 Found 383 hits with Last Name = 'stoddart' and Initial = 'la'
Found 383 hits with Last Name = 'stoddart' and Initial = 'la' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Mu-type opioid receptor
(Homo sapiens (Human)) | BDBM50001714
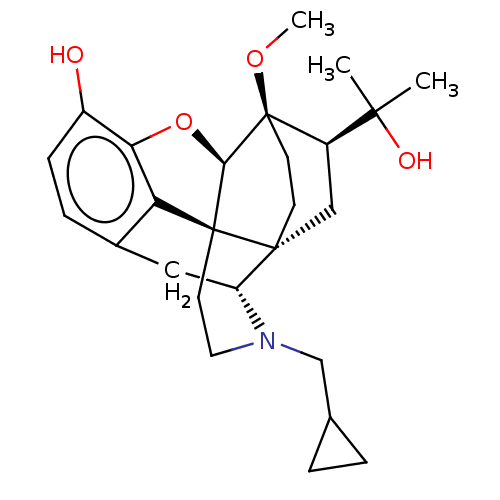
(2-[3-cyclopropylmethyl-11-hydroxy-15-methoxy-(1S,2...)Show SMILES CO[C@]12CC[C@@]3(C[C@@H]1C(C)(C)O)[C@H]1Cc4ccc(O)c5O[C@@H]2[C@]3(CCN1CC1CC1)c45 |THB:8:7:22.21:4.3| Show InChI InChI=1S/C26H35NO4/c1-23(2,29)18-13-24-8-9-26(18,30-3)22-25(24)10-11-27(14-15-4-5-15)19(24)12-16-6-7-17(28)21(31-22)20(16)25/h6-7,15,18-19,22,28-29H,4-5,8-14H2,1-3H3/t18-,19-,22-,24-,25+,26-/m1/s1 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
KEGG
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.123 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Nottingham
Curated by ChEMBL
| Assay Description
Displacement of 2-((1E,3E,5E)-5-(1-Ethyl-3,3-dimethyl-5-sulfoindolin-2-ylidene)-penta-1,3-dien-1-yl)-1-(6-((6-((6S,7R,7aR,12bS)-9-hydroxy-7-methoxy-3... |
J Med Chem 58: 9754-67 (2015)
Article DOI: 10.1021/acs.jmedchem.5b01664
BindingDB Entry DOI: 10.7270/Q25M67K5 |
More data for this
Ligand-Target Pair | |
Histamine H1 receptor
(Homo sapiens (Human)) | BDBM112780
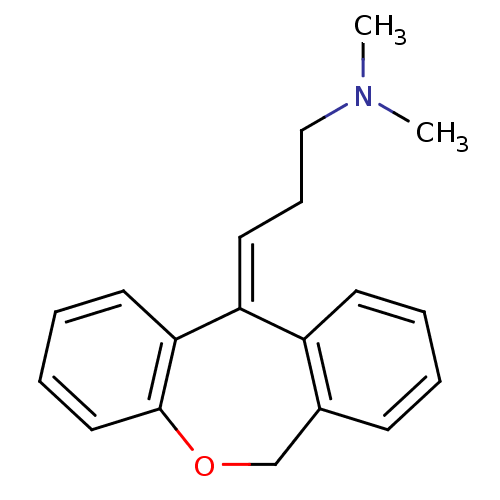
(US8629135, SW-07)Show InChI InChI=1S/C19H21NO/c1-20(2)13-7-11-17-16-9-4-3-8-15(16)14-21-19-12-6-5-10-18(17)19/h3-6,8-12H,7,13-14H2,1-2H3/b17-11+ | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
KEGG
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| 0.178 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00125
BindingDB Entry DOI: 10.7270/Q29W0KK1 |
More data for this
Ligand-Target Pair | |
Mu-type opioid receptor
(Homo sapiens (Human)) | BDBM50001714

(2-[3-cyclopropylmethyl-11-hydroxy-15-methoxy-(1S,2...)Show SMILES CO[C@]12CC[C@@]3(C[C@@H]1C(C)(C)O)[C@H]1Cc4ccc(O)c5O[C@@H]2[C@]3(CCN1CC1CC1)c45 |THB:8:7:22.21:4.3| Show InChI InChI=1S/C26H35NO4/c1-23(2,29)18-13-24-8-9-26(18,30-3)22-25(24)10-11-27(14-15-4-5-15)19(24)12-16-6-7-17(28)21(31-22)20(16)25/h6-7,15,18-19,22,28-29H,4-5,8-14H2,1-3H3/t18-,19-,22-,24-,25+,26-/m1/s1 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
KEGG
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Nottingham
Curated by ChEMBL
| Assay Description
Displacement of [3H]DAMGO from human MOR expressed in HEK293 cells preincubated for 1 hr followed by radioligand addition measured after 1 hr by liqu... |
J Med Chem 58: 9754-67 (2015)
Article DOI: 10.1021/acs.jmedchem.5b01664
BindingDB Entry DOI: 10.7270/Q25M67K5 |
More data for this
Ligand-Target Pair | |
Mu-type opioid receptor
(Homo sapiens (Human)) | BDBM60212

((4R,4aS,7aR,12bS)-3-(cyclopropylmethyl)-4a,9-bis(o...)Show SMILES Oc1ccc2C[C@H]3N(CC4CC4)CC[C@@]45[C@@H](Oc1c24)C(=O)CC[C@@]35O Show InChI InChI=1S/C20H23NO4/c22-13-4-3-12-9-15-20(24)6-5-14(23)18-19(20,16(12)17(13)25-18)7-8-21(15)10-11-1-2-11/h3-4,11,15,18,22,24H,1-2,5-10H2/t15-,18+,19+,20-/m1/s1 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
DrugBank
MCE
KEGG
PC cid
PC sid
UniChem
Similars
| DrugBank
Article
PubMed
| 0.200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Nottingham
Curated by ChEMBL
| Assay Description
Displacement of [3H]DAMGO from human MOR expressed in HEK293 cells preincubated for 1 hr followed by radioligand addition measured after 1 hr by liqu... |
J Med Chem 58: 9754-67 (2015)
Article DOI: 10.1021/acs.jmedchem.5b01664
BindingDB Entry DOI: 10.7270/Q25M67K5 |
More data for this
Ligand-Target Pair | |
Mu-type opioid receptor
(Homo sapiens (Human)) | BDBM60212

