 Found 1346 hits with Last Name = 'tanaka' and Initial = 'a'
Found 1346 hits with Last Name = 'tanaka' and Initial = 'a' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Vasopressin V1b receptor
(RAT) | BDBM35667
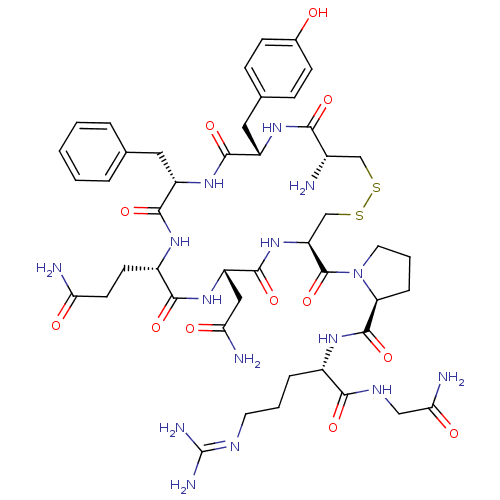
(AVP | CHEMBL373742 | US10131692, 44 (AVP) | [3H]Ar...)Show SMILES [#7]-[#6@H]-1-[#6]-[#16]-[#16]-[#6]-[#6@H](-[#7]-[#6](=O)-[#6@H](-[#6]-[#6](-[#7])=O)-[#7]-[#6](=O)-[#6@H](-[#6]-[#6]-[#6](-[#7])=O)-[#7]-[#6](=O)-[#6@H](-[#6]-c2ccccc2)-[#7]-[#6](=O)-[#6@H](-[#6]-c2ccc(-[#8])cc2)-[#7]-[#6]-1=O)-[#6](=O)-[#7]-1-[#6]-[#6]-[#6]-[#6@H]-1-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6]-[#6]\[#7]=[#6](\[#7])-[#7])-[#6](=O)-[#7]-[#6]-[#6](-[#7])=O Show InChI InChI=1S/C46H65N15O12S2/c47-27-22-74-75-23-33(45(73)61-17-5-9-34(61)44(72)56-28(8-4-16-53-46(51)52)39(67)54-21-37(50)65)60-43(71)32(20-36(49)64)59-40(68)29(14-15-35(48)63)55-41(69)31(18-24-6-2-1-3-7-24)58-42(70)30(57-38(27)66)19-25-10-12-26(62)13-11-25/h1-3,6-7,10-13,27-34,62H,4-5,8-9,14-23,47H2,(H2,48,63)(H2,49,64)(H2,50,65)(H,54,67)(H,55,69)(H,56,72)(H,57,66)(H,58,70)(H,59,68)(H,60,71)(H4,51,52,53)/t27-,28-,29-,30-,31-,32-,33-,34-/m0/s1 | UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MMDB
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 0.230 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Yamanouchi Pharmaceutical Co., Ltd.
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 282: 301-8 (1997)
BindingDB Entry DOI: 10.7270/Q25T3J1N |
More data for this
Ligand-Target Pair | |
Vasopressin V1a receptor
(RAT) | BDBM85095
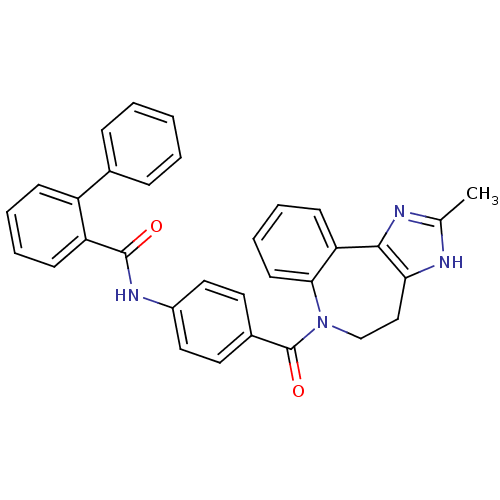
(CAS_151171 | CONIVAPTAN | NSC_151171 | YM087)Show SMILES Cc1nc-2c(CCN(C(=O)c3ccc(NC(=O)c4ccccc4-c4ccccc4)cc3)c3ccccc-23)[nH]1 Show InChI InChI=1S/C32H26N4O2/c1-21-33-28-19-20-36(29-14-8-7-13-27(29)30(28)34-21)32(38)23-15-17-24(18-16-23)35-31(37)26-12-6-5-11-25(26)22-9-3-2-4-10-22/h2-18H,19-20H2,1H3,(H,33,34)(H,35,37) | UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
DrugBank
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 0.240 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Yamanouchi Pharmaceutical Co., Ltd.
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 282: 301-8 (1997)
BindingDB Entry DOI: 10.7270/Q25T3J1N |
More data for this
Ligand-Target Pair | |
Prostaglandin E2 receptor EP4 subtype
(Homo sapiens (Human)) | BDBM50165949
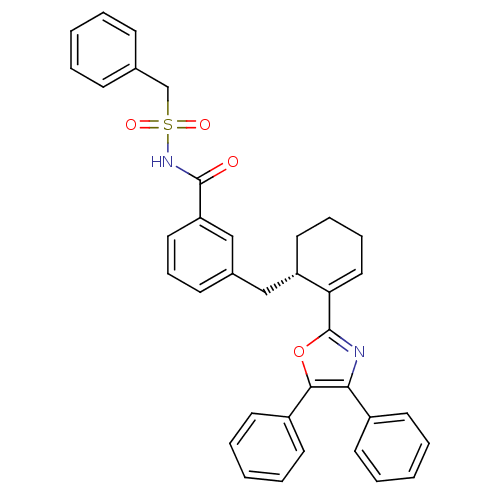
(C-Phenyl-N-{3-[2-((S)-5-phenyl-4-phenyl-oxazol-2-y...)Show SMILES O=C(NS(=O)(=O)Cc1ccccc1)c1cccc(C[C@@H]2CCCC=C2c2nc(c(o2)-c2ccccc2)-c2ccccc2)c1 |c:24| Show InChI InChI=1S/C36H32N2O4S/c39-35(38-43(40,41)25-26-13-4-1-5-14-26)31-21-12-15-27(24-31)23-30-20-10-11-22-32(30)36-37-33(28-16-6-2-7-17-28)34(42-36)29-18-8-3-9-19-29/h1-9,12-19,21-22,24,30H,10-11,20,23,25H2,(H,38,39)/t30-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Fujisawa Pharmaceutical Co., Ltd.
Curated by ChEMBL
| Assay Description
Inhibition of [3H]-PGE-2 binding to human prostanoid EP4 receptor |
J Med Chem 48: 3103-6 (2005)
Article DOI: 10.1021/jm050085k
BindingDB Entry DOI: 10.7270/Q2XD116M |
More data for this
Ligand-Target Pair | |
Vasopressin V1a receptor
(RAT) | BDBM85096
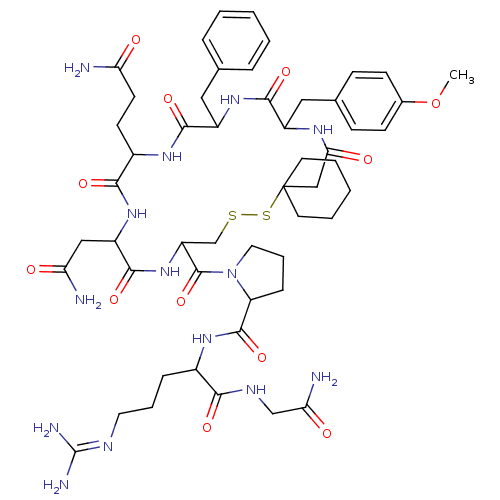
(CAS_105077 | NSC_105077 | d(CH2)5Tyr(Me)AVP)Show SMILES [#6]-[#8]-c1ccc(-[#6]-[#6]-2-[#7]-[#6](=O)-[#6]C3([#6]-[#6]-[#6]-[#6]-[#6]3)[#16]-[#16]-[#6]-[#6](-[#7]-[#6](=O)-[#6](-[#6]-[#6](-[#7])=O)-[#7]-[#6](=O)-[#6](-[#6]-[#6]-[#6](-[#7])=O)-[#7]-[#6](=O)-[#6](-[#6]-c3ccccc3)-[#7]-[#6]-2=O)-[#6](=O)-[#7]-2-[#6]-[#6]-[#6]-[#6]-2-[#6](=O)-[#7]-[#6](-[#6]-[#6]-[#6]\[#7]=[#6](\[#7])-[#7])-[#6](=O)-[#7]-[#6]-[#6](-[#7])=O)cc1 Show InChI InChI=1S/C52H74N14O12S2/c1-78-32-16-14-31(15-17-32)25-35-46(73)63-36(24-30-10-4-2-5-11-30)47(74)61-34(18-19-40(53)67)45(72)64-37(26-41(54)68)48(75)65-38(29-79-80-52(27-43(70)60-35)20-6-3-7-21-52)50(77)66-23-9-13-39(66)49(76)62-33(12-8-22-58-51(56)57)44(71)59-28-42(55)69/h2,4-5,10-11,14-17,33-39H,3,6-9,12-13,18-29H2,1H3,(H2,53,67)(H2,54,68)(H2,55,69)(H,59,71)(H,60,70)(H,61,74)(H,62,76)(H,63,73)(H,64,72)(H,65,75)(H4,56,57,58) | UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
UniChem
Similars
| PubMed
| 0.330 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Yamanouchi Pharmaceutical Co., Ltd.
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 282: 301-8 (1997)
BindingDB Entry DOI: 10.7270/Q25T3J1N |
More data for this
Ligand-Target Pair | |
Vasopressin V2 receptor
(Rattus norvegicus (Rat)) | BDBM85095

