 Found 510 hits with Last Name = 'st-gallay' and Initial = 's'
Found 510 hits with Last Name = 'st-gallay' and Initial = 's' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Monocarboxylate transporter 1
(Homo sapiens (Human)) | BDBM21995
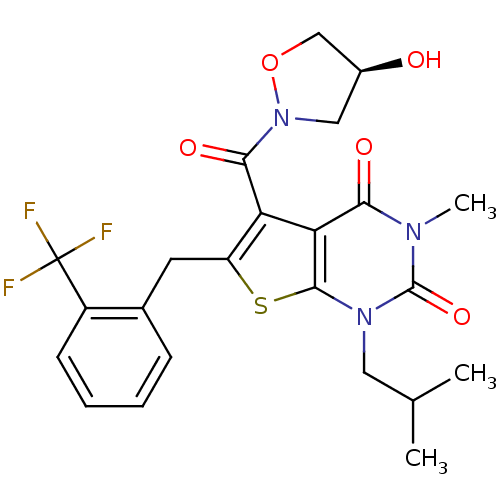
(5-{[(4S)-4-hydroxy-1,2-oxazolidin-2-yl]carbonyl}-3...)Show SMILES CC(C)Cn1c2sc(Cc3ccccc3C(F)(F)F)c(C(=O)N3C[C@H](O)CO3)c2c(=O)n(C)c1=O |r| Show InChI InChI=1S/C23H24F3N3O5S/c1-12(2)9-28-21-18(19(31)27(3)22(28)33)17(20(32)29-10-14(30)11-34-29)16(35-21)8-13-6-4-5-7-15(13)23(24,25)26/h4-7,12,14,30H,8-11H2,1-3H3/t14-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.0900 | -56.8 | n/a | n/a | n/a | n/a | n/a | 7.8 | 22 |
AstraZeneca
| Assay Description
Jurkat cell membranes were incubated with [3H]-labeled ligand in the absence or presence of increasing concentrations of test compound. The reagents ... |
J Med Chem 50: 254-63 (2007)
Article DOI: 10.1021/jm060995h
BindingDB Entry DOI: 10.7270/Q2GT5KGF |
More data for this
Ligand-Target Pair | |
Monocarboxylate transporter 1
(Homo sapiens (Human)) | BDBM21992
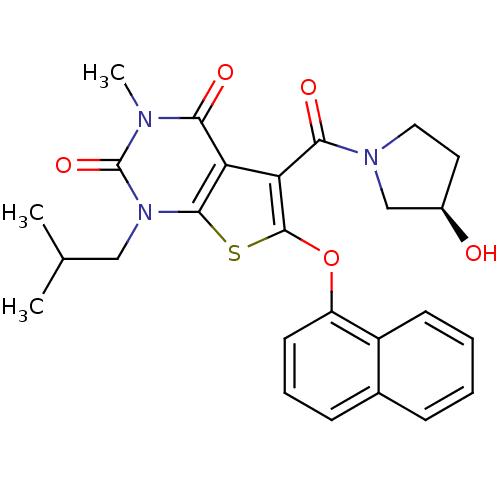
(5-{[(3R)-3-hydroxypyrrolidin-1-yl]carbonyl}-3-meth...)Show SMILES CC(C)Cn1c2sc(Oc3cccc4ccccc34)c(C(=O)N3CC[C@@H](O)C3)c2c(=O)n(C)c1=O |r| Show InChI InChI=1S/C26H27N3O5S/c1-15(2)13-29-24-20(22(31)27(3)26(29)33)21(23(32)28-12-11-17(30)14-28)25(35-24)34-19-10-6-8-16-7-4-5-9-18(16)19/h4-10,15,17,30H,11-14H2,1-3H3/t17-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.310 | -53.7 | n/a | n/a | n/a | n/a | n/a | 7.8 | 22 |
AstraZeneca
| Assay Description
Jurkat cell membranes were incubated with [3H]-labeled ligand in the absence or presence of increasing concentrations of test compound. The reagents ... |
J Med Chem 50: 254-63 (2007)
Article DOI: 10.1021/jm060995h
BindingDB Entry DOI: 10.7270/Q2GT5KGF |
More data for this
Ligand-Target Pair | |
Monocarboxylate transporter 1
(Homo sapiens (Human)) | BDBM21996
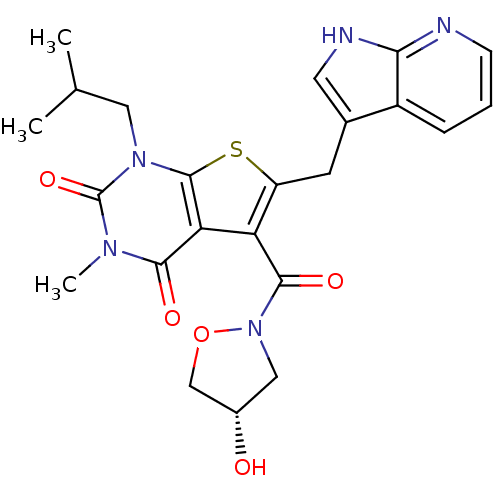
(5-{[(4S)-4-hydroxy-1,2-oxazolidin-2-yl]carbonyl}-3...)Show SMILES CC(C)Cn1c2sc(Cc3c[nH]c4ncccc34)c(C(=O)N3C[C@H](O)CO3)c2c(=O)n(C)c1=O |r| Show InChI InChI=1S/C23H25N5O5S/c1-12(2)9-27-22-18(20(30)26(3)23(27)32)17(21(31)28-10-14(29)11-33-28)16(34-22)7-13-8-25-19-15(13)5-4-6-24-19/h4-6,8,12,14,29H,7,9-11H2,1-3H3,(H,24,25)/t14-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.430 | -52.9 | n/a | n/a | n/a | n/a | n/a | 7.8 | 22 |
AstraZeneca
| Assay Description
Jurkat cell membranes were incubated with [3H]-labeled ligand in the absence or presence of increasing concentrations of test compound. The reagents ... |
J Med Chem 50: 254-63 (2007)
Article DOI: 10.1021/jm060995h
BindingDB Entry DOI: 10.7270/Q2GT5KGF |
More data for this
Ligand-Target Pair | |
Monocarboxylate transporter 1
(Homo sapiens (Human)) | BDBM21994

(5-{[(4S)-4-hydroxy-1,2-oxazolidin-2-yl]carbonyl}-3...)Show SMILES CC(C)Cn1c2sc(Cc3ccnc4ccccc34)c(C(=O)N3C[C@H](O)CO3)c2c(=O)n(C)c1=O |r| Show InChI InChI=1S/C25H26N4O5S/c1-14(2)11-28-24-21(22(31)27(3)25(28)33)20(23(32)29-12-16(30)13-34-29)19(35-24)10-15-8-9-26-18-7-5-4-6-17(15)18/h4-9,14,16,30H,10-13H2,1-3H3/t16-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.520 | -52.5 | n/a | n/a | n/a | n/a | n/a | 7.8 | 22 |
AstraZeneca
| Assay Description
Jurkat cell membranes were incubated with [3H]-labeled ligand in the absence or presence of increasing concentrations of test compound. The reagents ... |
J Med Chem 50: 254-63 (2007)
Article DOI: 10.1021/jm060995h
BindingDB Entry DOI: 10.7270/Q2GT5KGF |
More data for this
Ligand-Target Pair | |
Monocarboxylate transporter 1
(Homo sapiens (Human)) | BDBM21987