((4R,4aS,7aR,12bS)-3-(cyclopropylmethyl)-4a,9-bis(o...)Show SMILES Oc1ccc2C[C@H]3N(CC4CC4)CC[C@@]45[C@@H](Oc1c24)C(=O)CC[C@@]35O Show InChI InChI=1S/C20H23NO4/c22-13-4-3-12-9-15-20(24)6-5-14(23)18-19(20,16(12)17(13)25-18)7-8-21(15)10-11-1-2-11/h3-4,11,15,18,22,24H,1-2,5-10H2/t15-,18+,19+,20-/m1/s1 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
DrugBank
MCE
KEGG
PC cid
PC sid
UniChem
Similars
| DrugBank
Article
PubMed
| 0.275 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Nottingham
Curated by ChEMBL
| Assay Description
Displacement of 2-((1E,3E,5E)-5-(1-Ethyl-3,3-dimethyl-5-sulfoindolin-2-ylidene)-penta-1,3-dien-1-yl)-1-(6-((6-((6S,7R,7aR,12bS)-9-hydroxy-7-methoxy-3... |
J Med Chem 58: 9754-67 (2015)
Article DOI: 10.1021/acs.jmedchem.5b01664
BindingDB Entry DOI: 10.7270/Q25M67K5 |
More data for this
Ligand-Target Pair | |
Adenosine receptor A1
(Homo sapiens (Human)) | BDBM50583640
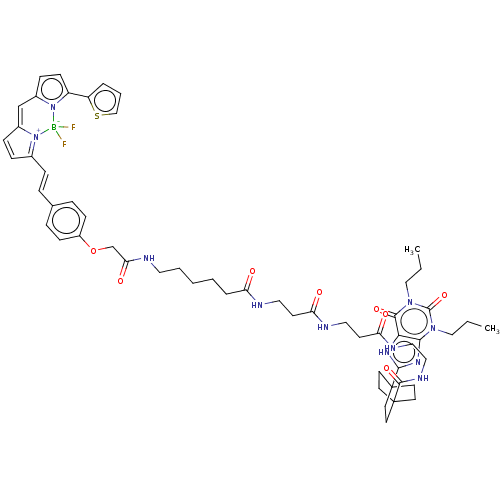
(CHEMBL5075285)Show SMILES CCCn1c2nc([nH]c2c(=O)n(CCC)c1=O)C12CCC(CC1)(CC2)C(=O)NCCNC(=O)CCNC(=O)CCNC(=O)CCCCCNC(=O)COc1ccc(\C=C\C2=[N+]3C(C=C2)=Cc2ccc(-c4cccs4)n2[B-]3(F)F)cc1 |c:66,68,t:63| | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.288 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Displacement of [3H]DPCPX from human A1 adenosine receptor expressed in Flp-In-CHO cells by radioligand competitive binding assay |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.0c02067
BindingDB Entry DOI: 10.7270/Q2CV4NM3 |
More data for this
Ligand-Target Pair | |
Adenosine receptor A1
(Homo sapiens (Human)) | BDBM50583641
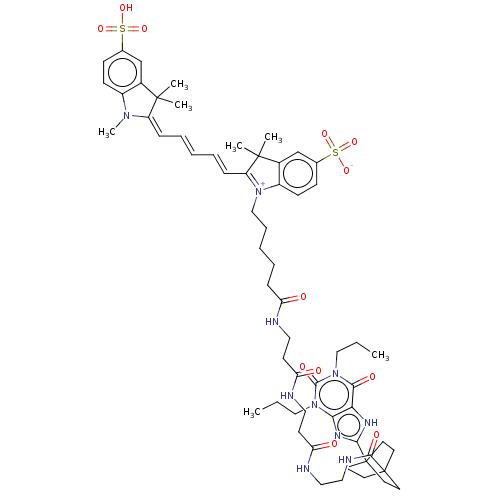
(CHEMBL5086197)Show SMILES CCCn1c2nc([nH]c2c(=O)n(CCC)c1=O)C12CCC(CC1)(CC2)C(=O)NCCNC(=O)CCNC(=O)CCNC(=O)CCCCC[N+]1=C(\C=C\C=C\C=C2\N(C)c3ccc(cc3C2(C)C)S(O)(=O)=O)C(C)(C)c2cc(ccc12)S([O-])(=O)=O |c:52| | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.708 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Binding affinity to NanoLuc human A1 adenosine receptor expressed in HEK293-A cells in prescence of SLV320 by NanoBRET competitive binding assay |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.0c02067
BindingDB Entry DOI: 10.7270/Q2CV4NM3 |
More data for this
Ligand-Target Pair | |
Adenosine receptor A1
(Homo sapiens (Human)) | BDBM50581950

(CHEMBL4204703)Show SMILES O[C@H]1CC[C@@H](CC1)Nc1nc(nc2[nH]ccc12)-c1ccccc1 |r,wU:4.7,wD:1.0,(5.09,-4.45,;6.42,-5.22,;6.43,-6.76,;7.77,-7.53,;9.09,-6.74,;9.09,-5.21,;7.76,-4.45,;10.43,-7.5,;10.44,-9.04,;9.12,-9.82,;9.13,-11.35,;10.47,-12.12,;11.8,-11.34,;13.26,-11.81,;14.16,-10.57,;13.25,-9.33,;11.79,-9.81,;7.8,-12.13,;6.47,-11.36,;5.14,-12.13,;5.14,-13.68,;6.47,-14.45,;7.81,-13.68,)| | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Displacement of [3H]DPCPX from human A1 adenosine receptor expressed in CHO-K1 cells by radioligand binding assay |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.0c02067
BindingDB Entry DOI: 10.7270/Q2CV4NM3 |
More data for this
Ligand-Target Pair | |
Mu-type opioid receptor
(Homo sapiens (Human)) | BDBM50000788

((morphine)4-methyl-(1S,5R,13R,14S,17R)-12-oxa-4-az...)Show SMILES Oc1ccc2C[C@H]3N(CC=C)CC[C@@]45[C@@H](Oc1c24)C(=O)CC[C@@]35O |r| Show InChI InChI=1S/C19H21NO4/c1-2-8-20-9-7-18-15-11-3-4-12(21)16(15)24-17(18)13(22)5-6-19(18,23)14(20)10-11/h2-4,14,17,21,23H,1,5-10H2/t14-,17+,18+,19-/m1/s1 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
DrugBank
MCE
KEGG
PC cid
PC sid
UniChem
Patents
Similars
| DrugBank
Article
PubMed
| 1.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Nottingham
Curated by ChEMBL
| Assay Description
Displacement of 2-((1E,3E,5E)-5-(1-Ethyl-3,3-dimethyl-5-sulfoindolin-2-ylidene)-penta-1,3-dien-1-yl)-1-(6-((6-((6S,7R,7aR,12bS)-9-hydroxy-7-methoxy-3... |
J Med Chem 58: 9754-67 (2015)
Article DOI: 10.1021/acs.jmedchem.5b01664
BindingDB Entry DOI: 10.7270/Q25M67K5 |
More data for this
Ligand-Target Pair | |
Mu-type opioid receptor
(Homo sapiens (Human)) | BDBM50000788

((morphine)4-methyl-(1S,5R,13R,14S,17R)-12-oxa-4-az...)Show SMILES Oc1ccc2C[C@H]3N(CC=C)CC[C@@]45[C@@H](Oc1c24)C(=O)CC[C@@]35O |r| Show InChI InChI=1S/C19H21NO4/c1-2-8-20-9-7-18-15-11-3-4-12(21)16(15)24-17(18)13(22)5-6-19(18,23)14(20)10-11/h2-4,14,17,21,23H,1,5-10H2/t14-,17+,18+,19-/m1/s1 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
DrugBank
MCE
KEGG
PC cid
PC sid
UniChem
Patents
Similars
| DrugBank
Article
PubMed
| 1.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Nottingham
Curated by ChEMBL
| Assay Description
Displacement of [3H]DAMGO from human MOR expressed in HEK293 cells preincubated for 1 hr followed by radioligand addition measured after 1 hr by liqu... |
J Med Chem 58: 9754-67 (2015)
Article DOI: 10.1021/acs.jmedchem.5b01664
BindingDB Entry DOI: 10.7270/Q25M67K5 |
More data for this
Ligand-Target Pair | |
Mu-type opioid receptor
(Homo sapiens (Human)) | BDBM21015

