

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Orexin receptor type 2 (Homo sapiens (Human)) | BDBM50093793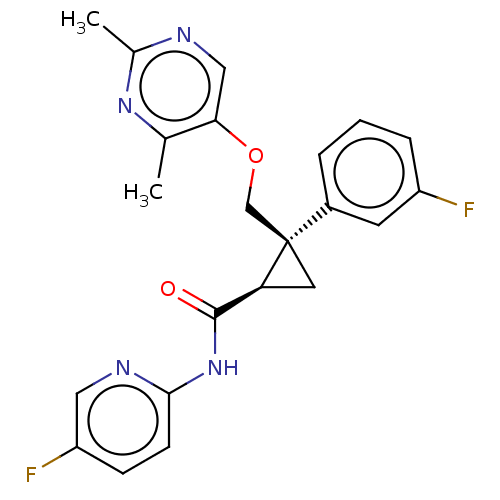 (E-2006 | Lemborexant) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid PDB UniChem | PDB Article PubMed | 0.440 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Eisai Co., Ltd. Curated by ChEMBL | Assay Description Binding affinity to human OX2R expressed in HEK-293 cells assessed as inhibition of orexin A-induced calcium accumulation by FLIPR assay | J Med Chem 58: 4648-64 (2015) Article DOI: 10.1021/acs.jmedchem.5b00217 BindingDB Entry DOI: 10.7270/Q2125VD7 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Neuropeptide Y receptor type 5 (Homo sapiens (Human)) | BDBM50091652 (CHEMBL269503 | PYY | PYY, rat | Peptide YY(PYY)(YP...) | KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Meiji Seika Kaisha, Ltd. Curated by PDSP Ki Database | J Pharmacol Exp Ther 317: 562-70 (2006) Article DOI: 10.1124/jpet.105.099705 BindingDB Entry DOI: 10.7270/Q25Q4TPT | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Orexin receptor type 2 (Homo sapiens (Human)) | BDBM50440024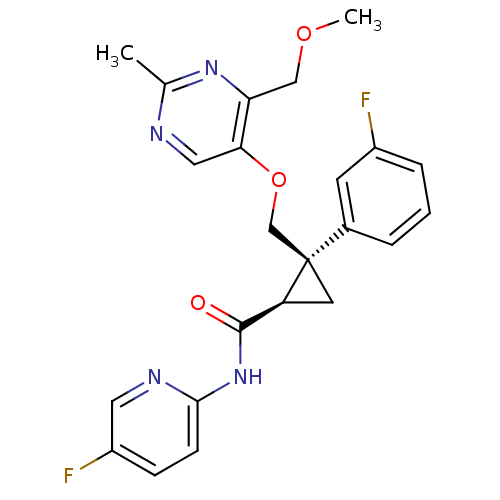 (CHEMBL2425785) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Eisai Co. , Ltd. Curated by ChEMBL | Assay Description Displacement of [125I]-orexin-A from human OX2 receptor expressed in CHO cells after 30 mins by liquid scintillation counting analysis | J Med Chem 56: 6371-85 (2013) Article DOI: 10.1021/jm400772t BindingDB Entry DOI: 10.7270/Q2QF8V91 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Orexin/Hypocretin receptor type 1 (Homo sapiens (Human)) | BDBM50440024 (CHEMBL2425785) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Eisai Co. , Ltd. Curated by ChEMBL | Assay Description Displacement of [125I]-orexin-A from human OX1 receptor expressed in CHO cells after 30 mins by liquid scintillation counting analysis | J Med Chem 56: 6371-85 (2013) Article DOI: 10.1021/jm400772t BindingDB Entry DOI: 10.7270/Q2QF8V91 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Orexin receptor type 2 (Homo sapiens (Human)) | BDBM50440021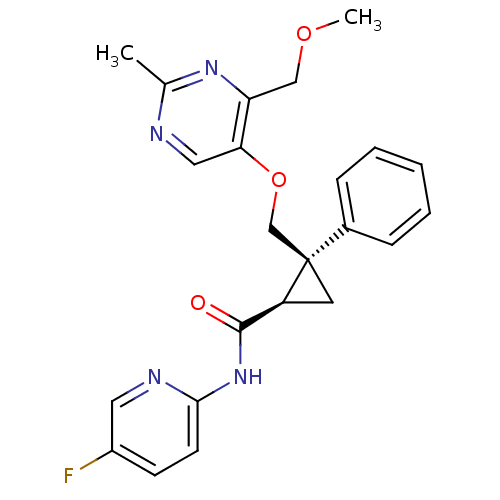 (CHEMBL2425788) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Eisai Co. , Ltd. Curated by ChEMBL | Assay Description Displacement of [125I]-orexin-A from human OX2 receptor expressed in CHO cells after 30 mins by liquid scintillation counting analysis | J Med Chem 56: 6371-85 (2013) Article DOI: 10.1021/jm400772t BindingDB Entry DOI: 10.7270/Q2QF8V91 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Type-2 angiotensin II receptor (RAT) | BDBM82260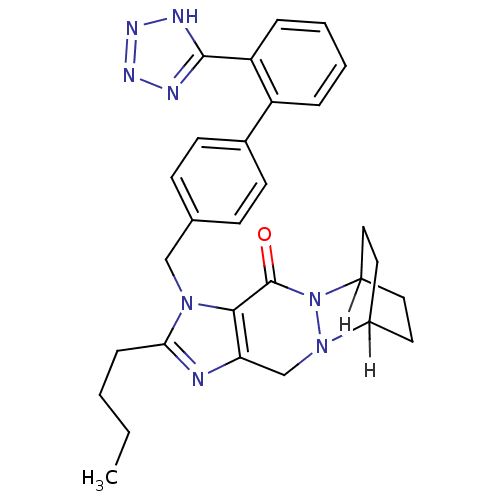 (U-97018) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | PubMed | 1.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Upjohn Pharmaceuticals Limited Curated by PDSP Ki Database | J Pharmacol Exp Ther 274: 1042-53 (1995) BindingDB Entry DOI: 10.7270/Q20G3HN9 | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Type-2 angiotensin II receptor (RAT) | BDBM82259 (CAS_123856 | L-158,809 | NSC_123856) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | PubMed | 1.