 Found 3968 hits with Last Name = 'sutherlin' and Initial = 'd'
Found 3968 hits with Last Name = 'sutherlin' and Initial = 'd' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit delta isoform
(Homo sapiens (Human)) | BDBM50438628
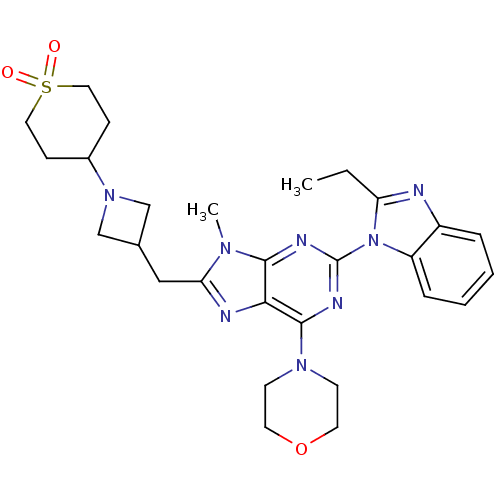
(CHEMBL2414299)Show SMILES CCc1nc2ccccc2n1-c1nc(N2CCOCC2)c2nc(CC3CN(C3)C3CCS(=O)(=O)CC3)n(C)c2n1 Show InChI InChI=1S/C28H36N8O3S/c1-3-23-29-21-6-4-5-7-22(21)36(23)28-31-26-25(27(32-28)34-10-12-39-13-11-34)30-24(33(26)2)16-19-17-35(18-19)20-8-14-40(37,38)15-9-20/h4-7,19-20H,3,8-18H2,1-2H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.230 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech
Curated by ChEMBL
| Assay Description
Inhibition of PI3Kdelta (unknown origin) assessed as formation of PIP3 by competitive fluorescence polarization assay |
Bioorg Med Chem Lett 23: 4953-9 (2013)
Article DOI: 10.1016/j.bmcl.2013.06.052
BindingDB Entry DOI: 10.7270/Q2ZG6TP9 |
More data for this
Ligand-Target Pair | |
Sodium channel protein type 9 subunit alpha
(Homo sapiens (Human)) | BDBM50521576
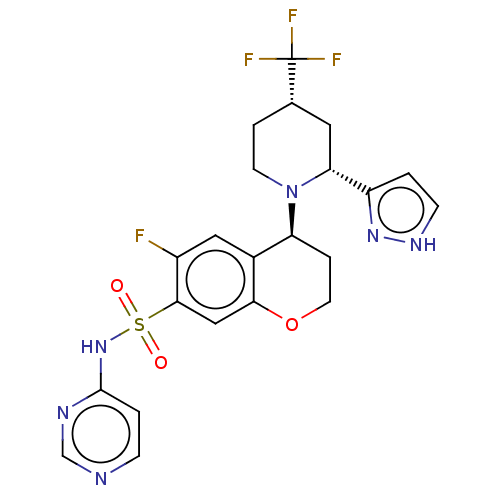
(CHEMBL4462278)Show SMILES Fc1cc2[C@H](CCOc2cc1S(=O)(=O)Nc1ccncn1)N1CC[C@@H](C[C@@H]1c1cc[nH]n1)C(F)(F)F |r| Show InChI InChI=1S/C22H22F4N6O3S/c23-15-10-14-17(32-7-3-13(22(24,25)26)9-18(32)16-1-6-29-30-16)4-8-35-19(14)11-20(15)36(33,34)31-21-2-5-27-12-28-21/h1-2,5-6,10-13,17-18H,3-4,7-9H2,(H,29,30)(H,27,28,31)/t13-,17-,18+/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.320 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech
Curated by ChEMBL
| Assay Description
Displacement of [3H]GX-545 from full length human Nav1.7 VSD4 domain expressed in HEK cell membranes measured after 20 hrs by liquid scintillation co... |
J Med Chem 62: 4091-4109 (2019)
Article DOI: 10.1021/acs.jmedchem.9b00141
BindingDB Entry DOI: 10.7270/Q2B85CJ3 |
More data for this
Ligand-Target Pair | |
Coagulation factor VII
(Homo sapiens (Human)) | BDBM14714
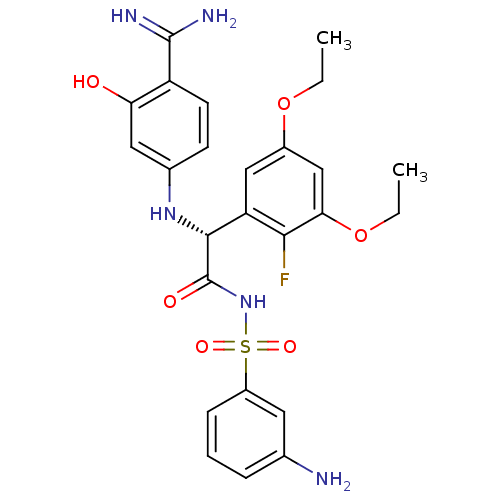
((2R)-N-[(3-aminobenzene)sulfonyl]-2-[(4-carbamimid...)Show SMILES CCOc1cc(OCC)c(F)c(c1)[C@@H](Nc1ccc(C(N)=N)c(O)c1)C(=O)NS(=O)(=O)c1cccc(N)c1 |r| Show InChI InChI=1S/C25H28FN5O6S/c1-3-36-16-12-19(22(26)21(13-16)37-4-2)23(30-15-8-9-18(24(28)29)20(32)11-15)25(33)31-38(34,35)17-7-5-6-14(27)10-17/h5-13,23,30,32H,3-4,27H2,1-2H3,(H3,28,29)(H,31,33)/t23-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| MMDB
PC cid
PC sid
PDB
UniChem
| MMDB
PDB
Article
PubMed
| 0.350 | -53.4 | n/a | n/a | n/a | n/a | n/a | 7.8 | 22 |
Genentech
| Assay Description
The enzyme reactions were initiated by the addition of substrate, and the color developed from the release of p-nitroanilide from each chromogenic su... |
J Biol Chem 280: 9160-9 (2005)
Article DOI: 10.1074/jbc.M409068200
BindingDB Entry DOI: 10.7270/Q2JM27W3 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit delta isoform
(Homo sapiens (Human)) | BDBM50438634
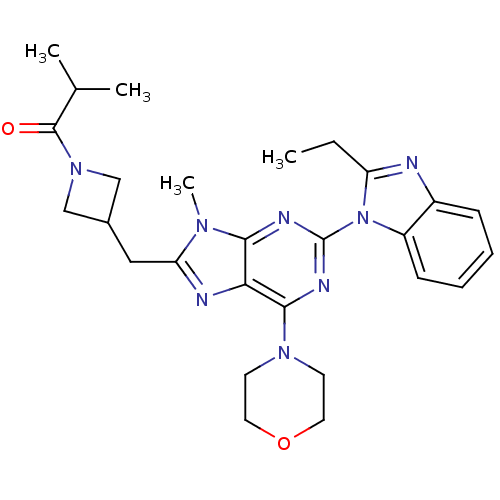
(CHEMBL2414301)Show SMILES CCc1nc2ccccc2n1-c1nc(N2CCOCC2)c2nc(CC3CN(C3)C(=O)C(C)C)n(C)c2n1 Show InChI InChI=1S/C27H34N8O2/c1-5-21-28-19-8-6-7-9-20(19)35(21)27-30-24-23(25(31-27)33-10-12-37-13-11-33)29-22(32(24)4)14-18-15-34(16-18)26(36)17(2)3/h6-9,17-18H,5,10-16H2,1-4H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.360 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech
Curated by ChEMBL
| Assay Description
Inhibition of PI3Kdelta (unknown origin) assessed as formation of PIP3 by competitive fluorescence polarization assay |
Bioorg Med Chem Lett 23: 4953-9 (2013)
Article DOI: 10.1016/j.bmcl.2013.06.052
BindingDB Entry DOI: 10.7270/Q2ZG6TP9 |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM50363990
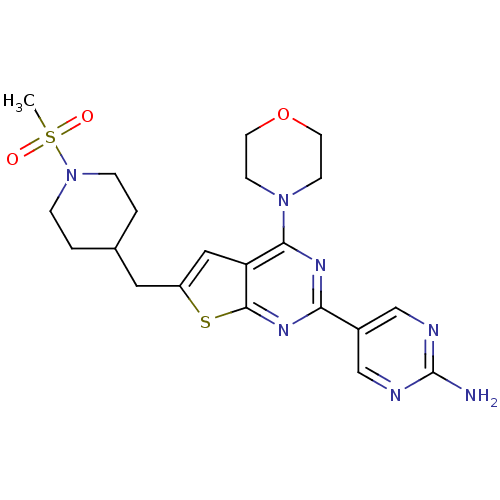
(CHEMBL1949915)Show SMILES CS(=O)(=O)N1CCC(Cc2cc3c(nc(nc3s2)-c2cnc(N)nc2)N2CCOCC2)CC1 Show InChI InChI=1S/C21H27N7O3S2/c1-33(29,30)28-4-2-14(3-5-28)10-16-11-17-19(27-6-8-31-9-7-27)25-18(26-20(17)32-16)15-12-23-21(22)24-13-15/h11-14H,2-10H2,1H3,(H2,22,23,24) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech
Curated by ChEMBL
| Assay Description
Apparent binding affinity to PI3Kalpha using PIP3 as substrate after 30 mins by fluorescence polarization assay |
J Med Chem 54: 7815-33 (2011)
Article DOI: 10.1021/jm2007084
BindingDB Entry DOI: 10.7270/Q2057GCQ |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit delta isoform
(Homo sapiens (Human)) | BDBM50403075
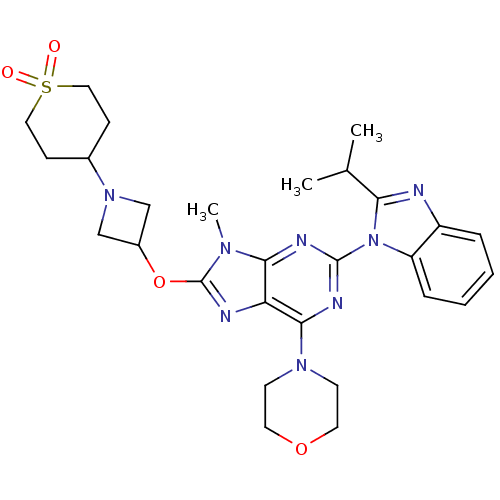
(CHEMBL2216902)Show SMILES CC(C)c1nc2ccccc2n1-c1nc(N2CCOCC2)c2nc(OC3CN(C3)C3CCS(=O)(=O)CC3)n(C)c2n1 Show InChI InChI=1S/C28H36N8O4S/c1-18(2)24-29-21-6-4-5-7-22(21)36(24)27-31-25-23(26(32-27)34-10-12-39-13-11-34)30-28(33(25)3)40-20-16-35(17-20)19-8-14-41(37,38)15-9-19/h4-7,18-20H,8-17H2,1-3H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.470 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech
Curated by ChEMBL
| Assay Description
Inhibition of PI3Kdelta (unknown origin) assessed as formation of PIP3 by competitive fluorescence polarization assay |
Bioorg Med Chem Lett 23: 4953-9 (2013)
Article DOI: 10.1016/j.bmcl.2013.06.052
BindingDB Entry DOI: 10.7270/Q2ZG6TP9 |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit delta isoform
(Homo sapiens (Human)) | BDBM172639
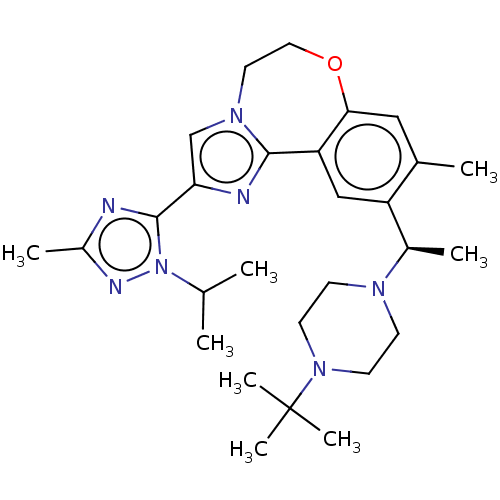
(US9090628, 299)Show SMILES CC(C)n1nc(C)nc1-c1cn2CCOc3cc(C)c(cc3-c2n1)[C@@H](C)N1CCN(CC1)C(C)(C)C |r| Show InChI InChI=1S/C28H41N7O/c1-18(2)35-27(29-21(5)31-35)24-17-33-13-14-36-25-15-19(3)22(16-23(25)26(33)30-24)20(4)32-9-11-34(12-10-32)28(6,7)8/h15-18,20H,9-14H2,1-8H3/t20-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.480 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech
Curated by ChEMBL
| Assay Description
Binding affinity to PI3Kdelta (unknown origin) |
ACS Med Chem Lett 8: 936-940 (2017)
Article DOI: 10.1021/acsmedchemlett.7b00170
BindingDB Entry DOI: 10.7270/Q2CV4M7R |
More data for this
Ligand-Target Pair | |
Sodium channel protein type 9 subunit alpha
(Homo sapiens (Human)) | BDBM50521582
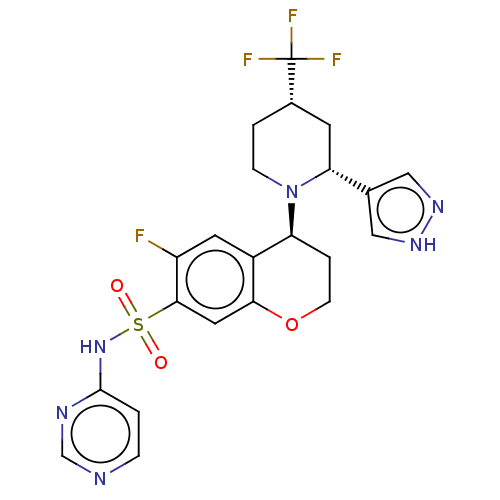
(CHEMBL4543884)Show SMILES Fc1cc2[C@H](CCOc2cc1S(=O)(=O)Nc1ccncn1)N1CC[C@@H](C[C@@H]1c1cn[nH]c1)C(F)(F)F |r| Show InChI InChI=1S/C22H22F4N6O3S/c23-16-8-15-17(32-5-2-14(22(24,25)26)7-18(32)13-10-29-30-11-13)3-6-35-19(15)9-20(16)36(33,34)31-21-1-4-27-12-28-21/h1,4,8-12,14,17-18H,2-3,5-7H2,(H,29,30)(H,27,28,31)/t14-,17-,18+/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.510 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech
Curated by ChEMBL
| Assay Description
Displacement of [3H]GX-545 from full length human Nav1.7 VSD4 domain expressed in HEK cell membranes measured after 20 hrs by liquid scintillation co... |
J Med Chem 62: 4091-4109 (2019)
Article DOI: 10.1021/acs.jmedchem.9b00141
BindingDB Entry DOI: 10.7270/Q2B85CJ3 |
More data for this
Ligand-Target Pair | |
Sodium channel protein type 9 subunit alpha
(Homo sapiens (Human)) | BDBM50466967
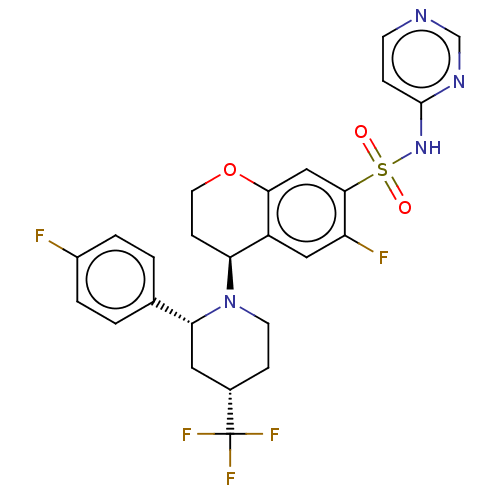
(CHEMBL4282994)Show SMILES Fc1ccc(cc1)[C@H]1C[C@H](CCN1[C@H]1CCOc2cc(c(F)cc12)S(=O)(=O)Nc1ccncn1)C(F)(F)F |r| Show InChI InChI=1S/C25H23F5N4O3S/c26-17-3-1-15(2-4-17)21-11-16(25(28,29)30)6-9-34(21)20-7-10-37-22-13-23(19(27)12-18(20)22)38(35,36)33-24-5-8-31-14-32-24/h1-5,8,12-14,16,20-21H,6-7,9-11H2,(H,31,32,33)/t16-,20-,21+/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.530 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech
Curated by ChEMBL
| Assay Description
Displacement of [3H]GX-545 from full length human Nav1.7 VSD4 domain expressed in HEK cell membranes measured after 20 hrs by liquid scintillation co... |
J Med Chem 62: 4091-4109 (2019)
Article DOI: 10.1021/acs.jmedchem.9b00141
BindingDB Entry DOI: 10.7270/Q2B85CJ3 |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit delta isoform
(Homo sapiens (Human)) | BDBM50403074
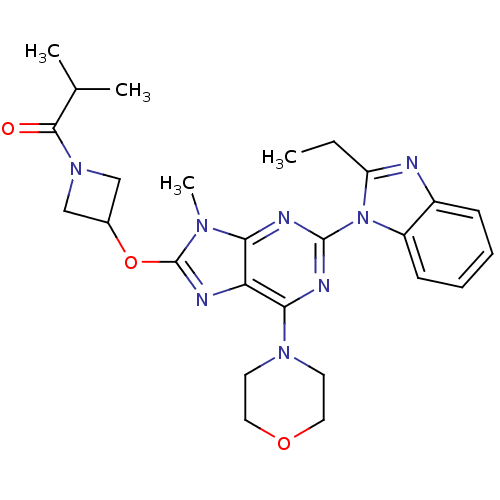
(CHEMBL2216903)Show SMILES CCc1nc2ccccc2n1-c1nc(N2CCOCC2)c2nc(OC3CN(C3)C(=O)C(C)C)n(C)c2n1 Show InChI InChI=1S/C26H32N8O3/c1-5-20-27-18-8-6-7-9-19(18)34(20)25-29-22-21(23(30-25)32-10-12-36-13-11-32)28-26(31(22)4)37-17-14-33(15-17)24(35)16(2)3/h6-9,16-17H,5,10-15H2,1-4H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.580 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech
Curated by ChEMBL
| Assay Description
Inhibition of PI3Kdelta (unknown origin) assessed as formation of PIP3 by competitive fluorescence polarization assay |
Bioorg Med Chem Lett 23: 4953-9 (2013)
Article DOI: 10.1016/j.bmcl.2013.06.052
BindingDB Entry DOI: 10.7270/Q2ZG6TP9 |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit delta isoform
(Homo sapiens (Human)) | BDBM50438630
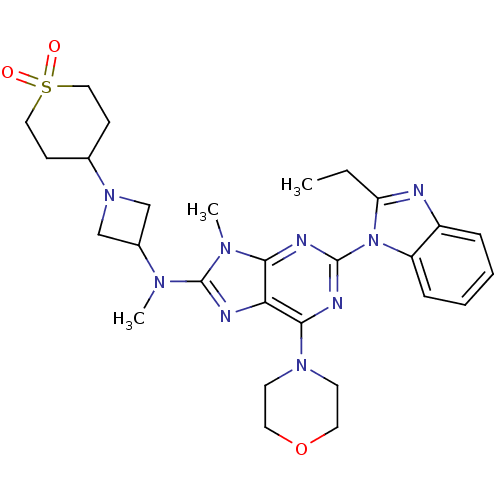
(CHEMBL2414297)Show SMILES CCc1nc2ccccc2n1-c1nc(N2CCOCC2)c2nc(N(C)C3CN(C3)C3CCS(=O)(=O)CC3)n(C)c2n1 Show InChI InChI=1S/C28H37N9O3S/c1-4-23-29-21-7-5-6-8-22(21)37(23)27-31-25-24(26(32-27)35-11-13-40-14-12-35)30-28(34(25)3)33(2)20-17-36(18-20)19-9-15-41(38,39)16-10-19/h5-8,19-20H,4,9-18H2,1-3H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.590 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech
Curated by ChEMBL
| Assay Description
Inhibition of PI3Kdelta (unknown origin) assessed as formation of PIP3 by competitive fluorescence polarization assay |
Bioorg Med Chem Lett 23: 4953-9 (2013)
Article DOI: 10.1016/j.bmcl.2013.06.052
BindingDB Entry DOI: 10.7270/Q2ZG6TP9 |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM50347092
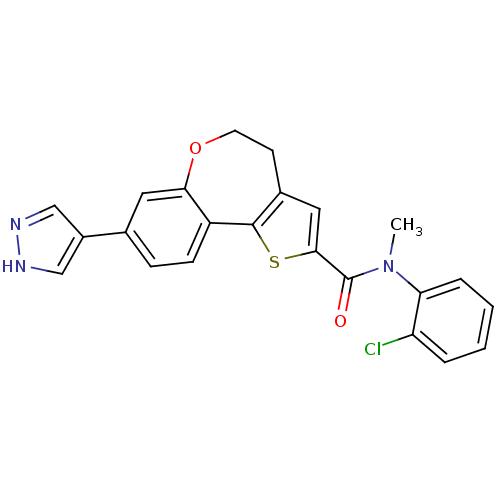
