 Found 785 hits with Last Name = 'swain' and Initial = 'a'
Found 785 hits with Last Name = 'swain' and Initial = 'a' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Histamine H4 receptor
(Homo sapiens (Human)) | BDBM50356880
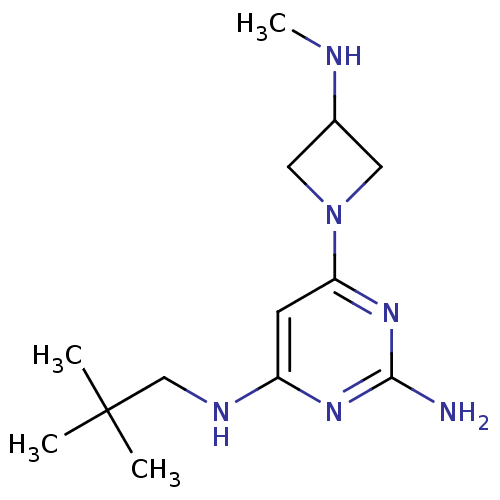
(CHEMBL1915536)Show InChI InChI=1S/C13H24N6/c1-13(2,3)8-16-10-5-11(18-12(14)17-10)19-6-9(7-19)15-4/h5,9,15H,6-8H2,1-4H3,(H3,14,16,17,18) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.0340 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Antagonist activity at full length human H4R expressed in HEK293 cells assessed as reversal of forskolin-induced cAMP production by CRE-beta-lactamas... |
Bioorg Med Chem Lett 21: 6596-602 (2011)
Article DOI: 10.1016/j.bmcl.2011.07.125
BindingDB Entry DOI: 10.7270/Q20C4W6C |
More data for this
Ligand-Target Pair | |
Neuronal acetylcholine receptor subunit alpha-4/beta-2
(Rattus norvegicus (Rat)) | BDBM50066789
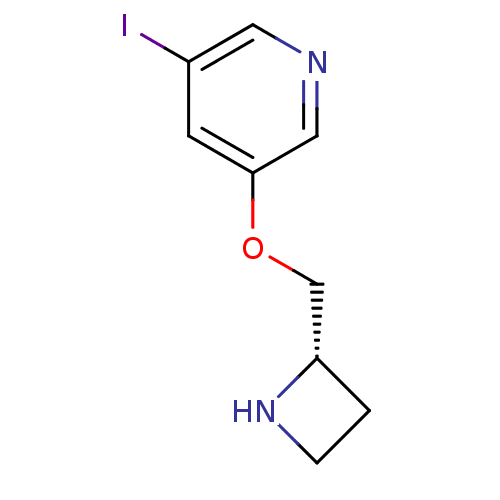
(3-((S)-1-Azetidin-2-ylmethoxy)-5-iodo-pyridine | A...)Show InChI InChI=1S/C9H11IN2O/c10-7-3-9(5-11-4-7)13-6-8-1-2-12-8/h3-5,8,12H,1-2,6H2/t8-/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.0500 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Displacement of [3H]epibatidine from alpha4beta2 nAChR in rat brain membrane |
J Med Chem 56: 593-624 (2013)
Article DOI: 10.1021/jm3011433
BindingDB Entry DOI: 10.7270/Q23B61GZ |
More data for this
Ligand-Target Pair | |
Neuronal acetylcholine receptor subunit alpha-4/beta-2
(Homo sapiens (Human)) | BDBM50166908

(5,8,14-triazatetracyclo[10.3.1.02,11.04,9]hexadeca...)Show InChI InChI=1S/C13H13N3/c1-2-16-13-5-11-9-3-8(6-14-7-9)10(11)4-12(13)15-1/h1-2,4-5,8-9,14H,3,6-7H2 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| 0.0600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Binding affinity to alpha4beta2 nAChR in rat cortex |
J Med Chem 56: 593-624 (2013)
Article DOI: 10.1021/jm3011433
BindingDB Entry DOI: 10.7270/Q23B61GZ |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Gamma-aminobutyric acid receptor subunit alpha-5
(Homo sapiens (Human)) | BDBM50067424
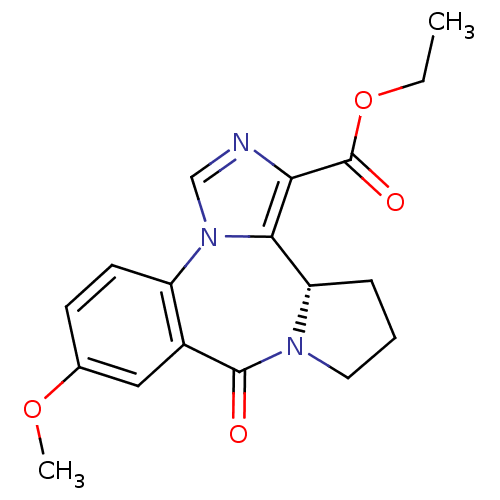
((S)-9-Methoxy-7-oxo-3b,4,5,6-tetrahydro-7H-2,6a,11...)Show SMILES CCOC(=O)c1ncn-2c1[C@@H]1CCCN1C(=O)c1cc(OC)ccc-21 Show InChI InChI=1S/C18H19N3O4/c1-3-25-18(23)15-16-14-5-4-8-20(14)17(22)12-9-11(24-2)6-7-13(12)21(16)10-19-15/h6-7,9-10,14H,3-5,8H2,1-2H3/t14-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| 0.450 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Positive allosteric modulation of GABAA alpha5 (unknown origin) |
J Med Chem 56: 593-624 (2013)
Article DOI: 10.1021/jm3011433
BindingDB Entry DOI: 10.7270/Q23B61GZ |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Gamma-aminobutyric acid receptor subunit alpha-3
(Homo sapiens (Human)) | BDBM50142570
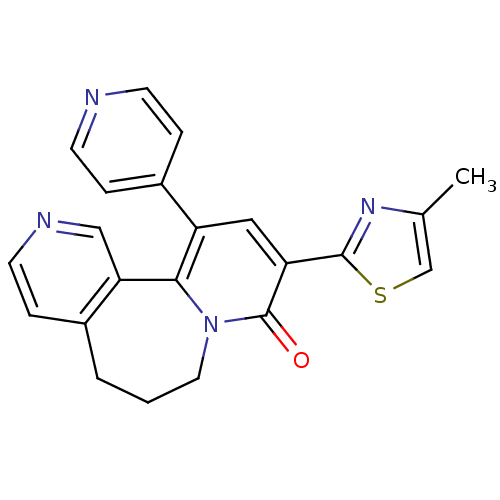
(9-(4-Methyl-thiazol-2-yl)-11-pyridin-4-yl-6,7-dihy...)Show SMILES Cc1csc(n1)-c1cc(-c2ccncc2)c2-c3cnccc3CCCn2c1=O Show InChI InChI=1S/C22H18N4OS/c1-14-13-28-21(25-14)18-11-17(16-4-7-23-8-5-16)20-19-12-24-9-6-15(19)3-2-10-26(20)22(18)27/h4-9,11-13H,2-3,10H2,1H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Positive allosteric modulation of GABAA alpha3 (unknown origin) |
J Med Chem 56: 593-624 (2013)
Article DOI: 10.1021/jm3011433
BindingDB Entry DOI: 10.7270/Q23B61GZ |
More data for this
Ligand-Target Pair | |
Gamma-aminobutyric acid receptor subunit alpha-2
(Homo sapiens (Human)) | BDBM50142570

