

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Histamine H3 receptor (Homo sapiens (Human)) | BDBM50277100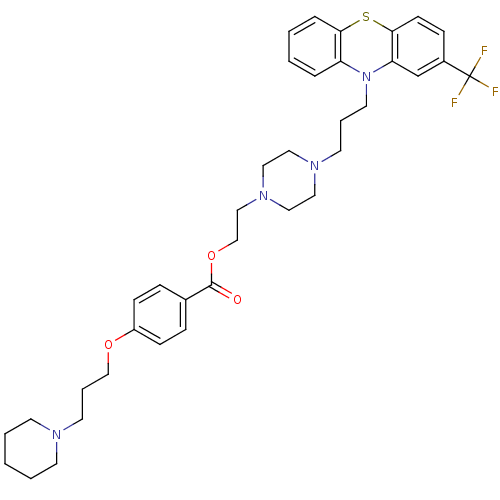 (4-(3-Piperidin-1-yl-propoxy)-benzoic acid 2-{4-[3-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | 0.0420 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johann Wolfgang Goethe Universit£t Curated by ChEMBL | Assay Description Displacement of [125I]iodoproxyfan from human histamine H3 receptor expressed in CHO/HEK293 cells | Bioorg Med Chem Lett 19: 538-42 (2008) Article DOI: 10.1016/j.bmcl.2008.09.012 BindingDB Entry DOI: 10.7270/Q25H7H6W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Homo sapiens (Human)) | BDBM50278495 (4-chloro-1-(4-(3-(piperidin-1-yl)propoxy)benzyl)pi...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.0680 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johann Wolfgang Goethe-Universität Curated by ChEMBL | Assay Description Displacement of [125I]iodoproxyfan from human histamine H3 receptor expressed in HEL293 cells | Bioorg Med Chem Lett 19: 2172-5 (2009) Article DOI: 10.1016/j.bmcl.2009.02.110 BindingDB Entry DOI: 10.7270/Q2H41RBM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Homo sapiens (Human)) | BDBM50110291 ((7-Chloro-quinolin-4-yl)-[4-(3-piperidin-1-yl-prop...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 0.0860 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Freie Universität Berlin Curated by ChEMBL | Assay Description Affinity for displacement of [125I]-iodoproxyfan from human histamine H3 receptors stably expressed in CHO cells | J Med Chem 45: 1128-41 (2002) BindingDB Entry DOI: 10.7270/Q2H70F4V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Homo sapiens (Human)) | BDBM50110288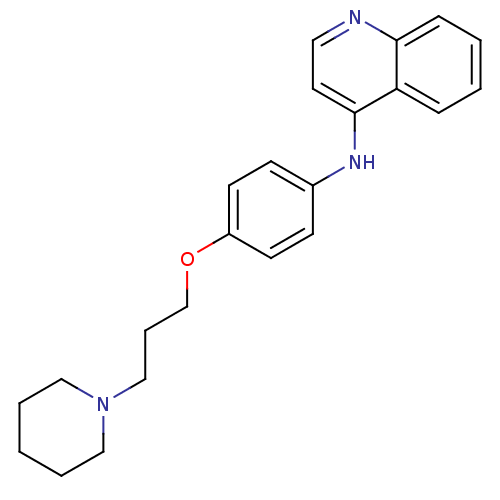 (CHEMBL15153 | N-(4-(3-(piperidin-1-yl)propoxy)phen...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.0910 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Freie Universität Berlin Curated by ChEMBL | Assay Description Affinity for displacement of [125I]-iodoproxyfan from human histamine H3 receptors stably expressed in CHO cells | J Med Chem 45: 1128-41 (2002) BindingDB Entry DOI: 10.7270/Q2H70F4V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Homo sapiens (Human)) | BDBM50278350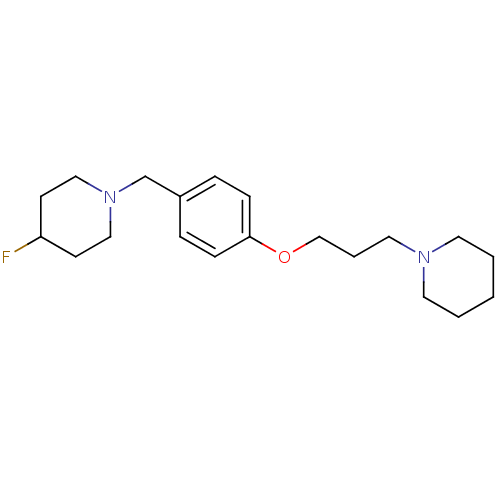 (4-fluoro-1-(4-(3-(piperidin-1-yl)propoxy)benzyl)pi...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.0933 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johann Wolfgang Goethe University Curated by ChEMBL | Assay Description Displacement of [3H]N-alpha-methylhistamine from recombinant human histamine H3 receptor expressed in HEK293 cells after 90 mins | Bioorg Med Chem Lett 24: 2236-9 (2014) Article DOI: 10.1016/j.bmcl.2014.03.098 BindingDB Entry DOI: 10.7270/Q2MP54TD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Homo sapiens (Human)) | BDBM50278350 (4-fluoro-1-(4-(3-(piperidin-1-yl)propoxy)benzyl)pi...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.0940 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johann Wolfgang Goethe-Universität Curated by ChEMBL | Assay Description Displacement of [125I]iodoproxyfan from human histamine H3 receptor expressed in HEL293 cells | Bioorg Med Chem Lett 19: 2172-5 (2009) Article DOI: 10.1016/j.bmcl.2009.02.110 BindingDB Entry DOI: 10.7270/Q2H41RBM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Homo sapiens (Human)) | BDBM50278350 (4-fluoro-1-(4-(3-(piperidin-1-yl)propoxy)benzyl)pi...