(CAS_151171 | CONIVAPTAN | NSC_151171 | YM087)Show SMILES Cc1nc-2c(CCN(C(=O)c3ccc(NC(=O)c4ccccc4-c4ccccc4)cc3)c3ccccc-23)[nH]1 Show InChI InChI=1S/C32H26N4O2/c1-21-33-28-19-20-36(29-14-8-7-13-27(29)30(28)34-21)32(38)23-15-17-24(18-16-23)35-31(37)26-12-6-5-11-25(26)22-9-3-2-4-10-22/h2-18H,19-20H2,1H3,(H,33,34)(H,35,37) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
DrugBank
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 0.380 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Yamanouchi Pharmaceutical Co., Ltd.
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 282: 301-8 (1997)
BindingDB Entry DOI: 10.7270/Q25T3J1N |
More data for this
Ligand-Target Pair | |
Vasopressin V2 receptor
(Rattus norvegicus (Rat)) | BDBM35667

(AVP | CHEMBL373742 | US10131692, 44 (AVP) | [3H]Ar...)Show SMILES [#7]-[#6@H]-1-[#6]-[#16]-[#16]-[#6]-[#6@H](-[#7]-[#6](=O)-[#6@H](-[#6]-[#6](-[#7])=O)-[#7]-[#6](=O)-[#6@H](-[#6]-[#6]-[#6](-[#7])=O)-[#7]-[#6](=O)-[#6@H](-[#6]-c2ccccc2)-[#7]-[#6](=O)-[#6@H](-[#6]-c2ccc(-[#8])cc2)-[#7]-[#6]-1=O)-[#6](=O)-[#7]-1-[#6]-[#6]-[#6]-[#6@H]-1-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6]-[#6]\[#7]=[#6](\[#7])-[#7])-[#6](=O)-[#7]-[#6]-[#6](-[#7])=O Show InChI InChI=1S/C46H65N15O12S2/c47-27-22-74-75-23-33(45(73)61-17-5-9-34(61)44(72)56-28(8-4-16-53-46(51)52)39(67)54-21-37(50)65)60-43(71)32(20-36(49)64)59-40(68)29(14-15-35(48)63)55-41(69)31(18-24-6-2-1-3-7-24)58-42(70)30(57-38(27)66)19-25-10-12-26(62)13-11-25/h1-3,6-7,10-13,27-34,62H,4-5,8-9,14-23,47H2,(H2,48,63)(H2,49,64)(H2,50,65)(H,54,67)(H,55,69)(H,56,72)(H,57,66)(H,58,70)(H,59,68)(H,60,71)(H4,51,52,53)/t27-,28-,29-,30-,31-,32-,33-,34-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MMDB
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 0.460 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Yamanouchi Pharmaceutical Co., Ltd.
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 282: 301-8 (1997)
BindingDB Entry DOI: 10.7270/Q25T3J1N |
More data for this
Ligand-Target Pair | |
Vasopressin V1a receptor
(RAT) | BDBM85095

(CAS_151171 | CONIVAPTAN | NSC_151171 | YM087)Show SMILES Cc1nc-2c(CCN(C(=O)c3ccc(NC(=O)c4ccccc4-c4ccccc4)cc3)c3ccccc-23)[nH]1 Show InChI InChI=1S/C32H26N4O2/c1-21-33-28-19-20-36(29-14-8-7-13-27(29)30(28)34-21)32(38)23-15-17-24(18-16-23)35-31(37)26-12-6-5-11-25(26)22-9-3-2-4-10-22/h2-18H,19-20H2,1H3,(H,33,34)(H,35,37) | UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
DrugBank
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 0.480 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Yamanouchi Pharmaceutical Co., Ltd.
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 282: 301-8 (1997)
BindingDB Entry DOI: 10.7270/Q25T3J1N |
More data for this
Ligand-Target Pair | |
Vasopressin V1a receptor
(RAT) | BDBM35667

(AVP | CHEMBL373742 | US10131692, 44 (AVP) | [3H]Ar...)Show SMILES [#7]-[#6@H]-1-[#6]-[#16]-[#16]-[#6]-[#6@H](-[#7]-[#6](=O)-[#6@H](-[#6]-[#6](-[#7])=O)-[#7]-[#6](=O)-[#6@H](-[#6]-[#6]-[#6](-[#7])=O)-[#7]-[#6](=O)-[#6@H](-[#6]-c2ccccc2)-[#7]-[#6](=O)-[#6@H](-[#6]-c2ccc(-[#8])cc2)-[#7]-[#6]-1=O)-[#6](=O)-[#7]-1-[#6]-[#6]-[#6]-[#6@H]-1-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6]-[#6]\[#7]=[#6](\[#7])-[#7])-[#6](=O)-[#7]-[#6]-[#6](-[#7])=O Show InChI InChI=1S/C46H65N15O12S2/c47-27-22-74-75-23-33(45(73)61-17-5-9-34(61)44(72)56-28(8-4-16-53-46(51)52)39(67)54-21-37(50)65)60-43(71)32(20-36(49)64)59-40(68)29(14-15-35(48)63)55-41(69)31(18-24-6-2-1-3-7-24)58-42(70)30(57-38(27)66)19-25-10-12-26(62)13-11-25/h1-3,6-7,10-13,27-34,62H,4-5,8-9,14-23,47H2,(H2,48,63)(H2,49,64)(H2,50,65)(H,54,67)(H,55,69)(H,56,72)(H,57,66)(H,58,70)(H,59,68)(H,60,71)(H4,51,52,53)/t27-,28-,29-,30-,31-,32-,33-,34-/m0/s1 | UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MMDB
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 0.620 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Yamanouchi Pharmaceutical Co., Ltd.
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 282: 301-8 (1997)
BindingDB Entry DOI: 10.7270/Q25T3J1N |
More data for this
Ligand-Target Pair | |
Prostaglandin E2 receptor EP4 subtype
(Homo sapiens (Human)) | BDBM50165945
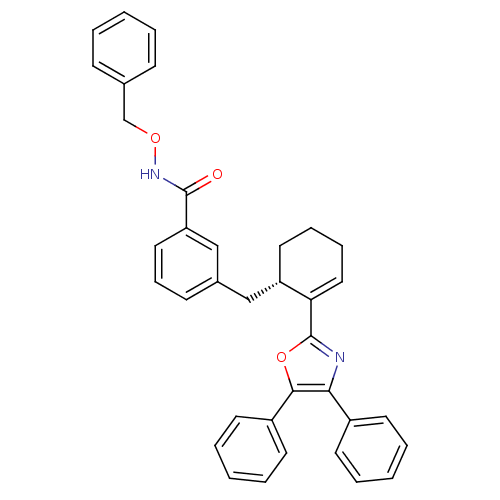
(CHEMBL371394 | N-Benzyloxy-3-[(S)-2-(4,5-diphenyl-...)Show SMILES O=C(NOCc1ccccc1)c1cccc(C[C@@H]2CCCC=C2c2nc(c(o2)-c2ccccc2)-c2ccccc2)c1 |c:22| Show InChI InChI=1S/C36H32N2O3/c39-35(38-40-25-26-13-4-1-5-14-26)31-21-12-15-27(24-31)23-30-20-10-11-22-32(30)36-37-33(28-16-6-2-7-17-28)34(41-36)29-18-8-3-9-19-29/h1-9,12-19,21-22,24,30H,10-11,20,23,25H2,(H,38,39)/t30-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.680 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Fujisawa Pharmaceutical Co., Ltd.
Curated by ChEMBL
| Assay Description
Inhibition of [3H]-PGE-2 binding to human prostanoid EP4 receptor |
J Med Chem 48: 3103-6 (2005)
Article DOI: 10.1021/jm050085k
BindingDB Entry DOI: 10.7270/Q2XD116M |
More data for this
Ligand-Target Pair | |
Prostaglandin E2 receptor EP4 subtype
(Rattus norvegicus) | BDBM50165949

(C-Phenyl-N-{3-[2-((S)-5-phenyl-4-phenyl-oxazol-2-y...)Show SMILES O=C(NS(=O)(=O)Cc1ccccc1)c1cccc(C[C@@H]2CCCC=C2c2nc(c(o2)-c2ccccc2)-c2ccccc2)c1 |c:24| Show InChI InChI=1S/C36H32N2O4S/c39-35(38-43(40,41)25-26-13-4-1-5-14-26)31-21-12-15-27(24-31)23-30-20-10-11-22-32(30)36-37-33(28-16-6-2-7-17-28)34(42-36)29-18-8-3-9-19-29/h1-9,12-19,21-22,24,30H,10-11,20,23,25H2,(H,38,39)/t30-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.740 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Fujisawa Pharmaceutical Co., Ltd.
Curated by ChEMBL
| Assay Description
Inhibition of rat prostanoid EP4 receptor |
J Med Chem 48: 3103-6 (2005)
Article DOI: 10.1021/jm050085k
BindingDB Entry DOI: 10.7270/Q2XD116M |
More data for this
Ligand-Target Pair | |
Oxytocin receptor
(RAT) | BDBM50013775

