 Found 89 hits with Last Name = 'taramelli' and Initial = 'd'
Found 89 hits with Last Name = 'taramelli' and Initial = 'd' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Plasmepsin II
(Plasmodium falciparum) | BDBM50323737
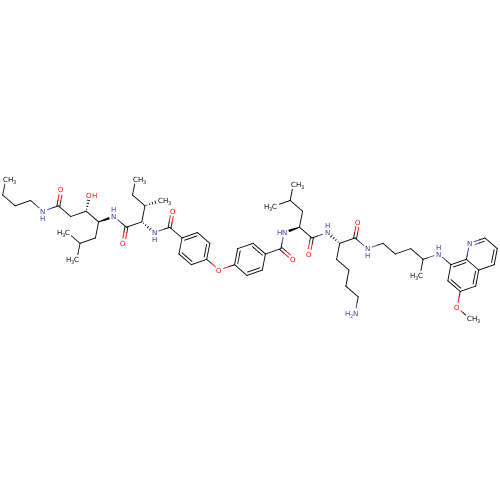
(CHEMBL1213687 | N-((2S)-1-((2S)-6-amino-1-(4-(6-me...)Show SMILES CCCCNC(=O)C[C@H](O)[C@H](CC(C)C)NC(=O)[C@@H](NC(=O)c1ccc(Oc2ccc(cc2)C(=O)N[C@@H](CC(C)C)C(=O)N[C@@H](CCCCN)C(=O)NCCCC(C)Nc2cc(OC)cc3cccnc23)cc1)[C@@H](C)CC |r| Show InChI InChI=1S/C59H87N9O9/c1-10-12-29-61-52(70)36-51(69)48(32-37(3)4)66-59(75)53(39(7)11-2)68-56(72)42-22-26-45(27-23-42)77-44-24-20-41(21-25-44)55(71)67-50(33-38(5)6)58(74)65-47(19-13-14-28-60)57(73)63-31-15-17-40(8)64-49-35-46(76-9)34-43-18-16-30-62-54(43)49/h16,18,20-27,30,34-35,37-40,47-48,50-51,53,64,69H,10-15,17,19,28-29,31-33,36,60H2,1-9H3,(H,61,70)(H,63,73)(H,65,74)(H,66,75)(H,67,71)(H,68,72)/t39-,40?,47-,48-,50-,51-,53-/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Milano
Curated by ChEMBL
| Assay Description
Inhibition of Plasmodium falciparum PLM2 using Lys-Glu-Phe-Val-Phe-NPhe-Ala-Leu-Lys as substrate by spectrophotometry |
Bioorg Med Chem Lett 22: 5915-8 (2012)
Article DOI: 10.1016/j.bmcl.2012.07.069
BindingDB Entry DOI: 10.7270/Q2M046HX |
More data for this
Ligand-Target Pair | |
Plasmepsin I
(Plasmodium falciparum) | BDBM50200018
((3S,4S)-4-((2S,3S)-2-(4-(4-((S)-((S)-1-(1-(4-(6-me...)Show SMILES CCCCNC(=O)C[C@H](O)[C@H](CC(C)C)NC(=O)[C@@H](NC(=O)c1ccc(Oc2ccc(cc2)C(=O)N[C@@H](C)C(=O)N[C@@H](C)C(=O)NCCCC(C)Nc2cc(OC)cc3cccnc23)cc1)C(C)CC |r| Show InChI InChI=1S/C53H74N8O9/c1-10-12-25-54-46(63)31-45(62)43(28-32(3)4)60-53(68)47(33(5)11-2)61-52(67)38-19-23-41(24-20-38)70-40-21-17-37(18-22-40)51(66)59-36(8)50(65)58-35(7)49(64)56-27-13-15-34(6)57-44-30-42(69-9)29-39-16-14-26-55-48(39)44/h14,16-24,26,29-30,32-36,43,45,47,57,62H,10-13,15,25,27-28,31H2,1-9H3,(H,54,63)(H,56,64)(H,58,65)(H,59,66)(H,60,68)(H,61,67)/t33?,34?,35-,36-,43-,45-,47-/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Milan
Curated by ChEMBL
| Assay Description
Inhibition of Plasmodium falciparum recombinant PLM1 |
J Med Chem 49: 7440-9 (2006)
Article DOI: 10.1021/jm061033d
BindingDB Entry DOI: 10.7270/Q29P319W |
More data for this
Ligand-Target Pair | |
Plasmepsin II
(Plasmodium falciparum) | BDBM50390636
(CHEMBL2069615)Show SMILES CCCCNC(=O)C[C@H](O)[C@H](CC(C)C)NC(=O)[C@@H](NC(=O)c1ccc(Oc2ccc(cc2)C(=O)NCc2ccccc2)cc1)[C@@H](C)CC |r| Show InChI InChI=1S/C39H52N4O6/c1-6-8-22-40-35(45)24-34(44)33(23-26(3)4)42-39(48)36(27(5)7-2)43-38(47)30-16-20-32(21-17-30)49-31-18-14-29(15-19-31)37(46)41-25-28-12-10-9-11-13-28/h9-21,26-27,33-34,36,44H,6-8,22-25H2,1-5H3,(H,40,45)(H,41,46)(H,42,48)(H,43,47)/t27-,33-,34-,36-/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Milano
Curated by ChEMBL
| Assay Description
Inhibition of Plasmodium falciparum PLM2 using Lys-Glu-Phe-Val-Phe-NPhe-Ala-Leu-Lys as substrate by spectrophotometry |
Bioorg Med Chem Lett 22: 5915-8 (2012)
Article DOI: 10.1016/j.bmcl.2012.07.069
BindingDB Entry DOI: 10.7270/Q2M046HX |
More data for this
Ligand-Target Pair | |
Plasmepsin II
(Plasmodium falciparum) | BDBM50390637
(CHEMBL2069616)Show SMILES CCCCNC(=O)C[C@H](O)[C@H](CC(C)C)NC(=O)[C@@H](NC(=O)c1ccc(Oc2ccc(cc2)C(=O)OCc2ccccc2)cc1)[C@@H](C)CC |r| Show InChI InChI=1S/C39H51N3O7/c1-6-8-22-40-35(44)24-34(43)33(23-26(3)4)41-38(46)36(27(5)7-2)42-37(45)29-14-18-31(19-15-29)49-32-20-16-30(17-21-32)39(47)48-25-28-12-10-9-11-13-28/h9-21,26-27,33-34,36,43H,6-8,22-25H2,1-5H3,(H,40,44)(H,41,46)(H,42,45)/t27-,33-,34-,36-/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Milano
Curated by ChEMBL
| Assay Description
Inhibition of Plasmodium falciparum PLM2 using Lys-Glu-Phe-Val-Phe-NPhe-Ala-Leu-Lys as substrate by spectrophotometry |
Bioorg Med Chem Lett 22: 5915-8 (2012)
Article DOI: 10.1016/j.bmcl.2012.07.069
BindingDB Entry DOI: 10.7270/Q2M046HX |
More data for this
Ligand-Target Pair | |
Plasmepsin II
(Plasmodium falciparum) | BDBM50390633
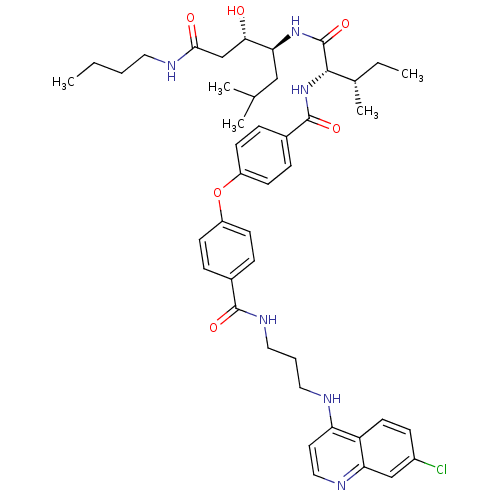
(CHEMBL2069612)Show SMILES CCCCNC(=O)C[C@H](O)[C@H](CC(C)C)NC(=O)[C@@H](NC(=O)c1ccc(Oc2ccc(cc2)C(=O)NCCCNc2ccnc3cc(Cl)ccc23)cc1)[C@@H](C)CC |r| Show InChI InChI=1S/C44H57ClN6O6/c1-6-8-21-48-40(53)27-39(52)38(25-28(3)4)50-44(56)41(29(5)7-2)51-43(55)31-12-17-34(18-13-31)57-33-15-10-30(11-16-33)42(54)49-23-9-22-46-36-20-24-47-37-26-32(45)14-19-35(36)37/h10-20,24,26,28-29,38-39,41,52H,6-9,21-23,25,27H2,1-5H3,(H,46,47)(H,48,53)(H,49,54)(H,50,56)(H,51,55)/t29-,38-,39-,41-/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 2.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Milano
Curated by ChEMBL
| Assay Description
Inhibition of Plasmodium falciparum PLM2 using Lys-Glu-Phe-Val-Phe-NPhe-Ala-Leu-Lys as substrate by spectrophotometry |
Bioorg Med Chem Lett 22: 5915-8 (2012)
Article DOI: 10.1016/j.bmcl.2012.07.069
BindingDB Entry DOI: 10.7270/Q2M046HX |
More data for this
Ligand-Target Pair | |
Plasmepsin II
(Plasmodium falciparum) | BDBM50390635
(CHEMBL2069614)Show SMILES CCCCNC(=O)C[C@H](O)[C@H](CC(C)C)NC(=O)[C@@H](NC(=O)c1ccc(Oc2ccc(cc2)C(=O)N[C@@H](CC(C)C)C(=O)N[C@@H](C)C(=O)NCCCNc2ccnc3ccccc23)cc1)[C@@H](C)CC |r| Show InChI InChI=1S/C53H74N8O8/c1-9-11-26-56-47(63)32-46(62)44(30-33(3)4)59-53(68)48(35(7)10-2)61-51(66)38-19-23-40(24-20-38)69-39-21-17-37(18-22-39)50(65)60-45(31-34(5)6)52(67)58-36(8)49(64)57-28-14-27-54-43-25-29-55-42-16-13-12-15-41(42)43/h12-13,15-25,29,33-36,44-46,48,62H,9-11,14,26-28,30-32H2,1-8H3,(H,54,55)(H,56,63)(H,57,64)(H,58,67)(H,59,68)(H,60,65)(H,61,66)/t35-,36-,44-,45-,46-,48-/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 2.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Milano
Curated by ChEMBL
| Assay Description
Inhibition of Plasmodium falciparum PLM2 using Lys-Glu-Phe-Val-Phe-NPhe-Ala-Leu-Lys as substrate by spectrophotometry |
Bioorg Med Chem Lett 22: 5915-8 (2012)
Article DOI: 10.1016/j.bmcl.2012.07.069
BindingDB Entry DOI: 10.7270/Q2M046HX |
More data for this
Ligand-Target Pair | |
Plasmepsin II
(Plasmodium falciparum) | BDBM50390634