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.0940 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johann Wolfgang Goethe University Curated by ChEMBL | Assay Description Displacement of [3H]N-alpha-methylhistamine from recombinant human histamine H3 receptor expressed in HEK293 cells after 90 mins | Bioorg Med Chem Lett 24: 2236-9 (2014) Article DOI: 10.1016/j.bmcl.2014.03.098 BindingDB Entry DOI: 10.7270/Q2MP54TD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Hrh3 protein (RAT) | BDBM22911 (2-(3H-imidazol-4-yl)ethylsulfanylmethanimidamide |...) | KEGG UniProtKB/TrEMBL GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid PDB UniChem Patents Similars | PubMed | 0.100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
U. 109 Curated by PDSP Ki Database | J Pharmacol Exp Ther 271: 452-9 (1994) BindingDB Entry DOI: 10.7270/Q23X8556 | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Rattus norvegicus (rat)) | BDBM50098203 (1-{4-[2-(1H-Imidazol-4-yl)-ethylsulfanyl]-phenyl}-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.110 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Freie Universität Berlin Curated by ChEMBL | Assay Description Inhibition of [125I]-iodoproxyfan from rat histamine H3 receptor expressed in CHO-K1 cells | Bioorg Med Chem Lett 11: 951-4 (2001) BindingDB Entry DOI: 10.7270/Q28P5ZRB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Rattus norvegicus (rat)) | BDBM50074629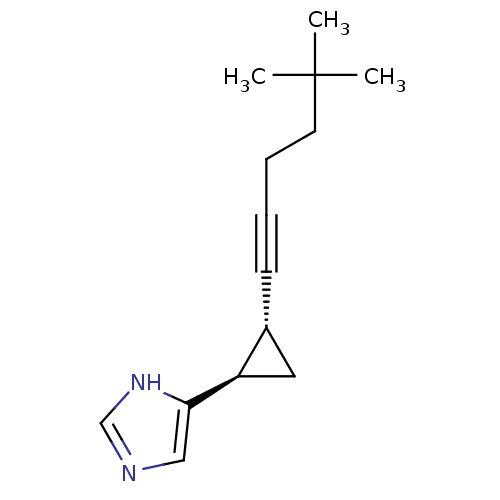 (4-[(1R,2R)-2-(5,5-Dimethyl-hex-1-ynyl)-cyclopropyl...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | PubMed | 0.120 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Freie Universität Berlin Curated by ChEMBL | Assay Description In vitro inhibitory activity against Histamine H3 receptor for K+ evoked depolarization-induced release of [3H]-histamine from synaptosomes of rat ce... | Bioorg Med Chem Lett 10: 2379-82 (2001) BindingDB Entry DOI: 10.7270/Q28G8JZ4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Homo sapiens (Human)) | BDBM50392759 (CHEMBL2151157) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | 0.126 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Catania Curated by ChEMBL | Assay Description Displacement of [3H]N-alpha-Methylhistamine from human recombinant H3 receptor expressed in HEK293 cells by competitive binding assay | Eur J Med Chem 86: 578-88 (2014) Article DOI: 10.1016/j.ejmech.2014.09.011 BindingDB Entry DOI: 10.7270/Q2222WCC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Hrh3 protein (RAT) | BDBM22911 (2-(3H-imidazol-4-yl)ethylsulfanylmethanimidamide |...) | KEGG UniProtKB/TrEMBL GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid PDB UniChem Patents Similars | PubMed | 0.150 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