((2S)-2-{2-[(2R)-2-[(2S)-2-amino-3-(4-hydroxyphenyl...)Show SMILES C[C@@H](NC(=O)[C@@H](N)Cc1ccc(O)cc1)C(=O)NCC(=O)N(C)[C@@H](Cc1ccccc1)C(=O)NCCO Show InChI InChI=1S/C26H35N5O6/c1-17(30-25(36)21(27)14-19-8-10-20(33)11-9-19)24(35)29-16-23(34)31(2)22(26(37)28-12-13-32)15-18-6-4-3-5-7-18/h3-11,17,21-22,32-33H,12-16,27H2,1-2H3,(H,28,37)(H,29,35)(H,30,36)/t17-,21+,22+/m1/s1 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Nottingham
Curated by ChEMBL
| Assay Description
Displacement of [3H]DAMGO from human MOR expressed in HEK293 cells preincubated for 1 hr followed by radioligand addition measured after 1 hr by liqu... |
J Med Chem 58: 9754-67 (2015)
Article DOI: 10.1021/acs.jmedchem.5b01664
BindingDB Entry DOI: 10.7270/Q25M67K5 |
More data for this
Ligand-Target Pair | |
Adenosine receptor A1
(Homo sapiens (Human)) | BDBM50583644
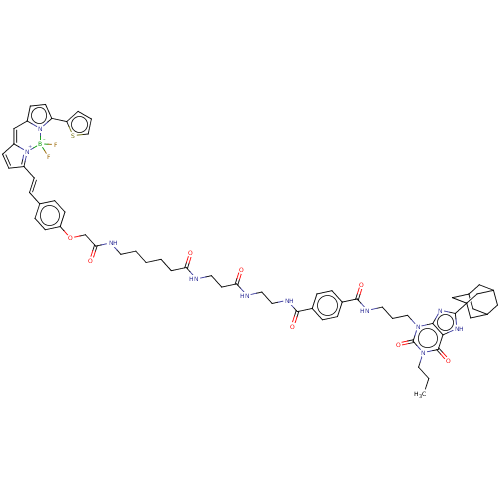
(CHEMBL5080679)Show SMILES CCCn1c(=O)n(CCCNC(=O)c2ccc(cc2)C(=O)NCCNC(=O)CCNC(=O)CCCCCNC(=O)COc2ccc(\C=C\C3=[N+]4C(C=C3)=Cc3ccc(-c5cccs5)n3[B-]4(F)F)cc2)c2nc([nH]c2c1=O)C12CC3CC(CC(C3)C1)C2 |c:52,54,t:49,TLB:79:80:77.78.83:84,THB:81:80:77:83.82.84,81:82:77:85.79.80,79:78:84:85.80.81| | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 1.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Binding affinity to NanoLuc human A1 adenosine receptor expressed in HEK293-A cells in prescence of SLV320 by NanoBRET competitive binding assay |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.0c02067
BindingDB Entry DOI: 10.7270/Q2CV4NM3 |
More data for this
Ligand-Target Pair | |
Mu-type opioid receptor
(Homo sapiens (Human)) | BDBM50135225
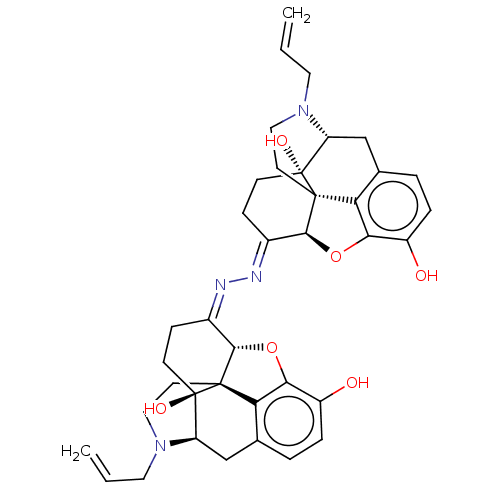
(Naloxonazine)Show SMILES [H][C@@]12Oc3c4c(C[C@@]5([H])N(CC=C)CC[C@@]14[C@@]5(O)CC\C2=N/N=C1\CC[C@@]2(O)[C@@]4([H])Cc5ccc(O)c6O[C@]1([H])[C@]2(CCN4CC=C)c56)ccc3O |r,TLB:44:43:26:47.31.30,THB:10:9:16:4.5.6| Show InChI InChI=1S/C38H42N4O6/c1-3-15-41-17-13-35-29-21-5-7-25(43)31(29)47-33(35)23(9-11-37(35,45)27(41)19-21)39-40-24-10-12-38(46)28-20-22-6-8-26(44)32-30(22)36(38,34(24)48-32)14-18-42(28)16-4-2/h3-8,27-28,33-34,43-46H,1-2,9-20H2/b39-23+,40-24+/t27-,28-,33+,34+,35+,36+,37-,38-/m1/s1 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Nottingham
Curated by ChEMBL
| Assay Description
Displacement of 2-((1E,3E,5E)-5-(1-Ethyl-3,3-dimethyl-5-sulfoindolin-2-ylidene)-penta-1,3-dien-1-yl)-1-(6-((6-((6S,7R,7aR,12bS)-9-hydroxy-7-methoxy-3... |
J Med Chem 58: 9754-67 (2015)
Article DOI: 10.1021/acs.jmedchem.5b01664
BindingDB Entry DOI: 10.7270/Q25M67K5 |
More data for this
Ligand-Target Pair | |
Mu-type opioid receptor
(Homo sapiens (Human)) | BDBM50026603

(Buprenorphine | CHEBI:3216)Show SMILES [H][C@@]12Oc3c4c(C[C@@]5([H])N(CC6CC6)CC[C@@]14[C@@]51CC[C@@]2(OC)[C@]([H])(C1)[C@](C)(O)C(C)(C)C)ccc3O |r,TLB:25:17:4.5.6:9.15.14,18:17:4.5.6:9.15.14,THB:10:9:17:4.5.6,3:4:17:9.15.14,26:23:16.1:18.19| Show InChI InChI=1S/C29H41NO4/c1-25(2,3)26(4,32)20-15-27-10-11-29(20,33-5)24-28(27)12-13-30(16-17-6-7-17)21(27)14-18-8-9-19(31)23(34-24)22(18)28/h8-9,17,20-21,24,31-32H,6-7,10-16H2,1-5H3/t20-,21-,24-,26+,27-,28+,29-/m1/s1 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| DrugBank
PC cid
PC sid
UniChem
Patents
Similars
| DrugBank
Article
PubMed
| 1.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Nottingham
Curated by ChEMBL
| Assay Description
Displacement of [3H]diprenorphine from human MOR expressed in CHO-FlpIn cell membranes after 1 hr by liquid scintillation counting analysis |
J Med Chem 58: 9754-67 (2015)
Article DOI: 10.1021/acs.jmedchem.5b01664
BindingDB Entry DOI: 10.7270/Q25M67K5 |
More data for this
Ligand-Target Pair | |
Mu-type opioid receptor
(Homo sapiens (Human)) | BDBM50059841

((S)-1-[(S)-2-[2-((S)-2-{(R)-2-[(S)-2-Amino-3-(4-hy...)Show SMILES C[C@@H](NC(=O)[C@@H](N)Cc1ccc(O)cc1)C(=O)N[C@@H](Cc1ccccc1)C(=O)NCC(=O)N[C@@H](Cc1ccc(O)cc1)C(=O)N1CCC[C@H]1C(=O)N[C@@H](CO)C(N)=O |r| Show InChI InChI=1S/C40H50N8O10/c1-23(44-37(55)29(41)18-25-9-13-27(50)14-10-25)36(54)46-30(19-24-6-3-2-4-7-24)38(56)43-21-34(52)45-31(20-26-11-15-28(51)16-12-26)40(58)48-17-5-8-33(48)39(57)47-32(22-49)35(42)53/h2-4,6-7,9-16,23,29-33,49-51H,5,8,17-22,41H2,1H3,(H2,42,53)(H,43,56)(H,44,55)(H,45,52)(H,46,54)(H,47,57)/t23-,29+,30+,31+,32+,33+/m1/s1 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 1.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Nottingham
Curated by ChEMBL
| Assay Description
Displacement of [3H]DAMGO from human MOR expressed in HEK293 cells preincubated for 1 hr followed by radioligand addition measured after 1 hr by liqu... |
J Med Chem 58: 9754-67 (2015)
Article DOI: 10.1021/acs.jmedchem.5b01664
BindingDB Entry DOI: 10.7270/Q25M67K5 |
More data for this
Ligand-Target Pair | |
Adenosine receptor A1
(Homo sapiens (Human)) | BDBM50581950

(CHEMBL4204703)Show SMILES O[C@H]1CC[C@@H](CC1)Nc1nc(nc2[nH]ccc12)-c1ccccc1 |r,wU:4.7,wD:1.0,(5.09,-4.45,;6.42,-5.22,;6.43,-6.76,;7.77,-7.53,;9.09,-6.74,;9.09,-5.21,;7.76,-4.45,;10.43,-7.5,;10.44,-9.04,;9.12,-9.82,;9.13,-11.35,;10.47,-12.12,;11.8,-11.34,;13.26,-11.81,;14.16,-10.57,;13.25,-9.33,;11.79,-9.81,;7.8,-12.13,;6.47,-11.36,;5.14,-12.13,;5.14,-13.68,;6.47,-14.45,;7.81,-13.68,)| | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| 1.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Displacement of [3H]DPCPX from human A1 adenosine receptor expressed in Flp-In-CHO cells by radioligand competitive binding assay |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.0c02067
BindingDB Entry DOI: 10.7270/Q2CV4NM3 |
More data for this
Ligand-Target Pair | |
Mu-type opioid receptor
(Homo sapiens (Human)) | BDBM50135222

