

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 72 kDa type IV collagenase (Homo sapiens (Human)) | BDBM50082556 ((S)-2,2-Dimethyl-4-[4-(pyridin-4-yloxy)-benzenesul...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE MMDB PC cid PC sid PDB UniChem Patents Similars | PubMed | n/a | n/a | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a |
Pharmacia Curated by ChEMBL | Assay Description 50 percent inhibition of human Matrix metalloprotease-2 by the cleavage of fluorogenic peptide MCA-Pro-Leu-Gly-Leu-Dpa-ala-Arg-NH2 | Bioorg Med Chem Lett 10: 2815-7 (2000) BindingDB Entry DOI: 10.7270/Q2ZK5H6Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Collagenase 3 (Homo sapiens (Human)) | BDBM50078668 (3-(4-Phenylsulfanyl-benzenesulfonyl)-cyclohexaneth...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents | PubMed | n/a | n/a | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a |
Searle Discovery Research Curated by ChEMBL | Assay Description Inhibition of human recombinant matrix metalloproteinase-13 (hMMP-13) | Bioorg Med Chem Lett 9: 1757-60 (1999) BindingDB Entry DOI: 10.7270/Q2ZK5FVN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 72 kDa type IV collagenase (Homo sapiens (Human)) | BDBM11863 (4-({[4-(4-chlorophenoxy)benzene]sulfonyl}methyl)-N...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE MMDB PC cid PC sid PDB UniChem Patents Similars | PubMed | n/a | n/a | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a |
Pharmacia Curated by ChEMBL | Assay Description 50 percent inhibition of human Matrix metalloprotease-2 by the cleavage of fluorogenic peptide MCA-Pro-Leu-Gly-Leu-Dpa-ala-Arg-NH2 | Bioorg Med Chem Lett 10: 2815-7 (2000) BindingDB Entry DOI: 10.7270/Q2ZK5H6Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Collagenase 3 (Homo sapiens (Human)) | BDBM50082556 ((S)-2,2-Dimethyl-4-[4-(pyridin-4-yloxy)-benzenesul...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE MMDB PC cid PC sid PDB UniChem Patents Similars | PubMed | n/a | n/a | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Pharmacia Curated by ChEMBL | Assay Description 50 percent inhibition of human Matrix metalloprotease-13 by the cleavage of fluorogenic peptide MCA-Pro-Leu-Gly-Leu-Dpa-ala-Arg-NH2 | Bioorg Med Chem Lett 10: 2815-7 (2000) BindingDB Entry DOI: 10.7270/Q2ZK5H6Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Collagenase 3 (Homo sapiens (Human)) | BDBM50076589 (3-(4-Phenoxy-benzenesulfonyl)-propane-1-thiol | CH...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Searle Discovery Research Curated by ChEMBL | Assay Description Inhibition of human recombinant matrix metalloproteinase-13 (hMMP-13) | Bioorg Med Chem Lett 9: 1757-60 (1999) BindingDB Entry DOI: 10.7270/Q2ZK5FVN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Collagenase 3 (Homo sapiens (Human)) | BDBM11863 (4-({[4-(4-chlorophenoxy)benzene]sulfonyl}methyl)-N...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE MMDB PC cid PC sid PDB UniChem Patents Similars | MMDB PDB PubMed | n/a | n/a | 0.600 | n/a | n/a | n/a | n/a | n/a | n/a |
