

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Prostaglandin G/H synthase 2 (Homo sapiens (Human)) | BDBM50297677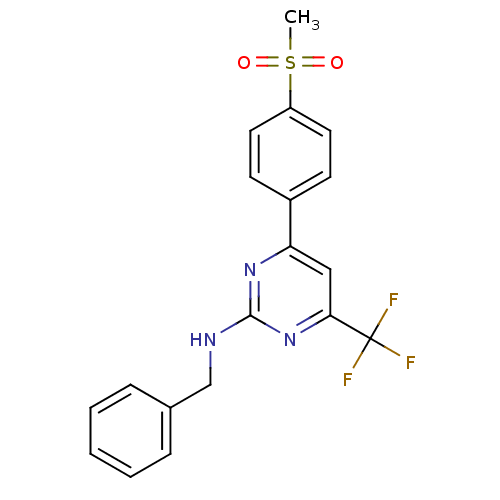 (CHEMBL551829 | N-benzyl-4-(4-(methylsulfonyl)pheny...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.25 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Inhibition of human COX2 expressed in baculovirus-infected SF9 cells assessed as inhibition of arachidonic acid-stimulated PGE2 production treated 1 ... | Bioorg Med Chem Lett 19: 4504-8 (2009) Article DOI: 10.1016/j.bmcl.2009.02.085 BindingDB Entry DOI: 10.7270/Q20Z7396 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin G/H synthase 2 (Homo sapiens (Human)) | BDBM50297675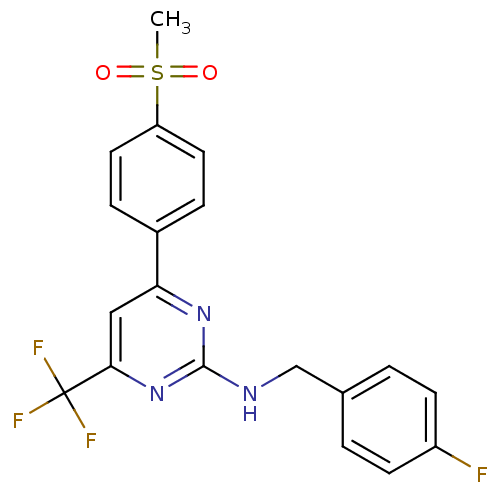 (CHEMBL551148 | N-(4-fluorobenzyl)-4-(4-(methylsulf...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.280 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Inhibition of human COX2 expressed in baculovirus-infected SF9 cells assessed as inhibition of arachidonic acid-stimulated PGE2 production treated 1 ... | Bioorg Med Chem Lett 19: 4504-8 (2009) Article DOI: 10.1016/j.bmcl.2009.02.085 BindingDB Entry DOI: 10.7270/Q20Z7396 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin G/H synthase 2 (Homo sapiens (Human)) | BDBM50297669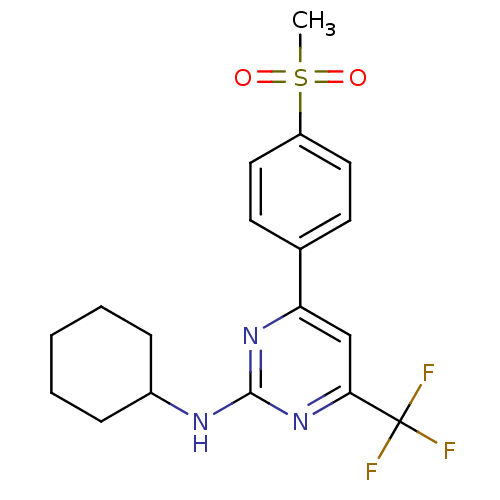 (CHEMBL561891 | N-cyclohexyl-4-(4-(methylsulfonyl)p...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Inhibition of human COX2 expressed in african green monkey COS cells assessed as inhibition of arachidonic acid-stimulated PGE2 production treated 1 ... | Bioorg Med Chem Lett 19: 4504-8 (2009) Article DOI: 10.1016/j.bmcl.2009.02.085 BindingDB Entry DOI: 10.7270/Q20Z7396 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin G/H synthase 2 (Homo sapiens (Human)) | BDBM50297672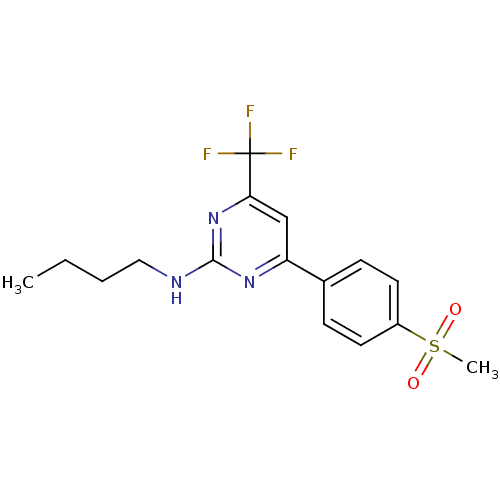 (CHEMBL549393 | N-butyl-4-(4-(methylsulfonyl)phenyl...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.30 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Inhibition of human COX2 expressed in baculovirus-infected SF9 cells assessed as inhibition of arachidonic acid-stimulated PGE2 production treated 1 ... | Bioorg Med Chem Lett 19: 4504-8 (2009) Article DOI: 10.1016/j.bmcl.2009.02.085 BindingDB Entry DOI: 10.7270/Q20Z7396 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin G/H synthase 2 (Homo sapiens (Human)) | BDBM50297671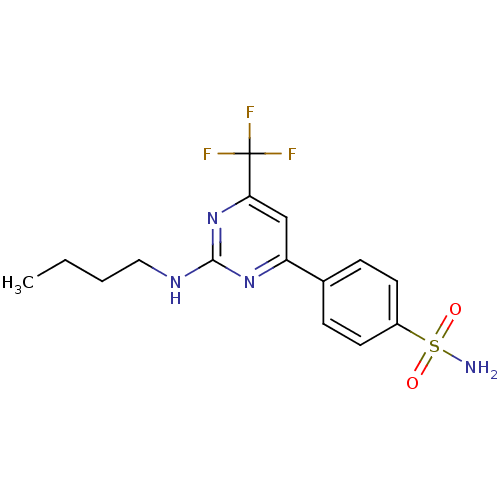 (4-(2-(butylamino)-6-(trifluoromethyl)pyrimidin-4-y...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | <1.40 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Inhibition of human COX2 expressed in african green monkey COS cells assessed as inhibition of arachidonic acid-stimulated PGE2 production treated 1 ... | Bioorg Med Chem Lett 19: 4504-8 (2009) Article DOI: 10.1016/j.bmcl.2009.02.085 BindingDB Entry DOI: 10.7270/Q20Z7396 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin G/H synthase 2 (Homo sapiens (Human)) | BDBM50297670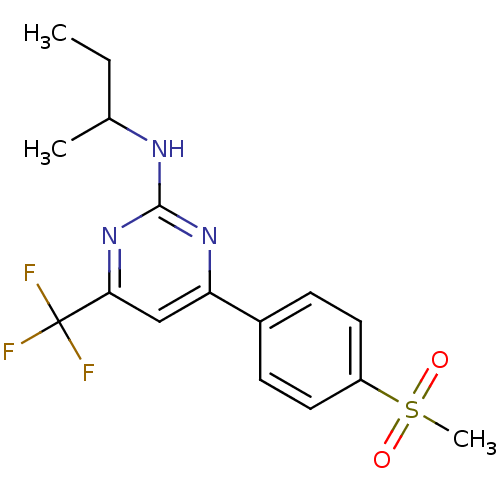 (CHEMBL539663 | GW-637185X | N-sec-butyl-4-(4-(meth...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Inhibition of human COX2 expressed in baculovirus-infected SF9 cells assessed as inhibition of arachidonic acid-stimulated PGE2 production treated 1 ... | Bioorg Med Chem Lett 19: 4504-8 (2009) Article DOI: 10.1016/j.bmcl.2009.02.085 BindingDB Entry DOI: 10.7270/Q20Z7396 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin G/H synthase 2 (Homo sapiens (Human)) | BDBM50297668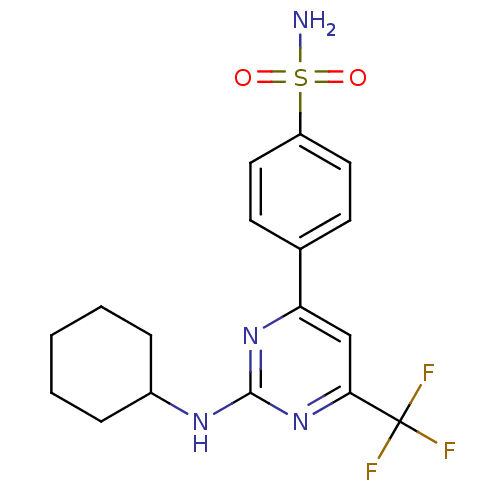 (4-(2-(cyclohexylamino)-6-(trifluoromethyl)pyrimidi...