 Found 19105 hits with Last Name = 'su' and Initial = 'r'
Found 19105 hits with Last Name = 'su' and Initial = 'r' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Histamine H3 receptor
(Homo sapiens (Human)) | BDBM50138119
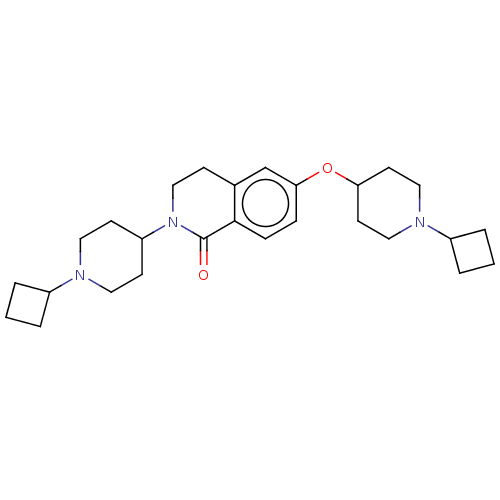
(CHEMBL3753475)Show SMILES O=C1N(CCc2cc(OC3CCN(CC3)C3CCC3)ccc12)C1CCN(CC1)C1CCC1 Show InChI InChI=1S/C27H39N3O2/c31-27-26-8-7-25(32-24-12-16-29(17-13-24)22-5-2-6-22)19-20(26)9-18-30(27)23-10-14-28(15-11-23)21-3-1-4-21/h7-8,19,21-24H,1-6,9-18H2 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.0300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Suven Life Sciences Ltd
Curated by ChEMBL
| Assay Description
Binding affinity to recombinant human H3 receptor |
Eur J Med Chem 108: 655-62 (2016)
Article DOI: 10.1016/j.ejmech.2015.12.005
BindingDB Entry DOI: 10.7270/Q2F191KF |
More data for this
Ligand-Target Pair | |
D(2) dopamine receptor
(Homo sapiens (Human)) | BDBM21397

(8-[4-(4-fluorophenyl)-4-keto-butyl]-1-phenyl-1,3,8...)Show SMILES Fc1ccc(cc1)C(=O)CCCN1CCC2(CC1)N(CNC2=O)c1ccccc1 Show InChI InChI=1S/C23H26FN3O2/c24-19-10-8-18(9-11-19)21(28)7-4-14-26-15-12-23(13-16-26)22(29)25-17-27(23)20-5-2-1-3-6-20/h1-3,5-6,8-11H,4,7,12-17H2,(H,25,29) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 0.0300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Toronto
Curated by PDSP Ki Database
| |
Nature 350: 610-4 (1991)
Article DOI: 10.1038/350610a0
BindingDB Entry DOI: 10.7270/Q24T6GVD |
More data for this
Ligand-Target Pair | |
Sodium-dependent dopamine transporter
(Rattus norvegicus (rat)) | BDBM50007404

(1-(3-Chloro-phenyl)-4-phenethyl-piperazine | CHEMB...)Show InChI InChI=1S/C18H21ClN2/c19-17-7-4-8-18(15-17)21-13-11-20(12-14-21)10-9-16-5-2-1-3-6-16/h1-8,15H,9-14H2 | Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Patents
| Article
PubMed
| 0.0400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Maryland
Curated by ChEMBL
| Assay Description
Displacement of [3H]WIN 35428 from dopamine transporter in rat striatum after 120 mins by liquid scintillation counting analysis |
Bioorg Med Chem Lett 23: 6920-6922 (2013)
Article DOI: 10.1016/j.bmcl.2013.09.038
BindingDB Entry DOI: 10.7270/Q2J969B5 |
More data for this
Ligand-Target Pair | |
Neuronal acetylcholine receptor subunit alpha-4/beta-2
(Homo sapiens (Human)) | BDBM221048
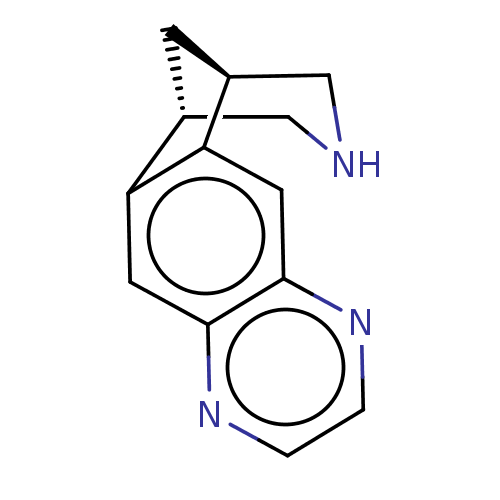
(US9284322, varenicline | US9303017, Varenicline)Show InChI InChI=1S/C13H13N3/c1-2-16-13-5-11-9-3-8(6-14-7-9)10(11)4-12(13)15-1/h1-2,4-5,8-9,14H,3,6-7H2/t8-,9+ | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| Purchase
DrugBank
MCE
PC cid
PC sid
PDB
UniChem
| PDB
Article
PubMed
| 0.0400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Suven Life Sciences Ltd.
Curated by ChEMBL
| Assay Description
Displacement of [3H]cytisine from human alpha4beta2 nAChR expressed in CHOK1 cell membrane by microbeta scintillation counting method |
J Med Chem 63: 2833-2853 (2020)
Article DOI: 10.1021/acs.jmedchem.9b00790
BindingDB Entry DOI: 10.7270/Q29027BJ |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Kappa-type opioid receptor
(Homo sapiens (Human)) | BDBM50007344
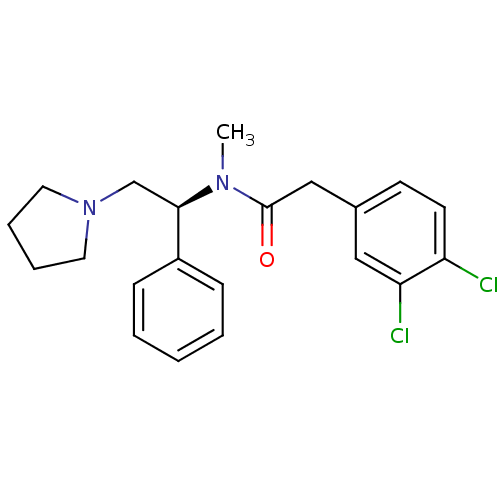
((S)-2-(3,4-Dichloro-phenyl)-N-methyl-N-(1-phenyl-2...)Show SMILES CN([C@H](CN1CCCC1)c1ccccc1)C(=O)Cc1ccc(Cl)c(Cl)c1 |r| Show InChI InChI=1S/C21H24Cl2N2O/c1-24(21(26)14-16-9-10-18(22)19(23)13-16)20(15-25-11-5-6-12-25)17-7-3-2-4-8-17/h2-4,7-10,13,20H,5-6,11-12,14-15H2,1H3/t20-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.0400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Adolor Corporation
Curated by ChEMBL
| Assay Description
Inhibitory constant against human Opioid receptor kappa using [3H]-diprenorphine as radio ligand |
Bioorg Med Chem Lett 15: 2647-52 (2005)
Article DOI: 10.1016/j.bmcl.2005.03.020
BindingDB Entry DOI: 10.7270/Q2PR7VH4 |
More data for this
Ligand-Target Pair | |
D(4) dopamine receptor
(RAT) | BDBM21397

(8-[4-(4-fluorophenyl)-4-keto-butyl]-1-phenyl-1,3,8...)Show SMILES Fc1ccc(cc1)C(=O)CCCN1CCC2(CC1)N(CNC2=O)c1ccccc1 Show InChI InChI=1S/C23H26FN3O2/c24-19-10-8-18(9-11-19)21(28)7-4-14-26-15-12-23(13-16-26)22(29)25-17-27(23)20-5-2-1-3-6-20/h1-3,5-6,8-11H,4,7,12-17H2,(H,25,29) | Reactome pathway
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 0.0500 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Toronto
Curated by PDSP Ki Database
| |
Nature 350: 610-4 (1991)
Article DOI: 10.1038/350610a0
BindingDB Entry DOI: 10.7270/Q24T6GVD |
More data for this
Ligand-Target Pair | |
D(2) dopamine receptor
(Homo sapiens (Human)) | BDBM21397

(8-[4-(4-fluorophenyl)-4-keto-butyl]-1-phenyl-1,3,8...)Show SMILES Fc1ccc(cc1)C(=O)CCCN1CCC2(CC1)N(CNC2=O)c1ccccc1 Show InChI InChI=1S/C23H26FN3O2/c24-19-10-8-18(9-11-19)21(28)7-4-14-26-15-12-23(13-16-26)22(29)25-17-27(23)20-5-2-1-3-6-20/h1-3,5-6,8-11H,4,7,12-17H2,(H,25,29) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 0.0500 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Toronto
Curated by PDSP Ki Database
| |
Nature 350: 610-4 (1991)
Article DOI: 10.1038/350610a0
BindingDB Entry DOI: 10.7270/Q24T6GVD |
More data for this
Ligand-Target Pair | |
D(2) dopamine receptor
(Homo sapiens (Human)) | BDBM21397