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Upjohn Pharmaceuticals Limited Curated by PDSP Ki Database | J Pharmacol Exp Ther 274: 1042-53 (1995) BindingDB Entry DOI: 10.7270/Q20G3HN9 | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor X (Oryctolagus cuniculus) | BDBM35743 (CHEMBL479863 | cis-1,2-diaminocyclohexane derivati...) | PDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL MMDB PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | 1.74 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Daiichi Sankyo Co., Ltd. Curated by ChEMBL | Assay Description Inhibition of rabbit factor 10a by Lineweaver-Burk plot | Bioorg Med Chem 17: 1193-206 (2009) Article DOI: 10.1016/j.bmc.2008.12.037 BindingDB Entry DOI: 10.7270/Q2V69JGR | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Prolactin-releasing peptide receptor (Homo sapiens (Human)) | BDBM195624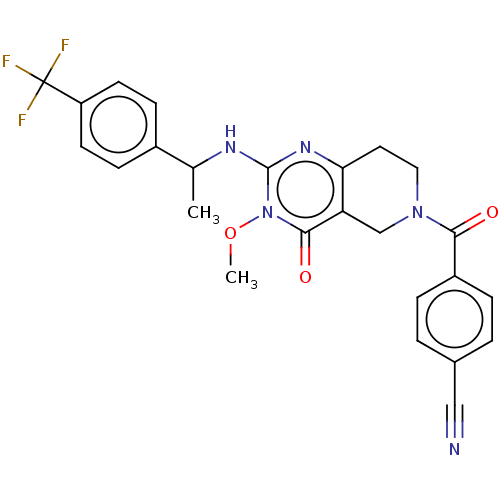 (US9206173, 2386) | UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 2 | -49.7 | n/a | n/a | n/a | n/a | n/a | 7.4 | 25 |
OTSUKA PHARMACEUTICAL CO., LTD. US Patent | Assay Description Receptor-expressing HEK 293 cell membrane fraction was analyzed by modifying the methods of C. J. Langmead et al. (see Langmead C J, Szekeres P G; Ch... | US Patent US9206173 (2015) BindingDB Entry DOI: 10.7270/Q23X85FS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Orexin/Hypocretin receptor type 1 (Homo sapiens (Human)) | BDBM50093788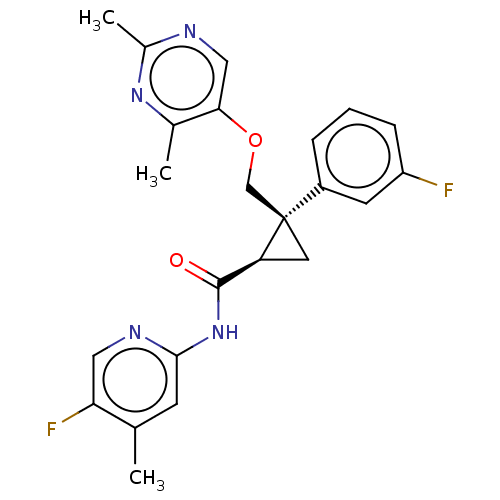 (CHEMBL3585957) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Eisai Co., Ltd. Curated by ChEMBL | Assay Description Binding affinity to human OX1R by radioligand displacement binding assay | J Med Chem 58: 4648-64 (2015) Article DOI: 10.1021/acs.jmedchem.5b00217 BindingDB Entry DOI: 10.7270/Q2125VD7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Orexin receptor type 2 (Homo sapiens (Human)) | BDBM50440025 (CHEMBL2425784) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Eisai Co. , Ltd. Curated by ChEMBL | Assay Description Displacement of [125I]-orexin-A from human OX2 receptor expressed in CHO cells after 30 mins by liquid scintillation counting analysis | J Med Chem 56: 6371-85 (2013) Article DOI: 10.1021/jm400772t BindingDB Entry DOI: 10.7270/Q2QF8V91 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Orexin receptor type 2 (Homo sapiens (Human)) | BDBM50093815 (CHEMBL3585948) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Eisai Co., Ltd. Curated by ChEMBL | Assay Description Binding affinity to human OX2R by radioligand displacement binding assay | J Med Chem 58: 4648-64 (2015) Article DOI: 10.1021/acs.jmedchem.5b00217 BindingDB Entry DOI: 10.7270/Q2125VD7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prolactin-releasing peptide receptor (Homo sapiens (Human)) | BDBM195673 (US9206173, 2435) | UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 2 | -49.7 | n/a | n/a | n/a | n/a | n/a | 7.4 | 25 |
OTSUKA PHARMACEUTICAL CO., LTD. US Patent | Assay Description Receptor-expressing HEK 293 cell membrane fraction was analyzed by modifying the methods of C. J. Langmead et al. (see Langmead C J, Szekeres P G; Ch... | US Patent US9206173 (2015) BindingDB Entry DOI: 10.7270/Q23X85FS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prolactin-releasing peptide receptor (Homo sapiens (Human)) | BDBM195644 (US9206173, 2406) | UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 2 | -49.7 | n/a | n/a | n/a | n/a | n/a | 7.4 | 25 |
