 Found 403 hits with Last Name = 'stevenson' and Initial = 'as'
Found 403 hits with Last Name = 'stevenson' and Initial = 'as' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Bifunctional epoxide hydrolase 2
(Homo sapiens (Human)) | BDBM50295535
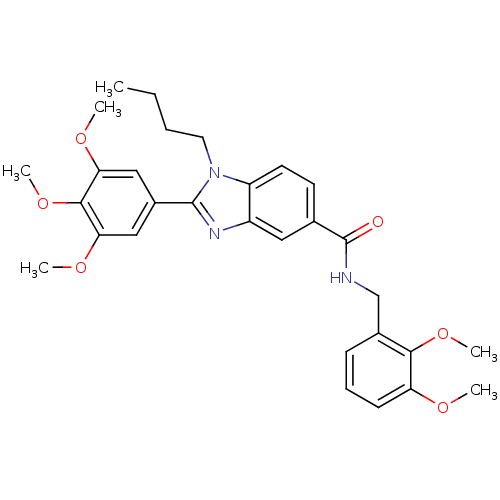
(1-butyl-N-(2,3-dimethoxybenzyl)-2-(3,4,5-trimethox...)Show SMILES CCCCn1c(nc2cc(ccc12)C(=O)NCc1cccc(OC)c1OC)-c1cc(OC)c(OC)c(OC)c1 Show InChI InChI=1S/C30H35N3O6/c1-7-8-14-33-23-13-12-19(30(34)31-18-20-10-9-11-24(35-2)27(20)38-5)15-22(23)32-29(33)21-16-25(36-3)28(39-6)26(17-21)37-4/h9-13,15-17H,7-8,14,18H2,1-6H3,(H,31,34) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of human sEH in HEK293 cells assessed as conversion of 14,15-epoxyeicosatrienoic acid to 14,15-dihydroepoxyeicosatrienoic acid |
J Med Chem 52: 5009-12 (2010)
Article DOI: 10.1021/jm900725r
BindingDB Entry DOI: 10.7270/Q2TX3G8W |
More data for this
Ligand-Target Pair | |
Bifunctional epoxide hydrolase 2
(Homo sapiens (Human)) | BDBM25737

(12-[(adamantan-1-ylcarbamoyl)amino]dodecanoic acid...)Show SMILES OC(=O)CCCCCCCCCCCNC(=O)NC12CC3CC(CC(C3)C1)C2 |TLB:25:20:27:24.23.26,25:24:20.21.19:27,THB:23:22:19:24.25.26,23:24:19:22.21.27| Show InChI InChI=1S/C23H40N2O3/c26-21(27)10-8-6-4-2-1-3-5-7-9-11-24-22(28)25-23-15-18-12-19(16-23)14-20(13-18)17-23/h18-20H,1-17H2,(H,26,27)(H2,24,25,28) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1.60 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of human sEH |
J Med Chem 52: 5009-12 (2010)
Article DOI: 10.1021/jm900725r
BindingDB Entry DOI: 10.7270/Q2TX3G8W |
More data for this
Ligand-Target Pair | |
Bifunctional epoxide hydrolase 2
(Homo sapiens (Human)) | BDBM50295541
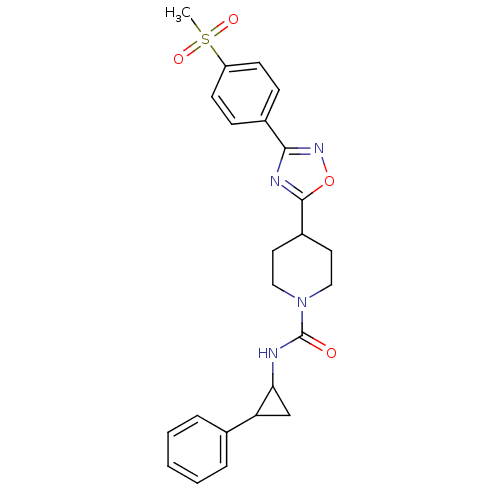
(4-(3-(4-(methylsulfonyl)phenyl)-1,2,4-oxadiazol-5-...)Show SMILES CS(=O)(=O)c1ccc(cc1)-c1noc(n1)C1CCN(CC1)C(=O)NC1CC1c1ccccc1 Show InChI InChI=1S/C24H26N4O4S/c1-33(30,31)19-9-7-17(8-10-19)22-26-23(32-27-22)18-11-13-28(14-12-18)24(29)25-21-15-20(21)16-5-3-2-4-6-16/h2-10,18,20-21H,11-15H2,1H3,(H,25,29) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of human sEH |
J Med Chem 52: 5009-12 (2010)
Article DOI: 10.1021/jm900725r
BindingDB Entry DOI: 10.7270/Q2TX3G8W |
More data for this
Ligand-Target Pair | |
Bifunctional epoxide hydrolase 2
(Homo sapiens (Human)) | BDBM50295542

(4-(5-(1-(2-phenylcyclopropylcarbamoyl)piperidin-4-...)Show SMILES OC(=O)c1ccc(cc1)-c1noc(n1)C1CCN(CC1)C(=O)NC1CC1c1ccccc1 Show InChI InChI=1S/C24H24N4O4/c29-23(30)18-8-6-16(7-9-18)21-26-22(32-27-21)17-10-12-28(13-11-17)24(31)25-20-14-19(20)15-4-2-1-3-5-15/h1-9,17,19-20H,10-14H2,(H,25,31)(H,29,30) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of human sEH |
J Med Chem 52: 5009-12 (2010)
Article DOI: 10.1021/jm900725r
BindingDB Entry DOI: 10.7270/Q2TX3G8W |
More data for this
Ligand-Target Pair | |
Bifunctional epoxide hydrolase 2
(Rattus norvegicus) | BDBM25737

(12-[(adamantan-1-ylcarbamoyl)amino]dodecanoic acid...)Show SMILES OC(=O)CCCCCCCCCCCNC(=O)NC12CC3CC(CC(C3)C1)C2 |TLB:25:20:27:24.23.26,25:24:20.21.19:27,THB:23:22:19:24.25.26,23:24:19:22.21.27| Show InChI InChI=1S/C23H40N2O3/c26-21(27)10-8-6-4-2-1-3-5-7-9-11-24-22(28)25-23-15-18-12-19(16-23)14-20(13-18)17-23/h18-20H,1-17H2,(H,26,27)(H2,24,25,28) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of rat sEH |
J Med Chem 52: 5009-12 (2010)
Article DOI: 10.1021/jm900725r
BindingDB Entry DOI: 10.7270/Q2TX3G8W |
More data for this
Ligand-Target Pair | |
Bifunctional epoxide hydrolase 2
(Rattus norvegicus) | BDBM50295540

