 Found 519 hits with Last Name = 'starnotti' and Initial = 'm'
Found 519 hits with Last Name = 'starnotti' and Initial = 'm' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Carbonic anhydrase 2
(Homo sapiens (Human)) | BDBM50144828

(5-(2,3,5,6-Tetrafluoro-benzenesulfonylamino)-[1,3,...)Show SMILES NS(=O)(=O)c1nnc(NS(=O)(=O)c2c(F)c(F)cc(F)c2F)s1 Show InChI InChI=1S/C8H4F4N4O4S3/c9-2-1-3(10)5(12)6(4(2)11)23(19,20)16-7-14-15-8(21-7)22(13,17)18/h1H,(H,14,16)(H2,13,17,18) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.700 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Firenze
Curated by ChEMBL
| Assay Description
In vitro inhibitory activity against human carbonic anhydrase II (hCAII) |
J Med Chem 47: 2796-804 (2004)
Article DOI: 10.1021/jm031116j
BindingDB Entry DOI: 10.7270/Q26974BC |
More data for this
Ligand-Target Pair | 
3D Structure (docked) |
Carbonic anhydrase 2
(Homo sapiens (Human)) | BDBM50135615
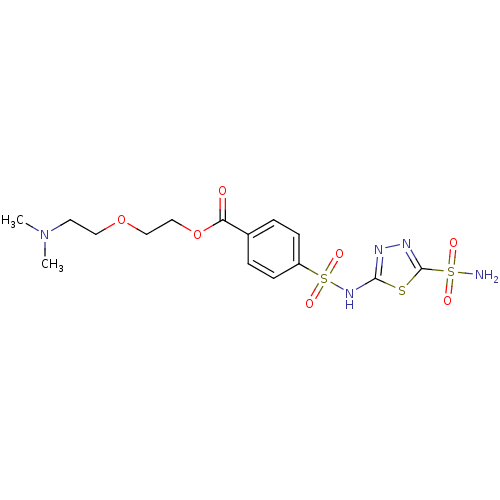
(4-(5-Sulfamoyl-[1,3,4]thiadiazol-2-ylsulfamoyl)-be...)Show SMILES CN(C)CCOCCOC(=O)c1ccc(cc1)S(=O)(=O)Nc1nnc(s1)S(N)(=O)=O Show InChI InChI=1S/C15H21N5O7S3/c1-20(2)7-8-26-9-10-27-13(21)11-3-5-12(6-4-11)30(24,25)19-14-17-18-15(28-14)29(16,22)23/h3-6H,7-10H2,1-2H3,(H,17,19)(H2,16,22,23) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 1.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi
Curated by ChEMBL
| Assay Description
Inhibitory activity against human carbonic anhydrase II |
Bioorg Med Chem Lett 13: 2867-73 (2003)
BindingDB Entry DOI: 10.7270/Q2BR8SQ8 |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 2
(Homo sapiens (Human)) | BDBM50146749

(2,3,5,6-Tetrafluoro-N-[3-methyl-5-sulfamoyl-3H-[1,...)Show SMILES Cn1nc(s\c1=N\C(=O)c1c(F)c(F)cc(F)c1F)S(N)(=O)=O Show InChI InChI=1S/C10H6F4N4O3S2/c1-18-9(22-10(17-18)23(15,20)21)16-8(19)5-6(13)3(11)2-4(12)7(5)14/h2H,1H3,(H2,15,20,21)/b16-9+ | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Firenze
Curated by ChEMBL
| Assay Description
In vitro inhibitory activity against human carbonic anhydrase II (hCAII) |
J Med Chem 47: 2796-804 (2004)
Article DOI: 10.1021/jm031116j
BindingDB Entry DOI: 10.7270/Q26974BC |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 2
(Homo sapiens (Human)) | BDBM11636