(8-[4-(4-fluorophenyl)-4-keto-butyl]-1-phenyl-1,3,8...)Show SMILES Fc1ccc(cc1)C(=O)CCCN1CCC2(CC1)N(CNC2=O)c1ccccc1 Show InChI InChI=1S/C23H26FN3O2/c24-19-10-8-18(9-11-19)21(28)7-4-14-26-15-12-23(13-16-26)22(29)25-17-27(23)20-5-2-1-3-6-20/h1-3,5-6,8-11H,4,7,12-17H2,(H,25,29) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 0.0530 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Toronto
Curated by PDSP Ki Database
| |
Nature 350: 610-4 (1991)
Article DOI: 10.1038/350610a0
BindingDB Entry DOI: 10.7270/Q24T6GVD |
More data for this
Ligand-Target Pair | |
Dopamine receptor D4
(CANINE) | BDBM50005120
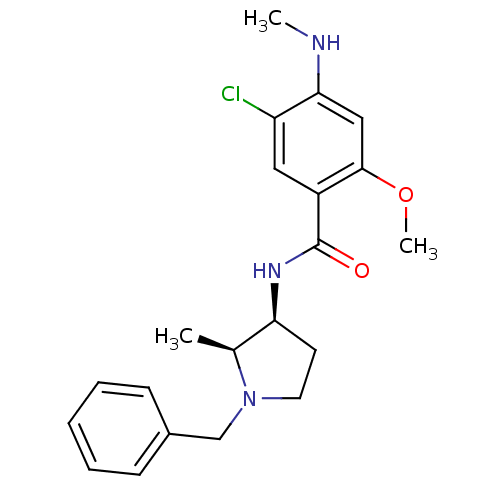
(CHEMBL274491 | N-((2S,3S)-1-Benzyl-2-methyl-pyrrol...)Show SMILES CNc1cc(OC)c(cc1Cl)C(=O)N[C@H]1CCN(Cc2ccccc2)[C@H]1C Show InChI InChI=1S/C21H26ClN3O2/c1-14-18(9-10-25(14)13-15-7-5-4-6-8-15)24-21(26)16-11-17(22)19(23-2)12-20(16)27-3/h4-8,11-12,14,18,23H,9-10,13H2,1-3H3,(H,24,26)/t14-,18-/m0/s1 | UniProtKB/TrEMBL
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.0600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Toronto
Curated by PDSP Ki Database
| |
Nature 350: 610-4 (1991)
Article DOI: 10.1038/350610a0
BindingDB Entry DOI: 10.7270/Q24T6GVD |
More data for this
Ligand-Target Pair | |
Gastrin/cholecystokinin type B receptor
(RAT) | BDBM50056101
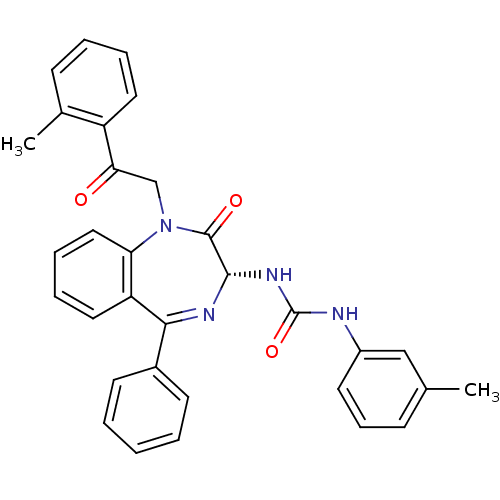
(1-[(R)-2-Oxo-1-(2-oxo-2-o-tolyl-ethyl)-5-phenyl-2,...)Show SMILES Cc1cccc(NC(=O)N[C@@H]2N=C(c3ccccc3)c3ccccc3N(CC(=O)c3ccccc3C)C2=O)c1 |t:11| Show InChI InChI=1S/C32H28N4O3/c1-21-11-10-15-24(19-21)33-32(39)35-30-31(38)36(20-28(37)25-16-7-6-12-22(25)2)27-18-9-8-17-26(27)29(34-30)23-13-4-3-5-14-23/h3-19,30H,20H2,1-2H3,(H2,33,35,39)/t30-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 0.0680 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Yamanouchi Pharmaceutical Co. Ltd.
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 269: 725-31 (1994)
BindingDB Entry DOI: 10.7270/Q2WD3Z2F |
More data for this
Ligand-Target Pair | |
D(2) dopamine receptor
(Rattus norvegicus (rat)) | BDBM21397

(8-[4-(4-fluorophenyl)-4-keto-butyl]-1-phenyl-1,3,8...)Show SMILES Fc1ccc(cc1)C(=O)CCCN1CCC2(CC1)N(CNC2=O)c1ccccc1 Show InChI InChI=1S/C23H26FN3O2/c24-19-10-8-18(9-11-19)21(28)7-4-14-26-15-12-23(13-16-26)22(29)25-17-27(23)20-5-2-1-3-6-20/h1-3,5-6,8-11H,4,7,12-17H2,(H,25,29) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 0.0690 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Toronto
Curated by PDSP Ki Database
| |
Nature 350: 610-4 (1991)
Article DOI: 10.1038/350610a0
BindingDB Entry DOI: 10.7270/Q24T6GVD |
More data for this
Ligand-Target Pair | |
Mu-type opioid receptor
(Homo sapiens (Human)) | BDBM50037134

((+)-trans-3-Cyclopropylmethyl-2,3,4,4aalpha,5,6,7,...)Show SMILES Oc1cccc2c1O[C@H]1CCC[C@H]3CN(CC4CC4)CC[C@]213 Show InChI InChI=1S/C19H25NO2/c21-16-5-2-4-15-18(16)22-17-6-1-3-14-12-20(11-13-7-8-13)10-9-19(14,15)17/h2,4-5,13-14,17,21H,1,3,6-12H2/t14-,17-,19-/m0/s1 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.0800 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Taiwan University
Curated by ChEMBL
| Assay Description
Displacement of [125I]OXY from human mu opioid receptor expressed in CHO cells |
J Med Chem 53: 1392-6 (2010)
Article DOI: 10.1021/jm901503e
BindingDB Entry DOI: 10.7270/Q2RX9C5Z |
More data for this
Ligand-Target Pair | |
D(4) dopamine receptor
(Homo sapiens (Human)) | BDBM21397

(8-[4-(4-fluorophenyl)-4-keto-butyl]-1-phenyl-1,3,8...)Show SMILES Fc1ccc(cc1)C(=O)CCCN1CCC2(CC1)N(CNC2=O)c1ccccc1 Show InChI InChI=1S/C23H26FN3O2/c24-19-10-8-18(9-11-19)21(28)7-4-14-26-15-12-23(13-16-26)22(29)25-17-27(23)20-5-2-1-3-6-20/h1-3,5-6,8-11H,4,7,12-17H2,(H,25,29) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 0.0800 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Toronto
Curated by PDSP Ki Database
| |
Nature 350: 610-4 (1991)
Article DOI: 10.1038/350610a0
BindingDB Entry DOI: 10.7270/Q24T6GVD |
More data for this
Ligand-Target Pair | |
P2Y purinoceptor 14
(Homo sapiens (Human)) | BDBM50512416

(CHEMBL4447162)Show SMILES Nc1ccc2c(-c3ccc(cc3C(O)=O)C(=O)NCCCCCCn3cc(CCCCN4CCC(CC4)c4ccc(cc4)-c4cc(cc5cc(ccc45)-c4ccc(cc4)C(F)(F)F)C(O)=O)nn3)c3ccc(=N)c(c3oc2c1S(O)(=O)=O)S(O)(=O)=O |(41.72,-13.14,;41.86,-14.67,;40.6,-15.57,;40.74,-17.1,;42.13,-17.74,;42.28,-19.27,;41.03,-20.17,;41.18,-21.7,;39.93,-22.59,;38.53,-21.95,;38.38,-20.42,;39.63,-19.53,;39.47,-17.99,;37.94,-17.87,;39.87,-16.5,;37.28,-22.84,;37.42,-24.37,;35.88,-22.2,;34.62,-23.09,;33.22,-22.45,;31.97,-23.35,;30.57,-22.71,;29.31,-23.6,;27.91,-22.96,;26.66,-23.85,;25.2,-23.36,;24.28,-24.6,;22.74,-24.59,;21.98,-23.25,;20.44,-23.23,;19.69,-21.89,;18.15,-21.87,;17.36,-23.2,;15.83,-23.18,;15.07,-21.84,;15.84,-20.51,;17.39,-20.53,;13.54,-21.84,;12.76,-23.17,;11.22,-23.15,;10.46,-21.81,;11.23,-20.49,;12.77,-20.49,;8.92,-21.8,;8.16,-20.45,;6.61,-20.45,;5.83,-21.78,;6.59,-23.12,;5.82,-24.44,;6.58,-25.78,;8.12,-25.8,;8.9,-24.47,;8.14,-23.13,;5.79,-27.1,;4.25,-27.09,;3.47,-28.41,;4.23,-29.76,;5.78,-29.76,;6.55,-28.44,;3.45,-31.08,;1.91,-31.07,;4.21,-32.42,;2.67,-32.41,;5.84,-19.11,;4.3,-19.1,;6.62,-17.78,;25.17,-25.85,;26.64,-25.39,;43.68,-19.91,;43.82,-21.43,;45.21,-22.07,;46.46,-21.19,;47.86,-21.84,;46.33,-19.66,;44.93,-19.02,;44.8,-17.49,;43.39,-16.85,;43.26,-15.32,;44.51,-14.44,;45.91,-15.09,;43.74,-13.1,;45.28,-13.09,;47.58,-18.77,;48.98,-19.41,;46.8,-17.43,;48.35,-17.43,)| Show InChI InChI=1S/C62H58F3N7O12S2/c63-62(64,65)44-16-12-37(13-17-44)40-14-18-46-42(31-40)32-43(60(74)75)34-50(46)39-10-8-36(9-11-39)38-24-29-71(30-25-38)27-6-3-7-45-35-72(70-69-45)28-5-2-1-4-26-68-59(73)41-15-19-47(51(33-41)61(76)77)54-48-20-22-52(66)57(85(78,79)80)55(48)84-56-49(54)21-23-53(67)58(56)86(81,82)83/h8-23,31-35,38,66H,1-7,24-30,67H2,(H,68,73)(H,74,75)(H,76,77)(H,78,79,80)(H,81,82,83) | Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.0800 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute of Diabetes and Digestive and Kidney Diseases
Curated by ChEMBL
| Assay Description
Antagonist activity at human P2Y14R expressed in CHO cells assessed as inhibition of UDPG-mediated reduction of forskolin-induced [3H]cAMP production... |
J Med Chem 59: 6149-68 (2016)
Article DOI: 10.1021/acs.jmedchem.6b00044
BindingDB Entry DOI: 10.7270/Q2DJ5K34 |
More data for this
Ligand-Target Pair | |
P2Y purinoceptor 14
(Homo sapiens (Human)) | BDBM50512416