Pharmacia Curated by ChEMBL | Assay Description 50 percent inhibition of human Matrix metalloprotease-13 by the cleavage of fluorogenic peptide MCA-Pro-Leu-Gly-Leu-Dpa-ala-Arg-NH2 | Bioorg Med Chem Lett 10: 2815-7 (2000) BindingDB Entry DOI: 10.7270/Q2ZK5H6Z | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| 72 kDa type IV collagenase (Homo sapiens (Human)) | BDBM50063917 ((2S,3R)-N(4)-[(2S)-3,3-dimethyl-1-(methylamino)-1-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE MMDB PC cid PC sid PDB UniChem Patents Similars | DrugBank PubMed | n/a | n/a | 0.75 | n/a | n/a | n/a | n/a | n/a | n/a |
Pharmacia Curated by ChEMBL | Assay Description 50 percent inhibition of human Matrix metalloprotease-2 by the cleavage of fluorogenic peptide MCA-Pro-Leu-Gly-Leu-Dpa-ala-Arg-NH2 | Bioorg Med Chem Lett 10: 2815-7 (2000) BindingDB Entry DOI: 10.7270/Q2ZK5H6Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Collagenase 3 (Homo sapiens (Human)) | BDBM50078667 (3-(4-Phenoxy-benzenesulfonyl)-cyclohexanethiol | C...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Searle Discovery Research Curated by ChEMBL | Assay Description Inhibition of human recombinant matrix metalloproteinase-13 (hMMP-13) | Bioorg Med Chem Lett 9: 1757-60 (1999) BindingDB Entry DOI: 10.7270/Q2ZK5FVN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 72 kDa type IV collagenase (Homo sapiens (Human)) | BDBM50095222 (CHEMBL83209 | N-Hydroxy-2-[4-(4-trifluoromethyl-ph...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 1.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Pharmacia Curated by ChEMBL | Assay Description 50 percent inhibition of human Matrix metalloprotease-2 by the cleavage of fluorogenic peptide MCA-Pro-Leu-Gly-Leu-Dpa-ala-Arg-NH2 | Bioorg Med Chem Lett 10: 2815-7 (2000) BindingDB Entry DOI: 10.7270/Q2ZK5H6Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor VII (Homo sapiens (Human)) | BDBM50167837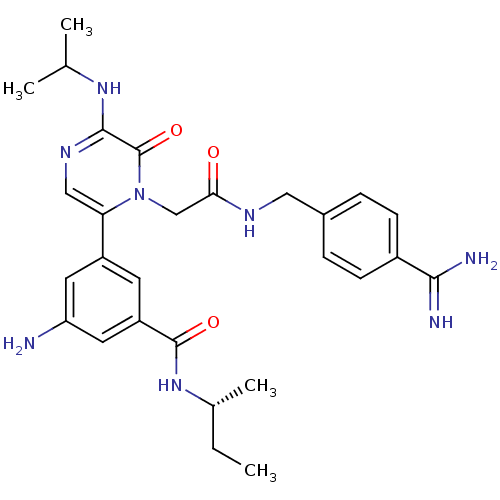 (3-Amino-N-((R)-sec-butyl)-5-{1-[(4-carbamimidoyl-b...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 1.70 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Corp. Curated by ChEMBL | Assay Description Inhibitory concentration against Coagulation factor VIIa | Bioorg Med Chem Lett 15: 3006-11 (2005) Article DOI: 10.1016/j.bmcl.2005.04.037 BindingDB Entry DOI: 10.7270/Q2SB459T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Collagenase 3 (Homo sapiens (Human)) | BDBM50063917 ((2S,3R)-N(4)-[(2S)-3,3-dimethyl-1-(methylamino)-1-...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE MMDB PC cid PC sid PDB UniChem Patents Similars | DrugBank PubMed | n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Pharmacia Curated by ChEMBL | Assay Description 50 percent inhibition of human Matrix metalloprotease-13 by the cleavage of fluorogenic peptide MCA-Pro-Leu-Gly-Leu-Dpa-ala-Arg-NH2 | Bioorg Med Chem Lett 10: 2815-7 (2000) BindingDB Entry DOI: 10.7270/Q2ZK5H6Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 72 kDa type IV collagenase (Homo sapiens (Human)) | BDBM50095218 (CHEMBL310008 | N-Hydroxy-2-[4-(4-trifluoromethoxy-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Pharmacia Curated by ChEMBL | Assay Description 50 percent inhibition of human Matrix metalloprotease-2 by the cleavage of fluorogenic peptide MCA-Pro-Leu-Gly-Leu-Dpa-ala-Arg-NH2 | Bioorg Med Chem Lett 10: 2815-7 (2000) BindingDB Entry DOI: 10.7270/Q2ZK5H6Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Collagenase 3 (Homo sapiens (Human)) | BDBM50076593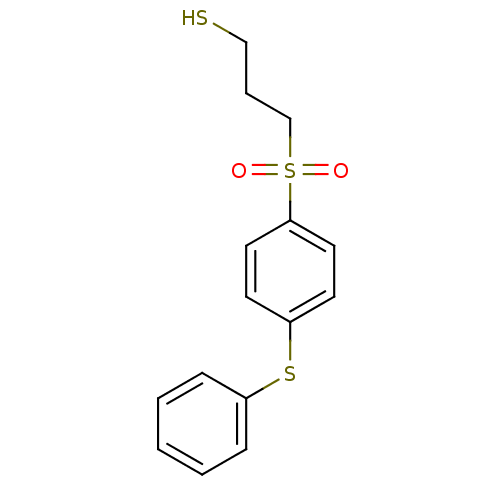 (3-(4-Phenylsulfanyl-benzenesulfonyl)-propane-1-thi...