U. 109 Curated by PDSP Ki Database | J Pharmacol Exp Ther 271: 452-9 (1994) BindingDB Entry DOI: 10.7270/Q23X8556 | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (GUINEA PIG) | BDBM50247053 (1-(3-(3-(4-chlorophenyl)propoxy)propyl)piperidine ...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | 0.160 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Jagiellonian University Medical College Curated by ChEMBL | Assay Description Antagonist activity at human histamine H3 receptor expressed in HEK293 cells by [35S]GTPgammaS binding assay | Bioorg Med Chem 17: 3037-42 (2009) Article DOI: 10.1016/j.bmc.2009.03.014 BindingDB Entry DOI: 10.7270/Q2SF2X3H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Homo sapiens (Human)) | BDBM50535225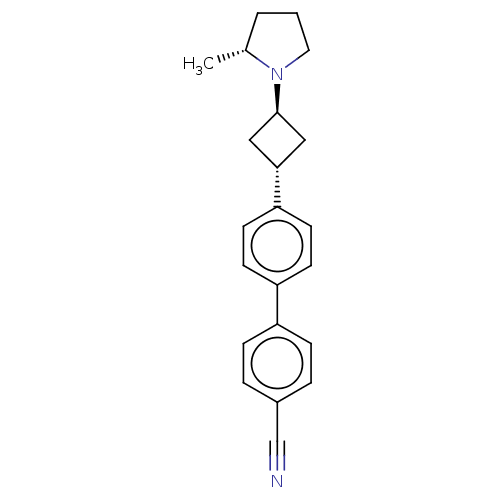 (CHEMBL4516622) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.170 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Jagiellonian University Medical College Curated by ChEMBL | Assay Description Binding affinity to human H3R | Bioorg Med Chem 25: 5341-5354 (2017) Article DOI: 10.1016/j.bmc.2017.07.058 BindingDB Entry DOI: 10.7270/Q29W0K0D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(3) dopamine receptor (Homo sapiens (Human)) | BDBM50142788 (CHEMBL3759491) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bioprojet-Biotech Curated by ChEMBL | Assay Description Displacement of [3H]-spiperone from human dopamine D3 receptor expressed in CHO cell membranes | Bioorg Med Chem Lett 26: 885-8 (2016) Article DOI: 10.1016/j.bmcl.2015.12.068 BindingDB Entry DOI: 10.7270/Q270838N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Hrh3 protein (RAT) | BDBM22916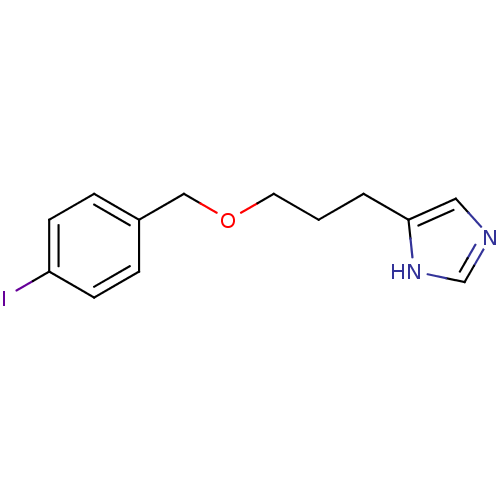 (5-{3-[(4-iodophenyl)methoxy]propyl}-1H-imidazole |...) | KEGG UniProtKB/TrEMBL GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
U. 109 Curated by PDSP Ki Database | J Pharmacol Exp Ther 271: 452-9 (1994) BindingDB Entry DOI: 10.7270/Q23X8556 | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Homo sapiens (Human)) | BDBM50278449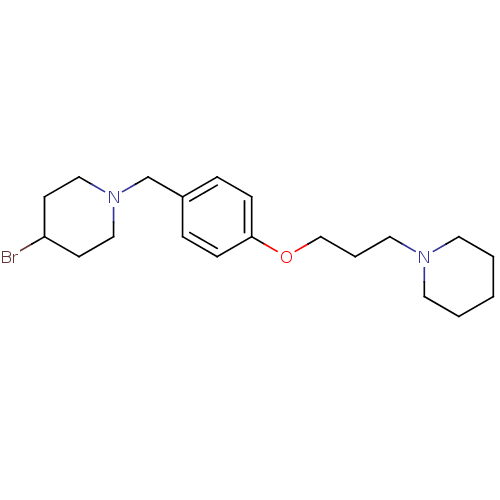 (4-bromo-1-(4-(3-(piperidin-1-yl)propoxy)benzyl)pip...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johann Wolfgang Goethe-Universität Curated by ChEMBL | Assay Description Displacement of [125I]iodoproxyfan from human histamine H3 receptor expressed in HEL293 cells | Bioorg Med Chem Lett 19: 2172-5 (2009) Article DOI: 10.1016/j.bmcl.2009.02.110 BindingDB Entry DOI: 10.7270/Q2H41RBM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Homo sapiens (Human)) | BDBM50013722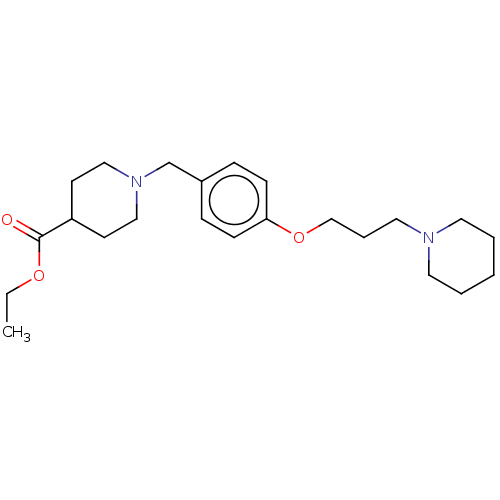 (CHEMBL3264546) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.229 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johann Wolfgang Goethe University Curated by ChEMBL | Assay Description Displacement of [3H]N-alpha-methylhistamine from recombinant human histamine H3 receptor expressed in HEK293 cells after 90 mins | Bioorg Med Chem Lett 24: 2236-9 (2014) Article DOI: 10.1016/j.bmcl.2014.03.098 BindingDB Entry DOI: 10.7270/Q2MP54TD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Homo sapiens (Human)) | BDBM50001752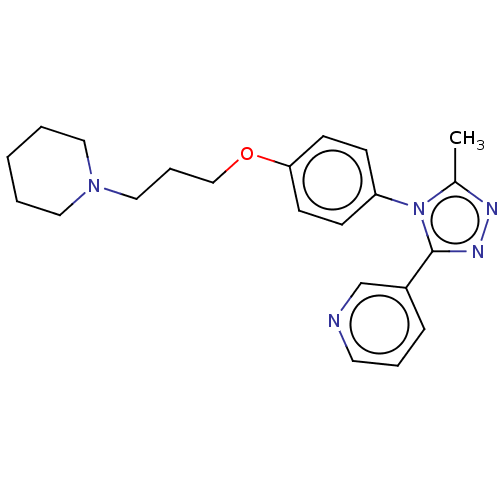 (CHEMBL3238445) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | 0.240 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
