 Found 315 hits with Last Name = 'tasaka' and Initial = 'a'
Found 315 hits with Last Name = 'tasaka' and Initial = 'a' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Androgen receptor
(Homo sapiens (Human)) | BDBM50068091
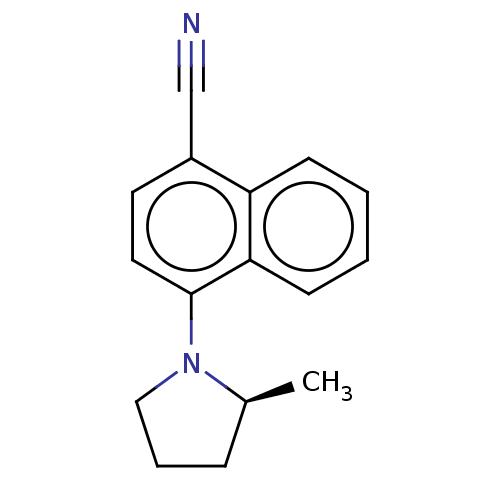
(CHEMBL3402221)Show InChI InChI=1S/C16H16N2/c1-12-5-4-10-18(12)16-9-8-13(11-17)14-6-2-3-7-15(14)16/h2-3,6-9,12H,4-5,10H2,1H3/t12-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.840 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company Ltd
Curated by ChEMBL
| Assay Description
Displacement of [3H]mibolerone from androgen receptor (unknown origin) after 3 hrs |
Bioorg Med Chem 23: 2568-78 (2015)
Article DOI: 10.1016/j.bmc.2015.03.032
BindingDB Entry DOI: 10.7270/Q2JM2C94 |
More data for this
Ligand-Target Pair | |
Androgen receptor
(Homo sapiens (Human)) | BDBM50068096
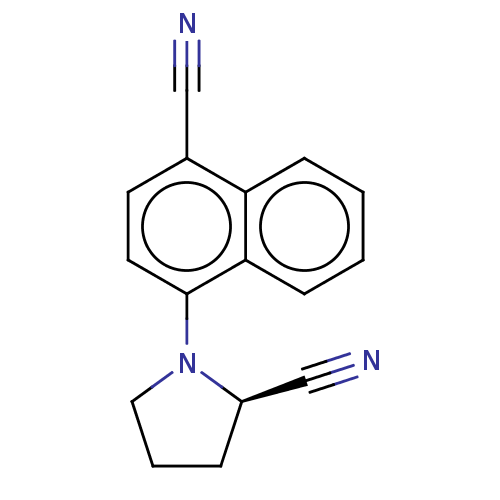
(CHEMBL3402227)Show InChI InChI=1S/C16H13N3/c17-10-12-7-8-16(15-6-2-1-5-14(12)15)19-9-3-4-13(19)11-18/h1-2,5-8,13H,3-4,9H2/t13-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company Ltd
Curated by ChEMBL
| Assay Description
Displacement of [3H]mibolerone from androgen receptor (unknown origin) after 3 hrs |
Bioorg Med Chem 23: 2568-78 (2015)
Article DOI: 10.1016/j.bmc.2015.03.032
BindingDB Entry DOI: 10.7270/Q2JM2C94 |
More data for this
Ligand-Target Pair | |
Androgen receptor
(Homo sapiens (Human)) | BDBM50068093
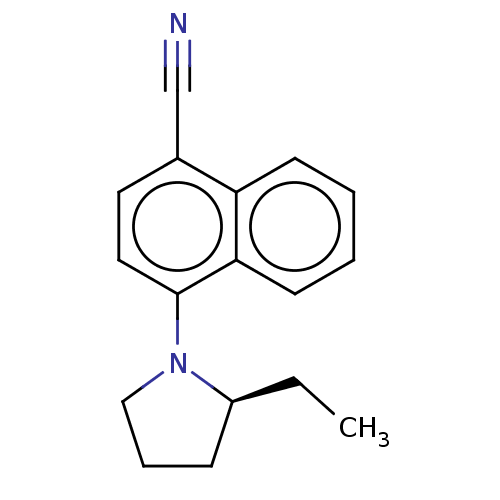
(CHEMBL3402224)Show InChI InChI=1S/C17H18N2/c1-2-14-6-5-11-19(14)17-10-9-13(12-18)15-7-3-4-8-16(15)17/h3-4,7-10,14H,2,5-6,11H2,1H3/t14-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.10 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company Ltd
Curated by ChEMBL
| Assay Description
Displacement of [3H]mibolerone from androgen receptor (unknown origin) after 3 hrs |
Bioorg Med Chem 23: 2568-78 (2015)
Article DOI: 10.1016/j.bmc.2015.03.032
BindingDB Entry DOI: 10.7270/Q2JM2C94 |
More data for this
Ligand-Target Pair | |
Androgen receptor
(Homo sapiens (Human)) | BDBM50068101
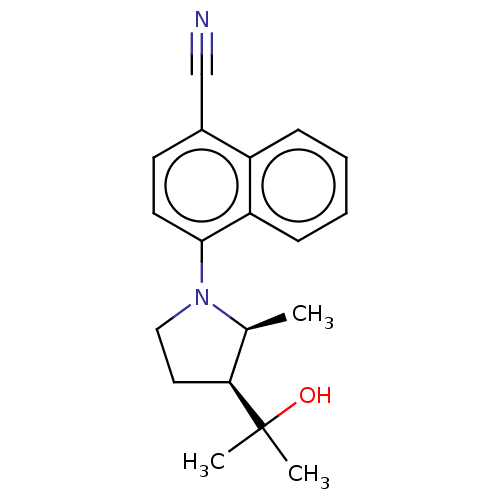