OTSUKA PHARMACEUTICAL CO., LTD. US Patent | Assay Description Receptor-expressing HEK 293 cell membrane fraction was analyzed by modifying the methods of C. J. Langmead et al. (see Langmead C J, Szekeres P G; Ch... | US Patent US9206173 (2015) BindingDB Entry DOI: 10.7270/Q23X85FS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Orexin receptor type 2 (Homo sapiens (Human)) | BDBM50440022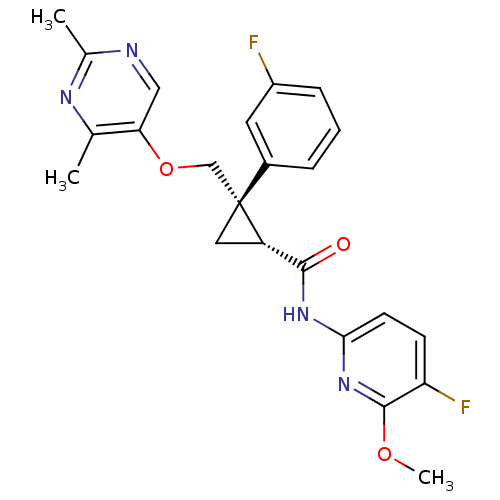 (CHEMBL2425787) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Eisai Co. , Ltd. Curated by ChEMBL | Assay Description Displacement of [125I]-orexin-A from human OX2 receptor expressed in CHO cells after 30 mins by liquid scintillation counting analysis | J Med Chem 56: 6371-85 (2013) Article DOI: 10.1021/jm400772t BindingDB Entry DOI: 10.7270/Q2QF8V91 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuropeptide Y receptor type 1 (Homo sapiens (Human)) | BDBM50091652 (CHEMBL269503 | PYY | PYY, rat | Peptide YY(PYY)(YP...) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Meiji Seika Kaisha, Ltd. Curated by PDSP Ki Database | J Pharmacol Exp Ther 317: 562-70 (2006) Article DOI: 10.1124/jpet.105.099705 BindingDB Entry DOI: 10.7270/Q25Q4TPT | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuropeptide Y receptor type 2 (Homo sapiens (Human)) | BDBM50091652 (CHEMBL269503 | PYY | PYY, rat | Peptide YY(PYY)(YP...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 2.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Meiji Seika Kaisha, Ltd. Curated by PDSP Ki Database | J Pharmacol Exp Ther 317: 562-70 (2006) Article DOI: 10.1124/jpet.105.099705 BindingDB Entry DOI: 10.7270/Q25Q4TPT | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor X (Homo sapiens (Human)) | BDBM35743 (CHEMBL479863 | cis-1,2-diaminocyclohexane derivati...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL MMDB PC cid PC sid PDB UniChem Patents Similars | MMDB PDB Article PubMed | 2.85 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Daiichi Sankyo Co., Ltd. Curated by ChEMBL | Assay Description Inhibition of human factor 10a by Lineweaver-Burk plot | Bioorg Med Chem 17: 1193-206 (2009) Article DOI: 10.1016/j.bmc.2008.12.037 BindingDB Entry DOI: 10.7270/Q2V69JGR | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Orexin receptor type 2 (Homo sapiens (Human)) | BDBM50093793 (E-2006 | Lemborexant) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid PDB UniChem | PDB Article PubMed | 3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Eisai Co., Ltd. Curated by ChEMBL | Assay Description Binding affinity to human OX2R by radioligand displacement binding assay | J Med Chem 58: 4648-64 (2015) Article DOI: 10.1021/acs.jmedchem.5b00217 BindingDB Entry DOI: 10.7270/Q2125VD7 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Orexin receptor type 2 (Homo sapiens (Human)) | BDBM50093791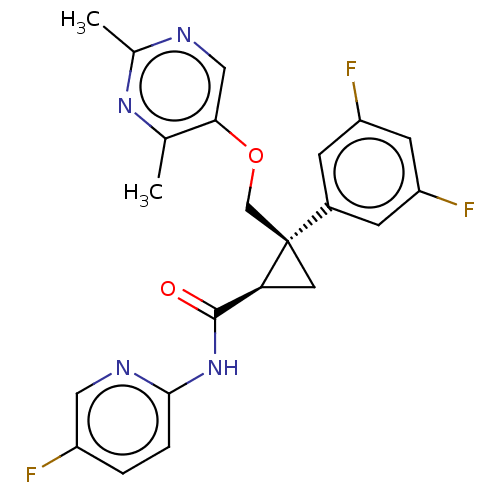 (CHEMBL3585955) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Eisai