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.40 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Inhibition of human COX2 expressed in baculovirus-infected SF9 cells assessed as inhibition of arachidonic acid-stimulated PGE2 production treated 1 ... | Bioorg Med Chem Lett 19: 4504-8 (2009) Article DOI: 10.1016/j.bmcl.2009.02.085 BindingDB Entry DOI: 10.7270/Q20Z7396 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin G/H synthase 2 (Homo sapiens (Human)) | BDBM50297664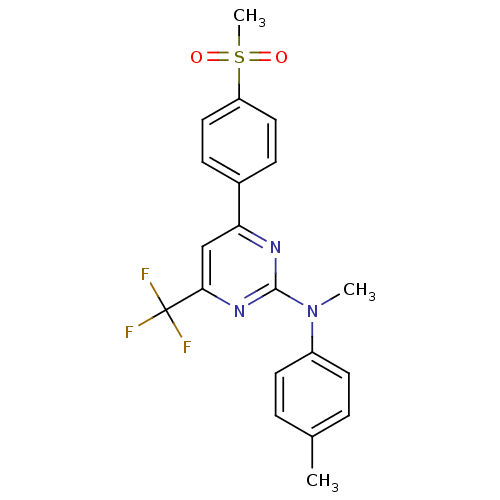 (CHEMBL559613 | N-methyl-4-(4-(methylsulfonyl)pheny...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Inhibition of human COX2 expressed in baculovirus-infected SF9 cells assessed as inhibition of arachidonic acid-stimulated PGE2 production treated 1 ... | Bioorg Med Chem Lett 19: 4504-8 (2009) Article DOI: 10.1016/j.bmcl.2009.02.085 BindingDB Entry DOI: 10.7270/Q20Z7396 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin G/H synthase 2 (Homo sapiens (Human)) | BDBM50297673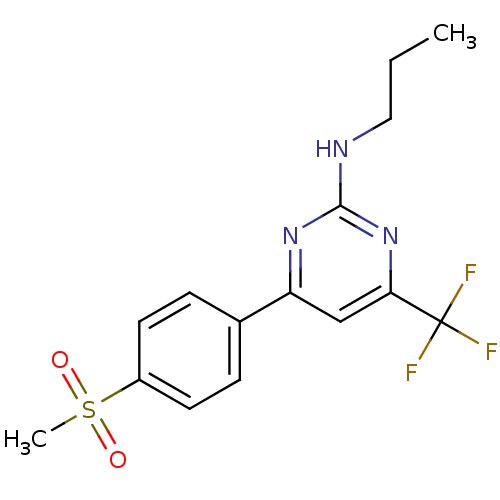 (4-(4-(methylsulfonyl)phenyl)-N-propyl-6-(trifluoro...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Inhibition of human COX2 expressed in baculovirus-infected SF9 cells assessed as inhibition of arachidonic acid-stimulated PGE2 production treated 1 ... | Bioorg Med Chem Lett 19: 4504-8 (2009) Article DOI: 10.1016/j.bmcl.2009.02.085 BindingDB Entry DOI: 10.7270/Q20Z7396 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin G/H synthase 2 (Homo sapiens (Human)) | BDBM50297665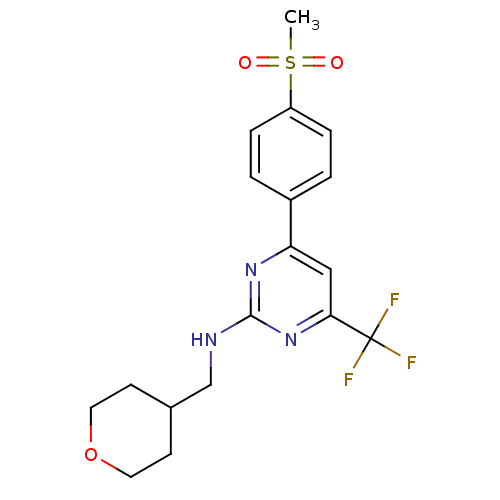 (4-(4-(methylsulfonyl)phenyl)-N-((tetrahydro-2H-pyr...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Inhibition of human COX2 expressed in baculovirus-infected SF9 cells assessed as inhibition of arachidonic acid-stimulated PGE2 production treated 1 ... | Bioorg Med Chem Lett 19: 4504-8 (2009) Article DOI: 10.1016/j.bmcl.2009.02.085 BindingDB Entry DOI: 10.7270/Q20Z7396 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin G/H synthase 2 (Homo sapiens (Human)) | BDBM50297680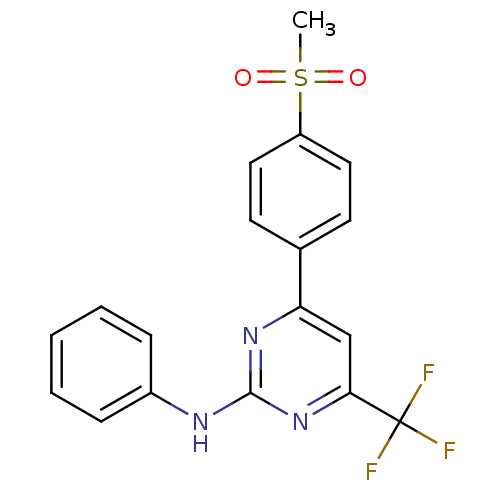 (4-(4-(methylsulfonyl)phenyl)-N-phenyl-6-(trifluoro...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 5.60 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Inhibition of human COX2 expressed in baculovirus-infected SF9 cells assessed as inhibition of arachidonic acid-stimulated PGE2 production treated 1 ... | Bioorg Med Chem Lett 19: 4504-8 (2009) Article DOI: 10.1016/j.bmcl.2009.02.085 BindingDB Entry DOI: 10.7270/Q20Z7396 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin G/H synthase 2 (Homo sapiens (Human)) | BDBM50297678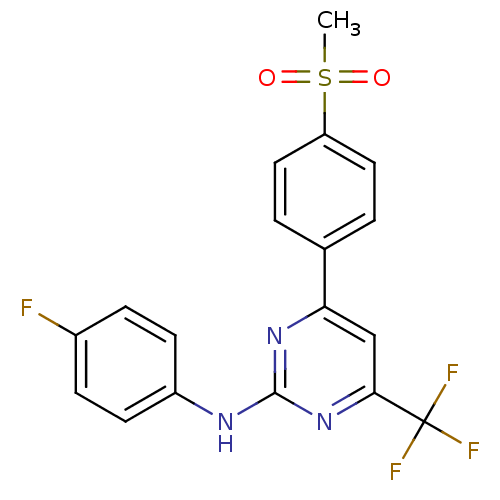 (CHEMBL561086 | N-(4-fluorophenyl)-4-(4-(methylsulf...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 7.20 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Inhibition of human COX2 expressed in baculovirus-infected SF9 cells assessed as inhibition of arachidonic acid-stimulated PGE2 production treated 1 ... | Bioorg Med Chem Lett 19: 4504-8 (2009) Article DOI: 10.1016/j.bmcl.2009.02.085 BindingDB Entry DOI: 10.7270/Q20Z7396 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin G/H synthase 2 (Homo sapiens (Human)) | BDBM50297677 (CHEMBL551829 | N-benzyl-4-(4-(methylsulfonyl)pheny...