

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Mitogen-activated protein kinase 1 (Homo sapiens (Human)) | BDBM35653 (pyrimidylpyrrole, 11a) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | <2 | <-50.5 | n/a | n/a | n/a | n/a | n/a | 7.5 | 30 |
Vertex | Assay Description A coupled spectrophotometric assay was used in which ADP generated by kinase was converted to ATP with the concomitant production of pyruvate from PE... | J Med Chem 52: 6362-8 (2009) Article DOI: 10.1021/jm900630q BindingDB Entry DOI: 10.7270/Q2D798SS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mitogen-activated protein kinase 1 (Homo sapiens (Human)) | BDBM15645 (N-((S)-1-(3-Chloro-4-fluorophenyl)-2-hydroxyethyl)...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase DrugBank MMDB PC cid PC sid PDB UniChem Similars | MMDB PDB Article PubMed | 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vertex | Assay Description A coupled spectrophotometric assay was used in which ADP generated by ERK2 was converted to ATP by pyruvate kinase with the production of pyruvate fr... | J Med Chem 50: 1280-7 (2007) Article DOI: 10.1021/jm061381f BindingDB Entry DOI: 10.7270/Q2BR8QFV | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Mitogen-activated protein kinase 1 (Homo sapiens (Human)) | BDBM35650 (pyrimidylpyrrole, 10c) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | <2 | <-50.5 | n/a | n/a | n/a | n/a | n/a | 7.5 | 30 |
Vertex | Assay Description A coupled spectrophotometric assay was used in which ADP generated by kinase was converted to ATP with the concomitant production of pyruvate from PE... | J Med Chem 52: 6362-8 (2009) Article DOI: 10.1021/jm900630q BindingDB Entry DOI: 10.7270/Q2D798SS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mitogen-activated protein kinase 1 (Homo sapiens (Human)) | BDBM35663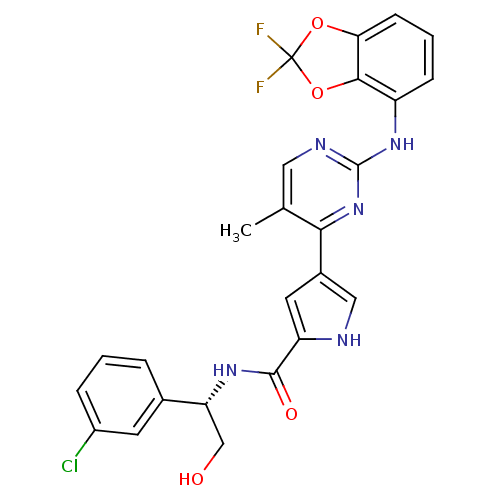 (pyrimidylpyrrole, 11k) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | <2 | <-50.5 | n/a | n/a | n/a | n/a | n/a | 7.5 | 30 |
Vertex | Assay Description A coupled spectrophotometric assay was used in which ADP generated by kinase was converted to ATP with the concomitant production of pyruvate from PE... | J Med Chem 52: 6362-8 (2009) Article DOI: 10.1021/jm900630q BindingDB Entry DOI: 10.7270/Q2D798SS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mitogen-activated protein kinase 1 (Homo sapiens (Human)) | BDBM35641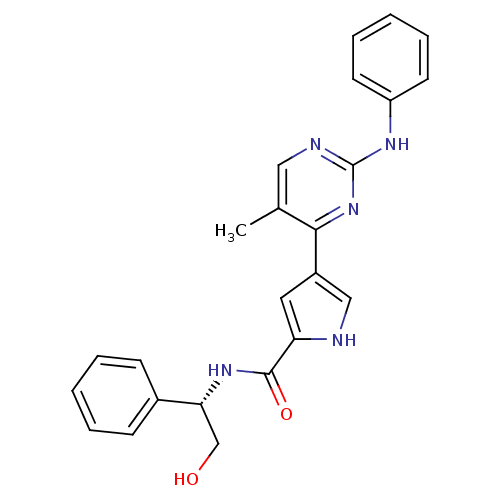 (erk000040 | pyrimidylpyrrole, 2) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | MMDB PC cid PC sid PDB UniChem Similars | MMDB PDB Article PubMed | <2 | <-50.5 | n/a | n/a | n/a | n/a | n/a | 7.5 | 30 |
Vertex | Assay Description A coupled spectrophotometric assay was used in which ADP generated by kinase was converted to ATP with the concomitant production of pyruvate from PE... | J Med Chem 52: 6362-8 (2009) Article DOI: 10.1021/jm900630q BindingDB Entry DOI: 10.7270/Q2D798SS | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Mitogen-activated protein kinase 1 (Homo sapiens (Human)) | BDBM35642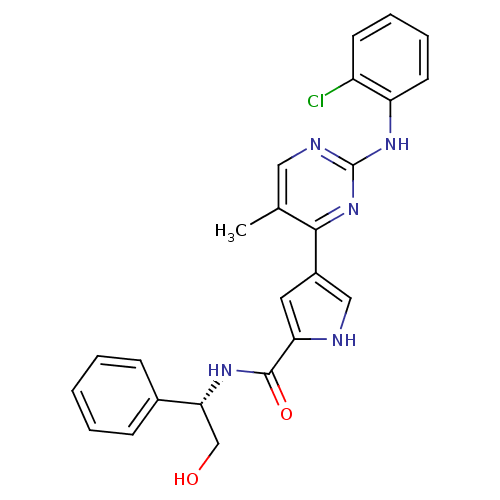 (pyrimidylpyrrole, 9a) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | MMDB PC cid PC sid PDB UniChem Similars | MMDB PDB Article PubMed | <2 | <-50.5 | n/a | n/a | n/a | n/a | n/a | 7.5 | 30 |
Vertex | Assay Description A coupled spectrophotometric assay was used in which ADP generated by kinase was converted to ATP with the concomitant production of pyruvate from PE... | J Med Chem 52: 6362-8 (2009) Article DOI: 10.1021/jm900630q BindingDB Entry DOI: 10.7270/Q2D798SS | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Mitogen-activated protein kinase 1 (Homo sapiens (Human)) | BDBM35643 (pyrimidylpyrrole, 9b) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | <2 | <-50.5 | n/a | n/a | n/a | n/a | n/a | 7.5 | 30 |
