

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Histamine receptor H3 (Macaca mulatta (Rhesus macaque)) | BDBM50443217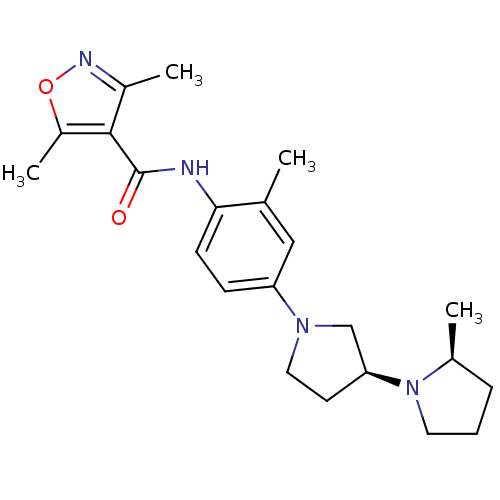 (CHEMBL3087669) | PDB UniProtKB/TrEMBL GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Sanofi US Curated by ChEMBL | Assay Description Displacement of [3H]N-alpha-methylhistamine from recombinant rhesus monkey histamine H3 receptor transfected in CHO cells after 1 hr by scintillation... | Bioorg Med Chem Lett 23: 6269-73 (2013) Article DOI: 10.1016/j.bmcl.2013.09.081 BindingDB Entry DOI: 10.7270/Q27D2WK2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Rattus norvegicus (rat)) | BDBM50443217 (CHEMBL3087669) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | 0.900 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Sanofi US Curated by ChEMBL | Assay Description Displacement of [3H]-methylhistamine from rat histamine H3 receptor (445 amino acid residues) transfected in human 293 cells after 1 hr by scintillat... | Bioorg Med Chem Lett 23: 6269-73 (2013) Article DOI: 10.1016/j.bmcl.2013.09.081 BindingDB Entry DOI: 10.7270/Q27D2WK2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine receptor H3 (Macaca mulatta (Rhesus macaque)) | BDBM50443216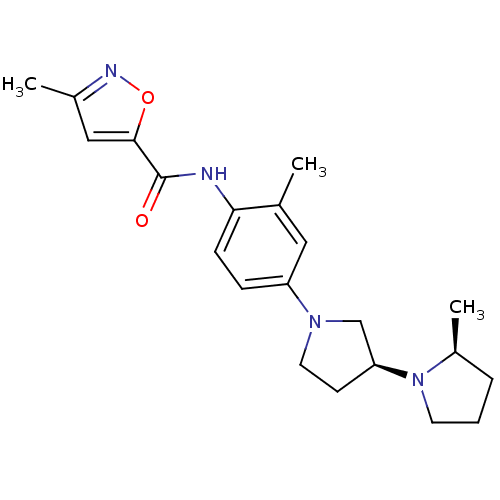 (CHEMBL3087667) | PDB UniProtKB/TrEMBL GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | 1.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Sanofi US Curated by ChEMBL | Assay Description Displacement of [3H]N-alpha-methylhistamine from recombinant rhesus monkey histamine H3 receptor transfected in CHO cells after 1 hr by scintillation... | Bioorg Med Chem Lett 23: 6269-73 (2013) Article DOI: 10.1016/j.bmcl.2013.09.081 BindingDB Entry DOI: 10.7270/Q27D2WK2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine receptor H3 (Macaca mulatta (Rhesus macaque)) | BDBM50443227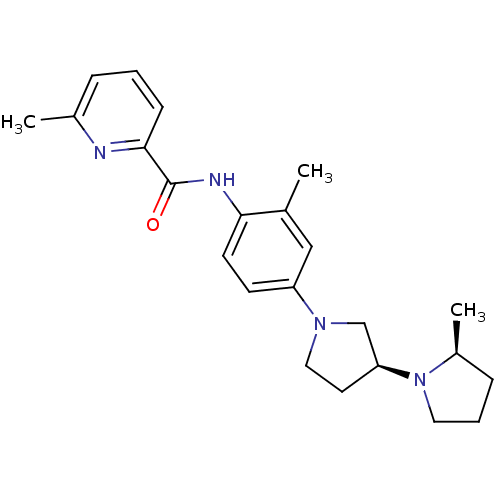 (CHEMBL3087356) | PDB UniProtKB/TrEMBL GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | 1.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Sanofi US Curated by ChEMBL | Assay Description Displacement of [3H]N-alpha-methylhistamine from recombinant rhesus monkey histamine H3 receptor transfected in CHO cells after 1 hr by scintillation... | Bioorg Med Chem Lett 23: 6269-73 (2013) Article DOI: 10.1016/j.bmcl.2013.09.081 BindingDB Entry DOI: 10.7270/Q27D2WK2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine receptor H3 (Macaca mulatta (Rhesus macaque)) | BDBM50443226 (CHEMBL3087357) | PDB UniProtKB/TrEMBL GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | 1.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Sanofi US Curated by ChEMBL | Assay Description Displacement of [3H]N-alpha-methylhistamine from recombinant rhesus monkey histamine H3 receptor transfected in CHO cells after 1 hr by scintillation... | Bioorg Med Chem Lett 23: 6269-73 (2013) Article DOI: 10.1016/j.bmcl.2013.09.081 BindingDB Entry DOI: 10.7270/Q27D2WK2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine receptor H3 (Macaca mulatta (Rhesus macaque)) | BDBM50437067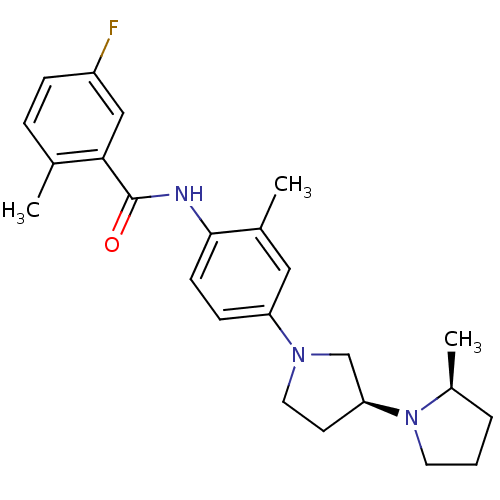 (CHEMBL2403550) | PDB UniProtKB/TrEMBL GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 2.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Sanofi US Curated by ChEMBL | Assay Description Displacement of [3H]N-alpha-methylhistamine from recombinant rhesus monkey histamine H3 receptor transfected in CHO cells after 1 hr by scintillation... | Bioorg Med Chem Lett 23: 6269-73 (2013) Article DOI: 10.1016/j.bmcl.2013.09.081 BindingDB Entry DOI: 10.7270/Q27D2WK2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine receptor H3 (Macaca mulatta (Rhesus macaque)) | BDBM50443225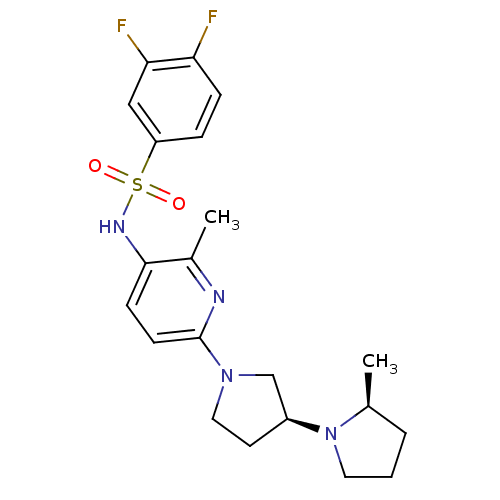 (CHEMBL3087671) | PDB UniProtKB/TrEMBL GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | 2.