

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| C-X-C chemokine receptor type 2 (Homo sapiens (Human)) | BDBM50371784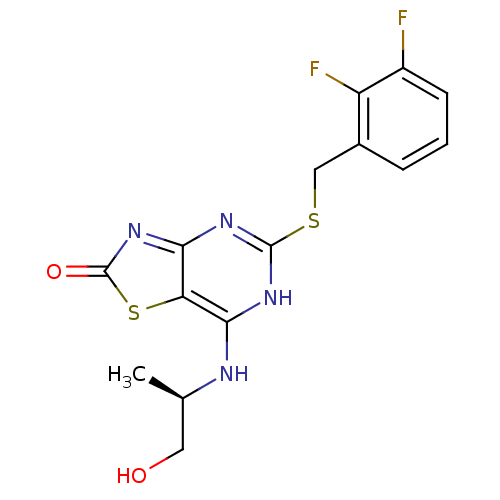 (CHEMBL446458) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Curated by ChEMBL | Assay Description Displacement of [125I]IL-8 from human recombinant CXCR2 receptor expressed in HEK293 cells by scintillation counting analysis | Bioorg Med Chem Lett 25: 1616-20 (2015) Article DOI: 10.1016/j.bmcl.2015.01.067 BindingDB Entry DOI: 10.7270/Q2F47QT4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-X-C chemokine receptor type 2 (Homo sapiens (Human)) | BDBM50065327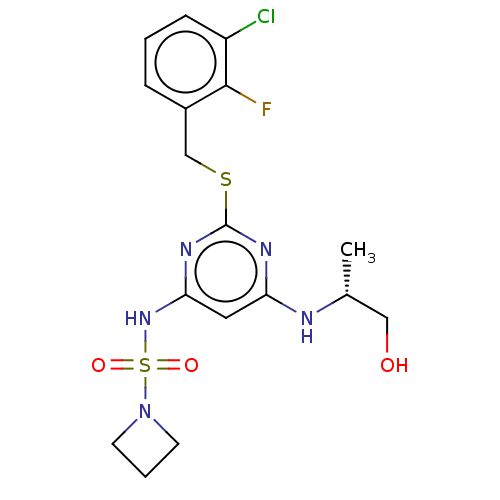 (CHEMBL3403851) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.5 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Curated by ChEMBL | Assay Description Displacement of [125I]IL-8 from human recombinant CXCR2 receptor expressed in HEK293 cells by scintillation counting analysis | Bioorg Med Chem Lett 25: 1616-20 (2015) Article DOI: 10.1016/j.bmcl.2015.01.067 BindingDB Entry DOI: 10.7270/Q2F47QT4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-X-C chemokine receptor type 2 (Homo sapiens (Human)) | BDBM50065330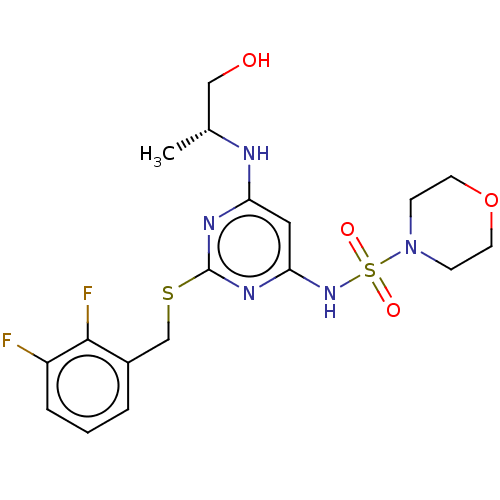 (CHEMBL3403854) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Curated by ChEMBL | Assay Description Displacement of [125I]IL-8 from human recombinant CXCR2 receptor expressed in HEK293 cells by scintillation counting analysis | Bioorg Med Chem Lett 25: 1616-20 (2015) Article DOI: 10.1016/j.bmcl.2015.01.067 BindingDB Entry DOI: 10.7270/Q2F47QT4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-X-C chemokine receptor type 2 (Homo sapiens (Human)) | BDBM50371783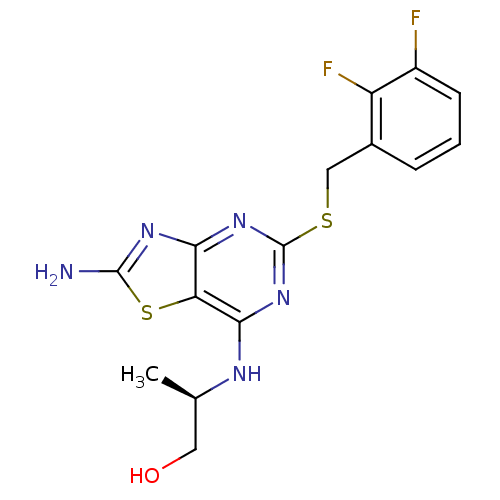 (CHEMBL397237) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Curated by ChEMBL | Assay Description Displacement of [125I]IL-8 from human recombinant CXCR2 receptor expressed in HEK293 cells by scintillation counting analysis | Bioorg Med Chem Lett 25: 1616-20 (2015) Article DOI: 10.1016/j.bmcl.2015.01.067 BindingDB Entry DOI: 10.7270/Q2F47QT4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-X-C chemokine receptor type 2 (Homo sapiens (Human)) | BDBM50065326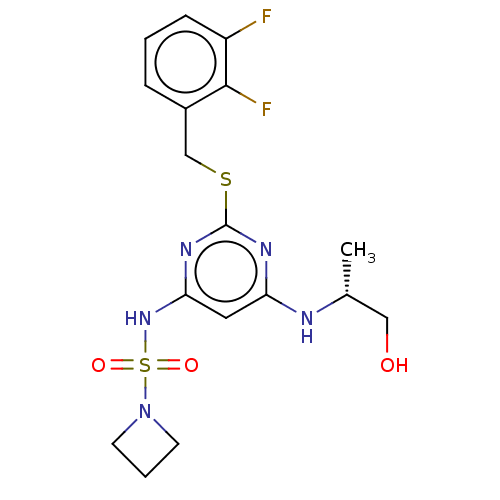 (CHEMBL3403850) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Curated by ChEMBL | Assay Description Displacement of [125I]IL-8 from human recombinant CXCR2 receptor expressed in HEK293 cells by scintillation counting analysis | Bioorg Med Chem Lett 25: 1616-20 (2015) Article DOI: 10.1016/j.bmcl.2015.01.067 BindingDB Entry DOI: 10.7270/Q2F47QT4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-X-C chemokine receptor type 2 (Homo sapiens (Human)) | BDBM50065324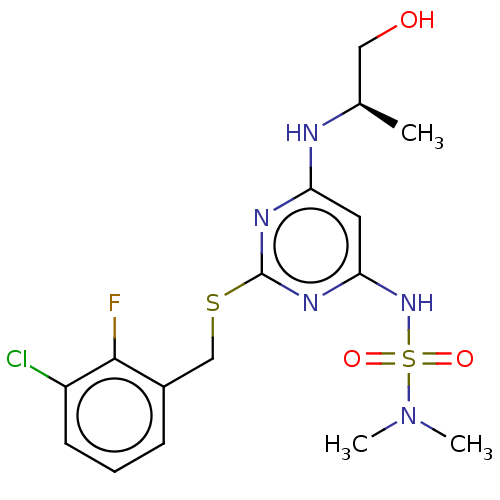 (CHEMBL3403848) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Curated by ChEMBL | Assay Description Displacement of [125I]IL-8 from human recombinant CXCR2 receptor expressed in HEK293 cells by scintillation counting analysis | Bioorg Med Chem Lett 25: 1616-20 (2015) Article DOI: 10.1016/j.bmcl.2015.01.067 BindingDB Entry DOI: 10.7270/Q2F47QT4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-X-C chemokine receptor type 2 (Homo sapiens (Human)) | BDBM50065331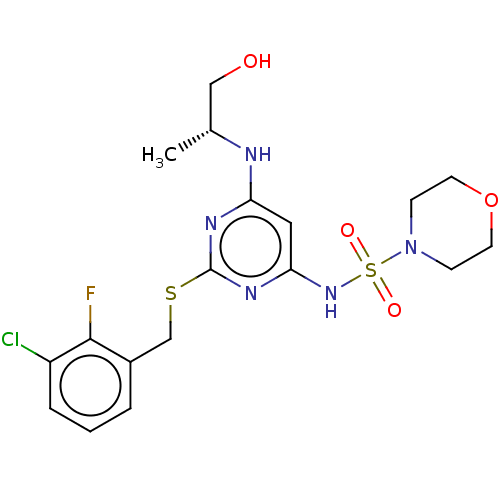 (CHEMBL3403855) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Curated by ChEMBL | Assay Description Displacement of [125I]IL-8 from human recombinant CXCR2 receptor expressed in HEK293 cells by scintillation counting analysis | Bioorg Med Chem Lett 25: 1616-20 (2015) Article DOI: 10.1016/j.bmcl.2015.01.067 BindingDB Entry DOI: 10.7270/Q2F47QT4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Myeloperoxidase (Homo sapiens (Human)) | BDBM312172 (Alternative Preparation | US10016430, Example 3 | ...