United Arab Emirates University Curated by ChEMBL | Assay Description Displacement of [3H]N-alpha-methylhistamine from human H3 receptor expressed in HEK-293 cell membrane after 90 mins by liquid scintillation counting ... | Eur J Med Chem 77: 269-79 (2014) Article DOI: 10.1016/j.ejmech.2014.03.014 BindingDB Entry DOI: 10.7270/Q29K4CR6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Homo sapiens (Human)) | BDBM50278448 (4,4-difluoro-1-(4-(3-(piperidin-1-yl)propoxy)benzy...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.240 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johann Wolfgang Goethe-Universität Curated by ChEMBL | Assay Description Displacement of [125I]iodoproxyfan from human histamine H3 receptor expressed in HEL293 cells | Bioorg Med Chem Lett 19: 2172-5 (2009) Article DOI: 10.1016/j.bmcl.2009.02.110 BindingDB Entry DOI: 10.7270/Q2H41RBM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Homo sapiens (Human)) | BDBM50277045 (CHEMBL459350 | [3-(10,11-Dihydro-dibenzo[a,d]cyclo...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.25 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johann Wolfgang Goethe Universit£t Curated by ChEMBL | Assay Description Displacement of [125I]iodoproxyfan from human histamine H3 receptor expressed in CHO/HEK293 cells | Bioorg Med Chem Lett 19: 538-42 (2008) Article DOI: 10.1016/j.bmcl.2008.09.012 BindingDB Entry DOI: 10.7270/Q25H7H6W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Homo sapiens (Human)) | BDBM50278445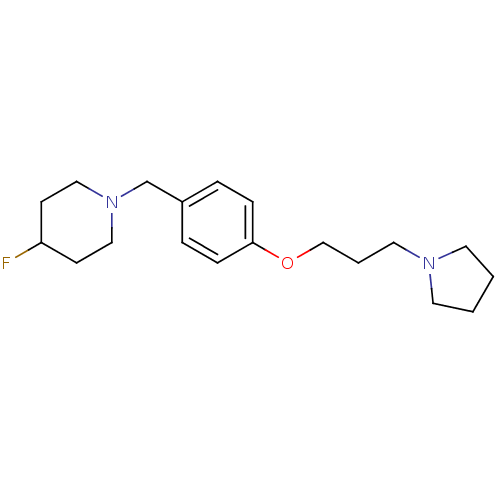 (4-fluoro-1-(4-(3-(pyrrolidin-1-yl)propoxy)benzyl)p...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.260 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johann Wolfgang Goethe-Universität Curated by ChEMBL | Assay Description Displacement of [125I]iodoproxyfan from human histamine H3 receptor expressed in HEL293 cells | Bioorg Med Chem Lett 19: 2172-5 (2009) Article DOI: 10.1016/j.bmcl.2009.02.110 BindingDB Entry DOI: 10.7270/Q2H41RBM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Rattus norvegicus (rat)) | BDBM50091385 (1-{4-[3-(1H-Imidazol-4-yl)-propoxy]-phenyl}-ethano...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.260 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Freie Universität Berlin Curated by ChEMBL | Assay Description Histamine H3 receptor antagonist activity in an assay with K+ evoked depolarization-induced release of [3H]-histamine from rat synaptosomes. | J Med Chem 43: 3335-43 (2000) BindingDB Entry DOI: 10.7270/Q2RR1XGV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Homo sapiens (Human)) | BDBM50159110 (1-(3-(4-(piperidin-1-ylmethyl)phenoxy)propyl)piper...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | 0.275 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Binding affinity to human H3R | Citation and Details Article DOI: 10.1016/j.ejmech.2020.113041 BindingDB Entry DOI: 10.7270/Q2MP570W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Homo sapiens (Human)) | BDBM50278400 ((S)-1-(3-(4-((3-fluoropyrrolidin-1-yl)methyl)pheno...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.280 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johann Wolfgang Goethe-Universität Curated by ChEMBL | Assay Description Displacement of [125I]iodoproxyfan from human histamine H3 receptor expressed in HEL293 cells | Bioorg Med Chem Lett 19: 2172-5 (2009) Article DOI: 10.1016/j.bmcl.2009.02.110 BindingDB Entry DOI: 10.7270/Q2H41RBM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Homo sapiens (Human)) | BDBM50278444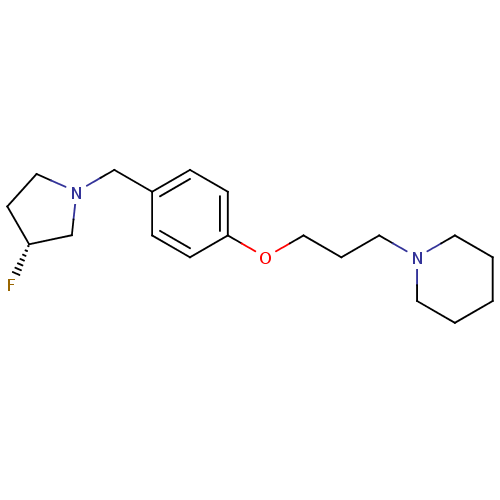 ((R)-1-(3-(4-((3-fluoropyrrolidin-1-yl)methyl)pheno...