

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Genome polyprotein/Non-structural protein 4A (Hepatitis C virus) | BDBM50110121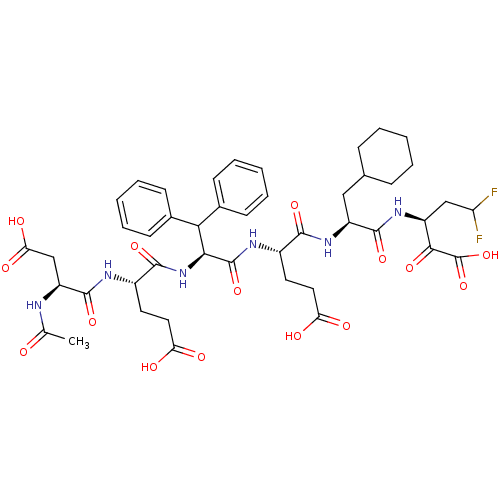 (3-[2-(2-{2-[2-(2-Acetylamino-3-carboxy-propionylam...) | PDB UniProtKB/TrEMBL GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.0100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
IRBM, MRL Rome Curated by ChEMBL | Assay Description Inhibitory activity against hepatitis C virus (HCV) NS3/NS4A serine protease | Bioorg Med Chem Lett 12: 705-8 (2002) BindingDB Entry DOI: 10.7270/Q24X572V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Platelet-activating factor acetylhydrolase (Homo sapiens (Human)) | BDBM50117772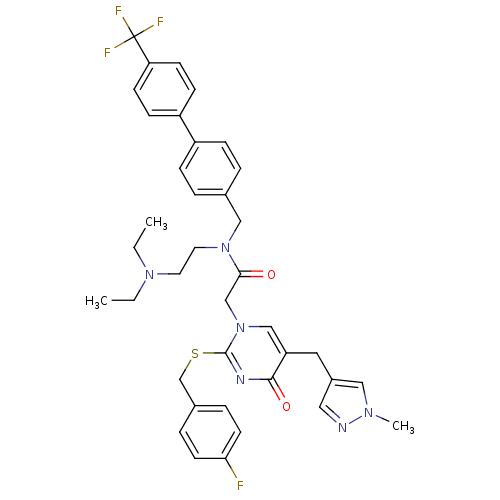 (CHEMBL10921 | N-(2-Diethylamino-ethyl)-2-[2-(4-flu...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | PubMed | 0.0300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Steady state and transient kinetics to a freely reversible, non-covalently bound, human recombinant Phospholipase A2 (rhLp-PLA2) was determined | Bioorg Med Chem Lett 12: 2603-6 (2002) BindingDB Entry DOI: 10.7270/Q2G44PNN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Platelet-activating factor acetylhydrolase (Homo sapiens (Human)) | BDBM50125265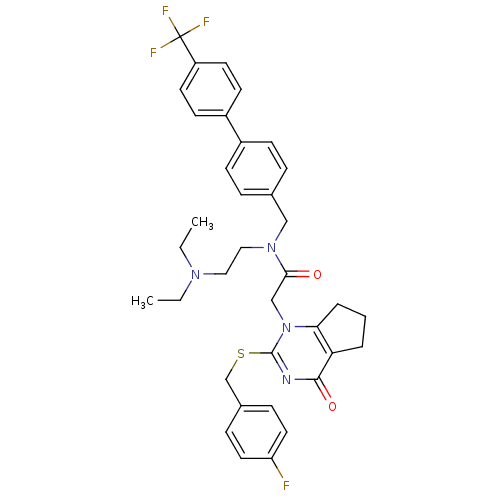 (CHEMBL204021 | N-(2-Diethylamino-ethyl)-2-[2-(4-fl...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid PDB UniChem Patents Similars | PDB PubMed | 0.110 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Inhibitory activity against recombinant human Lp-PLA2 | Bioorg Med Chem Lett 13: 1067-70 (2003) BindingDB Entry DOI: 10.7270/Q2S75FPD | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Genome polyprotein/Non-structural protein 4A (Hepatitis C virus) | BDBM50110117 (4-(2-Acetylamino-3-carboxy-propionylamino)-4-(1-{3...) | PDB UniProtKB/TrEMBL GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
IRBM, MRL Rome Curated by ChEMBL | Assay Description Inhibitory activity against hepatitis C virus (HCV) NS3/NS4A serine protease | Bioorg Med Chem Lett 12: 705-8 (2002) BindingDB Entry DOI: 10.7270/Q24X572V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein/Non-structural protein 4A (Hepatitis C virus) | BDBM50150997 (4-{2-[(S)-4,4-Difluoro-2-({(2S,4R)-1-((S)-2-isobut...) | PDB UniProtKB/TrEMBL GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibitory activity against Hepatitis C virus Non structural protein 3 serine protease/Non structural protein 4A serine protease | Bioorg Med Chem Lett 14: 4575-9 (2004) Article DOI: 10.1016/j.bmcl.2004.05.093 BindingDB Entry DOI: 10.7270/Q27S7PHC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein/Non-structural protein 4A (Hepatitis C virus) | BDBM50150993 (4-[2-(4,4-Difluoro-2-{[(S)-(R)-1-(2-isobutoxycarbo...) | PDB UniProtKB/TrEMBL GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibitory activity against Hepatitis C virus Non structural protein 3 serine protease/Non structural protein 4A serine protease | Bioorg Med Chem Lett 14: 4575-9 (2004) Article DOI: 10.1016/j.bmcl.2004.05.093 BindingDB Entry DOI: 10.7270/Q27S7PHC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein/Non-structural protein 4A (Hepatitis C virus) | BDBM50110125 (2-[2-(2-{2-[2-(2-Acetylamino-3-carboxy-propionylam...) | PDB UniProtKB/TrEMBL GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
