

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| N-lysine methyltransferase SMYD2 (Homo sapiens (Human)) | BDBM50180955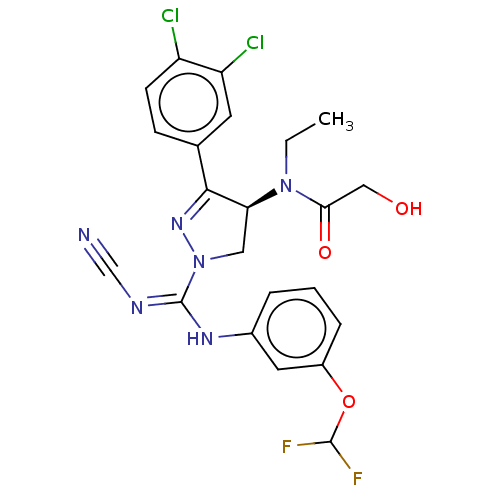 (CHEMBL3818617) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 8 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
BAYER Pharma AG Curated by ChEMBL | Assay Description Competitive inhibition of full length 6xHis-tagged SMYD2 (unknown origin) expressed in Escherichia coli using varying levels of Btn-Ahx-GSRAHSSHLKSKK... | J Med Chem 59: 4578-600 (2016) Article DOI: 10.1021/acs.jmedchem.5b01890 BindingDB Entry DOI: 10.7270/Q2RN39S5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| N-lysine methyltransferase SMYD2 (Homo sapiens (Human)) | BDBM50180955 (CHEMBL3818617) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 28 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
BAYER Pharma AG Curated by ChEMBL | Assay Description Uncompetitive inhibition of full length 6xHis-tagged SMYD2 (unknown origin) expressed in Escherichia coli using fixed levels of Btn-Ahx-GSRAHSSHLKSKK... | J Med Chem 59: 4578-600 (2016) Article DOI: 10.1021/acs.jmedchem.5b01890 BindingDB Entry DOI: 10.7270/Q2RN39S5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| N-lysine methyltransferase SMYD2 (Homo sapiens (Human)) | BDBM283143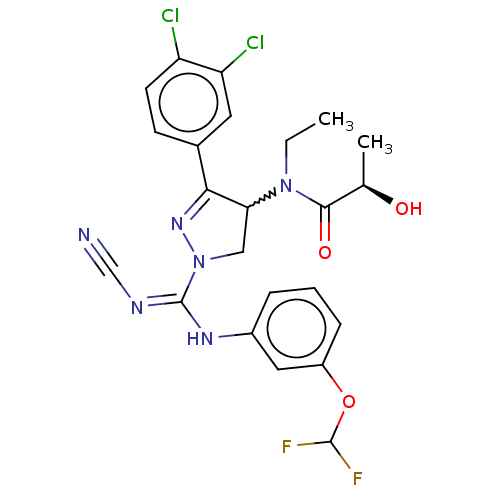 ((2R)—N-[1-{N′-cyano-N-[3-(difluorometho...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 2.64 | n/a | n/a | n/a | n/a | n/a | n/a |
Bayer Pharma Aktiengesellschaft US Patent | Assay Description For the detection of SMYD2 cellular methylation activity an In Cell Western (ICW) assay was established. This assay allows rapid processing of multip... | US Patent US10023539 (2018) BindingDB Entry DOI: 10.7270/Q2MG7RKD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| N-lysine methyltransferase SMYD2 (Homo sapiens (Human)) | BDBM283194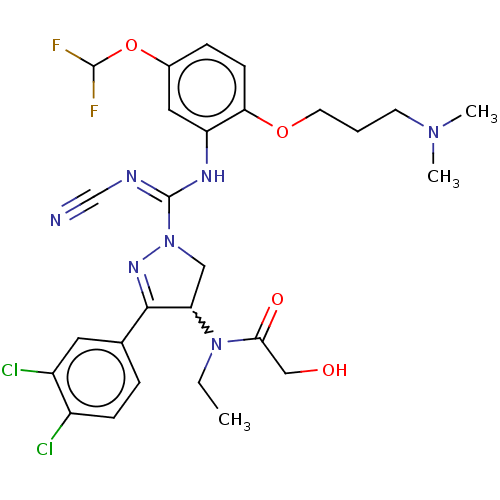 (Rac-N-[1-(N′-cyano-N-{5-(difluoromethoxy)-2-...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 5.55 | n/a | n/a | n/a | n/a | 9.0 | n/a |
Bayer Pharma Aktiengesellschaft US Patent | Assay Description SMYD2 inhibitory activities of the compounds described in the present invention were quantified using a scintillation proximity assay (SPA) which mea... | US Patent US10023539 (2018) BindingDB Entry DOI: 10.7270/Q2MG7RKD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| N-lysine methyltransferase SMYD2 (Homo sapiens (Human)) | BDBM283173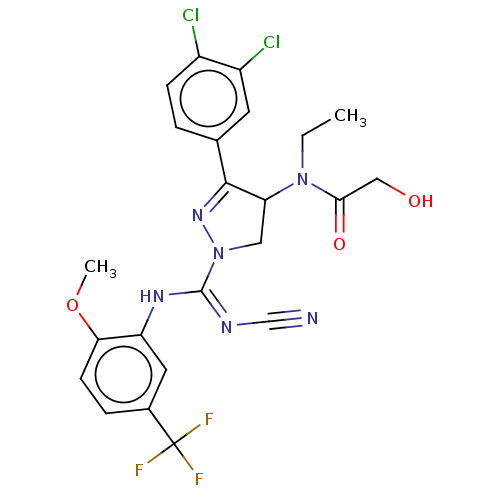 (N-[1-{N′-cyano-N-[2-methoxy-5-(trifluorometh...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 6.65 | n/a | n/a | n/a | n/a | 9.0 | n/a |
Bayer Pharma Aktiengesellschaft US Patent | Assay Description SMYD2 inhibitory activities of the compounds described in the present invention were quantified using a scintillation proximity assay (SPA) which mea... | US Patent US10023539 (2018) BindingDB Entry DOI: 10.7270/Q2MG7RKD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| N-lysine methyltransferase SMYD2 (Homo sapiens (Human)) | BDBM283196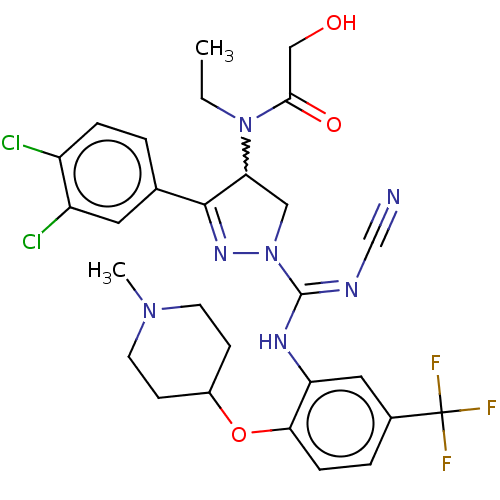 (Rac-N-[1-(N′-cyano-N-{2-[(1-methylpiperidin-...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 9.09 | n/a | n/a | n/a | n/a | 9.0 | n/a |