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.280 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johann Wolfgang Goethe-Universität Curated by ChEMBL | Assay Description Displacement of [125I]iodoproxyfan from human histamine H3 receptor expressed in HEL293 cells | Bioorg Med Chem Lett 19: 2172-5 (2009) Article DOI: 10.1016/j.bmcl.2009.02.110 BindingDB Entry DOI: 10.7270/Q2H41RBM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Homo sapiens (Human)) | BDBM50580518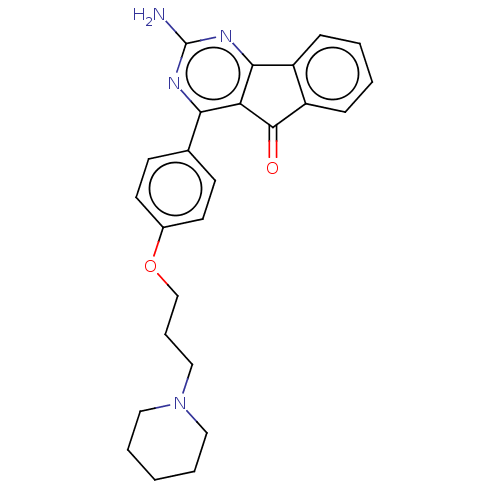 (CHEMBL5089580) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.280 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Displacement of [3H] N-alpha methylhistamine from human recombinant histamine H3 receptor expressed in HEK293 cells measured after 90 mins | Citation and Details Article DOI: 10.1021/acs.jmedchem.0c00914 BindingDB Entry DOI: 10.7270/Q22F7S8G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Homo sapiens (Human)) | BDBM50247053 (1-(3-(3-(4-chlorophenyl)propoxy)propyl)piperidine ...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Jagiellonian University Medical College Curated by ChEMBL | Assay Description Inhibition of human H3 receptor | Eur J Med Chem 152: 223-234 (2018) Article DOI: 10.1016/j.ejmech.2018.04.043 BindingDB Entry DOI: 10.7270/Q2697632 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(3) dopamine receptor (Homo sapiens (Human)) | BDBM50221380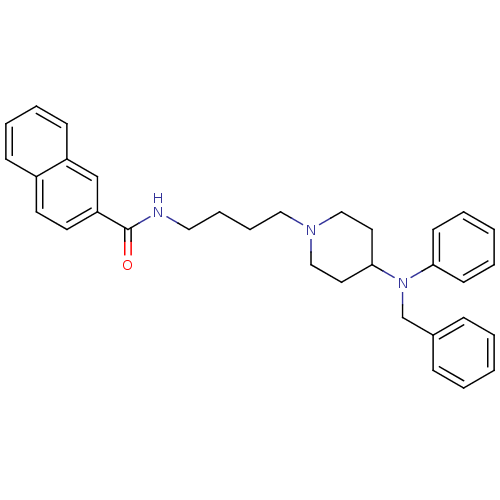 (CHEMBL239923 | N-(4-{4-[benzyl(phenyl)amino]piperi...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johann Wolfgang Goethe-University Curated by ChEMBL | Assay Description Displacement of [3H]spiperone from human D3 receptor expressed in CHO cells | Bioorg Med Chem 15: 7258-73 (2007) Article DOI: 10.1016/j.bmc.2007.08.034 BindingDB Entry DOI: 10.7270/Q21C1WMX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Homo sapiens (Human)) | BDBM50247053 (1-(3-(3-(4-chlorophenyl)propoxy)propyl)piperidine ...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Binding affinity to human H3R | Citation and Details Article DOI: 10.1016/j.ejmech.2020.113041 BindingDB Entry DOI: 10.7270/Q2MP570W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Rattus norvegicus (rat)) | BDBM50091373 (1-{2-Fluoro-4-[3-(1H-imidazol-4-yl)-propoxy]-pheny...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Freie Universität Berlin Curated by ChEMBL | Assay Description Histamine H3 receptor antagonist activity in an assay with K+ evoked depolarization-induced release of [3H]-histamine from rat synaptosomes. | J Med Chem 43: 3335-43 (2000) BindingDB Entry DOI: 10.7270/Q2RR1XGV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Homo sapiens (Human)) | BDBM50247053 (1-(3-(3-(4-chlorophenyl)propoxy)propyl)piperidine ...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Jagiellonian University Medical College Curated by ChEMBL | Assay Description Binding affinity to human histamine H3 receptor | Bioorg Med Chem 25: 2701-2712 (2017) Article DOI: 10.1016/j.bmc.2017.03.031 BindingDB Entry DOI: 10.7270/Q2833VPK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(2) dopamine receptor (Homo sapiens (Human)) | BDBM50130293 (7-{4-[4-(2,3-dichlorophenyl)piperazin-1-yl]butoxy}...