 Found 24 hits with Last Name = 'sweeny' and Initial = 'dj'
Found 24 hits with Last Name = 'sweeny' and Initial = 'dj' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Platelet-activating factor receptor
(RAT) | BDBM50286012
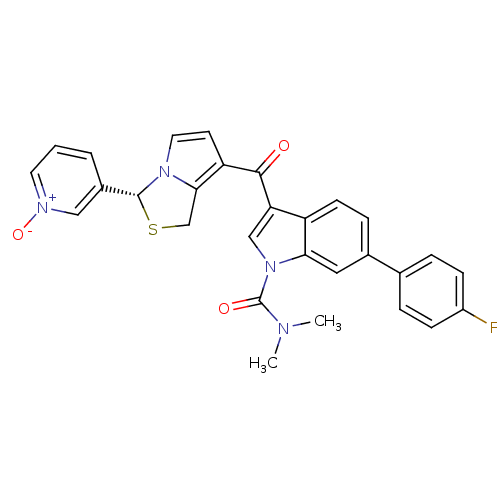
(3-{(R)-7-[1-Dimethylcarbamoyl-6-(4-fluoro-phenyl)-...)Show SMILES CN(C)C(=O)n1cc(C(=O)c2ccn3[C@H](SCc23)c2ccc[n+]([O-])c2)c2ccc(cc12)-c1ccc(F)cc1 Show InChI InChI=1S/C29H23FN4O3S/c1-31(2)29(36)34-16-24(22-10-7-19(14-25(22)34)18-5-8-21(30)9-6-18)27(35)23-11-13-33-26(23)17-38-28(33)20-4-3-12-32(37)15-20/h3-16,28H,17H2,1-2H3/t28-/m1/s1 | Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| 2.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of platelet activating factor(PAF) by binding to PAF receptor |
Bioorg Med Chem Lett 5: 2909-2912 (1995)
Article DOI: 10.1016/0960-894X(95)00510-Z
BindingDB Entry DOI: 10.7270/Q2S46RXM |
More data for this
Ligand-Target Pair | |
Platelet-activating factor receptor
(RAT) | BDBM50035397
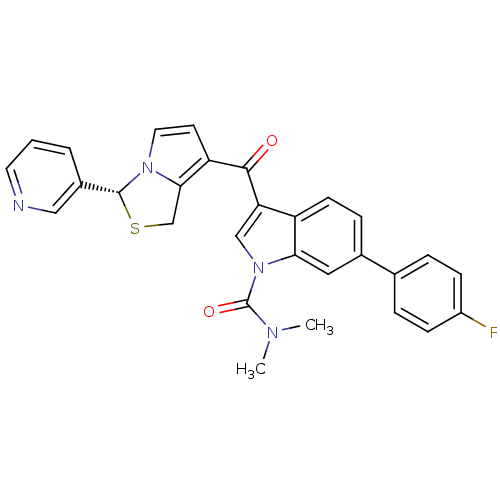
(6-(4-Fluoro-phenyl)-3-((R)-3-pyridin-3-yl-1H-pyrro...)Show SMILES CN(C)C(=O)n1cc(C(=O)c2ccn3[C@H](SCc23)c2cccnc2)c2ccc(cc12)-c1ccc(F)cc1 Show InChI InChI=1S/C29H23FN4O2S/c1-32(2)29(36)34-16-24(22-10-7-19(14-25(22)34)18-5-8-21(30)9-6-18)27(35)23-11-13-33-26(23)17-37-28(33)20-4-3-12-31-15-20/h3-16,28H,17H2,1-2H3/t28-/m1/s1 | Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
| 3.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of platelet activating factor (PAF) by binding to PAF receptor |
Bioorg Med Chem Lett 5: 2909-2912 (1995)
Article DOI: 10.1016/0960-894X(95)00510-Z
BindingDB Entry DOI: 10.7270/Q2S46RXM |
More data for this
Ligand-Target Pair | |
Platelet-activating factor receptor
(RAT) | BDBM50286013
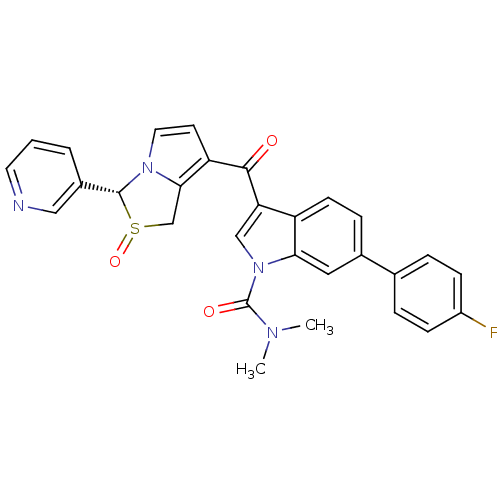
((cis)-(2S,3R)-7-[1-Dimethylcarbamoyl-6-(4-fluoro-p...)Show SMILES CN(C)C(=O)n1cc(C(=O)c2ccn3[C@@H](c4cccnc4)S(=O)Cc23)c2ccc(cc12)-c1ccc(F)cc1 |r| Show InChI InChI=1S/C29H23FN4O3S/c1-32(2)29(36)34-16-24(22-10-7-19(14-25(22)34)18-5-8-21(30)9-6-18)27(35)23-11-13-33-26(23)17-38(37)28(33)20-4-3-12-31-15-20/h3-16,28H,17H2,1-2H3/t28-,38?/m1/s1 | Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| 68 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of platelet activating factor(PAF) by binding to PAF receptor |