Co., Ltd. Curated by ChEMBL | Assay Description Binding affinity to human OX2R by radioligand displacement binding assay | J Med Chem 58: 4648-64 (2015) Article DOI: 10.1021/acs.jmedchem.5b00217 BindingDB Entry DOI: 10.7270/Q2125VD7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Orexin/Hypocretin receptor type 1 (Homo sapiens (Human)) | BDBM50093795 (CHEMBL3585952) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Eisai Co., Ltd. Curated by ChEMBL | Assay Description Binding affinity to human OX1R by radioligand displacement binding assay | J Med Chem 58: 4648-64 (2015) Article DOI: 10.1021/acs.jmedchem.5b00217 BindingDB Entry DOI: 10.7270/Q2125VD7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prolactin-releasing peptide receptor (Homo sapiens (Human)) | BDBM195733 (US9206173, 2495) | UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 3 | -48.6 | n/a | n/a | n/a | n/a | n/a | 7.4 | 25 |
OTSUKA PHARMACEUTICAL CO., LTD. US Patent | Assay Description Receptor-expressing HEK 293 cell membrane fraction was analyzed by modifying the methods of C. J. Langmead et al. (see Langmead C J, Szekeres P G; Ch... | US Patent US9206173 (2015) BindingDB Entry DOI: 10.7270/Q23X85FS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prolactin-releasing peptide receptor (Homo sapiens (Human)) | BDBM195701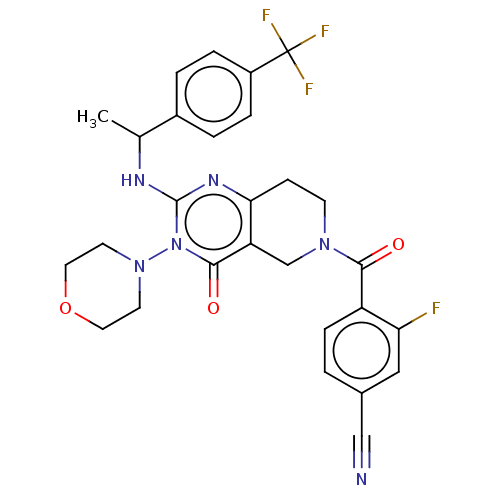 (US9206173, 2463) | UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 3 | -48.6 | n/a | n/a | n/a | n/a | n/a | 7.4 | 25 |
OTSUKA PHARMACEUTICAL CO., LTD. US Patent | Assay Description Receptor-expressing HEK 293 cell membrane fraction was analyzed by modifying the methods of C. J. Langmead et al. (see Langmead C J, Szekeres P G; Ch... | US Patent US9206173 (2015) BindingDB Entry DOI: 10.7270/Q23X85FS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Orexin/Hypocretin receptor type 1 (Homo sapiens (Human)) | BDBM50440025 (CHEMBL2425784) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Eisai Co. , Ltd. Curated by ChEMBL | Assay Description Displacement of [125I]-orexin-A from human OX1 receptor expressed in CHO cells after 30 mins by liquid scintillation counting analysis | J Med Chem 56: 6371-85 (2013) Article DOI: 10.1021/jm400772t BindingDB Entry DOI: 10.7270/Q2QF8V91 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Orexin receptor type 2 (Homo sapiens (Human)) | BDBM50093788 (CHEMBL3585957) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Eisai Co., Ltd. Curated by ChEMBL | Assay Description Binding affinity to human OX2R by radioligand displacement binding assay | J Med Chem 58: 4648-64 (2015) Article DOI: 10.1021/acs.jmedchem.5b00217 BindingDB Entry DOI: 10.7270/Q2125VD7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prolactin-releasing peptide receptor (Homo sapiens (Human)) | BDBM195690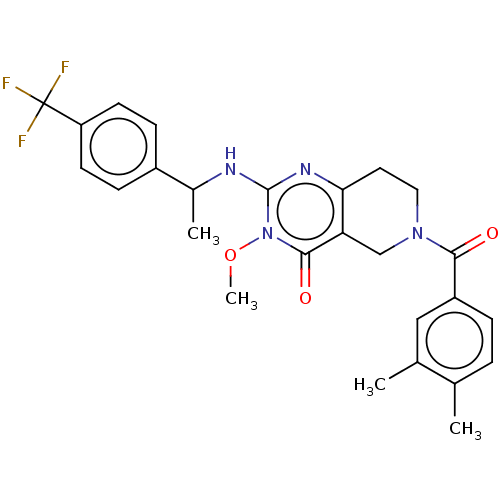 (US9206173, 2452) | UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 4 | -47.9 | n/a | n/a | n/a | n/a | n/a | 7.4 | 25 |