Vertex | Assay Description A coupled spectrophotometric assay was used in which ADP generated by kinase was converted to ATP with the concomitant production of pyruvate from PE... | J Med Chem 52: 6362-8 (2009) Article DOI: 10.1021/jm900630q BindingDB Entry DOI: 10.7270/Q2D798SS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mitogen-activated protein kinase 1 (Homo sapiens (Human)) | BDBM35649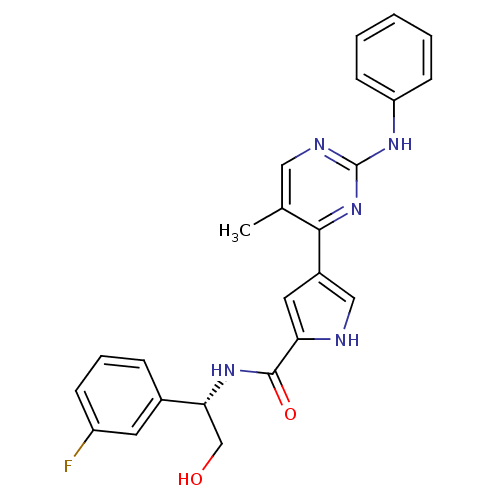 (erk000537 | pyrimidylpyrrole, 10b) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | <2 | <-50.5 | n/a | n/a | n/a | n/a | n/a | 7.5 | 30 |
Vertex | Assay Description A coupled spectrophotometric assay was used in which ADP generated by kinase was converted to ATP with the concomitant production of pyruvate from PE... | J Med Chem 52: 6362-8 (2009) Article DOI: 10.1021/jm900630q BindingDB Entry DOI: 10.7270/Q2D798SS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mitogen-activated protein kinase 1 (Homo sapiens (Human)) | BDBM35651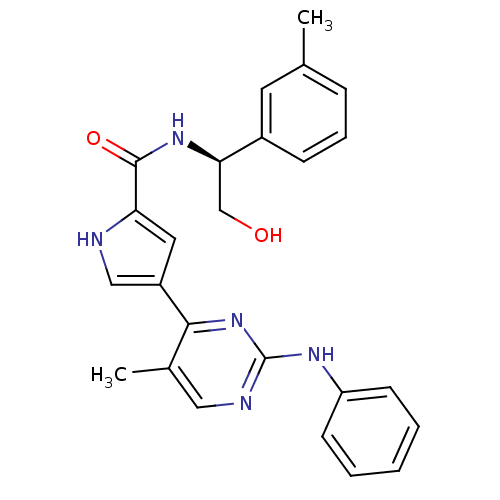 (pyrimidylpyrrole, 10d) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | <2 | <-50.5 | n/a | n/a | n/a | n/a | n/a | 7.5 | 30 |
Vertex | Assay Description A coupled spectrophotometric assay was used in which ADP generated by kinase was converted to ATP with the concomitant production of pyruvate from PE... | J Med Chem 52: 6362-8 (2009) Article DOI: 10.1021/jm900630q BindingDB Entry DOI: 10.7270/Q2D798SS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mitogen-activated protein kinase 1 (Homo sapiens (Human)) | BDBM35645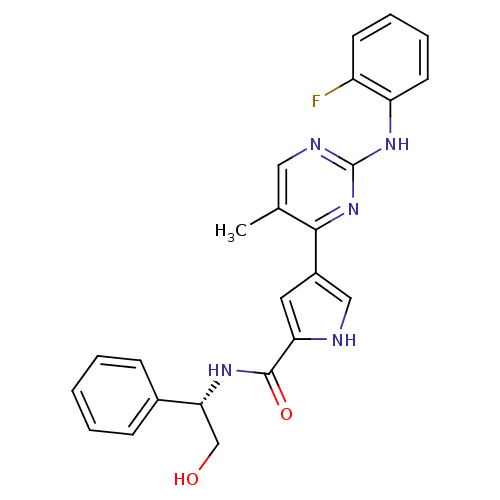 (pyrimidylpyrrole, 9d) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | <2 | <-50.5 | n/a | n/a | n/a | n/a | n/a | 7.5 | 30 |
Vertex | Assay Description A coupled spectrophotometric assay was used in which ADP generated by kinase was converted to ATP with the concomitant production of pyruvate from PE... | J Med Chem 52: 6362-8 (2009) Article DOI: 10.1021/jm900630q BindingDB Entry DOI: 10.7270/Q2D798SS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mitogen-activated protein kinase 1 (Homo sapiens (Human)) | BDBM35662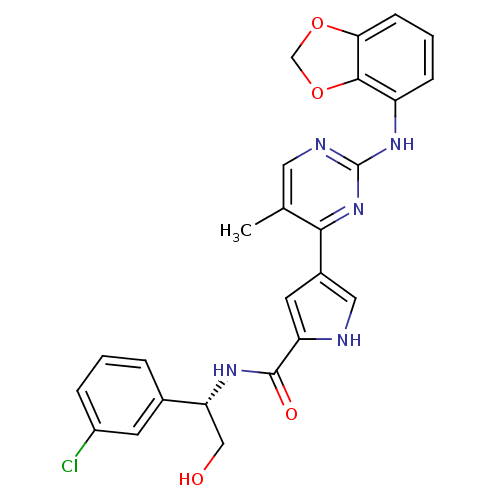 (pyrimidylpyrrole, 11j) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | <2 | <-50.5 | n/a | n/a | n/a | n/a | n/a | 7.5 | 30 |
Vertex | Assay Description A coupled spectrophotometric assay was used in which ADP generated by kinase was converted to ATP with the concomitant production of pyruvate from PE... | J Med Chem 52: 6362-8 (2009) Article DOI: 10.1021/jm900630q BindingDB Entry DOI: 10.7270/Q2D798SS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mitogen-activated protein kinase 1 (Homo sapiens (Human)) | BDBM35654 (erk000526 | pyrimidylpyrrole, 11b) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | <2 | <-50.5 | n/a | n/a | n/a | n/a | n/a | 7.5 | 30 |
Vertex | Assay Description A coupled spectrophotometric assay was used in which ADP generated by kinase was converted to ATP with the concomitant production of pyruvate from PE... | J Med Chem 52: 6362-8 (2009) Article DOI: 10.1021/jm900630q BindingDB Entry DOI: 10.7270/Q2D798SS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glycogen synthase kinase-3 beta (Homo sapiens (Human)) | BDBM35653 (pyrimidylpyrrole, 11a) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | <2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vertex | Assay Description A coupled spectrophotometric assay was used in which ADP generated by kinase was converted to ATP with the concomitant production of pyruvate from PE... | J Med Chem 52: 6362-8 (2009) Article DOI: 10.1021/jm900630q BindingDB Entry DOI: 10.7270/Q2D798SS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mitogen-activated protein kinase 1 (Homo sapiens (Human)) | BDBM35657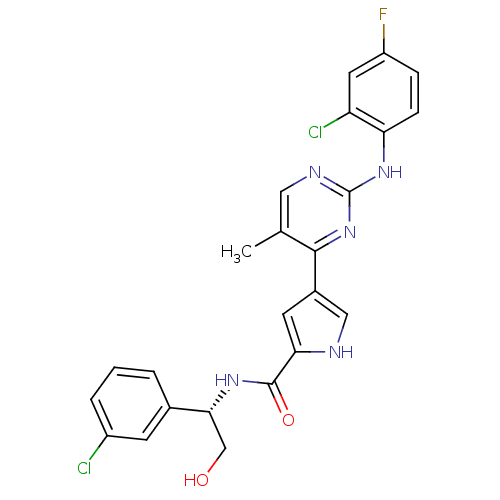 (pyrimidylpyrrole, 11e) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid PDB UniChem Similars | PDB Article PubMed | <2 | <-50.5 | n/a | n/a | n/a | n/a | n/a | 7.5 | 30 |