(CHEMBL309431 | N-[2-(diethylamino)ethyl]-4-[(5-sul...)Show SMILES CCN(CC)CCNC(=O)c1ccc(cc1)S(=O)(=O)Nc1nnc(s1)S(N)(=O)=O Show InChI InChI=1S/C15H22N6O5S3/c1-3-21(4-2)10-9-17-13(22)11-5-7-12(8-6-11)29(25,26)20-14-18-19-15(27-14)28(16,23)24/h5-8H,3-4,9-10H2,1-2H3,(H,17,22)(H,18,20)(H2,16,23,24) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 1.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi
Curated by ChEMBL
| Assay Description
Inhibitory activity against human carbonic anhydrase II |
Bioorg Med Chem Lett 13: 2867-73 (2003)
BindingDB Entry DOI: 10.7270/Q2BR8SQ8 |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 2
(Homo sapiens (Human)) | BDBM50135629
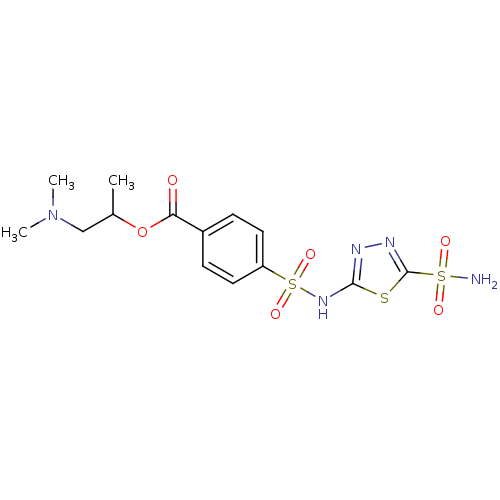
(4-(5-Sulfamoyl-[1,3,4]thiadiazol-2-ylsulfamoyl)-be...)Show SMILES CC(CN(C)C)OC(=O)c1ccc(cc1)S(=O)(=O)Nc1nnc(s1)S(N)(=O)=O Show InChI InChI=1S/C14H19N5O6S3/c1-9(8-19(2)3)25-12(20)10-4-6-11(7-5-10)28(23,24)18-13-16-17-14(26-13)27(15,21)22/h4-7,9H,8H2,1-3H3,(H,16,18)(H2,15,21,22) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 1.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi
Curated by ChEMBL
| Assay Description
Inhibitory activity against human carbonic anhydrase II |
Bioorg Med Chem Lett 13: 2867-73 (2003)
BindingDB Entry DOI: 10.7270/Q2BR8SQ8 |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 12
(Homo sapiens (Human)) | BDBM50171020
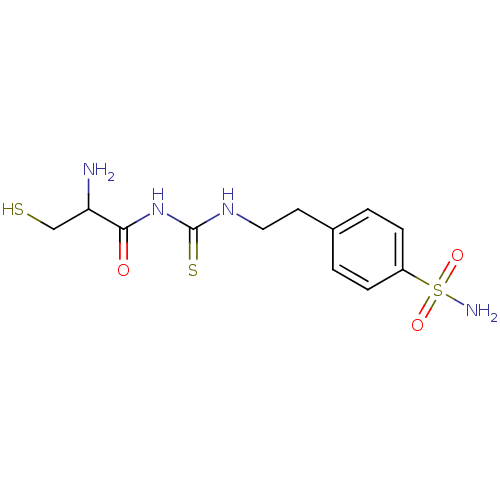
(CHEMBL181082 | N-{2-[4-(aminosulfonyl)phenyl]ethyl...)Show InChI InChI=1S/C12H18N4O3S3/c13-10(7-20)11(17)16-12(21)15-6-5-8-1-3-9(4-2-8)22(14,18)19/h1-4,10,20H,5-7,13H2,(H2,14,18,19)(H2,15,16,17,21) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universita degli Studi
Curated by ChEMBL
| Assay Description
Inhibitory activity against catalytic domain of human carbonic anhydrase XII |
Bioorg Med Chem Lett 15: 3821-7 (2005)
Article DOI: 10.1016/j.bmcl.2005.06.054
BindingDB Entry DOI: 10.7270/Q2H132SW |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 2
(Homo sapiens (Human)) | BDBM50135609
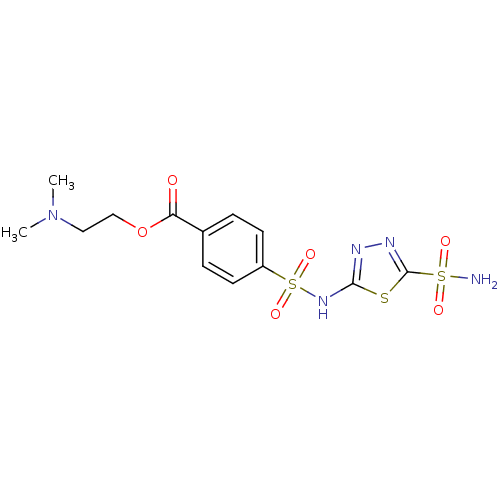
(4-(5-Sulfamoyl-[1,3,4]thiadiazol-2-ylsulfamoyl)-be...)Show SMILES CN(C)CCOC(=O)c1ccc(cc1)S(=O)(=O)Nc1nnc(s1)S(N)(=O)=O Show InChI InChI=1S/C13H17N5O6S3/c1-18(2)7-8-24-11(19)9-3-5-10(6-4-9)27(22,23)17-12-15-16-13(25-12)26(14,20)21/h3-6H,7-8H2,1-2H3,(H,15,17)(H2,14,20,21) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 1.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi
Curated by ChEMBL
| Assay Description
Inhibitory activity against human carbonic anhydrase II |
Bioorg Med Chem Lett 13: 2867-73 (2003)
BindingDB Entry DOI: 10.7270/Q2BR8SQ8 |
More data for this
Ligand-Target Pair | 
3D Structure (docked) |
Carbonic anhydrase 2
(Homo sapiens (Human)) | BDBM50144813
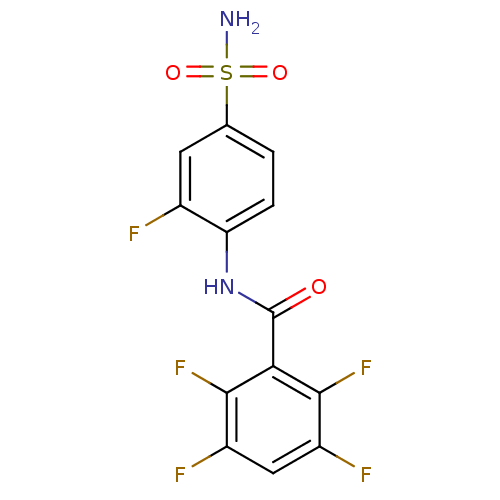
(2,3,5,6-Tetrafluoro-N-(2-fluoro-4-sulfamoyl-phenyl...)Show SMILES NS(=O)(=O)c1ccc(NC(=O)c2c(F)c(F)cc(F)c2F)c(F)c1 Show InChI InChI=1S/C13H7F5N2O3S/c14-6-3-5(24(19,22)23)1-2-9(6)20-13(21)10-11(17)7(15)4-8(16)12(10)18/h1-4H,(H,20,21)(H2,19,22,23) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Firenze
Curated by ChEMBL
| Assay Description
In vitro inhibitory activity against human carbonic anhydrase II (hCAII) |
J Med Chem 47: 2796-804 (2004)
Article DOI: 10.1021/jm031116j
BindingDB Entry DOI: 10.7270/Q26974BC |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 2
(Homo sapiens (Human)) | BDBM50135617
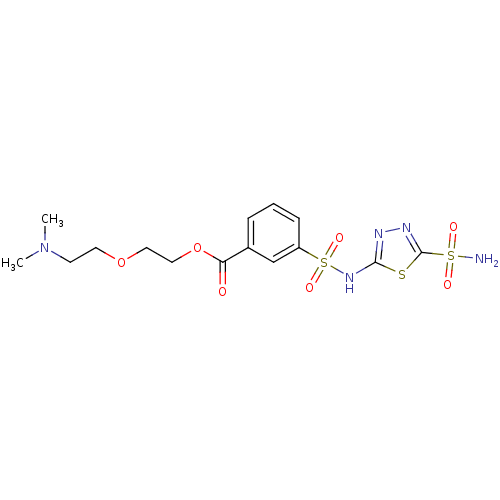
(3-(5-Sulfamoyl-[1,3,4]thiadiazol-2-ylsulfamoyl)-be...)Show SMILES CN(C)CCOCCOC(=O)c1cccc(c1)S(=O)(=O)Nc1nnc(s1)S(N)(=O)=O Show InChI InChI=1S/C15H21N5O7S3/c1-20(2)6-7-26-8-9-27-13(21)11-4-3-5-12(10-11)30(24,25)19-14-17-18-15(28-14)29(16,22)23/h3-5,10H,6-9H2,1-2H3,(H,17,19)(H2,16,22,23) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 1.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi
Curated by ChEMBL
| Assay Description
Inhibitory activity against human carbonic anhydrase II |
Bioorg Med Chem Lett 13: 2867-73 (2003)
BindingDB Entry DOI: 10.7270/Q2BR8SQ8 |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 2
(Homo sapiens (Human)) | BDBM50135623

