 Found 15 hits with Last Name = 'tan' and Initial = 'rx'
Found 15 hits with Last Name = 'tan' and Initial = 'rx' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Xanthine dehydrogenase/oxidase
(Homo sapiens (Human)) | BDBM50269378
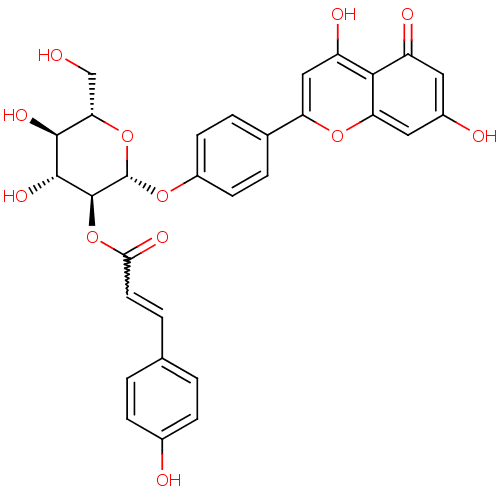
(CHEMBL447311 | apigenin-4'-O-(2''-O-p-coumaroyl)-b...)Show SMILES OC[C@@H]1O[C@H](Oc2ccc(cc2)-c2cc(O)c3c(cc(O)cc3=O)o2)[C@@H](OC(=O)C=Cc2ccc(O)cc2)[C@H](O)[C@H]1O |r,w:29.31| Show InChI InChI=1S/C30H26O12/c31-14-24-27(37)28(38)29(42-25(36)10-3-15-1-6-17(32)7-2-15)30(41-24)39-19-8-4-16(5-9-19)22-13-21(35)26-20(34)11-18(33)12-23(26)40-22/h1-13,24,27-33,35,37-38H,14H2/t24-,27-,28+,29-,30-/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.44E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Nanjing University
Curated by ChEMBL
| Assay Description
Inhibition of xanthine oxidase (unknown origin) by competitive Lineweaver-burk plot |
J Nat Prod 69: 1089-91 (2006)
Article DOI: 10.1021/np060038a
BindingDB Entry DOI: 10.7270/Q2RV0NG6 |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM50199522

((+)-huperzine A | (+-)-HA | (-)-1-Amino-13-ethylid...)Show SMILES C\C=C1/[C@@H]2Cc3[nH]c(=O)ccc3[C@@]1(N)CC(C)=C2 |r,c:18,THB:1:2:14.15.17:5.11.4| Show InChI InChI=1S/C15H18N2O/c1-3-11-10-6-9(2)8-15(11,16)12-4-5-14(18)17-13(12)7-10/h3-6,10H,7-8,16H2,1-2H3,(H,17,18)/b11-3+/t10-,15+/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| n/a | n/a | 4.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Nanjing University
Curated by ChEMBL
| Assay Description
Inhibition of AChE (unknown origin) using acetylthiocholine iodide as substrate after 5 mins by spectrophotometric analysis |
J Nat Prod 82: 792-797 (2019)
Article DOI: 10.1021/acs.jnatprod.8b00705
BindingDB Entry DOI: 10.7270/Q279486D |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Xanthine dehydrogenase/oxidase
(Homo sapiens (Human)) | BDBM35440
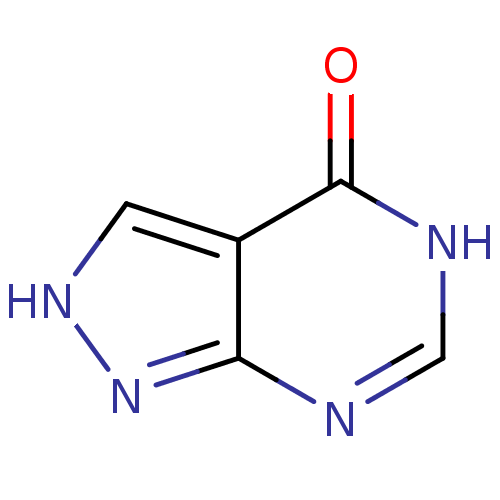
(ALLOPURINOL | MLS000069453 | SMR000059083 | cid_20...)Show InChI InChI=1S/C5H4N4O/c10-5-3-1-8-9-4(3)6-2-7-5/h1-2H,(H2,6,7,8,9,10) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 9.82E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Nanjing University