((oxytocin-OT) cyclo[Cys-Tyr-Ile-Gln-Asn-Cys]-Pro-L...)Show SMILES CCC(C)[C@@H]1NC(=O)[C@H](Cc2ccc(O)cc2)NC(=O)[C@H](N)CSSCC(NC(=O)[C@H](CC(N)=O)NC(=O)[C@H](CCC(N)=O)NC1=O)C(=O)N1CCCC1C(=O)N[C@@H](CC(C)C)C(=O)NCC(N)=O Show InChI InChI=1S/C43H66N12O12S2/c1-5-22(4)35-42(66)49-26(12-13-32(45)57)38(62)51-29(17-33(46)58)39(63)53-30(20-69-68-19-25(44)36(60)50-28(40(64)54-35)16-23-8-10-24(56)11-9-23)43(67)55-14-6-7-31(55)41(65)52-27(15-21(2)3)37(61)48-18-34(47)59/h8-11,21-22,25-31,35,56H,5-7,12-20,44H2,1-4H3,(H2,45,57)(H2,46,58)(H2,47,59)(H,48,61)(H,49,66)(H,50,60)(H,51,62)(H,52,65)(H,53,63)(H,54,64)/t22?,25-,26+,27+,28+,29+,30?,31?,35+/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 0.890 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Yamanouchi Pharmaceutical Co., Ltd.
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 282: 301-8 (1997)
BindingDB Entry DOI: 10.7270/Q25T3J1N |
More data for this
Ligand-Target Pair | |
Prostaglandin E2 receptor EP4 subtype
(Homo sapiens (Human)) | BDBM50165948

(6-{(S)-2-[(Benzofuran-2-carbonyl)-amino]-5-benzylo...)Show SMILES OC(=O)CCCCCNC(=O)[C@H](CCCNC(=O)OCc1ccccc1)NC(=O)c1cc2ccccc2o1 Show InChI InChI=1S/C28H33N3O7/c32-25(33)15-5-2-8-16-29-26(34)22(31-27(35)24-18-21-12-6-7-14-23(21)38-24)13-9-17-30-28(36)37-19-20-10-3-1-4-11-20/h1,3-4,6-7,10-12,14,18,22H,2,5,8-9,13,15-17,19H2,(H,29,34)(H,30,36)(H,31,35)(H,32,33)/t22-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.910 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Fujisawa Pharmaceutical Co., Ltd.
Curated by ChEMBL
| Assay Description
Inhibition of [3H]-PGE-2 binding to human prostanoid EP4 receptor |
J Med Chem 48: 3103-6 (2005)
Article DOI: 10.1021/jm050085k
BindingDB Entry DOI: 10.7270/Q2XD116M |
More data for this
Ligand-Target Pair | |
Vasopressin V1a receptor
(RAT) | BDBM35667

(AVP | CHEMBL373742 | US10131692, 44 (AVP) | [3H]Ar...)Show SMILES [#7]-[#6@H]-1-[#6]-[#16]-[#16]-[#6]-[#6@H](-[#7]-[#6](=O)-[#6@H](-[#6]-[#6](-[#7])=O)-[#7]-[#6](=O)-[#6@H](-[#6]-[#6]-[#6](-[#7])=O)-[#7]-[#6](=O)-[#6@H](-[#6]-c2ccccc2)-[#7]-[#6](=O)-[#6@H](-[#6]-c2ccc(-[#8])cc2)-[#7]-[#6]-1=O)-[#6](=O)-[#7]-1-[#6]-[#6]-[#6]-[#6@H]-1-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6]-[#6]\[#7]=[#6](\[#7])-[#7])-[#6](=O)-[#7]-[#6]-[#6](-[#7])=O Show InChI InChI=1S/C46H65N15O12S2/c47-27-22-74-75-23-33(45(73)61-17-5-9-34(61)44(72)56-28(8-4-16-53-46(51)52)39(67)54-21-37(50)65)60-43(71)32(20-36(49)64)59-40(68)29(14-15-35(48)63)55-41(69)31(18-24-6-2-1-3-7-24)58-42(70)30(57-38(27)66)19-25-10-12-26(62)13-11-25/h1-3,6-7,10-13,27-34,62H,4-5,8-9,14-23,47H2,(H2,48,63)(H2,49,64)(H2,50,65)(H,54,67)(H,55,69)(H,56,72)(H,57,66)(H,58,70)(H,59,68)(H,60,71)(H4,51,52,53)/t27-,28-,29-,30-,31-,32-,33-,34-/m0/s1 | UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MMDB
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 1.09 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Yamanouchi Pharmaceutical Co., Ltd.
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 282: 301-8 (1997)
BindingDB Entry DOI: 10.7270/Q25T3J1N |
More data for this
Ligand-Target Pair | |
Vasopressin V1a receptor
(RAT) | BDBM85096

(CAS_105077 | NSC_105077 | d(CH2)5Tyr(Me)AVP)Show SMILES [#6]-[#8]-c1ccc(-[#6]-[#6]-2-[#7]-[#6](=O)-[#6]C3([#6]-[#6]-[#6]-[#6]-[#6]3)[#16]-[#16]-[#6]-[#6](-[#7]-[#6](=O)-[#6](-[#6]-[#6](-[#7])=O)-[#7]-[#6](=O)-[#6](-[#6]-[#6]-[#6](-[#7])=O)-[#7]-[#6](=O)-[#6](-[#6]-c3ccccc3)-[#7]-[#6]-2=O)-[#6](=O)-[#7]-2-[#6]-[#6]-[#6]-[#6]-2-[#6](=O)-[#7]-[#6](-[#6]-[#6]-[#6]\[#7]=[#6](\[#7])-[#7])-[#6](=O)-[#7]-[#6]-[#6](-[#7])=O)cc1 Show InChI InChI=1S/C52H74N14O12S2/c1-78-32-16-14-31(15-17-32)25-35-46(73)63-36(24-30-10-4-2-5-11-30)47(74)61-34(18-19-40(53)67)45(72)64-37(26-41(54)68)48(75)65-38(29-79-80-52(27-43(70)60-35)20-6-3-7-21-52)50(77)66-23-9-13-39(66)49(76)62-33(12-8-22-58-51(56)57)44(71)59-28-42(55)69/h2,4-5,10-11,14-17,33-39H,3,6-9,12-13,18-29H2,1H3,(H2,53,67)(H2,54,68)(H2,55,69)(H,59,71)(H,60,70)(H,61,74)(H,62,76)(H,63,73)(H,64,72)(H,65,75)(H4,56,57,58) | UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
UniChem
Similars
| PubMed
| 1.79 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Yamanouchi Pharmaceutical Co., Ltd.
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 282: 301-8 (1997)
BindingDB Entry DOI: 10.7270/Q25T3J1N |
More data for this
Ligand-Target Pair | |
Vasopressin V2 receptor
(Rattus norvegicus (Rat)) | BDBM85094
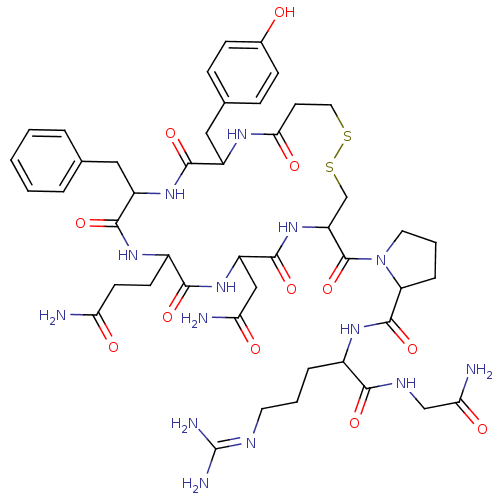
(CAS_62357-86-2 | NSC_64759 | dDAVP)Show SMILES [#7]-[#6](=O)-[#6]-[#6]-[#6]-1-[#7]-[#6](=O)-[#6](-[#6]-c2ccccc2)-[#7]-[#6](=O)-[#6](-[#6]-c2ccc(-[#8])cc2)-[#7]-[#6](=O)-[#6]-[#6]-[#16]-[#16]-[#6]-[#6](-[#7]-[#6](=O)-[#6](-[#6]-[#6](-[#7])=O)-[#7]-[#6]-1=O)-[#6](=O)-[#7]-1-[#6]-[#6]-[#6]-[#6]-1-[#6](=O)-[#7]-[#6](-[#6]-[#6]-[#6]\[#7]=[#6](\[#7])-[#7])-[#6](=O)-[#7]-[#6]-[#6](-[#7])=O Show InChI InChI=1S/C46H64N14O12S2/c47-35(62)15-14-29-40(67)58-32(22-36(48)63)43(70)59-33(45(72)60-18-5-9-34(60)44(71)56-28(8-4-17-52-46(50)51)39(66)53-23-37(49)64)24-74-73-19-16-38(65)54-30(21-26-10-12-27(61)13-11-26)41(68)57-31(42(69)55-29)20-25-6-2-1-3-7-25/h1-3,6-7,10-13,28-34,61H,4-5,8-9,14-24H2,(H2,47,62)(H2,48,63)(H2,49,64)(H,53,66)(H,54,65)(H,55,69)(H,56,71)(H,57,68)(H,58,67)(H,59,70)(H4,50,51,52) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
UniChem
Similars
| PubMed
| 1.93 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Yamanouchi Pharmaceutical Co., Ltd.
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 282: 301-8 (1997)
BindingDB Entry DOI: 10.7270/Q25T3J1N |
More data for this
Ligand-Target Pair | |
Vasopressin V2 receptor
(Rattus norvegicus (Rat)) | BDBM85095

