 Found 1238 hits with Last Name = 'su' and Initial = 'hp'
Found 1238 hits with Last Name = 'su' and Initial = 'hp' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
cGMP-dependent 3',5'-cyclic phosphodiesterase
(Homo sapiens (Human)) | BDBM50452209

(CHEMBL4207777 | US10647727, Example 9)Show SMILES Cn1ncc2c(NC3(CC3)c3ccc(cc3)C(F)(F)F)nc(Cl)nc12 Show InChI InChI=1S/C16H13ClF3N5/c1-25-13-11(8-21-25)12(22-14(17)23-13)24-15(6-7-15)9-2-4-10(5-3-9)16(18,19)20/h2-5,8H,6-7H2,1H3,(H,22,23,24) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 4.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck
Curated by ChEMBL
| Assay Description
Binding affinity to PDE2 (unknown origin) by SPR analysis |
Bioorg Med Chem Lett 27: 5167-5171 (2017)
Article DOI: 10.1016/j.bmcl.2017.10.054
BindingDB Entry DOI: 10.7270/Q28C9ZV3 |
More data for this
Ligand-Target Pair | |
cGMP-dependent 3',5'-cyclic phosphodiesterase
(Homo sapiens (Human)) | BDBM50452200
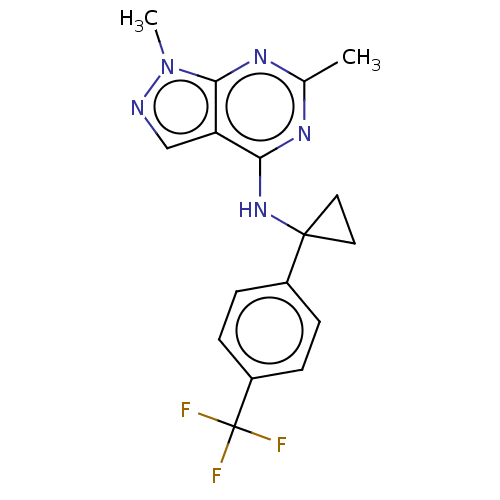
(CHEMBL4212416 | US10647727, Example 20)Show SMILES Cc1nc(NC2(CC2)c2ccc(cc2)C(F)(F)F)c2cnn(C)c2n1 Show InChI InChI=1S/C17H16F3N5/c1-10-22-14(13-9-21-25(2)15(13)23-10)24-16(7-8-16)11-3-5-12(6-4-11)17(18,19)20/h3-6,9H,7-8H2,1-2H3,(H,22,23,24) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 5.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck
Curated by ChEMBL
| Assay Description
Binding affinity to PDE2 (unknown origin) by SPR analysis |
Bioorg Med Chem Lett 27: 5167-5171 (2017)
Article DOI: 10.1016/j.bmcl.2017.10.054
BindingDB Entry DOI: 10.7270/Q28C9ZV3 |
More data for this
Ligand-Target Pair | |
cGMP-dependent 3',5'-cyclic phosphodiesterase
(Homo sapiens (Human)) | BDBM50452211

(CHEMBL4216198)Show SMILES FC(F)(F)c1ccc(cc1)C1(CC1)Nc1nc(Cl)nc2[nH]ncc12 Show InChI InChI=1S/C15H11ClF3N5/c16-13-21-11(10-7-20-24-12(10)22-13)23-14(5-6-14)8-1-3-9(4-2-8)15(17,18)19/h1-4,7H,5-6H2,(H2,20,21,22,23,24) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
PDB
UniChem
| PDB
Article
PubMed
| 14 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck
Curated by ChEMBL
| Assay Description
Binding affinity to PDE2 (unknown origin) by SPR analysis |
Bioorg Med Chem Lett 27: 5167-5171 (2017)
Article DOI: 10.1016/j.bmcl.2017.10.054
BindingDB Entry DOI: 10.7270/Q28C9ZV3 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
cGMP-dependent 3',5'-cyclic phosphodiesterase
(Homo sapiens (Human)) | BDBM50452216
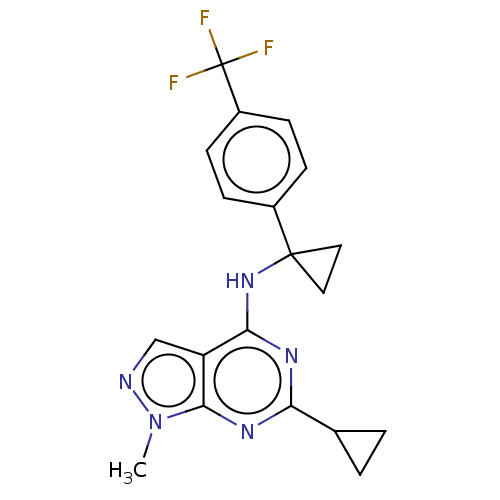
(CHEMBL4215537 | US10647727, Example 13)Show SMILES Cn1ncc2c(NC3(CC3)c3ccc(cc3)C(F)(F)F)nc(nc12)C1CC1 Show InChI InChI=1S/C19H18F3N5/c1-27-17-14(10-23-27)16(24-15(25-17)11-2-3-11)26-18(8-9-18)12-4-6-13(7-5-12)19(20,21)22/h4-7,10-11H,2-3,8-9H2,1H3,(H,24,25,26) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck
Curated by ChEMBL
| Assay Description
Binding affinity to PDE2 (unknown origin) by SPR analysis |
Bioorg Med Chem Lett 27: 5167-5171 (2017)
Article DOI: 10.1016/j.bmcl.2017.10.054
BindingDB Entry DOI: 10.7270/Q28C9ZV3 |
More data for this
Ligand-Target Pair | |
cGMP-dependent 3',5'-cyclic phosphodiesterase
(Homo sapiens (Human)) | BDBM50452208

(CHEMBL4215607)Show SMILES Cn1ncc2c(nc(Cl)nc12)N1CCCC1c1ccc(cc1)C(F)(F)F Show InChI InChI=1S/C17H15ClF3N5/c1-25-14-12(9-22-25)15(24-16(18)23-14)26-8-2-3-13(26)10-4-6-11(7-5-10)17(19,20)21/h4-7,9,13H,2-3,8H2,1H3 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 97 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck
Curated by ChEMBL
| Assay Description
Binding affinity to PDE2 (unknown origin) by SPR analysis |
Bioorg Med Chem Lett 27: 5167-5171 (2017)
Article DOI: 10.1016/j.bmcl.2017.10.054
BindingDB Entry DOI: 10.7270/Q28C9ZV3 |
More data for this
Ligand-Target Pair | |
cGMP-dependent 3',5'-cyclic phosphodiesterase
(Homo sapiens (Human)) | BDBM50452212

(CHEMBL4210651)Show SMILES Cn1ncc2c(nc(Cl)nc12)N1CCCCC1c1ccc(cc1)C(F)(F)F Show InChI InChI=1S/C18H17ClF3N5/c1-26-15-13(10-23-26)16(25-17(19)24-15)27-9-3-2-4-14(27)11-5-7-12(8-6-11)18(20,21)22/h5-8,10,14H,2-4,9H2,1H3 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 152 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck
Curated by ChEMBL
| Assay Description
Binding affinity to PDE2 (unknown origin) by SPR analysis |
Bioorg Med Chem Lett 27: 5167-5171 (2017)
Article DOI: 10.1016/j.bmcl.2017.10.054
BindingDB Entry DOI: 10.7270/Q28C9ZV3 |
More data for this
Ligand-Target Pair | |
cGMP-dependent 3',5'-cyclic phosphodiesterase
(Homo sapiens (Human)) | BDBM50452210

(CHEMBL4202746 | US10647727, Example 12)Show SMILES Cn1ncc2c(NC3(CC3)c3ccc(cc3)C(F)(F)F)nc(O)nc12 Show InChI InChI=1S/C16H14F3N5O/c1-24-13-11(8-20-24)12(21-14(25)22-13)23-15(6-7-15)9-2-4-10(5-3-9)16(17,18)19/h2-5,8H,6-7H2,1H3,(H2,21,22,23,25) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 195 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck
Curated by ChEMBL
| Assay Description
Binding affinity to PDE2 (unknown origin) by SPR analysis |
Bioorg Med Chem Lett 27: 5167-5171 (2017)
Article DOI: 10.1016/j.bmcl.2017.10.054
BindingDB Entry DOI: 10.7270/Q28C9ZV3 |
More data for this
Ligand-Target Pair | |
cGMP-dependent 3',5'-cyclic phosphodiesterase
(Homo sapiens (Human)) | BDBM50452215