(9-(4-Methyl-thiazol-2-yl)-11-pyridin-4-yl-6,7-dihy...)Show SMILES Cc1csc(n1)-c1cc(-c2ccncc2)c2-c3cnccc3CCCn2c1=O Show InChI InChI=1S/C22H18N4OS/c1-14-13-28-21(25-14)18-11-17(16-4-7-23-8-5-16)20-19-12-24-9-6-15(19)3-2-10-26(20)22(18)27/h4-9,11-13H,2-3,10H2,1H3 | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Positive allosteric modulation of GABAA alpha2 (unknown origin) |
J Med Chem 56: 593-624 (2013)
Article DOI: 10.1021/jm3011433
BindingDB Entry DOI: 10.7270/Q23B61GZ |
More data for this
Ligand-Target Pair | |
Gamma-aminobutyric acid receptor subunit alpha-3
(Homo sapiens (Human)) | BDBM50179998
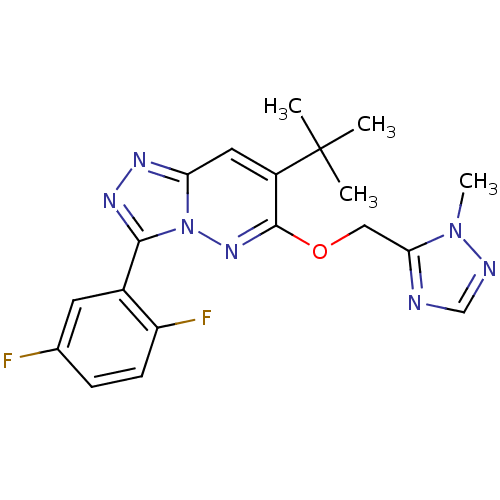
(7-tert-butyl-3-(2,5-difluorophenyl)-6-((2-methyl-2...)Show SMILES Cn1ncnc1COc1nn2c(nnc2cc1C(C)(C)C)-c1cc(F)ccc1F Show InChI InChI=1S/C19H19F2N7O/c1-19(2,3)13-8-15-24-25-17(12-7-11(20)5-6-14(12)21)28(15)26-18(13)29-9-16-22-10-23-27(16)4/h5-8,10H,9H2,1-4H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.700 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Positive allosteric modulation of GABAA alpha3 (unknown origin) |
J Med Chem 56: 593-624 (2013)
Article DOI: 10.1021/jm3011433
BindingDB Entry DOI: 10.7270/Q23B61GZ |
More data for this
Ligand-Target Pair | |
Gamma-aminobutyric acid receptor subunit alpha-2
(Homo sapiens (Human)) | BDBM50179998

(7-tert-butyl-3-(2,5-difluorophenyl)-6-((2-methyl-2...)Show SMILES Cn1ncnc1COc1nn2c(nnc2cc1C(C)(C)C)-c1cc(F)ccc1F Show InChI InChI=1S/C19H19F2N7O/c1-19(2,3)13-8-15-24-25-17(12-7-11(20)5-6-14(12)21)28(15)26-18(13)29-9-16-22-10-23-27(16)4/h5-8,10H,9H2,1-4H3 | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.700 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Positive allosteric modulation of GABAA alpha2 (unknown origin) |
J Med Chem 56: 593-624 (2013)
Article DOI: 10.1021/jm3011433
BindingDB Entry DOI: 10.7270/Q23B61GZ |
More data for this
Ligand-Target Pair | |
Gamma-aminobutyric acid receptor subunit alpha-3
(Homo sapiens (Human)) | BDBM50156083

(3-tert-Butyl-7-(5-methyl-isoxazol-3-yl)-2-(2-methy...)Show SMILES Cc1cc(no1)-c1nncc2c(c(OCc3ncnn3C)nn12)C(C)(C)C Show InChI InChI=1S/C17H20N8O2/c1-10-6-11(23-27-10)15-21-19-7-12-14(17(2,3)4)16(22-25(12)15)26-8-13-18-9-20-24(13)5/h6-7,9H,8H2,1-5H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.800 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Positive allosteric modulation of GABAA alpha3 (unknown origin) |
J Med Chem 56: 593-624 (2013)
Article DOI: 10.1021/jm3011433
BindingDB Entry DOI: 10.7270/Q23B61GZ |
More data for this
Ligand-Target Pair | |
Gamma-aminobutyric acid receptor subunit alpha-1
(Homo sapiens (Human)) | BDBM50156083

(3-tert-Butyl-7-(5-methyl-isoxazol-3-yl)-2-(2-methy...)Show SMILES Cc1cc(no1)-c1nncc2c(c(OCc3ncnn3C)nn12)C(C)(C)C Show InChI InChI=1S/C17H20N8O2/c1-10-6-11(23-27-10)15-21-19-7-12-14(17(2,3)4)16(22-25(12)15)26-8-13-18-9-20-24(13)5/h6-7,9H,8H2,1-5H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.800 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Positive allosteric modulation of GABAA alpha1 (unknown origin) |
J Med Chem 56: 593-624 (2013)
Article DOI: 10.1021/jm3011433
BindingDB Entry DOI: 10.7270/Q23B61GZ |
More data for this
Ligand-Target Pair | |
Gamma-aminobutyric acid receptor subunit alpha-2
(Homo sapiens (Human)) | BDBM50156083

(3-tert-Butyl-7-(5-methyl-isoxazol-3-yl)-2-(2-methy...)Show SMILES Cc1cc(no1)-c1nncc2c(c(OCc3ncnn3C)nn12)C(C)(C)C Show InChI InChI=1S/C17H20N8O2/c1-10-6-11(23-27-10)15-21-19-7-12-14(17(2,3)4)16(22-25(12)15)26-8-13-18-9-20-24(13)5/h6-7,9H,8H2,1-5H3 | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.800 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Positive allosteric modulation of GABAA alpha2 (unknown origin) |
J Med Chem 56: 593-624 (2013)
Article DOI: 10.1021/jm3011433
BindingDB Entry DOI: 10.7270/Q23B61GZ |
More data for this
Ligand-Target Pair | |
Gamma-aminobutyric acid receptor subunit alpha-1
(Homo sapiens (Human)) | BDBM50142570

