 Found 3606 hits with Last Name = 'sweeney' and Initial = 'zk'
Found 3606 hits with Last Name = 'sweeney' and Initial = 'zk' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit delta isoform
(Homo sapiens (Human)) | BDBM50438628
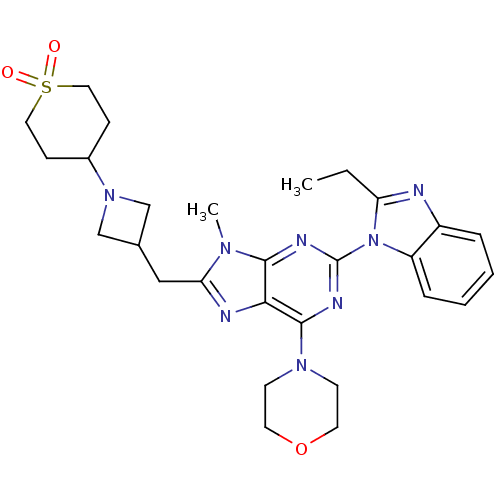
(CHEMBL2414299)Show SMILES CCc1nc2ccccc2n1-c1nc(N2CCOCC2)c2nc(CC3CN(C3)C3CCS(=O)(=O)CC3)n(C)c2n1 Show InChI InChI=1S/C28H36N8O3S/c1-3-23-29-21-6-4-5-7-22(21)36(23)28-31-26-25(27(32-28)34-10-12-39-13-11-34)30-24(33(26)2)16-19-17-35(18-19)20-8-14-40(37,38)15-9-20/h4-7,19-20H,3,8-18H2,1-2H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.230 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech
Curated by ChEMBL
| Assay Description
Inhibition of PI3Kdelta (unknown origin) assessed as formation of PIP3 by competitive fluorescence polarization assay |
Bioorg Med Chem Lett 23: 4953-9 (2013)
Article DOI: 10.1016/j.bmcl.2013.06.052
BindingDB Entry DOI: 10.7270/Q2ZG6TP9 |
More data for this
Ligand-Target Pair | |
Leucine-rich repeat serine/threonine-protein kinase 2
(Homo sapiens (Human)) | BDBM182716
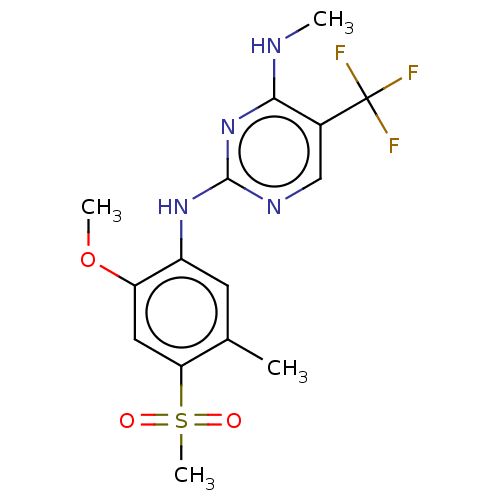
(US9145402, 23 | US9145402, 36)Show SMILES CNc1nc(Nc2cc(C)c(cc2OC)S(C)(=O)=O)ncc1C(F)(F)F Show InChI InChI=1S/C15H17F3N4O3S/c1-8-5-10(11(25-3)6-12(8)26(4,23)24)21-14-20-7-9(15(16,17)18)13(19-2)22-14/h5-7H,1-4H3,(H2,19,20,21,22) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| 0.300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech, Inc.
US Patent
| Assay Description
his assay was used to determine a compound's potency in inhibiting activity of LRRK2 by determining, Kiapp, IC50, or percent inhibition values. In 38... |
US Patent US9145402 (2015)
BindingDB Entry DOI: 10.7270/Q2J67FQ3 |
More data for this
Ligand-Target Pair | |
Leucine-rich repeat serine/threonine-protein kinase 2
(Homo sapiens (Human)) | BDBM196757

(US9212173, 35)Show SMILES CCNc1nc(Nc2cn(nc2C)C(C)(C)c2ccnn2C)ncc1C(F)(F)F Show InChI InChI=1S/C18H23F3N8/c1-6-22-15-12(18(19,20)21)9-23-16(26-15)25-13-10-29(27-11(13)2)17(3,4)14-7-8-24-28(14)5/h7-10H,6H2,1-5H3,(H2,22,23,25,26) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| 0.300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech, Inc.
US Patent
| Assay Description
This assay was used to determine a compound's potency in inhibiting activity of LRRK2 by determining, Kiapp, IC50, or percent inhibition values. In 3... |
US Patent US9212173 (2015)
BindingDB Entry DOI: 10.7270/Q2222SKT |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit delta isoform
(Homo sapiens (Human)) | BDBM50438634
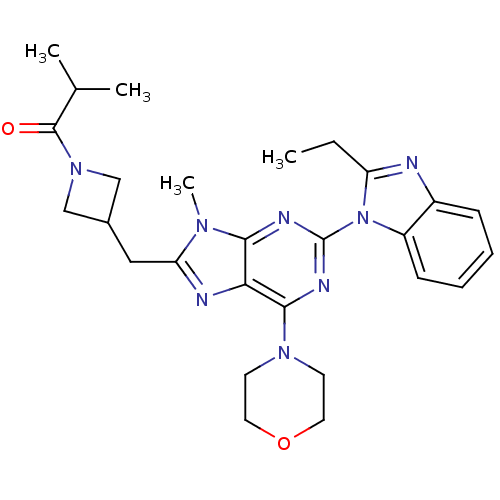
(CHEMBL2414301)Show SMILES CCc1nc2ccccc2n1-c1nc(N2CCOCC2)c2nc(CC3CN(C3)C(=O)C(C)C)n(C)c2n1 Show InChI InChI=1S/C27H34N8O2/c1-5-21-28-19-8-6-7-9-20(19)35(21)27-30-24-23(25(31-27)33-10-12-37-13-11-33)29-22(32(24)4)14-18-15-34(16-18)26(36)17(2)3/h6-9,17-18H,5,10-16H2,1-4H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.360 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech
Curated by ChEMBL
| Assay Description
Inhibition of PI3Kdelta (unknown origin) assessed as formation of PIP3 by competitive fluorescence polarization assay |
Bioorg Med Chem Lett 23: 4953-9 (2013)
Article DOI: 10.1016/j.bmcl.2013.06.052
BindingDB Entry DOI: 10.7270/Q2ZG6TP9 |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit delta isoform
(Homo sapiens (Human)) | BDBM50403075
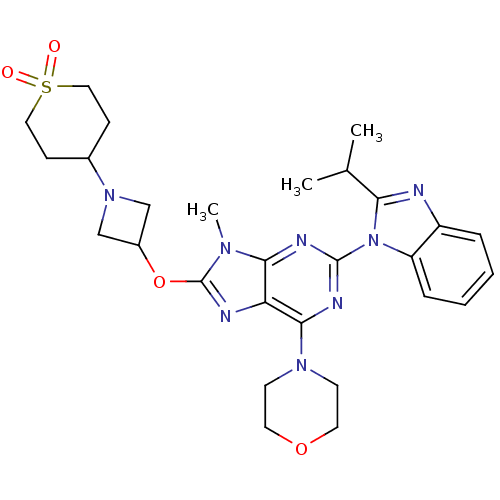
(CHEMBL2216902)Show SMILES CC(C)c1nc2ccccc2n1-c1nc(N2CCOCC2)c2nc(OC3CN(C3)C3CCS(=O)(=O)CC3)n(C)c2n1 Show InChI InChI=1S/C28H36N8O4S/c1-18(2)24-29-21-6-4-5-7-22(21)36(24)27-31-25-23(26(32-27)34-10-12-39-13-11-34)30-28(33(25)3)40-20-16-35(17-20)19-8-14-41(37,38)15-9-19/h4-7,18-20H,8-17H2,1-3H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.470 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech
Curated by ChEMBL
| Assay Description
Inhibition of PI3Kdelta (unknown origin) assessed as formation of PIP3 by competitive fluorescence polarization assay |
Bioorg Med Chem Lett 23: 4953-9 (2013)
Article DOI: 10.1016/j.bmcl.2013.06.052
BindingDB Entry DOI: 10.7270/Q2ZG6TP9 |
More data for this
Ligand-Target Pair | |
Leucine-rich repeat serine/threonine-protein kinase 2
(Homo sapiens (Human)) | BDBM50507669

(CHEMBL4539170)Show InChI InChI=1S/C17H22N6O/c1-3-12-10-14(22-23(12)2)16-15-13(20-21-16)4-7-18-17(15)19-11-5-8-24-9-6-11/h4,7,10-11H,3,5-6,8-9H2,1-2H3,(H,18,19)(H,20,21) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human GST-tagged LRRK2 G2019S mutant catalytic domain (970 to 2527 residues) expressed in insect cells using RtGWWRFYTLRRAR... |
Bioorg Med Chem Lett 29: 674-680 (2019)
Article DOI: 10.1016/j.bmcl.2018.10.017
BindingDB Entry DOI: 10.7270/Q2HH6PCX |
More data for this
Ligand-Target Pair | |
Leucine-rich repeat serine/threonine-protein kinase 2
(Homo sapiens (Human)) | BDBM196754
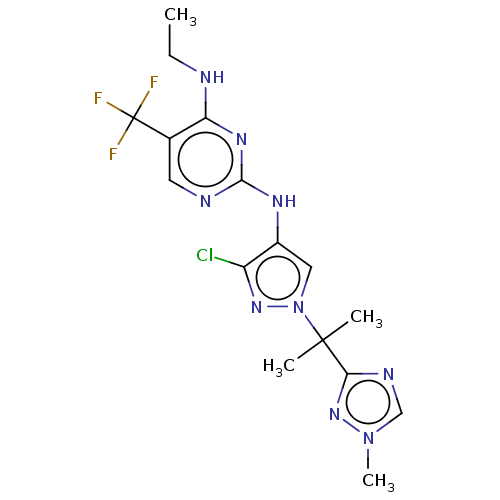
(US9212173, 32)Show SMILES CCNc1nc(Nc2cn(nc2Cl)C(C)(C)c2ncn(C)n2)ncc1C(F)(F)F Show InChI InChI=1S/C16H19ClF3N9/c1-5-21-12-9(16(18,19)20)6-22-14(25-12)24-10-7-29(26-11(10)17)15(2,3)13-23-8-28(4)27-13/h6-8H,5H2,1-4H3,(H2,21,22,24,25) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| 0.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech, Inc.