(CHEMBL2069613)Show SMILES CCCCNC(=O)C[C@H](O)[C@H](CC(C)C)NC(=O)[C@@H](NC(=O)c1ccc(Oc2ccc(cc2)C(=O)N[C@@H](C)C(=O)NCCCNc2ccnc3cc(Cl)ccc23)cc1)[C@@H](C)CC |r| Show InChI InChI=1S/C47H62ClN7O7/c1-7-9-22-51-42(57)28-41(56)40(26-29(3)4)54-47(61)43(30(5)8-2)55-46(60)33-13-18-36(19-14-33)62-35-16-11-32(12-17-35)45(59)53-31(6)44(58)52-24-10-23-49-38-21-25-50-39-27-34(48)15-20-37(38)39/h11-21,25,27,29-31,40-41,43,56H,7-10,22-24,26,28H2,1-6H3,(H,49,50)(H,51,57)(H,52,58)(H,53,59)(H,54,61)(H,55,60)/t30-,31-,40-,41-,43-/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 3.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Milano
Curated by ChEMBL
| Assay Description
Inhibition of Plasmodium falciparum PLM2 using Lys-Glu-Phe-Val-Phe-NPhe-Ala-Leu-Lys as substrate by spectrophotometry |
Bioorg Med Chem Lett 22: 5915-8 (2012)
Article DOI: 10.1016/j.bmcl.2012.07.069
BindingDB Entry DOI: 10.7270/Q2M046HX |
More data for this
Ligand-Target Pair | |
Plasmepsin II
(Plasmodium falciparum) | BDBM50200018

((3S,4S)-4-((2S,3S)-2-(4-(4-((S)-((S)-1-(1-(4-(6-me...)Show SMILES CCCCNC(=O)C[C@H](O)[C@H](CC(C)C)NC(=O)[C@@H](NC(=O)c1ccc(Oc2ccc(cc2)C(=O)N[C@@H](C)C(=O)N[C@@H](C)C(=O)NCCCC(C)Nc2cc(OC)cc3cccnc23)cc1)C(C)CC |r| Show InChI InChI=1S/C53H74N8O9/c1-10-12-25-54-46(63)31-45(62)43(28-32(3)4)60-53(68)47(33(5)11-2)61-52(67)38-19-23-41(24-20-38)70-40-21-17-37(18-22-40)51(66)59-36(8)50(65)58-35(7)49(64)56-27-13-15-34(6)57-44-30-42(69-9)29-39-16-14-26-55-48(39)44/h14,16-24,26,29-30,32-36,43,45,47,57,62H,10-13,15,25,27-28,31H2,1-9H3,(H,54,63)(H,56,64)(H,58,65)(H,59,66)(H,60,68)(H,61,67)/t33?,34?,35-,36-,43-,45-,47-/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 3.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Milan
Curated by ChEMBL
| Assay Description
Inhibition of Plasmodium falciparum recombinant PLM2 |
J Med Chem 49: 7440-9 (2006)
Article DOI: 10.1021/jm061033d
BindingDB Entry DOI: 10.7270/Q29P319W |
More data for this
Ligand-Target Pair | |
Plasmepsin II
(Plasmodium falciparum) | BDBM50200015
((S)-2-(2S-(4-(4-(((2S,3S)-1-((3S,4S)-1-(butylamino...)Show SMILES CCCCNC(=O)C[C@H](O)[C@H](CC(C)C)NC(=O)[C@@H](NC(=O)c1ccc(Oc2ccc(cc2)C(=O)N[C@@H](CC(C)C)C(=O)N[C@@H](CCCCN)C(=O)OCCC)cc1)C(C)CC |r| Show InChI InChI=1S/C47H74N6O9/c1-9-12-25-49-41(55)29-40(54)38(27-30(4)5)51-46(59)42(32(8)11-3)53-44(57)34-18-22-36(23-19-34)62-35-20-16-33(17-21-35)43(56)52-39(28-31(6)7)45(58)50-37(15-13-14-24-48)47(60)61-26-10-2/h16-23,30-32,37-40,42,54H,9-15,24-29,48H2,1-8H3,(H,49,55)(H,50,58)(H,51,59)(H,52,56)(H,53,57)/t32?,37-,38-,39-,40-,42-/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 9.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Milan
Curated by ChEMBL
| Assay Description
Inhibition of Plasmodium falciparum recombinant PLM2 |
J Med Chem 49: 7440-9 (2006)
Article DOI: 10.1021/jm061033d
BindingDB Entry DOI: 10.7270/Q29P319W |
More data for this
Ligand-Target Pair | |
Plasmepsin II
(Plasmodium falciparum) | BDBM50200020

((3S,4S)-4-((2S,3S)-2-(4-(4-((4-(6-methoxyquinolin-...)Show SMILES CCCCNC(=O)C[C@H](O)[C@H](CC(C)C)NC(=O)[C@@H](NC(=O)c1ccc(Oc2ccc(cc2)C(=O)NCCCC(C)Nc2cc(OC)cc3cccnc23)cc1)C(C)CC |r| Show InChI InChI=1S/C47H64N6O7/c1-8-10-23-48-42(55)29-41(54)39(26-30(3)4)52-47(58)43(31(5)9-2)53-46(57)34-17-21-37(22-18-34)60-36-19-15-33(16-20-36)45(56)50-25-11-13-32(6)51-40-28-38(59-7)27-35-14-12-24-49-44(35)40/h12,14-22,24,27-28,30-32,39,41,43,51,54H,8-11,13,23,25-26,29H2,1-7H3,(H,48,55)(H,50,56)(H,52,58)(H,53,57)/t31?,32?,39-,41-,43-/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 11 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Milan
Curated by ChEMBL
| Assay Description
Inhibition of Plasmodium falciparum recombinant PLM2 |
J Med Chem 49: 7440-9 (2006)
Article DOI: 10.1021/jm061033d
BindingDB Entry DOI: 10.7270/Q29P319W |
More data for this
Ligand-Target Pair | |
Plasmepsin I
(Plasmodium falciparum) | BDBM50200015

((S)-2-(2S-(4-(4-(((2S,3S)-1-((3S,4S)-1-(butylamino...)Show SMILES CCCCNC(=O)C[C@H](O)[C@H](CC(C)C)NC(=O)[C@@H](NC(=O)c1ccc(Oc2ccc(cc2)C(=O)N[C@@H](CC(C)C)C(=O)N[C@@H](CCCCN)C(=O)OCCC)cc1)C(C)CC |r| Show InChI InChI=1S/C47H74N6O9/c1-9-12-25-49-41(55)29-40(54)38(27-30(4)5)51-46(59)42(32(8)11-3)53-44(57)34-18-22-36(23-19-34)62-35-20-16-33(17-21-35)43(56)52-39(28-31(6)7)45(58)50-37(15-13-14-24-48)47(60)61-26-10-2/h16-23,30-32,37-40,42,54H,9-15,24-29,48H2,1-8H3,(H,49,55)(H,50,58)(H,51,59)(H,52,56)(H,53,57)/t32?,37-,38-,39-,40-,42-/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 11 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Milan
Curated by ChEMBL
| Assay Description
Inhibition of Plasmodium falciparum recombinant PLM1 |
J Med Chem 49: 7440-9 (2006)
Article DOI: 10.1021/jm061033d
BindingDB Entry DOI: 10.7270/Q29P319W |
More data for this
Ligand-Target Pair | |
Plasmepsin II
(Plasmodium falciparum) | BDBM50200019
((S)-2-(2S-(4-(4-(((2S,3S)-1-((3S,4S)-1-(butylamino...)Show SMILES CCCCNC(=O)C[C@H](O)[C@H](CC(C)C)NC(=O)[C@@H](NC(=O)c1ccc(Oc2ccc(cc2)C(=O)N[C@@H](CC(C)C)C(=O)N[C@@H](CCCCNC(=O)OC(C)(C)C)C(=O)OCCC)cc1)C(C)CC |r| Show InChI InChI=1S/C52H82N6O11/c1-12-15-27-53-44(60)32-43(59)41(30-33(4)5)56-49(64)45(35(8)14-3)58-47(62)37-21-25-39(26-22-37)68-38-23-19-36(20-24-38)46(61)57-42(31-34(6)7)48(63)55-40(50(65)67-29-13-2)18-16-17-28-54-51(66)69-52(9,10)11/h19-26,33-35,40-43,45,59H,12-18,27-32H2,1-11H3,(H,53,60)(H,54,66)(H,55,63)(H,56,64)(H,57,61)(H,58,62)/t35?,40-,41-,42-,43-,45-/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 11 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Milan
Curated by ChEMBL
| Assay Description
Inhibition of Plasmodium falciparum recombinant PLM2 |
J Med Chem 49: 7440-9 (2006)
Article DOI: 10.1021/jm061033d
BindingDB Entry DOI: 10.7270/Q29P319W |
More data for this
Ligand-Target Pair | |
Plasmepsin II
(Plasmodium falciparum) | BDBM50200017