(9-(4-Methyl-thiazol-2-yl)-11-pyridin-4-yl-6,7-dihy...)Show SMILES Cc1csc(n1)-c1cc(-c2ccncc2)c2-c3cnccc3CCCn2c1=O Show InChI InChI=1S/C22H18N4OS/c1-14-13-28-21(25-14)18-11-17(16-4-7-23-8-5-16)20-19-12-24-9-6-15(19)3-2-10-26(20)22(18)27/h4-9,11-13H,2-3,10H2,1H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 1.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Positive allosteric modulation of GABAA alpha1 (unknown origin) |
J Med Chem 56: 593-624 (2013)
Article DOI: 10.1021/jm3011433
BindingDB Entry DOI: 10.7270/Q23B61GZ |
More data for this
Ligand-Target Pair | |
Gamma-aminobutyric acid receptor subunit alpha-5
(Homo sapiens (Human)) | BDBM50156083

(3-tert-Butyl-7-(5-methyl-isoxazol-3-yl)-2-(2-methy...)Show SMILES Cc1cc(no1)-c1nncc2c(c(OCc3ncnn3C)nn12)C(C)(C)C Show InChI InChI=1S/C17H20N8O2/c1-10-6-11(23-27-10)15-21-19-7-12-14(17(2,3)4)16(22-25(12)15)26-8-13-18-9-20-24(13)5/h6-7,9H,8H2,1-5H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 1.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Positive allosteric modulation of GABAA alpha5 (unknown origin) |
J Med Chem 56: 593-624 (2013)
Article DOI: 10.1021/jm3011433
BindingDB Entry DOI: 10.7270/Q23B61GZ |
More data for this
Ligand-Target Pair | |
Histamine H4 receptor
(Homo sapiens (Human)) | BDBM50356881

(CHEMBL1915537)Show InChI InChI=1S/C14H26N6/c1-14(2,3)5-6-17-11-7-12(19-13(15)18-11)20-8-10(9-20)16-4/h7,10,16H,5-6,8-9H2,1-4H3,(H3,15,17,18,19) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 1.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Antagonist activity at full length human H4R expressed in HEK293 cells assessed as reversal of forskolin-induced cAMP production by CRE-beta-lactamas... |
Bioorg Med Chem Lett 21: 6596-602 (2011)
Article DOI: 10.1016/j.bmcl.2011.07.125
BindingDB Entry DOI: 10.7270/Q20C4W6C |
More data for this
Ligand-Target Pair | |
Histamine H4 receptor
(Homo sapiens (Human)) | BDBM50356884

(CHEMBL1915540)Show InChI InChI=1S/C13H22N6/c1-15-10-4-5-19(8-10)12-6-11(17-13(14)18-12)16-7-9-2-3-9/h6,9-10,15H,2-5,7-8H2,1H3,(H3,14,16,17,18)/t10-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 1.56 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Antagonist activity at full length human H4R expressed in HEK293 cells assessed as reversal of forskolin-induced cAMP production by CRE-beta-lactamas... |
Bioorg Med Chem Lett 21: 6596-602 (2011)
Article DOI: 10.1016/j.bmcl.2011.07.125
BindingDB Entry DOI: 10.7270/Q20C4W6C |
More data for this
Ligand-Target Pair | |
Histamine H4 receptor
(Homo sapiens (Human)) | BDBM50356880

(CHEMBL1915536)Show InChI InChI=1S/C13H24N6/c1-13(2,3)8-16-10-5-11(18-12(14)17-10)19-6-9(7-19)15-4/h5,9,15H,6-8H2,1-4H3,(H3,14,16,17,18) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 1.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Displacement of [3H]-histamine from full length human H4R expressed in HEK293 cells |
Bioorg Med Chem Lett 21: 6596-602 (2011)
Article DOI: 10.1016/j.bmcl.2011.07.125
BindingDB Entry DOI: 10.7270/Q20C4W6C |
More data for this
Ligand-Target Pair | |
Gamma-aminobutyric acid receptor subunit alpha-5
(Homo sapiens (Human)) | BDBM50179998

(7-tert-butyl-3-(2,5-difluorophenyl)-6-((2-methyl-2...)Show SMILES Cn1ncnc1COc1nn2c(nnc2cc1C(C)(C)C)-c1cc(F)ccc1F Show InChI InChI=1S/C19H19F2N7O/c1-19(2,3)13-8-15-24-25-17(12-7-11(20)5-6-14(12)21)28(15)26-18(13)29-9-16-22-10-23-27(16)4/h5-8,10H,9H2,1-4H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 2.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Positive allosteric modulation of GABAA alpha5 (unknown origin) |
J Med Chem 56: 593-624 (2013)
Article DOI: 10.1021/jm3011433
BindingDB Entry DOI: 10.7270/Q23B61GZ |
More data for this
Ligand-Target Pair | |
Histamine H4 receptor
(Homo sapiens (Human)) | BDBM50356884

(CHEMBL1915540)Show InChI InChI=1S/C13H22N6/c1-15-10-4-5-19(8-10)12-6-11(17-13(14)18-12)16-7-9-2-3-9/h6,9-10,15H,2-5,7-8H2,1H3,(H3,14,16,17,18)/t10-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 2.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Displacement of [3H]-histamine from full length human H4R expressed in HEK293 cells |
Bioorg Med Chem Lett 21: 6596-602 (2011)
Article DOI: 10.1016/j.bmcl.2011.07.125
BindingDB Entry DOI: 10.7270/Q20C4W6C |
More data for this
Ligand-Target Pair | |
Histamine H4 receptor
(Mus musculus (mouse)) | BDBM22566

(5-chloro-2-[(4-methylpiperazin-1-yl)carbonyl]-1H-i...)Show InChI InChI=1S/C14H16ClN3O/c1-17-4-6-18(7-5-17)14(19)13-9-10-8-11(15)2-3-12(10)16-13/h2-3,8-9,16H,4-7H2,1H3 | Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 3.89 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Displacement of [3H]-histamine from mouse H4R expressed in HEK293T cells |
Bioorg Med Chem Lett 21: 6596-602 (2011)
Article DOI: 10.1016/j.bmcl.2011.07.125
BindingDB Entry DOI: 10.7270/Q20C4W6C |
More data for this
Ligand-Target Pair | |
Histamine H4 receptor
(Homo sapiens (Human)) | BDBM22566

(5-chloro-2-[(4-methylpiperazin-1-yl)carbonyl]-1H-i...)Show InChI InChI=1S/C14H16ClN3O/c1-17-4-6-18(7-5-17)14(19)13-9-10-8-11(15)2-3-12(10)16-13/h2-3,8-9,16H,4-7H2,1H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Binding affinity to human histamine H4 receptor |
Bioorg Med Chem Lett 22: 1156-9 (2012)
Article DOI: 10.1016/j.bmcl.2011.11.098
BindingDB Entry DOI: 10.7270/Q2Q81DJD |
More data for this
Ligand-Target Pair | |
Histamine H4 receptor
(Rattus norvegicus (rat)) | BDBM22566