(4-[3-Methyl-5-sulfamoyl-3H-[1,3,4]thiadiazol-(2E)-...)Show SMILES CN(C)CCOCCOC(=O)c1ccc(cc1)S(=O)(=O)\N=c1\sc(nn1C)S(N)(=O)=O Show InChI InChI=1S/C16H23N5O7S3/c1-20(2)8-9-27-10-11-28-14(22)12-4-6-13(7-5-12)31(25,26)19-15-21(3)18-16(29-15)30(17,23)24/h4-7H,8-11H2,1-3H3,(H2,17,23,24)/b19-15+ | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 1.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi
Curated by ChEMBL
| Assay Description
Inhibitory activity against human carbonic anhydrase II |
Bioorg Med Chem Lett 13: 2867-73 (2003)
BindingDB Entry DOI: 10.7270/Q2BR8SQ8 |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 12
(Homo sapiens (Human)) | BDBM50171013
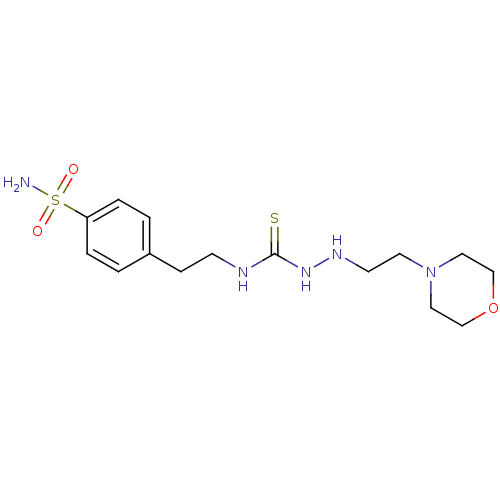
(CHEMBL538564 | N-{2-[4-(aminosulfonyl)phenyl]ethyl...)Show InChI InChI=1S/C15H25N5O3S2/c16-25(21,22)14-3-1-13(2-4-14)5-6-17-15(24)19-18-7-8-20-9-11-23-12-10-20/h1-4,18H,5-12H2,(H2,16,21,22)(H2,17,19,24) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universita degli Studi
Curated by ChEMBL
| Assay Description
Inhibitory activity against catalytic domain of human carbonic anhydrase XII |
Bioorg Med Chem Lett 15: 3821-7 (2005)
Article DOI: 10.1016/j.bmcl.2005.06.054
BindingDB Entry DOI: 10.7270/Q2H132SW |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 2
(Homo sapiens (Human)) | BDBM50135606
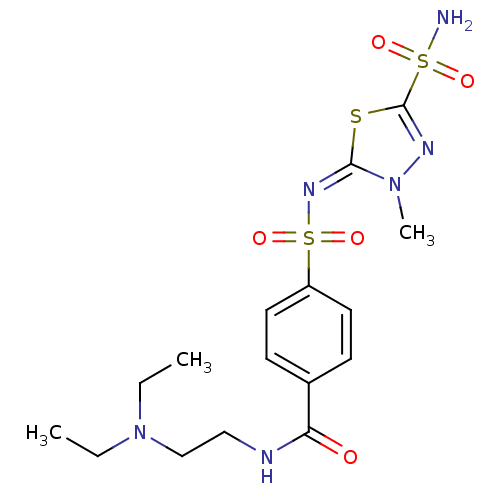
(CHEMBL99337 | N-(2-Diethylamino-ethyl)-4-[3-methyl...)Show SMILES CCN(CC)CCNC(=O)c1ccc(cc1)S(=O)(=O)\N=c1\sc(nn1C)S(N)(=O)=O Show InChI InChI=1S/C16H24N6O5S3/c1-4-22(5-2)11-10-18-14(23)12-6-8-13(9-7-12)30(26,27)20-15-21(3)19-16(28-15)29(17,24)25/h6-9H,4-5,10-11H2,1-3H3,(H,18,23)(H2,17,24,25)/b20-15+ | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 1.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi
Curated by ChEMBL
| Assay Description
Inhibitory activity against human carbonic anhydrase II |
Bioorg Med Chem Lett 13: 2867-73 (2003)
BindingDB Entry DOI: 10.7270/Q2BR8SQ8 |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 2
(Homo sapiens (Human)) | BDBM50135610
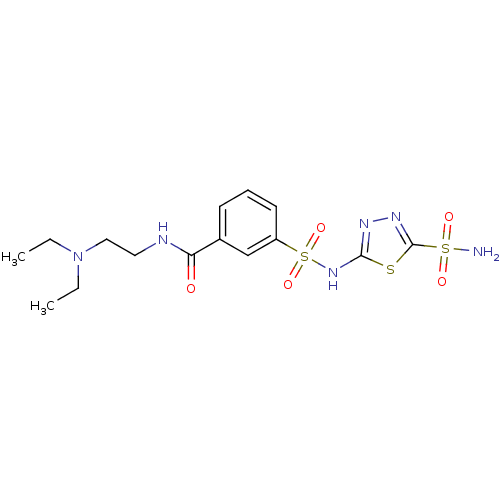
(CHEMBL100329 | N-(2-Diethylamino-ethyl)-3-(5-sulfa...)Show SMILES CCN(CC)CCNC(=O)c1cccc(c1)S(=O)(=O)Nc1nnc(s1)S(N)(=O)=O Show InChI InChI=1S/C15H22N6O5S3/c1-3-21(4-2)9-8-17-13(22)11-6-5-7-12(10-11)29(25,26)20-14-18-19-15(27-14)28(16,23)24/h5-7,10H,3-4,8-9H2,1-2H3,(H,17,22)(H,18,20)(H2,16,23,24) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 1.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi
Curated by ChEMBL
| Assay Description
Inhibitory activity against human carbonic anhydrase II |
Bioorg Med Chem Lett 13: 2867-73 (2003)
BindingDB Entry DOI: 10.7270/Q2BR8SQ8 |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 12
(Homo sapiens (Human)) | BDBM50171011
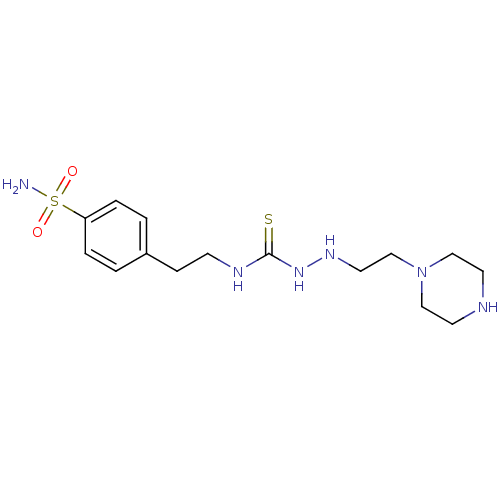
(CHEMBL180177 | Thioureido sulfonamide)Show InChI InChI=1S/C15H26N6O2S2/c16-25(22,23)14-3-1-13(2-4-14)5-6-18-15(24)20-19-9-12-21-10-7-17-8-11-21/h1-4,17,19H,5-12H2,(H2,16,22,23)(H2,18,20,24) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universita degli Studi
Curated by ChEMBL
| Assay Description
Inhibitory activity against catalytic domain of human carbonic anhydrase XII |
Bioorg Med Chem Lett 15: 3821-7 (2005)
Article DOI: 10.1016/j.bmcl.2005.06.054
BindingDB Entry DOI: 10.7270/Q2H132SW |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 2
(Homo sapiens (Human)) | BDBM50135603

(4-[3-Methyl-5-sulfamoyl-3H-[1,3,4]thiadiazol-(2E)-...)Show SMILES CC(CN(C)C)OC(=O)c1ccc(cc1)S(=O)(=O)\N=c1\sc(nn1C)S(N)(=O)=O Show InChI InChI=1S/C15H21N5O6S3/c1-10(9-19(2)3)26-13(21)11-5-7-12(8-6-11)29(24,25)18-14-20(4)17-15(27-14)28(16,22)23/h5-8,10H,9H2,1-4H3,(H2,16,22,23)/b18-14+ | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 1.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi
Curated by ChEMBL
| Assay Description
Inhibitory activity against human carbonic anhydrase II |
Bioorg Med Chem Lett 13: 2867-73 (2003)
BindingDB Entry DOI: 10.7270/Q2BR8SQ8 |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 12
(Homo sapiens (Human)) | BDBM50171014
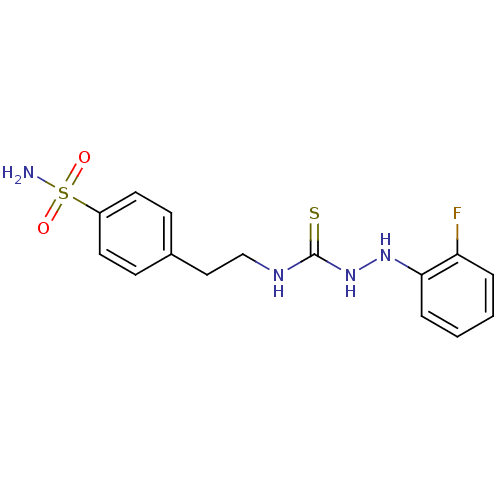
(CHEMBL179355 | N-{2-[4-(aminosulfonyl)phenyl]ethyl...)Show InChI InChI=1S/C15H17FN4O2S2/c16-13-3-1-2-4-14(13)19-20-15(23)18-10-9-11-5-7-12(8-6-11)24(17,21)22/h1-8,19H,9-10H2,(H2,17,21,22)(H2,18,20,23) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universita degli Studi
Curated by ChEMBL
| Assay Description
Inhibitory activity against catalytic domain of human carbonic anhydrase XII |
Bioorg Med Chem Lett 15: 3821-7 (2005)
Article DOI: 10.1016/j.bmcl.2005.06.054
BindingDB Entry DOI: 10.7270/Q2H132SW |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 2
(Homo sapiens (Human)) | BDBM50135619
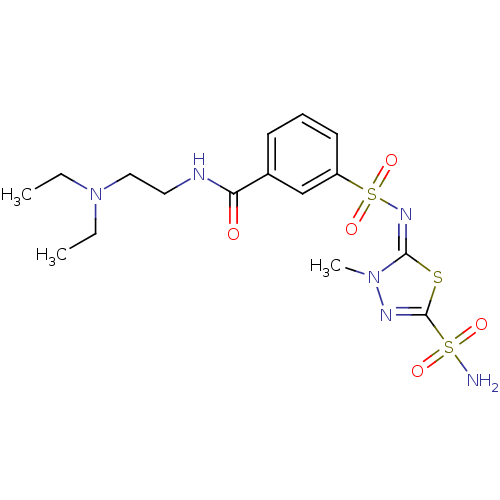
(CHEMBL317383 | N-(2-Diethylamino-ethyl)-3-[3-methy...)Show SMILES CCN(CC)CCNC(=O)c1cccc(c1)S(=O)(=O)\N=c1\sc(nn1C)S(N)(=O)=O Show InChI InChI=1S/C16H24N6O5S3/c1-4-22(5-2)10-9-18-14(23)12-7-6-8-13(11-12)30(26,27)20-15-21(3)19-16(28-15)29(17,24)25/h6-8,11H,4-5,9-10H2,1-3H3,(H,18,23)(H2,17,24,25)/b20-15+ | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 1.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi
Curated by ChEMBL
| Assay Description
Inhibitory activity against human carbonic anhydrase II |
Bioorg Med Chem Lett 13: 2867-73 (2003)
BindingDB Entry DOI: 10.7270/Q2BR8SQ8 |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 2
(Homo sapiens (Human)) | BDBM50135613