(CHEMBL561788 | N-(2-phenylcyclopropyl)-4-(3-(2-(tr...)Show SMILES FC(F)(F)c1ccccc1-c1noc(n1)C1CCN(CC1)C(=O)NC1CC1c1ccccc1 Show InChI InChI=1S/C24H23F3N4O2/c25-24(26,27)19-9-5-4-8-17(19)21-29-22(33-30-21)16-10-12-31(13-11-16)23(32)28-20-14-18(20)15-6-2-1-3-7-15/h1-9,16,18,20H,10-14H2,(H,28,32) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of rat sEH |
J Med Chem 52: 5009-12 (2010)
Article DOI: 10.1021/jm900725r
BindingDB Entry DOI: 10.7270/Q2TX3G8W |
More data for this
Ligand-Target Pair | |
Bifunctional epoxide hydrolase 2
(Homo sapiens (Human)) | BDBM50295543

(CHEMBL564757 | N-(2-phenylcyclopropyl)-4-(3-(pyrid...)Show SMILES O=C(NC1CC1c1ccccc1)N1CCC(CC1)c1nc(no1)-c1cccnc1 Show InChI InChI=1S/C22H23N5O2/c28-22(24-19-13-18(19)15-5-2-1-3-6-15)27-11-8-16(9-12-27)21-25-20(26-29-21)17-7-4-10-23-14-17/h1-7,10,14,16,18-19H,8-9,11-13H2,(H,24,28) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 7 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of human sEH |
J Med Chem 52: 5009-12 (2010)
Article DOI: 10.1021/jm900725r
BindingDB Entry DOI: 10.7270/Q2TX3G8W |
More data for this
Ligand-Target Pair | |
Bifunctional epoxide hydrolase 2
(Homo sapiens (Human)) | BDBM50295549
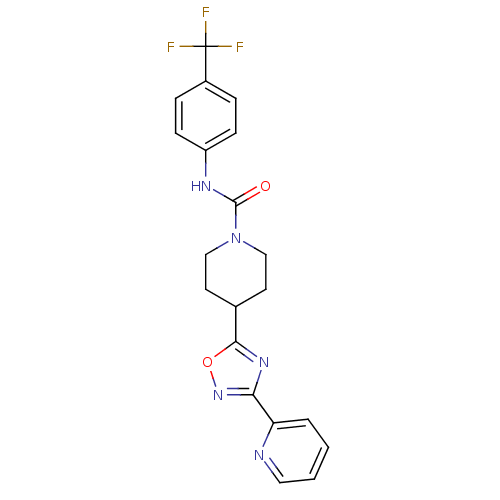
(4-(3-(pyridin-2-yl)-1,2,4-oxadiazol-5-yl)-N-(4-(tr...)Show SMILES FC(F)(F)c1ccc(NC(=O)N2CCC(CC2)c2nc(no2)-c2ccccn2)cc1 Show InChI InChI=1S/C20H18F3N5O2/c21-20(22,23)14-4-6-15(7-5-14)25-19(29)28-11-8-13(9-12-28)18-26-17(27-30-18)16-3-1-2-10-24-16/h1-7,10,13H,8-9,11-12H2,(H,25,29) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 7 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of human sEH in HEK293 cells assessed as conversion of 14,15-epoxyeicosatrienoic acid to 14,15-dihydroepoxyeicosatrienoic acid |
J Med Chem 52: 5009-12 (2010)
Article DOI: 10.1021/jm900725r
BindingDB Entry DOI: 10.7270/Q2TX3G8W |
More data for this
Ligand-Target Pair | |
Bifunctional epoxide hydrolase 2
(Homo sapiens (Human)) | BDBM50295549

(4-(3-(pyridin-2-yl)-1,2,4-oxadiazol-5-yl)-N-(4-(tr...)Show SMILES FC(F)(F)c1ccc(NC(=O)N2CCC(CC2)c2nc(no2)-c2ccccn2)cc1 Show InChI InChI=1S/C20H18F3N5O2/c21-20(22,23)14-4-6-15(7-5-14)25-19(29)28-11-8-13(9-12-28)18-26-17(27-30-18)16-3-1-2-10-24-16/h1-7,10,13H,8-9,11-12H2,(H,25,29) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 7 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of human sEH |
J Med Chem 52: 5009-12 (2010)
Article DOI: 10.1021/jm900725r
BindingDB Entry DOI: 10.7270/Q2TX3G8W |
More data for this
Ligand-Target Pair | |
Bifunctional epoxide hydrolase 2
(Homo sapiens (Human)) | BDBM50295548

(CHEMBL551921 | N-(2-phenylcyclopropyl)-4-(3-(quino...)Show SMILES O=C(NC1CC1c1ccccc1)N1CCC(CC1)c1nc(no1)-c1ccc2ccccc2n1 Show InChI InChI=1S/C26H25N5O2/c32-26(28-23-16-20(23)17-6-2-1-3-7-17)31-14-12-19(13-15-31)25-29-24(30-33-25)22-11-10-18-8-4-5-9-21(18)27-22/h1-11,19-20,23H,12-16H2,(H,28,32) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 7 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of human sEH |
J Med Chem 52: 5009-12 (2010)
Article DOI: 10.1021/jm900725r
BindingDB Entry DOI: 10.7270/Q2TX3G8W |
More data for this
Ligand-Target Pair | |
Bifunctional epoxide hydrolase 2
(Homo sapiens (Human)) | BDBM50295540

(CHEMBL561788 | N-(2-phenylcyclopropyl)-4-(3-(2-(tr...)Show SMILES FC(F)(F)c1ccccc1-c1noc(n1)C1CCN(CC1)C(=O)NC1CC1c1ccccc1 Show InChI InChI=1S/C24H23F3N4O2/c25-24(26,27)19-9-5-4-8-17(19)21-29-22(33-30-21)16-10-12-31(13-11-16)23(32)28-20-14-18(20)15-6-2-1-3-7-15/h1-9,16,18,20H,10-14H2,(H,28,32) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 7 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of human sEH in HEK293 cells assessed as conversion of 14,15-epoxyeicosatrienoic acid to 14,15-dihydroepoxyeicosatrienoic acid |
J Med Chem 52: 5009-12 (2010)
Article DOI: 10.1021/jm900725r
BindingDB Entry DOI: 10.7270/Q2TX3G8W |
More data for this
Ligand-Target Pair | |
Bifunctional epoxide hydrolase 2
(Mus musculus (Mouse)) | BDBM25737

