 Found 10512 hits with Last Name = 'tan' and Initial = 'l'
Found 10512 hits with Last Name = 'tan' and Initial = 'l' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
5'-nucleotidase
(Homo sapiens (Human)) | BDBM50527134

(CHEMBL4471306 | US20230295213, Compound a)Show SMILES C[C@H](Nc1cc(Cl)nc2n(ncc12)[C@@H]1O[C@H](COP(O)(=O)CP(O)(O)=O)[C@@H](O)[C@H]1O)c1ccccc1F |r| Show InChI InChI=1S/C20H24ClFN4O9P2/c1-10(11-4-2-3-5-13(11)22)24-14-6-16(21)25-19-12(14)7-23-26(19)20-18(28)17(27)15(35-20)8-34-37(32,33)9-36(29,30)31/h2-7,10,15,17-18,20,27-28H,8-9H2,1H3,(H,24,25)(H,32,33)(H2,29,30,31)/t10-,15+,17+,18+,20+/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
PDB
UniChem
| PDB
Article
PubMed
| 0.00500 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Competitive reversible inhibition of human C-terminal His6-tagged CD73 expressed in HEK293 cells using AMP as substrate preincubated with substrate f... |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.0c00525
BindingDB Entry DOI: 10.7270/Q29W0K29 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Dihydrofolate reductase
(Candida albicans) | BDBM50049912

(7-(1,1-Dimethyl-propyl)-8-methyl-7H-pyrrolo[3,2-f]...)Show InChI InChI=1S/C16H21N5/c1-5-16(3,4)21-9(2)8-10-12(21)7-6-11-13(10)14(17)20-15(18)19-11/h6-8H,5H2,1-4H3,(H4,17,18,19,20) | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.00710 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Wellcome Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of Dihydrofolate reductase enzyme from Candida albicans |
J Med Chem 39: 892-903 (1996)
Article DOI: 10.1021/jm9505122
BindingDB Entry DOI: 10.7270/Q2XD10RC |
More data for this
Ligand-Target Pair | |
D(2) dopamine receptor
(Rattus norvegicus (rat)) | BDBM50007518

((S)-3-chloro-5-ethyl-N-((1-ethylpyrrolidin-2-yl)me...)Show InChI InChI=1S/C17H25ClN2O3/c1-4-11-9-13(18)16(23-3)14(15(11)21)17(22)19-10-12-7-6-8-20(12)5-2/h9,12,21H,4-8,10H2,1-3H3,(H,19,22)/t12-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PubMed
| 0.0200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Washington University
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 268: 495-502 (1994)
BindingDB Entry DOI: 10.7270/Q2S75DWP |
More data for this
Ligand-Target Pair | |
D(2) dopamine receptor
(Rattus norvegicus (rat)) | BDBM50005120
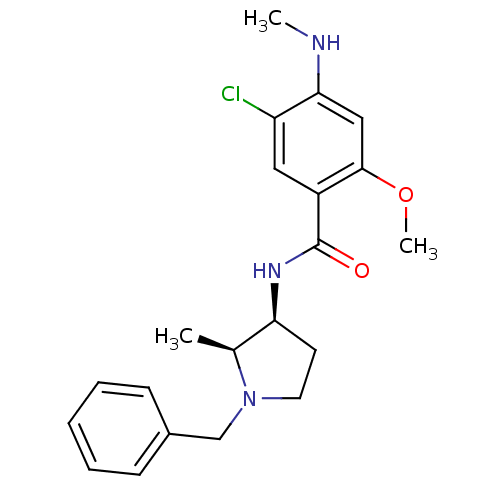
(CHEMBL274491 | N-((2S,3S)-1-Benzyl-2-methyl-pyrrol...)Show SMILES CNc1cc(OC)c(cc1Cl)C(=O)N[C@H]1CCN(Cc2ccccc2)[C@H]1C Show InChI InChI=1S/C21H26ClN3O2/c1-14-18(9-10-25(14)13-15-7-5-4-6-8-15)24-21(26)16-11-17(22)19(23-2)12-20(16)27-3/h4-8,11-12,14,18,23H,9-10,13H2,1-3H3,(H,24,26)/t14-,18-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 0.0200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Washington University
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 268: 495-502 (1994)
BindingDB Entry DOI: 10.7270/Q2S75DWP |
More data for this
Ligand-Target Pair | |
Dihydrofolate reductase
(Homo sapiens (Human)) | BDBM18224

(6-[(2,5-dimethoxyphenyl)methyl]-5-methylpyrido[2,3...)Show InChI InChI=1S/C17H19N5O2/c1-9-11(6-10-7-12(23-2)4-5-13(10)24-3)8-20-16-14(9)15(18)21-17(19)22-16/h4-5,7-8H,6H2,1-3H3,(H4,18,19,20,21,22) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| MMDB
PDB
Article
PubMed
| 0.0250 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Wellcome Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant Dihydrofolate reductase enzyme |
J Med Chem 39: 892-903 (1996)
Article DOI: 10.1021/jm9505122
BindingDB Entry DOI: 10.7270/Q2XD10RC |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Dihydrofolate reductase
(Candida albicans) | BDBM50049905
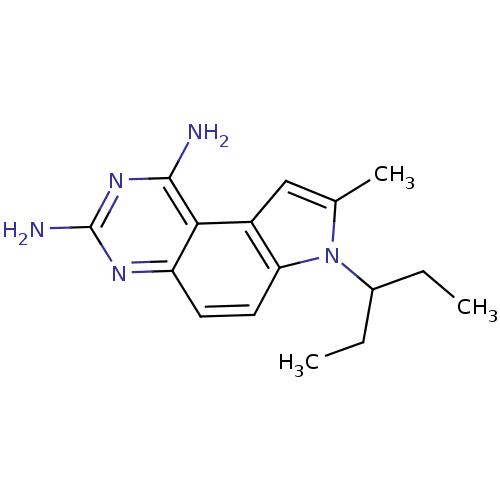
(7-(1-Ethyl-propyl)-8-methyl-7H-pyrrolo[3,2-f]quina...)Show InChI InChI=1S/C16H21N5/c1-4-10(5-2)21-9(3)8-11-13(21)7-6-12-14(11)15(17)20-16(18)19-12/h6-8,10H,4-5H2,1-3H3,(H4,17,18,19,20) | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.0300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Wellcome Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of Dihydrofolate reductase enzyme from Candida albicans |
J Med Chem 39: 892-903 (1996)
Article DOI: 10.1021/jm9505122
BindingDB Entry DOI: 10.7270/Q2XD10RC |
More data for this
Ligand-Target Pair | |
D(2) dopamine receptor
(Rattus norvegicus (rat)) | BDBM50005120

(CHEMBL274491 | N-((2S,3S)-1-Benzyl-2-methyl-pyrrol...)Show SMILES CNc1cc(OC)c(cc1Cl)C(=O)N[C@H]1CCN(Cc2ccccc2)[C@H]1C Show InChI InChI=1S/C21H26ClN3O2/c1-14-18(9-10-25(14)13-15-7-5-4-6-8-15)24-21(26)16-11-17(22)19(23-2)12-20(16)27-3/h4-8,11-12,14,18,23H,9-10,13H2,1-3H3,(H,24,26)/t14-,18-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 0.0300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Washington University
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 268: 495-502 (1994)
BindingDB Entry DOI: 10.7270/Q2S75DWP |
More data for this
Ligand-Target Pair | |
D(4) dopamine receptor
(Homo sapiens (Human)) | BDBM50005120

(CHEMBL274491 | N-((2S,3S)-1-Benzyl-2-methyl-pyrrol...)Show SMILES CNc1cc(OC)c(cc1Cl)C(=O)N[C@H]1CCN(Cc2ccccc2)[C@H]1C Show InChI InChI=1S/C21H26ClN3O2/c1-14-18(9-10-25(14)13-15-7-5-4-6-8-15)24-21(26)16-11-17(22)19(23-2)12-20(16)27-3/h4-8,11-12,14,18,23H,9-10,13H2,1-3H3,(H,24,26)/t14-,18-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 0.0300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Washington University
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 268: 495-502 (1994)
BindingDB Entry DOI: 10.7270/Q2S75DWP |
More data for this
Ligand-Target Pair | |
D(4) dopamine receptor
(Homo sapiens (Human)) | BDBM50005120

(CHEMBL274491 | N-((2S,3S)-1-Benzyl-2-methyl-pyrrol...)Show SMILES CNc1cc(OC)c(cc1Cl)C(=O)N[C@H]1CCN(Cc2ccccc2)[C@H]1C Show InChI InChI=1S/C21H26ClN3O2/c1-14-18(9-10-25(14)13-15-7-5-4-6-8-15)24-21(26)16-11-17(22)19(23-2)12-20(16)27-3/h4-8,11-12,14,18,23H,9-10,13H2,1-3H3,(H,24,26)/t14-,18-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 0.0300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Washington University
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 268: 495-502 (1994)
BindingDB Entry DOI: 10.7270/Q2S75DWP |
More data for this
Ligand-Target Pair | |
D(3) dopamine receptor
(Rattus norvegicus (Rat)) | BDBM50005120

