

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Histone deacetylase 1 (Homo sapiens (Human)) | BDBM24621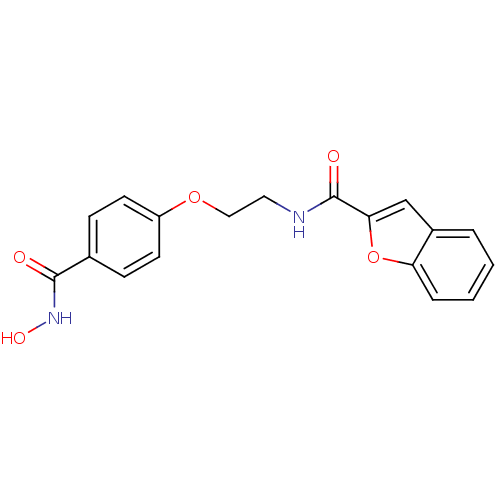 (CG-003 | N-{2-[4-(hydroxycarbamoyl)phenoxy]ethyl}-...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents | Article PubMed | 4 | -47.5 | n/a | n/a | n/a | n/a | n/a | 7.4 | 22 |
Celera Genomics | Assay Description HDAC activity was measured using a continuous trypsin-coupled assay. For inhibitor characterization, measurements were done using 96-well assay plat... | Mol Cancer Ther 5: 1309-17 (2006) Article DOI: 10.1158/1535-7163.MCT-05-0442 BindingDB Entry DOI: 10.7270/Q2DF6PJJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 1 (Homo sapiens (Human)) | BDBM24620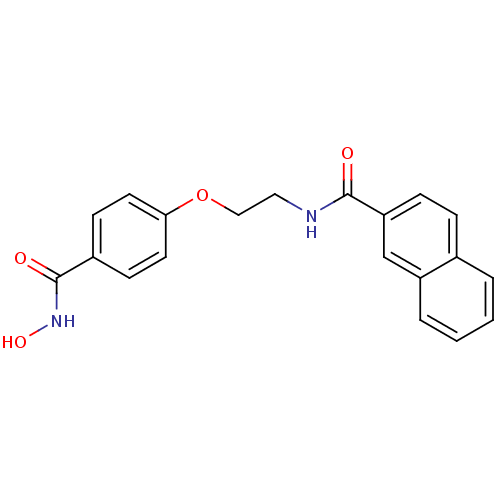 (CG-002 | N-{2-[4-(hydroxycarbamoyl)phenoxy]ethyl}n...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 6 | -46.5 | n/a | n/a | n/a | n/a | n/a | 7.4 | 22 |
Celera Genomics | Assay Description HDAC activity was measured using a continuous trypsin-coupled assay. For inhibitor characterization, measurements were done using 96-well assay plat... | Mol Cancer Ther 5: 1309-17 (2006) Article DOI: 10.1158/1535-7163.MCT-05-0442 BindingDB Entry DOI: 10.7270/Q2DF6PJJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lysine-specific histone demethylase 1A (Homo sapiens (Human)) | BDBM424912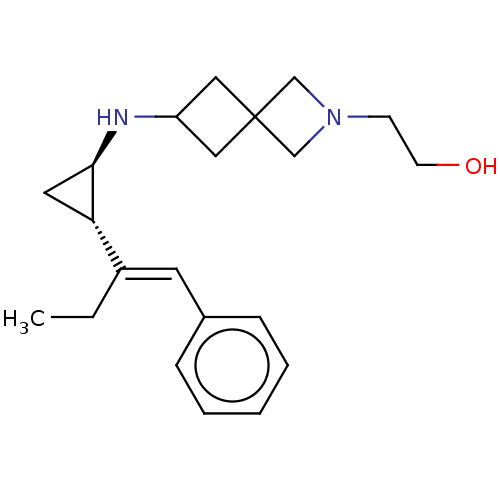 (2-(6-(((1R,2S)-2-((E)-1-phenylbut-1-en-2-yl)cyclop...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 6.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Constellation Pharmaceuticals Curated by ChEMBL | Assay Description Time dependant inhibition of recombinant N-terminal His-tagged LSD1 (unknown origin) expressed in Escherichia coli expression system assessed as inhi... | ACS Med Chem Lett 11: 1213-1220 (2020) Article DOI: 10.1021/acsmedchemlett.0c00060 BindingDB Entry DOI: 10.7270/Q22N55VZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 1 (Homo sapiens (Human)) | BDBM24622 (3-[(dimethylamino)methyl]-N-{2-[4-(hydroxycarbamoy...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem Patents Similars | Article PubMed | 7 | -46.1 | n/a | n/a | n/a | n/a | n/a | 7.4 | 22 |
Celera Genomics | Assay Description HDAC activity was measured using a continuous trypsin-coupled assay. For inhibitor characterization, measurements were done using 96-well assay plat... | Mol Cancer Ther 5: 1309-17 (2006) Article DOI: 10.1158/1535-7163.MCT-05-0442 BindingDB Entry DOI: 10.7270/Q2DF6PJJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 3 (Homo sapiens (Human)) | BDBM24622 (3-[(dimethylamino)methyl]-N-{2-[4-(hydroxycarbamoy...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem Patents Similars | Article PubMed | 8.20 | -45.7 | n/a | n/a | n/a | n/a | n/a | 7.4 | 22 |
Celera Genomics | Assay Description HDAC activity was measured using a continuous trypsin-coupled assay. For inhibitor characterization, measurements were done using 96-well assay plat... | Mol Cancer Ther 5: 1309-17 (2006) Article DOI: 10.1158/1535-7163.MCT-05-0442 BindingDB Entry DOI: 10.7270/Q2DF6PJJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lysine-specific histone demethylase 1A (Homo sapiens (Human)) | BDBM50158869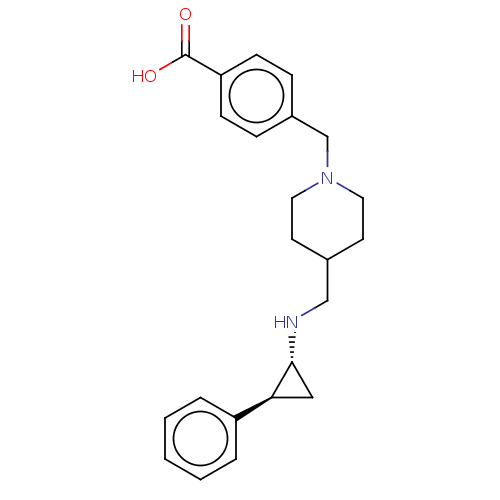 (CHEMBL3786182 | US10836743, Compound GSK-2879552 |...