US Patent
| Assay Description
This assay was used to determine a compound's potency in inhibiting activity of LRRK2 by determining, Kiapp, IC50, or percent inhibition values. In 3... |
US Patent US9212173 (2015)
BindingDB Entry DOI: 10.7270/Q2222SKT |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit delta isoform
(Homo sapiens (Human)) | BDBM50403074
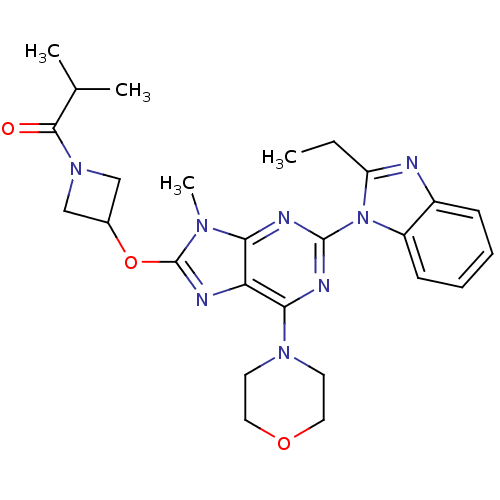
(CHEMBL2216903)Show SMILES CCc1nc2ccccc2n1-c1nc(N2CCOCC2)c2nc(OC3CN(C3)C(=O)C(C)C)n(C)c2n1 Show InChI InChI=1S/C26H32N8O3/c1-5-20-27-18-8-6-7-9-19(18)34(20)25-29-22-21(23(30-25)32-10-12-36-13-11-32)28-26(31(22)4)37-17-14-33(15-17)24(35)16(2)3/h6-9,16-17H,5,10-15H2,1-4H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.580 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech
Curated by ChEMBL
| Assay Description
Inhibition of PI3Kdelta (unknown origin) assessed as formation of PIP3 by competitive fluorescence polarization assay |
Bioorg Med Chem Lett 23: 4953-9 (2013)
Article DOI: 10.1016/j.bmcl.2013.06.052
BindingDB Entry DOI: 10.7270/Q2ZG6TP9 |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit delta isoform
(Homo sapiens (Human)) | BDBM50438630
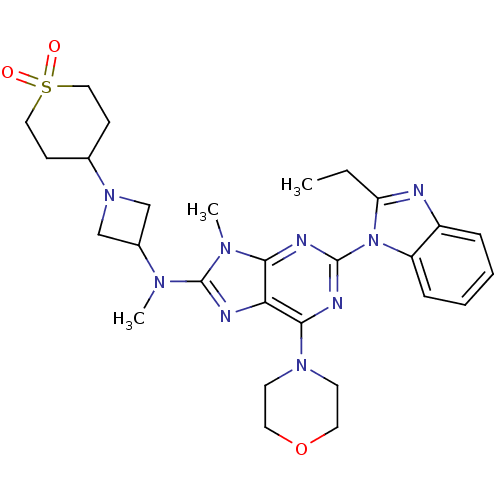
(CHEMBL2414297)Show SMILES CCc1nc2ccccc2n1-c1nc(N2CCOCC2)c2nc(N(C)C3CN(C3)C3CCS(=O)(=O)CC3)n(C)c2n1 Show InChI InChI=1S/C28H37N9O3S/c1-4-23-29-21-7-5-6-8-22(21)37(23)27-31-25-24(26(32-27)35-11-13-40-14-12-35)30-28(34(25)3)33(2)20-17-36(18-20)19-9-15-41(38,39)16-10-19/h5-8,19-20H,4,9-18H2,1-3H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.590 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech
Curated by ChEMBL
| Assay Description
Inhibition of PI3Kdelta (unknown origin) assessed as formation of PIP3 by competitive fluorescence polarization assay |
Bioorg Med Chem Lett 23: 4953-9 (2013)
Article DOI: 10.1016/j.bmcl.2013.06.052
BindingDB Entry DOI: 10.7270/Q2ZG6TP9 |
More data for this
Ligand-Target Pair | |
Leucine-rich repeat serine/threonine-protein kinase 2
(Homo sapiens (Human)) | BDBM129199
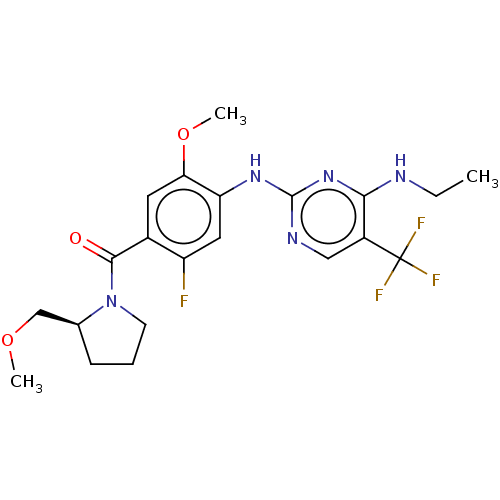
(US8802674, 306)Show SMILES CCNc1nc(Nc2cc(F)c(cc2OC)C(=O)N2CCC[C@H]2COC)ncc1C(F)(F)F |r| Show InChI InChI=1S/C21H25F4N5O3/c1-4-26-18-14(21(23,24)25)10-27-20(29-18)28-16-9-15(22)13(8-17(16)33-3)19(31)30-7-5-6-12(30)11-32-2/h8-10,12H,4-7,11H2,1-3H3,(H2,26,27,28,29)/t12-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| 0.600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech, Inc.
US Patent
| Assay Description
This assay was used to determine a compound's potency in inhibiting activity of LRRK2 by determining, Kiapp, IC50, or percent inhibition values. ... |
US Patent US8802674 (2014)
BindingDB Entry DOI: 10.7270/Q2GF0S6N |
More data for this
Ligand-Target Pair | |
Leucine-rich repeat serine/threonine-protein kinase 2
(Homo sapiens (Human)) | BDBM129179
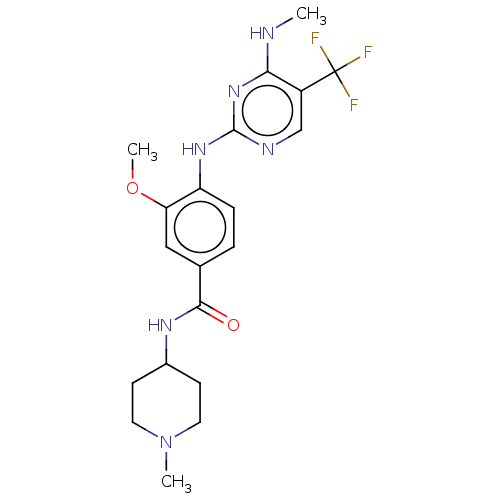
(US8802674, 282)Show SMILES CNc1nc(Nc2ccc(cc2OC)C(=O)NC2CCN(C)CC2)ncc1C(F)(F)F Show InChI InChI=1S/C20H25F3N6O2/c1-24-17-14(20(21,22)23)11-25-19(28-17)27-15-5-4-12(10-16(15)31-3)18(30)26-13-6-8-29(2)9-7-13/h4-5,10-11,13H,6-9H2,1-3H3,(H,26,30)(H2,24,25,27,28) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| 0.600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech, Inc.
US Patent
| Assay Description
This assay was used to determine a compound's potency in inhibiting activity of LRRK2 by determining, Kiapp, IC50, or percent inhibition values. ... |
US Patent US8802674 (2014)
BindingDB Entry DOI: 10.7270/Q2GF0S6N |
More data for this
Ligand-Target Pair | |
Leucine-rich repeat serine/threonine-protein kinase 2
(Homo sapiens (Human)) | BDBM196762

(US9212173, 40)Show SMILES CNc1nc(Nc2cnn(C3CCOC[C@@H]3F)c2C)ncc1C(F)(F)F |r| Show InChI InChI=1S/C15H18F4N6O/c1-8-11(6-22-25(8)12-3-4-26-7-10(12)16)23-14-21-5-9(15(17,18)19)13(20-2)24-14/h5-6,10,12H,3-4,7H2,1-2H3,(H2,20,21,23,24)/t10-,12?/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| 0.600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech, Inc.