(CHEMBL274491 | N-((2S,3S)-1-Benzyl-2-methyl-pyrrol...)Show SMILES CNc1cc(OC)c(cc1Cl)C(=O)N[C@H]1CCN(Cc2ccccc2)[C@H]1C Show InChI InChI=1S/C21H26ClN3O2/c1-14-18(9-10-25(14)13-15-7-5-4-6-8-15)24-21(26)16-11-17(22)19(23-2)12-20(16)27-3/h4-8,11-12,14,18,23H,9-10,13H2,1-3H3,(H,24,26)/t14-,18-/m0/s1 | KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 0.0500 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Washington University
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 268: 495-502 (1994)
BindingDB Entry DOI: 10.7270/Q2S75DWP |
More data for this
Ligand-Target Pair | |
Kappa-type opioid receptor
(Cavia porcellus (domestic guinea pig)) | BDBM50026614
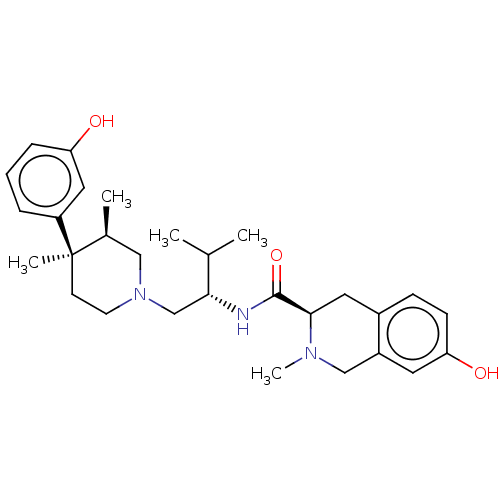
(CHEMBL575508)Show SMILES CC(C)[C@@H](CN1CC[C@](C)([C@@H](C)C1)c1cccc(O)c1)NC(=O)[C@H]1Cc2ccc(O)cc2CN1C |r| Show InChI InChI=1S/C29H41N3O3/c1-19(2)26(30-28(35)27-14-21-9-10-25(34)13-22(21)17-31(27)5)18-32-12-11-29(4,20(3)16-32)23-7-6-8-24(33)15-23/h6-10,13,15,19-20,26-27,33-34H,11-12,14,16-18H2,1-5H3,(H,30,35)/t20-,26+,27+,29+/m0/s1 | PDB
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.0530 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Research Triangle Institute
Curated by ChEMBL
| Assay Description
Inhibition of [3H]U-69593 binding to Opioid receptor kappa 1 of guinea pig brain |
J Med Chem 46: 3127-37 (2003)
Article DOI: 10.1021/jm030094y
BindingDB Entry DOI: 10.7270/Q2319WM4 |
More data for this
Ligand-Target Pair | |
D(3) dopamine receptor
(Rattus norvegicus (Rat)) | BDBM50005120

(CHEMBL274491 | N-((2S,3S)-1-Benzyl-2-methyl-pyrrol...)Show SMILES CNc1cc(OC)c(cc1Cl)C(=O)N[C@H]1CCN(Cc2ccccc2)[C@H]1C Show InChI InChI=1S/C21H26ClN3O2/c1-14-18(9-10-25(14)13-15-7-5-4-6-8-15)24-21(26)16-11-17(22)19(23-2)12-20(16)27-3/h4-8,11-12,14,18,23H,9-10,13H2,1-3H3,(H,24,26)/t14-,18-/m0/s1 | KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 0.0600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Washington University
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 268: 495-502 (1994)
BindingDB Entry DOI: 10.7270/Q2S75DWP |
More data for this
Ligand-Target Pair | |
D(2) dopamine receptor
(Rattus norvegicus (rat)) | BDBM50007518

