

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Polyphenol oxidase 2 (Agaricus bisporus (Common mushroom)) | BDBM50330795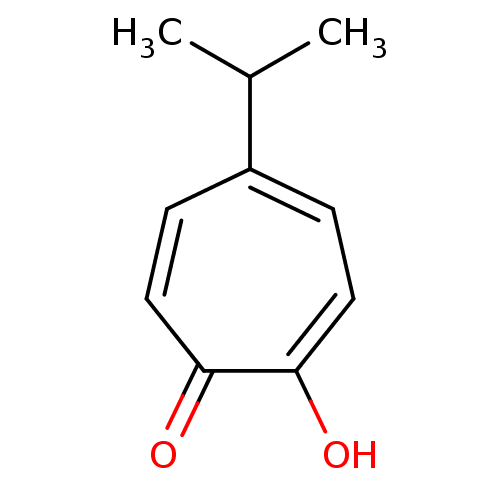 (2-hydroxy-5-isopropyl-2,4,6-cycloheptatrien-1-one ...) | UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL KEGG PC cid PC sid UniChem Patents Similars | Article PubMed | 20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Institute for Theoretical Medicine, Inc. Curated by ChEMBL | Assay Description Competitive inhibition of mushroom tyrosinase after 15 mins by Lineweaver-Bulk plot analysis | Bioorg Med Chem 18: 8112-8 (2010) Article DOI: 10.1016/j.bmc.2010.08.056 BindingDB Entry DOI: 10.7270/Q2JH3MF5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Polyphenol oxidase 2 (Agaricus bisporus (Common mushroom)) | BDBM50330794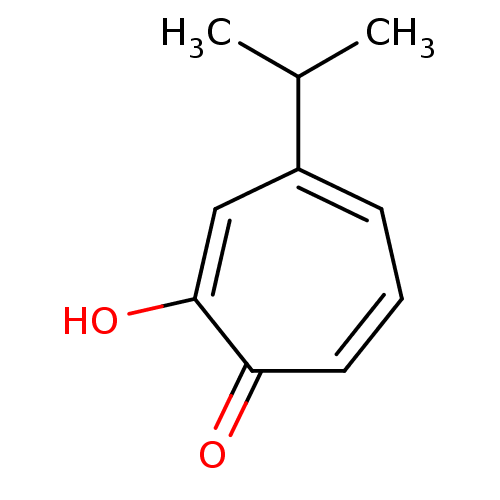 (2-Hydroxy-4-isopropyl-cyclohepta-2,4,6-trienone | ...) | UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG PC cid PC sid UniChem Patents Similars | Article PubMed | 60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Institute for Theoretical Medicine, Inc. Curated by ChEMBL | Assay Description Competitive inhibition of mushroom tyrosinase after 15 mins by Lineweaver-Bulk plot analysis | Bioorg Med Chem 18: 8112-8 (2010) Article DOI: 10.1016/j.bmc.2010.08.056 BindingDB Entry DOI: 10.7270/Q2JH3MF5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Polyphenol oxidase 2 (Agaricus bisporus (Common mushroom)) | BDBM50330793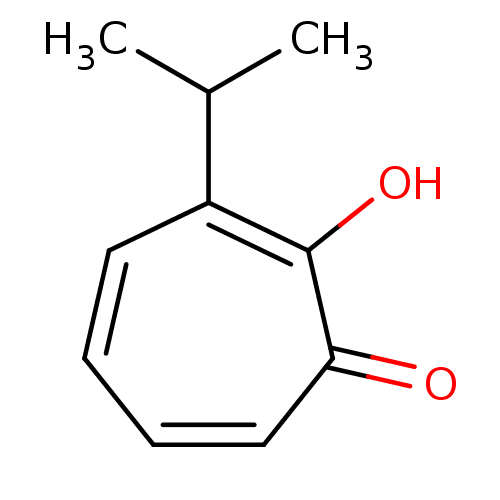 (2-hydroxy-3-isopropyl-2,4,6-cycloheptatrien-1-one ...) | UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents | Article PubMed | 3.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Institute for Theoretical Medicine, Inc. Curated by ChEMBL | Assay Description Competitive inhibition of mushroom tyrosinase after 15 mins by Lineweaver-Bulk plot analysis | Bioorg Med Chem 18: 8112-8 (2010) Article DOI: 10.1016/j.bmc.2010.08.056 BindingDB Entry DOI: 10.7270/Q2JH3MF5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Ubiquitin carboxyl-terminal hydrolase 5 (Homo sapiens (Human)) | BDBM50437693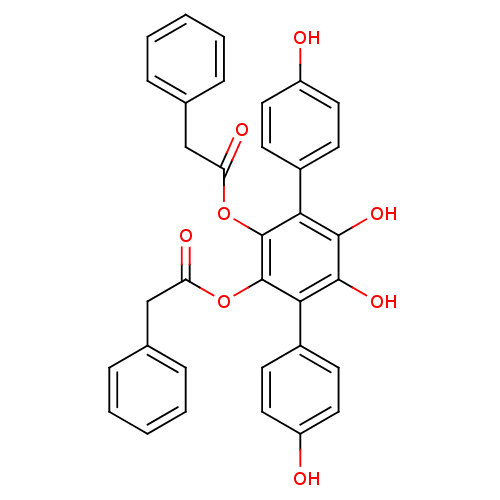 (VIALININ A) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem Similars | Article PubMed | 4.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Tokyo University of Agriculture Curated by ChEMBL | Assay Description Inhibition of wild type human USP5 expressed in Escherichia coli BL21(DE3) using Ub-AMC as substrate after 60 mins by fluorometric analysis | Bioorg Med Chem Lett 23: 4328-31 (2013) Article DOI: 10.1016/j.bmcl.2013.05.093 BindingDB Entry DOI: 10.7270/Q2T15528 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Ubiquitin carboxyl-terminal hydrolase 5 (Homo sapiens (Human)) | BDBM50437693 (VIALININ A) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem Similars | Article PubMed | 1.25E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Tokyo University of Agriculture Curated by ChEMBL | Assay Description Competitive inhibition of wild type human USP5 expressed in Escherichia coli BL21(DE3) using Ub-AMC as substrate | Bioorg Med Chem Lett 23: 4328-31 (2013) Article DOI: 10.1016/j.bmcl.2013.05.093 BindingDB Entry DOI: 10.7270/Q2T15528 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholine receptor subunit alpha/beta/delta/gamma (Torpedo californica) | BDBM50089157 ((3R,5S,7aR)-3-Heptyl-5-methyl-hexahydro-pyrrolizin...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 5.