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | Article PubMed | 9.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Constellation Pharmaceuticals Curated by ChEMBL | Assay Description Time dependant inhibition of recombinant N-terminal His-tagged LSD1 (unknown origin) expressed in Escherichia coli expression system assessed as inhi... | ACS Med Chem Lett 11: 1213-1220 (2020) Article DOI: 10.1021/acsmedchemlett.0c00060 BindingDB Entry DOI: 10.7270/Q22N55VZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 6 (Homo sapiens (Human)) | BDBM24622 (3-[(dimethylamino)methyl]-N-{2-[4-(hydroxycarbamoy...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem Patents Similars | Article PubMed | 17 | -43.9 | n/a | n/a | n/a | n/a | n/a | 7.4 | 22 |
Celera Genomics | Assay Description HDAC activity was measured using a continuous trypsin-coupled assay. For inhibitor characterization, measurements were done using 96-well assay plat... | Mol Cancer Ther 5: 1309-17 (2006) Article DOI: 10.1158/1535-7163.MCT-05-0442 BindingDB Entry DOI: 10.7270/Q2DF6PJJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 2 (Homo sapiens (Human)) | BDBM24622 (3-[(dimethylamino)methyl]-N-{2-[4-(hydroxycarbamoy...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem Patents Similars | Article PubMed | 19 | -43.6 | n/a | n/a | n/a | n/a | n/a | 7.4 | 22 |
Celera Genomics | Assay Description HDAC activity was measured using a continuous trypsin-coupled assay. For inhibitor characterization, measurements were done using 96-well assay plat... | Mol Cancer Ther 5: 1309-17 (2006) Article DOI: 10.1158/1535-7163.MCT-05-0442 BindingDB Entry DOI: 10.7270/Q2DF6PJJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase BTK (Homo sapiens (Human)) | BDBM468103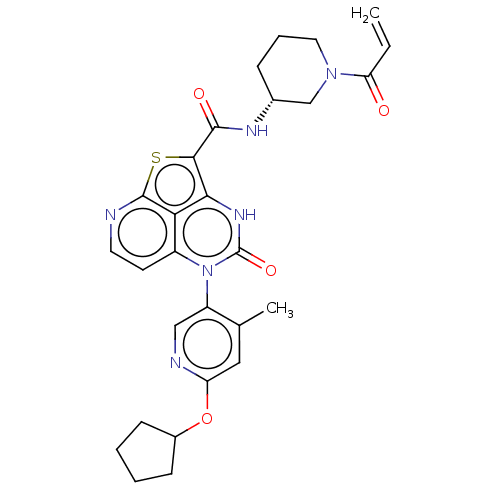 ((R)-N-(1-Acryloylpiperidin-3-yl)-5-(*S)-(6-(cyclop...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 22 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Citation and Details Article DOI: 10.1021/acs.jmedchem.2c01026 BindingDB Entry DOI: 10.7270/Q2MK6HZ3 | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Polyamine deacetylase HDAC10 (Homo sapiens (Human)) | BDBM24622 (3-[(dimethylamino)methyl]-N-{2-[4-(hydroxycarbamoy...) | NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem Patents Similars | Article PubMed | 24 | -43.1 | n/a | n/a | n/a | n/a | n/a | 7.4 | 22 |
Celera Genomics | Assay Description HDAC activity was measured using a continuous trypsin-coupled assay. For inhibitor characterization, measurements were done using 96-well assay plat... | Mol Cancer Ther 5: 1309-17 (2006) Article DOI: 10.1158/1535-7163.MCT-05-0442 BindingDB Entry DOI: 10.7270/Q2DF6PJJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase BTK (Homo sapiens (Human)) | BDBM468000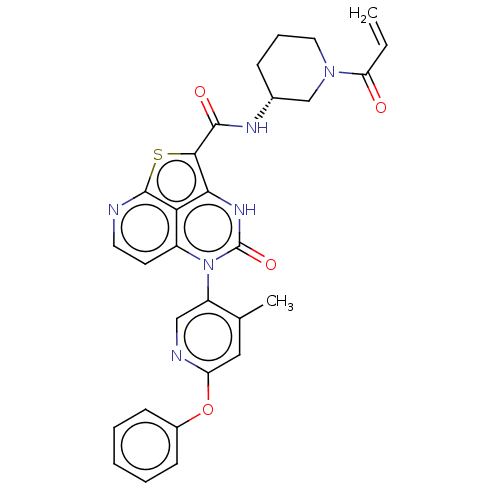 ((R)-N-(1-Acryloylpiperidin-3-yl)-5-(*S)-(4-methyl-...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 28 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Citation and Details Article DOI: 10.1021/acs.jmedchem.2c01026 BindingDB Entry DOI: 10.7270/Q2MK6HZ3 | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase BTK (Homo sapiens (Human)) | BDBM467718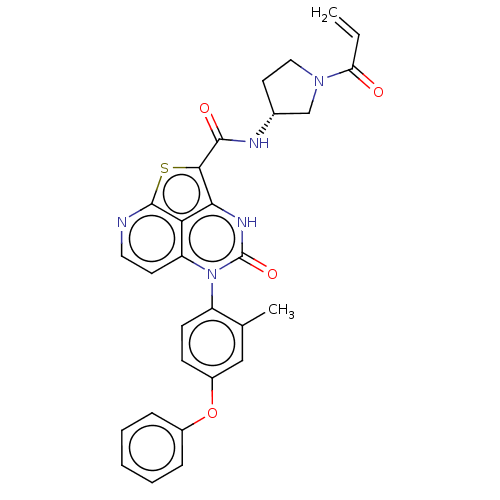 ((R)-N-(1-Acryloylpyrrolidin-3-yl)-5-(2-methyl-4-(2...