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents | PubMed | n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Searle Discovery Research Curated by ChEMBL | Assay Description Inhibition of human recombinant matrix metalloproteinase-13 (hMMP-13) | Bioorg Med Chem Lett 9: 1757-60 (1999) BindingDB Entry DOI: 10.7270/Q2ZK5FVN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Interstitial collagenase (Homo sapiens (Human)) | BDBM50063917 ((2S,3R)-N(4)-[(2S)-3,3-dimethyl-1-(methylamino)-1-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE MMDB PC cid PC sid PDB UniChem Patents Similars | DrugBank PubMed | n/a | n/a | 2.90 | n/a | n/a | n/a | n/a | n/a | n/a |
Pharmacia Curated by ChEMBL | Assay Description 50 percent inhibition of human Matrix metalloprotease-1 by the cleavage of fluorogenic peptide MCA-Pro-Leu-Gly-Leu-Dpa-ala-Arg-NH2 | Bioorg Med Chem Lett 10: 2815-7 (2000) BindingDB Entry DOI: 10.7270/Q2ZK5H6Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 72 kDa type IV collagenase (Homo sapiens (Human)) | BDBM50095225 (2-Fluoro-N-hydroxy-6-[4-(4-trifluoromethyl-phenoxy...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 3.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Pharmacia Curated by ChEMBL | Assay Description 50 percent inhibition of human Matrix metalloprotease-2 by the cleavage of fluorogenic peptide MCA-Pro-Leu-Gly-Leu-Dpa-ala-Arg-NH2 | Bioorg Med Chem Lett 10: 2815-7 (2000) BindingDB Entry DOI: 10.7270/Q2ZK5H6Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neutrophil collagenase (Homo sapiens (Human)) | BDBM50076589 (3-(4-Phenoxy-benzenesulfonyl)-propane-1-thiol | CH...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
Searle Discovery Research Curated by ChEMBL | Assay Description Inhibition of human recombinant matrix metalloproteinase-8 (hMMP-8) | Bioorg Med Chem Lett 9: 1757-60 (1999) BindingDB Entry DOI: 10.7270/Q2ZK5FVN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor VII (Homo sapiens (Human)) | BDBM50167830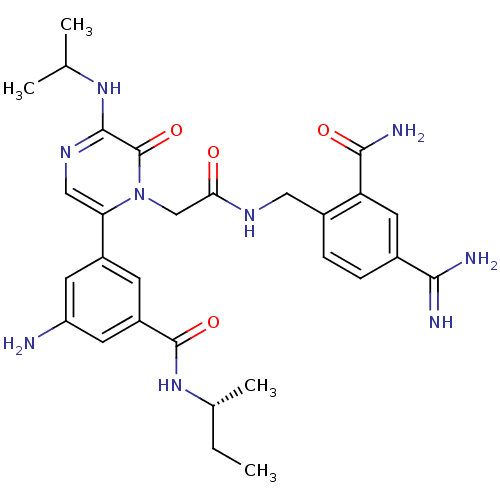 (3-amino-N-[(2R)-butan-2-yl]-5-[1-({[(4-carbamimido...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 6.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Corp. Curated by ChEMBL | Assay Description Inhibitory concentration against Coagulation factor VIIa | Bioorg Med Chem Lett 15: 3006-11 (2005) Article DOI: 10.1016/j.bmcl.2005.04.037 BindingDB Entry DOI: 10.7270/Q2SB459T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neutrophil collagenase (Homo sapiens (Human)) | BDBM50078667 (3-(4-Phenoxy-benzenesulfonyl)-cyclohexanethiol | C...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 8 | n/a | n/a | n/a | n/a | n/a | n/a |