(3-[3-Methyl-5-sulfamoyl-3H-[1,3,4]thiadiazol-(2E)-...)Show SMILES CN(C)CCOCCOC(=O)c1cccc(c1)S(=O)(=O)\N=c1\sc(nn1C)S(N)(=O)=O Show InChI InChI=1S/C16H23N5O7S3/c1-20(2)7-8-27-9-10-28-14(22)12-5-4-6-13(11-12)31(25,26)19-15-21(3)18-16(29-15)30(17,23)24/h4-6,11H,7-10H2,1-3H3,(H2,17,23,24)/b19-15+ | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi
Curated by ChEMBL
| Assay Description
Inhibitory activity against human carbonic anhydrase II |
Bioorg Med Chem Lett 13: 2867-73 (2003)
BindingDB Entry DOI: 10.7270/Q2BR8SQ8 |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 2
(Homo sapiens (Human)) | BDBM50135620

(3-(5-Sulfamoyl-[1,3,4]thiadiazol-2-ylsulfamoyl)-be...)Show SMILES CC(CN(C)C)OC(=O)c1cccc(c1)S(=O)(=O)Nc1nnc(s1)S(N)(=O)=O Show InChI InChI=1S/C14H19N5O6S3/c1-9(8-19(2)3)25-12(20)10-5-4-6-11(7-10)28(23,24)18-13-16-17-14(26-13)27(15,21)22/h4-7,9H,8H2,1-3H3,(H,16,18)(H2,15,21,22) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi
Curated by ChEMBL
| Assay Description
Inhibitory activity against human carbonic anhydrase II |
Bioorg Med Chem Lett 13: 2867-73 (2003)
BindingDB Entry DOI: 10.7270/Q2BR8SQ8 |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 12
(Homo sapiens (Human)) | BDBM50171016
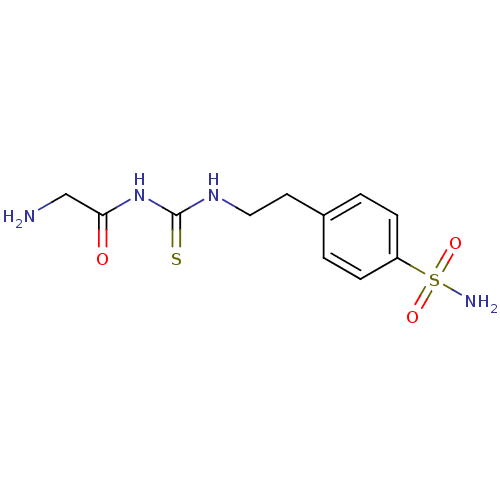
(4-{2-[3-(2-Amino-acetyl)-thioureido]-ethyl}-benzen...)Show InChI InChI=1S/C11H16N4O3S2/c12-7-10(16)15-11(19)14-6-5-8-1-3-9(4-2-8)20(13,17)18/h1-4H,5-7,12H2,(H2,13,17,18)(H2,14,15,16,19) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universita degli Studi
Curated by ChEMBL
| Assay Description
Inhibitory activity against catalytic domain of human carbonic anhydrase XII |
Bioorg Med Chem Lett 15: 3821-7 (2005)
Article DOI: 10.1016/j.bmcl.2005.06.054
BindingDB Entry DOI: 10.7270/Q2H132SW |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 2
(Homo sapiens (Human)) | BDBM50144820

(2,3,5,6-Tetrafluoro-N-(5-sulfamoyl-[1,3,4]thiadiaz...)Show SMILES NS(=O)(=O)c1nnc(NC(=O)c2c(F)c(F)cc(F)c2F)s1 Show InChI InChI=1S/C9H4F4N4O3S2/c10-2-1-3(11)6(13)4(5(2)12)7(18)15-8-16-17-9(21-8)22(14,19)20/h1H,(H2,14,19,20)(H,15,16,18) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 2.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Firenze
Curated by ChEMBL
| Assay Description
In vitro inhibitory activity against human carbonic anhydrase II (hCAII) |
J Med Chem 47: 2796-804 (2004)
Article DOI: 10.1021/jm031116j
BindingDB Entry DOI: 10.7270/Q26974BC |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 12
(Homo sapiens (Human)) | BDBM50171023
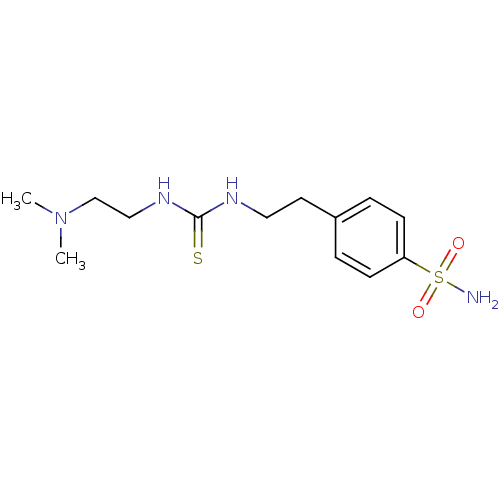
(4-{2-[3-(2-Dimethylamino-ethyl)-thioureido]-ethyl}...)Show InChI InChI=1S/C13H22N4O2S2/c1-17(2)10-9-16-13(20)15-8-7-11-3-5-12(6-4-11)21(14,18)19/h3-6H,7-10H2,1-2H3,(H2,14,18,19)(H2,15,16,20) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| 2.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universita degli Studi
Curated by ChEMBL
| Assay Description
Inhibitory activity against catalytic domain of human carbonic anhydrase XII |
Bioorg Med Chem Lett 15: 3821-7 (2005)
Article DOI: 10.1016/j.bmcl.2005.06.054
BindingDB Entry DOI: 10.7270/Q2H132SW |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 2
(Homo sapiens (Human)) | BDBM50135626

(3-(5-Sulfamoyl-[1,3,4]thiadiazol-2-ylsulfamoyl)-be...)Show SMILES CN(C)CCOC(=O)c1cccc(c1)S(=O)(=O)Nc1nnc(s1)S(N)(=O)=O Show InChI InChI=1S/C13H17N5O6S3/c1-18(2)6-7-24-11(19)9-4-3-5-10(8-9)27(22,23)17-12-15-16-13(25-12)26(14,20)21/h3-5,8H,6-7H2,1-2H3,(H,15,17)(H2,14,20,21) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 2.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi
Curated by ChEMBL
| Assay Description
Inhibitory activity against human carbonic anhydrase II |
Bioorg Med Chem Lett 13: 2867-73 (2003)
BindingDB Entry DOI: 10.7270/Q2BR8SQ8 |
More data for this
Ligand-Target Pair | 
3D Structure (docked) |
Carbonic anhydrase 2
(Homo sapiens (Human)) | BDBM50135611

(3-[3-Methyl-5-sulfamoyl-3H-[1,3,4]thiadiazol-(2E)-...)Show SMILES CC(CN(C)C)OC(=O)c1cccc(c1)S(=O)(=O)\N=c1\sc(nn1C)S(N)(=O)=O Show InChI InChI=1S/C15H21N5O6S3/c1-10(9-19(2)3)26-13(21)11-6-5-7-12(8-11)29(24,25)18-14-20(4)17-15(27-14)28(16,22)23/h5-8,10H,9H2,1-4H3,(H2,16,22,23)/b18-14+ | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 2.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi
Curated by ChEMBL
| Assay Description
Inhibitory activity against human carbonic anhydrase II |
Bioorg Med Chem Lett 13: 2867-73 (2003)
BindingDB Entry DOI: 10.7270/Q2BR8SQ8 |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 12
(Homo sapiens (Human)) | BDBM50171025
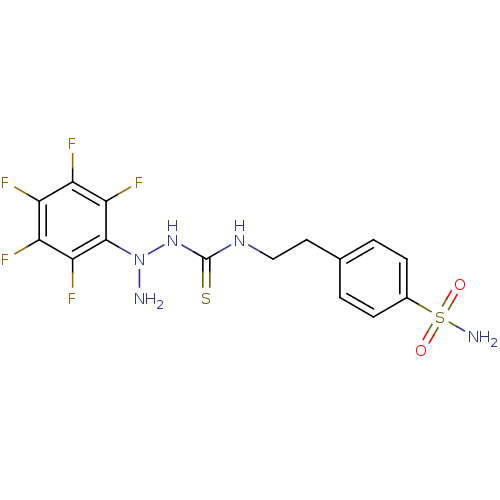
(CHEMBL182197 | N-{2-[4-(aminosulfonyl)phenyl]ethyl...)Show SMILES NN(NC(=S)NCCc1ccc(cc1)S(N)(=O)=O)c1c(F)c(F)c(F)c(F)c1F Show InChI InChI=1S/C15H14F5N5O2S2/c16-9-10(17)12(19)14(13(20)11(9)18)25(21)24-15(28)23-6-5-7-1-3-8(4-2-7)29(22,26)27/h1-4H,5-6,21H2,(H2,22,26,27)(H2,23,24,28) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 2.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universita degli Studi
Curated by ChEMBL
| Assay Description
Inhibitory activity against catalytic domain of human carbonic anhydrase XII |
Bioorg Med Chem Lett 15: 3821-7 (2005)
Article DOI: 10.1016/j.bmcl.2005.06.054
BindingDB Entry DOI: 10.7270/Q2H132SW |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 2
(Homo sapiens (Human)) | BDBM50135622