IRBM, MRL Rome Curated by ChEMBL | Assay Description Inhibitory activity against hepatitis C virus (HCV) NS3/NS4A serine protease | Bioorg Med Chem Lett 12: 705-8 (2002) BindingDB Entry DOI: 10.7270/Q24X572V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Platelet-activating factor acetylhydrolase (Homo sapiens (Human)) | BDBM50085629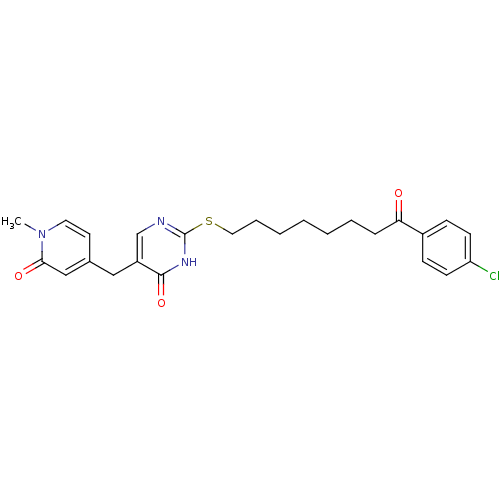 (2-[8-(4-Chloro-phenyl)-8-oxo-octylsulfanyl]-5-(1-m...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 41 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
SmithKline Beecham Pharmaceuticals Curated by ChEMBL | Assay Description The compound was tested for inhibition of Lipoprotein-associated phospholipase A2 (Lp-PLA2) | Bioorg Med Chem Lett 10: 395-8 (2000) BindingDB Entry DOI: 10.7270/Q25X285S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein/Non-structural protein 4A (Hepatitis C virus) | BDBM50150992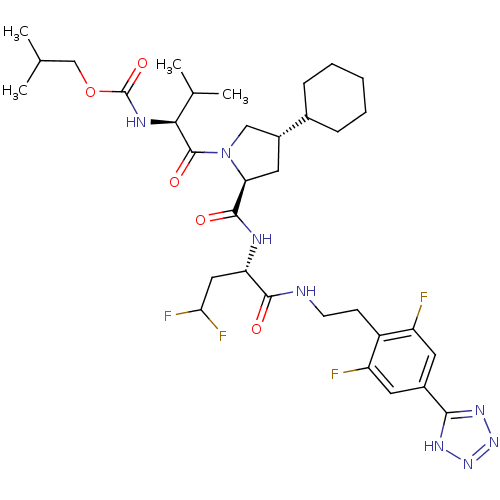 (CHEMBL366279 | {(S)-1-[(2S,4S)-4-Cyclohexyl-2-((S)...) | PDB UniProtKB/TrEMBL GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | 100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibitory activity against Hepatitis C virus Non structural protein 3 serine protease/Non structural protein 4A serine protease | Bioorg Med Chem Lett 14: 4575-9 (2004) Article DOI: 10.1016/j.bmcl.2004.05.093 BindingDB Entry DOI: 10.7270/Q27S7PHC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein/Non-structural protein 4A (Hepatitis C virus) | BDBM50150991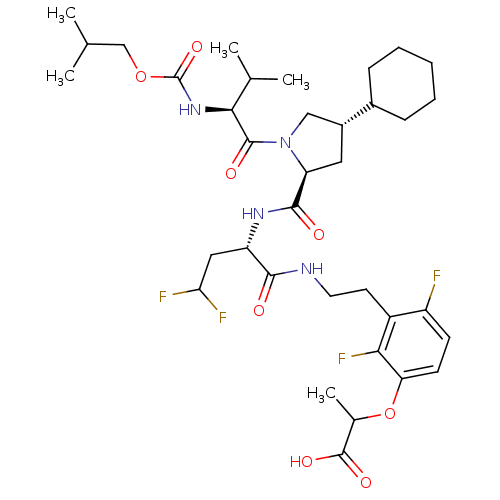 (2-{3-[2-((S)-2-{[(2S,4S)-4-Cyclohexyl-1-((S)-2-iso...) | PDB UniProtKB/TrEMBL GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 120 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibitory activity against Hepatitis C virus Non structural protein 3 serine protease/Non structural protein 4A serine protease | Bioorg Med Chem Lett 14: 4575-9 (2004) Article DOI: 10.1016/j.bmcl.2004.05.093 BindingDB Entry DOI: 10.7270/Q27S7PHC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein/Non-structural protein 4A (Hepatitis C virus) | BDBM50150989 (CHEMBL362406 | {3-[2-((S)-2-{[(2S,4S)-4-Cyclohexyl...) | PDB UniProtKB/TrEMBL GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 190 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibitory activity against Hepatitis C virus Non structural protein 3 serine protease/Non structural protein 4A serine protease | Bioorg Med Chem Lett 14: 4575-9 (2004) Article DOI: 10.1016/j.bmcl.2004.05.093 BindingDB Entry DOI: 10.7270/Q27S7PHC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein/Non-structural protein 4A (Hepatitis C virus) | BDBM50150990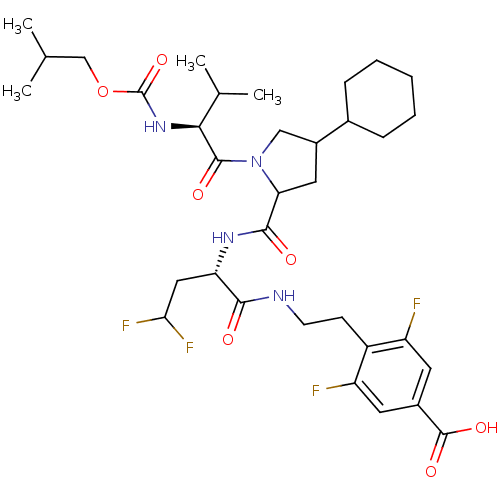 (4-[2-((S)-2-{[(R)-4-Cyclohexyl-1-((S)-2-isobutoxyc...) | PDB UniProtKB/TrEMBL GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 350 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibitory activity against