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Toyama Medical and Pharmaceutical University Curated by ChEMBL | Assay Description Compound was evaluated for inhibition of [3H]-TCP binding to Nicotinic acetylcholine receptor of Torpedo californica | Bioorg Med Chem Lett 10: 1293-5 (2000) BindingDB Entry DOI: 10.7270/Q2JH3KCV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholine receptor subunit alpha/beta/delta/gamma (Torpedo californica) | BDBM50089159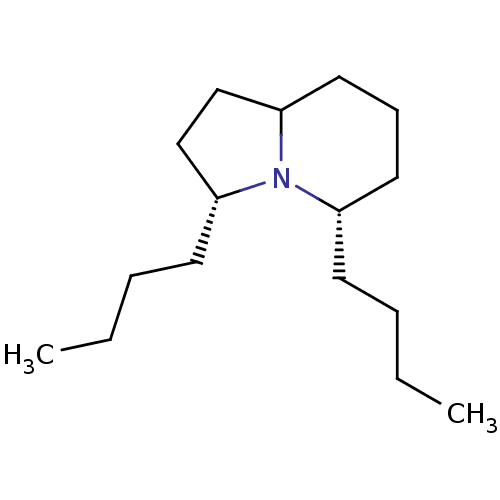 ((3R,5S)-3,5-Dibutyl-octahydro-indolizine | CHEMBL2...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 3.70E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Toyama Medical and Pharmaceutical University Curated by ChEMBL | Assay Description Compound was evaluated for inhibition of [3H]-TCP binding to Nicotinic acetylcholine receptor of Torpedo californica | Bioorg Med Chem Lett 10: 1293-5 (2000) BindingDB Entry DOI: 10.7270/Q2JH3KCV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholine receptor subunit alpha/beta/delta/gamma (Torpedo californica) | BDBM50089158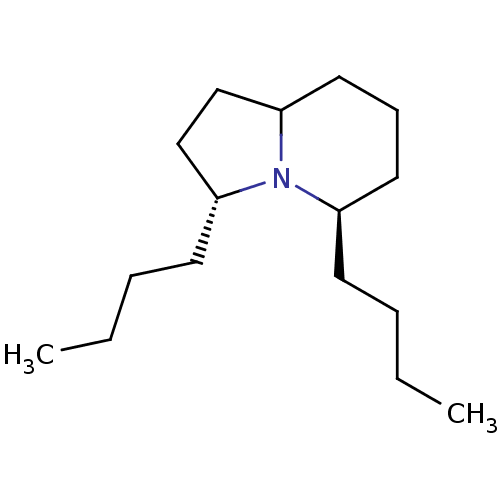 ((3R,5R)-3,5-Dibutyl-octahydro-indolizine | CHEMBL2...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 4.20E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Toyama Medical and Pharmaceutical University Curated by ChEMBL | Assay Description Compound was evaluated for inhibition of [3H]-TCP binding to Nicotinic acetylcholine receptor of Torpedo californica | Bioorg Med Chem Lett 10: 1293-5 (2000) BindingDB Entry DOI: 10.7270/Q2JH3KCV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholine receptor subunit alpha/beta/delta/gamma (Torpedo californica) | BDBM50089162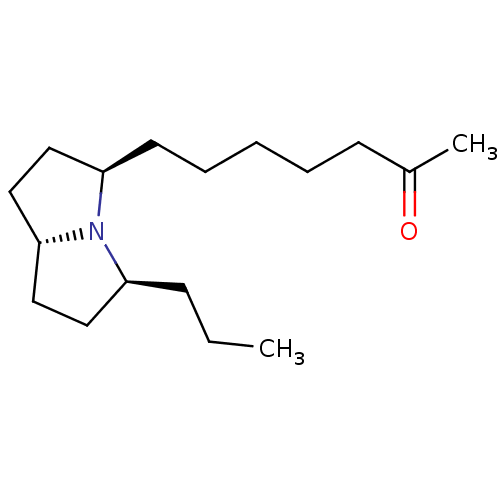 (7-((3R,5S,7aR)-5-Propyl-hexahydro-pyrrolizin-3-yl)...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | PubMed | 8.30E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Toyama Medical and Pharmaceutical University Curated by ChEMBL | Assay Description Compound was evaluated for inhibition of [3H]-TCP binding to Nicotinic acetylcholine receptor of Torpedo californica | Bioorg Med Chem Lett 10: 1293-5 (2000) BindingDB Entry DOI: 10.7270/Q2JH3KCV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholine receptor subunit alpha/beta/delta/gamma (Torpedo californica) | BDBM50089164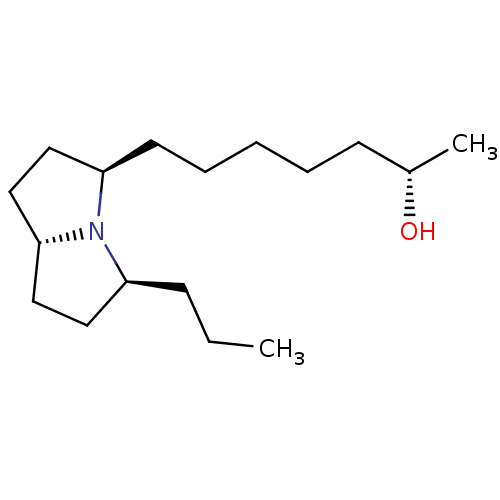 ((S)-7-((3R,5S,7aR)-5-Propyl-hexahydro-pyrrolizin-3...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 3.10E+6 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Toyama Medical and Pharmaceutical University Curated by ChEMBL | Assay Description Compound was evaluated for inhibition of [3H]-TCP binding to Nicotinic acetylcholine receptor of Torpedo californica | Bioorg Med Chem Lett 10: 1293-5 (2000) BindingDB Entry DOI: 10.7270/Q2JH3KCV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholine receptor subunit alpha/beta/delta/gamma (Torpedo californica) | BDBM50089163 ((R)-7-((3R,5S,7aR)-5-Propyl-hexahydro-pyrrolizin-3...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 3.10E+6 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Toyama Medical and Pharmaceutical University Curated by ChEMBL | Assay Description Compound was evaluated for inhibition of [3H]-TCP binding to Nicotinic acetylcholine receptor of Torpedo californica | Bioorg Med Chem Lett 10: 1293-5 (2000) BindingDB Entry DOI: 10.7270/Q2JH3KCV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholine receptor subunit alpha/beta/delta/gamma (Torpedo californica) | BDBM50089160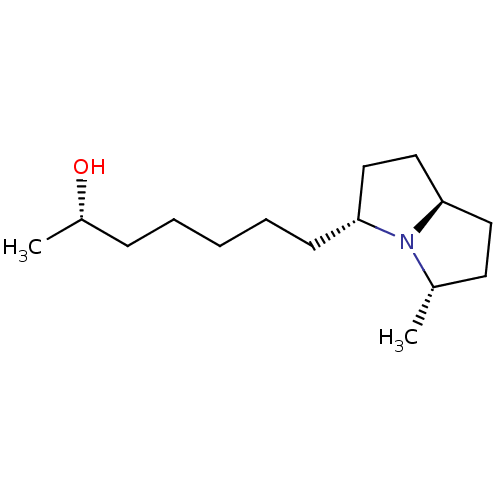 ((S)-7-((3R,5S,7aR)-5-Methyl-hexahydro-pyrrolizin-3...