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 8 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Inhibition of COX2 in human whole blood assessed as inhibition of lipopolysaccharide-stimulated PGE2 production after 24 hrs by enzyme immunoassay | Bioorg Med Chem Lett 19: 4504-8 (2009) Article DOI: 10.1016/j.bmcl.2009.02.085 BindingDB Entry DOI: 10.7270/Q20Z7396 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin G/H synthase 2 (Homo sapiens (Human)) | BDBM50297689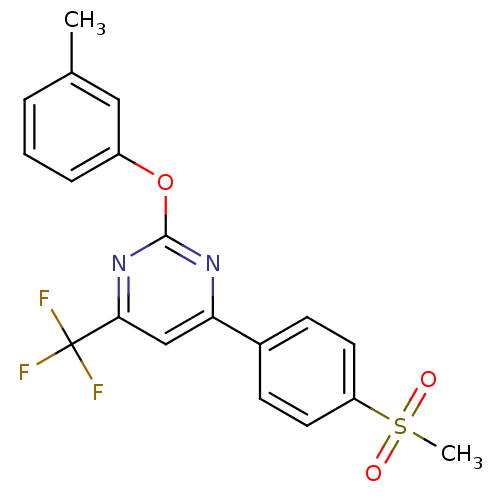 (4-(4-(methylsulfonyl)phenyl)-2-(m-tolyloxy)-6-(tri...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | <10 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Inhibition of human COX2 expressed in baculovirus-infected SF9 cells assessed as inhibition of arachidonic acid-stimulated PGE2 production treated 1 ... | Bioorg Med Chem Lett 19: 4504-8 (2009) Article DOI: 10.1016/j.bmcl.2009.02.085 BindingDB Entry DOI: 10.7270/Q20Z7396 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin G/H synthase 2 (Homo sapiens (Human)) | BDBM50297684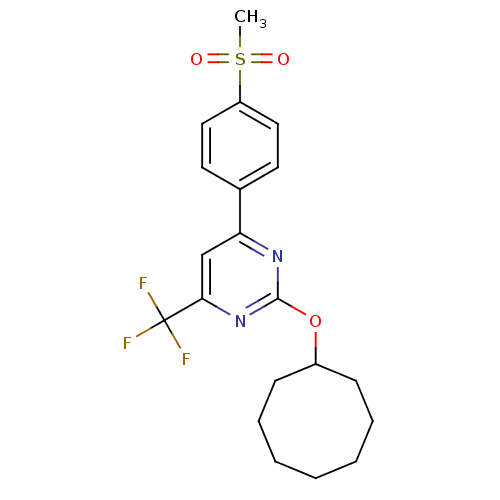 (2-(cyclooctyloxy)-4-(4-(methylsulfonyl)phenyl)-6-(...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | <10 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Inhibition of human COX2 expressed in baculovirus-infected SF9 cells assessed as inhibition of arachidonic acid-stimulated PGE2 production treated 1 ... | Bioorg Med Chem Lett 19: 4504-8 (2009) Article DOI: 10.1016/j.bmcl.2009.02.085 BindingDB Entry DOI: 10.7270/Q20Z7396 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin G/H synthase 2 (Homo sapiens (Human)) | BDBM50297676 (CHEMBL551830 | N-(4-methylbenzyl)-4-(4-(methylsulf...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | <10 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Inhibition of human COX2 expressed in african green monkey COS cells assessed as inhibition of arachidonic acid-stimulated PGE2 production treated 1 ... | Bioorg Med Chem Lett 19: 4504-8 (2009) Article DOI: 10.1016/j.bmcl.2009.02.085 BindingDB Entry DOI: 10.7270/Q20Z7396 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin G/H synthase 2 (Homo sapiens (Human)) | BDBM50297686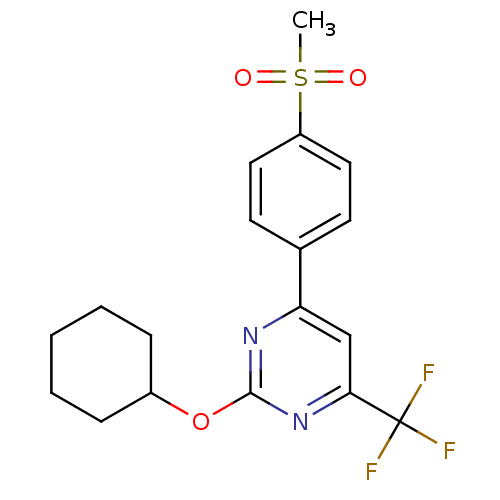 (2-(cyclohexyloxy)-4-(4-(methylsulfonyl)phenyl)-6-(...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | <10 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Inhibition of human COX2 expressed in baculovirus-infected SF9 cells assessed as inhibition of arachidonic acid-stimulated PGE2 production treated 1 ... | Bioorg Med Chem Lett 19: 4504-8 (2009) Article DOI: 10.1016/j.bmcl.2009.02.085 BindingDB Entry DOI: 10.7270/Q20Z7396 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin G/H synthase 2 (Homo sapiens (Human)) | BDBM50297690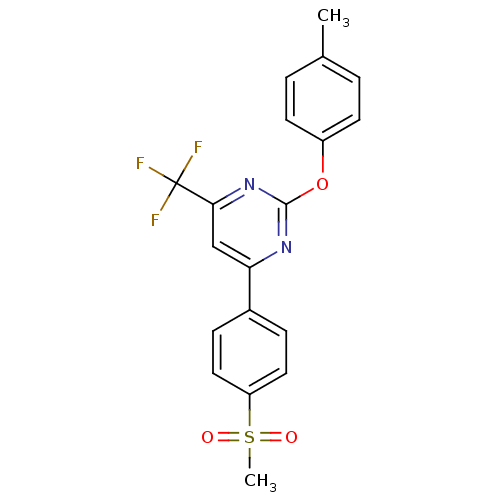 (4-(4-(methylsulfonyl)phenyl)-2-(p-tolyloxy)-6-(tri...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | <10 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Inhibition of human COX2 expressed in baculovirus-infected SF9 cells assessed as inhibition of arachidonic acid-stimulated PGE2 production treated 1 ... | Bioorg Med Chem Lett 19: 4504-8 (2009) Article DOI: 10.1016/j.bmcl.2009.02.085 BindingDB Entry DOI: 10.7270/Q20Z7396 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin G/H synthase 2 (Homo sapiens (Human)) | BDBM50297688 (2-cyclobutoxy-4-(4-(methylsulfonyl)phenyl)-6-(trif...