Bayer Pharma Aktiengesellschaft US Patent | Assay Description SMYD2 inhibitory activities of the compounds described in the present invention were quantified using a scintillation proximity assay (SPA) which mea... | US Patent US10023539 (2018) BindingDB Entry DOI: 10.7270/Q2MG7RKD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| N-lysine methyltransferase SMYD2 (Homo sapiens (Human)) | BDBM283172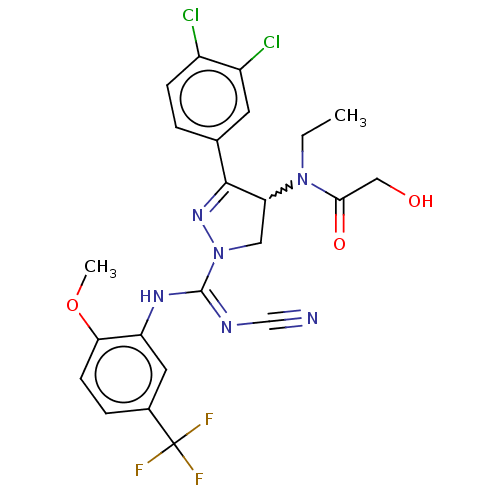 (Rac-N-[1-{N′-cyano-N-[2-methoxy-5-(trifluoro...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 9.98 | n/a | n/a | n/a | n/a | 9.0 | n/a |
Bayer Pharma Aktiengesellschaft US Patent | Assay Description SMYD2 inhibitory activities of the compounds described in the present invention were quantified using a scintillation proximity assay (SPA) which mea... | US Patent US10023539 (2018) BindingDB Entry DOI: 10.7270/Q2MG7RKD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Proteinase-activated receptor 1 (Homo sapiens (Human)) | BDBM50181506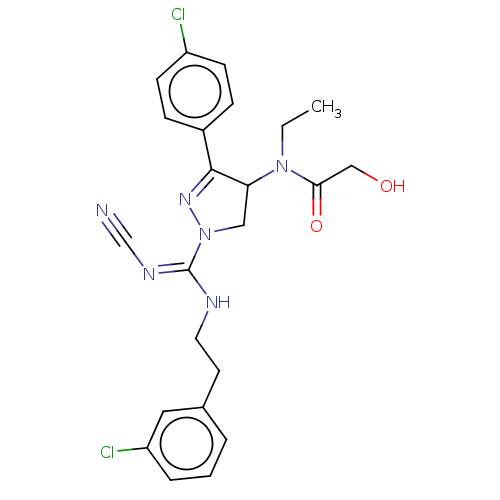 (CHEMBL3819038) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
BAYER Pharma AG Curated by ChEMBL | Assay Description Antagonist activity at PAR1 (unknown origin) | J Med Chem 59: 4578-600 (2016) Article DOI: 10.1021/acs.jmedchem.5b01890 BindingDB Entry DOI: 10.7270/Q2RN39S5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| N-lysine methyltransferase SMYD2 (Homo sapiens (Human)) | BDBM283169 (Rac-N-[1-{N′-cyano-N-[5-(difluoromethoxy)-2-...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 12.5 | n/a | n/a | n/a | n/a | 9.0 | n/a |
Bayer Pharma Aktiengesellschaft US Patent | Assay Description SMYD2 inhibitory activities of the compounds described in the present invention were quantified using a scintillation proximity assay (SPA) which mea... | US Patent US10023539 (2018) BindingDB Entry DOI: 10.7270/Q2MG7RKD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| N-lysine methyltransferase SMYD2 (Homo sapiens (Human)) | BDBM283143 ((2R)—N-[1-{N′-cyano-N-[3-(difluorometho...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 13 | n/a | n/a | n/a | n/a | 9.0 | n/a |
Bayer Pharma Aktiengesellschaft US Patent | Assay Description SMYD2 inhibitory activities of the compounds described in the present invention were quantified using a scintillation proximity assay (SPA) which mea... | US Patent US10023539 (2018) BindingDB Entry DOI: 10.7270/Q2MG7RKD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| N-lysine methyltransferase SMYD2 (Homo sapiens (Human)) | BDBM283139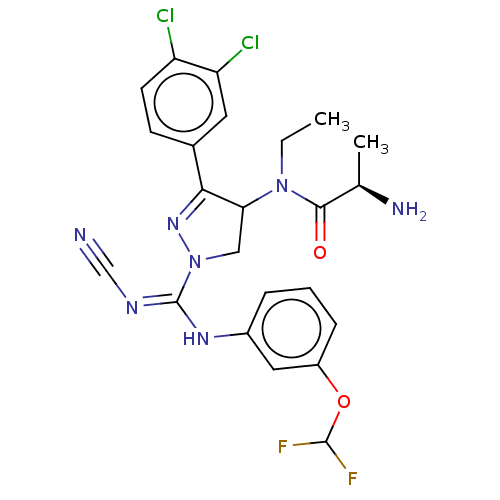 (N-[1-{N′-cyano-N-[3-(difluoromethoxy)phenyl]...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 13.2 | n/a | n/a | n/a | n/a | n/a | n/a |
Bayer Pharma Aktiengesellschaft US Patent | Assay Description For the detection of SMYD2 cellular methylation activity an In Cell Western (ICW) assay was established. This assay allows rapid processing of multip... | US Patent US10023539 (2018) BindingDB Entry DOI: 10.7270/Q2MG7RKD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| N-lysine methyltransferase SMYD2 (Homo sapiens (Human)) | BDBM283139 (N-[1-{N′-cyano-N-[3-(difluoromethoxy)phenyl]...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 13.5 | n/a | n/a | n/a | n/a | 9.0 | n/a |
Bayer Pharma Aktiengesellschaft US Patent | Assay Description SMYD2 inhibitory activities of the compounds described in the present invention were quantified using a scintillation proximity assay (SPA) which mea... | US Patent US10023539 (2018) BindingDB Entry DOI: 10.7270/Q2MG7RKD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| N-lysine methyltransferase SMYD2 (Homo sapiens (Human)) | BDBM50075102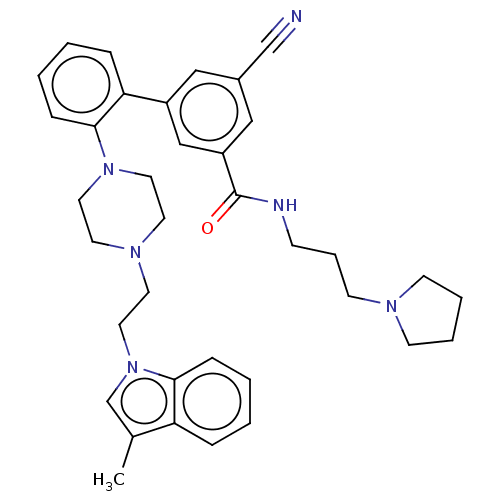 (CHEMBL3414623) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid PDB UniChem Similars | PDB Article PubMed | n/a | n/a | <15 | n/a | n/a | n/a | n/a | n/a | n/a |