Bioorg Med Chem Lett 5: 2909-2912 (1995)
Article DOI: 10.1016/0960-894X(95)00510-Z
BindingDB Entry DOI: 10.7270/Q2S46RXM |
More data for this
Ligand-Target Pair | |
Platelet-activating factor receptor
(RAT) | BDBM50286013

((cis)-(2S,3R)-7-[1-Dimethylcarbamoyl-6-(4-fluoro-p...)Show SMILES CN(C)C(=O)n1cc(C(=O)c2ccn3[C@@H](c4cccnc4)S(=O)Cc23)c2ccc(cc12)-c1ccc(F)cc1 |r| Show InChI InChI=1S/C29H23FN4O3S/c1-32(2)29(36)34-16-24(22-10-7-19(14-25(22)34)18-5-8-21(30)9-6-18)27(35)23-11-13-33-26(23)17-38(37)28(33)20-4-3-12-31-15-20/h3-16,28H,17H2,1-2H3/t28-,38?/m1/s1 | Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| 68 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of platelet activating factor(PAF) by binding to PAF receptor |
Bioorg Med Chem Lett 5: 2909-2912 (1995)
Article DOI: 10.1016/0960-894X(95)00510-Z
BindingDB Entry DOI: 10.7270/Q2S46RXM |
More data for this
Ligand-Target Pair | |
Neuraminidase
(Influenza A virus (A/Puerto Rico/8/34/Mount Sinai(...) | BDBM4994

((3R,4R,5S)-5-amino-4-acetamido-3-(pentan-3-yloxy)c...)Show SMILES CCC(CC)O[C@@H]1C=C(C[C@H](N)[C@H]1NC(C)=O)C(O)=O |r,c:7| Show InChI InChI=1S/C14H24N2O4/c1-4-10(5-2)20-12-7-9(14(18)19)6-11(15)13(12)16-8(3)17/h7,10-13H,4-6,15H2,1-3H3,(H,16,17)(H,18,19)/t11-,12+,13+/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | 6.5 | 37 |
Gilead Sciences Inc.
| Assay Description
A standard fluorimetric assay was used to measure influenza virus neuraminidase activity. The substrate 2 -(4-methylumbelliferyl)-alpha-D-acetylneura... |
Bioorg Med Chem Lett 9: 2811-4 (1999)
Article DOI: 10.1016/s0960-894x(99)00479-5
BindingDB Entry DOI: 10.7270/Q26H4FMM |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Neuraminidase
(Influenza B virus (B/Lee/40)) | BDBM4994

((3R,4R,5S)-5-amino-4-acetamido-3-(pentan-3-yloxy)c...)Show SMILES CCC(CC)O[C@@H]1C=C(C[C@H](N)[C@H]1NC(C)=O)C(O)=O |r,c:7| Show InChI InChI=1S/C14H24N2O4/c1-4-10(5-2)20-12-7-9(14(18)19)6-11(15)13(12)16-8(3)17/h7,10-13H,4-6,15H2,1-3H3,(H,16,17)(H,18,19)/t11-,12+,13+/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| n/a | n/a | 4 | n/a | n/a | n/a | n/a | 6.5 | 37 |
Gilead Sciences Inc.