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Sanofi US Curated by ChEMBL | Assay Description Displacement of [3H]N-alpha-methylhistamine from recombinant rhesus monkey histamine H3 receptor transfected in CHO cells after 1 hr by scintillation... | Bioorg Med Chem Lett 23: 6269-73 (2013) Article DOI: 10.1016/j.bmcl.2013.09.081 BindingDB Entry DOI: 10.7270/Q27D2WK2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine receptor H3 (Macaca mulatta (Rhesus macaque)) | BDBM50443224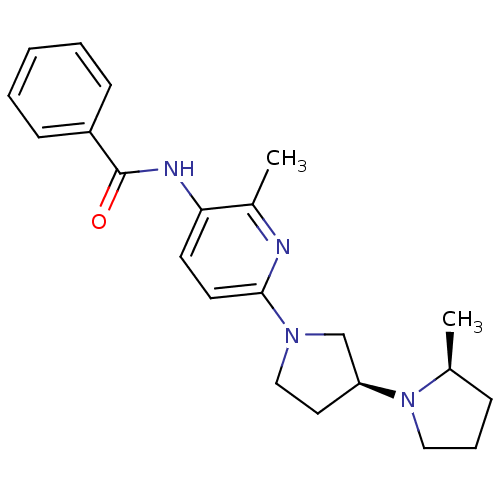 (CHEMBL3087670) | PDB UniProtKB/TrEMBL GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Sanofi US Curated by ChEMBL | Assay Description Displacement of [3H]N-alpha-methylhistamine from recombinant rhesus monkey histamine H3 receptor transfected in CHO cells after 1 hr by scintillation... | Bioorg Med Chem Lett 23: 6269-73 (2013) Article DOI: 10.1016/j.bmcl.2013.09.081 BindingDB Entry DOI: 10.7270/Q27D2WK2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine receptor H3 (Macaca mulatta (Rhesus macaque)) | BDBM50443223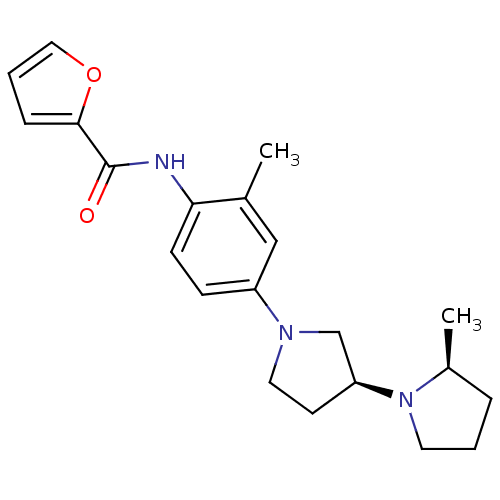 (CHEMBL3087358) | PDB UniProtKB/TrEMBL GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | 3.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Sanofi US Curated by ChEMBL | Assay Description Displacement of [3H]N-alpha-methylhistamine from recombinant rhesus monkey histamine H3 receptor transfected in CHO cells after 1 hr by scintillation... | Bioorg Med Chem Lett 23: 6269-73 (2013) Article DOI: 10.1016/j.bmcl.2013.09.081 BindingDB Entry DOI: 10.7270/Q27D2WK2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine receptor H3 (Macaca mulatta (Rhesus macaque)) | BDBM50443222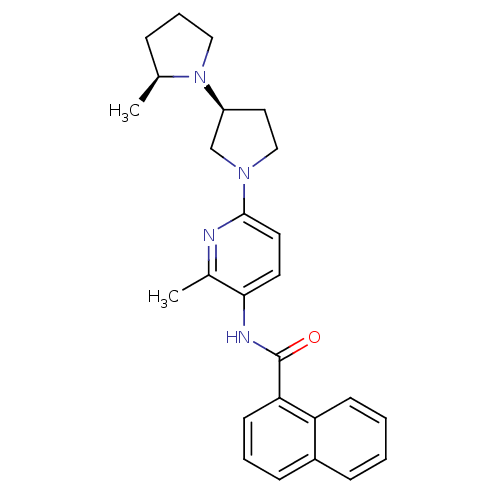 (CHEMBL3087673) | PDB UniProtKB/TrEMBL GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 4.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Sanofi US Curated by ChEMBL | Assay Description Displacement of [3H]N-alpha-methylhistamine from recombinant rhesus monkey histamine H3 receptor transfected in CHO cells after 1 hr by scintillation... | Bioorg Med Chem Lett 23: 6269-73 (2013) Article DOI: 10.1016/j.bmcl.2013.09.081 BindingDB Entry DOI: 10.7270/Q27D2WK2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine receptor H3 (Macaca mulatta (Rhesus macaque)) | BDBM200837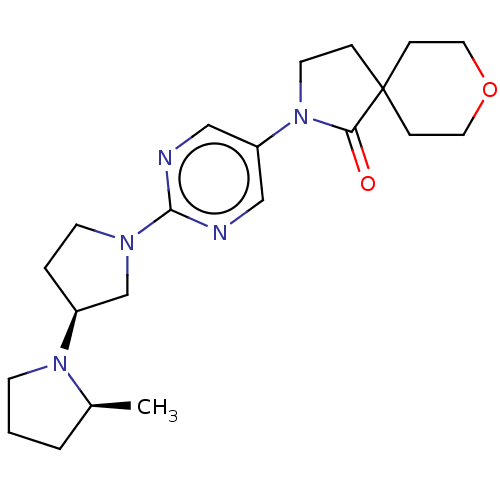 (2-[2-((2s,3's)-2-methyl-[1,3']bipyrrolidin...) | PDB UniProtKB/TrEMBL GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 4.21 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Sanofi US Patent | Assay Description Rhesus H3 radioligand binding assay was performed using rhesus H3 receptor membranes (prepared as described above), [3H]-Methylhistamine (Perkin Elme... | US Patent US9533995 (2017) BindingDB Entry DOI: 10.7270/Q25H7DFS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine receptor H3 (Macaca mulatta (Rhesus macaque)) | BDBM50443221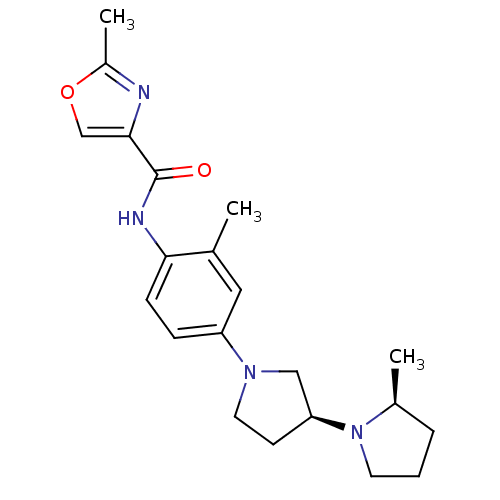 (CHEMBL3087668) | PDB UniProtKB/TrEMBL GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | 4.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Sanofi US Curated by ChEMBL | Assay Description Displacement of [3H]N-alpha-methylhistamine from recombinant rhesus monkey histamine H3 receptor transfected in CHO cells after 1 hr by scintillation... | Bioorg Med Chem Lett 23: 6269-73 (2013) Article DOI: 10.1016/j.bmcl.2013.09.081 BindingDB Entry DOI: 10.7270/Q27D2WK2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine receptor H3 (Macaca mulatta (Rhesus macaque)) | BDBM50443220 (CHEMBL3087359) | PDB UniProtKB/TrEMBL GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 4.