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 3.30E+6 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Toyama Medical and Pharmaceutical University Curated by ChEMBL | Assay Description Compound was evaluated for inhibition of [3H]-TCP binding to Nicotinic acetylcholine receptor of Torpedo californica | Bioorg Med Chem Lett 10: 1293-5 (2000) BindingDB Entry DOI: 10.7270/Q2JH3KCV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholine receptor subunit alpha/beta/delta/gamma (Torpedo californica) | BDBM50089161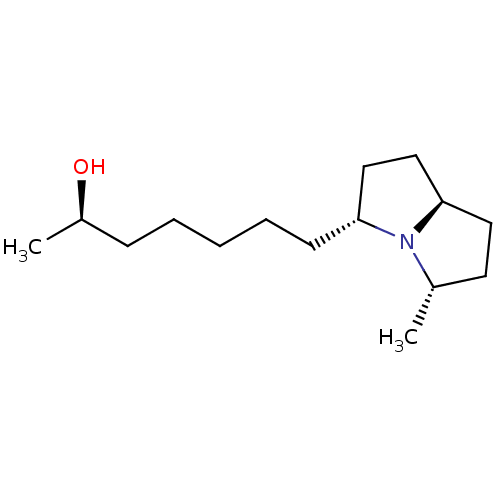 ((R)-7-((3R,5S,7aR)-5-Methyl-hexahydro-pyrrolizin-3...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 8.30E+6 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Toyama Medical and Pharmaceutical University Curated by ChEMBL | Assay Description Compound was evaluated for inhibition of [3H]-TCP binding to Nicotinic acetylcholine receptor of Torpedo californica | Bioorg Med Chem Lett 10: 1293-5 (2000) BindingDB Entry DOI: 10.7270/Q2JH3KCV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cysteinyl leukotriene receptor 1 (Homo sapiens (Human)) | BDBM50104905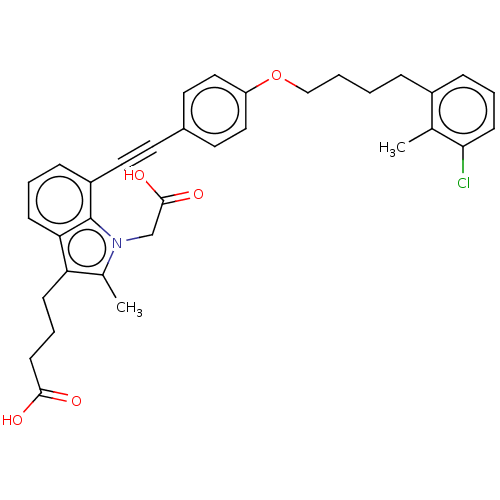 (CHEMBL3597618) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.220 | n/a | n/a | n/a | n/a | n/a | n/a |
Setsunan University Curated by ChEMBL | Assay Description Antagonist activity at human CysLT1 expressed in CHOK1 cells assessed as inhibition of LTD4-induced calcium mobilization preincubated for 30 mins pri... | J Med Chem 58: 6093-113 (2015) Article DOI: 10.1021/acs.jmedchem.5b00741 BindingDB Entry DOI: 10.7270/Q2QJ7K27 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM50271142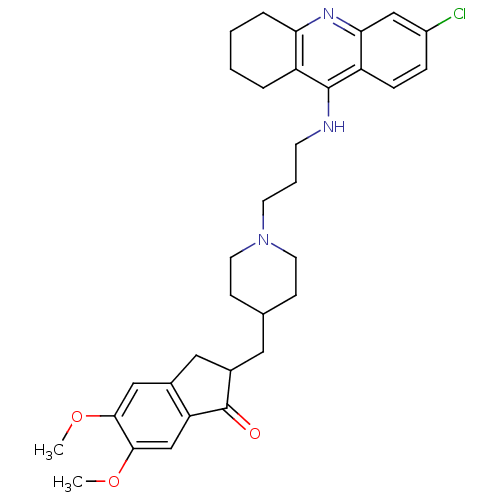 (6-Chloro-9-[(3-{4-[(5,6-Dimethoxy-1-oxoindan-2-yl)...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.270 | n/a | n/a | n/a | n/a | n/a | n/a |
University of S£o Paulo Curated by ChEMBL | Assay Description Binding affinity determined against ETA receptor in porcine aortic smooth muscle membrane | Eur J Med Chem 139: 773-791 (2017) Article DOI: 10.1016/j.ejmech.2017.08.051 BindingDB Entry DOI: 10.7270/Q2XS5XWN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cysteinyl leukotriene receptor 2 (Homo sapiens (Human)) | BDBM50066948 (CHEMBL3403187) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.310 | n/a | n/a | n/a | n/a | n/a | n/a |
Ono Pharmaceutical Co., Ltd Curated by ChEMBL | Assay Description Antagonist activity at human CysLT1 receptor expressed in CHO cells assessed as inhibition of LTD4-inudced intracellular calcium influx preincubated ... | Bioorg Med Chem 23: 2079-97 (2015) Article DOI: 10.1016/j.bmc.2015.03.011 BindingDB Entry DOI: 10.7270/Q2SB47FF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cysteinyl leukotriene receptor 1 (Homo sapiens (Human)) | BDBM50104904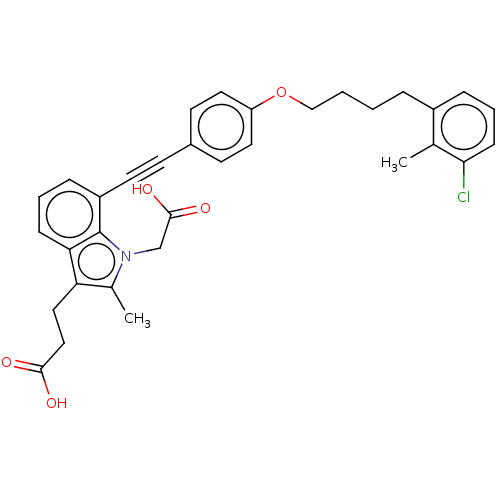 (CHEMBL3597617) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.320 | n/a | n/a | n/a | n/a | n/a | n/a |
Setsunan University Curated by ChEMBL | Assay Description Antagonist activity at human CysLT1 expressed in CHOK1 cells assessed as inhibition of LTD4-induced calcium mobilization preincubated for 30 mins pri... | J Med Chem 58: 6093-113 (2015) Article DOI: 10.1021/acs.jmedchem.5b00741 BindingDB Entry DOI: 10.7270/Q2QJ7K27 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neprilysin (Homo sapiens (Human)) | BDBM50354041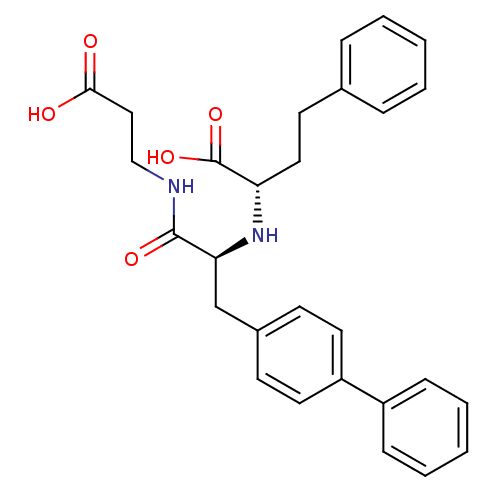 (CHEMBL1829584) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.360 | n/a | n/a | n/a | n/a | n/a | n/a |
Kao Corporation Curated by ChEMBL | Assay Description Inhibition of neutral endopeptidase in human fibroblasts homogenates using glutaryl-Ala-Ala-Phe-4-methoxy-2-naphtylamide as substrate after 1 hrs by ... | Bioorg Med Chem 19: 5935-47 (2011) Article DOI: 10.1016/j.bmc.2011.08.064 BindingDB Entry DOI: 10.7270/Q2154HF6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cysteinyl leukotriene receptor 1 (Homo sapiens (Human)) | BDBM50104883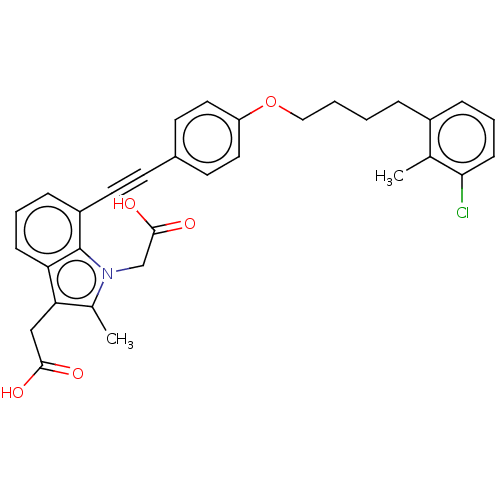 (CHEMBL3597616) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 0.470 | n/a | n/a | n/a | n/a | n/a | n/a |
