 Found 1544 hits with Last Name = 'suzuki' and Initial = 'n'
Found 1544 hits with Last Name = 'suzuki' and Initial = 'n' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Protein kinase C theta type
(Homo sapiens (Human)) | BDBM50391386

(CHEMBL2148106)Show SMILES CO[C@@H](CC[C@H](C)[C@H]1O[C@@]23C[C@H](OC(=O)C[C@@H](OC(=O)C[C@](O)(O2)[C@H](C)CC3(C)C)[C@@H](C)O)[C@@H]1C)c1cccc(O)c1 |r| Show InChI InChI=1S/C32H48O10/c1-18(11-12-24(38-7)22-9-8-10-23(34)13-22)29-20(3)26-16-32(41-29)30(5,6)15-19(2)31(37,42-32)17-28(36)39-25(21(4)33)14-27(35)40-26/h8-10,13,18-21,24-26,29,33-34,37H,11-12,14-17H2,1-7H3/t18-,19+,20-,21+,24-,25+,26?,29+,31-,32-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.110 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Kyoto University
Curated by ChEMBL
| Assay Description
Inhibition of [3H]PDBu binding to PKCtheta C1B domain |
J Med Chem 55: 5614-26 (2012)
Article DOI: 10.1021/jm300566h
BindingDB Entry DOI: 10.7270/Q200036J |
More data for this
Ligand-Target Pair | |
Protein kinase C eta type
(Homo sapiens (Human)) | BDBM50391386

(CHEMBL2148106)Show SMILES CO[C@@H](CC[C@H](C)[C@H]1O[C@@]23C[C@H](OC(=O)C[C@@H](OC(=O)C[C@](O)(O2)[C@H](C)CC3(C)C)[C@@H](C)O)[C@@H]1C)c1cccc(O)c1 |r| Show InChI InChI=1S/C32H48O10/c1-18(11-12-24(38-7)22-9-8-10-23(34)13-22)29-20(3)26-16-32(41-29)30(5,6)15-19(2)31(37,42-32)17-28(36)39-25(21(4)33)14-27(35)40-26/h8-10,13,18-21,24-26,29,33-34,37H,11-12,14-17H2,1-7H3/t18-,19+,20-,21+,24-,25+,26?,29+,31-,32-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.110 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Kyoto University
Curated by ChEMBL
| Assay Description
Inhibition of [3H]PDBu binding to PKCeta C1B domain |
J Med Chem 55: 5614-26 (2012)
Article DOI: 10.1021/jm300566h
BindingDB Entry DOI: 10.7270/Q200036J |
More data for this
Ligand-Target Pair | |
Protein kinase C beta type
(Homo sapiens (Human)) | BDBM50391386

(CHEMBL2148106)Show SMILES CO[C@@H](CC[C@H](C)[C@H]1O[C@@]23C[C@H](OC(=O)C[C@@H](OC(=O)C[C@](O)(O2)[C@H](C)CC3(C)C)[C@@H](C)O)[C@@H]1C)c1cccc(O)c1 |r| Show InChI InChI=1S/C32H48O10/c1-18(11-12-24(38-7)22-9-8-10-23(34)13-22)29-20(3)26-16-32(41-29)30(5,6)15-19(2)31(37,42-32)17-28(36)39-25(21(4)33)14-27(35)40-26/h8-10,13,18-21,24-26,29,33-34,37H,11-12,14-17H2,1-7H3/t18-,19+,20-,21+,24-,25+,26?,29+,31-,32-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.170 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Kyoto University
Curated by ChEMBL
| Assay Description
Inhibition of [3H]PDBu binding to PKCbeta C1A domain |
J Med Chem 55: 5614-26 (2012)
Article DOI: 10.1021/jm300566h
BindingDB Entry DOI: 10.7270/Q200036J |
More data for this
Ligand-Target Pair | |
Protein kinase C eta type
(Homo sapiens (Human)) | BDBM50493217

(DEMETHOXYDEBROMOAPLYSIATOXIN)Show SMILES [H][C@]12C[C@]3(O[C@]([H])([C@@H](C)CCCc4cccc(O)c4)[C@H]1C)O[C@@](O)(CC(=O)O[C@]([H])(CC(=O)O2)[C@@H](C)O)[C@H](C)CC3(C)C |r| Show InChI InChI=1S/C31H46O9/c1-18(9-7-10-22-11-8-12-23(33)13-22)28-20(3)25-16-31(39-28)29(5,6)15-19(2)30(36,40-31)17-27(35)37-24(21(4)32)14-26(34)38-25/h8,11-13,18-21,24-25,28,32-33,36H,7,9-10,14-17H2,1-6H3/t18-,19+,20-,21+,24+,25-,28+,30-,31-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.180 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Kyoto University
Curated by ChEMBL
| Assay Description
Displacement of [3H]PDBu from PKCeta C1B domain (unknown origin) |
Bioorg Med Chem Lett 23: 4319-23 (2013)
Article DOI: 10.1016/j.bmcl.2013.05.096
BindingDB Entry DOI: 10.7270/Q2VD72CX |
More data for this
Ligand-Target Pair | |
Protein kinase C theta type
(Homo sapiens (Human)) | BDBM50493217

(DEMETHOXYDEBROMOAPLYSIATOXIN)Show SMILES [H][C@]12C[C@]3(O[C@]([H])([C@@H](C)CCCc4cccc(O)c4)[C@H]1C)O[C@@](O)(CC(=O)O[C@]([H])(CC(=O)O2)[C@@H](C)O)[C@H](C)CC3(C)C |r| Show InChI InChI=1S/C31H46O9/c1-18(9-7-10-22-11-8-12-23(33)13-22)28-20(3)25-16-31(39-28)29(5,6)15-19(2)30(36,40-31)17-27(35)37-24(21(4)32)14-26(34)38-25/h8,11-13,18-21,24-25,28,32-33,36H,7,9-10,14-17H2,1-6H3/t18-,19+,20-,21+,24+,25-,28+,30-,31-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Kyoto University
Curated by ChEMBL
| Assay Description
Displacement of [3H]PDBu from PKCtheta C1B domain (unknown origin) |
Bioorg Med Chem Lett 23: 4319-23 (2013)
Article DOI: 10.1016/j.bmcl.2013.05.096
BindingDB Entry DOI: 10.7270/Q2VD72CX |
More data for this
Ligand-Target Pair | |
Protein kinase C delta type
(Homo sapiens (Human)) | BDBM50391386

(CHEMBL2148106)Show SMILES CO[C@@H](CC[C@H](C)[C@H]1O[C@@]23C[C@H](OC(=O)C[C@@H](OC(=O)C[C@](O)(O2)[C@H](C)CC3(C)C)[C@@H](C)O)[C@@H]1C)c1cccc(O)c1 |r| Show InChI InChI=1S/C32H48O10/c1-18(11-12-24(38-7)22-9-8-10-23(34)13-22)29-20(3)26-16-32(41-29)30(5,6)15-19(2)31(37,42-32)17-28(36)39-25(21(4)33)14-27(35)40-26/h8-10,13,18-21,24-26,29,33-34,37H,11-12,14-17H2,1-7H3/t18-,19+,20-,21+,24-,25+,26?,29+,31-,32-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Kyoto University
Curated by ChEMBL
| Assay Description
Inhibition of [3H]PDBu binding to PKCdelta C1B domain |
J Med Chem 55: 5614-26 (2012)
Article DOI: 10.1021/jm300566h
BindingDB Entry DOI: 10.7270/Q200036J |
More data for this
Ligand-Target Pair | |
Protein kinase C alpha type
(Homo sapiens (Human)) | BDBM50391386

(CHEMBL2148106)Show SMILES CO[C@@H](CC[C@H](C)[C@H]1O[C@@]23C[C@H](OC(=O)C[C@@H](OC(=O)C[C@](O)(O2)[C@H](C)CC3(C)C)[C@@H](C)O)[C@@H]1C)c1cccc(O)c1 |r| Show InChI InChI=1S/C32H48O10/c1-18(11-12-24(38-7)22-9-8-10-23(34)13-22)29-20(3)26-16-32(41-29)30(5,6)15-19(2)31(37,42-32)17-28(36)39-25(21(4)33)14-27(35)40-26/h8-10,13,18-21,24-26,29,33-34,37H,11-12,14-17H2,1-7H3/t18-,19+,20-,21+,24-,25+,26?,29+,31-,32-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.260 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Kyoto University
Curated by ChEMBL
| Assay Description
Inhibition of [3H]PDBu binding to PKCalpha C1A domain |
J Med Chem 55: 5614-26 (2012)
Article DOI: 10.1021/jm300566h
BindingDB Entry DOI: 10.7270/Q200036J |
More data for this
Ligand-Target Pair | |
Protein kinase C delta type
(Homo sapiens (Human)) | BDBM50493217