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG PC cid PC sid PDB UniChem Patents Similars | DrugBank Article PubMed | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Citation and Details Article DOI: 10.1016/j.ejmech.2022.114193 BindingDB Entry DOI: 10.7270/Q2WS8Z8T | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Homo sapiens (Human)) | BDBM50278446 ((R)-3-fluoro-1-(4-(3-(pyrrolidin-1-yl)propoxy)benz...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.310 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johann Wolfgang Goethe-Universität Curated by ChEMBL | Assay Description Displacement of [125I]iodoproxyfan from human histamine H3 receptor expressed in HEL293 cells | Bioorg Med Chem Lett 19: 2172-5 (2009) Article DOI: 10.1016/j.bmcl.2009.02.110 BindingDB Entry DOI: 10.7270/Q2H41RBM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Homo sapiens (Human)) | BDBM50580523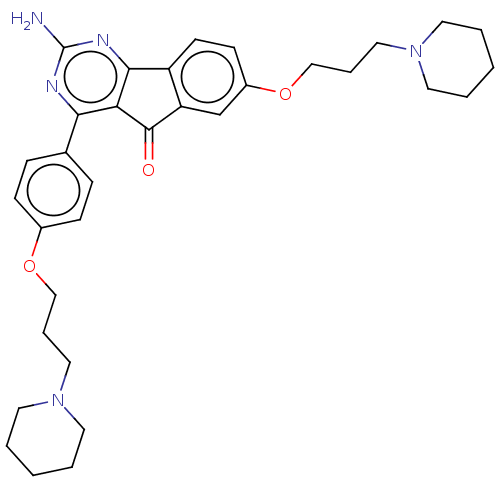 (CHEMBL5093850) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.310 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Displacement of [3H] N-alpha methylhistamine from human recombinant histamine H3 receptor expressed in HEK293 cells measured after 90 mins | Citation and Details Article DOI: 10.1021/acs.jmedchem.0c00914 BindingDB Entry DOI: 10.7270/Q22F7S8G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(3) dopamine receptor (Homo sapiens (Human)) | BDBM50132057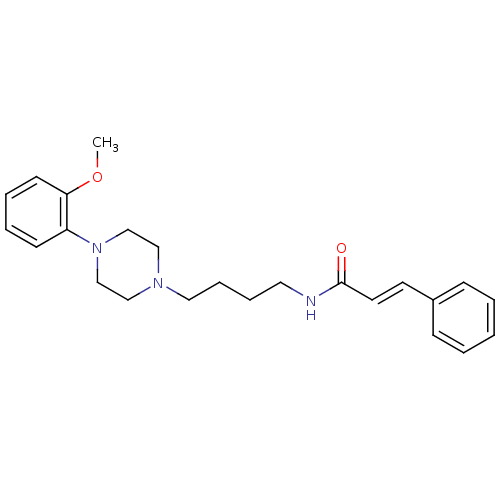 (CHEMBL1180430 | CHEMBL126128 | N-{4-[4-(2-Methoxy-...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.330 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johann Wolfgang Goethe-Universität Curated by ChEMBL | Assay Description Binding affinity for human dopamine receptor D3 by displacing [125I]-iodosulpiride expressed in CHO cells | J Med Chem 46: 3883-99 (2003) Article DOI: 10.1021/jm030836n BindingDB Entry DOI: 10.7270/Q23F4P18 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Homo sapiens (Human)) | BDBM50110300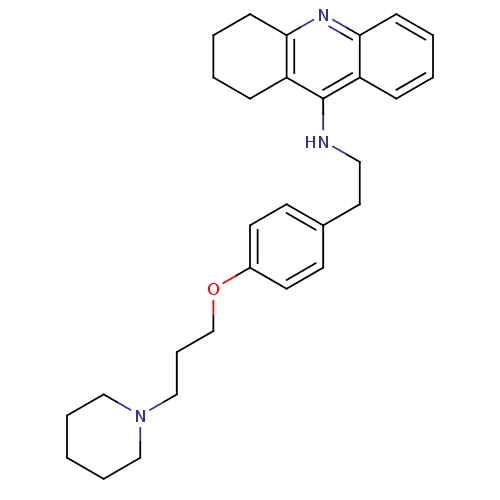 (CHEMBL15056 | N-(4-(3-(piperidin-1-yl)propoxy)phen...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.330 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Freie Universität Berlin Curated by ChEMBL | Assay Description Affinity for displacement of [125I]-iodoproxyfan from human histamine H3 receptors stably expressed in CHO cells | J Med Chem 45: 1128-41 (2002) BindingDB Entry DOI: 10.7270/Q2H70F4V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(3) dopamine receptor (Homo sapiens) | CHEMBL4293814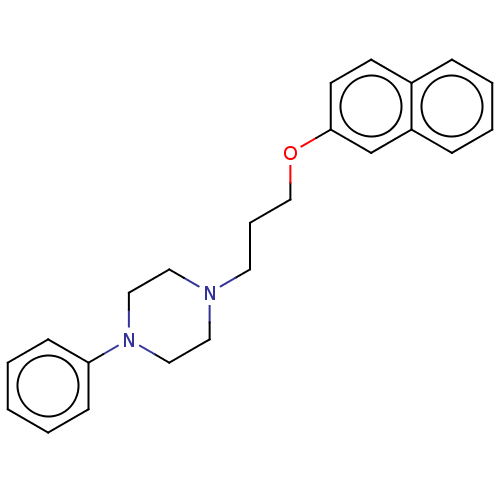 | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL UniChem | 0.340 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a | |