((3S,4S)-4-((2S,3S)-2-(4-(4-((S)-((S)-1-(6-amino-1-...)Show SMILES CCCCNC(=O)C[C@H](O)[C@H](CC(C)C)NC(=O)[C@@H](NC(=O)c1ccc(Oc2ccc(cc2)C(=O)N[C@@H](C)C(=O)N[C@@H](CCCCN)C(=O)NCCCC(C)Nc2cc(OC)cc3cccnc23)cc1)C(C)CC |r| Show InChI InChI=1S/C56H81N9O9/c1-9-11-28-58-49(67)34-48(66)46(31-35(3)4)64-56(72)50(36(5)10-2)65-54(70)40-21-25-43(26-22-40)74-42-23-19-39(20-24-42)53(69)62-38(7)52(68)63-45(18-12-13-27-57)55(71)60-30-14-16-37(6)61-47-33-44(73-8)32-41-17-15-29-59-51(41)47/h15,17,19-26,29,32-33,35-38,45-46,48,50,61,66H,9-14,16,18,27-28,30-31,34,57H2,1-8H3,(H,58,67)(H,60,71)(H,62,69)(H,63,68)(H,64,72)(H,65,70)/t36?,37?,38-,45-,46-,48-,50-/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 11 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Milan
Curated by ChEMBL
| Assay Description
Inhibition of Plasmodium falciparum recombinant PLM2 |
J Med Chem 49: 7440-9 (2006)
Article DOI: 10.1021/jm061033d
BindingDB Entry DOI: 10.7270/Q29P319W |
More data for this
Ligand-Target Pair | |
Plasmepsin II
(Plasmodium falciparum) | BDBM50200021
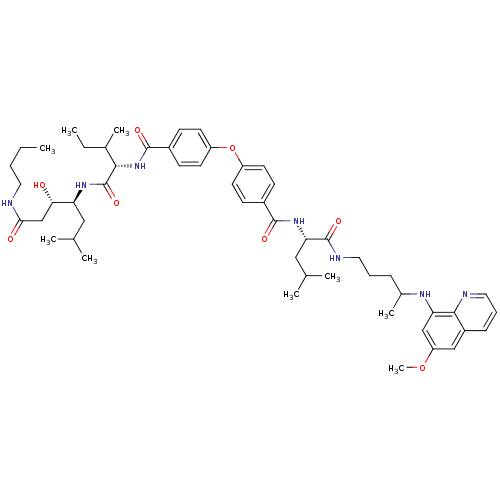
((3S,4S)-4-((2S,3S)-2-(4-(4-((S)-(1-(4-(6-methoxyqu...)Show SMILES CCCCNC(=O)C[C@H](O)[C@H](CC(C)C)NC(=O)[C@@H](NC(=O)c1ccc(Oc2ccc(cc2)C(=O)N[C@@H](CC(C)C)C(=O)NCCCC(C)Nc2cc(OC)cc3cccnc23)cc1)C(C)CC |r| Show InChI InChI=1S/C53H75N7O8/c1-10-12-25-54-47(62)32-46(61)43(28-33(3)4)58-53(66)48(35(7)11-2)60-51(64)38-19-23-41(24-20-38)68-40-21-17-37(18-22-40)50(63)59-45(29-34(5)6)52(65)56-27-13-15-36(8)57-44-31-42(67-9)30-39-16-14-26-55-49(39)44/h14,16-24,26,30-31,33-36,43,45-46,48,57,61H,10-13,15,25,27-29,32H2,1-9H3,(H,54,62)(H,56,65)(H,58,66)(H,59,63)(H,60,64)/t35?,36?,43-,45-,46-,48-/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 12 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Milan
Curated by ChEMBL
| Assay Description
Inhibition of Plasmodium falciparum recombinant PLM2 |
J Med Chem 49: 7440-9 (2006)
Article DOI: 10.1021/jm061033d
BindingDB Entry DOI: 10.7270/Q29P319W |
More data for this
Ligand-Target Pair | |
Plasmepsin I
(Plasmodium falciparum) | BDBM50200019

((S)-2-(2S-(4-(4-(((2S,3S)-1-((3S,4S)-1-(butylamino...)Show SMILES CCCCNC(=O)C[C@H](O)[C@H](CC(C)C)NC(=O)[C@@H](NC(=O)c1ccc(Oc2ccc(cc2)C(=O)N[C@@H](CC(C)C)C(=O)N[C@@H](CCCCNC(=O)OC(C)(C)C)C(=O)OCCC)cc1)C(C)CC |r| Show InChI InChI=1S/C52H82N6O11/c1-12-15-27-53-44(60)32-43(59)41(30-33(4)5)56-49(64)45(35(8)14-3)58-47(62)37-21-25-39(26-22-37)68-38-23-19-36(20-24-38)46(61)57-42(31-34(6)7)48(63)55-40(50(65)67-29-13-2)18-16-17-28-54-51(66)69-52(9,10)11/h19-26,33-35,40-43,45,59H,12-18,27-32H2,1-11H3,(H,53,60)(H,54,66)(H,55,63)(H,56,64)(H,57,61)(H,58,62)/t35?,40-,41-,42-,43-,45-/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 17 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Milan
Curated by ChEMBL
| Assay Description
Inhibition of Plasmodium falciparum recombinant PLM1 |
J Med Chem 49: 7440-9 (2006)
Article DOI: 10.1021/jm061033d
BindingDB Entry DOI: 10.7270/Q29P319W |
More data for this
Ligand-Target Pair | |
Plasmepsin II
(Plasmodium falciparum) | BDBM50200014
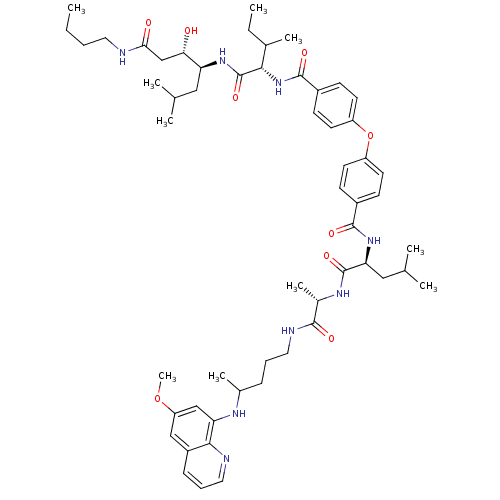
((3S,4S)-4-((2S,3S)-2-(4-(4-((S)-((S)-1-(1-(4-(6-me...)Show SMILES CCCCNC(=O)C[C@H](O)[C@H](CC(C)C)NC(=O)[C@@H](NC(=O)c1ccc(Oc2ccc(cc2)C(=O)N[C@@H](CC(C)C)C(=O)N[C@@H](C)C(=O)NCCCC(C)Nc2cc(OC)cc3cccnc23)cc1)C(C)CC |r| Show InChI InChI=1S/C56H80N8O9/c1-11-13-26-57-49(66)33-48(65)45(29-34(3)4)62-56(71)50(36(7)12-2)64-54(69)40-20-24-43(25-21-40)73-42-22-18-39(19-23-42)53(68)63-47(30-35(5)6)55(70)61-38(9)52(67)59-28-14-16-37(8)60-46-32-44(72-10)31-41-17-15-27-58-51(41)46/h15,17-25,27,31-32,34-38,45,47-48,50,60,65H,11-14,16,26,28-30,33H2,1-10H3,(H,57,66)(H,59,67)(H,61,70)(H,62,71)(H,63,68)(H,64,69)/t36?,37?,38-,45-,47-,48-,50-/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 26 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Milan
Curated by ChEMBL
| Assay Description
Inhibition of Plasmodium falciparum recombinant PLM2 |
J Med Chem 49: 7440-9 (2006)
Article DOI: 10.1021/jm061033d
BindingDB Entry DOI: 10.7270/Q29P319W |
More data for this
Ligand-Target Pair | |
Plasmepsin I
(Plasmodium falciparum) | BDBM50200017