(5-chloro-2-[(4-methylpiperazin-1-yl)carbonyl]-1H-i...)Show InChI InChI=1S/C14H16ClN3O/c1-17-4-6-18(7-5-17)14(19)13-9-10-8-11(15)2-3-12(10)16-13/h2-3,8-9,16H,4-7H2,1H3 | KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 4.27 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Displacement of [3H]-histamine from rat H4R expressed in HEK293T cells |
Bioorg Med Chem Lett 21: 6596-602 (2011)
Article DOI: 10.1016/j.bmcl.2011.07.125
BindingDB Entry DOI: 10.7270/Q20C4W6C |
More data for this
Ligand-Target Pair | |
Histamine H4 receptor
(Homo sapiens (Human)) | BDBM50362214
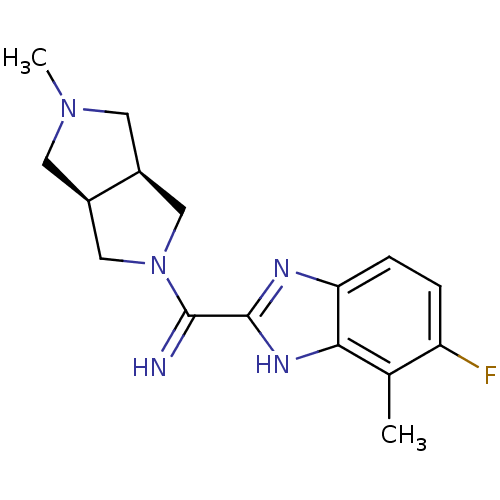
(CHEMBL1938975)Show SMILES CN1C[C@H]2CN(C[C@H]2C1)C(=N)c1nc2ccc(F)c(C)c2[nH]1 |r| Show InChI InChI=1S/C16H20FN5/c1-9-12(17)3-4-13-14(9)20-16(19-13)15(18)22-7-10-5-21(2)6-11(10)8-22/h3-4,10-11,18H,5-8H2,1-2H3,(H,19,20)/t10-,11+ | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 4.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Displacement of [3H]histamine from human recombinant histamine H4 receptor expressed in CHO cells coexpressing Ga15 by radioligand filtration binding... |
Bioorg Med Chem Lett 22: 1156-9 (2012)
Article DOI: 10.1016/j.bmcl.2011.11.098
BindingDB Entry DOI: 10.7270/Q2Q81DJD |
More data for this
Ligand-Target Pair | |
Histamine H4 receptor
(Homo sapiens (Human)) | BDBM50362216

(CHEMBL1938977)Show SMILES CN1C[C@H]2CN(C[C@H]2C1)C(=N)c1nc2ccc(F)cc2[nH]1 |r| Show InChI InChI=1S/C15H18FN5/c1-20-5-9-7-21(8-10(9)6-20)14(17)15-18-12-3-2-11(16)4-13(12)19-15/h2-4,9-10,17H,5-8H2,1H3,(H,18,19)/t9-,10+ | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 4.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Displacement of [3H]histamine from human recombinant histamine H4 receptor expressed in CHO cells coexpressing Ga15 by radioligand filtration binding... |
Bioorg Med Chem Lett 22: 1156-9 (2012)
Article DOI: 10.1016/j.bmcl.2011.11.098
BindingDB Entry DOI: 10.7270/Q2Q81DJD |
More data for this
Ligand-Target Pair | |
Histamine H4 receptor
(Homo sapiens (Human)) | BDBM50356881

(CHEMBL1915537)Show InChI InChI=1S/C14H26N6/c1-14(2,3)5-6-17-11-7-12(19-13(15)18-11)20-8-10(9-20)16-4/h7,10,16H,5-6,8-9H2,1-4H3,(H3,15,17,18,19) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 4.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Displacement of [3H]-histamine from full length human H4R expressed in HEK293 cells |
Bioorg Med Chem Lett 21: 6596-602 (2011)
Article DOI: 10.1016/j.bmcl.2011.07.125
BindingDB Entry DOI: 10.7270/Q20C4W6C |
More data for this
Ligand-Target Pair | |
Histamine H4 receptor
(Homo sapiens (Human)) | BDBM50362218
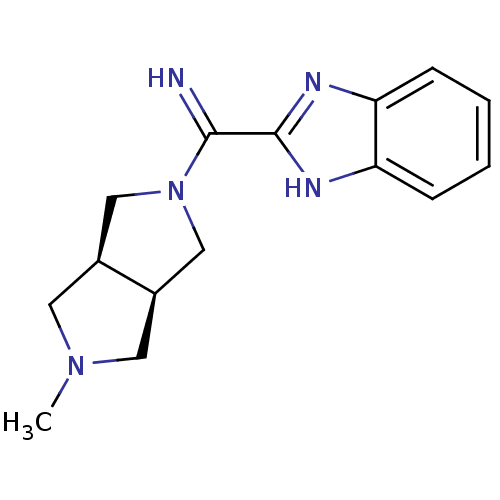
(CHEMBL1938979)Show SMILES CN1C[C@H]2CN(C[C@H]2C1)C(=N)c1nc2ccccc2[nH]1 |r| Show InChI InChI=1S/C15H19N5/c1-19-6-10-8-20(9-11(10)7-19)14(16)15-17-12-4-2-3-5-13(12)18-15/h2-5,10-11,16H,6-9H2,1H3,(H,17,18)/t10-,11+ | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 6.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Displacement of [3H]histamine from human recombinant histamine H4 receptor expressed in CHO cells coexpressing Ga15 by radioligand filtration binding... |
Bioorg Med Chem Lett 22: 1156-9 (2012)
Article DOI: 10.1016/j.bmcl.2011.11.098
BindingDB Entry DOI: 10.7270/Q2Q81DJD |
More data for this
Ligand-Target Pair | |
Histamine H4 receptor
(Homo sapiens (Human)) | BDBM22566

(5-chloro-2-[(4-methylpiperazin-1-yl)carbonyl]-1H-i...)Show InChI InChI=1S/C14H16ClN3O/c1-17-4-6-18(7-5-17)14(19)13-9-10-8-11(15)2-3-12(10)16-13/h2-3,8-9,16H,4-7H2,1H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 6.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Antagonist activity at human histamine H4 receptor expressed in HEK293 cells assessed as rev inhibition of forskolin-stimulated cAMP accumulation by ... |
Bioorg Med Chem Lett 22: 1156-9 (2012)
Article DOI: 10.1016/j.bmcl.2011.11.098
BindingDB Entry DOI: 10.7270/Q2Q81DJD |
More data for this
Ligand-Target Pair | |
Histamine H4 receptor
(Homo sapiens (Human)) | BDBM22566