(CHEMBL3746697)Show SMILES [H][C@@]12Oc3c4c(C[C@@]5([H])N(C)CC[C@@]14[C@@]51CC[C@@]2(OC)[C@H](C1)C(=O)NCCCCCCNC(=O)OC(C)(C)C)ccc3O |r,THB:10:9:14:6.4.5| Show InChI InChI=1S/C32H47N3O6/c1-29(2,3)41-28(38)34-16-9-7-6-8-15-33-26(37)21-19-30-12-13-32(21,39-5)27-31(30)14-17-35(4)23(30)18-20-10-11-22(36)25(40-27)24(20)31/h10-11,21,23,27,36H,6-9,12-19H2,1-5H3,(H,33,37)(H,34,38)/t21-,23-,27-,30-,31+,32-/m1/s1 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 2.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Nottingham
Curated by ChEMBL
| Assay Description
Displacement of [3H]diprenorphine from human MOR expressed in CHO-FlpIn cell membranes after 1 hr by liquid scintillation counting analysis |
J Med Chem 58: 9754-67 (2015)
Article DOI: 10.1021/acs.jmedchem.5b01664
BindingDB Entry DOI: 10.7270/Q25M67K5 |
More data for this
Ligand-Target Pair | |
Mu-type opioid receptor
(Homo sapiens (Human)) | BDBM50135225

(Naloxonazine)Show SMILES [H][C@@]12Oc3c4c(C[C@@]5([H])N(CC=C)CC[C@@]14[C@@]5(O)CC\C2=N/N=C1\CC[C@@]2(O)[C@@]4([H])Cc5ccc(O)c6O[C@]1([H])[C@]2(CCN4CC=C)c56)ccc3O |r,TLB:44:43:26:47.31.30,THB:10:9:16:4.5.6| Show InChI InChI=1S/C38H42N4O6/c1-3-15-41-17-13-35-29-21-5-7-25(43)31(29)47-33(35)23(9-11-37(35,45)27(41)19-21)39-40-24-10-12-38(46)28-20-22-6-8-26(44)32-30(22)36(38,34(24)48-32)14-18-42(28)16-4-2/h3-8,27-28,33-34,43-46H,1-2,9-20H2/b39-23+,40-24+/t27-,28-,33+,34+,35+,36+,37-,38-/m1/s1 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 3.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Nottingham
Curated by ChEMBL
| Assay Description
Displacement of [3H]DAMGO from human MOR expressed in HEK293 cells preincubated for 1 hr followed by radioligand addition measured after 1 hr by liqu... |
J Med Chem 58: 9754-67 (2015)
Article DOI: 10.1021/acs.jmedchem.5b01664
BindingDB Entry DOI: 10.7270/Q25M67K5 |
More data for this
Ligand-Target Pair | |
Histamine H1 receptor
(Homo sapiens (Human)) | BDBM50606429
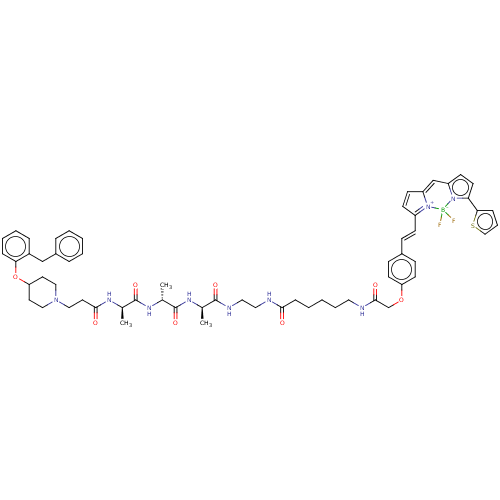
(CHEMBL5175488)Show SMILES C[C@@H](NC(=O)CCN1CCC(CC1)Oc1ccccc1Cc1ccccc1)C(=O)N[C@H](C)C(=O)N[C@H](C)C(=O)NCCNC(=O)CCCCCNC(=O)COc1ccc(\C=C\C2=[N+]3C(C=C2)=Cc2ccc(-c4cccs4)n2[B-]3(F)F)cc1 |r,c:67,69,t:64| | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00125
BindingDB Entry DOI: 10.7270/Q29W0KK1 |
More data for this
Ligand-Target Pair | |
Adenosine receptor A1
(Homo sapiens (Human)) | BDBM50583628
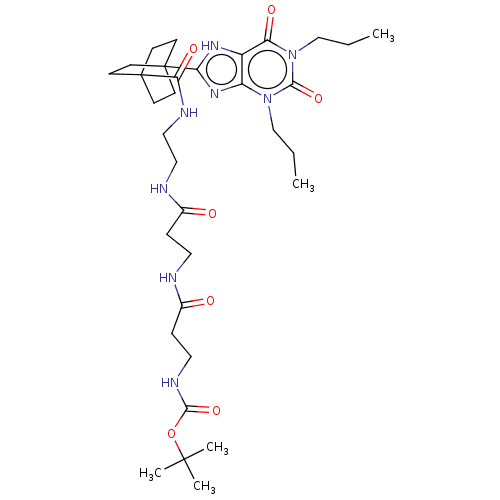
(CHEMBL5086635)Show SMILES CCCn1c2nc([nH]c2c(=O)n(CCC)c1=O)C12CCC(CC1)(CC2)C(=O)NCCNC(=O)CCNC(=O)CCNC(=O)OC(C)(C)C | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 4.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Displacement of [3H]DPCPX from human A1 adenosine receptor expressed in Flp-In-CHO cells by radioligand competitive binding assay |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.0c02067
BindingDB Entry DOI: 10.7270/Q2CV4NM3 |
More data for this
Ligand-Target Pair | |
Adenosine receptor A1
(Homo sapiens (Human)) | BDBM50583642

(CHEMBL5080416)Show SMILES CCCn1c(=O)n(CCCNC(=O)c2ccc(cc2)C(=O)NCCNC(=O)CCCCCNC(=O)COc2ccc(\C=C\C3=[N+]4C(C=C3)=Cc3ccc(-c5cccs5)n3[B-]4(F)F)cc2)c2nc([nH]c2c1=O)C12CC3CC(CC(C3)C1)C2 |c:47,49,t:44,TLB:74:75:72.73.78:79,THB:76:75:72:78.77.79,76:77:72:80.74.75,74:73:79:80.75.76| | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 5.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Displacement of [3H]DPCPX from human A1 adenosine receptor expressed in Flp-In-CHO cells by radioligand competitive binding assay |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.0c02067
BindingDB Entry DOI: 10.7270/Q2CV4NM3 |
More data for this
Ligand-Target Pair | |
Mu-type opioid receptor
(Homo sapiens (Human)) | BDBM50135224

(CHEMBL3747778)Show SMILES [H][C@@]12Oc3c4c(C[C@@]5([H])N(C)CC[C@@]14[C@]51C[C@H](C(=O)NCCCCCCNC(=O)OC(C)(C)C)[C@]2(OC)C=C1)ccc3O |r,wU:1.0,13.12,wD:34.38,16.19,14.42,7.7,c:41,THB:10:9:14:6.4.5,(-3.13,.26,;-2.17,.57,;-3.33,2.18,;-2.17,3.75,;-.8,2.95,;.55,3.75,;1.91,2.95,;1.91,1.38,;1.04,1.89,;3.29,.57,;4.08,-.36,;2.69,2.13,;.51,2.15,;-.8,1.38,;.55,.57,;.55,-.97,;-.8,-1.76,;-.79,-3.28,;.26,-3.89,;-2.11,-4.05,;-2.1,-5.57,;-3.41,-6.34,;-3.41,-7.86,;-4.72,-8.62,;-4.71,-10.14,;-6.03,-10.91,;-6.02,-12.43,;-7.33,-13.2,;-8.39,-12.6,;-7.33,-14.72,;-8.64,-15.49,;-8.64,-16.7,;-9.7,-14.88,;-9.69,-16.1,;-2.17,-.97,;-1.43,.37,;-2.07,1.4,;-.87,-.71,;-.87,.27,;.55,5.33,;-.8,6.12,;-2.17,5.33,;-3.22,5.94,)| Show InChI InChI=1S/C32H45N3O6/c1-29(2,3)41-28(38)34-16-9-7-6-8-15-33-26(37)21-19-30-12-13-32(21,39-5)27-31(30)14-17-35(4)23(30)18-20-10-11-22(36)25(40-27)24(20)31/h10-13,21,23,27,36H,6-9,14-19H2,1-5H3,(H,33,37)(H,34,38)/t21-,23-,27-,30-,31+,32-/m1/s1 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 7.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Nottingham
Curated by ChEMBL
| Assay Description
Displacement of [3H]diprenorphine from human MOR expressed in CHO-FlpIn cell membranes after 1 hr by liquid scintillation counting analysis |
J Med Chem 58: 9754-67 (2015)
Article DOI: 10.1021/acs.jmedchem.5b01664
BindingDB Entry DOI: 10.7270/Q25M67K5 |
More data for this
Ligand-Target Pair | |
Adenosine receptor A1
(Homo sapiens (Human)) | BDBM50583641