(DEMETHOXYDEBROMOAPLYSIATOXIN)Show SMILES [H][C@]12C[C@]3(O[C@]([H])([C@@H](C)CCCc4cccc(O)c4)[C@H]1C)O[C@@](O)(CC(=O)O[C@]([H])(CC(=O)O2)[C@@H](C)O)[C@H](C)CC3(C)C |r| Show InChI InChI=1S/C31H46O9/c1-18(9-7-10-22-11-8-12-23(33)13-22)28-20(3)25-16-31(39-28)29(5,6)15-19(2)30(36,40-31)17-27(35)37-24(21(4)32)14-26(34)38-25/h8,11-13,18-21,24-25,28,32-33,36H,7,9-10,14-17H2,1-6H3/t18-,19+,20-,21+,24+,25-,28+,30-,31-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.320 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Kyoto University
Curated by ChEMBL
| Assay Description
Displacement of [3H]PDBu from PKCdelta C1B domain (unknown origin) |
Bioorg Med Chem Lett 23: 4319-23 (2013)
Article DOI: 10.1016/j.bmcl.2013.05.096
BindingDB Entry DOI: 10.7270/Q2VD72CX |
More data for this
Ligand-Target Pair | |
Protein kinase C gamma type
(Homo sapiens (Human)) | BDBM50391386

(CHEMBL2148106)Show SMILES CO[C@@H](CC[C@H](C)[C@H]1O[C@@]23C[C@H](OC(=O)C[C@@H](OC(=O)C[C@](O)(O2)[C@H](C)CC3(C)C)[C@@H](C)O)[C@@H]1C)c1cccc(O)c1 |r| Show InChI InChI=1S/C32H48O10/c1-18(11-12-24(38-7)22-9-8-10-23(34)13-22)29-20(3)26-16-32(41-29)30(5,6)15-19(2)31(37,42-32)17-28(36)39-25(21(4)33)14-27(35)40-26/h8-10,13,18-21,24-26,29,33-34,37H,11-12,14-17H2,1-7H3/t18-,19+,20-,21+,24-,25+,26?,29+,31-,32-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.380 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Kyoto University
Curated by ChEMBL
| Assay Description
Inhibition of [3H]PDBu binding to PKCgamma C1A domain |
J Med Chem 55: 5614-26 (2012)
Article DOI: 10.1021/jm300566h
BindingDB Entry DOI: 10.7270/Q200036J |
More data for this
Ligand-Target Pair | |
Protein kinase C eta type
(Homo sapiens (Human)) | BDBM50391388
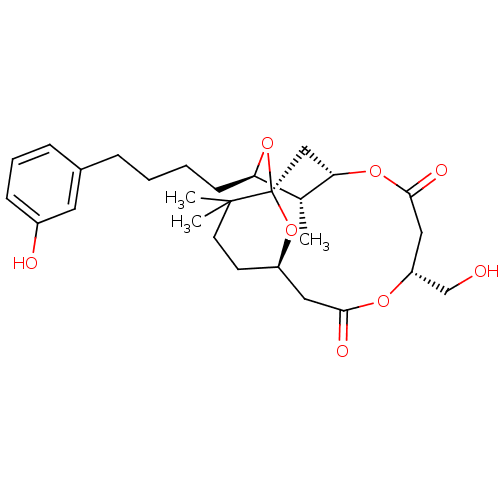
(CHEMBL2148108)Show SMILES C[C@@H]1[C@@H](CCCCc2cccc(O)c2)O[C@]23C[C@@H]1OC(=O)C[C@H](CO)OC(=O)C[C@@H](CCC2(C)C)O3 |r| Show InChI InChI=1S/C28H40O8/c1-18-23(10-5-4-7-19-8-6-9-20(30)13-19)36-28-16-24(18)34-26(32)15-22(17-29)33-25(31)14-21(35-28)11-12-27(28,2)3/h6,8-9,13,18,21-24,29-30H,4-5,7,10-12,14-17H2,1-3H3/t18-,21-,22-,23-,24?,28-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.450 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Kyoto University
Curated by ChEMBL
| Assay Description
Inhibition of [3H]PDBu binding to PKCeta C1B domain |
J Med Chem 55: 5614-26 (2012)
Article DOI: 10.1021/jm300566h
BindingDB Entry DOI: 10.7270/Q200036J |
More data for this
Ligand-Target Pair | |
Protein kinase C delta type
(Homo sapiens (Human)) | BDBM50391388

(CHEMBL2148108)Show SMILES C[C@@H]1[C@@H](CCCCc2cccc(O)c2)O[C@]23C[C@@H]1OC(=O)C[C@H](CO)OC(=O)C[C@@H](CCC2(C)C)O3 |r| Show InChI InChI=1S/C28H40O8/c1-18-23(10-5-4-7-19-8-6-9-20(30)13-19)36-28-16-24(18)34-26(32)15-22(17-29)33-25(31)14-21(35-28)11-12-27(28,2)3/h6,8-9,13,18,21-24,29-30H,4-5,7,10-12,14-17H2,1-3H3/t18-,21-,22-,23-,24?,28-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.460 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Kyoto University
Curated by ChEMBL
| Assay Description
Inhibition of [3H]PDBu binding to PKCdelta C1B domain |
J Med Chem 55: 5614-26 (2012)
Article DOI: 10.1021/jm300566h
BindingDB Entry DOI: 10.7270/Q200036J |
More data for this
Ligand-Target Pair | |
Protein kinase C theta type
(Homo sapiens (Human)) | BDBM50391388

(CHEMBL2148108)Show SMILES C[C@@H]1[C@@H](CCCCc2cccc(O)c2)O[C@]23C[C@@H]1OC(=O)C[C@H](CO)OC(=O)C[C@@H](CCC2(C)C)O3 |r| Show InChI InChI=1S/C28H40O8/c1-18-23(10-5-4-7-19-8-6-9-20(30)13-19)36-28-16-24(18)34-26(32)15-22(17-29)33-25(31)14-21(35-28)11-12-27(28,2)3/h6,8-9,13,18,21-24,29-30H,4-5,7,10-12,14-17H2,1-3H3/t18-,21-,22-,23-,24?,28-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.540 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Kyoto University
Curated by ChEMBL
| Assay Description
Inhibition of [3H]PDBu binding to PKCtheta C1B domain |
J Med Chem 55: 5614-26 (2012)
Article DOI: 10.1021/jm300566h
BindingDB Entry DOI: 10.7270/Q200036J |
More data for this
Ligand-Target Pair | |
Protein kinase C epsilon type
(Homo sapiens (Human)) | BDBM50391386

(CHEMBL2148106)Show SMILES CO[C@@H](CC[C@H](C)[C@H]1O[C@@]23C[C@H](OC(=O)C[C@@H](OC(=O)C[C@](O)(O2)[C@H](C)CC3(C)C)[C@@H](C)O)[C@@H]1C)c1cccc(O)c1 |r| Show InChI InChI=1S/C32H48O10/c1-18(11-12-24(38-7)22-9-8-10-23(34)13-22)29-20(3)26-16-32(41-29)30(5,6)15-19(2)31(37,42-32)17-28(36)39-25(21(4)33)14-27(35)40-26/h8-10,13,18-21,24-26,29,33-34,37H,11-12,14-17H2,1-7H3/t18-,19+,20-,21+,24-,25+,26?,29+,31-,32-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.630 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Kyoto University
Curated by ChEMBL
| Assay Description
Inhibition of [3H]PDBu binding to PKCepsilon C1B domain |
J Med Chem 55: 5614-26 (2012)
Article DOI: 10.1021/jm300566h
BindingDB Entry DOI: 10.7270/Q200036J |
More data for this
Ligand-Target Pair | |
Protein kinase C gamma type
(Homo sapiens (Human)) | BDBM50493217

(DEMETHOXYDEBROMOAPLYSIATOXIN)Show SMILES [H][C@]12C[C@]3(O[C@]([H])([C@@H](C)CCCc4cccc(O)c4)[C@H]1C)O[C@@](O)(CC(=O)O[C@]([H])(CC(=O)O2)[C@@H](C)O)[C@H](C)CC3(C)C |r| Show InChI InChI=1S/C31H46O9/c1-18(9-7-10-22-11-8-12-23(33)13-22)28-20(3)25-16-31(39-28)29(5,6)15-19(2)30(36,40-31)17-27(35)37-24(21(4)32)14-26(34)38-25/h8,11-13,18-21,24-25,28,32-33,36H,7,9-10,14-17H2,1-6H3/t18-,19+,20-,21+,24+,25-,28+,30-,31-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.690 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Kyoto University
Curated by ChEMBL
| Assay Description
Displacement of [3H]PDBu from PKCgamma C1A domain (unknown origin) |
Bioorg Med Chem Lett 23: 4319-23 (2013)
Article DOI: 10.1016/j.bmcl.2013.05.096
BindingDB Entry DOI: 10.7270/Q2VD72CX |
More data for this
Ligand-Target Pair | |
Protein kinase C alpha type
(Homo sapiens (Human)) | BDBM50493217

