

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Renin (Homo sapiens (Human)) | BDBM50003168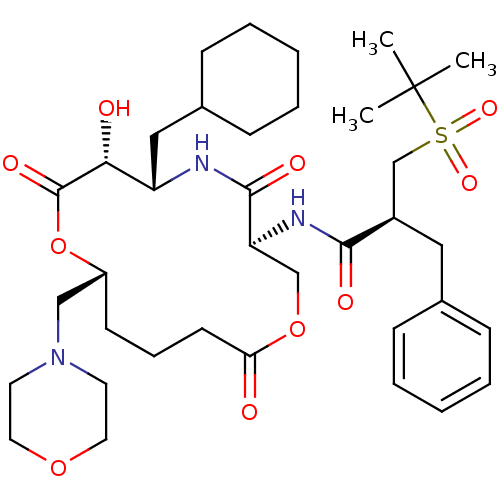 (2-Benzyl-N-(6-cyclohexylmethyl-7-hydroxy-10-morpho...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 0.200 | n/a | n/a | n/a | n/a | 7.4 | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibitory activity against human renin inhibition (at pH 7.4) | J Med Chem 35: 3755-73 (1992) BindingDB Entry DOI: 10.7270/Q26W9BPZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Renin (Homo sapiens (Human)) | BDBM50003195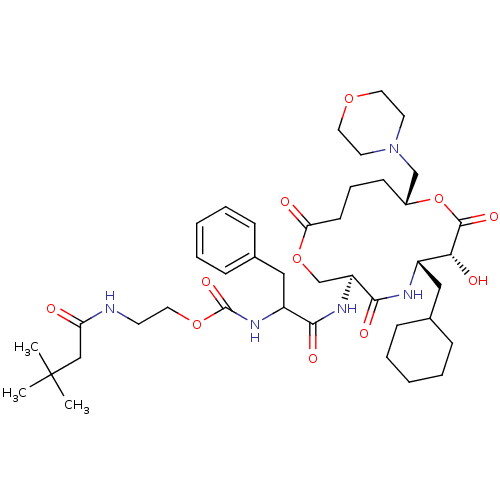 (CHEMBL126115 | [1-(6-Cyclohexylmethyl-7-hydroxy-10...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 0.230 | n/a | n/a | n/a | n/a | 7.4 | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibitory activity against human renin inhibition (at pH 7.4) | J Med Chem 35: 3755-73 (1992) BindingDB Entry DOI: 10.7270/Q26W9BPZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Melanocortin receptor 4 (Homo sapiens (Human)) | BDBM50119367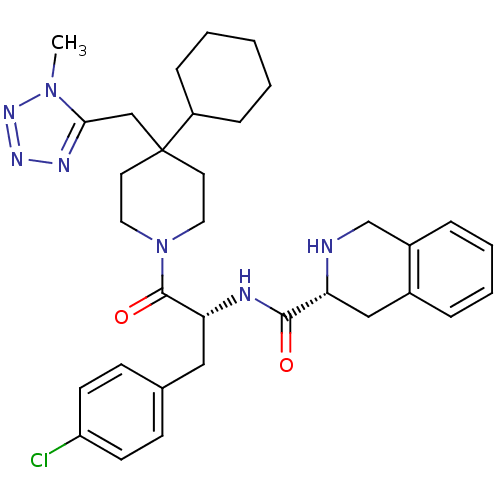 ((3R)-N-[(2R)-3-(4-chlorophenyl)-1-{4-cyclohexyl-4-...) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck & Co. Curated by ChEMBL | Assay Description Evaluated for binding affinity against Melanocortin-4 receptor by displacing [125I]-NDP-alpha-MSH radioligand expressed in CHO cells | J Med Chem 45: 4589-93 (2002) BindingDB Entry DOI: 10.7270/Q2GT5MH9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Melanocortin receptor 4 (Homo sapiens (Human)) | BDBM50168724 (1-[(R)-2-[((1S,2S)-1-Amino-1,2,3,4-tetrahydro-naph...) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 0.370 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Concentration required for 50% inhibition by displacement of of [125I]NDP-alpha-MSH of human MC4R expressed in CHO cells | Bioorg Med Chem Lett 15: 3430-3 (2005) Article DOI: 10.1016/j.bmcl.2005.05.012 BindingDB Entry DOI: 10.7270/Q2959H2N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Renin (Homo sapiens (Human)) | BDBM50003191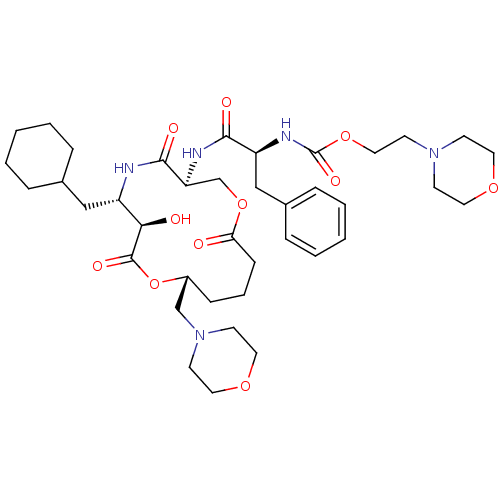 (CHEMBL338008 | [1-(6-Cyclohexylmethyl-7-hydroxy-10...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 0.410 | n/a | n/a | n/a | n/a | 7.4 | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibitory activity against human renin inhibition (at pH 7.4) | J Med Chem 35: 3755-73 (1992) BindingDB Entry DOI: 10.7270/Q26W9BPZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Melanocortin receptor 4 (Homo sapiens (Human)) | BDBM50329956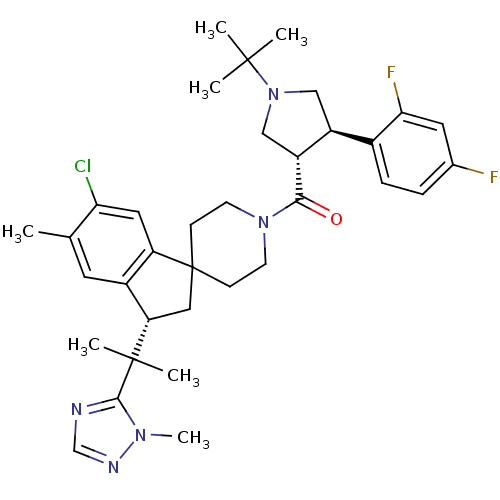 (((3S,4R)-1-tert-butyl-4-(2,4-difluorophenyl)pyrrol...) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.410 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibition of human MC4 receptor | Bioorg Med Chem Lett 20: 6524-32 (2010) Article DOI: 10.1016/j.bmcl.2010.09.049 BindingDB Entry DOI: 10.7270/Q24M94SF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Melanocortin receptor 4 (Homo sapiens (Human)) | BDBM50329959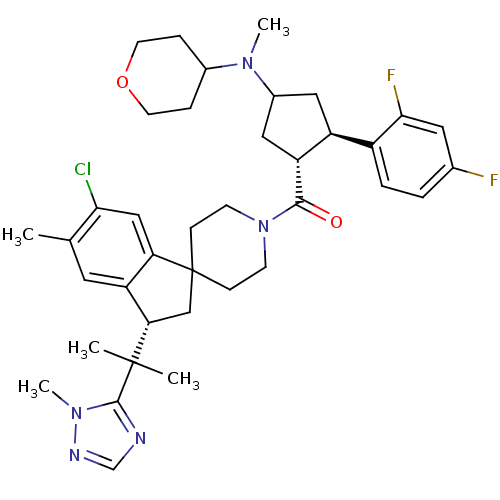 ((6-chloro-5-methyl-3-(2-(1-methyl-1H-1,2,4-triazol...) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.430 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibition of human MC4 receptor | Bioorg Med Chem Lett 20: 6524-32 (2010) Article DOI: 10.1016/j.bmcl.2010.09.049 BindingDB Entry DOI: 10.7270/Q24M94SF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Melanocortin receptor 4 (Homo sapiens (Human)) | BDBM50119371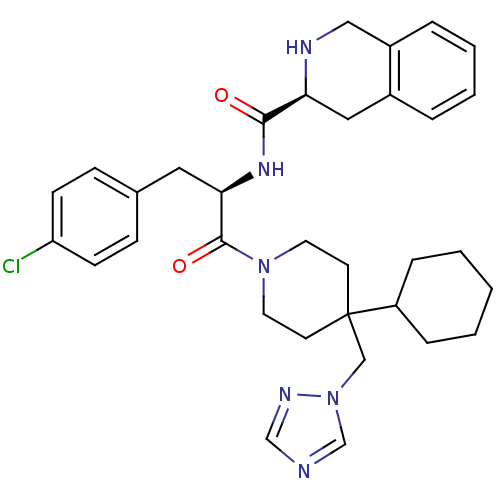 (1,2,3,4-Tetrahydro-isoquinoline-3-carboxylic acid ...) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck & Co. Curated by ChEMBL | Assay Description Evaluated for binding affinity against Melanocortin-4 receptor by displacing [125I]-NDP-alpha-MSH radioligand expressed in CHO cells | J Med Chem 45: 4589-93 (2002) BindingDB Entry DOI: 10.7270/Q2GT5MH9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Renin (Homo sapiens (Human)) | BDBM50003194 (CHEMBL124616 | [1-(6-Cyclohexylmethyl-7-hydroxy-10...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 0.5 | n/a | n/a | n/a | n/a | 7.4 | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibitory activity against human renin inhibition (at pH 7.4) | J Med Chem 35: 3755-73 (1992) BindingDB Entry DOI: 10.7270/Q26W9BPZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Renin (Homo sapiens (Human)) | BDBM50003174 (CHEMBL339289 | {2-[1-(6-Cyclohexylmethyl-7-hydroxy...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 0.5 | n/a | n/a | n/a | n/a | 7.4 | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibitory activity against human renin inhibition (at pH 7.4) | J Med Chem 35: 3755-73 (1992) BindingDB Entry DOI: 10.7270/Q26W9BPZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Melanocortin receptor 4 (Homo sapiens (Human)) | BDBM50348579 (CHEMBL1801215) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.530 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Displacement of [125I]-NDP-R-MSH from human MC4R expressed in CHO cells after 1.5 hrs by scintillation counting | Bioorg Med Chem Lett 20: 4399-405 (2010) Article DOI: 10.1016/j.bmcl.2010.06.062 BindingDB Entry DOI: 10.7270/Q23F4Q07 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Melanocortin receptor 4 (Homo sapiens (Human)) | BDBM50168725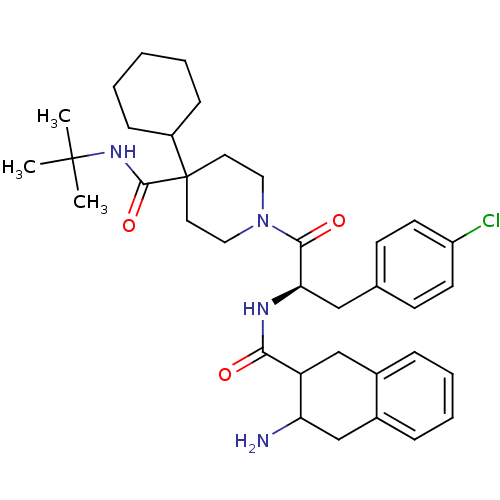 (1-[2-[((R)-3-Amino-1,2,3,4-tetrahydro-naphthalene-...) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.560 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Concentration required for 50% inhibition by displacement of of [125I]NDP-alpha-MSH of human MC4R expressed in CHO cells | Bioorg Med Chem Lett 15: 3430-3 (2005) Article DOI: 10.1016/j.bmcl.2005.05.012 BindingDB Entry DOI: 10.7270/Q2959H2N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Renin (Homo sapiens (Human)) | BDBM50003176 (2-[1-(6-Cyclohexylmethyl-7-hydroxy-10-morpholin-4-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 0.600 | n/a | n/a | n/a | n/a | 7.4 | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibitory activity against human renin inhibition (at pH 7.4) | J Med Chem 35: 3755-73 (1992) BindingDB Entry DOI: 10.7270/Q26W9BPZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Melanocortin receptor 4 (Rattus norvegicus) | BDBM50119368 ((R)-1,2,3,4-Tetrahydro-isoquinoline-3-carboxylic a...