BAYER Pharma AG Curated by ChEMBL | Assay Description Inhibition of SMYD2 (unknown origin) expressed in Escherichia coli BL21 (DE3) using Biotinaminohexanoyl-GSRAHSSHLKSKKGQSTSRH as substrate after 75 mi... | J Med Chem 59: 4578-600 (2016) Article DOI: 10.1021/acs.jmedchem.5b01890 BindingDB Entry DOI: 10.7270/Q2RN39S5 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| N-lysine methyltransferase SMYD2 (Homo sapiens (Human)) | BDBM283132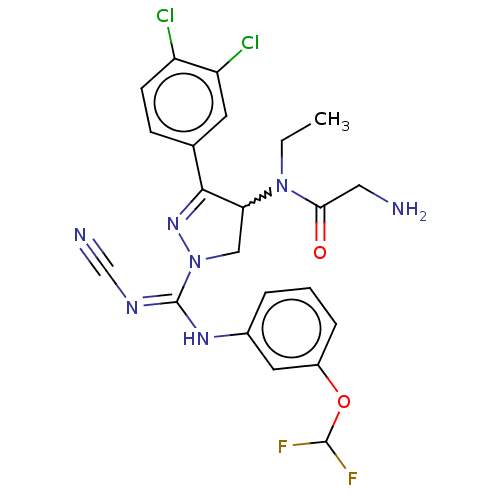 (Rac-N-[1-{N′-cyano-N-[3-(difluoromethoxy)phe...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 15 | n/a | n/a | n/a | n/a | 9.0 | n/a |
Bayer Pharma Aktiengesellschaft US Patent | Assay Description SMYD2 inhibitory activities of the compounds described in the present invention were quantified using a scintillation proximity assay (SPA) which mea... | US Patent US10023539 (2018) BindingDB Entry DOI: 10.7270/Q2MG7RKD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| N-lysine methyltransferase SMYD2 (Homo sapiens (Human)) | BDBM283195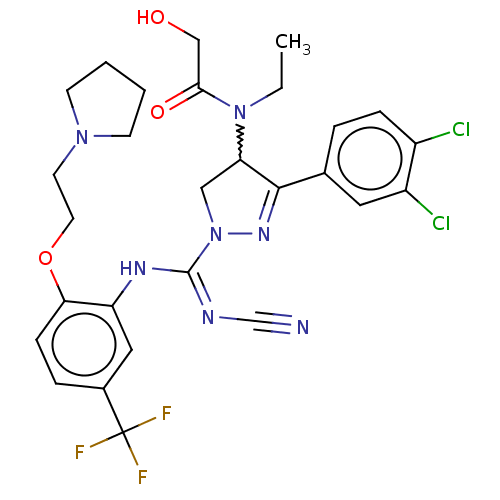 (Rac-N-[r-(N′-cyano-N-[2-[2-(pyrrolidin-1-yl)...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 15.8 | n/a | n/a | n/a | n/a | 9.0 | n/a |
Bayer Pharma Aktiengesellschaft US Patent | Assay Description SMYD2 inhibitory activities of the compounds described in the present invention were quantified using a scintillation proximity assay (SPA) which mea... | US Patent US10023539 (2018) BindingDB Entry DOI: 10.7270/Q2MG7RKD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| N-lysine methyltransferase SMYD2 (Homo sapiens (Human)) | BDBM50180967 (CHEMBL3818487) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 19 | n/a | n/a | n/a | n/a | n/a | n/a |
BAYER Pharma AG Curated by ChEMBL | Assay Description Inhibition of SMYD2 (unknown origin) using H4 as substrate after 2hrs in presence 3H-SAM of by scintillation proximity assay | J Med Chem 59: 4578-600 (2016) Article DOI: 10.1021/acs.jmedchem.5b01890 BindingDB Entry DOI: 10.7270/Q2RN39S5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| N-lysine methyltransferase SMYD2 (Homo sapiens (Human)) | BDBM283184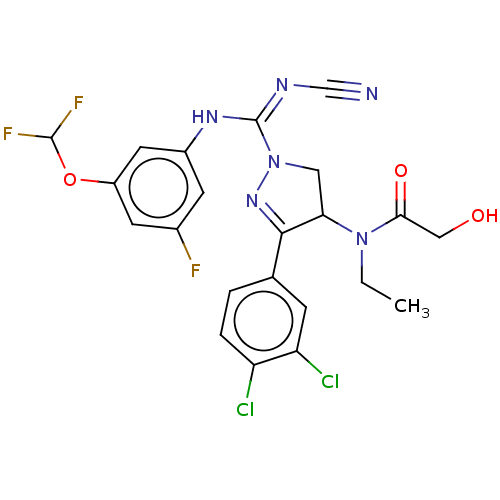 (N-[1-{N′-cyano-N-[3-(difluoromethoxy)-5-fluo...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 19.2 | n/a | n/a | n/a | n/a | 9.0 | n/a |
Bayer Pharma Aktiengesellschaft US Patent | Assay Description SMYD2 inhibitory activities of the compounds described in the present invention were quantified using a scintillation proximity assay (SPA) which mea... | US Patent US10023539 (2018) BindingDB Entry DOI: 10.7270/Q2MG7RKD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| N-lysine methyltransferase SMYD2 (Homo sapiens (Human)) | BDBM283172 (Rac-N-[1-{N′-cyano-N-[2-methoxy-5-(trifluoro...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 21.3 | n/a | n/a | n/a | n/a | n/a | n/a |
Bayer Pharma Aktiengesellschaft US Patent | Assay Description For the detection of SMYD2 cellular methylation activity an In Cell Western (ICW) assay was established. This assay allows rapid processing of multip... | US Patent US10023539 (2018) BindingDB Entry DOI: 10.7270/Q2MG7RKD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| N-lysine methyltransferase SMYD2 (Homo sapiens (Human)) | BDBM283205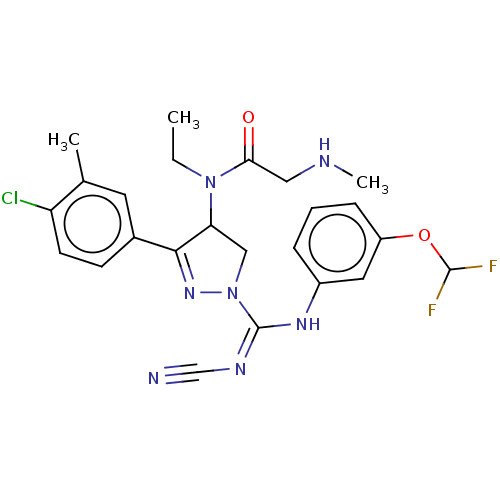 (N-[3-(4-Chloro-3-methylphenyl)-1-{N′-cyano-N...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 22.9 | n/a | n/a | n/a | n/a | 9.0 | n/a |