((3S,4S)-4-((2S,3S)-2-(4-(4-((S)-((S)-1-(6-amino-1-...)Show SMILES CCCCNC(=O)C[C@H](O)[C@H](CC(C)C)NC(=O)[C@@H](NC(=O)c1ccc(Oc2ccc(cc2)C(=O)N[C@@H](C)C(=O)N[C@@H](CCCCN)C(=O)NCCCC(C)Nc2cc(OC)cc3cccnc23)cc1)C(C)CC |r| Show InChI InChI=1S/C56H81N9O9/c1-9-11-28-58-49(67)34-48(66)46(31-35(3)4)64-56(72)50(36(5)10-2)65-54(70)40-21-25-43(26-22-40)74-42-23-19-39(20-24-42)53(69)62-38(7)52(68)63-45(18-12-13-27-57)55(71)60-30-14-16-37(6)61-47-33-44(73-8)32-41-17-15-29-59-51(41)47/h15,17,19-26,29,32-33,35-38,45-46,48,50,61,66H,9-14,16,18,27-28,30-31,34,57H2,1-8H3,(H,58,67)(H,60,71)(H,62,69)(H,63,68)(H,64,72)(H,65,70)/t36?,37?,38-,45-,46-,48-,50-/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 33 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Milan
Curated by ChEMBL
| Assay Description
Inhibition of Plasmodium falciparum recombinant PLM1 |
J Med Chem 49: 7440-9 (2006)
Article DOI: 10.1021/jm061033d
BindingDB Entry DOI: 10.7270/Q29P319W |
More data for this
Ligand-Target Pair | |
Plasmepsin I
(Plasmodium falciparum) | BDBM50200023

((3S,4S)-4-((2S,3S)-2-(4-(4-((S)-((S)-1-(1-(4-(6-me...)Show SMILES CCCCNC(=O)C[C@H](O)[C@H](CC(C)C)NC(=O)[C@@H](NC(=O)c1ccc(Oc2ccc(cc2)C(=O)N[C@@H](CC(C)C)C(=O)N[C@@H](CC(C)C)C(=O)NCCCC(C)Nc2cc(OC)cc3cccnc23)cc1)C(C)CC |r| Show InChI InChI=1S/C59H86N8O9/c1-12-14-27-60-52(69)35-51(68)47(30-36(3)4)64-59(74)53(39(9)13-2)67-56(71)42-21-25-45(26-22-42)76-44-23-19-41(20-24-44)55(70)65-50(32-38(7)8)58(73)66-49(31-37(5)6)57(72)62-29-15-17-40(10)63-48-34-46(75-11)33-43-18-16-28-61-54(43)48/h16,18-26,28,33-34,36-40,47,49-51,53,63,68H,12-15,17,27,29-32,35H2,1-11H3,(H,60,69)(H,62,72)(H,64,74)(H,65,70)(H,66,73)(H,67,71)/t39?,40?,47-,49-,50-,51-,53-/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 58 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Milan
Curated by ChEMBL
| Assay Description
Inhibition of Plasmodium falciparum recombinant PLM1 |
J Med Chem 49: 7440-9 (2006)
Article DOI: 10.1021/jm061033d
BindingDB Entry DOI: 10.7270/Q29P319W |
More data for this
Ligand-Target Pair | |
Plasmepsin II
(Plasmodium falciparum) | BDBM50146527

((3S,4S)-N-butyl-3-hydroxy-6-methyl-4-((2S,3S)-3-me...)Show SMILES CCCCNC(=O)C[C@H](O)[C@H](CC(C)C)NC(=O)[C@@H](NC(=O)COc1ccc2ccccc2c1)C(C)CC Show InChI InChI=1S/C30H45N3O5/c1-6-8-15-31-27(35)18-26(34)25(16-20(3)4)32-30(37)29(21(5)7-2)33-28(36)19-38-24-14-13-22-11-9-10-12-23(22)17-24/h9-14,17,20-21,25-26,29,34H,6-8,15-16,18-19H2,1-5H3,(H,31,35)(H,32,37)(H,33,36)/t21?,25-,26-,29-/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 59 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Milan
Curated by ChEMBL
| Assay Description
Inhibition of Plasmodium falciparum recombinant PLM2 |
J Med Chem 49: 7440-9 (2006)
Article DOI: 10.1021/jm061033d
BindingDB Entry DOI: 10.7270/Q29P319W |
More data for this
Ligand-Target Pair | |
Plasmepsin I
(Plasmodium falciparum) | BDBM50200014

((3S,4S)-4-((2S,3S)-2-(4-(4-((S)-((S)-1-(1-(4-(6-me...)Show SMILES CCCCNC(=O)C[C@H](O)[C@H](CC(C)C)NC(=O)[C@@H](NC(=O)c1ccc(Oc2ccc(cc2)C(=O)N[C@@H](CC(C)C)C(=O)N[C@@H](C)C(=O)NCCCC(C)Nc2cc(OC)cc3cccnc23)cc1)C(C)CC |r| Show InChI InChI=1S/C56H80N8O9/c1-11-13-26-57-49(66)33-48(65)45(29-34(3)4)62-56(71)50(36(7)12-2)64-54(69)40-20-24-43(25-21-40)73-42-22-18-39(19-23-42)53(68)63-47(30-35(5)6)55(70)61-38(9)52(67)59-28-14-16-37(8)60-46-32-44(72-10)31-41-17-15-27-58-51(41)46/h15,17-25,27,31-32,34-38,45,47-48,50,60,65H,11-14,16,26,28-30,33H2,1-10H3,(H,57,66)(H,59,67)(H,61,70)(H,62,71)(H,63,68)(H,64,69)/t36?,37?,38-,45-,47-,48-,50-/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 74 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Milan
Curated by ChEMBL
| Assay Description
Inhibition of Plasmodium falciparum recombinant PLM1 |
J Med Chem 49: 7440-9 (2006)
Article DOI: 10.1021/jm061033d
BindingDB Entry DOI: 10.7270/Q29P319W |
More data for this
Ligand-Target Pair | |
Plasmepsin I
(Plasmodium falciparum) | BDBM50200020

((3S,4S)-4-((2S,3S)-2-(4-(4-((4-(6-methoxyquinolin-...)Show SMILES CCCCNC(=O)C[C@H](O)[C@H](CC(C)C)NC(=O)[C@@H](NC(=O)c1ccc(Oc2ccc(cc2)C(=O)NCCCC(C)Nc2cc(OC)cc3cccnc23)cc1)C(C)CC |r| Show InChI InChI=1S/C47H64N6O7/c1-8-10-23-48-42(55)29-41(54)39(26-30(3)4)52-47(58)43(31(5)9-2)53-46(57)34-17-21-37(22-18-34)60-36-19-15-33(16-20-36)45(56)50-25-11-13-32(6)51-40-28-38(59-7)27-35-14-12-24-49-44(35)40/h12,14-22,24,27-28,30-32,39,41,43,51,54H,8-11,13,23,25-26,29H2,1-7H3,(H,48,55)(H,50,56)(H,52,58)(H,53,57)/t31?,32?,39-,41-,43-/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 81 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Milan
Curated by ChEMBL
| Assay Description
Inhibition of Plasmodium falciparum recombinant PLM1 |
J Med Chem 49: 7440-9 (2006)
Article DOI: 10.1021/jm061033d
BindingDB Entry DOI: 10.7270/Q29P319W |
More data for this
Ligand-Target Pair | |
Plasmepsin II
(Plasmodium falciparum) | BDBM50200023

((3S,4S)-4-((2S,3S)-2-(4-(4-((S)-((S)-1-(1-(4-(6-me...)Show SMILES CCCCNC(=O)C[C@H](O)[C@H](CC(C)C)NC(=O)[C@@H](NC(=O)c1ccc(Oc2ccc(cc2)C(=O)N[C@@H](CC(C)C)C(=O)N[C@@H](CC(C)C)C(=O)NCCCC(C)Nc2cc(OC)cc3cccnc23)cc1)C(C)CC |r| Show InChI InChI=1S/C59H86N8O9/c1-12-14-27-60-52(69)35-51(68)47(30-36(3)4)64-59(74)53(39(9)13-2)67-56(71)42-21-25-45(26-22-42)76-44-23-19-41(20-24-44)55(70)65-50(32-38(7)8)58(73)66-49(31-37(5)6)57(72)62-29-15-17-40(10)63-48-34-46(75-11)33-43-18-16-28-61-54(43)48/h16,18-26,28,33-34,36-40,47,49-51,53,63,68H,12-15,17,27,29-32,35H2,1-11H3,(H,60,69)(H,62,72)(H,64,74)(H,65,70)(H,66,73)(H,67,71)/t39?,40?,47-,49-,50-,51-,53-/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 103 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Milan
Curated by ChEMBL
| Assay Description
Inhibition of Plasmodium falciparum recombinant PLM2 |
J Med Chem 49: 7440-9 (2006)
Article DOI: 10.1021/jm061033d
BindingDB Entry DOI: 10.7270/Q29P319W |
More data for this
Ligand-Target Pair | |
Plasmepsin II
(Plasmodium falciparum) | BDBM50146524