(CHEMBL4212115 | US10647727, Example 2)Show SMILES C[C@@H](Nc1nc(Cl)nc2n(C)ncc12)c1ccc(cc1)C(F)(F)F |r| Show InChI InChI=1S/C15H13ClF3N5/c1-8(9-3-5-10(6-4-9)15(17,18)19)21-12-11-7-20-24(2)13(11)23-14(16)22-12/h3-8H,1-2H3,(H,21,22,23)/t8-/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
PDB
UniChem
| PDB
Article
PubMed
| 237 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck
Curated by ChEMBL
| Assay Description
Binding affinity to PDE2 (unknown origin) by SPR analysis |
Bioorg Med Chem Lett 27: 5167-5171 (2017)
Article DOI: 10.1016/j.bmcl.2017.10.054
BindingDB Entry DOI: 10.7270/Q28C9ZV3 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
cGMP-dependent 3',5'-cyclic phosphodiesterase
(Homo sapiens (Human)) | BDBM50452198

(CHEMBL4207234 | US10647727, Example 8)Show SMILES Cn1ncc2c(NC(C)(C)c3ccc(cc3)C(F)(F)F)nc(Cl)nc12 Show InChI InChI=1S/C16H15ClF3N5/c1-15(2,9-4-6-10(7-5-9)16(18,19)20)24-12-11-8-21-25(3)13(11)23-14(17)22-12/h4-8H,1-3H3,(H,22,23,24) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 642 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck
Curated by ChEMBL
| Assay Description
Binding affinity to PDE2 (unknown origin) by SPR analysis |
Bioorg Med Chem Lett 27: 5167-5171 (2017)
Article DOI: 10.1016/j.bmcl.2017.10.054
BindingDB Entry DOI: 10.7270/Q28C9ZV3 |
More data for this
Ligand-Target Pair | |
Prostaglandin D2 receptor
(Homo sapiens (Human)) | BDBM50460673
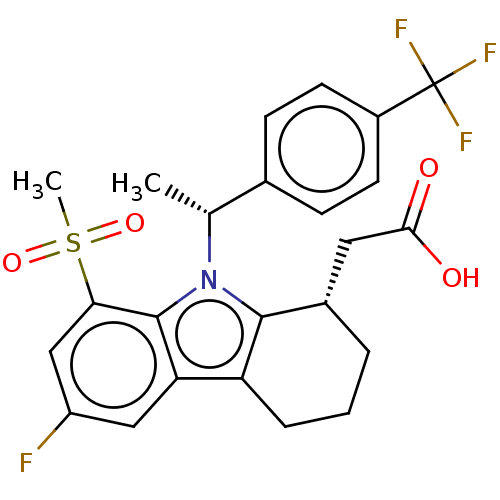
(CHEMBL4229054)Show SMILES C[C@H](c1ccc(cc1)C(F)(F)F)n1c2[C@H](CC(O)=O)CCCc2c2cc(F)cc(c12)S(C)(=O)=O |r| Show InChI InChI=1S/C24H23F4NO4S/c1-13(14-6-8-16(9-7-14)24(26,27)28)29-22-15(10-21(30)31)4-3-5-18(22)19-11-17(25)12-20(23(19)29)34(2,32)33/h6-9,11-13,15H,3-5,10H2,1-2H3,(H,30,31)/t13-,15+/m1/s1 | UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
PDB
UniChem
| Article
PubMed
| 674 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck & Co. Inc.
Curated by ChEMBL
| Assay Description
Antagonist activity at DP1 receptor (unknown origin) |
Bioorg Med Chem Lett 28: 1122-1126 (2018)
Article DOI: 10.1016/j.bmcl.2018.01.039
BindingDB Entry DOI: 10.7270/Q2QV3Q4C |
More data for this
Ligand-Target Pair | |
Prostaglandin D2 receptor
(Homo sapiens (Human)) | BDBM50460670
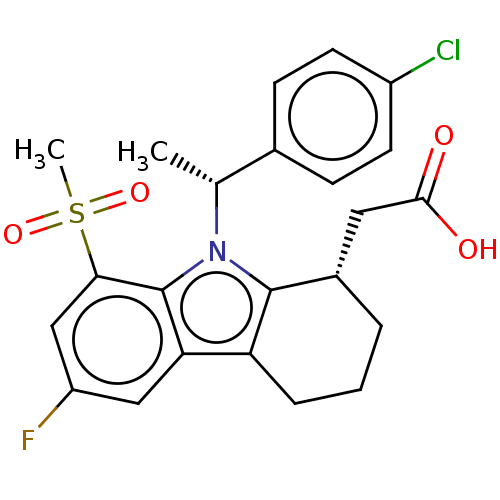
(CHEMBL4228792)Show SMILES C[C@H](c1ccc(Cl)cc1)n1c2[C@H](CC(O)=O)CCCc2c2cc(F)cc(c12)S(C)(=O)=O |r| Show InChI InChI=1S/C23H23ClFNO4S/c1-13(14-6-8-16(24)9-7-14)26-22-15(10-21(27)28)4-3-5-18(22)19-11-17(25)12-20(23(19)26)31(2,29)30/h6-9,11-13,15H,3-5,10H2,1-2H3,(H,27,28)/t13-,15+/m1/s1 | UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 873 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck & Co. Inc.
Curated by ChEMBL
| Assay Description
Antagonist activity at DP1 receptor (unknown origin) |
Bioorg Med Chem Lett 28: 1122-1126 (2018)
Article DOI: 10.1016/j.bmcl.2018.01.039
BindingDB Entry DOI: 10.7270/Q2QV3Q4C |
More data for this
Ligand-Target Pair | |
cGMP-dependent 3',5'-cyclic phosphodiesterase
(Homo sapiens (Human)) | BDBM50452206

(CHEMBL4209385 | US10647727, Example 4)Show InChI InChI=1S/C14H11ClF3N5/c1-23-12-10(7-20-23)11(21-13(15)22-12)19-6-8-2-4-9(5-3-8)14(16,17)18/h2-5,7H,6H2,1H3,(H,19,21,22) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 2.34E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck
Curated by ChEMBL
| Assay Description
Binding affinity to PDE2 (unknown origin) by SPR analysis |
Bioorg Med Chem Lett 27: 5167-5171 (2017)
Article DOI: 10.1016/j.bmcl.2017.10.054
BindingDB Entry DOI: 10.7270/Q28C9ZV3 |
More data for this
Ligand-Target Pair | |
cGMP-dependent 3',5'-cyclic phosphodiesterase
(Homo sapiens (Human)) | BDBM50452213
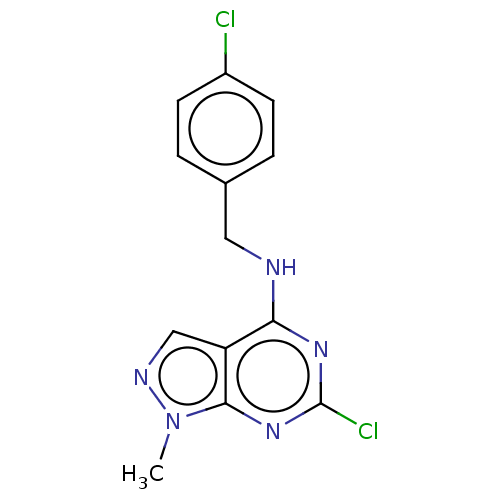
(CHEMBL4217749)Show InChI InChI=1S/C13H11Cl2N5/c1-20-12-10(7-17-20)11(18-13(15)19-12)16-6-8-2-4-9(14)5-3-8/h2-5,7H,6H2,1H3,(H,16,18,19) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 6.90E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck
Curated by ChEMBL
| Assay Description
Binding affinity to PDE2 (unknown origin) by SPR analysis |
Bioorg Med Chem Lett 27: 5167-5171 (2017)
Article DOI: 10.1016/j.bmcl.2017.10.054
BindingDB Entry DOI: 10.7270/Q28C9ZV3 |
More data for this
Ligand-Target Pair | |
cGMP-dependent 3',5'-cyclic phosphodiesterase
(Homo sapiens (Human)) | BDBM50452205