TBA | Citation and Details | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Homo sapiens (Human)) | BDBM50277046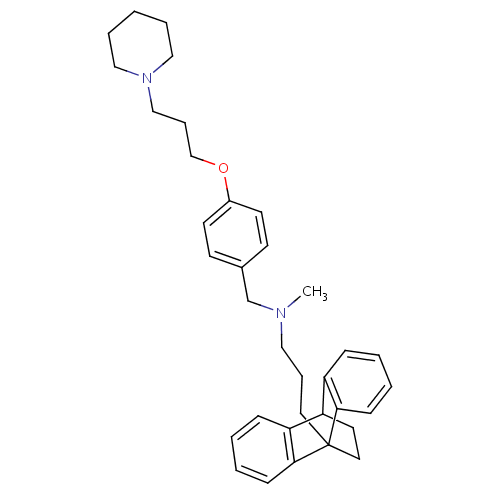 (CHEMBL517244 | methyl({4-[3-(piperidin-1-yl)propox...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | 0.358 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johann Wolfgang Goethe Universit£t Curated by ChEMBL | Assay Description Displacement of [125I]iodoproxyfan from human histamine H3 receptor expressed in CHO/HEK293 cells | Bioorg Med Chem Lett 19: 538-42 (2008) Article DOI: 10.1016/j.bmcl.2008.09.012 BindingDB Entry DOI: 10.7270/Q25H7H6W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(3) dopamine receptor (Homo sapiens (Human)) | BDBM50132040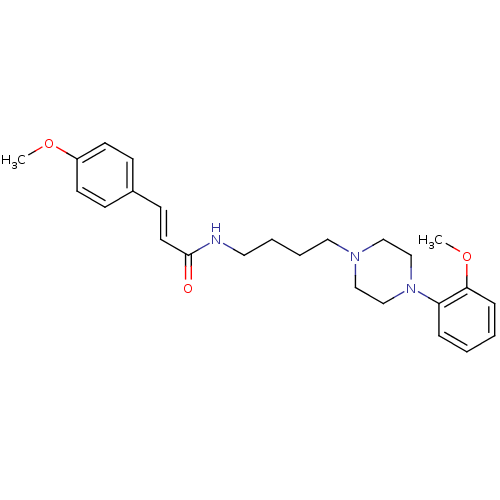 (3-(4-Methoxy-phenyl)-N-{4-[4-(2-methoxy-phenyl)-pi...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.380 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johann Wolfgang Goethe-Universität Curated by ChEMBL | Assay Description Binding affinity for human dopamine receptor D3 by displacing [125I]-iodosulpiride expressed in CHO cells | J Med Chem 46: 3883-99 (2003) Article DOI: 10.1021/jm030836n BindingDB Entry DOI: 10.7270/Q23F4P18 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(4) dopamine receptor (Homo sapiens (Human)) | BDBM50077001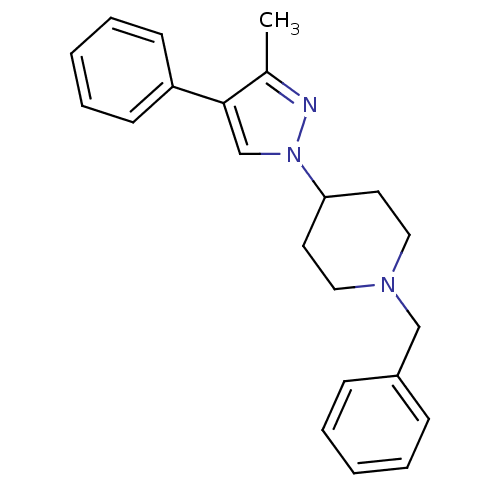 (1-Benzyl-4-(3-methyl-4-phenyl-pyrazol-1-yl)-piperi...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 0.390 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johann Wolfgang Goethe-Universität Curated by ChEMBL | Assay Description Binding affinity of compound towards human Dopamine receptor D4 by displacing [125I]-iodosulpiride expressed in CHO cells | J Med Chem 46: 3883-99 (2003) Article DOI: 10.1021/jm030836n BindingDB Entry DOI: 10.7270/Q23F4P18 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Homo sapiens (Human)) | BDBM50158595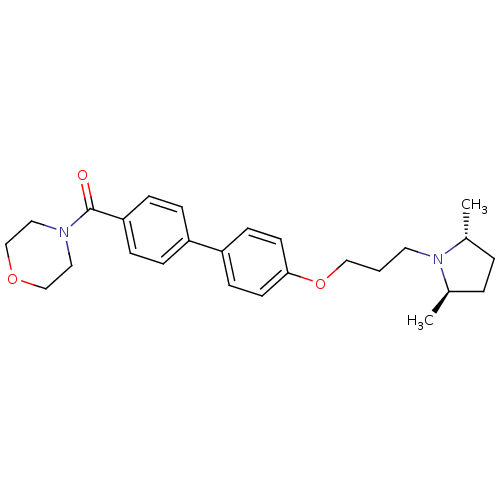 ((4'-(3-((2R,5R)-2,5-dimethylpyrrolidin-1-yl)propox...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 0.420 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Jagiellonian University Medical College Curated by ChEMBL | Assay Description Binding affinity to human H3R expressed in rat C6 cells | Bioorg Med Chem 25: 5341-5354 (2017) Article DOI: 10.1016/j.bmc.2017.07.058 BindingDB Entry DOI: 10.7270/Q29W0K0D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(3) dopamine receptor (Homo sapiens (Human)) | BDBM50132031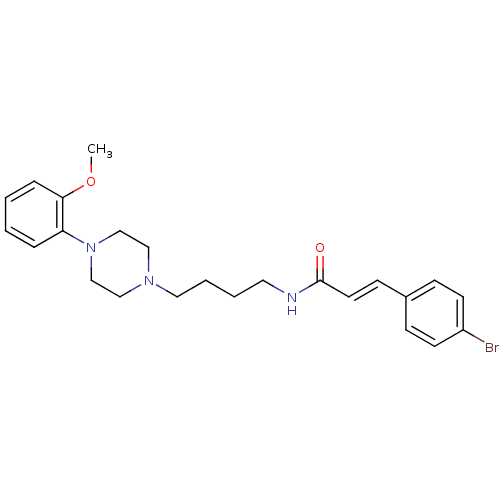 (3-(4-Bromo-phenyl)-N-{4-[4-(2-methoxy-phenyl)-pipe...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.430 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johann Wolfgang Goethe-Universität Curated by ChEMBL | Assay Description Binding affinity for human dopamine receptor D3 by displacing [125I]-iodosulpiride expressed in CHO cells | J Med Chem 46: 3883-99 (2003) Article DOI: 10.1021/jm030836n BindingDB Entry DOI: 10.7270/Q23F4P18 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Rattus norvegicus (rat)) | BDBM50091396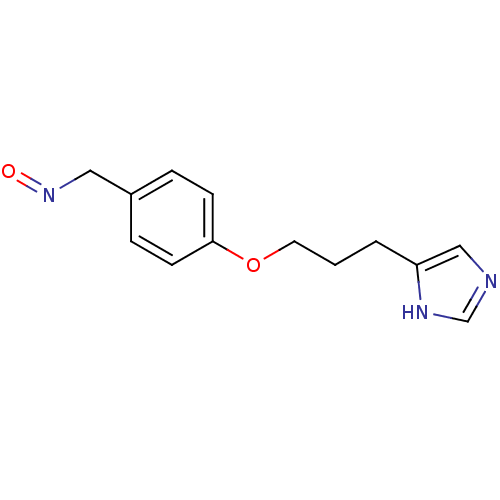 (4-[3-(1H-Imidazol-4-yl)-propoxy]-benzaldehyde oxim...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.440 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Freie Universität Berlin Curated by ChEMBL | Assay Description Histamine H3 receptor antagonist activity in an assay with K+ evoked depolarization-induced release of [3H]-histamine from rat synaptosomes. | J Med Chem 43: 3335-43 (2000) BindingDB Entry DOI: 10.7270/Q2RR1XGV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(3) dopamine receptor (Homo sapiens (Human)) | BDBM50132047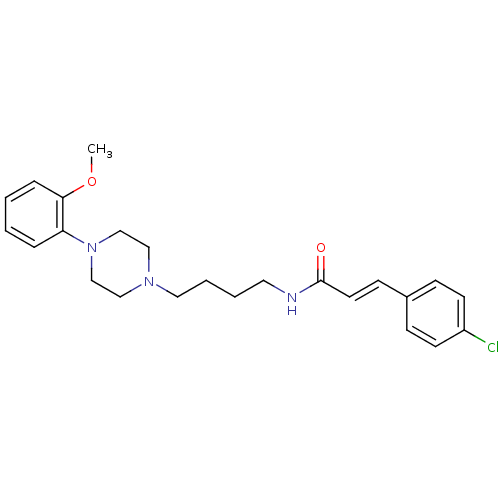 (3-(4-Chloro-phenyl)-N-{4-[4-(2-methoxy-phenyl)-pip...