 Found 13012 hits with Last Name = 'tan' and Initial = 'y'
Found 13012 hits with Last Name = 'tan' and Initial = 'y' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Protease
(Human immunodeficiency virus 1 (HIV-1)) | BDBM50480930
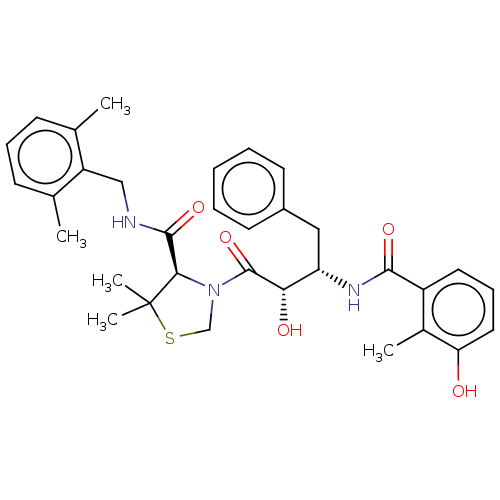
(CHEMBL584130 | KNI-814)Show SMILES Cc1cccc(C)c1CNC(=O)[C@H]1N(CSC1(C)C)C(=O)[C@@H](O)[C@H](Cc1ccccc1)NC(=O)c1cccc(O)c1C |r| Show InChI InChI=1S/C33H39N3O5S/c1-20-11-9-12-21(2)25(20)18-34-31(40)29-33(4,5)42-19-36(29)32(41)28(38)26(17-23-13-7-6-8-14-23)35-30(39)24-15-10-16-27(37)22(24)3/h6-16,26,28-29,37-38H,17-19H2,1-5H3,(H,34,40)(H,35,39)/t26-,28-,29+/m0/s1 | PDB
UniProtKB/TrEMBL
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.00240 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Kyoto Pharmaceutical University
Curated by ChEMBL
| Assay Description
Inhibition of HIV1 recombinant protease after 15 mins by fluorescence assay |
J Med Chem 52: 7604-17 (2009)
Article DOI: 10.1021/jm9005115
BindingDB Entry DOI: 10.7270/Q2FR00F2 |
More data for this
Ligand-Target Pair | |
Endothelin receptor type B
(Sus scrofa) | BDBM50000558

(CHEMBL437472 | ET-1 | Endothelin -1 | Endothelin 1...)Show SMILES CC[C@H](C)[C@H](NC(=O)[C@H](CC(O)=O)NC(=O)[C@H](CC(C)C)NC(=O)[C@H](Cc1cnc[nH]1)NC(=O)[C@@H]1CSSC[C@@H](N)C(=O)N[C@H](CO)C(=O)N[C@H]2CSSC[C@@H](NC(=O)[C@H](CCC(O)=O)NC(=O)[C@H](CCCCN)NC(=O)[C@@H](CC(O)=O)NC(=O)[C@H](CCSC)NC(=O)[C@@H](CC(C)C)NC(=O)[C@H](CO)NC(=O)[C@@H](CO)NC2=O)C(=O)N[C@@H](C(C)C)C(=O)N[C@H](Cc2ccc(O)cc2)C(=O)N[C@@H](Cc2ccccc2)C(=O)N1)C(=O)N[C@@H]([C@@H](C)CC)C(=O)N[C@@H](Cc1c[nH]c2ccccc12)C(O)=O Show InChI InChI=1S/C109H159N25O32S5/c1-12-56(9)87(107(163)125-76(109(165)166)39-60-43-113-65-24-18-17-23-63(60)65)134-108(164)88(57(10)13-2)133-99(155)75(42-85(143)144)123-94(150)70(36-54(5)6)118-97(153)73(40-61-44-112-52-114-61)121-103(159)80-49-169-168-48-64(111)89(145)126-77(45-135)102(158)131-81-50-170-171-51-82(105(161)132-86(55(7)8)106(162)124-72(38-59-26-28-62(138)29-27-59)95(151)120-71(96(152)130-80)37-58-21-15-14-16-22-58)129-91(147)67(30-31-83(139)140)116-90(146)66(25-19-20-33-110)115-98(154)74(41-84(141)142)122-92(148)68(32-34-167-11)117-93(149)69(35-53(3)4)119-100(156)78(46-136)127-101(157)79(47-137)128-104(81)160/h14-18,21-24,26-29,43-44,52-57,64,66-82,86-88,113,135-138H,12-13,19-20,25,30-42,45-51,110-111H2,1-11H3,(H,112,114)(H,115,154)(H,116,146)(H,117,149)(H,118,153)(H,119,156)(H,120,151)(H,121,159)(H,122,148)(H,123,150)(H,124,162)(H,125,163)(H,126,145)(H,127,157)(H,128,160)(H,129,147)(H,130,152)(H,131,158)(H,132,161)(H,133,155)(H,134,164)(H,139,140)(H,141,142)(H,143,144)(H,165,166)/t56-,57-,64+,66-,67-,68-,69+,70-,71-,72+,73-,74+,75-,76-,77+,78-,79+,80-,81-,82+,86-,87-,88-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| 0.00800 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Ability to inhibit [125I]ET-3 binding to the Endothelin B receptor from porcine lung membranes was evaluated |
Bioorg Med Chem Lett 6: 2323-2328 (1996)
Article DOI: 10.1016/0960-894X(96)00421-0
BindingDB Entry DOI: 10.7270/Q21J9B8K |
More data for this
Ligand-Target Pair | |
Protease
(Human immunodeficiency virus 1 (HIV-1)) | BDBM580

((4R)-3-[(2S,3S)-2-hydroxy-3-[(3-hydroxy-2-methylph...)Show SMILES Cc1ccccc1CNC(=O)[C@H]1N(CSC1(C)C)C(=O)[C@@H](O)[C@H](Cc1ccccc1)NC(=O)c1cccc(O)c1C |r| Show InChI InChI=1S/C32H37N3O5S/c1-20-11-8-9-14-23(20)18-33-30(39)28-32(3,4)41-19-35(28)31(40)27(37)25(17-22-12-6-5-7-13-22)34-29(38)24-15-10-16-26(36)21(24)2/h5-16,25,27-28,36-37H,17-19H2,1-4H3,(H,33,39)(H,34,38)/t25-,27-,28+/m0/s1 | PDB
UniProtKB/TrEMBL
B.MOAD
GoogleScholar
AffyNet 
| MCE
MMDB
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| 0.0310 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Kyoto Pharmaceutical University
Curated by ChEMBL
| Assay Description
Inhibition of HIV1 recombinant protease after 15 mins by fluorescence assay |
J Med Chem 52: 7604-17 (2009)
Article DOI: 10.1021/jm9005115
BindingDB Entry DOI: 10.7270/Q2FR00F2 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Kappa-type opioid receptor
(Cavia porcellus (domestic guinea pig)) | BDBM50491882
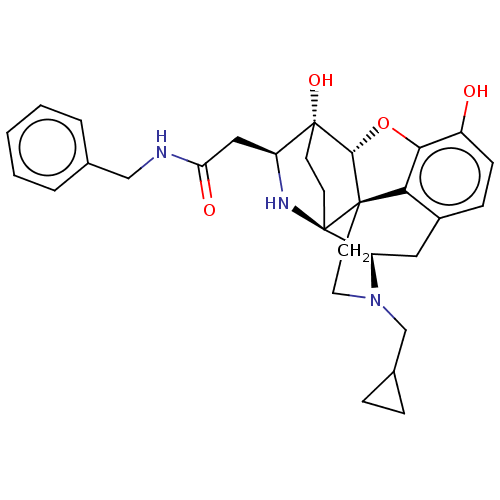
(CHEMBL3215908)Show SMILES Cl.Cl.[H][C@@]12Oc3c4c(C[C@@]5([H])N(CC6CC6)CC[C@]14[C@@]51CC[C@@]2(O)[C@H](CC(=O)NCc2ccccc2)N1)ccc3O |r,THB:25:24:3.18:20.21,12:11:19:6.8.7| Show InChI InChI=1S/C30H35N3O4.2ClH/c34-21-9-8-20-14-23-30-11-10-29(36,22(32-30)15-24(35)31-16-18-4-2-1-3-5-18)27-28(30,25(20)26(21)37-27)12-13-33(23)17-19-6-7-19;;/h1-5,8-9,19,22-23,27,32,34,36H,6-7,10-17H2,(H,31,35);2*1H/t22-,23+,27+,28+,29+,30+;;/m0../s1 | PDB
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.0370 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Kitasato University
Curated by ChEMBL
| Assay Description
Displacement of [3H]U-69,593 from kappa opioid receptor in guinea pig cerebellum |
Bioorg Med Chem 21: 3032-50 (2013)
Article DOI: 10.1016/j.bmc.2013.03.026
BindingDB Entry DOI: 10.7270/Q2JQ13XJ |
More data for this
Ligand-Target Pair | |
Kappa-type opioid receptor
(Cavia porcellus (domestic guinea pig)) | BDBM50102826
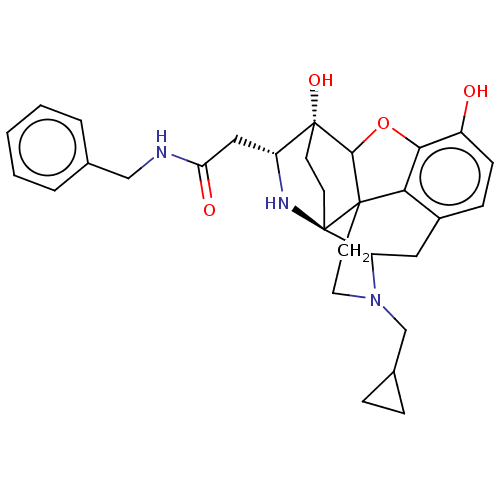
(CHEMBL3339374)Show SMILES Oc1ccc2CC3N(CC4CC4)CCC45C(Oc1c24)[C@@]1(O)CC[C@@]35N[C@@H]1CC(=O)NCc1ccccc1 |r,TLB:26:25:14.15:22.21,THB:3:4:23:7.12.13,17:18:23:7.12.13,22:23:4.18.5:7.12.13,24:23:4.18.5:7.12.13,8:7:4.18.5:23| Show InChI InChI=1S/C30H35N3O4/c34-21-9-8-20-14-23-30-11-10-29(36,22(32-30)15-24(35)31-16-18-4-2-1-3-5-18)27-28(30,25(20)26(21)37-27)12-13-33(23)17-19-6-7-19/h1-5,8-9,19,22-23,27,32,34,36H,6-7,10-17H2,(H,31,35)/t22-,23?,27?,28?,29-,30-/m1/s1 | PDB
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.0370 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Kitasato University
Curated by ChEMBL
| Assay Description
Displacement of [3H]U-69593 from kappa opioid receptor in guinea pig cerebellum |
Bioorg Med Chem Lett 24: 4980-3 (2014)
Article DOI: 10.1016/j.bmcl.2014.09.029
BindingDB Entry DOI: 10.7270/Q2154JTJ |
More data for this
Ligand-Target Pair | |
Endothelin-1 receptor
(Sus scrofa) | BDBM50000558