(CHEMBL3402232)Show SMILES C[C@H]1[C@H](CCN1c1ccc(C#N)c2ccccc12)C(C)(C)O |r| Show InChI InChI=1S/C19H22N2O/c1-13-17(19(2,3)22)10-11-21(13)18-9-8-14(12-20)15-6-4-5-7-16(15)18/h4-9,13,17,22H,10-11H2,1-3H3/t13-,17-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.40 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company Ltd
Curated by ChEMBL
| Assay Description
Displacement of [3H]mibolerone from androgen receptor (unknown origin) after 3 hrs |
Bioorg Med Chem 23: 2568-78 (2015)
Article DOI: 10.1016/j.bmc.2015.03.032
BindingDB Entry DOI: 10.7270/Q2JM2C94 |
More data for this
Ligand-Target Pair | |
Androgen receptor
(Homo sapiens (Human)) | BDBM50360442
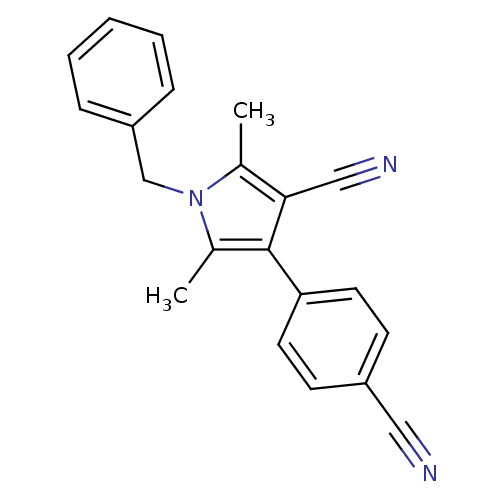
(CHEMBL1934442)Show InChI InChI=1S/C21H17N3/c1-15-20(13-23)21(19-10-8-17(12-22)9-11-19)16(2)24(15)14-18-6-4-3-5-7-18/h3-11H,14H2,1-2H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.40 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company Ltd
Curated by ChEMBL
| Assay Description
Displacement of [17-alpha-methyl-3H]-mibolerone from human androgen receptor expressed in HEK293 derived FreeStyle293F cells after 3 hrs |
Bioorg Med Chem 20: 422-34 (2011)
Article DOI: 10.1016/j.bmc.2011.10.067
BindingDB Entry DOI: 10.7270/Q2251JM1 |
More data for this
Ligand-Target Pair | |
Androgen receptor
(Homo sapiens (Human)) | BDBM50068094
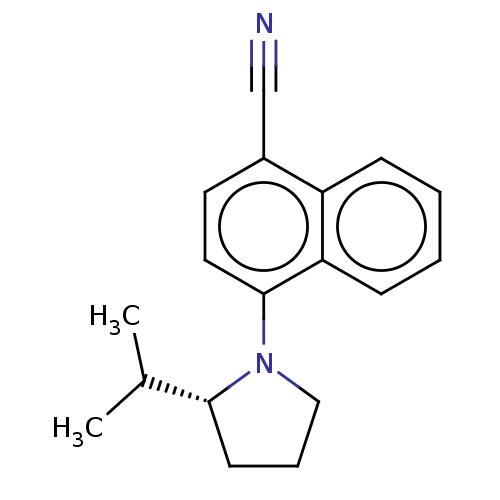
(CHEMBL3402225)Show InChI InChI=1S/C18H20N2/c1-13(2)17-8-5-11-20(17)18-10-9-14(12-19)15-6-3-4-7-16(15)18/h3-4,6-7,9-10,13,17H,5,8,11H2,1-2H3/t17-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.40 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company Ltd
Curated by ChEMBL
| Assay Description
Displacement of [3H]mibolerone from androgen receptor (unknown origin) after 3 hrs |
Bioorg Med Chem 23: 2568-78 (2015)
Article DOI: 10.1016/j.bmc.2015.03.032
BindingDB Entry DOI: 10.7270/Q2JM2C94 |
More data for this
Ligand-Target Pair | |
Androgen receptor
(Homo sapiens (Human)) | BDBM18161