(6-[(6-fluoroquinolin-4-yl)methyl]-5-{[(3R)-3-hydro...)Show SMILES CC(C)Cn1c2sc(Cc3ccnc4ccc(F)cc34)c(C(=O)N3CC[C@@H](O)C3)c2c(=O)n(C)c1=O |r| Show InChI InChI=1S/C26H27FN4O4S/c1-14(2)12-31-25-22(23(33)29(3)26(31)35)21(24(34)30-9-7-17(32)13-30)20(36-25)10-15-6-8-28-19-5-4-16(27)11-18(15)19/h4-6,8,11,14,17,32H,7,9-10,12-13H2,1-3H3/t17-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.810 | -51.4 | n/a | n/a | n/a | n/a | n/a | 7.8 | 22 |
AstraZeneca
| Assay Description
Jurkat cell membranes were incubated with [3H]-labeled ligand in the absence or presence of increasing concentrations of test compound. The reagents ... |
J Med Chem 50: 254-63 (2007)
Article DOI: 10.1021/jm060995h
BindingDB Entry DOI: 10.7270/Q2GT5KGF |
More data for this
Ligand-Target Pair | |
Monocarboxylate transporter 1
(Homo sapiens (Human)) | BDBM21998
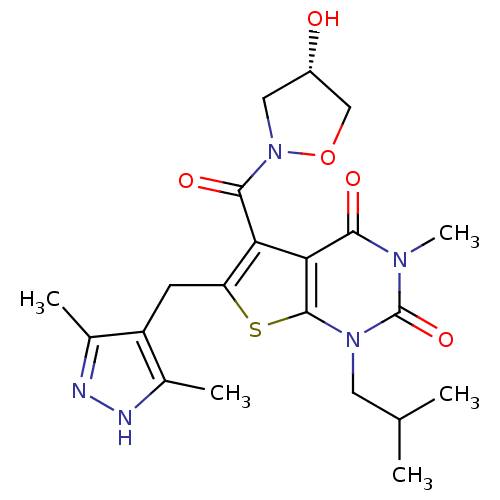
(6-[(3,5-dimethyl-1H-pyrazol-4-yl)methyl]-5-{[(4S)-...)Show SMILES CC(C)Cn1c2sc(Cc3c(C)n[nH]c3C)c(C(=O)N3C[C@H](O)CO3)c2c(=O)n(C)c1=O |r| Show InChI InChI=1S/C21H27N5O5S/c1-10(2)7-25-20-17(18(28)24(5)21(25)30)16(19(29)26-8-13(27)9-31-26)15(32-20)6-14-11(3)22-23-12(14)4/h10,13,27H,6-9H2,1-5H3,(H,22,23)/t13-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 1.20 | -50.4 | n/a | n/a | n/a | n/a | n/a | 7.8 | 22 |
AstraZeneca
| Assay Description
Jurkat cell membranes were incubated with [3H]-labeled ligand in the absence or presence of increasing concentrations of test compound. The reagents ... |
J Med Chem 50: 254-63 (2007)
Article DOI: 10.1021/jm060995h
BindingDB Entry DOI: 10.7270/Q2GT5KGF |
More data for this
Ligand-Target Pair | |
Monocarboxylate transporter 1
(Homo sapiens (Human)) | BDBM21997
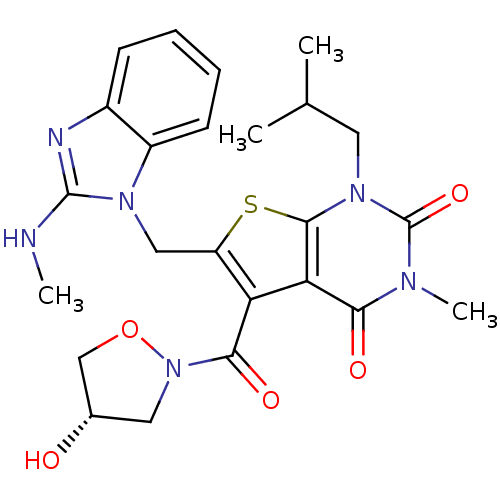
(5-{[(4S)-4-hydroxy-1,2-oxazolidin-2-yl]carbonyl}-3...)Show SMILES CNc1nc2ccccc2n1Cc1sc2n(CC(C)C)c(=O)n(C)c(=O)c2c1C(=O)N1C[C@H](O)CO1 |r| Show InChI InChI=1S/C24H28N6O5S/c1-13(2)9-29-22-19(20(32)27(4)24(29)34)18(21(33)30-10-14(31)12-35-30)17(36-22)11-28-16-8-6-5-7-15(16)26-23(28)25-3/h5-8,13-14,31H,9-12H2,1-4H3,(H,25,26)/t14-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 1.70 | -49.6 | n/a | n/a | n/a | n/a | n/a | 7.8 | 22 |
AstraZeneca
| Assay Description
Jurkat cell membranes were incubated with [3H]-labeled ligand in the absence or presence of increasing concentrations of test compound. The reagents ... |
J Med Chem 50: 254-63 (2007)
Article DOI: 10.1021/jm060995h
BindingDB Entry DOI: 10.7270/Q2GT5KGF |
More data for this
Ligand-Target Pair | |
Monocarboxylate transporter 1
(Homo sapiens (Human)) | BDBM21993

(6-[(4,5-dichloro-2-methyl-1H-imidazol-1-yl)methyl]...)Show SMILES CON(C)C(=O)c1c(Cn2c(C)nc(Cl)c2Cl)sc2n(CC(C)C)c(=O)n(C)c(=O)c12 Show InChI InChI=1S/C19H23Cl2N5O4S/c1-9(2)7-26-18-13(16(27)23(4)19(26)29)12(17(28)24(5)30-6)11(31-18)8-25-10(3)22-14(20)15(25)21/h9H,7-8H2,1-6H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 3.20 | -48.0 | n/a | n/a | n/a | n/a | n/a | 7.8 | 22 |
AstraZeneca
| Assay Description
Jurkat cell membranes were incubated with [3H]-labeled ligand in the absence or presence of increasing concentrations of test compound. The reagents ... |
J Med Chem 50: 254-63 (2007)
Article DOI: 10.1021/jm060995h
BindingDB Entry DOI: 10.7270/Q2GT5KGF |
More data for this
Ligand-Target Pair | |
Monocarboxylate transporter 1
(Homo sapiens (Human)) | BDBM21985
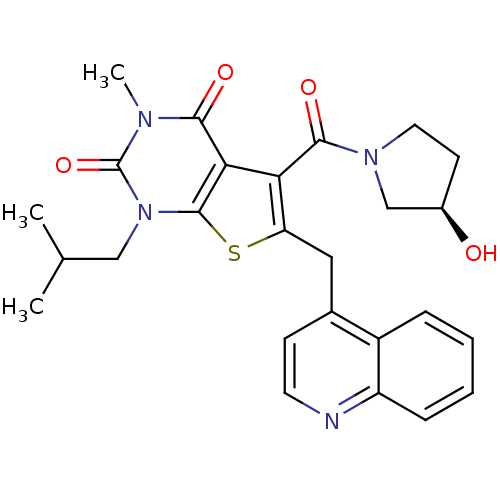
(5-{[(3R)-3-hydroxypyrrolidin-1-yl]carbonyl}-3-meth...)Show SMILES CC(C)Cn1c2sc(Cc3ccnc4ccccc34)c(C(=O)N3CC[C@@H](O)C3)c2c(=O)n(C)c1=O |r| Show InChI InChI=1S/C26H28N4O4S/c1-15(2)13-30-25-22(23(32)28(3)26(30)34)21(24(33)29-11-9-17(31)14-29)20(35-25)12-16-8-10-27-19-7-5-4-6-18(16)19/h4-8,10,15,17,31H,9,11-14H2,1-3H3/t17-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 4.80 | -47.0 | n/a | n/a | n/a | n/a | n/a | 7.8 | 22 |
AstraZeneca
| Assay Description
Jurkat cell membranes were incubated with [3H]-labeled ligand in the absence or presence of increasing concentrations of test compound. The reagents ... |
J Med Chem 50: 254-63 (2007)
Article DOI: 10.1021/jm060995h
BindingDB Entry DOI: 10.7270/Q2GT5KGF |
More data for this
Ligand-Target Pair | |
Monocarboxylate transporter 1
(Homo sapiens (Human)) | BDBM21991