Curated by ChEMBL
| Assay Description
Inhibition of xanthine oxidase (unknown origin) |
J Nat Prod 69: 1089-91 (2006)
Article DOI: 10.1021/np060038a
BindingDB Entry DOI: 10.7270/Q2RV0NG6 |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM50537211
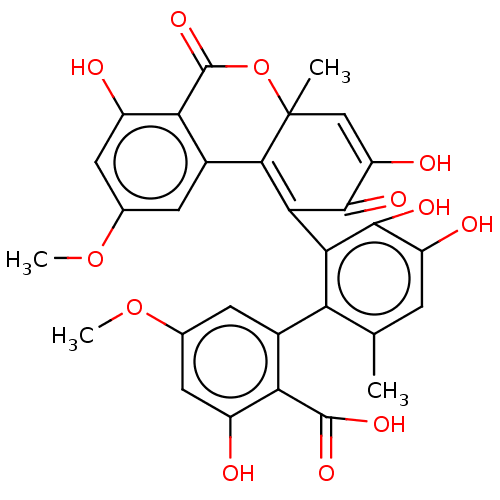
(CHEMBL4571704)Show SMILES COc1cc(O)c2C(=O)OC3(C)C=C(O)C(=O)C(=C3c2c1)c1c(O)c(O)cc(C)c1-c1cc(OC)cc(O)c1C(O)=O |c:17,t:12,(48.21,-31.33,;46.86,-30.54,;45.5,-31.33,;45.5,-32.83,;44.19,-33.58,;44.19,-35.15,;42.86,-32.81,;41.55,-33.57,;41.55,-35.13,;40.23,-32.81,;40.23,-31.31,;38.91,-32.07,;38.91,-30.54,;38.91,-28.99,;37.58,-28.22,;40.24,-28.22,;40.24,-26.68,;41.57,-28.98,;41.57,-30.54,;42.89,-31.3,;44.2,-30.57,;44.09,-27.53,;45.43,-28.3,;45.43,-29.83,;46.76,-27.53,;48.09,-28.3,;46.76,-26,;45.43,-25.23,;45.43,-23.68,;44.09,-26.01,;41.96,-24.78,;40.56,-25.58,;39.18,-24.78,;37.83,-25.57,;36.51,-24.8,;39.18,-23.19,;40.56,-22.39,;40.56,-20.84,;41.94,-23.19,;43.29,-22.41,;44.62,-23.18,;43.29,-20.86,)| Show InChI InChI=1S/C30H24O12/c1-11-5-18(33)26(35)23(20(11)14-6-12(40-3)8-16(31)21(14)28(37)38)24-25-15-7-13(41-4)9-17(32)22(15)29(39)42-30(25,2)10-19(34)27(24)36/h5-10,31-35H,1-4H3,(H,37,38) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.55E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Nanjing University
Curated by ChEMBL
| Assay Description
Inhibition of AChE (unknown origin) using acetylthiocholine iodide as substrate after 5 mins by spectrophotometric analysis |
J Nat Prod 82: 792-797 (2019)
Article DOI: 10.1021/acs.jnatprod.8b00705
BindingDB Entry DOI: 10.7270/Q279486D |
More data for this
Ligand-Target Pair | |
Xanthine dehydrogenase/oxidase
(Homo sapiens (Human)) | BDBM50241852

(CHEMBL469613 | Rubrofusarin B)Show InChI InChI=1S/C16H14O5/c1-8-4-11(17)15-13(21-8)6-9-5-10(19-2)7-12(20-3)14(9)16(15)18/h4-7,18H,1-3H3 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.68E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Nanjing University
Curated by ChEMBL
| Assay Description
Inhibition of xanthine oxidase (unknown origin) |
J Nat Prod 68: 1106-8 (2005)
