

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Apoptosis regulator Bcl-2 (Homo sapiens (Human)) | US20240043404, Example 377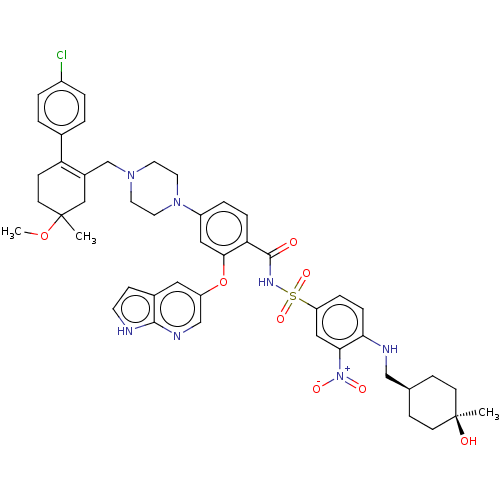 (4-(4-{[2-(4-chlorophenyl)-5-methoxy-5-methylcycloh...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | <0.00100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a | |
TBA | Citation and Details | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Apoptosis regulator Bcl-2 (Homo sapiens (Human)) | US20240043404, Example 378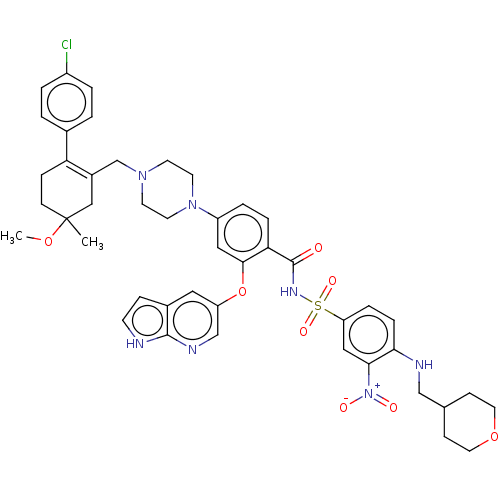 (US10213433, Compound 378 | US11369599, Compound 37...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | <0.00100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a | |
TBA | Citation and Details | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Apoptosis regulator Bcl-2 (Homo sapiens (Human)) | BDBM189797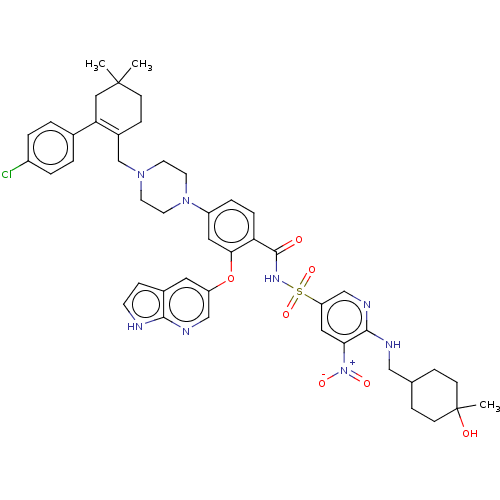 (US11369599, Compound 376 | US9174982, 371 | US9174...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | <0.00100 | <-68.5 | n/a | n/a | n/a | n/a | n/a | n/a | 25 |
ABBVIE INC.; GENENTECH, INC.; THE WALTER AND ELIZA HALL INSTITUTE OF MEDICAL RESEARCH US Patent | Assay Description Representative compounds were serially diluted in dimethyl sulfoxide (DMSO) starting at 50 uM (2x starting concentration; 10% DMSO) and 10 uL were tr... | US Patent US9174982 (2015) BindingDB Entry DOI: 10.7270/Q2RB73D5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Apoptosis regulator Bcl-2 (Homo sapiens (Human)) | BDBM189799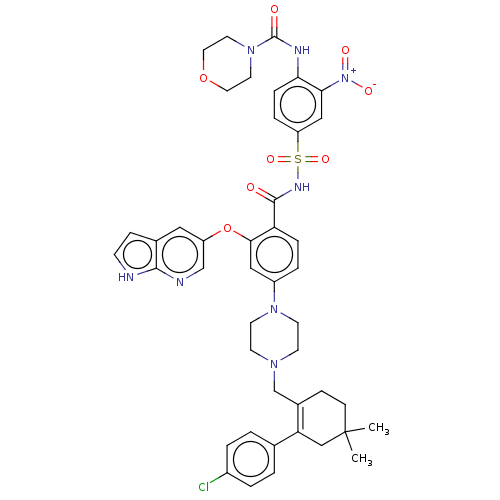 (US10213433, Compound 373 | US11369599, Compound 37...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | <0.00100 | <-68.5 | n/a | n/a | n/a | n/a | n/a | n/a | 25 |
ABBVIE INC.; GENENTECH, INC.; THE WALTER AND ELIZA HALL INSTITUTE OF MEDICAL RESEARCH US Patent | Assay Description Representative compounds were serially diluted in dimethyl sulfoxide (DMSO) starting at 50 uM (2x starting concentration; 10% DMSO) and 10 uL were tr... | US Patent US9174982 (2015) BindingDB Entry DOI: 10.7270/Q2RB73D5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Apoptosis regulator Bcl-2 (Homo sapiens (Human)) | BDBM189800 (US10213433, Compound 374 | US11369599, Compound 37...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | <0.00100 | <-68.5 | n/a | n/a | n/a | n/a | n/a | n/a | 25 |
ABBVIE INC.; GENENTECH, INC.; THE WALTER AND ELIZA HALL INSTITUTE OF MEDICAL RESEARCH US Patent | Assay Description Representative compounds were serially diluted in dimethyl sulfoxide (DMSO) starting at 50 uM (2x starting concentration; 10% DMSO) and 10 uL were tr... | US Patent US9174982 (2015) BindingDB Entry DOI: 10.7270/Q2RB73D5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Apoptosis regulator Bcl-2 (Homo sapiens (Human)) | BDBM189797 (US11369599, Compound 376 | US9174982, 371 | US9174...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | <0.00100 | <-68.5 | n/a | n/a | n/a | n/a | n/a | n/a | 25 |
ABBVIE INC.; GENENTECH, INC.; THE WALTER AND ELIZA HALL INSTITUTE OF MEDICAL RESEARCH US Patent | Assay Description Representative compounds were serially diluted in dimethyl sulfoxide (DMSO) starting at 50 uM (2x starting concentration; 10% DMSO) and 10 uL were tr... | US Patent US9174982 (2015) BindingDB Entry DOI: 10.7270/Q2RB73D5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Apoptosis regulator Bcl-2 (Homo sapiens (Human)) | US20240043404, Example 376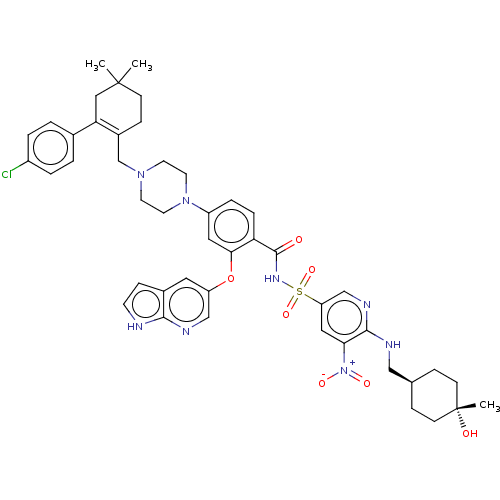 (4-(4-{[2-(4-chlorophenyl)-4,4-dimethylcyclohex-1-e...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | <0.00100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a | |