US Patent
| Assay Description
This assay was used to determine a compound's potency in inhibiting activity of LRRK2 by determining, Kiapp, IC50, or percent inhibition values. In 3... |
US Patent US9212173 (2015)
BindingDB Entry DOI: 10.7270/Q2222SKT |
More data for this
Ligand-Target Pair | |
Leucine-rich repeat serine/threonine-protein kinase 2
(Homo sapiens (Human)) | BDBM196759
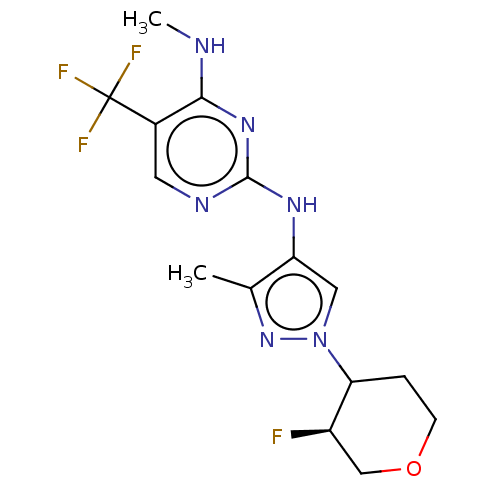
(US9212173, 37)Show SMILES CNc1nc(Nc2cn(nc2C)C2CCOC[C@H]2F)ncc1C(F)(F)F |r| Show InChI InChI=1S/C15H18F4N6O/c1-8-11(6-25(24-8)12-3-4-26-7-10(12)16)22-14-21-5-9(15(17,18)19)13(20-2)23-14/h5-6,10,12H,3-4,7H2,1-2H3,(H2,20,21,22,23)/t10-,12?/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| 0.600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech, Inc.
US Patent
| Assay Description
This assay was used to determine a compound's potency in inhibiting activity of LRRK2 by determining, Kiapp, IC50, or percent inhibition values. In 3... |
US Patent US9212173 (2015)
BindingDB Entry DOI: 10.7270/Q2222SKT |
More data for this
Ligand-Target Pair | |
Leucine-rich repeat serine/threonine-protein kinase 2
(Homo sapiens (Human)) | BDBM196752
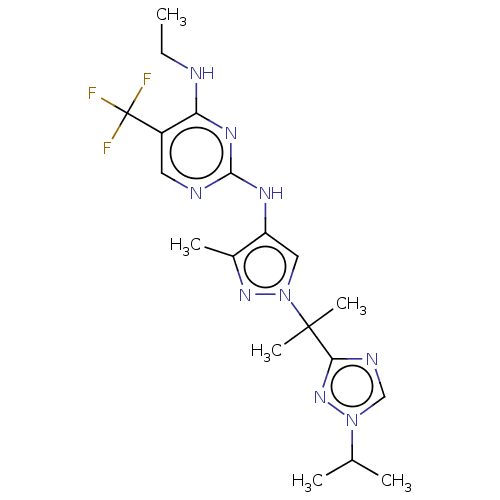
(US9212173, 30)Show SMILES CCNc1nc(Nc2cn(nc2C)C(C)(C)c2ncn(n2)C(C)C)ncc1C(F)(F)F Show InChI InChI=1S/C19H26F3N9/c1-7-23-15-13(19(20,21)22)8-24-17(27-15)26-14-9-31(28-12(14)4)18(5,6)16-25-10-30(29-16)11(2)3/h8-11H,7H2,1-6H3,(H2,23,24,26,27) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| 0.600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech, Inc.
US Patent
| Assay Description
This assay was used to determine a compound's potency in inhibiting activity of LRRK2 by determining, Kiapp, IC50, or percent inhibition values. In 3... |
US Patent US9212173 (2015)
BindingDB Entry DOI: 10.7270/Q2222SKT |
More data for this
Ligand-Target Pair | |
Leucine-rich repeat serine/threonine-protein kinase 2
(Homo sapiens (Human)) | BDBM50448127
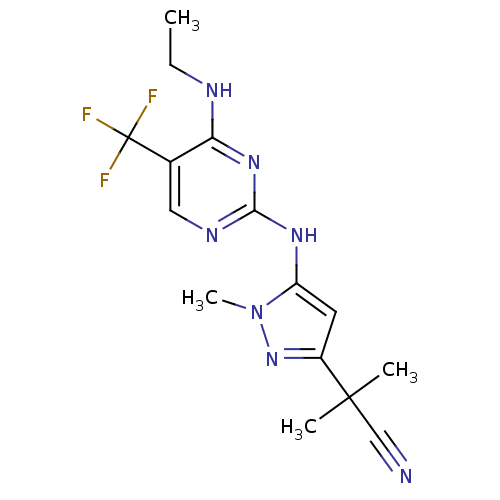
(CHEMBL3122119 | US9212173, 44)Show InChI InChI=1S/C15H18F3N7/c1-5-20-12-9(15(16,17)18)7-21-13(23-12)22-11-6-10(24-25(11)4)14(2,3)8-19/h6-7H,5H2,1-4H3,(H2,20,21,22,23) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| US Patent
| 0.700 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech, Inc.
US Patent
| Assay Description
This assay was used to determine a compound's potency in inhibiting activity of LRRK2 by determining, Kiapp, IC50, or percent inhibition values. In 3... |
US Patent US9212173 (2015)
BindingDB Entry DOI: 10.7270/Q2222SKT |
More data for this
Ligand-Target Pair | |
Leucine-rich repeat serine/threonine-protein kinase 2
(Homo sapiens (Human)) | BDBM50448118
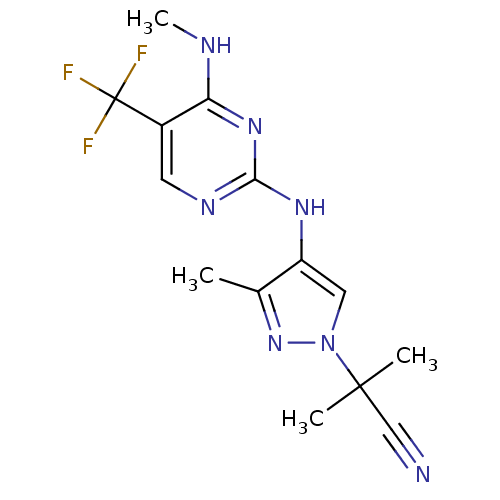
(CHEMBL3122113 | US10590114, No. 80 | US11111235, N...)Show InChI InChI=1S/C14H16F3N7/c1-8-10(6-24(23-8)13(2,3)7-18)21-12-20-5-9(14(15,16)17)11(19-4)22-12/h5-6H,1-4H3,(H2,19,20,21,22) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| 0.700 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech
Curated by ChEMBL
| Assay Description
Inhibition of LRRK2 (unknown origin) |
J Med Chem 57: 921-36 (2014)
Article DOI: 10.1021/jm401654j
BindingDB Entry DOI: 10.7270/Q2N0181N |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Leucine-rich repeat serine/threonine-protein kinase 2
(Homo sapiens (Human)) | BDBM50448127

(CHEMBL3122119 | US9212173, 44)Show InChI InChI=1S/C15H18F3N7/c1-5-20-12-9(15(16,17)18)7-21-13(23-12)22-11-6-10(24-25(11)4)14(2,3)8-19/h6-7H,5H2,1-4H3,(H2,20,21,22,23) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| 0.700 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech
Curated by ChEMBL
| Assay Description
Inhibition of LRRK2 (unknown origin) |
J Med Chem 57: 921-36 (2014)
Article DOI: 10.1021/jm401654j
BindingDB Entry DOI: 10.7270/Q2N0181N |
More data for this
Ligand-Target Pair | |
Leucine-rich repeat serine/threonine-protein kinase 2
(Homo sapiens (Human)) | BDBM129173
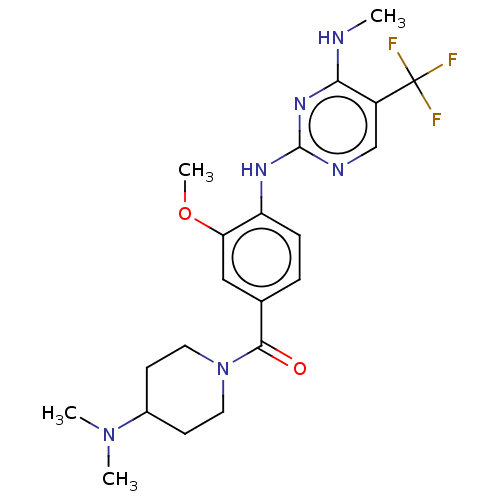
(US8802674, 276)Show SMILES CNc1nc(Nc2ccc(cc2OC)C(=O)N2CCC(CC2)N(C)C)ncc1C(F)(F)F Show InChI InChI=1S/C21H27F3N6O2/c1-25-18-15(21(22,23)24)12-26-20(28-18)27-16-6-5-13(11-17(16)32-4)19(31)30-9-7-14(8-10-30)29(2)3/h5-6,11-12,14H,7-10H2,1-4H3,(H2,25,26,27,28) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| 0.800 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech, Inc.