Vertex | Assay Description A coupled spectrophotometric assay was used in which ADP generated by kinase was converted to ATP with the concomitant production of pyruvate from PE... | J Med Chem 52: 6362-8 (2009) Article DOI: 10.1021/jm900630q BindingDB Entry DOI: 10.7270/Q2D798SS | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Glycogen synthase kinase-3 beta (Homo sapiens (Human)) | BDBM35647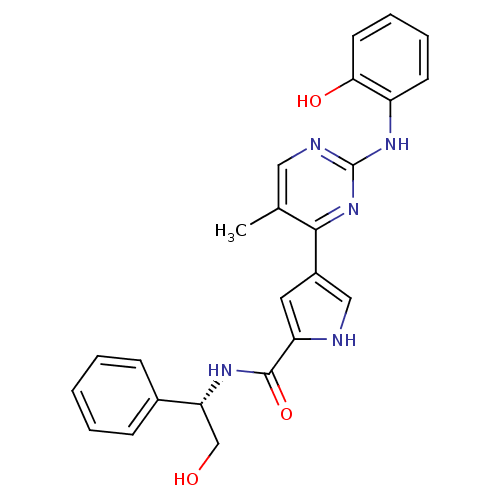 (erk000636 | pyrimidylpyrrole, 9f) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | <2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vertex | Assay Description A coupled spectrophotometric assay was used in which ADP generated by kinase was converted to ATP with the concomitant production of pyruvate from PE... | J Med Chem 52: 6362-8 (2009) Article DOI: 10.1021/jm900630q BindingDB Entry DOI: 10.7270/Q2D798SS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mitogen-activated protein kinase 1 (Homo sapiens (Human)) | BDBM35659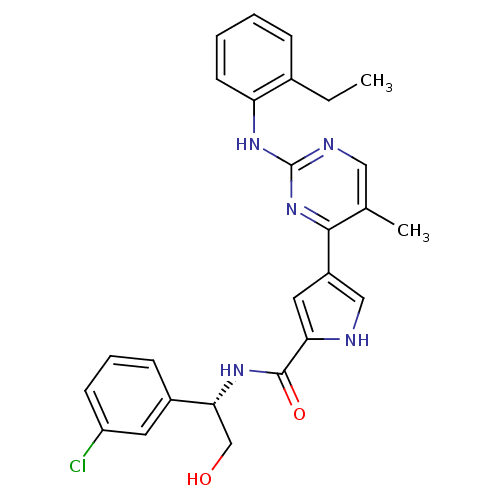 (pyrimidylpyrrole, 11g) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | <2 | <-50.5 | n/a | n/a | n/a | n/a | n/a | 7.5 | 30 |
Vertex | Assay Description A coupled spectrophotometric assay was used in which ADP generated by kinase was converted to ATP with the concomitant production of pyruvate from PE... | J Med Chem 52: 6362-8 (2009) Article DOI: 10.1021/jm900630q BindingDB Entry DOI: 10.7270/Q2D798SS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mitogen-activated protein kinase 1 (Homo sapiens (Human)) | BDBM35660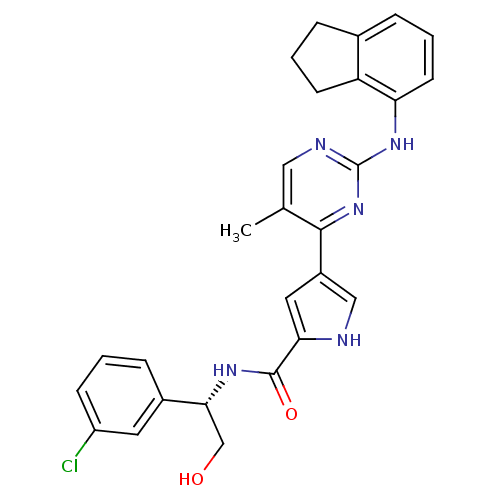 (pyrimidylpyrrole, 11h) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | <2 | <-50.5 | n/a | n/a | n/a | n/a | n/a | 7.5 | 30 |
Vertex | Assay Description A coupled spectrophotometric assay was used in which ADP generated by kinase was converted to ATP with the concomitant production of pyruvate from PE... | J Med Chem 52: 6362-8 (2009) Article DOI: 10.1021/jm900630q BindingDB Entry DOI: 10.7270/Q2D798SS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mitogen-activated protein kinase 1 (Homo sapiens (Human)) | BDBM35644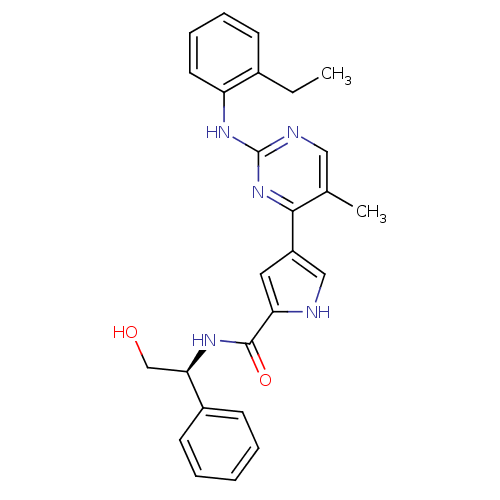 (erk000524 | pyrimidylpyrrole, 9c) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 3 | -49.5 | n/a | n/a | n/a | n/a | n/a | 7.5 | 30 |
Vertex | Assay Description A coupled spectrophotometric assay was used in which ADP generated by kinase was converted to ATP with the concomitant production of pyruvate from PE... | J Med Chem 52: 6362-8 (2009) Article DOI: 10.1021/jm900630q BindingDB Entry DOI: 10.7270/Q2D798SS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mitogen-activated protein kinase 1 (Homo sapiens (Human)) | BDBM35665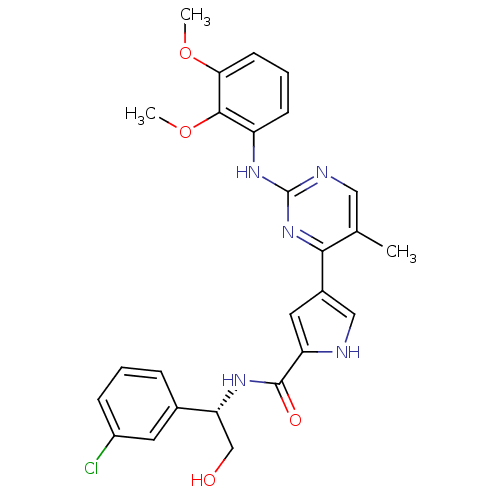 (erk000617 | pyrimidylpyrrole, 11m) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 3 | -49.5 | n/a | n/a | n/a | n/a | n/a | 7.5 | 30 |