| Assay Description
A standard fluorimetric assay was used to measure influenza virus neuraminidase activity. The substrate 2 -(4-methylumbelliferyl)-alpha-D-acetylneura... |
Bioorg Med Chem Lett 9: 2811-4 (1999)
Article DOI: 10.1016/s0960-894x(99)00479-5
BindingDB Entry DOI: 10.7270/Q26H4FMM |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Neuraminidase
(Influenza A virus (A/Puerto Rico/8/34/Mount Sinai(...) | BDBM5248

((3R,4R,5S)-5-amino-4-acetamido-3-{[(3R)-1-hydroxyp...)Show SMILES [H][C@@](CC)(CCO)O[C@@H]1C=C(C[C@H](N)[C@H]1NC(C)=O)C(O)=O |r,c:9| Show InChI InChI=1S/C14H24N2O5/c1-3-10(4-5-17)21-12-7-9(14(19)20)6-11(15)13(12)16-8(2)18/h7,10-13,17H,3-6,15H2,1-2H3,(H,16,18)(H,19,20)/t10-,11+,12-,13-/m1/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 30 | n/a | n/a | n/a | n/a | 6.5 | 37 |
Gilead Sciences Inc.
| Assay Description
A standard fluorimetric assay was used to measure influenza virus neuraminidase activity. The substrate 2 -(4-methylumbelliferyl)-alpha-D-acetylneura... |
Bioorg Med Chem Lett 9: 2811-4 (1999)
Article DOI: 10.1016/s0960-894x(99)00479-5
BindingDB Entry DOI: 10.7270/Q26H4FMM |
More data for this
Ligand-Target Pair | |
Neuraminidase
(Influenza A virus (A/Puerto Rico/8/34/Mount Sinai(...) | BDBM5249
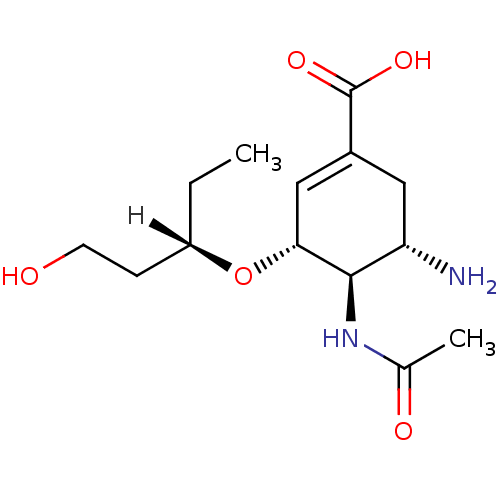
((3R,4R,5S)-5-amino-4-acetamido-3-{[(3S)-1-hydroxyp...)Show SMILES [H][C@](CC)(CCO)O[C@@H]1C=C(C[C@H](N)[C@H]1NC(C)=O)C(O)=O |r,c:9| Show InChI InChI=1S/C14H24N2O5/c1-3-10(4-5-17)21-12-7-9(14(19)20)6-11(15)13(12)16-8(2)18/h7,10-13,17H,3-6,15H2,1-2H3,(H,16,18)(H,19,20)/t10-,11-,12+,13+/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 40 | n/a | n/a | n/a | n/a | 6.5 | 37 |
Gilead Sciences Inc.