((S)-3-chloro-5-ethyl-N-((1-ethylpyrrolidin-2-yl)me...)Show InChI InChI=1S/C17H25ClN2O3/c1-4-11-9-13(18)16(23-3)14(15(11)21)17(22)19-10-12-7-6-8-20(12)5-2/h9,12,21H,4-8,10H2,1-3H3,(H,19,22)/t12-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PubMed
| 0.0600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Washington University
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 268: 495-502 (1994)
BindingDB Entry DOI: 10.7270/Q2S75DWP |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M3
(Homo sapiens (Human)) | BDBM103760
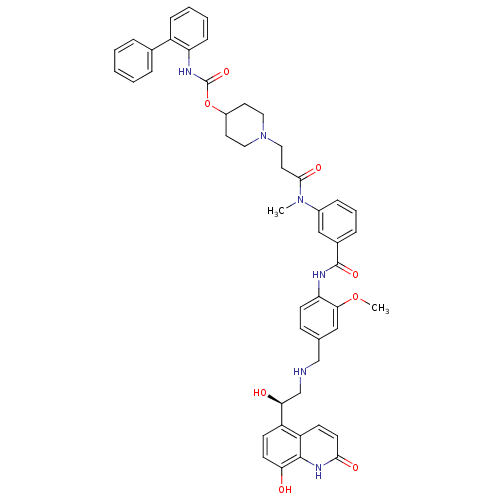
(US8551978, I-29 | US8816088, I-29 | US9394275, I-2...)Show SMILES COc1cc(CNC[C@H](O)c2ccc(O)c3[nH]c(=O)ccc23)ccc1NC(=O)c1cccc(c1)N(C)C(=O)CCN1CCC(CC1)OC(=O)Nc1ccccc1-c1ccccc1 |r| Show InChI InChI=1S/C48H50N6O8/c1-53(45(58)23-26-54-24-21-35(22-25-54)62-48(60)51-39-14-7-6-13-36(39)32-9-4-3-5-10-32)34-12-8-11-33(28-34)47(59)50-40-18-15-31(27-43(40)61-2)29-49-30-42(56)37-16-19-41(55)46-38(37)17-20-44(57)52-46/h3-20,27-28,35,42,49,55-56H,21-26,29-30H2,1-2H3,(H,50,59)(H,51,60)(H,52,57)/t42-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| 0.100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Theravance, Inc.
US Patent
| Assay Description
Radioligand binding assay using muscarinic receptors. |
US Patent US8551978 (2013)
BindingDB Entry DOI: 10.7270/Q2Q81BPC |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M3
(Homo sapiens (Human)) | BDBM6354
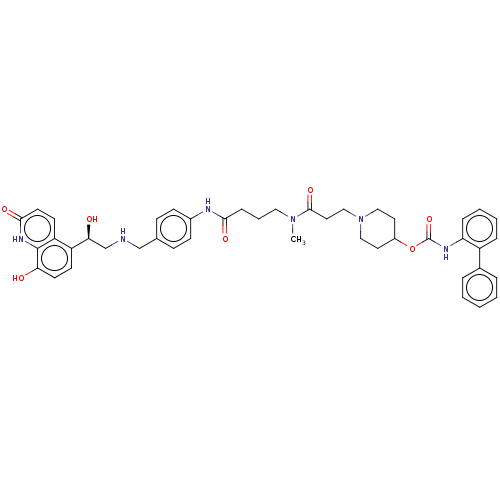
(US8551978, I-1 | US8816088, 1 | US9394275, I-1 | U...)Show SMILES CN(CCCC(=O)Nc1ccc(CNC[C@H](O)c2ccc(O)c3[nH]c(=O)ccc23)cc1)C(=O)CCN1CCC(CC1)OC(=O)Nc1ccccc1-c1ccccc1 |r| Show InChI InChI=1S/C44H50N6O7/c1-49(42(55)23-27-50-25-21-33(22-26-50)57-44(56)47-37-11-6-5-10-34(37)31-8-3-2-4-9-31)24-7-12-40(53)46-32-15-13-30(14-16-32)28-45-29-39(52)35-17-19-38(51)43-36(35)18-20-41(54)48-43/h2-6,8-11,13-20,33,39,45,51-52H,7,12,21-29H2,1H3,(H,46,53)(H,47,56)(H,48,54)/t39-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| 0.100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Theravance Respiratory Company, LLC
US Patent
| Assay Description
Radioligand binding assays for cloned muscarinic receptors were performed in 96-well microtiter plates in a total assay volume of 100 uL. CHO cell me... |
US Patent US8816088 (2014)
BindingDB Entry DOI: 10.7270/Q2TQ6068 |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M3
(Homo sapiens (Human)) | BDBM6363
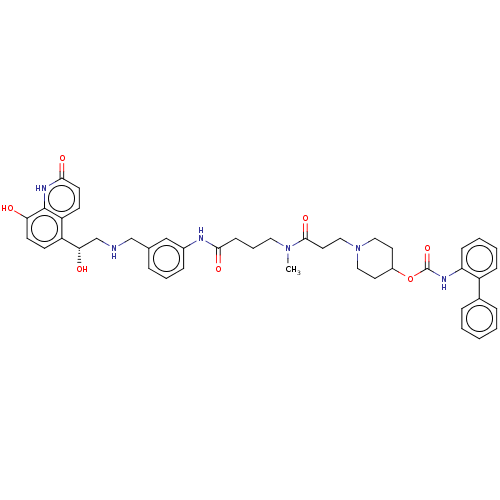
(US8816088, 7)Show SMILES CN(CCCC(=O)Nc1cccc(CNC[C@H](O)c2ccc(O)c3[nH]c(=O)ccc23)c1)C(=O)CCN1CCC(CC1)OC(=O)Nc1ccccc1-c1ccccc1 |r| Show InChI InChI=1S/C44H50N6O7/c1-49(42(55)22-26-50-24-20-33(21-25-50)57-44(56)47-37-14-6-5-13-34(37)31-10-3-2-4-11-31)23-8-15-40(53)46-32-12-7-9-30(27-32)28-45-29-39(52)35-16-18-38(51)43-36(35)17-19-41(54)48-43/h2-7,9-14,16-19,27,33,39,45,51-52H,8,15,20-26,28-29H2,1H3,(H,46,53)(H,47,56)(H,48,54)/t39-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| 0.100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Theravance Respiratory Company, LLC
US Patent
| Assay Description
Radioligand binding assays for cloned muscarinic receptors were performed in 96-well microtiter plates in a total assay volume of 100 uL. CHO cell me... |
US Patent US8816088 (2014)
BindingDB Entry DOI: 10.7270/Q2TQ6068 |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M3
(Homo sapiens (Human)) | BDBM6382
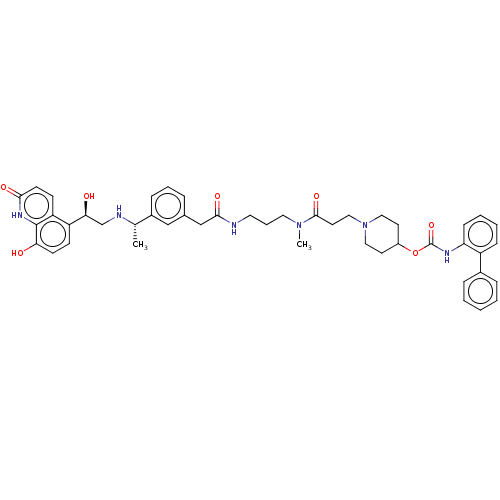
(US8816088, 13)Show SMILES C[C@H](NC[C@H](O)c1ccc(O)c2[nH]c(=O)ccc12)c1cccc(CC(=O)NCCCN(C)C(=O)CCN2CCC(CC2)OC(=O)Nc2ccccc2-c2ccccc2)c1 |r| Show InChI InChI=1S/C46H54N6O7/c1-31(48-30-41(54)37-16-18-40(53)45-38(37)17-19-42(55)50-45)34-13-8-10-32(28-34)29-43(56)47-23-9-24-51(2)44(57)22-27-52-25-20-35(21-26-52)59-46(58)49-39-15-7-6-14-36(39)33-11-4-3-5-12-33/h3-8,10-19,28,31,35,41,48,53-54H,9,20-27,29-30H2,1-2H3,(H,47,56)(H,49,58)(H,50,55)/t31-,41-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| 0.100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Theravance Respiratory Company, LLC
US Patent
| Assay Description
Radioligand binding assays for cloned muscarinic receptors were performed in 96-well microtiter plates in a total assay volume of 100 uL. CHO cell me... |
US Patent US8816088 (2014)
BindingDB Entry DOI: 10.7270/Q2TQ6068 |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M3
(Homo sapiens (Human)) | BDBM6384
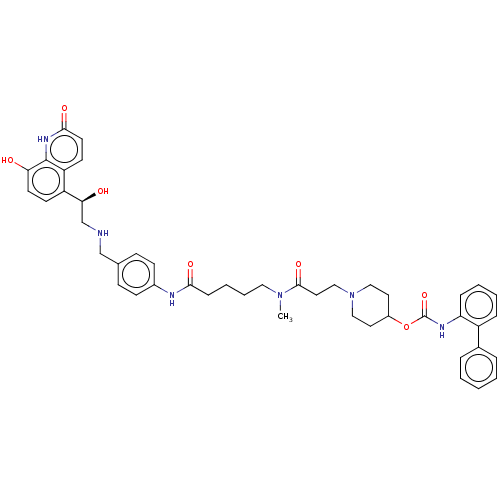
(US8551978, I-14 | US8816088, 14 | US8816088, I-15 ...)Show SMILES CN(CCCCC(=O)Nc1ccc(CNC[C@H](O)c2ccc(O)c3[nH]c(=O)ccc23)cc1)C(=O)CCN1CCC(CC1)OC(=O)Nc1ccccc1-c1ccccc1 |r| Show InChI InChI=1S/C45H52N6O7/c1-50(43(56)24-28-51-26-22-34(23-27-51)58-45(57)48-38-12-6-5-11-35(38)32-9-3-2-4-10-32)25-8-7-13-41(54)47-33-16-14-31(15-17-33)29-46-30-40(53)36-18-20-39(52)44-37(36)19-21-42(55)49-44/h2-6,9-12,14-21,34,40,46,52-53H,7-8,13,22-30H2,1H3,(H,47,54)(H,48,57)(H,49,55)/t40-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| 0.100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Theravance Respiratory Company, LLC
US Patent
| Assay Description
Radioligand binding assays for cloned muscarinic receptors were performed in 96-well microtiter plates in a total assay volume of 100 uL. CHO cell me... |
US Patent US8816088 (2014)
BindingDB Entry DOI: 10.7270/Q2TQ6068 |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M3
(Homo sapiens (Human)) | BDBM6387
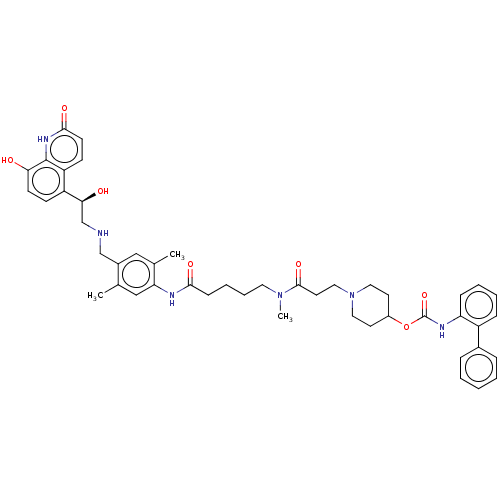
(US8816088, 18 | US9394275, I-18 | US9572802, Compo...)Show SMILES CN(CCCCC(=O)Nc1cc(C)c(CNC[C@H](O)c2ccc(O)c3[nH]c(=O)ccc23)cc1C)C(=O)CCN1CCC(CC1)OC(=O)Nc1ccccc1-c1ccccc1 |r| Show InChI InChI=1S/C47H56N6O7/c1-31-28-40(32(2)27-34(31)29-48-30-42(55)37-16-18-41(54)46-38(37)17-19-44(57)51-46)49-43(56)15-9-10-23-52(3)45(58)22-26-53-24-20-35(21-25-53)60-47(59)50-39-14-8-7-13-36(39)33-11-5-4-6-12-33/h4-8,11-14,16-19,27-28,35,42,48,54-55H,9-10,15,20-26,29-30H2,1-3H3,(H,49,56)(H,50,59)(H,51,57)/t42-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| 0.100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Theravance Respiratory Company, LLC
US Patent
| Assay Description
Radioligand binding assays for cloned muscarinic receptors were performed in 96-well microtiter plates in a total assay volume of 100 uL. CHO cell me... |
US Patent US8816088 (2014)
BindingDB Entry DOI: 10.7270/Q2TQ6068 |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M3
(Homo sapiens (Human)) | BDBM6427
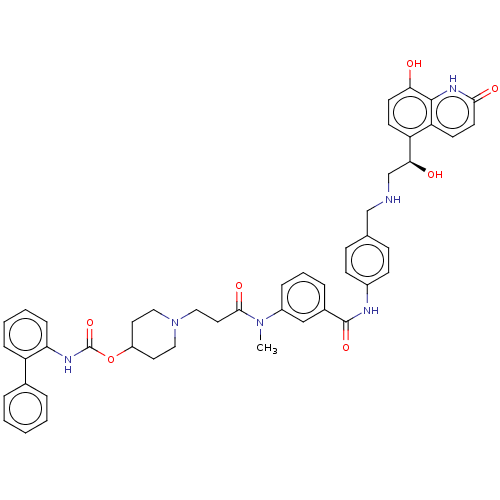
(US8551978, I-25 | US8816088, 25 | US9572802, Compo...)Show SMILES CN(C(=O)CCN1CCC(CC1)OC(=O)Nc1ccccc1-c1ccccc1)c1cccc(c1)C(=O)Nc1ccc(CNC[C@H](O)c2ccc(O)c3[nH]c(=O)ccc23)cc1 |r| Show InChI InChI=1S/C47H48N6O7/c1-52(44(57)24-27-53-25-22-36(23-26-53)60-47(59)50-40-13-6-5-12-37(40)32-8-3-2-4-9-32)35-11-7-10-33(28-35)46(58)49-34-16-14-31(15-17-34)29-48-30-42(55)38-18-20-41(54)45-39(38)19-21-43(56)51-45/h2-21,28,36,42,48,54-55H,22-27,29-30H2,1H3,(H,49,58)(H,50,59)(H,51,56)/t42-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| 0.100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Theravance Respiratory Company, LLC
US Patent
| Assay Description
Radioligand binding assays for cloned muscarinic receptors were performed in 96-well microtiter plates in a total assay volume of 100 uL. CHO cell me... |
US Patent US8816088 (2014)
BindingDB Entry DOI: 10.7270/Q2TQ6068 |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M3
(Homo sapiens (Human)) | BDBM6449
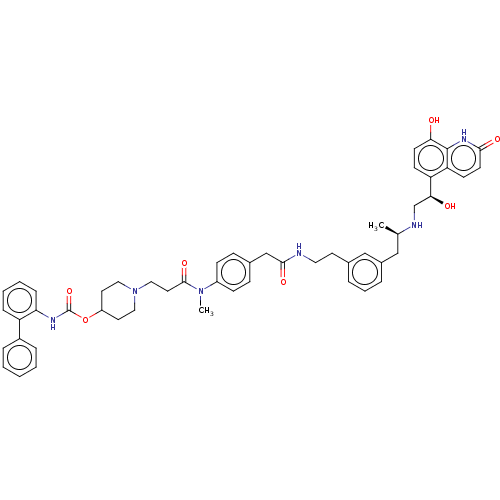
(US8551978, I-87 | US8816088, 87 | US9394275, I-7 |...)Show SMILES C[C@H](Cc1cccc(CCNC(=O)Cc2ccc(cc2)N(C)C(=O)CCN2CCC(CC2)OC(=O)Nc2ccccc2-c2ccccc2)c1)NC[C@H](O)c1ccc(O)c2[nH]c(=O)ccc12 |r| Show InChI InChI=1S/C52H58N6O7/c1-35(54-34-47(60)43-19-21-46(59)51-44(43)20-22-48(61)56-51)31-38-10-8-9-36(32-38)23-27-53-49(62)33-37-15-17-40(18-16-37)57(2)50(63)26-30-58-28-24-41(25-29-58)65-52(64)55-45-14-7-6-13-42(45)39-11-4-3-5-12-39/h3-22,32,35,41,47,54,59-60H,23-31,33-34H2,1-2H3,(H,53,62)(H,55,64)(H,56,61)/t35-,47+/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| 0.100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Theravance Respiratory Company, LLC