(CHEMBL437472 | ET-1 | Endothelin -1 | Endothelin 1...)Show SMILES CC[C@H](C)[C@H](NC(=O)[C@H](CC(O)=O)NC(=O)[C@H](CC(C)C)NC(=O)[C@H](Cc1cnc[nH]1)NC(=O)[C@@H]1CSSC[C@@H](N)C(=O)N[C@H](CO)C(=O)N[C@H]2CSSC[C@@H](NC(=O)[C@H](CCC(O)=O)NC(=O)[C@H](CCCCN)NC(=O)[C@@H](CC(O)=O)NC(=O)[C@H](CCSC)NC(=O)[C@@H](CC(C)C)NC(=O)[C@H](CO)NC(=O)[C@@H](CO)NC2=O)C(=O)N[C@@H](C(C)C)C(=O)N[C@H](Cc2ccc(O)cc2)C(=O)N[C@@H](Cc2ccccc2)C(=O)N1)C(=O)N[C@@H]([C@@H](C)CC)C(=O)N[C@@H](Cc1c[nH]c2ccccc12)C(O)=O Show InChI InChI=1S/C109H159N25O32S5/c1-12-56(9)87(107(163)125-76(109(165)166)39-60-43-113-65-24-18-17-23-63(60)65)134-108(164)88(57(10)13-2)133-99(155)75(42-85(143)144)123-94(150)70(36-54(5)6)118-97(153)73(40-61-44-112-52-114-61)121-103(159)80-49-169-168-48-64(111)89(145)126-77(45-135)102(158)131-81-50-170-171-51-82(105(161)132-86(55(7)8)106(162)124-72(38-59-26-28-62(138)29-27-59)95(151)120-71(96(152)130-80)37-58-21-15-14-16-22-58)129-91(147)67(30-31-83(139)140)116-90(146)66(25-19-20-33-110)115-98(154)74(41-84(141)142)122-92(148)68(32-34-167-11)117-93(149)69(35-53(3)4)119-100(156)78(46-136)127-101(157)79(47-137)128-104(81)160/h14-18,21-24,26-29,43-44,52-57,64,66-82,86-88,113,135-138H,12-13,19-20,25,30-42,45-51,110-111H2,1-11H3,(H,112,114)(H,115,154)(H,116,146)(H,117,149)(H,118,153)(H,119,156)(H,120,151)(H,121,159)(H,122,148)(H,123,150)(H,124,162)(H,125,163)(H,126,145)(H,127,157)(H,128,160)(H,129,147)(H,130,152)(H,131,158)(H,132,161)(H,133,155)(H,134,164)(H,139,140)(H,141,142)(H,143,144)(H,165,166)/t56-,57-,64+,66-,67-,68-,69+,70-,71-,72+,73-,74+,75-,76-,77+,78-,79+,80-,81-,82+,86-,87-,88-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| 0.0400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Ability to inhibit [125I]ET1 binding to the Endothelin A receptor from porcine lung membranes was evaluated |
Bioorg Med Chem Lett 6: 2323-2328 (1996)
Article DOI: 10.1016/0960-894X(96)00421-0
BindingDB Entry DOI: 10.7270/Q21J9B8K |
More data for this
Ligand-Target Pair | |
Kappa-type opioid receptor
(Cavia porcellus (domestic guinea pig)) | BDBM50102833
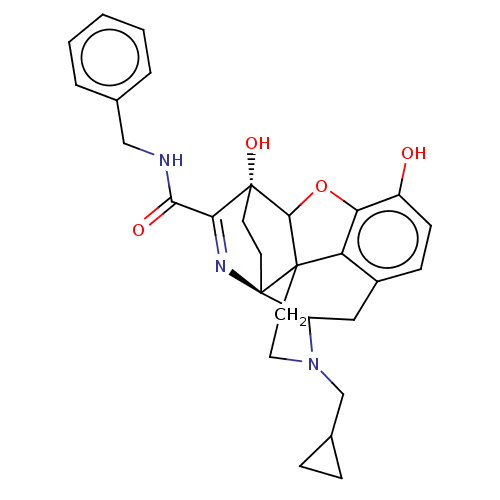
(CHEMBL3339378)Show SMILES Oc1ccc2CC3N(CC4CC4)CCC45C(Oc1c24)[C@@]1(O)CC[C@@]35N=C1C(=O)NCc1ccccc1 |r,c:30,TLB:26:25:14.15:22.21,THB:3:4:23:7.12.13,8:7:4.18.5:23,17:18:23:7.12.13,24:23:4.18.5:7.12.13,22:23:4.18.5:7.12.13| Show InChI InChI=1S/C29H31N3O4/c33-20-9-8-19-14-21-29-11-10-28(35,24(31-29)25(34)30-15-17-4-2-1-3-5-17)26-27(29,22(19)23(20)36-26)12-13-32(21)16-18-6-7-18/h1-5,8-9,18,21,26,33,35H,6-7,10-16H2,(H,30,34)/t21?,26?,27?,28-,29-/m1/s1 | PDB
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.0430 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Kitasato University
Curated by ChEMBL
| Assay Description
Displacement of [3H]U-69593 from kappa opioid receptor in guinea pig cerebellum |
Bioorg Med Chem Lett 24: 4980-3 (2014)
Article DOI: 10.1016/j.bmcl.2014.09.029
BindingDB Entry DOI: 10.7270/Q2154JTJ |
More data for this
Ligand-Target Pair | |
Mu-type opioid receptor
(MOUSE) | BDBM50380909
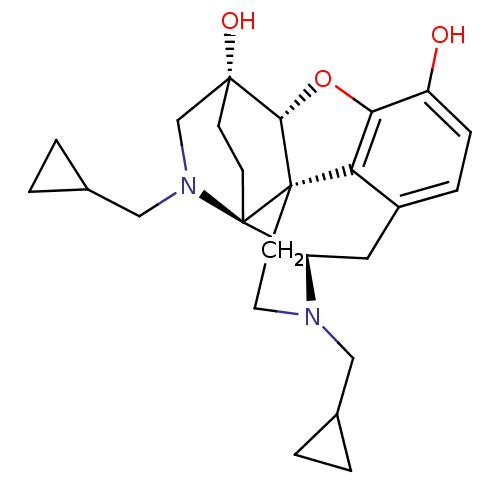
(CHEMBL2016678)Show SMILES Oc1ccc2C[C@H]3N(CC4CC4)CC[C@@]45[C@@H](Oc1c24)[C@@]1(O)CC[C@@]35N(CC2CC2)C1 |r,THB:25:24:22.21:14.15| Show InChI InChI=1S/C25H32N2O3/c28-18-6-5-17-11-19-25-8-7-23(29,14-27(25)13-16-3-4-16)22-24(25,20(17)21(18)30-22)9-10-26(19)12-15-1-2-15/h5-6,15-16,19,22,28-29H,1-4,7-14H2/t19-,22+,23-,24+,25-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.0490 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Kitasato University
Curated by ChEMBL
| Assay Description
Displacement of [3H]DAMGO from mouse brain mu opioid receptor |
Bioorg Med Chem Lett 22: 2689-92 (2012)
Article DOI: 10.1016/j.bmcl.2012.03.001
BindingDB Entry DOI: 10.7270/Q24X58T4 |
More data for this
Ligand-Target Pair | |
Mu-type opioid receptor
(MOUSE) | BDBM50491882

(CHEMBL3215908)Show SMILES Cl.Cl.[H][C@@]12Oc3c4c(C[C@@]5([H])N(CC6CC6)CC[C@]14[C@@]51CC[C@@]2(O)[C@H](CC(=O)NCc2ccccc2)N1)ccc3O |r,THB:25:24:3.18:20.21,12:11:19:6.8.7| Show InChI InChI=1S/C30H35N3O4.2ClH/c34-21-9-8-20-14-23-30-11-10-29(36,22(32-30)15-24(35)31-16-18-4-2-1-3-5-18)27-28(30,25(20)26(21)37-27)12-13-33(23)17-19-6-7-19;;/h1-5,8-9,19,22-23,27,32,34,36H,6-7,10-17H2,(H,31,35);2*1H/t22-,23+,27+,28+,29+,30+;;/m0../s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.0520 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Kitasato University
Curated by ChEMBL
| Assay Description
Displacement of [3H]DAMGO from mu opioid receptor in mouse whole brain devoid of cerebellum |
Bioorg Med Chem 21: 3032-50 (2013)
Article DOI: 10.1016/j.bmc.2013.03.026
BindingDB Entry DOI: 10.7270/Q2JQ13XJ |
More data for this
Ligand-Target Pair | |
Mu-type opioid receptor
(MOUSE) | BDBM50102826