(12-[(adamantan-1-ylcarbamoyl)amino]dodecanoic acid...)Show SMILES OC(=O)CCCCCCCCCCCNC(=O)NC12CC3CC(CC(C3)C1)C2 |TLB:25:20:27:24.23.26,25:24:20.21.19:27,THB:23:22:19:24.25.26,23:24:19:22.21.27| Show InChI InChI=1S/C23H40N2O3/c26-21(27)10-8-6-4-2-1-3-5-7-9-11-24-22(28)25-23-15-18-12-19(16-23)14-20(13-18)17-23/h18-20H,1-17H2,(H,26,27)(H2,24,25,28) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 7 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of mouse sEH |
J Med Chem 52: 5009-12 (2010)
Article DOI: 10.1021/jm900725r
BindingDB Entry DOI: 10.7270/Q2TX3G8W |
More data for this
Ligand-Target Pair | |
Bifunctional epoxide hydrolase 2
(Homo sapiens (Human)) | BDBM50295548

(CHEMBL551921 | N-(2-phenylcyclopropyl)-4-(3-(quino...)Show SMILES O=C(NC1CC1c1ccccc1)N1CCC(CC1)c1nc(no1)-c1ccc2ccccc2n1 Show InChI InChI=1S/C26H25N5O2/c32-26(28-23-16-20(23)17-6-2-1-3-7-17)31-14-12-19(13-15-31)25-29-24(30-33-25)22-11-10-18-8-4-5-9-21(18)27-22/h1-11,19-20,23H,12-16H2,(H,28,32) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 7 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of human sEH in HEK293 cells assessed as conversion of 14,15-epoxyeicosatrienoic acid to 14,15-dihydroepoxyeicosatrienoic acid |
J Med Chem 52: 5009-12 (2010)
Article DOI: 10.1021/jm900725r
BindingDB Entry DOI: 10.7270/Q2TX3G8W |
More data for this
Ligand-Target Pair | |
Bifunctional epoxide hydrolase 2
(Homo sapiens (Human)) | BDBM50295539

(4-(benzo[d]oxazol-2-yl)-N-(2-phenylcyclopropyl)pip...)Show InChI InChI=1S/C22H23N3O2/c26-22(24-19-14-17(19)15-6-2-1-3-7-15)25-12-10-16(11-13-25)21-23-18-8-4-5-9-20(18)27-21/h1-9,16-17,19H,10-14H2,(H,24,26) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 7.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of human sEH |
J Med Chem 52: 5009-12 (2010)
Article DOI: 10.1021/jm900725r
BindingDB Entry DOI: 10.7270/Q2TX3G8W |
More data for this
Ligand-Target Pair | |
Bifunctional epoxide hydrolase 2
(Homo sapiens (Human)) | BDBM50295539

(4-(benzo[d]oxazol-2-yl)-N-(2-phenylcyclopropyl)pip...)Show InChI InChI=1S/C22H23N3O2/c26-22(24-19-14-17(19)15-6-2-1-3-7-15)25-12-10-16(11-13-25)21-23-18-8-4-5-9-20(18)27-21/h1-9,16-17,19H,10-14H2,(H,24,26) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 8 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of human sEH in HEK293 cells assessed as conversion of 14,15-epoxyeicosatrienoic acid to 14,15-dihydroepoxyeicosatrienoic acid |
J Med Chem 52: 5009-12 (2010)
Article DOI: 10.1021/jm900725r
BindingDB Entry DOI: 10.7270/Q2TX3G8W |
More data for this
Ligand-Target Pair | |
Bifunctional epoxide hydrolase 2
(Homo sapiens (Human)) | BDBM50295532
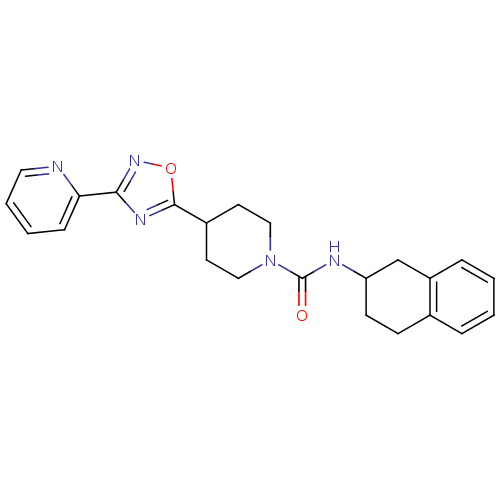
(4-(3-(pyridin-2-yl)-1,2,4-oxadiazol-5-yl)-N-(1,2,3...)Show SMILES O=C(NC1CCc2ccccc2C1)N1CCC(CC1)c1nc(no1)-c1ccccn1 Show InChI InChI=1S/C23H25N5O2/c29-23(25-19-9-8-16-5-1-2-6-18(16)15-19)28-13-10-17(11-14-28)22-26-21(27-30-22)20-7-3-4-12-24-20/h1-7,12,17,19H,8-11,13-15H2,(H,25,29) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 8 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of human sEH |
J Med Chem 52: 5009-12 (2010)
Article DOI: 10.1021/jm900725r
BindingDB Entry DOI: 10.7270/Q2TX3G8W |
More data for this
Ligand-Target Pair | |
Bifunctional epoxide hydrolase 2
(Rattus norvegicus) | BDBM50295548

(CHEMBL551921 | N-(2-phenylcyclopropyl)-4-(3-(quino...)Show SMILES O=C(NC1CC1c1ccccc1)N1CCC(CC1)c1nc(no1)-c1ccc2ccccc2n1 Show InChI InChI=1S/C26H25N5O2/c32-26(28-23-16-20(23)17-6-2-1-3-7-17)31-14-12-19(13-15-31)25-29-24(30-33-25)22-11-10-18-8-4-5-9-21(18)27-22/h1-11,19-20,23H,12-16H2,(H,28,32) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 8 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of rat sEH |
J Med Chem 52: 5009-12 (2010)
Article DOI: 10.1021/jm900725r
BindingDB Entry DOI: 10.7270/Q2TX3G8W |
More data for this
Ligand-Target Pair | |
Bifunctional epoxide hydrolase 2
(Homo sapiens (Human)) | BDBM50295534