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Sanofi US Curated by ChEMBL | Assay Description Displacement of [3H]N-alpha-methylhistamine from recombinant rhesus monkey histamine H3 receptor transfected in CHO cells after 1 hr by scintillation... | Bioorg Med Chem Lett 23: 6269-73 (2013) Article DOI: 10.1016/j.bmcl.2013.09.081 BindingDB Entry DOI: 10.7270/Q27D2WK2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine receptor H3 (Macaca mulatta (Rhesus macaque)) | BDBM50443219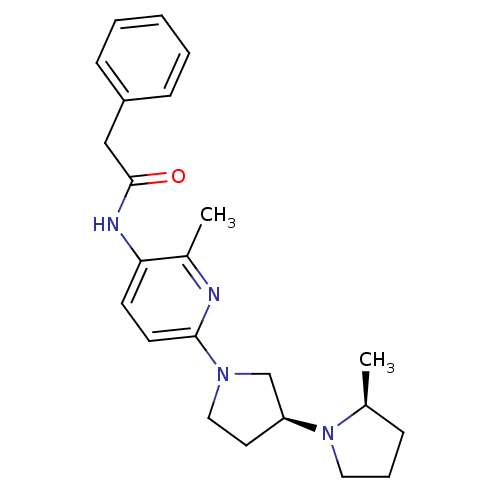 (CHEMBL3087672) | PDB UniProtKB/TrEMBL GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | 4.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Sanofi US Curated by ChEMBL | Assay Description Displacement of [3H]N-alpha-methylhistamine from recombinant rhesus monkey histamine H3 receptor transfected in CHO cells after 1 hr by scintillation... | Bioorg Med Chem Lett 23: 6269-73 (2013) Article DOI: 10.1016/j.bmcl.2013.09.081 BindingDB Entry DOI: 10.7270/Q27D2WK2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine receptor H3 (Macaca mulatta (Rhesus macaque)) | BDBM200827 (2-[2-methyl-6-((2s,3's)-2-methyl-[1,3']bip...) | PDB UniProtKB/TrEMBL GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 7.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Sanofi US Patent | Assay Description Rhesus H3 radioligand binding assay was performed using rhesus H3 receptor membranes (prepared as described above), [3H]-Methylhistamine (Perkin Elme... | US Patent US9533995 (2017) BindingDB Entry DOI: 10.7270/Q25H7DFS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine receptor H3 (Macaca mulatta (Rhesus macaque)) | BDBM200831 (2-[2-methyl-6-((2s,3's)-2-methyl-[1,3']bip...) | PDB UniProtKB/TrEMBL GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 7.57 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Sanofi US Patent | Assay Description Rhesus H3 radioligand binding assay was performed using rhesus H3 receptor membranes (prepared as described above), [3H]-Methylhistamine (Perkin Elme... | US Patent US9533995 (2017) BindingDB Entry DOI: 10.7270/Q25H7DFS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine receptor H3 (Macaca mulatta (Rhesus macaque)) | BDBM50443218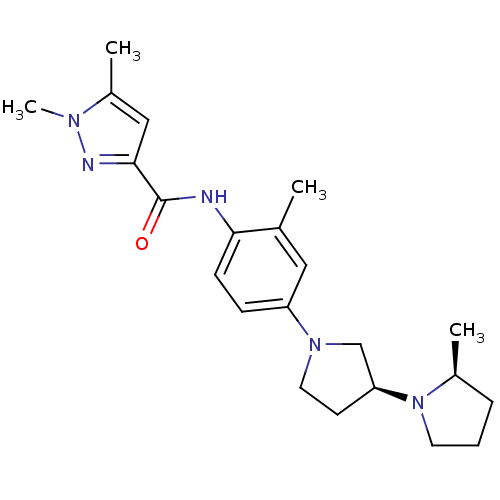 (CHEMBL3087360) | PDB UniProtKB/TrEMBL GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 11 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Sanofi US Curated by ChEMBL | Assay Description Displacement of [3H]N-alpha-methylhistamine from recombinant rhesus monkey histamine H3 receptor transfected in CHO cells after 1 hr by scintillation... | Bioorg Med Chem Lett 23: 6269-73 (2013) Article DOI: 10.1016/j.bmcl.2013.09.081 BindingDB Entry DOI: 10.7270/Q27D2WK2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine receptor H3 (Macaca mulatta (Rhesus macaque)) | BDBM200828 (2-[2-methyl-6-((2s,3'r)-2-methyl-[1,3']bip...) | PDB UniProtKB/TrEMBL GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 17.