US Patent
| Assay Description
This assay was used to determine a compound's potency in inhibiting activity of LRRK2 by determining, Kiapp, IC50, or percent inhibition values. ... |
US Patent US8802674 (2014)
BindingDB Entry DOI: 10.7270/Q2GF0S6N |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit delta isoform
(Homo sapiens (Human)) | BDBM50438633
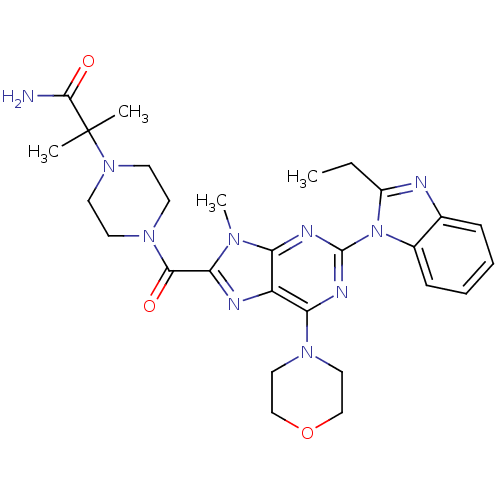
(CHEMBL2414294)Show SMILES CCc1nc2ccccc2n1-c1nc(N2CCOCC2)c2nc(C(=O)N3CCN(CC3)C(C)(C)C(N)=O)n(C)c2n1 Show InChI InChI=1S/C28H36N10O3/c1-5-20-30-18-8-6-7-9-19(18)38(20)27-32-22-21(23(33-27)35-14-16-41-17-15-35)31-24(34(22)4)25(39)36-10-12-37(13-11-36)28(2,3)26(29)40/h6-9H,5,10-17H2,1-4H3,(H2,29,40) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.800 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech
Curated by ChEMBL
| Assay Description
Inhibition of PI3Kdelta (unknown origin) assessed as formation of PIP3 by competitive fluorescence polarization assay |
Bioorg Med Chem Lett 23: 4953-9 (2013)
Article DOI: 10.1016/j.bmcl.2013.06.052
BindingDB Entry DOI: 10.7270/Q2ZG6TP9 |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit delta isoform
(Homo sapiens (Human)) | BDBM50438635
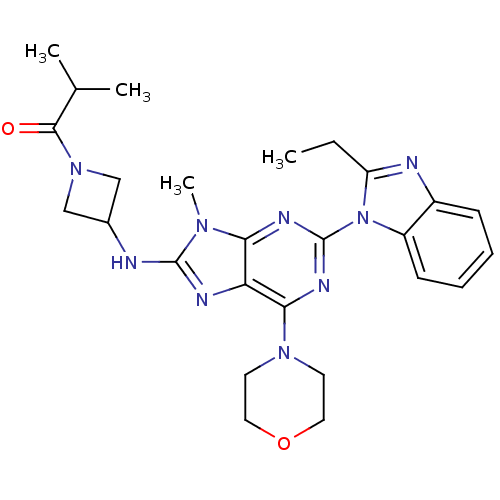
(CHEMBL2414300)Show SMILES CCc1nc2ccccc2n1-c1nc(N2CCOCC2)c2nc(NC3CN(C3)C(=O)C(C)C)n(C)c2n1 Show InChI InChI=1S/C26H33N9O2/c1-5-20-28-18-8-6-7-9-19(18)35(20)26-30-22-21(23(31-26)33-10-12-37-13-11-33)29-25(32(22)4)27-17-14-34(15-17)24(36)16(2)3/h6-9,16-17H,5,10-15H2,1-4H3,(H,27,29) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.880 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech
Curated by ChEMBL
| Assay Description
Inhibition of PI3Kdelta (unknown origin) assessed as formation of PIP3 by competitive fluorescence polarization assay |
Bioorg Med Chem Lett 23: 4953-9 (2013)
Article DOI: 10.1016/j.bmcl.2013.06.052
BindingDB Entry DOI: 10.7270/Q2ZG6TP9 |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit delta isoform
(Homo sapiens (Human)) | BDBM50438629
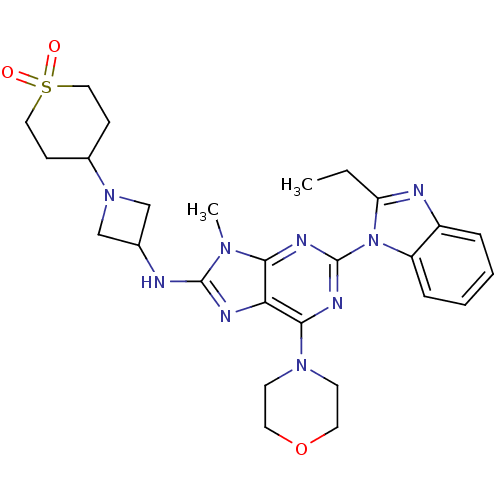
(CHEMBL2414298)Show SMILES CCc1nc2ccccc2n1-c1nc(N2CCOCC2)c2nc(NC3CN(C3)C3CCS(=O)(=O)CC3)n(C)c2n1 Show InChI InChI=1S/C27H35N9O3S/c1-3-22-29-20-6-4-5-7-21(20)36(22)27-31-24-23(25(32-27)34-10-12-39-13-11-34)30-26(33(24)2)28-18-16-35(17-18)19-8-14-40(37,38)15-9-19/h4-7,18-19H,3,8-17H2,1-2H3,(H,28,30) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.900 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech
Curated by ChEMBL
| Assay Description
Inhibition of PI3Kdelta (unknown origin) assessed as formation of PIP3 by competitive fluorescence polarization assay |
Bioorg Med Chem Lett 23: 4953-9 (2013)
Article DOI: 10.1016/j.bmcl.2013.06.052
BindingDB Entry DOI: 10.7270/Q2ZG6TP9 |
More data for this
Ligand-Target Pair | |
Leucine-rich repeat serine/threonine-protein kinase 2
(Homo sapiens (Human)) | BDBM50448126
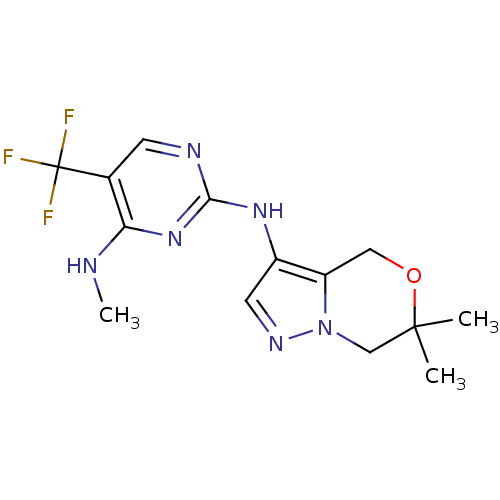
(CHEMBL3122105 | US9212186, 22)Show InChI InChI=1S/C14H17F3N6O/c1-13(2)7-23-10(6-24-13)9(5-20-23)21-12-19-4-8(14(15,16)17)11(18-3)22-12/h4-5H,6-7H2,1-3H3,(H2,18,19,21,22) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.900 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech
Curated by ChEMBL
| Assay Description
Inhibition of LRRK2 (unknown origin) |
J Med Chem 57: 921-36 (2014)
Article DOI: 10.1021/jm401654j
BindingDB Entry DOI: 10.7270/Q2N0181N |
More data for this
Ligand-Target Pair | |
Leucine-rich repeat serine/threonine-protein kinase 2
(Homo sapiens (Human)) | BDBM196742
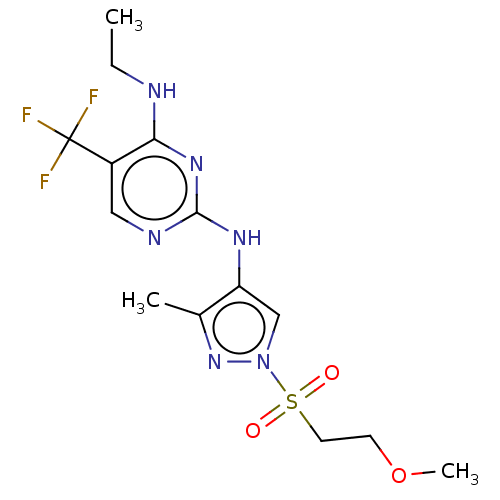
(US9212173, 20)Show SMILES CCNc1nc(Nc2cn(nc2C)S(=O)(=O)CCOC)ncc1C(F)(F)F Show InChI InChI=1S/C14H19F3N6O3S/c1-4-18-12-10(14(15,16)17)7-19-13(21-12)20-11-8-23(22-9(11)2)27(24,25)6-5-26-3/h7-8H,4-6H2,1-3H3,(H2,18,19,20,21) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| 0.960 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech, Inc.
US Patent
| Assay Description
This assay was used to determine a compound's potency in inhibiting activity of LRRK2 by determining, Kiapp, IC50, or percent inhibition values. In 3... |
US Patent US9212173 (2015)
BindingDB Entry DOI: 10.7270/Q2222SKT |
More data for this
Ligand-Target Pair | |
Leucine-rich repeat serine/threonine-protein kinase 2
(Homo sapiens (Human)) | BDBM196741
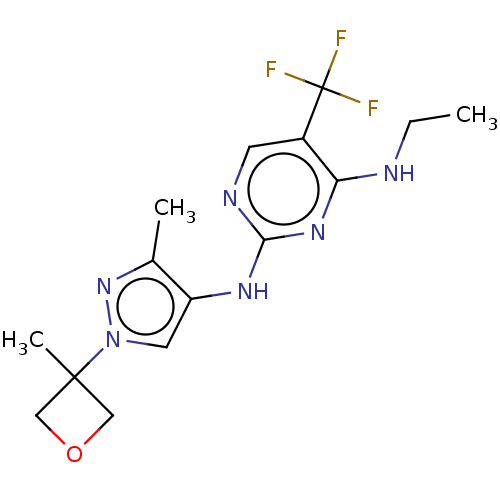
(US9212173, 19)Show InChI InChI=1S/C15H19F3N6O/c1-4-19-12-10(15(16,17)18)5-20-13(22-12)21-11-6-24(23-9(11)2)14(3)7-25-8-14/h5-6H,4,7-8H2,1-3H3,(H2,19,20,21,22) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| 0.975 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech, Inc.
US Patent
| Assay Description
This assay was used to determine a compound's potency in inhibiting activity of LRRK2 by determining, Kiapp, IC50, or percent inhibition values. In 3... |
US Patent US9212173 (2015)
BindingDB Entry DOI: 10.7270/Q2222SKT |
More data for this
Ligand-Target Pair | |
Leucine-rich repeat serine/threonine-protein kinase 2
(Homo sapiens (Human)) | BDBM128254

(US8796296, 9)Show SMILES CNc1nc(Nc2cc3CN(C)C(=O)c3cc2Cl)ncc1C(F)(F)F Show InChI InChI=1S/C15H13ClF3N5O/c1-20-12-9(15(17,18)19)5-21-14(23-12)22-11-3-7-6-24(2)13(25)8(7)4-10(11)16/h3-5H,6H2,1-2H3,(H2,20,21,22,23) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech, Inc.
