/Q2P55MS6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM50096628 (3-(4-Amino-cyclohexyl)-2-oxo-3-[((6S,8aS)-4-oxo-2-...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.260 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
BioChem Pharma Inc. Curated by ChEMBL | Assay Description In vitro activity of the compound against human alpha thrombin was determined | Bioorg Med Chem Lett 11: 287-90 (2001) BindingDB Entry DOI: 10.7270/Q2P55MS6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM50096620 ((6S,8aS)-4-Oxo-2-phenylmethanesulfonyl-octahydro-p...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.330 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
BioChem Pharma Inc. Curated by ChEMBL | Assay Description In vitro activity of the compound against human alpha thrombin was determined | Bioorg Med Chem Lett 11: 287-90 (2001) BindingDB Entry DOI: 10.7270/Q2P55MS6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM50072536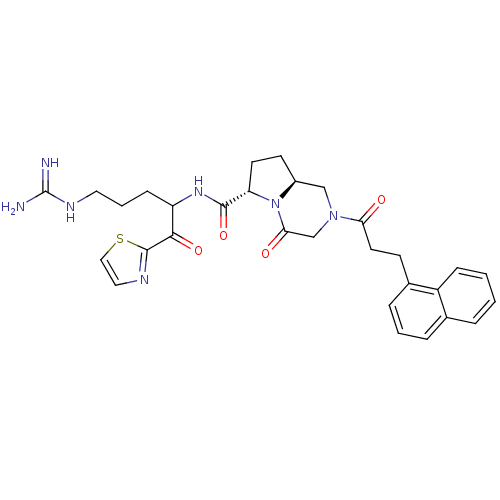 ((6S,8aS)-2-(3-Naphthalen-1-yl-propionyl)-4-oxo-oct...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.350 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
BioChem Therapeutic Inc. Curated by ChEMBL | Assay Description Binding affinity of the compound against thrombin | Bioorg Med Chem Lett 8: 3193-8 (1999) BindingDB Entry DOI: 10.7270/Q2HT2NGF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM50072524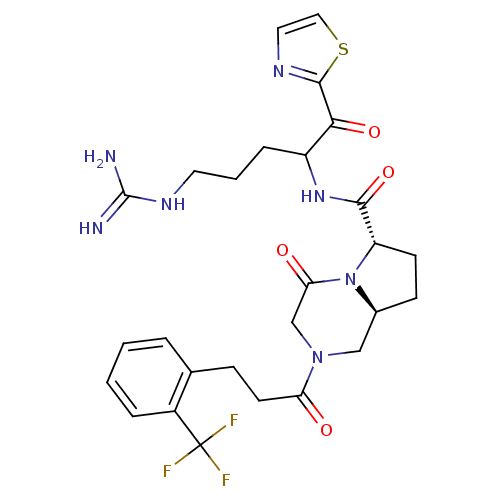 ((6S,8aS)-4-Oxo-2-[3-(2-trifluoromethyl-phenyl)-pro...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
BioChem Therapeutic Inc. Curated by ChEMBL | Assay Description Binding affinity of the compound against thrombin | Bioorg Med Chem Lett 8: 3193-8 (1999) BindingDB Entry DOI: 10.7270/Q2HT2NGF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM50072534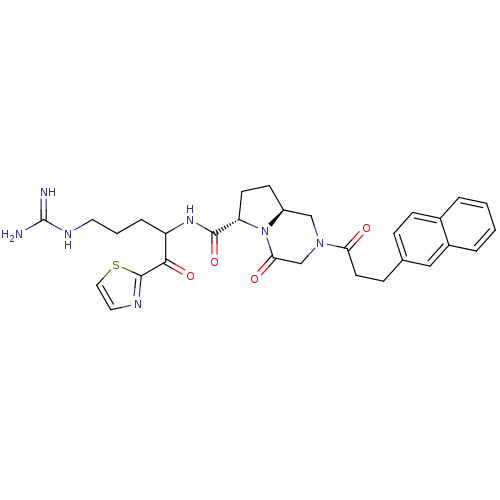 ((6S,8aS)-2-(3-Naphthalen-2-yl-propionyl)-4-oxo-oct...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
BioChem Therapeutic Inc. Curated by ChEMBL | Assay Description Binding affinity of the compound against thrombin | Bioorg Med Chem Lett 8: 3193-8 (1999) BindingDB Entry DOI: 10.7270/Q2HT2NGF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM50072537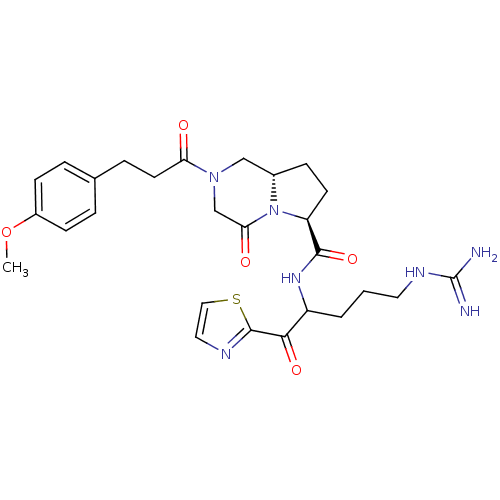 ((6S,8aS)-2-[3-(4-Methoxy-phenyl)-propionyl]-4-oxo-...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