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem | US Patent | n/a | n/a | 7 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca AB US Patent | Assay Description MPO: The pharmacological activity of compounds disclosed herein was tested in the following screen (Test A) in which the compounds were tested in the... | US Patent US10016430 (2018) BindingDB Entry DOI: 10.7270/Q2WW7M2N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Myeloperoxidase (Homo sapiens (Human)) | BDBM312171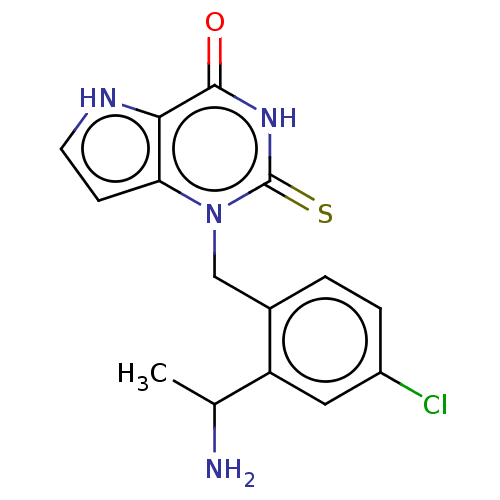 (1-[2-(1-Aminoethyl)-4-chlorobenzyl]-2-thioxo-1,2,3...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 7 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca AB US Patent | Assay Description MPO: The pharmacological activity of compounds disclosed herein was tested in the following screen (Test A) in which the compounds were tested in the... | US Patent US10016430 (2018) BindingDB Entry DOI: 10.7270/Q2WW7M2N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Myeloperoxidase (Homo sapiens (Human)) | BDBM312171 (1-[2-(1-Aminoethyl)-4-chlorobenzyl]-2-thioxo-1,2,3...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 7 | n/a | n/a | n/a | n/a | n/a | n/a |
ASTRAZENECA AB US Patent | Assay Description Methods for the determination of MPO inhibitory activity are disclosed in WO 02/090575. The pharmacological activity of compounds disclosed herein wa... | US Patent US11000525 (2021) BindingDB Entry DOI: 10.7270/Q2QJ7MD4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Myeloperoxidase (Homo sapiens (Human)) | BDBM312172 (Alternative Preparation | US10016430, Example 3 | ...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem | US Patent | n/a | n/a | 7 | n/a | n/a | n/a | n/a | n/a | n/a |
ASTRAZENECA AB US Patent | Assay Description Methods for the determination of MPO inhibitory activity are disclosed in WO 02/090575. The pharmacological activity of compounds disclosed herein wa... | US Patent US11000525 (2021) BindingDB Entry DOI: 10.7270/Q2QJ7MD4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Myeloperoxidase (Homo sapiens (Human)) | BDBM312172 (Alternative Preparation | US10016430, Example 3 | ...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem | US Patent | n/a | n/a | 7 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca AB US Patent | Assay Description The pharmacological activity of compounds disclosed herein was tested in the following screen (Test A) in which the compounds were tested in the pres... | US Patent US9616063 (2017) BindingDB Entry DOI: 10.7270/Q2Q24296 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Myeloperoxidase (Homo sapiens (Human)) | BDBM312171 (1-[2-(1-Aminoethyl)-4-chlorobenzyl]-2-thioxo-1,2,3...