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Sanofi US Patent | Assay Description Rhesus H3 radioligand binding assay was performed using rhesus H3 receptor membranes (prepared as described above), [3H]-Methylhistamine (Perkin Elme... | US Patent US9533995 (2017) BindingDB Entry DOI: 10.7270/Q25H7DFS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine receptor H3 (Macaca mulatta (Rhesus macaque)) | BDBM200833 (2-[5-((2s,3's)-2-methyl-[1,3']bipyrrolidin...) | PDB UniProtKB/TrEMBL GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 22.3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Sanofi US Patent | Assay Description Rhesus H3 radioligand binding assay was performed using rhesus H3 receptor membranes (prepared as described above), [3H]-Methylhistamine (Perkin Elme... | US Patent US9533995 (2017) BindingDB Entry DOI: 10.7270/Q25H7DFS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine receptor H3 (Macaca mulatta (Rhesus macaque)) | BDBM200830 (2-[5-((2s,3's)-2-methyl-[1,3']bipyrrolidin...) | PDB UniProtKB/TrEMBL GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 26.3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Sanofi US Patent | Assay Description Rhesus H3 radioligand binding assay was performed using rhesus H3 receptor membranes (prepared as described above), [3H]-Methylhistamine (Perkin Elme... | US Patent US9533995 (2017) BindingDB Entry DOI: 10.7270/Q25H7DFS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine receptor H3 (Macaca mulatta (Rhesus macaque)) | BDBM200815 (1 2-[2-Methyl-6-((2S,3′R)-2-methyl-[1,3̸...) | PDB UniProtKB/TrEMBL GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 37.6 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Sanofi US Patent | Assay Description Rhesus H3 radioligand binding assay was performed using rhesus H3 receptor membranes (prepared as described above), [3H]-Methylhistamine (Perkin Elme... | US Patent US9533995 (2017) BindingDB Entry DOI: 10.7270/Q25H7DFS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine receptor H3 (Macaca mulatta (Rhesus macaque)) | BDBM200829 (2-[2-methyl-6-((2s,3'r)-2-methyl-[1,3']bip...) | PDB UniProtKB/TrEMBL GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 38.9 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Sanofi US Patent | Assay Description Rhesus H3 radioligand binding assay was performed using rhesus H3 receptor membranes (prepared as described above), [3H]-Methylhistamine (Perkin Elme... | US Patent US9533995 (2017) BindingDB Entry DOI: 10.7270/Q25H7DFS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine receptor H3 (Macaca mulatta (Rhesus macaque)) | BDBM200832 (2-[2-((2s,3's)-2-methyl-[1,3']bipyrrolidin...) | PDB UniProtKB/TrEMBL GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 48.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Sanofi US Patent | Assay Description Rhesus H3 radioligand binding assay was performed using rhesus H3 receptor membranes (prepared as described above), [3H]-Methylhistamine (Perkin Elme... | US Patent US9533995 (2017) BindingDB Entry DOI: 10.7270/Q25H7DFS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine receptor H3 (Macaca mulatta (Rhesus macaque)) | BDBM200823 (2-[2-methyl-6-((2s,3'r)-2-methyl-[1,3']bip...) | PDB UniProtKB/TrEMBL GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 56.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Sanofi US Patent | Assay Description Rhesus H3 radioligand binding assay was performed using rhesus H3 receptor membranes (prepared as described above), [3H]-Methylhistamine (Perkin Elme... | US Patent US9533995 (2017) BindingDB Entry DOI: 10.7270/Q25H7DFS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine receptor H3 (Macaca mulatta (Rhesus macaque)) | BDBM200821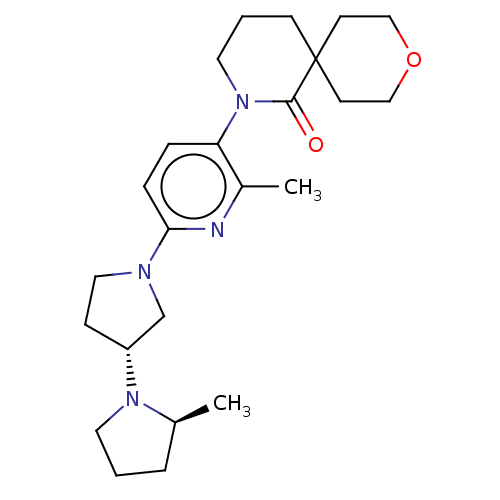 (2-[2-methyl-6-((2s,3'r)-2-methyl-[1,3']bip...) | PDB UniProtKB/TrEMBL GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 95.1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Sanofi US Patent | Assay Description Rhesus H3 radioligand binding assay was performed using rhesus H3 receptor membranes (prepared as described above), [3H]-Methylhistamine (Perkin Elme... | US Patent US9533995 (2017) BindingDB Entry DOI: 10.7270/Q25H7DFS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine receptor H3 (Macaca mulatta (Rhesus macaque)) | BDBM200836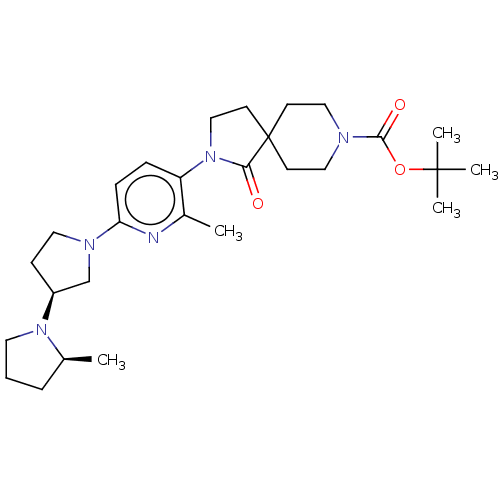 (2-[2-methyl-6-((2s,3's)-2-methyl-[1,3']bip...) | PDB UniProtKB/TrEMBL GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 95.