OTSUKA PHARMACEUTICAL CO., LTD. US Patent | Assay Description Receptor-expressing HEK 293 cell membrane fraction was analyzed by modifying the methods of C. J. Langmead et al. (see Langmead C J, Szekeres P G; Ch... | US Patent US9206173 (2015) BindingDB Entry DOI: 10.7270/Q23X85FS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Orexin receptor type 2 (Homo sapiens (Human)) | BDBM50093790 (CHEMBL3585956) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Eisai Co., Ltd. Curated by ChEMBL | Assay Description Binding affinity to human OX2R by radioligand displacement binding assay | J Med Chem 58: 4648-64 (2015) Article DOI: 10.1021/acs.jmedchem.5b00217 BindingDB Entry DOI: 10.7270/Q2125VD7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Orexin receptor type 2 (Homo sapiens (Human)) | BDBM50093798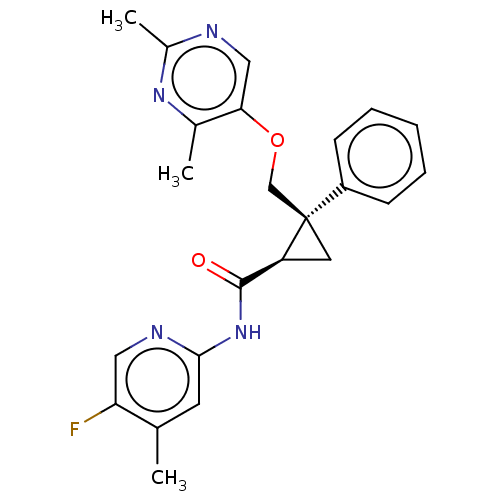 (CHEMBL3585951) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Eisai Co., Ltd. Curated by ChEMBL | Assay Description Binding affinity to human OX2R by radioligand displacement binding assay | J Med Chem 58: 4648-64 (2015) Article DOI: 10.1021/acs.jmedchem.5b00217 BindingDB Entry DOI: 10.7270/Q2125VD7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Free fatty acid receptor 1 (Rattus norvegicus) | BDBM50386646 (CHEMBL2048622) | KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | 4.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company, Ltd. Curated by ChEMBL | Assay Description Displacement of 3-[4-({2',6'-dimethyl-6-[(4-[3H])phenylmethoxy]biphenyl-3-yl}methoxy)phenyl] propanoic acid from rat GPR40 receptor expressed in CHO ... | J Med Chem 55: 3756-76 (2012) Article DOI: 10.1021/jm2016123 BindingDB Entry DOI: 10.7270/Q2N58NDH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuropeptide Y receptor type 5 (Homo sapiens (Human)) | BDBM86732 (3-(5,6,7,8-tetrahydro-9-isopropyl-carbazol-3-yl)-1...) | KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 4.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Meiji Seika Kaisha, Ltd. Curated by PDSP Ki Database | J Pharmacol Exp Ther 317: 562-70 (2006) Article DOI: 10.1124/jpet.105.099705 BindingDB Entry DOI: 10.7270/Q25Q4TPT | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Free fatty acid receptor 1 (Rattus norvegicus) | BDBM50386648 (CHEMBL2048625) | KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 4.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company, Ltd. Curated by ChEMBL | Assay Description Displacement of 3-[4-({2',6'-dimethyl-6-[(4-[3H])phenylmethoxy]biphenyl-3-yl}methoxy)phenyl] propanoic acid from rat GPR40 receptor expressed in CHO ... | J Med Chem 55: 3756-76 (2012) Article DOI: 10.1021/jm2016123 BindingDB Entry DOI: 10.7270/Q2N58NDH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Free fatty acid receptor 1 (Rattus norvegicus) | BDBM50386644 (CHEMBL2048618) | KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 4.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company, Ltd. Curated by ChEMBL | Assay Description Displacement of 3-[4-({2',6'-dimethyl-6-[(4-[3H])phenylmethoxy]biphenyl-3-yl}methoxy)phenyl] propanoic acid from rat GPR40 receptor expressed in CHO ... | J Med Chem 55: 3756-76 (2012) Article DOI: 10.1021/jm2016123 BindingDB Entry DOI: 10.7270/Q2N58NDH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Free fatty acid receptor 1 (Rattus norvegicus) | BDBM50386650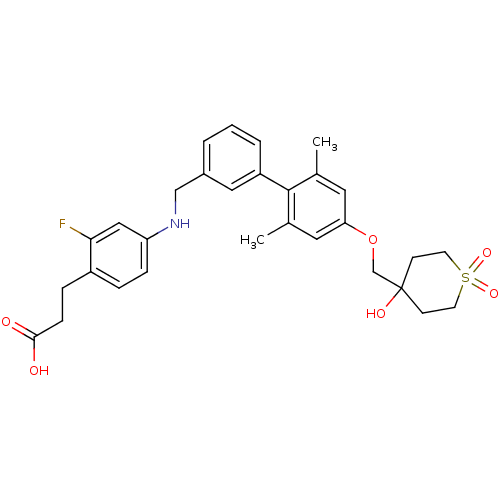 (CHEMBL2048627) | KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 4.