 Found 695 hits with Last Name = 'swarbrick' and Initial = 'me'
Found 695 hits with Last Name = 'swarbrick' and Initial = 'me' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Prostaglandin E2 receptor EP4 subtype
(Homo sapiens (Human)) | BDBM50461458
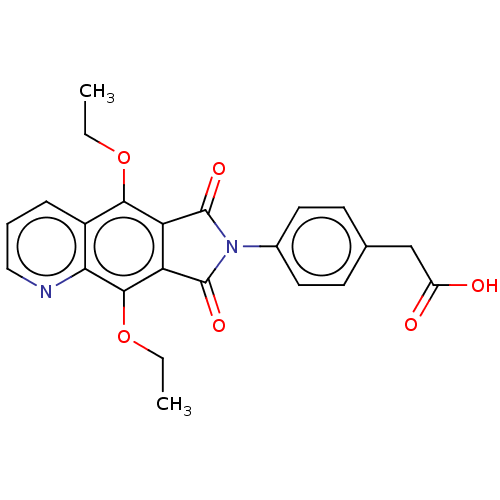
(CHEMBL4229200)Show SMILES CCOc1c2C(=O)N(C(=O)c2c(OCC)c2ncccc12)c1ccc(CC(O)=O)cc1 Show InChI InChI=1S/C23H20N2O6/c1-3-30-20-15-6-5-11-24-19(15)21(31-4-2)18-17(20)22(28)25(23(18)29)14-9-7-13(8-10-14)12-16(26)27/h5-11H,3-4,12H2,1-2H3,(H,26,27) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| KEGG
PC cid
PC sid
UniChem
| Article
PubMed
| 32 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Displacement of [3H]PGE2 from human EP4 receptor expressed in HEK 293 (EBNA) cell membranes incubated for 60 mins by scintillation counting method |
Bioorg Med Chem Lett 28: 1892-1896 (2018)
Article DOI: 10.1016/j.bmcl.2018.03.091
BindingDB Entry DOI: 10.7270/Q21Z472W |
More data for this
Ligand-Target Pair | |
Prostaglandin E2 receptor EP4 subtype
(Homo sapiens (Human)) | BDBM50461462
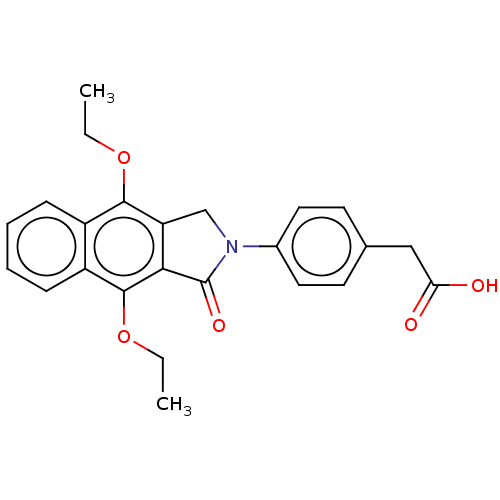
(CHEMBL4225786)Show SMILES CCOc1c2CN(C(=O)c2c(OCC)c2ccccc12)c1ccc(CC(O)=O)cc1 Show InChI InChI=1S/C24H23NO5/c1-3-29-22-17-7-5-6-8-18(17)23(30-4-2)21-19(22)14-25(24(21)28)16-11-9-15(10-12-16)13-20(26)27/h5-12H,3-4,13-14H2,1-2H3,(H,26,27) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| KEGG
PC cid
PC sid
UniChem
| Article
PubMed
| 79 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Displacement of [3H]PGE2 from human EP4 receptor expressed in HEK 293 (EBNA) cell membranes incubated for 60 mins by scintillation counting method |
Bioorg Med Chem Lett 28: 1892-1896 (2018)
Article DOI: 10.1016/j.bmcl.2018.03.091
BindingDB Entry DOI: 10.7270/Q21Z472W |
More data for this
Ligand-Target Pair | |
Prostaglandin E2 receptor EP4 subtype
(Homo sapiens (Human)) | BDBM50461457
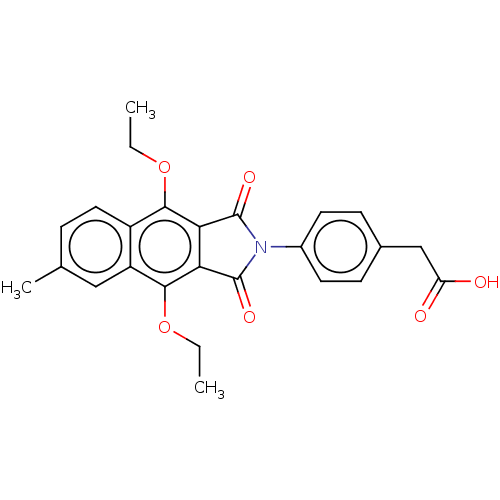
(CHEMBL4225963)Show SMILES CCOc1c2C(=O)N(C(=O)c2c(OCC)c2cc(C)ccc12)c1ccc(CC(O)=O)cc1 Show InChI InChI=1S/C25H23NO6/c1-4-31-22-17-11-6-14(3)12-18(17)23(32-5-2)21-20(22)24(29)26(25(21)30)16-9-7-15(8-10-16)13-19(27)28/h6-12H,4-5,13H2,1-3H3,(H,27,28) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| KEGG
PC cid
PC sid
UniChem
| Article
PubMed
| 158 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Displacement of [3H]PGE2 from human EP4 receptor expressed in HEK 293 (EBNA) cell membranes incubated for 60 mins by scintillation counting method |
Bioorg Med Chem Lett 28: 1892-1896 (2018)
Article DOI: 10.1016/j.bmcl.2018.03.091
BindingDB Entry DOI: 10.7270/Q21Z472W |
More data for this
Ligand-Target Pair | |
Prostaglandin E2 receptor EP4 subtype
(Homo sapiens (Human)) | BDBM50461454

(CHEMBL4224936)Show SMILES CCOc1c2C(=O)N(C(=O)c2c(OCC)c2ccccc12)c1ccc(CC(O)=O)cc1 Show InChI InChI=1S/C24H21NO6/c1-3-30-21-16-7-5-6-8-17(16)22(31-4-2)20-19(21)23(28)25(24(20)29)15-11-9-14(10-12-15)13-18(26)27/h5-12H,3-4,13H2,1-2H3,(H,26,27) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| KEGG
PC cid
PC sid
UniChem
| Article
PubMed
| 158 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Displacement of [3H]PGE2 from human EP4 receptor expressed in HEK 293 (EBNA) cell membranes incubated for 60 mins by scintillation counting method |
Bioorg Med Chem Lett 28: 1892-1896 (2018)
Article DOI: 10.1016/j.bmcl.2018.03.091
BindingDB Entry DOI: 10.7270/Q21Z472W |
More data for this
Ligand-Target Pair | |
Prostaglandin E2 receptor EP4 subtype
(Homo sapiens (Human)) | BDBM50461450

(CHEMBL4226720)Show SMILES CCOc1c2CN(C(=O)c2c(OCC)c2ccccc12)c1ccc(cc1)C(C)C(O)=O Show InChI InChI=1S/C25H25NO5/c1-4-30-22-18-8-6-7-9-19(18)23(31-5-2)21-20(22)14-26(24(21)27)17-12-10-16(11-13-17)15(3)25(28)29/h6-13,15H,4-5,14H2,1-3H3,(H,28,29) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Displacement of [3H]PGE2 from human EP4 receptor expressed in HEK 293 (EBNA) cell membranes incubated for 60 mins by scintillation counting method |
Bioorg Med Chem Lett 28: 1892-1896 (2018)
Article DOI: 10.1016/j.bmcl.2018.03.091
BindingDB Entry DOI: 10.7270/Q21Z472W |
More data for this
Ligand-Target Pair | |
Prostaglandin E2 receptor EP4 subtype
(Homo sapiens (Human)) | BDBM50461451

