 Found 192 hits with Last Name = 'stojan' and Initial = 'j'
Found 192 hits with Last Name = 'stojan' and Initial = 'j' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Cholinesterase
(Homo sapiens (Human)) | BDBM50247011

(CHEMBL4080419)Show SMILES Cl.CN(C)CCN(CC1CCCN(C1)C1Cc2ccccc2C1)C(=O)c1ccc2ccccc2c1 Show InChI InChI=1S/C30H37N3O.ClH/c1-31(2)16-17-33(30(34)28-14-13-24-9-3-4-10-25(24)18-28)22-23-8-7-15-32(21-23)29-19-26-11-5-6-12-27(26)20-29;/h3-6,9-14,18,23,29H,7-8,15-17,19-22H2,1-2H3;1H | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.0110 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Ljubljana
Curated by ChEMBL
| Assay Description
Competitive inhibition of recombinant human BChE using butyrylthiocholine iodide as substrate at pH 8 by stopped flow assay |
J Med Chem 61: 119-139 (2018)
Article DOI: 10.1021/acs.jmedchem.7b01086
BindingDB Entry DOI: 10.7270/Q2S75JRJ |
More data for this
Ligand-Target Pair | |
Cholinesterase
(Homo sapiens (Human)) | BDBM50247011

(CHEMBL4080419)Show SMILES Cl.CN(C)CCN(CC1CCCN(C1)C1Cc2ccccc2C1)C(=O)c1ccc2ccccc2c1 Show InChI InChI=1S/C30H37N3O.ClH/c1-31(2)16-17-33(30(34)28-14-13-24-9-3-4-10-25(24)18-28)22-23-8-7-15-32(21-23)29-19-26-11-5-6-12-27(26)20-29;/h3-6,9-14,18,23,29H,7-8,15-17,19-22H2,1-2H3;1H | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.0110 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Ljubljana
Curated by ChEMBL
| Assay Description
Competitive inhibition of recombinant human BChE using butyrylthiocholine iodide as substrate at pH 8 by stopped flow assay |
J Med Chem 61: 119-139 (2018)
Article DOI: 10.1021/acs.jmedchem.7b01086
BindingDB Entry DOI: 10.7270/Q2S75JRJ |
More data for this
Ligand-Target Pair | |
Cholinesterase
(Homo sapiens (Human)) | BDBM8963

(CHEMBL32823 | Homodimeric Tacrine Analog 3b | N-[7...)Show SMILES C(CCCNc1c2CCCCc2nc2ccccc12)CCCNc1c2CCCCc2nc2ccccc12 Show InChI InChI=1S/C33H40N4/c1(2-12-22-34-32-24-14-4-8-18-28(24)36-29-19-9-5-15-25(29)32)3-13-23-35-33-26-16-6-10-20-30(26)37-31-21-11-7-17-27(31)33/h4,6,8,10,14,16,18,20H,1-3,5,7,9,11-13,15,17,19,21-23H2,(H,34,36)(H,35,37) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| 0.163 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Ljubljana
Curated by ChEMBL
| Assay Description
Competitive inhibition of recombinant human BChE using butyrylthiocholine iodide as substrate at pH 8 by stopped flow assay |
J Med Chem 61: 119-139 (2018)
Article DOI: 10.1021/acs.jmedchem.7b01086
BindingDB Entry DOI: 10.7270/Q2S75JRJ |
More data for this
Ligand-Target Pair | |
Cholinesterase
(Homo sapiens (Human)) | BDBM50604405
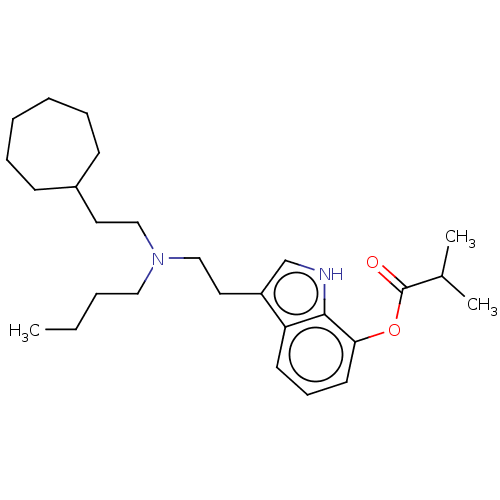
(CHEMBL5186889)Show SMILES CCCCN(CCC1CCCCCC1)CCc1c[nH]c2c(OC(=O)C(C)C)cccc12 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.180 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.ejmech.2022.114248
BindingDB Entry DOI: 10.7270/Q2125XRJ |
More data for this
Ligand-Target Pair | |
Cholinesterase
(Homo sapiens (Human)) | BDBM50604405

(CHEMBL5186889)Show SMILES CCCCN(CCC1CCCCCC1)CCc1c[nH]c2c(OC(=O)C(C)C)cccc12 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.180 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.ejmech.2022.114248
BindingDB Entry DOI: 10.7270/Q2125XRJ |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM50138279

(CHEMBL3752467)Show SMILES C(CCCNCc1cc2ccccc2o1)CCCNc1c2CCCCc2nc2ccccc12 Show InChI InChI=1S/C29H35N3O/c1(2-10-18-30-21-23-20-22-12-4-9-17-28(22)33-23)3-11-19-31-29-24-13-5-7-15-26(24)32-27-16-8-6-14-25(27)29/h4-5,7,9,12-13,15,17,20,30H,1-3,6,8,10-11,14,16,18-19,21H2,(H,31,32) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| 0.720 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
China Pharmaceutical University
Curated by ChEMBL
| Assay Description
Mixed-type inhibition of recombinant human AChE using acetylthiocholine iodide as substrate assessed as enzyme-inhibitor complex by Lineweaver-Burk d... |
J Med Chem 59: 114-31 (2016)
Article DOI: 10.1021/acs.jmedchem.5b01119
BindingDB Entry DOI: 10.7270/Q2S75J5X |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM50138279