(CHEMBL3339374)Show SMILES Oc1ccc2CC3N(CC4CC4)CCC45C(Oc1c24)[C@@]1(O)CC[C@@]35N[C@@H]1CC(=O)NCc1ccccc1 |r,TLB:26:25:14.15:22.21,THB:3:4:23:7.12.13,17:18:23:7.12.13,22:23:4.18.5:7.12.13,24:23:4.18.5:7.12.13,8:7:4.18.5:23| Show InChI InChI=1S/C30H35N3O4/c34-21-9-8-20-14-23-30-11-10-29(36,22(32-30)15-24(35)31-16-18-4-2-1-3-5-18)27-28(30,25(20)26(21)37-27)12-13-33(23)17-19-6-7-19/h1-5,8-9,19,22-23,27,32,34,36H,6-7,10-17H2,(H,31,35)/t22-,23?,27?,28?,29-,30-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.0520 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Kitasato University
Curated by ChEMBL
| Assay Description
Displacement of [3H]DAMGO from mu opioid receptor in mouse brain membranes without cerebellum |
Bioorg Med Chem Lett 24: 4980-3 (2014)
Article DOI: 10.1016/j.bmcl.2014.09.029
BindingDB Entry DOI: 10.7270/Q2154JTJ |
More data for this
Ligand-Target Pair | |
Mu-type opioid receptor
(MOUSE) | BDBM50491892

(CHEMBL3216338)Show SMILES Cl.Cl.[H][C@@]12Oc3c4c(C[C@@]5([H])N(CC6CC6)CC[C@@]14[C@@]51CC[C@@]2(O)[C@@H](N1)C(=O)Nc1ccccc1)ccc3O |r,THB:26:24:3.18:20.21,12:11:19:6.8.7| Show InChI InChI=1S/C28H31N3O4.2ClH/c32-19-9-8-17-14-20-28-11-10-27(34,23(30-28)24(33)29-18-4-2-1-3-5-18)25-26(28,21(17)22(19)35-25)12-13-31(20)15-16-6-7-16;;/h1-5,8-9,16,20,23,25,30,32,34H,6-7,10-15H2,(H,29,33);2*1H/t20-,23+,25-,26+,27-,28-;;/m1../s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.0750 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Kitasato University
Curated by ChEMBL
| Assay Description
Displacement of [3H]DAMGO from mu opioid receptor in mouse whole brain devoid of cerebellum |
Bioorg Med Chem 21: 3032-50 (2013)
Article DOI: 10.1016/j.bmc.2013.03.026
BindingDB Entry DOI: 10.7270/Q2JQ13XJ |
More data for this
Ligand-Target Pair | |
Endothelin receptor type B
(Sus scrofa) | BDBM50287890
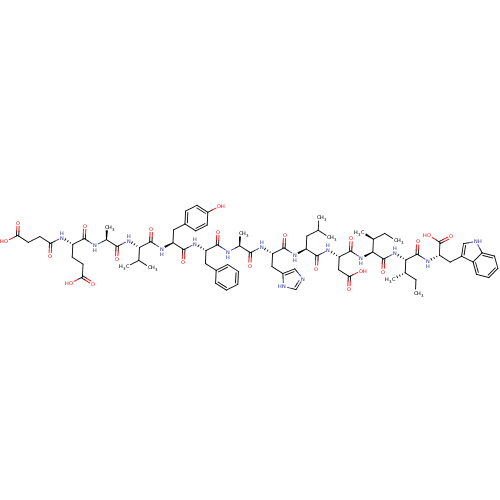
(CHEMBL427778 | Suc-Glu-Ala-Val-Tyr-Phe-Ala-His-Leu...)Show SMILES CC[C@H](C)[C@H](NC(=O)[C@H](CC(O)=O)NC(=O)[C@H](CC(C)C)NC(=O)[C@H](Cc1cnc[nH]1)NC(=O)[C@H](C)NC(=O)[C@H](Cc1ccccc1)NC(=O)[C@H](Cc1ccc(O)cc1)NC(=O)[C@@H](NC(=O)[C@H](C)NC(=O)[C@H](CCC(O)=O)NC(=O)CCC(O)=O)C(C)C)C(=O)N[C@@H]([C@@H](C)CC)C(=O)N[C@@H](Cc1c[nH]c2ccccc12)C(O)=O Show InChI InChI=1S/C77H105N15O21/c1-11-41(7)64(75(110)89-58(77(112)113)33-47-36-79-51-21-17-16-20-50(47)51)92-76(111)65(42(8)12-2)91-73(108)57(35-62(99)100)87-70(105)53(30-39(3)4)85-72(107)56(34-48-37-78-38-80-48)84-66(101)43(9)82-69(104)54(31-45-18-14-13-15-19-45)86-71(106)55(32-46-22-24-49(93)25-23-46)88-74(109)63(40(5)6)90-67(102)44(10)81-68(103)52(26-28-60(95)96)83-59(94)27-29-61(97)98/h13-25,36-44,52-58,63-65,79,93H,11-12,26-35H2,1-10H3,(H,78,80)(H,81,103)(H,82,104)(H,83,94)(H,84,101)(H,85,107)(H,86,106)(H,87,105)(H,88,109)(H,89,110)(H,90,102)(H,91,108)(H,92,111)(H,95,96)(H,97,98)(H,99,100)(H,112,113)/t41-,42-,43-,44-,52-,53-,54-,55-,56-,57-,58-,63-,64-,65-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| 0.0770 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Ability to inhibit [125I]ET-3 binding to the Endothelin B receptor from porcine lung membranes was evaluated |
Bioorg Med Chem Lett 6: 2323-2328 (1996)
Article DOI: 10.1016/0960-894X(96)00421-0
BindingDB Entry DOI: 10.7270/Q21J9B8K |
More data for this
Ligand-Target Pair | |
Mu-type opioid receptor
(MOUSE) | BDBM50491883