(CHEMBL4226523)Show SMILES CC(C)Oc1c2CN(C(=O)c2c(OC(C)C)c2ccccc12)c1ccc(CC(O)=O)cc1 Show InChI InChI=1S/C26H27NO5/c1-15(2)31-24-19-7-5-6-8-20(19)25(32-16(3)4)23-21(24)14-27(26(23)30)18-11-9-17(10-12-18)13-22(28)29/h5-12,15-16H,13-14H2,1-4H3,(H,28,29) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| KEGG
PC cid
PC sid
UniChem
| Article
PubMed
| 398 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Displacement of [3H]PGE2 from human EP4 receptor expressed in HEK 293 (EBNA) cell membranes incubated for 60 mins by scintillation counting method |
Bioorg Med Chem Lett 28: 1892-1896 (2018)
Article DOI: 10.1016/j.bmcl.2018.03.091
BindingDB Entry DOI: 10.7270/Q21Z472W |
More data for this
Ligand-Target Pair | |
Prostaglandin E2 receptor EP4 subtype
(Homo sapiens (Human)) | BDBM50461453
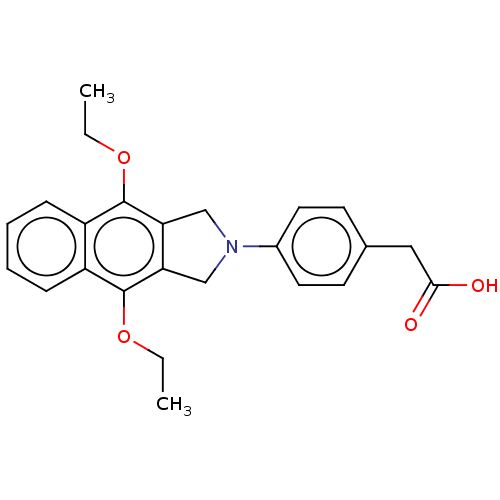
(CHEMBL4226984)Show SMILES CCOc1c2CN(Cc2c(OCC)c2ccccc12)c1ccc(CC(O)=O)cc1 Show InChI InChI=1S/C24H25NO4/c1-3-28-23-18-7-5-6-8-19(18)24(29-4-2)21-15-25(14-20(21)23)17-11-9-16(10-12-17)13-22(26)27/h5-12H,3-4,13-15H2,1-2H3,(H,26,27) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| KEGG
PC cid
PC sid
UniChem
| Article
PubMed
| 631 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Displacement of [3H]PGE2 from human EP4 receptor expressed in HEK 293 (EBNA) cell membranes incubated for 60 mins by scintillation counting method |
Bioorg Med Chem Lett 28: 1892-1896 (2018)
Article DOI: 10.1016/j.bmcl.2018.03.091
BindingDB Entry DOI: 10.7270/Q21Z472W |
More data for this
Ligand-Target Pair | |
Prostaglandin E2 receptor EP4 subtype
(Homo sapiens (Human)) | BDBM50461463

(CHEMBL4225442)Show SMILES CCCOc1c2CN(C(=O)c2c(OCCC)c2ccccc12)c1ccc(CC(O)=O)cc1 Show InChI InChI=1S/C26H27NO5/c1-3-13-31-24-19-7-5-6-8-20(19)25(32-14-4-2)23-21(24)16-27(26(23)30)18-11-9-17(10-12-18)15-22(28)29/h5-12H,3-4,13-16H2,1-2H3,(H,28,29) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| KEGG
PC cid
PC sid
UniChem
| Article
PubMed
| 631 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Displacement of [3H]PGE2 from human EP4 receptor expressed in HEK 293 (EBNA) cell membranes incubated for 60 mins by scintillation counting method |
Bioorg Med Chem Lett 28: 1892-1896 (2018)
Article DOI: 10.1016/j.bmcl.2018.03.091
BindingDB Entry DOI: 10.7270/Q21Z472W |
More data for this
Ligand-Target Pair | |
Prostaglandin E2 receptor EP4 subtype
(Homo sapiens (Human)) | BDBM50461468
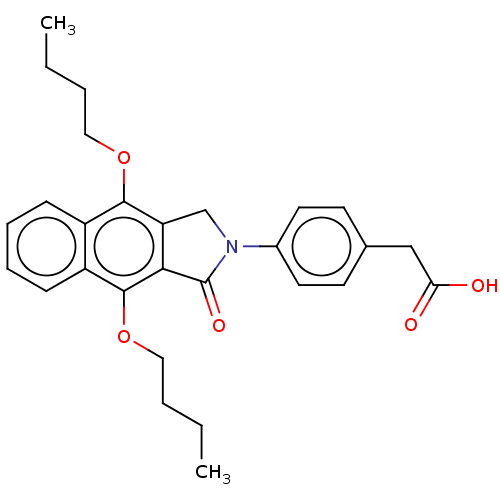
(CHEMBL4227243)Show SMILES CCCCOc1c2CN(C(=O)c2c(OCCCC)c2ccccc12)c1ccc(CC(O)=O)cc1 Show InChI InChI=1S/C28H31NO5/c1-3-5-15-33-26-21-9-7-8-10-22(21)27(34-16-6-4-2)25-23(26)18-29(28(25)32)20-13-11-19(12-14-20)17-24(30)31/h7-14H,3-6,15-18H2,1-2H3,(H,30,31) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| KEGG
PC cid
PC sid
UniChem
| Article
PubMed
| 2.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Displacement of [3H]PGE2 from human EP4 receptor expressed in HEK 293 (EBNA) cell membranes incubated for 60 mins by scintillation counting method |
Bioorg Med Chem Lett 28: 1892-1896 (2018)
Article DOI: 10.1016/j.bmcl.2018.03.091
BindingDB Entry DOI: 10.7270/Q21Z472W |
More data for this
Ligand-Target Pair | |
Prostaglandin E2 receptor EP4 subtype
(Homo sapiens (Human)) | BDBM50461456