(CHEMBL562822 | N-((1R,2S)-2-phenylcyclopropyl)-4-(...)Show SMILES O=C(N[C@@H]1C[C@H]1c1ccccc1)N1CCC(CC1)c1nc(no1)-c1ccccn1 |r| Show InChI InChI=1S/C22H23N5O2/c28-22(24-19-14-17(19)15-6-2-1-3-7-15)27-12-9-16(10-13-27)21-25-20(26-29-21)18-8-4-5-11-23-18/h1-8,11,16-17,19H,9-10,12-14H2,(H,24,28)/t17-,19+/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 9 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of human sEH |
J Med Chem 52: 5009-12 (2010)
Article DOI: 10.1021/jm900725r
BindingDB Entry DOI: 10.7270/Q2TX3G8W |
More data for this
Ligand-Target Pair | |
Bifunctional epoxide hydrolase 2
(Homo sapiens (Human)) | BDBM50295540

(CHEMBL561788 | N-(2-phenylcyclopropyl)-4-(3-(2-(tr...)Show SMILES FC(F)(F)c1ccccc1-c1noc(n1)C1CCN(CC1)C(=O)NC1CC1c1ccccc1 Show InChI InChI=1S/C24H23F3N4O2/c25-24(26,27)19-9-5-4-8-17(19)21-29-22(33-30-21)16-10-12-31(13-11-16)23(32)28-20-14-18(20)15-6-2-1-3-7-15/h1-9,16,18,20H,10-14H2,(H,28,32) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 9 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of human sEH |
J Med Chem 52: 5009-12 (2010)
Article DOI: 10.1021/jm900725r
BindingDB Entry DOI: 10.7270/Q2TX3G8W |
More data for this
Ligand-Target Pair | |
Bifunctional epoxide hydrolase 2
(Homo sapiens (Human)) | BDBM50295547
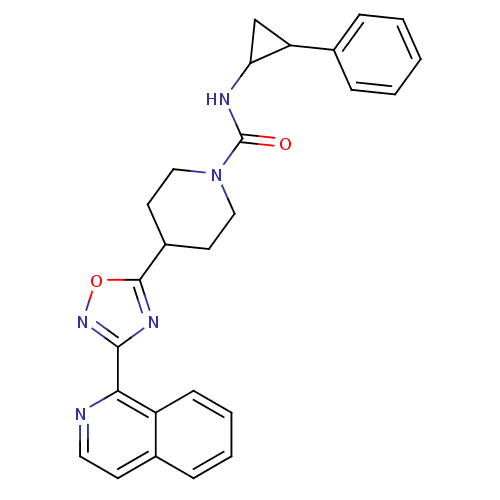
(4-(3-(isoquinolin-1-yl)-1,2,4-oxadiazol-5-yl)-N-(2...)Show SMILES O=C(NC1CC1c1ccccc1)N1CCC(CC1)c1nc(no1)-c1nccc2ccccc12 Show InChI InChI=1S/C26H25N5O2/c32-26(28-22-16-21(22)17-6-2-1-3-7-17)31-14-11-19(12-15-31)25-29-24(30-33-25)23-20-9-5-4-8-18(20)10-13-27-23/h1-10,13,19,21-22H,11-12,14-16H2,(H,28,32) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of human sEH |
J Med Chem 52: 5009-12 (2010)
Article DOI: 10.1021/jm900725r
BindingDB Entry DOI: 10.7270/Q2TX3G8W |
More data for this
Ligand-Target Pair | |
Bifunctional epoxide hydrolase 2
(Rattus norvegicus) | BDBM50295534

(CHEMBL562822 | N-((1R,2S)-2-phenylcyclopropyl)-4-(...)Show SMILES O=C(N[C@@H]1C[C@H]1c1ccccc1)N1CCC(CC1)c1nc(no1)-c1ccccn1 |r| Show InChI InChI=1S/C22H23N5O2/c28-22(24-19-14-17(19)15-6-2-1-3-7-15)27-12-9-16(10-13-27)21-25-20(26-29-21)18-8-4-5-11-23-18/h1-8,11,16-17,19H,9-10,12-14H2,(H,24,28)/t17-,19+/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of rat sEH |
J Med Chem 52: 5009-12 (2010)
Article DOI: 10.1021/jm900725r
BindingDB Entry DOI: 10.7270/Q2TX3G8W |
More data for this
Ligand-Target Pair | |
Bifunctional epoxide hydrolase 2
(Rattus norvegicus) | BDBM50295543

(CHEMBL564757 | N-(2-phenylcyclopropyl)-4-(3-(pyrid...)Show SMILES O=C(NC1CC1c1ccccc1)N1CCC(CC1)c1nc(no1)-c1cccnc1 Show InChI InChI=1S/C22H23N5O2/c28-22(24-19-13-18(19)15-5-2-1-3-6-15)27-11-8-16(9-12-27)21-25-20(26-29-21)17-7-4-10-23-14-17/h1-7,10,14,16,18-19H,8-9,11-13H2,(H,24,28) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of rat sEH |
J Med Chem 52: 5009-12 (2010)
Article DOI: 10.1021/jm900725r
BindingDB Entry DOI: 10.7270/Q2TX3G8W |
More data for this
Ligand-Target Pair | |
Bifunctional epoxide hydrolase 2
(Rattus norvegicus) | BDBM50295535