((1S,2S,7S,10R,11S,14S,15S)-14-hydroxy-2,15-dimethy...)Show SMILES [H][C@@]12CC[C@H](O)[C@@]1(C)CC[C@@]1([H])[C@@]2([H])CC[C@@]2([H])CC(=O)CC[C@]12C |r| Show InChI InChI=1S/C19H30O2/c1-18-9-7-13(20)11-12(18)3-4-14-15-5-6-17(21)19(15,2)10-8-16(14)18/h12,14-17,21H,3-11H2,1-2H3/t12-,14-,15-,16-,17-,18-,19-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| n/a | n/a | 2.10 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company Ltd
Curated by ChEMBL
| Assay Description
Displacement of [17-alpha-methyl-3H]-mibolerone from human androgen receptor expressed in HEK293 derived FreeStyle293F cells after 3 hrs |
Bioorg Med Chem 20: 422-34 (2011)
Article DOI: 10.1016/j.bmc.2011.10.067
BindingDB Entry DOI: 10.7270/Q2251JM1 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Androgen receptor
(Homo sapiens (Human)) | BDBM50068097
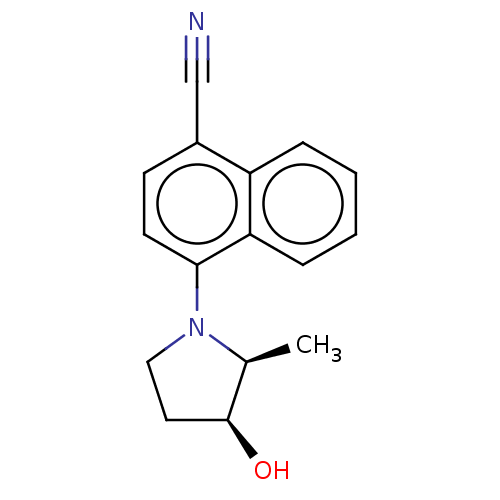
(CHEMBL3402228)Show SMILES C[C@H]1[C@@H](O)CCN1c1ccc(C#N)c2ccccc12 |r| Show InChI InChI=1S/C16H16N2O/c1-11-16(19)8-9-18(11)15-7-6-12(10-17)13-4-2-3-5-14(13)15/h2-7,11,16,19H,8-9H2,1H3/t11-,16-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company Ltd
Curated by ChEMBL
| Assay Description
Displacement of [3H]mibolerone from androgen receptor (unknown origin) after 3 hrs |
Bioorg Med Chem 23: 2568-78 (2015)
Article DOI: 10.1016/j.bmc.2015.03.032
BindingDB Entry DOI: 10.7270/Q2JM2C94 |
More data for this
Ligand-Target Pair | |
Androgen receptor
(Homo sapiens (Human)) | BDBM50068098
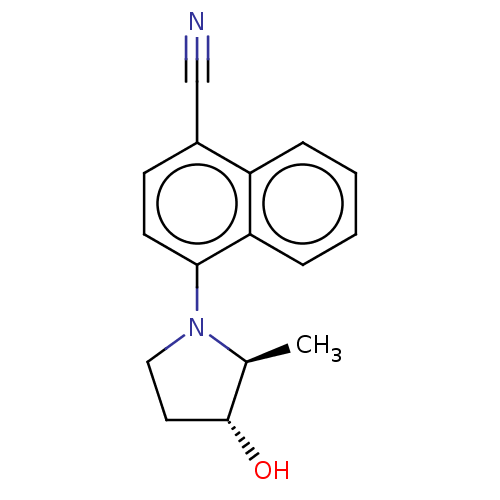
(CHEMBL3402229)Show InChI InChI=1S/C16H16N2O/c1-11-16(19)8-9-18(11)15-7-6-12(10-17)13-4-2-3-5-14(13)15/h2-7,11,16,19H,8-9H2,1H3/t11-,16+/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.40 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company Ltd
Curated by ChEMBL
| Assay Description
Displacement of [3H]mibolerone from androgen receptor (unknown origin) after 3 hrs |
Bioorg Med Chem 23: 2568-78 (2015)
Article DOI: 10.1016/j.bmc.2015.03.032
BindingDB Entry DOI: 10.7270/Q2JM2C94 |
More data for this
Ligand-Target Pair | |
Androgen receptor
(Homo sapiens (Human)) | BDBM50415097
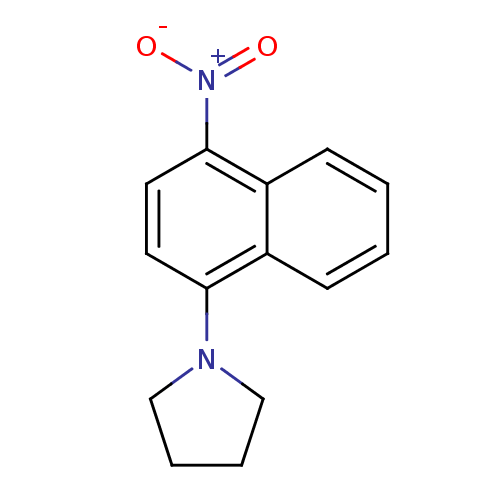