(5-chloro-2-[(4-methylpiperazin-1-yl)carbonyl]-1H-i...)Show InChI InChI=1S/C14H16ClN3O/c1-17-4-6-18(7-5-17)14(19)13-9-10-8-11(15)2-3-12(10)16-13/h2-3,8-9,16H,4-7H2,1H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 6.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Antagonist activity at human histamine H4 receptor expressed in HEK293 cells assessed as rev inhibition of forskolin-stimulated cAMP accumulation by ... |
Bioorg Med Chem Lett 22: 1156-9 (2012)
Article DOI: 10.1016/j.bmcl.2011.11.098
BindingDB Entry DOI: 10.7270/Q2Q81DJD |
More data for this
Ligand-Target Pair | |
Histamine H4 receptor
(Homo sapiens (Human)) | BDBM22566

(5-chloro-2-[(4-methylpiperazin-1-yl)carbonyl]-1H-i...)Show InChI InChI=1S/C14H16ClN3O/c1-17-4-6-18(7-5-17)14(19)13-9-10-8-11(15)2-3-12(10)16-13/h2-3,8-9,16H,4-7H2,1H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 6.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Antagonist activity at full length human H4R expressed in HEK293 cells assessed as reversal of forskolin-induced cAMP production by CRE-beta-lactamas... |
Bioorg Med Chem Lett 21: 6596-602 (2011)
Article DOI: 10.1016/j.bmcl.2011.07.125
BindingDB Entry DOI: 10.7270/Q20C4W6C |
More data for this
Ligand-Target Pair | |
Histamine H4 receptor
(Homo sapiens (Human)) | BDBM22566

(5-chloro-2-[(4-methylpiperazin-1-yl)carbonyl]-1H-i...)Show InChI InChI=1S/C14H16ClN3O/c1-17-4-6-18(7-5-17)14(19)13-9-10-8-11(15)2-3-12(10)16-13/h2-3,8-9,16H,4-7H2,1H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 8 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Displacement of [3H]-histamine from full length human H4R expressed in HEK293 cells |
Bioorg Med Chem Lett 21: 6596-602 (2011)
Article DOI: 10.1016/j.bmcl.2011.07.125
BindingDB Entry DOI: 10.7270/Q20C4W6C |
More data for this
Ligand-Target Pair | |
Histamine H4 receptor
(Homo sapiens (Human)) | BDBM22566

(5-chloro-2-[(4-methylpiperazin-1-yl)carbonyl]-1H-i...)Show InChI InChI=1S/C14H16ClN3O/c1-17-4-6-18(7-5-17)14(19)13-9-10-8-11(15)2-3-12(10)16-13/h2-3,8-9,16H,4-7H2,1H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 8 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Displacement of [3H]histamine from human recombinant histamine H4 receptor expressed in CHO cells coexpressing Ga15 by radioligand filtration binding... |
Bioorg Med Chem Lett 22: 1156-9 (2012)
Article DOI: 10.1016/j.bmcl.2011.11.098
BindingDB Entry DOI: 10.7270/Q2Q81DJD |
More data for this
Ligand-Target Pair | |
Histamine H4 receptor
(Homo sapiens (Human)) | BDBM22566

(5-chloro-2-[(4-methylpiperazin-1-yl)carbonyl]-1H-i...)Show InChI InChI=1S/C14H16ClN3O/c1-17-4-6-18(7-5-17)14(19)13-9-10-8-11(15)2-3-12(10)16-13/h2-3,8-9,16H,4-7H2,1H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 8 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Displacement of [3H]histamine from human recombinant histamine H4 receptor expressed in CHO cells coexpressing Ga15 by radioligand filtration binding... |
Bioorg Med Chem Lett 22: 1156-9 (2012)
Article DOI: 10.1016/j.bmcl.2011.11.098
BindingDB Entry DOI: 10.7270/Q2Q81DJD |
More data for this
Ligand-Target Pair | |
Histamine H4 receptor
(Homo sapiens (Human)) | BDBM50362216

(CHEMBL1938977)Show SMILES CN1C[C@H]2CN(C[C@H]2C1)C(=N)c1nc2ccc(F)cc2[nH]1 |r| Show InChI InChI=1S/C15H18FN5/c1-20-5-9-7-21(8-10(9)6-20)14(17)15-18-12-3-2-11(16)4-13(12)19-15/h2-4,9-10,17H,5-8H2,1H3,(H,18,19)/t9-,10+ | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 9.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Antagonist activity at human histamine H4 receptor expressed in HEK293 cells assessed as rev inhibition of forskolin-stimulated cAMP accumulation by ... |
Bioorg Med Chem Lett 22: 1156-9 (2012)
Article DOI: 10.1016/j.bmcl.2011.11.098
BindingDB Entry DOI: 10.7270/Q2Q81DJD |
More data for this
Ligand-Target Pair | |
Histamine H4 receptor
(Homo sapiens (Human)) | BDBM50362214

(CHEMBL1938975)Show SMILES CN1C[C@H]2CN(C[C@H]2C1)C(=N)c1nc2ccc(F)c(C)c2[nH]1 |r| Show InChI InChI=1S/C16H20FN5/c1-9-12(17)3-4-13-14(9)20-16(19-13)15(18)22-7-10-5-21(2)6-11(10)8-22/h3-4,10-11,18H,5-8H2,1-2H3,(H,19,20)/t10-,11+ | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 9.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Antagonist activity at human histamine H4 receptor expressed in HEK293 cells assessed as rev inhibition of forskolin-stimulated cAMP accumulation by ... |
Bioorg Med Chem Lett 22: 1156-9 (2012)
Article DOI: 10.1016/j.bmcl.2011.11.098
BindingDB Entry DOI: 10.7270/Q2Q81DJD |
More data for this
Ligand-Target Pair | |
Histamine H4 receptor
(Homo sapiens (Human)) | BDBM50356888
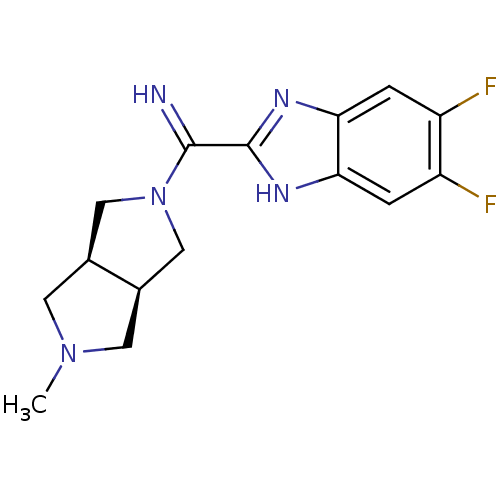
(CHEMBL1915348)Show SMILES CN1C[C@H]2CN(C[C@H]2C1)C(=N)c1nc2cc(F)c(F)cc2[nH]1 |r| Show InChI InChI=1S/C15H17F2N5/c1-21-4-8-6-22(7-9(8)5-21)14(18)15-19-12-2-10(16)11(17)3-13(12)20-15/h2-3,8-9,18H,4-7H2,1H3,(H,19,20)/t8-,9+ | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 9.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Displacement of [3H]histamine from human recombinant histamine H4 receptor expressed in CHO cells coexpressing Ga15 by radioligand filtration binding... |
Bioorg Med Chem Lett 22: 1156-9 (2012)
Article DOI: 10.1016/j.bmcl.2011.11.098
BindingDB Entry DOI: 10.7270/Q2Q81DJD |
More data for this
Ligand-Target Pair | |
Histamine H4 receptor
(Homo sapiens (Human)) | BDBM50356888