Hepatitis C virus Non structural protein 3 serine protease/Non structural protein 4A serine protease | Bioorg Med Chem Lett 14: 4575-9 (2004) Article DOI: 10.1016/j.bmcl.2004.05.093 BindingDB Entry DOI: 10.7270/Q27S7PHC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein/Non-structural protein 4A (Hepatitis C virus) | BDBM50150986 ((E)-3-{3-[2-((S)-4,4-Difluoro-2-{[(2S,4S)-1-((S)-2...) | PDB UniProtKB/TrEMBL GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 350 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibitory activity against Hepatitis C virus Non structural protein 3 serine protease/Non structural protein 4A serine protease | Bioorg Med Chem Lett 14: 4575-9 (2004) Article DOI: 10.1016/j.bmcl.2004.05.093 BindingDB Entry DOI: 10.7270/Q27S7PHC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein/Non-structural protein 4A (Hepatitis C virus) | BDBM50150994 ((E)-3-{3-[2-((S)-4,4-Difluoro-2-{[(2S,4S)-1-((S)-2...) | PDB UniProtKB/TrEMBL GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibitory activity against Hepatitis C virus Non structural protein 3 serine protease/Non structural protein 4A serine protease | Bioorg Med Chem Lett 14: 4575-9 (2004) Article DOI: 10.1016/j.bmcl.2004.05.093 BindingDB Entry DOI: 10.7270/Q27S7PHC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein/Non-structural protein 4A (Hepatitis C virus) | BDBM50150984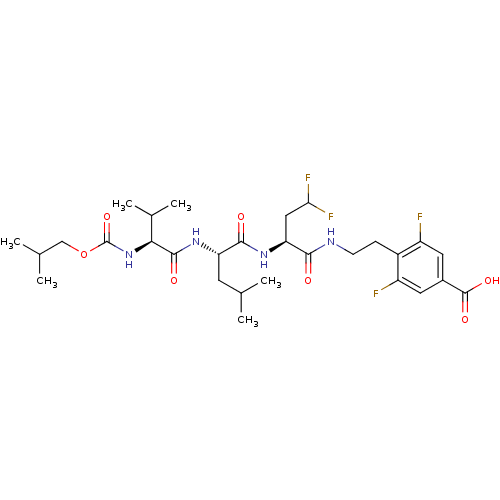 (4-(2-{(S)-4,4-Difluoro-2-[(S)-2-((S)-2-isobutoxyca...) | PDB UniProtKB/TrEMBL GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibitory activity against Hepatitis C virus Non structural protein 3 serine protease/Non structural protein 4A serine protease | Bioorg Med Chem Lett 14: 4575-9 (2004) Article DOI: 10.1016/j.bmcl.2004.05.093 BindingDB Entry DOI: 10.7270/Q27S7PHC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein/Non-structural protein 4A (Hepatitis C virus) | BDBM50150995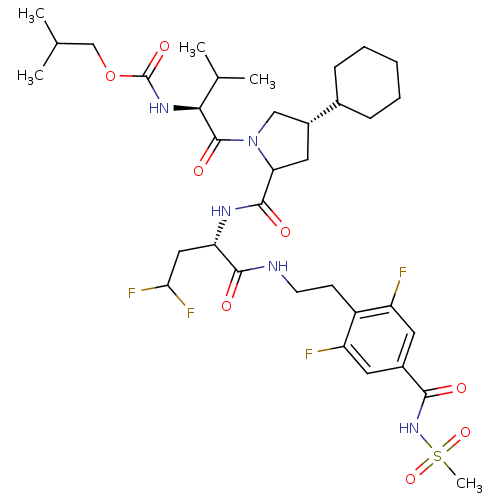 (CHEMBL263183 | [(S)-1-((2S,4S)-4-Cyclohexyl-2-{(S)...) | PDB UniProtKB/TrEMBL GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | 450 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibitory activity against Hepatitis C virus Non structural protein 3 serine protease/Non structural protein 4A serine protease | Bioorg Med Chem Lett 14: 4575-9 (2004) Article DOI: 10.1016/j.bmcl.2004.05.093 BindingDB Entry DOI: 10.7270/Q27S7PHC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein/Non-structural protein 4A (Hepatitis C virus) | BDBM50144349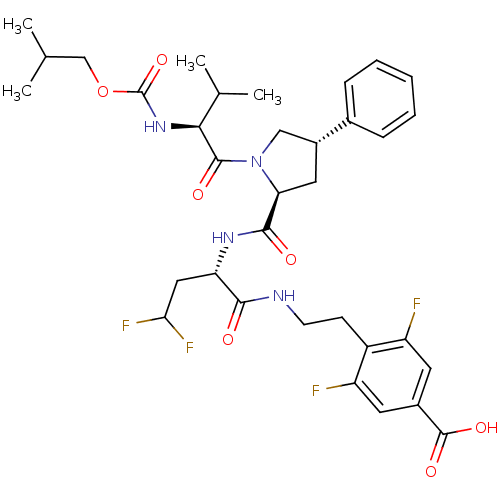 (4-[2-((S)-4,4-Difluoro-2-{[(2S,4S)-1-(2-isobutoxyc...) | PDB UniProtKB/TrEMBL GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 450 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibitory activity against Hepatitis C virus Non structural protein 3 serine protease/Non structural protein 4A serine protease | Bioorg Med Chem Lett 14: 4575-9 (2004) Article DOI: 10.1016/j.bmcl.2004.05.093 BindingDB Entry DOI: 10.7270/Q27S7PHC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein/Non-structural protein 4A (Hepatitis C virus) | BDBM50150983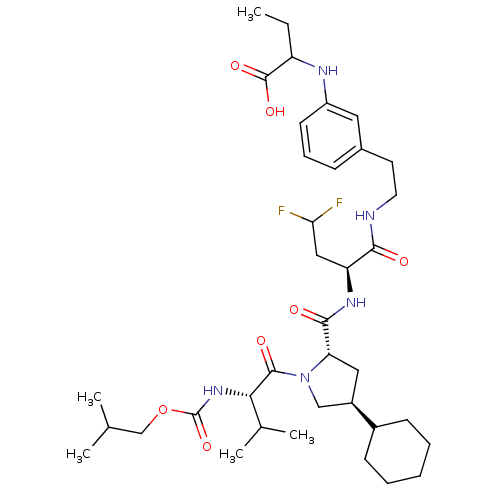 (2-{3-[2-((S)-2-{[(2S,4S)-4-Cyclohexyl-1-((S)-2-iso...) | PDB UniProtKB/TrEMBL GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 500 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibitory activity against Hepatitis C virus Non structural protein 3 serine protease/Non structural protein 4A serine protease | Bioorg Med Chem Lett 14: 4575-9 (2004) Article DOI: 10.1016/j.bmcl.2004.05.093 BindingDB Entry DOI: 10.7270/Q27S7PHC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein/Non-structural protein 4A (Hepatitis C virus) | BDBM50150996 (CHEMBL181465 | {3-[2-((S)-2-{[(2S,4S)-4-Cyclohexyl...) | PDB UniProtKB/TrEMBL GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 650 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibitory activity against Hepatitis C virus Non structural protein 3 serine protease/Non structural protein 4A serine protease | Bioorg Med Chem Lett 14: 4575-9 (2004) Article DOI: 10.1016/j.bmcl.2004.05.093 BindingDB Entry DOI: 10.7270/Q27S7PHC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein/Non-structural protein 4A (Hepatitis C virus) | BDBM50150985 (CHEMBL362404 | {3-[2-((S)-4,4-Difluoro-2-{[(2S,4S)...) | PDB UniProtKB/TrEMBL GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | 1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibitory activity against Hepatitis C virus Non structural protein 3 serine protease/Non structural protein 4A serine protease | Bioorg Med Chem Lett 14: 4575-9 (2004) Article DOI: 10.1016/j.bmcl.2004.05.093 BindingDB Entry DOI: 10.7270/Q27S7PHC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein/Non-structural protein 4A (Hepatitis C virus) | BDBM50144357 (3-Chloro-4-(2-{(S)-4,4-difluoro-2-[(S)-2-((S)-2-is...) | PDB UniProtKB/TrEMBL GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibitory activity against Hepatitis C virus Non structural protein 3 serine protease/Non structural protein 4A serine protease | Bioorg Med Chem Lett 14: 4575-9 (2004) Article DOI: 10.1016/j.bmcl.2004.05.093 BindingDB Entry DOI: 10.7270/Q27S7PHC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein/Non-structural protein 4A (Hepatitis C virus) | BDBM50150988 ((E)-3-{3-[2-((S)-4,4-Difluoro-2-{[(2S,4S)-1-((S)-2...) | PDB UniProtKB/TrEMBL GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibitory activity against Hepatitis C virus Non structural protein 3 serine protease/Non structural protein 4A serine protease | Bioorg Med Chem Lett 14: 4575-9 (2004) Article DOI: 10.1016/j.bmcl.2004.05.093 BindingDB Entry DOI: 10.7270/Q27S7PHC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H1 receptor (Homo sapiens (Human)) | BDBM50313618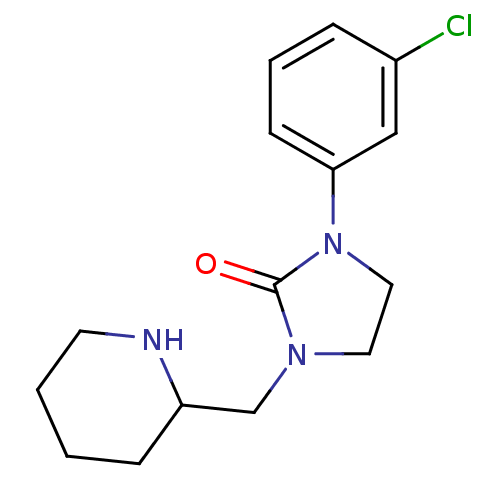 (1-(3-chlorophenyl)-3-(piperidin-2-ylmethyl)imidazo...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 2.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Activity at histamine H1 receptor | Bioorg Med Chem Lett 20: 2013-6 (2010) Article DOI: 10.1016/j.bmcl.2010.01.090 BindingDB Entry DOI: 10.7270/Q27P8ZJG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H1 receptor (Homo sapiens (Human)) | BDBM50313619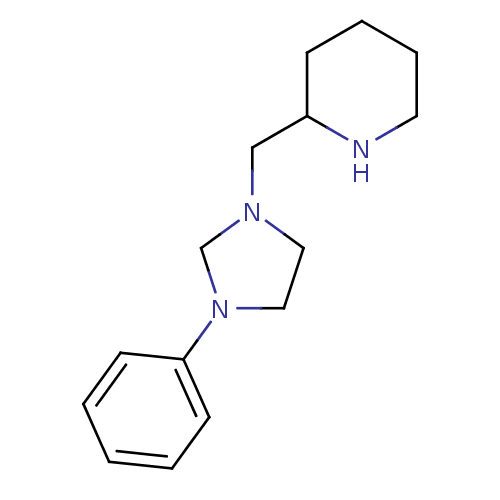 (2-((3-phenylimidazolidin-1-yl)methyl)piperidine | ...