US Patent
| Assay Description
Radioligand binding assays for cloned muscarinic receptors were performed in 96-well microtiter plates in a total assay volume of 100 uL. CHO cell me... |
US Patent US8816088 (2014)
BindingDB Entry DOI: 10.7270/Q2TQ6068 |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M3
(Homo sapiens (Human)) | BDBM103790
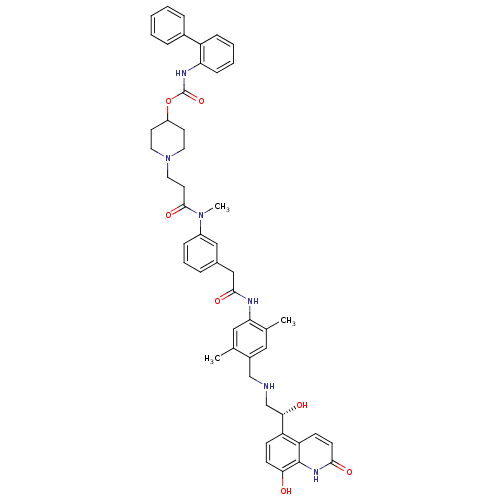
(US8551978, I-60 | US8816088, I-60 | US9394275, I-6...)Show SMILES CN(C(=O)CCN1CCC(CC1)OC(=O)Nc1ccccc1-c1ccccc1)c1cccc(CC(=O)Nc2cc(C)c(CNC[C@H](O)c3ccc(O)c4[nH]c(=O)ccc34)cc2C)c1 |r| Show InChI InChI=1S/C50H54N6O7/c1-32-27-43(33(2)26-36(32)30-51-31-45(58)40-16-18-44(57)49-41(40)17-19-46(59)54-49)52-47(60)29-34-10-9-13-37(28-34)55(3)48(61)22-25-56-23-20-38(21-24-56)63-50(62)53-42-15-8-7-14-39(42)35-11-5-4-6-12-35/h4-19,26-28,38,45,51,57-58H,20-25,29-31H2,1-3H3,(H,52,60)(H,53,62)(H,54,59)/t45-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| 0.100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Theravance, Inc.
US Patent
| Assay Description
Radioligand binding assay using muscarinic receptors. |
US Patent US8551978 (2013)
BindingDB Entry DOI: 10.7270/Q2Q81BPC |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M3
(Homo sapiens (Human)) | BDBM103791
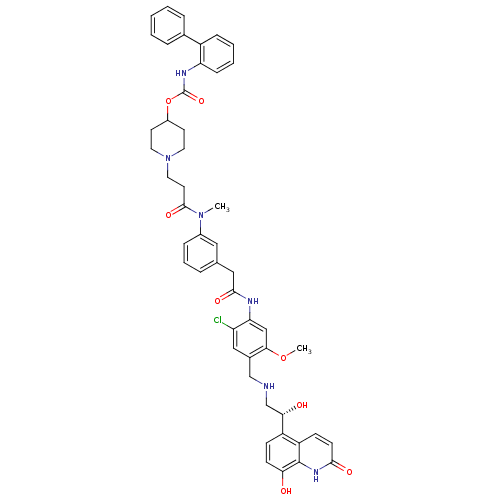
(US8551978, I-61 | US8816088, I-61 | US9394275, I-6...)Show SMILES COc1cc(NC(=O)Cc2cccc(c2)N(C)C(=O)CCN2CCC(CC2)OC(=O)Nc2ccccc2-c2ccccc2)c(Cl)cc1CNC[C@H](O)c1ccc(O)c2[nH]c(=O)ccc12 |r| Show InChI InChI=1S/C49H51ClN6O8/c1-55(47(61)21-24-56-22-19-35(20-23-56)64-49(62)53-40-14-7-6-13-36(40)32-10-4-3-5-11-32)34-12-8-9-31(25-34)26-46(60)52-41-28-44(63-2)33(27-39(41)50)29-51-30-43(58)37-15-17-42(57)48-38(37)16-18-45(59)54-48/h3-18,25,27-28,35,43,51,57-58H,19-24,26,29-30H2,1-2H3,(H,52,60)(H,53,62)(H,54,59)/t43-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| 0.100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Theravance, Inc.
US Patent
| Assay Description
Radioligand binding assay using muscarinic receptors. |
US Patent US8551978 (2013)
BindingDB Entry DOI: 10.7270/Q2Q81BPC |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M3
(Homo sapiens (Human)) | BDBM103792
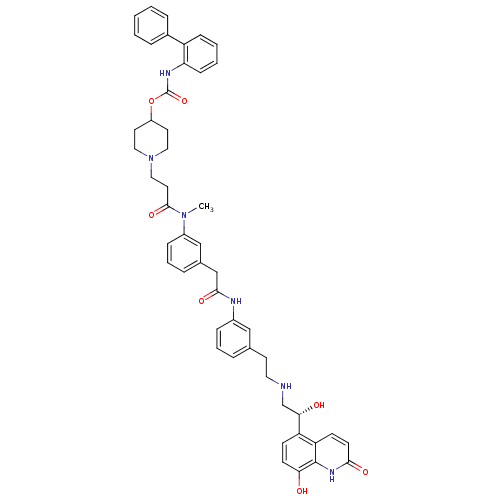
(US8551978, I-62 | US8816088, I-62 | US9394275, I-6...)Show SMILES CN(C(=O)CCN1CCC(CC1)OC(=O)Nc1ccccc1-c1ccccc1)c1cccc(CC(=O)Nc2cccc(CCNC[C@H](O)c3ccc(O)c4[nH]c(=O)ccc34)c2)c1 |r| Show InChI InChI=1S/C49H52N6O7/c1-54(47(60)24-28-55-26-22-38(23-27-55)62-49(61)52-42-16-6-5-15-39(42)35-11-3-2-4-12-35)37-14-8-10-34(30-37)31-46(59)51-36-13-7-9-33(29-36)21-25-50-32-44(57)40-17-19-43(56)48-41(40)18-20-45(58)53-48/h2-20,29-30,38,44,50,56-57H,21-28,31-32H2,1H3,(H,51,59)(H,52,61)(H,53,58)/t44-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| 0.100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Theravance, Inc.
US Patent
| Assay Description
Radioligand binding assay using muscarinic receptors. |
US Patent US8551978 (2013)
BindingDB Entry DOI: 10.7270/Q2Q81BPC |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M3
(Homo sapiens (Human)) | BDBM103793
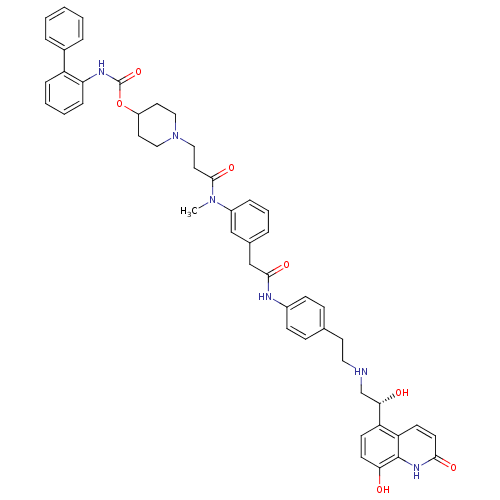
(US8551978, I-63 | US8816088, I-63 | US9394275, I-6...)Show SMILES CN(C(=O)CCN1CCC(CC1)OC(=O)Nc1ccccc1-c1ccccc1)c1cccc(CC(=O)Nc2ccc(CCNC[C@H](O)c3ccc(O)c4[nH]c(=O)ccc34)cc2)c1 |r| Show InChI InChI=1S/C49H52N6O7/c1-54(47(60)25-29-55-27-23-38(24-28-55)62-49(61)52-42-13-6-5-12-39(42)35-9-3-2-4-10-35)37-11-7-8-34(30-37)31-46(59)51-36-16-14-33(15-17-36)22-26-50-32-44(57)40-18-20-43(56)48-41(40)19-21-45(58)53-48/h2-21,30,38,44,50,56-57H,22-29,31-32H2,1H3,(H,51,59)(H,52,61)(H,53,58)/t44-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| 0.100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Theravance, Inc.
US Patent
| Assay Description
Radioligand binding assay using muscarinic receptors. |
US Patent US8551978 (2013)
BindingDB Entry DOI: 10.7270/Q2Q81BPC |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M3
(Homo sapiens (Human)) | BDBM103795
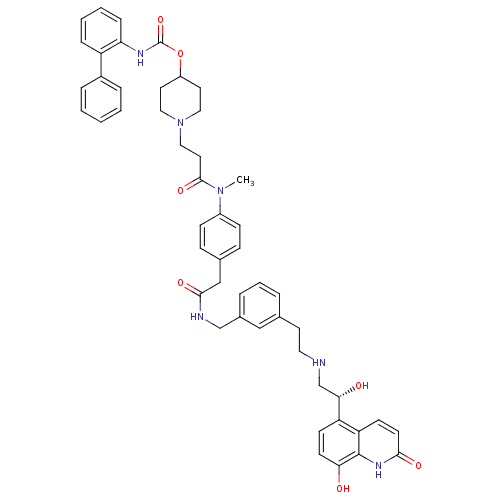
(US8551978, I-65 | US8816088, I-65 | US9394275, I-6...)Show SMILES CN(C(=O)CCN1CCC(CC1)OC(=O)Nc1ccccc1-c1ccccc1)c1ccc(CC(=O)NCc2cccc(CCNC[C@H](O)c3ccc(O)c4[nH]c(=O)ccc34)c2)cc1 |r| Show InChI InChI=1S/C50H54N6O7/c1-55(48(61)25-29-56-27-23-39(24-28-56)63-50(62)53-43-13-6-5-12-40(43)37-10-3-2-4-11-37)38-16-14-35(15-17-38)31-47(60)52-32-36-9-7-8-34(30-36)22-26-51-33-45(58)41-18-20-44(57)49-42(41)19-21-46(59)54-49/h2-21,30,39,45,51,57-58H,22-29,31-33H2,1H3,(H,52,60)(H,53,62)(H,54,59)/t45-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| 0.100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Theravance, Inc.
US Patent
| Assay Description
Radioligand binding assay using muscarinic receptors. |
US Patent US8551978 (2013)
BindingDB Entry DOI: 10.7270/Q2Q81BPC |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M3
(Homo sapiens (Human)) | BDBM103796
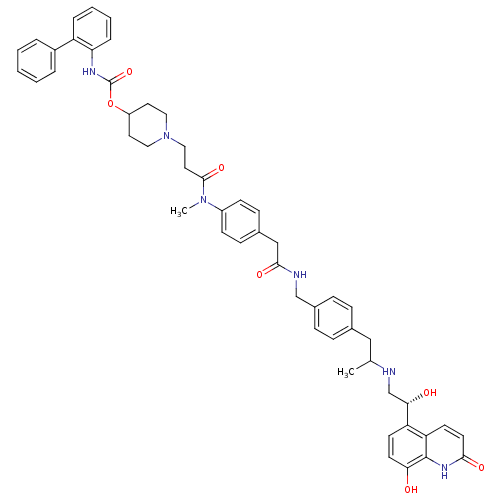
(US8551978, 322 | US8551978, 323 | US8816088, I-66 ...)Show SMILES CC(Cc1ccc(CNC(=O)Cc2ccc(cc2)N(C)C(=O)CCN2CCC(CC2)OC(=O)Nc2ccccc2-c2ccccc2)cc1)NC[C@H](O)c1ccc(O)c2[nH]c(=O)ccc12 |r| Show InChI InChI=1S/C51H56N6O7/c1-34(52-33-46(59)42-20-22-45(58)50-43(42)21-23-47(60)55-50)30-35-12-14-37(15-13-35)32-53-48(61)31-36-16-18-39(19-17-36)56(2)49(62)26-29-57-27-24-40(25-28-57)64-51(63)54-44-11-7-6-10-41(44)38-8-4-3-5-9-38/h3-23,34,40,46,52,58-59H,24-33H2,1-2H3,(H,53,61)(H,54,63)(H,55,60)/t34?,46-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| 0.100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Theravance, Inc.
US Patent
| Assay Description
Radioligand binding assay using muscarinic receptors. |
US Patent US8551978 (2013)
BindingDB Entry DOI: 10.7270/Q2Q81BPC |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M3
(Homo sapiens (Human)) | BDBM103798
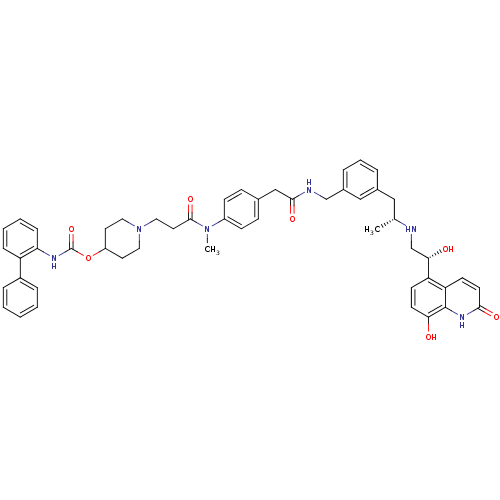
(US8551978, I-68 | US8816088, I-68 | US9394275, I-6...)Show SMILES C[C@H](Cc1cccc(CNC(=O)Cc2ccc(cc2)N(C)C(=O)CCN2CCC(CC2)OC(=O)Nc2ccccc2-c2ccccc2)c1)NC[C@H](O)c1ccc(O)c2[nH]c(=O)ccc12 |r| Show InChI InChI=1S/C51H56N6O7/c1-34(52-33-46(59)42-19-21-45(58)50-43(42)20-22-47(60)55-50)29-36-9-8-10-37(30-36)32-53-48(61)31-35-15-17-39(18-16-35)56(2)49(62)25-28-57-26-23-40(24-27-57)64-51(63)54-44-14-7-6-13-41(44)38-11-4-3-5-12-38/h3-22,30,34,40,46,52,58-59H,23-29,31-33H2,1-2H3,(H,53,61)(H,54,63)(H,55,60)/t34-,46+/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| 0.100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Theravance, Inc.
US Patent
| Assay Description
Radioligand binding assay using muscarinic receptors. |
US Patent US8551978 (2013)
BindingDB Entry DOI: 10.7270/Q2Q81BPC |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M3
(Homo sapiens (Human)) | BDBM103799
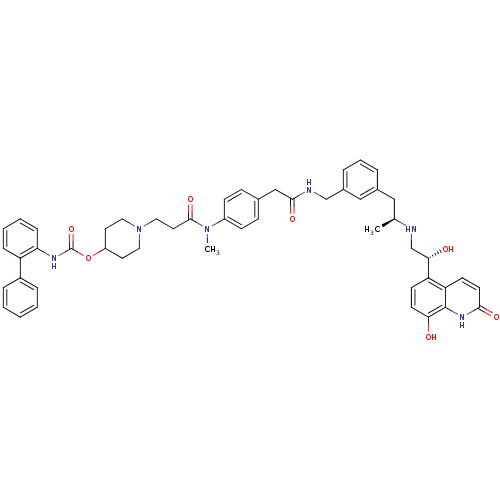
(US8551978, I-69 | US8816088, I-69 | US9394275, I-6...)Show SMILES C[C@@H](Cc1cccc(CNC(=O)Cc2ccc(cc2)N(C)C(=O)CCN2CCC(CC2)OC(=O)Nc2ccccc2-c2ccccc2)c1)NC[C@H](O)c1ccc(O)c2[nH]c(=O)ccc12 |r| Show InChI InChI=1S/C51H56N6O7/c1-34(52-33-46(59)42-19-21-45(58)50-43(42)20-22-47(60)55-50)29-36-9-8-10-37(30-36)32-53-48(61)31-35-15-17-39(18-16-35)56(2)49(62)25-28-57-26-23-40(24-27-57)64-51(63)54-44-14-7-6-13-41(44)38-11-4-3-5-12-38/h3-22,30,34,40,46,52,58-59H,23-29,31-33H2,1-2H3,(H,53,61)(H,54,63)(H,55,60)/t34-,46-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| 0.100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Theravance, Inc.
US Patent
| Assay Description
Radioligand binding assay using muscarinic receptors. |
US Patent US8551978 (2013)
BindingDB Entry DOI: 10.7270/Q2Q81BPC |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M3
(Homo sapiens (Human)) | BDBM103801
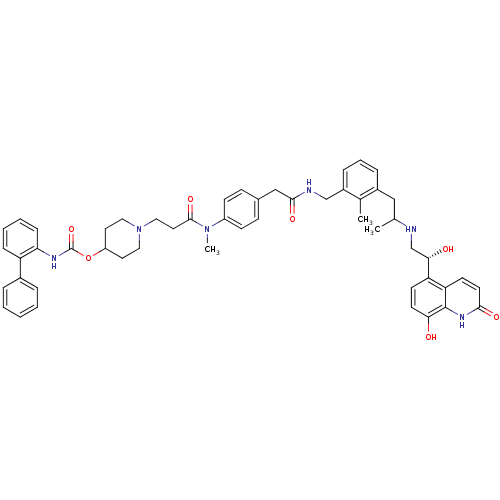