Setsunan University Curated by ChEMBL | Assay Description Antagonist activity at human CysLT1 expressed in CHOK1 cells assessed as inhibition of LTD4-induced calcium mobilization preincubated for 30 mins pri... | J Med Chem 58: 6093-113 (2015) Article DOI: 10.1021/acs.jmedchem.5b00741 BindingDB Entry DOI: 10.7270/Q2QJ7K27 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cysteinyl leukotriene receptor 2 (Homo sapiens (Human)) | BDBM50066911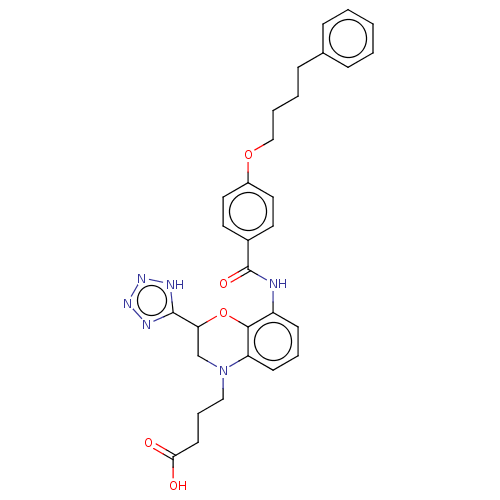 (CHEMBL3401689) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.490 | n/a | n/a | n/a | n/a | n/a | n/a |
Ono Pharmaceutical Co., Ltd Curated by ChEMBL | Assay Description Antagonist activity at human CysLT2 receptor expressed in HEK293 cells assessed as inhibition of LTD4-inudced intracellular calcium influx preincubat... | Bioorg Med Chem 23: 2079-97 (2015) Article DOI: 10.1016/j.bmc.2015.03.011 BindingDB Entry DOI: 10.7270/Q2SB47FF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neprilysin (Homo sapiens (Human)) | BDBM50354042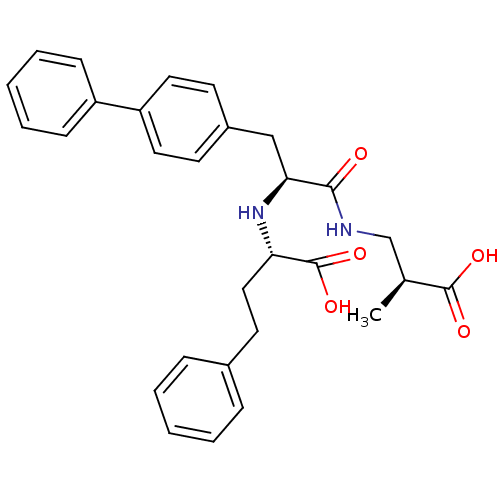 (CHEMBL1829585) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.510 | n/a | n/a | n/a | n/a | n/a | n/a |
Kao Corporation Curated by ChEMBL | Assay Description Inhibition of neutral endopeptidase in human fibroblasts homogenates using glutaryl-Ala-Ala-Phe-4-methoxy-2-naphtylamide as substrate after 1 hrs by ... | Bioorg Med Chem 19: 5935-47 (2011) Article DOI: 10.1016/j.bmc.2011.08.064 BindingDB Entry DOI: 10.7270/Q2154HF6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium channel protein type 9 subunit alpha/subunit beta-1/subunit beta-2 (Mus musculus) | BDBM50257179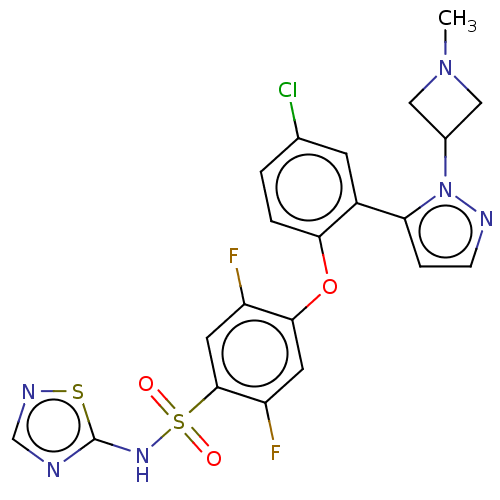 (CHEMBL2325622) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.550 | n/a | n/a | n/a | n/a | n/a | n/a |
Daiichi Sankyo Co., Ltd. Curated by ChEMBL | Assay Description Inhibition of mouse NaV1.7/beta1/beta2 expressed in HEK293A cells by Ionworks high-throughput electrophysiology method | J Med Chem 63: 10204-10220 (2020) Article DOI: 10.1021/acs.jmedchem.0c00259 BindingDB Entry DOI: 10.7270/Q2Q52T67 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cysteinyl leukotriene receptor 1 (Homo sapiens (Human)) | BDBM50104872 (CHEMBL3597535) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.560 | n/a | n/a | n/a | n/a | n/a | n/a |
Setsunan University Curated by ChEMBL | Assay Description Antagonist activity at human CysLT1 expressed in CHOK1 cells assessed as inhibition of LTD4-induced calcium mobilization preincubated for 30 mins pri... | J Med Chem 58: 6093-113 (2015) Article DOI: 10.1021/acs.jmedchem.5b00741 BindingDB Entry DOI: 10.7270/Q2QJ7K27 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cysteinyl leukotriene receptor 1 (Homo sapiens (Human)) | BDBM50066947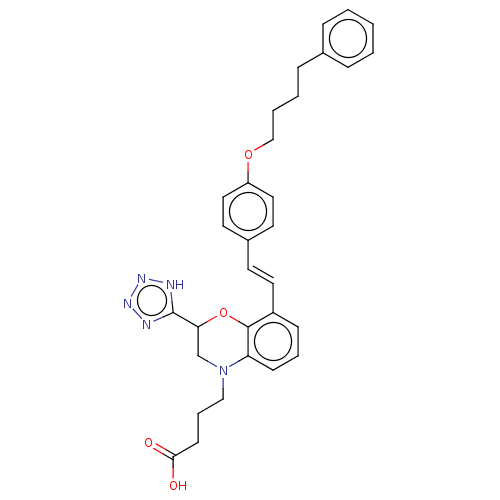 (CHEMBL3403186) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.590 | n/a | n/a | n/a | n/a | n/a | n/a |
Ono Pharmaceutical Co., Ltd Curated by ChEMBL | Assay Description Antagonist activity at human CysLT1 receptor expressed in CHO cells assessed as inhibition of LTD4-inudced intracellular calcium influx preincubated ... | Bioorg Med Chem 23: 2079-97 (2015) Article DOI: 10.1016/j.bmc.2015.03.011 BindingDB Entry DOI: 10.7270/Q2SB47FF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cysteinyl leukotriene receptor 2 (Homo sapiens (Human)) | BDBM50033089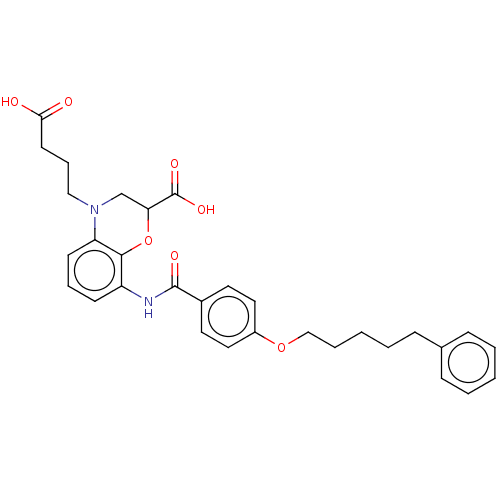 (CHEMBL3342959) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.620 | n/a | n/a | n/a | n/a | n/a | n/a |