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | 2.51E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Activity at histamine H1 receptor | Bioorg Med Chem Lett 20: 2013-6 (2010) Article DOI: 10.1016/j.bmcl.2010.01.090 BindingDB Entry DOI: 10.7270/Q27P8ZJG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein/Non-structural protein 4A (Hepatitis C virus) | BDBM50150987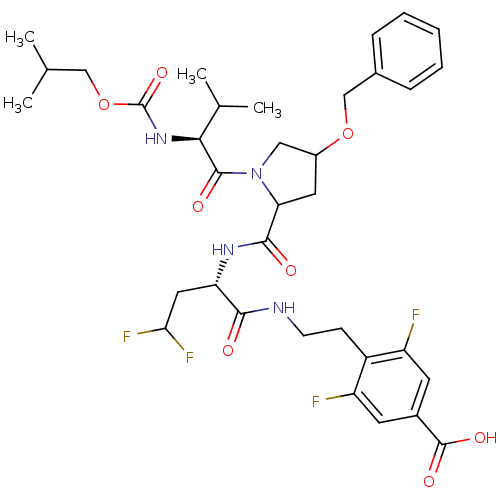 (4-[2-((S)-2-{[(R)-4-Benzyloxy-1-((S)-2-isobutoxyca...) | PDB UniProtKB/TrEMBL GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 4.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibitory activity against Hepatitis C virus Non structural protein 3 serine protease/Non structural protein 4A serine protease | Bioorg Med Chem Lett 14: 4575-9 (2004) Article DOI: 10.1016/j.bmcl.2004.05.093 BindingDB Entry DOI: 10.7270/Q27S7PHC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein/Non-structural protein 4A (Hepatitis C virus) | BDBM50150987 (4-[2-((S)-2-{[(R)-4-Benzyloxy-1-((S)-2-isobutoxyca...) | PDB UniProtKB/TrEMBL GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 4.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibitory activity against Hepatitis C virus Non structural protein 3 serine protease/Non structural protein 4A serine protease | Bioorg Med Chem Lett 14: 4575-9 (2004) Article DOI: 10.1016/j.bmcl.2004.05.093 BindingDB Entry DOI: 10.7270/Q27S7PHC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Platelet-activating factor acetylhydrolase (Homo sapiens (Human)) | BDBM50117772 (CHEMBL10921 | N-(2-Diethylamino-ethyl)-2-[2-(4-flu...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 0.00600 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Inhibition of the Phospholipase A2 (Lp-PLA2) enzyme in whole human plasma | Bioorg Med Chem Lett 12: 2603-6 (2002) BindingDB Entry DOI: 10.7270/Q2G44PNN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Platelet-activating factor acetylhydrolase (Homo sapiens (Human)) | BDBM50117772 (CHEMBL10921 | N-(2-Diethylamino-ethyl)-2-[2-(4-flu...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 0.0600 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Inhibitory activity against recombinant human Lp-PLA2 | Bioorg Med Chem Lett 13: 1067-70 (2003) BindingDB Entry DOI: 10.7270/Q2S75FPD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Platelet-activating factor acetylhydrolase (Homo sapiens (Human)) | BDBM50093917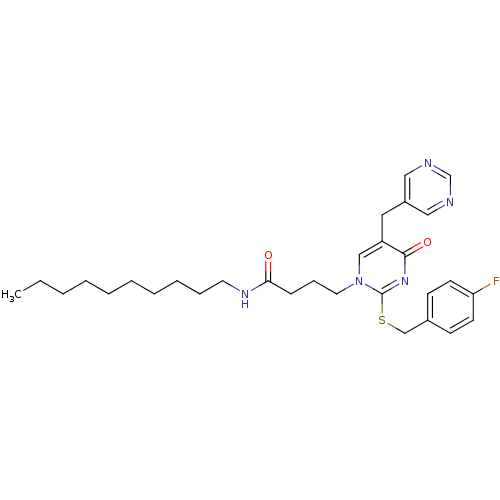 (CHEMBL87729 | N-Decyl-4-[2-(4-fluoro-benzylsulfany...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 0.100 | n/a | n/a | n/a | n/a | n/a | n/a |
SmithKline Beecham Pharmaceuticals Curated by ChEMBL | Assay Description Inhibition of lipoprotein associated phospholipase A2 in human plasma | Bioorg Med Chem Lett 10: 2557-61 (2001) BindingDB Entry DOI: 10.7270/Q2B857C6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Platelet-activating factor acetylhydrolase (Homo sapiens (Human)) | BDBM50117785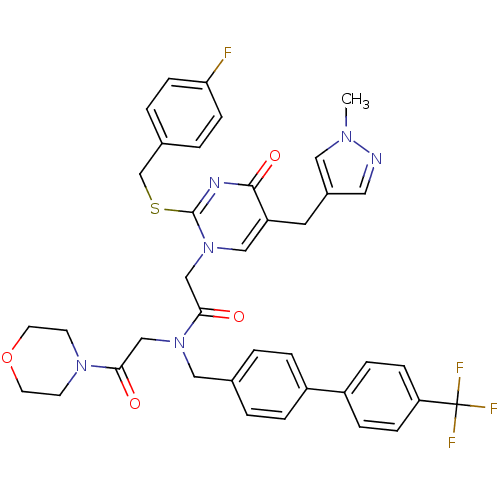 (2-[2-(4-Fluoro-benzylsulfanyl)-5-(1-methyl-1H-pyra...