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | <10 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Inhibition of human COX2 expressed in baculovirus-infected SF9 cells assessed as inhibition of arachidonic acid-stimulated PGE2 production treated 1 ... | Bioorg Med Chem Lett 19: 4504-8 (2009) Article DOI: 10.1016/j.bmcl.2009.02.085 BindingDB Entry DOI: 10.7270/Q20Z7396 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin G/H synthase 2 (Homo sapiens (Human)) | BDBM50297685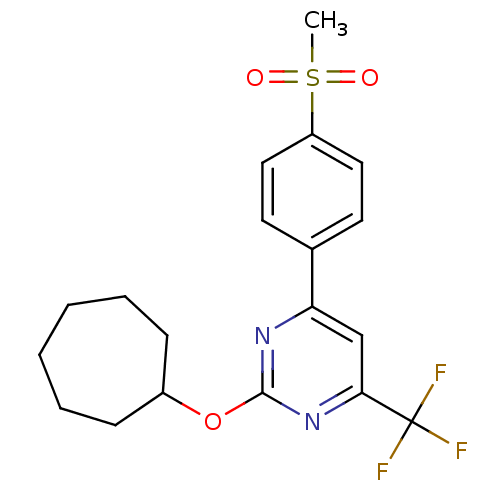 (2-(cycloheptyloxy)-4-(4-(methylsulfonyl)phenyl)-6-...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | <10 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Inhibition of human COX2 expressed in baculovirus-infected SF9 cells assessed as inhibition of arachidonic acid-stimulated PGE2 production treated 1 ... | Bioorg Med Chem Lett 19: 4504-8 (2009) Article DOI: 10.1016/j.bmcl.2009.02.085 BindingDB Entry DOI: 10.7270/Q20Z7396 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin G/H synthase 2 (Homo sapiens (Human)) | BDBM50297687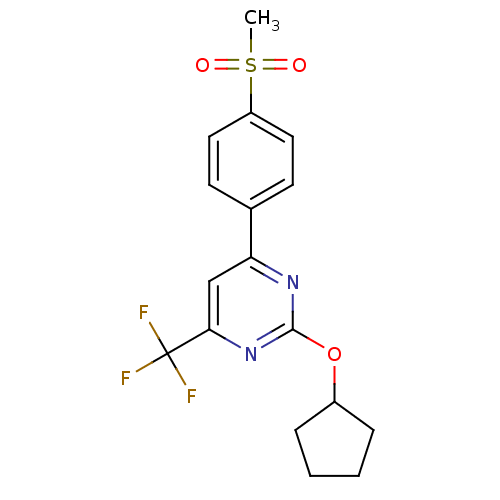 (2-(cyclopentyloxy)-4-(4-(methylsulfonyl)phenyl)-6-...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | <10 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Inhibition of human COX2 expressed in baculovirus-infected SF9 cells assessed as inhibition of arachidonic acid-stimulated PGE2 production treated 1 ... | Bioorg Med Chem Lett 19: 4504-8 (2009) Article DOI: 10.1016/j.bmcl.2009.02.085 BindingDB Entry DOI: 10.7270/Q20Z7396 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin G/H synthase 2 (Homo sapiens (Human)) | BDBM50297679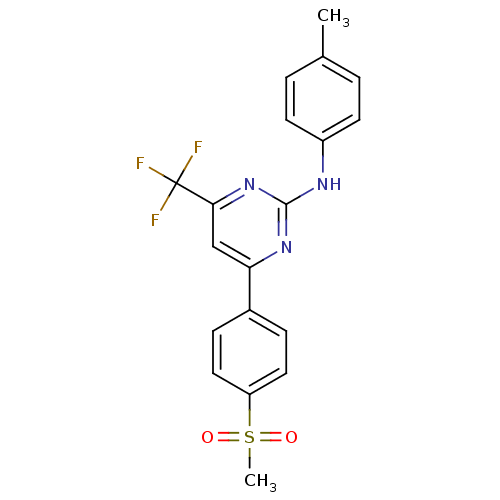 (4-(4-(methylsulfonyl)phenyl)-N-p-tolyl-6-(trifluor...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 11 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Inhibition of human COX2 expressed in baculovirus-infected SF9 cells assessed as inhibition of arachidonic acid-stimulated PGE2 production treated 1 ... | Bioorg Med Chem Lett 19: 4504-8 (2009) Article DOI: 10.1016/j.bmcl.2009.02.085 BindingDB Entry DOI: 10.7270/Q20Z7396 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin G/H synthase 2 (Homo sapiens (Human)) | BDBM50297670 (CHEMBL539663 | GW-637185X | N-sec-butyl-4-(4-(meth...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 12 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Inhibition of human COX2 expressed in baculovirus-infected SF9 cells assessed as inhibition of arachidonic acid-stimulated PGE2 production treated 1 ... | Bioorg Med Chem Lett 19: 4504-8 (2009) Article DOI: 10.1016/j.bmcl.2009.02.085 BindingDB Entry DOI: 10.7270/Q20Z7396 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin G/H synthase 2 (Homo sapiens (Human)) | BDBM50297661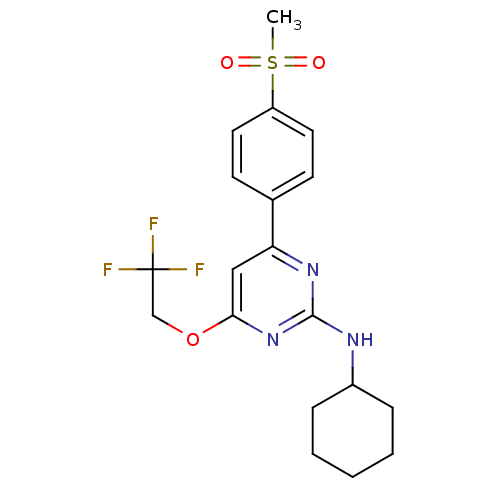 (CHEMBL560284 | N-cyclohexyl-4-(4-(methylsulfonyl)p...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 16 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Inhibition of human COX2 expressed in baculovirus-infected SF9 cells assessed as inhibition of arachidonic acid-stimulated PGE2 production treated 1 ... | Bioorg Med Chem Lett 19: 4504-8 (2009) Article DOI: 10.1016/j.bmcl.2009.02.085 BindingDB Entry DOI: 10.7270/Q20Z7396 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin G/H synthase 2 (Homo sapiens (Human)) | BDBM50297666 (4-(4-(methylsulfonyl)phenyl)-N-(tetrahydro-2H-pyra...