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem | Article PubMed | 29 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Citation and Details Article DOI: 10.1021/acs.jmedchem.2c01026 BindingDB Entry DOI: 10.7270/Q2MK6HZ3 | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase BTK (Homo sapiens (Human)) | BDBM467367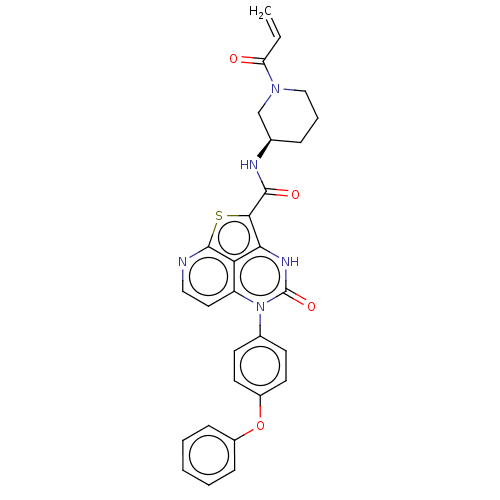 ((R)-N-(1-Acryloylpiperidin-3-yl)-4-oxo-5-(4-phenox...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 32 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Citation and Details Article DOI: 10.1021/acs.jmedchem.2c01026 BindingDB Entry DOI: 10.7270/Q2MK6HZ3 | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 1 (Homo sapiens (Human)) | BDBM24618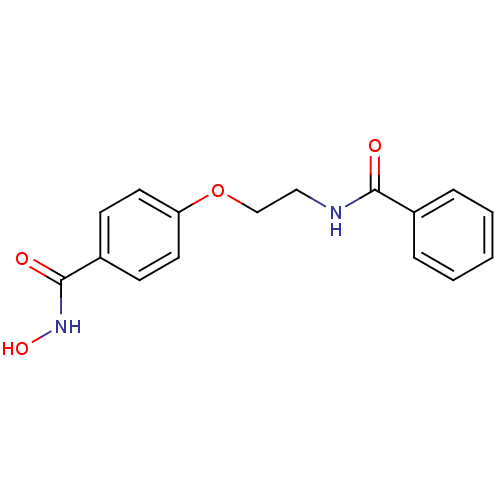 (CG-001 | N-hydroxy-4-[2-(phenylformamido)ethoxy]be...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | 43 | -41.6 | n/a | n/a | n/a | n/a | n/a | 7.4 | 22 |
Celera Genomics | Assay Description HDAC activity was measured using a continuous trypsin-coupled assay. For inhibitor characterization, measurements were done using 96-well assay plat... | Mol Cancer Ther 5: 1309-17 (2006) Article DOI: 10.1158/1535-7163.MCT-05-0442 BindingDB Entry DOI: 10.7270/Q2DF6PJJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase BTK (Homo sapiens (Human)) | BDBM50601991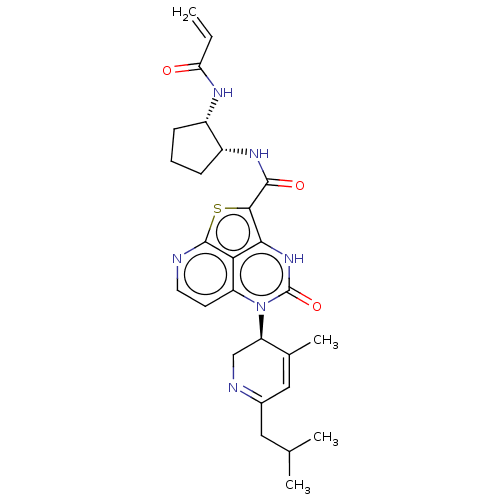 (CHEMBL5206366) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 49 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Citation and Details Article DOI: 10.1021/acs.jmedchem.2c01026 BindingDB Entry DOI: 10.7270/Q2MK6HZ3 | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase BTK (Homo sapiens (Human)) | BDBM468010 ((R)-N-(1-Acryloylpiperidin-3-yl)-5-(6-cyclobutoxy-...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 64 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Citation and Details Article DOI: 10.1021/acs.jmedchem.2c01026 BindingDB Entry DOI: 10.7270/Q2MK6HZ3 | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase BTK (Homo sapiens (Human)) | BDBM467836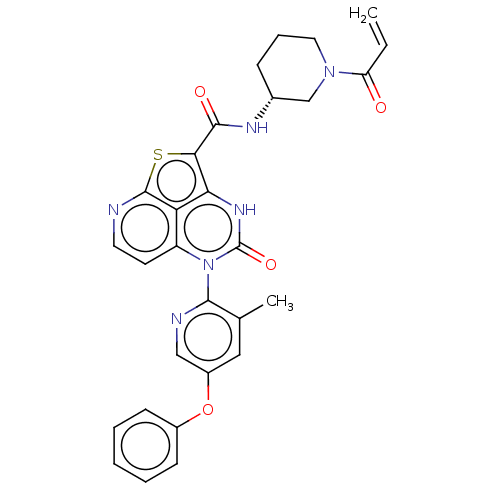 ((R)-N-(1-Acryloylpiperidin-3-yl)-5-(3-methyl-5-phe...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Citation and Details Article DOI: 10.1021/acs.jmedchem.2c01026 BindingDB Entry DOI: 10.7270/Q2MK6HZ3 | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase BTK (Homo sapiens (Human)) | BDBM468007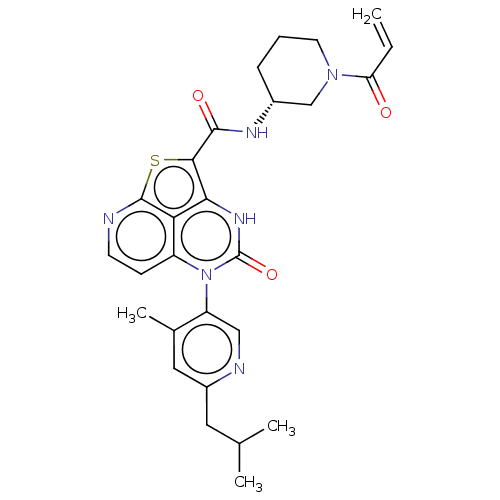 ((R)-N-(1-Acryloylpiperidin-3-yl)-5-(*S)-(6-isobuty...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 145 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Citation and Details Article DOI: 10.1021/acs.jmedchem.2c01026 BindingDB Entry DOI: 10.7270/Q2MK6HZ3 | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase BTK (Homo sapiens (Human)) | BDBM468124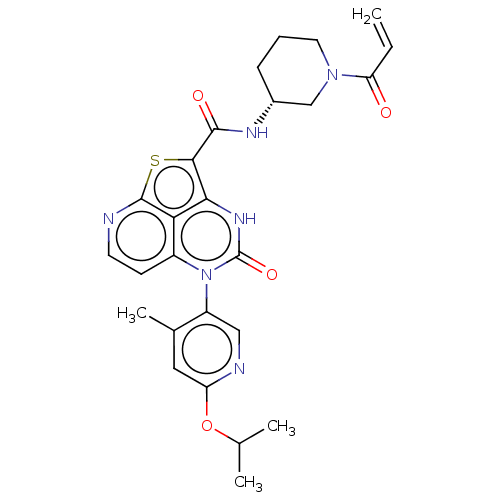 ((R)-N-(1-Acryloylpiperidin-3-yl)-5-(*S)-(6-isoprop...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 208 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Citation and Details Article DOI: 10.1021/acs.jmedchem.2c01026 BindingDB Entry DOI: 10.7270/Q2MK6HZ3 | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 8 (Homo sapiens (Human)) | BDBM24622 (3-[(dimethylamino)methyl]-N-{2-[4-(hydroxycarbamoy...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem Patents Similars | Article PubMed | 280 | -37.0 | n/a | n/a | n/a | n/a | n/a | 7.4 | 22 |