| Assay Description
A standard fluorimetric assay was used to measure influenza virus neuraminidase activity. The substrate 2 -(4-methylumbelliferyl)-alpha-D-acetylneura... |
Bioorg Med Chem Lett 9: 2811-4 (1999)
Article DOI: 10.1016/s0960-894x(99)00479-5
BindingDB Entry DOI: 10.7270/Q26H4FMM |
More data for this
Ligand-Target Pair | |
Neuraminidase
(Influenza B virus (B/Lee/40)) | BDBM5248

((3R,4R,5S)-5-amino-4-acetamido-3-{[(3R)-1-hydroxyp...)Show SMILES [H][C@@](CC)(CCO)O[C@@H]1C=C(C[C@H](N)[C@H]1NC(C)=O)C(O)=O |r,c:9| Show InChI InChI=1S/C14H24N2O5/c1-3-10(4-5-17)21-12-7-9(14(19)20)6-11(15)13(12)16-8(2)18/h7,10-13,17H,3-6,15H2,1-2H3,(H,16,18)(H,19,20)/t10-,11+,12-,13-/m1/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 85 | n/a | n/a | n/a | n/a | 6.5 | 37 |
Gilead Sciences Inc.
| Assay Description
A standard fluorimetric assay was used to measure influenza virus neuraminidase activity. The substrate 2 -(4-methylumbelliferyl)-alpha-D-acetylneura... |
Bioorg Med Chem Lett 9: 2811-4 (1999)
Article DOI: 10.1016/s0960-894x(99)00479-5
BindingDB Entry DOI: 10.7270/Q26H4FMM |
More data for this
Ligand-Target Pair | |
Neuraminidase
(Influenza B virus (B/Lee/40)) | BDBM5249

((3R,4R,5S)-5-amino-4-acetamido-3-{[(3S)-1-hydroxyp...)Show SMILES [H][C@](CC)(CCO)O[C@@H]1C=C(C[C@H](N)[C@H]1NC(C)=O)C(O)=O |r,c:9| Show InChI InChI=1S/C14H24N2O5/c1-3-10(4-5-17)21-12-7-9(14(19)20)6-11(15)13(12)16-8(2)18/h7,10-13,17H,3-6,15H2,1-2H3,(H,16,18)(H,19,20)/t10-,11-,12+,13+/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 400 | n/a | n/a | n/a | n/a | 6.5 | 37 |
Gilead Sciences Inc.
| Assay Description
A standard fluorimetric assay was used to measure influenza virus neuraminidase activity. The substrate 2 -(4-methylumbelliferyl)-alpha-D-acetylneura... |
Bioorg Med Chem Lett 9: 2811-4 (1999)
Article DOI: 10.1016/s0960-894x(99)00479-5
BindingDB Entry DOI: 10.7270/Q26H4FMM |
More data for this
Ligand-Target Pair | |
Neuraminidase
(Influenza A virus (A/Puerto Rico/8/34/Mount Sinai(...) | BDBM5246
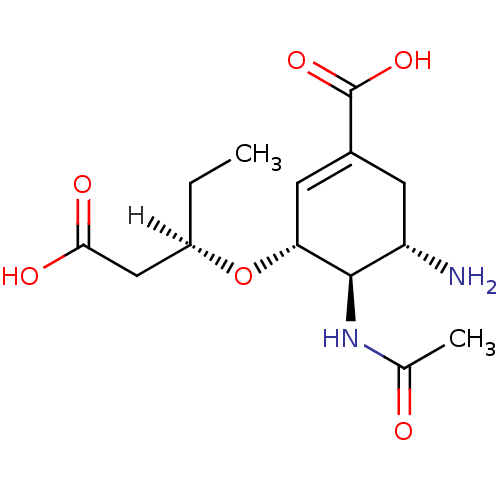
((3R,4R,5S)-5-amino-3-{[(2R)-1-carboxybutan-2-yl]ox...)Show SMILES [H][C@@](CC)(CC(O)=O)O[C@@H]1C=C(C[C@H](N)[C@H]1NC(C)=O)C(O)=O |r,c:10| Show InChI InChI=1S/C14H22N2O6/c1-3-9(6-12(18)19)22-11-5-8(14(20)21)4-10(15)13(11)16-7(2)17/h5,9-11,13H,3-4,6,15H2,1-2H3,(H,16,17)(H,18,19)(H,20,21)/t9-,10+,11-,13-/m1/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 450 | n/a | n/a | n/a | n/a | 6.5 | 37 |
Gilead Sciences Inc.