(CHEMBL4212801)Show InChI InChI=1S/C13H11Cl2N5/c1-20-12-10(7-17-20)11(18-13(15)19-12)16-6-8-3-2-4-9(14)5-8/h2-5,7H,6H2,1H3,(H,16,18,19) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 7.81E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck
Curated by ChEMBL
| Assay Description
Binding affinity to PDE2 (unknown origin) by SPR analysis |
Bioorg Med Chem Lett 27: 5167-5171 (2017)
Article DOI: 10.1016/j.bmcl.2017.10.054
BindingDB Entry DOI: 10.7270/Q28C9ZV3 |
More data for this
Ligand-Target Pair | |
cGMP-dependent 3',5'-cyclic phosphodiesterase
(Homo sapiens (Human)) | BDBM50452207

(CHEMBL4207765)Show InChI InChI=1S/C15H13ClF3N5/c1-24-13-11(8-21-24)12(22-14(16)23-13)20-7-6-9-2-4-10(5-3-9)15(17,18)19/h2-5,8H,6-7H2,1H3,(H,20,22,23) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 1.01E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck
Curated by ChEMBL
| Assay Description
Binding affinity to PDE2 (unknown origin) by SPR analysis |
Bioorg Med Chem Lett 27: 5167-5171 (2017)
Article DOI: 10.1016/j.bmcl.2017.10.054
BindingDB Entry DOI: 10.7270/Q28C9ZV3 |
More data for this
Ligand-Target Pair | |
cGMP-dependent 3',5'-cyclic phosphodiesterase
(Homo sapiens (Human)) | BDBM50452214
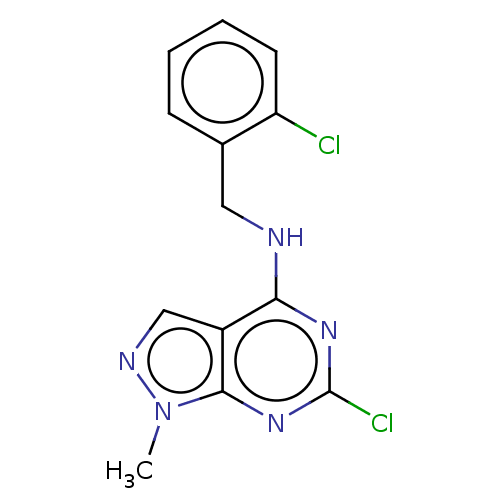
(CHEMBL4214287)Show InChI InChI=1S/C13H11Cl2N5/c1-20-12-9(7-17-20)11(18-13(15)19-12)16-6-8-4-2-3-5-10(8)14/h2-5,7H,6H2,1H3,(H,16,18,19) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 1.17E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck
Curated by ChEMBL
| Assay Description
Binding affinity to PDE2 (unknown origin) by SPR analysis |
Bioorg Med Chem Lett 27: 5167-5171 (2017)
Article DOI: 10.1016/j.bmcl.2017.10.054
BindingDB Entry DOI: 10.7270/Q28C9ZV3 |
More data for this
Ligand-Target Pair | |
cGMP-dependent 3',5'-cyclic phosphodiesterase
(Homo sapiens (Human)) | BDBM50452199
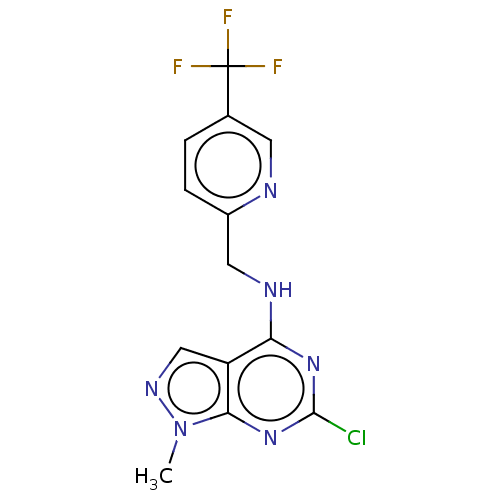
(CHEMBL4202883)Show InChI InChI=1S/C13H10ClF3N6/c1-23-11-9(6-20-23)10(21-12(14)22-11)19-5-8-3-2-7(4-18-8)13(15,16)17/h2-4,6H,5H2,1H3,(H,19,21,22) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 1.50E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck
Curated by ChEMBL
| Assay Description
Binding affinity to PDE2 (unknown origin) by SPR analysis |
Bioorg Med Chem Lett 27: 5167-5171 (2017)
Article DOI: 10.1016/j.bmcl.2017.10.054
BindingDB Entry DOI: 10.7270/Q28C9ZV3 |
More data for this
Ligand-Target Pair | |
cGMP-dependent 3',5'-cyclic phosphodiesterase
(Homo sapiens (Human)) | BDBM50452203
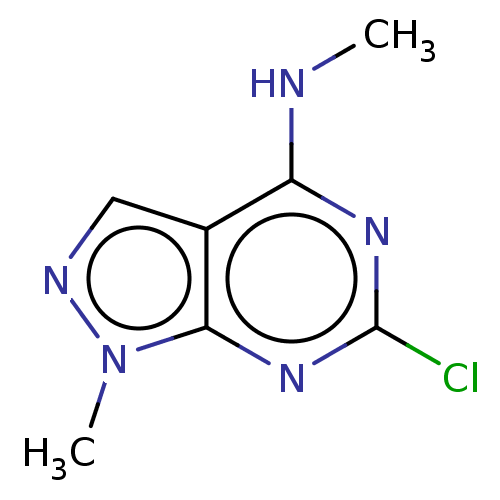
(CHEMBL4217231)Show InChI InChI=1S/C7H8ClN5/c1-9-5-4-3-10-13(2)6(4)12-7(8)11-5/h3H,1-2H3,(H,9,11,12) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
PDB
UniChem
| PDB
Article
PubMed
| 2.24E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck
Curated by ChEMBL
| Assay Description
Binding affinity to PDE2 (unknown origin) by SPR analysis |
Bioorg Med Chem Lett 27: 5167-5171 (2017)
Article DOI: 10.1016/j.bmcl.2017.10.054
BindingDB Entry DOI: 10.7270/Q28C9ZV3 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Protease
(Human immunodeficiency virus 1 (HIV-1)) | BDBM50482331

(CHEMBL1170704)Show SMILES COC(=O)N[C@@H](C(c1ccccc1)c1ccccc1)C(=O)N[C@@H](C)CCC[C@@H](CO)N(CCC(C)C)S(=O)(=O)c1ccc(N)cc1 |r| Show InChI InChI=1S/C35H48N4O6S/c1-25(2)22-23-39(46(43,44)31-20-18-29(36)19-21-31)30(24-40)17-11-12-26(3)37-34(41)33(38-35(42)45-4)32(27-13-7-5-8-14-27)28-15-9-6-10-16-28/h5-10,13-16,18-21,25-26,30,32-33,40H,11-12,17,22-24,36H2,1-4H3,(H,37,41)(H,38,42)/t26-,30-,33-/m0/s1 | PDB
UniProtKB/TrEMBL
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.00700 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of HIV1 protease |
Bioorg Med Chem Lett 20: 4065-8 (2010)
Article DOI: 10.1016/j.bmcl.2010.05.082
BindingDB Entry DOI: 10.7270/Q2SJ1PFR |
More data for this
Ligand-Target Pair | |
Protease
(Human immunodeficiency virus 1 (HIV-1)) | BDBM50280116
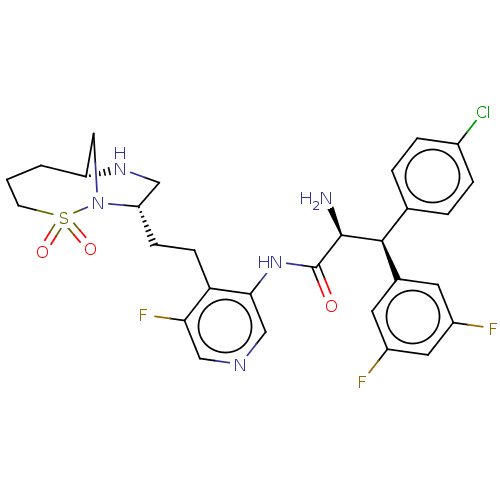
(CHEMBL4177355)Show SMILES [H][C@@]12CN([C@@H](CCc3c(F)cncc3NC(=O)[C@@H](N)[C@@H](c3ccc(Cl)cc3)c3cc(F)cc(F)c3)CN1)S(=O)(=O)CCC2 |r| Show InChI InChI=1S/C29H31ClF3N5O3S/c30-19-5-3-17(4-6-19)27(18-10-20(31)12-21(32)11-18)28(34)29(39)37-26-15-35-14-25(33)24(26)8-7-23-13-36-22-2-1-9-42(40,41)38(23)16-22/h3-6,10-12,14-15,22-23,27-28,36H,1-2,7-9,13,16,34H2,(H,37,39)/t22-,23+,27+,28+/m1/s1 | PDB
UniProtKB/TrEMBL
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.0120 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck & Co. Inc
Curated by ChEMBL
| Assay Description
Inhibition of HIV1 protease expressed in Escherichia coli using Val-Ser-Gln-Asn-(beta-naphtyl)Ala-Pro-Ile-Val as substrate preincubated for 30 mins f... |
ACS Med Chem Lett 8: 1292-1297 (2017)
Article DOI: 10.1021/acsmedchemlett.7b00386
BindingDB Entry DOI: 10.7270/Q2K35X57 |
More data for this
Ligand-Target Pair | |
Protease
(Human immunodeficiency virus 1 (HIV-1)) | BDBM50482336