Ono Pharmaceutical Co., Ltd. Curated by ChEMBL | Assay Description Antagonist activity at human CysLT2 receptor expressed in HEK293 cells assessed as inhibition of LTD4-inudced intracellular calcium influx preincubat... | ACS Med Chem Lett 5: 1230-4 (2014) Article DOI: 10.1021/ml500298y BindingDB Entry DOI: 10.7270/Q2QV3P3H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cysteinyl leukotriene receptor 1 (Homo sapiens (Human)) | BDBM50104864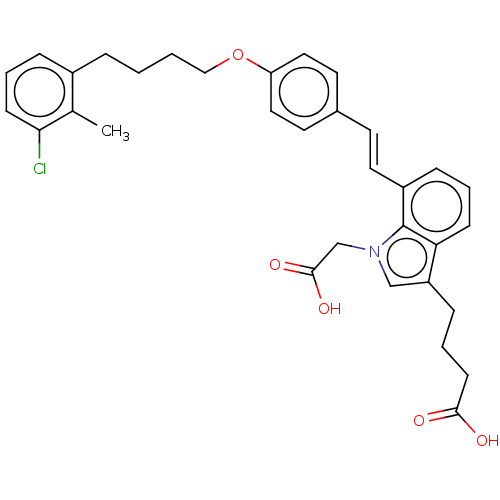 (CHEMBL3597527) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.620 | n/a | n/a | n/a | n/a | n/a | n/a |
Setsunan University Curated by ChEMBL | Assay Description Antagonist activity at human CysLT1 expressed in CHOK1 cells assessed as inhibition of LTD4-induced calcium mobilization preincubated for 30 mins pri... | J Med Chem 58: 6093-113 (2015) Article DOI: 10.1021/acs.jmedchem.5b00741 BindingDB Entry DOI: 10.7270/Q2QJ7K27 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cysteinyl leukotriene receptor 1 (Homo sapiens (Human)) | BDBM50066911 (CHEMBL3401689) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.790 | n/a | n/a | n/a | n/a | n/a | n/a |
Ono Pharmaceutical Co., Ltd Curated by ChEMBL | Assay Description Antagonist activity at human CysLT2 receptor expressed in HEK293 cells assessed as inhibition of LTD4-inudced intracellular calcium influx preincubat... | Bioorg Med Chem 23: 2079-97 (2015) Article DOI: 10.1016/j.bmc.2015.03.011 BindingDB Entry DOI: 10.7270/Q2SB47FF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cysteinyl leukotriene receptor 1 (Homo sapiens (Human)) | BDBM50104910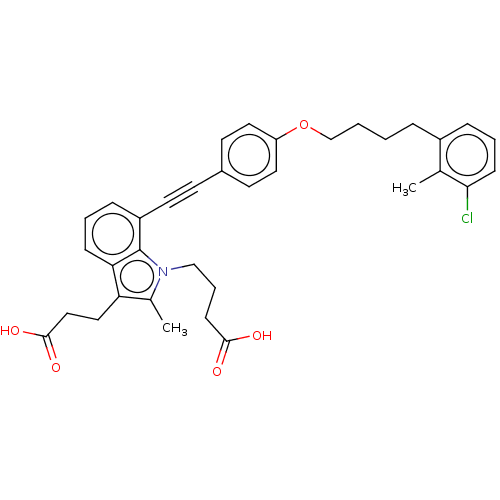 (CHEMBL3597623) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.790 | n/a | n/a | n/a | n/a | n/a | n/a |
Setsunan University Curated by ChEMBL | Assay Description Antagonist activity at human CysLT1 expressed in CHOK1 cells assessed as inhibition of LTD4-induced calcium mobilization preincubated for 30 mins pri... | J Med Chem 58: 6093-113 (2015) Article DOI: 10.1021/acs.jmedchem.5b00741 BindingDB Entry DOI: 10.7270/Q2QJ7K27 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cysteinyl leukotriene receptor 1 (Homo sapiens (Human)) | BDBM50104869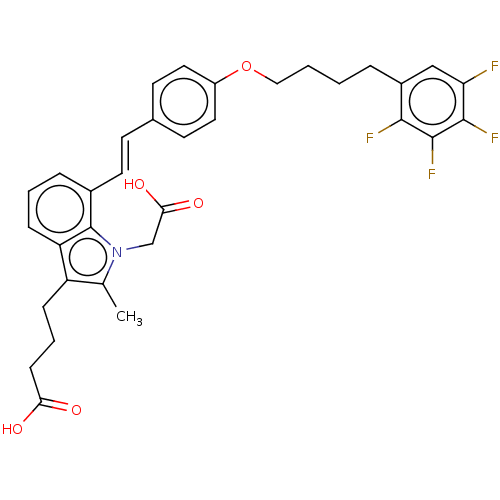 (CHEMBL3597532) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.800 | n/a | n/a | n/a | n/a | n/a | n/a |
Setsunan University Curated by ChEMBL | Assay Description Antagonist activity at human CysLT1 expressed in CHOK1 cells assessed as inhibition of LTD4-induced calcium mobilization preincubated for 30 mins pri... | J Med Chem 58: 6093-113 (2015) Article DOI: 10.1021/acs.jmedchem.5b00741 BindingDB Entry DOI: 10.7270/Q2QJ7K27 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cysteinyl leukotriene receptor 2 (Homo sapiens (Human)) | BDBM50033094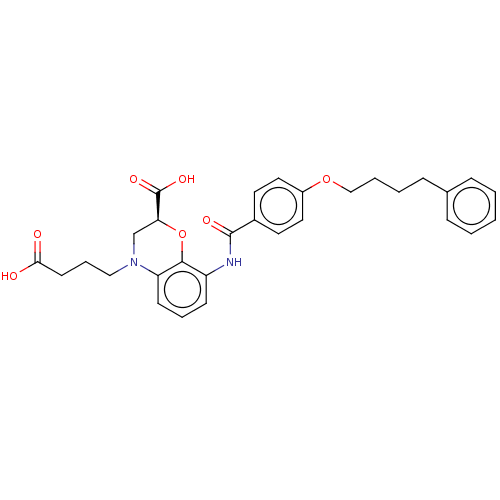 (CHEMBL3342964) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.870 | n/a | n/a | n/a | n/a | n/a | n/a |
Ono Pharmaceutical Co., Ltd. Curated by ChEMBL | Assay Description Antagonist activity at human CysLT2 receptor expressed in HEK293 cells assessed as inhibition of LTD4-inudced intracellular calcium influx preincubat... | ACS Med Chem Lett 5: 1230-4 (2014) Article DOI: 10.1021/ml500298y BindingDB Entry DOI: 10.7270/Q2QV3P3H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cysteinyl leukotriene receptor 2 (Homo sapiens (Human)) | BDBM50104905 (CHEMBL3597618) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.880 | n/a | n/a | n/a | n/a | n/a | n/a |