(CHEMBL1796757)Show SMILES CN(C(=O)c1cc2CCOc3cc(ccc3-c2s1)-c1cn[nH]c1)c1ccccc1Cl Show InChI InChI=1S/C23H18ClN3O2S/c1-27(19-5-3-2-4-18(19)24)23(28)21-11-15-8-9-29-20-10-14(16-12-25-26-13-16)6-7-17(20)22(15)30-21/h2-7,10-13H,8-9H2,1H3,(H,25,26) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of recombinant PI3Kalpha assessed as inhibition of PIP3 formation by fluorescence polarization assay |
Bioorg Med Chem Lett 21: 4054-8 (2011)
Article DOI: 10.1016/j.bmcl.2011.04.124
BindingDB Entry DOI: 10.7270/Q29G5N57 |
More data for this
Ligand-Target Pair | |
Sodium channel protein type 9 subunit alpha
(Homo sapiens (Human)) | BDBM50521579
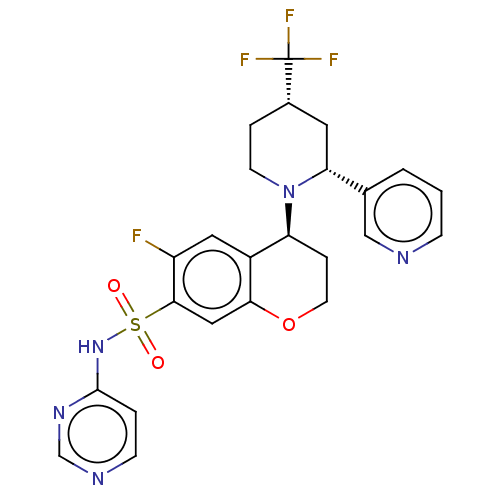
(CHEMBL4543422)Show SMILES Fc1cc2[C@H](CCOc2cc1S(=O)(=O)Nc1ccncn1)N1CC[C@@H](C[C@@H]1c1cccnc1)C(F)(F)F |r| Show InChI InChI=1S/C24H23F4N5O3S/c25-18-11-17-19(33-8-4-16(24(26,27)28)10-20(33)15-2-1-6-29-13-15)5-9-36-21(17)12-22(18)37(34,35)32-23-3-7-30-14-31-23/h1-3,6-7,11-14,16,19-20H,4-5,8-10H2,(H,30,31,32)/t16-,19-,20+/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.680 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech
Curated by ChEMBL
| Assay Description
Displacement of [3H]GX-545 from full length human Nav1.7 VSD4 domain expressed in HEK cell membranes measured after 20 hrs by liquid scintillation co... |
J Med Chem 62: 4091-4109 (2019)
Article DOI: 10.1021/acs.jmedchem.9b00141
BindingDB Entry DOI: 10.7270/Q2B85CJ3 |
More data for this
Ligand-Target Pair | |
Sodium channel protein type 9 subunit alpha
(Homo sapiens (Human)) | BDBM50521586
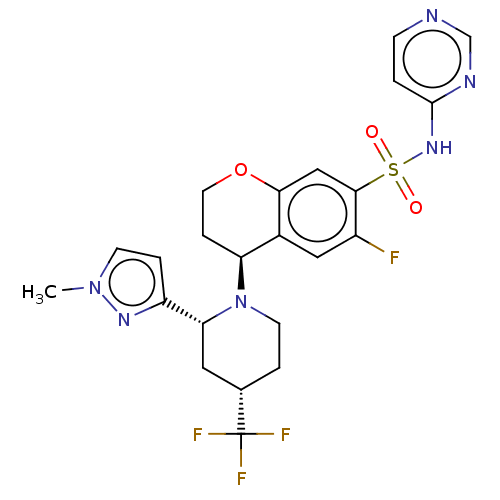
(CHEMBL4473871)Show SMILES Cn1ccc(n1)[C@H]1C[C@H](CCN1[C@H]1CCOc2cc(c(F)cc12)S(=O)(=O)Nc1ccncn1)C(F)(F)F |r| Show InChI InChI=1S/C23H24F4N6O3S/c1-32-7-4-17(30-32)19-10-14(23(25,26)27)3-8-33(19)18-5-9-36-20-12-21(16(24)11-15(18)20)37(34,35)31-22-2-6-28-13-29-22/h2,4,6-7,11-14,18-19H,3,5,8-10H2,1H3,(H,28,29,31)/t14-,18-,19+/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.760 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech
Curated by ChEMBL
| Assay Description
Displacement of [3H]GX-545 from full length human Nav1.7 VSD4 domain expressed in HEK cell membranes measured after 20 hrs by liquid scintillation co... |
J Med Chem 62: 4091-4109 (2019)
Article DOI: 10.1021/acs.jmedchem.9b00141
BindingDB Entry DOI: 10.7270/Q2B85CJ3 |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit delta isoform
(Homo sapiens (Human)) | BDBM172634
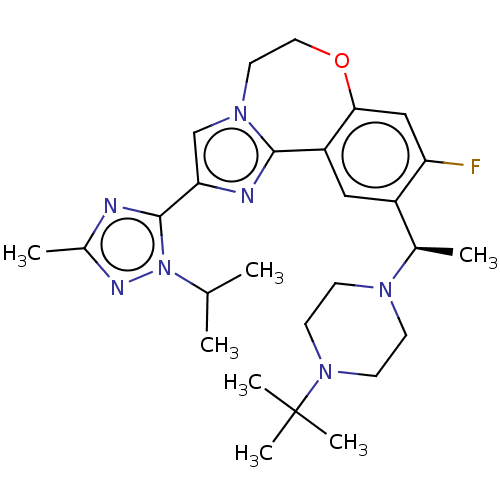
(US9090628, 294)Show SMILES CC(C)n1nc(C)nc1-c1cn2CCOc3cc(F)c(cc3-c2n1)[C@@H](C)N1CCN(CC1)C(C)(C)C |r| Show InChI InChI=1S/C27H38FN7O/c1-17(2)35-26(29-19(4)31-35)23-16-33-12-13-36-24-15-22(28)20(14-21(24)25(33)30-23)18(3)32-8-10-34(11-9-32)27(5,6)7/h14-18H,8-13H2,1-7H3/t18-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.780 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech
Curated by ChEMBL
| Assay Description
Binding affinity to PI3Kdelta (unknown origin) |
ACS Med Chem Lett 8: 936-940 (2017)
Article DOI: 10.1021/acsmedchemlett.7b00170
BindingDB Entry DOI: 10.7270/Q2CV4M7R |
More data for this
Ligand-Target Pair | |
Sodium channel protein type 9 subunit alpha
(Homo sapiens (Human)) | BDBM50521571
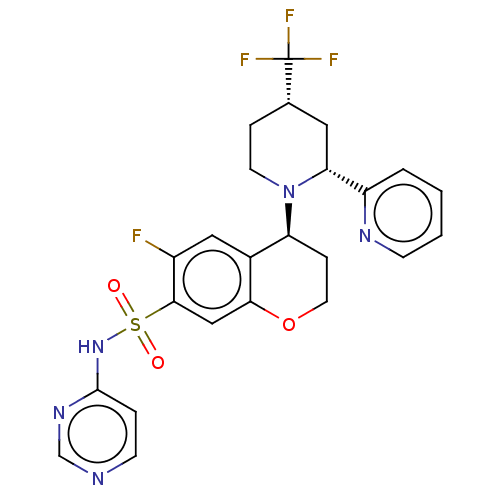
(CHEMBL4464990)Show SMILES Fc1cc2[C@H](CCOc2cc1S(=O)(=O)Nc1ccncn1)N1CC[C@@H](C[C@@H]1c1ccccn1)C(F)(F)F |r| Show InChI InChI=1S/C24H23F4N5O3S/c25-17-12-16-19(33-9-5-15(24(26,27)28)11-20(33)18-3-1-2-7-30-18)6-10-36-21(16)13-22(17)37(34,35)32-23-4-8-29-14-31-23/h1-4,7-8,12-15,19-20H,5-6,9-11H2,(H,29,31,32)/t15-,19-,20+/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| 0.790 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech
Curated by ChEMBL
| Assay Description
Displacement of [3H]GX-545 from full length human Nav1.7 VSD4 domain expressed in HEK cell membranes measured after 20 hrs by liquid scintillation co... |
J Med Chem 62: 4091-4109 (2019)
Article DOI: 10.1021/acs.jmedchem.9b00141
BindingDB Entry DOI: 10.7270/Q2B85CJ3 |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit delta isoform
(Homo sapiens (Human)) | BDBM50438633
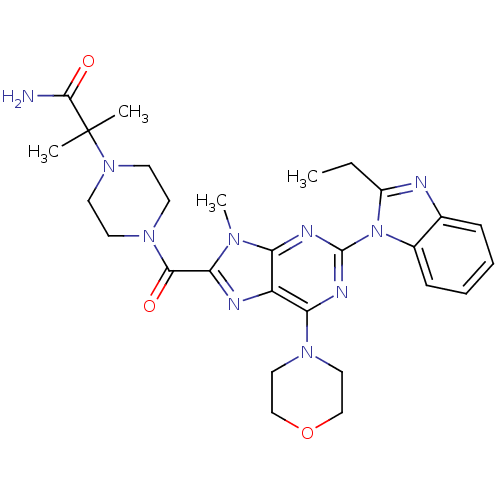
(CHEMBL2414294)Show SMILES CCc1nc2ccccc2n1-c1nc(N2CCOCC2)c2nc(C(=O)N3CCN(CC3)C(C)(C)C(N)=O)n(C)c2n1 Show InChI InChI=1S/C28H36N10O3/c1-5-20-30-18-8-6-7-9-19(18)38(20)27-32-22-21(23(33-27)35-14-16-41-17-15-35)31-24(34(22)4)25(39)36-10-12-37(13-11-36)28(2,3)26(29)40/h6-9H,5,10-17H2,1-4H3,(H2,29,40) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.800 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech
Curated by ChEMBL
| Assay Description
Inhibition of PI3Kdelta (unknown origin) assessed as formation of PIP3 by competitive fluorescence polarization assay |
Bioorg Med Chem Lett 23: 4953-9 (2013)
Article DOI: 10.1016/j.bmcl.2013.06.052
BindingDB Entry DOI: 10.7270/Q2ZG6TP9 |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit delta isoform
(Homo sapiens (Human)) | BDBM50278665
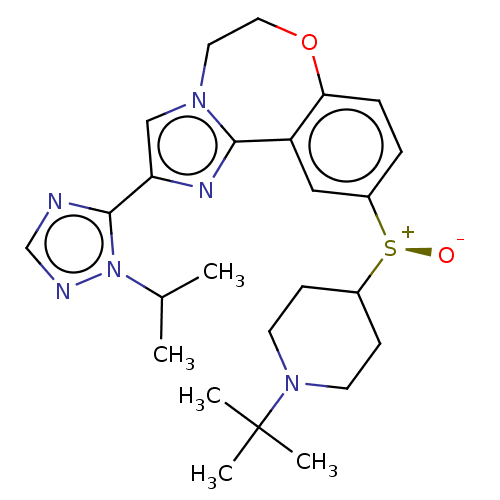