(CHEMBL3216579)Show SMILES Cl.Cl.[H][C@@]12Oc3c4c(C[C@@]5([H])N(CC6CC6)CC[C@]14[C@@]51CC[C@@]2(O)[C@H](CC(=O)Nc2ccccc2)N1)ccc3O |r,THB:25:24:3.18:20.21,12:11:19:6.8.7| Show InChI InChI=1S/C29H33N3O4.2ClH/c33-20-9-8-18-14-22-29-11-10-28(35,21(31-29)15-23(34)30-19-4-2-1-3-5-19)26-27(29,24(18)25(20)36-26)12-13-32(22)16-17-6-7-17;;/h1-5,8-9,17,21-22,26,31,33,35H,6-7,10-16H2,(H,30,34);2*1H/t21-,22+,26+,27+,28+,29+;;/m0../s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.0810 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Kitasato University
Curated by ChEMBL
| Assay Description
Displacement of [3H]DAMGO from mu opioid receptor in mouse whole brain devoid of cerebellum |
Bioorg Med Chem 21: 3032-50 (2013)
Article DOI: 10.1016/j.bmc.2013.03.026
BindingDB Entry DOI: 10.7270/Q2JQ13XJ |
More data for this
Ligand-Target Pair | |
Mu-type opioid receptor
(MOUSE) | BDBM50380906
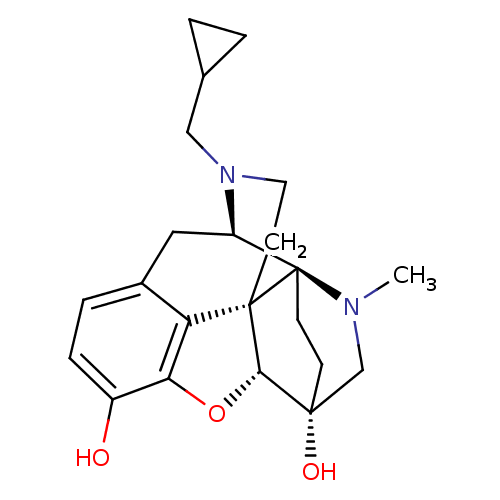
(CHEMBL2016675)Show SMILES CN1C[C@]2(O)CC[C@]11[C@H]3Cc4ccc(O)c5O[C@@H]2[C@]1(CCN3CC1CC1)c45 |r,TLB:0:1:6.5:18.17| Show InChI InChI=1S/C22H28N2O3/c1-23-12-20(26)6-7-22(23)16-10-14-4-5-15(25)18-17(14)21(22,19(20)27-18)8-9-24(16)11-13-2-3-13/h4-5,13,16,19,25-26H,2-3,6-12H2,1H3/t16-,19+,20-,21+,22-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.0970 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Kitasato University
Curated by ChEMBL
| Assay Description
Displacement of [3H]DAMGO from mouse brain mu opioid receptor |
Bioorg Med Chem Lett 22: 2689-92 (2012)
Article DOI: 10.1016/j.bmcl.2012.03.001
BindingDB Entry DOI: 10.7270/Q24X58T4 |
More data for this
Ligand-Target Pair | |
Adenosine receptor A2a
(Homo sapiens (Human)) | BDBM50161342
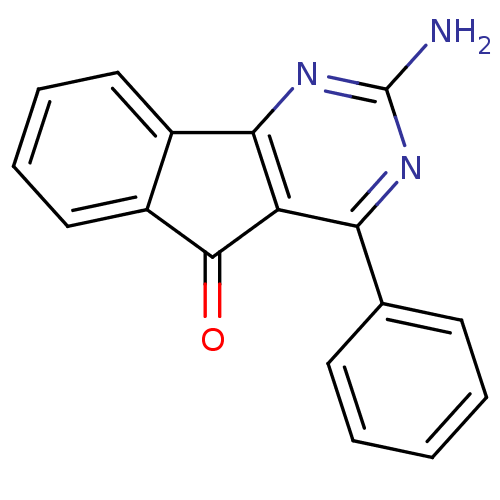
(2-Amino-4-phenyl-indeno[1,2-d]pyrimidin-5-one | 2-...)Show InChI InChI=1S/C17H11N3O/c18-17-19-14(10-6-2-1-3-7-10)13-15(20-17)11-8-4-5-9-12(11)16(13)21/h1-9H,(H2,18,19,20) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research and Development
Curated by ChEMBL
| Assay Description
Antagonist activity at adenosine A2A receptor |
Bioorg Med Chem Lett 20: 2864-7 (2010)
Article DOI: 10.1016/j.bmcl.2010.03.042
BindingDB Entry DOI: 10.7270/Q2GB246H |
More data for this
Ligand-Target Pair | |
Adenosine receptor A2a
(Homo sapiens (Human)) | BDBM50317007
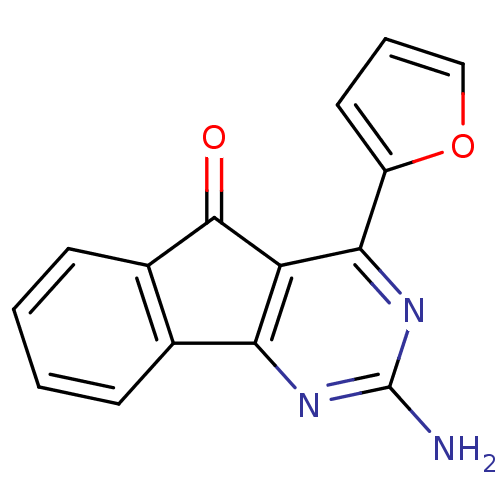
(2-amino-4-(furan-2-yl)-5H-indeno[1,2-d]pyrimidin-5...)Show InChI InChI=1S/C15H9N3O2/c16-15-17-12-8-4-1-2-5-9(8)14(19)11(12)13(18-15)10-6-3-7-20-10/h1-7H,(H2,16,17,18) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research and Development
Curated by ChEMBL
| Assay Description
Antagonist activity at adenosine A2A receptor |
Bioorg Med Chem Lett 20: 2864-7 (2010)
Article DOI: 10.1016/j.bmcl.2010.03.042
BindingDB Entry DOI: 10.7270/Q2GB246H |
More data for this
Ligand-Target Pair | |
Delta-type opioid receptor
(MOUSE) | BDBM50253136
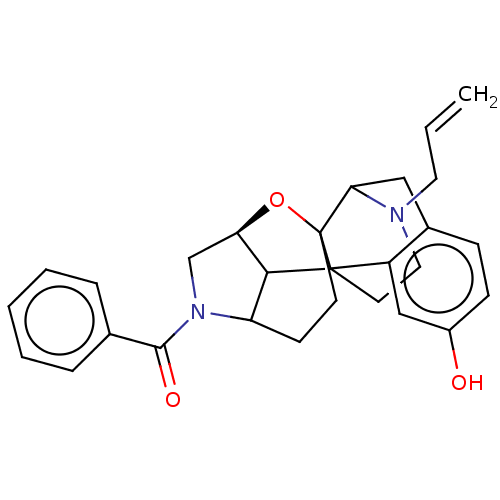
(CHEMBL4075409)Show SMILES [H][C@]12CN(C3CCC4(O1)C1Cc5ccc(O)cc5C4(CCN1CC=C)C23)C(=O)c1ccccc1 |r,TLB:3:4:18:8.1,2:1:18:5.6.4,6:7:21.20.19:10.17.11,17:18:8.1:5.6.4,16:17:21.20.19:7,THB:19:18:8.1:5.6.4| Show InChI InChI=1S/C28H30N2O3/c1-2-13-29-14-12-27-21-16-20(31)9-8-19(21)15-24(29)28(27)11-10-22-25(27)23(33-28)17-30(22)26(32)18-6-4-3-5-7-18/h2-9,16,22-25,31H,1,10-15,17H2/t22?,23-,24?,25?,27?,28?/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.109 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Laboratory of Medicinal Chemistry, School of Pharmacy, Kitasato University, 5-9-1, Shirokane, Minato-ku, Tokyo 108-8641, Japan; Discovery Research Laboratories, Nippon Chemiphar Co., Ltd., 1-22, Hiko
Curated by ChEMBL
| Assay Description
Displacement of [3H]DPDPE from delta opioid receptor in mouse whole brain without cerebellum membrane |
Bioorg Med Chem Lett 27: 2742-2745 (2017)
Article DOI: 10.1016/j.bmcl.2017.04.059
BindingDB Entry DOI: 10.7270/Q2H134GP |
More data for this
Ligand-Target Pair | |
Kappa-type opioid receptor
(Cavia porcellus (domestic guinea pig)) | BDBM50102828
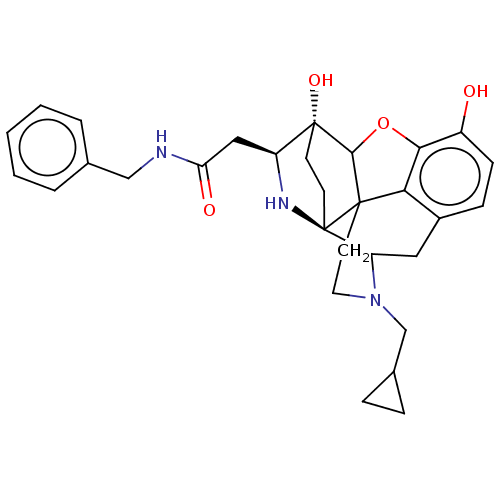
(CHEMBL3339372)Show SMILES Oc1ccc2CC3N(CC4CC4)CCC45C(Oc1c24)[C@@]1(O)CC[C@@]35N[C@H]1CC(=O)NCc1ccccc1 |r,TLB:26:25:14.15:22.21,THB:3:4:23:7.12.13,17:18:23:7.12.13,22:23:4.18.5:7.12.13,24:23:4.18.5:7.12.13,8:7:4.18.5:23| Show InChI InChI=1S/C30H35N3O4/c34-21-9-8-20-14-23-30-11-10-29(36,22(32-30)15-24(35)31-16-18-4-2-1-3-5-18)27-28(30,25(20)26(21)37-27)12-13-33(23)17-19-6-7-19/h1-5,8-9,19,22-23,27,32,34,36H,6-7,10-17H2,(H,31,35)/t22-,23?,27?,28?,29+,30+/m0/s1 | PDB
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.110 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Kitasato University
Curated by ChEMBL
| Assay Description
Displacement of [3H]U-69593 from kappa opioid receptor in guinea pig cerebellum |
Bioorg Med Chem Lett 24: 4980-3 (2014)
Article DOI: 10.1016/j.bmcl.2014.09.029
BindingDB Entry DOI: 10.7270/Q2154JTJ |
More data for this
Ligand-Target Pair | |
Kappa-type opioid receptor
(Cavia porcellus (domestic guinea pig)) | BDBM50491892

(CHEMBL3216338)Show SMILES Cl.Cl.[H][C@@]12Oc3c4c(C[C@@]5([H])N(CC6CC6)CC[C@@]14[C@@]51CC[C@@]2(O)[C@@H](N1)C(=O)Nc1ccccc1)ccc3O |r,THB:26:24:3.18:20.21,12:11:19:6.8.7| Show InChI InChI=1S/C28H31N3O4.2ClH/c32-19-9-8-17-14-20-28-11-10-27(34,23(30-28)24(33)29-18-4-2-1-3-5-18)25-26(28,21(17)22(19)35-25)12-13-31(20)15-16-6-7-16;;/h1-5,8-9,16,20,23,25,30,32,34H,6-7,10-15H2,(H,29,33);2*1H/t20-,23+,25-,26+,27-,28-;;/m1../s1 | PDB
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.110 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Kitasato University
Curated by ChEMBL
| Assay Description
Displacement of [3H]U-69,593 from kappa opioid receptor in guinea pig cerebellum |
Bioorg Med Chem 21: 3032-50 (2013)
Article DOI: 10.1016/j.bmc.2013.03.026
BindingDB Entry DOI: 10.7270/Q2JQ13XJ |
More data for this
Ligand-Target Pair | |
Kappa-type opioid receptor
(Cavia porcellus (domestic guinea pig)) | BDBM50491884

(CHEMBL3216801)Show SMILES Cl.Cl.[H][C@@]12Oc3c4c(C[C@@]5([H])N(CC6CC6)CC[C@]14[C@@]51CC[C@@]2(O)[C@@H](CC(=O)NCc2ccccc2)N1)ccc3O |r,THB:25:24:3.18:20.21,12:11:19:6.8.7| Show InChI InChI=1S/C30H35N3O4.2ClH/c34-21-9-8-20-14-23-30-11-10-29(36,22(32-30)15-24(35)31-16-18-4-2-1-3-5-18)27-28(30,25(20)26(21)37-27)12-13-33(23)17-19-6-7-19;;/h1-5,8-9,19,22-23,27,32,34,36H,6-7,10-17H2,(H,31,35);2*1H/t22-,23-,27-,28-,29-,30-;;/m1../s1 | PDB
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.110 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Kitasato University
Curated by ChEMBL
| Assay Description
Displacement of [3H]U-69,593 from kappa opioid receptor in guinea pig cerebellum |
Bioorg Med Chem 21: 3032-50 (2013)
Article DOI: 10.1016/j.bmc.2013.03.026
BindingDB Entry DOI: 10.7270/Q2JQ13XJ |
More data for this
Ligand-Target Pair | |
Mu-type opioid receptor
(MOUSE) | BDBM50491897

(CHEMBL2387739)Show SMILES Cl.[H][C@@]12Oc3c4c(C[C@@]5([H])N(CC6CC6)CC[C@]14[C@@]51CC[C@@]2(O)[C@H](CC(=O)Nc2ccccc2)N1C(C)=O)ccc3O |r,THB:34:18:5.7.6:16.10.15,35:34:2.17:19.20,4:5:18:16.10.15,24:23:2.17:19.20| Show InChI InChI=1S/C31H35N3O5.ClH/c1-18(35)34-23(16-25(37)32-21-5-3-2-4-6-21)30(38)11-12-31(34)24-15-20-9-10-22(36)27-26(20)29(31,28(30)39-27)13-14-33(24)17-19-7-8-19;/h2-6,9-10,19,23-24,28,36,38H,7-8,11-17H2,1H3,(H,32,37);1H/t23-,24+,28+,29+,30+,31+;/m0./s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.110 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Kitasato University
Curated by ChEMBL
| Assay Description
Displacement of [3H]DAMGO from mu opioid receptor in mouse whole brain devoid of cerebellum |
Bioorg Med Chem 21: 3032-50 (2013)
Article DOI: 10.1016/j.bmc.2013.03.026
BindingDB Entry DOI: 10.7270/Q2JQ13XJ |
More data for this
Ligand-Target Pair | |
Kappa-type opioid receptor
(Cavia porcellus (domestic guinea pig)) | BDBM50491883