(CHEMBL3752467)Show SMILES C(CCCNCc1cc2ccccc2o1)CCCNc1c2CCCCc2nc2ccccc12 Show InChI InChI=1S/C29H35N3O/c1(2-10-18-30-21-23-20-22-12-4-9-17-28(22)33-23)3-11-19-31-29-24-13-5-7-15-26(24)32-27-16-8-6-14-25(27)29/h4-5,7,9,12-13,15,17,20,30H,1-3,6,8,10-11,14,16,18-19,21H2,(H,31,32) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
China Pharmaceutical University
Curated by ChEMBL
| Assay Description
Mixed-type inhibition of recombinant human AChE using acetylthiocholine iodide as substrate assessed as enzyme-substrate-inhibitor complex by Linewea... |
J Med Chem 59: 114-31 (2016)
Article DOI: 10.1021/acs.jmedchem.5b01119
BindingDB Entry DOI: 10.7270/Q2S75J5X |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Tetronarce californica (Pacific electric ray) (Tor...) | BDBM50138279

(CHEMBL3752467)Show SMILES C(CCCNCc1cc2ccccc2o1)CCCNc1c2CCCCc2nc2ccccc12 Show InChI InChI=1S/C29H35N3O/c1(2-10-18-30-21-23-20-22-12-4-9-17-28(22)33-23)3-11-19-31-29-24-13-5-7-15-26(24)32-27-16-8-6-14-25(27)29/h4-5,7,9,12-13,15,17,20,30H,1-3,6,8,10-11,14,16,18-19,21H2,(H,31,32) | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| 2.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
China Pharmaceutical University
Curated by ChEMBL
| Assay Description
Noncompetitive inhibition of Torpedo californica AChE using ATCh as substrate preincubated for 90 mins followed by substrate addition by potentiometr... |
J Med Chem 59: 114-31 (2016)
Article DOI: 10.1021/acs.jmedchem.5b01119
BindingDB Entry DOI: 10.7270/Q2S75J5X |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Acetylcholinesterase
(Tetronarce californica (Pacific electric ray) (Tor...) | BDBM50138279

(CHEMBL3752467)Show SMILES C(CCCNCc1cc2ccccc2o1)CCCNc1c2CCCCc2nc2ccccc12 Show InChI InChI=1S/C29H35N3O/c1(2-10-18-30-21-23-20-22-12-4-9-17-28(22)33-23)3-11-19-31-29-24-13-5-7-15-26(24)32-27-16-8-6-14-25(27)29/h4-5,7,9,12-13,15,17,20,30H,1-3,6,8,10-11,14,16,18-19,21H2,(H,31,32) | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| 2.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
China Pharmaceutical University
Curated by ChEMBL
| Assay Description
Competitive inhibition of Torpedo californica AChE using ATCh as substrate preincubated for 90 mins followed by substrate addition by potentiometric ... |
J Med Chem 59: 114-31 (2016)
Article DOI: 10.1021/acs.jmedchem.5b01119
BindingDB Entry DOI: 10.7270/Q2S75J5X |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Cholinesterase
(Homo sapiens (Human)) | BDBM50027375

(CHEMBL3338394)Show SMILES COCCN(C[C@@H]1CCCN(C1)C1Cc2ccccc2C1)C(=O)c1ccc2ccccc2c1 |r| Show InChI InChI=1S/C29H34N2O2/c1-33-16-15-31(29(32)27-13-12-23-8-2-3-9-24(23)17-27)21-22-7-6-14-30(20-22)28-18-25-10-4-5-11-26(25)19-28/h2-5,8-13,17,22,28H,6-7,14-16,18-21H2,1H3/t22-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| 2.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Ljubljana
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human BChE at 50 nM by stopped flow apparatus method |
J Med Chem 57: 8167-79 (2014)
Article DOI: 10.1021/jm501195e
BindingDB Entry DOI: 10.7270/Q22V2HQ7 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Cholinesterase
(Homo sapiens (Human)) | BDBM50604404
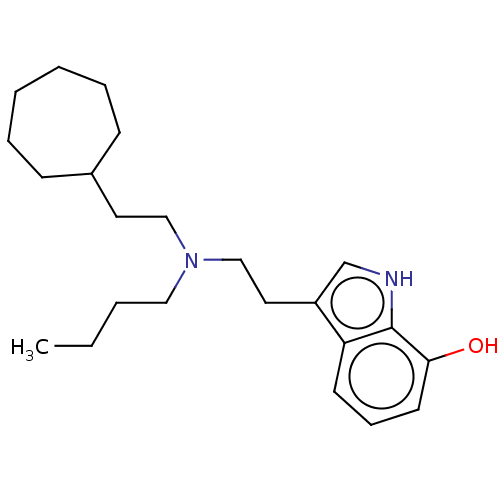
(CHEMBL5186857) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.ejmech.2022.114248
BindingDB Entry DOI: 10.7270/Q2125XRJ |
More data for this
Ligand-Target Pair | |
Cholinesterase
(Homo sapiens (Human)) | BDBM50604404

(CHEMBL5186857) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 6.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.ejmech.2022.114248
BindingDB Entry DOI: 10.7270/Q2125XRJ |
More data for this
Ligand-Target Pair | |
Cholinesterase
(Homo sapiens (Human)) | BDBM50604403