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid PDB UniChem Patents Similars | PDB PubMed | n/a | n/a | 0.600 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck & Co. Curated by ChEMBL | Assay Description Evaluated for binding affinity against rat Melanocortin-4 receptor (rMC4R) by displacing [125I]NDP-alpha-MSH radioligand expressed in CHO cells | J Med Chem 45: 4589-93 (2002) BindingDB Entry DOI: 10.7270/Q2GT5MH9 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Melanocortin receptor 4 (Homo sapiens (Human)) | BDBM50348554 (CHEMBL1801095) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.710 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Displacement of [125I]-NDP-R-MSH from human MC4R expressed in CHO cells after 1.5 hrs by scintillation counting | Bioorg Med Chem Lett 20: 4399-405 (2010) Article DOI: 10.1016/j.bmcl.2010.06.062 BindingDB Entry DOI: 10.7270/Q23F4Q07 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Melanocortin receptor 4 (Homo sapiens (Human)) | BDBM50168726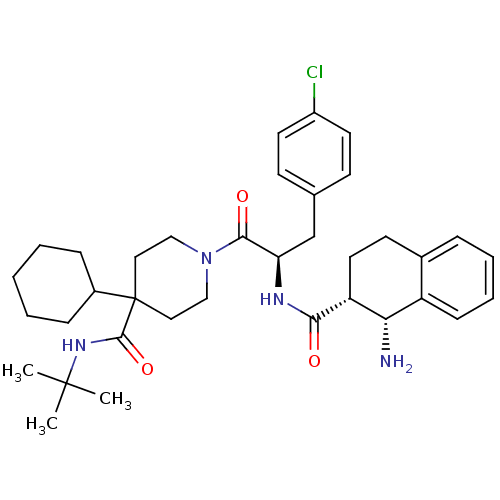 (1-[(R)-2-[((1R,2R)-1-Amino-1,2,3,4-tetrahydro-naph...) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 0.770 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Concentration required for 50% inhibition by displacement of of [125I]NDP-alpha-MSH of human MC4R expressed in CHO cells | Bioorg Med Chem Lett 15: 3430-3 (2005) Article DOI: 10.1016/j.bmcl.2005.05.012 BindingDB Entry DOI: 10.7270/Q2959H2N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Melanocortin receptor 4 (Homo sapiens (Human)) | BDBM50340458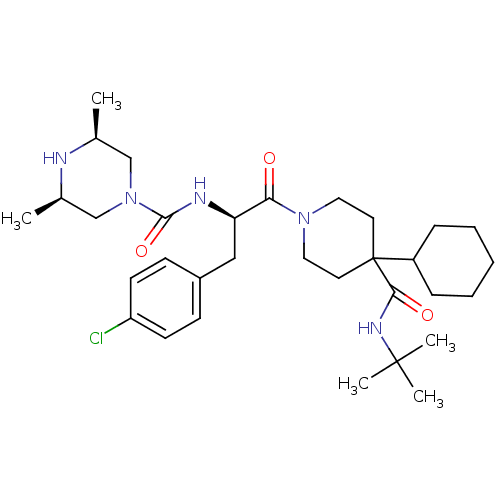 (CHEMBL1762009 | Cis-(3S,5R)-N-((R)-1-(4-(tert-buty...) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.780 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Displacement of [125I]NDP-alpha-MSH from human MC4 receptor expressed in CHO cells | Bioorg Med Chem Lett 21: 2330-4 (2011) Article DOI: 10.1016/j.bmcl.2011.02.090 BindingDB Entry DOI: 10.7270/Q22R3S0G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Melanocortin receptor 4 (Homo sapiens (Human)) | BDBM50329958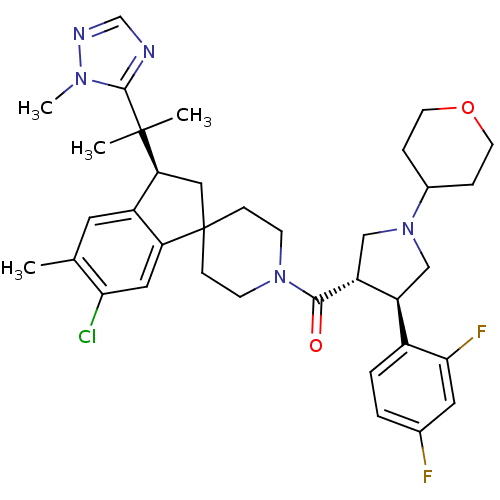 ((6-chloro-5-methyl-3-(2-(1-methyl-1H-1,2,4-triazol...) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 0.840 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibition of human MC4 receptor | Bioorg Med Chem Lett 20: 6524-32 (2010) Article DOI: 10.1016/j.bmcl.2010.09.049 BindingDB Entry DOI: 10.7270/Q24M94SF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Melanocortin receptor 4 (Homo sapiens (Human)) | BDBM50348574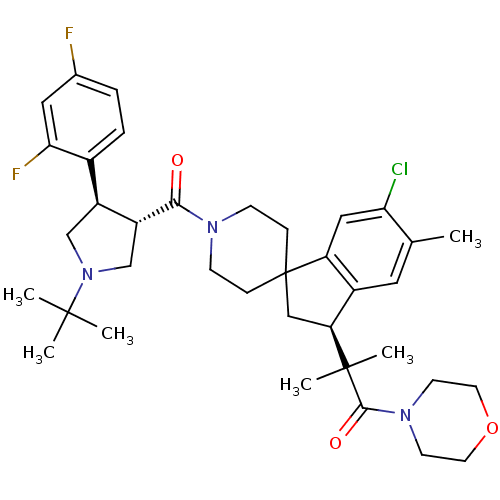 (CHEMBL1801146) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.850 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Displacement of [125I]-NDP-R-MSH from human MC4R expressed in CHO cells after 1.5 hrs by scintillation