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 7 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca AB US Patent | Assay Description The pharmacological activity of compounds disclosed herein was tested in the following screen (Test A) in which the compounds were tested in the pres... | US Patent US9616063 (2017) BindingDB Entry DOI: 10.7270/Q2Q24296 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-X-C chemokine receptor type 2 (Homo sapiens (Human)) | BDBM50065328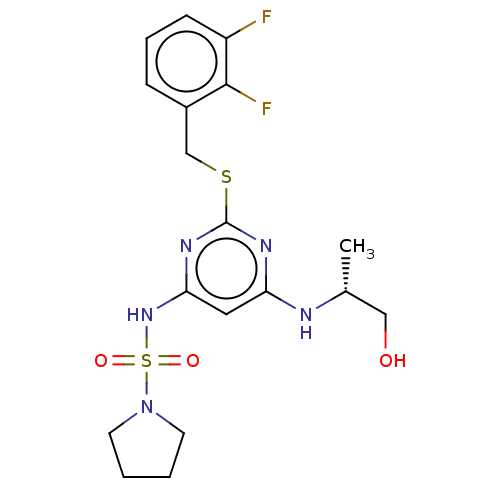 (CHEMBL3403852) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 7.90 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Curated by ChEMBL | Assay Description Displacement of [125I]IL-8 from human recombinant CXCR2 receptor expressed in HEK293 cells by scintillation counting analysis | Bioorg Med Chem Lett 25: 1616-20 (2015) Article DOI: 10.1016/j.bmcl.2015.01.067 BindingDB Entry DOI: 10.7270/Q2F47QT4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-X-C chemokine receptor type 2 (Homo sapiens (Human)) | BDBM50065325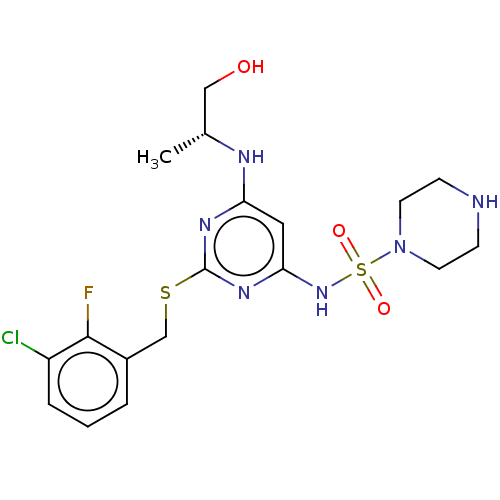 (CHEMBL3403849) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 7.90 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Curated by ChEMBL | Assay Description Displacement of [125I]IL-8 from human recombinant CXCR2 receptor expressed in HEK293 cells by scintillation counting analysis | Bioorg Med Chem Lett 25: 1616-20 (2015) Article DOI: 10.1016/j.bmcl.2015.01.067 BindingDB Entry DOI: 10.7270/Q2F47QT4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-X-C chemokine receptor type 2 (Homo sapiens (Human)) | BDBM50065329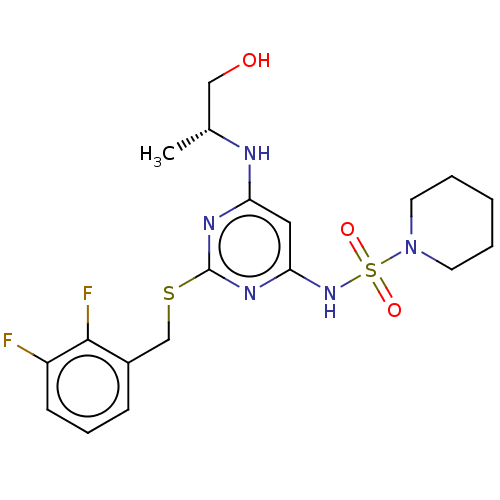 (CHEMBL3403853) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 7.90 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Curated by ChEMBL | Assay Description Displacement of [125I]IL-8 from human recombinant CXCR2 receptor expressed in HEK293 cells by scintillation counting analysis | Bioorg Med Chem Lett 25: 1616-20 (2015) Article DOI: 10.1016/j.bmcl.2015.01.067 BindingDB Entry DOI: 10.7270/Q2F47QT4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Myeloperoxidase (Homo sapiens (Human)) | BDBM312157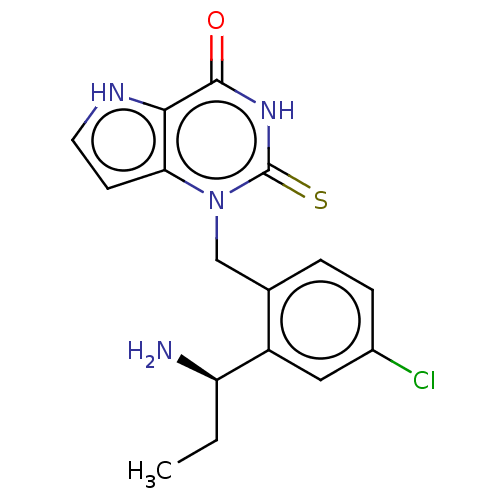 (1-{2-[(1R)-1-Aminopropyl]-4-chlorobenzyl}-2-thioxo...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 8.60 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca AB US Patent | Assay Description MPO: The pharmacological activity of compounds disclosed herein was tested in the following screen (Test A) in which the compounds were tested in the... | US Patent US10016430 (2018) BindingDB Entry DOI: 10.7270/Q2WW7M2N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Myeloperoxidase (Homo sapiens (Human)) | BDBM312157 (1-{2-[(1R)-1-Aminopropyl]-4-chlorobenzyl}-2-thioxo...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 8.60 | n/a | n/a | n/a | n/a | n/a | n/a |
ASTRAZENECA AB US Patent | Assay Description Methods for the determination of MPO inhibitory activity are disclosed in WO 02/090575. The pharmacological activity of compounds disclosed herein wa... | US Patent US11000525 (2021) BindingDB Entry DOI: 10.7270/Q2QJ7MD4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Myeloperoxidase (Homo sapiens (Human)) | BDBM312157 (1-{2-[(1R)-1-Aminopropyl]-4-chlorobenzyl}-2-thioxo...