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company, Ltd. Curated by ChEMBL | Assay Description Displacement of 3-[4-({2',6'-dimethyl-6-[(4-[3H])phenylmethoxy]biphenyl-3-yl}methoxy)phenyl] propanoic acid from rat GPR40 receptor expressed in CHO ... | J Med Chem 55: 3756-76 (2012) Article DOI: 10.1021/jm2016123 BindingDB Entry DOI: 10.7270/Q2N58NDH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Orexin receptor type 2 (Homo sapiens (Human)) | BDBM50292929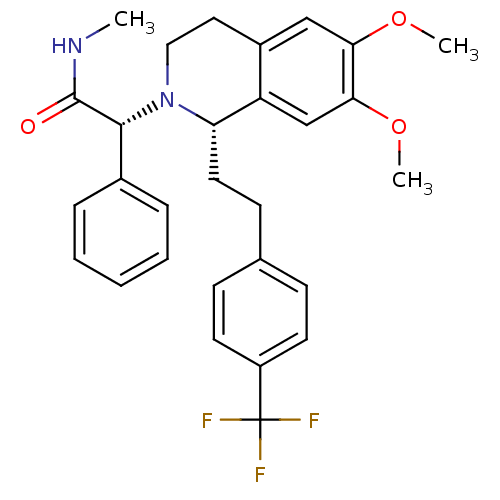 (CHEMBL455136 | almorexant) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | 4.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Eisai Co., Ltd Curated by ChEMBL | Assay Description Displacement of [125I]-Orexin A from human OX2R expressed in CHO cells after 30 mins by topcount analysis | Bioorg Med Chem 22: 6071-88 (2014) Article DOI: 10.1016/j.bmc.2014.08.034 BindingDB Entry DOI: 10.7270/Q20R9R0Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Orexin receptor type 2 (Homo sapiens (Human)) | BDBM50029064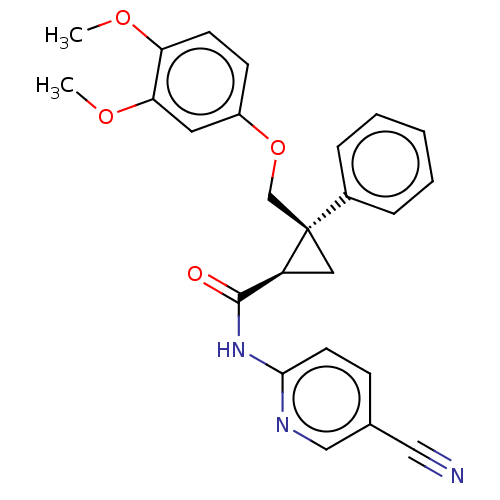 (CHEMBL3343259) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Eisai Co., Ltd. Curated by ChEMBL | Assay Description Binding affinity to human OX2R by radioligand displacement binding assay | J Med Chem 58: 4648-64 (2015) Article DOI: 10.1021/acs.jmedchem.5b00217 BindingDB Entry DOI: 10.7270/Q2125VD7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Orexin receptor type 2 (Homo sapiens (Human)) | BDBM50440023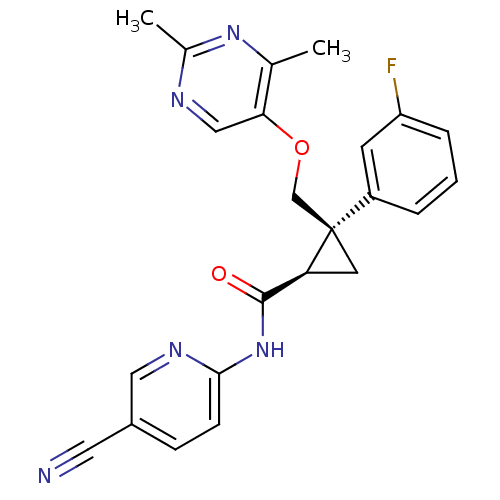 (CHEMBL2425786) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Eisai Co. , Ltd. Curated by ChEMBL | Assay Description Displacement of [125I]-orexin-A from human OX2 receptor expressed in CHO cells after 30 mins by liquid scintillation counting analysis | J Med Chem 56: 6371-85 (2013) Article DOI: 10.1021/jm400772t BindingDB Entry DOI: 10.7270/Q2QF8V91 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prolactin-releasing peptide receptor (Homo sapiens (Human)) | BDBM195749 (US9206173, 95) | UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 5 | -47.4 | n/a | n/a | n/a | n/a | n/a | 7.4 | 25 |
OTSUKA PHARMACEUTICAL CO., LTD. US Patent | Assay Description Receptor-expressing HEK 293 cell membrane fraction was analyzed by modifying the methods of C. J. Langmead et al. (see Langmead C J, Szekeres P G; Ch... | US Patent US9206173 (2015) BindingDB Entry DOI: 10.7270/Q23X85FS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prolactin-releasing peptide receptor (Homo sapiens (Human)) | BDBM195613 (US9206173, 1) | UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 5 | -47.4 | n/a | n/a | n/a | n/a | n/a | 7.4 | 25 |
OTSUKA PHARMACEUTICAL CO., LTD. US Patent | Assay Description Receptor-expressing HEK 293 cell membrane fraction was analyzed by modifying the methods of C. J. Langmead et al. (see Langmead C J, Szekeres P G; Ch... | US Patent US9206173 (2015) BindingDB Entry DOI: 10.7270/Q23X85FS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prolactin-releasing peptide receptor (Homo sapiens (Human)) | BDBM195671 (US9206173, 2433) | UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 5 | -47.4 | n/a | n/a | n/a | n/a | n/a | 7.4 | 25 |