Searle Discovery Research Curated by ChEMBL | Assay Description Inhibition of human recombinant matrix metalloproteinase-8 (hMMP-8) | Bioorg Med Chem Lett 9: 1757-60 (1999) BindingDB Entry DOI: 10.7270/Q2ZK5FVN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor VII (Homo sapiens (Human)) | BDBM50167833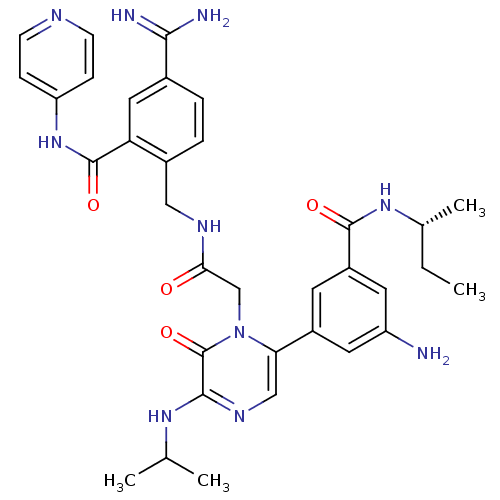 (3-amino-N-[(2R)-butan-2-yl]-5-(1-{[({4-carbamimido...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL MMDB PC cid PC sid PDB UniChem Similars | MMDB PDB Article PubMed | n/a | n/a | 9.40 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Corp. Curated by ChEMBL | Assay Description Inhibitory concentration against Coagulation factor VIIa | Bioorg Med Chem Lett 15: 3006-11 (2005) Article DOI: 10.1016/j.bmcl.2005.04.037 BindingDB Entry DOI: 10.7270/Q2SB459T | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Collagenase 3 (Homo sapiens (Human)) | BDBM50095225 (2-Fluoro-N-hydroxy-6-[4-(4-trifluoromethyl-phenoxy...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 12 | n/a | n/a | n/a | n/a | n/a | n/a |
Pharmacia Curated by ChEMBL | Assay Description 50 percent inhibition of human Matrix metalloprotease-13 by the cleavage of fluorogenic peptide MCA-Pro-Leu-Gly-Leu-Dpa-ala-Arg-NH2 | Bioorg Med Chem Lett 10: 2815-7 (2000) BindingDB Entry DOI: 10.7270/Q2ZK5H6Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor VII (Homo sapiens (Human)) | BDBM50167836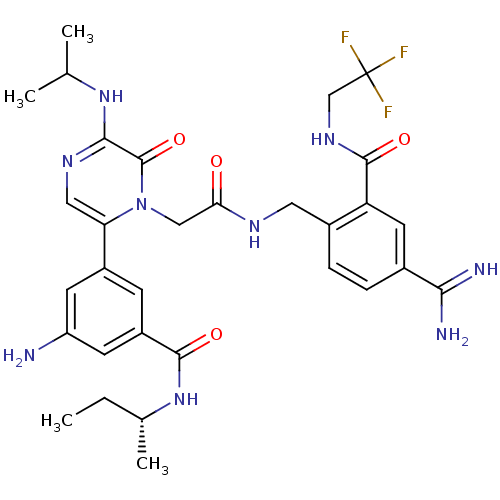 (3-amino-N-[(2R)-butan-2-yl]-5-(1-{[({4-carbamimido...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 12 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Corp. Curated by ChEMBL | Assay Description Inhibitory concentration against Coagulation factor VIIa | Bioorg Med Chem Lett 15: 3006-11 (2005) Article DOI: 10.1016/j.bmcl.2005.04.037 BindingDB Entry DOI: 10.7270/Q2SB459T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor VII (Homo sapiens (Human)) | BDBM14593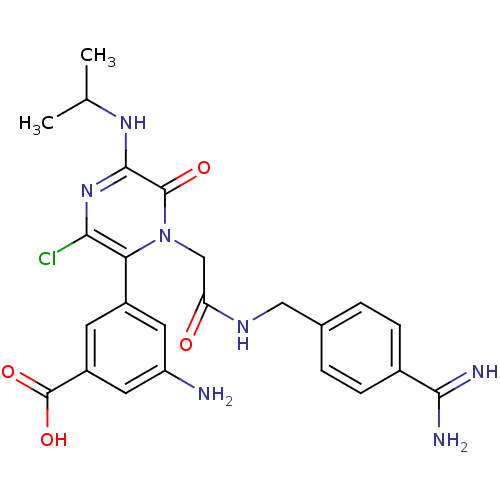 (3-amino-5-[1-({[(4-carbamimidoylphenyl)methyl]carb...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 16 | n/a | n/a | n/a | n/a | n/a | n/a |
Pharmacia Corporation | Assay Description Recombinant human Factor VIIa and soluble tissue factor were added to a 96-well assay plate containing substrate and inhibitor in reaction buffer. Th... | J Med Chem 46: 4050-62 (2003) Article DOI: 10.1021/jm030131l BindingDB Entry DOI: 10.7270/Q2FT8J9M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor VII (Homo sapiens (Human)) | BDBM14535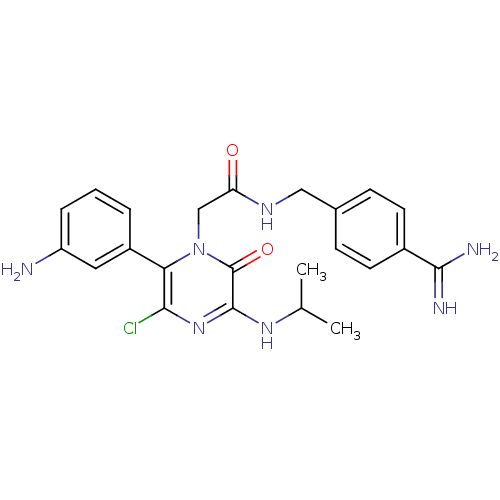 (2-[6-(3-aminophenyl)-5-chloro-2-oxo-3-(propan-2-yl...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 20 | n/a | n/a | n/a | n/a | n/a | n/a |
Pharmacia Corporation | Assay Description Recombinant human Factor VIIa and soluble tissue factor were added to a 96-well assay plate containing substrate and inhibitor in reaction buffer. Th... | J Med Chem 46: 4050-62 (2003) Article DOI: 10.1021/jm030131l BindingDB Entry DOI: 10.7270/Q2FT8J9M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor VII (Homo sapiens (Human)) | BDBM14543 (2-[6-(3-aminophenyl)-5-chloro-3-(cyclobutylamino)-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 20 | n/a | n/a | n/a | n/a | n/a | n/a |