Bayer Pharma Aktiengesellschaft US Patent | Assay Description SMYD2 inhibitory activities of the compounds described in the present invention were quantified using a scintillation proximity assay (SPA) which mea... | US Patent US10023539 (2018) BindingDB Entry DOI: 10.7270/Q2MG7RKD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| N-lysine methyltransferase SMYD2 (Homo sapiens (Human)) | BDBM283173 (N-[1-{N′-cyano-N-[2-methoxy-5-(trifluorometh...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 23.6 | n/a | n/a | n/a | n/a | n/a | n/a |
Bayer Pharma Aktiengesellschaft US Patent | Assay Description For the detection of SMYD2 cellular methylation activity an In Cell Western (ICW) assay was established. This assay allows rapid processing of multip... | US Patent US10023539 (2018) BindingDB Entry DOI: 10.7270/Q2MG7RKD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| N-lysine methyltransferase SMYD2 (Homo sapiens (Human)) | BDBM283197 (Rac-N-[1-(N′-cyano-N-{2-[(1-methylpiperidin-...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 26.1 | n/a | n/a | n/a | n/a | 9.0 | n/a |
Bayer Pharma Aktiengesellschaft US Patent | Assay Description SMYD2 inhibitory activities of the compounds described in the present invention were quantified using a scintillation proximity assay (SPA) which mea... | US Patent US10023539 (2018) BindingDB Entry DOI: 10.7270/Q2MG7RKD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| N-lysine methyltransferase SMYD2 (Homo sapiens (Human)) | BDBM50180955 (CHEMBL3818617) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 27 | n/a | n/a | n/a | n/a | n/a | n/a |
BAYER Pharma AG Curated by ChEMBL | Assay Description Inhibition of full length 6xHis-tagged SMYD2 (unknown origin) expressed in Escherichia coli using Btn-Ahx GSRAHSSHLKSKKGQSTSRH-amide as substrate aft... | J Med Chem 59: 4578-600 (2016) Article DOI: 10.1021/acs.jmedchem.5b01890 BindingDB Entry DOI: 10.7270/Q2RN39S5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| N-lysine methyltransferase SMYD2 (Homo sapiens (Human)) | BDBM283169 (Rac-N-[1-{N′-cyano-N-[5-(difluoromethoxy)-2-...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 27.7 | n/a | n/a | n/a | n/a | n/a | n/a |
Bayer Pharma Aktiengesellschaft US Patent | Assay Description For the detection of SMYD2 cellular methylation activity an In Cell Western (ICW) assay was established. This assay allows rapid processing of multip... | US Patent US10023539 (2018) BindingDB Entry DOI: 10.7270/Q2MG7RKD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| N-lysine methyltransferase SMYD2 (Homo sapiens (Human)) | BDBM50181508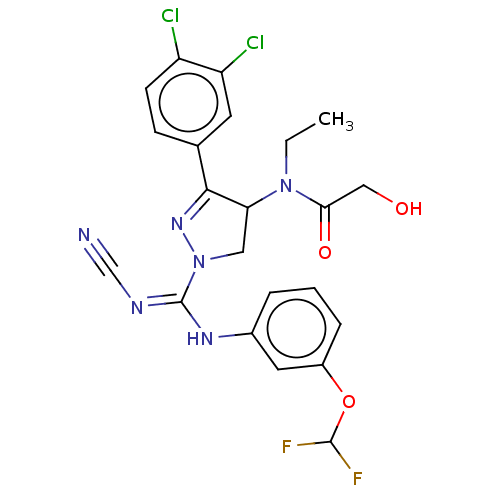 (CHEMBL3818083 | US10023539, Example 4.2) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 28.2 | n/a | n/a | n/a | n/a | 9.0 | n/a |
Bayer Pharma Aktiengesellschaft US Patent | Assay Description SMYD2 inhibitory activities of the compounds described in the present invention were quantified using a scintillation proximity assay (SPA) which mea... | US Patent US10023539 (2018) BindingDB Entry DOI: 10.7270/Q2MG7RKD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| N-lysine methyltransferase SMYD2 (Homo sapiens (Human)) | BDBM50181508 (CHEMBL3818083 | US10023539, Example 4.2) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 28.2 | n/a | n/a | n/a | n/a | 9.0 | n/a |