(CHEMBL4228095)Show SMILES CCCCCCOc1c2CN(C(=O)c2c(OCCCCCC)c2ccccc12)c1ccc(CC(O)=O)cc1 Show InChI InChI=1S/C32H39NO5/c1-3-5-7-11-19-37-30-25-13-9-10-14-26(25)31(38-20-12-8-6-4-2)29-27(30)22-33(32(29)36)24-17-15-23(16-18-24)21-28(34)35/h9-10,13-18H,3-8,11-12,19-22H2,1-2H3,(H,34,35) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| KEGG
PC cid
PC sid
UniChem
| Article
PubMed
| <6.31E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Displacement of [3H]PGE2 from human EP4 receptor expressed in HEK 293 (EBNA) cell membranes incubated for 60 mins by scintillation counting method |
Bioorg Med Chem Lett 28: 1892-1896 (2018)
Article DOI: 10.1016/j.bmcl.2018.03.091
BindingDB Entry DOI: 10.7270/Q21Z472W |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 2
(Homo sapiens (Human)) | BDBM50297677
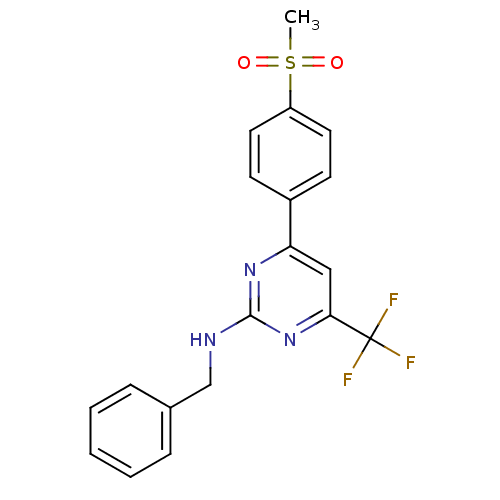
(CHEMBL551829 | N-benzyl-4-(4-(methylsulfonyl)pheny...)Show SMILES CS(=O)(=O)c1ccc(cc1)-c1cc(nc(NCc2ccccc2)n1)C(F)(F)F Show InChI InChI=1S/C19H16F3N3O2S/c1-28(26,27)15-9-7-14(8-10-15)16-11-17(19(20,21)22)25-18(24-16)23-12-13-5-3-2-4-6-13/h2-11H,12H2,1H3,(H,23,24,25) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.25 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibition of human COX2 expressed in baculovirus-infected SF9 cells assessed as inhibition of arachidonic acid-stimulated PGE2 production treated 1 ... |
Bioorg Med Chem Lett 19: 4504-8 (2009)
Article DOI: 10.1016/j.bmcl.2009.02.085
BindingDB Entry DOI: 10.7270/Q20Z7396 |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 2
(Homo sapiens (Human)) | BDBM50297675
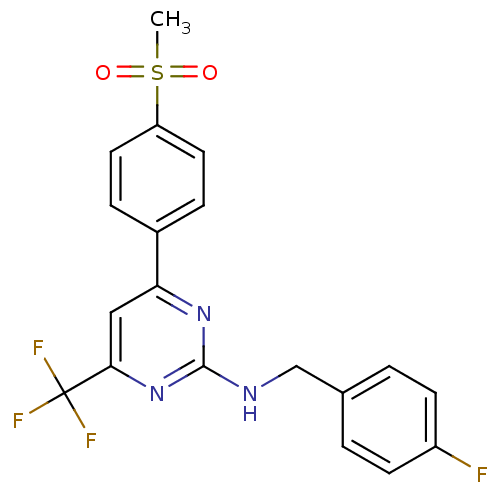
(CHEMBL551148 | N-(4-fluorobenzyl)-4-(4-(methylsulf...)Show SMILES CS(=O)(=O)c1ccc(cc1)-c1cc(nc(NCc2ccc(F)cc2)n1)C(F)(F)F Show InChI InChI=1S/C19H15F4N3O2S/c1-29(27,28)15-8-4-13(5-9-15)16-10-17(19(21,22)23)26-18(25-16)24-11-12-2-6-14(20)7-3-12/h2-10H,11H2,1H3,(H,24,25,26) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.280 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibition of human COX2 expressed in baculovirus-infected SF9 cells assessed as inhibition of arachidonic acid-stimulated PGE2 production treated 1 ... |
Bioorg Med Chem Lett 19: 4504-8 (2009)
Article DOI: 10.1016/j.bmcl.2009.02.085
BindingDB Entry DOI: 10.7270/Q20Z7396 |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 2
(Homo sapiens (Human)) | BDBM50297669
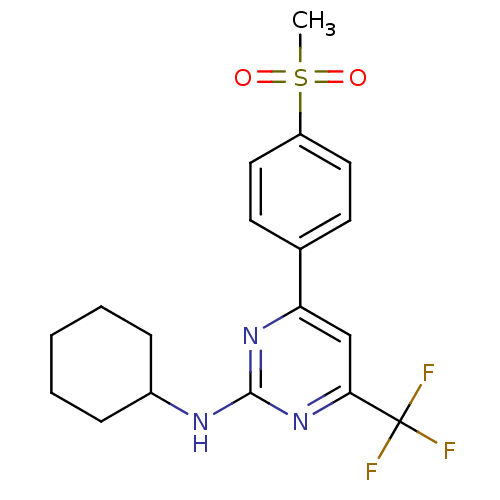
(CHEMBL561891 | N-cyclohexyl-4-(4-(methylsulfonyl)p...)Show SMILES CS(=O)(=O)c1ccc(cc1)-c1cc(nc(NC2CCCCC2)n1)C(F)(F)F Show InChI InChI=1S/C18H20F3N3O2S/c1-27(25,26)14-9-7-12(8-10-14)15-11-16(18(19,20)21)24-17(23-15)22-13-5-3-2-4-6-13/h7-11,13H,2-6H2,1H3,(H,22,23,24) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibition of human COX2 expressed in african green monkey COS cells assessed as inhibition of arachidonic acid-stimulated PGE2 production treated 1 ... |
Bioorg Med Chem Lett 19: 4504-8 (2009)
Article DOI: 10.1016/j.bmcl.2009.02.085
BindingDB Entry DOI: 10.7270/Q20Z7396 |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4-kinase alpha
(Homo sapiens (Human)) | BDBM50107257
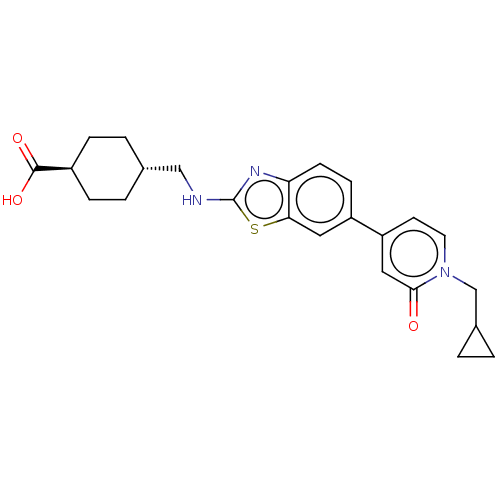
(CHEMBL3600785)Show SMILES OC(=O)[C@H]1CC[C@H](CNc2nc3ccc(cc3s2)-c2ccn(CC3CC3)c(=O)c2)CC1 |r,wU:6.6,wD:3.2,(11.72,2.53,;11.11,1.45,;11.74,.39,;9.57,1.44,;8.78,2.76,;7.24,2.75,;6.49,1.4,;4.95,1.38,;4.2,.04,;2.66,.02,;1.76,-1.24,;.3,-.77,;-1.03,-1.55,;-2.38,-.77,;-2.38,.77,;-1.03,1.55,;.3,.77,;1.76,1.24,;-3.71,1.53,;-5.05,.76,;-6.38,1.53,;-6.38,3.07,;-7.72,3.83,;-9.05,3.06,;-10.51,3.1,;-9.73,1.77,;-5.05,3.84,;-5.05,5.07,;-3.72,3.07,;7.28,.08,;8.82,.1,)| Show InChI InChI=1S/C24H27N3O3S/c28-22-12-19(9-10-27(22)14-16-1-2-16)18-7-8-20-21(11-18)31-24(26-20)25-13-15-3-5-17(6-4-15)23(29)30/h7-12,15-17H,1-6,13-14H2,(H,25,26)(H,29,30)/t15-,17- | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of N-terminal FLAG-tagged human full length recombinant PI4K3alpha using D-myo-phosphatidylinositol substrate and ATP incubated for 45 min... |
Bioorg Med Chem Lett 25: 3189-93 (2015)
Article DOI: 10.1016/j.bmcl.2015.05.093
BindingDB Entry DOI: 10.7270/Q2KK9DKR |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 2
(Homo sapiens (Human)) | BDBM50297672
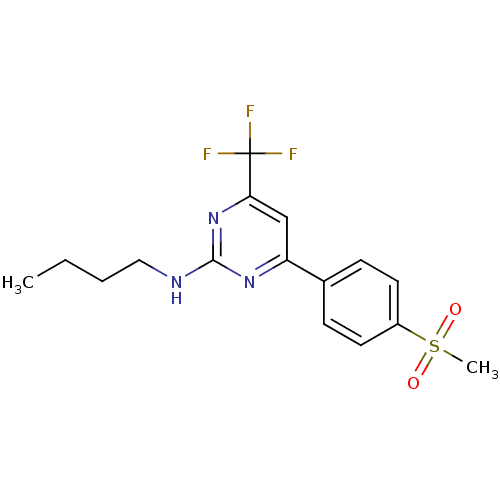
(CHEMBL549393 | N-butyl-4-(4-(methylsulfonyl)phenyl...)Show SMILES CCCCNc1nc(cc(n1)C(F)(F)F)-c1ccc(cc1)S(C)(=O)=O Show InChI InChI=1S/C16H18F3N3O2S/c1-3-4-9-20-15-21-13(10-14(22-15)16(17,18)19)11-5-7-12(8-6-11)25(2,23)24/h5-8,10H,3-4,9H2,1-2H3,(H,20,21,22) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.30 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibition of human COX2 expressed in baculovirus-infected SF9 cells assessed as inhibition of arachidonic acid-stimulated PGE2 production treated 1 ... |