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 18 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Inhibition of human COX2 expressed in baculovirus-infected SF9 cells assessed as inhibition of arachidonic acid-stimulated PGE2 production treated 1 ... | Bioorg Med Chem Lett 19: 4504-8 (2009) Article DOI: 10.1016/j.bmcl.2009.02.085 BindingDB Entry DOI: 10.7270/Q20Z7396 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin G/H synthase 2 (Homo sapiens (Human)) | BDBM50297681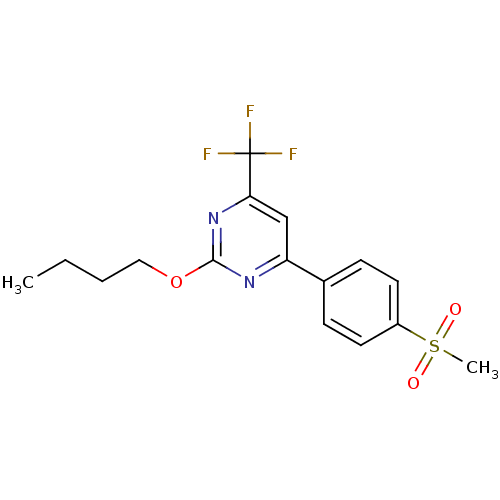 (2-butoxy-4-(4-(methylsulfonyl)phenyl)-6-(trifluoro...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 22 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Inhibition of human COX2 expressed in baculovirus-infected SF9 cells assessed as inhibition of arachidonic acid-stimulated PGE2 production treated 1 ... | Bioorg Med Chem Lett 19: 4504-8 (2009) Article DOI: 10.1016/j.bmcl.2009.02.085 BindingDB Entry DOI: 10.7270/Q20Z7396 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin G/H synthase 2 (Homo sapiens (Human)) | BDBM50297683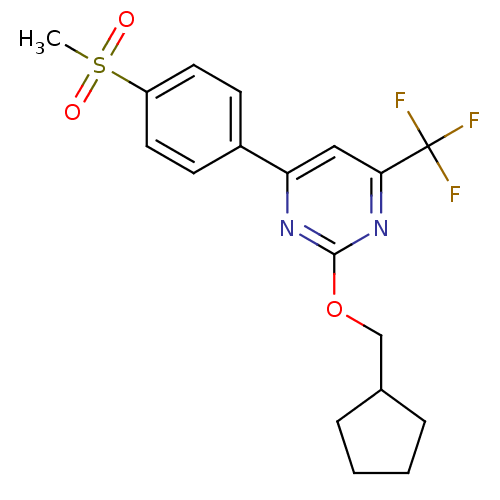 (2-(cyclopentylmethoxy)-4-(4-(methylsulfonyl)phenyl...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 22 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Inhibition of human COX2 expressed in baculovirus-infected SF9 cells assessed as inhibition of arachidonic acid-stimulated PGE2 production treated 1 ... | Bioorg Med Chem Lett 19: 4504-8 (2009) Article DOI: 10.1016/j.bmcl.2009.02.085 BindingDB Entry DOI: 10.7270/Q20Z7396 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin G/H synthase 2 (Homo sapiens (Human)) | BDBM50297667 (CHEMBL550473 | N-(cyclohexylmethyl)-4-(4-(methylsu...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 29 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Inhibition of human COX2 expressed in baculovirus-infected SF9 cells assessed as inhibition of arachidonic acid-stimulated PGE2 production treated 1 ... | Bioorg Med Chem Lett 19: 4504-8 (2009) Article DOI: 10.1016/j.bmcl.2009.02.085 BindingDB Entry DOI: 10.7270/Q20Z7396 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin G/H synthase 2 (Homo sapiens (Human)) | BDBM22369 (4-(4-methanesulfonylphenyl)-3-phenyl-2,5-dihydrofu...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | n/a | n/a | 32 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Inhibition of human COX2 expressed in african green monkey COS cells assessed as inhibition of arachidonic acid-stimulated PGE2 production treated 1 ... | Bioorg Med Chem Lett 19: 4504-8 (2009) Article DOI: 10.1016/j.bmcl.2009.02.085 BindingDB Entry DOI: 10.7270/Q20Z7396 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Prostaglandin G/H synthase 2 (Homo sapiens (Human)) | BDBM50297691 (2-tert-butoxy-4-(4-(methylsulfonyl)phenyl)-6-(trif...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 33 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Inhibition of human COX2 expressed in baculovirus-infected SF9 cells assessed as inhibition of arachidonic acid-stimulated PGE2 production treated 1 ... | Bioorg Med Chem Lett 19: 4504-8 (2009) Article DOI: 10.1016/j.bmcl.2009.02.085 BindingDB Entry DOI: 10.7270/Q20Z7396 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin G/H synthase 2 (Homo sapiens (Human)) | BDBM50297682 (2-(cyclohexylmethoxy)-4-(4-(methylsulfonyl)phenyl)...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 45 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Inhibition of human COX2 expressed in baculovirus-infected SF9 cells assessed as inhibition of arachidonic acid-stimulated PGE2 production treated 1 ... | Bioorg Med Chem Lett 19: 4504-8 (2009) Article DOI: 10.1016/j.bmcl.2009.02.085 BindingDB Entry DOI: 10.7270/Q20Z7396 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin G/H synthase 2 (Homo sapiens (Human)) | BDBM50297669 (CHEMBL561891 | N-cyclohexyl-4-(4-(methylsulfonyl)p...