| Assay Description
A standard fluorimetric assay was used to measure influenza virus neuraminidase activity. The substrate 2 -(4-methylumbelliferyl)-alpha-D-acetylneura... |
Bioorg Med Chem Lett 9: 2811-4 (1999)
Article DOI: 10.1016/s0960-894x(99)00479-5
BindingDB Entry DOI: 10.7270/Q26H4FMM |
More data for this
Ligand-Target Pair | |
Neuraminidase
(Influenza A virus (A/Puerto Rico/8/34/Mount Sinai(...) | BDBM5247

((3R,4R,5S)-5-amino-3-{[(2S)-1-carboxybutan-2-yl]ox...)Show SMILES [H][C@](CC)(CC(O)=O)O[C@@H]1C=C(C[C@H](N)[C@H]1NC(C)=O)C(O)=O |r,c:10| Show InChI InChI=1S/C14H22N2O6/c1-3-9(6-12(18)19)22-11-5-8(14(20)21)4-10(15)13(11)16-7(2)17/h5,9-11,13H,3-4,6,15H2,1-2H3,(H,16,17)(H,18,19)(H,20,21)/t9-,10-,11+,13+/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.15E+3 | n/a | n/a | n/a | n/a | 6.5 | 37 |
Gilead Sciences Inc.
| Assay Description
A standard fluorimetric assay was used to measure influenza virus neuraminidase activity. The substrate 2 -(4-methylumbelliferyl)-alpha-D-acetylneura... |
Bioorg Med Chem Lett 9: 2811-4 (1999)
Article DOI: 10.1016/s0960-894x(99)00479-5
BindingDB Entry DOI: 10.7270/Q26H4FMM |
More data for this
Ligand-Target Pair | |
Cytochrome P450 3A4
(Homo sapiens (Human)) | BDBM327994
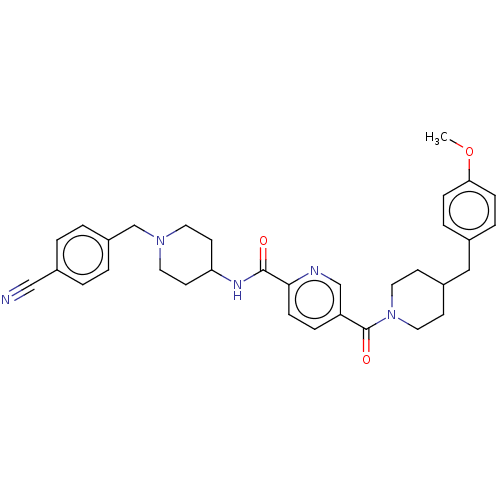
(N-(1-(4- cyanobenzyl)piperidin-4- yl)-5-(4-(4- met...)Show SMILES COc1ccc(CC2CCN(CC2)C(=O)c2ccc(nc2)C(=O)NC2CCN(Cc3ccc(cc3)C#N)CC2)cc1 Show InChI InChI=1S/C33H37N5O3/c1-41-30-9-6-24(7-10-30)20-25-12-18-38(19-13-25)33(40)28-8-11-31(35-22-28)32(39)36-29-14-16-37(17-15-29)23-27-4-2-26(21-34)3-5-27/h2-11,22,25,29H,12-20,23H2,1H3,(H,36,39) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 2.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.bmc.2022.116951
BindingDB Entry DOI: 10.7270/Q2HX1HM7 |
More data for this
Ligand-Target Pair | |
Cytochrome P450 3A4
(Homo sapiens (Human)) | BDBM327998
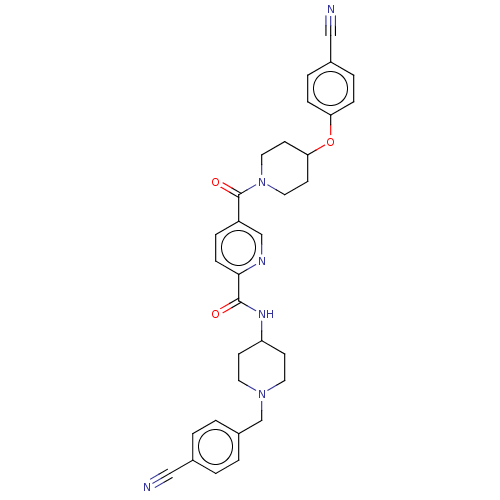