US Patent
| Assay Description
his assay was used to determine a compound's potency in inhibiting activity of LRRK2 by determining, Kiapp, IC50, or percent inhibition values. In 38... |
US Patent US8796296 (2014)
BindingDB Entry DOI: 10.7270/Q28W3C0R |
More data for this
Ligand-Target Pair | |
Leucine-rich repeat serine/threonine-protein kinase 2
(Homo sapiens (Human)) | BDBM128273
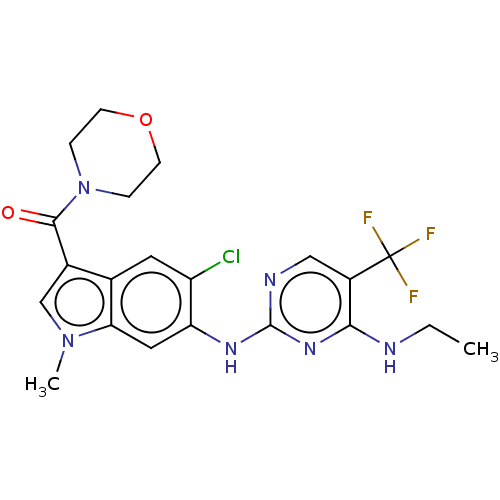
(US8796296, 29)Show SMILES CCNc1nc(Nc2cc3n(C)cc(C(=O)N4CCOCC4)c3cc2Cl)ncc1C(F)(F)F Show InChI InChI=1S/C21H22ClF3N6O2/c1-3-26-18-14(21(23,24)25)10-27-20(29-18)28-16-9-17-12(8-15(16)22)13(11-30(17)2)19(32)31-4-6-33-7-5-31/h8-11H,3-7H2,1-2H3,(H2,26,27,28,29) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech, Inc.
US Patent
| Assay Description
his assay was used to determine a compound's potency in inhibiting activity of LRRK2 by determining, Kiapp, IC50, or percent inhibition values. In 38... |
US Patent US8796296 (2014)
BindingDB Entry DOI: 10.7270/Q28W3C0R |
More data for this
Ligand-Target Pair | |
Leucine-rich repeat serine/threonine-protein kinase 2
(Homo sapiens (Human)) | BDBM128275
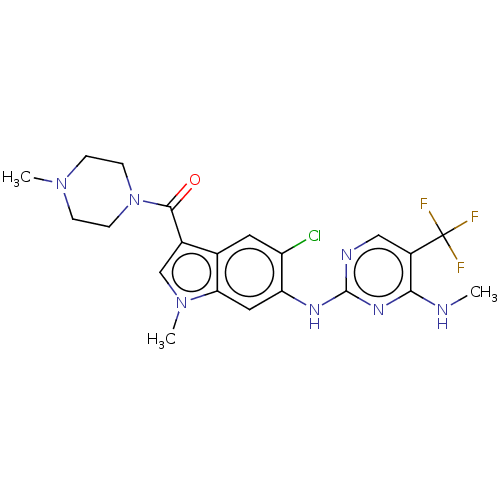
(US8796296, 31)Show SMILES CNc1nc(Nc2cc3n(C)cc(C(=O)N4CCN(C)CC4)c3cc2Cl)ncc1C(F)(F)F Show InChI InChI=1S/C21H23ClF3N7O/c1-26-18-14(21(23,24)25)10-27-20(29-18)28-16-9-17-12(8-15(16)22)13(11-31(17)3)19(33)32-6-4-30(2)5-7-32/h8-11H,4-7H2,1-3H3,(H2,26,27,28,29) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech, Inc.
US Patent
| Assay Description
his assay was used to determine a compound's potency in inhibiting activity of LRRK2 by determining, Kiapp, IC50, or percent inhibition values. In 38... |
US Patent US8796296 (2014)
BindingDB Entry DOI: 10.7270/Q28W3C0R |
More data for this
Ligand-Target Pair | |
Leucine-rich repeat serine/threonine-protein kinase 2
(Homo sapiens (Human)) | BDBM128276
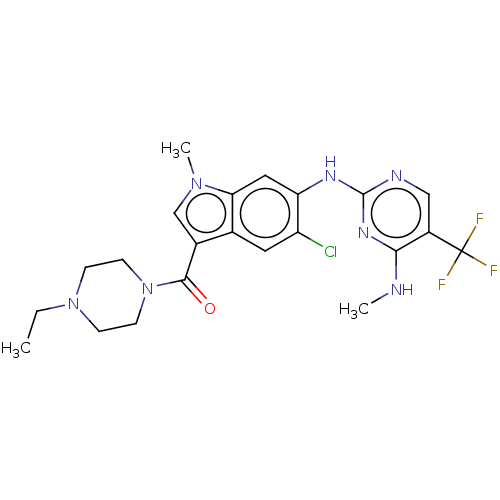
(US8796296, 32)Show SMILES CCN1CCN(CC1)C(=O)c1cn(C)c2cc(Nc3ncc(c(NC)n3)C(F)(F)F)c(Cl)cc12 Show InChI InChI=1S/C22H25ClF3N7O/c1-4-32-5-7-33(8-6-32)20(34)14-12-31(3)18-10-17(16(23)9-13(14)18)29-21-28-11-15(22(24,25)26)19(27-2)30-21/h9-12H,4-8H2,1-3H3,(H2,27,28,29,30) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech, Inc.
US Patent
| Assay Description
his assay was used to determine a compound's potency in inhibiting activity of LRRK2 by determining, Kiapp, IC50, or percent inhibition values. In 38... |
US Patent US8796296 (2014)
BindingDB Entry DOI: 10.7270/Q28W3C0R |
More data for this
Ligand-Target Pair | |
Leucine-rich repeat serine/threonine-protein kinase 2
(Homo sapiens (Human)) | BDBM50396148

(CHEMBL2171745 | US8802674, 50)Show SMILES CNc1nc(Nc2ccc(cc2OC)C(=O)N2CCOCC2)ncc1C(F)(F)F Show InChI InChI=1S/C18H20F3N5O3/c1-22-15-12(18(19,20)21)10-23-17(25-15)24-13-4-3-11(9-14(13)28-2)16(27)26-5-7-29-8-6-26/h3-4,9-10H,5-8H2,1-2H3,(H2,22,23,24,25) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech
Curated by ChEMBL
| Assay Description
Inhibition of LRRK2 |
J Med Chem 55: 5536-45 (2012)
Article DOI: 10.1021/jm300452p
BindingDB Entry DOI: 10.7270/Q2RR20CQ |
More data for this
Ligand-Target Pair | |
Leucine-rich repeat serine/threonine-protein kinase 2
(Homo sapiens (Human)) | BDBM127895
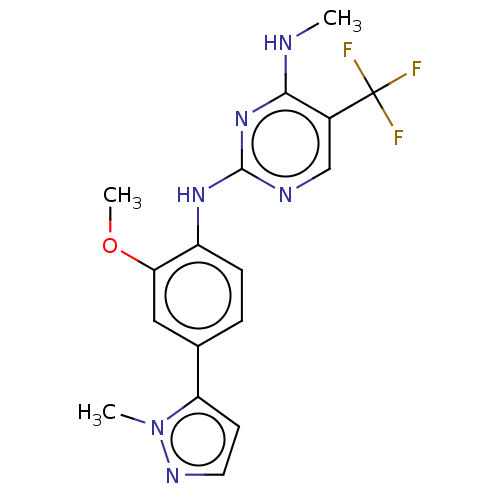
(US8791130, 25)Show InChI InChI=1S/C17H17F3N6O/c1-21-15-11(17(18,19)20)9-22-16(25-15)24-12-5-4-10(8-14(12)27-3)13-6-7-23-26(13)2/h4-9H,1-3H3,(H2,21,22,24,25) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech, Inc.
US Patent
| Assay Description
This assay was used to determine a compound's potency in inhibiting activity of LRRK2 by determining, Kiapp, IC50, or percent inhibition values. ... |
US Patent US8791130 (2014)
BindingDB Entry DOI: 10.7270/Q2K9367R |
More data for this
Ligand-Target Pair | |
Leucine-rich repeat serine/threonine-protein kinase 2
(Homo sapiens (Human)) | BDBM127896
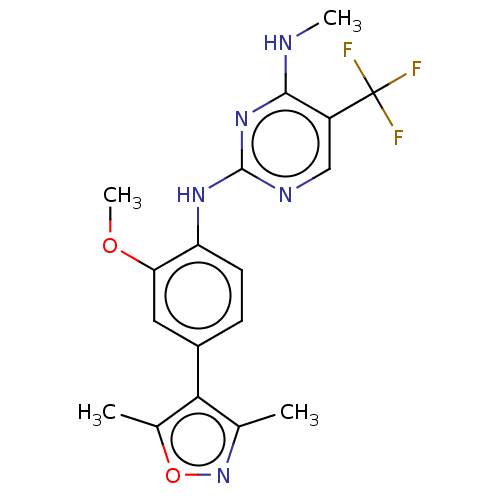
(US8791130, 26)Show SMILES CNc1nc(Nc2ccc(cc2OC)-c2c(C)noc2C)ncc1C(F)(F)F Show InChI InChI=1S/C18H18F3N5O2/c1-9-15(10(2)28-26-9)11-5-6-13(14(7-11)27-4)24-17-23-8-12(18(19,20)21)16(22-3)25-17/h5-8H,1-4H3,(H2,22,23,24,25) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech, Inc.