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 8.60 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca AB US Patent | Assay Description The pharmacological activity of compounds disclosed herein was tested in the following screen (Test A) in which the compounds were tested in the pres... | US Patent US9616063 (2017) BindingDB Entry DOI: 10.7270/Q2Q24296 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-X-C chemokine receptor type 2 (Homo sapiens (Human)) | BDBM50065267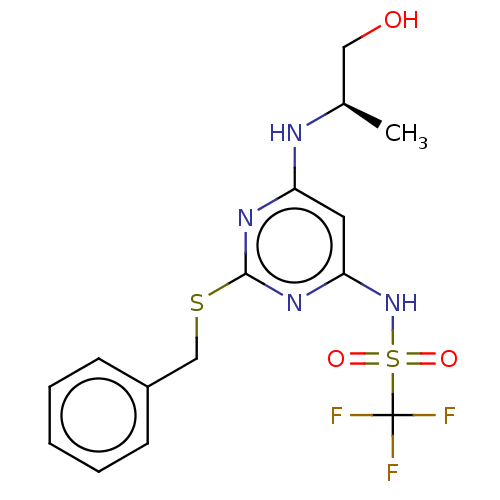 (CHEMBL3403840) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Curated by ChEMBL | Assay Description Displacement of [125I]IL-8 from human recombinant CXCR2 receptor expressed in HEK293 cells by scintillation counting analysis | Bioorg Med Chem Lett 25: 1616-20 (2015) Article DOI: 10.1016/j.bmcl.2015.01.067 BindingDB Entry DOI: 10.7270/Q2F47QT4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Myeloperoxidase (Homo sapiens (Human)) | BDBM312256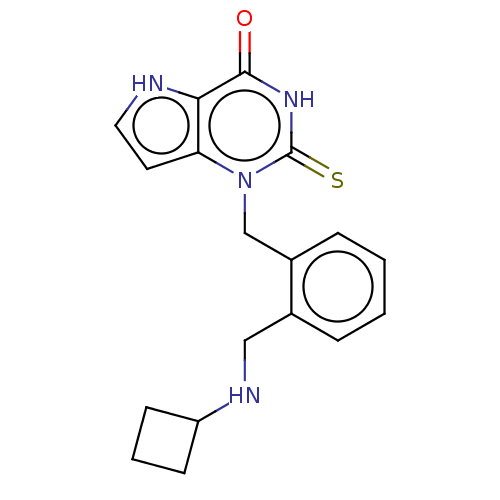 (1-{2-[(Cyclobutylamino)methyl]benzyl}-2-thioxo-1,2...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca AB US Patent | Assay Description The pharmacological activity of compounds disclosed herein was tested in the following screen (Test A) in which the compounds were tested in the pres... | US Patent US9616063 (2017) BindingDB Entry DOI: 10.7270/Q2Q24296 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Myeloperoxidase (Homo sapiens (Human)) | BDBM312256 (1-{2-[(Cyclobutylamino)methyl]benzyl}-2-thioxo-1,2...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
ASTRAZENECA AB US Patent | Assay Description Methods for the determination of MPO inhibitory activity are disclosed in WO 02/090575. The pharmacological activity of compounds disclosed herein wa... | US Patent US11000525 (2021) BindingDB Entry DOI: 10.7270/Q2QJ7MD4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Myeloperoxidase (Homo sapiens (Human)) | BDBM312256 (1-{2-[(Cyclobutylamino)methyl]benzyl}-2-thioxo-1,2...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca AB US Patent | Assay Description MPO: The pharmacological activity of compounds disclosed herein was tested in the following screen (Test A) in which the compounds were tested in the... | US Patent US10016430 (2018) BindingDB Entry DOI: 10.7270/Q2WW7M2N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Myeloperoxidase (Homo sapiens (Human)) | BDBM312245 (1-{4-Chloro-2-[(methylamino)methyl]benzyl}-2-thiox...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 13 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca AB US Patent | Assay Description The pharmacological activity of compounds disclosed herein was tested in the following screen (Test A) in which the compounds were tested in the pres... | US Patent US9616063 (2017) BindingDB Entry DOI: 10.7270/Q2Q24296 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-X-C chemokine receptor type 2 (Homo sapiens (Human)) | BDBM50065256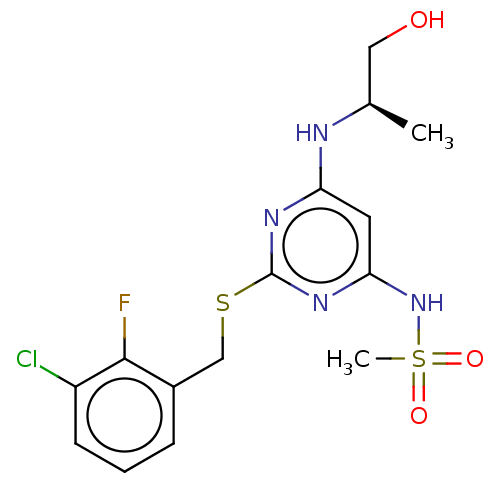 (CHEMBL3403835) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 13 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Curated by ChEMBL | Assay Description Displacement of [125I]IL-8 from human recombinant CXCR2 receptor expressed in HEK293 cells by scintillation counting analysis | Bioorg Med Chem Lett 25: 1616-20 (2015) Article DOI: 10.1016/j.bmcl.2015.01.067 BindingDB Entry DOI: 10.7270/Q2F47QT4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-X-C chemokine receptor type 2 (Homo sapiens (Human)) | BDBM50065323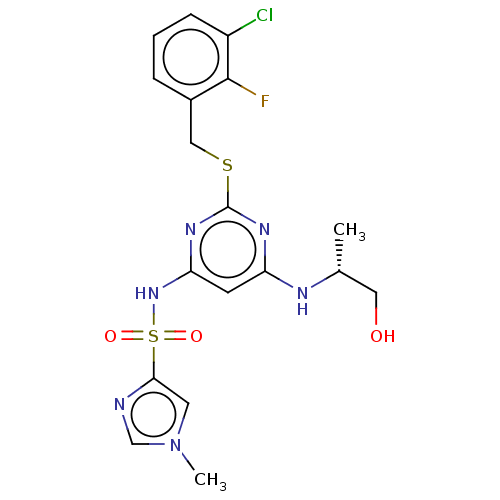 (CHEMBL3403847) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 13 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Curated by ChEMBL | Assay Description Displacement of [125I]IL-8 from human recombinant CXCR2 receptor expressed in HEK293 cells by scintillation counting analysis | Bioorg Med Chem Lett 25: 1616-20 (2015) Article DOI: 10.1016/j.bmcl.2015.01.067 BindingDB Entry DOI: 10.7270/Q2F47QT4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Myeloperoxidase (Homo sapiens (Human)) | BDBM312245 (1-{4-Chloro-2-[(methylamino)methyl]benzyl}-2-thiox...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 13 | n/a | n/a | n/a | n/a | n/a | n/a |
ASTRAZENECA AB US Patent | Assay Description Methods for the determination of MPO inhibitory activity are disclosed in WO 02/090575. The pharmacological activity of compounds disclosed herein wa... | US Patent US11000525 (2021) BindingDB Entry DOI: 10.7270/Q2QJ7MD4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Myeloperoxidase (Homo sapiens (Human)) | BDBM312245 (1-{4-Chloro-2-[(methylamino)methyl]benzyl}-2-thiox...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 13 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca AB US Patent | Assay Description MPO: The pharmacological activity of compounds disclosed herein was tested in the following screen (Test A) in which the compounds were tested in the... | US Patent US10016430 (2018) BindingDB Entry DOI: 10.7270/Q2WW7M2N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Myeloperoxidase (Homo sapiens (Human)) | BDBM312257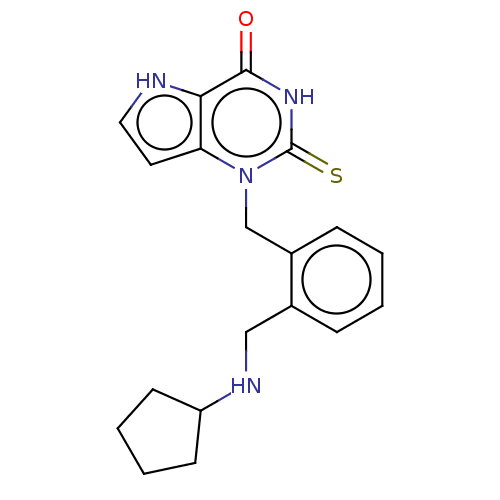 (1-{2-[(Cyclopentylamino)methyl]benzyl}-2-thioxo-1,...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 14 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca AB US Patent | Assay Description The pharmacological activity of compounds disclosed herein was tested in the following screen (Test A) in which the compounds were tested in the pres... | US Patent US9616063 (2017) BindingDB Entry DOI: 10.7270/Q2Q24296 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Myeloperoxidase (Homo sapiens (Human)) | BDBM312257 (1-{2-[(Cyclopentylamino)methyl]benzyl}-2-thioxo-1,...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 14 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca AB US Patent | Assay Description MPO: The pharmacological activity of compounds disclosed herein was tested in the following screen (Test A) in which the compounds were tested in the... | US Patent US10016430 (2018) BindingDB Entry DOI: 10.7270/Q2WW7M2N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Myeloperoxidase (Homo sapiens (Human)) | BDBM312257 (1-{2-[(Cyclopentylamino)methyl]benzyl}-2-thioxo-1,...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 14 | n/a | n/a | n/a | n/a | n/a | n/a |
ASTRAZENECA AB US Patent | Assay Description Methods for the determination of MPO inhibitory activity are disclosed in WO 02/090575. The pharmacological activity of compounds disclosed herein wa... | US Patent US11000525 (2021) BindingDB Entry DOI: 10.7270/Q2QJ7MD4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Myeloperoxidase (Homo sapiens (Human)) | BDBM312244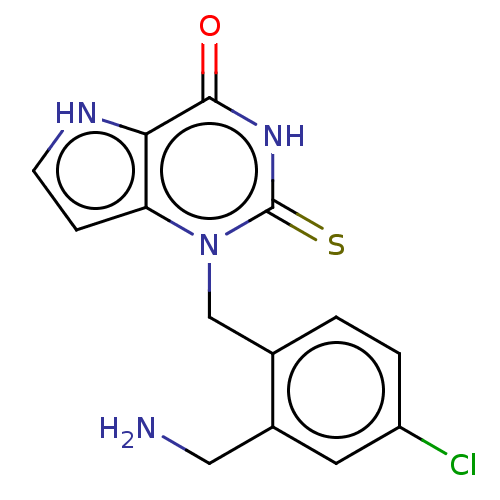 (1-[2-(Aminomethyl)-4-chlorobenzyl]-2-thioxo-1,2,3,...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 15 | n/a | n/a | n/a | n/a | n/a | n/a |