Vertex | Assay Description A coupled spectrophotometric assay was used in which ADP generated by kinase was converted to ATP with the concomitant production of pyruvate from PE... | J Med Chem 52: 6362-8 (2009) Article DOI: 10.1021/jm900630q BindingDB Entry DOI: 10.7270/Q2D798SS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mitogen-activated protein kinase 1 (Homo sapiens (Human)) | BDBM35646 (erk000506 | pyrimidylpyrrole, 9e) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 4 | -48.7 | n/a | n/a | n/a | n/a | n/a | 7.5 | 30 |
Vertex | Assay Description A coupled spectrophotometric assay was used in which ADP generated by kinase was converted to ATP with the concomitant production of pyruvate from PE... | J Med Chem 52: 6362-8 (2009) Article DOI: 10.1021/jm900630q BindingDB Entry DOI: 10.7270/Q2D798SS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mitogen-activated protein kinase 1 (Homo sapiens (Human)) | BDBM35647 (erk000636 | pyrimidylpyrrole, 9f) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 4 | -48.7 | n/a | n/a | n/a | n/a | n/a | 7.5 | 30 |
Vertex | Assay Description A coupled spectrophotometric assay was used in which ADP generated by kinase was converted to ATP with the concomitant production of pyruvate from PE... | J Med Chem 52: 6362-8 (2009) Article DOI: 10.1021/jm900630q BindingDB Entry DOI: 10.7270/Q2D798SS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| DNA gyrase subunit A/B (Escherichia coli (strain K12)) | BDBM24616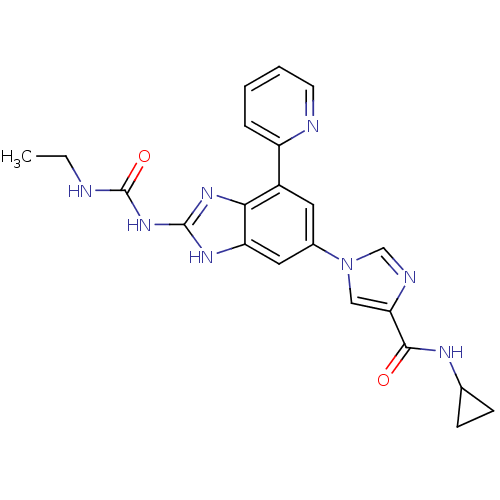 (1-(6-(4-(Cyclopropylcarbamoyl)-1H-imidazol-1-yl)-4...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Patents | Article PubMed | <4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vertex | Assay Description Enzymatic hydrolysis of ATP to ADP was coupled to the conversion of NADH to NAD+. The decrease in NADH absorbance was monitored at 340 nm for 20 min ... | J Med Chem 51: 5243-63 (2008) Article DOI: 10.1021/jm800318d BindingDB Entry DOI: 10.7270/Q2J67F7T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| DNA gyrase subunit A/B (Escherichia coli (strain K12)) | BDBM24614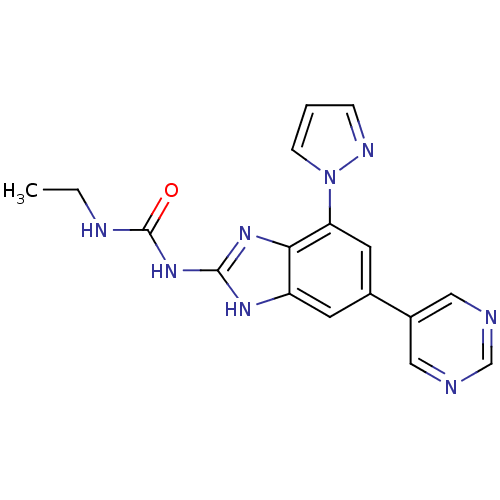 (3-ethyl-1-[7-(1H-pyrazol-1-yl)-5-(pyrimidin-5-yl)-...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | <4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vertex | Assay Description Enzymatic hydrolysis of ATP to ADP was coupled to the conversion of NADH to NAD+. The decrease in NADH absorbance was monitored at 340 nm for 20 min ... | J Med Chem 51: 5243-63 (2008) Article DOI: 10.1021/jm800318d BindingDB Entry DOI: 10.7270/Q2J67F7T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| DNA gyrase subunit A/B (Escherichia coli (strain K12)) | BDBM24615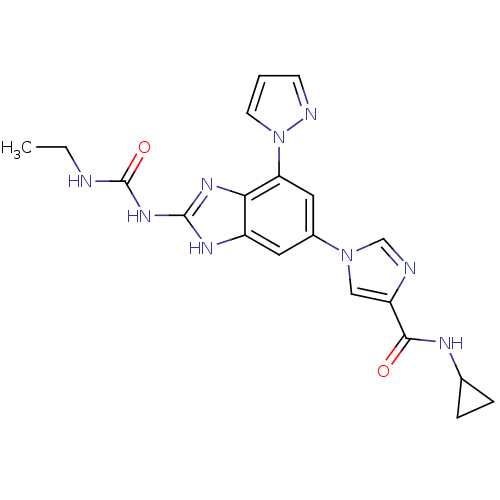 (Benzimidazole urea analogue, 19 | N-cyclopropyl-1-...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Patents | Article PubMed | <4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vertex | Assay Description Enzymatic hydrolysis of ATP to ADP was coupled to the conversion of NADH to NAD+. The decrease in NADH absorbance was monitored at 340 nm for 20 min ... | J Med Chem 51: 5243-63 (2008) Article DOI: 10.1021/jm800318d BindingDB Entry DOI: 10.7270/Q2J67F7T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| DNA gyrase subunit A/B (Escherichia coli (strain K12)) | BDBM24609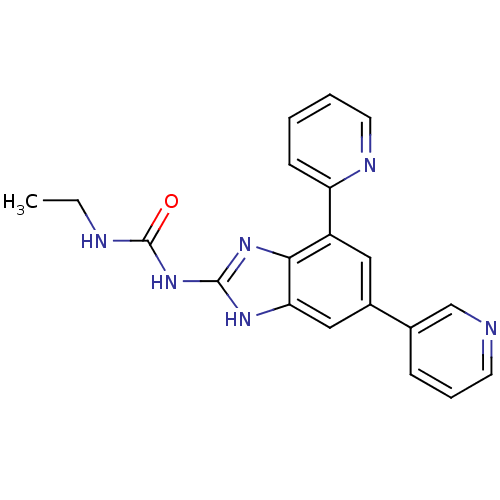 (3-ethyl-1-[7-(pyridin-2-yl)-5-(pyridin-3-yl)-1H-1,...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | <4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vertex | Assay Description Enzymatic hydrolysis of ATP to ADP was coupled to the conversion of NADH to NAD+. The decrease in NADH absorbance was monitored at 340 nm for 20 min ... | J Med Chem 51: 5243-63 (2008) Article DOI: 10.1021/jm800318d BindingDB Entry DOI: 10.7270/Q2J67F7T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| DNA gyrase subunit A/B (Escherichia coli (strain K12)) | BDBM24608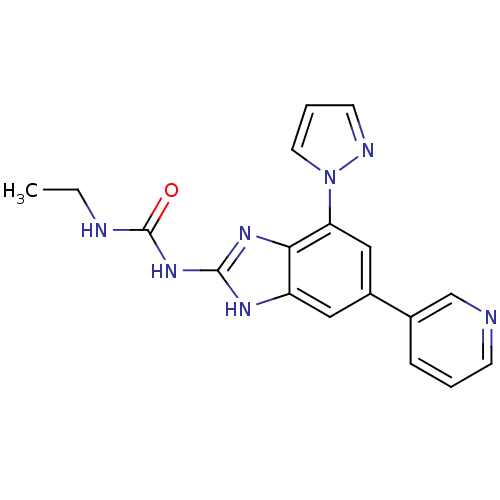 (3-ethyl-1-[7-(1H-pyrazol-1-yl)-5-(pyridin-3-yl)-1H...