counting | Bioorg Med Chem Lett 20: 4399-405 (2010) Article DOI: 10.1016/j.bmcl.2010.06.062 BindingDB Entry DOI: 10.7270/Q23F4Q07 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Melanocortin receptor 4 (Homo sapiens (Human)) | BDBM50348580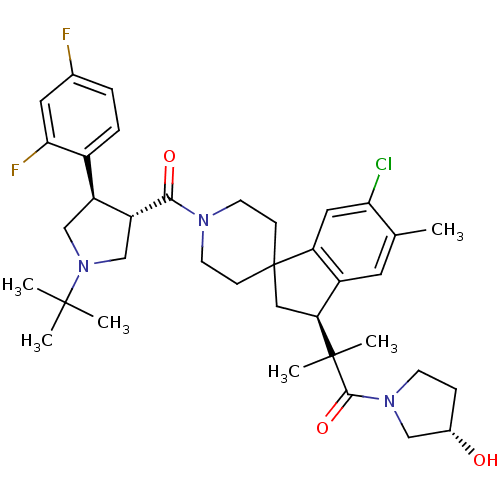 (CHEMBL1801216) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.890 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Displacement of [125I]-NDP-R-MSH from human MC4R expressed in CHO cells after 1.5 hrs by scintillation counting | Bioorg Med Chem Lett 20: 4399-405 (2010) Article DOI: 10.1016/j.bmcl.2010.06.062 BindingDB Entry DOI: 10.7270/Q23F4Q07 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Melanocortin receptor 4 (Homo sapiens (Human)) | BDBM50348572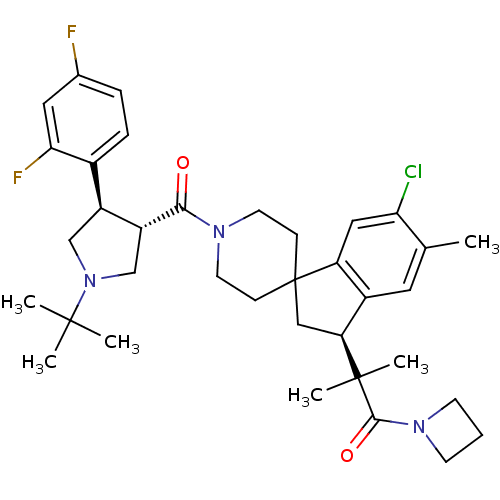 (CHEMBL1801144) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.10 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Displacement of [125I]-NDP-R-MSH from human MC4R expressed in CHO cells after 1.5 hrs by scintillation counting | Bioorg Med Chem Lett 20: 4399-405 (2010) Article DOI: 10.1016/j.bmcl.2010.06.062 BindingDB Entry DOI: 10.7270/Q23F4Q07 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Melanocortin receptor 4 (Homo sapiens (Human)) | BDBM50168727 (1-[(R)-2-[((1S,2S)-1-Amino-1-methyl-1,2,3,4-tetrah...) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 1.10 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Concentration required for 50% inhibition by displacement of of [125I]NDP-alpha-MSH of human MC4R expressed in CHO cells | Bioorg Med Chem Lett 15: 3430-3 (2005) Article DOI: 10.1016/j.bmcl.2005.05.012 BindingDB Entry DOI: 10.7270/Q2959H2N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Renin (Homo sapiens (Human)) | BDBM50003165 (1N-[1-[12-cyclohexylmethyl-11-hydroxy-8-(1,4-oxazi...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 1.10 | n/a | n/a | n/a | n/a | 7.4 | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibitory activity against human renin inhibition (at pH 7.4) | J Med Chem 35: 3755-73 (1992) BindingDB Entry DOI: 10.7270/Q26W9BPZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Melanocortin receptor 4 (Homo sapiens (Human)) | BDBM50024350 (CHEMBL2113041) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.10 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Concentration required for 50% inhibition by displacement of of [125I]NDP-alpha-MSH of human MC4R expressed in CHO cells | Bioorg Med Chem Lett 15: 3430-3 (2005) Article DOI: 10.1016/j.bmcl.2005.05.012 BindingDB Entry DOI: 10.7270/Q2959H2N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Melanocortin receptor 4 (Homo sapiens (Human)) | BDBM50119368 ((R)-1,2,3,4-Tetrahydro-isoquinoline-3-carboxylic a...) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid PDB UniChem Patents Similars | PDB PubMed | n/a | n/a | 1.