(1-butyl-N-(2,3-dimethoxybenzyl)-2-(3,4,5-trimethox...)Show SMILES CCCCn1c(nc2cc(ccc12)C(=O)NCc1cccc(OC)c1OC)-c1cc(OC)c(OC)c(OC)c1 Show InChI InChI=1S/C30H35N3O6/c1-7-8-14-33-23-13-12-19(30(34)31-18-20-10-9-11-24(35-2)27(20)38-5)15-22(23)32-29(33)21-16-25(36-3)28(39-6)26(17-21)37-4/h9-13,15-17H,7-8,14,18H2,1-6H3,(H,31,34) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of rat sEH |
J Med Chem 52: 5009-12 (2010)
Article DOI: 10.1021/jm900725r
BindingDB Entry DOI: 10.7270/Q2TX3G8W |
More data for this
Ligand-Target Pair | |
Bifunctional epoxide hydrolase 2
(Homo sapiens (Human)) | BDBM50295545
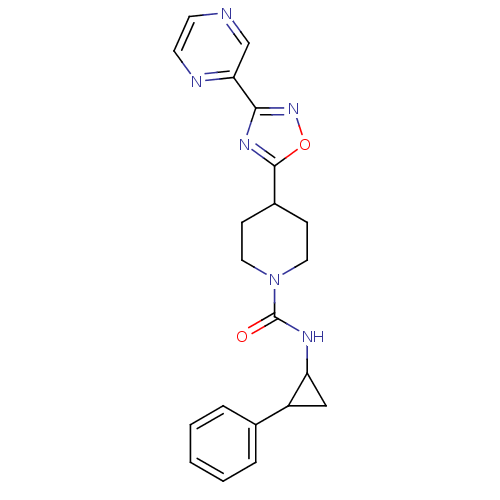
(CHEMBL557032 | N-(2-phenylcyclopropyl)-4-(3-(pyraz...)Show SMILES O=C(NC1CC1c1ccccc1)N1CCC(CC1)c1nc(no1)-c1cnccn1 Show InChI InChI=1S/C21H22N6O2/c28-21(24-17-12-16(17)14-4-2-1-3-5-14)27-10-6-15(7-11-27)20-25-19(26-29-20)18-13-22-8-9-23-18/h1-5,8-9,13,15-17H,6-7,10-12H2,(H,24,28) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 11 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of human sEH in HEK293 cells assessed as conversion of 14,15-epoxyeicosatrienoic acid to 14,15-dihydroepoxyeicosatrienoic acid |
J Med Chem 52: 5009-12 (2010)
Article DOI: 10.1021/jm900725r
BindingDB Entry DOI: 10.7270/Q2TX3G8W |
More data for this
Ligand-Target Pair | |
Bifunctional epoxide hydrolase 2
(Homo sapiens (Human)) | BDBM50295541

(4-(3-(4-(methylsulfonyl)phenyl)-1,2,4-oxadiazol-5-...)Show SMILES CS(=O)(=O)c1ccc(cc1)-c1noc(n1)C1CCN(CC1)C(=O)NC1CC1c1ccccc1 Show InChI InChI=1S/C24H26N4O4S/c1-33(30,31)19-9-7-17(8-10-19)22-26-23(32-27-22)18-11-13-28(14-12-18)24(29)25-21-15-20(21)16-5-3-2-4-6-16/h2-10,18,20-21H,11-15H2,1H3,(H,25,29) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 11 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of human sEH in HEK293 cells assessed as conversion of 14,15-epoxyeicosatrienoic acid to 14,15-dihydroepoxyeicosatrienoic acid |
J Med Chem 52: 5009-12 (2010)
Article DOI: 10.1021/jm900725r
BindingDB Entry DOI: 10.7270/Q2TX3G8W |
More data for this
Ligand-Target Pair | |
Bifunctional epoxide hydrolase 2
(Rattus norvegicus) | BDBM50295541

(4-(3-(4-(methylsulfonyl)phenyl)-1,2,4-oxadiazol-5-...)Show SMILES CS(=O)(=O)c1ccc(cc1)-c1noc(n1)C1CCN(CC1)C(=O)NC1CC1c1ccccc1 Show InChI InChI=1S/C24H26N4O4S/c1-33(30,31)19-9-7-17(8-10-19)22-26-23(32-27-22)18-11-13-28(14-12-18)24(29)25-21-15-20(21)16-5-3-2-4-6-16/h2-10,18,20-21H,11-15H2,1H3,(H,25,29) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 12 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of rat sEH |
J Med Chem 52: 5009-12 (2010)
Article DOI: 10.1021/jm900725r
BindingDB Entry DOI: 10.7270/Q2TX3G8W |
More data for this
Ligand-Target Pair | |
Bifunctional epoxide hydrolase 2
(Homo sapiens (Human)) | BDBM50295545

(CHEMBL557032 | N-(2-phenylcyclopropyl)-4-(3-(pyraz...)Show SMILES O=C(NC1CC1c1ccccc1)N1CCC(CC1)c1nc(no1)-c1cnccn1 Show InChI InChI=1S/C21H22N6O2/c28-21(24-17-12-16(17)14-4-2-1-3-5-14)27-10-6-15(7-11-27)20-25-19(26-29-20)18-13-22-8-9-23-18/h1-5,8-9,13,15-17H,6-7,10-12H2,(H,24,28) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 13 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of human sEH |
J Med Chem 52: 5009-12 (2010)
Article DOI: 10.1021/jm900725r
BindingDB Entry DOI: 10.7270/Q2TX3G8W |
More data for this
Ligand-Target Pair | |
Bifunctional epoxide hydrolase 2
(Homo sapiens (Human)) | BDBM50295543

(CHEMBL564757 | N-(2-phenylcyclopropyl)-4-(3-(pyrid...)Show SMILES O=C(NC1CC1c1ccccc1)N1CCC(CC1)c1nc(no1)-c1cccnc1 Show InChI InChI=1S/C22H23N5O2/c28-22(24-19-13-18(19)15-5-2-1-3-6-15)27-11-8-16(9-12-27)21-25-20(26-29-21)17-7-4-10-23-14-17/h1-7,10,14,16,18-19H,8-9,11-13H2,(H,24,28) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 13 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of human sEH in HEK293 cells assessed as conversion of 14,15-epoxyeicosatrienoic acid to 14,15-dihydroepoxyeicosatrienoic acid |
J Med Chem 52: 5009-12 (2010)
Article DOI: 10.1021/jm900725r
BindingDB Entry DOI: 10.7270/Q2TX3G8W |
More data for this
Ligand-Target Pair | |
Bifunctional epoxide hydrolase 2
(Homo sapiens (Human)) | BDBM50295544
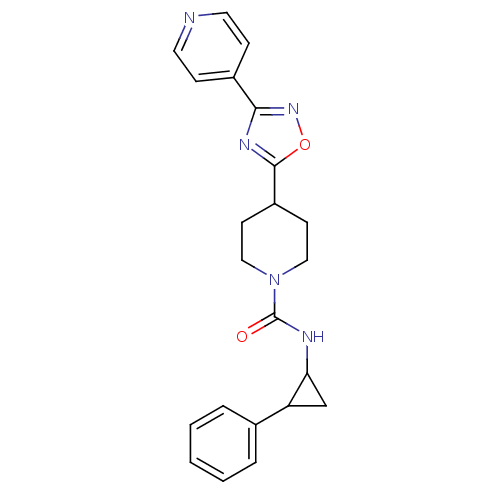
(CHEMBL556487 | N-(2-phenylcyclopropyl)-4-(3-(pyrid...)Show SMILES O=C(NC1CC1c1ccccc1)N1CCC(CC1)c1nc(no1)-c1ccncc1 Show InChI InChI=1S/C22H23N5O2/c28-22(24-19-14-18(19)15-4-2-1-3-5-15)27-12-8-17(9-13-27)21-25-20(26-29-21)16-6-10-23-11-7-16/h1-7,10-11,17-19H,8-9,12-14H2,(H,24,28) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 14 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of human sEH |
J Med Chem 52: 5009-12 (2010)
Article DOI: 10.1021/jm900725r
BindingDB Entry DOI: 10.7270/Q2TX3G8W |
More data for this
Ligand-Target Pair | |
Bifunctional epoxide hydrolase 2
(Homo sapiens (Human)) | BDBM50295534