(CHEMBL5086197)Show SMILES CCCn1c2nc([nH]c2c(=O)n(CCC)c1=O)C12CCC(CC1)(CC2)C(=O)NCCNC(=O)CCNC(=O)CCNC(=O)CCCCC[N+]1=C(\C=C\C=C\C=C2\N(C)c3ccc(cc3C2(C)C)S(O)(=O)=O)C(C)(C)c2cc(ccc12)S([O-])(=O)=O |c:52| | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 7.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Binding affinity to NanoLuc human A1 adenosine receptor expressed in HEK293-A cells in prescence of DPCPX by NanoBRET competitive binding assay |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.0c02067
BindingDB Entry DOI: 10.7270/Q2CV4NM3 |
More data for this
Ligand-Target Pair | |
Adenosine receptor A1
(Homo sapiens (Human)) | BDBM50583633
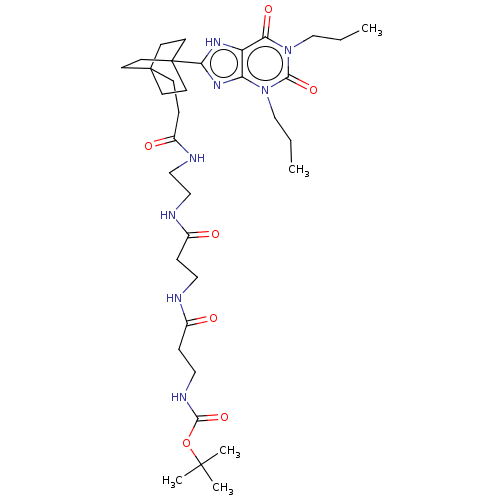
(CHEMBL5074153)Show SMILES CCCn1c2nc([nH]c2c(=O)n(CCC)c1=O)C12CCC(CCC(=O)NCCNC(=O)CCNC(=O)CCNC(=O)OC(C)(C)C)(CC1)CC2 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 7.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Displacement of [3H]DPCPX from human A1 adenosine receptor expressed in Flp-In-CHO cells by radioligand competitive binding assay |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.0c02067
BindingDB Entry DOI: 10.7270/Q2CV4NM3 |
More data for this
Ligand-Target Pair | |
Adenosine receptor A1
(Homo sapiens (Human)) | BDBM50583647

(CHEMBL5081913)Show SMILES CCCn1c2nc([nH]c2c(=O)n(CCC)c1=O)-c1ccc(OCC(=O)NCCNC(=O)CCCCNC(=O)COc2ccc(\C=C\C3=[N+]4C(C=C3)=Cc3ccc(-c5cccs5)n3[B-]4(F)F)cc2)cc1 |c:51,53,t:48,(40.32,-39.99,;40.32,-38.44,;38.98,-37.67,;38.97,-36.12,;40.32,-35.35,;41.79,-35.82,;42.68,-34.57,;41.77,-33.33,;40.31,-33.81,;38.97,-33.03,;38.97,-31.49,;37.64,-33.81,;36.3,-33.04,;36.29,-31.5,;34.95,-30.73,;37.64,-35.36,;36.3,-36.13,;44.21,-34.55,;44.99,-35.87,;46.52,-35.86,;47.27,-34.52,;48.81,-34.5,;49.6,-35.83,;51.13,-35.81,;51.89,-34.47,;51.92,-37.13,;53.46,-37.12,;54.24,-38.44,;55.78,-38.42,;56.56,-39.75,;55.81,-41.09,;58.1,-39.73,;58.89,-41.05,;60.43,-41.03,;61.21,-42.36,;62.75,-42.34,;63.53,-43.66,;62.78,-45,;65.07,-43.65,;65.85,-44.97,;67.39,-44.95,;68.17,-46.28,;69.71,-46.26,;70.46,-44.92,;72,-44.9,;72.79,-46.22,;74.32,-46.2,;75.82,-46.55,;75.96,-48.09,;74.54,-48.7,;73.52,-47.53,;77.36,-48.75,;78.63,-47.86,;80.13,-48.2,;80.93,-46.88,;79.91,-45.71,;80.26,-44.21,;81.68,-43.6,;81.54,-42.06,;80.03,-41.71,;79.24,-43.04,;78.49,-46.32,;77.07,-45.65,;75.99,-44.57,;77.99,-44.41,;69.67,-43.59,;68.14,-43.62,;46.48,-33.2,;44.95,-33.22,)| | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 9.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Binding affinity to N-terminal NLuc tagged human A1 adenosine receptor expressed in Flp-In-CHO cells by competitive binding assay |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.0c02067
BindingDB Entry DOI: 10.7270/Q2CV4NM3 |
More data for this
Ligand-Target Pair | |
Adenosine receptor A1
(Homo sapiens (Human)) | BDBM50583644

(CHEMBL5080679)Show SMILES CCCn1c(=O)n(CCCNC(=O)c2ccc(cc2)C(=O)NCCNC(=O)CCNC(=O)CCCCCNC(=O)COc2ccc(\C=C\C3=[N+]4C(C=C3)=Cc3ccc(-c5cccs5)n3[B-]4(F)F)cc2)c2nc([nH]c2c1=O)C12CC3CC(CC(C3)C1)C2 |c:52,54,t:49,TLB:79:80:77.78.83:84,THB:81:80:77:83.82.84,81:82:77:85.79.80,79:78:84:85.80.81| | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 9.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Displacement of [3H]DPCPX from human A1 adenosine receptor expressed in Flp-In-CHO cells by radioligand competitive binding assay |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.0c02067
BindingDB Entry DOI: 10.7270/Q2CV4NM3 |
More data for this
Ligand-Target Pair | |
Adenosine receptor A1
(Homo sapiens (Human)) | BDBM50583630
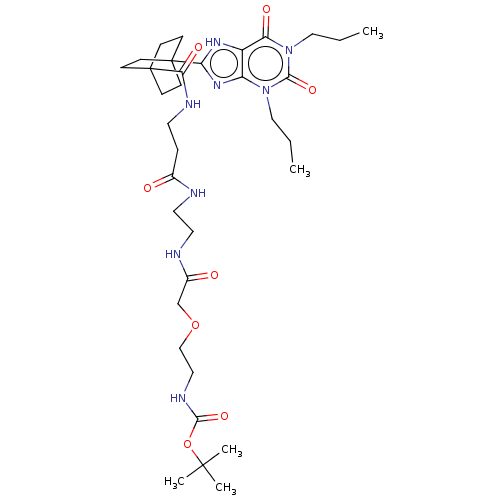
(CHEMBL5077560)Show SMILES CCCn1c2nc([nH]c2c(=O)n(CCC)c1=O)C12CCC(CC1)(CC2)C(=O)NCCC(=O)NCCNC(=O)COCCNC(=O)OC(C)(C)C | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 11 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Displacement of [3H]DPCPX from human A1 adenosine receptor expressed in Flp-In-CHO cells by radioligand competitive binding assay |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.0c02067
BindingDB Entry DOI: 10.7270/Q2CV4NM3 |
More data for this
Ligand-Target Pair | |
Adenosine receptor A1
(Homo sapiens (Human)) | BDBM50583634

(CHEMBL5090230)Show SMILES CCCn1c2nc([nH]c2c(=O)n(CCC)c1=O)C12CCC(CCC(=O)NCCNC(=O)COCCOCCNC(=O)OC(C)(C)C)(CC1)CC2 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 13 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Displacement of [3H]DPCPX from human A1 adenosine receptor expressed in Flp-In-CHO cells by radioligand competitive binding assay |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.0c02067
BindingDB Entry DOI: 10.7270/Q2CV4NM3 |
More data for this
Ligand-Target Pair | |
Adenosine receptor A1
(Homo sapiens (Human)) | BDBM50583631