(5-{[(3R,4S)-3,4-dihydroxypyrrolidin-1-yl]carbonyl}...)Show SMILES CC(C)Cn1c2sc(Cc3ccnc4ccccc34)c(C(=O)N3C[C@H](O)[C@H](O)C3)c2c(=O)n(C)c1=O |r| Show InChI InChI=1S/C26H28N4O5S/c1-14(2)11-30-25-22(23(33)28(3)26(30)35)21(24(34)29-12-18(31)19(32)13-29)20(36-25)10-15-8-9-27-17-7-5-4-6-16(15)17/h4-9,14,18-19,31-32H,10-13H2,1-3H3/t18-,19+ | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 29 | -42.6 | n/a | n/a | n/a | n/a | n/a | 7.8 | 22 |
AstraZeneca
| Assay Description
Jurkat cell membranes were incubated with [3H]-labeled ligand in the absence or presence of increasing concentrations of test compound. The reagents ... |
J Med Chem 50: 254-63 (2007)
Article DOI: 10.1021/jm060995h
BindingDB Entry DOI: 10.7270/Q2GT5KGF |
More data for this
Ligand-Target Pair | |
Monocarboxylate transporter 1
(Homo sapiens (Human)) | BDBM21990
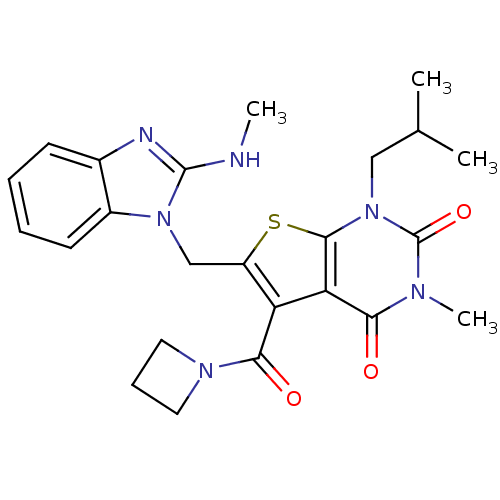
(5-(azetidin-1-ylcarbonyl)-3-methyl-6-{[2-(methylam...)Show SMILES CNc1nc2ccccc2n1Cc1sc2n(CC(C)C)c(=O)n(C)c(=O)c2c1C(=O)N1CCC1 Show InChI InChI=1S/C24H28N6O3S/c1-14(2)12-30-22-19(20(31)27(4)24(30)33)18(21(32)28-10-7-11-28)17(34-22)13-29-16-9-6-5-8-15(16)26-23(29)25-3/h5-6,8-9,14H,7,10-13H2,1-4H3,(H,25,26) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 59 | -40.8 | n/a | n/a | n/a | n/a | n/a | 7.8 | 22 |
AstraZeneca
| Assay Description
Jurkat cell membranes were incubated with [3H]-labeled ligand in the absence or presence of increasing concentrations of test compound. The reagents ... |
J Med Chem 50: 254-63 (2007)
Article DOI: 10.1021/jm060995h
BindingDB Entry DOI: 10.7270/Q2GT5KGF |
More data for this
Ligand-Target Pair | |
Monocarboxylate transporter 1
(Homo sapiens (Human)) | BDBM21988
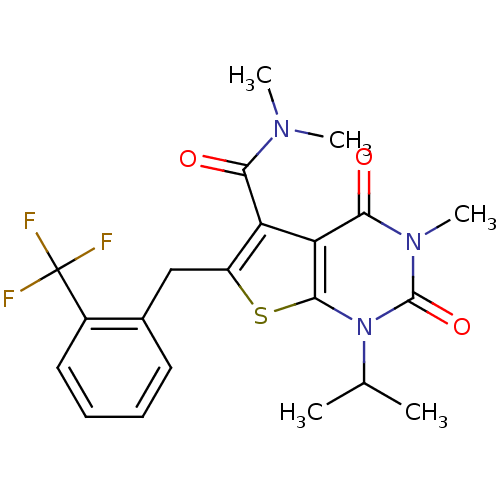
(N,N,3-trimethyl-2,4-dioxo-1-(propan-2-yl)-6-{[2-(t...)Show SMILES CC(C)n1c2sc(Cc3ccccc3C(F)(F)F)c(C(=O)N(C)C)c2c(=O)n(C)c1=O Show InChI InChI=1S/C21H22F3N3O3S/c1-11(2)27-19-16(18(29)26(5)20(27)30)15(17(28)25(3)4)14(31-19)10-12-8-6-7-9-13(12)21(22,23)24/h6-9,11H,10H2,1-5H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 117 | -39.2 | n/a | n/a | n/a | n/a | n/a | 7.8 | 22 |
AstraZeneca
| Assay Description
Jurkat cell membranes were incubated with [3H]-labeled ligand in the absence or presence of increasing concentrations of test compound. The reagents ... |
J Med Chem 50: 254-63 (2007)
Article DOI: 10.1021/jm060995h
BindingDB Entry DOI: 10.7270/Q2GT5KGF |
More data for this
Ligand-Target Pair | |
Monocarboxylate transporter 1
(Homo sapiens (Human)) | BDBM21989

(3-{[3-methyl-2,4-dioxo-1-(propan-2-yl)-6-{[2-(trif...)Show SMILES CC(C)n1c2sc(Cc3ccccc3C(F)(F)F)c(C(=O)N3CCS(=O)(=O)C3)c2c(=O)n(C)c1=O Show InChI InChI=1S/C22H22F3N3O5S2/c1-12(2)28-20-17(18(29)26(3)21(28)31)16(19(30)27-8-9-35(32,33)11-27)15(34-20)10-13-6-4-5-7-14(13)22(23,24)25/h4-7,12H,8-11H2,1-3H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| >190 | >-38.0 | n/a | n/a | n/a | n/a | n/a | 7.8 | 22 |
AstraZeneca
| Assay Description
Jurkat cell membranes were incubated with [3H]-labeled ligand in the absence or presence of increasing concentrations of test compound. The reagents ... |
J Med Chem 50: 254-63 (2007)
Article DOI: 10.1021/jm060995h
BindingDB Entry DOI: 10.7270/Q2GT5KGF |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase 1
(Homo sapiens (Human)) | BDBM50265958
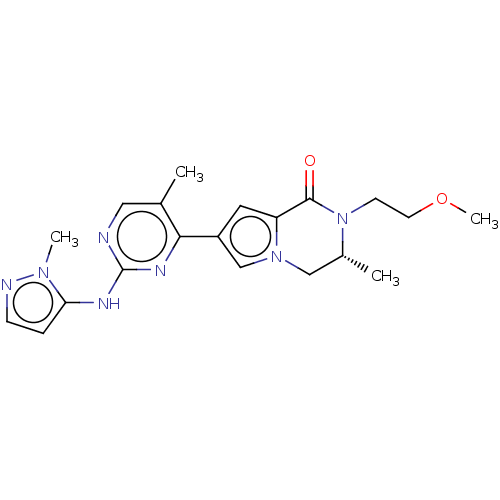
(CHEMBL4086507)Show SMILES COCCN1[C@H](C)Cn2cc(cc2C1=O)-c1nc(Nc2ccnn2C)ncc1C |r| Show InChI InChI=1S/C20H25N7O2/c1-13-10-21-20(23-17-5-6-22-25(17)3)24-18(13)15-9-16-19(28)27(7-8-29-4)14(2)11-26(16)12-15/h5-6,9-10,12,14H,7-8,11H2,1-4H3,(H,21,23,24)/t14-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of ERK2 (unknown origin) in the presence of 1 mM ATP |
J Med Chem 60: 3438-3450 (2017)
Article DOI: 10.1021/acs.jmedchem.7b00267
BindingDB Entry DOI: 10.7270/Q2FJ2K8J |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase 1
(Homo sapiens (Human)) | BDBM50265968