TBA | Citation and Details | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Apoptosis regulator Bcl-2 (Homo sapiens (Human)) | US20240043404, Example 374 (US10213433, Compound 374 | US11369599, Compound 37...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | <0.00100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a | |
TBA | Citation and Details | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Apoptosis regulator Bcl-2 (Homo sapiens (Human)) | US20240043404, Example 373 (US10213433, Compound 373 | US11369599, Compound 37...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | <0.00100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a | |
TBA | Citation and Details | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Apoptosis regulator Bcl-2 (Homo sapiens (Human)) | US20240043404, Example 371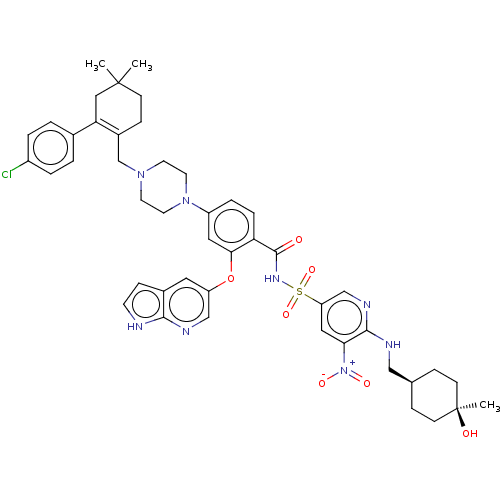 (4-(4-{[2-(4-chlorophenyl)-4,4-dimethylcyclohex-1-e...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | <0.00100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a | |
TBA | Citation and Details | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Apoptosis regulator Bcl-2 (Homo sapiens (Human)) | BDBM144940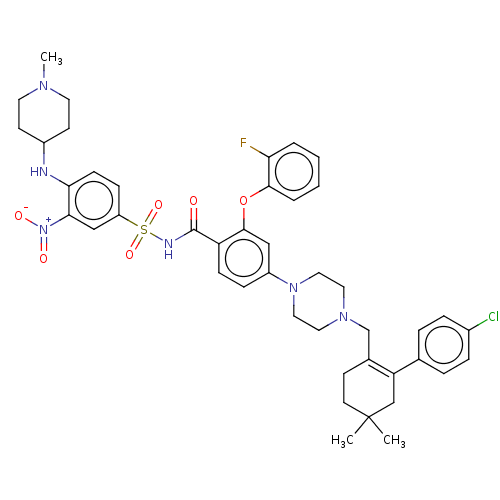 (US8952157, 122 | US9303025, 122) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | <0.00100 | <-68.5 | n/a | n/a | n/a | n/a | n/a | n/a | 25 |
AbbVie Inc. US Patent | Assay Description Representative compounds were serially diluted in dimethyl sulfoxide (DMSO) starting at 50 μM (2× starting concentration; 10% DMSO) and 10 μ... | US Patent US9303025 (2016) BindingDB Entry DOI: 10.7270/Q2XD10JP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Apoptosis regulator Bcl-2 (Homo sapiens (Human)) | BDBM189804 (US10213433, Compound 378 | US11369599, Compound 37...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | <0.00100 | <-68.5 | n/a | n/a | n/a | n/a | n/a | n/a | 25 |
ABBVIE INC.; GENENTECH, INC.; THE WALTER AND ELIZA HALL INSTITUTE OF MEDICAL RESEARCH US Patent | Assay Description Representative compounds were serially diluted in dimethyl sulfoxide (DMSO) starting at 50 uM (2x starting concentration; 10% DMSO) and 10 uL were tr... | US Patent US9174982 (2015) BindingDB Entry DOI: 10.7270/Q2RB73D5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Apoptosis regulator Bcl-2 (Homo sapiens (Human)) | BDBM144940 (US8952157, 122 | US9303025, 122) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | <0.00100 | <-68.5 | n/a | n/a | n/a | n/a | n/a | n/a | 25 |
AbbVie Inc.; The Walter and Eliza Hall Institute of Medical Research US Patent | Assay Description Representative compounds were serially diluted in dimethyl sulfoxide (DMSO) starting at 50 uM (2x starting concentration; 10% DMSO) and 10 uL were tr... | US Patent US8952157 (2015) BindingDB Entry DOI: 10.7270/Q2QN65G9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Apoptosis regulator Bcl-2 (Mus musculus (Mouse)) | BDBM178562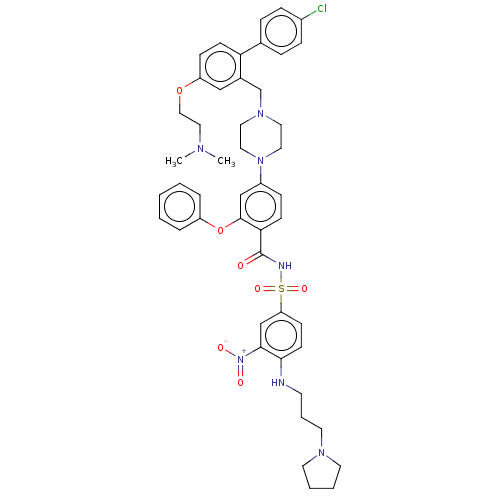 (US9125913, 121) | PDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 0.00100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AbbVie Inc. US Patent | Assay Description The inhibition constant (Ki) is the dissociation constant of an enzyme-inhibitor complex or a protein/small molecule complex, wherein the small molec... | US Patent US9125913 (2015) BindingDB Entry DOI: 10.7270/Q29Z93PP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Apoptosis regulator Bcl-2 (Homo sapiens (Human)) | BDBM189803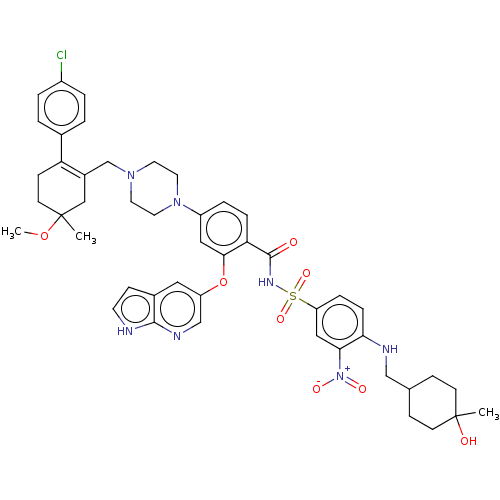 (US11369599, Compound 377 | US9174982, 377) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | <0.00100 | <-68.5 | n/a | n/a | n/a | n/a | n/a | n/a | 25 |