(CHEMBL1170709)Show SMILES COC(=O)N[C@@H](C(c1ccccc1)c1ccccc1)C(=O)N[C@H](CO)CCC[C@@H](CO)N(CCC(C)C)S(=O)(=O)c1ccc(N)cc1 |r| Show InChI InChI=1S/C35H48N4O7S/c1-25(2)21-22-39(47(44,45)31-19-17-28(36)18-20-31)30(24-41)16-10-15-29(23-40)37-34(42)33(38-35(43)46-3)32(26-11-6-4-7-12-26)27-13-8-5-9-14-27/h4-9,11-14,17-20,25,29-30,32-33,40-41H,10,15-16,21-24,36H2,1-3H3,(H,37,42)(H,38,43)/t29-,30-,33-/m0/s1 | PDB
UniProtKB/TrEMBL
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.0130 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of HIV1 protease |
Bioorg Med Chem Lett 20: 4065-8 (2010)
Article DOI: 10.1016/j.bmcl.2010.05.082
BindingDB Entry DOI: 10.7270/Q2SJ1PFR |
More data for this
Ligand-Target Pair | |
Protease
(Human immunodeficiency virus 1 (HIV-1)) | BDBM8125

((3R,3aS,6aR)-hexahydrofuro[2,3-b]furan-3-yl N-[(2S...)Show SMILES [H][C@@]1(CO[C@@]2([H])OCC[C@@]12[H])OC(=O)N[C@@H](Cc1ccccc1)[C@H](O)CN(CC(C)C)S(=O)(=O)c1ccc(N)cc1 |r| Show InChI InChI=1S/C27H37N3O7S/c1-18(2)15-30(38(33,34)21-10-8-20(28)9-11-21)16-24(31)23(14-19-6-4-3-5-7-19)29-27(32)37-25-17-36-26-22(25)12-13-35-26/h3-11,18,22-26,31H,12-17,28H2,1-2H3,(H,29,32)/t22-,23-,24+,25-,26+/m0/s1 | PDB
UniProtKB/TrEMBL
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| n/a | n/a | 0.0130 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck & Co. Inc
Curated by ChEMBL
| Assay Description
Inhibition of GAR transformylase from Lactobacillus casei |
ACS Med Chem Lett 8: 1292-1297 (2017)
Article DOI: 10.1021/acsmedchemlett.7b00386
BindingDB Entry DOI: 10.7270/Q2K35X57 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Protease
(Human immunodeficiency virus 1 (HIV-1)) | BDBM50482332
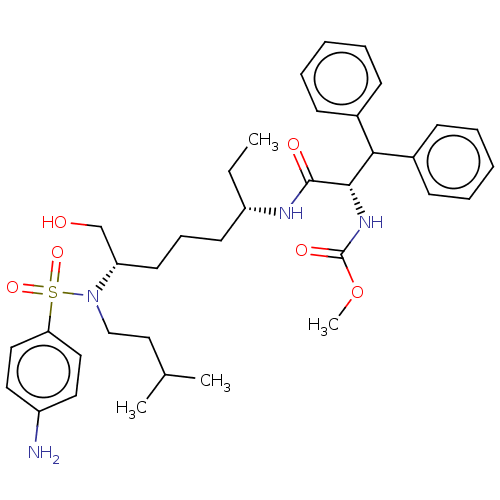
(CHEMBL1170706)Show SMILES CC[C@@H](CCC[C@@H](CO)N(CCC(C)C)S(=O)(=O)c1ccc(N)cc1)NC(=O)[C@@H](NC(=O)OC)C(c1ccccc1)c1ccccc1 |r| Show InChI InChI=1S/C36H50N4O6S/c1-5-30(17-12-18-31(25-41)40(24-23-26(2)3)47(44,45)32-21-19-29(37)20-22-32)38-35(42)34(39-36(43)46-4)33(27-13-8-6-9-14-27)28-15-10-7-11-16-28/h6-11,13-16,19-22,26,30-31,33-34,41H,5,12,17-18,23-25,37H2,1-4H3,(H,38,42)(H,39,43)/t30-,31-,34-/m0/s1 | PDB
UniProtKB/TrEMBL
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.0160 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of HIV1 protease |
Bioorg Med Chem Lett 20: 4065-8 (2010)
Article DOI: 10.1016/j.bmcl.2010.05.082
BindingDB Entry DOI: 10.7270/Q2SJ1PFR |
More data for this
Ligand-Target Pair | |
Protease
(Human immunodeficiency virus 1 (HIV-1)) | BDBM50482337

(CHEMBL1170703)Show SMILES COC(=O)N[C@@H](C(c1ccccc1)c1ccccc1)C(=O)NCCCC[C@@H](CO)N(CCC(C)C)S(=O)(=O)c1ccc(N)cc1 |r| Show InChI InChI=1S/C34H46N4O6S/c1-25(2)21-23-38(45(42,43)30-19-17-28(35)18-20-30)29(24-39)16-10-11-22-36-33(40)32(37-34(41)44-3)31(26-12-6-4-7-13-26)27-14-8-5-9-15-27/h4-9,12-15,17-20,25,29,31-32,39H,10-11,16,21-24,35H2,1-3H3,(H,36,40)(H,37,41)/t29-,32-/m0/s1 | PDB
UniProtKB/TrEMBL
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.0300 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of HIV1 protease |
Bioorg Med Chem Lett 20: 4065-8 (2010)
Article DOI: 10.1016/j.bmcl.2010.05.082
BindingDB Entry DOI: 10.7270/Q2SJ1PFR |
More data for this
Ligand-Target Pair | |
Protease
(Human immunodeficiency virus 1 (HIV-1)) | BDBM13934

(Atazanavir | BMS 232632 | CGP 73547 | CHEMBL1163 |...)Show SMILES COC(=O)N[C@H](C(=O)N[C@@H](Cc1ccccc1)[C@@H](O)CN(Cc1ccc(cc1)-c1ccccn1)NC(=O)[C@@H](NC(=O)OC)C(C)(C)C)C(C)(C)C |r| Show InChI InChI=1S/C38H52N6O7/c1-37(2,3)31(41-35(48)50-7)33(46)40-29(22-25-14-10-9-11-15-25)30(45)24-44(43-34(47)32(38(4,5)6)42-36(49)51-8)23-26-17-19-27(20-18-26)28-16-12-13-21-39-28/h9-21,29-32,45H,22-24H2,1-8H3,(H,40,46)(H,41,48)(H,42,49)(H,43,47)/t29-,30-,31+,32+/m0/s1 | PDB
UniProtKB/TrEMBL
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| n/a | n/a | 0.0400 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of wild-type HIV1 protease expressed in Escherichia coli assessed as reduction in product formation preincubated for 30 mins followed by a... |
ACS Med Chem Lett 7: 702-7 (2016)
Article DOI: 10.1021/acsmedchemlett.6b00135
BindingDB Entry DOI: 10.7270/Q28W3G74 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Protease
(Human immunodeficiency virus 1 (HIV-1)) | BDBM13934