US Patent
| Assay Description
This assay was used to determine a compound's potency in inhibiting activity of LRRK2 by determining, Kiapp, IC50, or percent inhibition values. ... |
US Patent US8791130 (2014)
BindingDB Entry DOI: 10.7270/Q2K9367R |
More data for this
Ligand-Target Pair | |
Leucine-rich repeat serine/threonine-protein kinase 2
(Homo sapiens (Human)) | BDBM127911

(US8791130, 41)Show SMILES CNc1nc(Nc2cc(F)c(cc2OC)-c2cnn(C)c2C(=O)N(C)C)ncc1C(F)(F)F Show InChI InChI=1S/C20H21F4N7O2/c1-25-17-12(20(22,23)24)9-26-19(29-17)28-14-7-13(21)10(6-15(14)33-5)11-8-27-31(4)16(11)18(32)30(2)3/h6-9H,1-5H3,(H2,25,26,28,29) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech, Inc.
US Patent
| Assay Description
This assay was used to determine a compound's potency in inhibiting activity of LRRK2 by determining, Kiapp, IC50, or percent inhibition values. ... |
US Patent US8791130 (2014)
BindingDB Entry DOI: 10.7270/Q2K9367R |
More data for this
Ligand-Target Pair | |
Leucine-rich repeat serine/threonine-protein kinase 2
(Homo sapiens (Human)) | BDBM127915
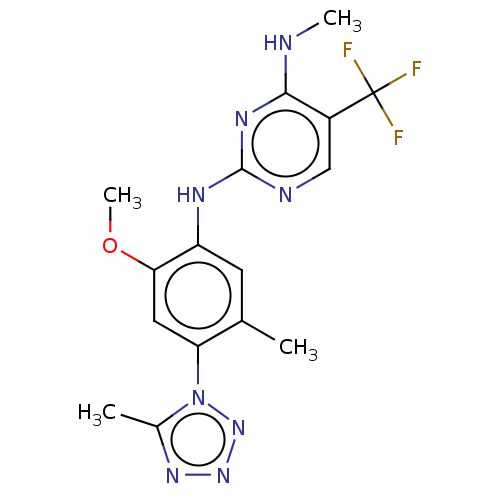
(US8791130, 45)Show SMILES CNc1nc(Nc2cc(C)c(cc2OC)-n2nnnc2C)ncc1C(F)(F)F Show InChI InChI=1S/C16H17F3N8O/c1-8-5-11(13(28-4)6-12(8)27-9(2)24-25-26-27)22-15-21-7-10(16(17,18)19)14(20-3)23-15/h5-7H,1-4H3,(H2,20,21,22,23) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech, Inc.
US Patent
| Assay Description
This assay was used to determine a compound's potency in inhibiting activity of LRRK2 by determining, Kiapp, IC50, or percent inhibition values. ... |
US Patent US8791130 (2014)
BindingDB Entry DOI: 10.7270/Q2K9367R |
More data for this
Ligand-Target Pair | |
Leucine-rich repeat serine/threonine-protein kinase 2
(Homo sapiens (Human)) | BDBM127916
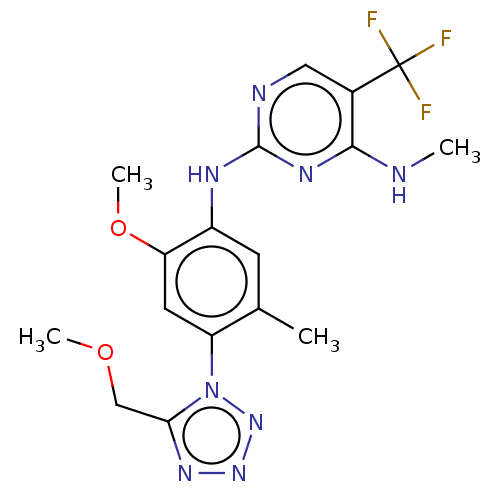
(US8791130, 46)Show SMILES CNc1nc(Nc2cc(C)c(cc2OC)-n2nnnc2COC)ncc1C(F)(F)F Show InChI InChI=1S/C17H19F3N8O2/c1-9-5-11(23-16-22-7-10(17(18,19)20)15(21-2)24-16)13(30-4)6-12(9)28-14(8-29-3)25-26-27-28/h5-7H,8H2,1-4H3,(H2,21,22,23,24) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech, Inc.
US Patent
| Assay Description
This assay was used to determine a compound's potency in inhibiting activity of LRRK2 by determining, Kiapp, IC50, or percent inhibition values. ... |
US Patent US8791130 (2014)
BindingDB Entry DOI: 10.7270/Q2K9367R |
More data for this
Ligand-Target Pair | |
Leucine-rich repeat serine/threonine-protein kinase 2
(Homo sapiens (Human)) | BDBM128247

(US8796296, 2)Show SMILES CNc1nc(Nc2cc3OC(C)(C)C(=O)N(C)c3cc2OC)ncc1Cl Show InChI InChI=1S/C17H20ClN5O3/c1-17(2)15(24)23(4)11-7-12(25-5)10(6-13(11)26-17)21-16-20-8-9(18)14(19-3)22-16/h6-8H,1-5H3,(H2,19,20,21,22) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech, Inc.
US Patent
| Assay Description
his assay was used to determine a compound's potency in inhibiting activity of LRRK2 by determining, Kiapp, IC50, or percent inhibition values. In 38... |
US Patent US8796296 (2014)
BindingDB Entry DOI: 10.7270/Q28W3C0R |
More data for this
Ligand-Target Pair | |
Leucine-rich repeat serine/threonine-protein kinase 2
(Homo sapiens (Human)) | BDBM128248

(US8796296, 3)Show SMILES CNc1nc(Nc2cc3oc(cc3cc2OC)C(=O)N(C)C)ncc1C(F)(F)F Show InChI InChI=1S/C18H18F3N5O3/c1-22-15-10(18(19,20)21)8-23-17(25-15)24-11-7-12-9(5-13(11)28-4)6-14(29-12)16(27)26(2)3/h5-8H,1-4H3,(H2,22,23,24,25) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech, Inc.
US Patent
| Assay Description
his assay was used to determine a compound's potency in inhibiting activity of LRRK2 by determining, Kiapp, IC50, or percent inhibition values. In 38... |
US Patent US8796296 (2014)
BindingDB Entry DOI: 10.7270/Q28W3C0R |
More data for this
Ligand-Target Pair | |
Leucine-rich repeat serine/threonine-protein kinase 2
(Homo sapiens (Human)) | BDBM50448117
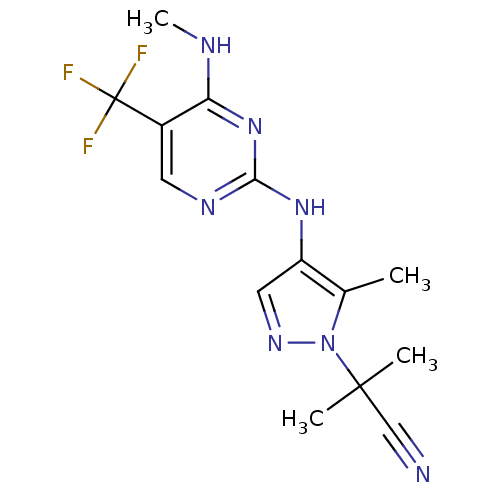
(CHEMBL3122114)Show InChI InChI=1S/C14H16F3N7/c1-8-10(6-21-24(8)13(2,3)7-18)22-12-20-5-9(14(15,16)17)11(19-4)23-12/h5-6H,1-4H3,(H2,19,20,22,23) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech
Curated by ChEMBL
| Assay Description
Inhibition of LRRK2 (unknown origin) |
J Med Chem 57: 921-36 (2014)
Article DOI: 10.1021/jm401654j
BindingDB Entry DOI: 10.7270/Q2N0181N |
More data for this
Ligand-Target Pair | |
Leucine-rich repeat serine/threonine-protein kinase 2
(Homo sapiens (Human)) | BDBM50398676
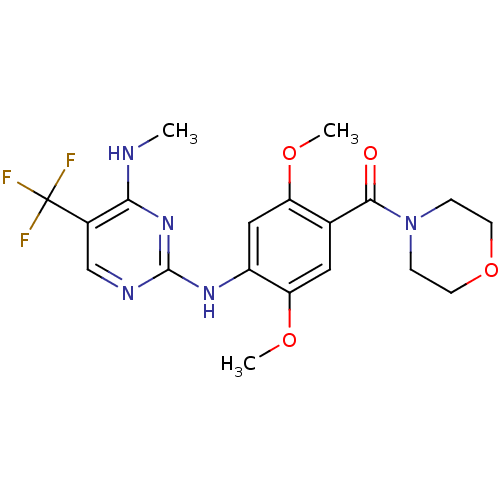
(CHEMBL2178125)Show SMILES CNc1nc(Nc2cc(OC)c(cc2OC)C(=O)N2CCOCC2)ncc1C(F)(F)F Show InChI InChI=1S/C19H22F3N5O4/c1-23-16-12(19(20,21)22)10-24-18(26-16)25-13-9-14(29-2)11(8-15(13)30-3)17(28)27-4-6-31-7-5-27/h8-10H,4-7H2,1-3H3,(H2,23,24,25,26) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech
Curated by ChEMBL
| Assay Description
Inhibition of LRRK2 using FAM-LRRKtide as substrate after 120 mins by microfluidic capillary electrophoresis assay |
J Med Chem 55: 9416-33 (2012)
Article DOI: 10.1021/jm301020q
BindingDB Entry DOI: 10.7270/Q2P55PN7 |
More data for this
Ligand-Target Pair | |
Leucine-rich repeat serine/threonine-protein kinase 2
(Homo sapiens (Human)) | BDBM182713
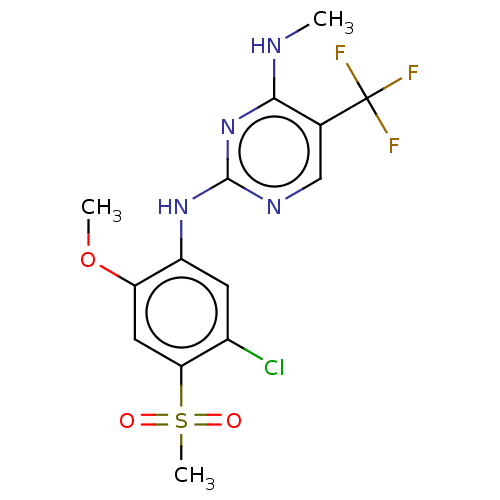
(US9145402, 20)Show SMILES CNc1nc(Nc2cc(Cl)c(cc2OC)S(C)(=O)=O)ncc1C(F)(F)F Show InChI InChI=1S/C14H14ClF3N4O3S/c1-19-12-7(14(16,17)18)6-20-13(22-12)21-9-4-8(15)11(26(3,23)24)5-10(9)25-2/h4-6H,1-3H3,(H2,19,20,21,22) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech, Inc.