((S)-3-(S)-Hydroxy-4-{(S)-3-methyl-2-[2-(naphthalen...)Show SMILES CCCCNC(=O)C[C@H](O)[C@H](Cc1ccccc1)NC(=O)[C@@H](NC(=O)COc1ccc2ccccc2c1)[C@@H](C)CC Show InChI InChI=1S/C33H43N3O5/c1-4-6-18-34-30(38)21-29(37)28(19-24-12-8-7-9-13-24)35-33(40)32(23(3)5-2)36-31(39)22-41-27-17-16-25-14-10-11-15-26(25)20-27/h7-17,20,23,28-29,32,37H,4-6,18-19,21-22H2,1-3H3,(H,34,38)(H,35,40)(H,36,39)/t23?,28-,29-,32-/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 110 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Milan
Curated by ChEMBL
| Assay Description
In vitro binding affinity towards Plasmodium falciparum plasmepsin-2 |
Bioorg Med Chem Lett 14: 2931-4 (2004)
Article DOI: 10.1016/j.bmcl.2004.03.030
BindingDB Entry DOI: 10.7270/Q2G44QVR |
More data for this
Ligand-Target Pair | |
Plasmepsin II
(Plasmodium falciparum) | BDBM50223627

(CHEMBL3215350)Show SMILES CCCCNC(=O)C[C@H](O)[C@H](CC(C)C)NC(=O)[C@@H](NC(=O)COc1ccc2ccccc2c1)[C@@H](C)CC Show InChI InChI=1S/C30H45N3O5/c1-6-8-15-31-27(35)18-26(34)25(16-20(3)4)32-30(37)29(21(5)7-2)33-28(36)19-38-24-14-13-22-11-9-10-12-23(22)17-24/h9-14,17,20-21,25-26,29,34H,6-8,15-16,18-19H2,1-5H3,(H,31,35)(H,32,37)(H,33,36)/t21-,25-,26-,29-/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
| Article
PubMed
| 180 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Milan
Curated by ChEMBL
| Assay Description
In vitro binding affinity towards Plasmodium falciparum plasmepsin-2 |
Bioorg Med Chem Lett 14: 2931-4 (2004)
Article DOI: 10.1016/j.bmcl.2004.03.030
BindingDB Entry DOI: 10.7270/Q2G44QVR |
More data for this
Ligand-Target Pair | |
Plasmepsin I
(Plasmodium falciparum) | BDBM50200016
((3S,4S)-4-((2S,3S)-2-(4-(4-((S)-((S)-1-(6-(tert-bu...)Show SMILES CCCCNC(=O)C[C@H](O)[C@H](CC(C)C)NC(=O)[C@@H](NC(=O)c1ccc(Oc2ccc(cc2)C(=O)N[C@@H](CC(C)C)C(=O)N[C@@H](CCCCNC(=O)OC(C)(C)C)C(=O)NCCCC(C)Nc2cc(OC)cc3cccnc23)cc1)C(C)CC |r| Show InChI InChI=1S/C64H95N9O11/c1-13-15-31-65-55(75)39-54(74)51(35-40(3)4)71-62(80)56(42(7)14-2)73-59(77)45-25-29-48(30-26-45)83-47-27-23-44(24-28-47)58(76)72-53(36-41(5)6)61(79)70-50(22-16-17-32-68-63(81)84-64(9,10)11)60(78)67-34-18-20-43(8)69-52-38-49(82-12)37-46-21-19-33-66-57(46)52/h19,21,23-30,33,37-38,40-43,50-51,53-54,56,69,74H,13-18,20,22,31-32,34-36,39H2,1-12H3,(H,65,75)(H,67,78)(H,68,81)(H,70,79)(H,71,80)(H,72,76)(H,73,77)/t42?,43?,50-,51-,53-,54-,56-/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 230 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Milan
Curated by ChEMBL
| Assay Description
Inhibition of Plasmodium falciparum recombinant PLM1 |
J Med Chem 49: 7440-9 (2006)
Article DOI: 10.1021/jm061033d
BindingDB Entry DOI: 10.7270/Q29P319W |
More data for this
Ligand-Target Pair | |
Plasmepsin II
(Plasmodium falciparum) | BDBM50200016

((3S,4S)-4-((2S,3S)-2-(4-(4-((S)-((S)-1-(6-(tert-bu...)Show SMILES CCCCNC(=O)C[C@H](O)[C@H](CC(C)C)NC(=O)[C@@H](NC(=O)c1ccc(Oc2ccc(cc2)C(=O)N[C@@H](CC(C)C)C(=O)N[C@@H](CCCCNC(=O)OC(C)(C)C)C(=O)NCCCC(C)Nc2cc(OC)cc3cccnc23)cc1)C(C)CC |r| Show InChI InChI=1S/C64H95N9O11/c1-13-15-31-65-55(75)39-54(74)51(35-40(3)4)71-62(80)56(42(7)14-2)73-59(77)45-25-29-48(30-26-45)83-47-27-23-44(24-28-47)58(76)72-53(36-41(5)6)61(79)70-50(22-16-17-32-68-63(81)84-64(9,10)11)60(78)67-34-18-20-43(8)69-52-38-49(82-12)37-46-21-19-33-66-57(46)52/h19,21,23-30,33,37-38,40-43,50-51,53-54,56,69,74H,13-18,20,22,31-32,34-36,39H2,1-12H3,(H,65,75)(H,67,78)(H,68,81)(H,70,79)(H,71,80)(H,72,76)(H,73,77)/t42?,43?,50-,51-,53-,54-,56-/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 403 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Milan
Curated by ChEMBL
| Assay Description
Inhibition of Plasmodium falciparum recombinant PLM2 |
J Med Chem 49: 7440-9 (2006)
Article DOI: 10.1021/jm061033d
BindingDB Entry DOI: 10.7270/Q29P319W |
More data for this
Ligand-Target Pair | |
Plasmepsin I
(Plasmodium falciparum) | BDBM50146527

((3S,4S)-N-butyl-3-hydroxy-6-methyl-4-((2S,3S)-3-me...)Show SMILES CCCCNC(=O)C[C@H](O)[C@H](CC(C)C)NC(=O)[C@@H](NC(=O)COc1ccc2ccccc2c1)C(C)CC Show InChI InChI=1S/C30H45N3O5/c1-6-8-15-31-27(35)18-26(34)25(16-20(3)4)32-30(37)29(21(5)7-2)33-28(36)19-38-24-14-13-22-11-9-10-12-23(22)17-24/h9-14,17,20-21,25-26,29,34H,6-8,15-16,18-19H2,1-5H3,(H,31,35)(H,32,37)(H,33,36)/t21?,25-,26-,29-/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 727 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Milan
Curated by ChEMBL
| Assay Description
Inhibition of Plasmodium falciparum recombinant PLM1 |
J Med Chem 49: 7440-9 (2006)
Article DOI: 10.1021/jm061033d
BindingDB Entry DOI: 10.7270/Q29P319W |
More data for this
Ligand-Target Pair | |
Plasmepsin I
(Plasmodium falciparum) | BDBM50200021

((3S,4S)-4-((2S,3S)-2-(4-(4-((S)-(1-(4-(6-methoxyqu...)Show SMILES CCCCNC(=O)C[C@H](O)[C@H](CC(C)C)NC(=O)[C@@H](NC(=O)c1ccc(Oc2ccc(cc2)C(=O)N[C@@H](CC(C)C)C(=O)NCCCC(C)Nc2cc(OC)cc3cccnc23)cc1)C(C)CC |r| Show InChI InChI=1S/C53H75N7O8/c1-10-12-25-54-47(62)32-46(61)43(28-33(3)4)58-53(66)48(35(7)11-2)60-51(64)38-19-23-41(24-20-38)68-40-21-17-37(18-22-40)50(63)59-45(29-34(5)6)52(65)56-27-13-15-36(8)57-44-31-42(67-9)30-39-16-14-26-55-49(39)44/h14,16-24,26,30-31,33-36,43,45-46,48,57,61H,10-13,15,25,27-29,32H2,1-9H3,(H,54,62)(H,56,65)(H,58,66)(H,59,63)(H,60,64)/t35?,36?,43-,45-,46-,48-/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 765 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Milan
Curated by ChEMBL
| Assay Description
Inhibition of Plasmodium falciparum recombinant PLM1 |
J Med Chem 49: 7440-9 (2006)
Article DOI: 10.1021/jm061033d
BindingDB Entry DOI: 10.7270/Q29P319W |
More data for this
Ligand-Target Pair | |
Botulinum neurotoxin type A
(Clostridium botulinum) | BDBM50343187

(CHEMBL1773155 | N,N'-Bis(3-aminopropyl)-3,9-dimeth...)Show SMILES Cc1cc(NCCCN)c2ccc3c(ccc4c(NCCCN)cc(C)nc34)c2n1 Show InChI InChI=1S/C24H30N6/c1-15-13-21(27-11-3-9-25)19-7-6-18-17(23(19)29-15)5-8-20-22(28-12-4-10-26)14-16(2)30-24(18)20/h5-8,13-14H,3-4,9-12,25-26H2,1-2H3,(H,27,29)(H,28,30) | PDB
MMDB
NCI pathway
Reactome pathway
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 8.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Belgrade
Curated by ChEMBL
| Assay Description
Inhibition of clostridium botulinum Botulinum neurotoxin type A light chain at 20 uM |
J Med Chem 54: 1157-69 (2011)
Article DOI: 10.1021/jm100938u
BindingDB Entry DOI: 10.7270/Q26T0MZR |
More data for this
Ligand-Target Pair | |
Botulinum neurotoxin type A
(Clostridium botulinum) | BDBM50343188