(CAS_151171 | CONIVAPTAN | NSC_151171 | YM087)Show SMILES Cc1nc-2c(CCN(C(=O)c3ccc(NC(=O)c4ccccc4-c4ccccc4)cc3)c3ccccc-23)[nH]1 Show InChI InChI=1S/C32H26N4O2/c1-21-33-28-19-20-36(29-14-8-7-13-27(29)30(28)34-21)32(38)23-15-17-24(18-16-23)35-31(37)26-12-6-5-11-25(26)22-9-3-2-4-10-22/h2-18H,19-20H2,1H3,(H,33,34)(H,35,37) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
DrugBank
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 3.04 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Yamanouchi Pharmaceutical Co., Ltd.
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 282: 301-8 (1997)
BindingDB Entry DOI: 10.7270/Q25T3J1N |
More data for this
Ligand-Target Pair | |
Vasopressin V2 receptor
(Rattus norvegicus (Rat)) | BDBM35667

(AVP | CHEMBL373742 | US10131692, 44 (AVP) | [3H]Ar...)Show SMILES [#7]-[#6@H]-1-[#6]-[#16]-[#16]-[#6]-[#6@H](-[#7]-[#6](=O)-[#6@H](-[#6]-[#6](-[#7])=O)-[#7]-[#6](=O)-[#6@H](-[#6]-[#6]-[#6](-[#7])=O)-[#7]-[#6](=O)-[#6@H](-[#6]-c2ccccc2)-[#7]-[#6](=O)-[#6@H](-[#6]-c2ccc(-[#8])cc2)-[#7]-[#6]-1=O)-[#6](=O)-[#7]-1-[#6]-[#6]-[#6]-[#6@H]-1-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6]-[#6]\[#7]=[#6](\[#7])-[#7])-[#6](=O)-[#7]-[#6]-[#6](-[#7])=O Show InChI InChI=1S/C46H65N15O12S2/c47-27-22-74-75-23-33(45(73)61-17-5-9-34(61)44(72)56-28(8-4-16-53-46(51)52)39(67)54-21-37(50)65)60-43(71)32(20-36(49)64)59-40(68)29(14-15-35(48)63)55-41(69)31(18-24-6-2-1-3-7-24)58-42(70)30(57-38(27)66)19-25-10-12-26(62)13-11-25/h1-3,6-7,10-13,27-34,62H,4-5,8-9,14-23,47H2,(H2,48,63)(H2,49,64)(H2,50,65)(H,54,67)(H,55,69)(H,56,72)(H,57,66)(H,58,70)(H,59,68)(H,60,71)(H4,51,52,53)/t27-,28-,29-,30-,31-,32-,33-,34-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MMDB
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 3.23 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Yamanouchi Pharmaceutical Co., Ltd.
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 282: 301-8 (1997)
BindingDB Entry DOI: 10.7270/Q25T3J1N |
More data for this
Ligand-Target Pair | |
Prostaglandin E2 receptor EP4 subtype
(Rattus norvegicus) | BDBM50165948

(6-{(S)-2-[(Benzofuran-2-carbonyl)-amino]-5-benzylo...)Show SMILES OC(=O)CCCCCNC(=O)[C@H](CCCNC(=O)OCc1ccccc1)NC(=O)c1cc2ccccc2o1 Show InChI InChI=1S/C28H33N3O7/c32-25(33)15-5-2-8-16-29-26(34)22(31-27(35)24-18-21-12-6-7-14-23(21)38-24)13-9-17-30-28(36)37-19-20-10-3-1-4-11-20/h1,3-4,6-7,10-12,14,18,22H,2,5,8-9,13,15-17,19H2,(H,29,34)(H,30,36)(H,31,35)(H,32,33)/t22-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 5.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Fujisawa Pharmaceutical Co., Ltd.
Curated by ChEMBL
| Assay Description
Inhibition of rat prostanoid EP4 receptor |
J Med Chem 48: 3103-6 (2005)
Article DOI: 10.1021/jm050085k
BindingDB Entry DOI: 10.7270/Q2XD116M |
More data for this
Ligand-Target Pair | |
Prostaglandin E2 receptor EP4 subtype
(Homo sapiens (Human)) | BDBM50165946
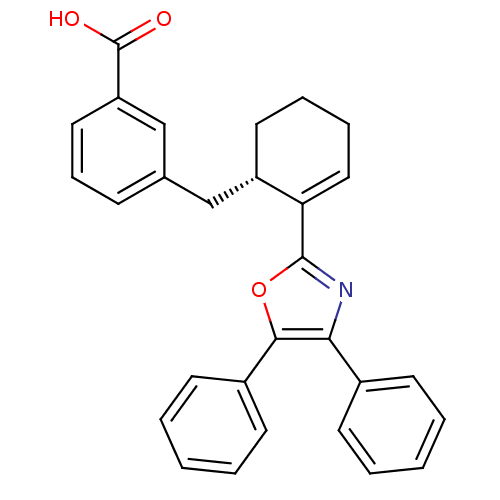
(3-[(S)-2-(4,5-Diphenyl-oxazol-2-yl)-cyclohex-2-eny...)Show SMILES OC(=O)c1cccc(C[C@@H]2CCCC=C2c2nc(c(o2)-c2ccccc2)-c2ccccc2)c1 |c:13| Show InChI InChI=1S/C29H25NO3/c31-29(32)24-16-9-10-20(19-24)18-23-15-7-8-17-25(23)28-30-26(21-11-3-1-4-12-21)27(33-28)22-13-5-2-6-14-22/h1-6,9-14,16-17,19,23H,7-8,15,18H2,(H,31,32)/t23-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 5.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Fujisawa Pharmaceutical Co., Ltd.
Curated by ChEMBL
| Assay Description
Inhibition of [3H]-PGE-2 binding to human prostanoid EP4 receptor |
J Med Chem 48: 3103-6 (2005)
Article DOI: 10.1021/jm050085k
BindingDB Entry DOI: 10.7270/Q2XD116M |
More data for this
Ligand-Target Pair | |
Prostacyclin receptor
(Homo sapiens (Human)) | BDBM50168287
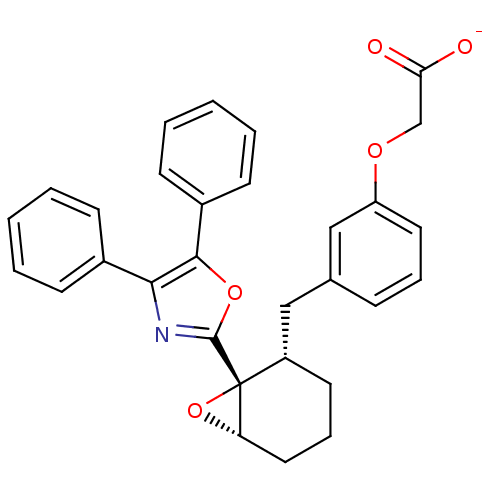
(CHEMBL363800 | Sodium; {3-[(1R,2S,6S)-1-(4,5-diphe...)Show SMILES [O-]C(=O)COc1cccc(C[C@@H]2CCC[C@@H]3O[C@]23c2nc(c(o2)-c2ccccc2)-c2ccccc2)c1 Show InChI InChI=1S/C30H27NO5/c32-26(33)19-34-24-15-7-9-20(18-24)17-23-14-8-16-25-30(23,36-25)29-31-27(21-10-3-1-4-11-21)28(35-29)22-12-5-2-6-13-22/h1-7,9-13,15,18,23,25H,8,14,16-17,19H2,(H,32,33)/p-1/t23-,25-,30+/m0/s1 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 6.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Fujisawa Pharmaceutical Co., Ltd.
Curated by ChEMBL
| Assay Description
Inhibition of [3H]-Iloprost binding to human Prostanoid IP receptor |
Bioorg Med Chem Lett 15: 3284-7 (2005)
Article DOI: 10.1016/j.bmcl.2005.04.076
BindingDB Entry DOI: 10.7270/Q2C53KCV |
More data for this
Ligand-Target Pair | |
Vasopressin V2 receptor
(Rattus norvegicus (Rat)) | BDBM35714
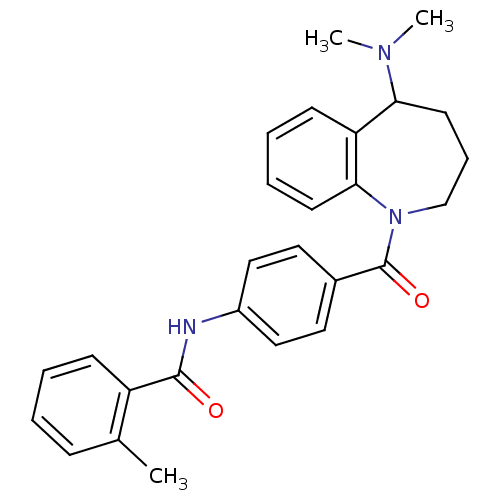
(CHEMBL420762 | Mozavaptan | N-[4-(5-Dimethylamino-...)Show SMILES CN(C)C1CCCN(C(=O)c2ccc(NC(=O)c3ccccc3C)cc2)c2ccccc12 Show InChI InChI=1S/C27H29N3O2/c1-19-9-4-5-10-22(19)26(31)28-21-16-14-20(15-17-21)27(32)30-18-8-13-24(29(2)3)23-11-6-7-12-25(23)30/h4-7,9-12,14-17,24H,8,13,18H2,1-3H3,(H,28,31) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 6.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Yamanouchi Pharmaceutical Co., Ltd.
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 282: 301-8 (1997)
BindingDB Entry DOI: 10.7270/Q25T3J1N |
More data for this
Ligand-Target Pair | |
Vasopressin V2 receptor
(Rattus norvegicus (Rat)) | BDBM85094