(CHEMBL4175041)Show SMILES CC(C)n1ncnc1-c1cn2CCOc3ccc(cc3-c2n1)[S@@+]([O-])C1CCN(CC1)C(C)(C)C |r| Show InChI InChI=1S/C25H34N6O2S/c1-17(2)31-24(26-16-27-31)21-15-29-12-13-33-22-7-6-19(14-20(22)23(29)28-21)34(32)18-8-10-30(11-9-18)25(3,4)5/h6-7,14-18H,8-13H2,1-5H3/t34-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.810 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech
Curated by ChEMBL
| Assay Description
Binding affinity to PI3Kdelta (unknown origin) |
ACS Med Chem Lett 8: 936-940 (2017)
Article DOI: 10.1021/acsmedchemlett.7b00170
BindingDB Entry DOI: 10.7270/Q2CV4M7R |
More data for this
Ligand-Target Pair | |
Sodium channel protein type 9 subunit alpha
(Homo sapiens (Human)) | BDBM50521573
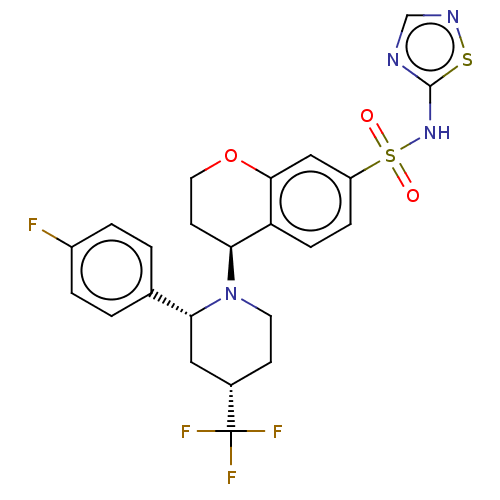
(CHEMBL4519170)Show SMILES Fc1ccc(cc1)[C@H]1C[C@H](CCN1[C@H]1CCOc2cc(ccc12)S(=O)(=O)Nc1ncns1)C(F)(F)F |r| Show InChI InChI=1S/C23H22F4N4O3S2/c24-16-3-1-14(2-4-16)20-11-15(23(25,26)27)7-9-31(20)19-8-10-34-21-12-17(5-6-18(19)21)36(32,33)30-22-28-13-29-35-22/h1-6,12-13,15,19-20H,7-11H2,(H,28,29,30)/t15-,19-,20+/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.850 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech
Curated by ChEMBL
| Assay Description
Displacement of [3H]GX-545 from full length human Nav1.7 VSD4 domain expressed in HEK cell membranes measured after 20 hrs by liquid scintillation co... |
J Med Chem 62: 4091-4109 (2019)
Article DOI: 10.1021/acs.jmedchem.9b00141
BindingDB Entry DOI: 10.7270/Q2B85CJ3 |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit delta isoform
(Homo sapiens (Human)) | BDBM50438635
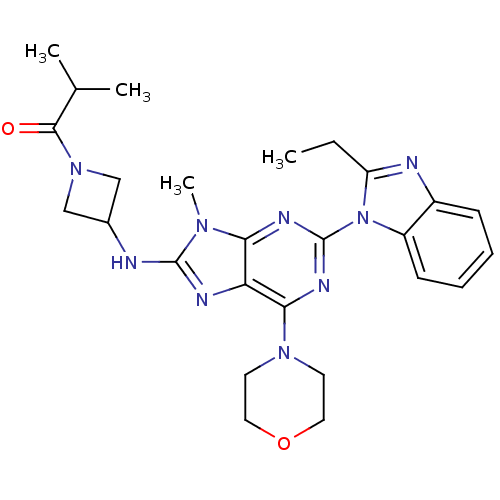
(CHEMBL2414300)Show SMILES CCc1nc2ccccc2n1-c1nc(N2CCOCC2)c2nc(NC3CN(C3)C(=O)C(C)C)n(C)c2n1 Show InChI InChI=1S/C26H33N9O2/c1-5-20-28-18-8-6-7-9-19(18)35(20)26-30-22-21(23(31-26)33-10-12-37-13-11-33)29-25(32(22)4)27-17-14-34(15-17)24(36)16(2)3/h6-9,16-17H,5,10-15H2,1-4H3,(H,27,29) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.880 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech
Curated by ChEMBL
| Assay Description
Inhibition of PI3Kdelta (unknown origin) assessed as formation of PIP3 by competitive fluorescence polarization assay |
Bioorg Med Chem Lett 23: 4953-9 (2013)
Article DOI: 10.1016/j.bmcl.2013.06.052
BindingDB Entry DOI: 10.7270/Q2ZG6TP9 |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit delta isoform
(Homo sapiens (Human)) | BDBM172640
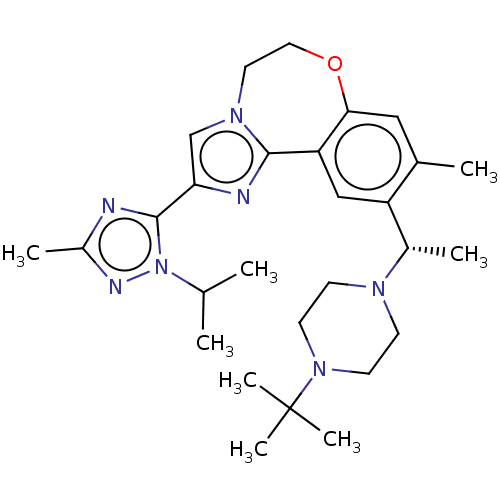
(US9090628, 300)Show SMILES CC(C)n1nc(C)nc1-c1cn2CCOc3cc(C)c(cc3-c2n1)[C@H](C)N1CCN(CC1)C(C)(C)C |r| Show InChI InChI=1S/C28H41N7O/c1-18(2)35-27(29-21(5)31-35)24-17-33-13-14-36-25-15-19(3)22(16-23(25)26(33)30-24)20(4)32-9-11-34(12-10-32)28(6,7)8/h15-18,20H,9-14H2,1-8H3/t20-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.880 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech
Curated by ChEMBL
| Assay Description
Binding affinity to PI3Kdelta (unknown origin) |
ACS Med Chem Lett 8: 936-940 (2017)
Article DOI: 10.1021/acsmedchemlett.7b00170
BindingDB Entry DOI: 10.7270/Q2CV4M7R |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit delta isoform
(Homo sapiens (Human)) | BDBM50438629
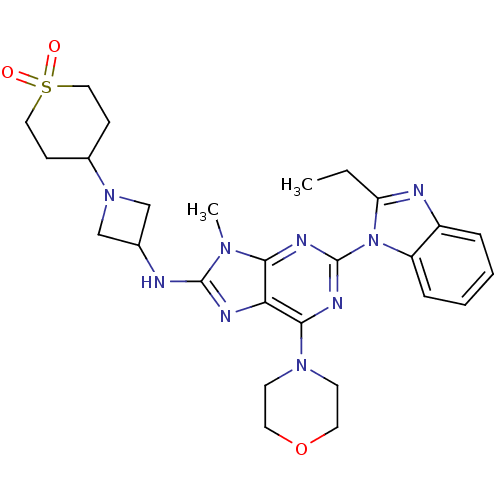
(CHEMBL2414298)Show SMILES CCc1nc2ccccc2n1-c1nc(N2CCOCC2)c2nc(NC3CN(C3)C3CCS(=O)(=O)CC3)n(C)c2n1 Show InChI InChI=1S/C27H35N9O3S/c1-3-22-29-20-6-4-5-7-21(20)36(22)27-31-24-23(25(32-27)34-10-12-39-13-11-34)30-26(33(24)2)28-18-16-35(17-18)19-8-14-40(37,38)15-9-19/h4-7,18-19H,3,8-17H2,1-2H3,(H,28,30) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.900 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech
Curated by ChEMBL
| Assay Description
Inhibition of PI3Kdelta (unknown origin) assessed as formation of PIP3 by competitive fluorescence polarization assay |
Bioorg Med Chem Lett 23: 4953-9 (2013)
Article DOI: 10.1016/j.bmcl.2013.06.052
BindingDB Entry DOI: 10.7270/Q2ZG6TP9 |
More data for this
Ligand-Target Pair | |
Sodium channel protein type 9 subunit alpha
(Homo sapiens (Human)) | BDBM50521569
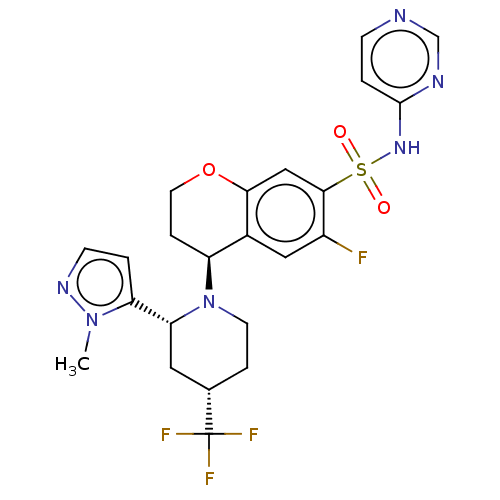
(CHEMBL4446868)Show SMILES Cn1nccc1[C@H]1C[C@H](CCN1[C@H]1CCOc2cc(c(F)cc12)S(=O)(=O)Nc1ccncn1)C(F)(F)F |r| Show InChI InChI=1S/C23H24F4N6O3S/c1-32-18(2-7-30-32)19-10-14(23(25,26)27)4-8-33(19)17-5-9-36-20-12-21(16(24)11-15(17)20)37(34,35)31-22-3-6-28-13-29-22/h2-3,6-7,11-14,17,19H,4-5,8-10H2,1H3,(H,28,29,31)/t14-,17-,19+/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.930 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech
Curated by ChEMBL
| Assay Description
Displacement of [3H]GX-545 from full length human Nav1.7 VSD4 domain expressed in HEK cell membranes measured after 20 hrs by liquid scintillation co... |
J Med Chem 62: 4091-4109 (2019)
Article DOI: 10.1021/acs.jmedchem.9b00141
BindingDB Entry DOI: 10.7270/Q2B85CJ3 |