(CHEMBL4065992)Show SMILES COCCN1[C@H](C)Cc2[nH]c(cc2C1=O)-c1nc(Nc2ccnn2C)ncc1C |r| Show InChI InChI=1S/C20H25N7O2/c1-12-11-21-20(24-17-5-6-22-26(17)3)25-18(12)16-10-14-15(23-16)9-13(2)27(19(14)28)7-8-29-4/h5-6,10-11,13,23H,7-9H2,1-4H3,(H,21,24,25)/t13-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| n/a | n/a | <0.300 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of ERK2 (unknown origin) in the presence of 60 uM ATP |
J Med Chem 60: 3438-3450 (2017)
Article DOI: 10.1021/acs.jmedchem.7b00267
BindingDB Entry DOI: 10.7270/Q2FJ2K8J |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Mitogen-activated protein kinase 1
(Homo sapiens (Human)) | BDBM50265962
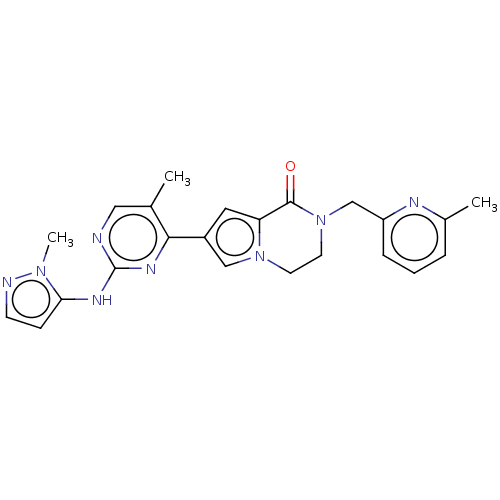
(CHEMBL4101072)Show SMILES Cc1cccc(CN2CCn3cc(cc3C2=O)-c2nc(Nc3ccnn3C)ncc2C)n1 Show InChI InChI=1S/C23H24N8O/c1-15-12-24-23(27-20-7-8-25-29(20)3)28-21(15)17-11-19-22(32)31(10-9-30(19)13-17)14-18-6-4-5-16(2)26-18/h4-8,11-13H,9-10,14H2,1-3H3,(H,24,27,28) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Patents
| Article
PubMed
| n/a | n/a | <0.300 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of ERK2 (unknown origin) in the presence of 60 uM ATP |
J Med Chem 60: 3438-3450 (2017)
Article DOI: 10.1021/acs.jmedchem.7b00267
BindingDB Entry DOI: 10.7270/Q2FJ2K8J |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase 1
(Homo sapiens (Human)) | BDBM50265961

(CHEMBL4090886)Show InChI InChI=1S/C19H23N7O2/c1-13-11-20-19(22-16-4-5-21-24(16)2)23-17(13)14-10-15-18(27)25(8-9-28-3)6-7-26(15)12-14/h4-5,10-12H,6-9H2,1-3H3,(H,20,22,23) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | <0.300 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of ERK2 (unknown origin) in the presence of 60 uM ATP |
J Med Chem 60: 3438-3450 (2017)
Article DOI: 10.1021/acs.jmedchem.7b00267
BindingDB Entry DOI: 10.7270/Q2FJ2K8J |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase 1
(Homo sapiens (Human)) | BDBM50265960

(CHEMBL4098608)Show SMILES COCCN1[C@@H](C)Cn2cc(cc2C1=O)-c1nc(Nc2ccnn2C)ncc1C |r| Show InChI InChI=1S/C20H25N7O2/c1-13-10-21-20(23-17-5-6-22-25(17)3)24-18(13)15-9-16-19(28)27(7-8-29-4)14(2)11-26(16)12-15/h5-6,9-10,12,14H,7-8,11H2,1-4H3,(H,21,23,24)/t14-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | <0.300 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of ERK2 (unknown origin) in the presence of 60 uM ATP |
J Med Chem 60: 3438-3450 (2017)
Article DOI: 10.1021/acs.jmedchem.7b00267
BindingDB Entry DOI: 10.7270/Q2FJ2K8J |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase 1
(Homo sapiens (Human)) | BDBM50265959
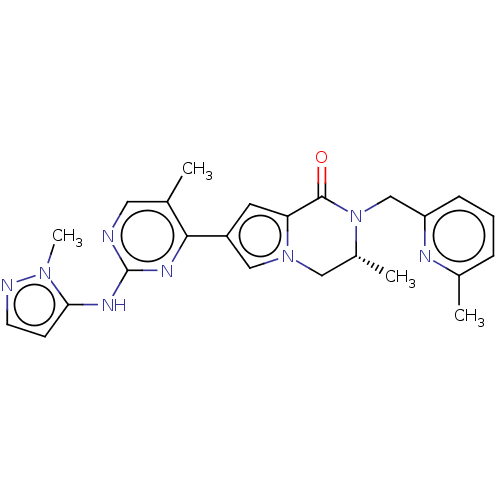
(CHEMBL4071576)Show SMILES C[C@@H]1Cn2cc(cc2C(=O)N1Cc1cccc(C)n1)-c1nc(Nc2ccnn2C)ncc1C |r| Show InChI InChI=1S/C24H26N8O/c1-15-11-25-24(28-21-8-9-26-30(21)4)29-22(15)18-10-20-23(33)32(17(3)12-31(20)13-18)14-19-7-5-6-16(2)27-19/h5-11,13,17H,12,14H2,1-4H3,(H,25,28,29)/t17-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | <0.300 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of ERK2 (unknown origin) in the presence of 60 uM ATP |
J Med Chem 60: 3438-3450 (2017)
Article DOI: 10.1021/acs.jmedchem.7b00267
BindingDB Entry DOI: 10.7270/Q2FJ2K8J |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase 1
(Homo sapiens (Human)) | BDBM50265958