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.440 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johann Wolfgang Goethe-Universität Curated by ChEMBL | Assay Description Binding affinity for human dopamine receptor D3 by displacing [125I]-iodosulpiride expressed in CHO cells | J Med Chem 46: 3883-99 (2003) Article DOI: 10.1021/jm030836n BindingDB Entry DOI: 10.7270/Q23F4P18 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Rattus norvegicus (rat)) | BDBM50091382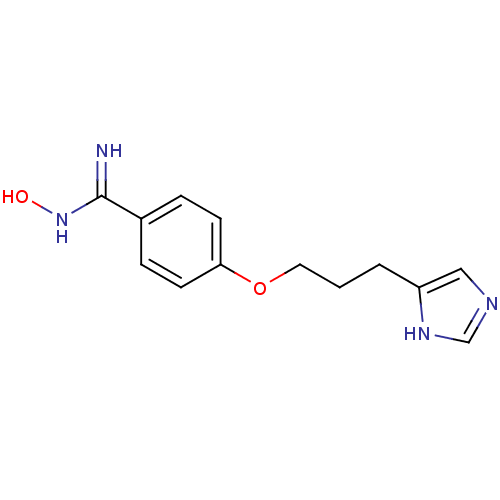 (CHEMBL324724 | N-Hydroxy-4-[3-(1H-imidazol-4-yl)-p...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.460 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Freie Universität Berlin Curated by ChEMBL | Assay Description Histamine H3 receptor antagonist activity in an assay with K+ evoked depolarization-induced release of [3H]-histamine from rat synaptosomes. | J Med Chem 43: 3335-43 (2000) BindingDB Entry DOI: 10.7270/Q2RR1XGV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(3) dopamine receptor (Homo sapiens (Human)) | BDBM50132060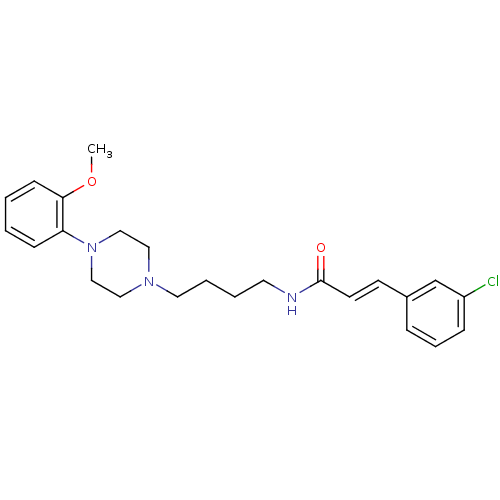 (3-(3-Chloro-phenyl)-N-{4-[4-(2-methoxy-phenyl)-pip...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.460 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johann Wolfgang Goethe-Universität Curated by ChEMBL | Assay Description Binding affinity for human dopamine receptor D3 by displacing [125I]-iodosulpiride expressed in CHO cells | J Med Chem 46: 3883-99 (2003) Article DOI: 10.1021/jm030836n BindingDB Entry DOI: 10.7270/Q23F4P18 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(3) dopamine receptor (Homo sapiens (Human)) | BDBM50132059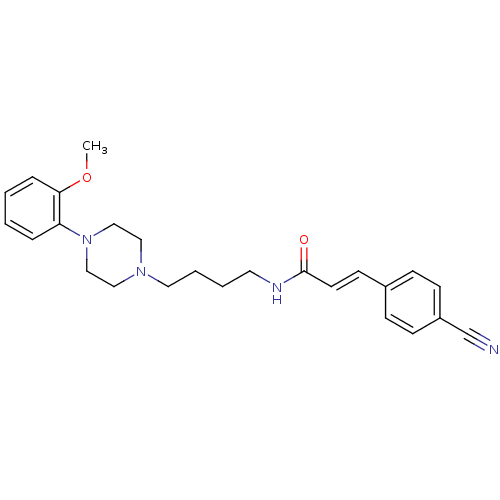 (3-(4-Cyano-phenyl)-N-{4-[4-(2-methoxy-phenyl)-pipe...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.460 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johann Wolfgang Goethe-Universität Curated by ChEMBL | Assay Description Binding affinity for human dopamine receptor D3 by displacing [125I]-iodosulpiride expressed in CHO cells | J Med Chem 46: 3883-99 (2003) Article DOI: 10.1021/jm030836n BindingDB Entry DOI: 10.7270/Q23F4P18 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Homo sapiens (Human)) | BDBM50013718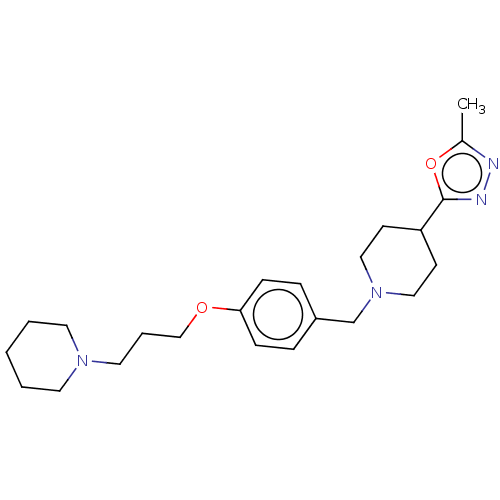 (CHEMBL3264547) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL MMDB PC cid PC sid UniChem Patents Similars | Article PubMed | 0.479 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johann Wolfgang Goethe University Curated by ChEMBL | Assay Description Displacement of [3H]N-alpha-methylhistamine from recombinant human histamine H3 receptor expressed in HEK293 cells after 90 mins | Bioorg Med Chem Lett 24: 2236-9 (2014) Article DOI: 10.1016/j.bmcl.2014.03.098 BindingDB Entry DOI: 10.7270/Q2MP54TD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Hrh3 protein (RAT) | BDBM22541 (Clobenpropit | N''-[(4-chlorophenyl)methyl]{[3-(1H...) | KEGG UniProtKB/TrEMBL GoogleScholar AffyNet | Purchase PC cid PC sid UniChem Patents Similars | PubMed | 0.480 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
U. 109 Curated by PDSP Ki Database | J Pharmacol Exp Ther 271: 452-9 (1994) BindingDB Entry DOI: 10.7270/Q23X8556 | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 2895 total ) | Next | Last >> |