(CHEMBL562822 | N-((1R,2S)-2-phenylcyclopropyl)-4-(...)Show SMILES O=C(N[C@@H]1C[C@H]1c1ccccc1)N1CCC(CC1)c1nc(no1)-c1ccccn1 |r| Show InChI InChI=1S/C22H23N5O2/c28-22(24-19-14-17(19)15-6-2-1-3-7-15)27-12-9-16(10-13-27)21-25-20(26-29-21)18-8-4-5-11-23-18/h1-8,11,16-17,19H,9-10,12-14H2,(H,24,28)/t17-,19+/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 14 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of human sEH |
J Med Chem 52: 5009-12 (2010)
Article DOI: 10.1021/jm900725r
BindingDB Entry DOI: 10.7270/Q2TX3G8W |
More data for this
Ligand-Target Pair | |
Bifunctional epoxide hydrolase 2
(Rattus norvegicus) | BDBM50295550

(4-(3-(pyridin-2-yl)-1,2,4-oxadiazol-5-yl)-N-(6,7,8...)Show SMILES O=C(NC1CCc2ccccc2CC1)N1CCC(CC1)c1nc(no1)-c1ccccn1 Show InChI InChI=1S/C24H27N5O2/c30-24(26-20-10-8-17-5-1-2-6-18(17)9-11-20)29-15-12-19(13-16-29)23-27-22(28-31-23)21-7-3-4-14-25-21/h1-7,14,19-20H,8-13,15-16H2,(H,26,30) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 15 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of rat sEH |
J Med Chem 52: 5009-12 (2010)
Article DOI: 10.1021/jm900725r
BindingDB Entry DOI: 10.7270/Q2TX3G8W |
More data for this
Ligand-Target Pair | |
Bifunctional epoxide hydrolase 2
(Rattus norvegicus) | BDBM50295544

(CHEMBL556487 | N-(2-phenylcyclopropyl)-4-(3-(pyrid...)Show SMILES O=C(NC1CC1c1ccccc1)N1CCC(CC1)c1nc(no1)-c1ccncc1 Show InChI InChI=1S/C22H23N5O2/c28-22(24-19-14-18(19)15-4-2-1-3-5-15)27-12-8-17(9-13-27)21-25-20(26-29-21)16-6-10-23-11-7-16/h1-7,10-11,17-19H,8-9,12-14H2,(H,24,28) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 15 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of rat sEH |
J Med Chem 52: 5009-12 (2010)
Article DOI: 10.1021/jm900725r
BindingDB Entry DOI: 10.7270/Q2TX3G8W |
More data for this
Ligand-Target Pair | |
Bifunctional epoxide hydrolase 2
(Rattus norvegicus) | BDBM50295539

(4-(benzo[d]oxazol-2-yl)-N-(2-phenylcyclopropyl)pip...)Show InChI InChI=1S/C22H23N3O2/c26-22(24-19-14-17(19)15-6-2-1-3-7-15)25-12-10-16(11-13-25)21-23-18-8-4-5-9-20(18)27-21/h1-9,16-17,19H,10-14H2,(H,24,26) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 16 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of rat sEH |
J Med Chem 52: 5009-12 (2010)
Article DOI: 10.1021/jm900725r
BindingDB Entry DOI: 10.7270/Q2TX3G8W |
More data for this
Ligand-Target Pair | |
Bifunctional epoxide hydrolase 2
(Rattus norvegicus) | BDBM50295545

(CHEMBL557032 | N-(2-phenylcyclopropyl)-4-(3-(pyraz...)Show SMILES O=C(NC1CC1c1ccccc1)N1CCC(CC1)c1nc(no1)-c1cnccn1 Show InChI InChI=1S/C21H22N6O2/c28-21(24-17-12-16(17)14-4-2-1-3-5-14)27-10-6-15(7-11-27)20-25-19(26-29-20)18-13-22-8-9-23-18/h1-5,8-9,13,15-17H,6-7,10-12H2,(H,24,28) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 17 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of rat sEH |
J Med Chem 52: 5009-12 (2010)
Article DOI: 10.1021/jm900725r
BindingDB Entry DOI: 10.7270/Q2TX3G8W |
More data for this
Ligand-Target Pair | |
Bifunctional epoxide hydrolase 2
(Rattus norvegicus) | BDBM50295549

(4-(3-(pyridin-2-yl)-1,2,4-oxadiazol-5-yl)-N-(4-(tr...)Show SMILES FC(F)(F)c1ccc(NC(=O)N2CCC(CC2)c2nc(no2)-c2ccccn2)cc1 Show InChI InChI=1S/C20H18F3N5O2/c21-20(22,23)14-4-6-15(7-5-14)25-19(29)28-11-8-13(9-12-28)18-26-17(27-30-18)16-3-1-2-10-24-16/h1-7,10,13H,8-9,11-12H2,(H,25,29) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 17 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of rat sEH |
J Med Chem 52: 5009-12 (2010)
Article DOI: 10.1021/jm900725r
BindingDB Entry DOI: 10.7270/Q2TX3G8W |
More data for this
Ligand-Target Pair | |
Bifunctional epoxide hydrolase 2
(Rattus norvegicus) | BDBM50295542

(4-(5-(1-(2-phenylcyclopropylcarbamoyl)piperidin-4-...)Show SMILES OC(=O)c1ccc(cc1)-c1noc(n1)C1CCN(CC1)C(=O)NC1CC1c1ccccc1 Show InChI InChI=1S/C24H24N4O4/c29-23(30)18-8-6-16(7-9-18)21-26-22(32-27-21)17-10-12-28(13-11-17)24(31)25-20-14-19(20)15-4-2-1-3-5-15/h1-9,17,19-20H,10-14H2,(H,25,31)(H,29,30) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 17 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of rat sEH |
J Med Chem 52: 5009-12 (2010)
Article DOI: 10.1021/jm900725r
BindingDB Entry DOI: 10.7270/Q2TX3G8W |
More data for this
Ligand-Target Pair | |
Bifunctional epoxide hydrolase 2
(Homo sapiens (Human)) | BDBM50295534