(CHEMBL4086507)Show SMILES COCCN1[C@H](C)Cn2cc(cc2C1=O)-c1nc(Nc2ccnn2C)ncc1C |r| Show InChI InChI=1S/C20H25N7O2/c1-13-10-21-20(23-17-5-6-22-25(17)3)24-18(13)15-9-16-19(28)27(7-8-29-4)14(2)11-26(16)12-15/h5-6,9-10,12,14H,7-8,11H2,1-4H3,(H,21,23,24)/t14-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | <0.300 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of ERK2 (unknown origin) in the presence of 60 uM ATP |
J Med Chem 60: 3438-3450 (2017)
Article DOI: 10.1021/acs.jmedchem.7b00267
BindingDB Entry DOI: 10.7270/Q2FJ2K8J |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase 1
(Homo sapiens (Human)) | BDBM50094465
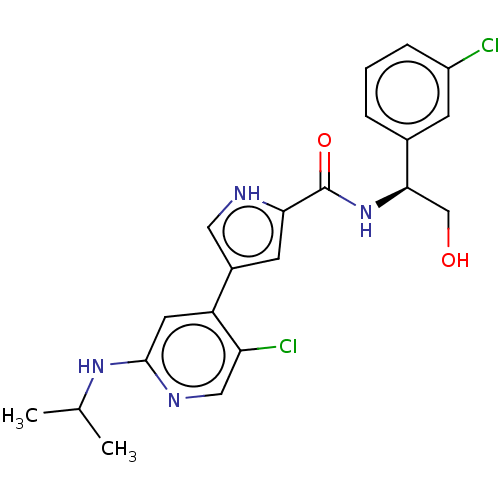
(CHEMBL3590106 | US10525036, Example BVD-523 | US10...)Show SMILES CC(C)Nc1cc(-c2c[nH]c(c2)C(=O)N[C@H](CO)c2cccc(Cl)c2)c(Cl)cn1 |r| Show InChI InChI=1S/C19H21NO3/c21-18(23-17-11-13-20-14-12-17)19(22,15-7-3-1-4-8-15)16-9-5-2-6-10-16/h1-10,17,20,22H,11-14H2 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| n/a | n/a | <0.300 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of ERK2 (unknown origin) in the presence of 1 mM ATP |
J Med Chem 60: 3438-3450 (2017)
Article DOI: 10.1021/acs.jmedchem.7b00267
BindingDB Entry DOI: 10.7270/Q2FJ2K8J |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Mitogen-activated protein kinase 1
(Homo sapiens (Human)) | BDBM50265934

(CHEMBL4060050)Show SMILES COCCN1[C@@H](C)Cc2[nH]c(cc2C1=O)-c1ccnc(Nc2ccnn2C)n1 |r| Show InChI InChI=1S/C19H23N7O2/c1-12-10-15-13(18(27)26(12)8-9-28-3)11-16(22-15)14-4-6-20-19(23-14)24-17-5-7-21-25(17)2/h4-7,11-12,22H,8-10H2,1-3H3,(H,20,23,24)/t12-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | <0.300 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of ERK2 (unknown origin) in the presence of 60 uM ATP |
J Med Chem 60: 3438-3450 (2017)
Article DOI: 10.1021/acs.jmedchem.7b00267
BindingDB Entry DOI: 10.7270/Q2FJ2K8J |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase 1
(Homo sapiens (Human)) | BDBM50265962

(CHEMBL4101072)Show SMILES Cc1cccc(CN2CCn3cc(cc3C2=O)-c2nc(Nc3ccnn3C)ncc2C)n1 Show InChI InChI=1S/C23H24N8O/c1-15-12-24-23(27-20-7-8-25-29(20)3)28-21(15)17-11-19-22(32)31(10-9-30(19)13-17)14-18-6-4-5-16(2)26-18/h4-8,11-13H,9-10,14H2,1-3H3,(H,24,27,28) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Patents
| Article
PubMed
| n/a | n/a | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of ERK2 (unknown origin) in the presence of 1 mM ATP |
J Med Chem 60: 3438-3450 (2017)
Article DOI: 10.1021/acs.jmedchem.7b00267
BindingDB Entry DOI: 10.7270/Q2FJ2K8J |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase 1
(Homo sapiens (Human)) | BDBM50265971

(CHEMBL4087393)Show SMILES COCCN1[C@@H](C)Cc2[nH]c(cc2C1=O)-c1nc(Nc2ccnn2C)ncc1C |r| Show InChI InChI=1S/C20H25N7O2/c1-12-11-21-20(24-17-5-6-22-26(17)3)25-18(12)16-10-14-15(23-16)9-13(2)27(19(14)28)7-8-29-4/h5-6,10-11,13,23H,7-9H2,1-4H3,(H,21,24,25)/t13-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| n/a | n/a | <0.300 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of ERK2 (unknown origin) in the presence of 60 uM ATP |
J Med Chem 60: 3438-3450 (2017)
Article DOI: 10.1021/acs.jmedchem.7b00267
BindingDB Entry DOI: 10.7270/Q2FJ2K8J |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Mitogen-activated protein kinase 1
(Homo sapiens (Human)) | BDBM50265973

(CHEMBL4075638)Show SMILES C[C@H]1Cn2cc(cc2C(=O)N1Cc1cccc(C)n1)-c1nc(Nc2ccnn2C)ncc1C |r| Show InChI InChI=1S/C24H26N8O/c1-15-11-25-24(28-21-8-9-26-30(21)4)29-22(15)18-10-20-23(33)32(17(3)12-31(20)13-18)14-19-7-5-6-16(2)27-19/h5-11,13,17H,12,14H2,1-4H3,(H,25,28,29)/t17-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | <0.300 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of ERK2 (unknown origin) in the presence of 60 uM ATP |
J Med Chem 60: 3438-3450 (2017)
Article DOI: 10.1021/acs.jmedchem.7b00267
BindingDB Entry DOI: 10.7270/Q2FJ2K8J |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase 1
(Homo sapiens (Human)) | BDBM50265960

(CHEMBL4098608)Show SMILES COCCN1[C@@H](C)Cn2cc(cc2C1=O)-c1nc(Nc2ccnn2C)ncc1C |r| Show InChI InChI=1S/C20H25N7O2/c1-13-10-21-20(23-17-5-6-22-25(17)3)24-18(13)15-9-16-19(28)27(7-8-29-4)14(2)11-26(16)12-15/h5-6,9-10,12,14H,7-8,11H2,1-4H3,(H,21,23,24)/t14-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | <0.300 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of ERK2 (unknown origin) in the presence of 1 mM ATP |
J Med Chem 60: 3438-3450 (2017)
Article DOI: 10.1021/acs.jmedchem.7b00267
BindingDB Entry DOI: 10.7270/Q2FJ2K8J |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase 1
(Homo sapiens (Human)) | BDBM50265961

(CHEMBL4090886)Show InChI InChI=1S/C19H23N7O2/c1-13-11-20-19(22-16-4-5-21-24(16)2)23-17(13)14-10-15-18(27)25(8-9-28-3)6-7-26(15)12-14/h4-5,10-12H,6-9H2,1-3H3,(H,20,22,23) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | <0.300 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of ERK2 (unknown origin) in the presence of 1 mM ATP |
J Med Chem 60: 3438-3450 (2017)
Article DOI: 10.1021/acs.jmedchem.7b00267
BindingDB Entry DOI: 10.7270/Q2FJ2K8J |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase 1
(Homo sapiens (Human)) | BDBM50265968