(CAS_62357-86-2 | NSC_64759 | dDAVP)Show SMILES [#7]-[#6](=O)-[#6]-[#6]-[#6]-1-[#7]-[#6](=O)-[#6](-[#6]-c2ccccc2)-[#7]-[#6](=O)-[#6](-[#6]-c2ccc(-[#8])cc2)-[#7]-[#6](=O)-[#6]-[#6]-[#16]-[#16]-[#6]-[#6](-[#7]-[#6](=O)-[#6](-[#6]-[#6](-[#7])=O)-[#7]-[#6]-1=O)-[#6](=O)-[#7]-1-[#6]-[#6]-[#6]-[#6]-1-[#6](=O)-[#7]-[#6](-[#6]-[#6]-[#6]\[#7]=[#6](\[#7])-[#7])-[#6](=O)-[#7]-[#6]-[#6](-[#7])=O Show InChI InChI=1S/C46H64N14O12S2/c47-35(62)15-14-29-40(67)58-32(22-36(48)63)43(70)59-33(45(72)60-18-5-9-34(60)44(71)56-28(8-4-17-52-46(50)51)39(66)53-23-37(49)64)24-74-73-19-16-38(65)54-30(21-26-10-12-27(61)13-11-26)41(68)57-31(42(69)55-29)20-25-6-2-1-3-7-25/h1-3,6-7,10-13,28-34,61H,4-5,8-9,14-24H2,(H2,47,62)(H2,48,63)(H2,49,64)(H,53,66)(H,54,65)(H,55,69)(H,56,71)(H,57,68)(H,58,67)(H,59,70)(H4,50,51,52) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
UniChem
Similars
| PubMed
| 7.94 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Yamanouchi Pharmaceutical Co., Ltd.
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 282: 301-8 (1997)
BindingDB Entry DOI: 10.7270/Q25T3J1N |
More data for this
Ligand-Target Pair | |
Prostaglandin E2 receptor EP4 subtype
(Homo sapiens (Human)) | BDBM50165951

(6-{(S)-2-[(Benzofuran-2-carbonyl)-amino]-6-benzylo...)Show SMILES OC(=O)CCCCCNC(=O)[C@H](CCCCNC(=O)OCc1ccccc1)NC(=O)c1cc2ccccc2o1 Show InChI InChI=1S/C29H35N3O7/c33-26(34)16-5-2-9-17-30-27(35)23(32-28(36)25-19-22-13-6-7-15-24(22)39-25)14-8-10-18-31-29(37)38-20-21-11-3-1-4-12-21/h1,3-4,6-7,11-13,15,19,23H,2,5,8-10,14,16-18,20H2,(H,30,35)(H,31,37)(H,32,36)(H,33,34)/t23-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 8.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Fujisawa Pharmaceutical Co., Ltd.
Curated by ChEMBL
| Assay Description
Inhibition of [3H]-PGE-2 binding to human prostanoid EP4 receptor |
J Med Chem 48: 3103-6 (2005)
Article DOI: 10.1021/jm050085k
BindingDB Entry DOI: 10.7270/Q2XD116M |
More data for this
Ligand-Target Pair | |
Prostacyclin receptor
(Homo sapiens (Human)) | BDBM50167887

((R)-2-(6-((diphenylcarbamoyloxy)methyl)-6-hydroxy-...)Show SMILES OC(=O)COc1cccc2C[C@@](O)(COC(=O)N(c3ccccc3)c3ccccc3)CCc12 Show InChI InChI=1S/C26H25NO6/c28-24(29)17-32-23-13-7-8-19-16-26(31,15-14-22(19)23)18-33-25(30)27(20-9-3-1-4-10-20)21-11-5-2-6-12-21/h1-13,31H,14-18H2,(H,28,29)/t26-/m1/s1 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 8.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Fujisawa Pharmaceutical Co., Ltd.
Curated by ChEMBL
| Assay Description
Displacement of [3H]iloprost from human Prostanoid IP receptor |
Bioorg Med Chem Lett 15: 3091-5 (2005)
Article DOI: 10.1016/j.bmcl.2005.04.047
BindingDB Entry DOI: 10.7270/Q2SF2WXV |
More data for this
Ligand-Target Pair | |
Lysine-specific histone demethylase 1A
(Homo sapiens (Human)) | BDBM50597540
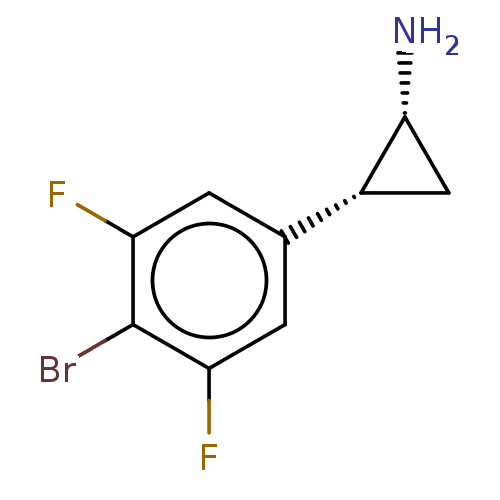
(CHEMBL5201156) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| 11 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acsmedchemlett.2c00294
BindingDB Entry DOI: 10.7270/Q28S4TZ1 |
More data for this
Ligand-Target Pair | |
Prostacyclin receptor
(Homo sapiens (Human)) | BDBM50167890
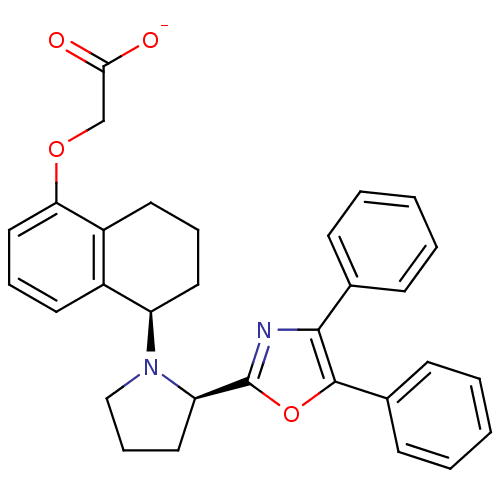
(CHEMBL190497 | FR-193262 | Sodium; {(R)-5-[(R)-2-(...)Show SMILES [O-]C(=O)COc1cccc2[C@@H](CCCc12)N1CCC[C@@H]1c1nc(c(o1)-c1ccccc1)-c1ccccc1 Show InChI InChI=1S/C31H30N2O4/c34-28(35)20-36-27-18-8-14-23-24(27)15-7-16-25(23)33-19-9-17-26(33)31-32-29(21-10-3-1-4-11-21)30(37-31)22-12-5-2-6-13-22/h1-6,8,10-14,18,25-26H,7,9,15-17,19-20H2,(H,34,35)/p-1/t25-,26-/m1/s1 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 12 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Fujisawa Pharmaceutical Co., Ltd.
Curated by ChEMBL
| Assay Description
Displacement of [3H]iloprost from human Prostanoid IP receptor |
Bioorg Med Chem Lett 15: 3091-5 (2005)
Article DOI: 10.1016/j.bmcl.2005.04.047
BindingDB Entry DOI: 10.7270/Q2SF2WXV |
More data for this
Ligand-Target Pair | |
Lysine-specific histone demethylase 1A
(Homo sapiens (Human)) | BDBM50597542

(CHEMBL5201621) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 13 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acsmedchemlett.2c00294
BindingDB Entry DOI: 10.7270/Q28S4TZ1 |
More data for this
Ligand-Target Pair | |
Vasopressin V1a receptor
(RAT) | BDBM50029644

(CHEMBL296908 | N-(3-(4-(4-(2-oxo-3,4-dihydroquinol...)Show SMILES CC(=O)NCCCOc1ccc(cc1)C(=O)N1CCC(CC1)N1C(=O)CCc2ccccc12 Show InChI InChI=1S/C26H31N3O4/c1-19(30)27-15-4-18-33-23-10-7-21(8-11-23)26(32)28-16-13-22(14-17-28)29-24-6-3-2-5-20(24)9-12-25(29)31/h2-3,5-8,10-11,22H,4,9,12-18H2,1H3,(H,27,30) | UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 14.9 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Yamanouchi Pharmaceutical Co., Ltd.
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 282: 301-8 (1997)
BindingDB Entry DOI: 10.7270/Q25T3J1N |
More data for this
Ligand-Target Pair | |
Vasopressin V1a receptor
(RAT) | BDBM50029644

(CHEMBL296908 | N-(3-(4-(4-(2-oxo-3,4-dihydroquinol...)Show SMILES CC(=O)NCCCOc1ccc(cc1)C(=O)N1CCC(CC1)N1C(=O)CCc2ccccc12 Show InChI InChI=1S/C26H31N3O4/c1-19(30)27-15-4-18-33-23-10-7-21(8-11-23)26(32)28-16-13-22(14-17-28)29-24-6-3-2-5-20(24)9-12-25(29)31/h2-3,5-8,10-11,22H,4,9,12-18H2,1H3,(H,27,30) | UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 23.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Yamanouchi Pharmaceutical Co., Ltd.
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 282: 301-8 (1997)
BindingDB Entry DOI: 10.7270/Q25T3J1N |
More data for this
Ligand-Target Pair | |
Vasopressin V1b receptor
(RAT) | BDBM85094