Article DOI: 10.1021/np050059p
BindingDB Entry DOI: 10.7270/Q2M908FF |
More data for this
Ligand-Target Pair | |
Urease
(Canavalia ensiformis (Jack bean) (Horse bean)) | BDBM50099857
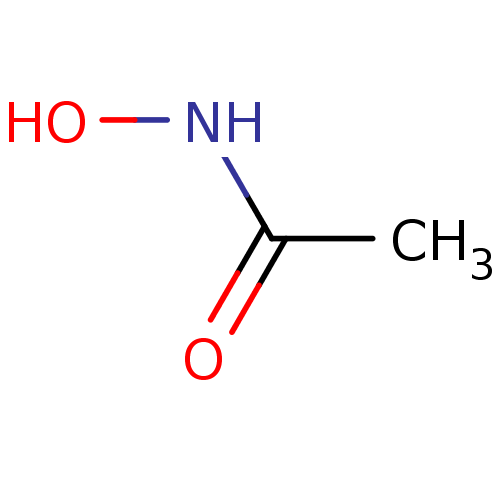
(ACETOHYDROXAMIC ACID (AHA) | AHA | Acethydroxamsae...)Show InChI InChI=1S/C2H5NO2/c1-2(4)3-5/h5H,1H3,(H,3,4) | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
| PDB
Article
PubMed
| n/a | n/a | 1.73E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Nanjing University
Curated by ChEMBL
| Assay Description
Inhibition of jack bean urease |
J Nat Prod 69: 1800-2 (2006)
Article DOI: 10.1021/np060242y
BindingDB Entry DOI: 10.7270/Q2348K4S |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Xanthine dehydrogenase/oxidase
(Homo sapiens (Human)) | BDBM50241853

(CHEMBL451678 | fonsecinone A)Show SMILES COc1cc(OC)c2c(O)c3c(oc(C)cc3=O)c(-c3c(OC)cc4cc(O)c5c(oc(C)cc5=O)c4c3OC)c2c1 |(9.32,-12.21,;9.32,-10.66,;10.66,-9.9,;10.67,-8.34,;12.01,-7.58,;12.02,-6.04,;10.69,-5.26,;13.34,-8.36,;14.67,-7.6,;14.68,-6.05,;16,-8.37,;16.01,-9.91,;17.33,-10.68,;18.66,-9.9,;20,-10.66,;18.65,-8.36,;17.32,-7.6,;17.31,-6.06,;14.67,-10.68,;14.67,-12.22,;13.33,-12.99,;12,-12.21,;10.66,-12.98,;13.33,-14.53,;14.67,-15.3,;14.66,-16.84,;16,-17.61,;16,-19.15,;17.33,-16.83,;17.33,-15.29,;18.66,-14.52,;19.98,-15.3,;21.32,-14.53,;19.98,-16.83,;18.65,-17.59,;18.65,-19.13,;16,-14.53,;16,-12.99,;17.34,-12.22,;18.67,-12.99,;13.34,-9.91,;12.01,-10.67,)| Show InChI InChI=1S/C32H26O10/c1-13-7-18(33)26-20(35)9-15-10-21(38-4)28(30(40-6)23(15)31(26)41-13)25-17-11-16(37-3)12-22(39-5)24(17)29(36)27-19(34)8-14(2)42-32(25)27/h7-12,35-36H,1-6H3 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.95E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Nanjing University
Curated by ChEMBL
| Assay Description
Inhibition of xanthine oxidase (unknown origin) |
J Nat Prod 68: 1106-8 (2005)
Article DOI: 10.1021/np050059p
BindingDB Entry DOI: 10.7270/Q2M908FF |
More data for this
Ligand-Target Pair | |
Xanthine dehydrogenase/oxidase
(Homo sapiens (Human)) | BDBM50269378