BioChem Therapeutic Inc. Curated by ChEMBL | Assay Description Binding affinity of the compound against thrombin | Bioorg Med Chem Lett 8: 3193-8 (1999) BindingDB Entry DOI: 10.7270/Q2HT2NGF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM50072527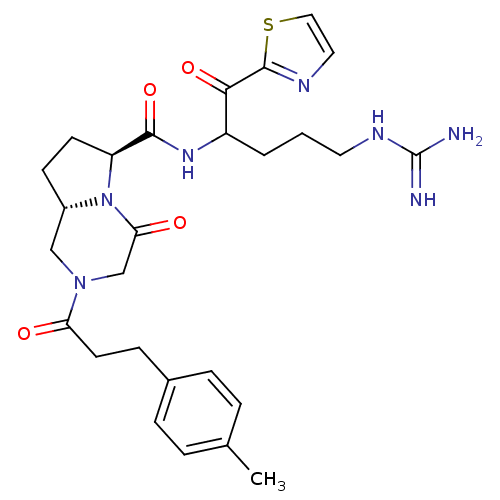 ((6S,8aS)-4-Oxo-2-(3-p-tolyl-propionyl)-octahydro-p...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
BioChem Therapeutic Inc. Curated by ChEMBL | Assay Description Binding affinity of the compound against thrombin | Bioorg Med Chem Lett 8: 3193-8 (1999) BindingDB Entry DOI: 10.7270/Q2HT2NGF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Rattus norvegicus) | BDBM50072740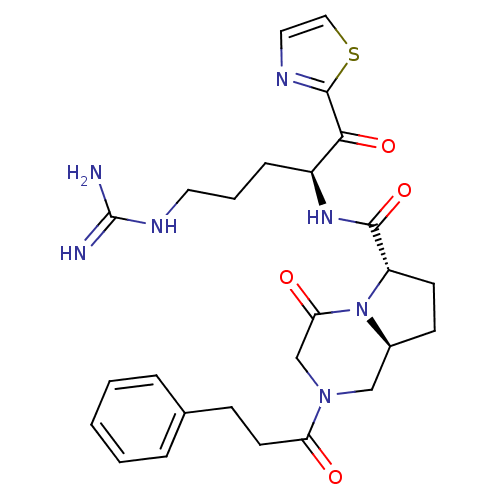 ((6S,8aS)-4-Oxo-2-(3-phenyl-propionyl)-octahydro-py...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 0.600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Shire BioChem Inc. Curated by ChEMBL | Assay Description In vitro ability of the compound to inhibit the amidolytic activity of thrombin | Bioorg Med Chem Lett 11: 3161-4 (2001) BindingDB Entry DOI: 10.7270/Q2JM28XZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM50072740 ((6S,8aS)-4-Oxo-2-(3-phenyl-propionyl)-octahydro-py...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 0.600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Warner-Lambert Company Curated by ChEMBL | Assay Description Inhibitory activity of the compound towards Thrombin | J Med Chem 43: 361-8 (2000) BindingDB Entry DOI: 10.7270/Q2P26XCW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM50072532 ((6S,8aS)-4-Oxo-2-(3-phenyl-propionyl)-octahydro-py...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 0.600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
BioChem Therapeutic Inc. Curated by ChEMBL | Assay Description Binding affinity of the compound against thrombin | Bioorg Med Chem Lett 8: 3193-8 (1999) BindingDB Entry DOI: 10.7270/Q2HT2NGF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM50096621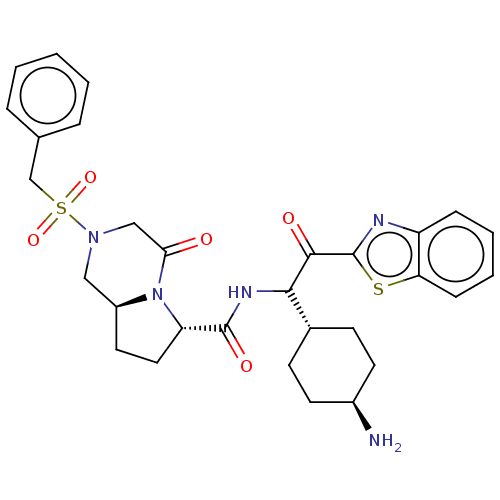 ((6S,8aS)-4-Oxo-2-phenylmethanesulfonyl-octahydro-p...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.720 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
BioChem Pharma Inc. Curated by ChEMBL | Assay Description In vitro activity of the compound against human alpha thrombin was determined | Bioorg Med Chem Lett 11: 287-90 (2001) BindingDB Entry DOI: 10.7270/Q2P55MS6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM50072520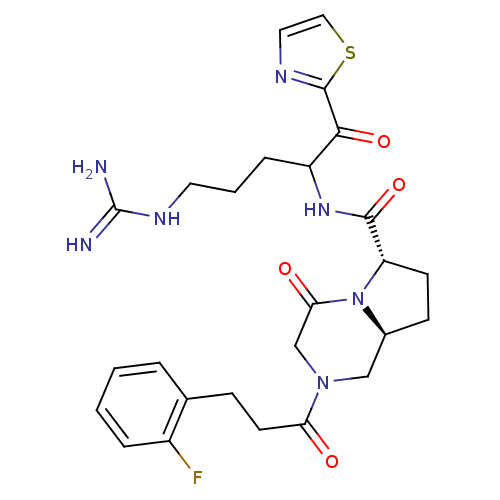 ((6S,8aS)-2-[3-(2-Fluoro-phenyl)-propionyl]-4-oxo-o...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.920 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