(CHEMBL1773156 | N,N'-Bis(2-aminoethyl)-3,9-dimethy...)Show SMILES Cc1cc(NCCN)c2ccc3c(ccc4c(NCCN)cc(C)nc34)c2n1 Show InChI InChI=1S/C22H26N6/c1-13-11-19(25-9-7-23)17-5-4-16-15(21(17)27-13)3-6-18-20(26-10-8-24)12-14(2)28-22(16)18/h3-6,11-12H,7-10,23-24H2,1-2H3,(H,25,27)(H,26,28) | PDB
MMDB
NCI pathway
Reactome pathway
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 8.70E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Belgrade
Curated by ChEMBL
| Assay Description
Inhibition of clostridium botulinum Botulinum neurotoxin type A light chain at 20 uM |
J Med Chem 54: 1157-69 (2011)
Article DOI: 10.1021/jm100938u
BindingDB Entry DOI: 10.7270/Q26T0MZR |
More data for this
Ligand-Target Pair | |
Plasmepsin II
(Plasmodium falciparum) | BDBM50366912
(CHEMBL1790774)Show SMILES CCCCNC(=O)C[C@H](O)[C@H](CC(C)C)NC(=O)[C@@H](NC(=O)c1ccc(Oc2ccc(cc2)C(=O)N[C@H](CC(C)C)C(=O)N[C@H](CCCCN)C(=O)NCCCC(C)Nc2cc(OC)cc3cccnc23)cc1)[C@H](C)CC Show InChI InChI=1S/C59H87N9O9/c1-10-12-29-61-52(70)36-51(69)48(32-37(3)4)66-59(75)53(39(7)11-2)68-56(72)42-22-26-45(27-23-42)77-44-24-20-41(21-25-44)55(71)67-50(33-38(5)6)58(74)65-47(19-13-14-28-60)57(73)63-31-15-17-40(8)64-49-35-46(76-9)34-43-18-16-30-62-54(43)49/h16,18,20-27,30,34-35,37-40,47-48,50-51,53,64,69H,10-15,17,19,28-29,31-33,36,60H2,1-9H3,(H,61,70)(H,63,73)(H,65,74)(H,66,75)(H,67,71)(H,68,72)/t39-,40?,47-,48+,50-,51+,53+/m1/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.590 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Milan
Curated by ChEMBL
| Assay Description
In vitro concentration required for inhibition of Plasmodium falciparum plasmepsin-2 |
Bioorg Med Chem Lett 14: 2931-4 (2004)
Article DOI: 10.1016/j.bmcl.2004.03.030
BindingDB Entry DOI: 10.7270/Q2G44QVR |
More data for this
Ligand-Target Pair | |
Plasmepsin II
(Plasmodium falciparum) | BDBM50366909
(CHEMBL1790773)Show SMILES CCCCNC(=O)C[C@H](O)[C@H](CC(C)C)NC(=O)[C@@H](NC(=O)c1ccc2cc(ccc2c1)C(=O)N[C@H](CC(C)C)C(=O)N[C@H](CCCCN)C(=O)NCCCC(C)Nc1cc(OC)cc2cccnc12)[C@H](C)CC Show InChI InChI=1S/C57H85N9O8/c1-10-12-25-59-50(68)34-49(67)46(28-35(3)4)64-57(73)51(37(7)11-2)66-54(70)43-23-21-39-30-42(22-20-40(39)31-43)53(69)65-48(29-36(5)6)56(72)63-45(19-13-14-24-58)55(71)61-27-15-17-38(8)62-47-33-44(74-9)32-41-18-16-26-60-52(41)47/h16,18,20-23,26,30-33,35-38,45-46,48-49,51,62,67H,10-15,17,19,24-25,27-29,34,58H2,1-9H3,(H,59,68)(H,61,71)(H,63,72)(H,64,73)(H,65,69)(H,66,70)/t37-,38?,45-,46+,48-,49+,51+/m1/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.5 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Milan
Curated by ChEMBL
| Assay Description
In vitro concentration required for inhibition of Plasmodium falciparum plasmepsin-2 |
Bioorg Med Chem Lett 14: 2931-4 (2004)
Article DOI: 10.1016/j.bmcl.2004.03.030
BindingDB Entry DOI: 10.7270/Q2G44QVR |
More data for this
Ligand-Target Pair | |
Plasmepsin II
(Plasmodium falciparum) | BDBM50366908
(CHEMBL1790772)Show SMILES CCCCNC(=O)C[C@H](O)[C@H](CC(C)C)NC(=O)[C@@H](NC(=O)c1ccc2cc(ccc2c1)C(=O)OCCCC(=O)N[C@H](CC(C)C)C(=O)N[C@H](CCCCN)C(=O)NCCCC(C)Nc1cc(OC)cc2cccnc12)[C@H](C)CC Show InChI InChI=1S/C61H91N9O10/c1-10-12-27-63-54(73)37-52(71)49(31-38(3)4)69-60(77)55(40(7)11-2)70-57(74)45-24-22-43-34-46(25-23-42(43)33-45)61(78)80-30-17-21-53(72)67-51(32-39(5)6)59(76)68-48(20-13-14-26-62)58(75)65-29-15-18-41(8)66-50-36-47(79-9)35-44-19-16-28-64-56(44)50/h16,19,22-25,28,33-36,38-41,48-49,51-52,55,66,71H,10-15,17-18,20-21,26-27,29-32,37,62H2,1-9H3,(H,63,73)(H,65,75)(H,67,72)(H,68,76)(H,69,77)(H,70,74)/t40-,41?,48-,49+,51-,52+,55+/m1/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 9.60 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Milan
Curated by ChEMBL
| Assay Description
In vitro concentration required for inhibition of Plasmodium falciparum plasmepsin-2 |
Bioorg Med Chem Lett 14: 2931-4 (2004)
Article DOI: 10.1016/j.bmcl.2004.03.030
BindingDB Entry DOI: 10.7270/Q2G44QVR |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50399931
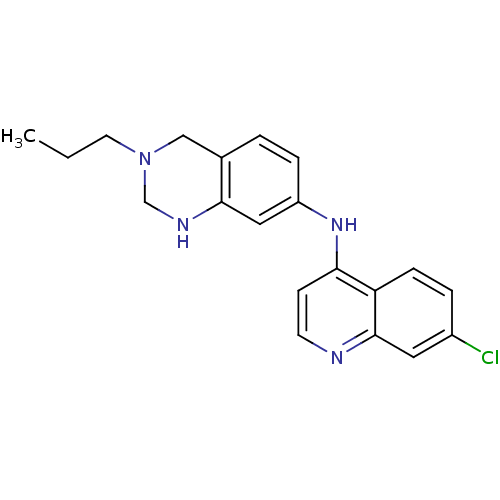
(CHEMBL2181207)Show InChI InChI=1S/C20H21ClN4/c1-2-9-25-12-14-3-5-16(11-19(14)23-13-25)24-18-7-8-22-20-10-15(21)4-6-17(18)20/h3-8,10-11,23H,2,9,12-13H2,1H3,(H,22,24) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 22 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Siena
Curated by ChEMBL
| Assay Description
Inhibition of human ERG expressed in rat after 2 to 3 days by patch clamp assay |
J Med Chem 55: 10387-404 (2012)
Article DOI: 10.1021/jm300831b
BindingDB Entry DOI: 10.7270/Q2N017PR |
More data for this
Ligand-Target Pair | |
Plasmepsin II
(Plasmodium falciparum) | BDBM50146530

((S)-2-[4-(4-{(S)-1-[(S)-1-((S)-2-Butylcarbamoyl-1-...)Show SMILES CCCCNC(=O)C[C@H](O)[C@H](CC(C)C)NC(=O)[C@@H](NC(=O)c1ccc(Oc2ccc(cc2)C(=O)N[C@@H](CC(C)C)C(O)=O)cc1)C(C)CC Show InChI InChI=1S/C38H56N4O8/c1-8-10-19-39-33(44)22-32(43)30(20-23(3)4)40-37(47)34(25(7)9-2)42-36(46)27-13-17-29(18-14-27)50-28-15-11-26(12-16-28)35(45)41-31(38(48)49)21-24(5)6/h11-18,23-25,30-32,34,43H,8-10,19-22H2,1-7H3,(H,39,44)(H,40,47)(H,41,45)(H,42,46)(H,48,49)/t25?,30-,31-,32-,34-/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 123 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Milan
Curated by ChEMBL
| Assay Description
In vitro concentration required for inhibition of Plasmodium falciparum plasmepsin-2 |
Bioorg Med Chem Lett 14: 2931-4 (2004)
Article DOI: 10.1016/j.bmcl.2004.03.030
BindingDB Entry DOI: 10.7270/Q2G44QVR |
More data for this
Ligand-Target Pair | |
Plasmepsin II
(Plasmodium falciparum) | BDBM50366910
(CHEMBL1790776)Show SMILES CCCCNC(=O)C[C@H](O)[C@H](CC(C)C)NC(=O)[C@@H](NC(=O)Cc1ccc(CC(=O)N[C@@H](CC(C)C)C(=O)N[C@@H](CCCCN)C(=O)NCCCC(C)Nc2cc(OC)cc3cccnc23)cc1)[C@H](C)CC Show InChI InChI=1S/C55H87N9O8/c1-10-12-25-57-48(66)34-47(65)44(28-35(3)4)63-55(71)51(37(7)11-2)64-50(68)31-40-22-20-39(21-23-40)30-49(67)61-46(29-36(5)6)54(70)62-43(19-13-14-24-56)53(69)59-27-15-17-38(8)60-45-33-42(72-9)32-41-18-16-26-58-52(41)45/h16,18,20-23,26,32-33,35-38,43-44,46-47,51,60,65H,10-15,17,19,24-25,27-31,34,56H2,1-9H3,(H,57,66)(H,59,69)(H,61,67)(H,62,70)(H,63,71)(H,64,68)/t37-,38?,43+,44+,46+,47+,51+/m1/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 135 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Milan
Curated by ChEMBL
| Assay Description
In vitro concentration required for inhibition of Plasmodium falciparum plasmepsin-2 |
Bioorg Med Chem Lett 14: 2931-4 (2004)
Article DOI: 10.1016/j.bmcl.2004.03.030
BindingDB Entry DOI: 10.7270/Q2G44QVR |
More data for this
Ligand-Target Pair | |
Cathepsin D
(Homo sapiens (Human)) | BDBM50390633