(CHEMBL5171829)Show SMILES CCCCN(CCC1CCCCCC1)CCc1c[nH]c2cc(OC(=O)N(C)C)ccc12 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| 17 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.ejmech.2022.114248
BindingDB Entry DOI: 10.7270/Q2125XRJ |
More data for this
Ligand-Target Pair | |
Cholinesterase
(Homo sapiens (Human)) | BDBM50027377
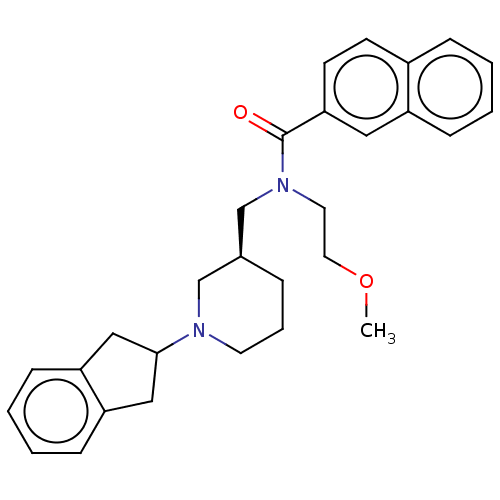
(CHEMBL3338395)Show SMILES COCCN(C[C@H]1CCCN(C1)C1Cc2ccccc2C1)C(=O)c1ccc2ccccc2c1 |r| Show InChI InChI=1S/C29H34N2O2/c1-33-16-15-31(29(32)27-13-12-23-8-2-3-9-24(23)17-27)21-22-7-6-14-30(20-22)28-18-25-10-4-5-11-26(25)19-28/h2-5,8-13,17,22,28H,6-7,14-16,18-21H2,1H3/t22-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 27 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Ljubljana
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human BChE at 50 nM by stopped flow apparatus method |
J Med Chem 57: 8167-79 (2014)
Article DOI: 10.1021/jm501195e
BindingDB Entry DOI: 10.7270/Q22V2HQ7 |
More data for this
Ligand-Target Pair | |
Cholinesterase
(Homo sapiens (Human)) | BDBM50604402

(CHEMBL5189102) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
PDB
UniChem
| PDB
Article
PubMed
| 62 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.ejmech.2022.114248
BindingDB Entry DOI: 10.7270/Q2125XRJ |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Cholinesterase
(Homo sapiens (Human)) | BDBM50604402

(CHEMBL5189102) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
PDB
UniChem
| PDB
Article
PubMed
| 252 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.ejmech.2022.114248
BindingDB Entry DOI: 10.7270/Q2125XRJ |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Cholinesterase
(Homo sapiens (Human)) | BDBM50138463

(CHEMBL3753957)Show SMILES COc1cccc2cc(CN(CCCCCCNc3c4CCCCc4nc4ccccc34)Cc3cc4cccc(OC)c4o3)oc12 Show InChI InChI=1S/C39H43N3O4/c1-43-35-19-11-13-27-23-29(45-38(27)35)25-42(26-30-24-28-14-12-20-36(44-2)39(28)46-30)22-10-4-3-9-21-40-37-31-15-5-7-17-33(31)41-34-18-8-6-16-32(34)37/h5,7,11-15,17,19-20,23-24H,3-4,6,8-10,16,18,21-22,25-26H2,1-2H3,(H,40,41) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.480 | n/a | n/a | n/a | n/a | n/a | n/a |
China Pharmaceutical University
Curated by ChEMBL
| Assay Description
Inhibition of human serum BChE using butyrylthiocholine iodide as substrate preincubated for 20 mins followed by substrate addition by Ellman's metho... |
J Med Chem 59: 114-31 (2016)
Article DOI: 10.1021/acs.jmedchem.5b01119
BindingDB Entry DOI: 10.7270/Q2S75J5X |
More data for this
Ligand-Target Pair | |
Cholinesterase
(Homo sapiens (Human)) | BDBM50138438

(CHEMBL3752969)Show SMILES COc1cccc2cc(CNCCCCCCNc3c4CCCCc4nc4ccccc34)oc12 Show InChI InChI=1S/C29H35N3O2/c1-33-27-16-10-11-21-19-22(34-29(21)27)20-30-17-8-2-3-9-18-31-28-23-12-4-6-14-25(23)32-26-15-7-5-13-24(26)28/h4,6,10-12,14,16,19,30H,2-3,5,7-9,13,15,17-18,20H2,1H3,(H,31,32) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.490 | n/a | n/a | n/a | n/a | n/a | n/a |
China Pharmaceutical University
Curated by ChEMBL
| Assay Description
Inhibition of human serum BChE using butyrylthiocholine iodide as substrate preincubated for 20 mins followed by substrate addition by Ellman's metho... |
J Med Chem 59: 114-31 (2016)
Article DOI: 10.1021/acs.jmedchem.5b01119
BindingDB Entry DOI: 10.7270/Q2S75J5X |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM8963

(CHEMBL32823 | Homodimeric Tacrine Analog 3b | N-[7...)Show SMILES C(CCCNc1c2CCCCc2nc2ccccc12)CCCNc1c2CCCCc2nc2ccccc12 Show InChI InChI=1S/C33H40N4/c1(2-12-22-34-32-24-14-4-8-18-28(24)36-29-19-9-5-15-25(29)32)3-13-23-35-33-26-16-6-10-20-30(26)37-31-21-11-7-17-27(31)33/h4,6,8,10,14,16,18,20H,1-3,5,7,9,11-13,15,17,19,21-23H2,(H,34,36)(H,35,37) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.810 | n/a | n/a | n/a | n/a | n/a | n/a |
China Pharmaceutical University
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human AChE using acetylthiocholine iodide as substrate preincubated for 20 mins followed by substrate addition by Ellman's ... |
J Med Chem 59: 114-31 (2016)
Article DOI: 10.1021/acs.jmedchem.5b01119
BindingDB Entry DOI: 10.7270/Q2S75J5X |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM50138279