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 62 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Inhibition of COX2 in human whole blood assessed as inhibition of lipopolysaccharide-stimulated PGE2 production after 24 hrs by enzyme immunoassay | Bioorg Med Chem Lett 19: 4504-8 (2009) Article DOI: 10.1016/j.bmcl.2009.02.085 BindingDB Entry DOI: 10.7270/Q20Z7396 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin G/H synthase 2 (Homo sapiens (Human)) | BDBM11639 (4-[5-(4-methylphenyl)-3-(trifluoromethyl)-1H-pyraz...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | DrugBank PDB Article PubMed | n/a | n/a | 63 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Inhibition of human COX2 expressed in baculovirus-infected SF9 cells assessed as inhibition of arachidonic acid-stimulated PGE2 production treated 1 ... | Bioorg Med Chem Lett 19: 4504-8 (2009) Article DOI: 10.1016/j.bmcl.2009.02.085 BindingDB Entry DOI: 10.7270/Q20Z7396 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Prostaglandin G/H synthase 2 (Homo sapiens (Human)) | BDBM50297692 (2-isopropoxy-4-(4-(methylsulfonyl)phenyl)-6-(trifl...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 63 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Inhibition of human COX2 expressed in baculovirus-infected SF9 cells assessed as inhibition of arachidonic acid-stimulated PGE2 production treated 1 ... | Bioorg Med Chem Lett 19: 4504-8 (2009) Article DOI: 10.1016/j.bmcl.2009.02.085 BindingDB Entry DOI: 10.7270/Q20Z7396 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin G/H synthase 2 (Homo sapiens (Human)) | BDBM11639 (4-[5-(4-methylphenyl)-3-(trifluoromethyl)-1H-pyraz...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | DrugBank PDB Article PubMed | n/a | n/a | 68 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Inhibition of human COX2 expressed in african green monkey COS cells assessed as inhibition of arachidonic acid-stimulated PGE2 production treated 1 ... | Bioorg Med Chem Lett 19: 4504-8 (2009) Article DOI: 10.1016/j.bmcl.2009.02.085 BindingDB Entry DOI: 10.7270/Q20Z7396 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Prostaglandin G/H synthase 2 (Homo sapiens (Human)) | BDBM50297672 (CHEMBL549393 | N-butyl-4-(4-(methylsulfonyl)phenyl...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 70 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Inhibition of COX2 in human whole blood assessed as inhibition of lipopolysaccharide-stimulated PGE2 production after 24 hrs by enzyme immunoassay | Bioorg Med Chem Lett 19: 4504-8 (2009) Article DOI: 10.1016/j.bmcl.2009.02.085 BindingDB Entry DOI: 10.7270/Q20Z7396 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin G/H synthase 2 (Homo sapiens (Human)) | BDBM22369 (4-(4-methanesulfonylphenyl)-3-phenyl-2,5-dihydrofu...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | n/a | n/a | 107 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Inhibition of human COX2 expressed in baculovirus-infected SF9 cells assessed as inhibition of arachidonic acid-stimulated PGE2 production treated 1 ... | Bioorg Med Chem Lett 19: 4504-8 (2009) Article DOI: 10.1016/j.bmcl.2009.02.085 BindingDB Entry DOI: 10.7270/Q20Z7396 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Prostaglandin G/H synthase 2 (Homo sapiens (Human)) | BDBM50297681 (2-butoxy-4-(4-(methylsulfonyl)phenyl)-6-(trifluoro...