(CHEMBL2069612)Show SMILES CCCCNC(=O)C[C@H](O)[C@H](CC(C)C)NC(=O)[C@@H](NC(=O)c1ccc(Oc2ccc(cc2)C(=O)NCCCNc2ccnc3cc(Cl)ccc23)cc1)[C@@H](C)CC |r| Show InChI InChI=1S/C44H57ClN6O6/c1-6-8-21-48-40(53)27-39(52)38(25-28(3)4)50-44(56)41(29(5)7-2)51-43(55)31-12-17-34(18-13-31)57-33-15-10-30(11-16-33)42(54)49-23-9-22-46-36-20-24-47-37-26-32(45)14-19-35(36)37/h10-20,24,26,28-29,38-39,41,52H,6-9,21-23,25,27H2,1-5H3,(H,46,47)(H,48,53)(H,49,54)(H,50,56)(H,51,55)/t29-,38-,39-,41-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 220 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Milano
Curated by ChEMBL
| Assay Description
Inhibition of human cathepsin D |
Bioorg Med Chem Lett 22: 5915-8 (2012)
Article DOI: 10.1016/j.bmcl.2012.07.069
BindingDB Entry DOI: 10.7270/Q2M046HX |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2D6
(Homo sapiens (Human)) | BDBM50247629
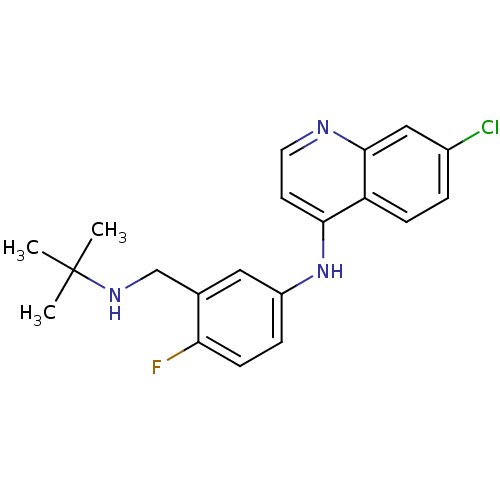
(CHEMBL453384 | N-(3-((tert-butylamino)methyl)-4-fl...)Show InChI InChI=1S/C20H21ClFN3/c1-20(2,3)24-12-13-10-15(5-7-17(13)22)25-18-8-9-23-19-11-14(21)4-6-16(18)19/h4-11,24H,12H2,1-3H3,(H,23,25) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 300 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Liverpool
Curated by ChEMBL
| Assay Description
Inhibition of human CYP2D6 |
J Med Chem 52: 1828-44 (2009)
Article DOI: 10.1021/jm8012757
BindingDB Entry DOI: 10.7270/Q21Z45C3 |
More data for this
Ligand-Target Pair | |
Cathepsin D
(Homo sapiens (Human)) | BDBM50390635

(CHEMBL2069614)Show SMILES CCCCNC(=O)C[C@H](O)[C@H](CC(C)C)NC(=O)[C@@H](NC(=O)c1ccc(Oc2ccc(cc2)C(=O)N[C@@H](CC(C)C)C(=O)N[C@@H](C)C(=O)NCCCNc2ccnc3ccccc23)cc1)[C@@H](C)CC |r| Show InChI InChI=1S/C53H74N8O8/c1-9-11-26-56-47(63)32-46(62)44(30-33(3)4)59-53(68)48(35(7)10-2)61-51(66)38-19-23-40(24-20-38)69-39-21-17-37(18-22-39)50(65)60-45(31-34(5)6)52(67)58-36(8)49(64)57-28-14-27-54-43-25-29-55-42-16-13-12-15-41(42)43/h12-13,15-25,29,33-36,44-46,48,62H,9-11,14,26-28,30-32H2,1-8H3,(H,54,55)(H,56,63)(H,57,64)(H,58,67)(H,59,68)(H,60,65)(H,61,66)/t35-,36-,44-,45-,46-,48-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 300 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Milano
Curated by ChEMBL
| Assay Description
Inhibition of human cathepsin D |
Bioorg Med Chem Lett 22: 5915-8 (2012)
Article DOI: 10.1016/j.bmcl.2012.07.069
BindingDB Entry DOI: 10.7270/Q2M046HX |
More data for this
Ligand-Target Pair | |
Plasmepsin II
(Plasmodium falciparum) | BDBM50366911

(CHEMBL1790775)Show SMILES CCCCNC(=O)C[C@H](O)[C@H](CC(C)C)NC(=O)[C@@H](NC(=O)CCC(=O)N[C@@H](CC(C)C)C(=O)N[C@@H](CCCCN)C(=O)NCCCC(C)Nc1cc(OC)cc2cccnc12)[C@H](C)CC Show InChI InChI=1S/C49H83N9O8/c1-10-12-23-51-44(62)30-41(59)38(26-31(3)4)57-49(65)45(33(7)11-2)58-43(61)21-20-42(60)55-40(27-32(5)6)48(64)56-37(19-13-14-22-50)47(63)53-25-15-17-34(8)54-39-29-36(66-9)28-35-18-16-24-52-46(35)39/h16,18,24,28-29,31-34,37-38,40-41,45,54,59H,10-15,17,19-23,25-27,30,50H2,1-9H3,(H,51,62)(H,53,63)(H,55,60)(H,56,64)(H,57,65)(H,58,61)/t33-,34?,37+,38+,40+,41+,45+/m1/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 396 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Milan
Curated by ChEMBL
| Assay Description
In vitro concentration required for inhibition of Plasmodium falciparum plasmepsin-2 |
Bioorg Med Chem Lett 14: 2931-4 (2004)
Article DOI: 10.1016/j.bmcl.2004.03.030
BindingDB Entry DOI: 10.7270/Q2G44QVR |
More data for this
Ligand-Target Pair | |
Cathepsin D
(Homo sapiens (Human)) | BDBM50390634

(CHEMBL2069613)Show SMILES CCCCNC(=O)C[C@H](O)[C@H](CC(C)C)NC(=O)[C@@H](NC(=O)c1ccc(Oc2ccc(cc2)C(=O)N[C@@H](C)C(=O)NCCCNc2ccnc3cc(Cl)ccc23)cc1)[C@@H](C)CC |r| Show InChI InChI=1S/C47H62ClN7O7/c1-7-9-22-51-42(57)28-41(56)40(26-29(3)4)54-47(61)43(30(5)8-2)55-46(60)33-13-18-36(19-14-33)62-35-16-11-32(12-17-35)45(59)53-31(6)44(58)52-24-10-23-49-38-21-25-50-39-27-34(48)15-20-37(38)39/h11-21,25,27,29-31,40-41,43,56H,7-10,22-24,26,28H2,1-6H3,(H,49,50)(H,51,57)(H,52,58)(H,53,59)(H,54,61)(H,55,60)/t30-,31-,40-,41-,43-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 540 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Milano
Curated by ChEMBL
| Assay Description
Inhibition of human cathepsin D |
Bioorg Med Chem Lett 22: 5915-8 (2012)
Article DOI: 10.1016/j.bmcl.2012.07.069
BindingDB Entry DOI: 10.7270/Q2M046HX |
More data for this
Ligand-Target Pair | |
Plasmepsin II
(Plasmodium falciparum) | BDBM50146528

(6-Amino-2-(S)-amino-hexanoic acid [4-(6-methoxy-qu...)Show SMILES COc1cc(NC(C)CCCNC(=O)[C@@H](N)CCCCN)c2ncccc2c1 Show InChI InChI=1S/C21H33N5O2/c1-15(7-5-12-25-21(27)18(23)9-3-4-10-22)26-19-14-17(28-2)13-16-8-6-11-24-20(16)19/h6,8,11,13-15,18,26H,3-5,7,9-10,12,22-23H2,1-2H3,(H,25,27)/t15?,18-/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | >1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Milan
Curated by ChEMBL
| Assay Description
In vitro concentration required for inhibition of Plasmodium falciparum plasmepsin-2 |
Bioorg Med Chem Lett 14: 2931-4 (2004)
Article DOI: 10.1016/j.bmcl.2004.03.030
BindingDB Entry DOI: 10.7270/Q2G44QVR |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50121975