(CHEMBL3752467)Show SMILES C(CCCNCc1cc2ccccc2o1)CCCNc1c2CCCCc2nc2ccccc12 Show InChI InChI=1S/C29H35N3O/c1(2-10-18-30-21-23-20-22-12-4-9-17-28(22)33-23)3-11-19-31-29-24-13-5-7-15-26(24)32-27-16-8-6-14-25(27)29/h4-5,7,9,12-13,15,17,20,30H,1-3,6,8,10-11,14,16,18-19,21H2,(H,31,32) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.860 | n/a | n/a | n/a | n/a | n/a | n/a |
China Pharmaceutical University
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human AChE using acetylthiocholine iodide as substrate preincubated for 20 mins followed by substrate addition by Ellman's ... |
J Med Chem 59: 114-31 (2016)
Article DOI: 10.1021/acs.jmedchem.5b01119
BindingDB Entry DOI: 10.7270/Q2S75J5X |
More data for this
Ligand-Target Pair | |
Cholinesterase
(Homo sapiens (Human)) | BDBM50604408
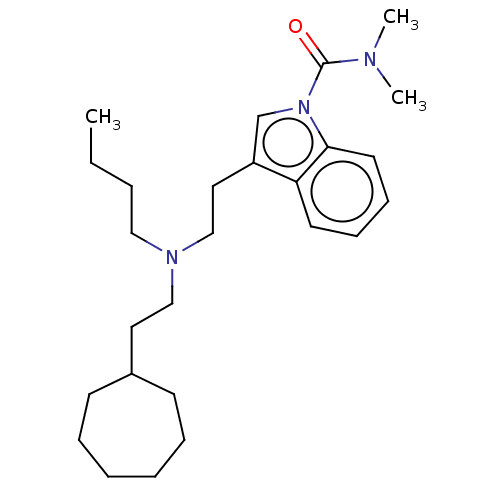
(CHEMBL5173013)Show SMILES CCCCN(CCC1CCCCCC1)CCc1cn(C(=O)N(C)C)c2ccccc12 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.870 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.ejmech.2022.114248
BindingDB Entry DOI: 10.7270/Q2125XRJ |
More data for this
Ligand-Target Pair | |
Cholinesterase
(Homo sapiens (Human)) | BDBM50247022

(CHEMBL4089230)Show SMILES CN(C)CCN(CC1CCCN(C1)C1Cc2ccccc2C1)C(=O)c1ccc2ccccc2c1 Show InChI InChI=1S/C30H37N3O/c1-31(2)16-17-33(30(34)28-14-13-24-9-3-4-10-25(24)18-28)22-23-8-7-15-32(21-23)29-19-26-11-5-6-12-27(26)20-29/h3-6,9-14,18,23,29H,7-8,15-17,19-22H2,1-2H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Ljubljana
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human BChE using butyrylthiocholine iodide as substrate preincubated for 300 secs followed by substrate addition measured f... |
J Med Chem 61: 119-139 (2018)
Article DOI: 10.1021/acs.jmedchem.7b01086
BindingDB Entry DOI: 10.7270/Q2S75JRJ |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Cholinesterase
(Homo sapiens (Human)) | BDBM50138440
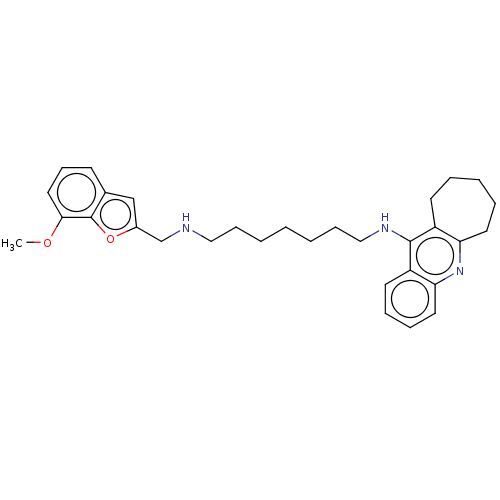
(CHEMBL3753405)Show SMILES COc1cccc2cc(CNCCCCCCCNc3c4CCCCCc4nc4ccccc34)oc12 Show InChI InChI=1S/C31H39N3O2/c1-35-29-18-12-13-23-21-24(36-31(23)29)22-32-19-10-3-2-4-11-20-33-30-25-14-6-5-7-16-27(25)34-28-17-9-8-15-26(28)30/h8-9,12-13,15,17-18,21,32H,2-7,10-11,14,16,19-20,22H2,1H3,(H,33,34) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.20 | n/a | n/a | n/a | n/a | n/a | n/a |
China Pharmaceutical University
Curated by ChEMBL
| Assay Description
Inhibition of human serum BChE using butyrylthiocholine iodide as substrate preincubated for 20 mins followed by substrate addition by Ellman's metho... |
J Med Chem 59: 114-31 (2016)
Article DOI: 10.1021/acs.jmedchem.5b01119
BindingDB Entry DOI: 10.7270/Q2S75J5X |
More data for this
Ligand-Target Pair | |
Cholinesterase
(Homo sapiens (Human)) | BDBM50138437

(CHEMBL3752356)Show SMILES COc1cccc2cc(CNCCCCCCNc3c4CCCc4nc4ccccc34)oc12 Show InChI InChI=1S/C28H33N3O2/c1-32-26-15-8-10-20-18-21(33-28(20)26)19-29-16-6-2-3-7-17-30-27-22-11-4-5-13-24(22)31-25-14-9-12-23(25)27/h4-5,8,10-11,13,15,18,29H,2-3,6-7,9,12,14,16-17,19H2,1H3,(H,30,31) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.30 | n/a | n/a | n/a | n/a | n/a | n/a |
China Pharmaceutical University
Curated by ChEMBL
| Assay Description
Inhibition of human serum BChE using butyrylthiocholine iodide as substrate preincubated for 20 mins followed by substrate addition by Ellman's metho... |
J Med Chem 59: 114-31 (2016)
Article DOI: 10.1021/acs.jmedchem.5b01119
BindingDB Entry DOI: 10.7270/Q2S75J5X |
More data for this
Ligand-Target Pair | |
Cholinesterase
(Homo sapiens (Human)) | BDBM50604399