Bioorg Med Chem Lett 19: 4504-8 (2009)
Article DOI: 10.1016/j.bmcl.2009.02.085
BindingDB Entry DOI: 10.7270/Q20Z7396 |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4-phosphate 5-kinase type-1 gamma
(Homo sapiens (Human)) | BDBM50599596
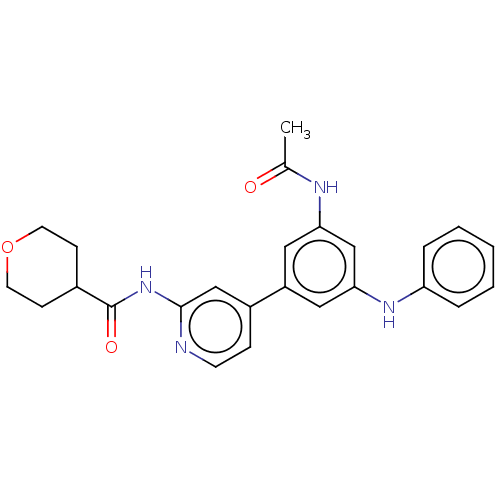
(CHEMBL5191668)Show SMILES CC(=O)Nc1cc(Nc2ccccc2)cc(c1)-c1ccnc(NC(=O)C2CCOCC2)c1 | Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.30 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.bmc.2021.116557
BindingDB Entry DOI: 10.7270/Q23J3J18 |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 2
(Homo sapiens (Human)) | BDBM50297671
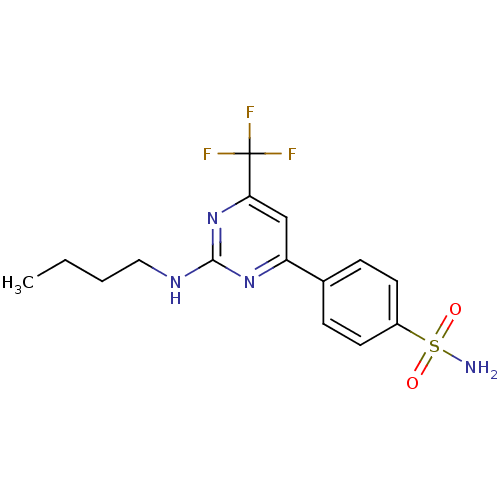
(4-(2-(butylamino)-6-(trifluoromethyl)pyrimidin-4-y...)Show SMILES CCCCNc1nc(cc(n1)C(F)(F)F)-c1ccc(cc1)S(N)(=O)=O Show InChI InChI=1S/C15H17F3N4O2S/c1-2-3-8-20-14-21-12(9-13(22-14)15(16,17)18)10-4-6-11(7-5-10)25(19,23)24/h4-7,9H,2-3,8H2,1H3,(H2,19,23,24)(H,20,21,22) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | <1.40 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibition of human COX2 expressed in african green monkey COS cells assessed as inhibition of arachidonic acid-stimulated PGE2 production treated 1 ... |
Bioorg Med Chem Lett 19: 4504-8 (2009)
Article DOI: 10.1016/j.bmcl.2009.02.085
BindingDB Entry DOI: 10.7270/Q20Z7396 |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4-phosphate 5-kinase type-1 gamma
(Homo sapiens (Human)) | BDBM50599618
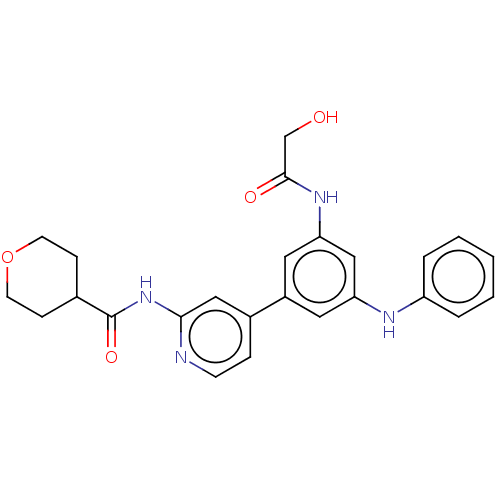
(CHEMBL5182242)Show SMILES OCC(=O)Nc1cc(Nc2ccccc2)cc(c1)-c1ccnc(NC(=O)C2CCOCC2)c1 | Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.bmc.2021.116557
BindingDB Entry DOI: 10.7270/Q23J3J18 |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4-kinase alpha
(Homo sapiens (Human)) | BDBM50107256
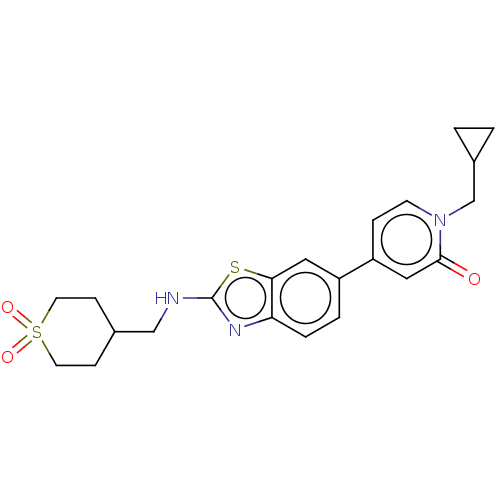
(CHEMBL3600784)Show SMILES O=c1cc(ccn1CC1CC1)-c1ccc2nc(NCC3CCS(=O)(=O)CC3)sc2c1 Show InChI InChI=1S/C22H25N3O3S2/c26-21-12-18(5-8-25(21)14-16-1-2-16)17-3-4-19-20(11-17)29-22(24-19)23-13-15-6-9-30(27,28)10-7-15/h3-5,8,11-12,15-16H,1-2,6-7,9-10,13-14H2,(H,23,24) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of N-terminal FLAG-tagged human full length recombinant PI4K3alpha using D-myo-phosphatidylinositol substrate and ATP incubated for 45 min... |
Bioorg Med Chem Lett 25: 3189-93 (2015)
Article DOI: 10.1016/j.bmcl.2015.05.093
BindingDB Entry DOI: 10.7270/Q2KK9DKR |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 2
(Homo sapiens (Human)) | BDBM50297670
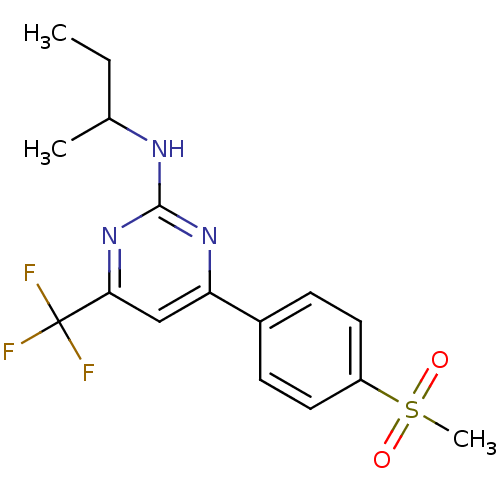
(CHEMBL539663 | GW-637185X | N-sec-butyl-4-(4-(meth...)Show SMILES CCC(C)Nc1nc(cc(n1)C(F)(F)F)-c1ccc(cc1)S(C)(=O)=O Show InChI InChI=1S/C16H18F3N3O2S/c1-4-10(2)20-15-21-13(9-14(22-15)16(17,18)19)11-5-7-12(8-6-11)25(3,23)24/h5-10H,4H2,1-3H3,(H,20,21,22) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibition of human COX2 expressed in baculovirus-infected SF9 cells assessed as inhibition of arachidonic acid-stimulated PGE2 production treated 1 ... |
Bioorg Med Chem Lett 19: 4504-8 (2009)
Article DOI: 10.1016/j.bmcl.2009.02.085
BindingDB Entry DOI: 10.7270/Q20Z7396 |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 2
(Homo sapiens (Human)) | BDBM50297668
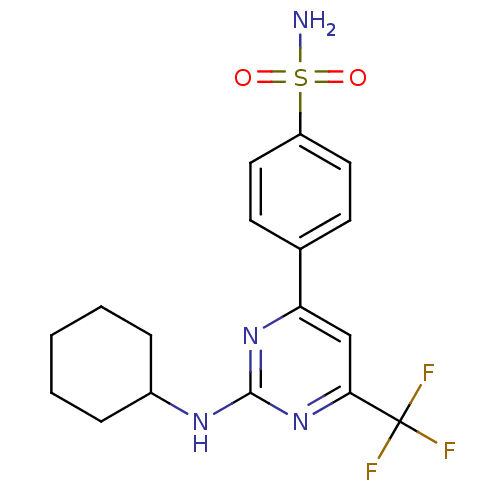
(4-(2-(cyclohexylamino)-6-(trifluoromethyl)pyrimidi...)Show SMILES NS(=O)(=O)c1ccc(cc1)-c1cc(nc(NC2CCCCC2)n1)C(F)(F)F Show InChI InChI=1S/C17H19F3N4O2S/c18-17(19,20)15-10-14(11-6-8-13(9-7-11)27(21,25)26)23-16(24-15)22-12-4-2-1-3-5-12/h6-10,12H,1-5H2,(H2,21,25,26)(H,22,23,24) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.40 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibition of human COX2 expressed in baculovirus-infected SF9 cells assessed as inhibition of arachidonic acid-stimulated PGE2 production treated 1 ... |
Bioorg Med Chem Lett 19: 4504-8 (2009)
Article DOI: 10.1016/j.bmcl.2009.02.085
BindingDB Entry DOI: 10.7270/Q20Z7396 |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 2
(Homo sapiens (Human)) | BDBM50297664
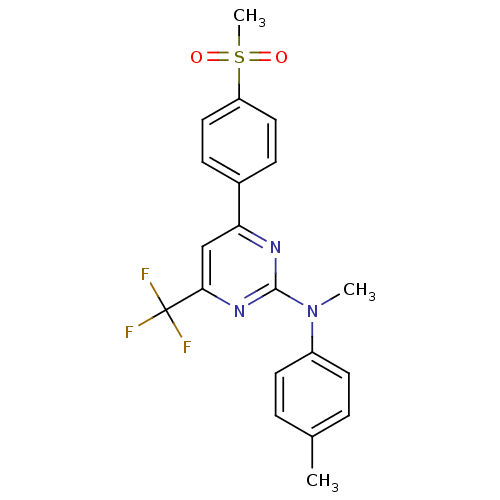
(CHEMBL559613 | N-methyl-4-(4-(methylsulfonyl)pheny...)Show SMILES CN(c1ccc(C)cc1)c1nc(cc(n1)C(F)(F)F)-c1ccc(cc1)S(C)(=O)=O Show InChI InChI=1S/C20H18F3N3O2S/c1-13-4-8-15(9-5-13)26(2)19-24-17(12-18(25-19)20(21,22)23)14-6-10-16(11-7-14)29(3,27)28/h4-12H,1-3H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibition of human COX2 expressed in baculovirus-infected SF9 cells assessed as inhibition of arachidonic acid-stimulated PGE2 production treated 1 ... |
Bioorg Med Chem Lett 19: 4504-8 (2009)
Article DOI: 10.1016/j.bmcl.2009.02.085
BindingDB Entry DOI: 10.7270/Q20Z7396 |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4-phosphate 5-kinase type-1 gamma
(Homo sapiens (Human)) | BDBM50599603