ABBVIE INC.; GENENTECH, INC.; THE WALTER AND ELIZA HALL INSTITUTE OF MEDICAL RESEARCH US Patent | Assay Description Representative compounds were serially diluted in dimethyl sulfoxide (DMSO) starting at 50 uM (2x starting concentration; 10% DMSO) and 10 uL were tr... | US Patent US9174982 (2015) BindingDB Entry DOI: 10.7270/Q2RB73D5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Apoptosis regulator Bcl-2 (Mus musculus (Mouse)) | BDBM178855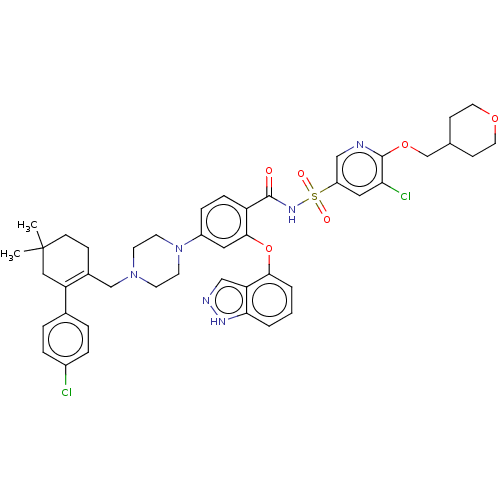 (US9125913, 427) | PDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 0.00200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AbbVie Inc. US Patent | Assay Description The inhibition constant (Ki) is the dissociation constant of an enzyme-inhibitor complex or a protein/small molecule complex, wherein the small molec... | US Patent US9125913 (2015) BindingDB Entry DOI: 10.7270/Q29Z93PP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Apoptosis regulator Bcl-2 (Homo sapiens (Human)) | US20240043404, Example 129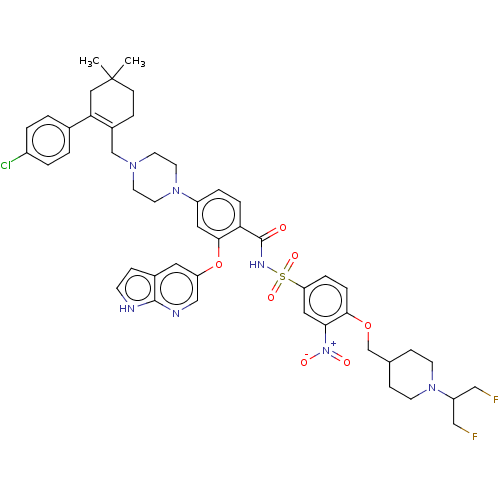 (US10213433, Compound 129 | US11369599, Compound 12...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | 0.00200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a | |
TBA | Citation and Details | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Apoptosis regulator Bcl-2 (Mus musculus (Mouse)) | BDBM178843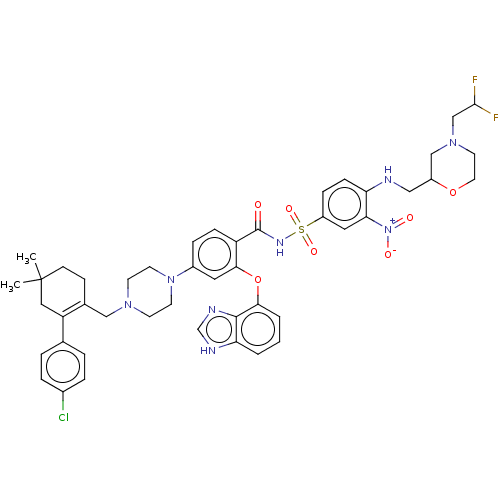 (US9125913, 415) | PDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 0.00200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AbbVie Inc. US Patent | Assay Description The inhibition constant (Ki) is the dissociation constant of an enzyme-inhibitor complex or a protein/small molecule complex, wherein the small molec... | US Patent US9125913 (2015) BindingDB Entry DOI: 10.7270/Q29Z93PP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Apoptosis regulator Bcl-2 (Mus musculus (Mouse)) | BDBM178836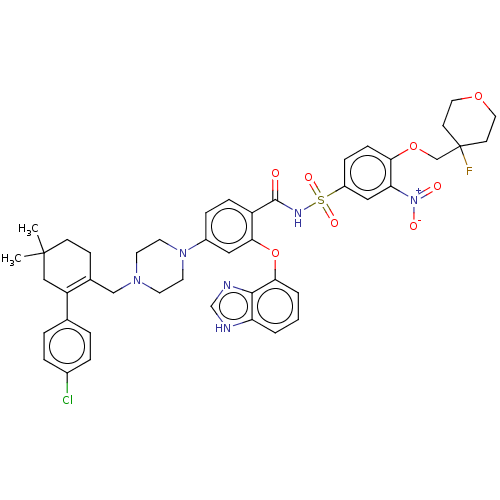 (US9125913, 408) | PDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 0.00200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AbbVie Inc. US Patent | Assay Description The inhibition constant (Ki) is the dissociation constant of an enzyme-inhibitor complex or a protein/small molecule complex, wherein the small molec... | US Patent US9125913 (2015) BindingDB Entry DOI: 10.7270/Q29Z93PP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Apoptosis regulator Bcl-2 (Homo sapiens (Human)) | BDBM189567 (US10213433, Compound 129 | US11369599, Compound 12...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 0.00200 | -66.8 | n/a | n/a | n/a | n/a | n/a | n/a | 25 |