Setsunan University Curated by ChEMBL | Assay Description Antagonist activity at human CysLT2 expressed in HEK293 cells assessed as inhibition of LTD4-induced calcium mobilization preincubated for 30 mins pr... | J Med Chem 58: 6093-113 (2015) Article DOI: 10.1021/acs.jmedchem.5b00741 BindingDB Entry DOI: 10.7270/Q2QJ7K27 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cysteinyl leukotriene receptor 2 (Homo sapiens (Human)) | BDBM50104883 (CHEMBL3597616) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 0.890 | n/a | n/a | n/a | n/a | n/a | n/a |
Setsunan University Curated by ChEMBL | Assay Description Antagonist activity at human CysLT2 expressed in HEK293 cells assessed as inhibition of LTD4-induced calcium mobilization preincubated for 30 mins pr... | J Med Chem 58: 6093-113 (2015) Article DOI: 10.1021/acs.jmedchem.5b00741 BindingDB Entry DOI: 10.7270/Q2QJ7K27 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cysteinyl leukotriene receptor 1 (Homo sapiens (Human)) | BDBM50033083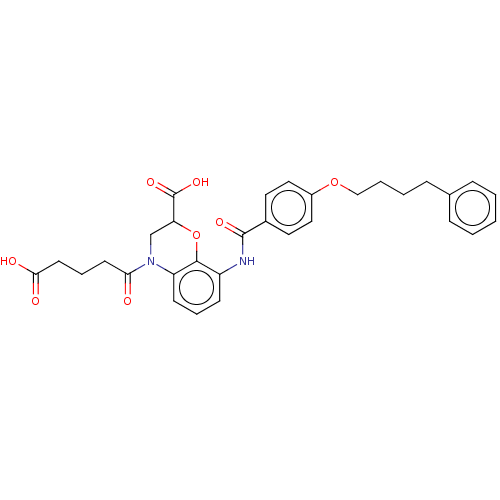 (CHEMBL3342953) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | <1 | n/a | n/a | n/a | n/a | n/a | n/a |
Ono Pharmaceutical Co., Ltd. Curated by ChEMBL | Assay Description Antagonist activity at human CysLT1 receptor expressed in CHO cells assessed as inhibition of LTD4-inudced intracellular calcium influx preincubated ... | ACS Med Chem Lett 5: 1230-4 (2014) Article DOI: 10.1021/ml500298y BindingDB Entry DOI: 10.7270/Q2QV3P3H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cysteinyl leukotriene receptor 1 (Homo sapiens (Human)) | BDBM50104839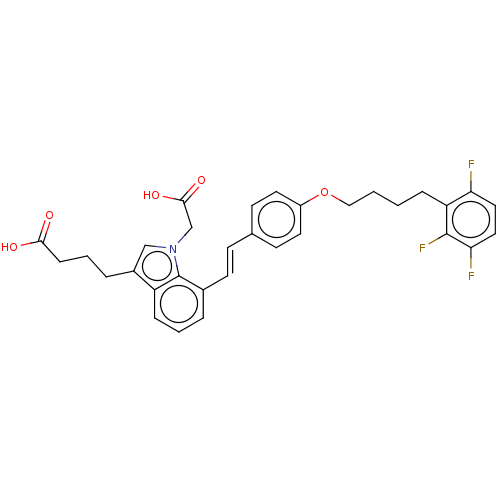 (CHEMBL3597525) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Setsunan University Curated by ChEMBL | Assay Description Antagonist activity at human CysLT1 expressed in CHOK1 cells assessed as inhibition of LTD4-induced calcium mobilization preincubated for 30 mins pri... | J Med Chem 58: 6093-113 (2015) Article DOI: 10.1021/acs.jmedchem.5b00741 BindingDB Entry DOI: 10.7270/Q2QJ7K27 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cysteinyl leukotriene receptor 2 (Homo sapiens (Human)) | BDBM50104867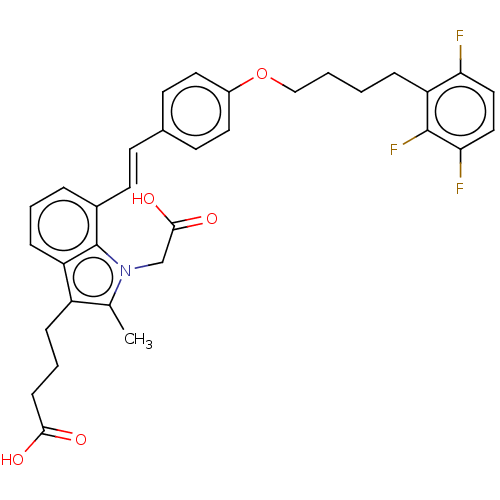 (CHEMBL3597530) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Setsunan University Curated by ChEMBL | Assay Description Antagonist activity at human CysLT2 expressed in HEK293 cells assessed as inhibition of LTD4-induced calcium mobilization preincubated for 30 mins pr... | J Med Chem 58: 6093-113 (2015) Article DOI: 10.1021/acs.jmedchem.5b00741 BindingDB Entry DOI: 10.7270/Q2QJ7K27 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cysteinyl leukotriene receptor 1 (Homo sapiens (Human)) | BDBM50104871 (CHEMBL3597534) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.10 | n/a | n/a | n/a | n/a | n/a | n/a |
Setsunan University Curated by ChEMBL | Assay Description Antagonist activity at human CysLT1 expressed in CHOK1 cells assessed as inhibition of LTD4-induced calcium mobilization preincubated for 30 mins pri... | J Med Chem 58: 6093-113 (2015) Article DOI: 10.1021/acs.jmedchem.5b00741 BindingDB Entry DOI: 10.7270/Q2QJ7K27 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cysteinyl leukotriene receptor 1 (Homo sapiens (Human)) | BDBM50104920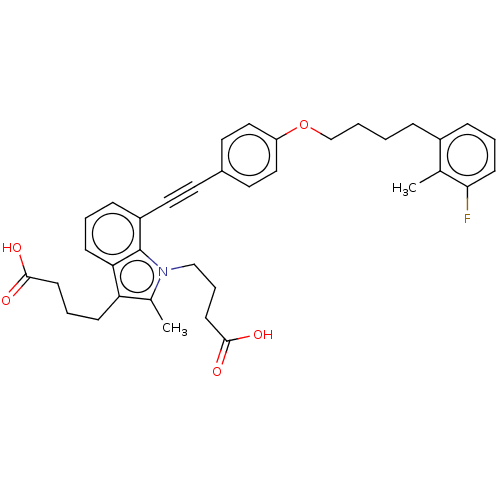 (CHEMBL3597633) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.10 | n/a | n/a | n/a | n/a | n/a | n/a |