(CHEMBL562822 | N-((1R,2S)-2-phenylcyclopropyl)-4-(...)Show SMILES O=C(N[C@@H]1C[C@H]1c1ccccc1)N1CCC(CC1)c1nc(no1)-c1ccccn1 |r| Show InChI InChI=1S/C22H23N5O2/c28-22(24-19-14-17(19)15-6-2-1-3-7-15)27-12-9-16(10-13-27)21-25-20(26-29-21)18-8-4-5-11-23-18/h1-8,11,16-17,19H,9-10,12-14H2,(H,24,28)/t17-,19+/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 18 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of human sEH in HEK293 cells assessed as conversion of 14,15-epoxyeicosatrienoic acid to 14,15-dihydroepoxyeicosatrienoic acid |
J Med Chem 52: 5009-12 (2010)
Article DOI: 10.1021/jm900725r
BindingDB Entry DOI: 10.7270/Q2TX3G8W |
More data for this
Ligand-Target Pair | |
Bifunctional epoxide hydrolase 2
(Rattus norvegicus) | BDBM50295534

(CHEMBL562822 | N-((1R,2S)-2-phenylcyclopropyl)-4-(...)Show SMILES O=C(N[C@@H]1C[C@H]1c1ccccc1)N1CCC(CC1)c1nc(no1)-c1ccccn1 |r| Show InChI InChI=1S/C22H23N5O2/c28-22(24-19-14-17(19)15-6-2-1-3-7-15)27-12-9-16(10-13-27)21-25-20(26-29-21)18-8-4-5-11-23-18/h1-8,11,16-17,19H,9-10,12-14H2,(H,24,28)/t17-,19+/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 18 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of rat sEH |
J Med Chem 52: 5009-12 (2010)
Article DOI: 10.1021/jm900725r
BindingDB Entry DOI: 10.7270/Q2TX3G8W |
More data for this
Ligand-Target Pair | |
Bifunctional epoxide hydrolase 2
(Rattus norvegicus) | BDBM50295547

(4-(3-(isoquinolin-1-yl)-1,2,4-oxadiazol-5-yl)-N-(2...)Show SMILES O=C(NC1CC1c1ccccc1)N1CCC(CC1)c1nc(no1)-c1nccc2ccccc12 Show InChI InChI=1S/C26H25N5O2/c32-26(28-22-16-21(22)17-6-2-1-3-7-17)31-14-11-19(12-15-31)25-29-24(30-33-25)23-20-9-5-4-8-18(20)10-13-27-23/h1-10,13,19,21-22H,11-12,14-16H2,(H,28,32) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 21 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of rat sEH |
J Med Chem 52: 5009-12 (2010)
Article DOI: 10.1021/jm900725r
BindingDB Entry DOI: 10.7270/Q2TX3G8W |
More data for this
Ligand-Target Pair | |
Bifunctional epoxide hydrolase 2
(Rattus norvegicus) | BDBM50295532

(4-(3-(pyridin-2-yl)-1,2,4-oxadiazol-5-yl)-N-(1,2,3...)Show SMILES O=C(NC1CCc2ccccc2C1)N1CCC(CC1)c1nc(no1)-c1ccccn1 Show InChI InChI=1S/C23H25N5O2/c29-23(25-19-9-8-16-5-1-2-6-18(16)15-19)28-13-10-17(11-14-28)22-26-21(27-30-22)20-7-3-4-12-24-20/h1-7,12,17,19H,8-11,13-15H2,(H,25,29) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 22 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of rat sEH |
J Med Chem 52: 5009-12 (2010)
Article DOI: 10.1021/jm900725r
BindingDB Entry DOI: 10.7270/Q2TX3G8W |
More data for this
Ligand-Target Pair | |
Bifunctional epoxide hydrolase 2
(Rattus norvegicus) | BDBM50295534

(CHEMBL562822 | N-((1R,2S)-2-phenylcyclopropyl)-4-(...)Show SMILES O=C(N[C@@H]1C[C@H]1c1ccccc1)N1CCC(CC1)c1nc(no1)-c1ccccn1 |r| Show InChI InChI=1S/C22H23N5O2/c28-22(24-19-14-17(19)15-6-2-1-3-7-15)27-12-9-16(10-13-27)21-25-20(26-29-21)18-8-4-5-11-23-18/h1-8,11,16-17,19H,9-10,12-14H2,(H,24,28)/t17-,19+/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 27 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of rat sEH |
J Med Chem 52: 5009-12 (2010)
Article DOI: 10.1021/jm900725r
BindingDB Entry DOI: 10.7270/Q2TX3G8W |
More data for this
Ligand-Target Pair | |
Bifunctional epoxide hydrolase 2
(Homo sapiens (Human)) | BDBM50295538

(4-(3-methyl-1,2,4-oxadiazol-5-yl)-N-(2-phenylcyclo...)Show InChI InChI=1S/C18H22N4O2/c1-12-19-17(24-21-12)14-7-9-22(10-8-14)18(23)20-16-11-15(16)13-5-3-2-4-6-13/h2-6,14-16H,7-11H2,1H3,(H,20,23) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 28 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of human sEH |
J Med Chem 52: 5009-12 (2010)
Article DOI: 10.1021/jm900725r
BindingDB Entry DOI: 10.7270/Q2TX3G8W |
More data for this
Ligand-Target Pair | |
Bifunctional epoxide hydrolase 2
(Homo sapiens (Human)) | BDBM50295544

(CHEMBL556487 | N-(2-phenylcyclopropyl)-4-(3-(pyrid...)Show SMILES O=C(NC1CC1c1ccccc1)N1CCC(CC1)c1nc(no1)-c1ccncc1 Show InChI InChI=1S/C22H23N5O2/c28-22(24-19-14-18(19)15-4-2-1-3-5-15)27-12-8-17(9-13-27)21-25-20(26-29-21)16-6-10-23-11-7-16/h1-7,10-11,17-19H,8-9,12-14H2,(H,24,28) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 30 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of human sEH in HEK293 cells assessed as conversion of 14,15-epoxyeicosatrienoic acid to 14,15-dihydroepoxyeicosatrienoic acid |
J Med Chem 52: 5009-12 (2010)
Article DOI: 10.1021/jm900725r
BindingDB Entry DOI: 10.7270/Q2TX3G8W |
More data for this
Ligand-Target Pair | |
Bifunctional epoxide hydrolase 2
(Homo sapiens (Human)) | BDBM50295546