(CHEMBL3216579)Show SMILES Cl.Cl.[H][C@@]12Oc3c4c(C[C@@]5([H])N(CC6CC6)CC[C@]14[C@@]51CC[C@@]2(O)[C@H](CC(=O)Nc2ccccc2)N1)ccc3O |r,THB:25:24:3.18:20.21,12:11:19:6.8.7| Show InChI InChI=1S/C29H33N3O4.2ClH/c33-20-9-8-18-14-22-29-11-10-28(35,21(31-29)15-23(34)30-19-4-2-1-3-5-19)26-27(29,24(18)25(20)36-26)12-13-32(22)16-17-6-7-17;;/h1-5,8-9,17,21-22,26,31,33,35H,6-7,10-16H2,(H,30,34);2*1H/t21-,22+,26+,27+,28+,29+;;/m0../s1 | PDB
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.110 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Kitasato University
Curated by ChEMBL
| Assay Description
Displacement of [3H]U-69,593 from kappa opioid receptor in guinea pig cerebellum |
Bioorg Med Chem 21: 3032-50 (2013)
Article DOI: 10.1016/j.bmc.2013.03.026
BindingDB Entry DOI: 10.7270/Q2JQ13XJ |
More data for this
Ligand-Target Pair | |
Kappa-type opioid receptor
(Homo sapiens (Human)) | BDBM50380911

(CHEMBL2016680)Show SMILES Oc1ccc2C[C@H]3N(CC4CC4)CC[C@@]45[C@@H](Oc1c24)[C@@]1(O)CC[C@@]35O[C@@H]1C(=O)Nc1ccccc1 |r,THB:26:25:22.21:14.15| Show InChI InChI=1S/C28H30N2O5/c31-19-9-8-17-14-20-28-11-10-27(33,23(35-28)24(32)29-18-4-2-1-3-5-18)25-26(28,21(17)22(19)34-25)12-13-30(20)15-16-6-7-16/h1-5,8-9,16,20,23,25,31,33H,6-7,10-15H2,(H,29,32)/t20-,23-,25-,26+,27-,28-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.110 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Kitasato University
Curated by ChEMBL
| Assay Description
Binding affinity to kappa opioid receptor |
Bioorg Med Chem Lett 22: 2689-92 (2012)
Article DOI: 10.1016/j.bmcl.2012.03.001
BindingDB Entry DOI: 10.7270/Q24X58T4 |
More data for this
Ligand-Target Pair | |
Kappa-type opioid receptor
(Cavia porcellus (domestic guinea pig)) | BDBM50102832
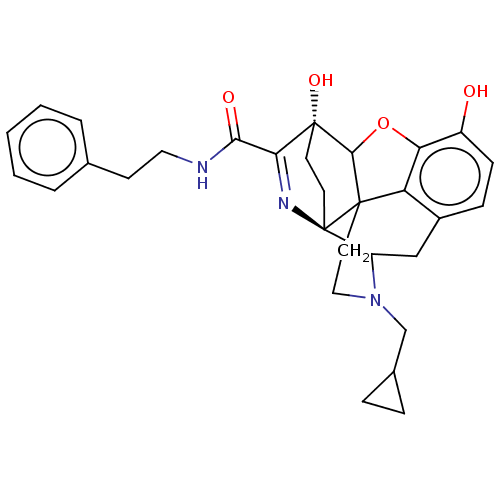
(CHEMBL3339379)Show SMILES Oc1ccc2CC3N(CC4CC4)CCC45C(Oc1c24)[C@@]1(O)CC[C@@]35N=C1C(=O)NCCc1ccccc1 |r,c:30,TLB:26:25:14.15:22.21,THB:3:4:23:7.12.13,8:7:4.18.5:23,17:18:23:7.12.13,24:23:4.18.5:7.12.13,22:23:4.18.5:7.12.13| Show InChI InChI=1S/C30H33N3O4/c34-21-9-8-20-16-22-30-12-11-29(36,25(32-30)26(35)31-14-10-18-4-2-1-3-5-18)27-28(30,23(20)24(21)37-27)13-15-33(22)17-19-6-7-19/h1-5,8-9,19,22,27,34,36H,6-7,10-17H2,(H,31,35)/t22?,27?,28?,29-,30-/m1/s1 | PDB
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.120 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Kitasato University
Curated by ChEMBL
| Assay Description
Displacement of [3H]U-69593 from kappa opioid receptor in guinea pig cerebellum |
Bioorg Med Chem Lett 24: 4980-3 (2014)
Article DOI: 10.1016/j.bmcl.2014.09.029
BindingDB Entry DOI: 10.7270/Q2154JTJ |
More data for this
Ligand-Target Pair | |
Kappa-type opioid receptor
(Cavia porcellus (domestic guinea pig)) | BDBM50380911

(CHEMBL2016680)Show SMILES Oc1ccc2C[C@H]3N(CC4CC4)CC[C@@]45[C@@H](Oc1c24)[C@@]1(O)CC[C@@]35O[C@@H]1C(=O)Nc1ccccc1 |r,THB:26:25:22.21:14.15| Show InChI InChI=1S/C28H30N2O5/c31-19-9-8-17-14-20-28-11-10-27(33,23(35-28)24(32)29-18-4-2-1-3-5-18)25-26(28,21(17)22(19)34-25)12-13-30(20)15-16-6-7-16/h1-5,8-9,16,20,23,25,31,33H,6-7,10-15H2,(H,29,32)/t20-,23-,25-,26+,27-,28-/m1/s1 | PDB
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.120 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Kitasato University
Curated by ChEMBL
| Assay Description
Displacement of [3H]U-69,593 from kappa opioid receptor in guinea pig cerebellum |
Bioorg Med Chem 21: 3032-50 (2013)
Article DOI: 10.1016/j.bmc.2013.03.026
BindingDB Entry DOI: 10.7270/Q2JQ13XJ |
More data for this
Ligand-Target Pair | |
Mu-type opioid receptor
(Homo sapiens (Human)) | BDBM50599576

(CHEMBL5200218)Show SMILES [H][C@@]12CC[C@@]3(O1)[C@@]1([H])Cc4ccc(O)cc4[C@@]3(CCN1CC1CC1)C[C@H]2NC(=O)CCc1ccccc1 |r,TLB:20:19:4:8.9.15| | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.126 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.bmc.2021.116552
BindingDB Entry DOI: 10.7270/Q2BZ6B34 |
More data for this
Ligand-Target Pair | |
Mu-type opioid receptor
(MOUSE) | BDBM50102828

(CHEMBL3339372)Show SMILES Oc1ccc2CC3N(CC4CC4)CCC45C(Oc1c24)[C@@]1(O)CC[C@@]35N[C@H]1CC(=O)NCc1ccccc1 |r,TLB:26:25:14.15:22.21,THB:3:4:23:7.12.13,17:18:23:7.12.13,22:23:4.18.5:7.12.13,24:23:4.18.5:7.12.13,8:7:4.18.5:23| Show InChI InChI=1S/C30H35N3O4/c34-21-9-8-20-14-23-30-11-10-29(36,22(32-30)15-24(35)31-16-18-4-2-1-3-5-18)27-28(30,25(20)26(21)37-27)12-13-33(23)17-19-6-7-19/h1-5,8-9,19,22-23,27,32,34,36H,6-7,10-17H2,(H,31,35)/t22-,23?,27?,28?,29+,30+/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.130 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Kitasato University
Curated by ChEMBL
| Assay Description
Displacement of [3H]DAMGO from mu opioid receptor in mouse brain membranes without cerebellum |
Bioorg Med Chem Lett 24: 4980-3 (2014)
Article DOI: 10.1016/j.bmcl.2014.09.029
BindingDB Entry DOI: 10.7270/Q2154JTJ |
More data for this
Ligand-Target Pair | |
Mu-type opioid receptor
(MOUSE) | BDBM50491885
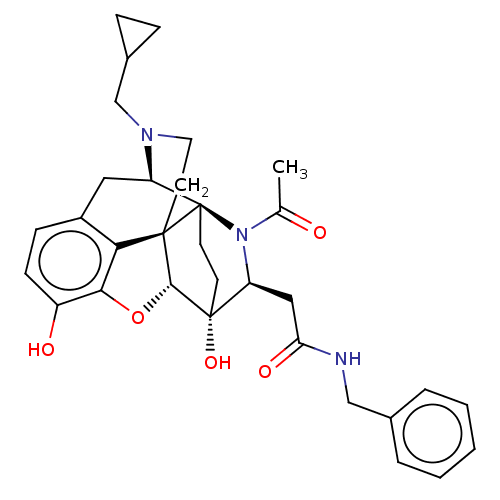
(CHEMBL2387740)Show SMILES Cl.[H][C@@]12Oc3c4c(C[C@@]5([H])N(CC6CC6)CC[C@]14[C@@]51CC[C@@]2(O)[C@H](CC(=O)NCc2ccccc2)N1C(C)=O)ccc3O |r,THB:35:18:5.7.6:16.10.15,4:5:18:16.10.15,24:23:2.17:19.20,36:35:2.17:19.20| Show InChI InChI=1S/C32H37N3O5.ClH/c1-19(36)35-24(16-26(38)33-17-20-5-3-2-4-6-20)31(39)11-12-32(35)25-15-22-9-10-23(37)28-27(22)30(32,29(31)40-28)13-14-34(25)18-21-7-8-21;/h2-6,9-10,21,24-25,29,37,39H,7-8,11-18H2,1H3,(H,33,38);1H/t24-,25+,29+,30+,31+,32+;/m0./s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.130 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Kitasato University
Curated by ChEMBL
| Assay Description
Displacement of [3H]DAMGO from mu opioid receptor in mouse whole brain devoid of cerebellum |
Bioorg Med Chem 21: 3032-50 (2013)
Article DOI: 10.1016/j.bmc.2013.03.026
BindingDB Entry DOI: 10.7270/Q2JQ13XJ |
More data for this
Ligand-Target Pair | |
Mu-type opioid receptor
(MOUSE) | BDBM50491884