(DEMETHOXYDEBROMOAPLYSIATOXIN)Show SMILES [H][C@]12C[C@]3(O[C@]([H])([C@@H](C)CCCc4cccc(O)c4)[C@H]1C)O[C@@](O)(CC(=O)O[C@]([H])(CC(=O)O2)[C@@H](C)O)[C@H](C)CC3(C)C |r| Show InChI InChI=1S/C31H46O9/c1-18(9-7-10-22-11-8-12-23(33)13-22)28-20(3)25-16-31(39-28)29(5,6)15-19(2)30(36,40-31)17-27(35)37-24(21(4)32)14-26(34)38-25/h8,11-13,18-21,24-25,28,32-33,36H,7,9-10,14-17H2,1-6H3/t18-,19+,20-,21+,24+,25-,28+,30-,31-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Kyoto University
Curated by ChEMBL
| Assay Description
Displacement of [3H]PDBu from PKCalpha C1A domain (unknown origin) |
Bioorg Med Chem Lett 23: 4319-23 (2013)
Article DOI: 10.1016/j.bmcl.2013.05.096
BindingDB Entry DOI: 10.7270/Q2VD72CX |
More data for this
Ligand-Target Pair | |
Protein kinase C epsilon type
(Homo sapiens (Human)) | BDBM50493217

(DEMETHOXYDEBROMOAPLYSIATOXIN)Show SMILES [H][C@]12C[C@]3(O[C@]([H])([C@@H](C)CCCc4cccc(O)c4)[C@H]1C)O[C@@](O)(CC(=O)O[C@]([H])(CC(=O)O2)[C@@H](C)O)[C@H](C)CC3(C)C |r| Show InChI InChI=1S/C31H46O9/c1-18(9-7-10-22-11-8-12-23(33)13-22)28-20(3)25-16-31(39-28)29(5,6)15-19(2)30(36,40-31)17-27(35)37-24(21(4)32)14-26(34)38-25/h8,11-13,18-21,24-25,28,32-33,36H,7,9-10,14-17H2,1-6H3/t18-,19+,20-,21+,24+,25-,28+,30-,31-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 1.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Kyoto University
Curated by ChEMBL
| Assay Description
Displacement of [3H]PDBu from PKCepsilon C1B domain (unknown origin) |
Bioorg Med Chem Lett 23: 4319-23 (2013)
Article DOI: 10.1016/j.bmcl.2013.05.096
BindingDB Entry DOI: 10.7270/Q2VD72CX |
More data for this
Ligand-Target Pair | |
Protein kinase C delta type
(Homo sapiens (Human)) | BDBM50491400
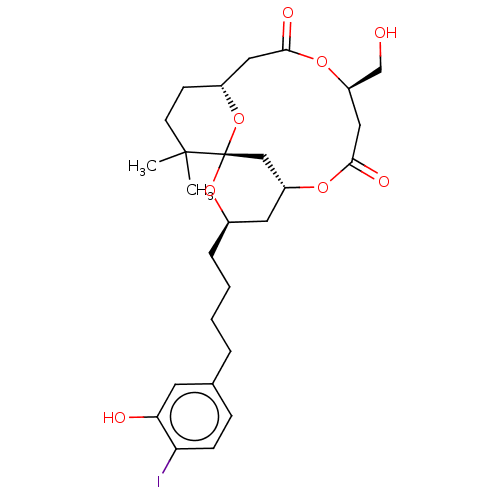
(CHEMBL2381165)Show SMILES [H][C@@]12CCC(C)(C)[C@]3(C[C@@]([H])(C[C@@H](CCCCc4ccc(I)c(O)c4)O3)OC(=O)C[C@H](CO)OC(=O)C1)O2 |r| Show InChI InChI=1S/C27H37IO8/c1-26(2)10-9-19-13-24(31)34-21(16-29)14-25(32)33-20-12-18(35-27(26,15-20)36-19)6-4-3-5-17-7-8-22(28)23(30)11-17/h7-8,11,18-21,29-30H,3-6,9-10,12-16H2,1-2H3/t18-,19-,20-,21-,27+/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 1.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Kyoto University
Curated by ChEMBL
| Assay Description
Inhibition of [3H]PDBu binding to PKCdelta-C1B domain peptide (unknown origin) |
Bioorg Med Chem 21: 2695-702 (2013)
Article DOI: 10.1016/j.bmc.2013.03.013
BindingDB Entry DOI: 10.7270/Q26M39RD |
More data for this
Ligand-Target Pair | |
Protein kinase C beta type
(Homo sapiens (Human)) | BDBM50493217

(DEMETHOXYDEBROMOAPLYSIATOXIN)Show SMILES [H][C@]12C[C@]3(O[C@]([H])([C@@H](C)CCCc4cccc(O)c4)[C@H]1C)O[C@@](O)(CC(=O)O[C@]([H])(CC(=O)O2)[C@@H](C)O)[C@H](C)CC3(C)C |r| Show InChI InChI=1S/C31H46O9/c1-18(9-7-10-22-11-8-12-23(33)13-22)28-20(3)25-16-31(39-28)29(5,6)15-19(2)30(36,40-31)17-27(35)37-24(21(4)32)14-26(34)38-25/h8,11-13,18-21,24-25,28,32-33,36H,7,9-10,14-17H2,1-6H3/t18-,19+,20-,21+,24+,25-,28+,30-,31-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 1.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Kyoto University
Curated by ChEMBL
| Assay Description
Displacement of [3H]PDBu from PKCbeta C1A domain (unknown origin) |
Bioorg Med Chem Lett 23: 4319-23 (2013)
Article DOI: 10.1016/j.bmcl.2013.05.096
BindingDB Entry DOI: 10.7270/Q2VD72CX |
More data for this
Ligand-Target Pair | |
Protein kinase C epsilon type
(Homo sapiens (Human)) | BDBM50391388

(CHEMBL2148108)Show SMILES C[C@@H]1[C@@H](CCCCc2cccc(O)c2)O[C@]23C[C@@H]1OC(=O)C[C@H](CO)OC(=O)C[C@@H](CCC2(C)C)O3 |r| Show InChI InChI=1S/C28H40O8/c1-18-23(10-5-4-7-19-8-6-9-20(30)13-19)36-28-16-24(18)34-26(32)15-22(17-29)33-25(31)14-21(35-28)11-12-27(28,2)3/h6,8-9,13,18,21-24,29-30H,4-5,7,10-12,14-17H2,1-3H3/t18-,21-,22-,23-,24?,28-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Kyoto University
Curated by ChEMBL
| Assay Description
Inhibition of [3H]PDBu binding to PKCepsilon C1B domain |
J Med Chem 55: 5614-26 (2012)
Article DOI: 10.1021/jm300566h
BindingDB Entry DOI: 10.7270/Q200036J |
More data for this
Ligand-Target Pair | |
Protein kinase C theta type
(Homo sapiens (Human)) | BDBM50391387
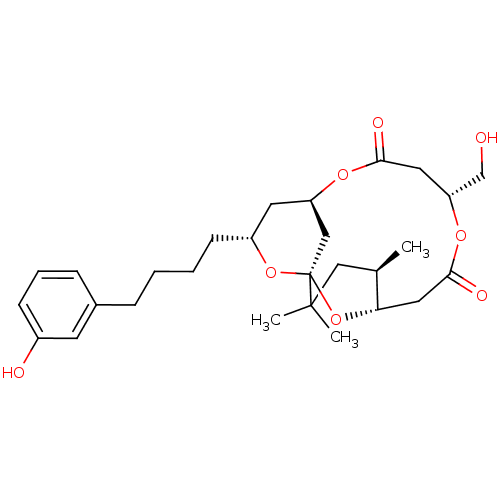
(CHEMBL2148107)Show SMILES C[C@@H]1CC(C)(C)[C@@]23C[C@@H](C[C@@H](CCCCc4cccc(O)c4)O2)OC(=O)C[C@H](CO)OC(=O)C[C@@H]1O3 |r| Show InChI InChI=1S/C28H40O8/c1-18-15-27(2,3)28-16-22(33-25(31)13-23(17-29)34-26(32)14-24(18)36-28)12-21(35-28)10-5-4-7-19-8-6-9-20(30)11-19/h6,8-9,11,18,21-24,29-30H,4-5,7,10,12-17H2,1-3H3/t18-,21-,22?,23-,24+,28+/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 2.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Kyoto University
Curated by ChEMBL
| Assay Description
Inhibition of [3H]PDBu binding to PKCtheta C1B domain |
J Med Chem 55: 5614-26 (2012)
Article DOI: 10.1021/jm300566h
BindingDB Entry DOI: 10.7270/Q200036J |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50127501
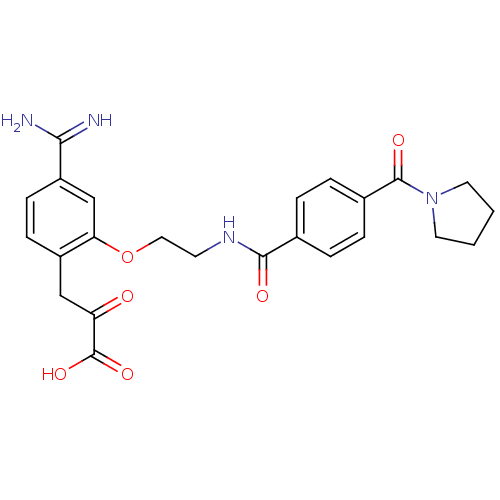
(3-(4-Carbamimidoyl-2-{2-[4-(pyrrolidine-1-carbonyl...)Show SMILES NC(=N)c1ccc(CC(=O)C(O)=O)c(OCCNC(=O)c2ccc(cc2)C(=O)N2CCCC2)c1 Show InChI InChI=1S/C24H26N4O6/c25-21(26)18-8-7-17(13-19(29)24(32)33)20(14-18)34-12-9-27-22(30)15-3-5-16(6-4-15)23(31)28-10-1-2-11-28/h3-8,14H,1-2,9-13H2,(H3,25,26)(H,27,30)(H,32,33) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Ajinomoto Company Inc.
Curated by ChEMBL
| Assay Description
Inhibitory activity against Coagulation factor X (fXa) |
J Med Chem 46: 1845-57 (2003)
Article DOI: 10.1021/jm020485x
BindingDB Entry DOI: 10.7270/Q2PZ586C |
More data for this
Ligand-Target Pair | |
Protein kinase C delta type
(Homo sapiens (Human)) | BDBM50391387