Setsunan University Curated by ChEMBL | Assay Description Antagonist activity at human CysLT1 expressed in CHOK1 cells assessed as inhibition of LTD4-induced calcium mobilization preincubated for 30 mins pri... | J Med Chem 58: 6093-113 (2015) Article DOI: 10.1021/acs.jmedchem.5b00741 BindingDB Entry DOI: 10.7270/Q2QJ7K27 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cysteinyl leukotriene receptor 1 (Homo sapiens (Human)) | BDBM50033060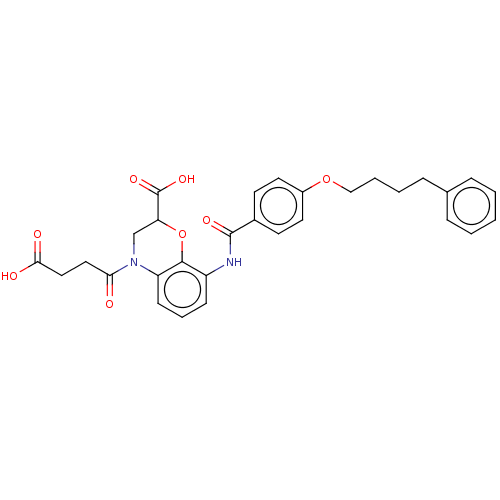 (CHEMBL3342952) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.40 | n/a | n/a | n/a | n/a | n/a | n/a |
Ono Pharmaceutical Co., Ltd. Curated by ChEMBL | Assay Description Antagonist activity at human CysLT1 receptor expressed in CHO cells assessed as inhibition of LTD4-inudced intracellular calcium influx preincubated ... | ACS Med Chem Lett 5: 1230-4 (2014) Article DOI: 10.1021/ml500298y BindingDB Entry DOI: 10.7270/Q2QV3P3H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cysteinyl leukotriene receptor 1 (Homo sapiens (Human)) | BDBM50104836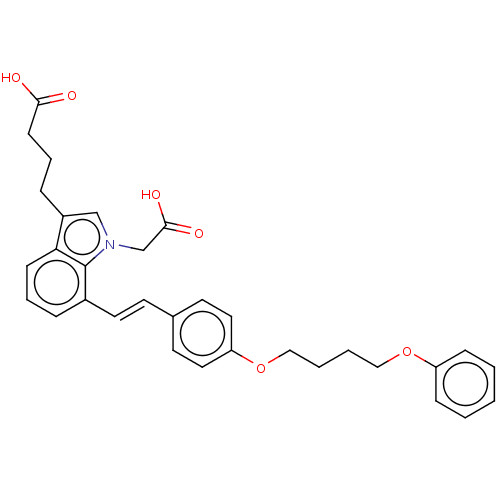 (CHEMBL3597522) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Setsunan University Curated by ChEMBL | Assay Description Antagonist activity at human CysLT1 expressed in CHOK1 cells assessed as inhibition of LTD4-induced calcium mobilization preincubated for 30 mins pri... | J Med Chem 58: 6093-113 (2015) Article DOI: 10.1021/acs.jmedchem.5b00741 BindingDB Entry DOI: 10.7270/Q2QJ7K27 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cysteinyl leukotriene receptor 2 (Homo sapiens (Human)) | BDBM50104869 (CHEMBL3597532) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Setsunan University Curated by ChEMBL | Assay Description Antagonist activity at human CysLT2 expressed in HEK293 cells assessed as inhibition of LTD4-induced calcium mobilization preincubated for 30 mins pr... | J Med Chem 58: 6093-113 (2015) Article DOI: 10.1021/acs.jmedchem.5b00741 BindingDB Entry DOI: 10.7270/Q2QJ7K27 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cysteinyl leukotriene receptor 1 (Homo sapiens (Human)) | BDBM50104911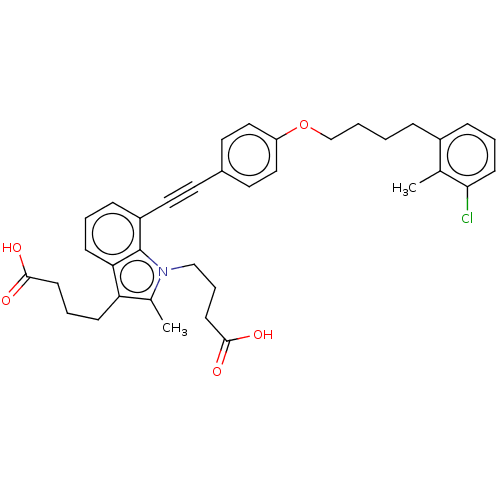 (CHEMBL3597624) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Setsunan University Curated by ChEMBL | Assay Description Antagonist activity at human CysLT1 expressed in CHOK1 cells assessed as inhibition of LTD4-induced calcium mobilization preincubated for 30 mins pri... | J Med Chem 58: 6093-113 (2015) Article DOI: 10.1021/acs.jmedchem.5b00741 BindingDB Entry DOI: 10.7270/Q2QJ7K27 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cysteinyl leukotriene receptor 1 (Homo sapiens (Human)) | BDBM50104870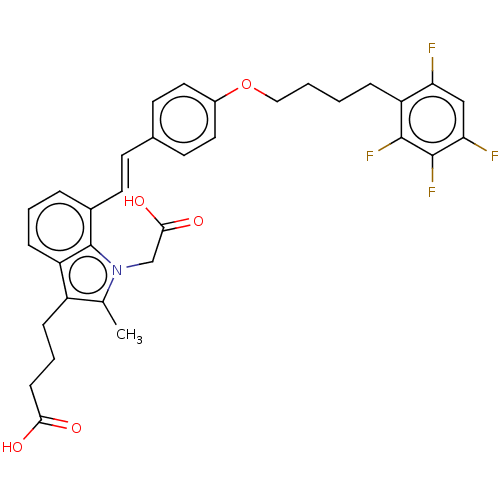 (CHEMBL3597533) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.60 | n/a | n/a | n/a | n/a | n/a | n/a |
Setsunan University Curated by ChEMBL | Assay Description Antagonist activity at human CysLT1 expressed in CHOK1 cells assessed as inhibition of LTD4-induced calcium mobilization preincubated for 30 mins pri... | J Med Chem 58: 6093-113 (2015) Article DOI: 10.1021/acs.jmedchem.5b00741 BindingDB Entry DOI: 10.7270/Q2QJ7K27 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cysteinyl leukotriene receptor 1 (Homo sapiens (Human)) | BDBM50104921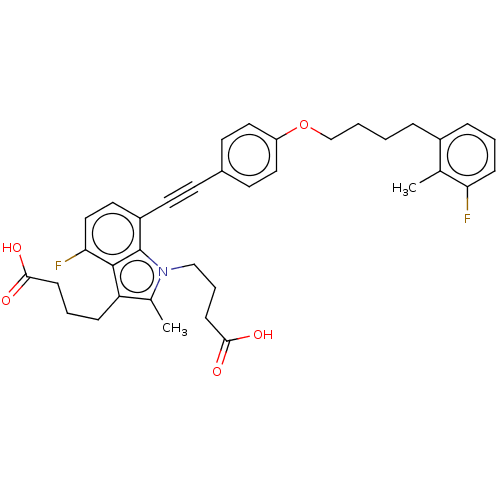 (CHEMBL3597634) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.70 | n/a | n/a | n/a | n/a | n/a | n/a |
Setsunan University Curated by ChEMBL | Assay Description Antagonist activity at human CysLT1 expressed in CHOK1 cells assessed as inhibition of LTD4-induced calcium mobilization preincubated for 30 mins pri... | J Med Chem 58: 6093-113 (2015) Article DOI: 10.1021/acs.jmedchem.5b00741 BindingDB Entry DOI: 10.7270/Q2QJ7K27 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium channel subunit beta-2 (Homo sapiens) | BDBM50257179 (CHEMBL2325622) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.90 | n/a | n/a | n/a | n/a | n/a | n/a |