Celera Genomics | Assay Description HDAC activity was measured using a continuous trypsin-coupled assay. For inhibitor characterization, measurements were done using 96-well assay plat... | Mol Cancer Ther 5: 1309-17 (2006) Article DOI: 10.1158/1535-7163.MCT-05-0442 BindingDB Entry DOI: 10.7270/Q2DF6PJJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase BTK (Homo sapiens (Human)) | BDBM467845 ((R)-N-(1-Acryloylpiperidin-3-yl)-4-oxo-5-(6-phenox...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 354 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Citation and Details Article DOI: 10.1021/acs.jmedchem.2c01026 BindingDB Entry DOI: 10.7270/Q2MK6HZ3 | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase BTK (Homo sapiens (Human)) | BDBM467852 ((R)-N-(1-Acryloylpiperidin-3-yl)-4-oxo-5-(5-phenox...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 456 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Citation and Details Article DOI: 10.1021/acs.jmedchem.2c01026 BindingDB Entry DOI: 10.7270/Q2MK6HZ3 | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase BTK (Homo sapiens (Human)) | BDBM468084 ((R)-N-(1-Acryloylpyrrolidin-3-yl)-5-(*S)-(6-isobut...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 2.55E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Citation and Details Article DOI: 10.1021/acs.jmedchem.2c01026 BindingDB Entry DOI: 10.7270/Q2MK6HZ3 | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 1 (Homo sapiens (Human)) | BDBM24623 (4-[2-({3-[(dimethylamino)methyl]-1-benzofuran-2-yl...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | >5.00E+4 | >-24.3 | n/a | n/a | n/a | n/a | n/a | 7.4 | 22 |
Celera Genomics | Assay Description HDAC activity was measured using a continuous trypsin-coupled assay. For inhibitor characterization, measurements were done using 96-well assay plat... | Mol Cancer Ther 5: 1309-17 (2006) Article DOI: 10.1158/1535-7163.MCT-05-0442 BindingDB Entry DOI: 10.7270/Q2DF6PJJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase BTK (Homo sapiens (Human)) | BDBM50601990 (CHEMBL5179679) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 2.43E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Citation and Details Article DOI: 10.1021/acs.jmedchem.2c01026 BindingDB Entry DOI: 10.7270/Q2MK6HZ3 | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase B-raf [V600E] (Homo sapiens (Human)) | BDBM87369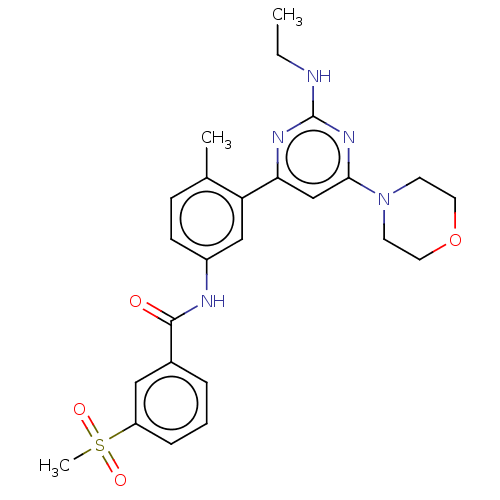 (US10245267, Example 704 | US10709712, Example 704 ...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 0.0200 | n/a | n/a | n/a | n/a | n/a | n/a |
NOVARTIS AG US Patent | Assay Description C-Raf TR refers to a truncated C-Raf protein, a Δ1-324 deletion mutant.C-Raf FL refers to the full-length C-Raf protein.Full length MEK1 with an... | US Patent US10709712 (2020) BindingDB Entry DOI: 10.7270/Q2280BP3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase B-raf [V600E] (Homo sapiens (Human)) | BDBM87369 (US10245267, Example 704 | US10709712, Example 704 ...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 0.0200 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company | Assay Description Raf and biotinylated Mek, kinase dead, were combined at 2× final concentrations in assay buffer (50 mM Tris, pH 7.5, 15 mM MgCl2, 0.01% BSA and 1 mM ... | Bioorg Med Chem Lett 17: 5115-20 (2007) BindingDB Entry DOI: 10.7270/Q2RB76WP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase B-raf [V600E] (Homo sapiens (Human)) | BDBM87369 (US10245267, Example 704 | US10709712, Example 704 ...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 0.0200 | n/a | n/a | n/a | n/a | 7.5 | 25 |