More data for this
Ligand-Target Pair | |
Sodium channel protein type 9 subunit alpha
(Homo sapiens (Human)) | BDBM50521568
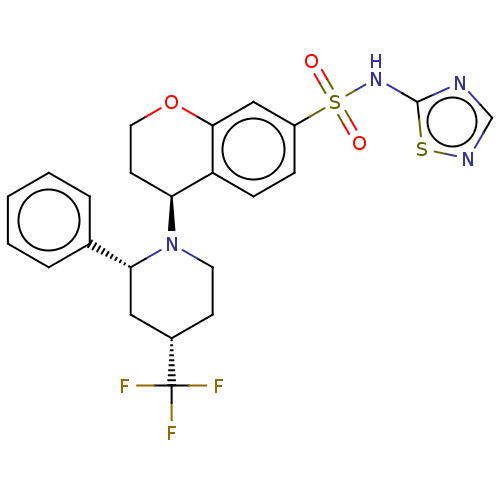
(CHEMBL4444408)Show SMILES FC(F)(F)[C@H]1CCN([C@H]2CCOc3cc(ccc23)S(=O)(=O)Nc2ncns2)[C@H](C1)c1ccccc1 |r| Show InChI InChI=1S/C23H23F3N4O3S2/c24-23(25,26)16-8-10-30(20(12-16)15-4-2-1-3-5-15)19-9-11-33-21-13-17(6-7-18(19)21)35(31,32)29-22-27-14-28-34-22/h1-7,13-14,16,19-20H,8-12H2,(H,27,28,29)/t16-,19-,20+/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.970 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech
Curated by ChEMBL
| Assay Description
Displacement of [3H]GX-545 from full length human Nav1.7 VSD4 domain expressed in HEK cell membranes measured after 20 hrs by liquid scintillation co... |
J Med Chem 62: 4091-4109 (2019)
Article DOI: 10.1021/acs.jmedchem.9b00141
BindingDB Entry DOI: 10.7270/Q2B85CJ3 |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM50347090
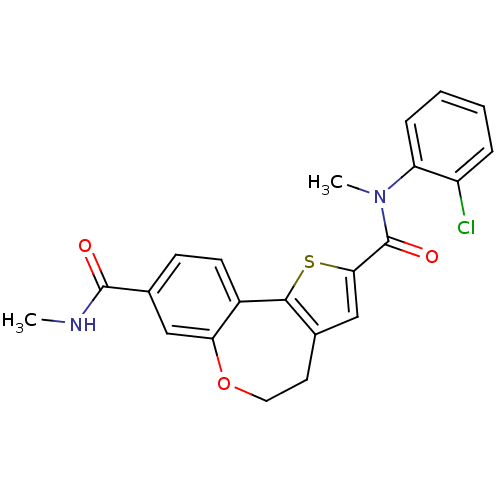
(CHEMBL1796276)Show SMILES CNC(=O)c1ccc2-c3sc(cc3CCOc2c1)C(=O)N(C)c1ccccc1Cl Show InChI InChI=1S/C22H19ClN2O3S/c1-24-21(26)14-7-8-15-18(11-14)28-10-9-13-12-19(29-20(13)15)22(27)25(2)17-6-4-3-5-16(17)23/h3-8,11-12H,9-10H2,1-2H3,(H,24,26) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech
Curated by ChEMBL
| Assay Description
Apparent binding affinity to PI3Kalpha using PIP3 as substrate after 30 mins by fluorescence polarization assay |
J Med Chem 54: 7815-33 (2011)
Article DOI: 10.1021/jm2007084
BindingDB Entry DOI: 10.7270/Q2057GCQ |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM50419769
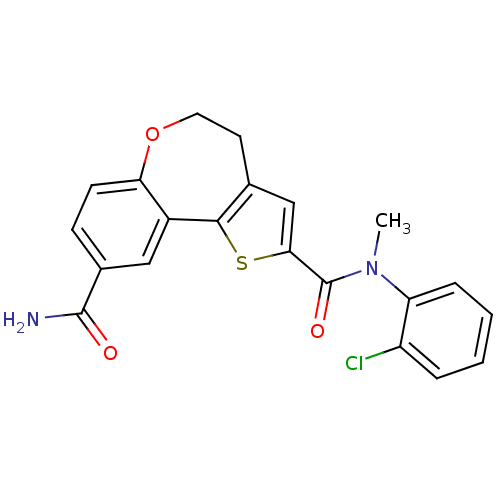
(CHEMBL1950034)Show SMILES CN(C(=O)c1cc2CCOc3ccc(cc3-c2s1)C(N)=O)c1ccccc1Cl Show InChI InChI=1S/C21H17ClN2O3S/c1-24(16-5-3-2-4-15(16)22)21(26)18-11-12-8-9-27-17-7-6-13(20(23)25)10-14(17)19(12)28-18/h2-7,10-11H,8-9H2,1H3,(H2,23,25) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech
Curated by ChEMBL
| Assay Description
Apparent binding affinity to PI3Kalpha using PIP3 as substrate after 30 mins by fluorescence polarization assay |
J Med Chem 54: 7815-33 (2011)
Article DOI: 10.1021/jm2007084
BindingDB Entry DOI: 10.7270/Q2057GCQ |
More data for this
Ligand-Target Pair | |
Sodium channel protein type 9 subunit alpha
(Homo sapiens (Human)) | BDBM50521577
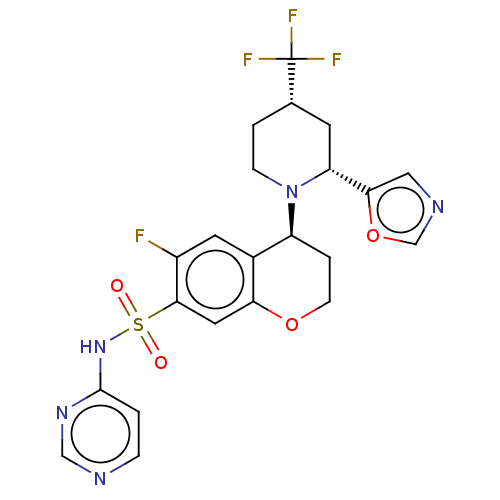
(CHEMBL4530176)Show SMILES Fc1cc2[C@H](CCOc2cc1S(=O)(=O)Nc1ccncn1)N1CC[C@@H](C[C@@H]1c1cnco1)C(F)(F)F |r| Show InChI InChI=1S/C22H21F4N5O4S/c23-15-8-14-16(31-5-2-13(22(24,25)26)7-17(31)19-10-28-12-35-19)3-6-34-18(14)9-20(15)36(32,33)30-21-1-4-27-11-29-21/h1,4,8-13,16-17H,2-3,5-7H2,(H,27,29,30)/t13-,16-,17+/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech
Curated by ChEMBL
| Assay Description
Displacement of [3H]GX-545 from full length human Nav1.7 VSD4 domain expressed in HEK cell membranes measured after 20 hrs by liquid scintillation co... |
J Med Chem 62: 4091-4109 (2019)
Article DOI: 10.1021/acs.jmedchem.9b00141
BindingDB Entry DOI: 10.7270/Q2B85CJ3 |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM50419754
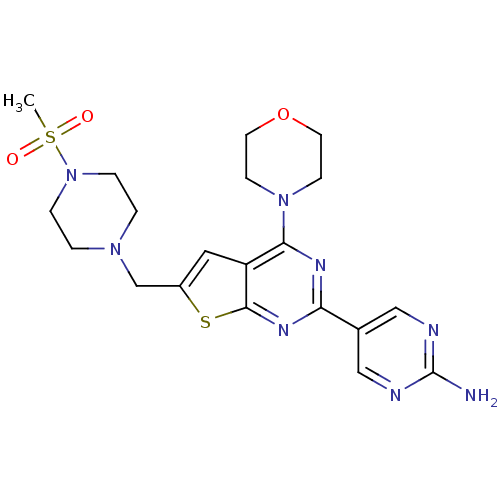
(CHEMBL1949910)Show SMILES CS(=O)(=O)N1CCN(Cc2cc3c(nc(nc3s2)-c2cnc(N)nc2)N2CCOCC2)CC1 Show InChI InChI=1S/C20H26N8O3S2/c1-33(29,30)28-4-2-26(3-5-28)13-15-10-16-18(27-6-8-31-9-7-27)24-17(25-19(16)32-15)14-11-22-20(21)23-12-14/h10-12H,2-9,13H2,1H3,(H2,21,22,23) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech
Curated by ChEMBL
| Assay Description
Apparent binding affinity to PI3Kalpha using PIP3 as substrate after 30 mins by fluorescence polarization assay |
J Med Chem 54: 7815-33 (2011)
Article DOI: 10.1021/jm2007084
BindingDB Entry DOI: 10.7270/Q2057GCQ |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM50419759
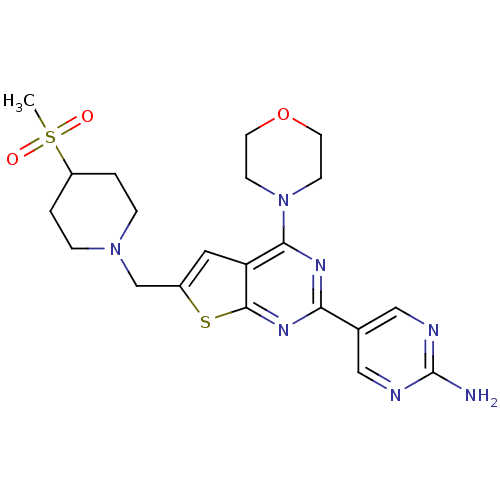
(CHEMBL1949916)Show SMILES CS(=O)(=O)C1CCN(Cc2cc3c(nc(nc3s2)-c2cnc(N)nc2)N2CCOCC2)CC1 Show InChI InChI=1S/C21H27N7O3S2/c1-33(29,30)16-2-4-27(5-3-16)13-15-10-17-19(28-6-8-31-9-7-28)25-18(26-20(17)32-15)14-11-23-21(22)24-12-14/h10-12,16H,2-9,13H2,1H3,(H2,22,23,24) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech
Curated by ChEMBL
| Assay Description
Apparent binding affinity to PI3Kalpha using PIP3 as substrate after 30 mins by fluorescence polarization assay |
J Med Chem 54: 7815-33 (2011)
Article DOI: 10.1021/jm2007084
BindingDB Entry DOI: 10.7270/Q2057GCQ |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM50419761

(CHEMBL1949919)Show SMILES CS(=O)(=O)c1cccc(c1)-c1cc2c(nc(nc2s1)-c1cnc(N)nc1)N1CCOCC1 Show InChI InChI=1S/C21H20N6O3S2/c1-32(28,29)15-4-2-3-13(9-15)17-10-16-19(27-5-7-30-8-6-27)25-18(26-20(16)31-17)14-11-23-21(22)24-12-14/h2-4,9-12H,5-8H2,1H3,(H2,22,23,24) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech
Curated by ChEMBL
| Assay Description