(CHEMBL2148107)Show SMILES C[C@@H]1CC(C)(C)[C@@]23C[C@@H](C[C@@H](CCCCc4cccc(O)c4)O2)OC(=O)C[C@H](CO)OC(=O)C[C@@H]1O3 |r| Show InChI InChI=1S/C28H40O8/c1-18-15-27(2,3)28-16-22(33-25(31)13-23(17-29)34-26(32)14-24(18)36-28)12-21(35-28)10-5-4-7-19-8-6-9-20(30)11-19/h6,8-9,11,18,21-24,29-30H,4-5,7,10,12-17H2,1-3H3/t18-,21-,22?,23-,24+,28+/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 3.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Kyoto University
Curated by ChEMBL
| Assay Description
Inhibition of [3H]PDBu binding to PKCdelta C1B domain |
J Med Chem 55: 5614-26 (2012)
Article DOI: 10.1021/jm300566h
BindingDB Entry DOI: 10.7270/Q200036J |
More data for this
Ligand-Target Pair | |
Protein kinase C delta type
(Homo sapiens (Human)) | BDBM50491401
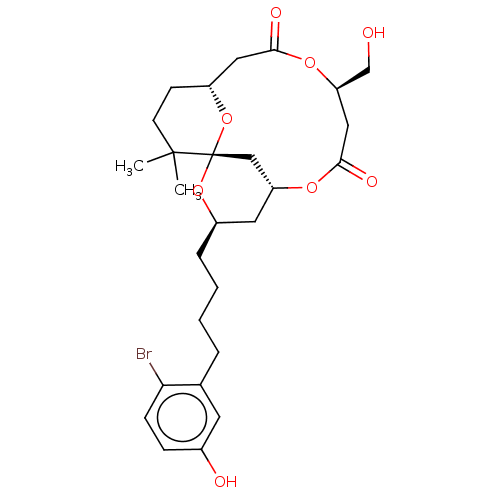
(CHEMBL2381162)Show SMILES [H][C@@]12CCC(C)(C)[C@]3(C[C@@]([H])(C[C@@H](CCCCc4cc(O)ccc4Br)O3)OC(=O)C[C@H](CO)OC(=O)C1)O2 |r| Show InChI InChI=1S/C27H37BrO8/c1-26(2)10-9-20-13-24(31)34-22(16-29)14-25(32)33-21-12-19(35-27(26,15-21)36-20)6-4-3-5-17-11-18(30)7-8-23(17)28/h7-8,11,19-22,29-30H,3-6,9-10,12-16H2,1-2H3/t19-,20-,21-,22-,27+/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 3.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Kyoto University
Curated by ChEMBL
| Assay Description
Inhibition of [3H]PDBu binding to PKCdelta-C1B domain peptide (unknown origin) |
Bioorg Med Chem 21: 2695-702 (2013)
Article DOI: 10.1016/j.bmc.2013.03.013
BindingDB Entry DOI: 10.7270/Q26M39RD |
More data for this
Ligand-Target Pair | |
Protein kinase C theta type
(Homo sapiens (Human)) | BDBM50327946
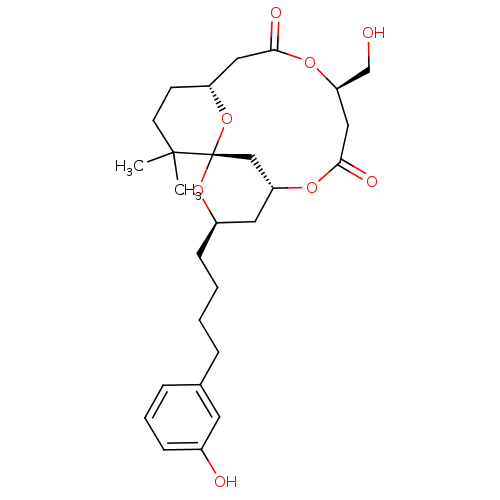
(1S,3R,5R,9R,13R)-9-Hydroxymethyl-3-[4-(3-hydroxy-p...)Show SMILES CC1(C)CC[C@@H]2CC(=O)O[C@@H](CO)CC(=O)O[C@@H]3C[C@@H](CCCCc4cccc(O)c4)O[C@@]1(C3)O2 |r| Show InChI InChI=1S/C27H38O8/c1-26(2)11-10-21-14-24(30)33-23(17-28)15-25(31)32-22-13-20(34-27(26,16-22)35-21)9-4-3-6-18-7-5-8-19(29)12-18/h5,7-8,12,20-23,28-29H,3-4,6,9-11,13-17H2,1-2H3/t20-,21-,22?,23-,27+/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Kyoto University
Curated by ChEMBL
| Assay Description
Inhibition of [3H]PDBu binding to PKCtheta C1B domain |
J Med Chem 55: 5614-26 (2012)
Article DOI: 10.1021/jm300566h
BindingDB Entry DOI: 10.7270/Q200036J |
More data for this
Ligand-Target Pair | |
Protein kinase C delta type
(Homo sapiens (Human)) | BDBM50491402

(CHEMBL2381163)Show SMILES [H][C@@]12CCC(C)(C)[C@]3(C[C@@]([H])(C[C@@H](CCCCc4cc(O)c(Br)cc4Br)O3)OC(=O)C[C@H](CO)OC(=O)C1)O2 |r| Show InChI InChI=1S/C27H36Br2O8/c1-26(2)8-7-18-11-24(32)35-20(15-30)12-25(33)34-19-10-17(36-27(26,14-19)37-18)6-4-3-5-16-9-23(31)22(29)13-21(16)28/h9,13,17-20,30-31H,3-8,10-12,14-15H2,1-2H3/t17-,18-,19-,20-,27+/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 4.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Kyoto University
Curated by ChEMBL
| Assay Description
Inhibition of [3H]PDBu binding to PKCdelta-C1B domain peptide (unknown origin) |
Bioorg Med Chem 21: 2695-702 (2013)
Article DOI: 10.1016/j.bmc.2013.03.013
BindingDB Entry DOI: 10.7270/Q26M39RD |
More data for this
Ligand-Target Pair | |
Free fatty acid receptor 1
(Rattus norvegicus) | BDBM50386646