(CHEMBL5190129)Show SMILES CCCCN(CCC1CCCCCC1)Cc1ccc(OC(=O)N(C)C)c2ccccc12 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.90 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.ejmech.2022.114248
BindingDB Entry DOI: 10.7270/Q2125XRJ |
More data for this
Ligand-Target Pair | |
Cholinesterase
(Homo sapiens (Human)) | BDBM50604386

(CHEMBL5189830)Show SMILES CN(C)C1CCC(CCN(CCCc2c[nH]c3ccccc23)CCC2CCCCCC2)CC1 |(9.28,-1.21,;8.19,-2.3,;8.59,-3.79,;6.7,-1.9,;6.3,-.42,;4.82,-.02,;3.73,-1.11,;2.24,-.71,;1.15,-1.8,;-.34,-1.4,;-1.43,-2.49,;-2.91,-2.09,;-4,-3.18,;-5.49,-2.78,;-6.11,-1.36,;-7.6,-1.51,;-7.94,-3.04,;-9.28,-3.81,;-9.28,-5.36,;-7.94,-6.12,;-6.62,-5.35,;-6.62,-3.81,;-.74,.09,;.35,1.18,;-.05,2.67,;-1.57,2.85,;-2.39,4.15,;-1.89,5.6,;-.43,6.12,;.88,5.3,;1.05,3.76,;4.12,-2.59,;5.61,-2.99,)| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.90 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.ejmech.2022.114248
BindingDB Entry DOI: 10.7270/Q2125XRJ |
More data for this
Ligand-Target Pair | |
Cholinesterase
(Homo sapiens (Human)) | BDBM50604386

(CHEMBL5189830)Show SMILES CN(C)C1CCC(CCN(CCCc2c[nH]c3ccccc23)CCC2CCCCCC2)CC1 |(9.28,-1.21,;8.19,-2.3,;8.59,-3.79,;6.7,-1.9,;6.3,-.42,;4.82,-.02,;3.73,-1.11,;2.24,-.71,;1.15,-1.8,;-.34,-1.4,;-1.43,-2.49,;-2.91,-2.09,;-4,-3.18,;-5.49,-2.78,;-6.11,-1.36,;-7.6,-1.51,;-7.94,-3.04,;-9.28,-3.81,;-9.28,-5.36,;-7.94,-6.12,;-6.62,-5.35,;-6.62,-3.81,;-.74,.09,;.35,1.18,;-.05,2.67,;-1.57,2.85,;-2.39,4.15,;-1.89,5.6,;-.43,6.12,;.88,5.3,;1.05,3.76,;4.12,-2.59,;5.61,-2.99,)| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.90 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.ejmech.2022.114248
BindingDB Entry DOI: 10.7270/Q2125XRJ |
More data for this
Ligand-Target Pair | |
Cholinesterase
(Homo sapiens (Human)) | BDBM50604373

(CHEMBL5190737) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.90 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.ejmech.2022.114248
BindingDB Entry DOI: 10.7270/Q2125XRJ |
More data for this
Ligand-Target Pair | |
Cholinesterase
(Homo sapiens (Human)) | BDBM50138279

(CHEMBL3752467)Show SMILES C(CCCNCc1cc2ccccc2o1)CCCNc1c2CCCCc2nc2ccccc12 Show InChI InChI=1S/C29H35N3O/c1(2-10-18-30-21-23-20-22-12-4-9-17-28(22)33-23)3-11-19-31-29-24-13-5-7-15-26(24)32-27-16-8-6-14-25(27)29/h4-5,7,9,12-13,15,17,20,30H,1-3,6,8,10-11,14,16,18-19,21H2,(H,31,32) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.20 | n/a | n/a | n/a | n/a | n/a | n/a |
China Pharmaceutical University
Curated by ChEMBL
| Assay Description
Inhibition of human serum BChE using butyrylthiocholine iodide as substrate preincubated for 20 mins followed by substrate addition by Ellman's metho... |
J Med Chem 59: 114-31 (2016)
Article DOI: 10.1021/acs.jmedchem.5b01119
BindingDB Entry DOI: 10.7270/Q2S75J5X |
More data for this
Ligand-Target Pair | |
Cholinesterase
(Homo sapiens (Human)) | BDBM50604405

(CHEMBL5186889)Show SMILES CCCCN(CCC1CCCCCC1)CCc1c[nH]c2c(OC(=O)C(C)C)cccc12 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 2.20 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.ejmech.2022.114248
BindingDB Entry DOI: 10.7270/Q2125XRJ |
More data for this
Ligand-Target Pair | |
Cholinesterase
(Homo sapiens (Human)) | BDBM50604398
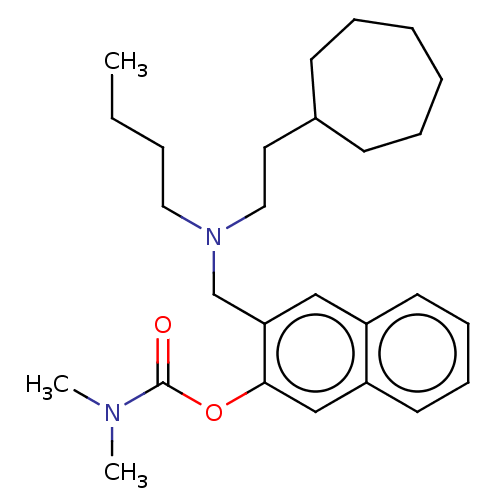
(CHEMBL5179799)Show SMILES CCCCN(CCC1CCCCCC1)Cc1cc2ccccc2cc1OC(=O)N(C)C | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 2.20 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.ejmech.2022.114248
BindingDB Entry DOI: 10.7270/Q2125XRJ |
More data for this
Ligand-Target Pair | |
Cholinesterase
(Homo sapiens (Human)) | BDBM50138462