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | <4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vertex | Assay Description Enzymatic hydrolysis of ATP to ADP was coupled to the conversion of NADH to NAD+. The decrease in NADH absorbance was monitored at 340 nm for 20 min ... | J Med Chem 51: 5243-63 (2008) Article DOI: 10.1021/jm800318d BindingDB Entry DOI: 10.7270/Q2J67F7T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| DNA gyrase subunit A/B (Escherichia coli (strain K12)) | BDBM24606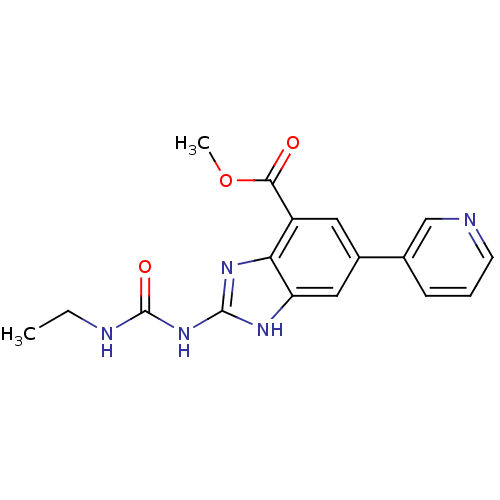 (Benzimidazole urea analogue, 10 | methyl 2-[(ethyl...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Patents | Article PubMed | <4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vertex | Assay Description Enzymatic hydrolysis of ATP to ADP was coupled to the conversion of NADH to NAD+. The decrease in NADH absorbance was monitored at 340 nm for 20 min ... | J Med Chem 51: 5243-63 (2008) Article DOI: 10.1021/jm800318d BindingDB Entry DOI: 10.7270/Q2J67F7T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| DNA gyrase subunit A/B (Escherichia coli (strain K12)) | BDBM24601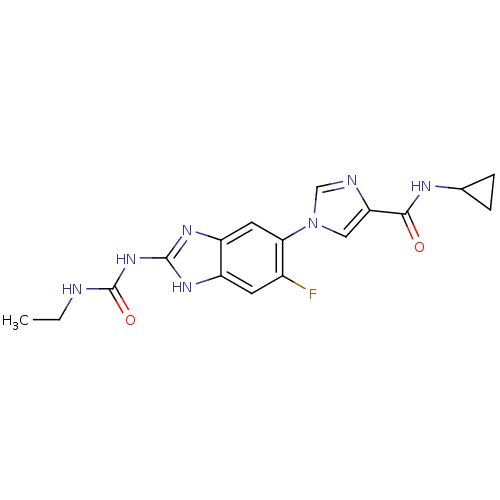 (Benzimidazole urea analogue, 5 | N-cyclopropyl-1-{...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Patents | Article PubMed | <4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vertex | Assay Description Enzymatic hydrolysis of ATP to ADP was coupled to the conversion of NADH to NAD+. The decrease in NADH absorbance was monitored at 340 nm for 20 min ... | J Med Chem 51: 5243-63 (2008) Article DOI: 10.1021/jm800318d BindingDB Entry DOI: 10.7270/Q2J67F7T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| DNA gyrase subunit A/B (Escherichia coli (strain K12)) | BDBM50497604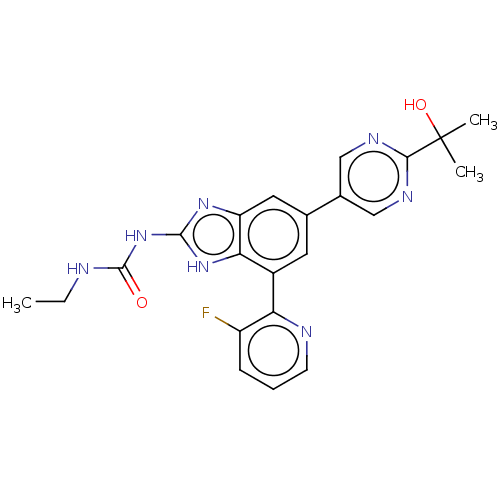 (CHEMBL3264033) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | <4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vertex Pharmaceuticals Inc Curated by ChEMBL | Assay Description Inhibition of aureus Escherichia coli DNA gyrase A2B2 using pBR322 plasmid DNA as substrate by coupled enzyme reaction assay | J Med Chem 57: 8792-816 (2014) Article DOI: 10.1021/jm500563g BindingDB Entry DOI: 10.7270/Q2TF01BF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| DNA gyrase subunit A/B (Escherichia coli (strain K12)) | BDBM24611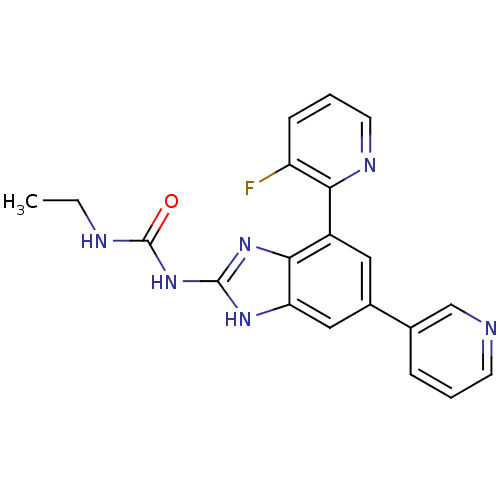 (3-ethyl-1-[7-(3-fluoropyridin-2-yl)-5-(pyridin-3-y...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | <4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vertex | Assay Description Enzymatic hydrolysis of ATP to ADP was coupled to the conversion of NADH to NAD+. The decrease in NADH absorbance was monitored at 340 nm for 20 min ... | J Med Chem 51: 5243-63 (2008) Article DOI: 10.1021/jm800318d BindingDB Entry DOI: 10.7270/Q2J67F7T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Ribosomal protein S6 kinase beta-1 (Homo sapiens (Human)) | BDBM50310456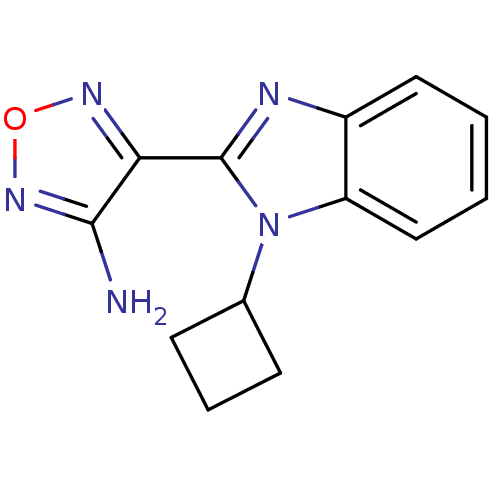 (4-(1-cyclobutyl-1H-benzo[d]imidazol-2-yl)-1,2,5-ox...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vertex Pharmaceuticals, Inc. Curated by ChEMBL | Assay Description Inhibition of p70S6K assessed as decrease in NADH absorbance at 340 nm in the presence of | Bioorg Med Chem Lett 19: 5191-4 (2009) Article DOI: 10.1016/j.bmcl.2009.07.022 BindingDB Entry DOI: 10.7270/Q2KP8287 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| DNA gyrase subunit A/B (Escherichia coli (strain K12)) | BDBM24617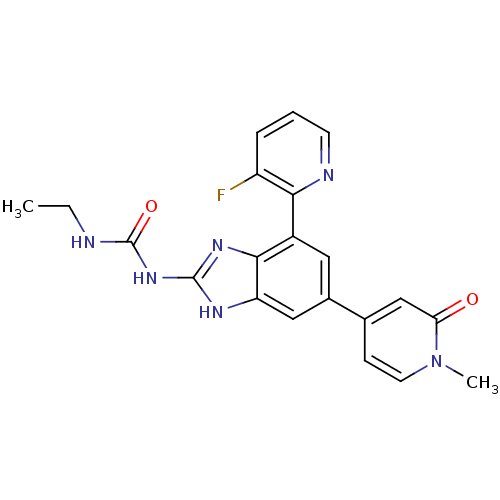 (3-ethyl-1-[7-(3-fluoropyridin-2-yl)-5-(1-methyl-2-...