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Sanofi US Patent | Assay Description Rhesus H3 radioligand binding assay was performed using rhesus H3 receptor membranes (prepared as described above), [3H]-Methylhistamine (Perkin Elme... | US Patent US9533995 (2017) BindingDB Entry DOI: 10.7270/Q25H7DFS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine receptor H3 (Macaca mulatta (Rhesus macaque)) | BDBM200834 (2-[5-((2s,3's)-2-methyl-[1,3']bipyrrolidin...) | PDB UniProtKB/TrEMBL GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 105 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Sanofi US Patent | Assay Description Rhesus H3 radioligand binding assay was performed using rhesus H3 receptor membranes (prepared as described above), [3H]-Methylhistamine (Perkin Elme... | US Patent US9533995 (2017) BindingDB Entry DOI: 10.7270/Q25H7DFS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine receptor H3 (Macaca mulatta (Rhesus macaque)) | BDBM200822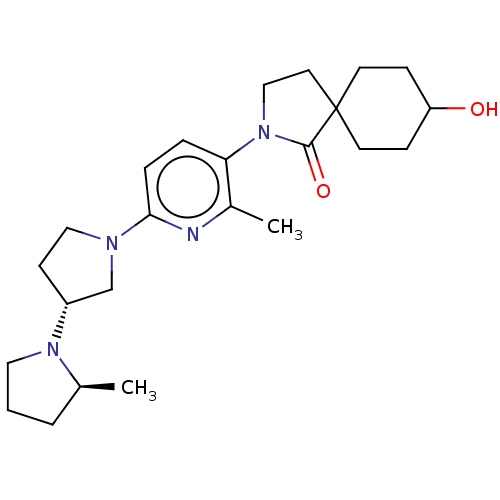 (8-hydroxy-2-[2-methyl-6-((2s,3'r)-2-methyl-[1,...) | PDB UniProtKB/TrEMBL GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 267 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Sanofi US Patent | Assay Description Rhesus H3 radioligand binding assay was performed using rhesus H3 receptor membranes (prepared as described above), [3H]-Methylhistamine (Perkin Elme... | US Patent US9533995 (2017) BindingDB Entry DOI: 10.7270/Q25H7DFS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin D2 receptor (Homo sapiens (Human)) | BDBM50417722 (CHEMBL1644207) | UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.0200 | n/a | n/a | n/a | n/a | n/a | n/a |
Sanofi Aventis US Curated by ChEMBL | Assay Description Antagonist activity at prostaglandin D2 receptor in human LS174T cells assessed as inhibition of PGD2-induced cAMP accumulation after 15 mins by scin... | Bioorg Med Chem Lett 21: 66-75 (2010) Article DOI: 10.1016/j.bmcl.2010.11.071 BindingDB Entry DOI: 10.7270/Q22F7PPM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin D2 receptor (Homo sapiens (Human)) | BDBM50417777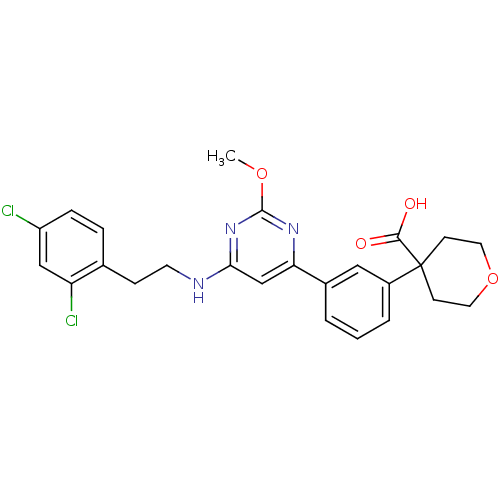 (CHEMBL1644214) | UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.0501 | n/a | n/a | n/a | n/a | n/a | n/a |
Sanofi Aventis US Curated by ChEMBL | Assay Description Antagonist activity at prostaglandin D2 receptor in human LS174T cells assessed as inhibition of PGD2-induced cAMP accumulation after 15 mins by scin... | Bioorg Med Chem Lett 21: 66-75 (2010) Article DOI: 10.1016/j.bmcl.2010.11.071 BindingDB Entry DOI: 10.7270/Q22F7PPM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin D2 receptor (Homo sapiens (Human)) | BDBM50417721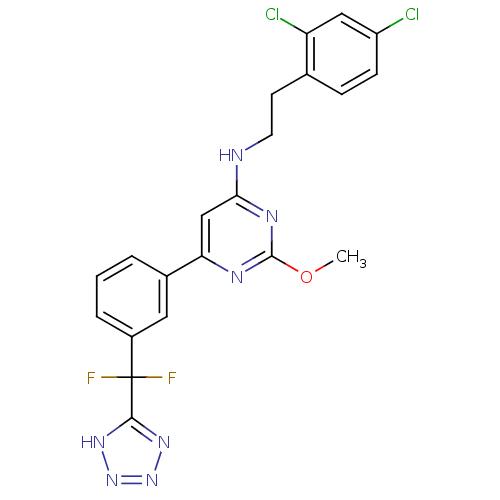 (CHEMBL1644206) | UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.100 | n/a | n/a | n/a | n/a | n/a | n/a |