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 0.100 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Inhibitory concentration against Lipoprotein-Associated Phospholipase A2 (Lp-PLA2) was estimated | Bioorg Med Chem Lett 12: 2603-6 (2002) BindingDB Entry DOI: 10.7270/Q2G44PNN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Platelet-activating factor acetylhydrolase (Homo sapiens (Human)) | BDBM50125266 (CHEMBL10441 | N-(2-Diethylamino-ethyl)-2-[2-(4-flu...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 0.100 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Inhibitory activity against recombinant human Lp-PLA2 | Bioorg Med Chem Lett 13: 1067-70 (2003) BindingDB Entry DOI: 10.7270/Q2S75FPD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Platelet-activating factor acetylhydrolase (Homo sapiens (Human)) | BDBM50117802 (CHEMBL87100 | N-(2-Ethylamino-ethyl)-2-[2-(4-fluor...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 0.120 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Inhibitory concentration against Lipoprotein-Associated Phospholipase A2 (Lp-PLA2) was estimated | Bioorg Med Chem Lett 12: 2603-6 (2002) BindingDB Entry DOI: 10.7270/Q2G44PNN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Platelet-activating factor acetylhydrolase (Homo sapiens (Human)) | BDBM50117788 (CHEMBL407553 | N-(4'-Chloro-biphenyl-4-ylmethyl)-N...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 0.120 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Inhibitory concentration against Lipoprotein-Associated Phospholipase A2 (Lp-PLA2) was estimated | Bioorg Med Chem Lett 12: 2603-6 (2002) BindingDB Entry DOI: 10.7270/Q2G44PNN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Platelet-activating factor acetylhydrolase (Homo sapiens (Human)) | BDBM50117778 (2-[2-(4-Fluoro-benzylsulfanyl)-5-(1-methyl-1H-pyra...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 0.170 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Inhibitory concentration against Lipoprotein-Associated Phospholipase A2 (Lp-PLA2) was estimated | Bioorg Med Chem Lett 12: 2603-6 (2002) BindingDB Entry DOI: 10.7270/Q2G44PNN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Platelet-activating factor acetylhydrolase (Homo sapiens (Human)) | BDBM50107480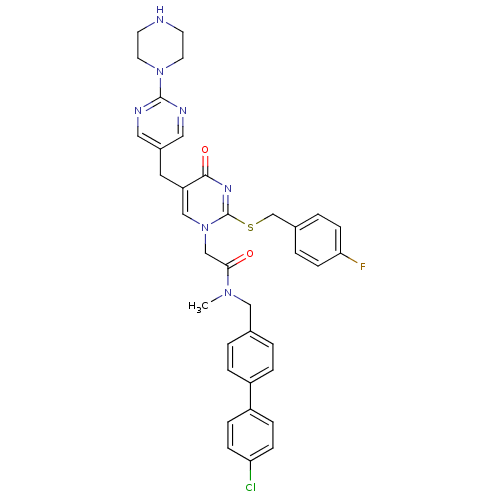 (CHEMBL348243 | N-(4'-Chloro-biphenyl-4-ylmethyl)-2...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Inhibitory activity against recombinant human Lp-PLA2 (Lp-PLA2). | Bioorg Med Chem Lett 12: 51-5 (2001) BindingDB Entry DOI: 10.7270/Q2862FR5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Platelet-activating factor acetylhydrolase (Homo sapiens (Human)) | BDBM50117801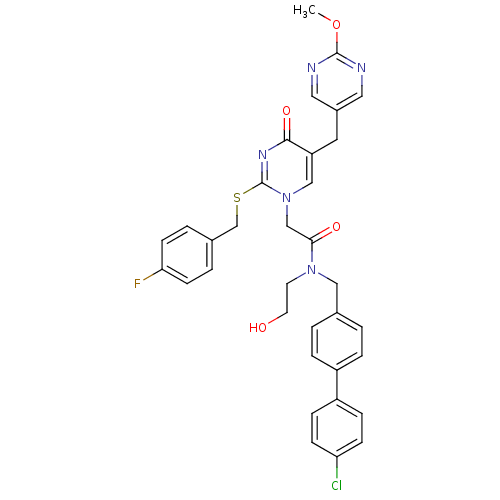 (CHEMBL87787 | N-(4'-Chloro-biphenyl-4-ylmethyl)-2-...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Inhibitory concentration against Lipoprotein-Associated Phospholipase A2 (Lp-PLA2) was estimated | Bioorg Med Chem Lett 12: 2603-6 (2002) BindingDB Entry DOI: 10.7270/Q2G44PNN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Platelet-activating factor acetylhydrolase (Homo sapiens (Human)) | BDBM50107505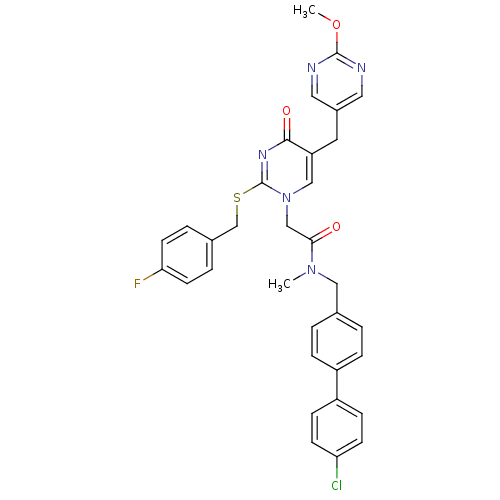 (CHEMBL79555 | N-(4'-Chloro-biphenyl-4-ylmethyl)-2-...