(CHEMBL570897)Show InChI InChI=1S/C14H14N2O2/c17-16(18)14-8-7-13(15-9-3-4-10-15)11-5-1-2-6-12(11)14/h1-2,5-8H,3-4,9-10H2 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 2.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company Ltd
Curated by ChEMBL
| Assay Description
Displacement of [3H]mibolerone from androgen receptor (unknown origin) after 3 hrs |
Bioorg Med Chem 23: 2568-78 (2015)
Article DOI: 10.1016/j.bmc.2015.03.032
BindingDB Entry DOI: 10.7270/Q2JM2C94 |
More data for this
Ligand-Target Pair | |
Androgen receptor
(Homo sapiens (Human)) | BDBM50415098

(CHEMBL570898)Show InChI InChI=1S/C15H14N2/c16-11-12-7-8-15(17-9-3-4-10-17)14-6-2-1-5-13(12)14/h1-2,5-8H,3-4,9-10H2 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company Ltd
Curated by ChEMBL
| Assay Description
Displacement of [3H]mibolerone from androgen receptor (unknown origin) after 3 hrs |
Bioorg Med Chem 23: 2568-78 (2015)
Article DOI: 10.1016/j.bmc.2015.03.032
BindingDB Entry DOI: 10.7270/Q2JM2C94 |
More data for this
Ligand-Target Pair | |
Androgen receptor
(Homo sapiens (Human)) | BDBM50360443
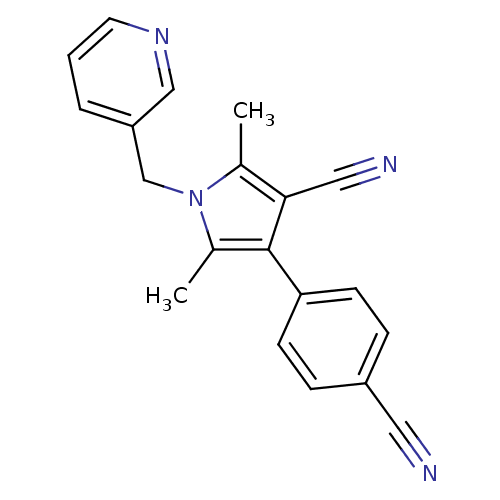
(CHEMBL1934443)Show InChI InChI=1S/C20H16N4/c1-14-19(11-22)20(18-7-5-16(10-21)6-8-18)15(2)24(14)13-17-4-3-9-23-12-17/h3-9,12H,13H2,1-2H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3.40 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company Ltd
Curated by ChEMBL
| Assay Description
Displacement of [17-alpha-methyl-3H]-mibolerone from human androgen receptor expressed in HEK293 derived FreeStyle293F cells after 3 hrs |
Bioorg Med Chem 20: 422-34 (2011)
Article DOI: 10.1016/j.bmc.2011.10.067
BindingDB Entry DOI: 10.7270/Q2251JM1 |
More data for this
Ligand-Target Pair | |
Androgen receptor
(Homo sapiens (Human)) | BDBM50360441

(CHEMBL1934441)Show SMILES COC(=O)c1c(C)n(Cc2ccccc2)c(C)c1-c1ccc(C#N)c(c1)C(F)(F)F Show InChI InChI=1S/C23H19F3N2O2/c1-14-20(17-9-10-18(12-27)19(11-17)23(24,25)26)21(22(29)30-3)15(2)28(14)13-16-7-5-4-6-8-16/h4-11H,13H2,1-3H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3.90 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company Ltd
Curated by ChEMBL
| Assay Description