(CHEMBL5094274)Show SMILES CCCn1c2nc([nH]c2c(=O)n(CCC)c1=O)C12CCC(CC1)(CC2)C(=O)NCCCNC(=O)CCNC(=O)CCNC(=O)OC(C)(C)C | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 13 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Displacement of [3H]DPCPX from human A1 adenosine receptor expressed in Flp-In-CHO cells by radioligand competitive binding assay |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.0c02067
BindingDB Entry DOI: 10.7270/Q2CV4NM3 |
More data for this
Ligand-Target Pair | |
Adenosine receptor A1
(Homo sapiens (Human)) | BDBM50583629
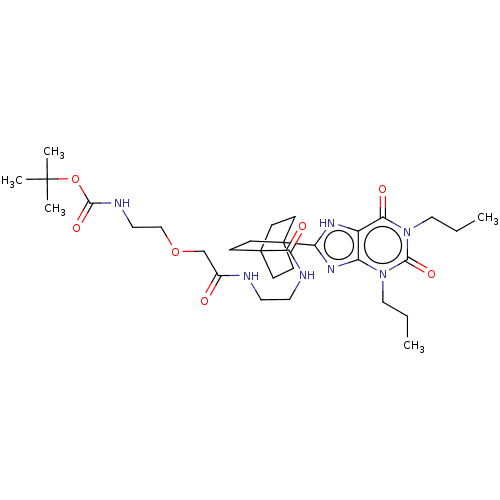
(CHEMBL5079788)Show SMILES CCCn1c2nc([nH]c2c(=O)n(CCC)c1=O)C12CCC(CC1)(CC2)C(=O)NCCNC(=O)COCCNC(=O)OC(C)(C)C | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 14 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Displacement of [3H]DPCPX from human A1 adenosine receptor expressed in Flp-In-CHO cells by radioligand competitive binding assay |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.0c02067
BindingDB Entry DOI: 10.7270/Q2CV4NM3 |
More data for this
Ligand-Target Pair | |
Adenosine receptor A1
(Homo sapiens (Human)) | BDBM50583637

(CHEMBL5081778)Show SMILES CCCn1c2nc([nH]c2c(=O)n(CCC)c1=O)C12CCC(CCC(=O)NCCNC(=O)COCCNC(=O)OC(C)(C)C)(CC1)CC2 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 15 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Displacement of [3H]DPCPX from human A1 adenosine receptor expressed in Flp-In-CHO cells by radioligand competitive binding assay |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.0c02067
BindingDB Entry DOI: 10.7270/Q2CV4NM3 |
More data for this
Ligand-Target Pair | |
Adenosine receptor A1
(Homo sapiens (Human)) | BDBM50583635
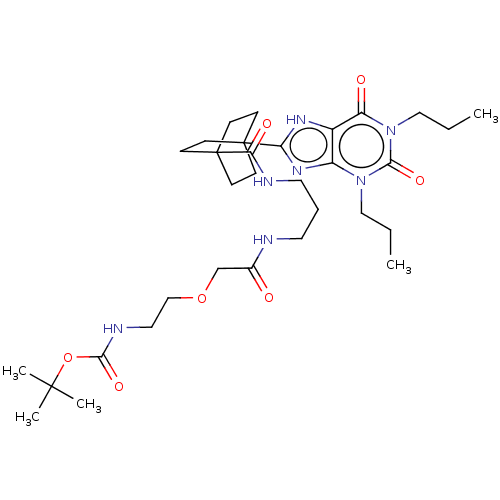
(CHEMBL5090546)Show SMILES CCCn1c2nc([nH]c2c(=O)n(CCC)c1=O)C12CCC(CC1)(CC2)C(=O)NCCCNC(=O)COCCNC(=O)OC(C)(C)C | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 16 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Displacement of [3H]DPCPX from human A1 adenosine receptor expressed in Flp-In-CHO cells by radioligand competitive binding assay |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.0c02067
BindingDB Entry DOI: 10.7270/Q2CV4NM3 |
More data for this
Ligand-Target Pair | |
Adenosine receptor A1
(Homo sapiens (Human)) | BDBM50583636

(CHEMBL5078265)Show SMILES CCCn1c2nc([nH]c2c(=O)n(CCC)c1=O)C12CCC(CC1)(CC2)C(=O)NCCNC(=O)COCCOCCNC(=O)OC(C)(C)C | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 17 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Displacement of [3H]DPCPX from human A1 adenosine receptor expressed in Flp-In-CHO cells by radioligand competitive binding assay |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.0c02067
BindingDB Entry DOI: 10.7270/Q2CV4NM3 |
More data for this
Ligand-Target Pair | |
Mu-type opioid receptor
(Homo sapiens (Human)) | BDBM50059841

((S)-1-[(S)-2-[2-((S)-2-{(R)-2-[(S)-2-Amino-3-(4-hy...)Show SMILES C[C@@H](NC(=O)[C@@H](N)Cc1ccc(O)cc1)C(=O)N[C@@H](Cc1ccccc1)C(=O)NCC(=O)N[C@@H](Cc1ccc(O)cc1)C(=O)N1CCC[C@H]1C(=O)N[C@@H](CO)C(N)=O |r| Show InChI InChI=1S/C40H50N8O10/c1-23(44-37(55)29(41)18-25-9-13-27(50)14-10-25)36(54)46-30(19-24-6-3-2-4-7-24)38(56)43-21-34(52)45-31(20-26-11-15-28(51)16-12-26)40(58)48-17-5-8-33(48)39(57)47-32(22-49)35(42)53/h2-4,6-7,9-16,23,29-33,49-51H,5,8,17-22,41H2,1H3,(H2,42,53)(H,43,56)(H,44,55)(H,45,52)(H,46,54)(H,47,57)/t23-,29+,30+,31+,32+,33+/m1/s1 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 19 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Nottingham
Curated by ChEMBL
| Assay Description
Displacement of 2-((1E,3E,5E)-5-(1-Ethyl-3,3-dimethyl-5-sulfoindolin-2-ylidene)-penta-1,3-dien-1-yl)-1-(6-((6-((6S,7R,7aR,12bS)-9-hydroxy-7-methoxy-3... |
J Med Chem 58: 9754-67 (2015)
Article DOI: 10.1021/acs.jmedchem.5b01664
BindingDB Entry DOI: 10.7270/Q25M67K5 |
More data for this
Ligand-Target Pair | |
Adenosine receptor A1
(Homo sapiens (Human)) | BDBM50583638
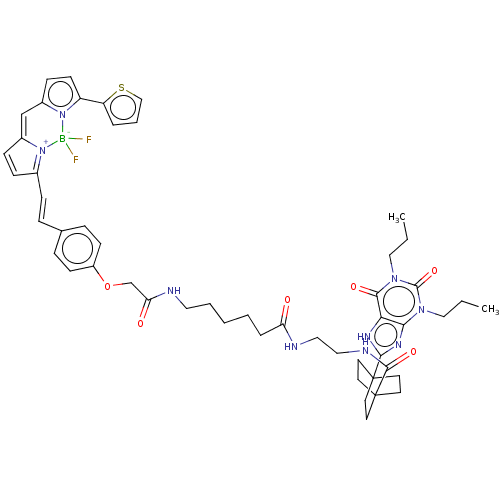
(CHEMBL5083466)Show SMILES CCCn1c2nc([nH]c2c(=O)n(CCC)c1=O)C12CCC(CC1)(CC2)C(=O)NCCNC(=O)CCCCCNC(=O)COc1ccc(\C=C\C2=[N+]3C(C=C2)=Cc2ccc(-c4cccs4)n2[B-]3(F)F)cc1 |c:56,58,t:53| | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Displacement of [3H]DPCPX from human A1 adenosine receptor expressed in Flp-In-CHO cells by radioligand competitive binding assay |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.0c02067
BindingDB Entry DOI: 10.7270/Q2CV4NM3 |
More data for this
Ligand-Target Pair | |
Adenosine receptor A1
(Homo sapiens (Human)) | BDBM50583644