US Patent
| Assay Description
his assay was used to determine a compound's potency in inhibiting activity of LRRK2 by determining, Kiapp, IC50, or percent inhibition values. In 38... |
US Patent US9145402 (2015)
BindingDB Entry DOI: 10.7270/Q2J67FQ3 |
More data for this
Ligand-Target Pair | |
Leucine-rich repeat serine/threonine-protein kinase 2
(Homo sapiens (Human)) | BDBM50398668
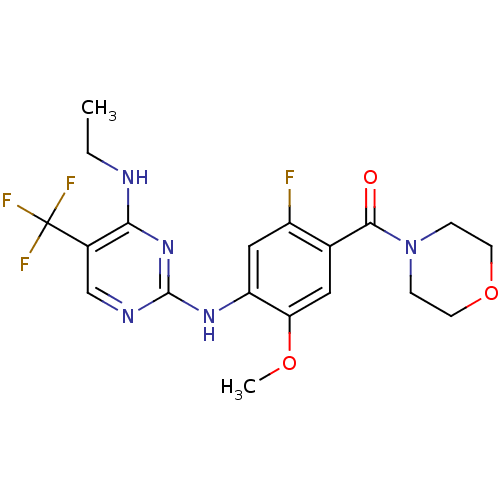
(CHEMBL2178134 | US8802674, 256)Show SMILES CCNc1nc(Nc2cc(F)c(cc2OC)C(=O)N2CCOCC2)ncc1C(F)(F)F Show InChI InChI=1S/C19H21F4N5O3/c1-3-24-16-12(19(21,22)23)10-25-18(27-16)26-14-9-13(20)11(8-15(14)30-2)17(29)28-4-6-31-7-5-28/h8-10H,3-7H2,1-2H3,(H2,24,25,26,27) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech
Curated by ChEMBL
| Assay Description
Inhibition of LRRK2 using FAM-LRRKtide as substrate after 120 mins by microfluidic capillary electrophoresis assay |
J Med Chem 55: 9416-33 (2012)
Article DOI: 10.1021/jm301020q
BindingDB Entry DOI: 10.7270/Q2P55PN7 |
More data for this
Ligand-Target Pair | |
Leucine-rich repeat serine/threonine-protein kinase 2
(Homo sapiens (Human)) | BDBM50398667

(CHEMBL2178135)Show SMILES COc1cc(C(=O)N2CCOCC2)c(F)cc1Nc1ncc(c(NC2CC2)n1)C(F)(F)F Show InChI InChI=1S/C20H21F4N5O3/c1-31-16-8-12(18(30)29-4-6-32-7-5-29)14(21)9-15(16)27-19-25-10-13(20(22,23)24)17(28-19)26-11-2-3-11/h8-11H,2-7H2,1H3,(H2,25,26,27,28) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech
Curated by ChEMBL
| Assay Description
Inhibition of LRRK2 using FAM-LRRKtide as substrate after 120 mins by microfluidic capillary electrophoresis assay |
J Med Chem 55: 9416-33 (2012)
Article DOI: 10.1021/jm301020q
BindingDB Entry DOI: 10.7270/Q2P55PN7 |
More data for this
Ligand-Target Pair | |
Leucine-rich repeat serine/threonine-protein kinase 2
(Homo sapiens (Human)) | BDBM50398662
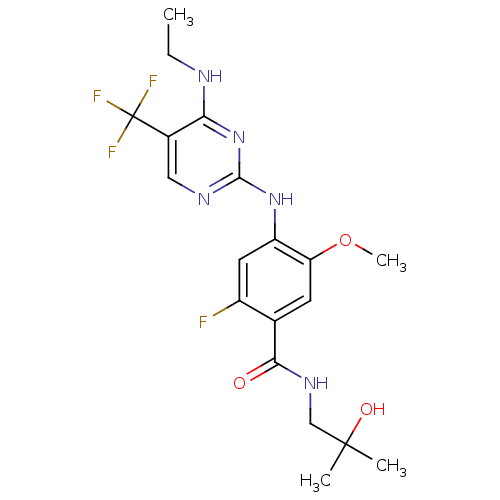
(CHEMBL2178140)Show SMILES CCNc1nc(Nc2cc(F)c(cc2OC)C(=O)NCC(C)(C)O)ncc1C(F)(F)F Show InChI InChI=1S/C19H23F4N5O3/c1-5-24-15-11(19(21,22)23)8-25-17(28-15)27-13-7-12(20)10(6-14(13)31-4)16(29)26-9-18(2,3)30/h6-8,30H,5,9H2,1-4H3,(H,26,29)(H2,24,25,27,28) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech
Curated by ChEMBL
| Assay Description
Inhibition of LRRK2 using FAM-LRRKtide as substrate after 120 mins by microfluidic capillary electrophoresis assay |
J Med Chem 55: 9416-33 (2012)
Article DOI: 10.1021/jm301020q
BindingDB Entry DOI: 10.7270/Q2P55PN7 |
More data for this
Ligand-Target Pair | |
Leucine-rich repeat serine/threonine-protein kinase 2
(Homo sapiens (Human)) | BDBM50396150
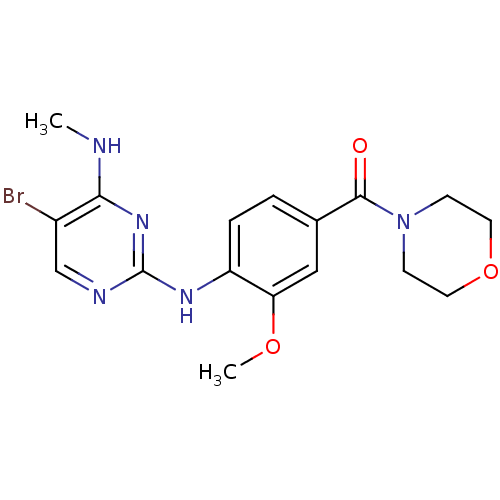
(CHEMBL2171743)Show InChI InChI=1S/C17H20BrN5O3/c1-19-15-12(18)10-20-17(22-15)21-13-4-3-11(9-14(13)25-2)16(24)23-5-7-26-8-6-23/h3-4,9-10H,5-8H2,1-2H3,(H2,19,20,21,22) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech
Curated by ChEMBL
| Assay Description
Inhibition of LRRK2 |
J Med Chem 55: 5536-45 (2012)
Article DOI: 10.1021/jm300452p
BindingDB Entry DOI: 10.7270/Q2RR20CQ |
More data for this
Ligand-Target Pair | |
Leucine-rich repeat serine/threonine-protein kinase 2
(Homo sapiens (Human)) | BDBM128274
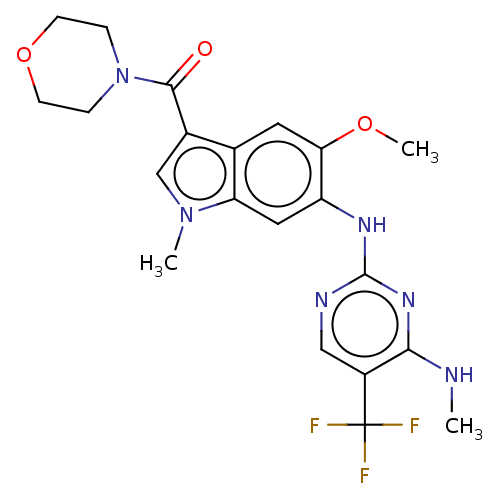
(US8796296, 30)Show SMILES CNc1nc(Nc2cc3n(C)cc(C(=O)N4CCOCC4)c3cc2OC)ncc1C(F)(F)F Show InChI InChI=1S/C21H23F3N6O3/c1-25-18-14(21(22,23)24)10-26-20(28-18)27-15-9-16-12(8-17(15)32-3)13(11-29(16)2)19(31)30-4-6-33-7-5-30/h8-11H,4-7H2,1-3H3,(H2,25,26,27,28) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech, Inc.