Sanofi Aventis US Curated by ChEMBL | Assay Description Antagonist activity at prostaglandin D2 receptor in human LS174T cells assessed as inhibition of PGD2-induced cAMP accumulation after 15 mins by scin... | Bioorg Med Chem Lett 21: 66-75 (2010) Article DOI: 10.1016/j.bmcl.2010.11.071 BindingDB Entry DOI: 10.7270/Q22F7PPM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin D2 receptor (Homo sapiens (Human)) | BDBM50417759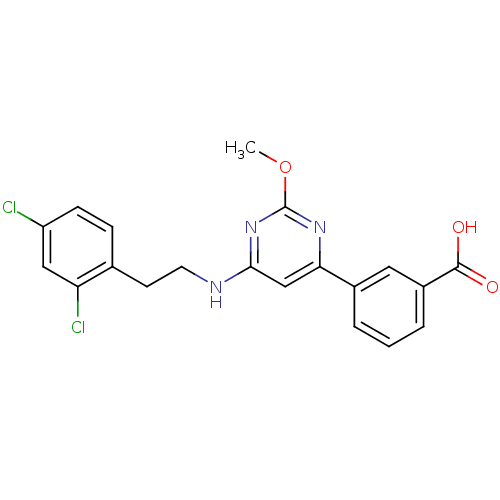 (CHEMBL1644213) | UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.302 | n/a | n/a | n/a | n/a | n/a | n/a |
Sanofi Aventis US Curated by ChEMBL | Assay Description Antagonist activity at prostaglandin D2 receptor in human LS174T cells assessed as inhibition of PGD2-induced cAMP accumulation after 15 mins by scin... | Bioorg Med Chem Lett 21: 66-75 (2010) Article DOI: 10.1016/j.bmcl.2010.11.071 BindingDB Entry DOI: 10.7270/Q22F7PPM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin D2 receptor (Homo sapiens (Human)) | BDBM50417747 (CHEMBL1644245) | UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.302 | n/a | n/a | n/a | n/a | n/a | n/a |
Sanofi Aventis US Curated by ChEMBL | Assay Description Antagonist activity at prostaglandin D2 receptor in human LS174T cells assessed as inhibition of PGD2-induced cAMP accumulation after 15 mins by scin... | Bioorg Med Chem Lett 21: 66-75 (2010) Article DOI: 10.1016/j.bmcl.2010.11.071 BindingDB Entry DOI: 10.7270/Q22F7PPM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin D2 receptor (Homo sapiens (Human)) | BDBM50417725 (CHEMBL1644211) | UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.398 | n/a | n/a | n/a | n/a | n/a | n/a |
Sanofi Aventis US Curated by ChEMBL | Assay Description Antagonist activity at prostaglandin D2 receptor in human LS174T cells assessed as inhibition of PGD2-induced cAMP accumulation after 15 mins by scin... | Bioorg Med Chem Lett 21: 66-75 (2010) Article DOI: 10.1016/j.bmcl.2010.11.071 BindingDB Entry DOI: 10.7270/Q22F7PPM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin D2 receptor (Homo sapiens (Human)) | BDBM50417764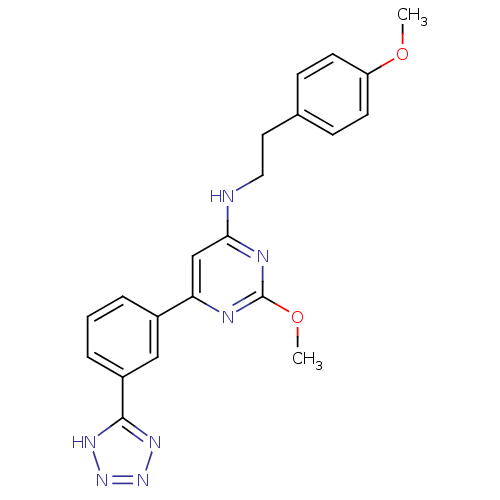 (CHEMBL1644227) | UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.398 | n/a | n/a | n/a | n/a | n/a | n/a |
Sanofi Aventis US Curated by ChEMBL | Assay Description Antagonist activity at prostaglandin D2 receptor in human LS174T cells assessed as inhibition of PGD2-induced cAMP accumulation after 15 mins by scin... | Bioorg Med Chem Lett 21: 66-75 (2010) Article DOI: 10.1016/j.bmcl.2010.11.071 BindingDB Entry DOI: 10.7270/Q22F7PPM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin D2 receptor (Homo sapiens (Human)) | BDBM50417771 (CHEMBL1644247) | UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.398 | n/a | n/a | n/a | n/a | n/a | n/a |
Sanofi Aventis US Curated by ChEMBL | Assay Description Antagonist activity at prostaglandin D2 receptor in human LS174T cells assessed as inhibition of PGD2-induced cAMP accumulation after 15 mins by scin... | Bioorg Med Chem Lett 21: 66-75 (2010) Article DOI: 10.1016/j.bmcl.2010.11.071 BindingDB Entry DOI: 10.7270/Q22F7PPM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin D2 receptor (Homo sapiens (Human)) | BDBM50417723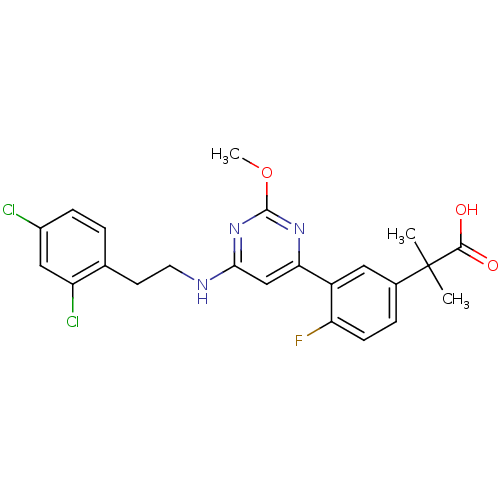 (CHEMBL1644208) | UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.501 | n/a | n/a | n/a | n/a | n/a | n/a |
Sanofi Aventis US Curated by ChEMBL | Assay Description Antagonist activity at prostaglandin D2 receptor in human LS174T cells assessed as inhibition of PGD2-induced cAMP accumulation after 15 mins by scin... | Bioorg Med Chem Lett 21: 66-75 (2010) Article DOI: 10.1016/j.bmcl.2010.11.071 BindingDB Entry DOI: 10.7270/Q22F7PPM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin D2 receptor (Homo sapiens (Human)) | BDBM50417726 (CHEMBL1644212) | UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.501 | n/a | n/a | n/a | n/a | n/a | n/a |