Displacement of [17-alpha-methyl-3H]-mibolerone from human androgen receptor expressed in HEK293 derived FreeStyle293F cells after 3 hrs |
Bioorg Med Chem 20: 422-34 (2011)
Article DOI: 10.1016/j.bmc.2011.10.067
BindingDB Entry DOI: 10.7270/Q2251JM1 |
More data for this
Ligand-Target Pair | |
Androgen receptor
(Homo sapiens (Human)) | BDBM50068100
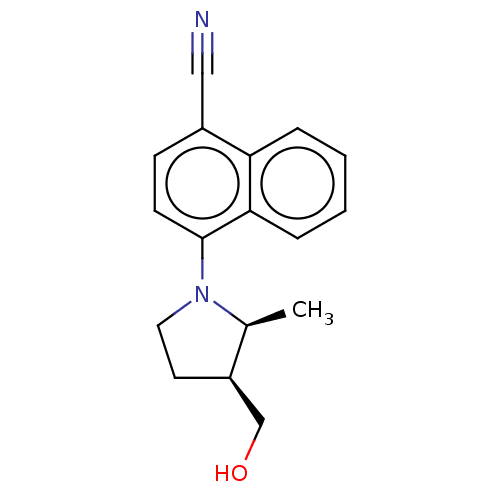
(CHEMBL3402231)Show SMILES C[C@H]1[C@@H](CO)CCN1c1ccc(C#N)c2ccccc12 |r| Show InChI InChI=1S/C17H18N2O/c1-12-14(11-20)8-9-19(12)17-7-6-13(10-18)15-4-2-3-5-16(15)17/h2-7,12,14,20H,8-9,11H2,1H3/t12-,14+/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 4.10 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company Ltd
Curated by ChEMBL
| Assay Description
Displacement of [3H]mibolerone from androgen receptor (unknown origin) after 3 hrs |
Bioorg Med Chem 23: 2568-78 (2015)
Article DOI: 10.1016/j.bmc.2015.03.032
BindingDB Entry DOI: 10.7270/Q2JM2C94 |
More data for this
Ligand-Target Pair | |
Androgen receptor
(Homo sapiens (Human)) | BDBM50360434
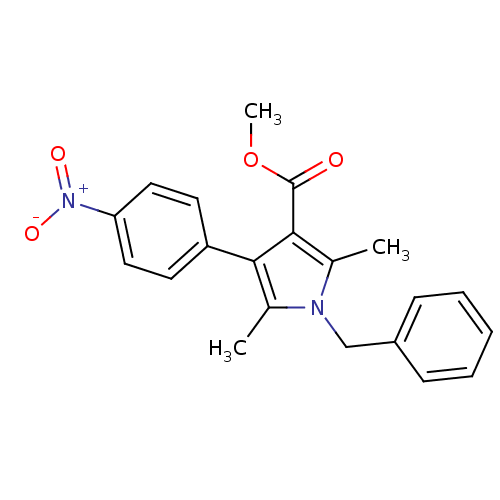
(CHEMBL1934434)Show SMILES COC(=O)c1c(C)n(Cc2ccccc2)c(C)c1-c1ccc(cc1)[N+]([O-])=O Show InChI InChI=1S/C21H20N2O4/c1-14-19(17-9-11-18(12-10-17)23(25)26)20(21(24)27-3)15(2)22(14)13-16-7-5-4-6-8-16/h4-12H,13H2,1-3H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company Ltd
Curated by ChEMBL
| Assay Description
Displacement of [17-alpha-methyl-3H]-mibolerone from human androgen receptor expressed in HEK293 derived FreeStyle293F cells after 3 hrs |
Bioorg Med Chem 20: 422-34 (2011)
Article DOI: 10.1016/j.bmc.2011.10.067
BindingDB Entry DOI: 10.7270/Q2251JM1 |
More data for this
Ligand-Target Pair | |
Steroid 17-alpha-hydroxylase/17,20 lyase
(Homo sapiens (Human)) | BDBM50358192
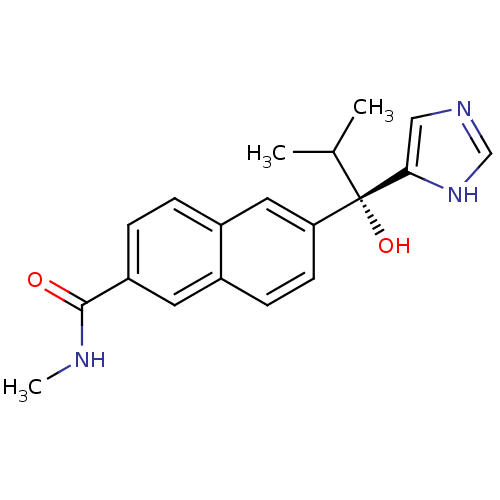
(CHEMBL1921975)Show SMILES CNC(=O)c1ccc2cc(ccc2c1)[C@@](O)(C(C)C)c1cnc[nH]1 |r| Show InChI InChI=1S/C19H21N3O2/c1-12(2)19(24,17-10-21-11-22-17)16-7-6-13-8-15(18(23)20-3)5-4-14(13)9-16/h4-12,24H,1-3H3,(H,20,23)(H,21,22)/t19-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 5.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company, Ltd.