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Inhibitory concentration against Lipoprotein-Associated Phospholipase A2 (Lp-PLA2) was estimated | Bioorg Med Chem Lett 12: 2603-6 (2002) BindingDB Entry DOI: 10.7270/Q2G44PNN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Platelet-activating factor acetylhydrolase (Homo sapiens (Human)) | BDBM50107512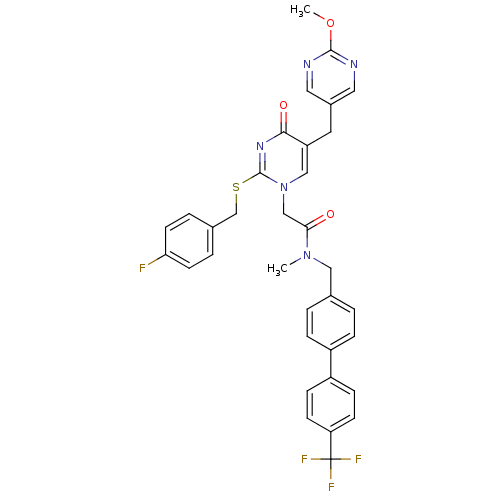 (2-[2-(4-Fluoro-benzylsulfanyl)-5-(2-methoxy-pyrimi...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Inhibitory activity against recombinant human Lp-PLA2 (Lp-PLA2). | Bioorg Med Chem Lett 12: 51-5 (2001) BindingDB Entry DOI: 10.7270/Q2862FR5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Platelet-activating factor acetylhydrolase (Homo sapiens (Human)) | BDBM50117794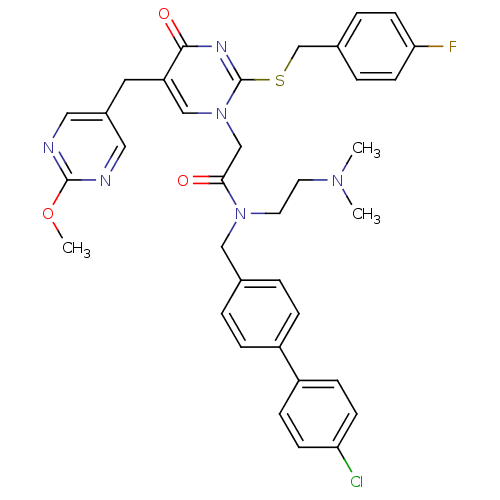 (CHEMBL314954 | N-(4'-Chloro-biphenyl-4-ylmethyl)-N...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Inhibitory concentration against Lipoprotein-Associated Phospholipase A2 (Lp-PLA2) was estimated | Bioorg Med Chem Lett 12: 2603-6 (2002) BindingDB Entry DOI: 10.7270/Q2G44PNN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Platelet-activating factor acetylhydrolase (Homo sapiens (Human)) | BDBM50117780 (CHEMBL85080 | N-(4'-Chloro-biphenyl-4-ylmethyl)-N-...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Inhibitory concentration against Lipoprotein-Associated Phospholipase A2 (Lp-PLA2) was estimated | Bioorg Med Chem Lett 12: 2603-6 (2002) BindingDB Entry DOI: 10.7270/Q2G44PNN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Platelet-activating factor acetylhydrolase (Homo sapiens (Human)) | BDBM50117791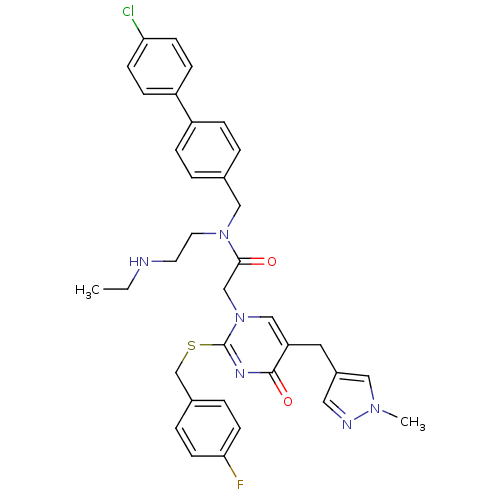 (CHEMBL87156 | N-(4'-Chloro-biphenyl-4-ylmethyl)-N-...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Inhibitory concentration against Lipoprotein-Associated Phospholipase A2 (Lp-PLA2) was estimated | Bioorg Med Chem Lett 12: 2603-6 (2002) BindingDB Entry DOI: 10.7270/Q2G44PNN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Platelet-activating factor acetylhydrolase (Homo sapiens (Human)) | BDBM50117790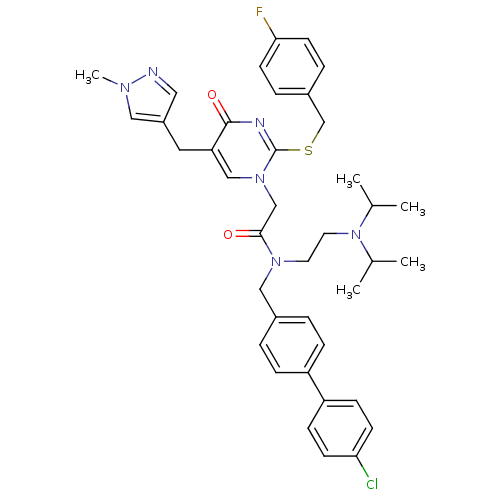 (CHEMBL315504 | N-(4'-Chloro-biphenyl-4-ylmethyl)-N...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Inhibitory concentration against Lipoprotein-Associated Phospholipase A2 (Lp-PLA2) was estimated | Bioorg Med Chem Lett 12: 2603-6 (2002) BindingDB Entry DOI: 10.7270/Q2G44PNN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Platelet-activating factor acetylhydrolase (Homo sapiens (Human)) | BDBM50117775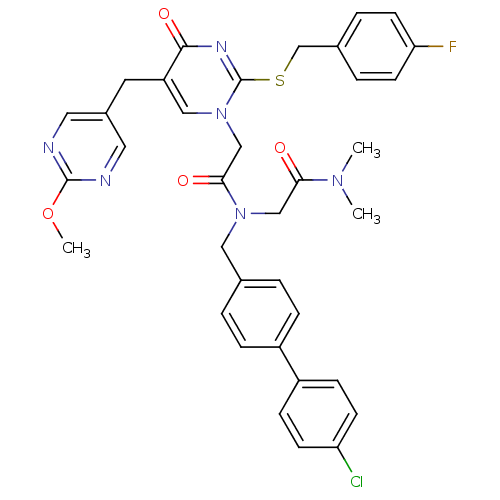 (CHEMBL84894 | N-(4'-Chloro-biphenyl-4-ylmethyl)-N-...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Inhibitory concentration against Lipoprotein-Associated Phospholipase A2 (Lp-PLA2) was estimated | Bioorg Med Chem Lett 12: 2603-6 (2002) BindingDB Entry DOI: 10.7270/Q2G44PNN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Platelet-activating factor acetylhydrolase (Homo sapiens (Human)) | BDBM50107505 (CHEMBL79555 | N-(4'-Chloro-biphenyl-4-ylmethyl)-2-...