(CHEMBL447311 | apigenin-4'-O-(2''-O-p-coumaroyl)-b...)Show SMILES OC[C@@H]1O[C@H](Oc2ccc(cc2)-c2cc(O)c3c(cc(O)cc3=O)o2)[C@@H](OC(=O)C=Cc2ccc(O)cc2)[C@H](O)[C@H]1O |r,w:29.31| Show InChI InChI=1S/C30H26O12/c31-14-24-27(37)28(38)29(42-25(36)10-3-15-1-6-17(32)7-2-15)30(41-24)39-19-8-4-16(5-9-19)22-13-21(35)26-20(34)11-18(33)12-23(26)40-22/h1-13,24,27-33,35,37-38H,14H2/t24-,27-,28+,29-,30-/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.40E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Nanjing University
Curated by ChEMBL
| Assay Description
Inhibition of xanthine oxidase (unknown origin) |
J Nat Prod 69: 1089-91 (2006)
Article DOI: 10.1021/np060038a
BindingDB Entry DOI: 10.7270/Q2RV0NG6 |
More data for this
Ligand-Target Pair | |
Xanthine dehydrogenase/oxidase
(Homo sapiens (Human)) | BDBM35440

(ALLOPURINOL | MLS000069453 | SMR000059083 | cid_20...)Show InChI InChI=1S/C5H4N4O/c10-5-3-1-8-9-4(3)6-2-7-5/h1-2H,(H2,6,7,8,9,10) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 2.42E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Nanjing University
Curated by ChEMBL
| Assay Description
Inhibition of xanthine oxidase (unknown origin) by spectrophotometry |
J Nat Prod 62: 1053-5 (1999)
Article DOI: 10.1021/np990009i
BindingDB Entry DOI: 10.7270/Q2MP531P |
More data for this
Ligand-Target Pair | |
Xanthine dehydrogenase/oxidase
(Homo sapiens (Human)) | BDBM50242286
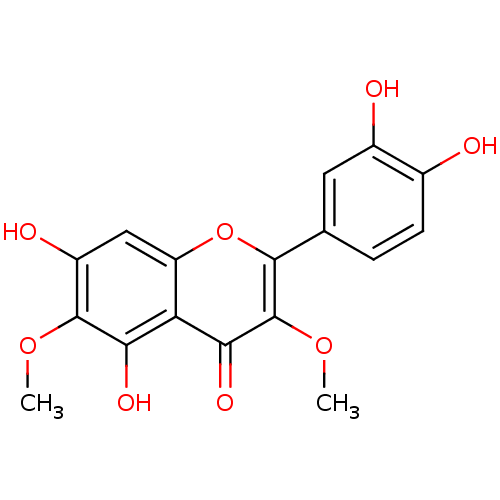
(2-(3,4-dihydroxyphenyl)-5,7-dihydroxy-3,6-dimethox...)Show SMILES COc1c(O)cc2oc(-c3ccc(O)c(O)c3)c(OC)c(=O)c2c1O Show InChI InChI=1S/C17H14O8/c1-23-16-10(20)6-11-12(13(16)21)14(22)17(24-2)15(25-11)7-3-4-8(18)9(19)5-7/h3-6,18-21H,1-2H3 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3.60E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Nanjing University
Curated by ChEMBL
| Assay Description
Inhibition of xanthine oxidase (unknown origin) by spectrophotometry |
J Nat Prod 62: 1053-5 (1999)
Article DOI: 10.1021/np990009i
BindingDB Entry DOI: 10.7270/Q2MP531P |
More data for this
Ligand-Target Pair | |
Xanthine dehydrogenase/oxidase
(Homo sapiens (Human)) | BDBM50240898
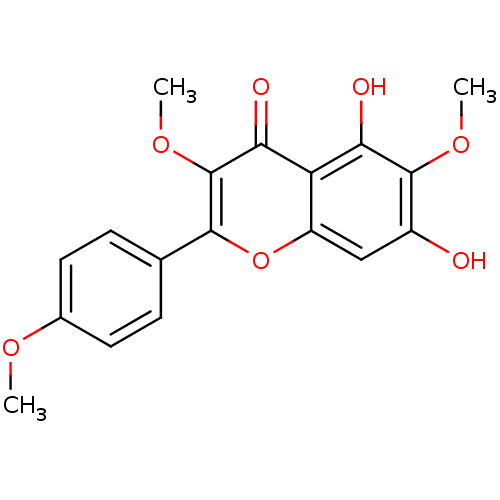