(US8551978, I-71 | US8816088, I-71 | US9394275, I-7...)Show SMILES CC(Cc1cccc(CNC(=O)Cc2ccc(cc2)N(C)C(=O)CCN2CCC(CC2)OC(=O)Nc2ccccc2-c2ccccc2)c1C)NC[C@H](O)c1ccc(O)c2[nH]c(=O)ccc12 |r| Show InChI InChI=1S/C52H58N6O7/c1-34(53-33-47(60)43-20-22-46(59)51-44(43)21-23-48(61)56-51)30-38-12-9-13-39(35(38)2)32-54-49(62)31-36-16-18-40(19-17-36)57(3)50(63)26-29-58-27-24-41(25-28-58)65-52(64)55-45-15-8-7-14-42(45)37-10-5-4-6-11-37/h4-23,34,41,47,53,59-60H,24-33H2,1-3H3,(H,54,62)(H,55,64)(H,56,61)/t34?,47-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| 0.100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Theravance, Inc.
US Patent
| Assay Description
Radioligand binding assay using muscarinic receptors. |
US Patent US8551978 (2013)
BindingDB Entry DOI: 10.7270/Q2Q81BPC |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M3
(Homo sapiens (Human)) | BDBM103807
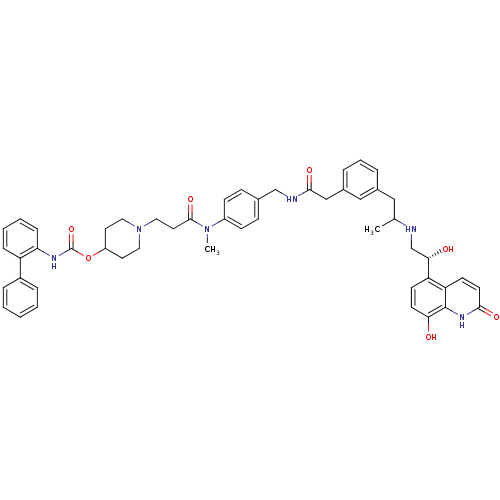
(US8551978, I-77 | US8816088, I-77 | US9394275, I-7...)Show SMILES CC(Cc1cccc(CC(=O)NCc2ccc(cc2)N(C)C(=O)CCN2CCC(CC2)OC(=O)Nc2ccccc2-c2ccccc2)c1)NC[C@H](O)c1ccc(O)c2[nH]c(=O)ccc12 |r| Show InChI InChI=1S/C51H56N6O7/c1-34(52-33-46(59)42-19-21-45(58)50-43(42)20-22-47(60)55-50)29-36-9-8-10-37(30-36)31-48(61)53-32-35-15-17-39(18-16-35)56(2)49(62)25-28-57-26-23-40(24-27-57)64-51(63)54-44-14-7-6-13-41(44)38-11-4-3-5-12-38/h3-22,30,34,40,46,52,58-59H,23-29,31-33H2,1-2H3,(H,53,61)(H,54,63)(H,55,60)/t34?,46-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| 0.100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Theravance, Inc.
US Patent
| Assay Description
Radioligand binding assay using muscarinic receptors. |
US Patent US8551978 (2013)
BindingDB Entry DOI: 10.7270/Q2Q81BPC |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M3
(Homo sapiens (Human)) | BDBM103809
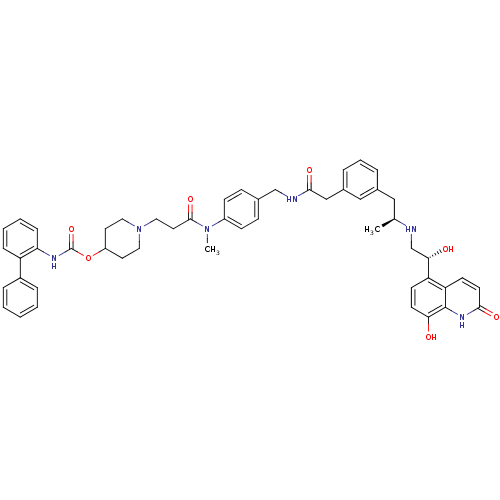
(US8551978, I-79 | US8816088, I-79 | US9394275, I-7...)Show SMILES C[C@@H](Cc1cccc(CC(=O)NCc2ccc(cc2)N(C)C(=O)CCN2CCC(CC2)OC(=O)Nc2ccccc2-c2ccccc2)c1)NC[C@H](O)c1ccc(O)c2[nH]c(=O)ccc12 |r| Show InChI InChI=1S/C51H56N6O7/c1-34(52-33-46(59)42-19-21-45(58)50-43(42)20-22-47(60)55-50)29-36-9-8-10-37(30-36)31-48(61)53-32-35-15-17-39(18-16-35)56(2)49(62)25-28-57-26-23-40(24-27-57)64-51(63)54-44-14-7-6-13-41(44)38-11-4-3-5-12-38/h3-22,30,34,40,46,52,58-59H,23-29,31-33H2,1-2H3,(H,53,61)(H,54,63)(H,55,60)/t34-,46-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| 0.100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Theravance, Inc.
US Patent
| Assay Description
Radioligand binding assay using muscarinic receptors. |
US Patent US8551978 (2013)
BindingDB Entry DOI: 10.7270/Q2Q81BPC |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M3
(Homo sapiens (Human)) | BDBM103811
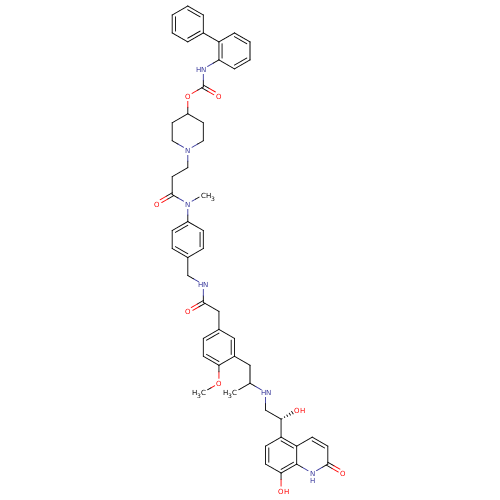
(US8551978, I-81 | US8816088, I-81 | US9394275, I-8...)Show SMILES COc1ccc(CC(=O)NCc2ccc(cc2)N(C)C(=O)CCN2CCC(CC2)OC(=O)Nc2ccccc2-c2ccccc2)cc1CC(C)NC[C@H](O)c1ccc(O)c2[nH]c(=O)ccc12 |r| Show InChI InChI=1S/C52H58N6O8/c1-34(53-33-46(60)42-18-20-45(59)51-43(42)19-22-48(61)56-51)29-38-30-36(15-21-47(38)65-3)31-49(62)54-32-35-13-16-39(17-14-35)57(2)50(63)25-28-58-26-23-40(24-27-58)66-52(64)55-44-12-8-7-11-41(44)37-9-5-4-6-10-37/h4-22,30,34,40,46,53,59-60H,23-29,31-33H2,1-3H3,(H,54,62)(H,55,64)(H,56,61)/t34?,46-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| 0.100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Theravance, Inc.
US Patent
| Assay Description
Radioligand binding assay using muscarinic receptors. |
US Patent US8551978 (2013)
BindingDB Entry DOI: 10.7270/Q2Q81BPC |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M3
(Homo sapiens (Human)) | BDBM103812
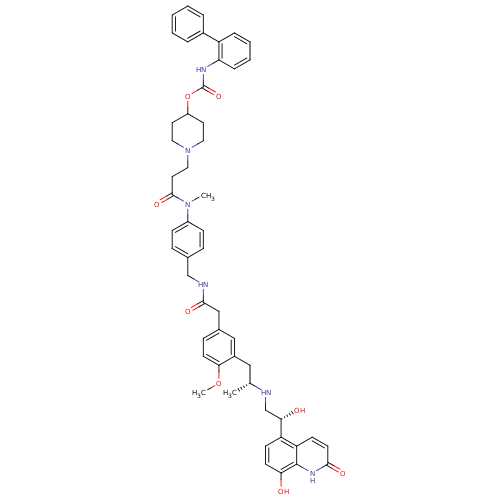
(US8551978, I-82 | US8816088, I-82 | US9394275, I-8...)Show SMILES COc1ccc(CC(=O)NCc2ccc(cc2)N(C)C(=O)CCN2CCC(CC2)OC(=O)Nc2ccccc2-c2ccccc2)cc1C[C@@H](C)NC[C@H](O)c1ccc(O)c2[nH]c(=O)ccc12 |r| Show InChI InChI=1S/C52H58N6O8/c1-34(53-33-46(60)42-18-20-45(59)51-43(42)19-22-48(61)56-51)29-38-30-36(15-21-47(38)65-3)31-49(62)54-32-35-13-16-39(17-14-35)57(2)50(63)25-28-58-26-23-40(24-27-58)66-52(64)55-44-12-8-7-11-41(44)37-9-5-4-6-10-37/h4-22,30,34,40,46,53,59-60H,23-29,31-33H2,1-3H3,(H,54,62)(H,55,64)(H,56,61)/t34-,46+/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| 0.100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Theravance, Inc.
US Patent
| Assay Description
Radioligand binding assay using muscarinic receptors. |
US Patent US8551978 (2013)
BindingDB Entry DOI: 10.7270/Q2Q81BPC |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M3
(Homo sapiens (Human)) | BDBM103814
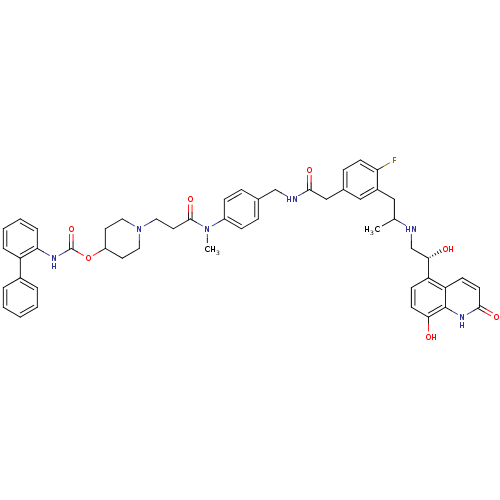
(US8551978, I-84 | US8816088, I-84 | US9394275, I-8...)Show SMILES CC(Cc1cc(CC(=O)NCc2ccc(cc2)N(C)C(=O)CCN2CCC(CC2)OC(=O)Nc2ccccc2-c2ccccc2)ccc1F)NC[C@H](O)c1ccc(O)c2[nH]c(=O)ccc12 |r| Show InChI InChI=1S/C51H55FN6O7/c1-33(53-32-46(60)41-17-20-45(59)50-42(41)18-21-47(61)56-50)28-37-29-35(14-19-43(37)52)30-48(62)54-31-34-12-15-38(16-13-34)57(2)49(63)24-27-58-25-22-39(23-26-58)65-51(64)55-44-11-7-6-10-40(44)36-8-4-3-5-9-36/h3-21,29,33,39,46,53,59-60H,22-28,30-32H2,1-2H3,(H,54,62)(H,55,64)(H,56,61)/t33?,46-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| 0.100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Theravance, Inc.
US Patent
| Assay Description
Radioligand binding assay using muscarinic receptors. |
US Patent US8551978 (2013)
BindingDB Entry DOI: 10.7270/Q2Q81BPC |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M3
(Homo sapiens (Human)) | BDBM103817
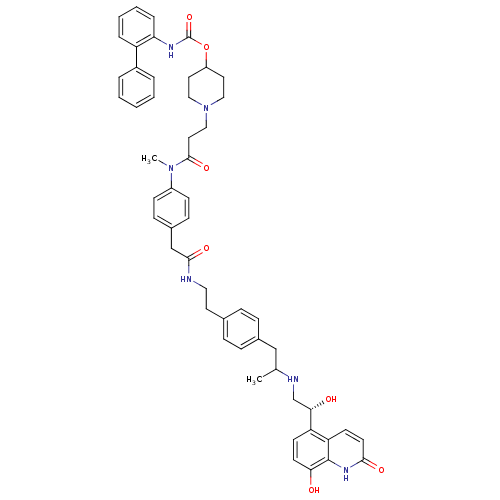
(US8551978, I-88 | US8816088, I-88 | US9394275, I-8...)Show SMILES CC(Cc1ccc(CCNC(=O)Cc2ccc(cc2)N(C)C(=O)CCN2CCC(CC2)OC(=O)Nc2ccccc2-c2ccccc2)cc1)NC[C@H](O)c1ccc(O)c2[nH]c(=O)ccc12 |r| Show InChI InChI=1S/C52H58N6O7/c1-35(54-34-47(60)43-20-22-46(59)51-44(43)21-23-48(61)56-51)32-37-14-12-36(13-15-37)24-28-53-49(62)33-38-16-18-40(19-17-38)57(2)50(63)27-31-58-29-25-41(26-30-58)65-52(64)55-45-11-7-6-10-42(45)39-8-4-3-5-9-39/h3-23,35,41,47,54,59-60H,24-34H2,1-2H3,(H,53,62)(H,55,64)(H,56,61)/t35?,47-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| 0.100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Theravance, Inc.
US Patent
| Assay Description
Radioligand binding assay using muscarinic receptors. |
US Patent US8551978 (2013)
BindingDB Entry DOI: 10.7270/Q2Q81BPC |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M3
(Homo sapiens (Human)) | BDBM103818
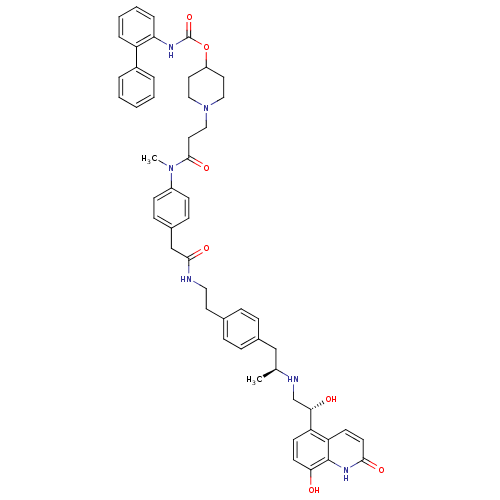
(US8551978, I-89 | US8816088, I-89 | US9394275, I-8...)Show SMILES C[C@@H](Cc1ccc(CCNC(=O)Cc2ccc(cc2)N(C)C(=O)CCN2CCC(CC2)OC(=O)Nc2ccccc2-c2ccccc2)cc1)NC[C@H](O)c1ccc(O)c2[nH]c(=O)ccc12 |r| Show InChI InChI=1S/C52H58N6O7/c1-35(54-34-47(60)43-20-22-46(59)51-44(43)21-23-48(61)56-51)32-37-14-12-36(13-15-37)24-28-53-49(62)33-38-16-18-40(19-17-38)57(2)50(63)27-31-58-29-25-41(26-30-58)65-52(64)55-45-11-7-6-10-42(45)39-8-4-3-5-9-39/h3-23,35,41,47,54,59-60H,24-34H2,1-2H3,(H,53,62)(H,55,64)(H,56,61)/t35-,47-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| 0.100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Theravance, Inc.
US Patent
| Assay Description
Radioligand binding assay using muscarinic receptors. |
US Patent US8551978 (2013)
BindingDB Entry DOI: 10.7270/Q2Q81BPC |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M3
(Homo sapiens (Human)) | BDBM103819
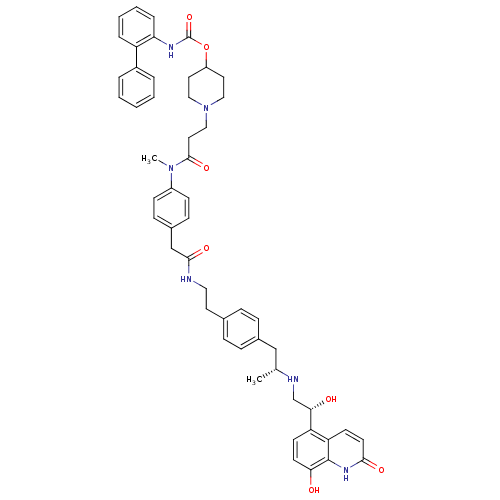
(US8551978, I-90 | US8816088, I-90 | US9394275, I-9...)Show SMILES C[C@H](Cc1ccc(CCNC(=O)Cc2ccc(cc2)N(C)C(=O)CCN2CCC(CC2)OC(=O)Nc2ccccc2-c2ccccc2)cc1)NC[C@H](O)c1ccc(O)c2[nH]c(=O)ccc12 |r| Show InChI InChI=1S/C52H58N6O7/c1-35(54-34-47(60)43-20-22-46(59)51-44(43)21-23-48(61)56-51)32-37-14-12-36(13-15-37)24-28-53-49(62)33-38-16-18-40(19-17-38)57(2)50(63)27-31-58-29-25-41(26-30-58)65-52(64)55-45-11-7-6-10-42(45)39-8-4-3-5-9-39/h3-23,35,41,47,54,59-60H,24-34H2,1-2H3,(H,53,62)(H,55,64)(H,56,61)/t35-,47+/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| 0.100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Theravance, Inc.
US Patent
| Assay Description
Radioligand binding assay using muscarinic receptors. |
US Patent US8551978 (2013)
BindingDB Entry DOI: 10.7270/Q2Q81BPC |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M3
(Homo sapiens (Human)) | BDBM103823