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | <4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vertex | Assay Description Enzymatic hydrolysis of ATP to ADP was coupled to the conversion of NADH to NAD+. The decrease in NADH absorbance was monitored at 340 nm for 20 min ... | J Med Chem 51: 5243-63 (2008) Article DOI: 10.1021/jm800318d BindingDB Entry DOI: 10.7270/Q2J67F7T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| DNA gyrase subunit A/B (Escherichia coli (strain K12)) | BDBM24607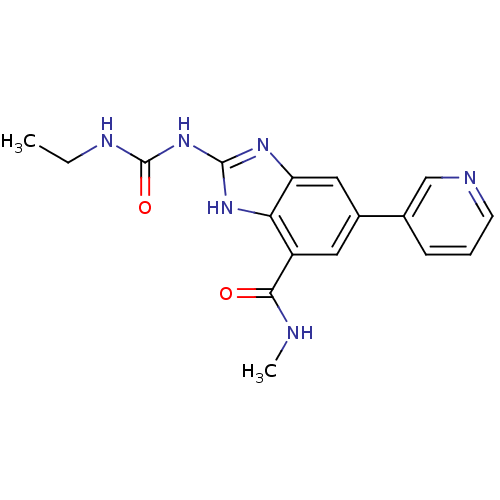 (2-[(ethylcarbamoyl)amino]-N-methyl-5-(pyridin-3-yl...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Patents | Article PubMed | 5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vertex | Assay Description Enzymatic hydrolysis of ATP to ADP was coupled to the conversion of NADH to NAD+. The decrease in NADH absorbance was monitored at 340 nm for 20 min ... | J Med Chem 51: 5243-63 (2008) Article DOI: 10.1021/jm800318d BindingDB Entry DOI: 10.7270/Q2J67F7T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| DNA gyrase subunit A/B (Escherichia coli (strain K12)) | BDBM50497603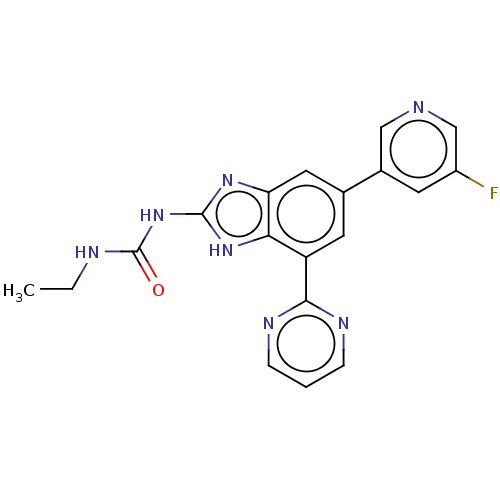 (CHEMBL3356986) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid PDB UniChem | Article PubMed | 5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vertex Pharmaceuticals Inc Curated by ChEMBL | Assay Description Inhibition of aureus Escherichia coli DNA gyrase A2B2 using pBR322 plasmid DNA as substrate by coupled enzyme reaction assay | J Med Chem 57: 8792-816 (2014) Article DOI: 10.1021/jm500563g BindingDB Entry DOI: 10.7270/Q2TF01BF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mitogen-activated protein kinase 1 (Homo sapiens (Human)) | BDBM35656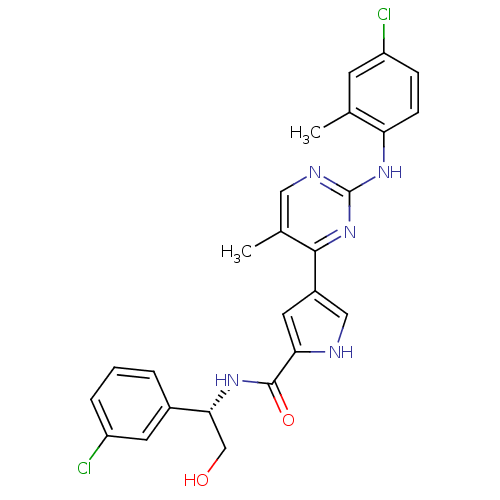 (erk000650 | pyrimidylpyrrole, 11d) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 5 | -48.2 | n/a | n/a | n/a | n/a | n/a | 7.5 | 30 |
Vertex | Assay Description A coupled spectrophotometric assay was used in which ADP generated by kinase was converted to ATP with the concomitant production of pyruvate from PE... | J Med Chem 52: 6362-8 (2009) Article DOI: 10.1021/jm900630q BindingDB Entry DOI: 10.7270/Q2D798SS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glycogen synthase kinase-3 beta (Homo sapiens (Human)) | BDBM35649 (erk000537 | pyrimidylpyrrole, 10b) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vertex | Assay Description A coupled spectrophotometric assay was used in which ADP generated by kinase was converted to ATP with the concomitant production of pyruvate from PE... | J Med Chem 52: 6362-8 (2009) Article DOI: 10.1021/jm900630q BindingDB Entry DOI: 10.7270/Q2D798SS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mitogen-activated protein kinase 1 (Homo sapiens (Human)) | BDBM35658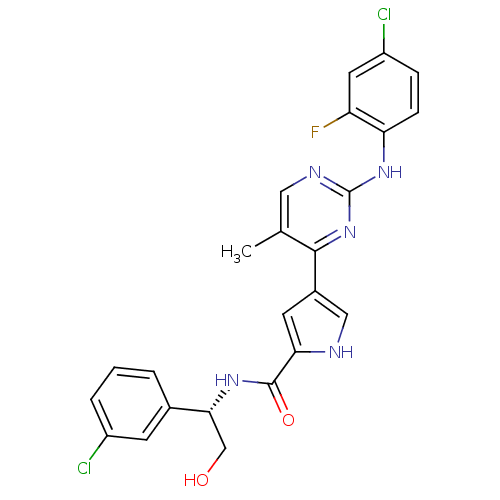 (erk000651 | pyrimidylpyrrole, 11f) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 5 | -48.2 | n/a | n/a | n/a | n/a | n/a | 7.5 | 30 |
Vertex | Assay Description A coupled spectrophotometric assay was used in which ADP generated by kinase was converted to ATP with the concomitant production of pyruvate from PE... | J Med Chem 52: 6362-8 (2009) Article DOI: 10.1021/jm900630q BindingDB Entry DOI: 10.7270/Q2D798SS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| DNA gyrase subunit A/B (Escherichia coli (strain K12)) | BDBM24610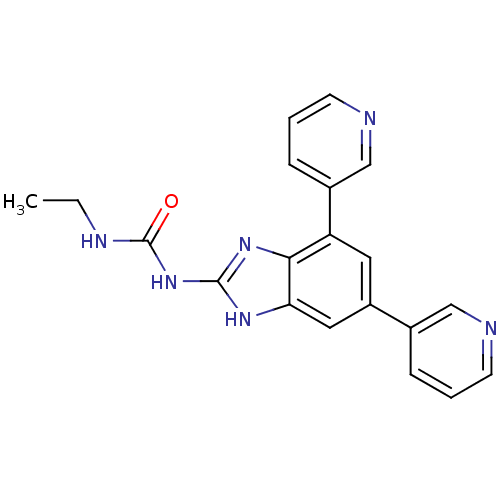 (1-[5,7-bis(pyridin-3-yl)-1H-1,3-benzodiazol-2-yl]-...