BioChem Therapeutic Inc. Curated by ChEMBL | Assay Description Binding affinity of the compound against thrombin | Bioorg Med Chem Lett 8: 3193-8 (1999) BindingDB Entry DOI: 10.7270/Q2HT2NGF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor X (Homo sapiens (Human)) | BDBM50111673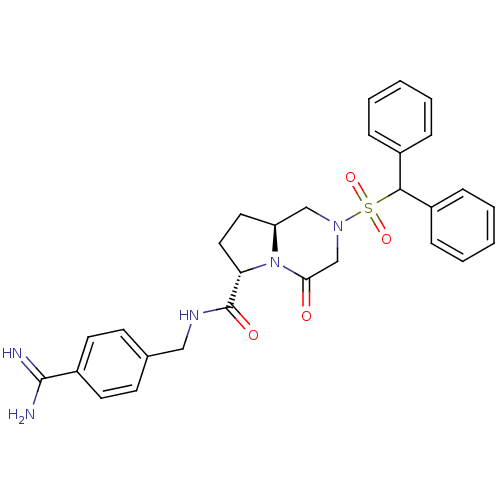 ((6S,8aS)-2-(Diphenyl-methanesulfonyl)-4-oxo-octahy...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Shire BioChem. Curated by ChEMBL | Assay Description Inhibition of amidolytic activity of thrombin | Bioorg Med Chem Lett 12: 1181-4 (2002) BindingDB Entry DOI: 10.7270/Q22V2FFT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor X (Homo sapiens (Human)) | BDBM50111661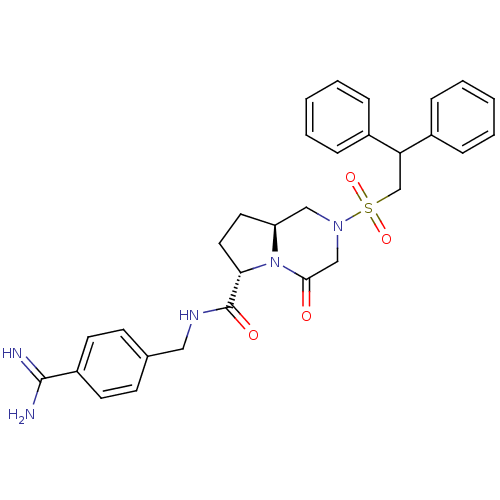 ((6S,8aS)-2-(2,2-Diphenyl-ethanesulfonyl)-4-oxo-oct...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Shire BioChem. Curated by ChEMBL | Assay Description Inhibition of amidolytic activity of thrombin | Bioorg Med Chem Lett 12: 1181-4 (2002) BindingDB Entry DOI: 10.7270/Q22V2FFT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM50072533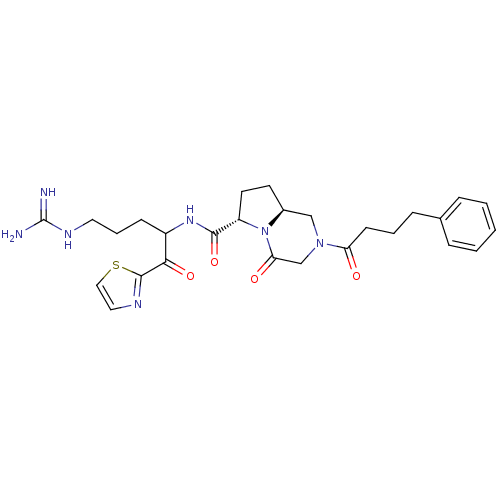 ((6S,8aS)-4-Oxo-2-(4-phenyl-butyryl)-octahydro-pyrr...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 1.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
BioChem Therapeutic Inc. Curated by ChEMBL | Assay Description Binding affinity of the compound against thrombin | Bioorg Med Chem Lett 8: 3193-8 (1999) BindingDB Entry DOI: 10.7270/Q2HT2NGF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor X (Homo sapiens (Human)) | BDBM50111665 ((6S,8aS)-2-(Diphenyl-methanesulfonyl)-4-oxo-octahy...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Shire BioChem. Curated by ChEMBL | Assay Description Inhibition of amidolytic activity of thrombin | Bioorg Med Chem Lett 12: 1181-4 (2002) BindingDB Entry DOI: 10.7270/Q22V2FFT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor X (Homo sapiens (Human)) | BDBM50111675 ((6S,8aS)-2-(2,2-Diphenyl-ethanesulfonyl)-4-oxo-oct...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Shire BioChem. Curated by ChEMBL | Assay Description Inhibition of amidolytic activity of thrombin | Bioorg Med Chem Lett 12: 1181-4 (2002) BindingDB Entry DOI: 10.7270/Q22V2FFT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor X (Homo sapiens (Human)) | BDBM50111658 ((6S,8aS)-2-(Diphenyl-methanesulfonyl)-4-oxo-octahy...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Shire BioChem. Curated by ChEMBL | Assay Description Inhibition of amidolytic activity of thrombin | Bioorg Med Chem Lett 12: 1181-4 (2002) BindingDB Entry DOI: 10.7270/Q22V2FFT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor X (Homo sapiens (Human)) | BDBM50111672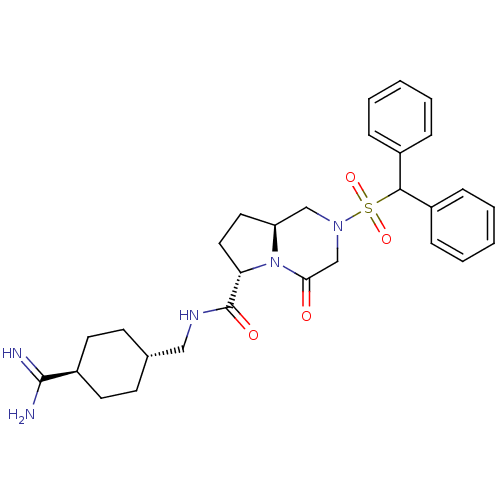 ((6S,8aS)-2-(Diphenyl-methanesulfonyl)-4-oxo-octahy...