Bayer Pharma Aktiengesellschaft US Patent | Assay Description SMYD2 inhibitory activities of the compounds described in the present invention were quantified using a scintillation proximity assay (SPA) which mea... | US Patent US10023539 (2018) BindingDB Entry DOI: 10.7270/Q2MG7RKD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| N-lysine methyltransferase SMYD2 (Homo sapiens (Human)) | BDBM283170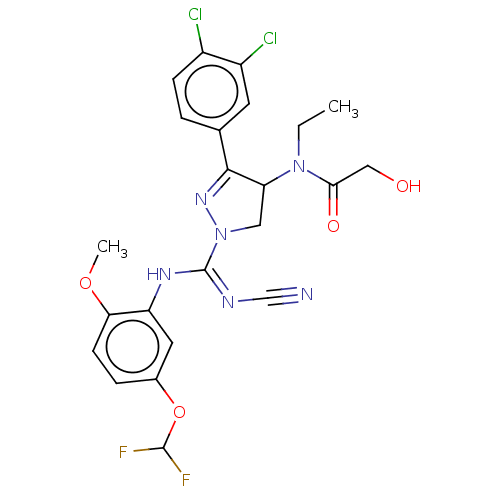 (N-[1-{N′-cyano-N-[5-(difluoromethoxy)-2-meth...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 28.7 | n/a | n/a | n/a | n/a | n/a | n/a |
Bayer Pharma Aktiengesellschaft US Patent | Assay Description For the detection of SMYD2 cellular methylation activity an In Cell Western (ICW) assay was established. This assay allows rapid processing of multip... | US Patent US10023539 (2018) BindingDB Entry DOI: 10.7270/Q2MG7RKD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Proteinase-activated receptor 1 (Homo sapiens (Human)) | BDBM50181504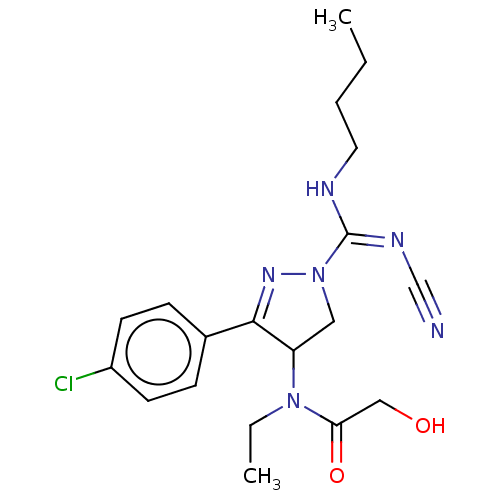 (CHEMBL3818898) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 30 | n/a | n/a | n/a | n/a | n/a | n/a |
BAYER Pharma AG Curated by ChEMBL | Assay Description Antagonist activity at PAR1 (unknown origin) | J Med Chem 59: 4578-600 (2016) Article DOI: 10.1021/acs.jmedchem.5b01890 BindingDB Entry DOI: 10.7270/Q2RN39S5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Proteinase-activated receptor 1 (Homo sapiens (Human)) | BDBM50181502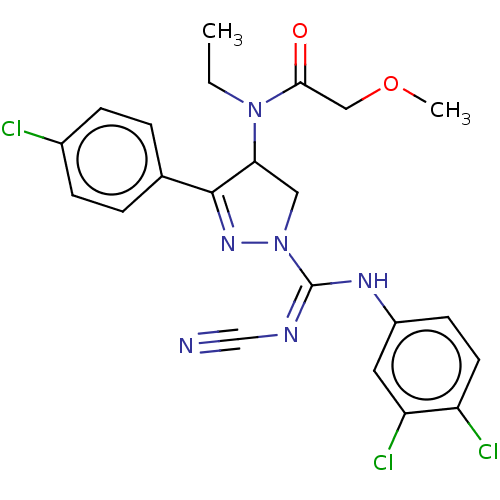 (CHEMBL3818510) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 30 | n/a | n/a | n/a | n/a | n/a | n/a |
BAYER Pharma AG Curated by ChEMBL | Assay Description Antagonist activity at PAR1 (unknown origin) | J Med Chem 59: 4578-600 (2016) Article DOI: 10.1021/acs.jmedchem.5b01890 BindingDB Entry DOI: 10.7270/Q2RN39S5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| N-lysine methyltransferase SMYD2 (Homo sapiens (Human)) | BDBM283170 (N-[1-{N′-cyano-N-[5-(difluoromethoxy)-2-meth...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 30 | n/a | n/a | n/a | n/a | 9.0 | n/a |
Bayer Pharma Aktiengesellschaft US Patent | Assay Description SMYD2 inhibitory activities of the compounds described in the present invention were quantified using a scintillation proximity assay (SPA) which mea... | US Patent US10023539 (2018) BindingDB Entry DOI: 10.7270/Q2MG7RKD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| N-lysine methyltransferase SMYD2 (Homo sapiens (Human)) | BDBM283189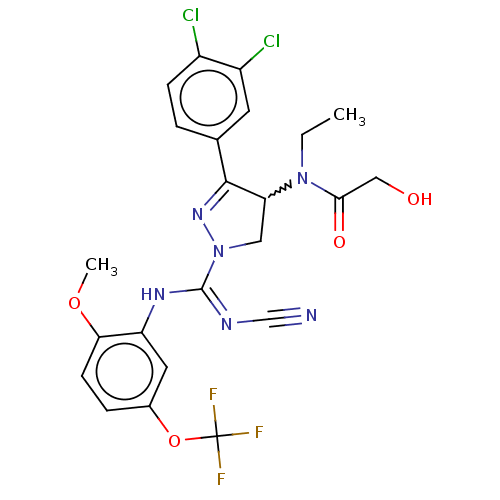 (Rac-N-[1-{N′-cyano-N-[2-methoxy-5-(trifluoro...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 30.6 | n/a | n/a | n/a | n/a | 9.0 | n/a |
Bayer Pharma Aktiengesellschaft US Patent | Assay Description SMYD2 inhibitory activities of the compounds described in the present invention were quantified using a scintillation proximity assay (SPA) which mea... | US Patent US10023539 (2018) BindingDB Entry DOI: 10.7270/Q2MG7RKD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| N-lysine methyltransferase SMYD2 (Homo sapiens (Human)) | BDBM283208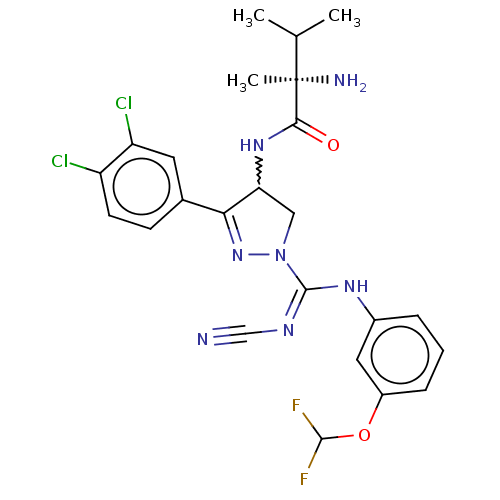 (N-[1-{N′-cyano-N-[3-(difluoromethoxy)phenyl]...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 32 | n/a | n/a | n/a | n/a | 9.0 | n/a |
Bayer Pharma Aktiengesellschaft US Patent | Assay Description SMYD2 inhibitory activities of the compounds described in the present invention were quantified using a scintillation proximity assay (SPA) which mea... | US Patent US10023539 (2018) BindingDB Entry DOI: 10.7270/Q2MG7RKD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| N-lysine methyltransferase SMYD2 (Homo sapiens (Human)) | BDBM283209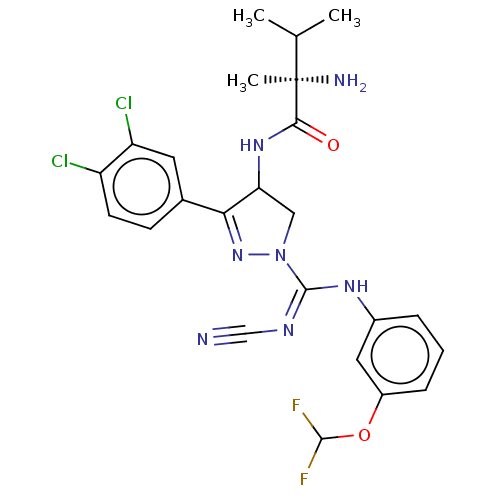 (N-[1-{N′-cyano-N-[3-(difluoromethoxy)phenyl]...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 35.6 | n/a | n/a | n/a | n/a | n/a | n/a |