ASTRAZENECA AB US Patent | Assay Description Methods for the determination of MPO inhibitory activity are disclosed in WO 02/090575. The pharmacological activity of compounds disclosed herein wa... | US Patent US11000525 (2021) BindingDB Entry DOI: 10.7270/Q2QJ7MD4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Myeloperoxidase (Homo sapiens (Human)) | BDBM312244 (1-[2-(Aminomethyl)-4-chlorobenzyl]-2-thioxo-1,2,3,...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 15 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca AB US Patent | Assay Description The pharmacological activity of compounds disclosed herein was tested in the following screen (Test A) in which the compounds were tested in the pres... | US Patent US9616063 (2017) BindingDB Entry DOI: 10.7270/Q2Q24296 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Myeloperoxidase (Homo sapiens (Human)) | BDBM312244 (1-[2-(Aminomethyl)-4-chlorobenzyl]-2-thioxo-1,2,3,...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 15 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca AB US Patent | Assay Description MPO: The pharmacological activity of compounds disclosed herein was tested in the following screen (Test A) in which the compounds were tested in the... | US Patent US10016430 (2018) BindingDB Entry DOI: 10.7270/Q2WW7M2N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Myeloperoxidase (Homo sapiens (Human)) | BDBM312176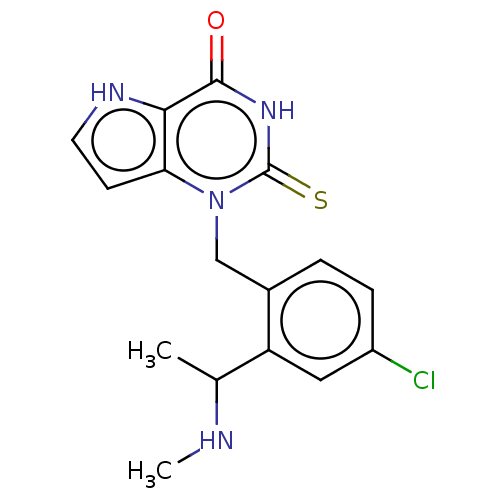 (1-{4-Chloro-2-[1-(methylamino)ethyl]benzyl}-2-thio...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 19 | n/a | n/a | n/a | n/a | n/a | n/a |
ASTRAZENECA AB US Patent | Assay Description Methods for the determination of MPO inhibitory activity are disclosed in WO 02/090575. The pharmacological activity of compounds disclosed herein wa... | US Patent US11000525 (2021) BindingDB Entry DOI: 10.7270/Q2QJ7MD4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Myeloperoxidase (Homo sapiens (Human)) | BDBM312176 (1-{4-Chloro-2-[1-(methylamino)ethyl]benzyl}-2-thio...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 19 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca AB US Patent | Assay Description MPO: The pharmacological activity of compounds disclosed herein was tested in the following screen (Test A) in which the compounds were tested in the... | US Patent US10016430 (2018) BindingDB Entry DOI: 10.7270/Q2WW7M2N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Myeloperoxidase (Homo sapiens (Human)) | BDBM312176 (1-{4-Chloro-2-[1-(methylamino)ethyl]benzyl}-2-thio...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 19 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca AB US Patent | Assay Description The pharmacological activity of compounds disclosed herein was tested in the following screen (Test A) in which the compounds were tested in the pres... | US Patent US9616063 (2017) BindingDB Entry DOI: 10.7270/Q2Q24296 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Myeloperoxidase (Homo sapiens (Human)) | BDBM312243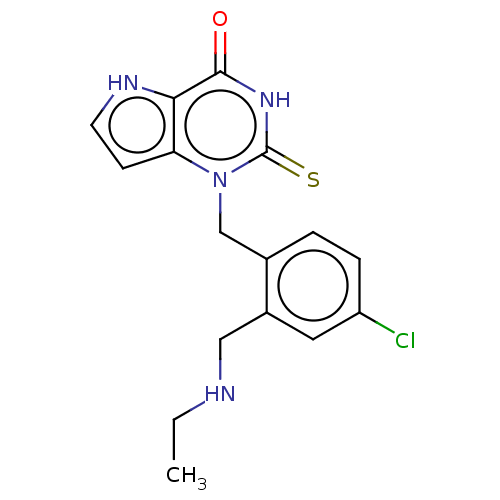 (1-{4-Chloro-2-[(ethylamino)methyl]benzyl}-2-thioxo...