Apparent binding affinity to PI3Kalpha using PIP3 as substrate after 30 mins by fluorescence polarization assay |
J Med Chem 54: 7815-33 (2011)
Article DOI: 10.1021/jm2007084
BindingDB Entry DOI: 10.7270/Q2057GCQ |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM50347087
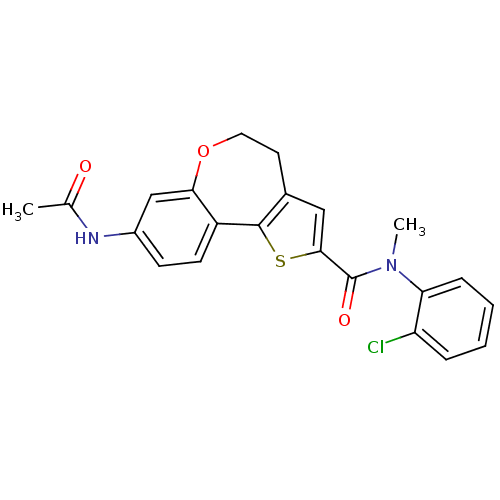
(CHEMBL1796273)Show SMILES CN(C(=O)c1cc2CCOc3cc(NC(C)=O)ccc3-c2s1)c1ccccc1Cl Show InChI InChI=1S/C22H19ClN2O3S/c1-13(26)24-15-7-8-16-19(12-15)28-10-9-14-11-20(29-21(14)16)22(27)25(2)18-6-4-3-5-17(18)23/h3-8,11-12H,9-10H2,1-2H3,(H,24,26) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech
Curated by ChEMBL
| Assay Description
Apparent binding affinity to PI3Kalpha using PIP3 as substrate after 30 mins by fluorescence polarization assay |
J Med Chem 54: 7815-33 (2011)
Article DOI: 10.1021/jm2007084
BindingDB Entry DOI: 10.7270/Q2057GCQ |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM50419770
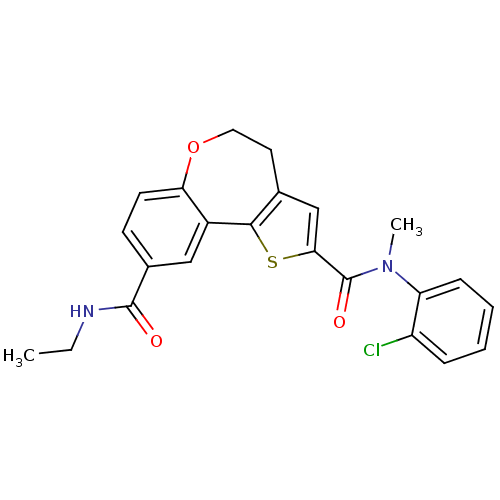
(CHEMBL1950035)Show SMILES CCNC(=O)c1ccc2OCCc3cc(sc3-c2c1)C(=O)N(C)c1ccccc1Cl Show InChI InChI=1S/C23H21ClN2O3S/c1-3-25-22(27)15-8-9-19-16(12-15)21-14(10-11-29-19)13-20(30-21)23(28)26(2)18-7-5-4-6-17(18)24/h4-9,12-13H,3,10-11H2,1-2H3,(H,25,27) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech
Curated by ChEMBL
| Assay Description
Apparent binding affinity to PI3Kalpha using PIP3 as substrate after 30 mins by fluorescence polarization assay |
J Med Chem 54: 7815-33 (2011)
Article DOI: 10.1021/jm2007084
BindingDB Entry DOI: 10.7270/Q2057GCQ |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit delta isoform
(Homo sapiens (Human)) | BDBM50438637
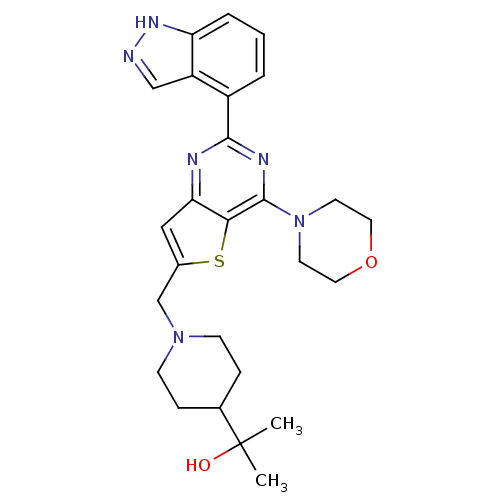
(CHEMBL2414238)Show SMILES CC(C)(O)C1CCN(Cc2cc3nc(nc(N4CCOCC4)c3s2)-c2cccc3[nH]ncc23)CC1 Show InChI InChI=1S/C26H32N6O2S/c1-26(2,33)17-6-8-31(9-7-17)16-18-14-22-23(35-18)25(32-10-12-34-13-11-32)29-24(28-22)19-4-3-5-21-20(19)15-27-30-21/h3-5,14-15,17,33H,6-13,16H2,1-2H3,(H,27,30) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech
Curated by ChEMBL
| Assay Description
Inhibition of PI3Kdelta (unknown origin) assessed as formation of PIP3 by competitive fluorescence polarization assay |
Bioorg Med Chem Lett 23: 4953-9 (2013)
Article DOI: 10.1016/j.bmcl.2013.06.052
BindingDB Entry DOI: 10.7270/Q2ZG6TP9 |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM50347089
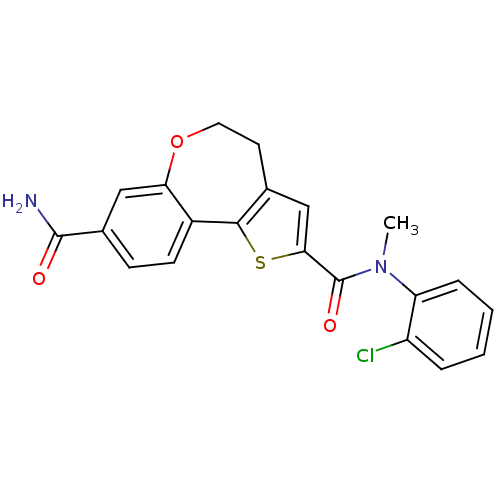
(CHEMBL1796275)Show SMILES CN(C(=O)c1cc2CCOc3cc(ccc3-c2s1)C(N)=O)c1ccccc1Cl Show InChI InChI=1S/C21H17ClN2O3S/c1-24(16-5-3-2-4-15(16)22)21(26)18-11-12-8-9-27-17-10-13(20(23)25)6-7-14(17)19(12)28-18/h2-7,10-11H,8-9H2,1H3,(H2,23,25) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 1.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of recombinant PI3Kalpha assessed as inhibition of PIP3 formation by fluorescence polarization assay |
Bioorg Med Chem Lett 21: 4054-8 (2011)
Article DOI: 10.1016/j.bmcl.2011.04.124
BindingDB Entry DOI: 10.7270/Q29G5N57 |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit delta isoform
(Homo sapiens (Human)) | BDBM50278667

(CHEMBL4167171)Show SMILES CC(C)n1ncnc1-c1cn2CCOc3ccc(cc3-c2n1)S(=O)(=O)N1CCN(CC1)C(C)(C)C Show InChI InChI=1S/C24H33N7O3S/c1-17(2)31-23(25-16-26-31)20-15-28-12-13-34-21-7-6-18(14-19(21)22(28)27-20)35(32,33)30-10-8-29(9-11-30)24(3,4)5/h6-7,14-17H,8-13H2,1-5H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech
Curated by ChEMBL
| Assay Description
Binding affinity to PI3Kdelta (unknown origin) |
ACS Med Chem Lett 8: 936-940 (2017)
Article DOI: 10.1021/acsmedchemlett.7b00170
BindingDB Entry DOI: 10.7270/Q2CV4M7R |
More data for this
Ligand-Target Pair | |
Sodium channel protein type 9 subunit alpha
(Homo sapiens (Human)) | BDBM50521585
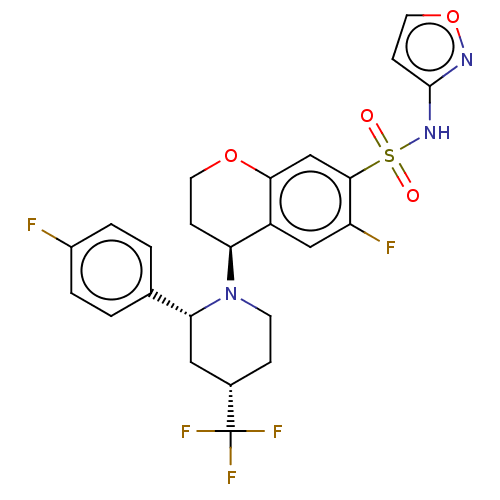
(CHEMBL4557850)Show SMILES Fc1ccc(cc1)[C@H]1C[C@H](CCN1[C@H]1CCOc2cc(c(F)cc12)S(=O)(=O)Nc1ccon1)C(F)(F)F |r| Show InChI InChI=1S/C24H22F5N3O4S/c25-16-3-1-14(2-4-16)20-11-15(24(27,28)29)5-8-32(20)19-6-9-35-21-13-22(18(26)12-17(19)21)37(33,34)31-23-7-10-36-30-23/h1-4,7,10,12-13,15,19-20H,5-6,8-9,11H2,(H,30,31)/t15-,19-,20+/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 1.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech
Curated by ChEMBL
| Assay Description
Displacement of [3H]GX-545 from full length human Nav1.7 VSD4 domain expressed in HEK cell membranes measured after 20 hrs by liquid scintillation co... |
J Med Chem 62: 4091-4109 (2019)
Article DOI: 10.1021/acs.jmedchem.9b00141
BindingDB Entry DOI: 10.7270/Q2B85CJ3 |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM50347088
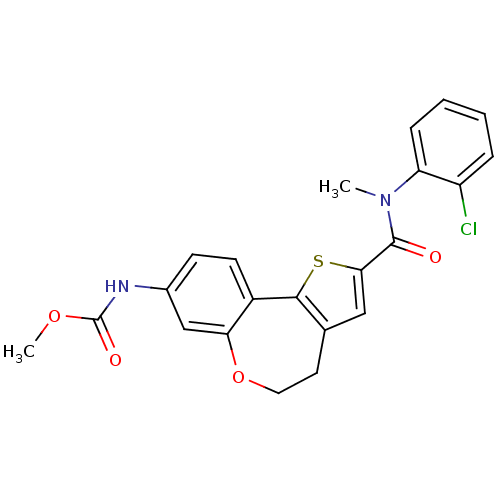
(CHEMBL1796274)Show SMILES COC(=O)Nc1ccc2-c3sc(cc3CCOc2c1)C(=O)N(C)c1ccccc1Cl Show InChI InChI=1S/C22H19ClN2O4S/c1-25(17-6-4-3-5-16(17)23)21(26)19-11-13-9-10-29-18-12-14(24-22(27)28-2)7-8-15(18)20(13)30-19/h3-8,11-12H,9-10H2,1-2H3,(H,24,27) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 1.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of recombinant PI3Kalpha assessed as inhibition of PIP3 formation by fluorescence polarization assay |