(CHEMBL3216801)Show SMILES Cl.Cl.[H][C@@]12Oc3c4c(C[C@@]5([H])N(CC6CC6)CC[C@]14[C@@]51CC[C@@]2(O)[C@@H](CC(=O)NCc2ccccc2)N1)ccc3O |r,THB:25:24:3.18:20.21,12:11:19:6.8.7| Show InChI InChI=1S/C30H35N3O4.2ClH/c34-21-9-8-20-14-23-30-11-10-29(36,22(32-30)15-24(35)31-16-18-4-2-1-3-5-18)27-28(30,25(20)26(21)37-27)12-13-33(23)17-19-6-7-19;;/h1-5,8-9,19,22-23,27,32,34,36H,6-7,10-17H2,(H,31,35);2*1H/t22-,23-,27-,28-,29-,30-;;/m1../s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.130 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Kitasato University
Curated by ChEMBL
| Assay Description
Displacement of [3H]DAMGO from mu opioid receptor in mouse whole brain devoid of cerebellum |
Bioorg Med Chem 21: 3032-50 (2013)
Article DOI: 10.1016/j.bmc.2013.03.026
BindingDB Entry DOI: 10.7270/Q2JQ13XJ |
More data for this
Ligand-Target Pair | |
Kappa-type opioid receptor
(Homo sapiens (Human)) | BDBM50274482
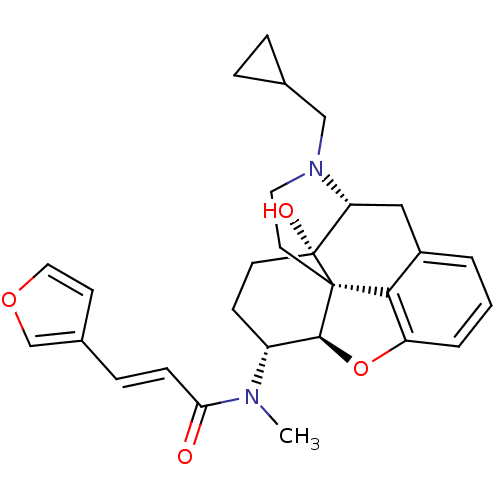
((2E)-N-[(5R,6R)-17-(Cyclopropylmethyl)-4,5-epoxy-1...)Show SMILES CN([C@@H]1CC[C@@]2(O)[C@H]3Cc4cccc5O[C@@H]1[C@]2(CCN3CC1CC1)c45)C(=O)\C=C\c1ccoc1 |r,TLB:4:5:9.24.8:17.19.18,THB:6:5:9.24.8:17.19.18| Show InChI InChI=1S/C28H32N2O4/c1-29(24(31)8-7-19-10-14-33-17-19)21-9-11-28(32)23-15-20-3-2-4-22-25(20)27(28,26(21)34-22)12-13-30(23)16-18-5-6-18/h2-4,7-8,10,14,17-18,21,23,26,32H,5-6,9,11-13,15-16H2,1H3/b8-7+/t21-,23-,26+,27+,28-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.130 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.bmc.2021.116552
BindingDB Entry DOI: 10.7270/Q2BZ6B34 |
More data for this
Ligand-Target Pair | |
Kappa-type opioid receptor
(Homo sapiens (Human)) | BDBM50599574
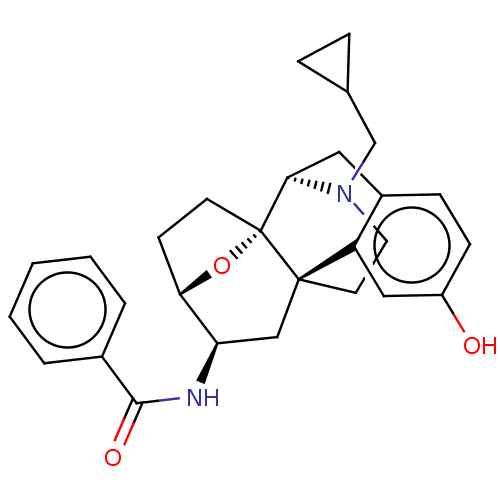
(CHEMBL5186730)Show SMILES [H][C@@]12CC[C@@]3(O1)[C@@]1([H])Cc4ccc(O)cc4[C@@]3(CCN1CC1CC1)C[C@H]2NC(=O)c1ccccc1 |r,TLB:20:19:4:8.9.15| | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.140 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.bmc.2021.116552
BindingDB Entry DOI: 10.7270/Q2BZ6B34 |
More data for this
Ligand-Target Pair | |
Mu-type opioid receptor
(MOUSE) | BDBM50102833

(CHEMBL3339378)Show SMILES Oc1ccc2CC3N(CC4CC4)CCC45C(Oc1c24)[C@@]1(O)CC[C@@]35N=C1C(=O)NCc1ccccc1 |r,c:30,TLB:26:25:14.15:22.21,THB:3:4:23:7.12.13,8:7:4.18.5:23,17:18:23:7.12.13,24:23:4.18.5:7.12.13,22:23:4.18.5:7.12.13| Show InChI InChI=1S/C29H31N3O4/c33-20-9-8-19-14-21-29-11-10-28(35,24(31-29)25(34)30-15-17-4-2-1-3-5-17)26-27(29,22(19)23(20)36-26)12-13-32(21)16-18-6-7-18/h1-5,8-9,18,21,26,33,35H,6-7,10-16H2,(H,30,34)/t21?,26?,27?,28-,29-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.140 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Kitasato University
Curated by ChEMBL
| Assay Description
Displacement of [3H]DAMGO from mu opioid receptor in mouse brain membranes without cerebellum |
Bioorg Med Chem Lett 24: 4980-3 (2014)
Article DOI: 10.1016/j.bmcl.2014.09.029
BindingDB Entry DOI: 10.7270/Q2154JTJ |
More data for this
Ligand-Target Pair | |
Mu-type opioid receptor
(MOUSE) | BDBM50102830
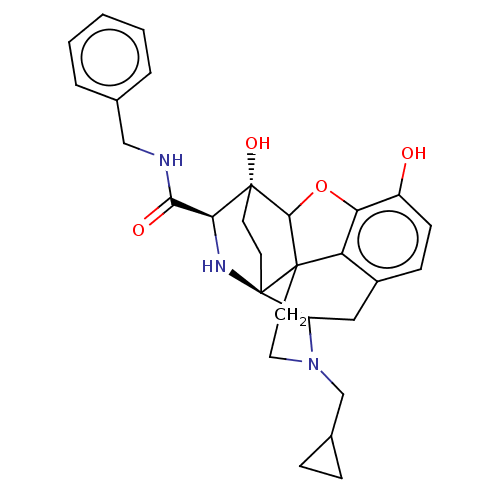
(CHEMBL3339381)Show SMILES Oc1ccc2CC3N(CC4CC4)CCC45C(Oc1c24)[C@@]1(O)CC[C@@]35N[C@H]1C(=O)NCc1ccccc1 |r,TLB:26:25:14.15:22.21,THB:3:4:23:7.12.13,8:7:4.18.5:23,17:18:23:7.12.13,24:23:4.18.5:7.12.13,22:23:4.18.5:7.12.13| Show InChI InChI=1S/C29H33N3O4/c33-20-9-8-19-14-21-29-11-10-28(35,24(31-29)25(34)30-15-17-4-2-1-3-5-17)26-27(29,22(19)23(20)36-26)12-13-32(21)16-18-6-7-18/h1-5,8-9,18,21,24,26,31,33,35H,6-7,10-16H2,(H,30,34)/t21?,24-,26?,27?,28+,29+/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.150 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Kitasato University
Curated by ChEMBL
| Assay Description
Displacement of [3H]DAMGO from mu opioid receptor in mouse brain membranes without cerebellum |
Bioorg Med Chem Lett 24: 4980-3 (2014)
Article DOI: 10.1016/j.bmcl.2014.09.029
BindingDB Entry DOI: 10.7270/Q2154JTJ |
More data for this
Ligand-Target Pair | |
Delta-type opioid receptor
(MOUSE) | BDBM50102826

(CHEMBL3339374)Show SMILES Oc1ccc2CC3N(CC4CC4)CCC45C(Oc1c24)[C@@]1(O)CC[C@@]35N[C@@H]1CC(=O)NCc1ccccc1 |r,TLB:26:25:14.15:22.21,THB:3:4:23:7.12.13,17:18:23:7.12.13,22:23:4.18.5:7.12.13,24:23:4.18.5:7.12.13,8:7:4.18.5:23| Show InChI InChI=1S/C30H35N3O4/c34-21-9-8-20-14-23-30-11-10-29(36,22(32-30)15-24(35)31-16-18-4-2-1-3-5-18)27-28(30,25(20)26(21)37-27)12-13-33(23)17-19-6-7-19/h1-5,8-9,19,22-23,27,32,34,36H,6-7,10-17H2,(H,31,35)/t22-,23?,27?,28?,29-,30-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.150 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Kitasato University
Curated by ChEMBL
| Assay Description
Displacement of [3H]DPDPE from delta opioid receptor in mouse brain membranes without cerebellum |
Bioorg Med Chem Lett 24: 4980-3 (2014)
Article DOI: 10.1016/j.bmcl.2014.09.029
BindingDB Entry DOI: 10.7270/Q2154JTJ |
More data for this
Ligand-Target Pair | |
Kappa-type opioid receptor
(Cavia porcellus (domestic guinea pig)) | BDBM50491880

(CHEMBL2387724)Show SMILES Cl.[H][C@@]12Oc3c4c(C[C@@]5([H])N(CC6CC6)CC[C@]14[C@@]51CC[C@@]2(O)[C@@H](CC(=O)NCc2ccccc2)N1C)ccc3O |r,THB:35:18:5.7.6:16.10.15,4:5:18:16.10.15,24:23:2.17:19.20,36:35:2.17:19.20| Show InChI InChI=1S/C31H37N3O4.ClH/c1-33-23(16-25(36)32-17-19-5-3-2-4-6-19)30(37)11-12-31(33)24-15-21-9-10-22(35)27-26(21)29(31,28(30)38-27)13-14-34(24)18-20-7-8-20;/h2-6,9-10,20,23-24,28,35,37H,7-8,11-18H2,1H3,(H,32,36);1H/t23-,24-,28-,29-,30-,31-;/m1./s1 | PDB
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.150 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Kitasato University
Curated by ChEMBL
| Assay Description
Displacement of [3H]U-69,593 from kappa opioid receptor in guinea pig cerebellum |
Bioorg Med Chem 21: 3032-50 (2013)
Article DOI: 10.1016/j.bmc.2013.03.026
BindingDB Entry DOI: 10.7270/Q2JQ13XJ |
More data for this
Ligand-Target Pair | |
Kappa-type opioid receptor
(Cavia porcellus (domestic guinea pig)) | BDBM50491890