(CHEMBL4065992)Show SMILES COCCN1[C@H](C)Cc2[nH]c(cc2C1=O)-c1nc(Nc2ccnn2C)ncc1C |r| Show InChI InChI=1S/C20H25N7O2/c1-12-11-21-20(24-17-5-6-22-26(17)3)25-18(12)16-10-14-15(23-16)9-13(2)27(19(14)28)7-8-29-4/h5-6,10-11,13,23H,7-9H2,1-4H3,(H,21,24,25)/t13-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| n/a | n/a | <0.300 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of ERK2 (unknown origin) in the presence of 1 mM ATP |
J Med Chem 60: 3438-3450 (2017)
Article DOI: 10.1021/acs.jmedchem.7b00267
BindingDB Entry DOI: 10.7270/Q2FJ2K8J |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Mitogen-activated protein kinase 1
(Homo sapiens (Human)) | BDBM50265971

(CHEMBL4087393)Show SMILES COCCN1[C@@H](C)Cc2[nH]c(cc2C1=O)-c1nc(Nc2ccnn2C)ncc1C |r| Show InChI InChI=1S/C20H25N7O2/c1-12-11-21-20(24-17-5-6-22-26(17)3)25-18(12)16-10-14-15(23-16)9-13(2)27(19(14)28)7-8-29-4/h5-6,10-11,13,23H,7-9H2,1-4H3,(H,21,24,25)/t13-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| n/a | n/a | <0.300 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of ERK2 (unknown origin) in the presence of 1 mM ATP |
J Med Chem 60: 3438-3450 (2017)
Article DOI: 10.1021/acs.jmedchem.7b00267
BindingDB Entry DOI: 10.7270/Q2FJ2K8J |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Mitogen-activated protein kinase 1
(Homo sapiens (Human)) | BDBM50265970

(CHEMBL4097186)Show SMILES O=C1N(Cc2ccccc2)CCc2[nH]c(cc12)-c1ccnc(NC2CCOCC2)n1 Show InChI InChI=1S/C23H25N5O2/c29-22-18-14-21(20-6-10-24-23(27-20)25-17-8-12-30-13-9-17)26-19(18)7-11-28(22)15-16-4-2-1-3-5-16/h1-6,10,14,17,26H,7-9,11-13,15H2,(H,24,25,27) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| n/a | n/a | <0.300 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of ERK2 (unknown origin) in the presence of 1 mM ATP |
J Med Chem 60: 3438-3450 (2017)
Article DOI: 10.1021/acs.jmedchem.7b00267
BindingDB Entry DOI: 10.7270/Q2FJ2K8J |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Mitogen-activated protein kinase 1
(Homo sapiens (Human)) | BDBM50265938
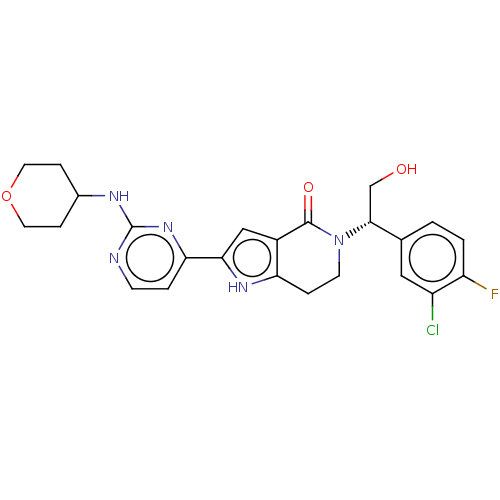
(CHEMBL4096522)Show SMILES OC[C@@H](N1CCc2[nH]c(cc2C1=O)-c1ccnc(NC2CCOCC2)n1)c1ccc(F)c(Cl)c1 |r| Show InChI InChI=1S/C24H25ClFN5O3/c25-17-11-14(1-2-18(17)26)22(13-32)31-8-4-19-16(23(31)33)12-21(29-19)20-3-7-27-24(30-20)28-15-5-9-34-10-6-15/h1-3,7,11-12,15,22,29,32H,4-6,8-10,13H2,(H,27,28,30)/t22-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | <0.310 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of ERK2 (unknown origin) in the presence of 60 uM ATP |
J Med Chem 60: 3438-3450 (2017)
Article DOI: 10.1021/acs.jmedchem.7b00267
BindingDB Entry DOI: 10.7270/Q2FJ2K8J |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase 1
(Homo sapiens (Human)) | BDBM50265970

(CHEMBL4097186)Show SMILES O=C1N(Cc2ccccc2)CCc2[nH]c(cc12)-c1ccnc(NC2CCOCC2)n1 Show InChI InChI=1S/C23H25N5O2/c29-22-18-14-21(20-6-10-24-23(27-20)25-17-8-12-30-13-9-17)26-19(18)7-11-28(22)15-16-4-2-1-3-5-16/h1-6,10,14,17,26H,7-9,11-13,15H2,(H,24,25,27) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| n/a | n/a | <0.320 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of ERK2 (unknown origin) in the presence of 60 uM ATP |
J Med Chem 60: 3438-3450 (2017)
Article DOI: 10.1021/acs.jmedchem.7b00267
BindingDB Entry DOI: 10.7270/Q2FJ2K8J |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Mitogen-activated protein kinase 1
(Homo sapiens (Human)) | BDBM50265934

(CHEMBL4060050)Show SMILES COCCN1[C@@H](C)Cc2[nH]c(cc2C1=O)-c1ccnc(Nc2ccnn2C)n1 |r| Show InChI InChI=1S/C19H23N7O2/c1-12-10-15-13(18(27)26(12)8-9-28-3)11-16(22-15)14-4-6-20-19(23-14)24-17-5-7-21-25(17)2/h4-7,11-12,22H,8-10H2,1-3H3,(H,20,23,24)/t12-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | <0.330 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of ERK2 (unknown origin) in the presence of 1 mM ATP |
J Med Chem 60: 3438-3450 (2017)
Article DOI: 10.1021/acs.jmedchem.7b00267
BindingDB Entry DOI: 10.7270/Q2FJ2K8J |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase 1
(Homo sapiens (Human)) | BDBM50265939

(CHEMBL4078489)Show SMILES OC[C@@H](N1CCc2[nH]c(cc2C1=O)-c1ccnc(NC2CCOCC2)n1)c1ccccc1 |r| Show InChI InChI=1S/C24H27N5O3/c30-15-22(16-4-2-1-3-5-16)29-11-7-19-18(23(29)31)14-21(27-19)20-6-10-25-24(28-20)26-17-8-12-32-13-9-17/h1-6,10,14,17,22,27,30H,7-9,11-13,15H2,(H,25,26,28)/t22-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | <0.340 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of ERK2 (unknown origin) in the presence of 60 uM ATP |
J Med Chem 60: 3438-3450 (2017)
Article DOI: 10.1021/acs.jmedchem.7b00267
BindingDB Entry DOI: 10.7270/Q2FJ2K8J |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase 1
(Homo sapiens (Human)) | BDBM50265963
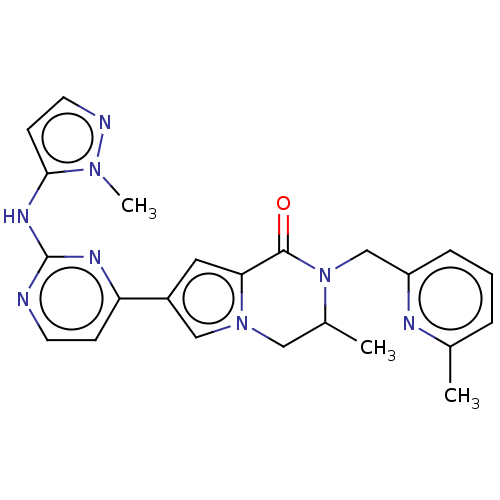
(CHEMBL4083098)Show SMILES CC1Cn2cc(cc2C(=O)N1Cc1cccc(C)n1)-c1ccnc(Nc2ccnn2C)n1 Show InChI InChI=1S/C23H24N8O/c1-15-5-4-6-18(26-15)14-31-16(2)12-30-13-17(11-20(30)22(31)32)19-7-9-24-23(27-19)28-21-8-10-25-29(21)3/h4-11,13,16H,12,14H2,1-3H3,(H,24,27,28) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | <0.390 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of ERK2 (unknown origin) in the presence of 60 uM ATP |
J Med Chem 60: 3438-3450 (2017)
Article DOI: 10.1021/acs.jmedchem.7b00267
BindingDB Entry DOI: 10.7270/Q2FJ2K8J |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase 1
(Homo sapiens (Human)) | BDBM50265939