US Patent
| Assay Description
his assay was used to determine a compound's potency in inhibiting activity of LRRK2 by determining, Kiapp, IC50, or percent inhibition values. In 38... |
US Patent US8796296 (2014)
BindingDB Entry DOI: 10.7270/Q28W3C0R |
More data for this
Ligand-Target Pair | |
Leucine-rich repeat serine/threonine-protein kinase 2
(Homo sapiens (Human)) | BDBM128256

(US8796296, 11)Show SMILES CNc1nc(Nc2ccc3C(=O)N(C)Cc3c2OC)ncc1C(F)(F)F Show InChI InChI=1S/C16H16F3N5O2/c1-20-13-10(16(17,18)19)6-21-15(23-13)22-11-5-4-8-9(12(11)26-3)7-24(2)14(8)25/h4-6H,7H2,1-3H3,(H2,20,21,22,23) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech, Inc.
US Patent
| Assay Description
his assay was used to determine a compound's potency in inhibiting activity of LRRK2 by determining, Kiapp, IC50, or percent inhibition values. In 38... |
US Patent US8796296 (2014)
BindingDB Entry DOI: 10.7270/Q28W3C0R |
More data for this
Ligand-Target Pair | |
Leucine-rich repeat serine/threonine-protein kinase 2
(Homo sapiens (Human)) | BDBM129180
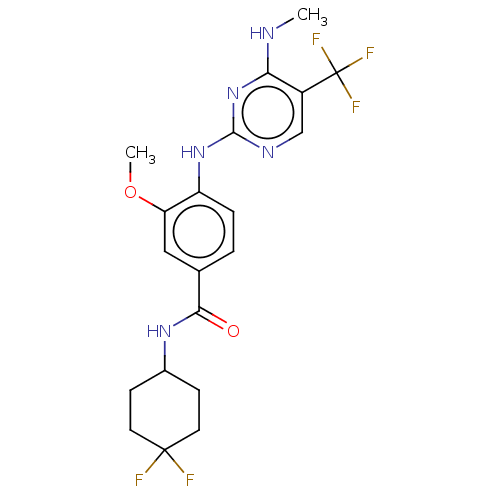
(US8802674, 283)Show SMILES CNc1nc(Nc2ccc(cc2OC)C(=O)NC2CCC(F)(F)CC2)ncc1C(F)(F)F Show InChI InChI=1S/C20H22F5N5O2/c1-26-16-13(20(23,24)25)10-27-18(30-16)29-14-4-3-11(9-15(14)32-2)17(31)28-12-5-7-19(21,22)8-6-12/h3-4,9-10,12H,5-8H2,1-2H3,(H,28,31)(H2,26,27,29,30) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| 1.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech, Inc.
US Patent
| Assay Description
This assay was used to determine a compound's potency in inhibiting activity of LRRK2 by determining, Kiapp, IC50, or percent inhibition values. ... |
US Patent US8802674 (2014)
BindingDB Entry DOI: 10.7270/Q2GF0S6N |
More data for this
Ligand-Target Pair | |
Leucine-rich repeat serine/threonine-protein kinase 2
(Homo sapiens (Human)) | BDBM196745
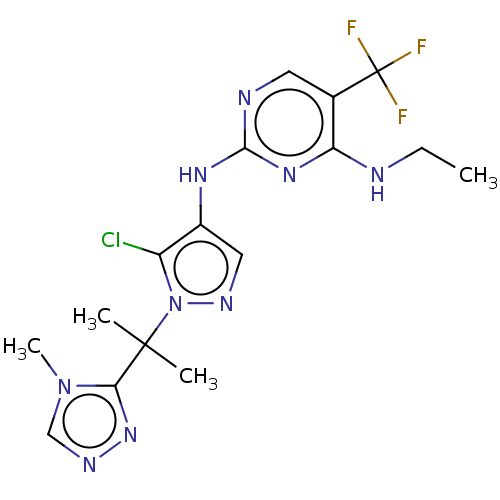
(US9212173, 23)Show SMILES CCNc1nc(Nc2cnn(c2Cl)C(C)(C)c2nncn2C)ncc1C(F)(F)F Show InChI InChI=1S/C16H19ClF3N9/c1-5-21-12-9(16(18,19)20)6-22-14(26-12)25-10-7-24-29(11(10)17)15(2,3)13-27-23-8-28(13)4/h6-8H,5H2,1-4H3,(H2,21,22,25,26) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| 1.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech, Inc.
US Patent
| Assay Description
This assay was used to determine a compound's potency in inhibiting activity of LRRK2 by determining, Kiapp, IC50, or percent inhibition values. In 3... |
US Patent US9212173 (2015)
BindingDB Entry DOI: 10.7270/Q2222SKT |
More data for this
Ligand-Target Pair | |
Leucine-rich repeat serine/threonine-protein kinase 2
(Homo sapiens (Human)) | BDBM196736
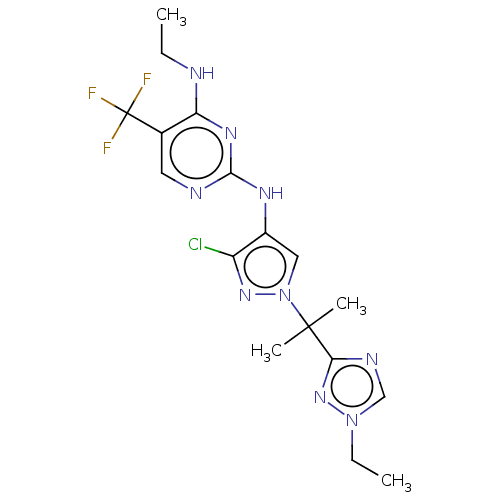
(US9212173, 14)Show SMILES CCNc1nc(Nc2cn(nc2Cl)C(C)(C)c2ncn(CC)n2)ncc1C(F)(F)F Show InChI InChI=1S/C17H21ClF3N9/c1-5-22-13-10(17(19,20)21)7-23-15(26-13)25-11-8-30(27-12(11)18)16(3,4)14-24-9-29(6-2)28-14/h7-9H,5-6H2,1-4H3,(H2,22,23,25,26) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| 1.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech, Inc.
US Patent
| Assay Description
This assay was used to determine a compound's potency in inhibiting activity of LRRK2 by determining, Kiapp, IC50, or percent inhibition values. In 3... |
US Patent US9212173 (2015)
BindingDB Entry DOI: 10.7270/Q2222SKT |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit delta isoform
(Homo sapiens (Human)) | BDBM50438637
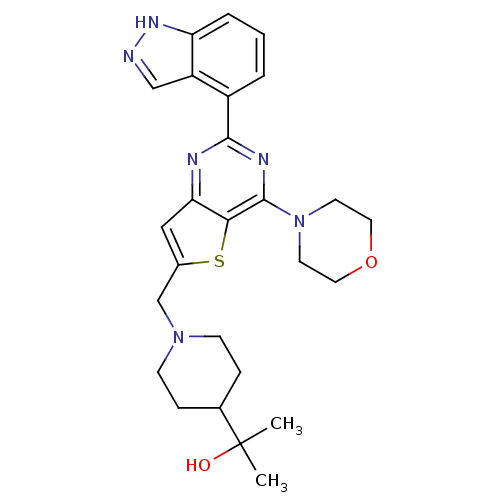
(CHEMBL2414238)Show SMILES CC(C)(O)C1CCN(Cc2cc3nc(nc(N4CCOCC4)c3s2)-c2cccc3[nH]ncc23)CC1 Show InChI InChI=1S/C26H32N6O2S/c1-26(2,33)17-6-8-31(9-7-17)16-18-14-22-23(35-18)25(32-10-12-34-13-11-32)29-24(28-22)19-4-3-5-21-20(19)15-27-30-21/h3-5,14-15,17,33H,6-13,16H2,1-2H3,(H,27,30) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech
Curated by ChEMBL
| Assay Description
Inhibition of PI3Kdelta (unknown origin) assessed as formation of PIP3 by competitive fluorescence polarization assay |
Bioorg Med Chem Lett 23: 4953-9 (2013)
Article DOI: 10.1016/j.bmcl.2013.06.052
BindingDB Entry DOI: 10.7270/Q2ZG6TP9 |
More data for this
Ligand-Target Pair | |
Leucine-rich repeat serine/threonine-protein kinase 2
(Homo sapiens (Human)) | BDBM50507673
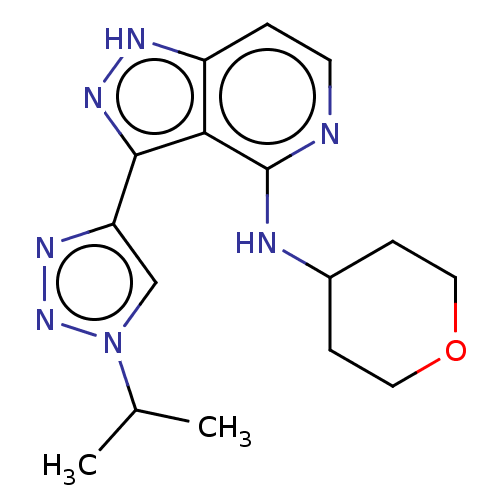
(CHEMBL4441650)Show InChI InChI=1S/C16H21N7O/c1-10(2)23-9-13(20-22-23)15-14-12(19-21-15)3-6-17-16(14)18-11-4-7-24-8-5-11/h3,6,9-11H,4-5,7-8H2,1-2H3,(H,17,18)(H,19,21) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 1.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human GST-tagged LRRK2 G2019S mutant catalytic domain (970 to 2527 residues) expressed in insect cells using RtGWWRFYTLRRAR... |
Bioorg Med Chem Lett 29: 674-680 (2019)
Article DOI: 10.1016/j.bmcl.2018.10.017
BindingDB Entry DOI: 10.7270/Q2HH6PCX |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data