(US8551978, I-94 | US8816088, I-94 | US9394275, I-9...)Show SMILES COc1ccc(CC(C)NC[C@H](O)c2ccc(O)c3[nH]c(=O)ccc23)cc1CC(=O)NCc1ccc(cc1)N(C)C(=O)CCN1CCC(CC1)OC(=O)Nc1ccccc1-c1ccccc1 |r| Show InChI InChI=1S/C52H58N6O8/c1-34(53-33-46(60)42-18-20-45(59)51-43(42)19-22-48(61)56-51)29-36-15-21-47(65-3)38(30-36)31-49(62)54-32-35-13-16-39(17-14-35)57(2)50(63)25-28-58-26-23-40(24-27-58)66-52(64)55-44-12-8-7-11-41(44)37-9-5-4-6-10-37/h4-22,30,34,40,46,53,59-60H,23-29,31-33H2,1-3H3,(H,54,62)(H,55,64)(H,56,61)/t34?,46-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| 0.100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Theravance, Inc.
US Patent
| Assay Description
Radioligand binding assay using muscarinic receptors. |
US Patent US8551978 (2013)
BindingDB Entry DOI: 10.7270/Q2Q81BPC |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M3
(Homo sapiens (Human)) | BDBM6427

(US8551978, I-25 | US8816088, 25 | US9572802, Compo...)Show SMILES CN(C(=O)CCN1CCC(CC1)OC(=O)Nc1ccccc1-c1ccccc1)c1cccc(c1)C(=O)Nc1ccc(CNC[C@H](O)c2ccc(O)c3[nH]c(=O)ccc23)cc1 |r| Show InChI InChI=1S/C47H48N6O7/c1-52(44(57)24-27-53-25-22-36(23-26-53)60-47(59)50-40-13-6-5-12-37(40)32-8-3-2-4-9-32)35-11-7-10-33(28-35)46(58)49-34-16-14-31(15-17-34)29-48-30-42(55)38-18-20-41(54)45-39(38)19-21-43(56)51-45/h2-21,28,36,42,48,54-55H,22-27,29-30H2,1H3,(H,49,58)(H,50,59)(H,51,56)/t42-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| 0.100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Theravance, Inc.
US Patent
| Assay Description
Radioligand binding assay using muscarinic receptors. |
US Patent US8551978 (2013)
BindingDB Entry DOI: 10.7270/Q2Q81BPC |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M3
(Homo sapiens (Human)) | BDBM6449