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Inhibitory activity against recombinant human Lp-PLA2 (Lp-PLA2). | Bioorg Med Chem Lett 12: 51-5 (2001) BindingDB Entry DOI: 10.7270/Q2862FR5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Platelet-activating factor acetylhydrolase (Homo sapiens (Human)) | BDBM50125267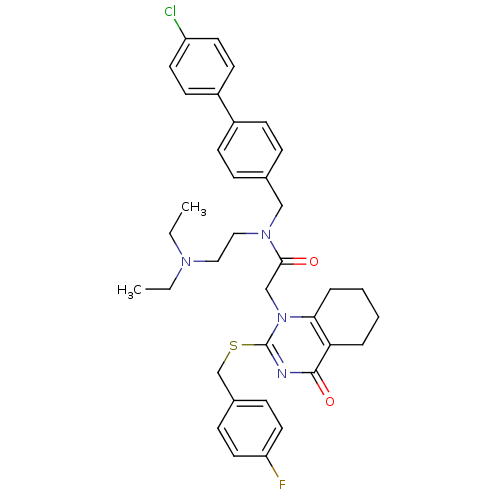 (CHEMBL10501 | N-(4'-Chloro-biphenyl-4-ylmethyl)-N-...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 0.25 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Inhibitory activity against recombinant human Lp-PLA2 | Bioorg Med Chem Lett 13: 1067-70 (2003) BindingDB Entry DOI: 10.7270/Q2S75FPD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Platelet-activating factor acetylhydrolase (Homo sapiens (Human)) | BDBM50125265 (CHEMBL204021 | N-(2-Diethylamino-ethyl)-2-[2-(4-fl...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid PDB UniChem Patents Similars | PDB PubMed | n/a | n/a | 0.25 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Inhibitory activity against recombinant human Lp-PLA2 by mechanistic studies | Bioorg Med Chem Lett 13: 1067-70 (2003) BindingDB Entry DOI: 10.7270/Q2S75FPD | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Platelet-activating factor acetylhydrolase (Homo sapiens (Human)) | BDBM50117773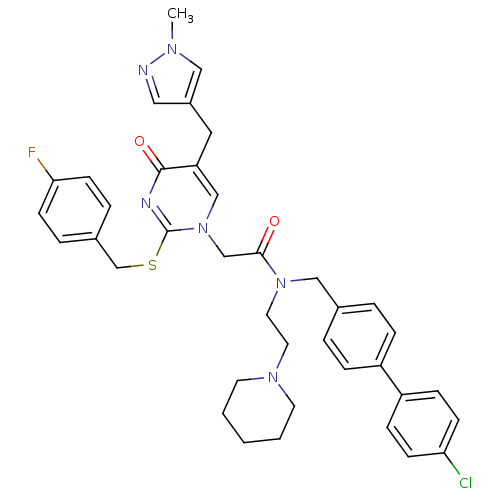 (CHEMBL85179 | N-(4'-Chloro-biphenyl-4-ylmethyl)-2-...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Inhibitory concentration against Lipoprotein-Associated Phospholipase A2 (Lp-PLA2) was estimated | Bioorg Med Chem Lett 12: 2603-6 (2002) BindingDB Entry DOI: 10.7270/Q2G44PNN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Platelet-activating factor acetylhydrolase (Homo sapiens (Human)) | BDBM50093916 (CHEMBL85885 | N-Dodecyl-2-[2-(4-fluoro-benzylsulfa...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a |
SmithKline Beecham Pharmaceuticals Curated by ChEMBL | Assay Description Inhibition of lipoprotein associated phospholipase A2 in human plasma | Bioorg Med Chem Lett 10: 2557-61 (2001) BindingDB Entry DOI: 10.7270/Q2B857C6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Platelet-activating factor acetylhydrolase (Homo sapiens (Human)) | BDBM50117800 (CHEMBL328527 | N-Dimethylcarbamoylmethyl-2-[2-(4-f...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Inhibitory concentration against Lipoprotein-Associated Phospholipase A2 (Lp-PLA2) was estimated | Bioorg Med Chem Lett 12: 2603-6 (2002) BindingDB Entry DOI: 10.7270/Q2G44PNN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Platelet-activating factor acetylhydrolase (Homo sapiens (Human)) | BDBM50117782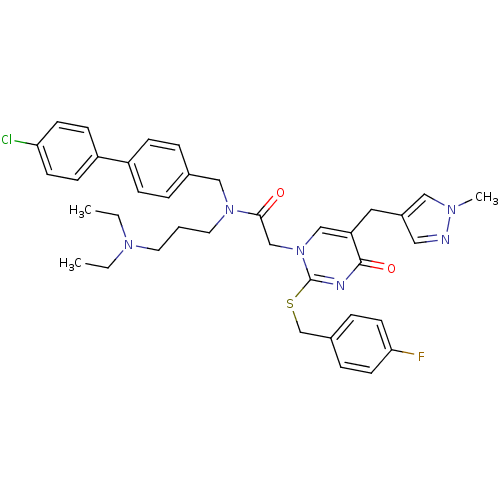 (CHEMBL315766 | N-(4'-Chloro-biphenyl-4-ylmethyl)-N...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 0.350 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Inhibitory concentration against Lipoprotein-Associated Phospholipase A2 (Lp-PLA2) was estimated | Bioorg Med Chem Lett 12: 2603-6 (2002) BindingDB Entry DOI: 10.7270/Q2G44PNN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 714 total ) | Next | Last >> |