ABBVIE INC.; GENENTECH, INC.; THE WALTER AND ELIZA HALL INSTITUTE OF MEDICAL RESEARCH US Patent | Assay Description Representative compounds were serially diluted in dimethyl sulfoxide (DMSO) starting at 50 uM (2x starting concentration; 10% DMSO) and 10 uL were tr... | US Patent US9174982 (2015) BindingDB Entry DOI: 10.7270/Q2RB73D5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Apoptosis regulator Bcl-2 (Mus musculus (Mouse)) | BDBM178856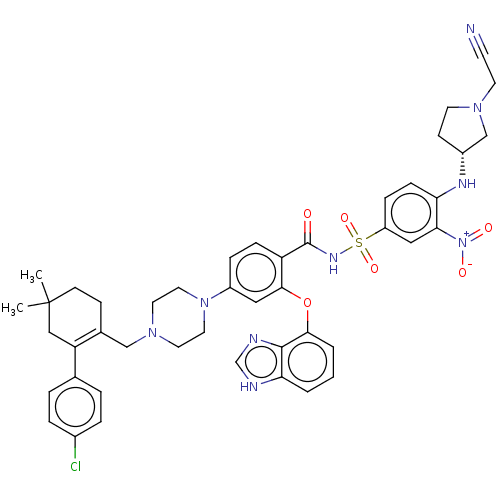 (US9125913, 428) | PDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 0.00300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AbbVie Inc. US Patent | Assay Description The inhibition constant (Ki) is the dissociation constant of an enzyme-inhibitor complex or a protein/small molecule complex, wherein the small molec... | US Patent US9125913 (2015) BindingDB Entry DOI: 10.7270/Q29Z93PP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Apoptosis regulator Bcl-2 (Mus musculus (Mouse)) | BDBM178839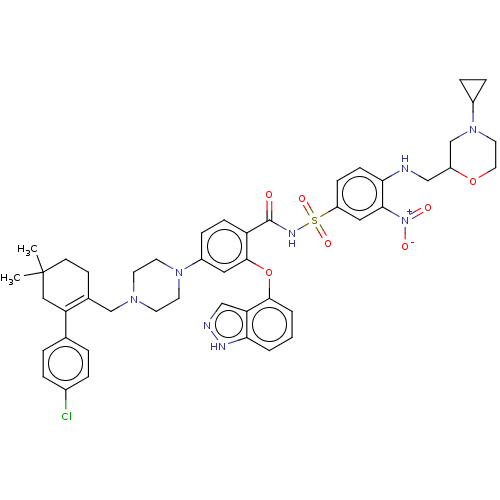 (US9125913, 411) | PDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 0.00300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AbbVie Inc. US Patent | Assay Description The inhibition constant (Ki) is the dissociation constant of an enzyme-inhibitor complex or a protein/small molecule complex, wherein the small molec... | US Patent US9125913 (2015) BindingDB Entry DOI: 10.7270/Q29Z93PP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Apoptosis regulator Bcl-2 (Mus musculus (Mouse)) | BDBM178831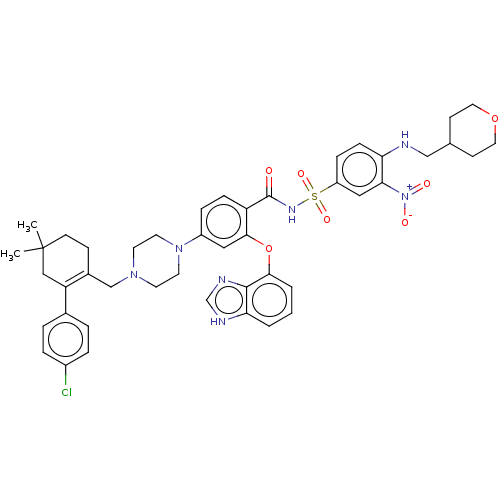 (US9125913, 403) | PDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 0.00300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AbbVie Inc. US Patent | Assay Description The inhibition constant (Ki) is the dissociation constant of an enzyme-inhibitor complex or a protein/small molecule complex, wherein the small molec... | US Patent US9125913 (2015) BindingDB Entry DOI: 10.7270/Q29Z93PP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Apoptosis regulator Bcl-2 (Mus musculus (Mouse)) | BDBM178566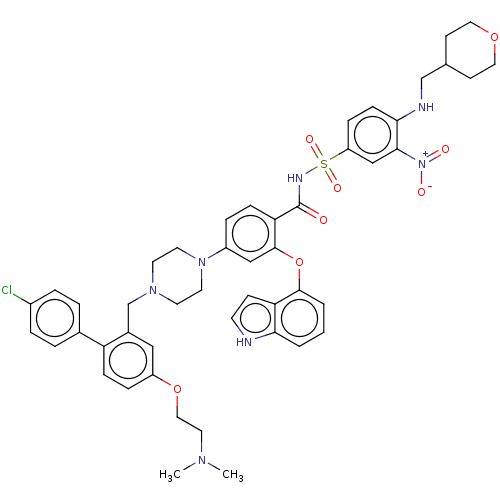 (US9125913, 125) | PDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 0.00300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AbbVie Inc. US Patent | Assay Description The inhibition constant (Ki) is the dissociation constant of an enzyme-inhibitor complex or a protein/small molecule complex, wherein the small molec... | US Patent US9125913 (2015) BindingDB Entry DOI: 10.7270/Q29Z93PP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Apoptosis regulator Bcl-2 (Mus musculus (Mouse)) | BDBM178853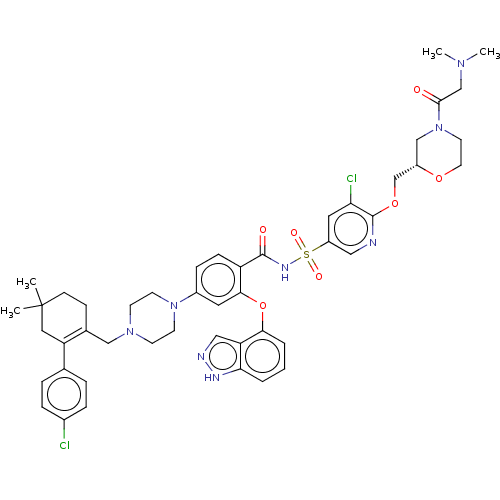 (US9125913, 425) | PDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 0.00300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AbbVie Inc. US Patent | Assay Description The inhibition constant (Ki) is the dissociation constant of an enzyme-inhibitor complex or a protein/small molecule complex, wherein the small molec... | US Patent US9125913 (2015) BindingDB Entry DOI: 10.7270/Q29Z93PP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Apoptosis regulator Bcl-2 (Mus musculus (Mouse)) | BDBM178846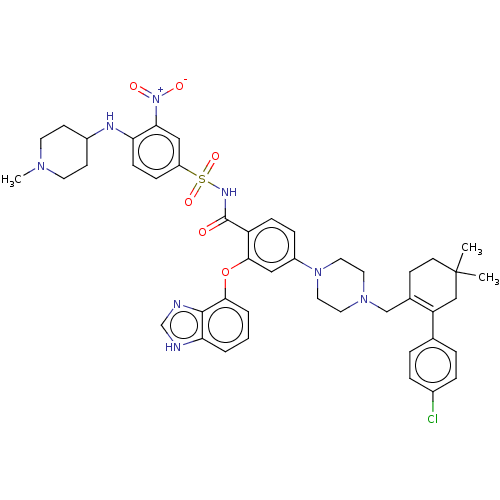 (US9125913, 418) | PDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 0.00400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AbbVie Inc. US Patent | Assay Description The inhibition constant (Ki) is the dissociation constant of an enzyme-inhibitor complex or a protein/small molecule complex, wherein the small molec... | US Patent US9125913 (2015) BindingDB Entry DOI: 10.7270/Q29Z93PP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor X (Homo sapiens (Human)) | BDBM17129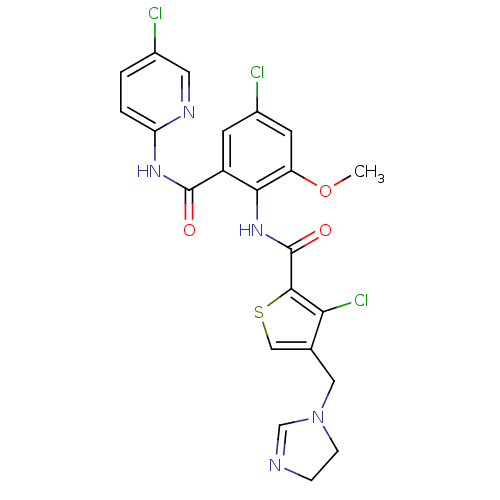 (3-chloro-N-{4-chloro-2-[(5-chloropyridin-2-yl)carb...