(CHEMBL2387726)Show SMILES Cl.[H][C@@]12Oc3c4c(C[C@@]5([H])N(CC6CC6)CC[C@]14[C@@]51CC[C@@]2(O)[C@H](CC(=O)Nc2ccccc2)N1C)ccc3O |r,THB:34:18:5.7.6:16.10.15,4:5:18:16.10.15,35:34:2.17:19.20,24:23:2.17:19.20| Show InChI InChI=1S/C30H35N3O4.ClH/c1-32-22(16-24(35)31-20-5-3-2-4-6-20)29(36)11-12-30(32)23-15-19-9-10-21(34)26-25(19)28(30,27(29)37-26)13-14-33(23)17-18-7-8-18;/h2-6,9-10,18,22-23,27,34,36H,7-8,11-17H2,1H3,(H,31,35);1H/t22-,23+,27+,28+,29+,30+;/m0./s1 | PDB
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.150 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Kitasato University
Curated by ChEMBL
| Assay Description
Displacement of [3H]U-69,593 from kappa opioid receptor in guinea pig cerebellum |
Bioorg Med Chem 21: 3032-50 (2013)
Article DOI: 10.1016/j.bmc.2013.03.026
BindingDB Entry DOI: 10.7270/Q2JQ13XJ |
More data for this
Ligand-Target Pair | |
Delta-type opioid receptor
(MOUSE) | BDBM50491882

(CHEMBL3215908)Show SMILES Cl.Cl.[H][C@@]12Oc3c4c(C[C@@]5([H])N(CC6CC6)CC[C@]14[C@@]51CC[C@@]2(O)[C@H](CC(=O)NCc2ccccc2)N1)ccc3O |r,THB:25:24:3.18:20.21,12:11:19:6.8.7| Show InChI InChI=1S/C30H35N3O4.2ClH/c34-21-9-8-20-14-23-30-11-10-29(36,22(32-30)15-24(35)31-16-18-4-2-1-3-5-18)27-28(30,25(20)26(21)37-27)12-13-33(23)17-19-6-7-19;;/h1-5,8-9,19,22-23,27,32,34,36H,6-7,10-17H2,(H,31,35);2*1H/t22-,23+,27+,28+,29+,30+;;/m0../s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.150 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Kitasato University
Curated by ChEMBL
| Assay Description
Displacement of [3H]DPDPE from delta opioid receptor in mouse whole brain devoid of cerebellum |
Bioorg Med Chem 21: 3032-50 (2013)
Article DOI: 10.1016/j.bmc.2013.03.026
BindingDB Entry DOI: 10.7270/Q2JQ13XJ |
More data for this
Ligand-Target Pair | |
Delta-type opioid receptor
(Homo sapiens (Human)) | BDBM50253129
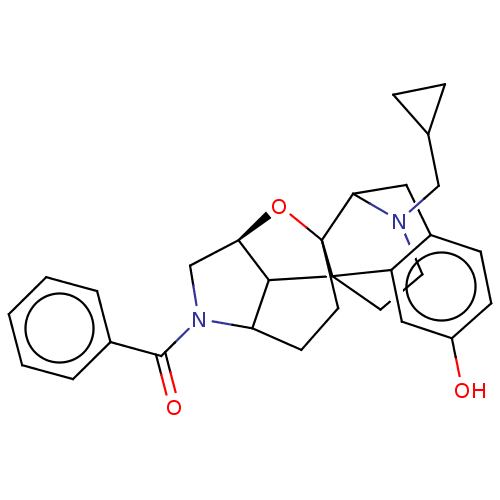
(CHEMBL4066752)Show SMILES [H][C@]12CN(C3CCC4(O1)C1Cc5ccc(O)cc5C4(CCN1CC1CC1)C23)C(=O)c1ccccc1 |r,TLB:3:4:18:8.1,2:1:18:5.6.4,6:7:21.20.19:10.17.11,17:18:8.1:5.6.4,22:21:7:10.17.11,16:17:21.20.19:7,THB:19:18:8.1:5.6.4| Show InChI InChI=1S/C29H32N2O3/c32-21-9-8-20-14-25-29-11-10-23-26(24(34-29)17-31(23)27(33)19-4-2-1-3-5-19)28(29,22(20)15-21)12-13-30(25)16-18-6-7-18/h1-5,8-9,15,18,23-26,32H,6-7,10-14,16-17H2/t23?,24-,25?,26?,28?,29?/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.154 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Laboratory of Medicinal Chemistry, School of Pharmacy, Kitasato University, 5-9-1, Shirokane, Minato-ku, Tokyo 108-8641, Japan; Discovery Research Laboratories, Nippon Chemiphar Co., Ltd., 1-22, Hiko
Curated by ChEMBL
| Assay Description
Displacement of [3H]DPDPE from human delta opioid receptor expressed in CHO cells |
Bioorg Med Chem Lett 27: 2742-2745 (2017)
Article DOI: 10.1016/j.bmcl.2017.04.059
BindingDB Entry DOI: 10.7270/Q2H134GP |
More data for this
Ligand-Target Pair | |
Delta-type opioid receptor
(MOUSE) | BDBM50491897

(CHEMBL2387739)Show SMILES Cl.[H][C@@]12Oc3c4c(C[C@@]5([H])N(CC6CC6)CC[C@]14[C@@]51CC[C@@]2(O)[C@H](CC(=O)Nc2ccccc2)N1C(C)=O)ccc3O |r,THB:34:18:5.7.6:16.10.15,35:34:2.17:19.20,4:5:18:16.10.15,24:23:2.17:19.20| Show InChI InChI=1S/C31H35N3O5.ClH/c1-18(35)34-23(16-25(37)32-21-5-3-2-4-6-21)30(38)11-12-31(34)24-15-20-9-10-22(36)27-26(20)29(31,28(30)39-27)13-14-33(24)17-19-7-8-19;/h2-6,9-10,19,23-24,28,36,38H,7-8,11-17H2,1H3,(H,32,37);1H/t23-,24+,28+,29+,30+,31+;/m0./s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.160 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Kitasato University
Curated by ChEMBL
| Assay Description
Displacement of [3H]DPDPE from delta opioid receptor in mouse whole brain devoid of cerebellum |
Bioorg Med Chem 21: 3032-50 (2013)
Article DOI: 10.1016/j.bmc.2013.03.026
BindingDB Entry DOI: 10.7270/Q2JQ13XJ |
More data for this
Ligand-Target Pair | |
Delta-type opioid receptor
(MOUSE) | BDBM50491879

(CHEMBL2387727)Show SMILES Cl.[H][C@@]12Oc3c4c(C[C@@]5([H])N(CC6CC6)CC[C@]14[C@@]51CC[C@@]2(O)[C@H](CC(=O)NCc2ccccc2)N1C)ccc3O |r,THB:35:18:5.7.6:16.10.15,4:5:18:16.10.15,24:23:2.17:19.20,36:35:2.17:19.20| Show InChI InChI=1S/C31H37N3O4.ClH/c1-33-23(16-25(36)32-17-19-5-3-2-4-6-19)30(37)11-12-31(33)24-15-21-9-10-22(35)27-26(21)29(31,28(30)38-27)13-14-34(24)18-20-7-8-20;/h2-6,9-10,20,23-24,28,35,37H,7-8,11-18H2,1H3,(H,32,36);1H/t23-,24+,28+,29+,30+,31+;/m0./s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.160 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Kitasato University
Curated by ChEMBL
| Assay Description
Displacement of [3H]DPDPE from delta opioid receptor in mouse whole brain devoid of cerebellum |
Bioorg Med Chem 21: 3032-50 (2013)
Article DOI: 10.1016/j.bmc.2013.03.026
BindingDB Entry DOI: 10.7270/Q2JQ13XJ |
More data for this
Ligand-Target Pair | |
Mu-type opioid receptor
(Homo sapiens (Human)) | BDBM50599574

(CHEMBL5186730)Show SMILES [H][C@@]12CC[C@@]3(O1)[C@@]1([H])Cc4ccc(O)cc4[C@@]3(CCN1CC1CC1)C[C@H]2NC(=O)c1ccccc1 |r,TLB:20:19:4:8.9.15| | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.165 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.bmc.2021.116552
BindingDB Entry DOI: 10.7270/Q2BZ6B34 |
More data for this
Ligand-Target Pair | |
Kappa-type opioid receptor
(Cavia porcellus (domestic guinea pig)) | BDBM50325534
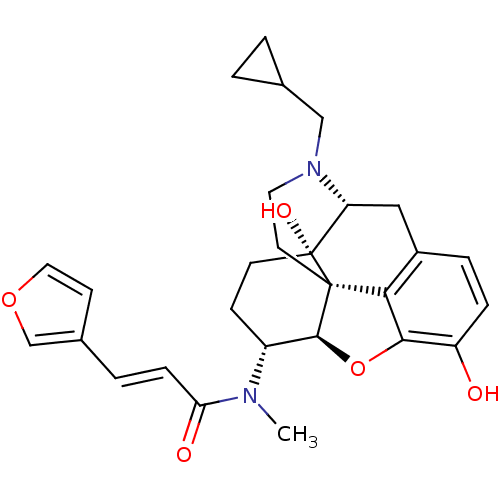
(CHEMBL267495 | nalfurafine)Show SMILES CN([C@@H]1CC[C@@]2(O)[C@H]3Cc4ccc(O)c5O[C@@H]1[C@]2(CCN3CC1CC1)c45)C(=O)\C=C\c1ccoc1 Show InChI InChI=1S/C28H32N2O5/c1-29(23(32)7-4-18-9-13-34-16-18)20-8-10-28(33)22-14-19-5-6-21(31)25-24(19)27(28,26(20)35-25)11-12-30(22)15-17-2-3-17/h4-7,9,13,16-17,20,22,26,31,33H,2-3,8,10-12,14-15H2,1H3/b7-4+/t20-,22-,26+,27+,28-/m1/s1 | PDB
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| 0.180 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Kitasato University
Curated by ChEMBL
| Assay Description
Displacement of [3H]U-69593 from kappa opioid receptor in guinea pig cerebellum |
Bioorg Med Chem Lett 24: 4980-3 (2014)
Article DOI: 10.1016/j.bmcl.2014.09.029
BindingDB Entry DOI: 10.7270/Q2154JTJ |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Mu-type opioid receptor
(MOUSE) | BDBM50491879