Bayer Pharma Aktiengesellschaft US Patent | Assay Description For the detection of SMYD2 cellular methylation activity an In Cell Western (ICW) assay was established. This assay allows rapid processing of multip... | US Patent US10023539 (2018) BindingDB Entry DOI: 10.7270/Q2MG7RKD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| N-lysine methyltransferase SMYD2 (Homo sapiens (Human)) | BDBM283208 (N-[1-{N′-cyano-N-[3-(difluoromethoxy)phenyl]...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 37.2 | n/a | n/a | n/a | n/a | n/a | n/a |
Bayer Pharma Aktiengesellschaft US Patent | Assay Description For the detection of SMYD2 cellular methylation activity an In Cell Western (ICW) assay was established. This assay allows rapid processing of multip... | US Patent US10023539 (2018) BindingDB Entry DOI: 10.7270/Q2MG7RKD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| N-lysine methyltransferase SMYD2 (Homo sapiens (Human)) | BDBM283209 (N-[1-{N′-cyano-N-[3-(difluoromethoxy)phenyl]...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 37.5 | n/a | n/a | n/a | n/a | 9.0 | n/a |
Bayer Pharma Aktiengesellschaft US Patent | Assay Description SMYD2 inhibitory activities of the compounds described in the present invention were quantified using a scintillation proximity assay (SPA) which mea... | US Patent US10023539 (2018) BindingDB Entry DOI: 10.7270/Q2MG7RKD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| N-lysine methyltransferase SMYD2 (Homo sapiens (Human)) | BDBM50180974 (CHEMBL3818092 | US10023539, Example 33.2) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 37.6 | n/a | n/a | n/a | n/a | 9.0 | n/a |
Bayer Pharma Aktiengesellschaft US Patent | Assay Description SMYD2 inhibitory activities of the compounds described in the present invention were quantified using a scintillation proximity assay (SPA) which mea... | US Patent US10023539 (2018) BindingDB Entry DOI: 10.7270/Q2MG7RKD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| N-lysine methyltransferase SMYD2 (Homo sapiens (Human)) | BDBM50180973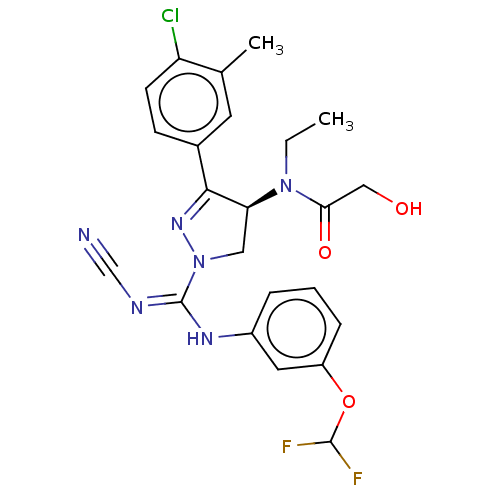 (CHEMBL3819284) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 38 | n/a | n/a | n/a | n/a | n/a | n/a |
BAYER Pharma AG Curated by ChEMBL | Assay Description Inhibition of SMYD2 (unknown origin) using H4 as substrate after 2hrs in presence 3H-SAM of by scintillation proximity assay | J Med Chem 59: 4578-600 (2016) Article DOI: 10.1021/acs.jmedchem.5b01890 BindingDB Entry DOI: 10.7270/Q2RN39S5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Proteinase-activated receptor 1 (Homo sapiens (Human)) | BDBM50181505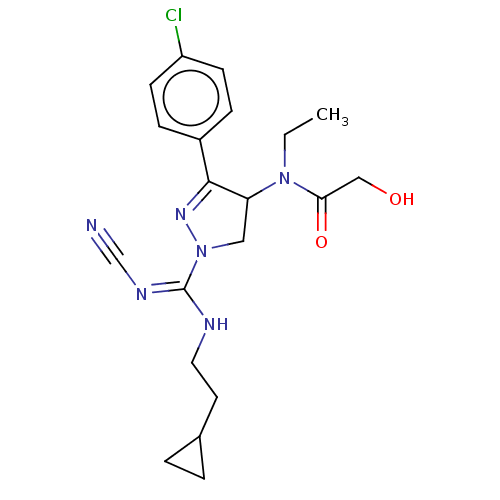 (CHEMBL3819204) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 40 | n/a | n/a | n/a | n/a | n/a | n/a |
BAYER Pharma AG Curated by ChEMBL | Assay Description Antagonist activity at PAR1 (unknown origin) | J Med Chem 59: 4578-600 (2016) Article DOI: 10.1021/acs.jmedchem.5b01890 BindingDB Entry DOI: 10.7270/Q2RN39S5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| N-lysine methyltransferase SMYD2 (Homo sapiens (Human)) | BDBM283204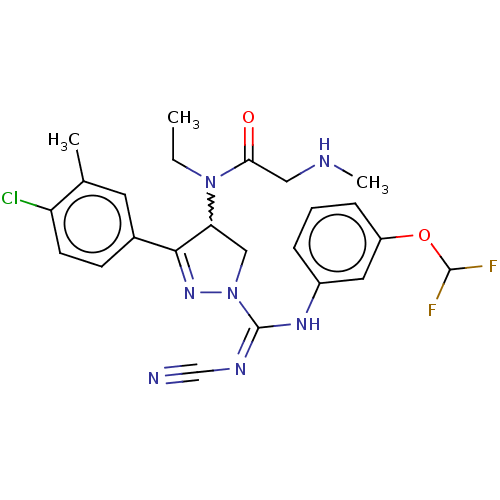 (US10023539, Example 36 | rac-N-[3-(4-Chloro-3-meth...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 40.7 | n/a | n/a | n/a | n/a | 9.0 | n/a |