(CHEMBL4078489)Show SMILES OC[C@@H](N1CCc2[nH]c(cc2C1=O)-c1ccnc(NC2CCOCC2)n1)c1ccccc1 |r| Show InChI InChI=1S/C24H27N5O3/c30-15-22(16-4-2-1-3-5-16)29-11-7-19-18(23(29)31)14-21(27-19)20-6-10-25-24(28-20)26-17-8-12-32-13-9-17/h1-6,10,14,17,22,27,30H,7-9,11-13,15H2,(H,25,26,28)/t22-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.430 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of ERK2 (unknown origin) in the presence of 1 mM ATP |
J Med Chem 60: 3438-3450 (2017)
Article DOI: 10.1021/acs.jmedchem.7b00267
BindingDB Entry DOI: 10.7270/Q2FJ2K8J |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase 1
(Homo sapiens (Human)) | BDBM50265936

(CHEMBL4068705)Show SMILES Cc1cccc(CN2CCc3[nH]c(cc3C2=O)-c2ccnc(NC3CCOCC3)n2)n1 Show InChI InChI=1S/C23H26N6O2/c1-15-3-2-4-17(25-15)14-29-10-6-19-18(22(29)30)13-21(27-19)20-5-9-24-23(28-20)26-16-7-11-31-12-8-16/h2-5,9,13,16,27H,6-8,10-12,14H2,1H3,(H,24,26,28) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.470 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of ERK2 (unknown origin) in the presence of 60 uM ATP |
J Med Chem 60: 3438-3450 (2017)
Article DOI: 10.1021/acs.jmedchem.7b00267
BindingDB Entry DOI: 10.7270/Q2FJ2K8J |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase 1
(Homo sapiens (Human)) | BDBM50265966

(CHEMBL4103768)Show SMILES Cn1c2CCN(Cc3ccccc3)C(=O)c2cc1-c1ccnc(NC2CCOCC2)n1 Show InChI InChI=1S/C24H27N5O2/c1-28-21-8-12-29(16-17-5-3-2-4-6-17)23(30)19(21)15-22(28)20-7-11-25-24(27-20)26-18-9-13-31-14-10-18/h2-7,11,15,18H,8-10,12-14,16H2,1H3,(H,25,26,27) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | <0.480 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of ERK2 (unknown origin) in the presence of 60 uM ATP |
J Med Chem 60: 3438-3450 (2017)
Article DOI: 10.1021/acs.jmedchem.7b00267
BindingDB Entry DOI: 10.7270/Q2FJ2K8J |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase 1
(Homo sapiens (Human)) | BDBM50265959

(CHEMBL4071576)Show SMILES C[C@@H]1Cn2cc(cc2C(=O)N1Cc1cccc(C)n1)-c1nc(Nc2ccnn2C)ncc1C |r| Show InChI InChI=1S/C24H26N8O/c1-15-11-25-24(28-21-8-9-26-30(21)4)29-22(15)18-10-20-23(33)32(17(3)12-31(20)13-18)14-19-7-5-6-16(2)27-19/h5-11,13,17H,12,14H2,1-4H3,(H,25,28,29)/t17-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of ERK2 (unknown origin) in the presence of 1 mM ATP |
J Med Chem 60: 3438-3450 (2017)
Article DOI: 10.1021/acs.jmedchem.7b00267
BindingDB Entry DOI: 10.7270/Q2FJ2K8J |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase 1
(Homo sapiens (Human)) | BDBM50265938

(CHEMBL4096522)Show SMILES OC[C@@H](N1CCc2[nH]c(cc2C1=O)-c1ccnc(NC2CCOCC2)n1)c1ccc(F)c(Cl)c1 |r| Show InChI InChI=1S/C24H25ClFN5O3/c25-17-11-14(1-2-18(17)26)22(13-32)31-8-4-19-16(23(31)33)12-21(29-19)20-3-7-27-24(30-20)28-15-5-9-34-10-6-15/h1-3,7,11-12,15,22,29,32H,4-6,8-10,13H2,(H,27,28,30)/t22-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.720 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of ERK2 (unknown origin) in the presence of 1 mM ATP |
J Med Chem 60: 3438-3450 (2017)
Article DOI: 10.1021/acs.jmedchem.7b00267
BindingDB Entry DOI: 10.7270/Q2FJ2K8J |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase 1
(Homo sapiens (Human)) | BDBM50265973

(CHEMBL4075638)Show SMILES C[C@H]1Cn2cc(cc2C(=O)N1Cc1cccc(C)n1)-c1nc(Nc2ccnn2C)ncc1C |r| Show InChI InChI=1S/C24H26N8O/c1-15-11-25-24(28-21-8-9-26-30(21)4)29-22(15)18-10-20-23(33)32(17(3)12-31(20)13-18)14-19-7-5-6-16(2)27-19/h5-11,13,17H,12,14H2,1-4H3,(H,25,28,29)/t17-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.900 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of ERK2 (unknown origin) in the presence of 1 mM ATP |
J Med Chem 60: 3438-3450 (2017)
Article DOI: 10.1021/acs.jmedchem.7b00267
BindingDB Entry DOI: 10.7270/Q2FJ2K8J |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase 1
(Homo sapiens (Human)) | BDBM50265936

(CHEMBL4068705)Show SMILES Cc1cccc(CN2CCc3[nH]c(cc3C2=O)-c2ccnc(NC3CCOCC3)n2)n1 Show InChI InChI=1S/C23H26N6O2/c1-15-3-2-4-17(25-15)14-29-10-6-19-18(22(29)30)13-21(27-19)20-5-9-24-23(28-20)26-16-7-11-31-12-8-16/h2-5,9,13,16,27H,6-8,10-12,14H2,1H3,(H,24,26,28) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of ERK2 (unknown origin) in the presence of 1 mM ATP |
J Med Chem 60: 3438-3450 (2017)
Article DOI: 10.1021/acs.jmedchem.7b00267
BindingDB Entry DOI: 10.7270/Q2FJ2K8J |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase 1
(Homo sapiens (Human)) | BDBM50265972