(4-[3-Methyl-5-sulfamoyl-3H-[1,3,4]thiadiazol-(2E)-...)Show SMILES CN(C)CCOC(=O)c1ccc(cc1)S(=O)(=O)\N=c1\sc(nn1C)S(N)(=O)=O Show InChI InChI=1S/C14H19N5O6S3/c1-18(2)8-9-25-12(20)10-4-6-11(7-5-10)28(23,24)17-13-19(3)16-14(26-13)27(15,21)22/h4-7H,8-9H2,1-3H3,(H2,15,21,22)/b17-13+ | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 2.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi
Curated by ChEMBL
| Assay Description
Inhibitory activity against human carbonic anhydrase II |
Bioorg Med Chem Lett 13: 2867-73 (2003)
BindingDB Entry DOI: 10.7270/Q2BR8SQ8 |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 2
(Homo sapiens (Human)) | BDBM50135614
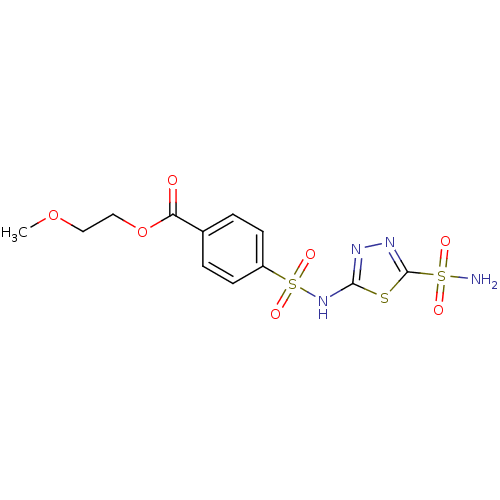
(4-(5-Sulfamoyl-[1,3,4]thiadiazol-2-ylsulfamoyl)-be...)Show SMILES COCCOC(=O)c1ccc(cc1)S(=O)(=O)Nc1nnc(s1)S(N)(=O)=O Show InChI InChI=1S/C12H14N4O7S3/c1-22-6-7-23-10(17)8-2-4-9(5-3-8)26(20,21)16-11-14-15-12(24-11)25(13,18)19/h2-5H,6-7H2,1H3,(H,14,16)(H2,13,18,19) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 2.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi
Curated by ChEMBL
| Assay Description
Inhibitory activity against human carbonic anhydrase II |
Bioorg Med Chem Lett 13: 2867-73 (2003)
BindingDB Entry DOI: 10.7270/Q2BR8SQ8 |
More data for this
Ligand-Target Pair | 
3D Structure (docked) |
Carbonic anhydrase 2
(Homo sapiens (Human)) | BDBM50135627
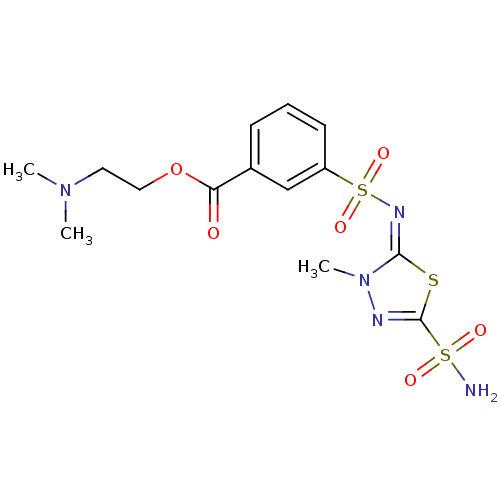
(3-[3-Methyl-5-sulfamoyl-3H-[1,3,4]thiadiazol-(2E)-...)Show SMILES CN(C)CCOC(=O)c1cccc(c1)S(=O)(=O)\N=c1\sc(nn1C)S(N)(=O)=O Show InChI InChI=1S/C14H19N5O6S3/c1-18(2)7-8-25-12(20)10-5-4-6-11(9-10)28(23,24)17-13-19(3)16-14(26-13)27(15,21)22/h4-6,9H,7-8H2,1-3H3,(H2,15,21,22)/b17-13+ | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 2.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi
Curated by ChEMBL
| Assay Description
Inhibitory activity against human carbonic anhydrase II |
Bioorg Med Chem Lett 13: 2867-73 (2003)
BindingDB Entry DOI: 10.7270/Q2BR8SQ8 |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 2
(Homo sapiens (Human)) | BDBM50135605

(3-(5-Sulfamoyl-[1,3,4]thiadiazol-2-ylsulfamoyl)-be...)Show SMILES COCCOC(=O)c1cccc(c1)S(=O)(=O)Nc1nnc(s1)S(N)(=O)=O Show InChI InChI=1S/C12H14N4O7S3/c1-22-5-6-23-10(17)8-3-2-4-9(7-8)26(20,21)16-11-14-15-12(24-11)25(13,18)19/h2-4,7H,5-6H2,1H3,(H,14,16)(H2,13,18,19) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 2.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi
Curated by ChEMBL
| Assay Description
Inhibitory activity against human carbonic anhydrase II |
Bioorg Med Chem Lett 13: 2867-73 (2003)
BindingDB Entry DOI: 10.7270/Q2BR8SQ8 |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 2
(Homo sapiens (Human)) | BDBM50135616
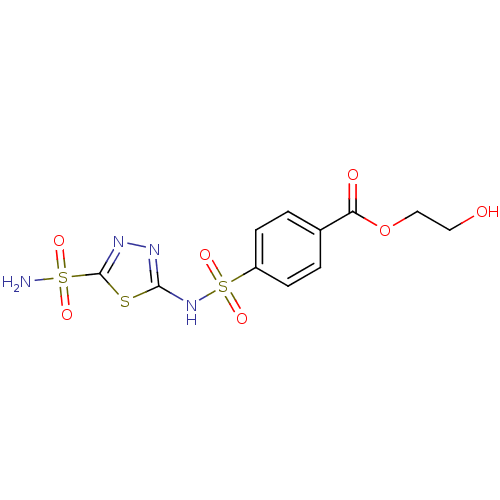
(4-(5-Sulfamoyl-[1,3,4]thiadiazol-2-ylsulfamoyl)-be...)Show SMILES NS(=O)(=O)c1nnc(NS(=O)(=O)c2ccc(cc2)C(=O)OCCO)s1 Show InChI InChI=1S/C11H12N4O7S3/c12-24(18,19)11-14-13-10(23-11)15-25(20,21)8-3-1-7(2-4-8)9(17)22-6-5-16/h1-4,16H,5-6H2,(H,13,15)(H2,12,18,19) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 2.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi
Curated by ChEMBL
| Assay Description
Inhibitory activity against human carbonic anhydrase II |
Bioorg Med Chem Lett 13: 2867-73 (2003)
BindingDB Entry DOI: 10.7270/Q2BR8SQ8 |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 12
(Homo sapiens (Human)) | BDBM10885

((4R)-4-(ethylamino)-2-(3-methoxypropyl)-1,1-dioxo-...)Show SMILES CCN[C@H]1CN(CCCOC)S(=O)(=O)c2sc(cc12)S(N)(=O)=O |r| Show InChI InChI=1S/C12H21N3O5S3/c1-3-14-10-8-15(5-4-6-20-2)23(18,19)12-9(10)7-11(21-12)22(13,16)17/h7,10,14H,3-6,8H2,1-2H3,(H2,13,16,17)/t10-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universita degli Studi
Curated by ChEMBL
| Assay Description
Inhibitory activity against catalytic domain of human carbonic anhydrase XII |
Bioorg Med Chem Lett 15: 3821-7 (2005)
Article DOI: 10.1016/j.bmcl.2005.06.054
BindingDB Entry DOI: 10.7270/Q2H132SW |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 2
(Homo sapiens (Human)) | BDBM10885