(US8551978, I-87 | US8816088, 87 | US9394275, I-7 |...)Show SMILES C[C@H](Cc1cccc(CCNC(=O)Cc2ccc(cc2)N(C)C(=O)CCN2CCC(CC2)OC(=O)Nc2ccccc2-c2ccccc2)c1)NC[C@H](O)c1ccc(O)c2[nH]c(=O)ccc12 |r| Show InChI InChI=1S/C52H58N6O7/c1-35(54-34-47(60)43-19-21-46(59)51-44(43)20-22-48(61)56-51)31-38-10-8-9-36(32-38)23-27-53-49(62)33-37-15-17-40(18-16-37)57(2)50(63)26-30-58-28-24-41(25-29-58)65-52(64)55-45-14-7-6-13-42(45)39-11-4-3-5-12-39/h3-22,32,35,41,47,54,59-60H,23-31,33-34H2,1-2H3,(H,53,62)(H,55,64)(H,56,61)/t35-,47+/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| 0.100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Theravance, Inc.
US Patent
| Assay Description
Radioligand binding assay using muscarinic receptors. |
US Patent US8551978 (2013)
BindingDB Entry DOI: 10.7270/Q2Q81BPC |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M3
(Homo sapiens (Human)) | BDBM6354

(US8551978, I-1 | US8816088, 1 | US9394275, I-1 | U...)Show SMILES CN(CCCC(=O)Nc1ccc(CNC[C@H](O)c2ccc(O)c3[nH]c(=O)ccc23)cc1)C(=O)CCN1CCC(CC1)OC(=O)Nc1ccccc1-c1ccccc1 |r| Show InChI InChI=1S/C44H50N6O7/c1-49(42(55)23-27-50-25-21-33(22-26-50)57-44(56)47-37-11-6-5-10-34(37)31-8-3-2-4-9-31)24-7-12-40(53)46-32-15-13-30(14-16-32)28-45-29-39(52)35-17-19-38(51)43-36(35)18-20-41(54)48-43/h2-6,8-11,13-20,33,39,45,51-52H,7,12,21-29H2,1H3,(H,46,53)(H,47,56)(H,48,54)/t39-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| 0.100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Theravance, Inc.
US Patent
| Assay Description
Radioligand binding assay using muscarinic receptors. |
US Patent US8551978 (2013)
BindingDB Entry DOI: 10.7270/Q2Q81BPC |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M3
(Homo sapiens (Human)) | BDBM38242
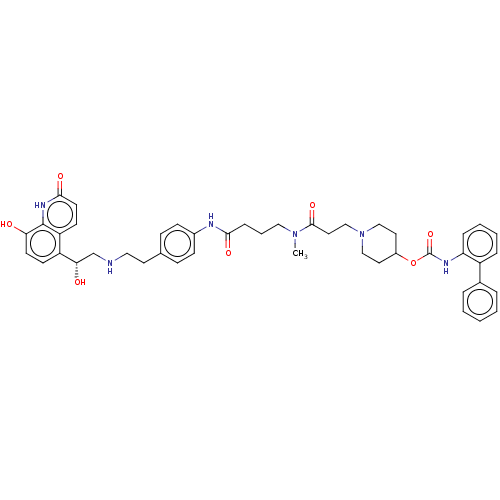
(US8551978, I-7 | US8551978, I-8 | US9394275, I-8 |...)Show SMILES CN(CCCC(=O)Nc1ccc(CCNC[C@H](O)c2ccc(O)c3[nH]c(=O)ccc23)cc1)C(=O)CCN1CCC(CC1)OC(=O)Nc1ccccc1-c1ccccc1 |r| Show InChI InChI=1S/C45H52N6O7/c1-50(43(56)24-29-51-27-22-34(23-28-51)58-45(57)48-38-11-6-5-10-35(38)32-8-3-2-4-9-32)26-7-12-41(54)47-33-15-13-31(14-16-33)21-25-46-30-40(53)36-17-19-39(52)44-37(36)18-20-42(55)49-44/h2-6,8-11,13-20,34,40,46,52-53H,7,12,21-30H2,1H3,(H,47,54)(H,48,57)(H,49,55)/t40-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| 0.100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Theravance, Inc.
US Patent
| Assay Description
Radioligand binding assay using muscarinic receptors. |
US Patent US8551978 (2013)
BindingDB Entry DOI: 10.7270/Q2Q81BPC |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M3
(Homo sapiens (Human)) | BDBM38243
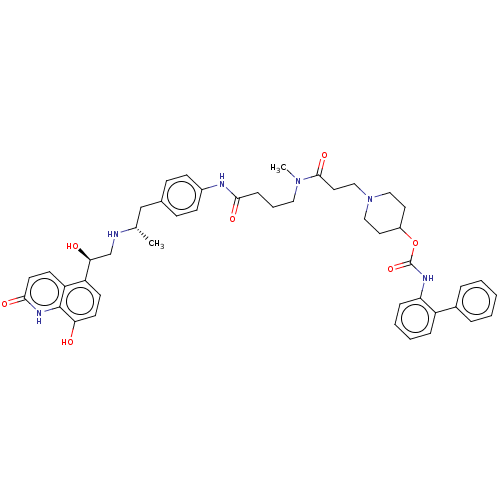
(US8551978, I-13)Show SMILES C[C@@H](Cc1ccc(NC(=O)CCCN(C)C(=O)CCN2CCC(CC2)OC(=O)Nc2ccccc2-c2ccccc2)cc1)NC[C@H](O)c1ccc(O)c2[nH]c(=O)ccc12 |r| Show InChI InChI=1S/C46H54N6O7/c1-31(47-30-41(54)37-18-20-40(53)45-38(37)19-21-43(56)50-45)29-32-14-16-34(17-15-32)48-42(55)13-8-25-51(2)44(57)24-28-52-26-22-35(23-27-52)59-46(58)49-39-12-7-6-11-36(39)33-9-4-3-5-10-33/h3-7,9-12,14-21,31,35,41,47,53-54H,8,13,22-30H2,1-2H3,(H,48,55)(H,49,58)(H,50,56)/t31-,41-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| 0.100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Theravance, Inc.
US Patent
| Assay Description
Radioligand binding assay using muscarinic receptors. |
US Patent US8551978 (2013)
BindingDB Entry DOI: 10.7270/Q2Q81BPC |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M3
(Homo sapiens (Human)) | BDBM6384