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 158 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Inhibition of COX2 in human whole blood assessed as inhibition of lipopolysaccharide-stimulated PGE2 production after 24 hrs by enzyme immunoassay | Bioorg Med Chem Lett 19: 4504-8 (2009) Article DOI: 10.1016/j.bmcl.2009.02.085 BindingDB Entry DOI: 10.7270/Q20Z7396 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin G/H synthase 1 (Homo sapiens (Human)) | BDBM50297668 (4-(2-(cyclohexylamino)-6-(trifluoromethyl)pyrimidi...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 167 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Inhibition of human COX1 expressed in african green monkey COS cells assessed as inhibition of arachidonic acid-stimulated PGE2 treated 1 hr before a... | Bioorg Med Chem Lett 19: 4504-8 (2009) Article DOI: 10.1016/j.bmcl.2009.02.085 BindingDB Entry DOI: 10.7270/Q20Z7396 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin G/H synthase 2 (Homo sapiens (Human)) | BDBM13063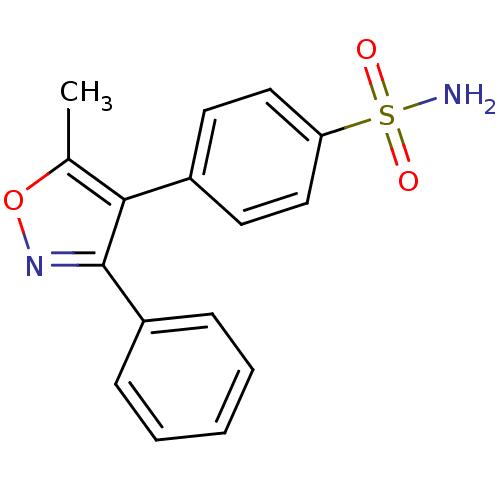 (4-(5-methyl-3-phenyl-1,2-oxazol-4-yl)benzene-1-sul...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 183 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Inhibition of human COX2 expressed in baculovirus-infected SF9 cells assessed as inhibition of arachidonic acid-stimulated PGE2 production treated 1 ... | Bioorg Med Chem Lett 19: 4504-8 (2009) Article DOI: 10.1016/j.bmcl.2009.02.085 BindingDB Entry DOI: 10.7270/Q20Z7396 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 3A4 (Homo sapiens (Human)) | BDBM50254851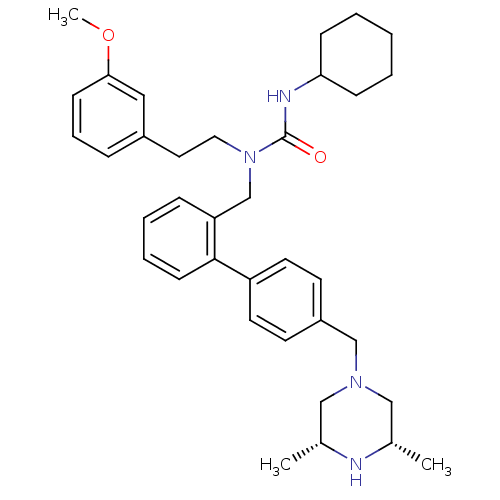 (3-Cyclohexyl-1-[4'-((3R,5S)-3,5-dimethyl-piperazin...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 200 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Inhibition of CYP3A4 (unknown origin) using diethoxyfluorescein substrate | Bioorg Med Chem Lett 18: 6429-36 (2008) Article DOI: 10.1016/j.bmcl.2008.10.072 BindingDB Entry DOI: 10.7270/Q2HQ3ZR3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin G/H synthase 2 (Homo sapiens (Human)) | BDBM50297670 (CHEMBL539663 | GW-637185X | N-sec-butyl-4-(4-(meth...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 206 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Inhibition of COX2 in human whole blood assessed as inhibition of lipopolysaccharide-stimulated PGE2 production after 24 hrs by enzyme immunoassay | Bioorg Med Chem Lett 19: 4504-8 (2009) Article DOI: 10.1016/j.bmcl.2009.02.085 BindingDB Entry DOI: 10.7270/Q20Z7396 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin G/H synthase 2 (Homo sapiens (Human)) | BDBM22369 (4-(4-methanesulfonylphenyl)-3-phenyl-2,5-dihydrofu...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | n/a | n/a | 260 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Inhibition of COX2 in human whole blood assessed as inhibition of lipopolysaccharide-stimulated PGE2 production after 24 hrs by enzyme immunoassay | Bioorg Med Chem Lett 19: 4504-8 (2009) Article DOI: 10.1016/j.bmcl.2009.02.085 BindingDB Entry DOI: 10.7270/Q20Z7396 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Prostaglandin G/H synthase 2 (Homo sapiens (Human)) | BDBM50297674 (4-(4-(methylsulfonyl)phenyl)-6-(trifluoromethyl)py...