(N-(1-(4- cyanobenzyl)piperidin-4- yl)-5-(4-(4- cya...)Show SMILES O=C(NC1CCN(Cc2ccc(cc2)C#N)CC1)c1ccc(cn1)C(=O)N1CCC(CC1)Oc1ccc(cc1)C#N Show InChI InChI=1S/C32H32N6O3/c33-19-23-1-3-25(4-2-23)22-37-15-11-27(12-16-37)36-31(39)30-10-7-26(21-35-30)32(40)38-17-13-29(14-18-38)41-28-8-5-24(20-34)6-9-28/h1-10,21,27,29H,11-18,22H2,(H,36,39) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 3.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.bmc.2022.116951
BindingDB Entry DOI: 10.7270/Q2HX1HM7 |
More data for this
Ligand-Target Pair | |
Cytochrome P450 3A4
(Homo sapiens (Human)) | BDBM328001
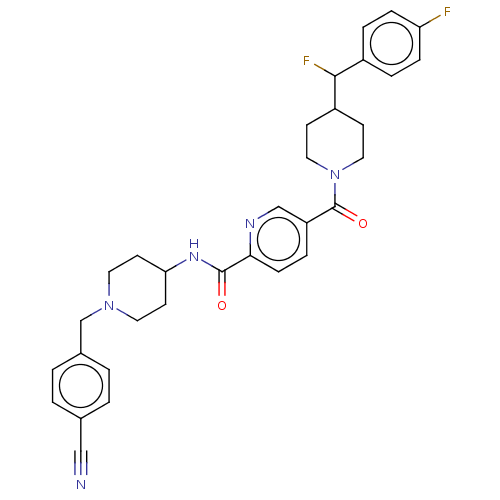
(N-(1-(4- cyanobenzyl)piperidin-4- yl)-5-(4-(fluoro...)Show SMILES FC(C1CCN(CC1)C(=O)c1ccc(nc1)C(=O)NC1CCN(Cc2ccc(cc2)C#N)CC1)c1ccc(F)cc1 Show InChI InChI=1S/C32H33F2N5O2/c33-27-8-5-24(6-9-27)30(34)25-11-17-39(18-12-25)32(41)26-7-10-29(36-20-26)31(40)37-28-13-15-38(16-14-28)21-23-3-1-22(19-35)2-4-23/h1-10,20,25,28,30H,11-18,21H2,(H,37,40) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 9.80E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.bmc.2022.116951
BindingDB Entry DOI: 10.7270/Q2HX1HM7 |
More data for this
Ligand-Target Pair | |
Cytochrome P450 3A4
(Homo sapiens (Human)) | BDBM328133

(N-(1-(4- cyanobenzyl)piperidin-4- yl)-5-(4-(2,4- d...)Show SMILES Fc1ccc(C(=O)C2CCN(CC2)C(=O)c2ccc(nc2)C(=O)NC2CCN(Cc3ccc(cc3)C#N)CC2)c(F)c1 Show InChI InChI=1S/C32H31F2N5O3/c33-25-6-7-27(28(34)17-25)30(40)23-9-15-39(16-10-23)32(42)24-5-8-29(36-19-24)31(41)37-26-11-13-38(14-12-26)20-22-3-1-21(18-35)2-4-22/h1-8,17,19,23,26H,9-16,20H2,(H,37,41) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.bmc.2022.116951
BindingDB Entry DOI: 10.7270/Q2HX1HM7 |
More data for this
Ligand-Target Pair | |
Cytochrome P450 3A4
(Homo sapiens (Human)) | BDBM328006
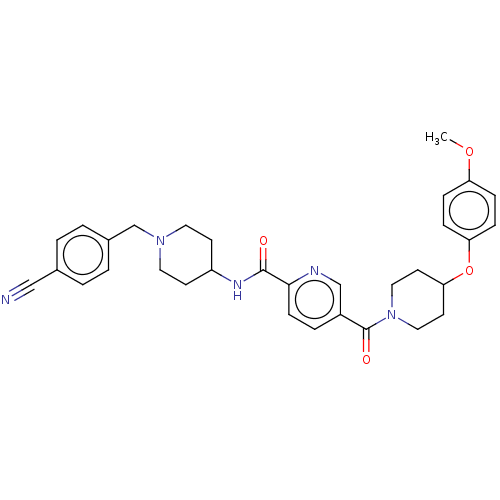
(N-(1-(4- cyanobenzyl)piperidin-4- yl)-5-(4-(4- met...)Show SMILES COc1ccc(OC2CCN(CC2)C(=O)c2ccc(nc2)C(=O)NC2CCN(Cc3ccc(cc3)C#N)CC2)cc1 Show InChI InChI=1S/C32H35N5O4/c1-40-27-7-9-28(10-8-27)41-29-14-18-37(19-15-29)32(39)25-6-11-30(34-21-25)31(38)35-26-12-16-36(17-13-26)22-24-4-2-23(20-33)3-5-24/h2-11,21,26,29H,12-19,22H2,1H3,(H,35,38) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.bmc.2022.116951