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck & Co. Curated by ChEMBL | Assay Description Evaluated for Functional Activity at Melanocortin-4 receptor as effective concentration at 50% maximum CMP accumulation | J Med Chem 45: 4589-93 (2002) BindingDB Entry DOI: 10.7270/Q2GT5MH9 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Melanocortin receptor 4 (Homo sapiens (Human)) | BDBM50119368 ((R)-1,2,3,4-Tetrahydro-isoquinoline-3-carboxylic a...) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | n/a | n/a | 1.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Concentration required for 50% inhibition by displacement of of [125I]NDP-alpha-MSH of human MC4R expressed in CHO cells | Bioorg Med Chem Lett 15: 3430-3 (2005) Article DOI: 10.1016/j.bmcl.2005.05.012 BindingDB Entry DOI: 10.7270/Q2959H2N | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Melanocortin receptor 4 (Homo sapiens (Human)) | BDBM50119368 ((R)-1,2,3,4-Tetrahydro-isoquinoline-3-carboxylic a...) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid PDB UniChem Patents Similars | PDB PubMed | n/a | n/a | 1.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck & Co. Curated by ChEMBL | Assay Description Evaluated for binding affinity against human Melanocortin-4 receptor (hMC4R) by displacing [125I]-NDP-alpha-MSH radioligand expressed in CHO cells | J Med Chem 45: 4589-93 (2002) BindingDB Entry DOI: 10.7270/Q2GT5MH9 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Melanocortin receptor 4 (Homo sapiens (Human)) | BDBM50119366 ((R)-N-((R)-3-(4-chlorophenyl)-1-(4-cyclohexyl-4-((...) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 1.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck & Co. Curated by ChEMBL | Assay Description Evaluated for binding affinity against Melanocortin-4 receptor by displacing [125I]-NDP-alpha-MSH radioligand expressed in CHO cells | J Med Chem 45: 4589-93 (2002) BindingDB Entry DOI: 10.7270/Q2GT5MH9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Renin (Homo sapiens (Human)) | BDBM50003192 (2-(1-Aza-bicyclo[2.2.2]oct-3-ylamino)-N-(6-cyclohe...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 1.30 | n/a | n/a | n/a | n/a | 7.4 | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibitory activity against human renin inhibition (at pH 7.4) | J Med Chem 35: 3755-73 (1992) BindingDB Entry DOI: 10.7270/Q26W9BPZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Renin (Homo sapiens (Human)) | BDBM50003181 ((diastereomer-1) (1-{6-Cyclohexylmethyl-7-hydroxy-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 1.30 | n/a | n/a | n/a | n/a | 7.4 | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibitory activity against human renin inhibition (at pH 7.4) | J Med Chem 35: 3755-73 (1992) BindingDB Entry DOI: 10.7270/Q26W9BPZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Renin (Homo sapiens (Human)) | BDBM50003181 ((diastereomer-1) (1-{6-Cyclohexylmethyl-7-hydroxy-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 1.30 | n/a | n/a | n/a | n/a | 7.4 | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibitory activity against human renin inhibition (at pH 7.4) | J Med Chem 35: 3755-73 (1992) BindingDB Entry DOI: 10.7270/Q26W9BPZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Melanocortin