(CHEMBL551517 | N-(2-phenylcyclopropyl)-4-(3-(pyrim...)Show SMILES O=C(NC1CC1c1ccccc1)N1CCC(CC1)c1nc(no1)-c1ncccn1 Show InChI InChI=1S/C21H22N6O2/c28-21(24-17-13-16(17)14-5-2-1-3-6-14)27-11-7-15(8-12-27)20-25-19(26-29-20)18-22-9-4-10-23-18/h1-6,9-10,15-17H,7-8,11-13H2,(H,24,28) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 33 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of human sEH |
J Med Chem 52: 5009-12 (2010)
Article DOI: 10.1021/jm900725r
BindingDB Entry DOI: 10.7270/Q2TX3G8W |
More data for this
Ligand-Target Pair | |
Bifunctional epoxide hydrolase 2
(Homo sapiens (Human)) | BDBM50295534

(CHEMBL562822 | N-((1R,2S)-2-phenylcyclopropyl)-4-(...)Show SMILES O=C(N[C@@H]1C[C@H]1c1ccccc1)N1CCC(CC1)c1nc(no1)-c1ccccn1 |r| Show InChI InChI=1S/C22H23N5O2/c28-22(24-19-14-17(19)15-6-2-1-3-7-15)27-12-9-16(10-13-27)21-25-20(26-29-21)18-8-4-5-11-23-18/h1-8,11,16-17,19H,9-10,12-14H2,(H,24,28)/t17-,19+/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 34 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of human sEH in HEK293 cells assessed as conversion of 14,15-epoxyeicosatrienoic acid to 14,15-dihydroepoxyeicosatrienoic acid |
J Med Chem 52: 5009-12 (2010)
Article DOI: 10.1021/jm900725r
BindingDB Entry DOI: 10.7270/Q2TX3G8W |
More data for this
Ligand-Target Pair | |
Bifunctional epoxide hydrolase 2
(Homo sapiens (Human)) | BDBM50295547

(4-(3-(isoquinolin-1-yl)-1,2,4-oxadiazol-5-yl)-N-(2...)Show SMILES O=C(NC1CC1c1ccccc1)N1CCC(CC1)c1nc(no1)-c1nccc2ccccc12 Show InChI InChI=1S/C26H25N5O2/c32-26(28-22-16-21(22)17-6-2-1-3-7-17)31-14-11-19(12-15-31)25-29-24(30-33-25)23-20-9-5-4-8-18(20)10-13-27-23/h1-10,13,19,21-22H,11-12,14-16H2,(H,28,32) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 35 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of human sEH in HEK293 cells assessed as conversion of 14,15-epoxyeicosatrienoic acid to 14,15-dihydroepoxyeicosatrienoic acid |
J Med Chem 52: 5009-12 (2010)
Article DOI: 10.1021/jm900725r
BindingDB Entry DOI: 10.7270/Q2TX3G8W |
More data for this
Ligand-Target Pair | |
Bifunctional epoxide hydrolase 2
(Rattus norvegicus) | BDBM50295546

(CHEMBL551517 | N-(2-phenylcyclopropyl)-4-(3-(pyrim...)Show SMILES O=C(NC1CC1c1ccccc1)N1CCC(CC1)c1nc(no1)-c1ncccn1 Show InChI InChI=1S/C21H22N6O2/c28-21(24-17-13-16(17)14-5-2-1-3-6-14)27-11-7-15(8-12-27)20-25-19(26-29-20)18-22-9-4-10-23-18/h1-6,9-10,15-17H,7-8,11-13H2,(H,24,28) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 35 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of rat sEH |
J Med Chem 52: 5009-12 (2010)
Article DOI: 10.1021/jm900725r
BindingDB Entry DOI: 10.7270/Q2TX3G8W |
More data for this
Ligand-Target Pair | |
Bifunctional epoxide hydrolase 2
(Homo sapiens (Human)) | BDBM50295550

(4-(3-(pyridin-2-yl)-1,2,4-oxadiazol-5-yl)-N-(6,7,8...)Show SMILES O=C(NC1CCc2ccccc2CC1)N1CCC(CC1)c1nc(no1)-c1ccccn1 Show InChI InChI=1S/C24H27N5O2/c30-24(26-20-10-8-17-5-1-2-6-18(17)9-11-20)29-15-12-19(13-16-29)23-27-22(28-31-23)21-7-3-4-14-25-21/h1-7,14,19-20H,8-13,15-16H2,(H,26,30) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 36 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of human sEH |
J Med Chem 52: 5009-12 (2010)
Article DOI: 10.1021/jm900725r
BindingDB Entry DOI: 10.7270/Q2TX3G8W |
More data for this
Ligand-Target Pair | |
Bifunctional epoxide hydrolase 2
(Rattus norvegicus) | BDBM50295538

(4-(3-methyl-1,2,4-oxadiazol-5-yl)-N-(2-phenylcyclo...)Show InChI InChI=1S/C18H22N4O2/c1-12-19-17(24-21-12)14-7-9-22(10-8-14)18(23)20-16-11-15(16)13-5-3-2-4-6-13/h2-6,14-16H,7-11H2,1H3,(H,20,23) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 48 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of rat sEH |
J Med Chem 52: 5009-12 (2010)
Article DOI: 10.1021/jm900725r
BindingDB Entry DOI: 10.7270/Q2TX3G8W |
More data for this
Ligand-Target Pair | |
Bifunctional epoxide hydrolase 2
(Homo sapiens (Human)) | BDBM50295532

(4-(3-(pyridin-2-yl)-1,2,4-oxadiazol-5-yl)-N-(1,2,3...)Show SMILES O=C(NC1CCc2ccccc2C1)N1CCC(CC1)c1nc(no1)-c1ccccn1 Show InChI InChI=1S/C23H25N5O2/c29-23(25-19-9-8-16-5-1-2-6-18(16)15-19)28-13-10-17(11-14-28)22-26-21(27-30-22)20-7-3-4-12-24-20/h1-7,12,17,19H,8-11,13-15H2,(H,25,29) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 49 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of human sEH in HEK293 cells assessed as conversion of 14,15-epoxyeicosatrienoic acid to 14,15-dihydroepoxyeicosatrienoic acid |
J Med Chem 52: 5009-12 (2010)
Article DOI: 10.1021/jm900725r
BindingDB Entry DOI: 10.7270/Q2TX3G8W |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data