(US8551978, I-14 | US8816088, 14 | US8816088, I-15 ...)Show SMILES CN(CCCCC(=O)Nc1ccc(CNC[C@H](O)c2ccc(O)c3[nH]c(=O)ccc23)cc1)C(=O)CCN1CCC(CC1)OC(=O)Nc1ccccc1-c1ccccc1 |r| Show InChI InChI=1S/C45H52N6O7/c1-50(43(56)24-28-51-26-22-34(23-27-51)58-45(57)48-38-12-6-5-11-35(38)32-9-3-2-4-10-32)25-8-7-13-41(54)47-33-16-14-31(15-17-33)29-46-30-40(53)36-18-20-39(52)44-37(36)19-21-42(55)49-44/h2-6,9-12,14-21,34,40,46,52-53H,7-8,13,22-30H2,1H3,(H,47,54)(H,48,57)(H,49,55)/t40-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| 0.100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Theravance, Inc.
US Patent
| Assay Description
Radioligand binding assay using muscarinic receptors. |
US Patent US8551978 (2013)
BindingDB Entry DOI: 10.7270/Q2Q81BPC |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M3
(Homo sapiens (Human)) | BDBM103738
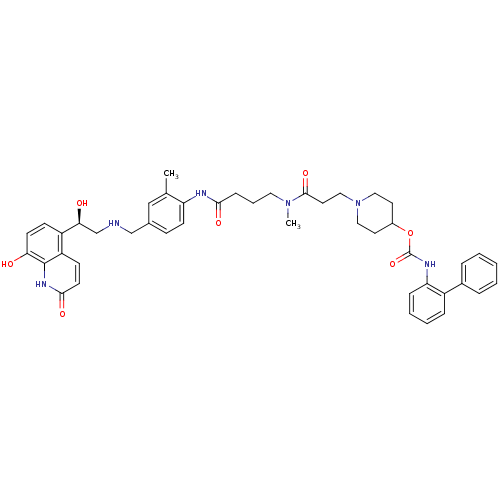
(US8551978, I-2 | US8816088, I-2 | US9394275, I-2 |...)Show SMILES CN(CCCC(=O)Nc1ccc(CNC[C@H](O)c2ccc(O)c3[nH]c(=O)ccc23)cc1C)C(=O)CCN1CCC(CC1)OC(=O)Nc1ccccc1-c1ccccc1 |r| Show InChI InChI=1S/C45H52N6O7/c1-30-27-31(28-46-29-40(53)35-15-18-39(52)44-36(35)16-19-42(55)49-44)14-17-37(30)47-41(54)13-8-23-50(2)43(56)22-26-51-24-20-33(21-25-51)58-45(57)48-38-12-7-6-11-34(38)32-9-4-3-5-10-32/h3-7,9-12,14-19,27,33,40,46,52-53H,8,13,20-26,28-29H2,1-2H3,(H,47,54)(H,48,57)(H,49,55)/t40-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| 0.100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Theravance, Inc.
US Patent
| Assay Description
Radioligand binding assay using muscarinic receptors. |
US Patent US8551978 (2013)
BindingDB Entry DOI: 10.7270/Q2Q81BPC |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M3
(Homo sapiens (Human)) | BDBM103739
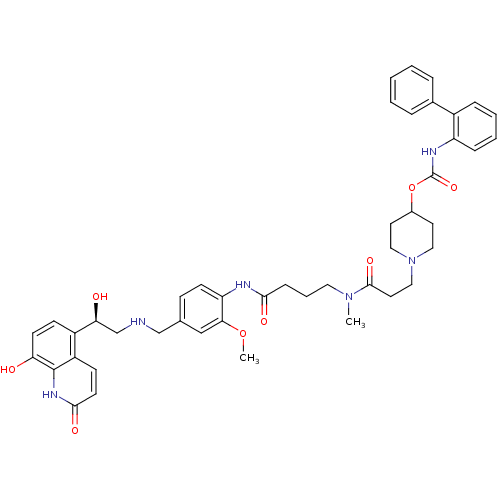
(US8551978, I-3 | US8816088, I-3 | US9394275, I-3 |...)Show SMILES COc1cc(CNC[C@H](O)c2ccc(O)c3[nH]c(=O)ccc23)ccc1NC(=O)CCCN(C)C(=O)CCN1CCC(CC1)OC(=O)Nc1ccccc1-c1ccccc1 |r| Show InChI InChI=1S/C45H52N6O8/c1-50(43(56)22-26-51-24-20-32(21-25-51)59-45(57)48-36-12-7-6-11-33(36)31-9-4-3-5-10-31)23-8-13-41(54)47-37-17-14-30(27-40(37)58-2)28-46-29-39(53)34-15-18-38(52)44-35(34)16-19-42(55)49-44/h3-7,9-12,14-19,27,32,39,46,52-53H,8,13,20-26,28-29H2,1-2H3,(H,47,54)(H,48,57)(H,49,55)/t39-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| 0.100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Theravance, Inc.
US Patent
| Assay Description
Radioligand binding assay using muscarinic receptors. |
US Patent US8551978 (2013)
BindingDB Entry DOI: 10.7270/Q2Q81BPC |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M3
(Homo sapiens (Human)) | BDBM103740
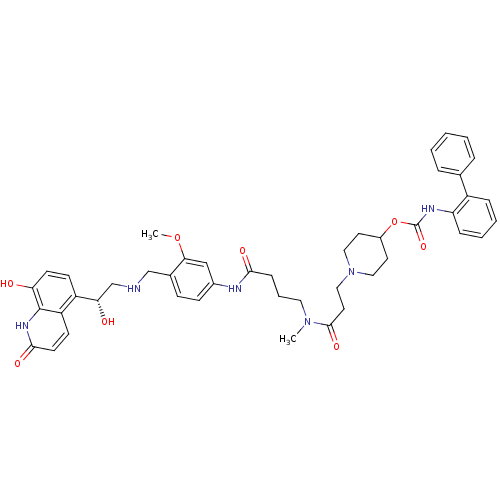
(US8551978, 266 | US8816088, I-4 | US9394275, I-4 |...)Show SMILES COc1cc(NC(=O)CCCN(C)C(=O)CCN2CCC(CC2)OC(=O)Nc2ccccc2-c2ccccc2)ccc1CNC[C@H](O)c1ccc(O)c2[nH]c(=O)ccc12 |r| Show InChI InChI=1S/C45H52N6O8/c1-50(43(56)22-26-51-24-20-33(21-25-51)59-45(57)48-37-12-7-6-11-34(37)30-9-4-3-5-10-30)23-8-13-41(54)47-32-15-14-31(40(27-32)58-2)28-46-29-39(53)35-16-18-38(52)44-36(35)17-19-42(55)49-44/h3-7,9-12,14-19,27,33,39,46,52-53H,8,13,20-26,28-29H2,1-2H3,(H,47,54)(H,48,57)(H,49,55)/t39-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| 0.100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Theravance, Inc.
US Patent
| Assay Description
Radioligand binding assay using muscarinic receptors. |
US Patent US8551978 (2013)
BindingDB Entry DOI: 10.7270/Q2Q81BPC |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M3
(Homo sapiens (Human)) | BDBM103741
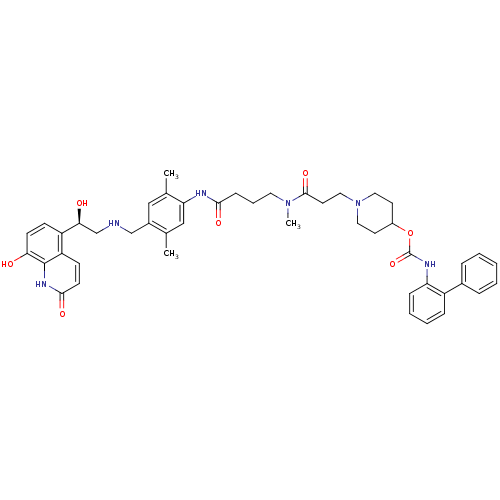
(US8551978, I-5 | US8816088, I-5 | US9394275, I-5 |...)Show SMILES CN(CCCC(=O)Nc1cc(C)c(CNC[C@H](O)c2ccc(O)c3[nH]c(=O)ccc23)cc1C)C(=O)CCN1CCC(CC1)OC(=O)Nc1ccccc1-c1ccccc1 |r| Show InChI InChI=1S/C46H54N6O7/c1-30-27-39(31(2)26-33(30)28-47-29-41(54)36-15-17-40(53)45-37(36)16-18-43(56)50-45)48-42(55)14-9-22-51(3)44(57)21-25-52-23-19-34(20-24-52)59-46(58)49-38-13-8-7-12-35(38)32-10-5-4-6-11-32/h4-8,10-13,15-18,26-27,34,41,47,53-54H,9,14,19-25,28-29H2,1-3H3,(H,48,55)(H,49,58)(H,50,56)/t41-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| 0.100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Theravance, Inc.
US Patent
| Assay Description
Radioligand binding assay using muscarinic receptors. |
US Patent US8551978 (2013)
BindingDB Entry DOI: 10.7270/Q2Q81BPC |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M3
(Homo sapiens (Human)) | BDBM103742

(US8551978, I-6 | US8816088, I-6 | US9394275, I-6 |...)Show SMILES COc1cc(NC(=O)CCCN(C)C(=O)CCN2CCC(CC2)OC(=O)Nc2ccccc2-c2ccccc2)c(Cl)cc1CNC[C@H](O)c1ccc(O)c2[nH]c(=O)ccc12 |r| Show InChI InChI=1S/C45H51ClN6O8/c1-51(43(57)20-24-52-22-18-31(19-23-52)60-45(58)49-36-12-7-6-11-32(36)29-9-4-3-5-10-29)21-8-13-41(55)48-37-26-40(59-2)30(25-35(37)46)27-47-28-39(54)33-14-16-38(53)44-34(33)15-17-42(56)50-44/h3-7,9-12,14-17,25-26,31,39,47,53-54H,8,13,18-24,27-28H2,1-2H3,(H,48,55)(H,49,58)(H,50,56)/t39-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| 0.100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Theravance, Inc.
US Patent
| Assay Description
Radioligand binding assay using muscarinic receptors. |
US Patent US8551978 (2013)
BindingDB Entry DOI: 10.7270/Q2Q81BPC |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data