Curated by ChEMBL
| Assay Description
Inhibition of 17,20-lyase activity of human CYP17A1 |
Bioorg Med Chem 19: 6383-99 (2011)
Article DOI: 10.1016/j.bmc.2011.08.066
BindingDB Entry DOI: 10.7270/Q2X63NDF |
More data for this
Ligand-Target Pair | |
Androgen receptor
(Homo sapiens (Human)) | BDBM50360429
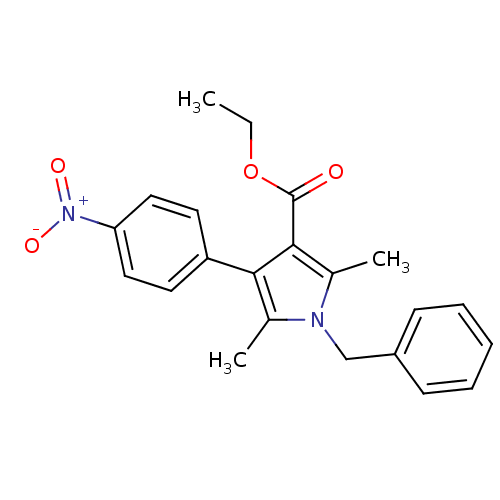
(CHEMBL1934430)Show SMILES CCOC(=O)c1c(C)n(Cc2ccccc2)c(C)c1-c1ccc(cc1)[N+]([O-])=O Show InChI InChI=1S/C22H22N2O4/c1-4-28-22(25)21-16(3)23(14-17-8-6-5-7-9-17)15(2)20(21)18-10-12-19(13-11-18)24(26)27/h5-13H,4,14H2,1-3H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 5.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company Ltd
Curated by ChEMBL
| Assay Description
Displacement of [17-alpha-methyl-3H]-mibolerone from human androgen receptor expressed in HEK293 derived FreeStyle293F cells after 3 hrs |
Bioorg Med Chem 20: 422-34 (2011)
Article DOI: 10.1016/j.bmc.2011.10.067
BindingDB Entry DOI: 10.7270/Q2251JM1 |
More data for this
Ligand-Target Pair | |
Steroid 17-alpha-hydroxylase/17,20 lyase
(Rattus norvegicus (Rat)) | BDBM50338363
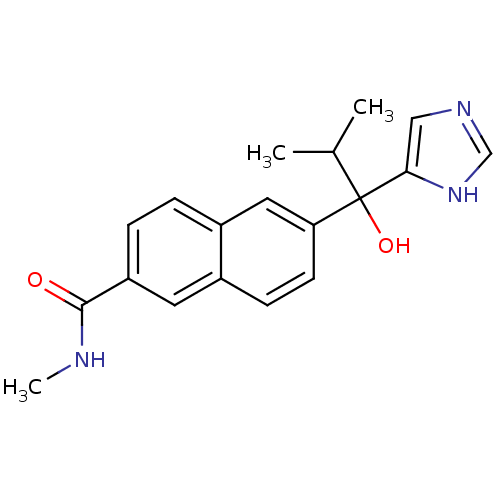
(CHEMBL1682893 | rac-6-[1-Hydroxy-1-(1H-imidazol-4-...)Show SMILES CNC(=O)c1ccc2cc(ccc2c1)C(O)(C(C)C)c1cnc[nH]1 Show InChI InChI=1S/C19H21N3O2/c1-12(2)19(24,17-10-21-11-22-17)16-7-6-13-8-15(18(23)20-3)5-4-14(13)9-16/h4-12,24H,1-3H3,(H,20,23)(H,21,22) | KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company, Ltd
Curated by ChEMBL
| Assay Description
Inhibition of 17,20 lyase activity in Sprague-Dawley rat testicular microsomes |
Bioorg Med Chem 19: 1751-70 (2011)
Article DOI: 10.1016/j.bmc.2011.01.017
BindingDB Entry DOI: 10.7270/Q2X92C9F |
More data for this
Ligand-Target Pair | |
Steroid 17-alpha-hydroxylase/17,20 lyase
(Rattus norvegicus (Rat)) | BDBM50338363

(CHEMBL1682893 | rac-6-[1-Hydroxy-1-(1H-imidazol-4-...)Show SMILES CNC(=O)c1ccc2cc(ccc2c1)C(O)(C(C)C)c1cnc[nH]1 Show InChI InChI=1S/C19H21N3O2/c1-12(2)19(24,17-10-21-11-22-17)16-7-6-13-8-15(18(23)20-3)5-4-14(13)9-16/h4-12,24H,1-3H3,(H,20,23)(H,21,22) | KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 6.10 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company, Ltd.