(CHEMBL2048622)Show SMILES CCS(=O)(=O)CCOc1cc(C)c(c(C)c1)-c1cccc(COc2ccc(CCC(O)=O)c(F)c2)c1 Show InChI InChI=1S/C28H31FO6S/c1-4-36(32,33)13-12-34-25-14-19(2)28(20(3)15-25)23-7-5-6-21(16-23)18-35-24-10-8-22(26(29)17-24)9-11-27(30)31/h5-8,10,14-17H,4,9,11-13,18H2,1-3H3,(H,30,31) | KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| 4.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company, Ltd.
Curated by ChEMBL
| Assay Description
Displacement of 3-[4-({2',6'-dimethyl-6-[(4-[3H])phenylmethoxy]biphenyl-3-yl}methoxy)phenyl] propanoic acid from rat GPR40 receptor expressed in CHO ... |
J Med Chem 55: 3756-76 (2012)
Article DOI: 10.1021/jm2016123
BindingDB Entry DOI: 10.7270/Q2N58NDH |
More data for this
Ligand-Target Pair | |
Protein kinase C eta type
(Homo sapiens (Human)) | BDBM50327946

(1S,3R,5R,9R,13R)-9-Hydroxymethyl-3-[4-(3-hydroxy-p...)Show SMILES CC1(C)CC[C@@H]2CC(=O)O[C@@H](CO)CC(=O)O[C@@H]3C[C@@H](CCCCc4cccc(O)c4)O[C@@]1(C3)O2 |r| Show InChI InChI=1S/C27H38O8/c1-26(2)11-10-21-14-24(30)33-23(17-28)15-25(31)32-22-13-20(34-27(26,16-22)35-21)9-4-3-6-18-7-5-8-19(29)12-18/h5,7-8,12,20-23,28-29H,3-4,6,9-11,13-17H2,1-2H3/t20-,21-,22?,23-,27+/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 4.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Kyoto University
Curated by ChEMBL
| Assay Description
Inhibition of [3H]PDBu binding to PKCeta C1B domain |
J Med Chem 55: 5614-26 (2012)
Article DOI: 10.1021/jm300566h
BindingDB Entry DOI: 10.7270/Q200036J |
More data for this
Ligand-Target Pair | |
Free fatty acid receptor 1
(Rattus norvegicus) | BDBM50386650
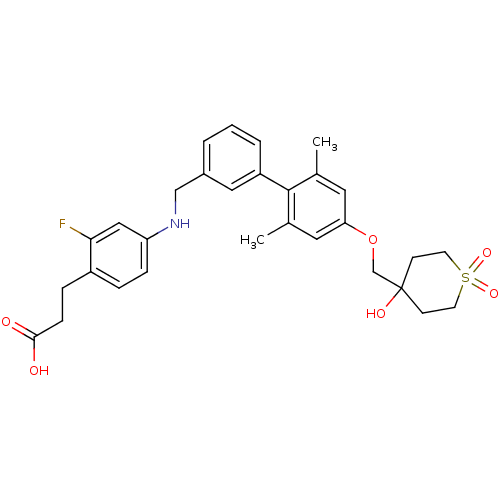
(CHEMBL2048627)Show SMILES Cc1cc(OCC2(O)CCS(=O)(=O)CC2)cc(C)c1-c1cccc(CNc2ccc(CCC(O)=O)c(F)c2)c1 Show InChI InChI=1S/C30H34FNO6S/c1-20-14-26(38-19-30(35)10-12-39(36,37)13-11-30)15-21(2)29(20)24-5-3-4-22(16-24)18-32-25-8-6-23(27(31)17-25)7-9-28(33)34/h3-6,8,14-17,32,35H,7,9-13,18-19H2,1-2H3,(H,33,34) | KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 4.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company, Ltd.
Curated by ChEMBL
| Assay Description
Displacement of 3-[4-({2',6'-dimethyl-6-[(4-[3H])phenylmethoxy]biphenyl-3-yl}methoxy)phenyl] propanoic acid from rat GPR40 receptor expressed in CHO ... |
J Med Chem 55: 3756-76 (2012)
Article DOI: 10.1021/jm2016123
BindingDB Entry DOI: 10.7270/Q2N58NDH |
More data for this
Ligand-Target Pair | |
Free fatty acid receptor 1
(Rattus norvegicus) | BDBM50386648

(CHEMBL2048625)Show SMILES CCS(=O)(=O)CCOc1cc(C)c(c(C)c1)-c1cccc(CNc2ccc(CCC(O)=O)c(F)c2)c1 Show InChI InChI=1S/C28H32FNO5S/c1-4-36(33,34)13-12-35-25-14-19(2)28(20(3)15-25)23-7-5-6-21(16-23)18-30-24-10-8-22(26(29)17-24)9-11-27(31)32/h5-8,10,14-17,30H,4,9,11-13,18H2,1-3H3,(H,31,32) | KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 4.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company, Ltd.
Curated by ChEMBL
| Assay Description
Displacement of 3-[4-({2',6'-dimethyl-6-[(4-[3H])phenylmethoxy]biphenyl-3-yl}methoxy)phenyl] propanoic acid from rat GPR40 receptor expressed in CHO ... |
J Med Chem 55: 3756-76 (2012)
Article DOI: 10.1021/jm2016123
BindingDB Entry DOI: 10.7270/Q2N58NDH |
More data for this
Ligand-Target Pair | |
Free fatty acid receptor 1
(Rattus norvegicus) | BDBM50386644

(CHEMBL2048618)Show SMILES CCOCCOc1cc(C)c(c(C)c1)-c1cccc(COc2ccc(CCC(O)=O)c(F)c2)c1 Show InChI InChI=1S/C28H31FO5/c1-4-32-12-13-33-25-14-19(2)28(20(3)15-25)23-7-5-6-21(16-23)18-34-24-10-8-22(26(29)17-24)9-11-27(30)31/h5-8,10,14-17H,4,9,11-13,18H2,1-3H3,(H,30,31) | KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 4.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company, Ltd.
Curated by ChEMBL
| Assay Description
Displacement of 3-[4-({2',6'-dimethyl-6-[(4-[3H])phenylmethoxy]biphenyl-3-yl}methoxy)phenyl] propanoic acid from rat GPR40 receptor expressed in CHO ... |
J Med Chem 55: 3756-76 (2012)
Article DOI: 10.1021/jm2016123
BindingDB Entry DOI: 10.7270/Q2N58NDH |
More data for this
Ligand-Target Pair | |
Protein kinase C delta type
(Homo sapiens (Human)) | BDBM50491404
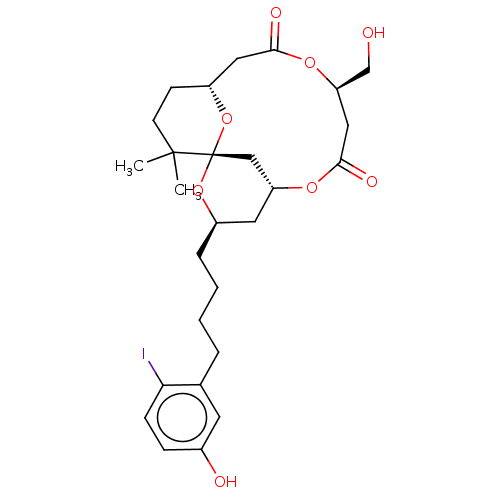
(CHEMBL2381166)Show SMILES [H][C@@]12CCC(C)(C)[C@]3(C[C@@]([H])(C[C@@H](CCCCc4cc(O)ccc4I)O3)OC(=O)C[C@H](CO)OC(=O)C1)O2 |r| Show InChI InChI=1S/C27H37IO8/c1-26(2)10-9-20-13-24(31)34-22(16-29)14-25(32)33-21-12-19(35-27(26,15-21)36-20)6-4-3-5-17-11-18(30)7-8-23(17)28/h7-8,11,19-22,29-30H,3-6,9-10,12-16H2,1-2H3/t19-,20-,21-,22-,27+/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 4.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Kyoto University
Curated by ChEMBL
| Assay Description
Inhibition of [3H]PDBu binding to PKCdelta-C1B domain peptide (unknown origin) |
Bioorg Med Chem 21: 2695-702 (2013)
Article DOI: 10.1016/j.bmc.2013.03.013
BindingDB Entry DOI: 10.7270/Q26M39RD |
More data for this
Ligand-Target Pair | |
Protein kinase C alpha type
(Homo sapiens (Human)) | BDBM50391388

(CHEMBL2148108)Show SMILES C[C@@H]1[C@@H](CCCCc2cccc(O)c2)O[C@]23C[C@@H]1OC(=O)C[C@H](CO)OC(=O)C[C@@H](CCC2(C)C)O3 |r| Show InChI InChI=1S/C28H40O8/c1-18-23(10-5-4-7-19-8-6-9-20(30)13-19)36-28-16-24(18)34-26(32)15-22(17-29)33-25(31)14-21(35-28)11-12-27(28,2)3/h6,8-9,13,18,21-24,29-30H,4-5,7,10-12,14-17H2,1-3H3/t18-,21-,22-,23-,24?,28-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 4.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Kyoto University
Curated by ChEMBL
| Assay Description
Inhibition of [3H]PDBu binding to PKCalpha C1A domain |
J Med Chem 55: 5614-26 (2012)
Article DOI: 10.1021/jm300566h
BindingDB Entry DOI: 10.7270/Q200036J |
More data for this
Ligand-Target Pair | |
Free fatty acid receptor 1
(Rattus norvegicus) | BDBM50386649