(CHEMBL5194835)Show SMILES CNC(=O)c1cc(Nc2ccccc2)cc(c1)-c1ccnc(NC(=O)C2CC2)c1 | Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 3.60 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.bmc.2021.116557
BindingDB Entry DOI: 10.7270/Q23J3J18 |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4-phosphate 5-kinase type-1 gamma
(Homo sapiens (Human)) | BDBM50599620

(CHEMBL5195505)Show SMILES CS(=O)(=O)Nc1cc(Nc2ccccc2)cc(c1)-c1ccnc(NC(=O)C2CCOCC2)c1 | Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 3.80 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.bmc.2021.116557
BindingDB Entry DOI: 10.7270/Q23J3J18 |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4-phosphate 5-kinase type-1 gamma
(Homo sapiens (Human)) | BDBM50599612

(CHEMBL5173406)Show SMILES NC(=O)CNC(=O)c1cc(Nc2ccccc2)cc(c1)-c1ccnc(NC(=O)C2CC2)c1 | Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 3.90 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.bmc.2021.116557
BindingDB Entry DOI: 10.7270/Q23J3J18 |
More data for this
Ligand-Target Pair | |
DNA polymerase theta
(Homo sapiens) | BDBM50591272
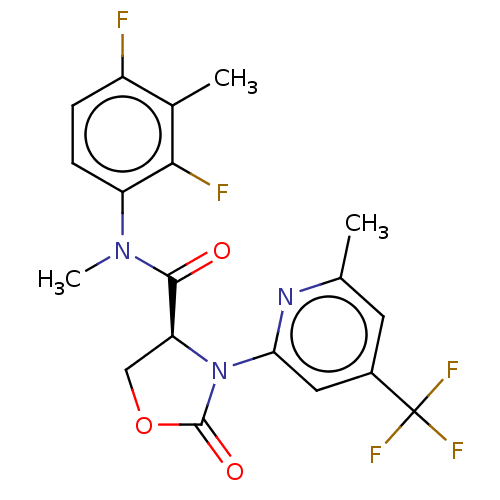
(CHEMBL5208956)Show SMILES CN(C(=O)[C@@H]1COC(=O)N1c1cc(cc(C)n1)C(F)(F)F)c1ccc(F)c(C)c1F |r| | PDB
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 4.10 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c01142
BindingDB Entry DOI: 10.7270/Q2Z03D46 |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 2
(Homo sapiens (Human)) | BDBM50297673
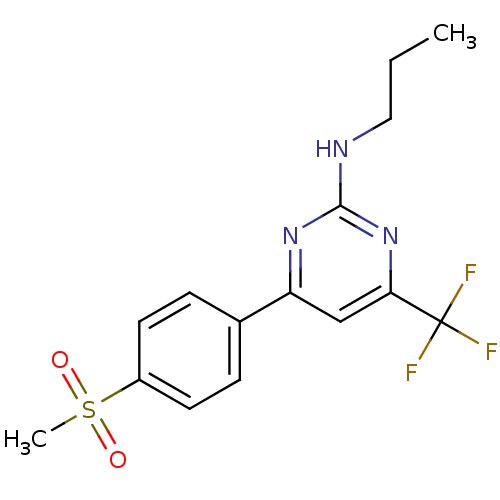
(4-(4-(methylsulfonyl)phenyl)-N-propyl-6-(trifluoro...)Show SMILES CCCNc1nc(cc(n1)C(F)(F)F)-c1ccc(cc1)S(C)(=O)=O Show InChI InChI=1S/C15H16F3N3O2S/c1-3-8-19-14-20-12(9-13(21-14)15(16,17)18)10-4-6-11(7-5-10)24(2,22)23/h4-7,9H,3,8H2,1-2H3,(H,19,20,21) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibition of human COX2 expressed in baculovirus-infected SF9 cells assessed as inhibition of arachidonic acid-stimulated PGE2 production treated 1 ... |
Bioorg Med Chem Lett 19: 4504-8 (2009)
Article DOI: 10.1016/j.bmcl.2009.02.085
BindingDB Entry DOI: 10.7270/Q20Z7396 |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4-kinase alpha
(Homo sapiens (Human)) | BDBM50107248