Sanofi Aventis US Curated by ChEMBL | Assay Description Antagonist activity at prostaglandin D2 receptor in human LS174T cells assessed as inhibition of PGD2-induced cAMP accumulation after 15 mins by scin... | Bioorg Med Chem Lett 21: 66-75 (2010) Article DOI: 10.1016/j.bmcl.2010.11.071 BindingDB Entry DOI: 10.7270/Q22F7PPM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin D2 receptor (Homo sapiens (Human)) | BDBM50417773 (CHEMBL1644253) | UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.501 | n/a | n/a | n/a | n/a | n/a | n/a |
Sanofi Aventis US Curated by ChEMBL | Assay Description Antagonist activity at prostaglandin D2 receptor in human LS174T cells assessed as inhibition of PGD2-induced cAMP accumulation after 15 mins by scin... | Bioorg Med Chem Lett 21: 66-75 (2010) Article DOI: 10.1016/j.bmcl.2010.11.071 BindingDB Entry DOI: 10.7270/Q22F7PPM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin D2 receptor (Homo sapiens (Human)) | BDBM50417728 (CHEMBL1644217) | UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.794 | n/a | n/a | n/a | n/a | n/a | n/a |
Sanofi Aventis US Curated by ChEMBL | Assay Description Antagonist activity at prostaglandin D2 receptor in human LS174T cells assessed as inhibition of PGD2-induced cAMP accumulation after 15 mins by scin... | Bioorg Med Chem Lett 21: 66-75 (2010) Article DOI: 10.1016/j.bmcl.2010.11.071 BindingDB Entry DOI: 10.7270/Q22F7PPM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin D2 receptor (Homo sapiens (Human)) | BDBM50417763 (CHEMBL1644224) | UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.891 | n/a | n/a | n/a | n/a | n/a | n/a |
Sanofi Aventis US Curated by ChEMBL | Assay Description Antagonist activity at prostaglandin D2 receptor in human LS174T cells assessed as inhibition of PGD2-induced cAMP accumulation after 15 mins by scin... | Bioorg Med Chem Lett 21: 66-75 (2010) Article DOI: 10.1016/j.bmcl.2010.11.071 BindingDB Entry DOI: 10.7270/Q22F7PPM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin D2 receptor (Homo sapiens (Human)) | BDBM50417754 (CHEMBL1644255) | UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.29 | n/a | n/a | n/a | n/a | n/a | n/a |
Sanofi Aventis US Curated by ChEMBL | Assay Description Antagonist activity at prostaglandin D2 receptor in human LS174T cells assessed as inhibition of PGD2-induced cAMP accumulation after 15 mins by scin... | Bioorg Med Chem Lett 21: 66-75 (2010) Article DOI: 10.1016/j.bmcl.2010.11.071 BindingDB Entry DOI: 10.7270/Q22F7PPM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin D2 receptor (Homo sapiens (Human)) | BDBM50417778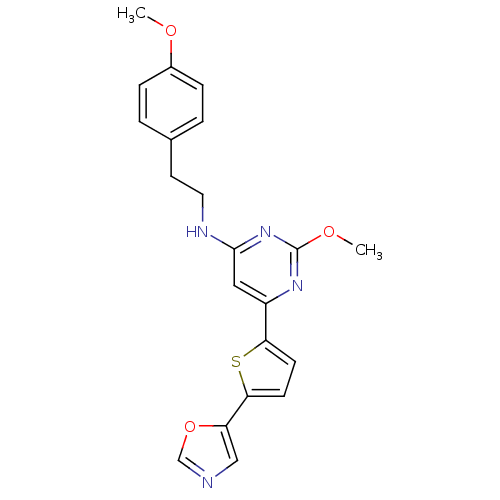 (CHEMBL1644260) | UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 2.29 | n/a | n/a | n/a | n/a | n/a | n/a |
Sanofi Aventis US Curated by ChEMBL | Assay Description Antagonist activity at prostaglandin D2 receptor in human LS174T cells assessed as inhibition of PGD2-induced cAMP accumulation after 15 mins by scin... | Bioorg Med Chem Lett 21: 66-75 (2010) Article DOI: 10.1016/j.bmcl.2010.11.071 BindingDB Entry DOI: 10.7270/Q22F7PPM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin D2 receptor (Homo sapiens (Human)) | BDBM50417774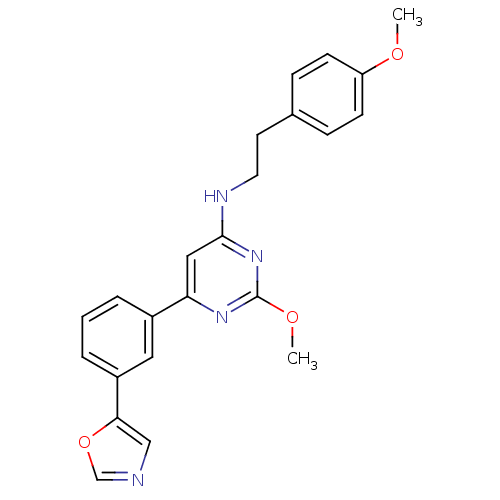 (CHEMBL1644256) | UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 2.57 | n/a | n/a | n/a | n/a | n/a | n/a |