((4R)-4-(ethylamino)-2-(3-methoxypropyl)-1,1-dioxo-...)Show SMILES CCN[C@H]1CN(CCCOC)S(=O)(=O)c2sc(cc12)S(N)(=O)=O |r| Show InChI InChI=1S/C12H21N3O5S3/c1-3-14-10-8-15(5-4-6-20-2)23(18,19)12-9(10)7-11(21-12)22(13,16)17/h7,10,14H,3-6,8H2,1-2H3,(H2,13,16,17)/t10-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
PubMed
| 3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi
Curated by ChEMBL
| Assay Description
Inhibitory activity against human carbonic anhydrase II |
Bioorg Med Chem Lett 13: 2867-73 (2003)
BindingDB Entry DOI: 10.7270/Q2BR8SQ8 |
More data for this
Ligand-Target Pair | 
3D Structure (docked) |
Carbonic anhydrase 1
(Homo sapiens (Human)) | BDBM10885

((4R)-4-(ethylamino)-2-(3-methoxypropyl)-1,1-dioxo-...)Show SMILES CCN[C@H]1CN(CCCOC)S(=O)(=O)c2sc(cc12)S(N)(=O)=O |r| Show InChI InChI=1S/C12H21N3O5S3/c1-3-14-10-8-15(5-4-6-20-2)23(18,19)12-9(10)7-11(21-12)22(13,16)17/h7,10,14H,3-6,8H2,1-2H3,(H2,13,16,17)/t10-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
PubMed
| 3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi
Curated by ChEMBL
| Assay Description
Compound was evaluated for binding affinity against human carbonic anhydrase II (hCA -II) |
Bioorg Med Chem Lett 11: 1787-91 (2001)
BindingDB Entry DOI: 10.7270/Q2K64JM5 |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 2
(Homo sapiens (Human)) | BDBM50101340

(4-Sulfamoyl-N-(5-sulfamoyl-[1,3,4]thiadiazol-2-yl)...)Show SMILES NS(=O)(=O)c1nnc(NC(=O)c2ccc(cc2)S(N)(=O)=O)s1 Show InChI InChI=1S/C9H9N5O5S3/c10-21(16,17)6-3-1-5(2-4-6)7(15)12-8-13-14-9(20-8)22(11,18)19/h1-4H,(H2,10,16,17)(H2,11,18,19)(H,12,13,15) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi
Curated by ChEMBL
| Assay Description
Binding affinity against human carbonic anhydrase II (hCA -II) |
Bioorg Med Chem Lett 11: 1787-91 (2001)
BindingDB Entry DOI: 10.7270/Q2K64JM5 |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 2
(Homo sapiens (Human)) | BDBM10885

((4R)-4-(ethylamino)-2-(3-methoxypropyl)-1,1-dioxo-...)Show SMILES CCN[C@H]1CN(CCCOC)S(=O)(=O)c2sc(cc12)S(N)(=O)=O |r| Show InChI InChI=1S/C12H21N3O5S3/c1-3-14-10-8-15(5-4-6-20-2)23(18,19)12-9(10)7-11(21-12)22(13,16)17/h7,10,14H,3-6,8H2,1-2H3,(H2,13,16,17)/t10-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
PubMed
| 3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ Montpellier II
Curated by ChEMBL
| Assay Description
Inhibition of human carbonic anhydrase II |
Bioorg Med Chem Lett 14: 225-9 (2003)
BindingDB Entry DOI: 10.7270/Q2WD414J |
More data for this
Ligand-Target Pair | 
3D Structure (docked) |
Carbonic anhydrase 12
(Homo sapiens (Human)) | BDBM50171012
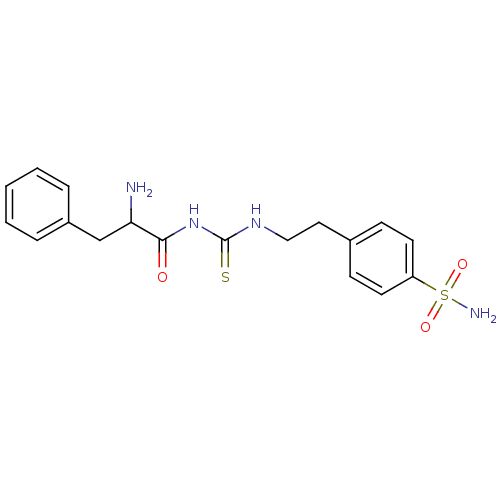
(CHEMBL180506 | N-{2-[4-(aminosulfonyl)phenyl]ethyl...)Show SMILES NC(Cc1ccccc1)C(=O)NC(=S)NCCc1ccc(cc1)S(N)(=O)=O Show InChI InChI=1S/C18H22N4O3S2/c19-16(12-14-4-2-1-3-5-14)17(23)22-18(26)21-11-10-13-6-8-15(9-7-13)27(20,24)25/h1-9,16H,10-12,19H2,(H2,20,24,25)(H2,21,22,23,26) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universita degli Studi
Curated by ChEMBL
| Assay Description
Inhibitory activity against catalytic domain of human carbonic anhydrase XII |
Bioorg Med Chem Lett 15: 3821-7 (2005)
Article DOI: 10.1016/j.bmcl.2005.06.054
BindingDB Entry DOI: 10.7270/Q2H132SW |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 12
(Homo sapiens (Human)) | BDBM50171024
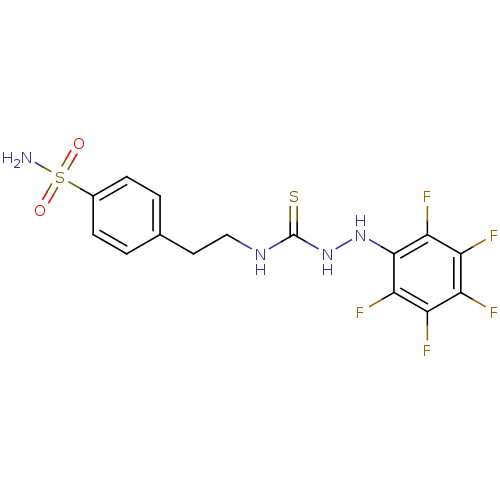
(CHEMBL361955 | N-{2-[4-(aminosulfonyl)phenyl]ethyl...)Show SMILES NS(=O)(=O)c1ccc(CCNC(=S)NNc2c(F)c(F)c(F)c(F)c2F)cc1 Show InChI InChI=1S/C15H13F5N4O2S2/c16-9-10(17)12(19)14(13(20)11(9)18)23-24-15(27)22-6-5-7-1-3-8(4-2-7)28(21,25)26/h1-4,23H,5-6H2,(H2,21,25,26)(H2,22,24,27) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universita degli Studi
Curated by ChEMBL
| Assay Description
Inhibitory activity against catalytic domain of human carbonic anhydrase XII |
Bioorg Med Chem Lett 15: 3821-7 (2005)
Article DOI: 10.1016/j.bmcl.2005.06.054
BindingDB Entry DOI: 10.7270/Q2H132SW |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 2
(Homo sapiens (Human)) | BDBM10885