(CHEMBL4447162)Show SMILES Nc1ccc2c(-c3ccc(cc3C(O)=O)C(=O)NCCCCCCn3cc(CCCCN4CCC(CC4)c4ccc(cc4)-c4cc(cc5cc(ccc45)-c4ccc(cc4)C(F)(F)F)C(O)=O)nn3)c3ccc(=N)c(c3oc2c1S(O)(=O)=O)S(O)(=O)=O |(41.72,-13.14,;41.86,-14.67,;40.6,-15.57,;40.74,-17.1,;42.13,-17.74,;42.28,-19.27,;41.03,-20.17,;41.18,-21.7,;39.93,-22.59,;38.53,-21.95,;38.38,-20.42,;39.63,-19.53,;39.47,-17.99,;37.94,-17.87,;39.87,-16.5,;37.28,-22.84,;37.42,-24.37,;35.88,-22.2,;34.62,-23.09,;33.22,-22.45,;31.97,-23.35,;30.57,-22.71,;29.31,-23.6,;27.91,-22.96,;26.66,-23.85,;25.2,-23.36,;24.28,-24.6,;22.74,-24.59,;21.98,-23.25,;20.44,-23.23,;19.69,-21.89,;18.15,-21.87,;17.36,-23.2,;15.83,-23.18,;15.07,-21.84,;15.84,-20.51,;17.39,-20.53,;13.54,-21.84,;12.76,-23.17,;11.22,-23.15,;10.46,-21.81,;11.23,-20.49,;12.77,-20.49,;8.92,-21.8,;8.16,-20.45,;6.61,-20.45,;5.83,-21.78,;6.59,-23.12,;5.82,-24.44,;6.58,-25.78,;8.12,-25.8,;8.9,-24.47,;8.14,-23.13,;5.79,-27.1,;4.25,-27.09,;3.47,-28.41,;4.23,-29.76,;5.78,-29.76,;6.55,-28.44,;3.45,-31.08,;1.91,-31.07,;4.21,-32.42,;2.67,-32.41,;5.84,-19.11,;4.3,-19.1,;6.62,-17.78,;25.17,-25.85,;26.64,-25.39,;43.68,-19.91,;43.82,-21.43,;45.21,-22.07,;46.46,-21.19,;47.86,-21.84,;46.33,-19.66,;44.93,-19.02,;44.8,-17.49,;43.39,-16.85,;43.26,-15.32,;44.51,-14.44,;45.91,-15.09,;43.74,-13.1,;45.28,-13.09,;47.58,-18.77,;48.98,-19.41,;46.8,-17.43,;48.35,-17.43,)| Show InChI InChI=1S/C62H58F3N7O12S2/c63-62(64,65)44-16-12-37(13-17-44)40-14-18-46-42(31-40)32-43(60(74)75)34-50(46)39-10-8-36(9-11-39)38-24-29-71(30-25-38)27-6-3-7-45-35-72(70-69-45)28-5-2-1-4-26-68-59(73)41-15-19-47(51(33-41)61(76)77)54-48-20-22-52(66)57(85(78,79)80)55(48)84-56-49(54)21-23-53(67)58(56)86(81,82)83/h8-23,31-35,38,66H,1-7,24-30,67H2,(H,68,73)(H,74,75)(H,76,77)(H,78,79,80)(H,81,82,83) | Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.0800 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute of Diabetes and Digestive and Kidney Diseases
Curated by ChEMBL
| Assay Description
Antagonist activity at human P2Y14R expressed in CHO cells assessed as inhibition of UDPG-mediated reduction of forskolin-induced [3H]cAMP production... |
J Med Chem 59: 6149-68 (2016)
Article DOI: 10.1021/acs.jmedchem.6b00044
BindingDB Entry DOI: 10.7270/Q2DJ5K34 |
More data for this
Ligand-Target Pair | |
Dopamine receptor D4
(CANINE) | BDBM50005120

(CHEMBL274491 | N-((2S,3S)-1-Benzyl-2-methyl-pyrrol...)Show SMILES CNc1cc(OC)c(cc1Cl)C(=O)N[C@H]1CCN(Cc2ccccc2)[C@H]1C Show InChI InChI=1S/C21H26ClN3O2/c1-14-18(9-10-25(14)13-15-7-5-4-6-8-15)24-21(26)16-11-17(22)19(23-2)12-20(16)27-3/h4-8,11-12,14,18,23H,9-10,13H2,1-3H3,(H,24,26)/t14-,18-/m0/s1 | UniProtKB/TrEMBL
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.0900 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Toronto
Curated by PDSP Ki Database
| |
Nature 350: 610-4 (1991)
Article DOI: 10.1038/350610a0
BindingDB Entry DOI: 10.7270/Q24T6GVD |
More data for this
Ligand-Target Pair | |
D(2) dopamine receptor
(Homo sapiens (Human)) | BDBM50005120

(CHEMBL274491 | N-((2S,3S)-1-Benzyl-2-methyl-pyrrol...)Show SMILES CNc1cc(OC)c(cc1Cl)C(=O)N[C@H]1CCN(Cc2ccccc2)[C@H]1C Show InChI InChI=1S/C21H26ClN3O2/c1-14-18(9-10-25(14)13-15-7-5-4-6-8-15)24-21(26)16-11-17(22)19(23-2)12-20(16)27-3/h4-8,11-12,14,18,23H,9-10,13H2,1-3H3,(H,24,26)/t14-,18-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.0900 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Toronto
Curated by PDSP Ki Database
| |
Nature 350: 610-4 (1991)
Article DOI: 10.1038/350610a0
BindingDB Entry DOI: 10.7270/Q24T6GVD |
More data for this
Ligand-Target Pair | |
Dopamine receptor D4
(CANINE) | BDBM50007518

((S)-3-chloro-5-ethyl-N-((1-ethylpyrrolidin-2-yl)me...)Show InChI InChI=1S/C17H25ClN2O3/c1-4-11-9-13(18)16(23-3)14(15(11)21)17(22)19-10-12-7-6-8-20(12)5-2/h9,12,21H,4-8,10H2,1-3H3,(H,19,22)/t12-/m0/s1 | UniProtKB/TrEMBL
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 0.0900 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Toronto
Curated by PDSP Ki Database
| |
Nature 350: 610-4 (1991)
Article DOI: 10.1038/350610a0
BindingDB Entry DOI: 10.7270/Q24T6GVD |
More data for this
Ligand-Target Pair | |
Neuronal acetylcholine receptor subunit alpha-7
(Homo sapiens (Human)) | BDBM50364875
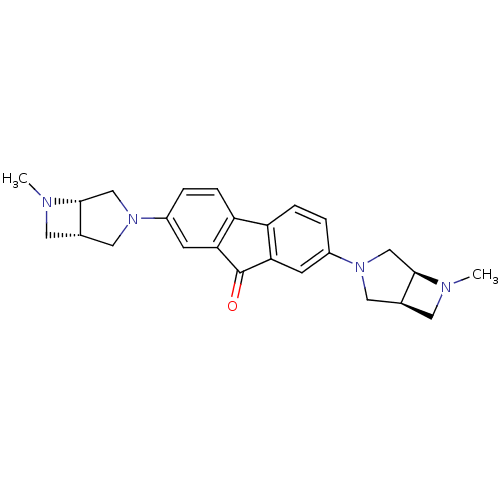
(CHEMBL1950156)Show SMILES CN1C[C@H]2CN(C[C@@H]12)c1ccc2-c3ccc(cc3C(=O)c2c1)N1C[C@@H]2CN(C)[C@@H]2C1 |r| Show InChI InChI=1S/C25H28N4O/c1-26-9-15-11-28(13-23(15)26)17-3-5-19-20-6-4-18(8-22(20)25(30)21(19)7-17)29-12-16-10-27(2)24(16)14-29/h3-8,15-16,23-24H,9-14H2,1-2H3/t15-,16-,23+,24+/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.0920 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The Johns Hopkins University School of Medicine
Curated by ChEMBL
| Assay Description
Binding affinity to alpha7 nAChR (unknown origin) |
J Med Chem 56: 7574-89 (2013)
Article DOI: 10.1021/jm401184f
BindingDB Entry DOI: 10.7270/Q2Z3215R |
More data for this
Ligand-Target Pair | |
Gastrin/cholecystokinin type B receptor
(RAT) | BDBM21147