(CHEMBL3754694)Show SMILES COc1cccc2cc(CN(CCCCCCNc3c4CCCc4nc4ccccc34)Cc3cc4cccc(OC)c4o3)oc12 Show InChI InChI=1S/C38H41N3O4/c1-42-34-18-9-12-26-22-28(44-37(26)34)24-41(25-29-23-27-13-10-19-35(43-2)38(27)45-29)21-8-4-3-7-20-39-36-30-14-5-6-16-32(30)40-33-17-11-15-31(33)36/h5-6,9-10,12-14,16,18-19,22-23H,3-4,7-8,11,15,17,20-21,24-25H2,1-2H3,(H,39,40) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.30 | n/a | n/a | n/a | n/a | n/a | n/a |
China Pharmaceutical University
Curated by ChEMBL
| Assay Description
Inhibition of human serum BChE using butyrylthiocholine iodide as substrate preincubated for 20 mins followed by substrate addition by Ellman's metho... |
J Med Chem 59: 114-31 (2016)
Article DOI: 10.1021/acs.jmedchem.5b01119
BindingDB Entry DOI: 10.7270/Q2S75J5X |
More data for this
Ligand-Target Pair | |
Cholinesterase
(Homo sapiens (Human)) | BDBM50604385

(CHEMBL5184276)Show SMILES [I-].CCCC[N+](C)(CCCc1c[nH]c2ccccc12)CCC1CCCCCC1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 2.30 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.ejmech.2022.114248
BindingDB Entry DOI: 10.7270/Q2125XRJ |
More data for this
Ligand-Target Pair | |
Cholinesterase
(Homo sapiens (Human)) | BDBM50138464

(CHEMBL3754778)Show SMILES COc1cccc2cc(CN(CCCCCCNc3c4CCCCCc4nc4ccccc34)Cc3cc4cccc(OC)c4o3)oc12 Show InChI InChI=1S/C40H45N3O4/c1-44-36-20-12-14-28-24-30(46-39(28)36)26-43(27-31-25-29-15-13-21-37(45-2)40(29)47-31)23-11-4-3-10-22-41-38-32-16-6-5-7-18-34(32)42-35-19-9-8-17-33(35)38/h8-9,12-15,17,19-21,24-25H,3-7,10-11,16,18,22-23,26-27H2,1-2H3,(H,41,42) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 2.40 | n/a | n/a | n/a | n/a | n/a | n/a |
China Pharmaceutical University
Curated by ChEMBL
| Assay Description
Inhibition of human serum BChE using butyrylthiocholine iodide as substrate preincubated for 20 mins followed by substrate addition by Ellman's metho... |
J Med Chem 59: 114-31 (2016)
Article DOI: 10.1021/acs.jmedchem.5b01119
BindingDB Entry DOI: 10.7270/Q2S75J5X |
More data for this
Ligand-Target Pair | |
Cholinesterase
(Homo sapiens (Human)) | BDBM50604376

(CHEMBL5206702) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 2.40 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.ejmech.2022.114248
BindingDB Entry DOI: 10.7270/Q2125XRJ |
More data for this
Ligand-Target Pair | |
Cholinesterase
(Homo sapiens (Human)) | BDBM50604394
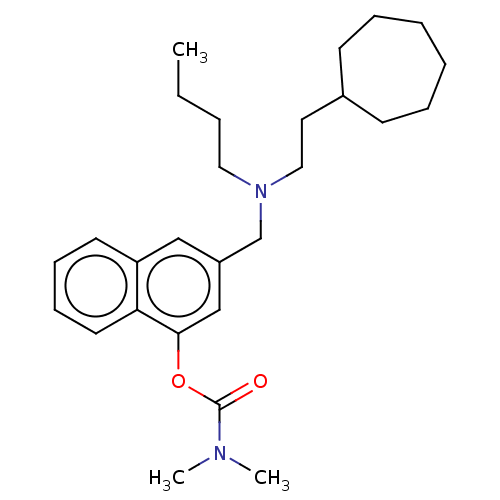
(CHEMBL5172799)Show SMILES CCCCN(CCC1CCCCCC1)Cc1cc(OC(=O)N(C)C)c2ccccc2c1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 2.40 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.ejmech.2022.114248
BindingDB Entry DOI: 10.7270/Q2125XRJ |
More data for this
Ligand-Target Pair | |
Cholinesterase
(Homo sapiens (Human)) | BDBM50138431

(CHEMBL3751941)Show SMILES C(CCCNc1c2CCCc2nc2ccccc12)CCNCc1cc2ccccc2o1 Show InChI InChI=1S/C27H31N3O/c1(7-16-28-19-21-18-20-10-3-6-15-26(20)31-21)2-8-17-29-27-22-11-4-5-13-24(22)30-25-14-9-12-23(25)27/h3-6,10-11,13,15,18,28H,1-2,7-9,12,14,16-17,19H2,(H,29,30) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.5 | n/a | n/a | n/a | n/a | n/a | n/a |
China Pharmaceutical University
Curated by ChEMBL
| Assay Description
Inhibition of human serum BChE using butyrylthiocholine iodide as substrate preincubated for 20 mins followed by substrate addition by Ellman's metho... |
J Med Chem 59: 114-31 (2016)
Article DOI: 10.1021/acs.jmedchem.5b01119
BindingDB Entry DOI: 10.7270/Q2S75J5X |
More data for this
Ligand-Target Pair | |
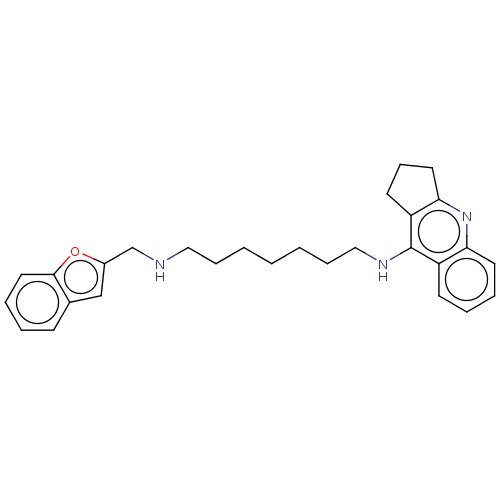
Cholinesterase
(Homo sapiens (Human)) | BDBM50138439
(CHEMBL3752543)Show SMILES C(CCCNCc1cc2ccccc2o1)CCCNc1c2CCCc2nc2ccccc12 Show InChI InChI=1S/C28H33N3O/c1(2-8-17-29-20-22-19-21-11-4-7-16-27(21)32-22)3-9-18-30-28-23-12-5-6-14-25(23)31-26-15-10-13-24(26)28/h4-7,11-12,14,16,19,29H,1-3,8-10,13,15,17-18,20H2,(H,30,31) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.5 | n/a | n/a | n/a | n/a | n/a | n/a |
China Pharmaceutical University
Curated by ChEMBL
| Assay Description
Inhibition of human serum BChE using butyrylthiocholine iodide as substrate preincubated for 20 mins followed by substrate addition by Ellman's metho... |
J Med Chem 59: 114-31 (2016)
Article DOI: 10.1021/acs.jmedchem.5b01119
BindingDB Entry DOI: 10.7270/Q2S75J5X |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM50138440