(CHEMBL2048626)Show SMILES Cc1cc(OC2CCS(=O)(=O)CC2)cc(C)c1-c1cccc(CNc2ccc(CCC(O)=O)c(F)c2)c1 Show InChI InChI=1S/C29H32FNO5S/c1-19-14-26(36-25-10-12-37(34,35)13-11-25)15-20(2)29(19)23-5-3-4-21(16-23)18-31-24-8-6-22(27(30)17-24)7-9-28(32)33/h3-6,8,14-17,25,31H,7,9-13,18H2,1-2H3,(H,32,33) | KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 5.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company, Ltd.
Curated by ChEMBL
| Assay Description
Displacement of 3-[4-({2',6'-dimethyl-6-[(4-[3H])phenylmethoxy]biphenyl-3-yl}methoxy)phenyl] propanoic acid from rat GPR40 receptor expressed in CHO ... |
J Med Chem 55: 3756-76 (2012)
Article DOI: 10.1021/jm2016123
BindingDB Entry DOI: 10.7270/Q2N58NDH |
More data for this
Ligand-Target Pair | |
Protein kinase C gamma type
(Homo sapiens (Human)) | BDBM50391388

(CHEMBL2148108)Show SMILES C[C@@H]1[C@@H](CCCCc2cccc(O)c2)O[C@]23C[C@@H]1OC(=O)C[C@H](CO)OC(=O)C[C@@H](CCC2(C)C)O3 |r| Show InChI InChI=1S/C28H40O8/c1-18-23(10-5-4-7-19-8-6-9-20(30)13-19)36-28-16-24(18)34-26(32)15-22(17-29)33-25(31)14-21(35-28)11-12-27(28,2)3/h6,8-9,13,18,21-24,29-30H,4-5,7,10-12,14-17H2,1-3H3/t18-,21-,22-,23-,24?,28-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 5.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Kyoto University
Curated by ChEMBL
| Assay Description
Inhibition of [3H]PDBu binding to PKCgamma C1A domain |
J Med Chem 55: 5614-26 (2012)
Article DOI: 10.1021/jm300566h
BindingDB Entry DOI: 10.7270/Q200036J |
More data for this
Ligand-Target Pair | |
Free fatty acid receptor 1
(Rattus norvegicus) | BDBM50386641
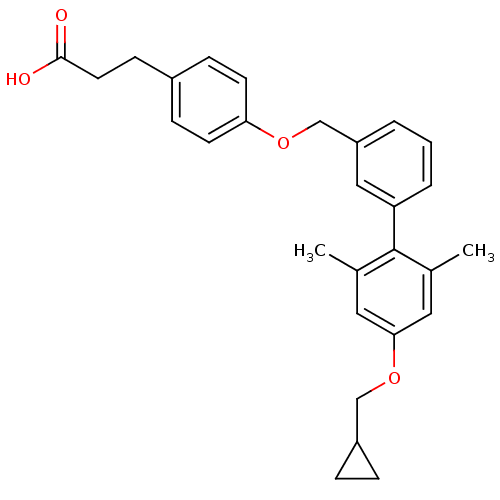
(CHEMBL2048615)Show SMILES Cc1cc(OCC2CC2)cc(C)c1-c1cccc(COc2ccc(CCC(O)=O)cc2)c1 Show InChI InChI=1S/C28H30O4/c1-19-14-26(32-17-22-6-7-22)15-20(2)28(19)24-5-3-4-23(16-24)18-31-25-11-8-21(9-12-25)10-13-27(29)30/h3-5,8-9,11-12,14-16,22H,6-7,10,13,17-18H2,1-2H3,(H,29,30) | KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 5.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company, Ltd.
Curated by ChEMBL
| Assay Description
Displacement of 3-[4-({2',6'-dimethyl-6-[(4-[3H])phenylmethoxy]biphenyl-3-yl}methoxy)phenyl] propanoic acid from rat GPR40 receptor expressed in CHO ... |
J Med Chem 55: 3756-76 (2012)
Article DOI: 10.1021/jm2016123
BindingDB Entry DOI: 10.7270/Q2N58NDH |
More data for this
Ligand-Target Pair | |
Protein kinase C eta type
(Homo sapiens (Human)) | BDBM50391387

(CHEMBL2148107)Show SMILES C[C@@H]1CC(C)(C)[C@@]23C[C@@H](C[C@@H](CCCCc4cccc(O)c4)O2)OC(=O)C[C@H](CO)OC(=O)C[C@@H]1O3 |r| Show InChI InChI=1S/C28H40O8/c1-18-15-27(2,3)28-16-22(33-25(31)13-23(17-29)34-26(32)14-24(18)36-28)12-21(35-28)10-5-4-7-19-8-6-9-20(30)11-19/h6,8-9,11,18,21-24,29-30H,4-5,7,10,12-17H2,1-3H3/t18-,21-,22?,23-,24+,28+/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 5.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Kyoto University
Curated by ChEMBL
| Assay Description
Inhibition of [3H]PDBu binding to PKCeta C1B domain |
J Med Chem 55: 5614-26 (2012)
Article DOI: 10.1021/jm300566h
BindingDB Entry DOI: 10.7270/Q200036J |
More data for this
Ligand-Target Pair | |
Free fatty acid receptor 1
(Rattus norvegicus) | BDBM50386651
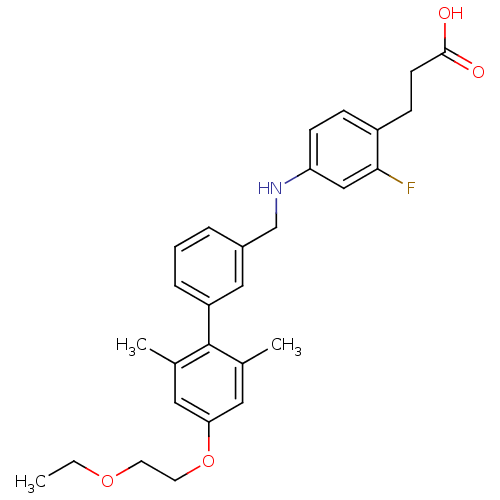
(CHEMBL2048628)Show SMILES CCOCCOc1cc(C)c(c(C)c1)-c1cccc(CNc2ccc(CCC(O)=O)c(F)c2)c1 Show InChI InChI=1S/C28H32FNO4/c1-4-33-12-13-34-25-14-19(2)28(20(3)15-25)23-7-5-6-21(16-23)18-30-24-10-8-22(26(29)17-24)9-11-27(31)32/h5-8,10,14-17,30H,4,9,11-13,18H2,1-3H3,(H,31,32) | KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| 6.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company, Ltd.
Curated by ChEMBL
| Assay Description
Displacement of 3-[4-({2',6'-dimethyl-6-[(4-[3H])phenylmethoxy]biphenyl-3-yl}methoxy)phenyl] propanoic acid from rat GPR40 receptor expressed in CHO ... |
J Med Chem 55: 3756-76 (2012)
Article DOI: 10.1021/jm2016123
BindingDB Entry DOI: 10.7270/Q2N58NDH |
More data for this
Ligand-Target Pair | |
Free fatty acid receptor 1
(Rattus norvegicus) | BDBM50386640

(CHEMBL2048614)Show SMILES COc1cc(C)c(c(C)c1)-c1cccc(COc2ccc(CCC(O)=O)cc2)c1 Show InChI InChI=1S/C25H26O4/c1-17-13-23(28-3)14-18(2)25(17)21-6-4-5-20(15-21)16-29-22-10-7-19(8-11-22)9-12-24(26)27/h4-8,10-11,13-15H,9,12,16H2,1-3H3,(H,26,27) | KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 6.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company, Ltd.
Curated by ChEMBL
| Assay Description
Displacement of 3-[4-({2',6'-dimethyl-6-[(4-[3H])phenylmethoxy]biphenyl-3-yl}methoxy)phenyl] propanoic acid from rat GPR40 receptor expressed in CHO ... |
J Med Chem 55: 3756-76 (2012)
Article DOI: 10.1021/jm2016123
BindingDB Entry DOI: 10.7270/Q2N58NDH |
More data for this
Ligand-Target Pair | |
Free fatty acid receptor 1
(Rattus norvegicus) | BDBM50386637