((3S)-3-[(2S)-2-[(2S)-2-{2-[(2S)-2-[(2S)-2-[(3S)-3-...)Show SMILES CSCC[C@H](NC(=O)[C@H](Cc1ccc(OS(O)(=O)=O)cc1)NC(=O)[C@@H](N)CC(O)=O)C(=O)NCC(=O)N[C@@H](Cc1c[nH]c2ccccc12)C(=O)N[C@@H](CCSC)C(=O)N[C@@H](CC(O)=O)C(=O)N[C@@H](Cc1ccccc1)C(N)=O Show InChI InChI=1S/C49H62N10O16S3/c1-76-18-16-34(55-47(69)37(58-44(66)32(50)23-41(61)62)21-28-12-14-30(15-13-28)75-78(72,73)74)45(67)53-26-40(60)54-38(22-29-25-52-33-11-7-6-10-31(29)33)48(70)56-35(17-19-77-2)46(68)59-39(24-42(63)64)49(71)57-36(43(51)65)20-27-8-4-3-5-9-27/h3-15,25,32,34-39,52H,16-24,26,50H2,1-2H3,(H2,51,65)(H,53,67)(H,54,60)(H,55,69)(H,56,70)(H,57,71)(H,58,66)(H,59,68)(H,61,62)(H,63,64)(H,72,73,74)/t32-,34-,35-,36-,37-,38-,39-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
PC cid
PC sid
UniChem
Similars
| PubMed
| 0.0970 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Yamanouchi Pharmaceutical Co. Ltd.
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 269: 725-31 (1994)
BindingDB Entry DOI: 10.7270/Q2WD3Z2F |
More data for this
Ligand-Target Pair | |
Kappa-type opioid receptor
(Homo sapiens (Human)) | BDBM50037134

((+)-trans-3-Cyclopropylmethyl-2,3,4,4aalpha,5,6,7,...)Show SMILES Oc1cccc2c1O[C@H]1CCC[C@H]3CN(CC4CC4)CC[C@]213 Show InChI InChI=1S/C19H25NO2/c21-16-5-2-4-15-18(16)22-17-6-1-3-14-12-20(11-13-7-8-13)10-9-19(14,15)17/h2,4-5,13-14,17,21H,1,3,6-12H2/t14-,17-,19-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Taiwan University
Curated by ChEMBL
| Assay Description
Displacement of [125I]OXY from human kappa opioid receptor expressed in CHO cells |
J Med Chem 53: 1392-6 (2010)
Article DOI: 10.1021/jm901503e
BindingDB Entry DOI: 10.7270/Q2RX9C5Z |
More data for this
Ligand-Target Pair | |
Histamine H3 receptor
(Homo sapiens (Human)) | BDBM50138124
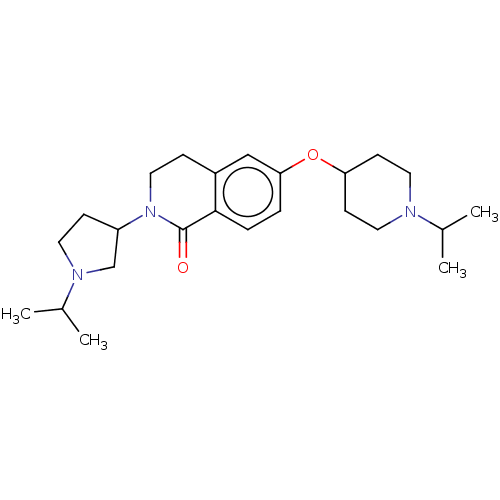
(CHEMBL3753901)Show SMILES CC(C)N1CCC(C1)N1CCc2cc(OC3CCN(CC3)C(C)C)ccc2C1=O Show InChI InChI=1S/C24H37N3O2/c1-17(2)25-12-9-21(10-13-25)29-22-5-6-23-19(15-22)7-14-27(24(23)28)20-8-11-26(16-20)18(3)4/h5-6,15,17-18,20-21H,7-14,16H2,1-4H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Suven Life Sciences Ltd
Curated by ChEMBL
| Assay Description
Binding affinity to recombinant human H3 receptor |
Eur J Med Chem 108: 655-62 (2016)
Article DOI: 10.1016/j.ejmech.2015.12.005
BindingDB Entry DOI: 10.7270/Q2F191KF |
More data for this
Ligand-Target Pair | |
Kappa-type opioid receptor
(Homo sapiens (Human)) | BDBM50037134

((+)-trans-3-Cyclopropylmethyl-2,3,4,4aalpha,5,6,7,...)Show SMILES Oc1cccc2c1O[C@H]1CCC[C@H]3CN(CC4CC4)CC[C@]213 Show InChI InChI=1S/C19H25NO2/c21-16-5-2-4-15-18(16)22-17-6-1-3-14-12-20(11-13-7-8-13)10-9-19(14,15)17/h2,4-5,13-14,17,21H,1,3,6-12H2/t14-,17-,19-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Taiwan University
Curated by ChEMBL
| Assay Description
Displacement of [125I]OXY from human kappa opioid receptor expressed in CHO cells |
J Med Chem 53: 1392-6 (2010)
Article DOI: 10.1021/jm901503e
BindingDB Entry DOI: 10.7270/Q2RX9C5Z |
More data for this
Ligand-Target Pair | |
Mu-type opioid receptor
(Homo sapiens (Human)) | BDBM50529518
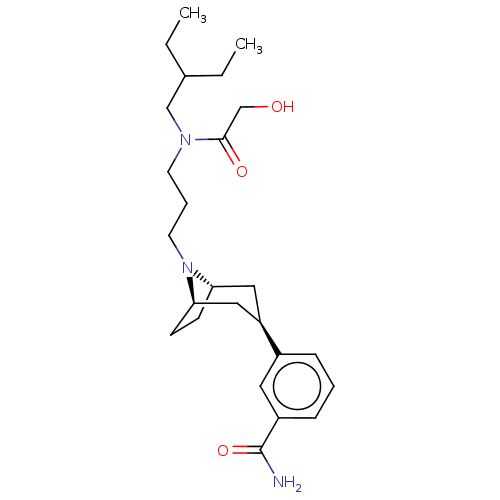
(CHEMBL4463675)Show SMILES [H][C@]12CC[C@]([H])(C[C@@H](C1)c1cccc(c1)C(N)=O)N2CCCN(CC(CC)CC)C(=O)CO |r,TLB:9:7:18:3.2| Show InChI InChI=1S/C25H39N3O3/c1-3-18(4-2)16-27(24(30)17-29)11-6-12-28-22-9-10-23(28)15-21(14-22)19-7-5-8-20(13-19)25(26)31/h5,7-8,13,18,21-23,29H,3-4,6,9-12,14-17H2,1-2H3,(H2,26,31)/t21-,22+,23- | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Theravance Biopharma US, Inc.
Curated by ChEMBL
| Assay Description
Displacement of [3H]-DPN from recombinant human mu opioid receptor expressed in CHO-K1 cell membranes by radioligand binding assay |
ACS Med Chem Lett 10: 1641-1647 (2019)
Article DOI: 10.1021/acsmedchemlett.9b00406
BindingDB Entry DOI: 10.7270/Q21C219X |
More data for this
Ligand-Target Pair | |
Mu-type opioid receptor
(Homo sapiens (Human)) | BDBM50529515