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 6 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vertex | Assay Description Enzymatic hydrolysis of ATP to ADP was coupled to the conversion of NADH to NAD+. The decrease in NADH absorbance was monitored at 340 nm for 20 min ... | J Med Chem 51: 5243-63 (2008) Article DOI: 10.1021/jm800318d BindingDB Entry DOI: 10.7270/Q2J67F7T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| DNA gyrase subunit A/B (Escherichia coli (strain K12)) | BDBM50497602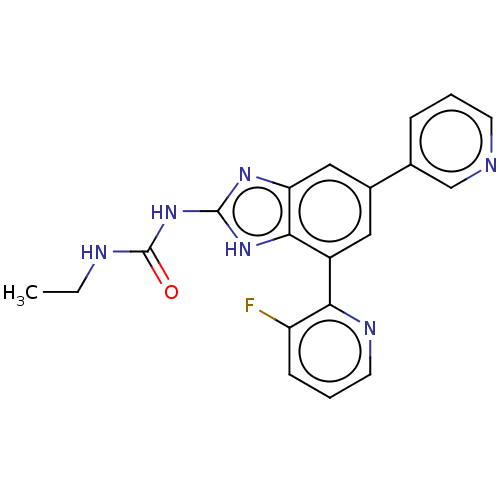 (CHEMBL222333 | VRT-752586) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 6 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vertex Pharmaceuticals Inc Curated by ChEMBL | Assay Description Inhibition of aureus Escherichia coli DNA gyrase A2B2 using pBR322 plasmid DNA as substrate by coupled enzyme reaction assay | J Med Chem 57: 8792-816 (2014) Article DOI: 10.1021/jm500563g BindingDB Entry DOI: 10.7270/Q2TF01BF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mitogen-activated protein kinase 1 (Homo sapiens (Human)) | BDBM15644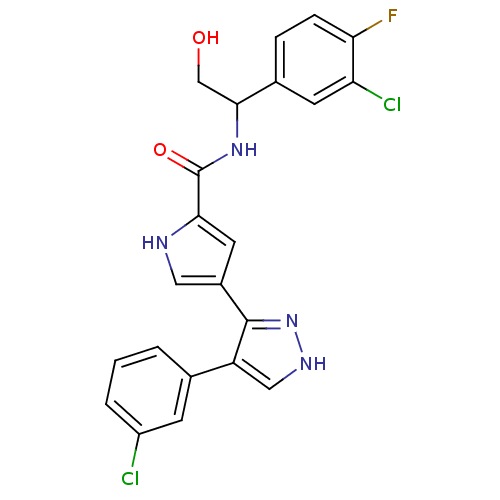 (N-(1-(3-Chloro-4-fluorophenyl)-2-hydroxyethyl)-4-(...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase PC cid PC sid PDB UniChem Similars | PDB Article PubMed | 6 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vertex | Assay Description A coupled spectrophotometric assay was used in which ADP generated by ERK2 was converted to ATP by pyruvate kinase with the production of pyruvate fr... | J Med Chem 50: 1280-7 (2007) Article DOI: 10.1021/jm061381f BindingDB Entry DOI: 10.7270/Q2BR8QFV | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| DNA gyrase subunit A/B (Staphylococcus aureus) | BDBM24609 (3-ethyl-1-[7-(pyridin-2-yl)-5-(pyridin-3-yl)-1H-1,...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | 7 | -47.3 | n/a | n/a | n/a | n/a | n/a | 7.5 | 30 |
Vertex | Assay Description Enzymatic hydrolysis of ATP to ADP was coupled to the conversion of NADH to NAD+. The decrease in NADH absorbance was monitored at 340 nm for 20 min ... | J Med Chem 51: 5243-63 (2008) Article DOI: 10.1021/jm800318d BindingDB Entry DOI: 10.7270/Q2J67F7T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glycogen synthase kinase-3 beta (Homo sapiens (Human)) | BDBM35641 (erk000040 | pyrimidylpyrrole, 2) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | MMDB PC cid PC sid PDB UniChem Similars | PDB Article PubMed | 7 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vertex | Assay Description A coupled spectrophotometric assay was used in which ADP generated by kinase was converted to ATP with the concomitant production of pyruvate from PE... | J Med Chem 52: 6362-8 (2009) Article DOI: 10.1021/jm900630q BindingDB Entry DOI: 10.7270/Q2D798SS | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Mitogen-activated protein kinase 1 (Homo sapiens (Human)) | BDBM15643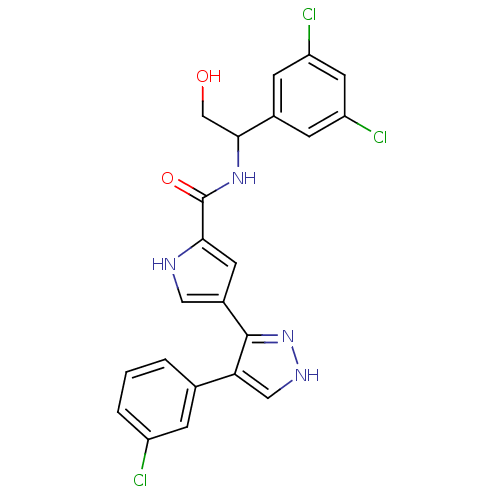 (4-(4-(3-Chlorophenyl)-1H-pyrazol-3-yl)-N-(1-(3,5-d...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 7 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vertex | Assay Description A coupled spectrophotometric assay was used in which ADP generated by ERK2 was converted to ATP by pyruvate kinase with the production of pyruvate fr... | J Med Chem 50: 1280-7 (2007) Article DOI: 10.1021/jm061381f BindingDB Entry DOI: 10.7270/Q2BR8QFV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| DNA topoisomerase 4 subunit A/B (Escherichia coli (strain K12)) | BDBM24616 (1-(6-(4-(Cyclopropylcarbamoyl)-1H-imidazol-1-yl)-4...