(Atazanavir | BMS 232632 | CGP 73547 | CHEMBL1163 |...)Show SMILES COC(=O)N[C@H](C(=O)N[C@@H](Cc1ccccc1)[C@@H](O)CN(Cc1ccc(cc1)-c1ccccn1)NC(=O)[C@@H](NC(=O)OC)C(C)(C)C)C(C)(C)C |r| Show InChI InChI=1S/C38H52N6O7/c1-37(2,3)31(41-35(48)50-7)33(46)40-29(22-25-14-10-9-11-15-25)30(45)24-44(43-34(47)32(38(4,5)6)42-36(49)51-8)23-26-17-19-27(20-18-26)28-16-12-13-21-39-28/h9-21,29-32,45H,22-24H2,1-8H3,(H,40,46)(H,41,48)(H,42,49)(H,43,47)/t29-,30-,31+,32+/m0/s1 | PDB
UniProtKB/TrEMBL
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| n/a | n/a | 0.0400 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck & Co. Inc
Curated by ChEMBL
| Assay Description
Inhibition of HIV1 protease expressed in Escherichia coli using Val-Ser-Gln-Asn-(beta-naphtyl)Ala-Pro-Ile-Val as substrate preincubated for 30 mins f... |
ACS Med Chem Lett 8: 1292-1297 (2017)
Article DOI: 10.1021/acsmedchemlett.7b00386
BindingDB Entry DOI: 10.7270/Q2K35X57 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Protease
(Human immunodeficiency virus 1 (HIV-1)) | BDBM50482333

(CHEMBL1170707)Show SMILES CCC[C@@H](CCC[C@@H](CO)N(CCC(C)C)S(=O)(=O)c1ccc(N)cc1)NC(=O)[C@@H](NC(=O)OC)C(c1ccccc1)c1ccccc1 |r| Show InChI InChI=1S/C37H52N4O6S/c1-5-13-31(18-12-19-32(26-42)41(25-24-27(2)3)48(45,46)33-22-20-30(38)21-23-33)39-36(43)35(40-37(44)47-4)34(28-14-8-6-9-15-28)29-16-10-7-11-17-29/h6-11,14-17,20-23,27,31-32,34-35,42H,5,12-13,18-19,24-26,38H2,1-4H3,(H,39,43)(H,40,44)/t31-,32-,35-/m0/s1 | PDB
UniProtKB/TrEMBL
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.100 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of HIV1 protease |
Bioorg Med Chem Lett 20: 4065-8 (2010)
Article DOI: 10.1016/j.bmcl.2010.05.082
BindingDB Entry DOI: 10.7270/Q2SJ1PFR |
More data for this
Ligand-Target Pair | |
Protease
(Human immunodeficiency virus 1 (HIV-1)) | BDBM50482334

(CHEMBL1170708)Show SMILES COC(=O)N[C@@H](C(c1ccccc1)c1ccccc1)C(=O)N[C@@H](CCC[C@@H](CO)N(CCC(C)C)S(=O)(=O)c1ccc(N)cc1)C(C)C |r| Show InChI InChI=1S/C37H52N4O6S/c1-26(2)23-24-41(48(45,46)32-21-19-30(38)20-22-32)31(25-42)17-12-18-33(27(3)4)39-36(43)35(40-37(44)47-5)34(28-13-8-6-9-14-28)29-15-10-7-11-16-29/h6-11,13-16,19-22,26-27,31,33-35,42H,12,17-18,23-25,38H2,1-5H3,(H,39,43)(H,40,44)/t31-,33-,35-/m0/s1 | PDB
UniProtKB/TrEMBL
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.100 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of HIV1 protease |
Bioorg Med Chem Lett 20: 4065-8 (2010)
Article DOI: 10.1016/j.bmcl.2010.05.082
BindingDB Entry DOI: 10.7270/Q2SJ1PFR |
More data for this
Ligand-Target Pair | |
MAP/microtubule affinity-regulating kinase 3
(Homo sapiens (Human)) | BDBM50536695
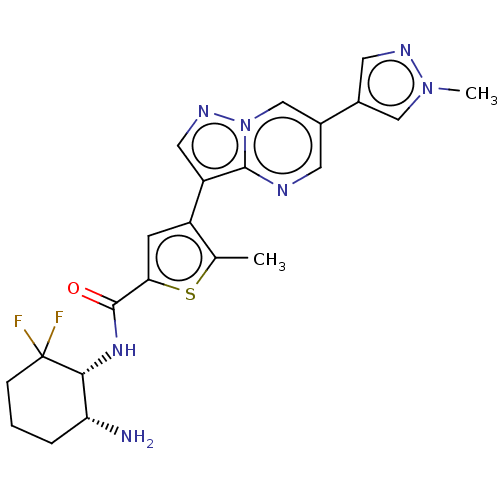
(CHEMBL4565845)Show SMILES Cc1sc(cc1-c1cnn2cc(cnc12)-c1cnn(C)c1)C(=O)N[C@@H]1[C@H](N)CCCC1(F)F |r| Show InChI InChI=1S/C22H23F2N7OS/c1-12-15(6-18(33-12)21(32)29-19-17(25)4-3-5-22(19,23)24)16-9-28-31-11-13(7-26-20(16)31)14-8-27-30(2)10-14/h6-11,17,19H,3-5,25H2,1-2H3,(H,29,32)/t17-,19-/m1/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | <0.25 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck and Co.
Curated by ChEMBL
| Assay Description
Inhibition of full-length recombinant human GST-tagged MARK3 expressed in baculovirus expression system using biotinylated-Cdc25C peptide substrate m... |
Bioorg Med Chem Lett 26: 4362-6 (2016)
Article DOI: 10.1016/j.bmcl.2016.02.003
BindingDB Entry DOI: 10.7270/Q2NP27X5 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase JAK2
(Homo sapiens (Human)) | BDBM50355409

(CHEMBL1835142)Show SMILES Cn1cc(cn1)-c1ccc2c(c1)[nH]c1c(cnc(N[C@H](C3CC3)C(F)(F)F)c21)C(N)=O |r| Show InChI InChI=1S/C21H19F3N6O/c1-30-9-12(7-27-30)11-4-5-13-15(6-11)28-17-14(19(25)31)8-26-20(16(13)17)29-18(10-2-3-10)21(22,23)24/h4-10,18,28H,2-3H2,1H3,(H2,25,31)(H,26,29)/t18-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck& Co.
Curated by ChEMBL
| Assay Description
Inhibition of JAK2 using biotin-EQEDEPEGDYFEWLE- NH2 as substrate |
J Med Chem 54: 7334-49 (2011)
Article DOI: 10.1021/jm200909u
BindingDB Entry DOI: 10.7270/Q2T43TGS |
More data for this
Ligand-Target Pair | |
Protease
(Human immunodeficiency virus 1 (HIV-1)) | BDBM50482338

(CHEMBL1170705)Show SMILES COC(=O)N[C@@H](C(c1ccccc1)c1ccccc1)C(=O)N[C@H](C)CCC[C@@H](CO)N(CCC(C)C)S(=O)(=O)c1ccc(N)cc1 |r| Show InChI InChI=1S/C35H48N4O6S/c1-25(2)22-23-39(46(43,44)31-20-18-29(36)19-21-31)30(24-40)17-11-12-26(3)37-34(41)33(38-35(42)45-4)32(27-13-7-5-8-14-27)28-15-9-6-10-16-28/h5-10,13-16,18-21,25-26,30,32-33,40H,11-12,17,22-24,36H2,1-4H3,(H,37,41)(H,38,42)/t26-,30+,33+/m1/s1 | PDB
UniProtKB/TrEMBL
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of HIV1 protease |
Bioorg Med Chem Lett 20: 4065-8 (2010)
Article DOI: 10.1016/j.bmcl.2010.05.082
BindingDB Entry DOI: 10.7270/Q2SJ1PFR |
More data for this
Ligand-Target Pair | |
Protease
(Human immunodeficiency virus 1 (HIV-1)) | BDBM50482335