NOVARTIS AG US Patent | Assay Description Raf and biotinylated Mek, kinase dead, were combined at 2x final concentrations in assay buffer (50 mM Tris, pH 7.5, 15 mM MgCl2, 0.01% BSA and 1 mM ... | US Patent US9694016 (2017) BindingDB Entry DOI: 10.7270/Q2MC8X6W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| RAF proto-oncogene serine/threonine-protein kinase (Homo sapiens (Human)) | BDBM202851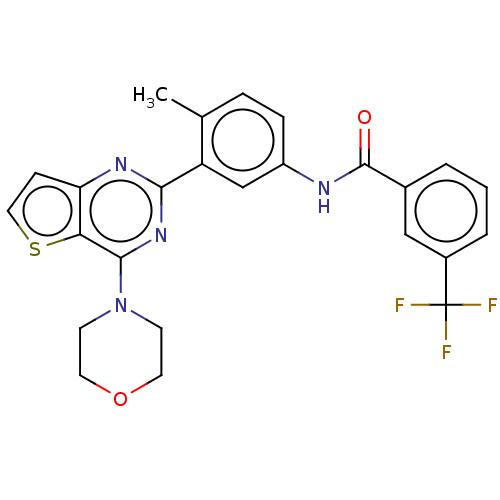 (US10245267, Example 829 | US10709712, Example 204 ...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.0390 | n/a | n/a | n/a | n/a | n/a | n/a |
NOVARTIS AG US Patent | Assay Description C-Raf TR refers to a truncated C-Raf protein, a Δ1-324 deletion mutant.C-Raf FL refers to the full-length C-Raf protein.Full length MEK1 with an... | US Patent US10709712 (2020) BindingDB Entry DOI: 10.7270/Q2280BP3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| RAF proto-oncogene serine/threonine-protein kinase [Y340E,Y341E] (Homo sapiens (Human)) | BDBM202851 (US10245267, Example 829 | US10709712, Example 204 ...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.0400 | n/a | n/a | n/a | n/a | 7.5 | 25 |
NOVARTIS AG US Patent | Assay Description Raf and biotinylated Mek, kinase dead, were combined at 2x final concentrations in assay buffer (50 mM Tris, pH 7.5, 15 mM MgCl2, 0.01% BSA and 1 mM ... | US Patent US9694016 (2017) BindingDB Entry DOI: 10.7270/Q2MC8X6W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| RAF proto-oncogene serine/threonine-protein kinase (Homo sapiens (Human)) | BDBM202851 (US10245267, Example 829 | US10709712, Example 204 ...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.0400 | n/a | n/a | n/a | n/a | n/a | n/a |
NOVARTIS AG US Patent | Assay Description C-Raf TR refers to a truncated C-Raf protein, a Δ1-324 deletion mutant.C-Raf FL refers to the full-length C-Raf protein.Full length MEK1 with an... | US Patent US10709712 (2020) BindingDB Entry DOI: 10.7270/Q2280BP3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| RAF proto-oncogene serine/threonine-protein kinase (Homo sapiens (Human)) | BDBM87817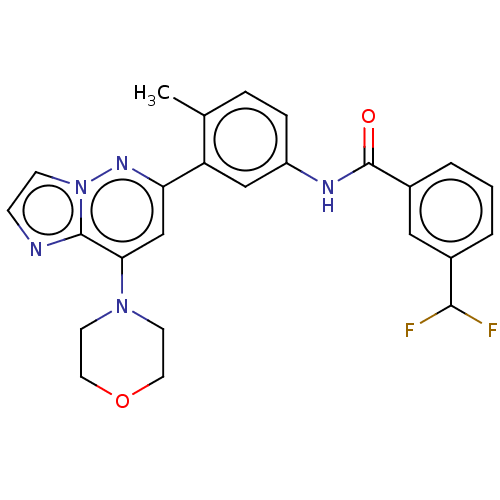 (US10245267, Example 847 | US10709712, Example 847 ...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 0.0400 | n/a | n/a | n/a | n/a | n/a | n/a |
NOVARTIS AG US Patent | Assay Description C-Raf TR refers to a truncated C-Raf protein, a Δ1-324 deletion mutant.C-Raf FL refers to the full-length C-Raf protein.Full length MEK1 with an... | US Patent US10709712 (2020) BindingDB Entry DOI: 10.7270/Q2280BP3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| RAF proto-oncogene serine/threonine-protein kinase (Homo sapiens (Human)) | BDBM87817 (US10245267, Example 847 | US10709712, Example 847 ...