((6-Methoxy-quinolin-4-yl)-(5-vinyl-1-aza-bicyclo[2...)Show SMILES COc1ccc2nccc([C@H](O)[C@H]3C[C@@H]4CCN3C[C@@H]4C=C)c2c1 |r,THB:20:19:12.13:16.15,10:12:18.19:16.15| Show InChI InChI=1S/C20H24N2O2/c1-3-13-12-22-9-7-14(13)10-19(22)20(23)16-6-8-21-18-5-4-15(24-2)11-17(16)18/h3-6,8,11,13-14,19-20,23H,1,7,9-10,12H2,2H3/t13-,14-,19+,20-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
Article
PubMed
| n/a | n/a | 2.67E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Chemistry
Curated by ChEMBL
| Assay Description
Inhibition of human ERG expressed in CHO cells by patch plate method |
J Med Chem 59: 264-81 (2016)
Article DOI: 10.1021/acs.jmedchem.5b01374
BindingDB Entry DOI: 10.7270/Q2CR5W7D |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2C8
(Homo sapiens (Human)) | BDBM50041457

(4-[(7-chloroquinolin-4-yl)amino]-2-[(diethylamino)...)Show InChI InChI=1S/C20H22ClN3O/c1-3-24(4-2)13-14-11-16(6-8-20(14)25)23-18-9-10-22-19-12-15(21)5-7-17(18)19/h5-12,25H,3-4,13H2,1-2H3,(H,22,23) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
Article
PubMed
| n/a | n/a | 2.70E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Liverpool
Curated by ChEMBL
| Assay Description
Inhibition of human CYP2C8 |
J Med Chem 52: 1828-44 (2009)
Article DOI: 10.1021/jm8012757
BindingDB Entry DOI: 10.7270/Q21Z45C3 |
More data for this
Ligand-Target Pair | |
Voltage-dependent L-type calcium channel subunit alpha-1C
(RAT) | BDBM50462274

(CHEMBL4246869)Show SMILES Clc1ccc(-c2ccc(CNCC3CCNCC3)o2)c(c1)-c1ccccc1 Show InChI InChI=1S/C23H25ClN2O/c24-19-6-8-21(22(14-19)18-4-2-1-3-5-18)23-9-7-20(27-23)16-26-15-17-10-12-25-13-11-17/h1-9,14,17,25-26H,10-13,15-16H2 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 3.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Siena
Curated by ChEMBL
| Assay Description
Inhibition of CaV1.2 channel in rat tail artery myocytes assessed as reduction in Ba2+ current at -50 mV holding potential by whole-cell patch-clamp ... |
Eur J Med Chem 150: 698-718 (2018)
Article DOI: 10.1016/j.ejmech.2018.03.024
BindingDB Entry DOI: 10.7270/Q2PC3518 |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50142038
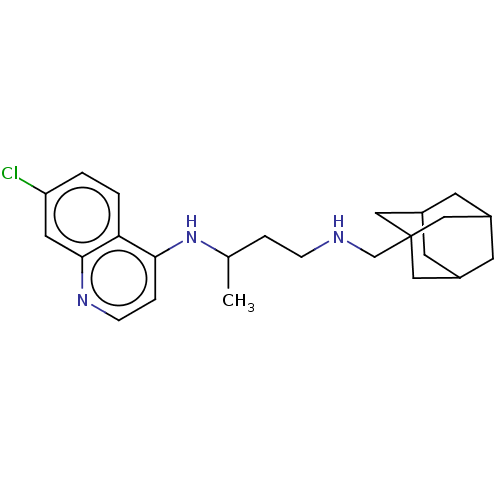
(CHEMBL3758349)Show SMILES CC(CCNCC12CC3CC(CC(C3)C1)C2)Nc1ccnc2cc(Cl)ccc12 |TLB:13:8:15:12.14.11,13:12:15:7.8.9,THB:11:10:7:13.12.14,11:12:7:15.9.10| Show InChI InChI=1S/C24H32ClN3/c1-16(28-22-5-7-27-23-11-20(25)2-3-21(22)23)4-6-26-15-24-12-17-8-18(13-24)10-19(9-17)14-24/h2-3,5,7,11,16-19,26H,4,6,8-10,12-15H2,1H3,(H,27,28) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | >5.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Chemistry
Curated by ChEMBL
| Assay Description
Inhibition of human ERG expressed in CHO cells by patch plate method |
J Med Chem 59: 264-81 (2016)
Article DOI: 10.1021/acs.jmedchem.5b01374
BindingDB Entry DOI: 10.7270/Q2CR5W7D |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50142039

(CHEMBL3758840)Show SMILES CC(CCNCC12CC3CC(CC(C3)C1)C2)Nc1c(F)cnc2cc(Cl)ccc12 |TLB:13:8:15:12.14.11,13:12:15:7.8.9,THB:11:10:7:13.12.14,11:12:7:15.9.10| Show InChI InChI=1S/C24H31ClFN3/c1-15(29-23-20-3-2-19(25)9-22(20)28-13-21(23)26)4-5-27-14-24-10-16-6-17(11-24)8-18(7-16)12-24/h2-3,9,13,15-18,27H,4-8,10-12,14H2,1H3,(H,28,29) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >5.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Chemistry
Curated by ChEMBL
| Assay Description
Inhibition of human ERG expressed in CHO cells by patch plate method |
J Med Chem 59: 264-81 (2016)
Article DOI: 10.1021/acs.jmedchem.5b01374
BindingDB Entry DOI: 10.7270/Q2CR5W7D |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2D6
(Homo sapiens (Human)) | BDBM50041457

(4-[(7-chloroquinolin-4-yl)amino]-2-[(diethylamino)...)Show InChI InChI=1S/C20H22ClN3O/c1-3-24(4-2)13-14-11-16(6-8-20(14)25)23-18-9-10-22-19-12-15(21)5-7-17(18)19/h5-12,25H,3-4,13H2,1-2H3,(H,22,23) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 6.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Liverpool
Curated by ChEMBL
| Assay Description
Inhibition of human CYP2D6 |
J Med Chem 52: 1828-44 (2009)
Article DOI: 10.1021/jm8012757
BindingDB Entry DOI: 10.7270/Q21Z45C3 |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2D6
(Homo sapiens (Human)) | BDBM50056190
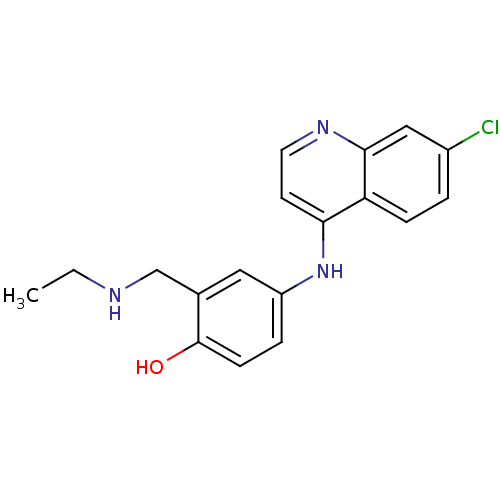
(4-(7-Chloro-quinolin-4-ylamino)-2-ethylaminomethyl...)Show InChI InChI=1S/C18H18ClN3O/c1-2-20-11-12-9-14(4-6-18(12)23)22-16-7-8-21-17-10-13(19)3-5-15(16)17/h3-10,20,23H,2,11H2,1H3,(H,21,22) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
MMDB
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 6.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Liverpool
Curated by ChEMBL
| Assay Description
Inhibition of human CYP2D6 |
J Med Chem 52: 1828-44 (2009)
Article DOI: 10.1021/jm8012757
BindingDB Entry DOI: 10.7270/Q21Z45C3 |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2D6
(Homo sapiens (Human)) | BDBM50134931
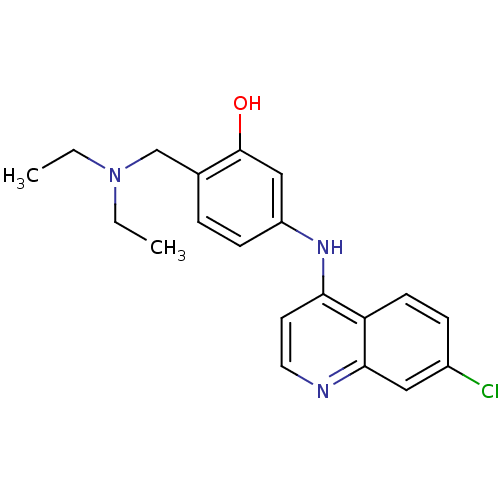
(5-(7-Chloro-quinolin-4-ylamino)-2-diethylaminometh...)Show InChI InChI=1S/C20H22ClN3O/c1-3-24(4-2)13-14-5-7-16(12-20(14)25)23-18-9-10-22-19-11-15(21)6-8-17(18)19/h5-12,25H,3-4,13H2,1-2H3,(H,22,23) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 7.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Liverpool
Curated by ChEMBL
| Assay Description
Inhibition of human CYP2D6 |
J Med Chem 52: 1828-44 (2009)
Article DOI: 10.1021/jm8012757
BindingDB Entry DOI: 10.7270/Q21Z45C3 |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data