(CHEMBL5080679)Show SMILES CCCn1c(=O)n(CCCNC(=O)c2ccc(cc2)C(=O)NCCNC(=O)CCNC(=O)CCCCCNC(=O)COc2ccc(\C=C\C3=[N+]4C(C=C3)=Cc3ccc(-c5cccs5)n3[B-]4(F)F)cc2)c2nc([nH]c2c1=O)C12CC3CC(CC(C3)C1)C2 |c:52,54,t:49,TLB:79:80:77.78.83:84,THB:81:80:77:83.82.84,81:82:77:85.79.80,79:78:84:85.80.81| | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 21 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Binding affinity to NanoLuc human A1 adenosine receptor expressed in HEK293-A cells in prescence of DPCPX by NanoBRET competitive binding assay |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.0c02067
BindingDB Entry DOI: 10.7270/Q2CV4NM3 |
More data for this
Ligand-Target Pair | |
Adenosine receptor A1
(Homo sapiens (Human)) | BDBM50583632
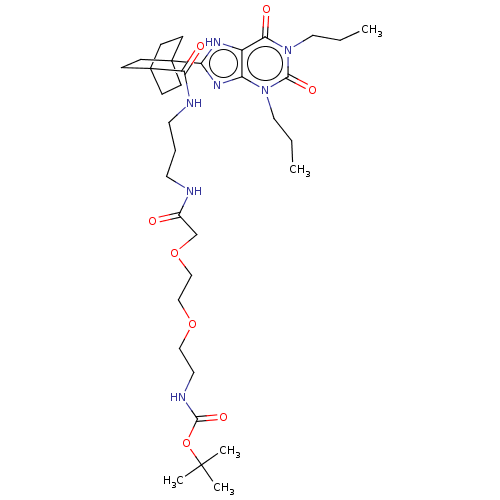
(CHEMBL5083938)Show SMILES CCCn1c2nc([nH]c2c(=O)n(CCC)c1=O)C12CCC(CC1)(CC2)C(=O)NCCCNC(=O)COCCOCCNC(=O)OC(C)(C)C | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 22 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Displacement of [3H]DPCPX from human A1 adenosine receptor expressed in Flp-In-CHO cells by radioligand competitive binding assay |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.0c02067
BindingDB Entry DOI: 10.7270/Q2CV4NM3 |
More data for this
Ligand-Target Pair | |
P2Y purinoceptor 2
(Homo sapiens (Human)) | BDBM50271558
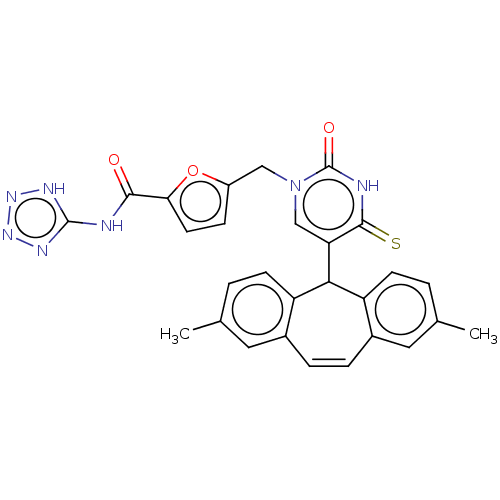
(CHEMBL4082045)Show SMILES Cc1ccc2C(c3ccc(C)cc3C=Cc2c1)c1cn(Cc2ccc(o2)C(=O)Nc2nnn[nH]2)c(=O)[nH]c1=S |c:14| Show InChI InChI=1S/C28H23N7O3S/c1-15-3-8-20-17(11-15)5-6-18-12-16(2)4-9-21(18)24(20)22-14-35(28(37)30-26(22)39)13-19-7-10-23(38-19)25(36)29-27-31-33-34-32-27/h3-12,14,24H,13H2,1-2H3,(H,30,37,39)(H2,29,31,32,33,34,36) | Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 22 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University Park Nottingham
Curated by ChEMBL
| Assay Description
Displacement of (E)-2-((5-(7-Chloro-2-((3-((2-(6-(2-(4-(2-(5,5-difluoro-7-(thiophen-2-yl)-5H-4-lambda4,5-lambda4-dipyrrolo[1,2-c:2,1-f ][1,3,2]diazab... |
J Med Chem 61: 3089-3113 (2018)
Article DOI: 10.1021/acs.jmedchem.8b00139
BindingDB Entry DOI: 10.7270/Q21C20CD |
More data for this
Ligand-Target Pair | |
Transmembrane domain-containing protein TMIGD3
(Homo sapiens (Human)) | BDBM50500484
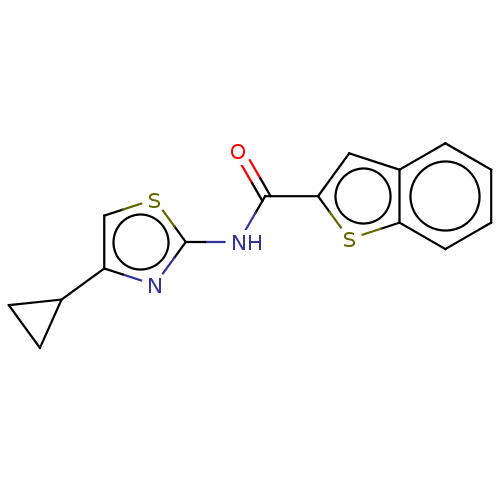
(CHEMBL3745868)Show InChI InChI=1S/C15H12N2OS2/c18-14(13-7-10-3-1-2-4-12(10)20-13)17-15-16-11(8-19-15)9-5-6-9/h1-4,7-9H,5-6H2,(H,16,17,18) | UniProtKB/SwissProt
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
UniChem
| Article
PubMed
| 33.9 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Stockholm University
Curated by ChEMBL
| Assay Description
Displacement of CA200645 from human adenosine A3 receptor expressed in CHO CRE-SPAP cells incubated for 1 hr by fluorescence analysis |
J Med Chem 58: 9578-90 (2015)
Article DOI: 10.1021/acs.jmedchem.5b01120
BindingDB Entry DOI: 10.7270/Q25M68QC |
More data for this
Ligand-Target Pair | |
Transmembrane domain-containing protein TMIGD3
(Homo sapiens (Human)) | BDBM50500484

(CHEMBL3745868)Show InChI InChI=1S/C15H12N2OS2/c18-14(13-7-10-3-1-2-4-12(10)20-13)17-15-16-11(8-19-15)9-5-6-9/h1-4,7-9H,5-6H2,(H,16,17,18) | UniProtKB/SwissProt
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
UniChem
| Article
PubMed
| 34 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Stockholm University
Curated by ChEMBL
| Assay Description
Displacement of CA200645 from human adenosine A3 receptor expressed in CHO CRE-SPAP cells incubated for 1 hr by fluorescence analysis |
J Med Chem 58: 9578-90 (2015)
Article DOI: 10.1021/acs.jmedchem.5b01120
BindingDB Entry DOI: 10.7270/Q25M68QC |
More data for this
Ligand-Target Pair | |
P2Y purinoceptor 2
(Homo sapiens (Human)) | BDBM50271558

(CHEMBL4082045)Show SMILES Cc1ccc2C(c3ccc(C)cc3C=Cc2c1)c1cn(Cc2ccc(o2)C(=O)Nc2nnn[nH]2)c(=O)[nH]c1=S |c:14| Show InChI InChI=1S/C28H23N7O3S/c1-15-3-8-20-17(11-15)5-6-18-12-16(2)4-9-21(18)24(20)22-14-35(28(37)30-26(22)39)13-19-7-10-23(38-19)25(36)29-27-31-33-34-32-27/h3-12,14,24H,13H2,1-2H3,(H,30,37,39)(H2,29,31,32,33,34,36) | Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 35 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University Park Nottingham
Curated by ChEMBL
| Assay Description
Displacement of N-(2-(3-((7-Chloro-4-(1-methyl-2-oxo-4-thioxo-1,2,3,4-tetrahydropyrimidin-5-yl)-4H-benzo[5,6]cyclohepta[1,2-d]thiazol-2-yl)-amino)pro... |
J Med Chem 61: 3089-3113 (2018)
Article DOI: 10.1021/acs.jmedchem.8b00139
BindingDB Entry DOI: 10.7270/Q21C20CD |
More data for this
Ligand-Target Pair | |
Adenosine receptor A1
(Rattus norvegicus (rat)) | BDBM50500487
(CHEMBL3747370)Show InChI InChI=1S/C11H10N2O2S/c14-10(8-3-4-15-5-8)13-11-12-9(6-16-11)7-1-2-7/h3-7H,1-2H2,(H,12,13,14) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
UniChem
| Article
PubMed
| 39 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Stockholm University
Curated by ChEMBL
| Assay Description
Displacement of [3H]DPCPX from rat cortical membrane adenosine A1 receptor by gamma-scintillation counting analysis |
J Med Chem 58: 9578-90 (2015)
Article DOI: 10.1021/acs.jmedchem.5b01120
BindingDB Entry DOI: 10.7270/Q25M68QC |
More data for this
Ligand-Target Pair | |
Adenosine receptor A1
(Homo sapiens (Human)) | BDBM21173