Daiichi Sankyo Co., Ltd. Curated by ChEMBL | Assay Description Inhibition of human NaV1.7/beta1/beta2 expressed in HEK293A cells by Ionworks high-throughput electrophysiology method | J Med Chem 63: 10204-10220 (2020) Article DOI: 10.1021/acs.jmedchem.0c00259 BindingDB Entry DOI: 10.7270/Q2Q52T67 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cysteinyl leukotriene receptor 1 (Homo sapiens (Human)) | BDBM50009073 (4-(5-cyclopentyloxycarbonylamino-1-methyl-1H-indol...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG PC cid PC sid PDB UniChem Patents Similars | DrugBank PDB Article PubMed | n/a | n/a | 1.90 | n/a | n/a | n/a | n/a | n/a | n/a |
Setsunan University Curated by ChEMBL | Assay Description Antagonist activity at human CysLT1 | J Med Chem 58: 6093-113 (2015) Article DOI: 10.1021/acs.jmedchem.5b00741 BindingDB Entry DOI: 10.7270/Q2QJ7K27 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Cysteinyl leukotriene receptor 1 (GUINEA PIG) | BDBM50104920 (CHEMBL3597633) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Setsunan University Curated by ChEMBL | Assay Description Antagonist activity at guinea pig CysLT1 expressed in CHOK1 cells assessed as inhibition of LTD4-induced calcium mobilization preincubated for 30 min... | J Med Chem 58: 6093-113 (2015) Article DOI: 10.1021/acs.jmedchem.5b00741 BindingDB Entry DOI: 10.7270/Q2QJ7K27 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neprilysin (Homo sapiens (Human)) | BDBM50251742 ((3R,4R,5R,6S)-3,4,5-trihydroxy-6-methyl-tetrahydro...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Kao Corporation Curated by ChEMBL | Assay Description Inhibition of neutral endopeptidase in human fibroblasts homogenates using glutaryl-Ala-Ala-Phe-4-methoxy-2-naphtylamide as substrate after 1 hrs by ... | Bioorg Med Chem 19: 5935-47 (2011) Article DOI: 10.1016/j.bmc.2011.08.064 BindingDB Entry DOI: 10.7270/Q2154HF6 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Cysteinyl leukotriene receptor 2 (Homo sapiens (Human)) | BDBM50104839 (CHEMBL3597525) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.10 | n/a | n/a | n/a | n/a | n/a | n/a |
Setsunan University Curated by ChEMBL | Assay Description Antagonist activity at human CysLT2 expressed in HEK293 cells assessed as inhibition of LTD4-induced calcium mobilization preincubated for 30 mins pr... | J Med Chem 58: 6093-113 (2015) Article DOI: 10.1021/acs.jmedchem.5b00741 BindingDB Entry DOI: 10.7270/Q2QJ7K27 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cysteinyl leukotriene receptor 2 (Homo sapiens (Human)) | BDBM50104870 (CHEMBL3597533) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Setsunan University Curated by ChEMBL | Assay Description Antagonist activity at human CysLT2 expressed in HEK293 cells assessed as inhibition of LTD4-induced calcium mobilization preincubated for 30 mins pr... | J Med Chem 58: 6093-113 (2015) Article DOI: 10.1021/acs.jmedchem.5b00741 BindingDB Entry DOI: 10.7270/Q2QJ7K27 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cysteinyl leukotriene receptor 1 (GUINEA PIG) | BDBM50104911 (CHEMBL3597624) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Setsunan University Curated by ChEMBL | Assay Description Antagonist activity at guinea pig CysLT1 expressed in CHOK1 cells assessed as inhibition of LTD4-induced calcium mobilization preincubated for 30 min... | J Med Chem 58: 6093-113 (2015) Article DOI: 10.1021/acs.jmedchem.5b00741 BindingDB Entry DOI: 10.7270/Q2QJ7K27 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 557 total ) | Next | Last >> |