(CHEMBL2387727)Show SMILES Cl.[H][C@@]12Oc3c4c(C[C@@]5([H])N(CC6CC6)CC[C@]14[C@@]51CC[C@@]2(O)[C@H](CC(=O)NCc2ccccc2)N1C)ccc3O |r,THB:35:18:5.7.6:16.10.15,4:5:18:16.10.15,24:23:2.17:19.20,36:35:2.17:19.20| Show InChI InChI=1S/C31H37N3O4.ClH/c1-33-23(16-25(36)32-17-19-5-3-2-4-6-19)30(37)11-12-31(33)24-15-21-9-10-22(35)27-26(21)29(31,28(30)38-27)13-14-34(24)18-20-7-8-20;/h2-6,9-10,20,23-24,28,35,37H,7-8,11-18H2,1H3,(H,32,36);1H/t23-,24+,28+,29+,30+,31+;/m0./s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.180 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Kitasato University
Curated by ChEMBL
| Assay Description
Displacement of [3H]DAMGO from mu opioid receptor in mouse whole brain devoid of cerebellum |
Bioorg Med Chem 21: 3032-50 (2013)
Article DOI: 10.1016/j.bmc.2013.03.026
BindingDB Entry DOI: 10.7270/Q2JQ13XJ |
More data for this
Ligand-Target Pair | |
Kappa-type opioid receptor
(Cavia porcellus (domestic guinea pig)) | BDBM50102830

(CHEMBL3339381)Show SMILES Oc1ccc2CC3N(CC4CC4)CCC45C(Oc1c24)[C@@]1(O)CC[C@@]35N[C@H]1C(=O)NCc1ccccc1 |r,TLB:26:25:14.15:22.21,THB:3:4:23:7.12.13,8:7:4.18.5:23,17:18:23:7.12.13,24:23:4.18.5:7.12.13,22:23:4.18.5:7.12.13| Show InChI InChI=1S/C29H33N3O4/c33-20-9-8-19-14-21-29-11-10-28(35,24(31-29)25(34)30-15-17-4-2-1-3-5-17)26-27(29,22(19)23(20)36-26)12-13-32(21)16-18-6-7-18/h1-5,8-9,18,21,24,26,31,33,35H,6-7,10-16H2,(H,30,34)/t21?,24-,26?,27?,28+,29+/m0/s1 | PDB
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.180 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Kitasato University
Curated by ChEMBL
| Assay Description
Displacement of [3H]U-69593 from kappa opioid receptor in guinea pig cerebellum |
Bioorg Med Chem Lett 24: 4980-3 (2014)
Article DOI: 10.1016/j.bmcl.2014.09.029
BindingDB Entry DOI: 10.7270/Q2154JTJ |
More data for this
Ligand-Target Pair | |
Protease
(Human immunodeficiency virus 1 (HIV-1)) | BDBM50480931
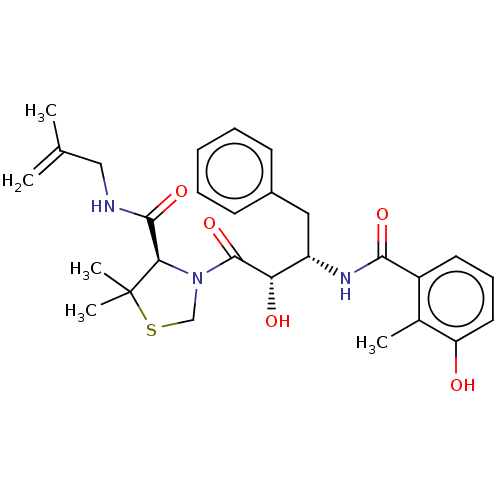
(CHEMBL575512 | KNI-1614)Show SMILES CC(=C)CNC(=O)[C@H]1N(CSC1(C)C)C(=O)[C@@H](O)[C@H](Cc1ccccc1)NC(=O)c1cccc(O)c1C |r| Show InChI InChI=1S/C28H35N3O5S/c1-17(2)15-29-26(35)24-28(4,5)37-16-31(24)27(36)23(33)21(14-19-10-7-6-8-11-19)30-25(34)20-12-9-13-22(32)18(20)3/h6-13,21,23-24,32-33H,1,14-16H2,2-5H3,(H,29,35)(H,30,34)/t21-,23-,24+/m0/s1 | PDB
UniProtKB/TrEMBL
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.190 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Kyoto Pharmaceutical University
Curated by ChEMBL
| Assay Description
Inhibition of HIV1 recombinant protease after 15 mins by fluorescence assay |
J Med Chem 52: 7604-17 (2009)
Article DOI: 10.1021/jm9005115
BindingDB Entry DOI: 10.7270/Q2FR00F2 |
More data for this
Ligand-Target Pair | |
Kappa-type opioid receptor
(Cavia porcellus (domestic guinea pig)) | BDBM50380909

(CHEMBL2016678)Show SMILES Oc1ccc2C[C@H]3N(CC4CC4)CC[C@@]45[C@@H](Oc1c24)[C@@]1(O)CC[C@@]35N(CC2CC2)C1 |r,THB:25:24:22.21:14.15| Show InChI InChI=1S/C25H32N2O3/c28-18-6-5-17-11-19-25-8-7-23(29,14-27(25)13-16-3-4-16)22-24(25,20(17)21(18)30-22)9-10-26(19)12-15-1-2-15/h5-6,15-16,19,22,28-29H,1-4,7-14H2/t19-,22+,23-,24+,25-/m1/s1 | PDB
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.190 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Kitasato University
Curated by ChEMBL
| Assay Description
Displacement of [3H]DPDPE from kappa opioid receptor in guinea pig cerebellum membrane |
Bioorg Med Chem Lett 22: 2689-92 (2012)
Article DOI: 10.1016/j.bmcl.2012.03.001
BindingDB Entry DOI: 10.7270/Q24X58T4 |
More data for this
Ligand-Target Pair | |
Mu-type opioid receptor
(MOUSE) | BDBM50491891

(CHEMBL3217022)Show SMILES Cl.Cl.[H][C@@]12Oc3c4c(C[C@@]5([H])N(CC6CC6)CC[C@@]14[C@@]51CC[C@@]2(O)[C@H](N1C)C(=O)Nc1ccccc1)ccc3O |r,TLB:26:25:3.18:20.21,THB:27:24:3.18:20.21,12:11:19:6.8.7| Show InChI InChI=1S/C29H33N3O4.2ClH/c1-31-24(25(34)30-19-5-3-2-4-6-19)28(35)11-12-29(31)21-15-18-9-10-20(33)23-22(18)27(29,26(28)36-23)13-14-32(21)16-17-7-8-17;;/h2-6,9-10,17,21,24,26,33,35H,7-8,11-16H2,1H3,(H,30,34);2*1H/t21-,24-,26-,27+,28-,29-;;/m1../s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.190 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Kitasato University
Curated by ChEMBL
| Assay Description
Displacement of [3H]DAMGO from mu opioid receptor in mouse whole brain devoid of cerebellum |
Bioorg Med Chem 21: 3032-50 (2013)
Article DOI: 10.1016/j.bmc.2013.03.026
BindingDB Entry DOI: 10.7270/Q2JQ13XJ |
More data for this
Ligand-Target Pair | |
Kappa-type opioid receptor
(Homo sapiens (Human)) | BDBM50599575

(CHEMBL5178511)Show SMILES [H][C@@]12CC[C@@]3(O1)[C@@]1([H])Cc4ccc(O)cc4[C@@]3(CCN1CC1CC1)C[C@H]2NC(=O)Cc1ccccc1 |r,TLB:20:19:4:8.9.15| | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.193 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.bmc.2021.116552
BindingDB Entry DOI: 10.7270/Q2BZ6B34 |
More data for this
Ligand-Target Pair | |
Kappa-type opioid receptor
(Homo sapiens (Human)) | BDBM50599576

(CHEMBL5200218)Show SMILES [H][C@@]12CC[C@@]3(O1)[C@@]1([H])Cc4ccc(O)cc4[C@@]3(CCN1CC1CC1)C[C@H]2NC(=O)CCc1ccccc1 |r,TLB:20:19:4:8.9.15| | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.197 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.bmc.2021.116552
BindingDB Entry DOI: 10.7270/Q2BZ6B34 |
More data for this
Ligand-Target Pair | |
Adenosine receptor A2a
(Homo sapiens (Human)) | BDBM50317008
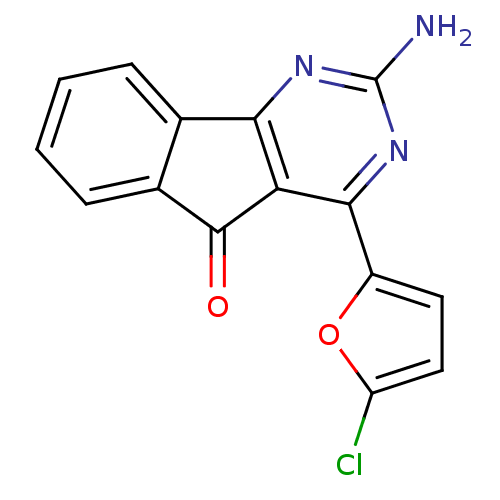
(2-amino-4-(5-chlorofuran-2-yl)-5H-indeno[1,2-d]pyr...)Show InChI InChI=1S/C15H8ClN3O2/c16-10-6-5-9(21-10)13-11-12(18-15(17)19-13)7-3-1-2-4-8(7)14(11)20/h1-6H,(H2,17,18,19) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research and Development
Curated by ChEMBL
| Assay Description
Antagonist activity at adenosine A2A receptor |
Bioorg Med Chem Lett 20: 2864-7 (2010)
Article DOI: 10.1016/j.bmcl.2010.03.042
BindingDB Entry DOI: 10.7270/Q2GB246H |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data