Pharmacia Corporation | Assay Description Recombinant human Factor VIIa and soluble tissue factor were added to a 96-well assay plate containing substrate and inhibitor in reaction buffer. Th... | J Med Chem 46: 4050-62 (2003) Article DOI: 10.1021/jm030131l BindingDB Entry DOI: 10.7270/Q2FT8J9M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor VII (Homo sapiens (Human)) | BDBM50167835 (3-amino-N-[(2R)-butan-2-yl]-5-[1-({[(4-carbamimido...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 20 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Corp. Curated by ChEMBL | Assay Description Inhibitory concentration against Coagulation factor VIIa | Bioorg Med Chem Lett 15: 3006-11 (2005) Article DOI: 10.1016/j.bmcl.2005.04.037 BindingDB Entry DOI: 10.7270/Q2SB459T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor VII (Homo sapiens (Human)) | BDBM50167831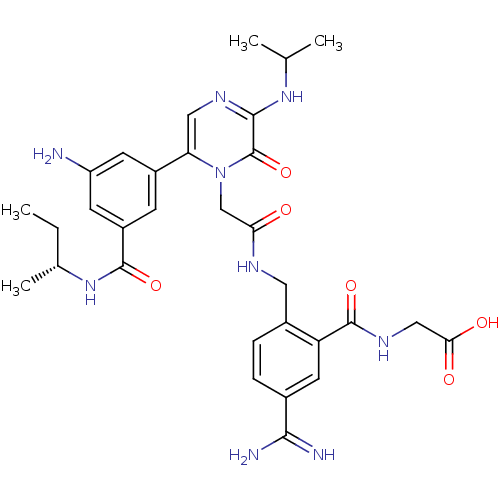 (CHEMBL189166 | {2-[(2-{6-[3-Amino-5-((R)-sec-butyl...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 21 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Corp. Curated by ChEMBL | Assay Description Inhibitory concentration against Coagulation factor VIIa | Bioorg Med Chem Lett 15: 3006-11 (2005) Article DOI: 10.1016/j.bmcl.2005.04.037 BindingDB Entry DOI: 10.7270/Q2SB459T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Collagenase 3 (Homo sapiens (Human)) | BDBM50078669 (3-(4-Methoxy-benzenesulfonyl)-cyclohexanethiol | C...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 22 | n/a | n/a | n/a | n/a | n/a | n/a |
Searle Discovery Research Curated by ChEMBL | Assay Description Inhibition of human recombinant matrix metalloproteinase-13 (hMMP-13) | Bioorg Med Chem Lett 9: 1757-60 (1999) BindingDB Entry DOI: 10.7270/Q2ZK5FVN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor VII (Homo sapiens (Human)) | BDBM50167832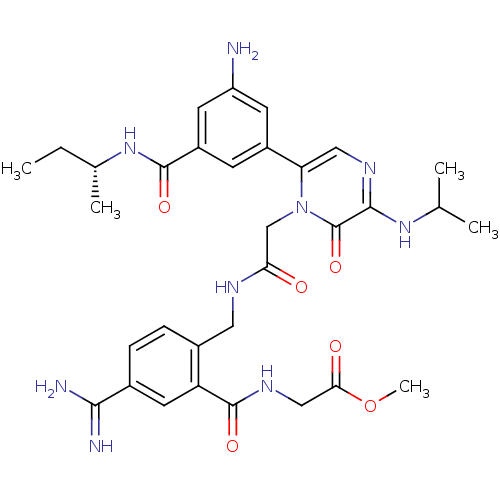 (CHEMBL189597 | {2-[(2-{6-[3-Amino-5-((R)-sec-butyl...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 23 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Corp. Curated by ChEMBL | Assay Description Inhibitory concentration against Coagulation factor VIIa | Bioorg Med Chem Lett 15: 3006-11 (2005) Article DOI: 10.1016/j.bmcl.2005.04.037 BindingDB Entry DOI: 10.7270/Q2SB459T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Interstitial collagenase (Homo sapiens (Human)) | BDBM50082556 ((S)-2,2-Dimethyl-4-[4-(pyridin-4-yloxy)-benzenesul...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE MMDB PC cid PC sid PDB UniChem Patents Similars | PubMed | n/a | n/a | 24 | n/a | n/a | n/a | n/a | n/a | n/a |
Pharmacia Curated by ChEMBL | Assay Description 50 percent inhibition of human Matrix metalloprotease-1 by the cleavage of fluorogenic peptide MCA-Pro-Leu-Gly-Leu-Dpa-ala-Arg-NH2 | Bioorg Med Chem Lett 10: 2815-7 (2000) BindingDB Entry DOI: 10.7270/Q2ZK5H6Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor X (Homo sapiens (Human)) | BDBM50130063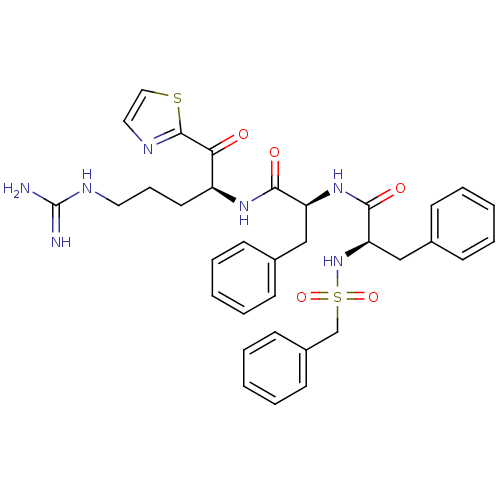 ((R)-N-{(S)-1-[(S)-4-Guanidino-1-(thiazole-2-carbon...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 27 | n/a | n/a | n/a | n/a | n/a | n/a |
Pharmacia Corporation Curated by ChEMBL | Assay Description Inhibition of coagulation factor X | Bioorg Med Chem Lett 13: 2363-7 (2003) BindingDB Entry DOI: 10.7270/Q2RX9CMS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor X (Homo sapiens (Human)) | BDBM50130063 ((R)-N-{(S)-1-[(S)-4-Guanidino-1-(thiazole-2-carbon...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 27 | n/a | n/a | n/a | n/a | n/a | n/a |