BindingDB Entry DOI: 10.7270/Q2HX1HM7 |
More data for this
Ligand-Target Pair | |
Cytochrome P450 3A4
(Homo sapiens (Human)) | BDBM328171

(N-(1-(4- cyanobenzyl)piperidin-4- yl)-6-(4-(4- met...)Show SMILES COc1ccc(cc1)C(=O)C1CCN(CC1)C(=O)c1ccc(cn1)C(=O)NC1CCN(Cc2ccc(cc2)C#N)CC1 Show InChI InChI=1S/C33H35N5O4/c1-42-29-9-6-25(7-10-29)31(39)26-12-18-38(19-13-26)33(41)30-11-8-27(21-35-30)32(40)36-28-14-16-37(17-15-28)22-24-4-2-23(20-34)3-5-24/h2-11,21,26,28H,12-19,22H2,1H3,(H,36,40) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.bmc.2022.116951
BindingDB Entry DOI: 10.7270/Q2HX1HM7 |
More data for this
Ligand-Target Pair | |
Cytochrome P450 3A4
(Homo sapiens (Human)) | BDBM328133

(N-(1-(4- cyanobenzyl)piperidin-4- yl)-5-(4-(2,4- d...)Show SMILES Fc1ccc(C(=O)C2CCN(CC2)C(=O)c2ccc(nc2)C(=O)NC2CCN(Cc3ccc(cc3)C#N)CC2)c(F)c1 Show InChI InChI=1S/C32H31F2N5O3/c33-25-6-7-27(28(34)17-25)30(40)23-9-15-39(16-10-23)32(42)24-5-8-29(36-19-24)31(41)37-26-11-13-38(14-12-26)20-22-3-1-21(18-35)2-4-22/h1-8,17,19,23,26H,9-16,20H2,(H,37,41) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.bmc.2022.116951
BindingDB Entry DOI: 10.7270/Q2HX1HM7 |
More data for this
Ligand-Target Pair | |
Cytochrome P450 3A4
(Homo sapiens (Human)) | BDBM328171

(N-(1-(4- cyanobenzyl)piperidin-4- yl)-6-(4-(4- met...)Show SMILES COc1ccc(cc1)C(=O)C1CCN(CC1)C(=O)c1ccc(cn1)C(=O)NC1CCN(Cc2ccc(cc2)C#N)CC1 Show InChI InChI=1S/C33H35N5O4/c1-42-29-9-6-25(7-10-29)31(39)26-12-18-38(19-13-26)33(41)30-11-8-27(21-35-30)32(40)36-28-14-16-37(17-15-28)22-24-4-2-23(20-34)3-5-24/h2-11,21,26,28H,12-19,22H2,1H3,(H,36,40) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.bmc.2022.116951
BindingDB Entry DOI: 10.7270/Q2HX1HM7 |
More data for this
Ligand-Target Pair | |
Cytochrome P450 3A4
(Homo sapiens (Human)) | BDBM328006