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 22 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca AB US Patent | Assay Description The pharmacological activity of compounds disclosed herein was tested in the following screen (Test A) in which the compounds were tested in the pres... | US Patent US9616063 (2017) BindingDB Entry DOI: 10.7270/Q2Q24296 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Myeloperoxidase (Homo sapiens (Human)) | BDBM312243 (1-{4-Chloro-2-[(ethylamino)methyl]benzyl}-2-thioxo...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 22 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca AB US Patent | Assay Description MPO: The pharmacological activity of compounds disclosed herein was tested in the following screen (Test A) in which the compounds were tested in the... | US Patent US10016430 (2018) BindingDB Entry DOI: 10.7270/Q2WW7M2N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Myeloperoxidase (Homo sapiens (Human)) | BDBM312243 (1-{4-Chloro-2-[(ethylamino)methyl]benzyl}-2-thioxo...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 22 | n/a | n/a | n/a | n/a | n/a | n/a |
ASTRAZENECA AB US Patent | Assay Description Methods for the determination of MPO inhibitory activity are disclosed in WO 02/090575. The pharmacological activity of compounds disclosed herein wa... | US Patent US11000525 (2021) BindingDB Entry DOI: 10.7270/Q2QJ7MD4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Myeloperoxidase (Homo sapiens (Human)) | BDBM312261 (1-{2-[(Propan-2-ylamino)methyl]benzyl}-2-thioxo-1,...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 24 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca AB US Patent | Assay Description The pharmacological activity of compounds disclosed herein was tested in the following screen (Test A) in which the compounds were tested in the pres... | US Patent US9616063 (2017) BindingDB Entry DOI: 10.7270/Q2Q24296 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Myeloperoxidase (Homo sapiens (Human)) | BDBM312261 (1-{2-[(Propan-2-ylamino)methyl]benzyl}-2-thioxo-1,...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 24 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca AB US Patent | Assay Description MPO: The pharmacological activity of compounds disclosed herein was tested in the following screen (Test A) in which the compounds were tested in the... | US Patent US10016430 (2018) BindingDB Entry DOI: 10.7270/Q2WW7M2N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Myeloperoxidase (Homo sapiens (Human)) | BDBM312261 (1-{2-[(Propan-2-ylamino)methyl]benzyl}-2-thioxo-1,...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 24 | n/a | n/a | n/a | n/a | n/a | n/a |
ASTRAZENECA AB US Patent | Assay Description Methods for the determination of MPO inhibitory activity are disclosed in WO 02/090575. The pharmacological activity of compounds disclosed herein wa... | US Patent US11000525 (2021) BindingDB Entry DOI: 10.7270/Q2QJ7MD4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-X-C chemokine receptor type 2 (Homo sapiens (Human)) | BDBM50065270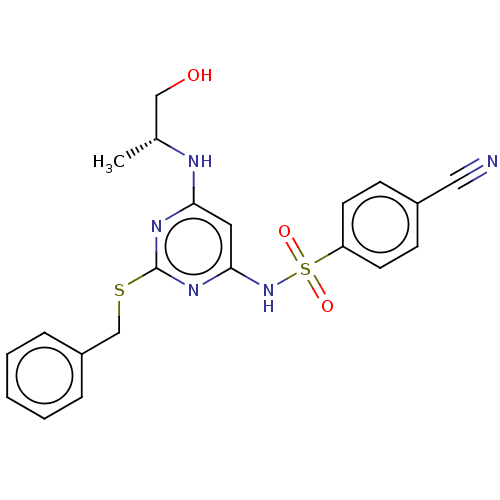 (CHEMBL3403843) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 25 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Curated by ChEMBL | Assay Description Displacement of [125I]IL-8 from human recombinant CXCR2 receptor expressed in HEK293 cells by scintillation counting analysis | Bioorg Med Chem Lett 25: 1616-20 (2015) Article DOI: 10.1016/j.bmcl.2015.01.067 BindingDB Entry DOI: 10.7270/Q2F47QT4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Myeloperoxidase (Homo sapiens (Human)) | BDBM312251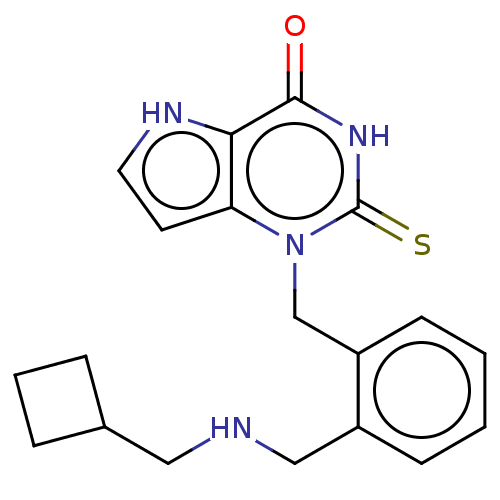 (1-(2-{[(Cyclobutylmethyl)amino]methyl}benzyl)-2-th...