Bayer Pharma Aktiengesellschaft US Patent | Assay Description SMYD2 inhibitory activities of the compounds described in the present invention were quantified using a scintillation proximity assay (SPA) which mea... | US Patent US10023539 (2018) BindingDB Entry DOI: 10.7270/Q2MG7RKD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| N-lysine methyltransferase SMYD2 (Homo sapiens (Human)) | BDBM50180968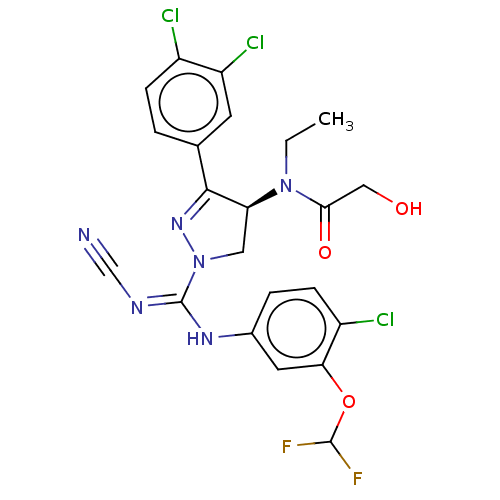 (CHEMBL3818080) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 44 | n/a | n/a | n/a | n/a | n/a | n/a |
BAYER Pharma AG Curated by ChEMBL | Assay Description Inhibition of SMYD2 (unknown origin) using H4 as substrate after 2hrs in presence 3H-SAM of by scintillation proximity assay | J Med Chem 59: 4578-600 (2016) Article DOI: 10.1021/acs.jmedchem.5b01890 BindingDB Entry DOI: 10.7270/Q2RN39S5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| N-lysine methyltransferase SMYD2 (Homo sapiens (Human)) | BDBM283150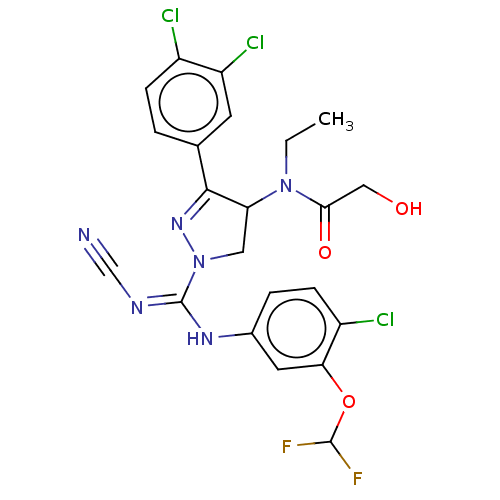 (N-[1-{N-[4-chloro-3-(difluoromethoxy)phenyl]-NR...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 44.1 | n/a | n/a | n/a | n/a | 9.0 | n/a |
Bayer Pharma Aktiengesellschaft US Patent | Assay Description SMYD2 inhibitory activities of the compounds described in the present invention were quantified using a scintillation proximity assay (SPA) which mea... | US Patent US10023539 (2018) BindingDB Entry DOI: 10.7270/Q2MG7RKD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| N-lysine methyltransferase SMYD2 (Homo sapiens (Human)) | BDBM50181508 (CHEMBL3818083 | US10023539, Example 4.2) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 46.7 | n/a | n/a | n/a | n/a | n/a | n/a |
Bayer Pharma Aktiengesellschaft US Patent | Assay Description For the detection of SMYD2 cellular methylation activity an In Cell Western (ICW) assay was established. This assay allows rapid processing of multip... | US Patent US10023539 (2018) BindingDB Entry DOI: 10.7270/Q2MG7RKD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| N-lysine methyltransferase SMYD2 (Homo sapiens (Human)) | BDBM283159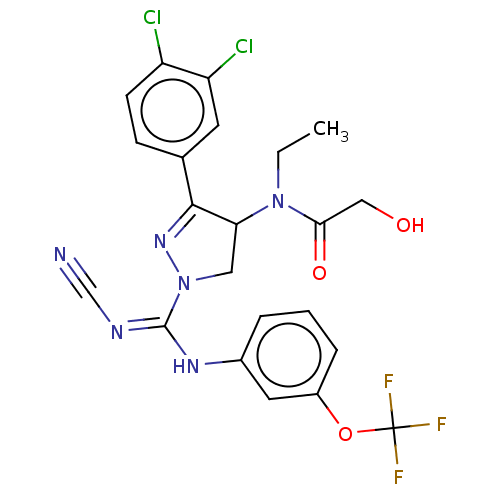 (N-[1-{N′-cyano-N-[3-(trifluoromethoxy)phenyl...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 50 | n/a | n/a | n/a | n/a | 9.0 | n/a |
Bayer Pharma Aktiengesellschaft US Patent | Assay Description SMYD2 inhibitory activities of the compounds described in the present invention were quantified using a scintillation proximity assay (SPA) which mea... | US Patent US10023539 (2018) BindingDB Entry DOI: 10.7270/Q2MG7RKD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Proteinase-activated receptor 1 (Homo sapiens (Human)) | BDBM50181513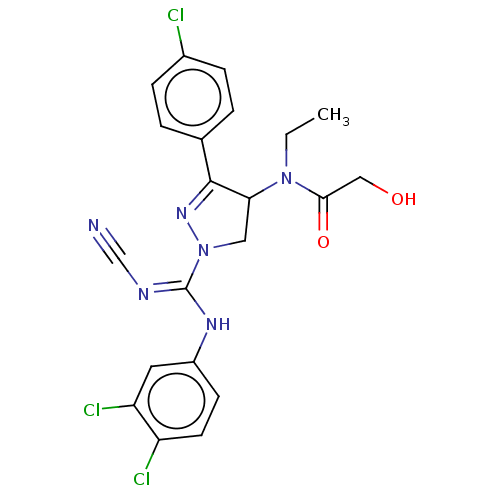 (CHEMBL3819237) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 50 | n/a | n/a | n/a | n/a | n/a | n/a |
BAYER Pharma AG Curated by ChEMBL | Assay Description Antagonist activity at PAR1 (unknown origin) | J Med Chem 59: 4578-600 (2016) Article DOI: 10.1021/acs.jmedchem.5b01890 BindingDB Entry DOI: 10.7270/Q2RN39S5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Proteinase-activated receptor 1 (Homo sapiens (Human)) | BDBM50181507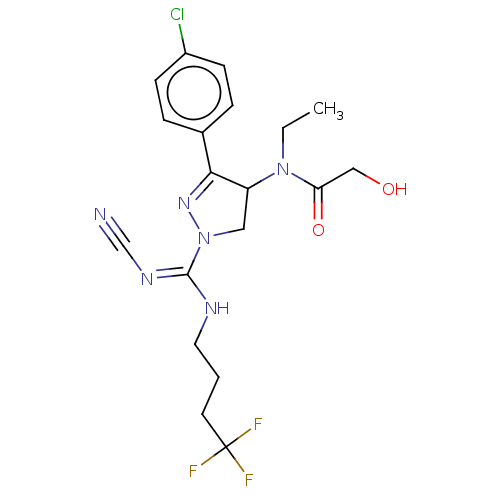 (CHEMBL3818421) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 50 | n/a | n/a | n/a | n/a | n/a | n/a |