Curated by ChEMBL
| Assay Description
Inhibition of 17,20-lyase activity of Sprague-Dawley rat testicular microsomal CYP17A1 using [1,2-3H]-17a-hydroxyprogesterone as substrate after 15 m... |
Bioorg Med Chem 19: 6383-99 (2011)
Article DOI: 10.1016/j.bmc.2011.08.066
BindingDB Entry DOI: 10.7270/Q2X63NDF |
More data for this
Ligand-Target Pair | |
Androgen receptor
(Homo sapiens (Human)) | BDBM50360438
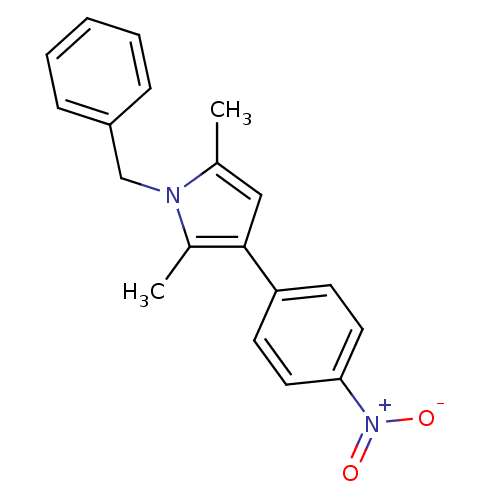
(CHEMBL1934438)Show SMILES Cc1cc(c(C)n1Cc1ccccc1)-c1ccc(cc1)[N+]([O-])=O Show InChI InChI=1S/C19H18N2O2/c1-14-12-19(17-8-10-18(11-9-17)21(22)23)15(2)20(14)13-16-6-4-3-5-7-16/h3-12H,13H2,1-2H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 6.70 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company Ltd
Curated by ChEMBL
| Assay Description
Displacement of [17-alpha-methyl-3H]-mibolerone from human androgen receptor expressed in HEK293 derived FreeStyle293F cells after 3 hrs |
Bioorg Med Chem 20: 422-34 (2011)
Article DOI: 10.1016/j.bmc.2011.10.067
BindingDB Entry DOI: 10.7270/Q2251JM1 |
More data for this
Ligand-Target Pair | |
Androgen receptor
(Homo sapiens (Human)) | BDBM50068089
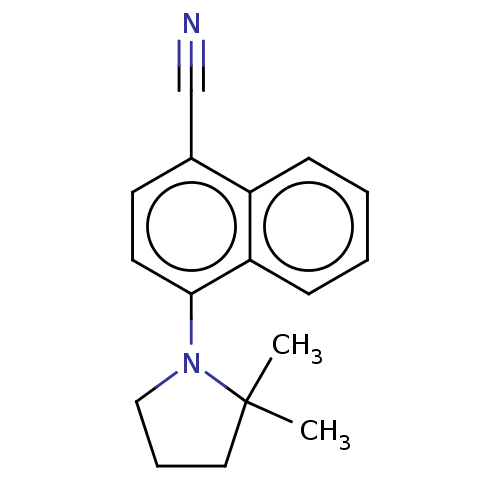
(CHEMBL3402223)Show InChI InChI=1S/C17H18N2/c1-17(2)10-5-11-19(17)16-9-8-13(12-18)14-6-3-4-7-15(14)16/h3-4,6-9H,5,10-11H2,1-2H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 7 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company Ltd
Curated by ChEMBL
| Assay Description
Displacement of [3H]mibolerone from androgen receptor (unknown origin) after 3 hrs |
Bioorg Med Chem 23: 2568-78 (2015)
Article DOI: 10.1016/j.bmc.2015.03.032
BindingDB Entry DOI: 10.7270/Q2JM2C94 |
More data for this
Ligand-Target Pair | |
Androgen receptor
(Homo sapiens (Human)) | BDBM50068102
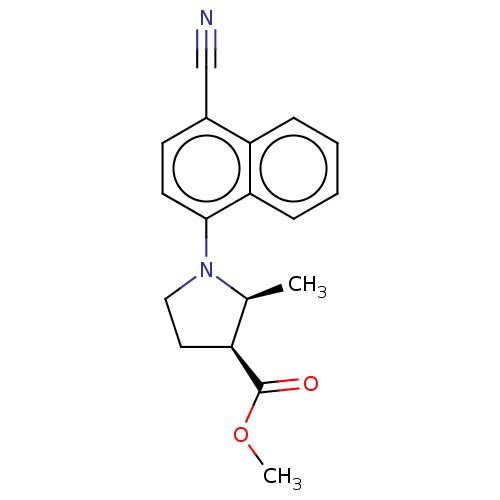
(CHEMBL3402233)Show SMILES COC(=O)[C@H]1CCN([C@H]1C)c1ccc(C#N)c2ccccc12 |r| Show InChI InChI=1S/C18H18N2O2/c1-12-14(18(21)22-2)9-10-20(12)17-8-7-13(11-19)15-5-3-4-6-16(15)17/h3-8,12,14H,9-10H2,1-2H3/t12-,14-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 7.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company Ltd