(CHEMBL3753405)Show SMILES COc1cccc2cc(CNCCCCCCCNc3c4CCCCCc4nc4ccccc34)oc12 Show InChI InChI=1S/C31H39N3O2/c1-35-29-18-12-13-23-21-24(36-31(23)29)22-32-19-10-3-2-4-11-20-33-30-25-14-6-5-7-16-27(25)34-28-17-9-8-15-26(28)30/h8-9,12-13,15,17-18,21,32H,2-7,10-11,14,16,19-20,22H2,1H3,(H,33,34) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.60 | n/a | n/a | n/a | n/a | n/a | n/a |
China Pharmaceutical University
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human AChE using acetylthiocholine iodide as substrate preincubated for 20 mins followed by substrate addition by Ellman's ... |
J Med Chem 59: 114-31 (2016)
Article DOI: 10.1021/acs.jmedchem.5b01119
BindingDB Entry DOI: 10.7270/Q2S75J5X |
More data for this
Ligand-Target Pair | |
Cholinesterase
(Homo sapiens (Human)) | BDBM50604410

(CHEMBL5199183)Show SMILES CCCCN(C)[C@H](CN(C)CCC1CCCCCC1)Cc1c[nH]c2ccccc12 |r| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.ejmech.2022.114248
BindingDB Entry DOI: 10.7270/Q2125XRJ |
More data for this
Ligand-Target Pair | |
Cholinesterase
(Homo sapiens (Human)) | BDBM50604368
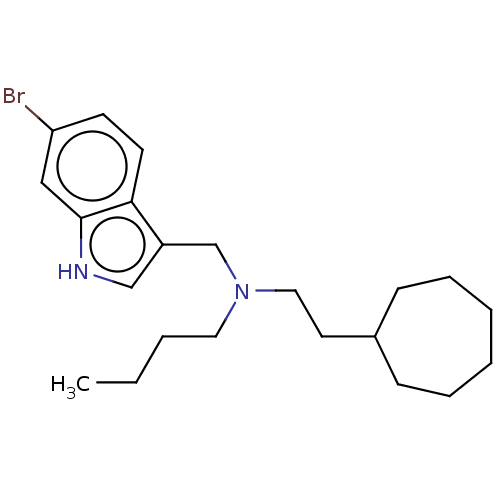
(CHEMBL5208984) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 3.60 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.ejmech.2022.114248
BindingDB Entry DOI: 10.7270/Q2125XRJ |
More data for this
Ligand-Target Pair | |
Cholinesterase
(Homo sapiens (Human)) | BDBM50604389

(CHEMBL5209876)Show SMILES CN(C)c1ccc(\C=C\CN(CCCc2c[nH]c3ccccc23)CCC2CCCCCC2)cc1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 4.60 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.ejmech.2022.114248
BindingDB Entry DOI: 10.7270/Q2125XRJ |
More data for this
Ligand-Target Pair | |
Cholinesterase
(Homo sapiens (Human)) | BDBM50604393
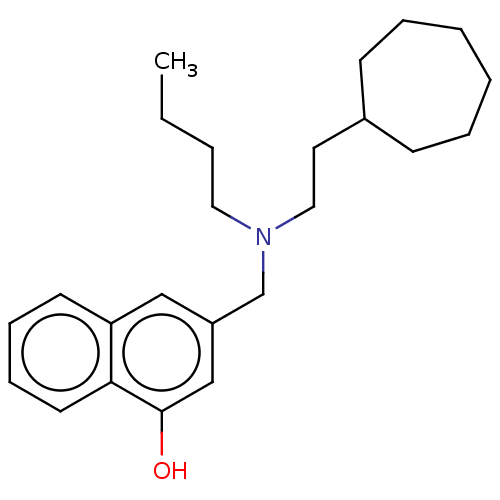
(CHEMBL5187323) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 4.70 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.ejmech.2022.114248
BindingDB Entry DOI: 10.7270/Q2125XRJ |
More data for this
Ligand-Target Pair | |
Cholinesterase
(Homo sapiens (Human)) | BDBM50138286