((4R)-4-(ethylamino)-2-(3-methoxypropyl)-1,1-dioxo-...)Show SMILES CCN[C@H]1CN(CCCOC)S(=O)(=O)c2sc(cc12)S(N)(=O)=O |r| Show InChI InChI=1S/C12H21N3O5S3/c1-3-14-10-8-15(5-4-6-20-2)23(18,19)12-9(10)7-11(21-12)22(13,16)17/h7,10,14H,3-6,8H2,1-2H3,(H2,13,16,17)/t10-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
Article
PubMed
| 3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universita degli Studi
Curated by ChEMBL
| Assay Description
Inhibitory activity against human carbonic anhydrase II |
Bioorg Med Chem Lett 15: 3821-7 (2005)
Article DOI: 10.1016/j.bmcl.2005.06.054
BindingDB Entry DOI: 10.7270/Q2H132SW |
More data for this
Ligand-Target Pair | 
3D Structure (docked) |
Carbonic anhydrase 2
(Homo sapiens (Human)) | BDBM50135625
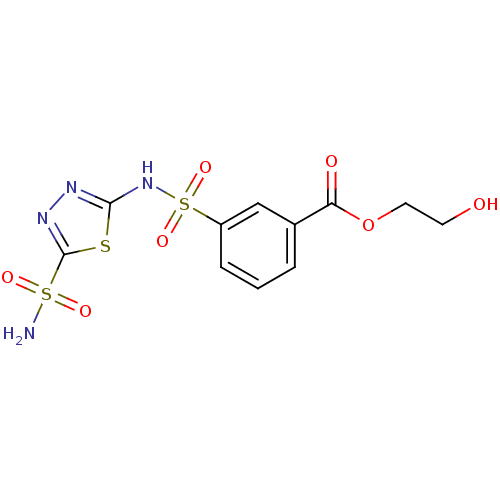
(3-(5-Sulfamoyl-[1,3,4]thiadiazol-2-ylsulfamoyl)-be...)Show SMILES NS(=O)(=O)c1nnc(NS(=O)(=O)c2cccc(c2)C(=O)OCCO)s1 Show InChI InChI=1S/C11H12N4O7S3/c12-24(18,19)11-14-13-10(23-11)15-25(20,21)8-3-1-2-7(6-8)9(17)22-5-4-16/h1-3,6,16H,4-5H2,(H,13,15)(H2,12,18,19) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 3.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi
Curated by ChEMBL
| Assay Description
Inhibitory activity against human carbonic anhydrase II |
Bioorg Med Chem Lett 13: 2867-73 (2003)
BindingDB Entry DOI: 10.7270/Q2BR8SQ8 |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 2
(Homo sapiens (Human)) | BDBM50135628
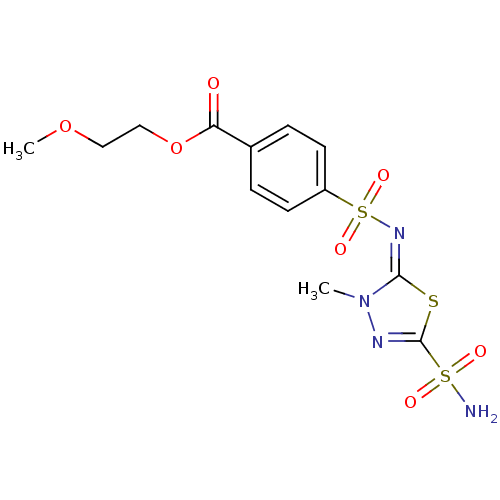
(4-[3-Methyl-5-sulfamoyl-3H-[1,3,4]thiadiazol-(2E)-...)Show SMILES COCCOC(=O)c1ccc(cc1)S(=O)(=O)\N=c1\sc(nn1C)S(N)(=O)=O Show InChI InChI=1S/C13H16N4O7S3/c1-17-12(25-13(15-17)26(14,19)20)16-27(21,22)10-5-3-9(4-6-10)11(18)24-8-7-23-2/h3-6H,7-8H2,1-2H3,(H2,14,19,20)/b16-12+ | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 3.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi
Curated by ChEMBL
| Assay Description
Inhibitory activity against human carbonic anhydrase II |
Bioorg Med Chem Lett 13: 2867-73 (2003)
BindingDB Entry DOI: 10.7270/Q2BR8SQ8 |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 2
(Homo sapiens (Human)) | BDBM50135607
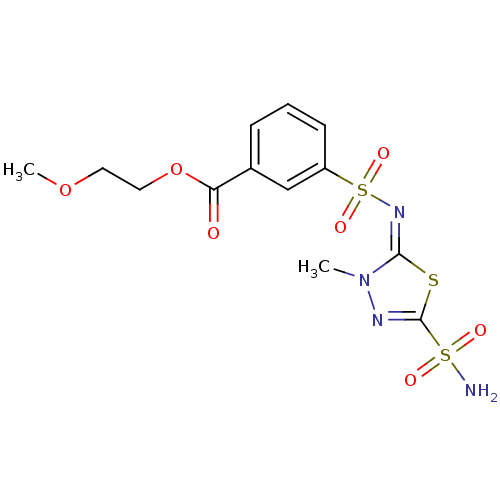
(3-[3-Methyl-5-sulfamoyl-3H-[1,3,4]thiadiazol-(2E)-...)Show SMILES COCCOC(=O)c1cccc(c1)S(=O)(=O)\N=c1\sc(nn1C)S(N)(=O)=O Show InChI InChI=1S/C13H16N4O7S3/c1-17-12(25-13(15-17)26(14,19)20)16-27(21,22)10-5-3-4-9(8-10)11(18)24-7-6-23-2/h3-5,8H,6-7H2,1-2H3,(H2,14,19,20)/b16-12+ | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 3.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi
Curated by ChEMBL
| Assay Description
Inhibitory activity against human carbonic anhydrase II |
Bioorg Med Chem Lett 13: 2867-73 (2003)
BindingDB Entry DOI: 10.7270/Q2BR8SQ8 |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 12
(Homo sapiens (Human)) | BDBM50171009
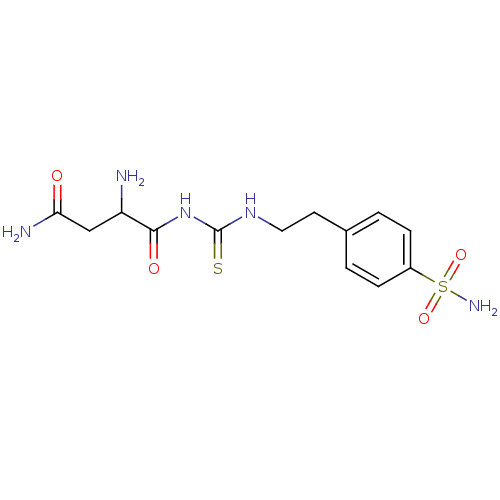
((3R)-3-{2-[({2-[4-(aminosulfonyl)phenyl]ethyl}amin...)Show SMILES NC(CC(N)=O)C(=O)NC(=S)NCCc1ccc(cc1)S(N)(=O)=O Show InChI InChI=1S/C13H19N5O4S2/c14-10(7-11(15)19)12(20)18-13(23)17-6-5-8-1-3-9(4-2-8)24(16,21)22/h1-4,10H,5-7,14H2,(H2,15,19)(H2,16,21,22)(H2,17,18,20,23) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 3.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universita degli Studi
Curated by ChEMBL
| Assay Description
Inhibitory activity against catalytic domain of human carbonic anhydrase XII |
Bioorg Med Chem Lett 15: 3821-7 (2005)
Article DOI: 10.1016/j.bmcl.2005.06.054
BindingDB Entry DOI: 10.7270/Q2H132SW |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 12
(Homo sapiens (Human)) | BDBM10881

(CHEMBL288100 | MZA3 | Methazolamide | Methazolamid...)Show InChI InChI=1S/C5H8N4O3S2/c1-3(10)7-4-9(2)8-5(13-4)14(6,11)12/h1-2H3,(H2,6,11,12) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 3.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universita degli Studi
Curated by ChEMBL
| Assay Description
Inhibitory activity against catalytic domain of human carbonic anhydrase XII |
Bioorg Med Chem Lett 15: 3821-7 (2005)
Article DOI: 10.1016/j.bmcl.2005.06.054
BindingDB Entry DOI: 10.7270/Q2H132SW |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 12
(Homo sapiens (Human)) | BDBM10884

((2S,4S)-4-(ethylamino)-2-methyl-1,1-dioxo-2H,3H,4H...)Show SMILES CCN[C@H]1C[C@H](C)S(=O)(=O)c2sc(cc12)S(N)(=O)=O |r| Show InChI InChI=1S/C10H16N2O4S3/c1-3-12-8-4-6(2)18(13,14)10-7(8)5-9(17-10)19(11,15)16/h5-6,8,12H,3-4H2,1-2H3,(H2,11,15,16)/t6-,8-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 3.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universita degli Studi
Curated by ChEMBL
| Assay Description
Inhibitory activity against catalytic domain of human carbonic anhydrase XII |
Bioorg Med Chem Lett 15: 3821-7 (2005)
Article DOI: 10.1016/j.bmcl.2005.06.054
BindingDB Entry DOI: 10.7270/Q2H132SW |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 2
(Homo sapiens (Human)) | BDBM50146761