(CHEMBL4074437)Show SMILES COCCN1CCc2[nH]c(cc2C1=O)-c1ccnc(NC2CCOCC2)n1 Show InChI InChI=1S/C19H25N5O3/c1-26-11-8-24-7-3-15-14(18(24)25)12-17(22-15)16-2-6-20-19(23-16)21-13-4-9-27-10-5-13/h2,6,12-13,22H,3-5,7-11H2,1H3,(H,20,21,23) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| n/a | n/a | <1.10 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of ERK2 (unknown origin) in the presence of 60 uM ATP |
J Med Chem 60: 3438-3450 (2017)
Article DOI: 10.1021/acs.jmedchem.7b00267
BindingDB Entry DOI: 10.7270/Q2FJ2K8J |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Mitogen-activated protein kinase 1
(Homo sapiens (Human)) | BDBM50265937
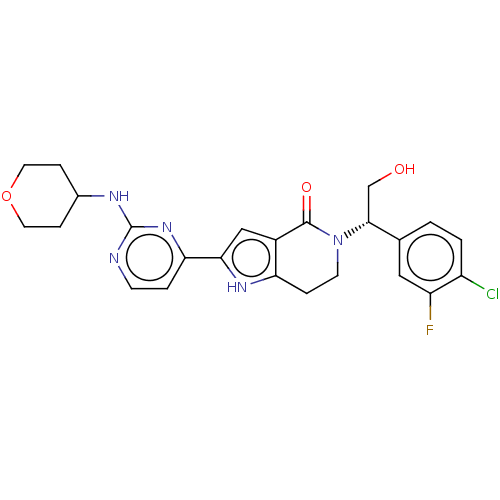
(CHEMBL4100076)Show SMILES OC[C@@H](N1CCc2[nH]c(cc2C1=O)-c1ccnc(NC2CCOCC2)n1)c1ccc(Cl)c(F)c1 |r| Show InChI InChI=1S/C24H25ClFN5O3/c25-17-2-1-14(11-18(17)26)22(13-32)31-8-4-19-16(23(31)33)12-21(29-19)20-3-7-27-24(30-20)28-15-5-9-34-10-6-15/h1-3,7,11-12,15,22,29,32H,4-6,8-10,13H2,(H,27,28,30)/t22-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.10 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of ERK2 (unknown origin) in the presence of 60 uM ATP |
J Med Chem 60: 3438-3450 (2017)
Article DOI: 10.1021/acs.jmedchem.7b00267
BindingDB Entry DOI: 10.7270/Q2FJ2K8J |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase 1
(Homo sapiens (Human)) | BDBM50265956

(CHEMBL4080944)Show InChI InChI=1S/C16H19N5O2/c22-15-11-9-14(20-12(11)1-5-17-15)13-2-6-18-16(21-13)19-10-3-7-23-8-4-10/h2,6,9-10,20H,1,3-5,7-8H2,(H,17,22)(H,18,19,21) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.20 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of ERK2 (unknown origin) in the presence of 60 uM ATP |
J Med Chem 60: 3438-3450 (2017)
Article DOI: 10.1021/acs.jmedchem.7b00267
BindingDB Entry DOI: 10.7270/Q2FJ2K8J |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase 1
(Homo sapiens (Human)) | BDBM50265967

(CHEMBL4079515)Show InChI InChI=1S/C17H21N5O2/c1-22-14-3-7-18-16(23)12(14)10-15(22)13-2-6-19-17(21-13)20-11-4-8-24-9-5-11/h2,6,10-11H,3-5,7-9H2,1H3,(H,18,23)(H,19,20,21) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.40 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of ERK2 (unknown origin) in the presence of 60 uM ATP |
J Med Chem 60: 3438-3450 (2017)
Article DOI: 10.1021/acs.jmedchem.7b00267
BindingDB Entry DOI: 10.7270/Q2FJ2K8J |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase 1
(Homo sapiens (Human)) | BDBM50265966

(CHEMBL4103768)Show SMILES Cn1c2CCN(Cc3ccccc3)C(=O)c2cc1-c1ccnc(NC2CCOCC2)n1 Show InChI InChI=1S/C24H27N5O2/c1-28-21-8-12-29(16-17-5-3-2-4-6-17)23(30)19(21)15-22(28)20-7-11-25-24(27-20)26-18-9-13-31-14-10-18/h2-7,11,15,18H,8-10,12-14,16H2,1H3,(H,25,26,27) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.60 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of ERK2 (unknown origin) in the presence of 1 mM ATP |
J Med Chem 60: 3438-3450 (2017)
Article DOI: 10.1021/acs.jmedchem.7b00267
BindingDB Entry DOI: 10.7270/Q2FJ2K8J |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase 1
(Homo sapiens (Human)) | BDBM50265969
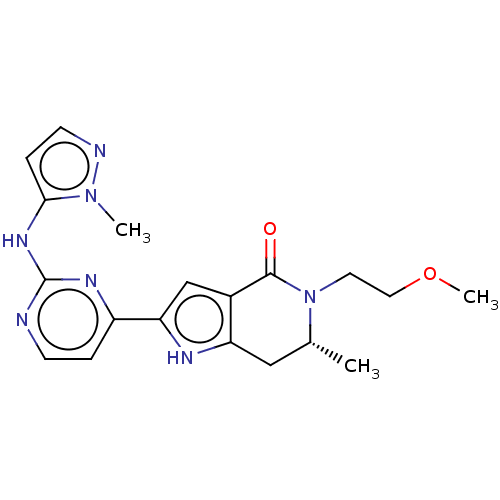
(CHEMBL4088284)Show SMILES COCCN1[C@H](C)Cc2[nH]c(cc2C1=O)-c1ccnc(Nc2ccnn2C)n1 |r| Show InChI InChI=1S/C19H23N7O2/c1-12-10-15-13(18(27)26(12)8-9-28-3)11-16(22-15)14-4-6-20-19(23-14)24-17-5-7-21-25(17)2/h4-7,11-12,22H,8-10H2,1-3H3,(H,20,23,24)/t12-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.90 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of ERK2 (unknown origin) in the presence of 60 uM ATP |
J Med Chem 60: 3438-3450 (2017)
Article DOI: 10.1021/acs.jmedchem.7b00267
BindingDB Entry DOI: 10.7270/Q2FJ2K8J |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase 1
(Homo sapiens (Human)) | BDBM50265935

(CHEMBL4074311)Show SMILES COCCN1CCc2[nH]c(cc2C1=O)-c1ccnc(Nc2ccnn2C)n1 Show InChI InChI=1S/C18H21N7O2/c1-24-16(4-7-20-24)23-18-19-6-3-14(22-18)15-11-12-13(21-15)5-8-25(17(12)26)9-10-27-2/h3-4,6-7,11,21H,5,8-10H2,1-2H3,(H,19,22,23) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| n/a | n/a | 2.5 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of ERK2 (unknown origin) in the presence of 1 mM ATP |
J Med Chem 60: 3438-3450 (2017)
Article DOI: 10.1021/acs.jmedchem.7b00267
BindingDB Entry DOI: 10.7270/Q2FJ2K8J |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Mitogen-activated protein kinase 1
(Homo sapiens (Human)) | BDBM50265974

(CHEMBL4082931)Show SMILES COCCN1CCc2c(cc(-c3ccnc(NC4CCOCC4)n3)n2C)C1=O Show InChI InChI=1S/C20H27N5O3/c1-24-17-4-8-25(9-12-27-2)19(26)15(17)13-18(24)16-3-7-21-20(23-16)22-14-5-10-28-11-6-14/h3,7,13-14H,4-6,8-12H2,1-2H3,(H,21,22,23) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.70 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of ERK2 (unknown origin) in the presence of 60 uM ATP |
J Med Chem 60: 3438-3450 (2017)
Article DOI: 10.1021/acs.jmedchem.7b00267
BindingDB Entry DOI: 10.7270/Q2FJ2K8J |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data