(5,7-Dihydroxy-3,6-dimethoxy-2-(4-methoxy-phenyl)-c...)Show SMILES COc1ccc(cc1)-c1oc2cc(O)c(OC)c(O)c2c(=O)c1OC Show InChI InChI=1S/C18H16O7/c1-22-10-6-4-9(5-7-10)16-18(24-3)15(21)13-12(25-16)8-11(19)17(23-2)14(13)20/h4-8,19-20H,1-3H3 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 3.65E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Nanjing University
Curated by ChEMBL
| Assay Description
Inhibition of xanthine oxidase (unknown origin) by spectrophotometry |
J Nat Prod 62: 1053-5 (1999)
Article DOI: 10.1021/np990009i
BindingDB Entry DOI: 10.7270/Q2MP531P |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM50537210
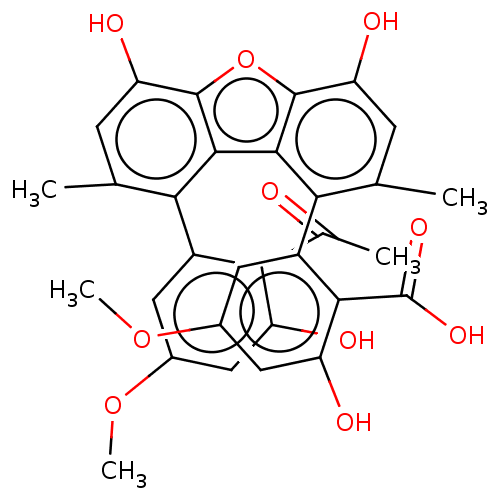
(CHEMBL4556664)Show SMILES COc1cc(O)c(C(C)=O)c(c1)-c1c(C)cc(O)c2oc3c(O)cc(C)c(-c4cc(OC)cc(O)c4C(O)=O)c3c12 |(26.02,-34.9,;26.03,-33.36,;27.37,-32.6,;28.7,-33.38,;30.05,-32.61,;31.37,-33.39,;30.05,-31.08,;31.38,-30.31,;32.71,-31.09,;31.38,-28.78,;28.73,-30.31,;27.38,-31.07,;28.69,-26.76,;30.22,-26.93,;31.54,-27.69,;31.12,-25.68,;30.5,-24.29,;31.41,-23.04,;28.98,-24.12,;28.08,-22.88,;26.61,-23.35,;25.28,-22.58,;25.27,-21.04,;23.95,-23.35,;23.95,-24.91,;22.62,-25.68,;25.28,-25.66,;25.28,-27.25,;26.66,-28.04,;26.67,-29.63,;26.65,-31.22,;25.27,-32,;25.29,-30.44,;23.9,-29.64,;22.52,-30.44,;23.9,-28.05,;22.52,-27.26,;21.14,-28.06,;23.58,-26.17,;26.61,-24.89,;28.08,-25.36,)| Show InChI InChI=1S/C31H26O10/c1-12-6-21(35)29-27(23(12)17-8-15(39-4)10-19(33)25(17)14(3)32)28-24(13(2)7-22(36)30(28)41-29)18-9-16(40-5)11-20(34)26(18)31(37)38/h6-11,33-36H,1-5H3,(H,37,38) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 6.83E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Nanjing University
Curated by ChEMBL
| Assay Description
Inhibition of AChE (unknown origin) using acetylthiocholine iodide as substrate after 5 mins by spectrophotometric analysis |
J Nat Prod 82: 792-797 (2019)
Article DOI: 10.1021/acs.jnatprod.8b00705
BindingDB Entry DOI: 10.7270/Q279486D |
More data for this
Ligand-Target Pair | |
Xanthine dehydrogenase/oxidase
(Homo sapiens (Human)) | BDBM50269380