(CHEMBL1915348)Show SMILES CN1C[C@H]2CN(C[C@H]2C1)C(=N)c1nc2cc(F)c(F)cc2[nH]1 |r| Show InChI InChI=1S/C15H17F2N5/c1-21-4-8-6-22(7-9(8)5-21)14(18)15-19-12-2-10(16)11(17)3-13(12)20-15/h2-3,8-9,18H,4-7H2,1H3,(H,19,20)/t8-,9+ | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Antagonist activity at full length human H4R expressed in HEK293 cells assessed as reversal of forskolin-induced cAMP production by CRE-beta-lactamas... |
Bioorg Med Chem Lett 21: 6596-602 (2011)
Article DOI: 10.1016/j.bmcl.2011.07.125
BindingDB Entry DOI: 10.7270/Q20C4W6C |
More data for this
Ligand-Target Pair | |
Histamine H4 receptor
(Homo sapiens (Human)) | BDBM50356888

(CHEMBL1915348)Show SMILES CN1C[C@H]2CN(C[C@H]2C1)C(=N)c1nc2cc(F)c(F)cc2[nH]1 |r| Show InChI InChI=1S/C15H17F2N5/c1-21-4-8-6-22(7-9(8)5-21)14(18)15-19-12-2-10(16)11(17)3-13(12)20-15/h2-3,8-9,18H,4-7H2,1H3,(H,19,20)/t8-,9+ | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Displacement of [3H]-histamine from full length human H4R expressed in HEK293 cells |
Bioorg Med Chem Lett 21: 6596-602 (2011)
Article DOI: 10.1016/j.bmcl.2011.07.125
BindingDB Entry DOI: 10.7270/Q20C4W6C |
More data for this
Ligand-Target Pair | |
Histamine H4 receptor
(Rattus norvegicus (rat)) | BDBM50356884

(CHEMBL1915540)Show InChI InChI=1S/C13H22N6/c1-15-10-4-5-19(8-10)12-6-11(17-13(14)18-12)16-7-9-2-3-9/h6,9-10,15H,2-5,7-8H2,1H3,(H3,14,16,17,18)/t10-/m1/s1 | KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 12.3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Displacement of [3H]-histamine from rat H4R expressed in HEK293T cells |
Bioorg Med Chem Lett 21: 6596-602 (2011)
Article DOI: 10.1016/j.bmcl.2011.07.125
BindingDB Entry DOI: 10.7270/Q20C4W6C |
More data for this
Ligand-Target Pair | |
Histamine H4 receptor
(Homo sapiens (Human)) | BDBM50362218

(CHEMBL1938979)Show SMILES CN1C[C@H]2CN(C[C@H]2C1)C(=N)c1nc2ccccc2[nH]1 |r| Show InChI InChI=1S/C15H19N5/c1-19-6-10-8-20(9-11(10)7-19)14(16)15-17-12-4-2-3-5-13(12)18-15/h2-5,10-11,16H,6-9H2,1H3,(H,17,18)/t10-,11+ | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 12.4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Antagonist activity at human histamine H4 receptor expressed in HEK293 cells assessed as rev inhibition of forskolin-stimulated cAMP accumulation by ... |
Bioorg Med Chem Lett 22: 1156-9 (2012)
Article DOI: 10.1016/j.bmcl.2011.11.098
BindingDB Entry DOI: 10.7270/Q2Q81DJD |
More data for this
Ligand-Target Pair | |
Histamine H4 receptor
(Homo sapiens (Human)) | BDBM50356882
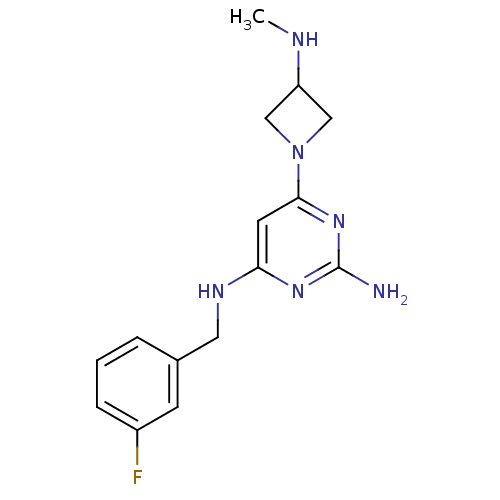
(CHEMBL1915538)Show InChI InChI=1S/C15H19FN6/c1-18-12-8-22(9-12)14-6-13(20-15(17)21-14)19-7-10-3-2-4-11(16)5-10/h2-6,12,18H,7-9H2,1H3,(H3,17,19,20,21) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 13 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Antagonist activity at full length human H4R expressed in HEK293 cells assessed as reversal of forskolin-induced cAMP production by CRE-beta-lactamas... |
Bioorg Med Chem Lett 21: 6596-602 (2011)
Article DOI: 10.1016/j.bmcl.2011.07.125
BindingDB Entry DOI: 10.7270/Q20C4W6C |
More data for this
Ligand-Target Pair | |
Sodium channel protein type 9 subunit alpha
(Homo sapiens (Human)) | BDBM185975
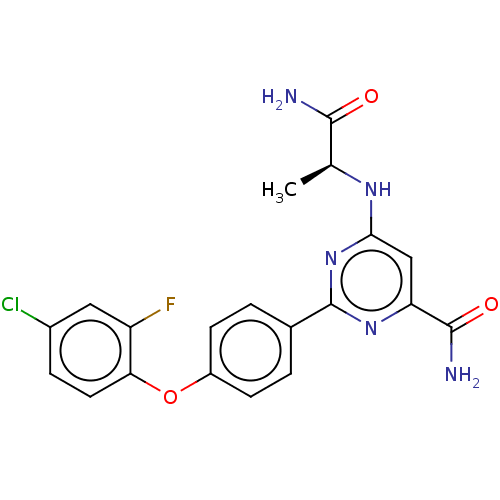
(US9163008, 64 | US9656968, Compound 64)Show SMILES C[C@H](Nc1cc(nc(n1)-c1ccc(Oc2ccc(Cl)cc2F)cc1)C(N)=O)C(N)=O |r| Show InChI InChI=1S/C20H17ClFN5O3/c1-10(18(23)28)25-17-9-15(19(24)29)26-20(27-17)11-2-5-13(6-3-11)30-16-7-4-12(21)8-14(16)22/h2-10H,1H3,(H2,23,28)(H2,24,29)(H,25,26,27)/t10-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 16 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human Nav1.7 expressed in HEK293 cells by electrophysiology method |
Bioorg Med Chem Lett 24: 3690-9 (2014)
Article DOI: 10.1016/j.bmcl.2014.06.038
BindingDB Entry DOI: 10.7270/Q2KS6VJM |
More data for this
Ligand-Target Pair | |
Histamine H4 receptor
(Homo sapiens (Human)) | BDBM50356885
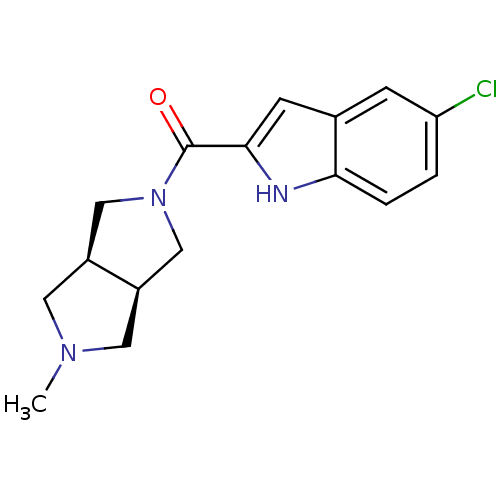
(CHEMBL1915345)Show SMILES CN1C[C@H]2CN(C[C@H]2C1)C(=O)c1cc2cc(Cl)ccc2[nH]1 |r| Show InChI InChI=1S/C16H18ClN3O/c1-19-6-11-8-20(9-12(11)7-19)16(21)15-5-10-4-13(17)2-3-14(10)18-15/h2-5,11-12,18H,6-9H2,1H3/t11-,12+ | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
| Article
PubMed
| 16 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Displacement of [3H]-histamine from full length human H4R expressed in HEK293 cells |
Bioorg Med Chem Lett 21: 6596-602 (2011)
Article DOI: 10.1016/j.bmcl.2011.07.125
BindingDB Entry DOI: 10.7270/Q20C4W6C |
More data for this
Ligand-Target Pair | |
Histamine H4 receptor
(Homo sapiens (Human)) | BDBM50356885