(CAS_62357-86-2 | NSC_64759 | dDAVP)Show SMILES [#7]-[#6](=O)-[#6]-[#6]-[#6]-1-[#7]-[#6](=O)-[#6](-[#6]-c2ccccc2)-[#7]-[#6](=O)-[#6](-[#6]-c2ccc(-[#8])cc2)-[#7]-[#6](=O)-[#6]-[#6]-[#16]-[#16]-[#6]-[#6](-[#7]-[#6](=O)-[#6](-[#6]-[#6](-[#7])=O)-[#7]-[#6]-1=O)-[#6](=O)-[#7]-1-[#6]-[#6]-[#6]-[#6]-1-[#6](=O)-[#7]-[#6](-[#6]-[#6]-[#6]\[#7]=[#6](\[#7])-[#7])-[#6](=O)-[#7]-[#6]-[#6](-[#7])=O Show InChI InChI=1S/C46H64N14O12S2/c47-35(62)15-14-29-40(67)58-32(22-36(48)63)43(70)59-33(45(72)60-18-5-9-34(60)44(71)56-28(8-4-17-52-46(50)51)39(66)53-23-37(49)64)24-74-73-19-16-38(65)54-30(21-26-10-12-27(61)13-11-26)41(68)57-31(42(69)55-29)20-25-6-2-1-3-7-25/h1-3,6-7,10-13,28-34,61H,4-5,8-9,14-24H2,(H2,47,62)(H2,48,63)(H2,49,64)(H,53,66)(H,54,65)(H,55,69)(H,56,71)(H,57,68)(H,58,67)(H,59,70)(H4,50,51,52) | UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
UniChem
Similars
| PubMed
| 25.1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Yamanouchi Pharmaceutical Co., Ltd.
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 282: 301-8 (1997)
BindingDB Entry DOI: 10.7270/Q25T3J1N |
More data for this
Ligand-Target Pair | |
Lysine-specific histone demethylase 1A
(Homo sapiens (Human)) | BDBM50597543

(CHEMBL5182939) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 27 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acsmedchemlett.2c00294
BindingDB Entry DOI: 10.7270/Q28S4TZ1 |
More data for this
Ligand-Target Pair | |
Vasopressin V1b receptor
(RAT) | BDBM85096

(CAS_105077 | NSC_105077 | d(CH2)5Tyr(Me)AVP)Show SMILES [#6]-[#8]-c1ccc(-[#6]-[#6]-2-[#7]-[#6](=O)-[#6]C3([#6]-[#6]-[#6]-[#6]-[#6]3)[#16]-[#16]-[#6]-[#6](-[#7]-[#6](=O)-[#6](-[#6]-[#6](-[#7])=O)-[#7]-[#6](=O)-[#6](-[#6]-[#6]-[#6](-[#7])=O)-[#7]-[#6](=O)-[#6](-[#6]-c3ccccc3)-[#7]-[#6]-2=O)-[#6](=O)-[#7]-2-[#6]-[#6]-[#6]-[#6]-2-[#6](=O)-[#7]-[#6](-[#6]-[#6]-[#6]\[#7]=[#6](\[#7])-[#7])-[#6](=O)-[#7]-[#6]-[#6](-[#7])=O)cc1 Show InChI InChI=1S/C52H74N14O12S2/c1-78-32-16-14-31(15-17-32)25-35-46(73)63-36(24-30-10-4-2-5-11-30)47(74)61-34(18-19-40(53)67)45(72)64-37(26-41(54)68)48(75)65-38(29-79-80-52(27-43(70)60-35)20-6-3-7-21-52)50(77)66-23-9-13-39(66)49(76)62-33(12-8-22-58-51(56)57)44(71)59-28-42(55)69/h2,4-5,10-11,14-17,33-39H,3,6-9,12-13,18-29H2,1H3,(H2,53,67)(H2,54,68)(H2,55,69)(H,59,71)(H,60,70)(H,61,74)(H,62,76)(H,63,73)(H,64,72)(H,65,75)(H4,56,57,58) | UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
UniChem
Similars
| PubMed
| 37.6 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Yamanouchi Pharmaceutical Co., Ltd.
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 282: 301-8 (1997)
BindingDB Entry DOI: 10.7270/Q25T3J1N |
More data for this
Ligand-Target Pair | |
Macrophage migration inhibitory factor
(Homo sapiens (Human)) | BDBM50095997
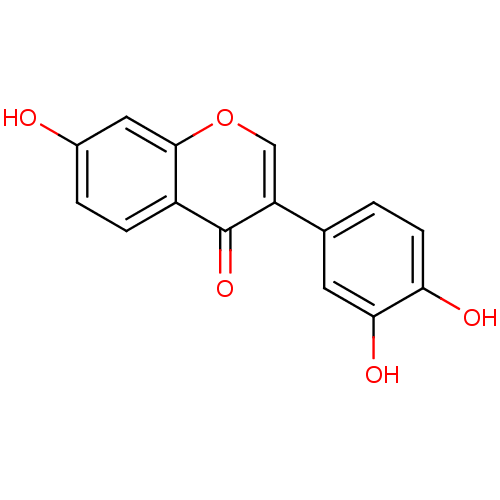
(3',4',7-trihydroxyisoflavone | CHEMBL13486)Show InChI InChI=1S/C15H10O5/c16-9-2-3-10-14(6-9)20-7-11(15(10)19)8-1-4-12(17)13(18)5-8/h1-7,16-18H | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| MMDB
PDB
PubMed
| 38 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Yamanouchi Pharmaceutical Company Ltd.
Curated by ChEMBL
| Assay Description
Inhibitory activity against tautomerase macrophage migration inhibitory factor (MIF) |
J Med Chem 44: 540-7 (2001)
BindingDB Entry DOI: 10.7270/Q2W66MGR |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Vasopressin V1b receptor
(RAT) | BDBM50013775

((oxytocin-OT) cyclo[Cys-Tyr-Ile-Gln-Asn-Cys]-Pro-L...)Show SMILES CCC(C)[C@@H]1NC(=O)[C@H](Cc2ccc(O)cc2)NC(=O)[C@H](N)CSSCC(NC(=O)[C@H](CC(N)=O)NC(=O)[C@H](CCC(N)=O)NC1=O)C(=O)N1CCCC1C(=O)N[C@@H](CC(C)C)C(=O)NCC(N)=O Show InChI InChI=1S/C43H66N12O12S2/c1-5-22(4)35-42(66)49-26(12-13-32(45)57)38(62)51-29(17-33(46)58)39(63)53-30(20-69-68-19-25(44)36(60)50-28(40(64)54-35)16-23-8-10-24(56)11-9-23)43(67)55-14-6-7-31(55)41(65)52-27(15-21(2)3)37(61)48-18-34(47)59/h8-11,21-22,25-31,35,56H,5-7,12-20,44H2,1-4H3,(H2,45,57)(H2,46,58)(H2,47,59)(H,48,61)(H,49,66)(H,50,60)(H,51,62)(H,52,65)(H,53,63)(H,54,64)/t22?,25-,26+,27+,28+,29+,30?,31?,35+/m1/s1 | UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 39.4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Yamanouchi Pharmaceutical Co., Ltd.
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 282: 301-8 (1997)
BindingDB Entry DOI: 10.7270/Q25T3J1N |
More data for this
Ligand-Target Pair | |
Vasopressin V2 receptor
(Rattus norvegicus (Rat)) | BDBM85096

(CAS_105077 | NSC_105077 | d(CH2)5Tyr(Me)AVP)Show SMILES [#6]-[#8]-c1ccc(-[#6]-[#6]-2-[#7]-[#6](=O)-[#6]C3([#6]-[#6]-[#6]-[#6]-[#6]3)[#16]-[#16]-[#6]-[#6](-[#7]-[#6](=O)-[#6](-[#6]-[#6](-[#7])=O)-[#7]-[#6](=O)-[#6](-[#6]-[#6]-[#6](-[#7])=O)-[#7]-[#6](=O)-[#6](-[#6]-c3ccccc3)-[#7]-[#6]-2=O)-[#6](=O)-[#7]-2-[#6]-[#6]-[#6]-[#6]-2-[#6](=O)-[#7]-[#6](-[#6]-[#6]-[#6]\[#7]=[#6](\[#7])-[#7])-[#6](=O)-[#7]-[#6]-[#6](-[#7])=O)cc1 Show InChI InChI=1S/C52H74N14O12S2/c1-78-32-16-14-31(15-17-32)25-35-46(73)63-36(24-30-10-4-2-5-11-30)47(74)61-34(18-19-40(53)67)45(72)64-37(26-41(54)68)48(75)65-38(29-79-80-52(27-43(70)60-35)20-6-3-7-21-52)50(77)66-23-9-13-39(66)49(76)62-33(12-8-22-58-51(56)57)44(71)59-28-42(55)69/h2,4-5,10-11,14-17,33-39H,3,6-9,12-13,18-29H2,1H3,(H2,53,67)(H2,54,68)(H2,55,69)(H,59,71)(H,60,70)(H,61,74)(H,62,76)(H,63,73)(H,64,72)(H,65,75)(H4,56,57,58) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
UniChem
Similars
| PubMed
| 39.6 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Yamanouchi Pharmaceutical Co., Ltd.
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 282: 301-8 (1997)
BindingDB Entry DOI: 10.7270/Q25T3J1N |
More data for this
Ligand-Target Pair | |
Prostacyclin receptor
(Homo sapiens (Human)) | BDBM50168291
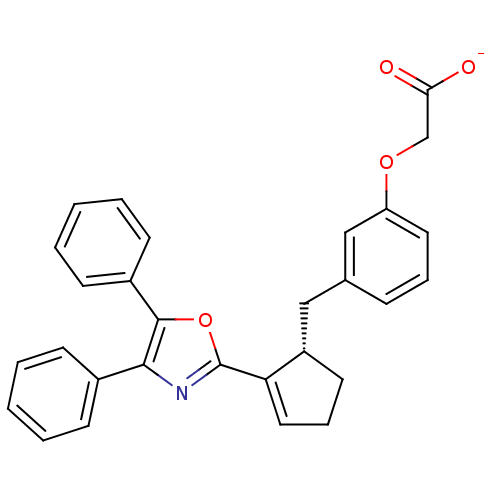
(CHEMBL363350 | Sodium; {3-[(S)-2-(4,5-diphenyl-oxa...)Show SMILES [O-]C(=O)COc1cccc(C[C@@H]2CCC=C2c2nc(c(o2)-c2ccccc2)-c2ccccc2)c1 |c:14| Show InChI InChI=1S/C29H25NO4/c31-26(32)19-33-24-15-7-9-20(18-24)17-23-14-8-16-25(23)29-30-27(21-10-3-1-4-11-21)28(34-29)22-12-5-2-6-13-22/h1-7,9-13,15-16,18,23H,8,14,17,19H2,(H,31,32)/p-1/t23-/m0/s1 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 41 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Fujisawa Pharmaceutical Co., Ltd.
Curated by ChEMBL
| Assay Description
Inhibition of [3H]-Iloprost binding to human Prostanoid IP receptor |
Bioorg Med Chem Lett 15: 3284-7 (2005)
Article DOI: 10.1016/j.bmcl.2005.04.076
BindingDB Entry DOI: 10.7270/Q2C53KCV |
More data for this
Ligand-Target Pair | |
Vasopressin V2 receptor
(Rattus norvegicus (Rat)) | BDBM35714