(MK-8122 | MX-100 | PL 100 (PHARMACEUTICAL) | PL-10...)Show SMILES COC(=O)N[C@@H](C(c1ccccc1)c1ccccc1)C(=O)NCCCC[C@@H](CO)N(CC(C)C)S(=O)(=O)c1ccc(N)cc1 |r| Show InChI InChI=1S/C33H44N4O6S/c1-24(2)22-37(44(41,42)29-19-17-27(34)18-20-29)28(23-38)16-10-11-21-35-32(39)31(36-33(40)43-3)30(25-12-6-4-7-13-25)26-14-8-5-9-15-26/h4-9,12-15,17-20,24,28,30-31,38H,10-11,16,21-23,34H2,1-3H3,(H,35,39)(H,36,40)/t28-,31-/m0/s1 | PDB
UniProtKB/TrEMBL
B.MOAD
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
PDB
UniChem
| PDB
Article
PubMed
| n/a | n/a | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of HIV1 protease |
Bioorg Med Chem Lett 20: 4065-8 (2010)
Article DOI: 10.1016/j.bmcl.2010.05.082
BindingDB Entry DOI: 10.7270/Q2SJ1PFR |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Protease
(Human immunodeficiency virus 1 (HIV-1)) | BDBM50190623
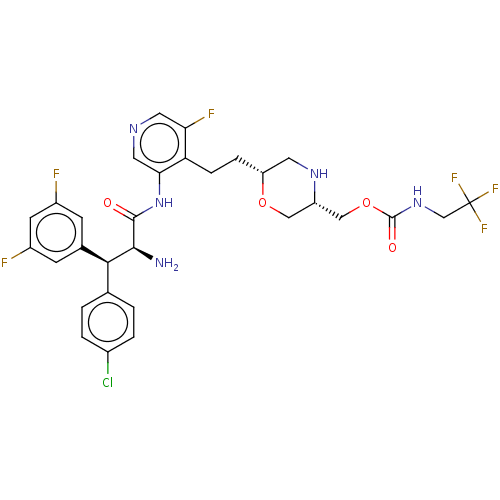
(CHEMBL3828743)Show SMILES N[C@@H]([C@@H](c1ccc(Cl)cc1)c1cc(F)cc(F)c1)C(=O)Nc1cncc(F)c1CC[C@@H]1CN[C@H](COC(=O)NCC(F)(F)F)CO1 |r| Show InChI InChI=1S/C30H30ClF6N5O4/c31-18-3-1-16(2-4-18)26(17-7-19(32)9-20(33)8-17)27(38)28(43)42-25-12-39-11-24(34)23(25)6-5-22-10-40-21(13-45-22)14-46-29(44)41-15-30(35,36)37/h1-4,7-9,11-12,21-22,26-27,40H,5-6,10,13-15,38H2,(H,41,44)(H,42,43)/t21-,22+,26-,27-/m0/s1 | PDB
UniProtKB/TrEMBL
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.700 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck & Co. Inc
Curated by ChEMBL
| Assay Description
Inhibition of HIV1 protease expressed in Escherichia coli using Val-Ser-Gln-Asn-(beta-naphtyl)Ala-Pro-Ile-Val as substrate preincubated for 30 mins f... |
ACS Med Chem Lett 8: 1292-1297 (2017)
Article DOI: 10.1021/acsmedchemlett.7b00386
BindingDB Entry DOI: 10.7270/Q2K35X57 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase JAK2
(Homo sapiens (Human)) | BDBM50355405
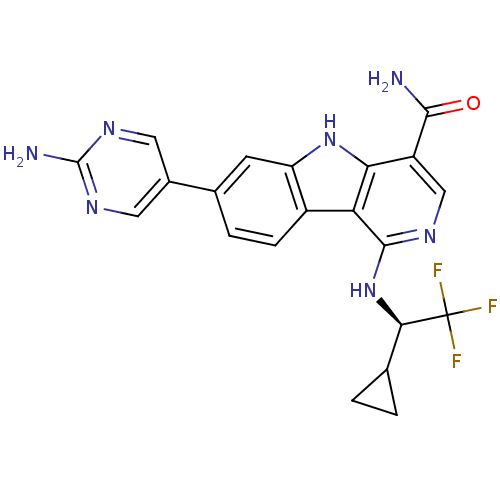
(CHEMBL1835153)Show SMILES NC(=O)c1cnc(N[C@H](C2CC2)C(F)(F)F)c2c3ccc(cc3[nH]c12)-c1cnc(N)nc1 |r| Show InChI InChI=1S/C21H18F3N7O/c22-21(23,24)17(9-1-2-9)31-19-15-12-4-3-10(11-6-28-20(26)29-7-11)5-14(12)30-16(15)13(8-27-19)18(25)32/h3-9,17,30H,1-2H2,(H2,25,32)(H,27,31)(H2,26,28,29)/t17-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 0.800 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck& Co.
Curated by ChEMBL
| Assay Description
Inhibition of JAK2 using biotin-EQEDEPEGDYFEWLE- NH2 as substrate |
J Med Chem 54: 7334-49 (2011)
Article DOI: 10.1021/jm200909u
BindingDB Entry DOI: 10.7270/Q2T43TGS |
More data for this
Ligand-Target Pair | |
Protease
(Human immunodeficiency virus 1 (HIV-1)) | BDBM50190623

(CHEMBL3828743)Show SMILES N[C@@H]([C@@H](c1ccc(Cl)cc1)c1cc(F)cc(F)c1)C(=O)Nc1cncc(F)c1CC[C@@H]1CN[C@H](COC(=O)NCC(F)(F)F)CO1 |r| Show InChI InChI=1S/C30H30ClF6N5O4/c31-18-3-1-16(2-4-18)26(17-7-19(32)9-20(33)8-17)27(38)28(43)42-25-12-39-11-24(34)23(25)6-5-22-10-40-21(13-45-22)14-46-29(44)41-15-30(35,36)37/h1-4,7-9,11-12,21-22,26-27,40H,5-6,10,13-15,38H2,(H,41,44)(H,42,43)/t21-,22+,26-,27-/m0/s1 | PDB
UniProtKB/TrEMBL
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.800 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of wild-type HIV1 protease expressed in Escherichia coli assessed as reduction in product formation preincubated for 30 mins followed by a... |
ACS Med Chem Lett 7: 702-7 (2016)
Article DOI: 10.1021/acsmedchemlett.6b00135
BindingDB Entry DOI: 10.7270/Q28W3G74 |
More data for this
Ligand-Target Pair | |
Ribosomal protein S6 kinase alpha-3
(Homo sapiens (Human)) | BDBM50536679
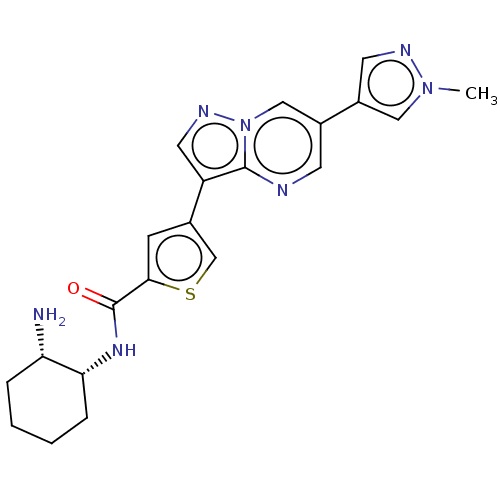
(CHEMBL4568087)Show SMILES Cn1cc(cn1)-c1cnc2c(cnn2c1)-c1csc(c1)C(=O)N[C@@H]1CCCC[C@@H]1N |r| Show InChI InChI=1S/C21H23N7OS/c1-27-10-15(8-24-27)14-7-23-20-16(9-25-28(20)11-14)13-6-19(30-12-13)21(29)26-18-5-3-2-4-17(18)22/h6-12,17-18H,2-5,22H2,1H3,(H,26,29)/t17-,18+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | <1 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck and Co.
Curated by ChEMBL
| Assay Description
Inhibition of full-length recombinant human His-tagged RPS6KA3 expressed in baculovirus expression system by Z'-LYTE assay |
Bioorg Med Chem Lett 26: 4362-6 (2016)
Article DOI: 10.1016/j.bmcl.2016.02.003
BindingDB Entry DOI: 10.7270/Q2NP27X5 |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase TBK1
(Homo sapiens (Human)) | BDBM50536679