(CHEMBL3919761)Show SMILES CS(=O)(=O)CC(=O)N(CCN1[C@H]2CC[C@@H]1C[C@@H](C2)c1cccc(c1)C(N)=O)CC1CCCCC1 |r| Show InChI InChI=1S/C26H39N3O4S/c1-34(32,33)18-25(30)28(17-19-6-3-2-4-7-19)12-13-29-23-10-11-24(29)16-22(15-23)20-8-5-9-21(14-20)26(27)31/h5,8-9,14,19,22-24H,2-4,6-7,10-13,15-18H2,1H3,(H2,27,31)/t22-,23+,24- | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Theravance Biopharma US, Inc.
Curated by ChEMBL
| Assay Description
Displacement of [3H]-DPN from recombinant human mu opioid receptor expressed in CHO-K1 cell membranes by radioligand binding assay |
ACS Med Chem Lett 10: 1641-1647 (2019)
Article DOI: 10.1021/acsmedchemlett.9b00406
BindingDB Entry DOI: 10.7270/Q21C219X |
More data for this
Ligand-Target Pair | |
Mu-type opioid receptor
(Homo sapiens (Human)) | BDBM50529514
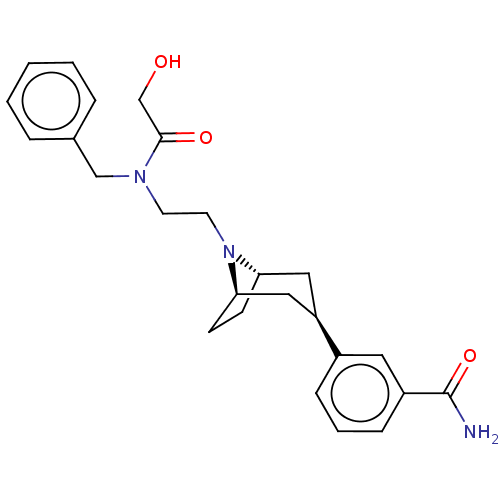
(CHEMBL3906031)Show SMILES NC(=O)c1cccc(c1)[C@@H]1C[C@@H]2CC[C@H](C1)N2CCN(Cc1ccccc1)C(=O)CO |r| Show InChI InChI=1S/C25H31N3O3/c26-25(31)20-8-4-7-19(13-20)21-14-22-9-10-23(15-21)28(22)12-11-27(24(30)17-29)16-18-5-2-1-3-6-18/h1-8,13,21-23,29H,9-12,14-17H2,(H2,26,31)/t21-,22+,23- | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Theravance Biopharma US, Inc.
Curated by ChEMBL
| Assay Description
Displacement of [3H]-DPN from recombinant human mu opioid receptor expressed in CHO-K1 cell membranes by radioligand binding assay |
ACS Med Chem Lett 10: 1641-1647 (2019)
Article DOI: 10.1021/acsmedchemlett.9b00406
BindingDB Entry DOI: 10.7270/Q21C219X |
More data for this
Ligand-Target Pair | |
Mu-type opioid receptor
(Homo sapiens (Human)) | BDBM50045758
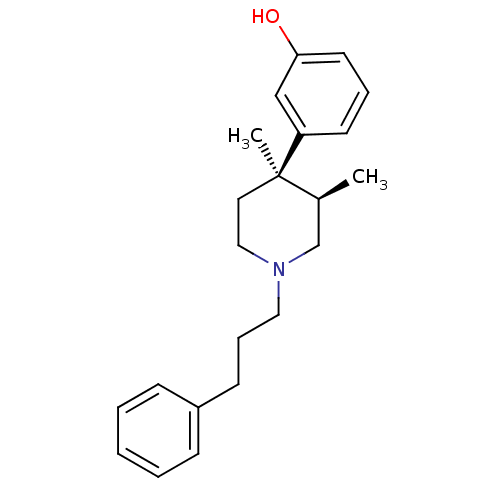
(3-[3,4-Dimethyl-1-(3-phenyl-propyl)-piperidin-4-yl...)Show SMILES C[C@H]1CN(CCCc2ccccc2)CC[C@@]1(C)c1cccc(O)c1 Show InChI InChI=1S/C22H29NO/c1-18-17-23(14-7-10-19-8-4-3-5-9-19)15-13-22(18,2)20-11-6-12-21(24)16-20/h3-6,8-9,11-12,16,18,24H,7,10,13-15,17H2,1-2H3/t18-,22+/m0/s1 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Theravance Biopharma US, Inc.
Curated by ChEMBL
| Assay Description
Displacement of [3H]-DPN from recombinant human mu opioid receptor expressed in CHO-K1 cell membranes by radioligand binding assay |
ACS Med Chem Lett 10: 1641-1647 (2019)
Article DOI: 10.1021/acsmedchemlett.9b00406
BindingDB Entry DOI: 10.7270/Q21C219X |
More data for this
Ligand-Target Pair | |
Kappa-type opioid receptor
(Cavia porcellus (domestic guinea pig)) | BDBM50529515

(CHEMBL3919761)Show SMILES CS(=O)(=O)CC(=O)N(CCN1[C@H]2CC[C@@H]1C[C@@H](C2)c1cccc(c1)C(N)=O)CC1CCCCC1 |r| Show InChI InChI=1S/C26H39N3O4S/c1-34(32,33)18-25(30)28(17-19-6-3-2-4-7-19)12-13-29-23-10-11-24(29)16-22(15-23)20-8-5-9-21(14-20)26(27)31/h5,8-9,14,19,22-24H,2-4,6-7,10-13,15-18H2,1H3,(H2,27,31)/t22-,23+,24- | PDB
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Theravance Biopharma US, Inc.
Curated by ChEMBL
| Assay Description
Displacement of [3H]-DPN from guinea pig kappa opioid receptor expressed in CHO-K1 cell membranes by radioligand binding assay |
ACS Med Chem Lett 10: 1641-1647 (2019)
Article DOI: 10.1021/acsmedchemlett.9b00406
BindingDB Entry DOI: 10.7270/Q21C219X |
More data for this
Ligand-Target Pair | |
Mu-type opioid receptor
(Homo sapiens (Human)) | BDBM50529522

(CHEMBL4471516)Show SMILES [H][C@]12CC[C@]([H])(C[C@@H](C1)c1cccc(c1)C(N)=O)N2CCN(CC1CCCCC1)C(=O)c1ccco1 |r,TLB:9:7:18:3.2| Show InChI InChI=1S/C28H37N3O3/c29-27(32)22-9-4-8-21(16-22)23-17-24-11-12-25(18-23)31(24)14-13-30(19-20-6-2-1-3-7-20)28(33)26-10-5-15-34-26/h4-5,8-10,15-16,20,23-25H,1-3,6-7,11-14,17-19H2,(H2,29,32)/t23-,24+,25- | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Theravance Biopharma US, Inc.
Curated by ChEMBL
| Assay Description
Displacement of [3H]-DPN from recombinant human mu opioid receptor expressed in CHO-K1 cell membranes by radioligand binding assay |
ACS Med Chem Lett 10: 1641-1647 (2019)
Article DOI: 10.1021/acsmedchemlett.9b00406
BindingDB Entry DOI: 10.7270/Q21C219X |
More data for this
Ligand-Target Pair | |
Mu-type opioid receptor
(Homo sapiens (Human)) | BDBM50529505
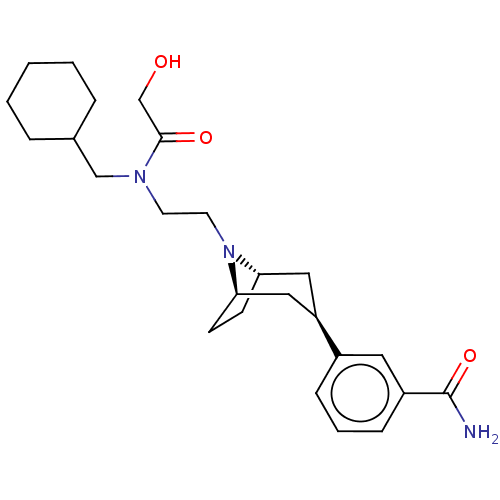
(CHEMBL3954068)Show SMILES NC(=O)c1cccc(c1)[C@@H]1C[C@@H]2CC[C@H](C1)N2CCN(CC1CCCCC1)C(=O)CO |r| Show InChI InChI=1S/C25H37N3O3/c26-25(31)20-8-4-7-19(13-20)21-14-22-9-10-23(15-21)28(22)12-11-27(24(30)17-29)16-18-5-2-1-3-6-18/h4,7-8,13,18,21-23,29H,1-3,5-6,9-12,14-17H2,(H2,26,31)/t21-,22+,23- | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Theravance Biopharma US, Inc.
Curated by ChEMBL
| Assay Description
Displacement of [3H]-DPN from recombinant human mu opioid receptor expressed in CHO-K1 cell membranes by radioligand binding assay |
ACS Med Chem Lett 10: 1641-1647 (2019)
Article DOI: 10.1021/acsmedchemlett.9b00406
BindingDB Entry DOI: 10.7270/Q21C219X |
More data for this
Ligand-Target Pair | |
Kappa-type opioid receptor
(Cavia porcellus (domestic guinea pig)) | BDBM50529522

(CHEMBL4471516)Show SMILES [H][C@]12CC[C@]([H])(C[C@@H](C1)c1cccc(c1)C(N)=O)N2CCN(CC1CCCCC1)C(=O)c1ccco1 |r,TLB:9:7:18:3.2| Show InChI InChI=1S/C28H37N3O3/c29-27(32)22-9-4-8-21(16-22)23-17-24-11-12-25(18-23)31(24)14-13-30(19-20-6-2-1-3-7-20)28(33)26-10-5-15-34-26/h4-5,8-10,15-16,20,23-25H,1-3,6-7,11-14,17-19H2,(H2,29,32)/t23-,24+,25- | PDB
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Theravance Biopharma US, Inc.
Curated by ChEMBL
| Assay Description
Displacement of [3H]-DPN from guinea pig kappa opioid receptor expressed in CHO-K1 cell membranes by radioligand binding assay |
ACS Med Chem Lett 10: 1641-1647 (2019)
Article DOI: 10.1021/acsmedchemlett.9b00406
BindingDB Entry DOI: 10.7270/Q21C219X |
More data for this
Ligand-Target Pair | |
Mu-type opioid receptor
(Homo sapiens (Human)) | BDBM50529509
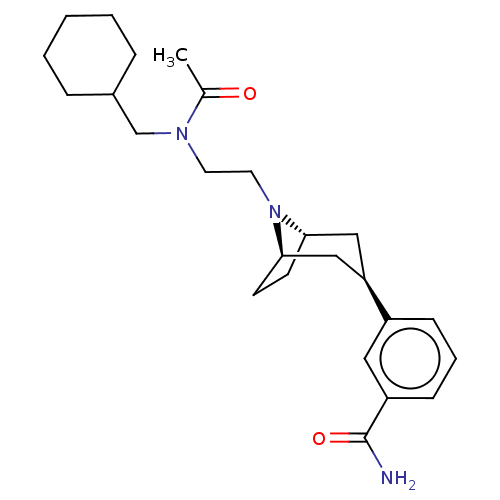
(CHEMBL4467513)Show SMILES [H][C@]12CC[C@]([H])(C[C@@H](C1)c1cccc(c1)C(N)=O)N2CCN(CC1CCCCC1)C(C)=O |r,TLB:9:7:18:3.2| Show InChI InChI=1S/C25H37N3O2/c1-18(29)27(17-19-6-3-2-4-7-19)12-13-28-23-10-11-24(28)16-22(15-23)20-8-5-9-21(14-20)25(26)30/h5,8-9,14,19,22-24H,2-4,6-7,10-13,15-17H2,1H3,(H2,26,30)/t22-,23+,24- | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Theravance Biopharma US, Inc.
Curated by ChEMBL
| Assay Description
Displacement of [3H]-DPN from recombinant human mu opioid receptor expressed in CHO-K1 cell membranes by radioligand binding assay |
ACS Med Chem Lett 10: 1641-1647 (2019)
Article DOI: 10.1021/acsmedchemlett.9b00406
BindingDB Entry DOI: 10.7270/Q21C219X |
More data for this
Ligand-Target Pair | |
Gastrin/cholecystokinin type B receptor
(RAT) | BDBM50056101