Pharmacia Corporation Curated by ChEMBL | Assay Description Inhibitory activity against coagulation factor X. | J Med Chem 46: 4043-9 (2003) Article DOI: 10.1021/jm030130t BindingDB Entry DOI: 10.7270/Q2BC4092 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Collagenase 3 (Homo sapiens (Human)) | BDBM50095222 (CHEMBL83209 | N-Hydroxy-2-[4-(4-trifluoromethyl-ph...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 28 | n/a | n/a | n/a | n/a | n/a | n/a |
Pharmacia Curated by ChEMBL | Assay Description 50 percent inhibition of human Matrix metalloprotease-13 by the cleavage of fluorogenic peptide MCA-Pro-Leu-Gly-Leu-Dpa-ala-Arg-NH2 | Bioorg Med Chem Lett 10: 2815-7 (2000) BindingDB Entry DOI: 10.7270/Q2ZK5H6Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine protease 1 (Homo sapiens (Human)) | BDBM14543 (2-[6-(3-aminophenyl)-5-chloro-3-(cyclobutylamino)-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 32 | n/a | n/a | n/a | n/a | n/a | n/a |
Pharmacia Corporation Curated by ChEMBL | Assay Description Inhibitory activity of the compound against trypsin | Bioorg Med Chem Lett 13: 2319-25 (2003) BindingDB Entry DOI: 10.7270/Q2G16068 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Collagenase 3 (Homo sapiens (Human)) | BDBM50095218 (CHEMBL310008 | N-Hydroxy-2-[4-(4-trifluoromethoxy-...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 33 | n/a | n/a | n/a | n/a | n/a | n/a |
Pharmacia Curated by ChEMBL | Assay Description 50 percent inhibition of human Matrix metalloprotease-13 by the cleavage of fluorogenic peptide MCA-Pro-Leu-Gly-Leu-Dpa-ala-Arg-NH2 | Bioorg Med Chem Lett 10: 2815-7 (2000) BindingDB Entry DOI: 10.7270/Q2ZK5H6Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 72 kDa type IV collagenase (Homo sapiens (Human)) | BDBM50095224 (CHEMBL94467 | N-Hydroxy-2-[4-(4-trifluoromethyl-be...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 35 | n/a | n/a | n/a | n/a | n/a | n/a |
Pharmacia Curated by ChEMBL | Assay Description 50 percent inhibition of human Matrix metalloprotease-2 by the cleavage of fluorogenic peptide MCA-Pro-Leu-Gly-Leu-Dpa-ala-Arg-NH2 | Bioorg Med Chem Lett 10: 2815-7 (2000) BindingDB Entry DOI: 10.7270/Q2ZK5H6Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neutrophil collagenase (Homo sapiens (Human)) | BDBM50076593 (3-(4-Phenylsulfanyl-benzenesulfonyl)-propane-1-thi...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents | PubMed | n/a | n/a | 36 | n/a | n/a | n/a | n/a | n/a | n/a |
Searle Discovery Research Curated by ChEMBL | Assay Description Inhibition of human recombinant matrix metalloproteinase-8 (hMMP-8) | Bioorg Med Chem Lett 9: 1757-60 (1999) BindingDB Entry DOI: 10.7270/Q2ZK5FVN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM14563 (N-[(4-carbamimidoylphenyl)methyl]-2-{5-chloro-6-et...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | <40 | n/a | n/a | n/a | n/a | n/a | n/a |
Pharmacia Corporation | Assay Description Human thrombin and substrate were added to a 96-well assay plate containing inhibitor in reaction buffer. The rate of hydrolysis of the substrate was... | J Med Chem 46: 4050-62 (2003) Article DOI: 10.1021/jm030131l BindingDB Entry DOI: 10.7270/Q2FT8J9M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM14520 (N-[(4-carbamimidoylphenyl)methyl]-2-(5-chloro-3-{[...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | <40 | n/a | n/a | n/a | n/a | n/a | n/a |