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | 0.00400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Berlex Biosciences | Assay Description The enzyme activities were determined kinetically as the initial rate of cleavage of a peptide p-nitroanilide. Km for enzyme and substrate was determ... | J Med Chem 50: 2967-80 (2007) Article DOI: 10.1021/jm070125f BindingDB Entry DOI: 10.7270/Q2J101F5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor X (Homo sapiens (Human)) | BDBM17122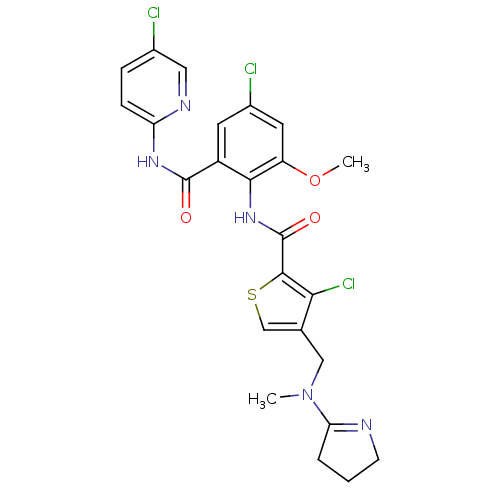 (3-chloro-N-{4-chloro-2-[(5-chloropyridin-2-yl)carb...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | 0.00400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Berlex Biosciences | Assay Description The enzyme activities were determined kinetically as the initial rate of cleavage of a peptide p-nitroanilide. Km for enzyme and substrate was determ... | J Med Chem 50: 2967-80 (2007) Article DOI: 10.1021/jm070125f BindingDB Entry DOI: 10.7270/Q2J101F5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor X (Homo sapiens (Human)) | BDBM17127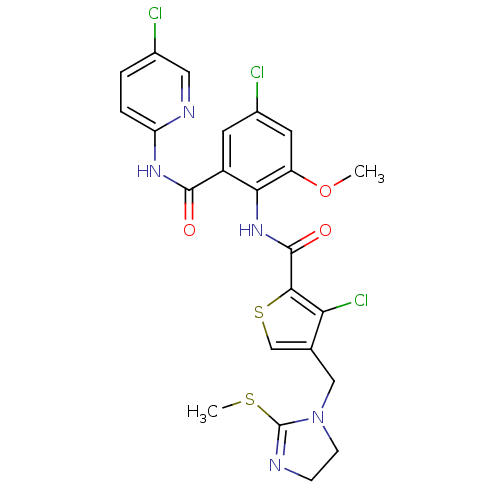 (3-chloro-N-{4-chloro-2-[(5-chloropyridin-2-yl)carb...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | 0.00400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Berlex Biosciences | Assay Description The enzyme activities were determined kinetically as the initial rate of cleavage of a peptide p-nitroanilide. Km for enzyme and substrate was determ... | J Med Chem 50: 2967-80 (2007) Article DOI: 10.1021/jm070125f BindingDB Entry DOI: 10.7270/Q2J101F5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Apoptosis regulator Bcl-2 (Mus musculus (Mouse)) | BDBM178883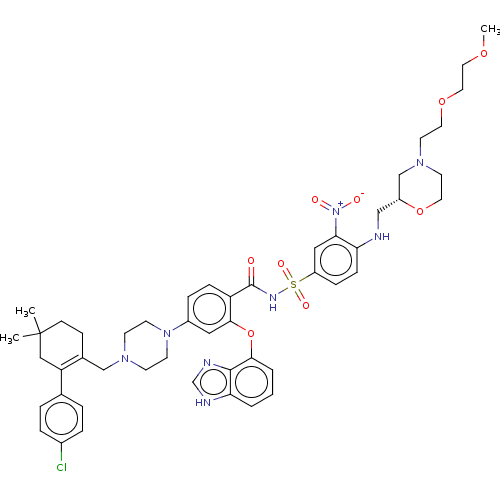 (US9125913, 455) | PDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 0.00400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AbbVie Inc. US Patent | Assay Description The inhibition constant (Ki) is the dissociation constant of an enzyme-inhibitor complex or a protein/small molecule complex, wherein the small molec... | US Patent US9125913 (2015) BindingDB Entry DOI: 10.7270/Q29Z93PP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Apoptosis regulator Bcl-2 (Homo sapiens (Human)) | BDBM144855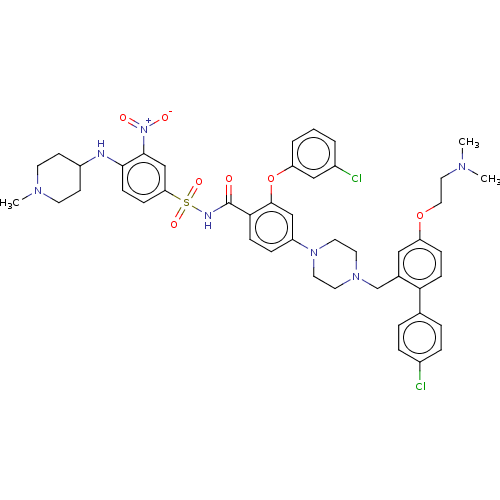 (US8952157, 37 | US9303025, 37) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 0.00500 | -64.5 | n/a | n/a | n/a | n/a | n/a | n/a | 25 |
AbbVie Inc.; The Walter and Eliza Hall Institute of Medical Research US Patent | Assay Description Representative compounds were serially diluted in dimethyl sulfoxide (DMSO) starting at 50 uM (2x starting concentration; 10% DMSO) and 10 uL were tr... | US Patent US8952157 (2015) BindingDB Entry DOI: 10.7270/Q2QN65G9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor X (Homo sapiens (Human)) | BDBM17135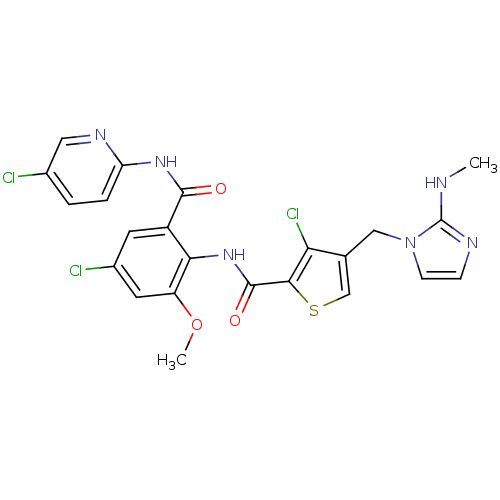 (3-chloro-N-{4-chloro-2-[(5-chloropyridin-2-yl)carb...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | MMDB PC cid PC sid PDB UniChem Patents Similars | MMDB PDB Article PubMed | 0.00500 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Berlex Biosciences | Assay Description The enzyme activities were determined kinetically as the initial rate of cleavage of a peptide p-nitroanilide. Km for enzyme and substrate was determ... | J Med Chem 50: 2967-80 (2007) Article DOI: 10.1021/jm070125f BindingDB Entry DOI: 10.7270/Q2J101F5 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Coagulation factor X (Homo sapiens (Human)) | BDBM17136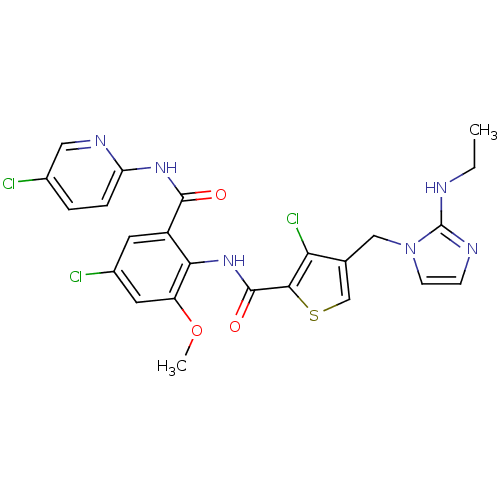 (3-chloro-N-{4-chloro-2-[(5-chloropyridin-2-yl)carb...