BAYER Pharma AG Curated by ChEMBL | Assay Description Antagonist activity at PAR1 (unknown origin) | J Med Chem 59: 4578-600 (2016) Article DOI: 10.1021/acs.jmedchem.5b01890 BindingDB Entry DOI: 10.7270/Q2RN39S5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| N-lysine methyltransferase SMYD2 (Homo sapiens (Human)) | BDBM50180971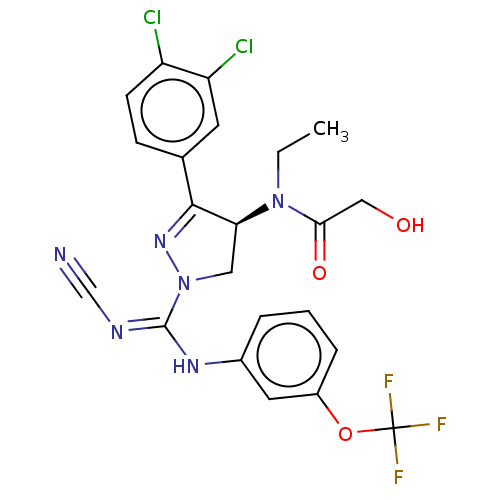 (CHEMBL3818322) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 50 | n/a | n/a | n/a | n/a | n/a | n/a |
BAYER Pharma AG Curated by ChEMBL | Assay Description Inhibition of SMYD2 (unknown origin) using H4 as substrate after 2hrs in presence 3H-SAM of by scintillation proximity assay | J Med Chem 59: 4578-600 (2016) Article DOI: 10.1021/acs.jmedchem.5b01890 BindingDB Entry DOI: 10.7270/Q2RN39S5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| N-lysine methyltransferase SMYD2 (Homo sapiens (Human)) | BDBM283179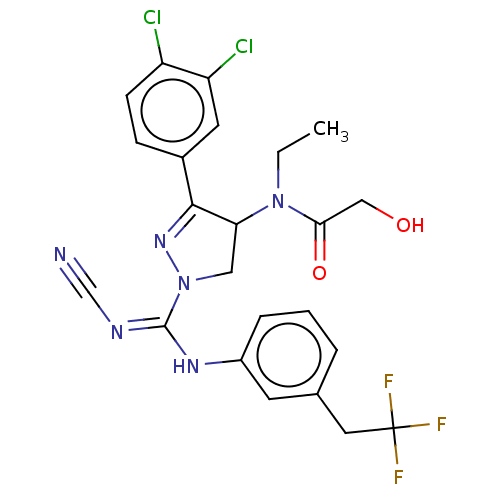 (N-[1-{N′-cyano-N-[3-(2,2,2-trifluoroethyl)ph...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 53 | n/a | n/a | n/a | n/a | 9.0 | n/a |
Bayer Pharma Aktiengesellschaft US Patent | Assay Description SMYD2 inhibitory activities of the compounds described in the present invention were quantified using a scintillation proximity assay (SPA) which mea... | US Patent US10023539 (2018) BindingDB Entry DOI: 10.7270/Q2MG7RKD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| N-lysine methyltransferase SMYD2 (Homo sapiens (Human)) | BDBM283156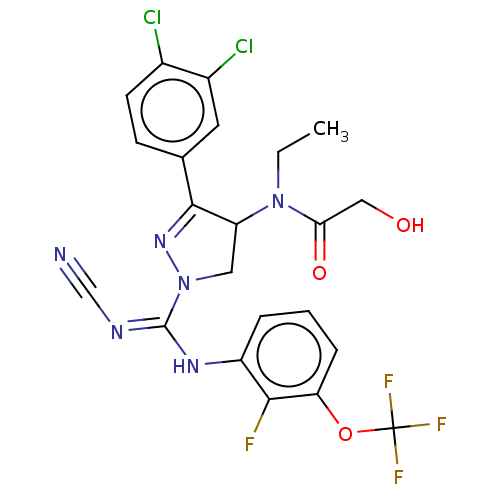 (N-[1-{N′-cyano-N-[2-fluoro-3-(trifluorometho...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 54.1 | n/a | n/a | n/a | n/a | 9.0 | n/a |
Bayer Pharma Aktiengesellschaft US Patent | Assay Description SMYD2 inhibitory activities of the compounds described in the present invention were quantified using a scintillation proximity assay (SPA) which mea... | US Patent US10023539 (2018) BindingDB Entry DOI: 10.7270/Q2MG7RKD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| N-lysine methyltransferase SMYD2 (Homo sapiens (Human)) | BDBM283168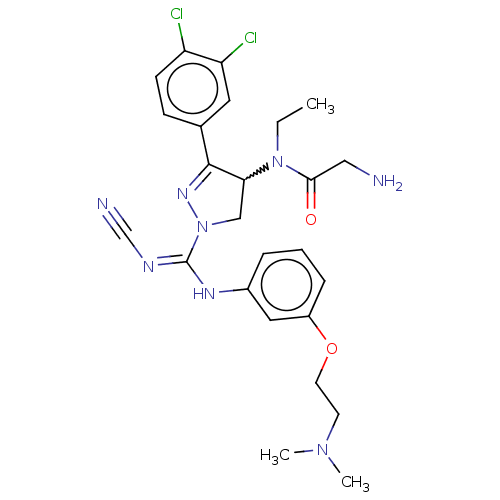 (Rac-N-[1-(N′-cyano-N-{3-[2-(dimethylamino)et...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 59.5 | n/a | n/a | n/a | n/a | 9.0 | n/a |
Bayer Pharma Aktiengesellschaft US Patent | Assay Description SMYD2 inhibitory activities of the compounds described in the present invention were quantified using a scintillation proximity assay (SPA) which mea... | US Patent US10023539 (2018) BindingDB Entry DOI: 10.7270/Q2MG7RKD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| N-lysine methyltransferase SMYD2 (Homo sapiens (Human)) | BDBM50180955 (CHEMBL3818617) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 60 | n/a | n/a | n/a | n/a | n/a | n/a |
BAYER Pharma AG Curated by ChEMBL | Assay Description Inhibition of N-terminal 2xc-myc-tagged human SMYD2 transfected in human MDA-MB-231 cells assessed as reduction in AHNAK methylation after 72 hrs by ... | J Med Chem 59: 4578-600 (2016) Article DOI: 10.1021/acs.jmedchem.5b01890 BindingDB Entry DOI: 10.7270/Q2RN39S5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Proteinase-activated receptor 1 (Homo sapiens (Human)) | BDBM50181511 (CHEMBL3819343) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 60 | n/a | n/a | n/a | n/a | n/a | n/a |
BAYER Pharma AG Curated by ChEMBL | Assay Description Antagonist activity at PAR1 (unknown origin) | J Med Chem 59: 4578-600 (2016) Article DOI: 10.1021/acs.jmedchem.5b01890 BindingDB Entry DOI: 10.7270/Q2RN39S5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 162 total ) | Next | Last >> |