(CHEMBL4568087)Show SMILES Cn1cc(cn1)-c1cnc2c(cnn2c1)-c1csc(c1)C(=O)N[C@@H]1CCCC[C@@H]1N |r| Show InChI InChI=1S/C21H23N7OS/c1-27-10-15(8-24-27)14-7-23-20-16(9-25-28(20)11-14)13-6-19(30-12-13)21(29)26-18-5-3-2-4-17(18)22/h6-12,17-18H,2-5,22H2,1H3,(H,26,29)/t17-,18+/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | <1 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck and Co.
Curated by ChEMBL
| Assay Description
Inhibition of full-length recombinant human GST-tagged TBK1 expressed in insect cells by Z'-LYTE assay |
Bioorg Med Chem Lett 26: 4362-6 (2016)
Article DOI: 10.1016/j.bmcl.2016.02.003
BindingDB Entry DOI: 10.7270/Q2NP27X5 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase JAK2
(Homo sapiens (Human)) | BDBM50355451

(CHEMBL1835137)Show SMILES C[C@H](Nc1ncc(C(N)=O)c2[nH]c3cc(ccc3c12)-c1cnn(C)c1)C1CC1 |r| Show InChI InChI=1S/C21H22N6O/c1-11(12-3-4-12)25-21-18-15-6-5-13(14-8-24-27(2)10-14)7-17(15)26-19(18)16(9-23-21)20(22)28/h5-12,26H,3-4H2,1-2H3,(H2,22,28)(H,23,25)/t11-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck& Co.
Curated by ChEMBL
| Assay Description
Inhibition of JAK2 using biotin-EQEDEPEGDYFEWLE- NH2 as substrate |
J Med Chem 54: 7334-49 (2011)
Article DOI: 10.1021/jm200909u
BindingDB Entry DOI: 10.7270/Q2T43TGS |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase JAK2
(Homo sapiens (Human)) | BDBM50355415

(CHEMBL1833986)Show SMILES NC(=O)c1cnc(NC(C2CC2)C2CC2)c2c3ccc(cc3[nH]c12)-c1cnc(N)nc1 Show InChI InChI=1S/C23H23N7O/c24-21(31)16-10-26-22(30-19(11-1-2-11)12-3-4-12)18-15-6-5-13(7-17(15)29-20(16)18)14-8-27-23(25)28-9-14/h5-12,19,29H,1-4H2,(H2,24,31)(H,26,30)(H2,25,27,28) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck& Co.
Curated by ChEMBL
| Assay Description
Inhibition of JAK2 using biotin-EQEDEPEGDYFEWLE- NH2 as substrate |
J Med Chem 54: 7334-49 (2011)
Article DOI: 10.1021/jm200909u
BindingDB Entry DOI: 10.7270/Q2T43TGS |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase BTK
(Homo sapiens (Human)) | BDBM50536679

(CHEMBL4568087)Show SMILES Cn1cc(cn1)-c1cnc2c(cnn2c1)-c1csc(c1)C(=O)N[C@@H]1CCCC[C@@H]1N |r| Show InChI InChI=1S/C21H23N7OS/c1-27-10-15(8-24-27)14-7-23-20-16(9-25-28(20)11-14)13-6-19(30-12-13)21(29)26-18-5-3-2-4-17(18)22/h6-12,17-18H,2-5,22H2,1H3,(H,26,29)/t17-,18+/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | <1 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck and Co.
Curated by ChEMBL
| Assay Description
Inhibition of full-length recombinant human His-tagged BTK expressed in baculovirus expression system by Z'-LYTE assay |
Bioorg Med Chem Lett 26: 4362-6 (2016)
Article DOI: 10.1016/j.bmcl.2016.02.003
BindingDB Entry DOI: 10.7270/Q2NP27X5 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase JAK2
(Homo sapiens (Human)) | BDBM50355416

(CHEMBL1835149)Show SMILES NC(=O)c1cnc(N[C@H](C2CC2)C(F)(F)F)c2c3ccc(cc3[nH]c12)-c1cn[nH]c1 |r| Show InChI InChI=1S/C20H17F3N6O/c21-20(22,23)17(9-1-2-9)29-19-15-12-4-3-10(11-6-26-27-7-11)5-14(12)28-16(15)13(8-25-19)18(24)30/h3-9,17,28H,1-2H2,(H2,24,30)(H,25,29)(H,26,27)/t17-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck& Co.
Curated by ChEMBL
| Assay Description
Inhibition of JAK2 using biotin-EQEDEPEGDYFEWLE- NH2 as substrate |
J Med Chem 54: 7334-49 (2011)
Article DOI: 10.1021/jm200909u
BindingDB Entry DOI: 10.7270/Q2T43TGS |
More data for this
Ligand-Target Pair | |
Testis-specific serine/threonine-protein kinase 1
(Homo sapiens (Human)) | BDBM50536679

(CHEMBL4568087)Show SMILES Cn1cc(cn1)-c1cnc2c(cnn2c1)-c1csc(c1)C(=O)N[C@@H]1CCCC[C@@H]1N |r| Show InChI InChI=1S/C21H23N7OS/c1-27-10-15(8-24-27)14-7-23-20-16(9-25-28(20)11-14)13-6-19(30-12-13)21(29)26-18-5-3-2-4-17(18)22/h6-12,17-18H,2-5,22H2,1H3,(H,26,29)/t17-,18+/m0/s1 | KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | <1 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck and Co.
Curated by ChEMBL
| Assay Description
Inhibition of full-length recombinant human His-tagged STK22D expressed in baculovirus expression system by Z'-LYTE assay |
Bioorg Med Chem Lett 26: 4362-6 (2016)
Article DOI: 10.1016/j.bmcl.2016.02.003
BindingDB Entry DOI: 10.7270/Q2NP27X5 |
More data for this
Ligand-Target Pair | |
MAP/microtubule affinity-regulating kinase 3
(Homo sapiens (Human)) | BDBM50536677
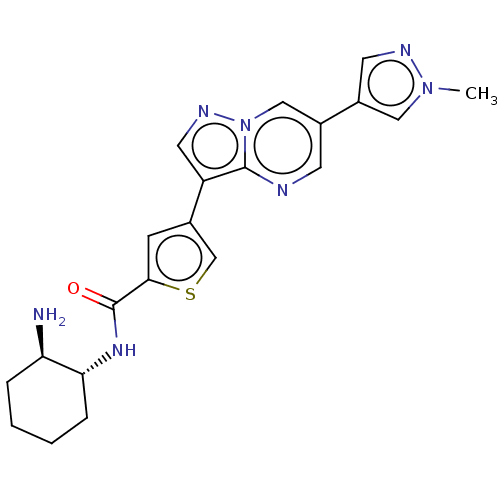
(CHEMBL4532781)Show SMILES Cn1cc(cn1)-c1cnc2c(cnn2c1)-c1csc(c1)C(=O)N[C@@H]1CCCC[C@H]1N |r| Show InChI InChI=1S/C21H23N7OS/c1-27-10-15(8-24-27)14-7-23-20-16(9-25-28(20)11-14)13-6-19(30-12-13)21(29)26-18-5-3-2-4-17(18)22/h6-12,17-18H,2-5,22H2,1H3,(H,26,29)/t17-,18-/m1/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck and Co.
Curated by ChEMBL
| Assay Description
Inhibition of full-length recombinant human GST-tagged MARK3 expressed in baculovirus expression system using biotinylated-Cdc25C peptide substrate m... |
Bioorg Med Chem Lett 26: 4362-6 (2016)
Article DOI: 10.1016/j.bmcl.2016.02.003
BindingDB Entry DOI: 10.7270/Q2NP27X5 |
More data for this
Ligand-Target Pair | |
MAP/microtubule affinity-regulating kinase 3
(Homo sapiens (Human)) | BDBM50536675
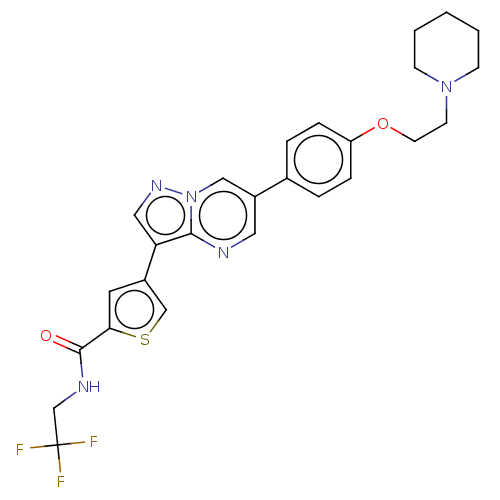
(CHEMBL4569508)Show SMILES FC(F)(F)CNC(=O)c1cc(cs1)-c1cnn2cc(cnc12)-c1ccc(OCCN2CCCCC2)cc1 Show InChI InChI=1S/C26H26F3N5O2S/c27-26(28,29)17-31-25(35)23-12-19(16-37-23)22-14-32-34-15-20(13-30-24(22)34)18-4-6-21(7-5-18)36-11-10-33-8-2-1-3-9-33/h4-7,12-16H,1-3,8-11,17H2,(H,31,35) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck and Co.
Curated by ChEMBL
| Assay Description
Inhibition of full-length recombinant human GST-tagged MARK3 expressed in baculovirus expression system using biotinylated-Cdc25C peptide substrate m... |
Bioorg Med Chem Lett 26: 4362-6 (2016)
Article DOI: 10.1016/j.bmcl.2016.02.003
BindingDB Entry DOI: 10.7270/Q2NP27X5 |
More data for this
Ligand-Target Pair | |
MAP/microtubule affinity-regulating kinase 3
(Homo sapiens (Human)) | BDBM50208911
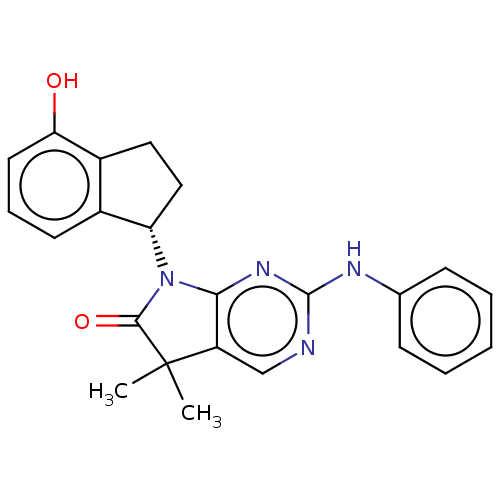
(CHEMBL3884319)Show SMILES CC1(C)C(=O)N([C@H]2CCc3c2cccc3O)c2nc(Nc3ccccc3)ncc12 |r| Show InChI InChI=1S/C23H22N4O2/c1-23(2)17-13-24-22(25-14-7-4-3-5-8-14)26-20(17)27(21(23)29)18-12-11-16-15(18)9-6-10-19(16)28/h3-10,13,18,28H,11-12H2,1-2H3,(H,24,25,26)/t18-/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck& Co.
Curated by ChEMBL
| Assay Description
Inhibition of full length human MARK3 using biotin labeled peptide substrate by HTRF based assay |
Bioorg Med Chem Lett 27: 114-120 (2017)
Article DOI: 10.1016/j.bmcl.2016.08.068
BindingDB Entry DOI: 10.7270/Q2N018JC |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase ABL1
(Homo sapiens (Human)) | BDBM50536679