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | 0.00500 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Berlex Biosciences | Assay Description The enzyme activities were determined kinetically as the initial rate of cleavage of a peptide p-nitroanilide. Km for enzyme and substrate was determ... | J Med Chem 50: 2967-80 (2007) Article DOI: 10.1021/jm070125f BindingDB Entry DOI: 10.7270/Q2J101F5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Apoptosis regulator Bcl-2 (Mus musculus (Mouse)) | BDBM178876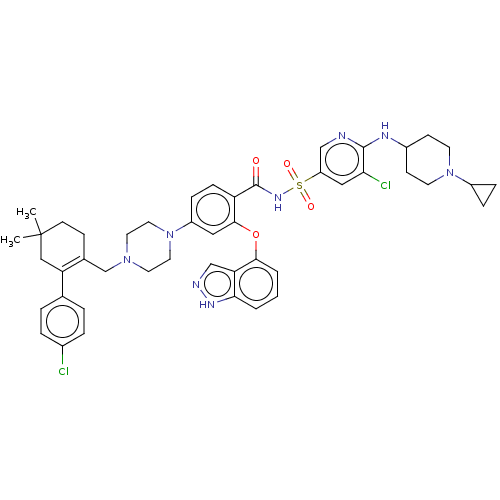 (US9125913, 448) | PDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 0.00500 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AbbVie Inc. US Patent | Assay Description The inhibition constant (Ki) is the dissociation constant of an enzyme-inhibitor complex or a protein/small molecule complex, wherein the small molec... | US Patent US9125913 (2015) BindingDB Entry DOI: 10.7270/Q29Z93PP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Apoptosis regulator Bcl-2 (Homo sapiens (Human)) | BDBM145154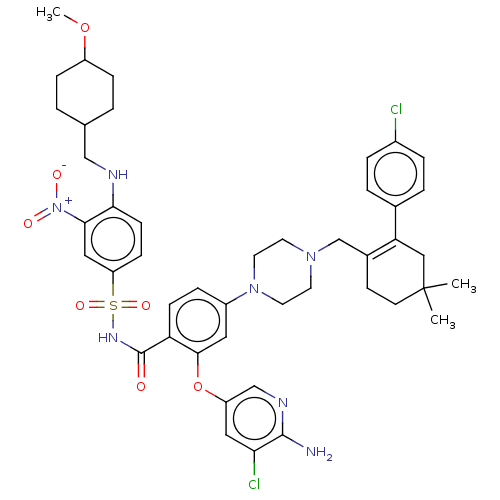 (US8952157, 345 | US9303025, 345) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 0.00500 | -64.5 | n/a | n/a | n/a | n/a | n/a | n/a | 25 |
AbbVie Inc. US Patent | Assay Description Representative compounds were serially diluted in dimethyl sulfoxide (DMSO) starting at 50 μM (2× starting concentration; 10% DMSO) and 10 μ... | US Patent US9303025 (2016) BindingDB Entry DOI: 10.7270/Q2XD10JP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Apoptosis regulator Bcl-2 (Mus musculus (Mouse)) | BDBM178639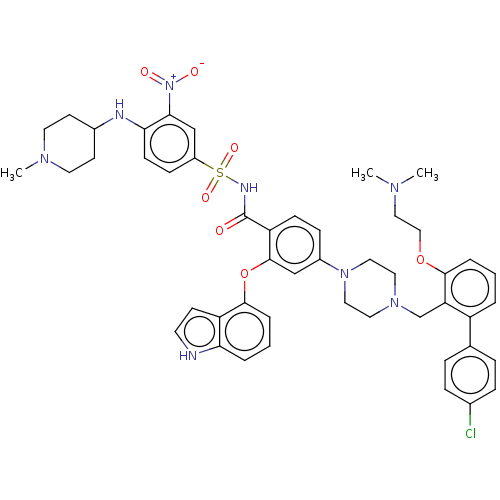 (US9125913, 205) | PDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 0.00500 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AbbVie Inc. US Patent | Assay Description The inhibition constant (Ki) is the dissociation constant of an enzyme-inhibitor complex or a protein/small molecule complex, wherein the small molec... | US Patent US9125913 (2015) BindingDB Entry DOI: 10.7270/Q29Z93PP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Apoptosis regulator Bcl-2 (Homo sapiens (Human)) | BDBM145154 (US8952157, 345 | US9303025, 345) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 0.00500 | -64.5 | n/a | n/a | n/a | n/a | n/a | n/a | 25 |
AbbVie Inc.; The Walter and Eliza Hall Institute of Medical Research US Patent | Assay Description Representative compounds were serially diluted in dimethyl sulfoxide (DMSO) starting at 50 uM (2x starting concentration; 10% DMSO) and 10 uL were tr... | US Patent US8952157 (2015) BindingDB Entry DOI: 10.7270/Q2QN65G9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Apoptosis regulator Bcl-2 (Homo sapiens (Human)) | BDBM144855 (US8952157, 37 | US9303025, 37) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 0.00500 | -64.5 | n/a | n/a | n/a | n/a | n/a | n/a | 25 |