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Shire BioChem. Curated by ChEMBL | Assay Description Inhibition of amidolytic activity of thrombin | Bioorg Med Chem Lett 12: 1181-4 (2002) BindingDB Entry DOI: 10.7270/Q22V2FFT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM50096622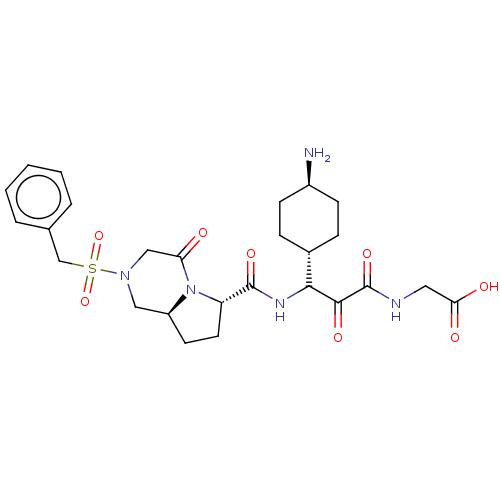 (CHEMBL3084834 | {3-(4-Amino-cyclohexyl)-2-oxo-3-[(...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 2.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
BioChem Pharma Inc. Curated by ChEMBL | Assay Description In vitro activity of the compound against human alpha thrombin was determined | Bioorg Med Chem Lett 11: 287-90 (2001) BindingDB Entry DOI: 10.7270/Q2P55MS6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM50072530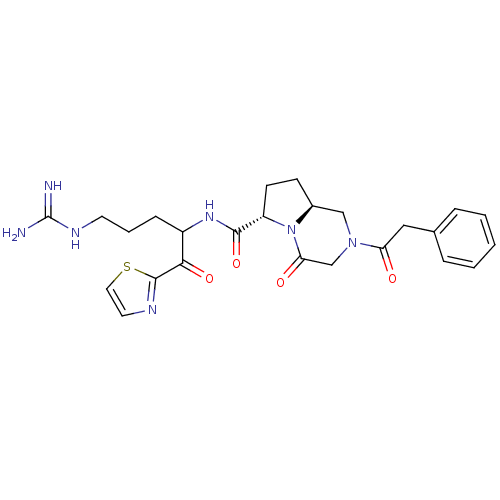 ((6S,8aS)-4-Oxo-2-phenylacetyl-octahydro-pyrrolo[1,...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 2.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
BioChem Therapeutic Inc. Curated by ChEMBL | Assay Description Binding affinity of the compound against thrombin | Bioorg Med Chem Lett 8: 3193-8 (1999) BindingDB Entry DOI: 10.7270/Q2HT2NGF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM50072519 ((6S,8aS)-2-[(S)-2-Amino-3-(4-fluoro-phenyl)-propio...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
BioChem Therapeutic Inc. Curated by ChEMBL | Assay Description Binding affinity of the compound against thrombin | Bioorg Med Chem Lett 8: 3193-8 (1999) BindingDB Entry DOI: 10.7270/Q2HT2NGF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM50072541 ((6S,8aS)-4-Oxo-2-(3-pyridin-2-yl-propionyl)-octahy...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
BioChem Therapeutic Inc. Curated by ChEMBL | Assay Description Binding affinity of the compound against thrombin | Bioorg Med Chem Lett 8: 3193-8 (1999) BindingDB Entry DOI: 10.7270/Q2HT2NGF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM50096630 ((6S,8aS)-4-Oxo-2-phenylmethanesulfonyl-octahydro-p...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 3.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