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 0.0400 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company | Assay Description Raf and biotinylated Mek, kinase dead, were combined at 2× final concentrations in assay buffer (50 mM Tris, pH 7.5, 15 mM MgCl2, 0.01% BSA and 1 mM ... | Bioorg Med Chem Lett 17: 5115-20 (2007) BindingDB Entry DOI: 10.7270/Q2RB76WP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| RAF proto-oncogene serine/threonine-protein kinase (Homo sapiens (Human)) | BDBM202851 (US10245267, Example 829 | US10709712, Example 204 ...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.0400 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company | Assay Description Raf and biotinylated Mek, kinase dead, were combined at 2× final concentrations in assay buffer (50 mM Tris, pH 7.5, 15 mM MgCl2, 0.01% BSA and 1 mM ... | Bioorg Med Chem Lett 17: 5115-20 (2007) BindingDB Entry DOI: 10.7270/Q2RB76WP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| RAF proto-oncogene serine/threonine-protein kinase [Y340E,Y341E] (Homo sapiens (Human)) | BDBM87817 (US10245267, Example 847 | US10709712, Example 847 ...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 0.0400 | n/a | n/a | n/a | n/a | 7.5 | 25 |
NOVARTIS AG US Patent | Assay Description Raf and biotinylated Mek, kinase dead, were combined at 2x final concentrations in assay buffer (50 mM Tris, pH 7.5, 15 mM MgCl2, 0.01% BSA and 1 mM ... | US Patent US9694016 (2017) BindingDB Entry DOI: 10.7270/Q2MC8X6W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| RAF proto-oncogene serine/threonine-protein kinase (Homo sapiens (Human)) | BDBM202729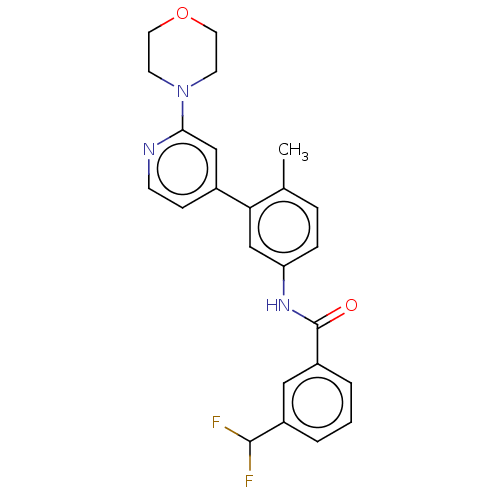 (US10245267, Example 75 | US10709712, Example 75 | ...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.0560 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company | Assay Description Raf and biotinylated Mek, kinase dead, were combined at 2× final concentrations in assay buffer (50 mM Tris, pH 7.5, 15 mM MgCl2, 0.01% BSA and 1 mM ... | Bioorg Med Chem Lett 17: 5115-20 (2007) BindingDB Entry DOI: 10.7270/Q2RB76WP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| RAF proto-oncogene serine/threonine-protein kinase (Homo sapiens (Human)) | BDBM202729 (US10245267, Example 75 | US10709712, Example 75 | ...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.0560 | n/a | n/a | n/a | n/a | n/a | n/a |
NOVARTIS AG US Patent | Assay Description C-Raf TR refers to a truncated C-Raf protein, a Δ1-324 deletion mutant.C-Raf FL refers to the full-length C-Raf protein.Full length MEK1 with an... | US Patent US10709712 (2020) BindingDB Entry DOI: 10.7270/Q2280BP3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| RAF proto-oncogene serine/threonine-protein kinase (Homo sapiens (Human)) | BDBM202729 (US10245267, Example 75 | US10709712, Example 75 | ...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.0600 | n/a | n/a | n/a | n/a | n/a | n/a |
NOVARTIS AG US Patent | Assay Description C-Raf TR refers to a truncated C-Raf protein, a Δ1-324 deletion mutant.C-Raf FL refers to the full-length C-Raf protein.Full length MEK1 with an... | US Patent US10709712 (2020) BindingDB Entry DOI: 10.7270/Q2280BP3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| RAF proto-oncogene serine/threonine-protein kinase (Homo sapiens (Human)) | BDBM202729 (US10245267, Example 75 | US10709712, Example 75 | ...