AbbVie Inc. US Patent | Assay Description Representative compounds were serially diluted in dimethyl sulfoxide (DMSO) starting at 50 μM (2× starting concentration; 10% DMSO) and 10 μ... | US Patent US9303025 (2016) BindingDB Entry DOI: 10.7270/Q2XD10JP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Apoptosis regulator Bcl-2 (Mus musculus (Mouse)) | BDBM178793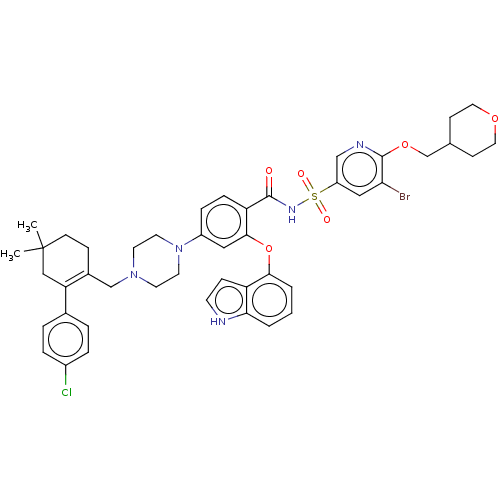 (US9125913, 364) | PDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 0.00600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AbbVie Inc. US Patent | Assay Description The inhibition constant (Ki) is the dissociation constant of an enzyme-inhibitor complex or a protein/small molecule complex, wherein the small molec... | US Patent US9125913 (2015) BindingDB Entry DOI: 10.7270/Q29Z93PP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor X (Homo sapiens (Human)) | BDBM17134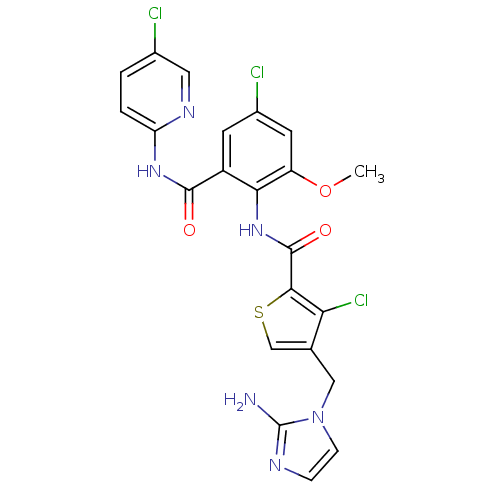 (4-[(2-amino-1H-imidazol-1-yl)methyl]-3-chloro-N-{4...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | 0.00600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Berlex Biosciences | Assay Description The enzyme activities were determined kinetically as the initial rate of cleavage of a peptide p-nitroanilide. Km for enzyme and substrate was determ... | J Med Chem 50: 2967-80 (2007) Article DOI: 10.1021/jm070125f BindingDB Entry DOI: 10.7270/Q2J101F5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor X (Homo sapiens (Human)) | BDBM17111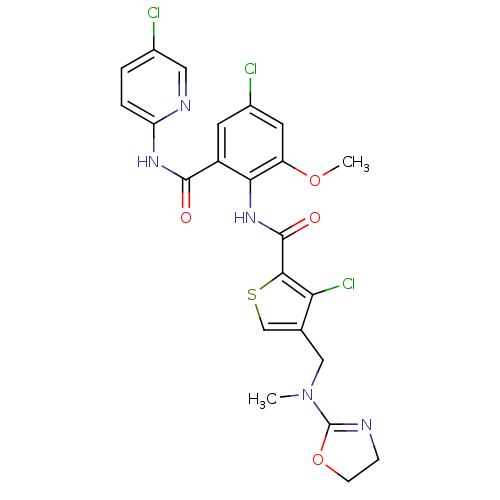 (3-chloro-N-{4-chloro-2-[(5-chloropyridin-2-yl)carb...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid PDB UniChem Similars | PDB Article PubMed | 0.00700 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Berlex Biosciences | Assay Description The enzyme activities were determined kinetically as the initial rate of cleavage of a peptide p-nitroanilide. Km for enzyme and substrate was determ... | J Med Chem 50: 2967-80 (2007) Article DOI: 10.1021/jm070125f BindingDB Entry DOI: 10.7270/Q2J101F5 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Apoptosis regulator Bcl-2 (Mus musculus (Mouse)) | BDBM178842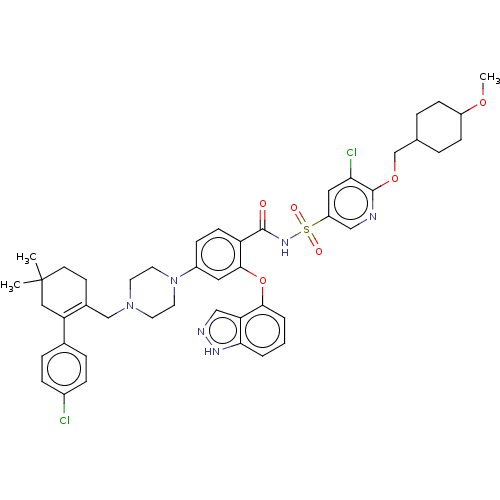 (US9125913, 414) | PDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 0.00700 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AbbVie Inc. US Patent | Assay Description The inhibition constant (Ki) is the dissociation constant of an enzyme-inhibitor complex or a protein/small molecule complex, wherein the small molec... | US Patent US9125913 (2015) BindingDB Entry DOI: 10.7270/Q29Z93PP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Apoptosis regulator Bcl-2 (Mus musculus (Mouse)) | BDBM178854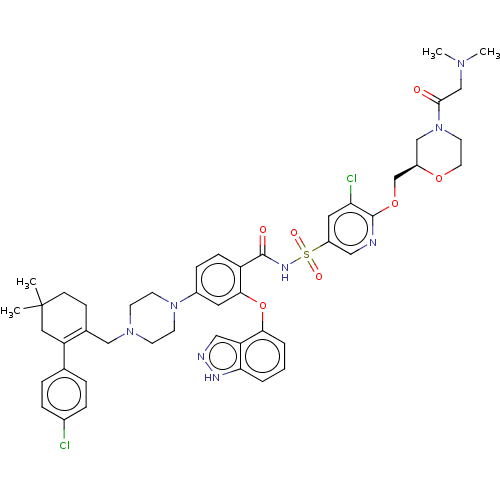 (US9125913, 426) | PDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 0.00700 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AbbVie Inc. US Patent | Assay Description The inhibition constant (Ki) is the dissociation constant of an enzyme-inhibitor complex or a protein/small molecule complex, wherein the small molec... | US Patent US9125913 (2015) BindingDB Entry DOI: 10.7270/Q29Z93PP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Apoptosis regulator Bcl-2 (Mus musculus (Mouse)) | BDBM178891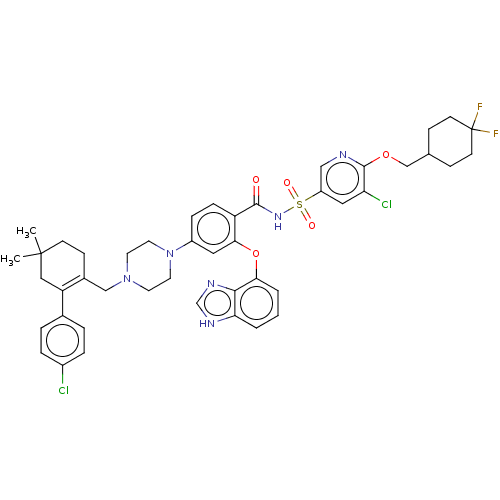 (US9125913, 463) | PDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 0.00700 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AbbVie Inc. US Patent | Assay Description The inhibition constant (Ki) is the dissociation constant of an enzyme-inhibitor complex or a protein/small molecule complex, wherein the small molec... | US Patent US9125913 (2015) BindingDB Entry DOI: 10.7270/Q29Z93PP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor X (Homo sapiens (Human)) | BDBM17137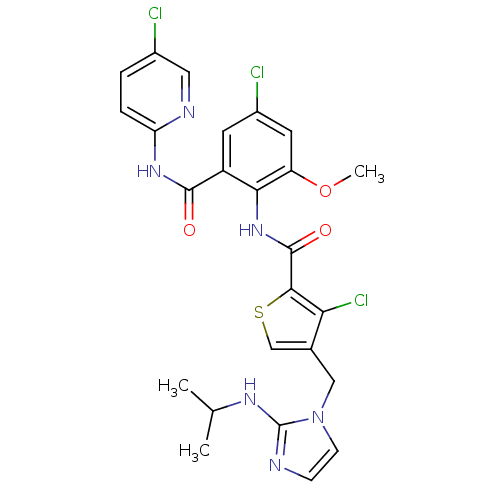 (3-chloro-N-{4-chloro-2-[(5-chloropyridin-2-yl)carb...