(1-[(R)-2-Oxo-1-(2-oxo-2-o-tolyl-ethyl)-5-phenyl-2,...)Show SMILES Cc1cccc(NC(=O)N[C@@H]2N=C(c3ccccc3)c3ccccc3N(CC(=O)c3ccccc3C)C2=O)c1 |t:11| Show InChI InChI=1S/C32H28N4O3/c1-21-11-10-15-24(19-21)33-32(39)35-30-31(38)36(20-28(37)25-16-7-6-12-22(25)2)27-18-9-8-17-26(27)29(34-30)23-13-4-3-5-14-23/h3-19,30H,20H2,1-2H3,(H2,33,35,39)/t30-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 0.110 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Yamanouchi Pharmaceutical Co. Ltd.
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 269: 725-31 (1994)
BindingDB Entry DOI: 10.7270/Q2WD3Z2F |
More data for this
Ligand-Target Pair | |
Sigma non-opioid intracellular receptor 1
(RAT) | BDBM50374600
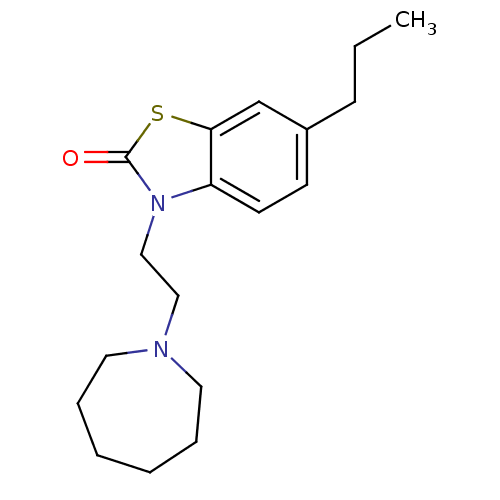
(CHEMBL272899)Show InChI InChI=1S/C18H26N2OS/c1-2-7-15-8-9-16-17(14-15)22-18(21)20(16)13-12-19-10-5-3-4-6-11-19/h8-9,14H,2-7,10-13H2,1H3 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.120 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Mississippi
Curated by PDSP Ki Database
| Assay Description
Displacement of [3H](+)-pentazocine from opioid sigma1 receptor in rat brain homogenate |
J Med Chem 51: 1482-6 (2008)
Article DOI: 10.1021/jm701357m
BindingDB Entry DOI: 10.7270/Q2736RTZ |
More data for this
Ligand-Target Pair | |
Sigma non-opioid intracellular receptor 1
(RAT) | BDBM50007410
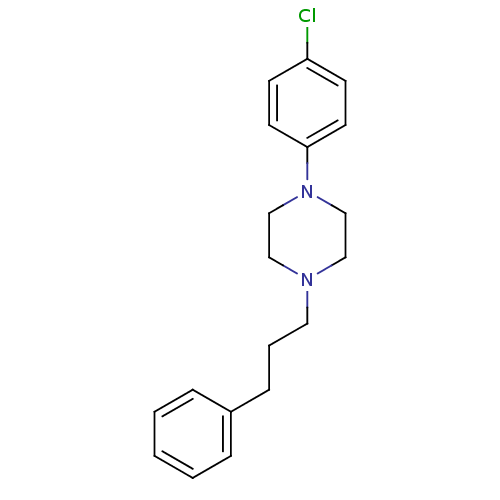
(1-(4-Chloro-phenyl)-4-(3-phenyl-propyl)-piperazine...)Show InChI InChI=1S/C19H23ClN2/c20-18-8-10-19(11-9-18)22-15-13-21(14-16-22)12-4-7-17-5-2-1-3-6-17/h1-3,5-6,8-11H,4,7,12-16H2 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.120 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Maryland
Curated by ChEMBL
| Assay Description
Displacement of [3H](+)-pentazocine from sigma1 receptor in rat brain after 120 mins by liquid scintillation counting analysis |
Bioorg Med Chem Lett 23: 6920-6922 (2013)
Article DOI: 10.1016/j.bmcl.2013.09.038
BindingDB Entry DOI: 10.7270/Q2J969B5 |
More data for this
Ligand-Target Pair | |
Kappa-type opioid receptor
(Cavia porcellus (domestic guinea pig)) | BDBM50529505

(CHEMBL3954068)Show SMILES NC(=O)c1cccc(c1)[C@@H]1C[C@@H]2CC[C@H](C1)N2CCN(CC1CCCCC1)C(=O)CO |r| Show InChI InChI=1S/C25H37N3O3/c26-25(31)20-8-4-7-19(13-20)21-14-22-9-10-23(15-21)28(22)12-11-27(24(30)17-29)16-18-5-2-1-3-6-18/h4,7-8,13,18,21-23,29H,1-3,5-6,9-12,14-17H2,(H2,26,31)/t21-,22+,23- | PDB
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.126 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Theravance Biopharma US, Inc.
Curated by ChEMBL
| Assay Description
Displacement of [3H]-DPN from guinea pig kappa opioid receptor expressed in CHO-K1 cell membranes by radioligand binding assay |
ACS Med Chem Lett 10: 1641-1647 (2019)
Article DOI: 10.1021/acsmedchemlett.9b00406
BindingDB Entry DOI: 10.7270/Q21C219X |
More data for this
Ligand-Target Pair | |
Mu-type opioid receptor
(Homo sapiens (Human)) | BDBM50529519