(CHEMBL1915345)Show SMILES CN1C[C@H]2CN(C[C@H]2C1)C(=O)c1cc2cc(Cl)ccc2[nH]1 |r| Show InChI InChI=1S/C16H18ClN3O/c1-19-6-11-8-20(9-12(11)7-19)16(21)15-5-10-4-13(17)2-3-14(10)18-15/h2-5,11-12,18H,6-9H2,1H3/t11-,12+ | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
| Article
PubMed
| 16 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Displacement of [3H]histamine from human recombinant histamine H4 receptor expressed in CHO cells coexpressing Ga15 by radioligand filtration binding... |
Bioorg Med Chem Lett 22: 1156-9 (2012)
Article DOI: 10.1016/j.bmcl.2011.11.098
BindingDB Entry DOI: 10.7270/Q2Q81DJD |
More data for this
Ligand-Target Pair | |
Histamine H4 receptor
(Homo sapiens (Human)) | BDBM50356888

(CHEMBL1915348)Show SMILES CN1C[C@H]2CN(C[C@H]2C1)C(=N)c1nc2cc(F)c(F)cc2[nH]1 |r| Show InChI InChI=1S/C15H17F2N5/c1-21-4-8-6-22(7-9(8)5-21)14(18)15-19-12-2-10(16)11(17)3-13(12)20-15/h2-3,8-9,18H,4-7H2,1H3,(H,19,20)/t8-,9+ | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 17.2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Antagonist activity at human histamine H4 receptor expressed in HEK293 cells assessed as rev inhibition of forskolin-stimulated cAMP accumulation by ... |
Bioorg Med Chem Lett 22: 1156-9 (2012)
Article DOI: 10.1016/j.bmcl.2011.11.098
BindingDB Entry DOI: 10.7270/Q2Q81DJD |
More data for this
Ligand-Target Pair | |
Histamine H4 receptor
(Homo sapiens (Human)) | BDBM50356882

(CHEMBL1915538)Show InChI InChI=1S/C15H19FN6/c1-18-12-8-22(9-12)14-6-13(20-15(17)21-14)19-7-10-3-2-4-11(16)5-10/h2-6,12,18H,7-9H2,1H3,(H3,17,19,20,21) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 18 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Displacement of [3H]-histamine from full length human H4R expressed in HEK293 cells |
Bioorg Med Chem Lett 21: 6596-602 (2011)
Article DOI: 10.1016/j.bmcl.2011.07.125
BindingDB Entry DOI: 10.7270/Q20C4W6C |
More data for this
Ligand-Target Pair | |
Sodium channel protein type 9 subunit alpha
(Homo sapiens (Human)) | BDBM50393023
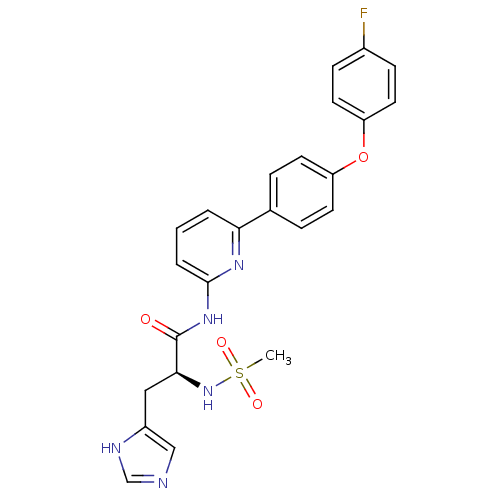
(CHEMBL2152688)Show SMILES CS(=O)(=O)N[C@@H](Cc1cnc[nH]1)C(=O)Nc1cccc(n1)-c1ccc(Oc2ccc(F)cc2)cc1 |r| Show InChI InChI=1S/C24H22FN5O4S/c1-35(32,33)30-22(13-18-14-26-15-27-18)24(31)29-23-4-2-3-21(28-23)16-5-9-19(10-6-16)34-20-11-7-17(25)8-12-20/h2-12,14-15,22,30H,13H2,1H3,(H,26,27)(H,28,29,31)/t22-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| 19 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human Nav1.7 expressed in HEK293 cells by electrophysiology method |
Bioorg Med Chem Lett 24: 3690-9 (2014)
Article DOI: 10.1016/j.bmcl.2014.06.038
BindingDB Entry DOI: 10.7270/Q2KS6VJM |
More data for this
Ligand-Target Pair | |
Histamine H4 receptor
(Homo sapiens (Human)) | BDBM50362217
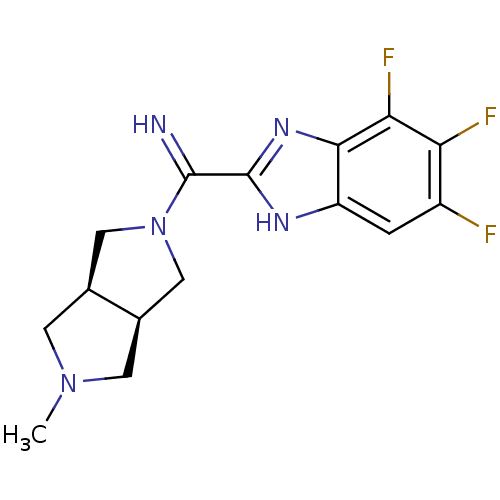
(CHEMBL1938978)Show SMILES CN1C[C@H]2CN(C[C@H]2C1)C(=N)c1nc2c(F)c(F)c(F)cc2[nH]1 |r| Show InChI InChI=1S/C15H16F3N5/c1-22-3-7-5-23(6-8(7)4-22)14(19)15-20-10-2-9(16)11(17)12(18)13(10)21-15/h2,7-8,19H,3-6H2,1H3,(H,20,21)/t7-,8+ | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Antagonist activity at human histamine H4 receptor expressed in HEK293 cells assessed as rev inhibition of forskolin-stimulated cAMP accumulation by ... |
Bioorg Med Chem Lett 22: 1156-9 (2012)
Article DOI: 10.1016/j.bmcl.2011.11.098
BindingDB Entry DOI: 10.7270/Q2Q81DJD |
More data for this
Ligand-Target Pair | |
Histamine H4 receptor
(Mus musculus (mouse)) | BDBM50356884