OTSUKA PHARMACEUTICAL CO., LTD. US Patent | Assay Description Receptor-expressing HEK 293 cell membrane fraction was analyzed by modifying the methods of C. J. Langmead et al. (see Langmead C J, Szekeres P G; Ch... | US Patent US9206173 (2015) BindingDB Entry DOI: 10.7270/Q23X85FS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Orexin receptor type 2 (Homo sapiens (Human)) | BDBM50029065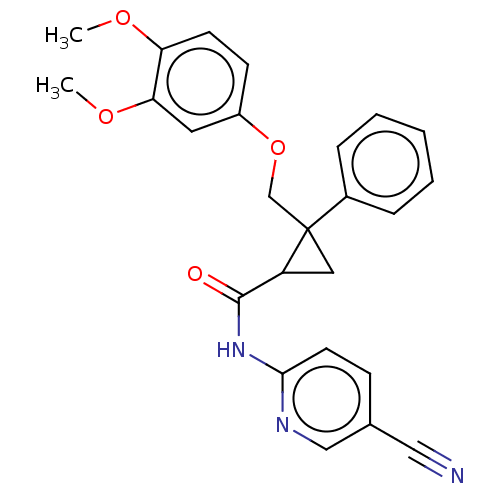 (CHEMBL3343260) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Eisai Co., Ltd Curated by ChEMBL | Assay Description Displacement of [125I]-Orexin A from human OX2R expressed in CHO cells after 30 mins by topcount analysis | Bioorg Med Chem 22: 6071-88 (2014) Article DOI: 10.1016/j.bmc.2014.08.034 BindingDB Entry DOI: 10.7270/Q20R9R0Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Free fatty acid receptor 1 (Rattus norvegicus) | BDBM50386649 (CHEMBL2048626) | KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 5.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company, Ltd. Curated by ChEMBL | Assay Description Displacement of 3-[4-({2',6'-dimethyl-6-[(4-[3H])phenylmethoxy]biphenyl-3-yl}methoxy)phenyl] propanoic acid from rat GPR40 receptor expressed in CHO ... | J Med Chem 55: 3756-76 (2012) Article DOI: 10.1021/jm2016123 BindingDB Entry DOI: 10.7270/Q2N58NDH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Free fatty acid receptor 1 (Rattus norvegicus) | BDBM50386641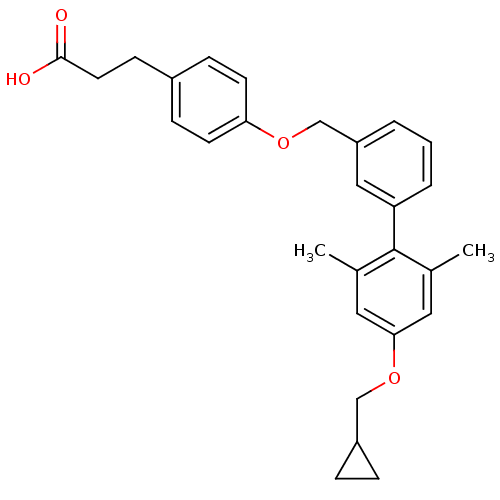 (CHEMBL2048615) | KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 5.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company, Ltd. Curated by ChEMBL | Assay Description Displacement of 3-[4-({2',6'-dimethyl-6-[(4-[3H])phenylmethoxy]biphenyl-3-yl}methoxy)phenyl] propanoic acid from rat GPR40 receptor expressed in CHO ... | J Med Chem 55: 3756-76 (2012) Article DOI: 10.1021/jm2016123 BindingDB Entry DOI: 10.7270/Q2N58NDH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Orexin/Hypocretin receptor type 1 (Homo sapiens (Human)) | BDBM50093793 (E-2006 | Lemborexant) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid PDB UniChem | PDB Article PubMed | 5.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Eisai Co., Ltd. Curated by ChEMBL | Assay Description Binding affinity to human OX1R expressed in HEK-293 cells assessed as inhibition of orexin A-induced calcium accumulation by FLIPR assay | J Med Chem 58: 4648-64 (2015) Article DOI: 10.1021/acs.jmedchem.5b00217 BindingDB Entry DOI: 10.7270/Q2125VD7 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Prolactin-releasing peptide receptor (Homo sapiens (Human)) | BDBM195640 (US9206173, 2402) | UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 6 | -46.9 | n/a | n/a | n/a | n/a | n/a | 7.4 | 25 |