Pharmacia Corporation | Assay Description Human thrombin and substrate were added to a 96-well assay plate containing inhibitor in reaction buffer. The rate of hydrolysis of the substrate was... | J Med Chem 46: 4050-62 (2003) Article DOI: 10.1021/jm030131l BindingDB Entry DOI: 10.7270/Q2FT8J9M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM14519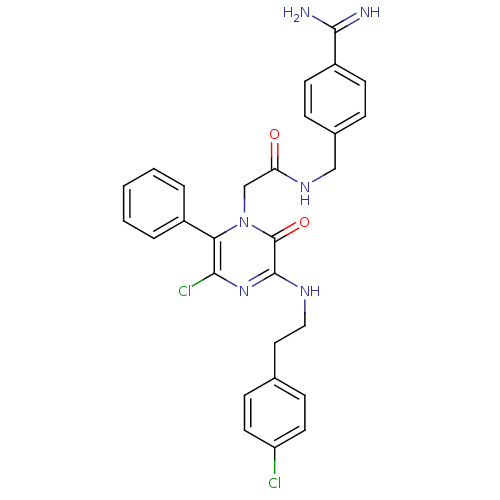 (N-[(4-carbamimidoylphenyl)methyl]-2-(5-chloro-3-{[...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | <40 | n/a | n/a | n/a | n/a | n/a | n/a |
Pharmacia Corporation | Assay Description Human thrombin and substrate were added to a 96-well assay plate containing inhibitor in reaction buffer. The rate of hydrolysis of the substrate was... | J Med Chem 46: 4050-62 (2003) Article DOI: 10.1021/jm030131l BindingDB Entry DOI: 10.7270/Q2FT8J9M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM50133093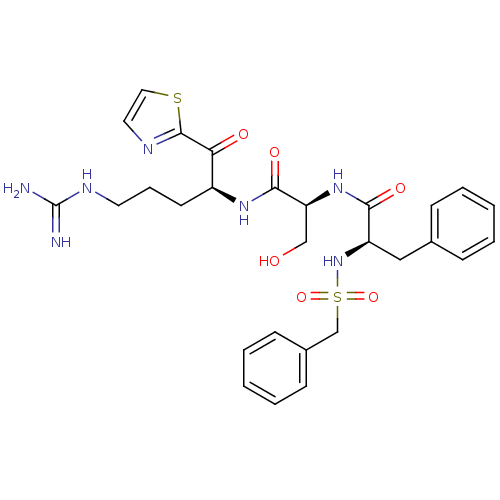 ((R)-N-{(S)-1-[(S)-4-guanidino-1-(thiazole-2-carbon...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 40 | n/a | n/a | n/a | n/a | n/a | n/a |
Pharmacia Corporation Curated by ChEMBL | Assay Description Inhibitory activity against thrombin (IIa). | J Med Chem 46: 4043-9 (2003) Article DOI: 10.1021/jm030130t BindingDB Entry DOI: 10.7270/Q2BC4092 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor VII (Homo sapiens (Human)) | BDBM14528 (2-[6-(3-aminophenyl)-5-chloro-3-(ethylamino)-2-oxo...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 40 | n/a | n/a | n/a | n/a | n/a | n/a |
Pharmacia Corporation | Assay Description Recombinant human Factor VIIa and soluble tissue factor were added to a 96-well assay plate containing substrate and inhibitor in reaction buffer. Th... | J Med Chem 46: 4050-62 (2003) Article DOI: 10.1021/jm030131l BindingDB Entry DOI: 10.7270/Q2FT8J9M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM50129981 (2-(5-Chloro-2-oxo-3-phenethylamino-6-phenyl-2H-pyr...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | <40 | n/a | n/a | n/a | n/a | n/a | n/a |
Pharmacia Corporation Curated by ChEMBL | Assay Description Inhibitory activity against Thrombin | Bioorg Med Chem Lett 13: 2319-25 (2003) BindingDB Entry DOI: 10.7270/Q2G16068 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor VII (Homo sapiens (Human)) | BDBM50130063 ((R)-N-{(S)-1-[(S)-4-Guanidino-1-(thiazole-2-carbon...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 42 | n/a | n/a | n/a | n/a | n/a | n/a |
Pharmacia Corporation Curated by ChEMBL | Assay Description Inhibition of tissue coagulation factor VII | Bioorg Med Chem Lett 13: 2363-7 (2003) BindingDB Entry DOI: 10.7270/Q2RX9CMS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor VII (Homo sapiens (Human)) | BDBM50130063 ((R)-N-{(S)-1-[(S)-4-Guanidino-1-(thiazole-2-carbon...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 42 | n/a | n/a | n/a | n/a | n/a | n/a |