(5-(3-Pentafluorophenyl-ureido)-[1,3,4]thiadiazole-...)Show SMILES NS(=O)(=O)c1nnc(NC(=O)Nc2c(F)c(F)c(F)c(F)c2F)s1 Show InChI InChI=1S/C9H4F5N5O3S2/c10-1-2(11)4(13)6(5(14)3(1)12)16-7(20)17-8-18-19-9(23-8)24(15,21)22/h(H2,15,21,22)(H2,16,17,18,20) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| 3.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Firenze
Curated by ChEMBL
| Assay Description
In vitro inhibitory activity against human carbonic anhydrase II (hCAII) |
J Med Chem 47: 2796-804 (2004)
Article DOI: 10.1021/jm031116j
BindingDB Entry DOI: 10.7270/Q26974BC |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 2
(Homo sapiens (Human)) | BDBM50146755
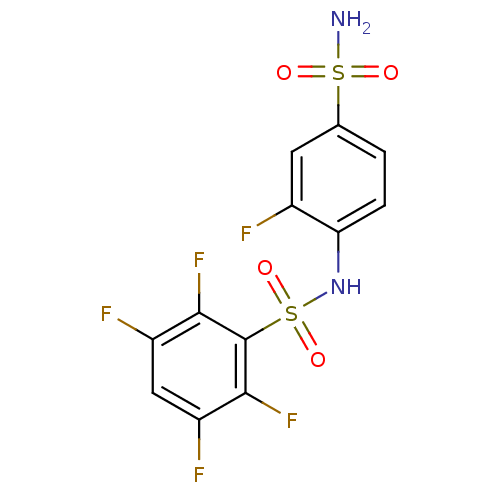
(2,3,5,6-Tetrafluoro-N-(2-fluoro-4-sulfamoyl-phenyl...)Show SMILES NS(=O)(=O)c1ccc(NS(=O)(=O)c2c(F)c(F)cc(F)c2F)c(F)c1 Show InChI InChI=1S/C12H7F5N2O4S2/c13-6-3-5(24(18,20)21)1-2-9(6)19-25(22,23)12-10(16)7(14)4-8(15)11(12)17/h1-4,19H,(H2,18,20,21) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 3.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Firenze
Curated by ChEMBL
| Assay Description
In vitro inhibitory activity against human carbonic anhydrase II (hCAII) |
J Med Chem 47: 2796-804 (2004)
Article DOI: 10.1021/jm031116j
BindingDB Entry DOI: 10.7270/Q26974BC |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 2
(Homo sapiens (Human)) | BDBM50135604
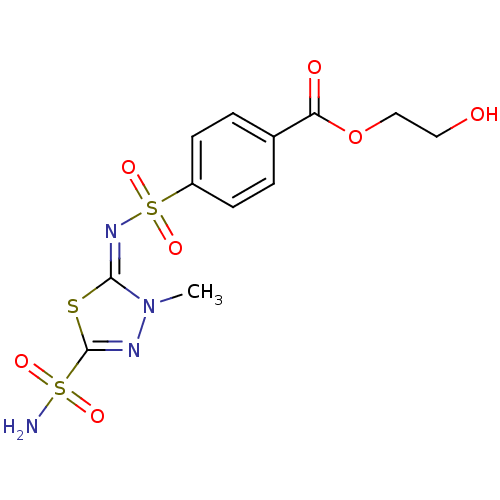
(4-[3-Methyl-5-sulfamoyl-3H-[1,3,4]thiadiazol-(2E)-...)Show SMILES Cn1nc(s\c1=N\S(=O)(=O)c1ccc(cc1)C(=O)OCCO)S(N)(=O)=O Show InChI InChI=1S/C12H14N4O7S3/c1-16-11(24-12(14-16)25(13,19)20)15-26(21,22)9-4-2-8(3-5-9)10(18)23-7-6-17/h2-5,17H,6-7H2,1H3,(H2,13,19,20)/b15-11+ | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi
Curated by ChEMBL
| Assay Description
Inhibitory activity against human carbonic anhydrase II |
Bioorg Med Chem Lett 13: 2867-73 (2003)
BindingDB Entry DOI: 10.7270/Q2BR8SQ8 |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 2
(Homo sapiens (Human)) | BDBM50135621

(4-(5-Sulfamoyl-[1,3,4]thiadiazol-2-ylsulfamoyl)-be...)Show SMILES NS(=O)(=O)c1nnc(NS(=O)(=O)c2ccc(cc2)C(O)=O)s1 Show InChI InChI=1S/C9H8N4O6S3/c10-21(16,17)9-12-11-8(20-9)13-22(18,19)6-3-1-5(2-4-6)7(14)15/h1-4H,(H,11,13)(H,14,15)(H2,10,16,17) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 4.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi
Curated by ChEMBL
| Assay Description
Inhibitory activity against human carbonic anhydrase II |
Bioorg Med Chem Lett 13: 2867-73 (2003)
BindingDB Entry DOI: 10.7270/Q2BR8SQ8 |
More data for this
Ligand-Target Pair | 
3D Structure (docked) |
Carbonic anhydrase 12
(Homo sapiens (Human)) | BDBM50171026
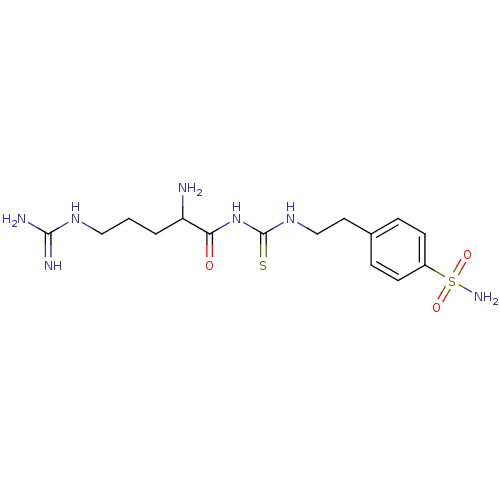
(4-{2-[3-(2-Amino-5-guanidino-pentanoyl)-thioureido...)Show SMILES NC(CCCNC(N)=N)C(=O)NC(=S)NCCc1ccc(cc1)S(N)(=O)=O Show InChI InChI=1S/C15H25N7O3S2/c16-12(2-1-8-20-14(17)18)13(23)22-15(26)21-9-7-10-3-5-11(6-4-10)27(19,24)25/h3-6,12H,1-2,7-9,16H2,(H4,17,18,20)(H2,19,24,25)(H2,21,22,23,26) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| 4.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universita degli Studi
Curated by ChEMBL
| Assay Description
Inhibitory activity against catalytic domain of human carbonic anhydrase XII |
Bioorg Med Chem Lett 15: 3821-7 (2005)
Article DOI: 10.1016/j.bmcl.2005.06.054
BindingDB Entry DOI: 10.7270/Q2H132SW |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 12
(Homo sapiens (Human)) | BDBM50171018
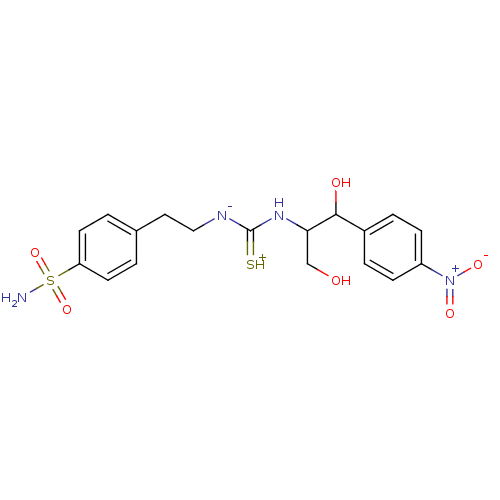
(4-(2-{3-[2-Hydroxy-1-hydroxymethyl-2-(4-nitro-phen...)Show SMILES NS(=O)(=O)c1ccc(CC[N-]C(=[SH+])NC(CO)C(O)c2ccc(cc2)[N+]([O-])=O)cc1 Show InChI InChI=1S/C18H22N4O6S2/c19-30(27,28)15-7-1-12(2-8-15)9-10-20-18(29)21-16(11-23)17(24)13-3-5-14(6-4-13)22(25)26/h1-8,16-17,23-24H,9-11H2,(H4,19,20,21,27,28,29) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 4.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universita degli Studi
Curated by ChEMBL
| Assay Description
Inhibitory activity against catalytic domain of human carbonic anhydrase XII |
Bioorg Med Chem Lett 15: 3821-7 (2005)
Article DOI: 10.1016/j.bmcl.2005.06.054
BindingDB Entry DOI: 10.7270/Q2H132SW |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data