(1,3-dipropyl-8-cyclopentylxanthine | 8-cyclopentyl...)Show InChI InChI=1S/C16H24N4O2/c1-3-9-19-14-12(15(21)20(10-4-2)16(19)22)17-13(18-14)11-7-5-6-8-11/h11H,3-10H2,1-2H3,(H,17,18) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
KEGG
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Displacement of [3H]DPCPX from human A1 adenosine receptor expressed in CHO-K1 cells by radioligand binding assay |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.0c02067
BindingDB Entry DOI: 10.7270/Q2CV4NM3 |
More data for this
Ligand-Target Pair | |
P2Y purinoceptor 2
(Homo sapiens (Human)) | BDBM50271558

(CHEMBL4082045)Show SMILES Cc1ccc2C(c3ccc(C)cc3C=Cc2c1)c1cn(Cc2ccc(o2)C(=O)Nc2nnn[nH]2)c(=O)[nH]c1=S |c:14| Show InChI InChI=1S/C28H23N7O3S/c1-15-3-8-20-17(11-15)5-6-18-12-16(2)4-9-21(18)24(20)22-14-35(28(37)30-26(22)39)13-19-7-10-23(38-19)25(36)29-27-31-33-34-32-27/h3-12,14,24H,13H2,1-2H3,(H,30,37,39)(H2,29,31,32,33,34,36) | Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 42 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University Park Nottingham
Curated by ChEMBL
| Assay Description
Displacement of (E)-2-((5-(7-Chloro-2-((3-((2-(6-(3-(5,5-difluoro-7,9-dimethyl-5H-5-lambda4,6-lambda4-dipyrrolo[1,2-c:2,1-f ][1,3,2]diazaborinin-3-yl... |
J Med Chem 61: 3089-3113 (2018)
Article DOI: 10.1021/acs.jmedchem.8b00139
BindingDB Entry DOI: 10.7270/Q21C20CD |
More data for this
Ligand-Target Pair | |
P2Y purinoceptor 2
(Homo sapiens (Human)) | BDBM50271558

(CHEMBL4082045)Show SMILES Cc1ccc2C(c3ccc(C)cc3C=Cc2c1)c1cn(Cc2ccc(o2)C(=O)Nc2nnn[nH]2)c(=O)[nH]c1=S |c:14| Show InChI InChI=1S/C28H23N7O3S/c1-15-3-8-20-17(11-15)5-6-18-12-16(2)4-9-21(18)24(20)22-14-35(28(37)30-26(22)39)13-19-7-10-23(38-19)25(36)29-27-31-33-34-32-27/h3-12,14,24H,13H2,1-2H3,(H,30,37,39)(H2,29,31,32,33,34,36) | Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 48 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University Park Nottingham
Curated by ChEMBL
| Assay Description
Displacement of N-(2-(2-(2-(3-((7-Chloro-4-(1-methyl-2-oxo-4-thioxo-1,2,3,4-tetrahydropyrimidin-5-yl)-4H-benzo[5,6]cyclohepta[1,2-d]thiazol-2-yl)amin... |
J Med Chem 61: 3089-3113 (2018)
Article DOI: 10.1021/acs.jmedchem.8b00139
BindingDB Entry DOI: 10.7270/Q21C20CD |
More data for this
Ligand-Target Pair | |
P2Y purinoceptor 2
(Homo sapiens (Human)) | BDBM50271600

(CHEMBL4086462)Show SMILES O[C@H]1[C@@H](O)[C@@H](O[C@@H]1COP([O-])(=O)OP([O-])(=O)C(Cl)(Cl)P([O-])([O-])=O)n1cc(C2c3ccccc3CCc3ccccc23)c(=S)[nH]c1=O |r| Show InChI InChI=1S/C25H27Cl2N2O13P3S.4Na/c26-25(27,43(33,34)35)44(36,37)42-45(38,39)40-12-18-20(30)21(31)23(41-18)29-11-17(22(46)28-24(29)32)19-15-7-3-1-5-13(15)9-10-14-6-2-4-8-16(14)19;;;;/h1-8,11,18-21,23,30-31H,9-10,12H2,(H,36,37)(H,38,39)(H,28,32,46)(H2,33,34,35);;;;/q;4*+1/p-4/t18-,20-,21-,23-;;;;/m1..../s1 | Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 83 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University Park Nottingham
Curated by ChEMBL
| Assay Description
Displacement of (E)-2-((5-(7-Chloro-2-((3-((2-(6-(3-(5,5-difluoro-7,9-dimethyl-5H-5-lambda4,6-lambda4-dipyrrolo[1,2-c:2,1-f ][1,3,2]diazaborinin-3-yl... |
J Med Chem 61: 3089-3113 (2018)
Article DOI: 10.1021/acs.jmedchem.8b00139
BindingDB Entry DOI: 10.7270/Q21C20CD |
More data for this
Ligand-Target Pair | |
Mu-type opioid receptor
(Homo sapiens (Human)) | BDBM21015

((2S)-2-{2-[(2R)-2-[(2S)-2-amino-3-(4-hydroxyphenyl...)Show SMILES C[C@@H](NC(=O)[C@@H](N)Cc1ccc(O)cc1)C(=O)NCC(=O)N(C)[C@@H](Cc1ccccc1)C(=O)NCCO Show InChI InChI=1S/C26H35N5O6/c1-17(30-25(36)21(27)14-19-8-10-20(33)11-9-19)24(35)29-16-23(34)31(2)22(26(37)28-12-13-32)15-18-6-4-3-5-7-18/h3-11,17,21-22,32-33H,12-16,27H2,1-2H3,(H,28,37)(H,29,35)(H,30,36)/t17-,21+,22+/m1/s1 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 87 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Nottingham
Curated by ChEMBL
| Assay Description
Displacement of 2-((1E,3E,5E)-5-(1-Ethyl-3,3-dimethyl-5-sulfoindolin-2-ylidene)-penta-1,3-dien-1-yl)-1-(6-((6-((6S,7R,7aR,12bS)-9-hydroxy-7-methoxy-3... |
J Med Chem 58: 9754-67 (2015)
Article DOI: 10.1021/acs.jmedchem.5b01664
BindingDB Entry DOI: 10.7270/Q25M67K5 |
More data for this
Ligand-Target Pair | |
Histamine H1 receptor
(Homo sapiens (Human)) | BDBM50606458
(CHEMBL5193095)Show SMILES CC(C)[C@H](NC(=O)CCN1CCC(CC1)Oc1ccccc1Cc1ccccc1)C(=O)N[C@@H](CO)C(=O)N[C@@H](CC(N)=O)C(=O)N[C@@H](Cc1ccccc1)C(N)=O |r| | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 89 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00125
BindingDB Entry DOI: 10.7270/Q29W0KK1 |
More data for this
Ligand-Target Pair | |
Histamine H1 receptor
(Homo sapiens (Human)) | BDBM50606458

(CHEMBL5193095)Show SMILES CC(C)[C@H](NC(=O)CCN1CCC(CC1)Oc1ccccc1Cc1ccccc1)C(=O)N[C@@H](CO)C(=O)N[C@@H](CC(N)=O)C(=O)N[C@@H](Cc1ccccc1)C(N)=O |r| | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 89 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00125
BindingDB Entry DOI: 10.7270/Q29W0KK1 |
More data for this
Ligand-Target Pair | |
Histamine H1 receptor
(Homo sapiens (Human)) | BDBM50606454

(CHEMBL5209546)Show SMILES CC(C)[C@H](NC(=O)CCN1CCC(CC1)Oc1ccccc1Cc1ccccc1)C(=O)N[C@@H](CO)C(=O)N[C@@H](CC(N)=O)C(=O)N[C@H](Cc1ccccc1)C(N)=O |r| | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 95 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00125
BindingDB Entry DOI: 10.7270/Q29W0KK1 |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data