(CHEMBL2048623)Show SMILES Cc1cc(OC2CCS(=O)(=O)CC2)cc(C)c1-c1cccc(COc2ccc(CCC(O)=O)c(F)c2)c1 Show InChI InChI=1S/C29H31FO6S/c1-19-14-26(36-24-10-12-37(33,34)13-11-24)15-20(2)29(19)23-5-3-4-21(16-23)18-35-25-8-6-22(27(30)17-25)7-9-28(31)32/h3-6,8,14-17,24H,7,9-13,18H2,1-2H3,(H,31,32) | KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 6.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company, Ltd.
Curated by ChEMBL
| Assay Description
Displacement of 3-[4-({2',6'-dimethyl-6-[(4-[3H])phenylmethoxy]biphenyl-3-yl}methoxy)phenyl] propanoic acid from rat GPR40 receptor expressed in CHO ... |
J Med Chem 55: 3756-76 (2012)
Article DOI: 10.1021/jm2016123
BindingDB Entry DOI: 10.7270/Q2N58NDH |
More data for this
Ligand-Target Pair | |
Free fatty acid receptor 1
(Rattus norvegicus) | BDBM50386639

(CHEMBL2048619)Show SMILES Cc1cc(OC2CCOCC2)cc(C)c1-c1cccc(COc2ccc(CCC(O)=O)c(F)c2)c1 Show InChI InChI=1S/C29H31FO5/c1-19-14-26(35-24-10-12-33-13-11-24)15-20(2)29(19)23-5-3-4-21(16-23)18-34-25-8-6-22(27(30)17-25)7-9-28(31)32/h3-6,8,14-17,24H,7,9-13,18H2,1-2H3,(H,31,32) | KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| 6.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company, Ltd.
Curated by ChEMBL
| Assay Description
Displacement of 3-[4-({2',6'-dimethyl-6-[(4-[3H])phenylmethoxy]biphenyl-3-yl}methoxy)phenyl] propanoic acid from rat GPR40 receptor expressed in CHO ... |
J Med Chem 55: 3756-76 (2012)
Article DOI: 10.1021/jm2016123
BindingDB Entry DOI: 10.7270/Q2N58NDH |
More data for this
Ligand-Target Pair | |
Free fatty acid receptor 1
(Rattus norvegicus) | BDBM50386638
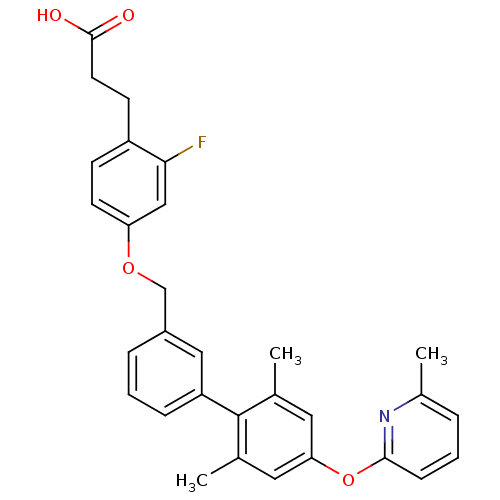
(CHEMBL2048620)Show SMILES Cc1cccc(Oc2cc(C)c(c(C)c2)-c2cccc(COc3ccc(CCC(O)=O)c(F)c3)c2)n1 Show InChI InChI=1S/C30H28FNO4/c1-19-14-26(36-28-9-4-6-21(3)32-28)15-20(2)30(19)24-8-5-7-22(16-24)18-35-25-12-10-23(27(31)17-25)11-13-29(33)34/h4-10,12,14-17H,11,13,18H2,1-3H3,(H,33,34) | KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| 6.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company, Ltd.
Curated by ChEMBL
| Assay Description
Displacement of 3-[4-({2',6'-dimethyl-6-[(4-[3H])phenylmethoxy]biphenyl-3-yl}methoxy)phenyl] propanoic acid from rat GPR40 receptor expressed in CHO ... |
J Med Chem 55: 3756-76 (2012)
Article DOI: 10.1021/jm2016123
BindingDB Entry DOI: 10.7270/Q2N58NDH |
More data for this
Ligand-Target Pair | |
Free fatty acid receptor 1
(Rattus norvegicus) | BDBM50386642

(CHEMBL2048616)Show SMILES Cc1cc(OCc2ccccc2)cc(C)c1-c1cccc(COc2ccc(CCC(O)=O)cc2)c1 Show InChI InChI=1S/C31H30O4/c1-22-17-29(35-20-25-7-4-3-5-8-25)18-23(2)31(22)27-10-6-9-26(19-27)21-34-28-14-11-24(12-15-28)13-16-30(32)33/h3-12,14-15,17-19H,13,16,20-21H2,1-2H3,(H,32,33) | KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 6.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company, Ltd.
Curated by ChEMBL
| Assay Description
Displacement of 3-[4-({2',6'-dimethyl-6-[(4-[3H])phenylmethoxy]biphenyl-3-yl}methoxy)phenyl] propanoic acid from rat GPR40 receptor expressed in CHO ... |
J Med Chem 55: 3756-76 (2012)
Article DOI: 10.1021/jm2016123
BindingDB Entry DOI: 10.7270/Q2N58NDH |
More data for this
Ligand-Target Pair | |
Protein kinase C delta type
(Homo sapiens (Human)) | BDBM50491406
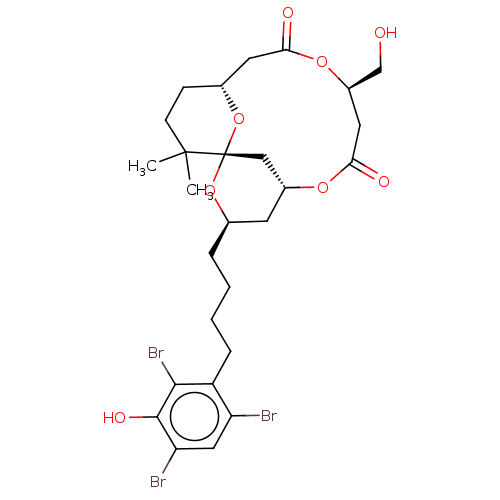
(CHEMBL2381164)Show SMILES [H][C@@]12CCC(C)(C)[C@]3(C[C@@]([H])(C[C@@H](CCCCc4c(Br)cc(Br)c(O)c4Br)O3)OC(=O)C[C@H](CO)OC(=O)C1)O2 |r| Show InChI InChI=1S/C27H35Br3O8/c1-26(2)8-7-16-10-22(32)36-18(14-31)11-23(33)35-17-9-15(37-27(26,13-17)38-16)5-3-4-6-19-20(28)12-21(29)25(34)24(19)30/h12,15-18,31,34H,3-11,13-14H2,1-2H3/t15-,16-,17-,18-,27+/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 6.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Kyoto University
Curated by ChEMBL
| Assay Description
Inhibition of [3H]PDBu binding to PKCdelta-C1B domain peptide (unknown origin) |
Bioorg Med Chem 21: 2695-702 (2013)
Article DOI: 10.1016/j.bmc.2013.03.013
BindingDB Entry DOI: 10.7270/Q26M39RD |
More data for this
Ligand-Target Pair | |
Free fatty acid receptor 1
(Homo sapiens (Human)) | BDBM50382530

(CHEMBL2022256)Show SMILES Cc1cccc(C)c1-c1cc(COc2ccc3C(CC(O)=O)COc3c2)ccc1OCc1ccccc1 |(29.58,-44.67,;29.58,-46.21,;28.25,-46.98,;28.26,-48.52,;29.6,-49.29,;30.93,-48.51,;32.27,-49.27,;30.92,-46.98,;32.25,-46.2,;33.58,-46.97,;34.91,-46.2,;36.24,-46.97,;37.58,-46.19,;38.91,-46.96,;38.91,-48.5,;40.25,-49.26,;41.58,-48.48,;43.05,-48.94,;43.53,-50.41,;45.04,-50.72,;45.53,-52.18,;46.06,-49.56,;43.94,-47.69,;43.03,-46.45,;41.57,-46.94,;40.23,-46.18,;34.9,-44.66,;33.56,-43.89,;32.23,-44.67,;30.89,-43.91,;30.88,-42.37,;29.54,-41.61,;29.53,-40.08,;28.19,-39.32,;26.87,-40.1,;26.88,-41.65,;28.22,-42.4,)| Show InChI InChI=1S/C32H30O5/c1-21-7-6-8-22(2)32(21)28-15-24(11-14-29(28)36-18-23-9-4-3-5-10-23)19-35-26-12-13-27-25(16-31(33)34)20-37-30(27)17-26/h3-15,17,25H,16,18-20H2,1-2H3,(H,33,34) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 6.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company Limited
Curated by ChEMBL
| Assay Description
Displacement of 3-[4-({2',6'-dimethyl-6-[(4-[3H])-phenylmethoxy]biphenyl-3-yl}methoxy)phenyl] propanoic acid from human GPR40 receptor expressed in C... |
J Med Chem 55: 1538-52 (2012)
Article DOI: 10.1021/jm2012968
BindingDB Entry DOI: 10.7270/Q2HQ40X4 |
More data for this
Ligand-Target Pair | |
Protein kinase C delta type
(Homo sapiens (Human)) | BDBM50327946