OTSUKA PHARMACEUTICAL CO., LTD. US Patent | Assay Description Receptor-expressing HEK 293 cell membrane fraction was analyzed by modifying the methods of C. J. Langmead et al. (see Langmead C J, Szekeres P G; Ch... | US Patent US9206173 (2015) BindingDB Entry DOI: 10.7270/Q23X85FS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Orexin receptor type 2 (Homo sapiens (Human)) | BDBM50093795 (CHEMBL3585952) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 6 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Eisai Co., Ltd. Curated by ChEMBL | Assay Description Binding affinity to human OX2R by radioligand displacement binding assay | J Med Chem 58: 4648-64 (2015) Article DOI: 10.1021/acs.jmedchem.5b00217 BindingDB Entry DOI: 10.7270/Q2125VD7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Orexin/Hypocretin receptor type 1 (Homo sapiens (Human)) | BDBM50093798 (CHEMBL3585951) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 6 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Eisai Co., Ltd. Curated by ChEMBL | Assay Description Binding affinity to human OX1R by radioligand displacement binding assay | J Med Chem 58: 4648-64 (2015) Article DOI: 10.1021/acs.jmedchem.5b00217 BindingDB Entry DOI: 10.7270/Q2125VD7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Orexin/Hypocretin receptor type 1 (Homo sapiens (Human)) | BDBM50093793 (E-2006 | Lemborexant) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid PDB UniChem | PDB Article PubMed | 6 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Eisai Co., Ltd. Curated by ChEMBL | Assay Description Binding affinity to human OX1R by radioligand displacement binding assay | J Med Chem 58: 4648-64 (2015) Article DOI: 10.1021/acs.jmedchem.5b00217 BindingDB Entry DOI: 10.7270/Q2125VD7 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Prolactin-releasing peptide receptor (Homo sapiens (Human)) | BDBM195694 (US9206173, 2456) | UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 6 | -46.9 | n/a | n/a | n/a | n/a | n/a | 7.4 | 25 |
OTSUKA PHARMACEUTICAL CO., LTD. US Patent | Assay Description Receptor-expressing HEK 293 cell membrane fraction was analyzed by modifying the methods of C. J. Langmead et al. (see Langmead C J, Szekeres P G; Ch... | US Patent US9206173 (2015) BindingDB Entry DOI: 10.7270/Q23X85FS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prolactin-releasing peptide receptor (Homo sapiens (Human)) | BDBM195706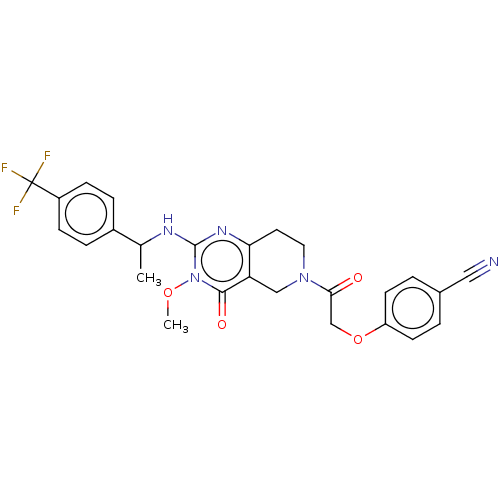 (US9206173, 2468) | UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 6 | -46.9 | n/a | n/a | n/a | n/a | n/a | 7.4 | 25 |
OTSUKA PHARMACEUTICAL CO., LTD. US Patent | Assay Description Receptor-expressing HEK 293 cell membrane fraction was analyzed by modifying the methods of C. J. Langmead et al. (see Langmead C J, Szekeres P G; Ch... | US Patent US9206173 (2015) BindingDB Entry DOI: 10.7270/Q23X85FS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prolactin-releasing peptide receptor (Homo sapiens (Human)) | BDBM195722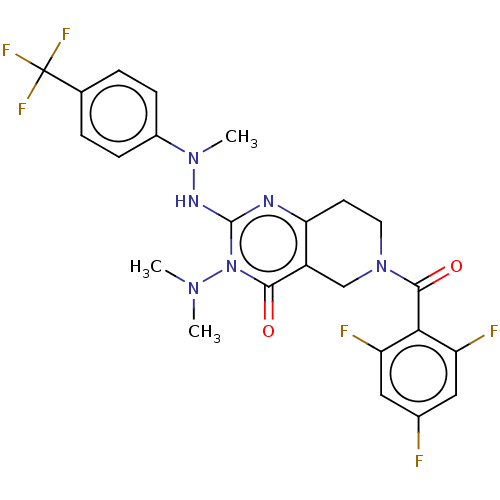 (US9206173, 2484) | UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 6 | -46.9 | n/a | n/a | n/a | n/a | n/a | 7.4 | 25 |
OTSUKA PHARMACEUTICAL CO., LTD. US Patent | Assay Description Receptor-expressing HEK 293 cell membrane fraction was analyzed by modifying the methods of C. J. Langmead et al. (see Langmead C J, Szekeres P G; Ch... | US Patent US9206173 (2015) BindingDB Entry DOI: 10.7270/Q23X85FS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 1827 total ) | Next | Last >> |