Bioorg Med Chem Lett 21: 4054-8 (2011)
Article DOI: 10.1016/j.bmcl.2011.04.124
BindingDB Entry DOI: 10.7270/Q29G5N57 |
More data for this
Ligand-Target Pair | |
Sodium channel protein type 9 subunit alpha
(Homo sapiens (Human)) | BDBM50521580
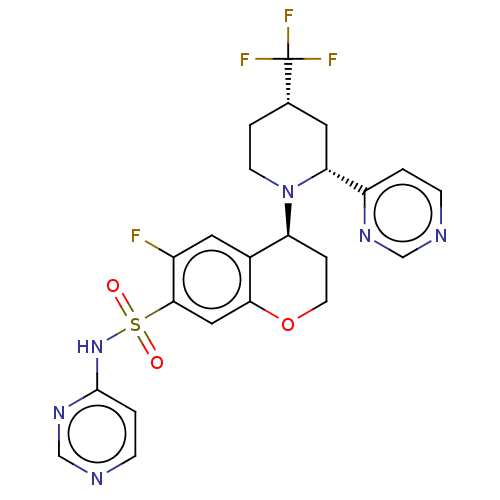
(CHEMBL4474681)Show SMILES Fc1cc2[C@H](CCOc2cc1S(=O)(=O)Nc1ccncn1)N1CC[C@@H](C[C@@H]1c1ccncn1)C(F)(F)F |r| Show InChI InChI=1S/C23H22F4N6O3S/c24-16-10-15-18(33-7-3-14(23(25,26)27)9-19(33)17-1-5-28-12-30-17)4-8-36-20(15)11-21(16)37(34,35)32-22-2-6-29-13-31-22/h1-2,5-6,10-14,18-19H,3-4,7-9H2,(H,29,31,32)/t14-,18-,19+/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 1.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech
Curated by ChEMBL
| Assay Description
Displacement of [3H]GX-545 from full length human Nav1.7 VSD4 domain expressed in HEK cell membranes measured after 20 hrs by liquid scintillation co... |
J Med Chem 62: 4091-4109 (2019)
Article DOI: 10.1021/acs.jmedchem.9b00141
BindingDB Entry DOI: 10.7270/Q2B85CJ3 |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit delta isoform
(Homo sapiens (Human)) | BDBM172467

(US9090628, 110)Show SMILES CC(C)n1ncnc1-c1cn2CCOc3ccc(cc3-c2n1)C(=O)N1CCN(CC1)C(C)(C)C Show InChI InChI=1S/C25H33N7O2/c1-17(2)32-23(26-16-27-32)20-15-30-12-13-34-21-7-6-18(14-19(21)22(30)28-20)24(33)29-8-10-31(11-9-29)25(3,4)5/h6-7,14-17H,8-13H2,1-5H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech
Curated by ChEMBL
| Assay Description
Binding affinity to PI3Kdelta (unknown origin) |
ACS Med Chem Lett 8: 936-940 (2017)
Article DOI: 10.1021/acsmedchemlett.7b00170
BindingDB Entry DOI: 10.7270/Q2CV4M7R |
More data for this
Ligand-Target Pair | |
Sodium channel protein type 9 subunit alpha
(Homo sapiens (Human)) | BDBM50521578

(CHEMBL4444085)Show SMILES Fc1cc2[C@H](CCOc2cc1S(=O)(=O)Nc1ccncn1)N1CC[C@@H](C[C@@H]1c1ccncc1)C(F)(F)F |r| Show InChI InChI=1S/C24H23F4N5O3S/c25-18-12-17-19(33-9-4-16(24(26,27)28)11-20(33)15-1-6-29-7-2-15)5-10-36-21(17)13-22(18)37(34,35)32-23-3-8-30-14-31-23/h1-3,6-8,12-14,16,19-20H,4-5,9-11H2,(H,30,31,32)/t16-,19-,20+/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 1.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech
Curated by ChEMBL
| Assay Description
Displacement of [3H]GX-545 from full length human Nav1.7 VSD4 domain expressed in HEK cell membranes measured after 20 hrs by liquid scintillation co... |
J Med Chem 62: 4091-4109 (2019)
Article DOI: 10.1021/acs.jmedchem.9b00141
BindingDB Entry DOI: 10.7270/Q2B85CJ3 |
More data for this
Ligand-Target Pair | |
Prolyl endopeptidase
(Homo sapiens (Human)) | BDBM50200728

((R)-1-(4-oxo-4-phenylbutanoyl)pyrrolidin-2-ylboron...)Show InChI InChI=1S/C14H18BNO4/c17-12(11-5-2-1-3-6-11)8-9-14(18)16-10-4-7-13(16)15(19)20/h1-3,5-6,13,19-20H,4,7-10H2/t13-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| 1.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech
Curated by ChEMBL
| Assay Description
Inhibitory constant against POP |
Bioorg Med Chem Lett 17: 1438-42 (2007)
Article DOI: 10.1016/j.bmcl.2006.11.072
BindingDB Entry DOI: 10.7270/Q2VH5NH1 |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit delta isoform
(Homo sapiens (Human)) | BDBM50396642
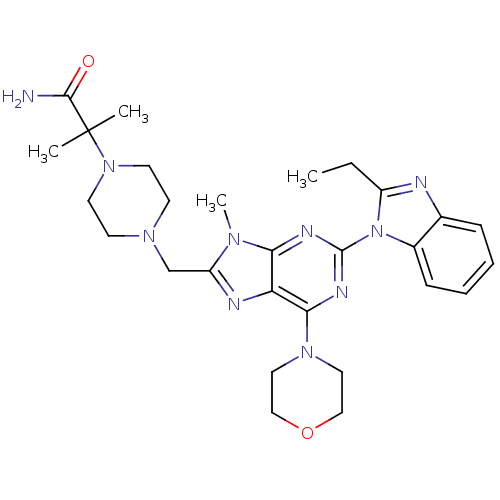
(CHEMBL2171939)Show SMILES CCc1nc2ccccc2n1-c1nc(N2CCOCC2)c2nc(CN3CCN(CC3)C(C)(C)C(N)=O)n(C)c2n1 Show InChI InChI=1S/C28H38N10O2/c1-5-21-30-19-8-6-7-9-20(19)38(21)27-32-24-23(25(33-27)36-14-16-40-17-15-36)31-22(34(24)4)18-35-10-12-37(13-11-35)28(2,3)26(29)39/h6-9H,5,10-18H2,1-4H3,(H2,29,39) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech
Curated by ChEMBL
| Assay Description
Inhibition of PI3Kdelta (unknown origin) assessed as formation of PIP3 by competitive fluorescence polarization assay |
Bioorg Med Chem Lett 23: 4953-9 (2013)
Article DOI: 10.1016/j.bmcl.2013.06.052
BindingDB Entry DOI: 10.7270/Q2ZG6TP9 |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM50347087

(CHEMBL1796273)Show SMILES CN(C(=O)c1cc2CCOc3cc(NC(C)=O)ccc3-c2s1)c1ccccc1Cl Show InChI InChI=1S/C22H19ClN2O3S/c1-13(26)24-15-7-8-16-19(12-15)28-10-9-14-11-20(29-21(14)16)22(27)25(2)18-6-4-3-5-17(18)23/h3-8,11-12H,9-10H2,1-2H3,(H,24,26) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 1.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of recombinant PI3Kalpha assessed as inhibition of PIP3 formation by fluorescence polarization assay |
Bioorg Med Chem Lett 21: 4054-8 (2011)
Article DOI: 10.1016/j.bmcl.2011.04.124
BindingDB Entry DOI: 10.7270/Q29G5N57 |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit delta isoform