BioChem Pharma Inc. Curated by ChEMBL | Assay Description In vitro activity of the compound against human alpha thrombin was determined | Bioorg Med Chem Lett 11: 287-90 (2001) BindingDB Entry DOI: 10.7270/Q2P55MS6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM50072736 ((6S,8aS)-4-Oxo-2-(3-phenyl-propionyl)-octahydro-py...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 3.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Warner-Lambert Company Curated by ChEMBL | Assay Description Inhibitory activity of the compound towards Thrombin | J Med Chem 43: 361-8 (2000) BindingDB Entry DOI: 10.7270/Q2P26XCW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM50072529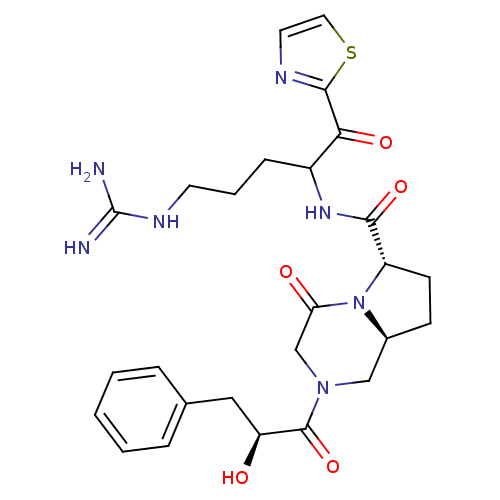 ((6S,8aS)-2-((S)-2-Hydroxy-3-phenyl-propionyl)-4-ox...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 4.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
BioChem Therapeutic Inc. Curated by ChEMBL | Assay Description Binding affinity of the compound against thrombin | Bioorg Med Chem Lett 8: 3193-8 (1999) BindingDB Entry DOI: 10.7270/Q2HT2NGF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor X (Homo sapiens (Human)) | BDBM50111676 ((6S,8aS)-2-(Diphenyl-methanesulfonyl)-4-oxo-octahy...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Shire BioChem. Curated by ChEMBL | Assay Description Inhibition of amidolytic activity of thrombin | Bioorg Med Chem Lett 12: 1181-4 (2002) BindingDB Entry DOI: 10.7270/Q22V2FFT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor X (Homo sapiens (Human)) | BDBM50111671 ((6S,8aS)-2-(2,2-Diphenyl-ethanesulfonyl)-4-oxo-oct...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Shire BioChem. Curated by ChEMBL | Assay Description Inhibition of amidolytic activity of thrombin | Bioorg Med Chem Lett 12: 1181-4 (2002) BindingDB Entry DOI: 10.7270/Q22V2FFT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM50072535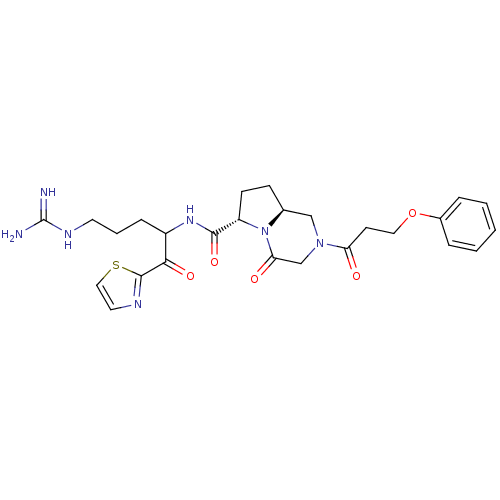 ((6S,8aS)-4-Oxo-2-(3-phenoxy-propionyl)-octahydro-p...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
BioChem Therapeutic Inc. Curated by ChEMBL | Assay Description Binding affinity of the compound against thrombin | Bioorg Med Chem Lett 8: 3193-8 (1999) BindingDB Entry DOI: 10.7270/Q2HT2NGF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM50096627 ((6S,8aS)-4-Oxo-2-phenylmethanesulfonyl-octahydro-p...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