(CHEMBL420762 | Mozavaptan | N-[4-(5-Dimethylamino-...)Show SMILES CN(C)C1CCCN(C(=O)c2ccc(NC(=O)c3ccccc3C)cc2)c2ccccc12 Show InChI InChI=1S/C27H29N3O2/c1-19-9-4-5-10-22(19)26(31)28-21-16-14-20(15-17-21)27(32)30-18-8-13-24(29(2)3)23-11-6-7-12-25(23)30/h4-7,9-12,14-17,24H,8,13,18H2,1-3H3,(H,28,31) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 42.3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Yamanouchi Pharmaceutical Co., Ltd.
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 282: 301-8 (1997)
BindingDB Entry DOI: 10.7270/Q25T3J1N |
More data for this
Ligand-Target Pair | |
Prostaglandin E2 receptor EP4 subtype
(Homo sapiens (Human)) | BDBM50165943

((S)-2-{(S)-5-Benzyloxycarbonylamino-2-[(1H-indole-...)Show SMILES OC(=O)[C@H](Cc1ccccc1)NC(=O)[C@H](CCCNC(=O)OCc1ccccc1)NC(=O)c1cc2ccccc2[nH]1 Show InChI InChI=1S/C31H32N4O6/c36-28(35-27(30(38)39)18-21-10-3-1-4-11-21)25(34-29(37)26-19-23-14-7-8-15-24(23)33-26)16-9-17-32-31(40)41-20-22-12-5-2-6-13-22/h1-8,10-15,19,25,27,33H,9,16-18,20H2,(H,32,40)(H,34,37)(H,35,36)(H,38,39)/t25-,27-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 43 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Fujisawa Pharmaceutical Co., Ltd.
Curated by ChEMBL
| Assay Description
Inhibition of [3H]-PGE-2 binding to human prostanoid EP4 receptor |
J Med Chem 48: 3103-6 (2005)
Article DOI: 10.1021/jm050085k
BindingDB Entry DOI: 10.7270/Q2XD116M |
More data for this
Ligand-Target Pair | |
Oxytocin receptor
(RAT) | BDBM85095

(CAS_151171 | CONIVAPTAN | NSC_151171 | YM087)Show SMILES Cc1nc-2c(CCN(C(=O)c3ccc(NC(=O)c4ccccc4-c4ccccc4)cc3)c3ccccc-23)[nH]1 Show InChI InChI=1S/C32H26N4O2/c1-21-33-28-19-20-36(29-14-8-7-13-27(29)30(28)34-21)32(38)23-15-17-24(18-16-23)35-31(37)26-12-6-5-11-25(26)22-9-3-2-4-10-22/h2-18H,19-20H2,1H3,(H,33,34)(H,35,37) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
DrugBank
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 44.4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Yamanouchi Pharmaceutical Co., Ltd.
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 282: 301-8 (1997)
BindingDB Entry DOI: 10.7270/Q25T3J1N |
More data for this
Ligand-Target Pair | |
Vasopressin V1a receptor
(RAT) | BDBM50013775

((oxytocin-OT) cyclo[Cys-Tyr-Ile-Gln-Asn-Cys]-Pro-L...)Show SMILES CCC(C)[C@@H]1NC(=O)[C@H](Cc2ccc(O)cc2)NC(=O)[C@H](N)CSSCC(NC(=O)[C@H](CC(N)=O)NC(=O)[C@H](CCC(N)=O)NC1=O)C(=O)N1CCCC1C(=O)N[C@@H](CC(C)C)C(=O)NCC(N)=O Show InChI InChI=1S/C43H66N12O12S2/c1-5-22(4)35-42(66)49-26(12-13-32(45)57)38(62)51-29(17-33(46)58)39(63)53-30(20-69-68-19-25(44)36(60)50-28(40(64)54-35)16-23-8-10-24(56)11-9-23)43(67)55-14-6-7-31(55)41(65)52-27(15-21(2)3)37(61)48-18-34(47)59/h8-11,21-22,25-31,35,56H,5-7,12-20,44H2,1-4H3,(H2,45,57)(H2,46,58)(H2,47,59)(H,48,61)(H,49,66)(H,50,60)(H,51,62)(H,52,65)(H,53,63)(H,54,64)/t22?,25-,26+,27+,28+,29+,30?,31?,35+/m1/s1 | UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 46.2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Yamanouchi Pharmaceutical Co., Ltd.
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 282: 301-8 (1997)
BindingDB Entry DOI: 10.7270/Q25T3J1N |
More data for this
Ligand-Target Pair | |
Prostaglandin E2 receptor EP4 subtype
(Homo sapiens (Human)) | BDBM50165944

(CHEMBL189378 | {3-[(S)-2-(4,5-Diphenyl-oxazol-2-yl...)Show SMILES OC(=O)Cc1cccc(C[C@@H]2CCCC=C2c2nc(c(o2)-c2ccccc2)-c2ccccc2)c1 |c:14| Show InChI InChI=1S/C30H27NO3/c32-27(33)20-22-11-9-10-21(18-22)19-25-16-7-8-17-26(25)30-31-28(23-12-3-1-4-13-23)29(34-30)24-14-5-2-6-15-24/h1-6,9-15,17-18,25H,7-8,16,19-20H2,(H,32,33)/t25-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 50 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Fujisawa Pharmaceutical Co., Ltd.
Curated by ChEMBL
| Assay Description
Inhibition of [3H]-PGE-2 binding to human prostanoid EP4 receptor |
J Med Chem 48: 3103-6 (2005)
Article DOI: 10.1021/jm050085k
BindingDB Entry DOI: 10.7270/Q2XD116M |
More data for this
Ligand-Target Pair | |
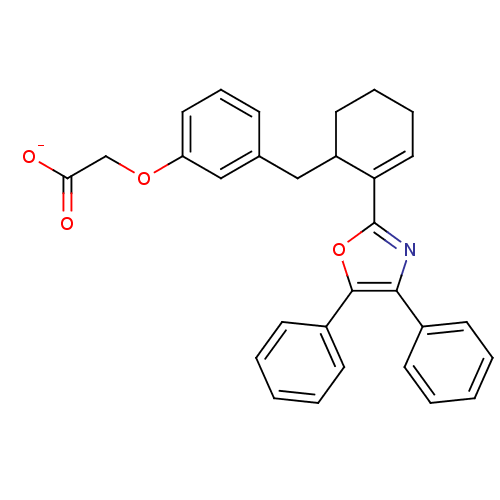
Prostacyclin receptor
(Homo sapiens (Human)) | BDBM50136234
(CHEMBL132589 | FR-181157 | Sodium; {3-[(R)-2-(4,5-...)Show SMILES [O-]C(=O)COc1cccc(CC2CCCC=C2c2nc(c(o2)-c2ccccc2)-c2ccccc2)c1 |c:15| Show InChI InChI=1S/C30H27NO4/c32-27(33)20-34-25-16-9-10-21(19-25)18-24-15-7-8-17-26(24)30-31-28(22-11-3-1-4-12-22)29(35-30)23-13-5-2-6-14-23/h1-6,9-14,16-17,19,24H,7-8,15,18,20H2,(H,32,33)/p-1 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 54 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Fujisawa Pharmaceutical Co., Ltd.
Curated by ChEMBL
| Assay Description
Inhibition of [3H]-Iloprost binding to human Prostanoid IP receptor |
Bioorg Med Chem Lett 15: 3284-7 (2005)
Article DOI: 10.1016/j.bmcl.2005.04.076
BindingDB Entry DOI: 10.7270/Q2C53KCV |
More data for this
Ligand-Target Pair | |
Prostacyclin receptor
(Homo sapiens (Human)) | BDBM50136234