(CHEMBL3910689)Show SMILES NC(=O)c1cccc(c1)[C@@H]1C[C@@H]2CC[C@H](C1)N2CCN(CC1CCC(F)(F)CC1)C(=O)CO |r| Show InChI InChI=1S/C25H35F2N3O3/c26-25(27)8-6-17(7-9-25)15-29(23(32)16-31)10-11-30-21-4-5-22(30)14-20(13-21)18-2-1-3-19(12-18)24(28)33/h1-3,12,17,20-22,31H,4-11,13-16H2,(H2,28,33)/t20-,21+,22- | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.126 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Theravance Biopharma US, Inc.
Curated by ChEMBL
| Assay Description
Displacement of [3H]-DPN from recombinant human mu opioid receptor expressed in CHO-K1 cell membranes by radioligand binding assay |
ACS Med Chem Lett 10: 1641-1647 (2019)
Article DOI: 10.1021/acsmedchemlett.9b00406
BindingDB Entry DOI: 10.7270/Q21C219X |
More data for this
Ligand-Target Pair | |
Mu-type opioid receptor
(Homo sapiens (Human)) | BDBM50529520
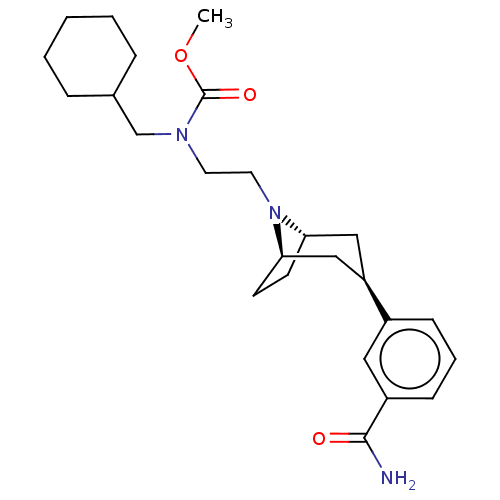
(CHEMBL4520212)Show SMILES [H][C@]12CC[C@]([H])(C[C@@H](C1)c1cccc(c1)C(N)=O)N2CCN(CC1CCCCC1)C(=O)OC |r,TLB:9:7:18:3.2| Show InChI InChI=1S/C25H37N3O3/c1-31-25(30)27(17-18-6-3-2-4-7-18)12-13-28-22-10-11-23(28)16-21(15-22)19-8-5-9-20(14-19)24(26)29/h5,8-9,14,18,21-23H,2-4,6-7,10-13,15-17H2,1H3,(H2,26,29)/t21-,22+,23- | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.126 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Theravance Biopharma US, Inc.
Curated by ChEMBL
| Assay Description
Displacement of [3H]-DPN from recombinant human mu opioid receptor expressed in CHO-K1 cell membranes by radioligand binding assay |
ACS Med Chem Lett 10: 1641-1647 (2019)
Article DOI: 10.1021/acsmedchemlett.9b00406
BindingDB Entry DOI: 10.7270/Q21C219X |
More data for this
Ligand-Target Pair | |
Mu-type opioid receptor
(Homo sapiens (Human)) | BDBM50529508
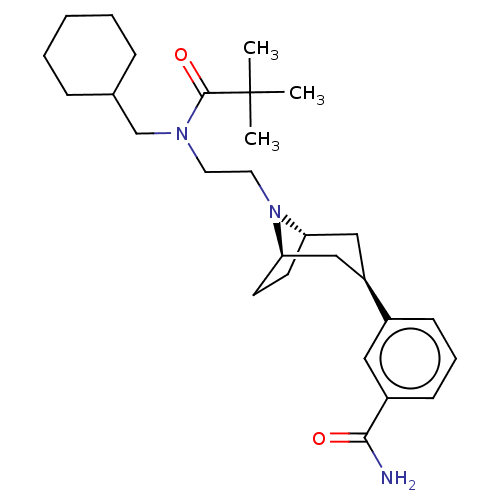
(CHEMBL4567073)Show SMILES [H][C@]12CC[C@]([H])(C[C@@H](C1)c1cccc(c1)C(N)=O)N2CCN(CC1CCCCC1)C(=O)C(C)(C)C |r,TLB:9:7:18:3.2| Show InChI InChI=1S/C28H43N3O2/c1-28(2,3)27(33)30(19-20-8-5-4-6-9-20)14-15-31-24-12-13-25(31)18-23(17-24)21-10-7-11-22(16-21)26(29)32/h7,10-11,16,20,23-25H,4-6,8-9,12-15,17-19H2,1-3H3,(H2,29,32)/t23-,24+,25- | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.126 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Theravance Biopharma US, Inc.
Curated by ChEMBL
| Assay Description
Displacement of [3H]-DPN from recombinant human mu opioid receptor expressed in CHO-K1 cell membranes by radioligand binding assay |
ACS Med Chem Lett 10: 1641-1647 (2019)
Article DOI: 10.1021/acsmedchemlett.9b00406
BindingDB Entry DOI: 10.7270/Q21C219X |
More data for this
Ligand-Target Pair | |
Kappa-type opioid receptor
(Cavia porcellus (domestic guinea pig)) | BDBM50529499
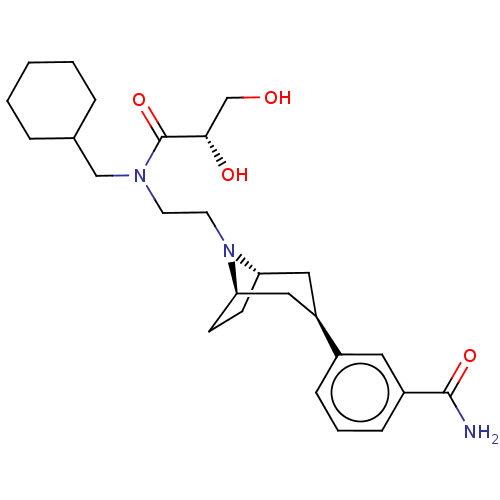
(Axelopran sulfate | TD-1211 Sulfate)Show SMILES OS(O)(=O)=O.[H][C@]12CC[C@]([H])(C[C@@H](C1)c1cccc(c1)C(N)=O)N2CCN(CC1CCCCC1)C(=O)[C@@H](O)CO |r,TLB:14:12:23:7.8| Show InChI InChI=1S/C26H39N3O4.H2O4S/c27-25(32)20-8-4-7-19(13-20)21-14-22-9-10-23(15-21)29(22)12-11-28(26(33)24(31)17-30)16-18-5-2-1-3-6-18;1-5(2,3)4/h4,7-8,13,18,21-24,30-31H,1-3,5-6,9-12,14-17H2,(H2,27,32);(H2,1,2,3,4)/t21-,22+,23-,24-;/m0./s1 | PDB
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.126 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Theravance Biopharma US, Inc.
Curated by ChEMBL
| Assay Description
Displacement of [3H]-DPN from guinea pig kappa opioid receptor expressed in CHO-K1 cell membranes by radioligand binding assay |
ACS Med Chem Lett 10: 1641-1647 (2019)
Article DOI: 10.1021/acsmedchemlett.9b00406
BindingDB Entry DOI: 10.7270/Q21C219X |
More data for this
Ligand-Target Pair | |
Kappa-type opioid receptor
(Cavia porcellus (domestic guinea pig)) | BDBM50529496
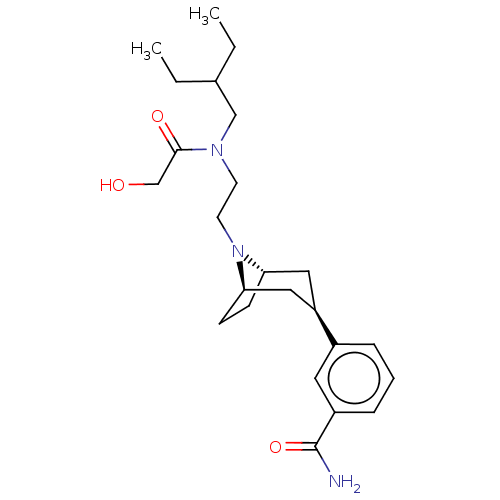
(CHEMBL4468796)Show SMILES [H][C@]12CC[C@]([H])(C[C@@H](C1)c1cccc(c1)C(N)=O)N2CCN(CC(CC)CC)C(=O)CO |r,TLB:9:7:18:3.2| Show InChI InChI=1S/C24H37N3O3/c1-3-17(4-2)15-26(23(29)16-28)10-11-27-21-8-9-22(27)14-20(13-21)18-6-5-7-19(12-18)24(25)30/h5-7,12,17,20-22,28H,3-4,8-11,13-16H2,1-2H3,(H2,25,30)/t20-,21+,22- | PDB
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.126 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Theravance Biopharma US, Inc.
Curated by ChEMBL
| Assay Description
Displacement of [3H]-DPN from guinea pig kappa opioid receptor expressed in CHO-K1 cell membranes by radioligand binding assay |
ACS Med Chem Lett 10: 1641-1647 (2019)
Article DOI: 10.1021/acsmedchemlett.9b00406
BindingDB Entry DOI: 10.7270/Q21C219X |
More data for this
Ligand-Target Pair | |
Kappa-type opioid receptor
(Cavia porcellus (domestic guinea pig)) | BDBM50529509

(CHEMBL4467513)Show SMILES [H][C@]12CC[C@]([H])(C[C@@H](C1)c1cccc(c1)C(N)=O)N2CCN(CC1CCCCC1)C(C)=O |r,TLB:9:7:18:3.2| Show InChI InChI=1S/C25H37N3O2/c1-18(29)27(17-19-6-3-2-4-7-19)12-13-28-23-10-11-24(28)16-22(15-23)20-8-5-9-21(14-20)25(26)30/h5,8-9,14,19,22-24H,2-4,6-7,10-13,15-17H2,1H3,(H2,26,30)/t22-,23+,24- | PDB
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.126 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Theravance Biopharma US, Inc.
Curated by ChEMBL
| Assay Description
Displacement of [3H]-DPN from guinea pig kappa opioid receptor expressed in CHO-K1 cell membranes by radioligand binding assay |
ACS Med Chem Lett 10: 1641-1647 (2019)
Article DOI: 10.1021/acsmedchemlett.9b00406
BindingDB Entry DOI: 10.7270/Q21C219X |
More data for this
Ligand-Target Pair | |
Sodium-dependent dopamine transporter
(Rattus norvegicus (rat)) | BDBM50494706
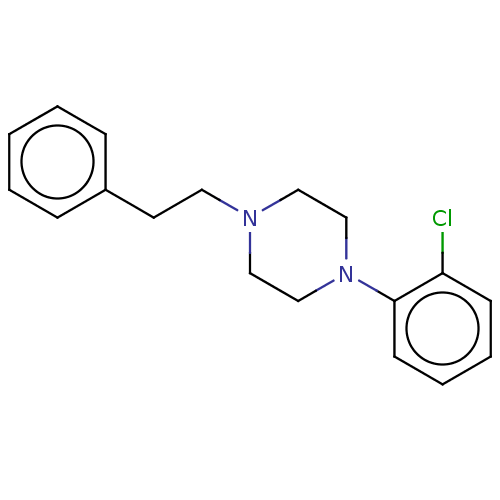
(CHEMBL3093268)Show InChI InChI=1S/C18H21ClN2/c19-17-8-4-5-9-18(17)21-14-12-20(13-15-21)11-10-16-6-2-1-3-7-16/h1-9H,10-15H2 | Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.130 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Maryland
Curated by ChEMBL
| Assay Description
Displacement of [3H]WIN 35428 from dopamine transporter in rat striatum after 120 mins by liquid scintillation counting analysis |
Bioorg Med Chem Lett 23: 6920-6922 (2013)
Article DOI: 10.1016/j.bmcl.2013.09.038
BindingDB Entry DOI: 10.7270/Q2J969B5 |
More data for this
Ligand-Target Pair | |
Cholinesterase
(Homo sapiens (Human)) | BDBM50234795
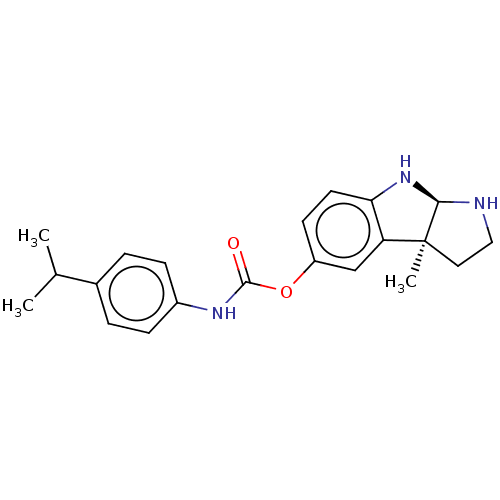
(CHEMBL4089082)Show SMILES [H][C@]12NCC[C@@]1(C)c1cc(OC(=O)Nc3ccc(cc3)C(C)C)ccc1N2 |r| Show InChI InChI=1S/C21H25N3O2/c1-13(2)14-4-6-15(7-5-14)23-20(25)26-16-8-9-18-17(12-16)21(3)10-11-22-19(21)24-18/h4-9,12-13,19,22,24H,10-11H2,1-3H3,(H,23,25)/t19-,21+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.131 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Martin-Luther University Halle-Wittenberg
Curated by ChEMBL
| Assay Description
Inhibition of human serum BChE using butyrylthiocholine iodide as substrate by Ellman's method |
Eur J Med Chem 126: 652-668 (2017)
Article DOI: 10.1016/j.ejmech.2016.11.056
BindingDB Entry DOI: 10.7270/Q2X0698K |
More data for this
Ligand-Target Pair | |
Neuronal acetylcholine receptor subunit alpha-4/beta-2
(Homo sapiens (Human)) | BDBM50538045
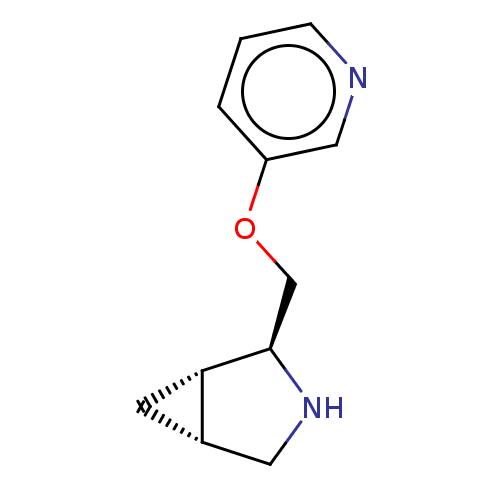
(CHEMBL4636163)Show SMILES Cl.[H][C@@]12C[C@]1([H])[C@@H](COc1cccnc1)NC2 |r| Show InChI InChI=1S/C11H14N2O.ClH/c1-2-9(6-12-3-1)14-7-11-10-4-8(10)5-13-11;/h1-3,6,8,10-11,13H,4-5,7H2;1H/t8-,10-,11+;/m0./s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.140 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Suven Life Sciences Ltd.
Curated by ChEMBL
| Assay Description
Displacement of [3H]cytisine from human alpha4beta2 nAChR expressed in CHOK1 cell membrane by microbeta scintillation counting method |
J Med Chem 63: 2833-2853 (2020)
Article DOI: 10.1021/acs.jmedchem.9b00790
BindingDB Entry DOI: 10.7270/Q29027BJ |
More data for this
Ligand-Target Pair | |
Sigma non-opioid intracellular receptor 1
(RAT) | BDBM50494706