(CHEMBL3600781)Show SMILES O=c1cc(ccn1CC1CC1)-c1ccc2nc(NCC3CCOCC3)sc2c1 Show InChI InChI=1S/C22H25N3O2S/c26-21-12-18(5-8-25(21)14-16-1-2-16)17-3-4-19-20(11-17)28-22(24-19)23-13-15-6-9-27-10-7-15/h3-5,8,11-12,15-16H,1-2,6-7,9-10,13-14H2,(H,23,24) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of N-terminal FLAG-tagged human full length recombinant PI4K3alpha using D-myo-phosphatidylinositol substrate and ATP incubated for 45 min... |
Bioorg Med Chem Lett 25: 3189-93 (2015)
Article DOI: 10.1016/j.bmcl.2015.05.093
BindingDB Entry DOI: 10.7270/Q2KK9DKR |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 2
(Homo sapiens (Human)) | BDBM50297665
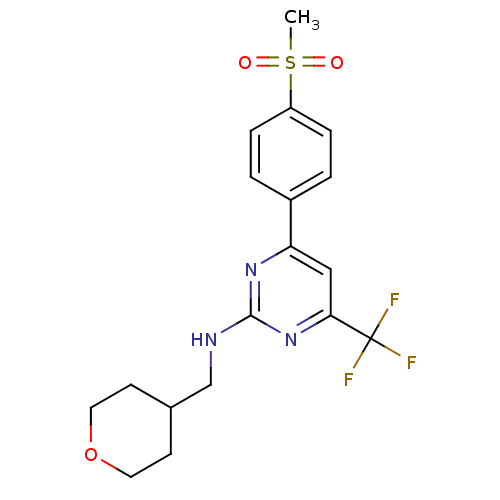
(4-(4-(methylsulfonyl)phenyl)-N-((tetrahydro-2H-pyr...)Show SMILES CS(=O)(=O)c1ccc(cc1)-c1cc(nc(NCC2CCOCC2)n1)C(F)(F)F Show InChI InChI=1S/C18H20F3N3O3S/c1-28(25,26)14-4-2-13(3-5-14)15-10-16(18(19,20)21)24-17(23-15)22-11-12-6-8-27-9-7-12/h2-5,10,12H,6-9,11H2,1H3,(H,22,23,24) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibition of human COX2 expressed in baculovirus-infected SF9 cells assessed as inhibition of arachidonic acid-stimulated PGE2 production treated 1 ... |
Bioorg Med Chem Lett 19: 4504-8 (2009)
Article DOI: 10.1016/j.bmcl.2009.02.085
BindingDB Entry DOI: 10.7270/Q20Z7396 |
More data for this
Ligand-Target Pair | |
DNA polymerase theta
(Homo sapiens) | BDBM50591280

(CHEMBL5205456)Show SMILES CN(C(=O)[C@@H]1COC(=O)N1c1cc(cc(C)n1)C(F)(F)F)c1ccc(F)c(Cl)c1F |r| | PDB
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 5.40 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c01142
BindingDB Entry DOI: 10.7270/Q2Z03D46 |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 2
(Homo sapiens (Human)) | BDBM50297680
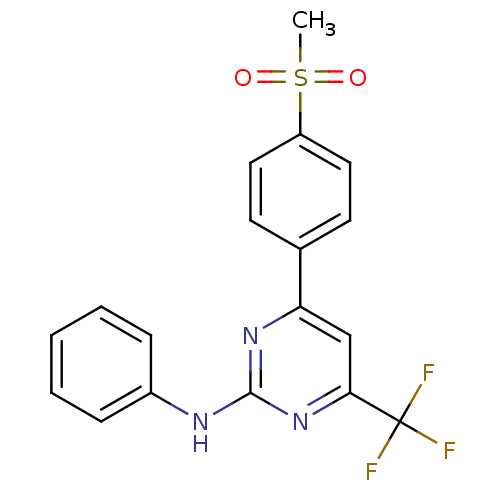
(4-(4-(methylsulfonyl)phenyl)-N-phenyl-6-(trifluoro...)Show SMILES CS(=O)(=O)c1ccc(cc1)-c1cc(nc(Nc2ccccc2)n1)C(F)(F)F Show InChI InChI=1S/C18H14F3N3O2S/c1-27(25,26)14-9-7-12(8-10-14)15-11-16(18(19,20)21)24-17(23-15)22-13-5-3-2-4-6-13/h2-11H,1H3,(H,22,23,24) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 5.60 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibition of human COX2 expressed in baculovirus-infected SF9 cells assessed as inhibition of arachidonic acid-stimulated PGE2 production treated 1 ... |
Bioorg Med Chem Lett 19: 4504-8 (2009)
Article DOI: 10.1016/j.bmcl.2009.02.085
BindingDB Entry DOI: 10.7270/Q20Z7396 |
More data for this
Ligand-Target Pair | |
DNA polymerase theta
(Homo sapiens) | BDBM50591283
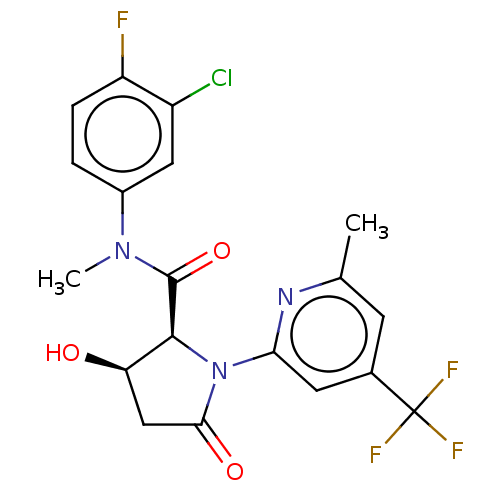
(CHEMBL5190089)Show SMILES CN(C(=O)[C@@H]1[C@H](O)CC(=O)N1c1cc(cc(C)n1)C(F)(F)F)c1ccc(F)c(Cl)c1 |r| | PDB
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 5.90 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c01142
BindingDB Entry DOI: 10.7270/Q2Z03D46 |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4-phosphate 5-kinase type-1 gamma
(Homo sapiens (Human)) | BDBM50599624

(CHEMBL5208791)Show SMILES CNc1cc(Nc2ccccc2)cc(c1)-c1ccnc(NC(=O)C2CCOCC2)c1 | Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 5.90 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.bmc.2021.116557
BindingDB Entry DOI: 10.7270/Q23J3J18 |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4-kinase alpha
(Homo sapiens (Human)) | BDBM50107243

(CHEMBL3600779)Show InChI InChI=1S/C16H15N3OS/c17-16-18-13-4-3-11(7-14(13)21-16)12-5-6-19(15(20)8-12)9-10-1-2-10/h3-8,10H,1-2,9H2,(H2,17,18) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 6.30 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of N-terminal FLAG-tagged human full length recombinant PI4K3alpha using D-myo-phosphatidylinositol substrate and ATP incubated for 45 min... |
Bioorg Med Chem Lett 25: 3189-93 (2015)
Article DOI: 10.1016/j.bmcl.2015.05.093
BindingDB Entry DOI: 10.7270/Q2KK9DKR |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4-phosphate 5-kinase type-1 gamma
(Homo sapiens (Human)) | BDBM50599613