(1S,3R,5R,9R,13R)-9-Hydroxymethyl-3-[4-(3-hydroxy-p...)Show SMILES CC1(C)CC[C@@H]2CC(=O)O[C@@H](CO)CC(=O)O[C@@H]3C[C@@H](CCCCc4cccc(O)c4)O[C@@]1(C3)O2 |r| Show InChI InChI=1S/C27H38O8/c1-26(2)11-10-21-14-24(30)33-23(17-28)15-25(31)32-22-13-20(34-27(26,16-22)35-21)9-4-3-6-18-7-5-8-19(29)12-18/h5,7-8,12,20-23,28-29H,3-4,6,9-11,13-17H2,1-2H3/t20-,21-,22?,23-,27+/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 7.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Kyoto University
Curated by ChEMBL
| Assay Description
Inhibition of [3H]PDBu binding to PKCdelta-C1B domain peptide (unknown origin) |
Bioorg Med Chem 21: 2695-702 (2013)
Article DOI: 10.1016/j.bmc.2013.03.013
BindingDB Entry DOI: 10.7270/Q26M39RD |
More data for this
Ligand-Target Pair | |
Protein kinase C delta type
(Homo sapiens (Human)) | BDBM50327946

(1S,3R,5R,9R,13R)-9-Hydroxymethyl-3-[4-(3-hydroxy-p...)Show SMILES CC1(C)CC[C@@H]2CC(=O)O[C@@H](CO)CC(=O)O[C@@H]3C[C@@H](CCCCc4cccc(O)c4)O[C@@]1(C3)O2 |r| Show InChI InChI=1S/C27H38O8/c1-26(2)11-10-21-14-24(30)33-23(17-28)15-25(31)32-22-13-20(34-27(26,16-22)35-21)9-4-3-6-18-7-5-8-19(29)12-18/h5,7-8,12,20-23,28-29H,3-4,6,9-11,13-17H2,1-2H3/t20-,21-,22?,23-,27+/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 7.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Kyoto University
Curated by ChEMBL
| Assay Description
Inhibition of [3H]PDBu binding to PKCdelta C1B domain |
J Med Chem 55: 5614-26 (2012)
Article DOI: 10.1021/jm300566h
BindingDB Entry DOI: 10.7270/Q200036J |
More data for this
Ligand-Target Pair | |
Free fatty acid receptor 1
(Rattus norvegicus) | BDBM50386647

(CHEMBL2048624)Show SMILES Cc1cc(OCC2(O)CCS(=O)(=O)CC2)cc(C)c1-c1cccc(COc2ccc(CCC(O)=O)c(F)c2)c1 Show InChI InChI=1S/C30H33FO7S/c1-20-14-26(38-19-30(34)10-12-39(35,36)13-11-30)15-21(2)29(20)24-5-3-4-22(16-24)18-37-25-8-6-23(27(31)17-25)7-9-28(32)33/h3-6,8,14-17,34H,7,9-13,18-19H2,1-2H3,(H,32,33) | KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 8.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company, Ltd.
Curated by ChEMBL
| Assay Description
Displacement of 3-[4-({2',6'-dimethyl-6-[(4-[3H])phenylmethoxy]biphenyl-3-yl}methoxy)phenyl] propanoic acid from rat GPR40 receptor expressed in CHO ... |
J Med Chem 55: 3756-76 (2012)
Article DOI: 10.1021/jm2016123
BindingDB Entry DOI: 10.7270/Q2N58NDH |
More data for this
Ligand-Target Pair | |
Melanin-concentrating hormone receptor 1
(RAT) | BDBM50150715
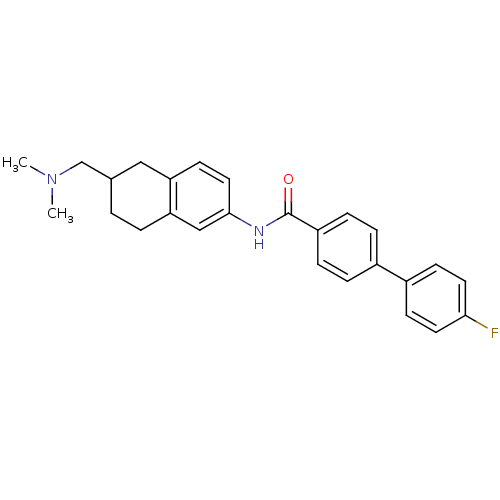
(4''-Fluoro-biphenyl-4-carboxylic acid (6-dimethyla...)Show SMILES CN(C)CC1CCc2cc(NC(=O)c3ccc(cc3)-c3ccc(F)cc3)ccc2C1 Show InChI InChI=1S/C26H27FN2O/c1-29(2)17-18-3-4-23-16-25(14-11-22(23)15-18)28-26(30)21-7-5-19(6-8-21)20-9-12-24(27)13-10-20/h5-14,16,18H,3-4,15,17H2,1-2H3,(H,28,30) | Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 8.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Chemical Industries, Ltd.
Curated by PDSP Ki Database
| |
Eur J Pharmacol 438: 129-35 (2002)
Article DOI: 10.1016/s0014-2999(02)01314-6
BindingDB Entry DOI: 10.7270/Q2GX495R |
More data for this
Ligand-Target Pair | |
Free fatty acid receptor 1
(Homo sapiens (Human)) | BDBM50386792
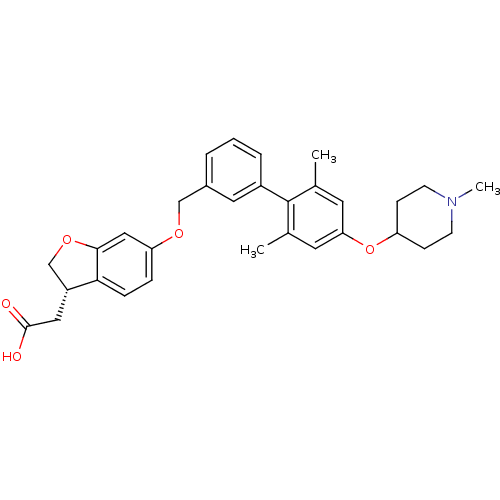
(CHEMBL2047161)Show SMILES CN1CCC(CC1)Oc1cc(C)c(c(C)c1)-c1cccc(COc2ccc3[C@H](CC(O)=O)COc3c2)c1 |r| Show InChI InChI=1S/C31H35NO5/c1-20-13-27(37-25-9-11-32(3)12-10-25)14-21(2)31(20)23-6-4-5-22(15-23)18-35-26-7-8-28-24(16-30(33)34)19-36-29(28)17-26/h4-8,13-15,17,24-25H,9-12,16,18-19H2,1-3H3,(H,33,34)/t24-/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 8.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company Limited
Curated by ChEMBL
| Assay Description
Displacement of 3-[4-({2',6'-dimethyl-6-[(4-[3H])-phenylmethoxy]biphenyl-3-yl}methoxy)phenyl]propanoic acid from human GPR40 expressed in CHO cells a... |
J Med Chem 55: 3960-74 (2012)
Article DOI: 10.1021/jm300170m
BindingDB Entry DOI: 10.7270/Q23779R6 |
More data for this
Ligand-Target Pair | |
Protein kinase C delta type
(Homo sapiens (Human)) | BDBM50391386

(CHEMBL2148106)Show SMILES CO[C@@H](CC[C@H](C)[C@H]1O[C@@]23C[C@H](OC(=O)C[C@@H](OC(=O)C[C@](O)(O2)[C@H](C)CC3(C)C)[C@@H](C)O)[C@@H]1C)c1cccc(O)c1 |r| Show InChI InChI=1S/C32H48O10/c1-18(11-12-24(38-7)22-9-8-10-23(34)13-22)29-20(3)26-16-32(41-29)30(5,6)15-19(2)31(37,42-32)17-28(36)39-25(21(4)33)14-27(35)40-26/h8-10,13,18-21,24-26,29,33-34,37H,11-12,14-17H2,1-7H3/t18-,19+,20-,21+,24-,25+,26?,29+,31-,32-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 9.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Kyoto University
Curated by ChEMBL
| Assay Description
Inhibition of [3H]PDBu binding to PKCdelta-C1A domain peptide (unknown origin) |
Bioorg Med Chem 21: 2695-702 (2013)
Article DOI: 10.1016/j.bmc.2013.03.013
BindingDB Entry DOI: 10.7270/Q26M39RD |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data