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Patents | Article PubMed | 7 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vertex | Assay Description Enzymatic hydrolysis of ATP to ADP was coupled to the conversion of NADH to NAD+. The decrease in NADH absorbance was monitored at 340 nm for 20 min ... | J Med Chem 51: 5243-63 (2008) Article DOI: 10.1021/jm800318d BindingDB Entry DOI: 10.7270/Q2J67F7T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Hepatocyte growth factor receptor (Homo sapiens (Human)) | BDBM50557859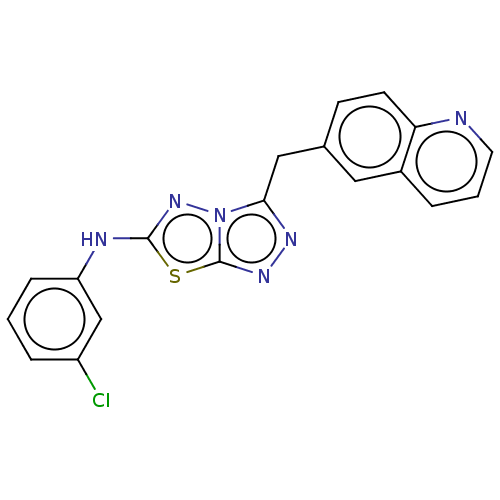 (CHEMBL4784332) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 7 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of c-Met (unknown origin) using poly Glu-Tyr as substrate preincubated for 10 mins followed by ATP addition by phosphoenolpyruvate/pyruvat... | Citation and Details Article DOI: 10.1021/acsmedchemlett.1c00094 BindingDB Entry DOI: 10.7270/Q28919JG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| DNA gyrase subunit A/B (Staphylococcus aureus) | BDBM24606 (Benzimidazole urea analogue, 10 | methyl 2-[(ethyl...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Patents | Article PubMed | 8 | -47.0 | n/a | n/a | n/a | n/a | n/a | 7.5 | 30 |
Vertex | Assay Description Enzymatic hydrolysis of ATP to ADP was coupled to the conversion of NADH to NAD+. The decrease in NADH absorbance was monitored at 340 nm for 20 min ... | J Med Chem 51: 5243-63 (2008) Article DOI: 10.1021/jm800318d BindingDB Entry DOI: 10.7270/Q2J67F7T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Hepatocyte growth factor receptor (Homo sapiens (Human)) | BDBM50557853 (CHEMBL4753090) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 8 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of c-Met (unknown origin) using poly Glu-Tyr as substrate preincubated for 10 mins followed by ATP addition by phosphoenolpyruvate/pyruvat... | Citation and Details Article DOI: 10.1021/acsmedchemlett.1c00094 BindingDB Entry DOI: 10.7270/Q28919JG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| DNA gyrase subunit A/B (Staphylococcus aureus) | BDBM50393079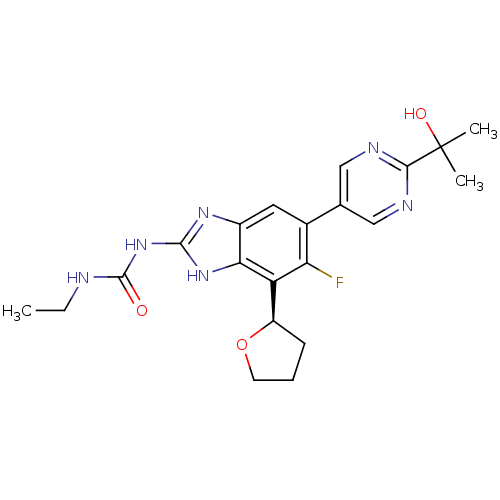 (CHEMBL2152855 | US9040542, 23) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | Article PubMed | 9 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vertex Pharmaceuticals Inc Curated by ChEMBL | Assay Description Inhibition of Staphylococcus aureus DNA gyrase using pBR322 plasmid DNA as substrate by coupled enzyme reaction assay | J Med Chem 57: 8792-816 (2014) Article DOI: 10.1021/jm500563g BindingDB Entry DOI: 10.7270/Q2TF01BF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| DNA gyrase subunit A/B (Staphylococcus aureus) | BDBM24601 (Benzimidazole urea analogue, 5 | N-cyclopropyl-1-{...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Patents | Article PubMed | 10 | -46.4 | n/a | n/a | n/a | n/a | n/a | 7.5 | 30 |
Vertex | Assay Description Enzymatic hydrolysis of ATP to ADP was coupled to the conversion of NADH to NAD+. The decrease in NADH absorbance was monitored at 340 nm for 20 min ... | J Med Chem 51: 5243-63 (2008) Article DOI: 10.1021/jm800318d BindingDB Entry DOI: 10.7270/Q2J67F7T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| DNA gyrase subunit A/B (Staphylococcus aureus) | BDBM24615 (Benzimidazole urea analogue, 19 | N-cyclopropyl-1-...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Patents | Article PubMed | 10 | -46.4 | n/a | n/a | n/a | n/a | n/a | 7.5 | 30 |
Vertex | Assay Description Enzymatic hydrolysis of ATP to ADP was coupled to the conversion of NADH to NAD+. The decrease in NADH absorbance was monitored at 340 nm for 20 min ... | J Med Chem 51: 5243-63 (2008) Article DOI: 10.1021/jm800318d BindingDB Entry DOI: 10.7270/Q2J67F7T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 1735 total ) | Next | Last >> |