(CHEMBL5202114)Show SMILES O=C(Nc1cc(ccn1)-c1cc(Nc2ccccc2)cc(c1)C(=O)NCC1OCCO1)C1CC1 | Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 6.70 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.bmc.2021.116557
BindingDB Entry DOI: 10.7270/Q23J3J18 |
More data for this
Ligand-Target Pair | |
DNA polymerase theta
(Homo sapiens) | BDBM50591246
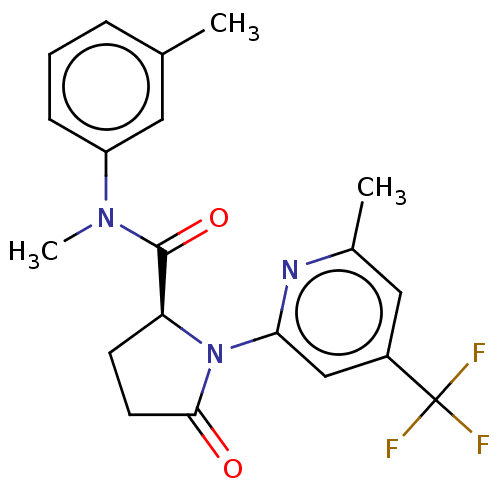
(CHEMBL5175531)Show SMILES CN(C(=O)[C@@H]1CCC(=O)N1c1cc(cc(C)n1)C(F)(F)F)c1cccc(C)c1 |r| | PDB
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 6.80 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c01142
BindingDB Entry DOI: 10.7270/Q2Z03D46 |
More data for this
Ligand-Target Pair | |
DNA polymerase theta
(Homo sapiens) | BDBM50591270
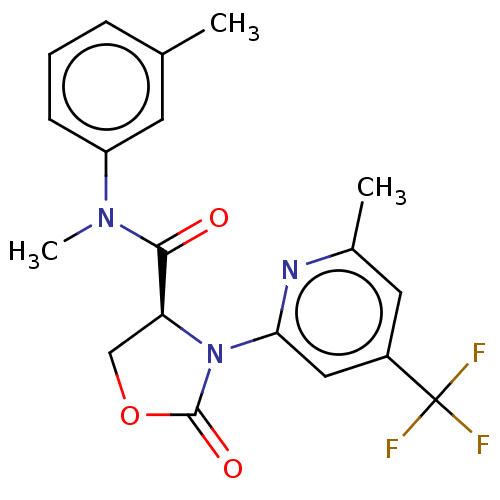
(CHEMBL5176919)Show SMILES CN(C(=O)[C@@H]1COC(=O)N1c1cc(cc(C)n1)C(F)(F)F)c1cccc(C)c1 |r| | PDB
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 7 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c01142
BindingDB Entry DOI: 10.7270/Q2Z03D46 |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 2
(Homo sapiens (Human)) | BDBM50297678
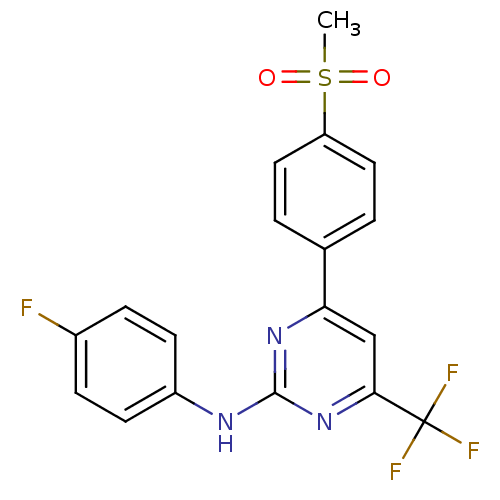
(CHEMBL561086 | N-(4-fluorophenyl)-4-(4-(methylsulf...)Show SMILES CS(=O)(=O)c1ccc(cc1)-c1cc(nc(Nc2ccc(F)cc2)n1)C(F)(F)F Show InChI InChI=1S/C18H13F4N3O2S/c1-28(26,27)14-8-2-11(3-9-14)15-10-16(18(20,21)22)25-17(24-15)23-13-6-4-12(19)5-7-13/h2-10H,1H3,(H,23,24,25) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 7.20 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibition of human COX2 expressed in baculovirus-infected SF9 cells assessed as inhibition of arachidonic acid-stimulated PGE2 production treated 1 ... |
Bioorg Med Chem Lett 19: 4504-8 (2009)
Article DOI: 10.1016/j.bmcl.2009.02.085
BindingDB Entry DOI: 10.7270/Q20Z7396 |
More data for this
Ligand-Target Pair | |
DNA polymerase theta
(Homo sapiens) | BDBM50591285
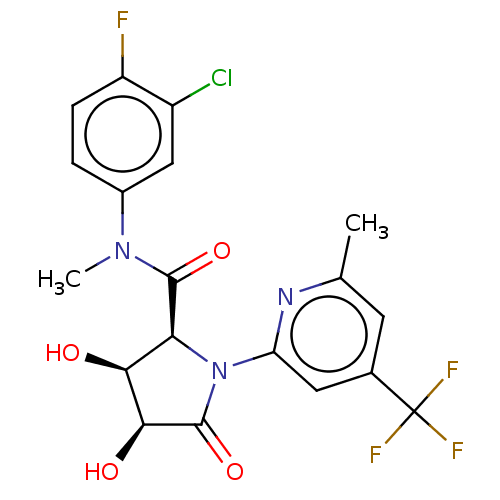
(CHEMBL5191330)Show SMILES CN(C(=O)[C@@H]1[C@H](O)[C@H](O)C(=O)N1c1cc(cc(C)n1)C(F)(F)F)c1ccc(F)c(Cl)c1 |r| | PDB
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 7.60 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c01142
BindingDB Entry DOI: 10.7270/Q2Z03D46 |
More data for this
Ligand-Target Pair | |
DNA polymerase theta
(Homo sapiens) | BDBM50591248

(CHEMBL5206992)Show SMILES CN(C(=O)[C@@H]1[C@H](O)CCN1c1nc(C)cc(c1C#N)C(F)(F)F)c1cccc(C)c1 |r| | PDB
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
PDB
UniChem
| Article
PubMed
| n/a | n/a | 7.90 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c01142
BindingDB Entry DOI: 10.7270/Q2Z03D46 |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4-kinase alpha
(Homo sapiens (Human)) | BDBM50107254

(CHEMBL3600782)Show SMILES C[C@@H](Nc1nc2ccc(cc2s1)-c1ccn(CC2CC2)c(=O)c1)C1CCOCC1 |r| Show InChI InChI=1S/C23H27N3O2S/c1-15(17-7-10-28-11-8-17)24-23-25-20-5-4-18(12-21(20)29-23)19-6-9-26(22(27)13-19)14-16-2-3-16/h4-6,9,12-13,15-17H,2-3,7-8,10-11,14H2,1H3,(H,24,25)/t15-/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 7.90 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of N-terminal FLAG-tagged human full length recombinant PI4K3alpha using D-myo-phosphatidylinositol substrate and ATP incubated for 45 min... |
Bioorg Med Chem Lett 25: 3189-93 (2015)
Article DOI: 10.1016/j.bmcl.2015.05.093
BindingDB Entry DOI: 10.7270/Q2KK9DKR |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 2
(Homo sapiens (Human)) | BDBM50297677

(CHEMBL551829 | N-benzyl-4-(4-(methylsulfonyl)pheny...)Show SMILES CS(=O)(=O)c1ccc(cc1)-c1cc(nc(NCc2ccccc2)n1)C(F)(F)F Show InChI InChI=1S/C19H16F3N3O2S/c1-28(26,27)15-9-7-14(8-10-15)16-11-17(19(20,21)22)25-18(24-16)23-12-13-5-3-2-4-6-13/h2-11H,12H2,1H3,(H,23,24,25) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 8 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibition of COX2 in human whole blood assessed as inhibition of lipopolysaccharide-stimulated PGE2 production after 24 hrs by enzyme immunoassay |
Bioorg Med Chem Lett 19: 4504-8 (2009)
Article DOI: 10.1016/j.bmcl.2009.02.085
BindingDB Entry DOI: 10.7270/Q20Z7396 |
More data for this
Ligand-Target Pair | |
DNA polymerase theta
(Homo sapiens) | BDBM50591255

(CHEMBL5180095)Show SMILES CC(C)N(C(=O)[C@@H]1CCCN1c1nc(C)cc(c1C#N)C(F)(F)F)c1cccc(C)c1 |r| | PDB
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 8.80 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c01142
BindingDB Entry DOI: 10.7270/Q2Z03D46 |
More data for this
Ligand-Target Pair | |
DNA polymerase theta
(Homo sapiens) | BDBM50591271