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 272 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Inhibition of human COX2 expressed in baculovirus-infected SF9 cells assessed as inhibition of arachidonic acid-stimulated PGE2 production treated 1 ... | Bioorg Med Chem Lett 19: 4504-8 (2009) Article DOI: 10.1016/j.bmcl.2009.02.085 BindingDB Entry DOI: 10.7270/Q20Z7396 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin G/H synthase 2 (Homo sapiens (Human)) | BDBM11639 (4-[5-(4-methylphenyl)-3-(trifluoromethyl)-1H-pyraz...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | DrugBank PDB Article PubMed | n/a | n/a | 336 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Inhibition of COX2 in human whole blood assessed as inhibition of lipopolysaccharide-stimulated PGE2 production after 24 hrs by enzyme immunoassay | Bioorg Med Chem Lett 19: 4504-8 (2009) Article DOI: 10.1016/j.bmcl.2009.02.085 BindingDB Entry DOI: 10.7270/Q20Z7396 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Prostaglandin G/H synthase 2 (Homo sapiens (Human)) | BDBM50072064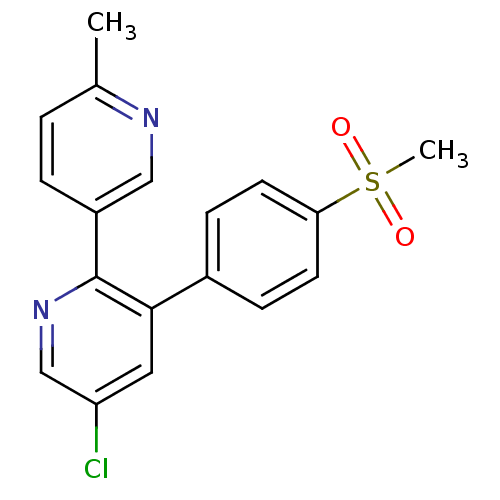 (5-Chloro-3-(4-methanesulfonyl-phenyl)-6''-methyl-[...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | DrugBank Article PubMed | n/a | n/a | 347 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Inhibition of human COX2 expressed in baculovirus-infected SF9 cells assessed as inhibition of arachidonic acid-stimulated PGE2 production treated 1 ... | Bioorg Med Chem Lett 19: 4504-8 (2009) Article DOI: 10.1016/j.bmcl.2009.02.085 BindingDB Entry DOI: 10.7270/Q20Z7396 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 3A4 (Homo sapiens (Human)) | BDBM50254851 (3-Cyclohexyl-1-[4'-((3R,5S)-3,5-dimethyl-piperazin...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 400 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Inhibition of CYP3A4 (unknown origin) using phenylpiperazinylmethylbenzylresofurin substrate | Bioorg Med Chem Lett 18: 6429-36 (2008) Article DOI: 10.1016/j.bmcl.2008.10.072 BindingDB Entry DOI: 10.7270/Q2HQ3ZR3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 3A4 (Homo sapiens (Human)) | BDBM50254968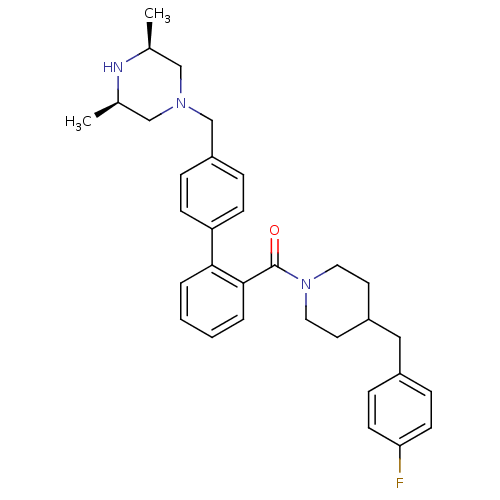 (CHEMBL479418 | [4'-((3R,5S)-3,5-Dimethyl-piperazin...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 500 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Inhibition of CYP3A4 (unknown origin) using phenylpiperazinylmethylbenzylresofurin substrate | Bioorg Med Chem Lett 18: 6429-36 (2008) Article DOI: 10.1016/j.bmcl.2008.10.072 BindingDB Entry DOI: 10.7270/Q2HQ3ZR3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin G/H synthase 2 (Homo sapiens (Human)) | BDBM50072064 (5-Chloro-3-(4-methanesulfonyl-phenyl)-6''-methyl-[...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | DrugBank Article PubMed | n/a | n/a | 554 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Inhibition of COX2 in human whole blood assessed as inhibition of lipopolysaccharide-stimulated PGE2 production after 24 hrs by enzyme immunoassay | Bioorg Med Chem Lett 19: 4504-8 (2009) Article DOI: 10.1016/j.bmcl.2009.02.085 BindingDB Entry DOI: 10.7270/Q20Z7396 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 3A4 (Homo sapiens (Human)) | BDBM50254968 (CHEMBL479418 | [4'-((3R,5S)-3,5-Dimethyl-piperazin...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 600 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Inhibition of CYP3A4 (unknown origin) using diethoxyfluorescein substrate | Bioorg Med Chem Lett 18: 6429-36 (2008) Article DOI: 10.1016/j.bmcl.2008.10.072 BindingDB Entry DOI: 10.7270/Q2HQ3ZR3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 190 total ) | Next | Last >> |