(Homo sapiens (Human)) | BDBM172643
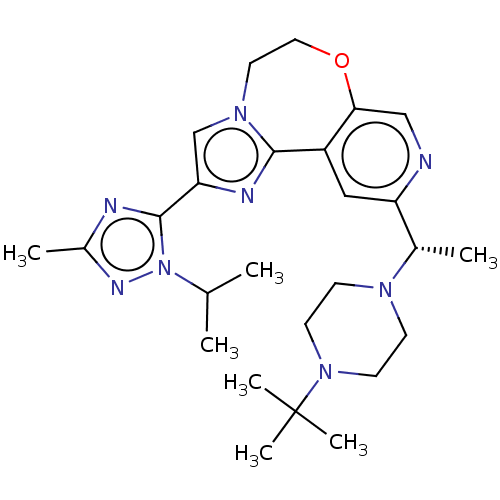
(US9090628, 303)Show SMILES CC(C)n1nc(C)nc1-c1cn2CCOc3cnc(cc3-c2n1)[C@H](C)N1CCN(CC1)C(C)(C)C |r| Show InChI InChI=1S/C26H38N8O/c1-17(2)34-25(28-19(4)30-34)22-16-32-12-13-35-23-15-27-21(14-20(23)24(32)29-22)18(3)31-8-10-33(11-9-31)26(5,6)7/h14-18H,8-13H2,1-7H3/t18-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech
Curated by ChEMBL
| Assay Description
Binding affinity to PI3Kdelta (unknown origin) |
ACS Med Chem Lett 8: 936-940 (2017)
Article DOI: 10.1021/acsmedchemlett.7b00170
BindingDB Entry DOI: 10.7270/Q2CV4M7R |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM50419758

(CHEMBL1949914)Show SMILES Nc1ncc(cn1)-c1nc(N2CCOCC2)c2cc(CC3CCN(CC(F)(F)F)CC3)sc2n1 Show InChI InChI=1S/C22H26F3N7OS/c23-22(24,25)13-31-3-1-14(2-4-31)9-16-10-17-19(32-5-7-33-8-6-32)29-18(30-20(17)34-16)15-11-27-21(26)28-12-15/h10-12,14H,1-9,13H2,(H2,26,27,28) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech
Curated by ChEMBL
| Assay Description
Apparent binding affinity to PI3Kalpha using PIP3 as substrate after 30 mins by fluorescence polarization assay |
J Med Chem 54: 7815-33 (2011)
Article DOI: 10.1021/jm2007084
BindingDB Entry DOI: 10.7270/Q2057GCQ |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM50419773
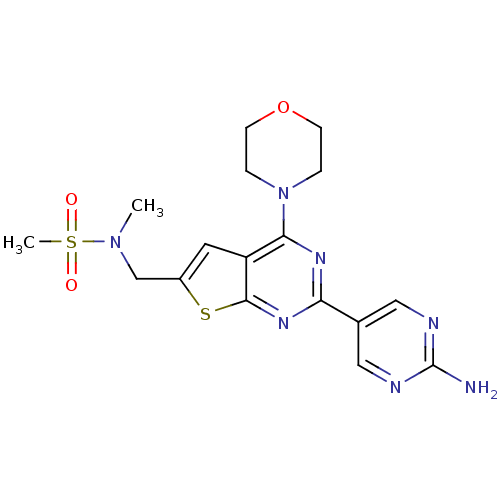
(CHEMBL1949917)Show SMILES CN(Cc1cc2c(nc(nc2s1)-c1cnc(N)nc1)N1CCOCC1)S(C)(=O)=O Show InChI InChI=1S/C17H21N7O3S2/c1-23(29(2,25)26)10-12-7-13-15(24-3-5-27-6-4-24)21-14(22-16(13)28-12)11-8-19-17(18)20-9-11/h7-9H,3-6,10H2,1-2H3,(H2,18,19,20) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech
Curated by ChEMBL
| Assay Description
Apparent binding affinity to PI3Kalpha using PIP3 as substrate after 30 mins by fluorescence polarization assay |
J Med Chem 54: 7815-33 (2011)
Article DOI: 10.1021/jm2007084
BindingDB Entry DOI: 10.7270/Q2057GCQ |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit delta isoform
(Homo sapiens (Human)) | BDBM50396628
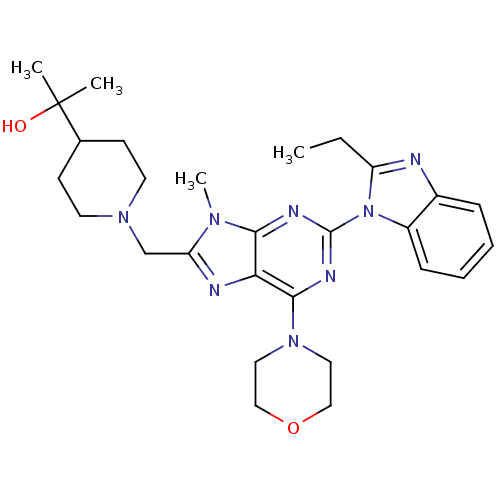
(CHEMBL2171944)Show SMILES CCc1nc2ccccc2n1-c1nc(N2CCOCC2)c2nc(CN3CCC(CC3)C(C)(C)O)n(C)c2n1 Show InChI InChI=1S/C28H38N8O2/c1-5-22-29-20-8-6-7-9-21(20)36(22)27-31-25-24(26(32-27)35-14-16-38-17-15-35)30-23(33(25)4)18-34-12-10-19(11-13-34)28(2,3)37/h6-9,19,37H,5,10-18H2,1-4H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech
Curated by ChEMBL
| Assay Description
Inhibition of PI3Kdelta (unknown origin) assessed as formation of PIP3 by competitive fluorescence polarization assay |
Bioorg Med Chem Lett 23: 4953-9 (2013)
Article DOI: 10.1016/j.bmcl.2013.06.052
BindingDB Entry DOI: 10.7270/Q2ZG6TP9 |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit delta isoform
(Homo sapiens (Human)) | BDBM50396633
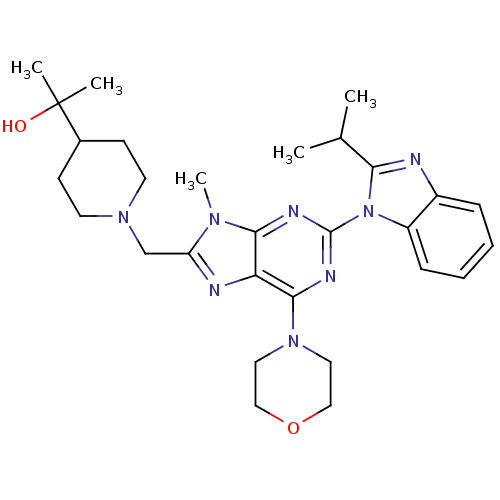
(CHEMBL2171952)Show SMILES CC(C)c1nc2ccccc2n1-c1nc(N2CCOCC2)c2nc(CN3CCC(CC3)C(C)(C)O)n(C)c2n1 Show InChI InChI=1S/C29H40N8O2/c1-19(2)25-30-21-8-6-7-9-22(21)37(25)28-32-26-24(27(33-28)36-14-16-39-17-15-36)31-23(34(26)5)18-35-12-10-20(11-13-35)29(3,4)38/h6-9,19-20,38H,10-18H2,1-5H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech
Curated by ChEMBL
| Assay Description
Inhibition of PI3Kdelta (unknown origin) assessed as formation of PIP3 by competitive fluorescence polarization assay |
Bioorg Med Chem Lett 23: 4953-9 (2013)
Article DOI: 10.1016/j.bmcl.2013.06.052
BindingDB Entry DOI: 10.7270/Q2ZG6TP9 |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM50347076

(CHEMBL1796761)Show SMILES CNC(=O)c1ccc2-c3sc(cc3CCOc2c1)C(=O)N(C)c1ccc(cc1Cl)C(=O)N(C)C Show InChI InChI=1S/C25H24ClN3O4S/c1-27-23(30)15-5-7-17-20(12-15)33-10-9-14-13-21(34-22(14)17)25(32)29(4)19-8-6-16(11-18(19)26)24(31)28(2)3/h5-8,11-13H,9-10H2,1-4H3,(H,27,30) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech
Curated by ChEMBL
| Assay Description
Apparent binding affinity to PI3Kalpha using PIP3 as substrate after 30 mins by fluorescence polarization assay |
J Med Chem 54: 7815-33 (2011)
Article DOI: 10.1021/jm2007084
BindingDB Entry DOI: 10.7270/Q2057GCQ |
More data for this
Ligand-Target Pair | |
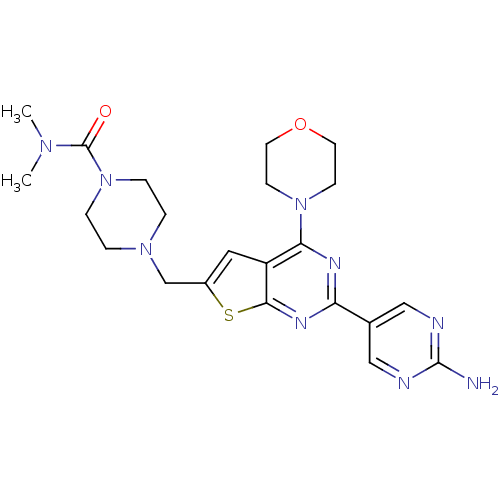
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM50419760
(CHEMBL1949918)Show SMILES CN(C)C(=O)N1CCN(Cc2cc3c(nc(nc3s2)-c2cnc(N)nc2)N2CCOCC2)CC1 Show InChI InChI=1S/C22H29N9O2S/c1-28(2)22(32)31-5-3-29(4-6-31)14-16-11-17-19(30-7-9-33-10-8-30)26-18(27-20(17)34-16)15-12-24-21(23)25-13-15/h11-13H,3-10,14H2,1-2H3,(H2,23,24,25) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech
Curated by ChEMBL
| Assay Description
Apparent binding affinity to PI3Kalpha using PIP3 as substrate after 30 mins by fluorescence polarization assay |
J Med Chem 54: 7815-33 (2011)
Article DOI: 10.1021/jm2007084
BindingDB Entry DOI: 10.7270/Q2057GCQ |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data