(CHEMBL454228 | apigenin-4'-O-(2'',6''-di-O-p-couma...)Show SMILES O[C@H]1[C@H](COC(=O)C=Cc2ccc(O)cc2)O[C@H](Oc2ccc(cc2)-c2cc(O)c3c(cc(O)cc3=O)o2)[C@@H](OC(=O)C=Cc2ccc(O)cc2)[C@@H]1O |r,w:7.6,42.45| Show InChI InChI=1S/C39H32O14/c40-24-9-1-21(2-10-24)5-15-33(45)49-20-32-36(47)37(48)38(53-34(46)16-6-22-3-11-25(41)12-4-22)39(52-32)50-27-13-7-23(8-14-27)30-19-29(44)35-28(43)17-26(42)18-31(35)51-30/h1-19,32,36-42,44,47-48H,20H2/t32-,36-,37+,38-,39-/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Nanjing University
Curated by ChEMBL
| Assay Description
Inhibition of xanthine oxidase (unknown origin) |
J Nat Prod 69: 1089-91 (2006)
Article DOI: 10.1021/np060038a
BindingDB Entry DOI: 10.7270/Q2RV0NG6 |
More data for this
Ligand-Target Pair | |
Xanthine dehydrogenase/oxidase
(Homo sapiens (Human)) | BDBM50269379
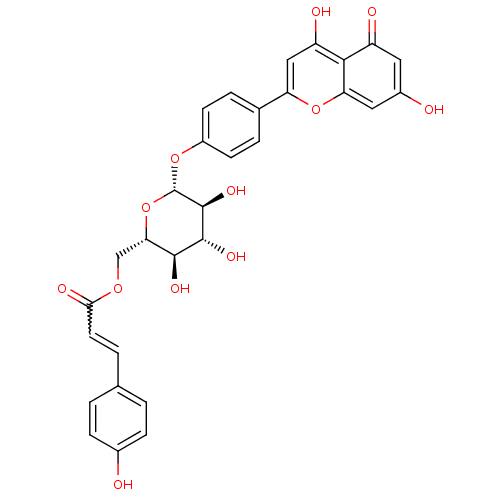
(CHEMBL503232 | apigenin-4'-O-(6''-O-p-coumaroyl)-b...)Show SMILES O[C@H]1[C@H](COC(=O)C=Cc2ccc(O)cc2)O[C@H](Oc2ccc(cc2)-c2cc(O)c3c(cc(O)cc3=O)o2)[C@@H](O)[C@@H]1O |r,w:7.6| Show InChI InChI=1S/C30H26O12/c31-17-6-1-15(2-7-17)3-10-25(35)39-14-24-27(36)28(37)29(38)30(42-24)40-19-8-4-16(5-9-19)22-13-21(34)26-20(33)11-18(32)12-23(26)41-22/h1-13,24,27-32,34,36-38H,14H2/t24-,27-,28+,29-,30-/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Nanjing University
Curated by ChEMBL
| Assay Description
Inhibition of xanthine oxidase (unknown origin) |
J Nat Prod 69: 1089-91 (2006)
Article DOI: 10.1021/np060038a
BindingDB Entry DOI: 10.7270/Q2RV0NG6 |
More data for this
Ligand-Target Pair | |
Urease
(Canavalia ensiformis (Jack bean) (Horse bean)) | BDBM50250383
(CHEMBL1939429 | CHEMBL490506 | shoreaphenol)Show SMILES Oc1ccc(cc1)-c1oc2cc(O)cc3c(O)c(-c4ccc(O)cc4)c4c(O)cc(O)cc4c1c23 Show InChI InChI=1S/C28H18O7/c29-15-5-1-13(2-6-15)23-24-19(9-17(31)11-21(24)33)26-25-20(27(23)34)10-18(32)12-22(25)35-28(26)14-3-7-16(30)8-4-14/h1-12,29-34H | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.27E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Nanjing University
Curated by ChEMBL
| Assay Description
Inhibition of jack bean urease |
J Nat Prod 69: 1800-2 (2006)
Article DOI: 10.1021/np060242y
BindingDB Entry DOI: 10.7270/Q2348K4S |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data