(CHEMBL132589 | FR-181157 | Sodium; {3-[(R)-2-(4,5-...)Show SMILES [O-]C(=O)COc1cccc(CC2CCCC=C2c2nc(c(o2)-c2ccccc2)-c2ccccc2)c1 |c:15| Show InChI InChI=1S/C30H27NO4/c32-27(33)20-34-25-16-9-10-21(19-25)18-24-15-7-8-17-26(24)30-31-28(22-11-3-1-4-12-22)29(35-30)23-13-5-2-6-14-23/h1-6,9-14,16-17,19,24H,7-8,15,18,20H2,(H,32,33)/p-1 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 54 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Fujisawa Pharmaceutical Co., Ltd.
Curated by ChEMBL
| Assay Description
Inhibition of [3H]-Iloprost binding to human prostanoid IP receptor |
J Med Chem 48: 3103-6 (2005)
Article DOI: 10.1021/jm050085k
BindingDB Entry DOI: 10.7270/Q2XD116M |
More data for this
Ligand-Target Pair | |
Prostacyclin receptor
(Homo sapiens (Human)) | BDBM50370452
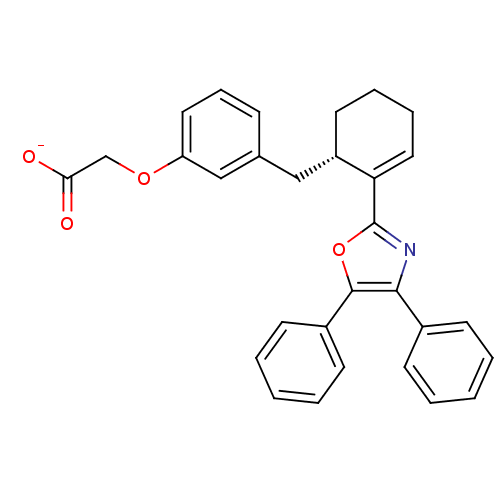
(CHEMBL132589 | FR-181157)Show SMILES [O-]C(=O)COc1cccc(C[C@@H]2CCCC=C2c2nc(c(o2)-c2ccccc2)-c2ccccc2)c1 |c:15| Show InChI InChI=1S/C30H27NO4/c32-27(33)20-34-25-16-9-10-21(19-25)18-24-15-7-8-17-26(24)30-31-28(22-11-3-1-4-12-22)29(35-30)23-13-5-2-6-14-23/h1-6,9-14,16-17,19,24H,7-8,15,18,20H2,(H,32,33)/p-1/t24-/m0/s1 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 54 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Fujisawa Pharmaceutical Co., Ltd.
Curated by ChEMBL
| Assay Description
Displacement of [3H]iloprost from human Prostanoid IP receptor |
Bioorg Med Chem Lett 15: 3091-5 (2005)
Article DOI: 10.1016/j.bmcl.2005.04.047
BindingDB Entry DOI: 10.7270/Q2SF2WXV |
More data for this
Ligand-Target Pair | |
Vasopressin V1a receptor
(RAT) | BDBM50013775

((oxytocin-OT) cyclo[Cys-Tyr-Ile-Gln-Asn-Cys]-Pro-L...)Show SMILES CCC(C)[C@@H]1NC(=O)[C@H](Cc2ccc(O)cc2)NC(=O)[C@H](N)CSSCC(NC(=O)[C@H](CC(N)=O)NC(=O)[C@H](CCC(N)=O)NC1=O)C(=O)N1CCCC1C(=O)N[C@@H](CC(C)C)C(=O)NCC(N)=O Show InChI InChI=1S/C43H66N12O12S2/c1-5-22(4)35-42(66)49-26(12-13-32(45)57)38(62)51-29(17-33(46)58)39(63)53-30(20-69-68-19-25(44)36(60)50-28(40(64)54-35)16-23-8-10-24(56)11-9-23)43(67)55-14-6-7-31(55)41(65)52-27(15-21(2)3)37(61)48-18-34(47)59/h8-11,21-22,25-31,35,56H,5-7,12-20,44H2,1-4H3,(H2,45,57)(H2,46,58)(H2,47,59)(H,48,61)(H,49,66)(H,50,60)(H,51,62)(H,52,65)(H,53,63)(H,54,64)/t22?,25-,26+,27+,28+,29+,30?,31?,35+/m1/s1 | UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 58.4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Yamanouchi Pharmaceutical Co., Ltd.
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 282: 301-8 (1997)
BindingDB Entry DOI: 10.7270/Q25T3J1N |
More data for this
Ligand-Target Pair | |
Prostacyclin receptor
(Homo sapiens (Human)) | BDBM50136234

(CHEMBL132589 | FR-181157 | Sodium; {3-[(R)-2-(4,5-...)Show SMILES [O-]C(=O)COc1cccc(CC2CCCC=C2c2nc(c(o2)-c2ccccc2)-c2ccccc2)c1 |c:15| Show InChI InChI=1S/C30H27NO4/c32-27(33)20-34-25-16-9-10-21(19-25)18-24-15-7-8-17-26(24)30-31-28(22-11-3-1-4-12-22)29(35-30)23-13-5-2-6-14-23/h1-6,9-14,16-17,19,24H,7-8,15,18,20H2,(H,32,33)/p-1 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Fujisawa Pharmaceutical Co., Ltd.
Curated by ChEMBL
| Assay Description
Displacement of [3H]iloprost from cloned human PGI2 receptor |
Bioorg Med Chem Lett 16: 4861-4 (2006)
Article DOI: 10.1016/j.bmcl.2006.06.076
BindingDB Entry DOI: 10.7270/Q2R49QD9 |
More data for this
Ligand-Target Pair | |
Lysine-specific histone demethylase 1A
(Homo sapiens (Human)) | BDBM50597536
(CHEMBL5206853) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 69 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acsmedchemlett.2c00294
BindingDB Entry DOI: 10.7270/Q28S4TZ1 |
More data for this
Ligand-Target Pair | |
Prostacyclin receptor
(Homo sapiens (Human)) | BDBM50191133

(CHEMBL439357 | sodium (R)-2-(3-((2-(4,5-diphenylox...)Show SMILES [O-]C(=O)COc1cccc(CN2CCC[C@@H]2c2nc(c(o2)-c2ccccc2)-c2ccccc2)c1 Show InChI InChI=1S/C28H26N2O4/c31-25(32)19-33-23-14-7-9-20(17-23)18-30-16-8-15-24(30)28-29-26(21-10-3-1-4-11-21)27(34-28)22-12-5-2-6-13-22/h1-7,9-14,17,24H,8,15-16,18-19H2,(H,31,32)/p-1/t24-/m1/s1 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 76 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Fujisawa Pharmaceutical Co., Ltd.
Curated by ChEMBL
| Assay Description
Displacement of [3H]iloprost from cloned human PGI2 receptor |
Bioorg Med Chem Lett 16: 4861-4 (2006)
Article DOI: 10.1016/j.bmcl.2006.06.076
BindingDB Entry DOI: 10.7270/Q2R49QD9 |
More data for this
Ligand-Target Pair | |
Prostaglandin E2 receptor EP4 subtype
(Homo sapiens (Human)) | BDBM50165950

(CHEMBL192303 | {(S)-4-[(S)-1-(Benzyl-methyl-carbam...)Show SMILES CN(Cc1ccccc1)C(=O)[C@H](Cc1ccccc1)NC(=O)[C@H](CCCNC(=O)OCc1ccccc1)NC(=O)c1cc2ccccc2[nH]1 Show InChI InChI=1S/C39H41N5O5/c1-44(26-29-16-7-3-8-17-29)38(47)35(24-28-14-5-2-6-15-28)43-36(45)33(42-37(46)34-25-31-20-11-12-21-32(31)41-34)22-13-23-40-39(48)49-27-30-18-9-4-10-19-30/h2-12,14-21,25,33,35,41H,13,22-24,26-27H2,1H3,(H,40,48)(H,42,46)(H,43,45)/t33-,35-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Fujisawa Pharmaceutical Co., Ltd.
Curated by ChEMBL
| Assay Description
Inhibition of [3H]-PGE-2 binding to human prostanoid EP4 receptor |
J Med Chem 48: 3103-6 (2005)
Article DOI: 10.1021/jm050085k
BindingDB Entry DOI: 10.7270/Q2XD116M |
More data for this
Ligand-Target Pair | |
Lysine-specific histone demethylase 1A
(Homo sapiens (Human)) | BDBM50596984
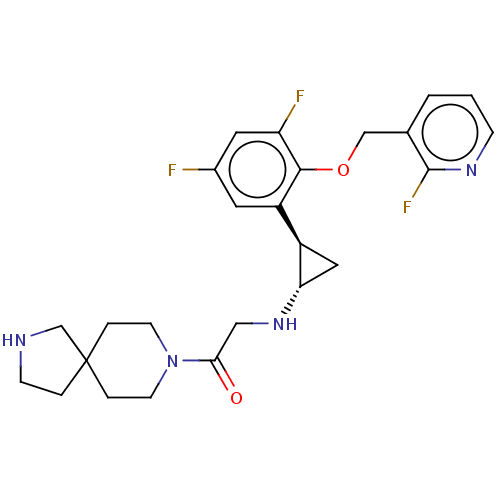
(CHEMBL5207485)Show SMILES Fc1cc(F)c(OCc2cccnc2F)c(c1)[C@H]1C[C@@H]1NCC(=O)N1CCC2(CCNC2)CC1 |r| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| 80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acsmedchemlett.2c00120
BindingDB Entry DOI: 10.7270/Q2FR01MQ |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data