receptor 4 (Homo sapiens (Human)) | BDBM50348581 (CHEMBL1801217) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.40 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Displacement of [125I]-NDP-R-MSH from human MC4R expressed in CHO cells after 1.5 hrs by scintillation counting | Bioorg Med Chem Lett 20: 4399-405 (2010) Article DOI: 10.1016/j.bmcl.2010.06.062 BindingDB Entry DOI: 10.7270/Q23F4Q07 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Melanocortin receptor 4 (Homo sapiens (Human)) | BDBM50348578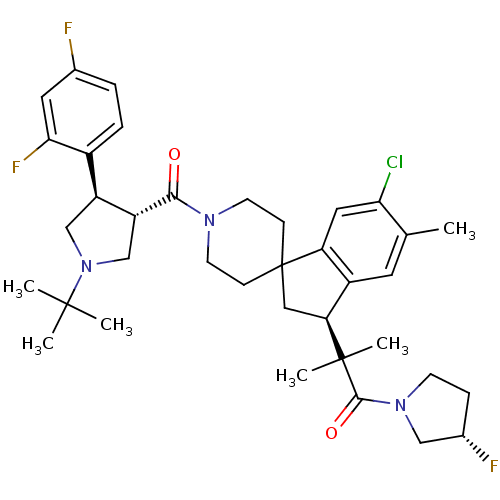 (CHEMBL1801150) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.40 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Displacement of [125I]-NDP-R-MSH from human MC4R expressed in CHO cells after 1.5 hrs by scintillation counting | Bioorg Med Chem Lett 20: 4399-405 (2010) Article DOI: 10.1016/j.bmcl.2010.06.062 BindingDB Entry DOI: 10.7270/Q23F4Q07 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Melanocortin receptor 4 (Homo sapiens (Human)) | BDBM50348577 (CHEMBL1801149) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.60 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Displacement of [125I]-NDP-R-MSH from human MC4R expressed in CHO cells after 1.5 hrs by scintillation counting | Bioorg Med Chem Lett 20: 4399-405 (2010) Article DOI: 10.1016/j.bmcl.2010.06.062 BindingDB Entry DOI: 10.7270/Q23F4Q07 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Melanocortin receptor 4 (Homo sapiens (Human)) | BDBM50348575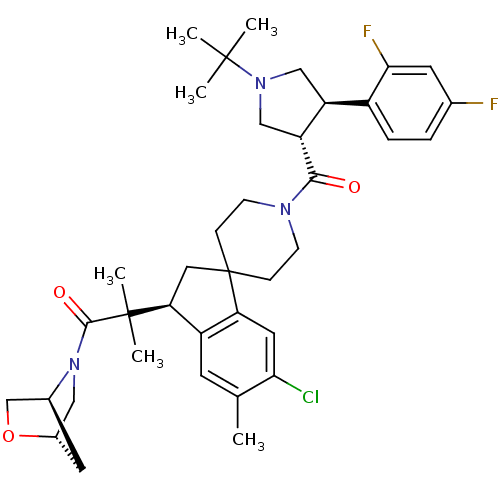 (CHEMBL1801147) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.60 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Displacement of [125I]-NDP-R-MSH from human MC4R expressed in CHO cells after 1.5 hrs by scintillation counting | Bioorg Med Chem Lett 20: 4399-405 (2010) Article DOI: 10.1016/j.bmcl.2010.06.062 BindingDB Entry DOI: 10.7270/Q23F4Q07 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Melanocortin receptor 4 (Homo sapiens (Human)) | BDBM50329957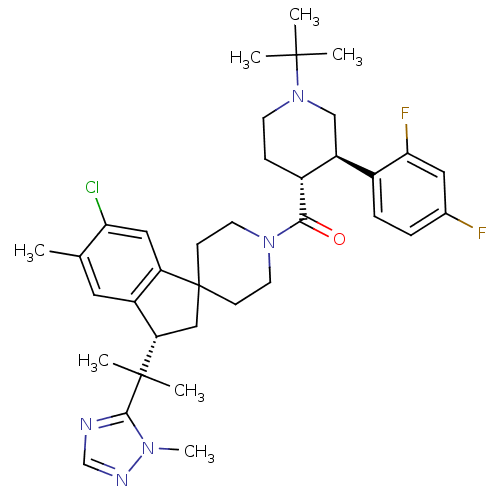 (((3R,4R)-1-tert-butyl-3-(2,4-difluorophenyl)piperi...) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.70 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibition of human MC4 receptor | Bioorg Med Chem Lett 20: 6524-32 (2010) Article DOI: 10.1016/j.bmcl.2010.09.049 BindingDB Entry DOI: 10.7270/Q24M94SF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Renin (Homo sapiens (Human)) | BDBM50230157 (CHEMBL3144232) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 1.70 | n/a | n/a | n/a | n/a | 7.4 | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibitory activity against human renin inhibition (at pH 7.4) | J Med Chem 35: 3755-73 (1992) BindingDB Entry DOI: 10.7270/Q26W9BPZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Melanocortin receptor 4 (Homo sapiens (Human)) | BDBM50348573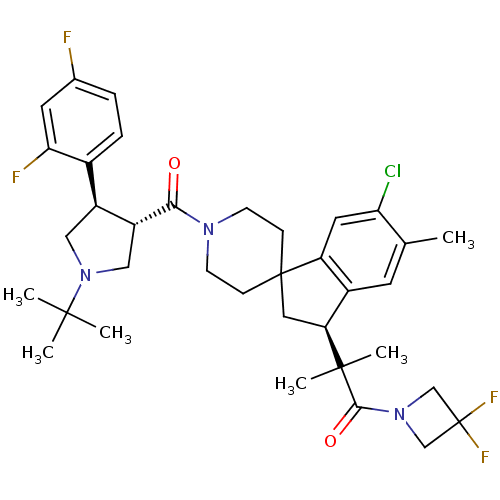 (CHEMBL1801145) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.80 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Displacement