BioChem Pharma Inc. Curated by ChEMBL | Assay Description In vitro activity of the compound against human alpha thrombin was determined | Bioorg Med Chem Lett 11: 287-90 (2001) BindingDB Entry DOI: 10.7270/Q2P55MS6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Rattus norvegicus) | BDBM50107062 ((6S,8aS)-4-Oxo-2-phenylmethanesulfonyl-octahydro-p...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 7 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Shire BioChem Inc. Curated by ChEMBL | Assay Description In vitro ability of the compound to inhibit the amidolytic activity of thrombin | Bioorg Med Chem Lett 11: 3161-4 (2001) BindingDB Entry DOI: 10.7270/Q2JM28XZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor X (Homo sapiens (Human)) | BDBM50111674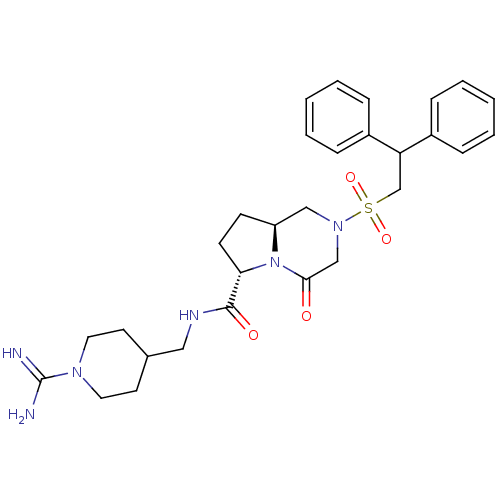 ((6S,8aS)-2-(2,2-Diphenyl-ethanesulfonyl)-4-oxo-oct...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 8 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Shire BioChem. Curated by ChEMBL | Assay Description Inhibition of amidolytic activity of thrombin | Bioorg Med Chem Lett 12: 1181-4 (2002) BindingDB Entry DOI: 10.7270/Q22V2FFT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Rattus norvegicus) | BDBM50107060 ((6S,8aS)-4-Oxo-2-phenylmethanesulfonyl-octahydro-p...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 8 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Shire BioChem Inc. Curated by ChEMBL | Assay Description In vitro ability of the compound to inhibit the amidolytic activity of thrombin | Bioorg Med Chem Lett 11: 3161-4 (2001) BindingDB Entry DOI: 10.7270/Q2JM28XZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor X (Homo sapiens (Human)) | BDBM50111669 ((6S,8aS)-2-(Diphenyl-methanesulfonyl)-4-oxo-octahy...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 9 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Shire BioChem. Curated by ChEMBL | Assay Description Inhibition of amidolytic activity of thrombin | Bioorg Med Chem Lett 12: 1181-4 (2002) BindingDB Entry DOI: 10.7270/Q22V2FFT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor X (Homo sapiens (Human)) | BDBM50111663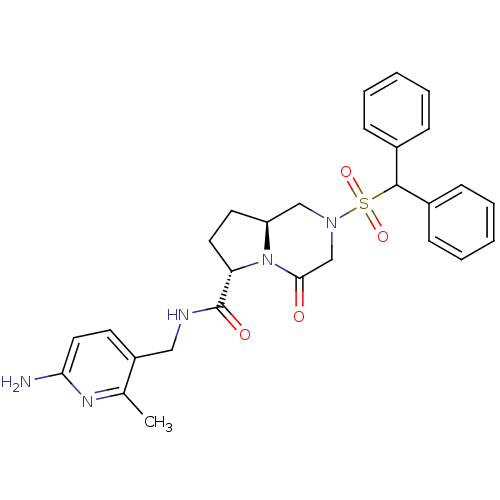 ((6S,8aS)-2-(Diphenyl-methanesulfonyl)-4-oxo-octahy...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Shire BioChem. Curated by ChEMBL | Assay Description Inhibition of amidolytic activity of thrombin | Bioorg Med Chem Lett 12: 1181-4 (2002) BindingDB Entry DOI: 10.7270/Q22V2FFT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine protease 1 (Homo sapiens (Human)) | BDBM50080895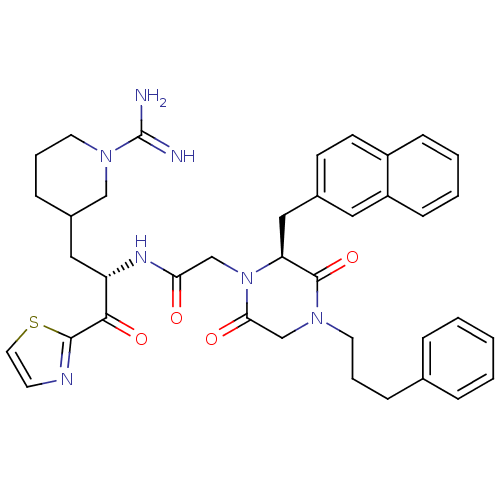 (CHEMBL311198 | N-[(S)-1-(1-Carbamimidoyl-piperidin...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 14 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Warner-Lambert Company Curated by ChEMBL | Assay Description Inhibitory activity of the compound against human thrombin | Bioorg Med Chem Lett 9: 2497-502 (1999) BindingDB Entry DOI: 10.7270/Q23T9GDD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Rattus norvegicus) | BDBM50107064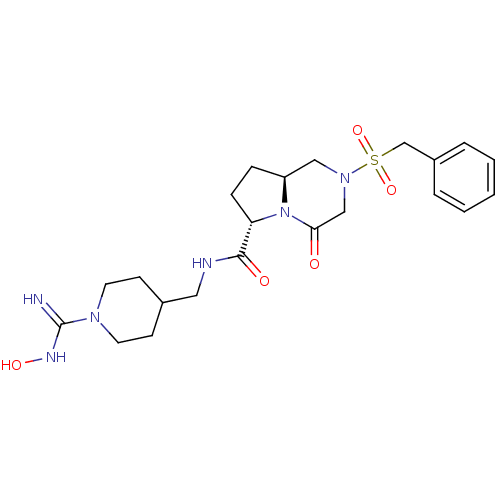 ((6S,8aS)-4-Oxo-2-phenylmethanesulfonyl-octahydro-p...