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.0600 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company | Assay Description Raf and biotinylated Mek, kinase dead, were combined at 2× final concentrations in assay buffer (50 mM Tris, pH 7.5, 15 mM MgCl2, 0.01% BSA and 1 mM ... | Bioorg Med Chem Lett 17: 5115-20 (2007) BindingDB Entry DOI: 10.7270/Q2RB76WP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| RAF proto-oncogene serine/threonine-protein kinase [Y340E,Y341E] (Homo sapiens (Human)) | BDBM202729 (US10245267, Example 75 | US10709712, Example 75 | ...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.0600 | n/a | n/a | n/a | n/a | 7.5 | 25 |
NOVARTIS AG US Patent | Assay Description Raf and biotinylated Mek, kinase dead, were combined at 2x final concentrations in assay buffer (50 mM Tris, pH 7.5, 15 mM MgCl2, 0.01% BSA and 1 mM ... | US Patent US9694016 (2017) BindingDB Entry DOI: 10.7270/Q2MC8X6W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| RAF proto-oncogene serine/threonine-protein kinase [Y340E,Y341E] (Homo sapiens (Human)) | BDBM202729 (US10245267, Example 75 | US10709712, Example 75 | ...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.0600 | n/a | n/a | n/a | n/a | 7.5 | 25 |
NOVARTIS AG US Patent | Assay Description Raf and biotinylated Mek, kinase dead, were combined at 2x final concentrations in assay buffer (50 mM Tris, pH 7.5, 15 mM MgCl2, 0.01% BSA and 1 mM ... | US Patent US9694016 (2017) BindingDB Entry DOI: 10.7270/Q2MC8X6W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| RAF proto-oncogene serine/threonine-protein kinase (Homo sapiens (Human)) | BDBM202850 (US10245267, Example 824 | US10709712, Example 203 ...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.0650 | n/a | n/a | n/a | n/a | n/a | n/a |
NOVARTIS AG US Patent | Assay Description C-Raf TR refers to a truncated C-Raf protein, a Δ1-324 deletion mutant.C-Raf FL refers to the full-length C-Raf protein.Full length MEK1 with an... | US Patent US10709712 (2020) BindingDB Entry DOI: 10.7270/Q2280BP3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| RAF proto-oncogene serine/threonine-protein kinase [Y340E,Y341E] (Homo sapiens (Human)) | BDBM202850 (US10245267, Example 824 | US10709712, Example 203 ...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.0700 | n/a | n/a | n/a | n/a | 7.5 | 25 |
NOVARTIS AG US Patent | Assay Description Raf and biotinylated Mek, kinase dead, were combined at 2x final concentrations in assay buffer (50 mM Tris, pH 7.5, 15 mM MgCl2, 0.01% BSA and 1 mM ... | US Patent US9694016 (2017) BindingDB Entry DOI: 10.7270/Q2MC8X6W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| RAF proto-oncogene serine/threonine-protein kinase (Homo sapiens (Human)) | BDBM88127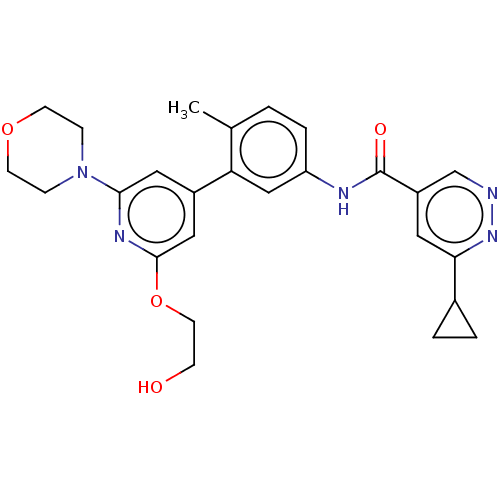 (US10245267, Example 1162 | US10709712, Example 116...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 0.0700 | n/a | n/a | n/a | n/a | n/a | n/a |
NOVARTIS AG US Patent | Assay Description C-Raf TR refers to a truncated C-Raf protein, a Δ1-324 deletion mutant.C-Raf FL refers to the full-length C-Raf protein.Full length MEK1 with an... | US Patent US10709712 (2020) BindingDB Entry DOI: 10.7270/Q2280BP3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| RAF proto-oncogene serine/threonine-protein kinase (Homo sapiens (Human)) | BDBM202850 (US10245267, Example 824 | US10709712, Example 203 ...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.0700 | n/a | n/a | n/a | n/a | n/a | n/a |