Sanofi Aventis US Curated by ChEMBL | Assay Description Antagonist activity at prostaglandin D2 receptor in human LS174T cells assessed as inhibition of PGD2-induced cAMP accumulation after 15 mins by scin... | Bioorg Med Chem Lett 21: 66-75 (2010) Article DOI: 10.1016/j.bmcl.2010.11.071 BindingDB Entry DOI: 10.7270/Q22F7PPM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin D2 receptor (Homo sapiens (Human)) | BDBM50417767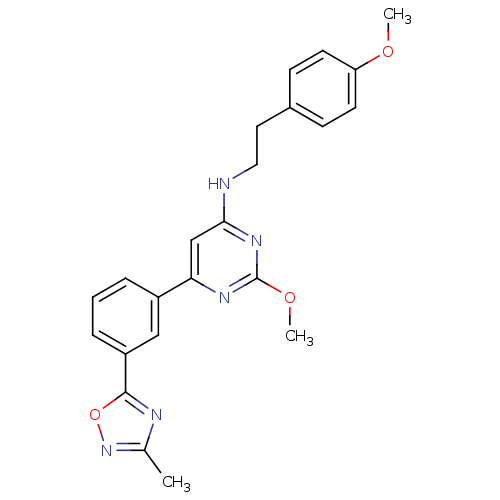 (CHEMBL1644235) | UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 2.57 | n/a | n/a | n/a | n/a | n/a | n/a |
Sanofi Aventis US Curated by ChEMBL | Assay Description Antagonist activity at prostaglandin D2 receptor in human LS174T cells assessed as inhibition of PGD2-induced cAMP accumulation after 15 mins by scin... | Bioorg Med Chem Lett 21: 66-75 (2010) Article DOI: 10.1016/j.bmcl.2010.11.071 BindingDB Entry DOI: 10.7270/Q22F7PPM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin D2 receptor (Homo sapiens (Human)) | BDBM50417719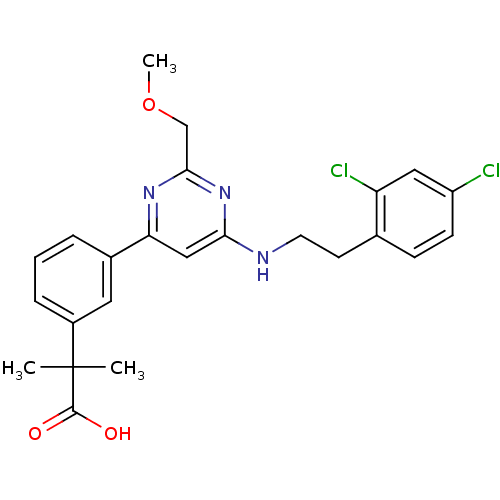 (CHEMBL1644204) | UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 2.69 | n/a | n/a | n/a | n/a | n/a | n/a |
Sanofi Aventis US Curated by ChEMBL | Assay Description Antagonist activity at prostaglandin D2 receptor in human LS174T cells assessed as inhibition of PGD2-induced cAMP accumulation after 15 mins by scin... | Bioorg Med Chem Lett 21: 66-75 (2010) Article DOI: 10.1016/j.bmcl.2010.11.071 BindingDB Entry DOI: 10.7270/Q22F7PPM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin D2 receptor (Homo sapiens (Human)) | BDBM50417768 (CHEMBL1644238) | UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.88 | n/a | n/a | n/a | n/a | n/a | n/a |
Sanofi Aventis US Curated by ChEMBL | Assay Description Antagonist activity at prostaglandin D2 receptor in human LS174T cells assessed as inhibition of PGD2-induced cAMP accumulation after 15 mins by scin... | Bioorg Med Chem Lett 21: 66-75 (2010) Article DOI: 10.1016/j.bmcl.2010.11.071 BindingDB Entry DOI: 10.7270/Q22F7PPM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin D2 receptor (Homo sapiens (Human)) | BDBM50417760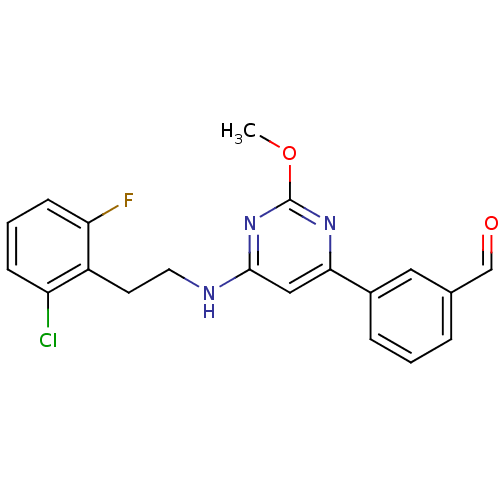 (CHEMBL1644216) | UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3.63 | n/a | n/a | n/a | n/a | n/a | n/a |
Sanofi Aventis US Curated by ChEMBL | Assay Description Antagonist activity at prostaglandin D2 receptor in human LS174T cells assessed as inhibition of PGD2-induced cAMP accumulation after 15 mins by scin... | Bioorg Med Chem Lett 21: 66-75 (2010) Article DOI: 10.1016/j.bmcl.2010.11.071 BindingDB Entry DOI: 10.7270/Q22F7PPM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin D2 receptor (Homo sapiens (Human)) | BDBM50417744 (CHEMBL1644240) | UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3.80 | n/a | n/a | n/a | n/a | n/a | n/a |
Sanofi Aventis US Curated by ChEMBL | Assay Description Antagonist activity at prostaglandin D2 receptor in human LS174T cells assessed as inhibition of PGD2-induced cAMP accumulation after 15 mins by scin... | Bioorg Med Chem Lett 21: 66-75 (2010) Article DOI: 10.1016/j.bmcl.2010.11.071 BindingDB Entry DOI: 10.7270/Q22F7PPM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin D2 receptor (Homo sapiens (Human)) | BDBM50417749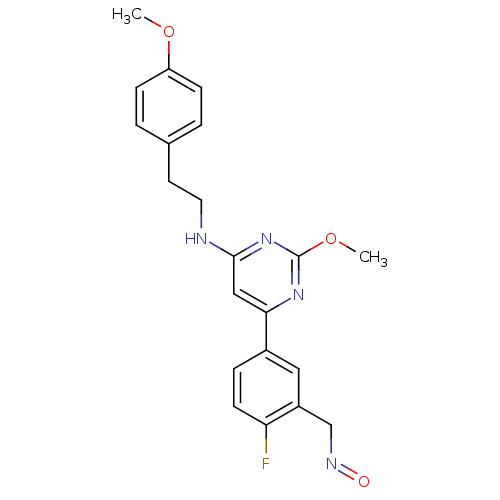 (CHEMBL1644248) | UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 4.47 | n/a | n/a | n/a | n/a | n/a | n/a |
Sanofi Aventis US Curated by ChEMBL | Assay Description Antagonist activity at prostaglandin D2 receptor in human LS174T cells assessed as inhibition of PGD2-induced cAMP accumulation after 15 mins by scin... | Bioorg Med Chem Lett 21: 66-75 (2010) Article DOI: 10.1016/j.bmcl.2010.11.071 BindingDB Entry DOI: 10.7270/Q22F7PPM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 201 total ) | Next | Last >> |