of [125I]-NDP-R-MSH from human MC4R expressed in CHO cells after 1.5 hrs by scintillation counting | Bioorg Med Chem Lett 20: 4399-405 (2010) Article DOI: 10.1016/j.bmcl.2010.06.062 BindingDB Entry DOI: 10.7270/Q23F4Q07 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Melanocortin receptor 4 (Homo sapiens (Human)) | BDBM50348576 (CHEMBL1801148) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.80 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Displacement of [125I]-NDP-R-MSH from human MC4R expressed in CHO cells after 1.5 hrs by scintillation counting | Bioorg Med Chem Lett 20: 4399-405 (2010) Article DOI: 10.1016/j.bmcl.2010.06.062 BindingDB Entry DOI: 10.7270/Q23F4Q07 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Melanocortin receptor 4 (Homo sapiens (Human)) | BDBM50329961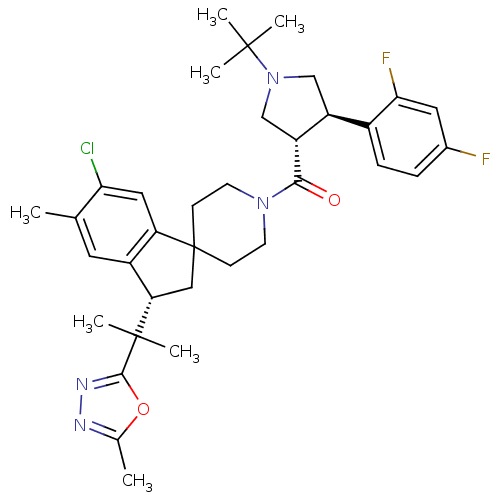 (((3S,4R)-1-tert-butyl-4-(2,4-difluorophenyl)pyrrol...) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.90 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibition of human MC4 receptor | Bioorg Med Chem Lett 20: 6524-32 (2010) Article DOI: 10.1016/j.bmcl.2010.09.049 BindingDB Entry DOI: 10.7270/Q24M94SF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Melanocortin receptor 4 (Homo sapiens (Human)) | BDBM50168723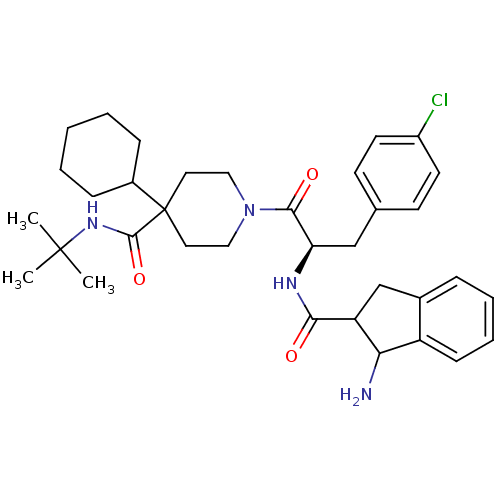 (1-[2-[((R)-1-Amino-indane-2-carbonyl)-amino]-3-(4-...) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 1.90 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Concentration required for 50% inhibition by displacement of of [125I]NDP-alpha-MSH of human MC4R expressed in CHO cells | Bioorg Med Chem Lett 15: 3430-3 (2005) Article DOI: 10.1016/j.bmcl.2005.05.012 BindingDB Entry DOI: 10.7270/Q2959H2N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Melanocortin receptor 4 (Homo sapiens (Human)) | BDBM50348584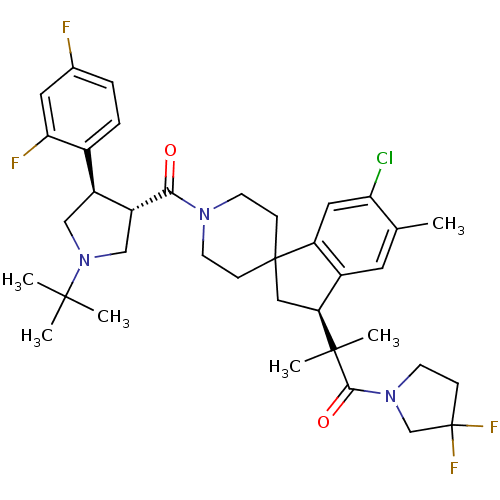 (CHEMBL1801214) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Displacement of [125I]-NDP-R-MSH from human MC4R expressed in CHO cells after 1.5 hrs by scintillation counting | Bioorg Med Chem Lett 20: 4399-405 (2010) Article DOI: 10.1016/j.bmcl.2010.06.062 BindingDB Entry DOI: 10.7270/Q23F4Q07 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Melanocortin receptor 4 (Homo sapiens (Human)) | BDBM50348585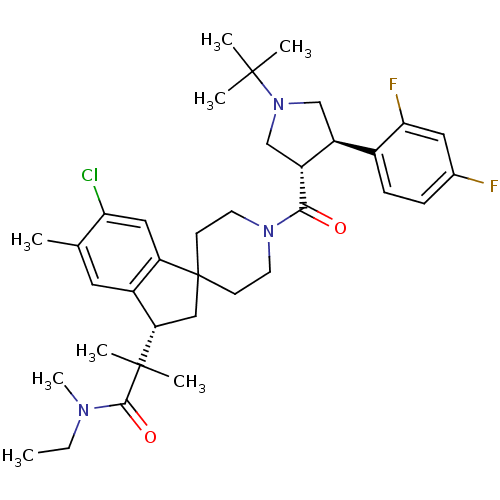 (CHEMBL1801142) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.10 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Displacement of [125I]-NDP-R-MSH from human MC4R expressed in CHO cells after 1.5 hrs by scintillation counting | Bioorg Med Chem Lett 20: 4399-405 (2010) Article DOI: 10.1016/j.bmcl.2010.06.062 BindingDB Entry DOI: 10.7270/Q23F4Q07 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Melanocortin receptor 4 (Homo sapiens (Human)) | BDBM50348560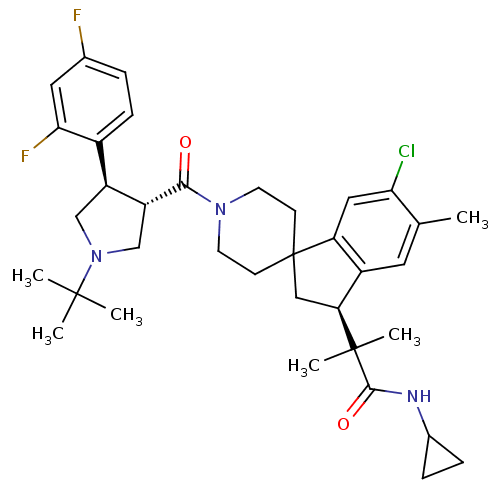 (CHEMBL1801118) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Displacement of [125I]-NDP-R-MSH from human MC4R expressed in CHO cells after 1.5 hrs by scintillation counting | Bioorg Med Chem Lett 20: 