(CHEMBL1915540)Show InChI InChI=1S/C13H22N6/c1-15-10-4-5-19(8-10)12-6-11(17-13(14)18-12)16-7-9-2-3-9/h6,9-10,15H,2-5,7-8H2,1H3,(H3,14,16,17,18)/t10-/m1/s1 | Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 20.9 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Displacement of [3H]-histamine from mouse H4R expressed in HEK293T cells |
Bioorg Med Chem Lett 21: 6596-602 (2011)
Article DOI: 10.1016/j.bmcl.2011.07.125
BindingDB Entry DOI: 10.7270/Q20C4W6C |
More data for this
Ligand-Target Pair | |
Histamine H4 receptor
(Homo sapiens (Human)) | BDBM50362215
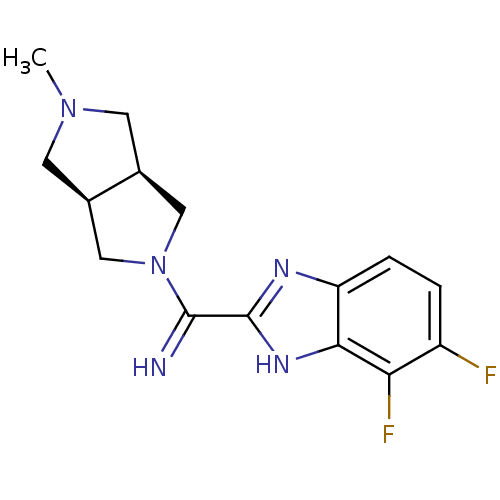
(CHEMBL1938976)Show SMILES CN1C[C@H]2CN(C[C@H]2C1)C(=N)c1nc2ccc(F)c(F)c2[nH]1 |r| Show InChI InChI=1S/C15H17F2N5/c1-21-4-8-6-22(7-9(8)5-21)14(18)15-19-11-3-2-10(16)12(17)13(11)20-15/h2-3,8-9,18H,4-7H2,1H3,(H,19,20)/t8-,9+ | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 24 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Antagonist activity at human histamine H4 receptor expressed in HEK293 cells assessed as rev inhibition of forskolin-stimulated cAMP accumulation by ... |
Bioorg Med Chem Lett 22: 1156-9 (2012)
Article DOI: 10.1016/j.bmcl.2011.11.098
BindingDB Entry DOI: 10.7270/Q2Q81DJD |
More data for this
Ligand-Target Pair | |
Gamma-aminobutyric acid receptor subunit alpha-3
(Homo sapiens (Human)) | BDBM50067424

((S)-9-Methoxy-7-oxo-3b,4,5,6-tetrahydro-7H-2,6a,11...)Show SMILES CCOC(=O)c1ncn-2c1[C@@H]1CCCN1C(=O)c1cc(OC)ccc-21 Show InChI InChI=1S/C18H19N3O4/c1-3-25-18(23)15-16-14-5-4-8-20(14)17(22)12-9-11(24-2)6-7-13(12)21(16)10-19-15/h6-7,9-10,14H,3-5,8H2,1-2H3/t14-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 25 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Positive allosteric modulation of GABAA alpha3 (unknown origin) |
J Med Chem 56: 593-624 (2013)
Article DOI: 10.1021/jm3011433
BindingDB Entry DOI: 10.7270/Q23B61GZ |
More data for this
Ligand-Target Pair | |
Histamine H4 receptor
(Homo sapiens (Human)) | BDBM50362213
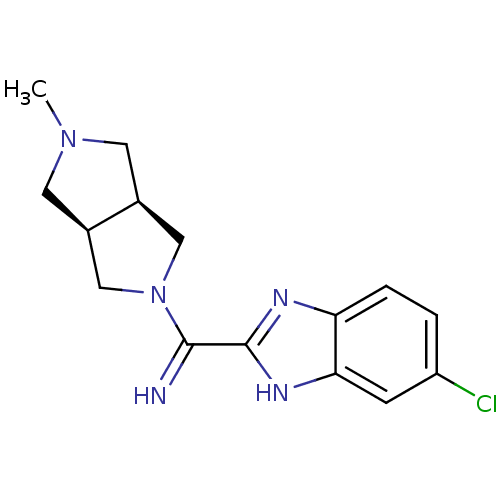
(CHEMBL1938974)Show SMILES CN1C[C@H]2CN(C[C@H]2C1)C(=N)c1nc2ccc(Cl)cc2[nH]1 |r| Show InChI InChI=1S/C15H18ClN5/c1-20-5-9-7-21(8-10(9)6-20)14(17)15-18-12-3-2-11(16)4-13(12)19-15/h2-4,9-10,17H,5-8H2,1H3,(H,18,19)/t9-,10+ | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 26 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Displacement of [3H]histamine from human recombinant histamine H4 receptor expressed in CHO cells coexpressing Ga15 by radioligand filtration binding... |
Bioorg Med Chem Lett 22: 1156-9 (2012)
Article DOI: 10.1016/j.bmcl.2011.11.098
BindingDB Entry DOI: 10.7270/Q2Q81DJD |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data