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 0.00800 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Berlex Biosciences | Assay Description The enzyme activities were determined kinetically as the initial rate of cleavage of a peptide p-nitroanilide. Km for enzyme and substrate was determ... | J Med Chem 50: 2967-80 (2007) Article DOI: 10.1021/jm070125f BindingDB Entry DOI: 10.7270/Q2J101F5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Apoptosis regulator Bcl-2 (Mus musculus (Mouse)) | BDBM178576 (US9125913, 135) | PDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 0.00800 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AbbVie Inc. US Patent | Assay Description The inhibition constant (Ki) is the dissociation constant of an enzyme-inhibitor complex or a protein/small molecule complex, wherein the small molec... | US Patent US9125913 (2015) BindingDB Entry DOI: 10.7270/Q29Z93PP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Apoptosis regulator Bcl-2 (Homo sapiens (Human)) | BDBM145152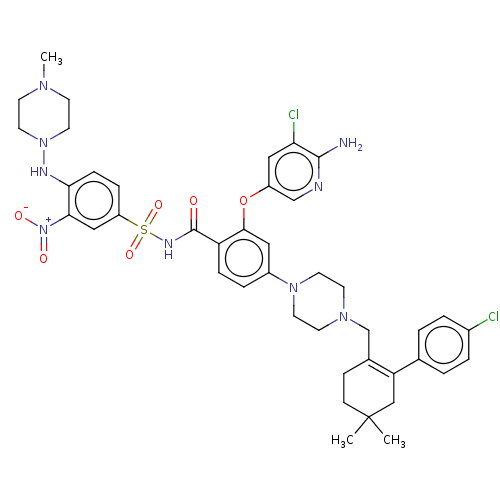 (US8952157, 343 | US9303025, 343) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 0.00900 | -63.0 | n/a | n/a | n/a | n/a | n/a | n/a | 25 |
AbbVie Inc.; The Walter and Eliza Hall Institute of Medical Research US Patent | Assay Description Representative compounds were serially diluted in dimethyl sulfoxide (DMSO) starting at 50 uM (2x starting concentration; 10% DMSO) and 10 uL were tr... | US Patent US8952157 (2015) BindingDB Entry DOI: 10.7270/Q2QN65G9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Apoptosis regulator Bcl-2 (Mus musculus (Mouse)) | BDBM178574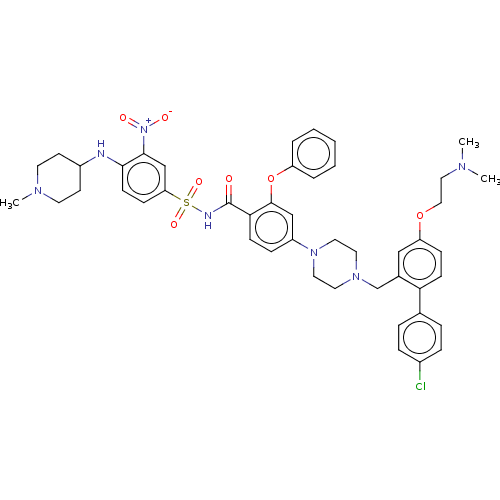 (US9125913, 133) | PDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 0.00900 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AbbVie Inc. US Patent | Assay Description The inhibition constant (Ki) is the dissociation constant of an enzyme-inhibitor complex or a protein/small molecule complex, wherein the small molec... | US Patent US9125913 (2015) BindingDB Entry DOI: 10.7270/Q29Z93PP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Apoptosis regulator Bcl-2 (Mus musculus (Mouse)) | BDBM178645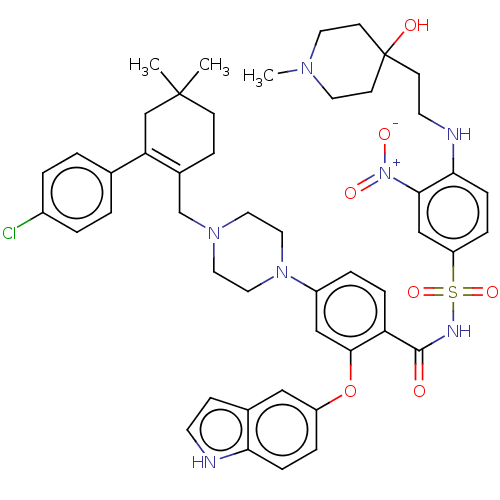 (US9125913, 211) | PDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 0.00900 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AbbVie Inc. US Patent | Assay Description The inhibition constant (Ki) is the dissociation constant of an enzyme-inhibitor complex or a protein/small molecule complex, wherein the small molec... | US Patent US9125913 (2015) BindingDB Entry DOI: 10.7270/Q29Z93PP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Apoptosis regulator Bcl-2 (Homo sapiens (Human)) | BDBM145152 (US8952157, 343 | US9303025, 343) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 0.00900 | -63.0 | n/a | n/a | n/a | n/a | n/a | n/a | 25 |
AbbVie Inc. US Patent | Assay Description Representative compounds were serially diluted in dimethyl sulfoxide (DMSO) starting at 50 μM (2× starting concentration; 10% DMSO) and 10 μ... | US Patent US9303025 (2016) BindingDB Entry DOI: 10.7270/Q2XD10JP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 4402 total ) | Next | Last >> |