(CHEMBL4568087)Show SMILES Cn1cc(cn1)-c1cnc2c(cnn2c1)-c1csc(c1)C(=O)N[C@@H]1CCCC[C@@H]1N |r| Show InChI InChI=1S/C21H23N7OS/c1-27-10-15(8-24-27)14-7-23-20-16(9-25-28(20)11-14)13-6-19(30-12-13)21(29)26-18-5-3-2-4-17(18)22/h6-12,17-18H,2-5,22H2,1H3,(H,26,29)/t17-,18+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck and Co.
Curated by ChEMBL
| Assay Description
Inhibition of full-length human His-tagged ABL1 expressed in baculovirus expression system by Z'-LYTE assay |
Bioorg Med Chem Lett 26: 4362-6 (2016)
Article DOI: 10.1016/j.bmcl.2016.02.003
BindingDB Entry DOI: 10.7270/Q2NP27X5 |
More data for this
Ligand-Target Pair | |
MAP/microtubule affinity-regulating kinase 3
(Homo sapiens (Human)) | BDBM50536692
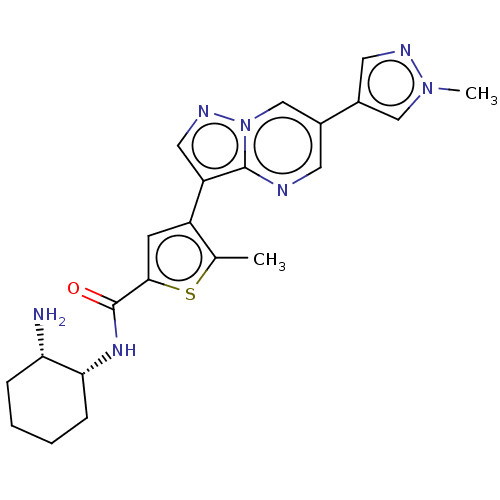
(CHEMBL4550486)Show SMILES Cc1sc(cc1-c1cnn2cc(cnc12)-c1cnn(C)c1)C(=O)N[C@@H]1CCCC[C@@H]1N |r| Show InChI InChI=1S/C22H25N7OS/c1-13-16(7-20(31-13)22(30)27-19-6-4-3-5-18(19)23)17-10-26-29-12-14(8-24-21(17)29)15-9-25-28(2)11-15/h7-12,18-19H,3-6,23H2,1-2H3,(H,27,30)/t18-,19+/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck and Co.
Curated by ChEMBL
| Assay Description
Inhibition of full-length recombinant human GST-tagged MARK3 expressed in baculovirus expression system using biotinylated-Cdc25C peptide substrate m... |
Bioorg Med Chem Lett 26: 4362-6 (2016)
Article DOI: 10.1016/j.bmcl.2016.02.003
BindingDB Entry DOI: 10.7270/Q2NP27X5 |
More data for this
Ligand-Target Pair | |
High affinity nerve growth factor receptor
(Homo sapiens (Human)) | BDBM50022671

(CHEMBL3298265)Show SMILES Cc1cn2cc(CNc3ncnc4ccc(nc34)N3CCC[C@@H]3c3cc(F)ccc3F)nc2s1 |r| Show InChI InChI=1S/C24H21F2N7S/c1-14-11-32-12-16(30-24(32)34-14)10-27-23-22-19(28-13-29-23)6-7-21(31-22)33-8-2-3-20(33)17-9-15(25)4-5-18(17)26/h4-7,9,11-13,20H,2-3,8,10H2,1H3,(H,27,28,29)/t20-/m1/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of purified TrkA cytoplasmic domain (unknown origin) by HTRF assay |
J Med Chem 57: 5800-16 (2014)
Article DOI: 10.1021/jm5006429
BindingDB Entry DOI: 10.7270/Q2BV7J69 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase JAK1
(Homo sapiens (Human)) | BDBM50355405

(CHEMBL1835153)Show SMILES NC(=O)c1cnc(N[C@H](C2CC2)C(F)(F)F)c2c3ccc(cc3[nH]c12)-c1cnc(N)nc1 |r| Show InChI InChI=1S/C21H18F3N7O/c22-21(23,24)17(9-1-2-9)31-19-15-12-4-3-10(11-6-28-20(26)29-7-11)5-14(12)30-16(15)13(8-27-19)18(25)32/h3-9,17,30H,1-2H2,(H2,25,32)(H,27,31)(H2,26,28,29)/t17-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck& Co.
Curated by ChEMBL
| Assay Description
Inhibition of JAK1 |
J Med Chem 54: 7334-49 (2011)
Article DOI: 10.1021/jm200909u
BindingDB Entry DOI: 10.7270/Q2T43TGS |
More data for this
Ligand-Target Pair | |
Death-associated protein kinase 1
(Homo sapiens (Human)) | BDBM50536679

(CHEMBL4568087)Show SMILES Cn1cc(cn1)-c1cnc2c(cnn2c1)-c1csc(c1)C(=O)N[C@@H]1CCCC[C@@H]1N |r| Show InChI InChI=1S/C21H23N7OS/c1-27-10-15(8-24-27)14-7-23-20-16(9-25-28(20)11-14)13-6-19(30-12-13)21(29)26-18-5-3-2-4-17(18)22/h6-12,17-18H,2-5,22H2,1H3,(H,26,29)/t17-,18+/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.90 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck and Co.
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human GST-tagged DAPK1 catalytic domain expressed in baculovirus expression system by Adapta assay |
Bioorg Med Chem Lett 26: 4362-6 (2016)
Article DOI: 10.1016/j.bmcl.2016.02.003
BindingDB Entry DOI: 10.7270/Q2NP27X5 |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data