4399-405 (2010) Article DOI: 10.1016/j.bmcl.2010.06.062 BindingDB Entry DOI: 10.7270/Q23F4Q07 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Melanocortin receptor 4 (Homo sapiens (Human)) | BDBM50348582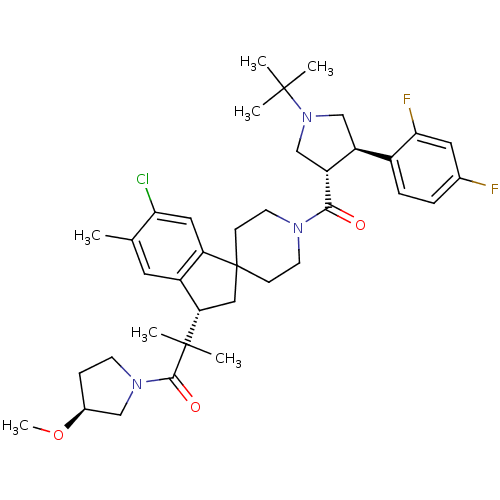 (CHEMBL1801218) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Displacement of [125I]-NDP-R-MSH from human MC4R expressed in CHO cells after 1.5 hrs by scintillation counting | Bioorg Med Chem Lett 20: 4399-405 (2010) Article DOI: 10.1016/j.bmcl.2010.06.062 BindingDB Entry DOI: 10.7270/Q23F4Q07 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Melanocortin receptor 4 (Homo sapiens (Human)) | BDBM50348569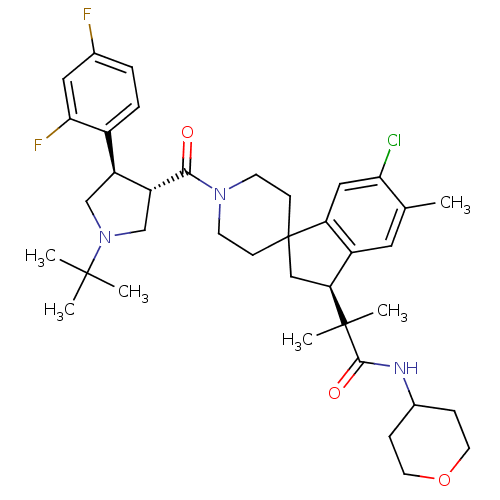 (CHEMBL1801127) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Displacement of [125I]-NDP-R-MSH from human MC4R expressed in CHO cells after 1.5 hrs by scintillation counting | Bioorg Med Chem Lett 20: 4399-405 (2010) Article DOI: 10.1016/j.bmcl.2010.06.062 BindingDB Entry DOI: 10.7270/Q23F4Q07 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Melanocortin receptor 4 (Homo sapiens (Human)) | BDBM50348553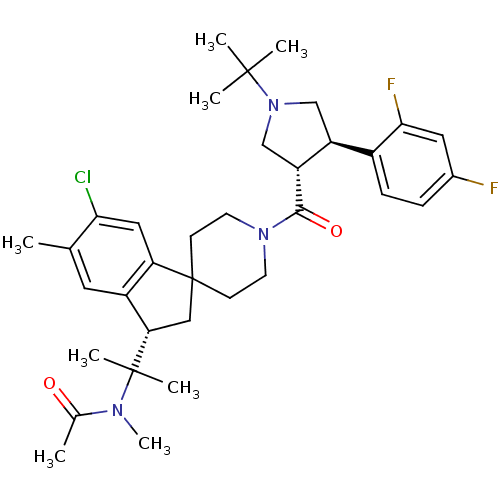 (CHEMBL1801093) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 2.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Displacement of [125I]-NDP-R-MSH from human MC4R expressed in CHO cells after 1.5 hrs by scintillation counting | Bioorg Med Chem Lett 20: 4399-405 (2010) Article DOI: 10.1016/j.bmcl.2010.06.062 BindingDB Entry DOI: 10.7270/Q23F4Q07 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Melanocortin receptor 4 (Homo sapiens (Human)) | BDBM50348568 (CHEMBL1801126) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.60 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Displacement of [125I]-NDP-R-MSH from human MC4R expressed in CHO cells after 1.5 hrs by scintillation counting | Bioorg Med Chem Lett 20: 4399-405 (2010) Article DOI: 10.1016/j.bmcl.2010.06.062 BindingDB Entry DOI: 10.7270/Q23F4Q07 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Melanocortin receptor 4 (Homo sapiens (Human)) | BDBM50348565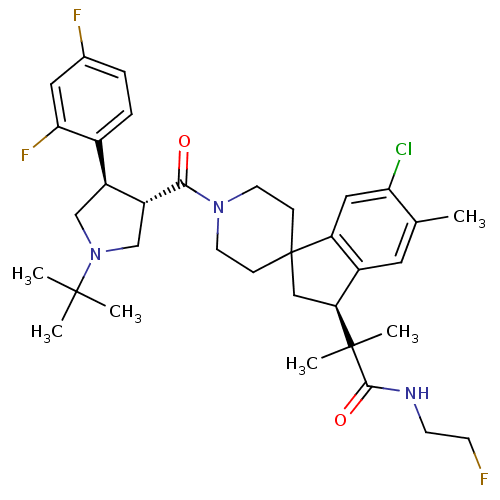 (CHEMBL1801123) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.60 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Displacement of [125I]-NDP-R-MSH from human MC4R expressed in CHO cells after 1.5 hrs by scintillation counting | Bioorg Med Chem Lett 20: 4399-405 (2010) Article DOI: 10.1016/j.bmcl.2010.06.062 BindingDB Entry DOI: 10.7270/Q23F4Q07 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Melanocortin receptor 4 (Homo sapiens (Human)) | BDBM50348556 (CHEMBL1801097) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.60 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Displacement of [125I]-NDP-R-MSH from human MC4R expressed in CHO cells after 1.5 hrs by scintillation counting | Bioorg Med Chem Lett 20: 4399-405 (2010) Article DOI: 10.1016/j.bmcl.2010.06.062 BindingDB Entry DOI: 10.7270/Q23F4Q07 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 780 total ) | Next | Last >> |