NOVARTIS AG US Patent | Assay Description C-Raf TR refers to a truncated C-Raf protein, a Δ1-324 deletion mutant.C-Raf FL refers to the full-length C-Raf protein.Full length MEK1 with an... | US Patent US10709712 (2020) BindingDB Entry DOI: 10.7270/Q2280BP3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| RAF proto-oncogene serine/threonine-protein kinase (Homo sapiens (Human)) | BDBM202850 (US10245267, Example 824 | US10709712, Example 203 ...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.0700 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company | Assay Description Raf and biotinylated Mek, kinase dead, were combined at 2× final concentrations in assay buffer (50 mM Tris, pH 7.5, 15 mM MgCl2, 0.01% BSA and 1 mM ... | Bioorg Med Chem Lett 17: 5115-20 (2007) BindingDB Entry DOI: 10.7270/Q2RB76WP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| RAF proto-oncogene serine/threonine-protein kinase (Homo sapiens (Human)) | BDBM88127 (US10245267, Example 1162 | US10709712, Example 116...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 0.0700 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company | Assay Description Raf and biotinylated Mek, kinase dead, were combined at 2× final concentrations in assay buffer (50 mM Tris, pH 7.5, 15 mM MgCl2, 0.01% BSA and 1 mM ... | Bioorg Med Chem Lett 17: 5115-20 (2007) BindingDB Entry DOI: 10.7270/Q2RB76WP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| RAF proto-oncogene serine/threonine-protein kinase [Y340E,Y341E] (Homo sapiens (Human)) | BDBM88127 (US10245267, Example 1162 | US10709712, Example 116...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 0.0700 | n/a | n/a | n/a | n/a | 7.5 | 25 |
NOVARTIS AG US Patent | Assay Description Raf and biotinylated Mek, kinase dead, were combined at 2x final concentrations in assay buffer (50 mM Tris, pH 7.5, 15 mM MgCl2, 0.01% BSA and 1 mM ... | US Patent US9694016 (2017) BindingDB Entry DOI: 10.7270/Q2MC8X6W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| RAF proto-oncogene serine/threonine-protein kinase [Y340E,Y341E] (Homo sapiens (Human)) | BDBM87741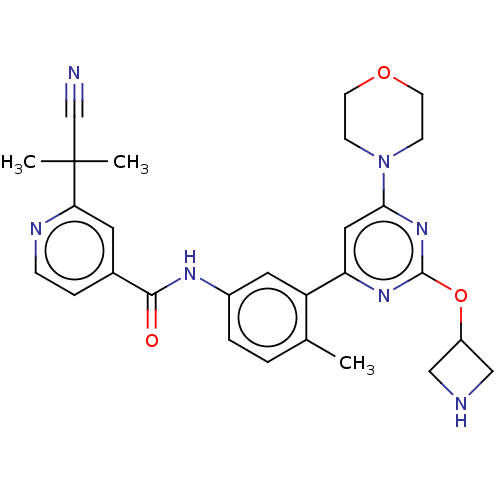 (US10245267, Example 777 | US10709712, Example 777 ...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 0.0800 | n/a | n/a | n/a | n/a | 7.5 | 25 |
NOVARTIS AG US Patent | Assay Description Raf and biotinylated Mek, kinase dead, were combined at 2x final concentrations in assay buffer (50 mM Tris, pH 7.5, 15 mM MgCl2, 0.01% BSA and 1 mM ... | US Patent US9694016 (2017) BindingDB Entry DOI: 10.7270/Q2MC8X6W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| RAF proto-oncogene serine/threonine-protein kinase [Y340E,Y341E] (Homo sapiens (Human)) | BDBM87829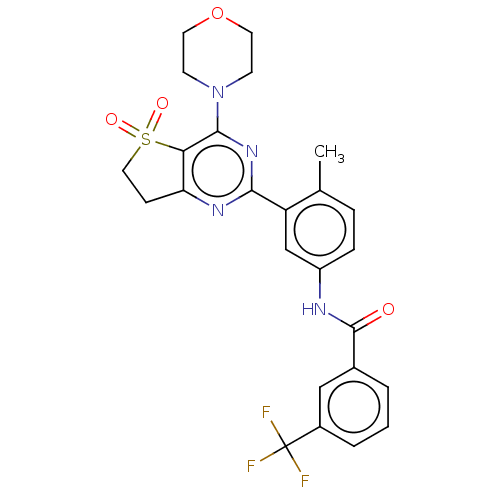 (US10245267, Example 857 | US10709712, Example 857 ...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 0.0800 | n/a | n/a | n/a | n/a | 7.5 | 25 |
NOVARTIS AG US Patent | Assay Description Raf and biotinylated Mek, kinase dead, were combined at 2x final concentrations in assay buffer (50 mM Tris, pH 7.5, 15 mM MgCl2, 0.01% BSA and 1 mM ... | US Patent US9694016 (2017) BindingDB Entry DOI: 10.7270/Q2MC8X6W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 9233 total ) | Next | Last >> |