Pharmacia Corporation Curated by ChEMBL | Assay Description Inhibitory activity against tissue coagulation factor VII. | J Med Chem 46: 4043-9 (2003) Article DOI: 10.1021/jm030130t BindingDB Entry DOI: 10.7270/Q2BC4092 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Collagenase 3 (Homo sapiens (Human)) | BDBM50076592 (3-(4-Methoxy-benzenesulfonyl)-propane-1-thiol | CH...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 50 | n/a | n/a | n/a | n/a | n/a | n/a |
Searle Discovery Research Curated by ChEMBL | Assay Description Inhibition of human recombinant matrix metalloproteinase-13 (hMMP-13) | Bioorg Med Chem Lett 9: 1757-60 (1999) BindingDB Entry DOI: 10.7270/Q2ZK5FVN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neutrophil collagenase (Homo sapiens (Human)) | BDBM50078668 (3-(4-Phenylsulfanyl-benzenesulfonyl)-cyclohexaneth...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents | PubMed | n/a | n/a | 50 | n/a | n/a | n/a | n/a | n/a | n/a |
Searle Discovery Research Curated by ChEMBL | Assay Description Inhibition of human recombinant matrix metalloproteinase-8 (hMMP-8) | Bioorg Med Chem Lett 9: 1757-60 (1999) BindingDB Entry DOI: 10.7270/Q2ZK5FVN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor VII (Homo sapiens (Human)) | BDBM14537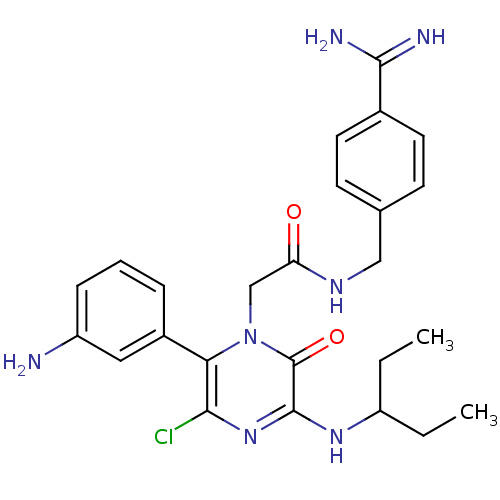 (2-[6-(3-aminophenyl)-5-chloro-2-oxo-3-(pentan-3-yl...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 50 | n/a | n/a | n/a | n/a | n/a | n/a |
Pharmacia Corporation | Assay Description Recombinant human Factor VIIa and soluble tissue factor were added to a 96-well assay plate containing substrate and inhibitor in reaction buffer. Th... | J Med Chem 46: 4050-62 (2003) Article DOI: 10.1021/jm030131l BindingDB Entry DOI: 10.7270/Q2FT8J9M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor VII (Homo sapiens (Human)) | BDBM14497 (2-Pyridone 14b | N-[(4-carbamimidoylphenyl)methyl]...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 52 | n/a | n/a | n/a | n/a | 8.0 | 22 |
Pharmacia Corporation | Assay Description Recombinant human Factor VIIa and soluble tissue factor were added to a 96-well assay plate containing substrate and inhibitor in reaction buffer. Th... | J Med Chem 46: 4696-701 (2003) Article DOI: 10.1021/jm0301686 BindingDB Entry DOI: 10.7270/Q2KK992S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM14562 (N-[(4-carbamimidoylphenyl)methyl]-2-{5-chloro-6-me...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 60 | n/a | n/a | n/a | n/a | n/a | n/a |
Pharmacia Corporation | Assay Description Human thrombin and substrate were added to a 96-well assay plate containing inhibitor in reaction buffer. The rate of hydrolysis of the substrate was... | J Med Chem 46: 4050-62 (2003) Article DOI: 10.1021/jm030131l BindingDB Entry DOI: 10.7270/Q2FT8J9M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor VII (Homo sapiens (Human)) | BDBM14526 (2-[6-(3-aminophenyl)-5-chloro-3-(methylamino)-2-ox...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 60 | n/a | n/a | n/a | n/a | n/a | n/a |
Pharmacia Corporation | Assay Description Recombinant human Factor VIIa and soluble tissue factor were added to a 96-well assay plate containing substrate and inhibitor in reaction buffer. Th... | J Med Chem 46: 4050-62 (2003) Article DOI: 10.1021/jm030131l BindingDB Entry DOI: 10.7270/Q2FT8J9M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 497 total ) | Next | Last >> |