(CHEMBL3752451)Show SMILES O=C(NCCCCCCNc1c2CCCCc2nc2ccccc12)c1cc2ccccc2o1 Show InChI InChI=1S/C28H31N3O2/c32-28(26-19-20-11-3-8-16-25(20)33-26)30-18-10-2-1-9-17-29-27-21-12-4-6-14-23(21)31-24-15-7-5-13-22(24)27/h3-4,6,8,11-12,14,16,19H,1-2,5,7,9-10,13,15,17-18H2,(H,29,31)(H,30,32) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 4.70 | n/a | n/a | n/a | n/a | n/a | n/a |
China Pharmaceutical University
Curated by ChEMBL
| Assay Description
Inhibition of human serum BChE using butyrylthiocholine iodide as substrate preincubated for 20 mins followed by substrate addition by Ellman's metho... |
J Med Chem 59: 114-31 (2016)
Article DOI: 10.1021/acs.jmedchem.5b01119
BindingDB Entry DOI: 10.7270/Q2S75J5X |
More data for this
Ligand-Target Pair | |
Cholinesterase
(Homo sapiens (Human)) | BDBM50604371

(CHEMBL5204132) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 4.70 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.ejmech.2022.114248
BindingDB Entry DOI: 10.7270/Q2125XRJ |
More data for this
Ligand-Target Pair | |
Cholinesterase
(Homo sapiens (Human)) | BDBM50604388

(CHEMBL5187420)Show SMILES C(CN(CCC1CCCCC1)CCC1CCCCCC1)Cc1c[nH]c2ccccc12 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 4.90 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.ejmech.2022.114248
BindingDB Entry DOI: 10.7270/Q2125XRJ |
More data for this
Ligand-Target Pair | |
Cholinesterase
(Homo sapiens (Human)) | BDBM50246979
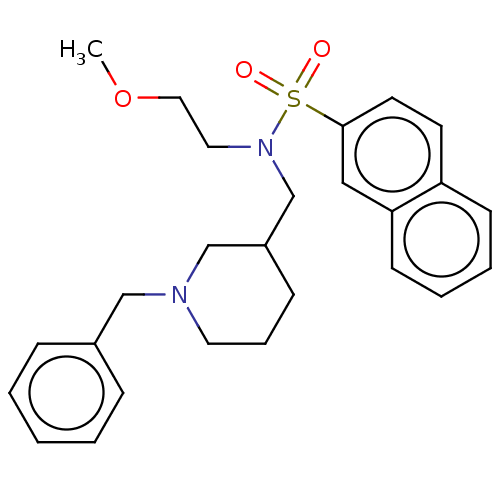
(CHEMBL4105611 | US20230331674, Table 1.1)Show SMILES COCCN(CC1CCCN(Cc2ccccc2)C1)S(=O)(=O)c1ccc2ccccc2c1 Show InChI InChI=1S/C26H32N2O3S/c1-31-17-16-28(32(29,30)26-14-13-24-11-5-6-12-25(24)18-26)21-23-10-7-15-27(20-23)19-22-8-3-2-4-9-22/h2-6,8-9,11-14,18,23H,7,10,15-17,19-21H2,1H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
PDB
UniChem
| PDB
Article
PubMed
| n/a | n/a | 4.90 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Ljubljana
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human BChE using butyrylthiocholine iodide as substrate preincubated for 300 secs followed by substrate addition measured f... |
J Med Chem 61: 119-139 (2018)
Article DOI: 10.1021/acs.jmedchem.7b01086
BindingDB Entry DOI: 10.7270/Q2S75JRJ |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Cholinesterase
(Homo sapiens (Human)) | BDBM50138284
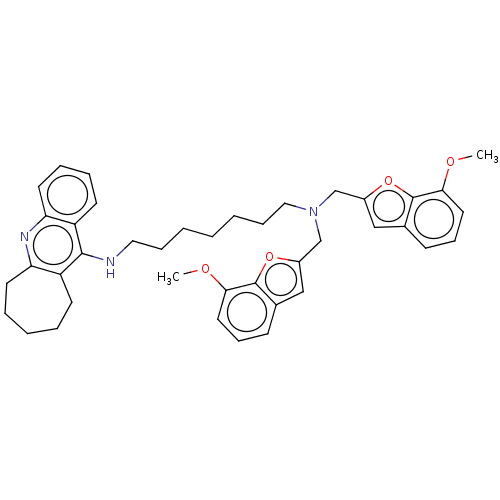
(CHEMBL3754005)Show SMILES COc1cccc2cc(CN(CCCCCCCNc3c4CCCCCc4nc4ccccc34)Cc3cc4cccc(OC)c4o3)oc12 Show InChI InChI=1S/C41H47N3O4/c1-45-37-21-13-15-29-25-31(47-40(29)37)27-44(28-32-26-30-16-14-22-38(46-2)41(30)48-32)24-12-5-3-4-11-23-42-39-33-17-7-6-8-19-35(33)43-36-20-10-9-18-34(36)39/h9-10,13-16,18,20-22,25-26H,3-8,11-12,17,19,23-24,27-28H2,1-2H3,(H,42,43) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 4.90 | n/a | n/a | n/a | n/a | n/a | n/a |
China Pharmaceutical University
Curated by ChEMBL
| Assay Description
Inhibition of human serum BChE using butyrylthiocholine iodide as substrate preincubated for 20 mins followed by substrate addition by Ellman's metho... |
J Med Chem 59: 114-31 (2016)
Article DOI: 10.1021/acs.jmedchem.5b01119
BindingDB Entry DOI: 10.7270/Q2S75J5X |
More data for this
Ligand-Target Pair | |
Cholinesterase
(Homo sapiens (Human)) | BDBM50604414

(CHEMBL5209132)Show SMILES CCCCN1CCN(CCCC2CCCCCC2)C[C@@H]1Cc1c[nH]c2ccccc12 |r| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.ejmech.2022.114248
BindingDB Entry DOI: 10.7270/Q2125XRJ |
More data for this
Ligand-Target Pair | |
Cholinesterase
(Homo sapiens (Human)) | BDBM50604377

(CHEMBL5169405) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.ejmech.2022.114248
BindingDB Entry DOI: 10.7270/Q2125XRJ |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data