(N-(1-(4- cyanobenzyl)piperidin-4- yl)-5-(4-(4- met...)Show SMILES COc1ccc(OC2CCN(CC2)C(=O)c2ccc(nc2)C(=O)NC2CCN(Cc3ccc(cc3)C#N)CC2)cc1 Show InChI InChI=1S/C32H35N5O4/c1-40-27-7-9-28(10-8-27)41-29-14-18-37(19-15-29)32(39)25-6-11-30(34-21-25)31(38)35-26-12-16-36(17-13-26)22-24-4-2-23(20-33)3-5-24/h2-11,21,26,29H,12-19,22H2,1H3,(H,35,38) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.bmc.2022.116951
BindingDB Entry DOI: 10.7270/Q2HX1HM7 |
More data for this
Ligand-Target Pair | |
Cytochrome P450 3A4
(Homo sapiens (Human)) | BDBM327994

(N-(1-(4- cyanobenzyl)piperidin-4- yl)-5-(4-(4- met...)Show SMILES COc1ccc(CC2CCN(CC2)C(=O)c2ccc(nc2)C(=O)NC2CCN(Cc3ccc(cc3)C#N)CC2)cc1 Show InChI InChI=1S/C33H37N5O3/c1-41-30-9-6-24(7-10-30)20-25-12-18-38(19-13-25)33(40)28-8-11-31(35-22-28)32(39)36-29-14-16-37(17-15-29)23-27-4-2-26(21-34)3-5-27/h2-11,22,25,29H,12-20,23H2,1H3,(H,36,39) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.bmc.2022.116951
BindingDB Entry DOI: 10.7270/Q2HX1HM7 |
More data for this
Ligand-Target Pair | |
Cytochrome P450 3A4
(Homo sapiens (Human)) | BDBM327927
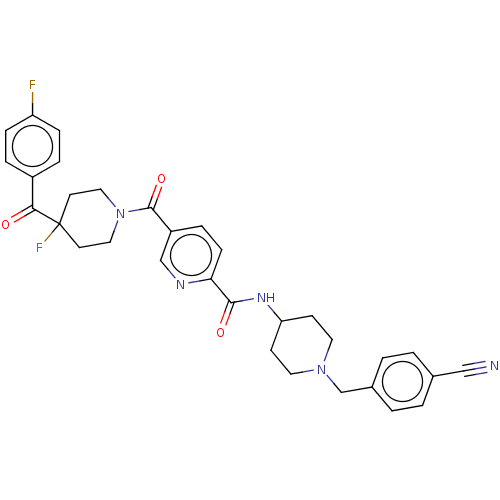
(N-(1-(4- cyanobenzyl)piperidin-4- yl)-5-(4-(4-fluo...)Show SMILES Fc1ccc(cc1)C(=O)C1(F)CCN(CC1)C(=O)c1ccc(nc1)C(=O)NC1CCN(Cc2ccc(cc2)C#N)CC1 Show InChI InChI=1S/C32H31F2N5O3/c33-26-8-5-24(6-9-26)29(40)32(34)13-17-39(18-14-32)31(42)25-7-10-28(36-20-25)30(41)37-27-11-15-38(16-12-27)21-23-3-1-22(19-35)2-4-23/h1-10,20,27H,11-18,21H2,(H,37,41) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.bmc.2022.116951
BindingDB Entry DOI: 10.7270/Q2HX1HM7 |
More data for this
Ligand-Target Pair | |
Cytochrome P450 3A4
(Homo sapiens (Human)) | BDBM327927

(N-(1-(4- cyanobenzyl)piperidin-4- yl)-5-(4-(4-fluo...)Show SMILES Fc1ccc(cc1)C(=O)C1(F)CCN(CC1)C(=O)c1ccc(nc1)C(=O)NC1CCN(Cc2ccc(cc2)C#N)CC1 Show InChI InChI=1S/C32H31F2N5O3/c33-26-8-5-24(6-9-26)29(40)32(34)13-17-39(18-14-32)31(42)25-7-10-28(36-20-25)30(41)37-27-11-15-38(16-12-27)21-23-3-1-22(19-35)2-4-23/h1-10,20,27H,11-18,21H2,(H,37,41) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.bmc.2022.116951
BindingDB Entry DOI: 10.7270/Q2HX1HM7 |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data