(CHEMBL3093268)Show InChI InChI=1S/C18H21ClN2/c19-17-8-4-5-9-18(17)21-14-12-20(13-15-21)11-10-16-6-2-1-3-7-16/h1-9H,10-15H2 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.150 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Maryland
Curated by ChEMBL
| Assay Description
Displacement of [3H](+)-pentazocine from sigma1 receptor in rat brain after 120 mins by liquid scintillation counting analysis |
Bioorg Med Chem Lett 23: 6920-6922 (2013)
Article DOI: 10.1016/j.bmcl.2013.09.038
BindingDB Entry DOI: 10.7270/Q2J969B5 |
More data for this
Ligand-Target Pair | |
Kappa-type opioid receptor
(Cavia porcellus (domestic guinea pig)) | BDBM50529507

(CHEMBL3971059)Show SMILES CC(C)NC(=O)N(CCN1[C@H]2CC[C@@H]1C[C@@H](C2)c1cccc(c1)C(N)=O)CC1CCCCC1 |r| Show InChI InChI=1S/C27H42N4O2/c1-19(2)29-27(33)30(18-20-7-4-3-5-8-20)13-14-31-24-11-12-25(31)17-23(16-24)21-9-6-10-22(15-21)26(28)32/h6,9-10,15,19-20,23-25H,3-5,7-8,11-14,16-18H2,1-2H3,(H2,28,32)(H,29,33)/t23-,24+,25- | PDB
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.158 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Theravance Biopharma US, Inc.
Curated by ChEMBL
| Assay Description
Displacement of [3H]-DPN from guinea pig kappa opioid receptor expressed in CHO-K1 cell membranes by radioligand binding assay |
ACS Med Chem Lett 10: 1641-1647 (2019)
Article DOI: 10.1021/acsmedchemlett.9b00406
BindingDB Entry DOI: 10.7270/Q21C219X |
More data for this
Ligand-Target Pair | |
Mu-type opioid receptor
(Homo sapiens (Human)) | BDBM60212

((4R,4aS,7aR,12bS)-3-(cyclopropylmethyl)-4a,9-bis(o...)Show SMILES Oc1ccc2C[C@H]3N(CC4CC4)CC[C@@]45[C@@H](Oc1c24)C(=O)CC[C@@]35O Show InChI InChI=1S/C20H23NO4/c22-13-4-3-12-9-15-20(24)6-5-14(23)18-19(20,16(12)17(13)25-18)7-8-21(15)10-11-1-2-11/h3-4,11,15,18,22,24H,1-2,5-10H2/t15-,18+,19+,20-/m1/s1 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
DrugBank
MCE
KEGG
PC cid
PC sid
UniChem
Similars
| DrugBank
Article
PubMed
| 0.158 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Theravance Biopharma US, Inc.
Curated by ChEMBL
| Assay Description
Displacement of [3H]-DPN from recombinant human mu opioid receptor expressed in CHO-K1 cell membranes by radioligand binding assay |
ACS Med Chem Lett 10: 1641-1647 (2019)
Article DOI: 10.1021/acsmedchemlett.9b00406
BindingDB Entry DOI: 10.7270/Q21C219X |
More data for this
Ligand-Target Pair | |
Kappa-type opioid receptor
(Cavia porcellus (domestic guinea pig)) | BDBM50529510
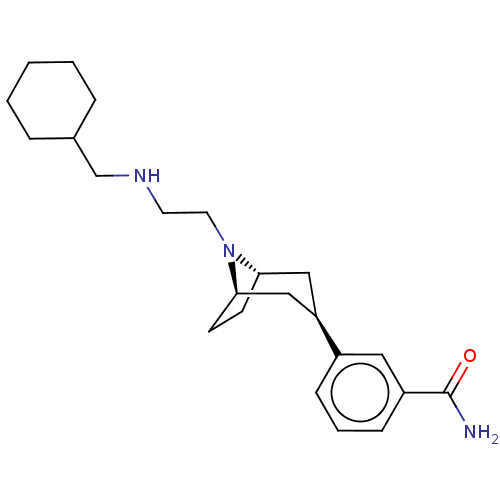
(CHEMBL4474822)Show SMILES [H][C@]12CC[C@]([H])(C[C@@H](C1)c1cccc(c1)C(N)=O)N2CCNCC1CCCCC1 |r,TLB:9:7:18:3.2| Show InChI InChI=1S/C23H35N3O/c24-23(27)19-8-4-7-18(13-19)20-14-21-9-10-22(15-20)26(21)12-11-25-16-17-5-2-1-3-6-17/h4,7-8,13,17,20-22,25H,1-3,5-6,9-12,14-16H2,(H2,24,27)/t20-,21+,22- | PDB
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.158 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Theravance Biopharma US, Inc.
Curated by ChEMBL
| Assay Description
Displacement of [3H]-DPN from guinea pig kappa opioid receptor expressed in CHO-K1 cell membranes by radioligand binding assay |
ACS Med Chem Lett 10: 1641-1647 (2019)
Article DOI: 10.1021/acsmedchemlett.9b00406
BindingDB Entry DOI: 10.7270/Q21C219X |
More data for this
Ligand-Target Pair | |
Mu-type opioid receptor
(Homo sapiens (Human)) | BDBM50529499

(Axelopran sulfate | TD-1211 Sulfate)Show SMILES OS(O)(=O)=O.[H][C@]12CC[C@]([H])(C[C@@H](C1)c1cccc(c1)C(N)=O)N2CCN(CC1CCCCC1)C(=O)[C@@H](O)CO |r,TLB:14:12:23:7.8| Show InChI InChI=1S/C26H39N3O4.H2O4S/c27-25(32)20-8-4-7-19(13-20)21-14-22-9-10-23(15-21)29(22)12-11-28(26(33)24(31)17-30)16-18-5-2-1-3-6-18;1-5(2,3)4/h4,7-8,13,18,21-24,30-31H,1-3,5-6,9-12,14-17H2,(H2,27,32);(H2,1,2,3,4)/t21-,22+,23-,24-;/m0./s1 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.158 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Theravance Biopharma US, Inc.
Curated by ChEMBL
| Assay Description
Displacement of [3H]-DPN from recombinant human mu opioid receptor expressed in CHO-K1 cell membranes by radioligand binding assay |
ACS Med Chem Lett 10: 1641-1647 (2019)
Article DOI: 10.1021/acsmedchemlett.9b00406
BindingDB Entry DOI: 10.7270/Q21C219X |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data