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 14 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Shire BioChem Inc. Curated by ChEMBL | Assay Description In vitro ability of the compound to inhibit the amidolytic activity of thrombin | Bioorg Med Chem Lett 11: 3161-4 (2001) BindingDB Entry DOI: 10.7270/Q2JM28XZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor X (Homo sapiens (Human)) | BDBM50111667 ((6S,8aS)-4-Oxo-2-(2-phenyl-ethanesulfonyl)-octahyd...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 14 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Shire BioChem. Curated by ChEMBL | Assay Description Inhibition of amidolytic activity of thrombin | Bioorg Med Chem Lett 12: 1181-4 (2002) BindingDB Entry DOI: 10.7270/Q22V2FFT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM50072521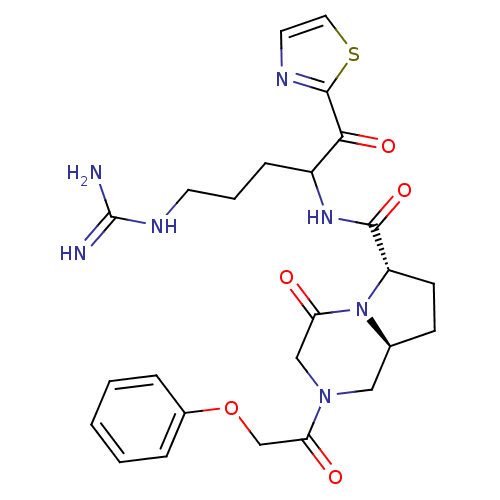 ((6S,8aS)-4-Oxo-2-(2-phenoxy-acetyl)-octahydro-pyrr...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 16 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
BioChem Therapeutic Inc. Curated by ChEMBL | Assay Description Binding affinity of the compound against thrombin | Bioorg Med Chem Lett 8: 3193-8 (1999) BindingDB Entry DOI: 10.7270/Q2HT2NGF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM50072538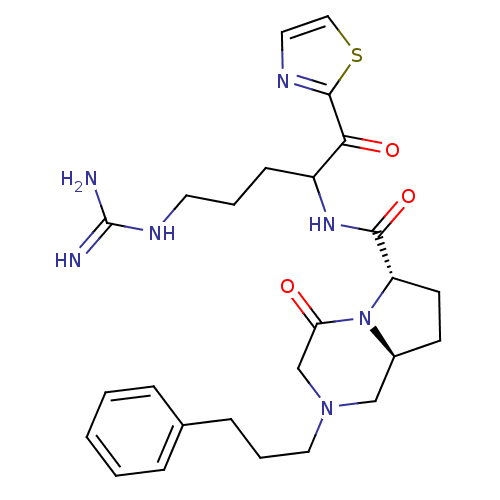 ((6S,8aS)-4-Oxo-2-(3-phenyl-propyl)-octahydro-pyrro...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 17 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
BioChem Therapeutic Inc. Curated by ChEMBL | Assay Description Binding affinity of the compound against thrombin | Bioorg Med Chem Lett 8: 3193-8 (1999) BindingDB Entry DOI: 10.7270/Q2HT2NGF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM50072526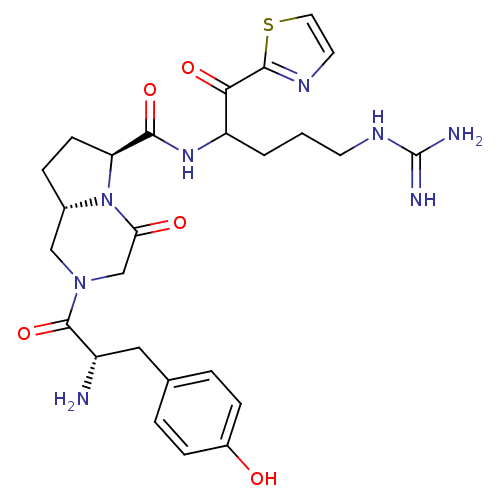 ((6S,8aS)-2-[(S)-2-Amino-3-(4-hydroxy-phenyl)-propi...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 18 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
BioChem Therapeutic Inc. Curated by ChEMBL | Assay Description Binding affinity of the compound against thrombin | Bioorg Med Chem Lett 8: 3193-8 (1999) BindingDB Entry DOI: 10.7270/Q2HT2NGF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM50072526 ((6S,8aS)-2-[(S)-2-Amino-3-(4-hydroxy-phenyl)-propi...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 18 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