(CHEMBL5200410)Show SMILES CN(C(=O)[C@@H]1COC(=O)N1c1cc(cc(C)n1)C(F)(F)F)c1ccc(F)c(C)c1 |r| | PDB
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 9.40 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c01142
BindingDB Entry DOI: 10.7270/Q2Z03D46 |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4-phosphate 5-kinase type-1 gamma
(Homo sapiens (Human)) | BDBM50599594
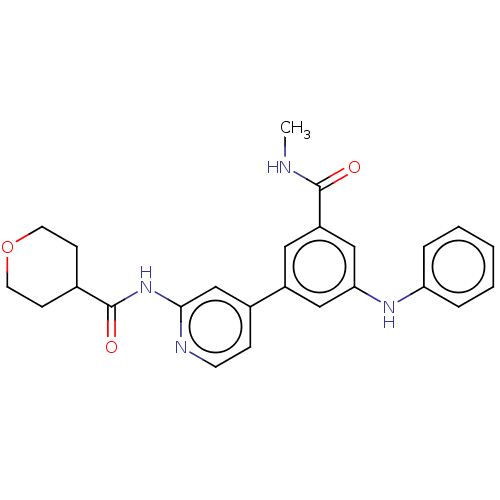
(CHEMBL5171272)Show SMILES CNC(=O)c1cc(Nc2ccccc2)cc(c1)-c1ccnc(NC(=O)C2CCOCC2)c1 | Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 9.5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.bmc.2021.116557
BindingDB Entry DOI: 10.7270/Q23J3J18 |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 2
(Homo sapiens (Human)) | BDBM50297684
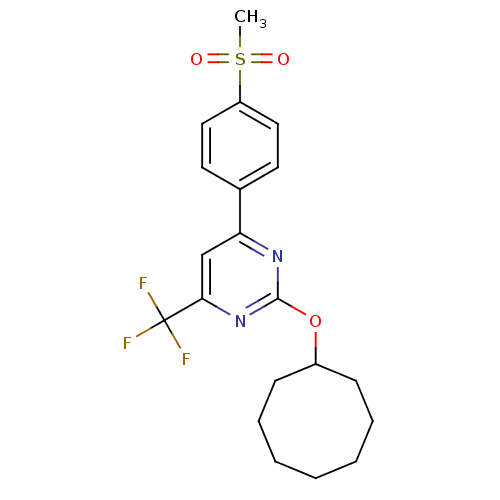
(2-(cyclooctyloxy)-4-(4-(methylsulfonyl)phenyl)-6-(...)Show SMILES CS(=O)(=O)c1ccc(cc1)-c1cc(nc(OC2CCCCCCC2)n1)C(F)(F)F Show InChI InChI=1S/C20H23F3N2O3S/c1-29(26,27)16-11-9-14(10-12-16)17-13-18(20(21,22)23)25-19(24-17)28-15-7-5-3-2-4-6-8-15/h9-13,15H,2-8H2,1H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | <10 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibition of human COX2 expressed in baculovirus-infected SF9 cells assessed as inhibition of arachidonic acid-stimulated PGE2 production treated 1 ... |
Bioorg Med Chem Lett 19: 4504-8 (2009)
Article DOI: 10.1016/j.bmcl.2009.02.085
BindingDB Entry DOI: 10.7270/Q20Z7396 |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 2
(Homo sapiens (Human)) | BDBM50297676

(CHEMBL551830 | N-(4-methylbenzyl)-4-(4-(methylsulf...)Show SMILES Cc1ccc(CNc2nc(cc(n2)C(F)(F)F)-c2ccc(cc2)S(C)(=O)=O)cc1 Show InChI InChI=1S/C20H18F3N3O2S/c1-13-3-5-14(6-4-13)12-24-19-25-17(11-18(26-19)20(21,22)23)15-7-9-16(10-8-15)29(2,27)28/h3-11H,12H2,1-2H3,(H,24,25,26) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | <10 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibition of human COX2 expressed in african green monkey COS cells assessed as inhibition of arachidonic acid-stimulated PGE2 production treated 1 ... |
Bioorg Med Chem Lett 19: 4504-8 (2009)
Article DOI: 10.1016/j.bmcl.2009.02.085
BindingDB Entry DOI: 10.7270/Q20Z7396 |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 2
(Homo sapiens (Human)) | BDBM50297686
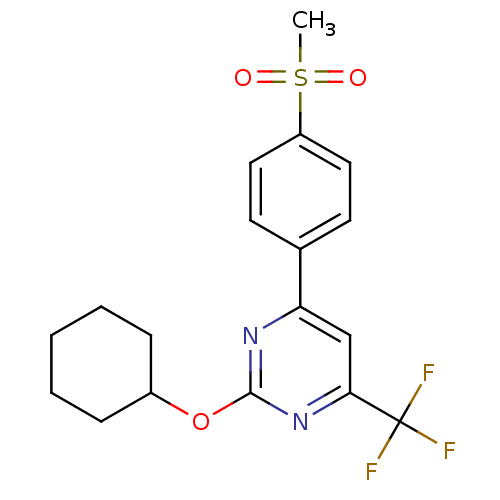
(2-(cyclohexyloxy)-4-(4-(methylsulfonyl)phenyl)-6-(...)Show SMILES CS(=O)(=O)c1ccc(cc1)-c1cc(nc(OC2CCCCC2)n1)C(F)(F)F Show InChI InChI=1S/C18H19F3N2O3S/c1-27(24,25)14-9-7-12(8-10-14)15-11-16(18(19,20)21)23-17(22-15)26-13-5-3-2-4-6-13/h7-11,13H,2-6H2,1H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | <10 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibition of human COX2 expressed in baculovirus-infected SF9 cells assessed as inhibition of arachidonic acid-stimulated PGE2 production treated 1 ... |
Bioorg Med Chem Lett 19: 4504-8 (2009)
Article DOI: 10.1016/j.bmcl.2009.02.085
BindingDB Entry DOI: 10.7270/Q20Z7396 |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 2
(Homo sapiens (Human)) | BDBM50297688

(2-cyclobutoxy-4-(4-(methylsulfonyl)phenyl)-6-(trif...)Show SMILES CS(=O)(=O)c1ccc(cc1)-c1cc(nc(OC2CCC2)n1)C(F)(F)F Show InChI InChI=1S/C16H15F3N2O3S/c1-25(22,23)12-7-5-10(6-8-12)13-9-14(16(17,18)19)21-15(20-13)24-11-3-2-4-11/h5-9,11H,2-4H2,1H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | <10 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibition of human COX2 expressed in baculovirus-infected SF9 cells assessed as inhibition of arachidonic acid-stimulated PGE2 production treated 1 ... |
Bioorg Med Chem Lett 19: 4504-8 (2009)
Article DOI: 10.1016/j.bmcl.2009.02.085
BindingDB Entry DOI: 10.7270/Q20Z7396 |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 2
(Homo sapiens (Human)) | BDBM50297685
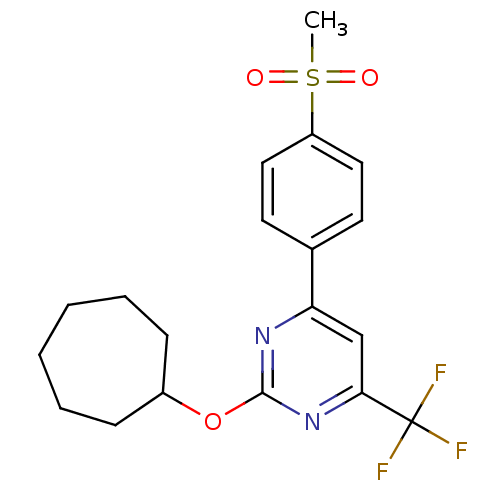
(2-(cycloheptyloxy)-4-(4-(methylsulfonyl)phenyl)-6-...)Show SMILES CS(=O)(=O)c1ccc(cc1)-c1cc(nc(OC2CCCCCC2)n1)C(F)(F)F Show InChI InChI=1S/C19H21F3N2O3S/c1-28(25,26)15-10-8-13(9-11-15)16-12-17(19(20,21)22)24-18(23-16)27-14-6-4-2-3-5-7-14/h8-12,14H,2-7H2,1H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | <10 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibition of human COX2 expressed in baculovirus-infected SF9 cells assessed as inhibition of arachidonic acid-stimulated PGE2 production treated 1 ... |
Bioorg Med Chem Lett 19: 4504-8 (2009)
Article DOI: 10.1016/j.bmcl.2009.02.085
BindingDB Entry DOI: 10.7270/Q20Z7396 |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data