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 29 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca AB US Patent | Assay Description The pharmacological activity of compounds disclosed herein was tested in the following screen (Test A) in which the compounds were tested in the pres... | US Patent US9616063 (2017) BindingDB Entry DOI: 10.7270/Q2Q24296 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Myeloperoxidase (Homo sapiens (Human)) | BDBM312251 (1-(2-{[(Cyclobutylmethyl)amino]methyl}benzyl)-2-th...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 29 | n/a | n/a | n/a | n/a | n/a | n/a |
ASTRAZENECA AB US Patent | Assay Description Methods for the determination of MPO inhibitory activity are disclosed in WO 02/090575. The pharmacological activity of compounds disclosed herein wa... | US Patent US11000525 (2021) BindingDB Entry DOI: 10.7270/Q2QJ7MD4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Myeloperoxidase (Homo sapiens (Human)) | BDBM312251 (1-(2-{[(Cyclobutylmethyl)amino]methyl}benzyl)-2-th...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 29 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca AB US Patent | Assay Description MPO: The pharmacological activity of compounds disclosed herein was tested in the following screen (Test A) in which the compounds were tested in the... | US Patent US10016430 (2018) BindingDB Entry DOI: 10.7270/Q2WW7M2N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-X-C chemokine receptor type 2 (Homo sapiens (Human)) | BDBM50065269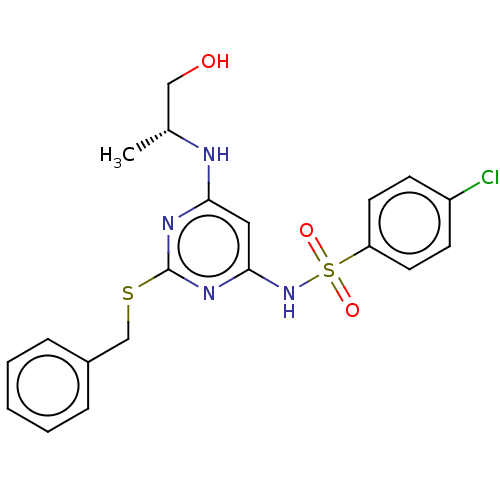 (CHEMBL3403842) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 32 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Curated by ChEMBL | Assay Description Displacement of [125I]IL-8 from human recombinant CXCR2 receptor expressed in HEK293 cells by scintillation counting analysis | Bioorg Med Chem Lett 25: 1616-20 (2015) Article DOI: 10.1016/j.bmcl.2015.01.067 BindingDB Entry DOI: 10.7270/Q2F47QT4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-X-C chemokine receptor type 2 (Homo sapiens (Human)) | BDBM50065254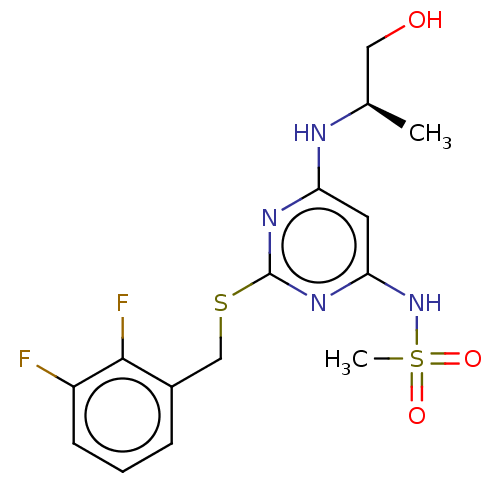 (CHEMBL3403834) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 32 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Curated by ChEMBL | Assay Description Displacement of [125I]IL-8 from human recombinant CXCR2 receptor expressed in HEK293 cells by scintillation counting analysis | Bioorg Med Chem Lett 25: 1616-20 (2015) Article DOI: 10.1016/j.bmcl.2015.01.067 BindingDB Entry DOI: 10.7270/Q2F47QT4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-X-C chemokine receptor type 2 (Homo sapiens (Human)) | BDBM50065322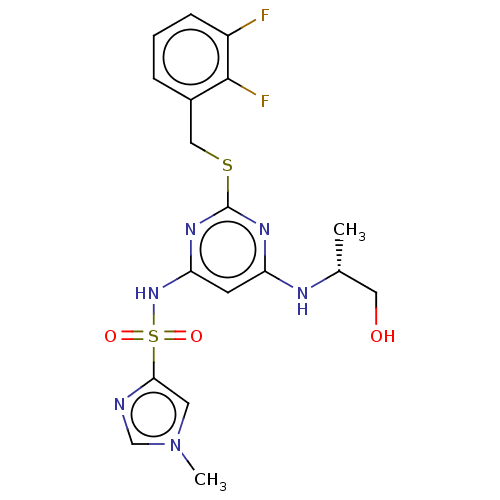 (CHEMBL3403846) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 32 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Curated by ChEMBL | Assay Description Displacement of [125I]IL-8 from human recombinant CXCR2 receptor expressed in HEK293 cells by scintillation counting analysis | Bioorg Med Chem Lett 25: 1616-20 (2015) Article DOI: 10.1016/j.bmcl.2015.01.067 BindingDB Entry DOI: 10.7270/Q2F47QT4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 120 total ) | Next | Last >> |