BioChem Therapeutic Inc. Curated by ChEMBL | Assay Description Binding affinity of the compound against thrombin | Bioorg Med Chem Lett 8: 3193-8 (1999) BindingDB Entry DOI: 10.7270/Q2HT2NGF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM50080908 (2-[(S)-2-Benzyl-3,6-dioxo-4-(3-phenyl-propyl)-pipe...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 24 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Warner-Lambert Company Curated by ChEMBL | Assay Description Binding affinity against thrombin | Bioorg Med Chem Lett 9: 2497-502 (1999) BindingDB Entry DOI: 10.7270/Q23T9GDD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM50072539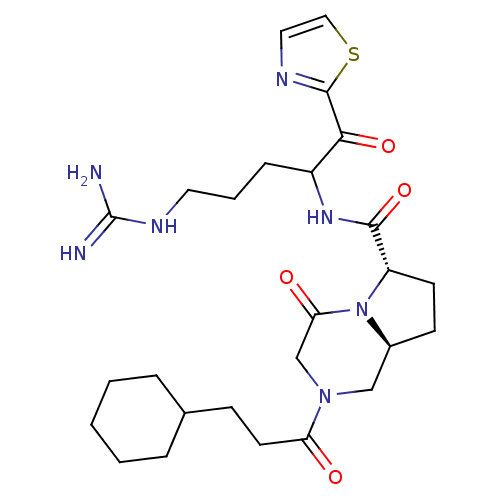 ((6S,8aS)-2-(3-Cyclohexyl-propionyl)-4-oxo-octahydr...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 24 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
BioChem Therapeutic Inc. Curated by ChEMBL | Assay Description Binding affinity of the compound against thrombin | Bioorg Med Chem Lett 8: 3193-8 (1999) BindingDB Entry DOI: 10.7270/Q2HT2NGF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM50072540 ((6S,8aS)-2-Benzoyl-4-oxo-octahydro-pyrrolo[1,2-a]p...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 25 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
BioChem Therapeutic Inc. Curated by ChEMBL | Assay Description Binding affinity of the compound against thrombin | Bioorg Med Chem Lett 8: 3193-8 (1999) BindingDB Entry DOI: 10.7270/Q2HT2NGF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor X (Homo sapiens (Human)) | BDBM50111668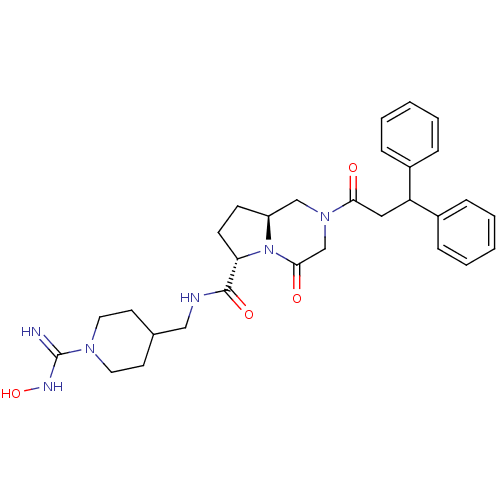 ((6S,8aS)-2-(3,3-Diphenyl-propionyl)-4-oxo-octahydr...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 25 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Shire BioChem. Curated by ChEMBL | Assay Description Inhibition of amidolytic activity of thrombin | Bioorg Med Chem Lett 12: 1181-4 (2002) BindingDB Entry DOI: 10.7270/Q22V2FFT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine protease 1 (Homo sapiens (Human)) | BDBM50096622 (CHEMBL3084834 | {3-(4-Amino-cyclohexyl)-2-oxo-3-[(...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 29 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
BioChem Pharma Inc. Curated by ChEMBL | Assay Description In vitro activity of the compound against human alpha trypsin was determined | Bioorg Med Chem Lett 11: 287-90 (2001) BindingDB Entry DOI: 10.7270/Q2P55MS6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 253 total ) | Next | Last >> |