Curated by ChEMBL
| Assay Description
Displacement of [3H]mibolerone from androgen receptor (unknown origin) after 3 hrs |
Bioorg Med Chem 23: 2568-78 (2015)
Article DOI: 10.1016/j.bmc.2015.03.032
BindingDB Entry DOI: 10.7270/Q2JM2C94 |
More data for this
Ligand-Target Pair | |
Steroid 17-alpha-hydroxylase/17,20 lyase
(Rattus norvegicus (Rat)) | BDBM50342169
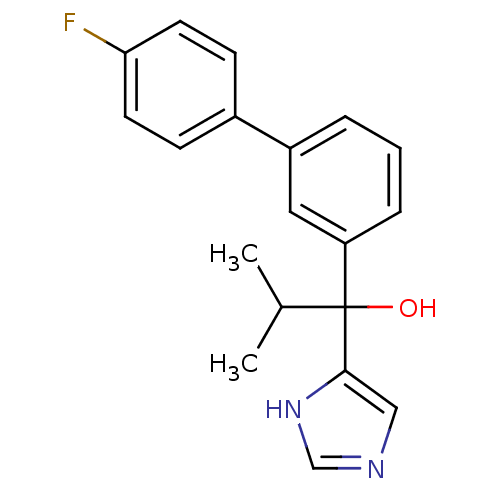
(CHEMBL1766170 | rac-1-(4'-Fluoro[1,1'-biphenyl]-3-...)Show SMILES CC(C)C(O)(c1cnc[nH]1)c1cccc(c1)-c1ccc(F)cc1 Show InChI InChI=1S/C19H19FN2O/c1-13(2)19(23,18-11-21-12-22-18)16-5-3-4-15(10-16)14-6-8-17(20)9-7-14/h3-13,23H,1-2H3,(H,21,22) | KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 8.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company, Ltd
Curated by ChEMBL
| Assay Description
Inhibition of 17,20 lyase activity in Sprague-Dawley rat testicular microsomes |
Bioorg Med Chem 19: 2428-42 (2011)
Article DOI: 10.1016/j.bmc.2011.02.009
BindingDB Entry DOI: 10.7270/Q2FJ2H3G |
More data for this
Ligand-Target Pair | |
Androgen receptor
(Homo sapiens (Human)) | BDBM50360444
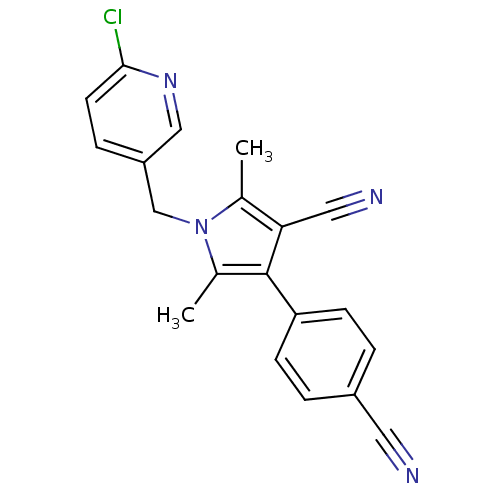
(CHEMBL1934444)Show SMILES Cc1c(C#N)c(c(C)n1Cc1ccc(Cl)nc1)-c1ccc(cc1)C#N Show InChI InChI=1S/C20H15ClN4/c1-13-18(10-23)20(17-6-3-15(9-22)4-7-17)14(2)25(13)12-16-5-8-19(21)24-11-16/h3-8,11H,12H2,1-2H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 9 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company Ltd
Curated by ChEMBL
| Assay Description
Displacement of [17-alpha-methyl-3H]-mibolerone from human androgen receptor expressed in HEK293 derived FreeStyle293F cells after 3 hrs |
Bioorg Med Chem 20: 422-34 (2011)
Article DOI: 10.1016/j.bmc.2011.10.067
BindingDB Entry DOI: 10.7270/Q2251JM1 |
More data for this
Ligand-Target Pair | |
Steroid 17-alpha-hydroxylase/17,20 lyase
(Rattus norvegicus (Rat)) | BDBM50342168
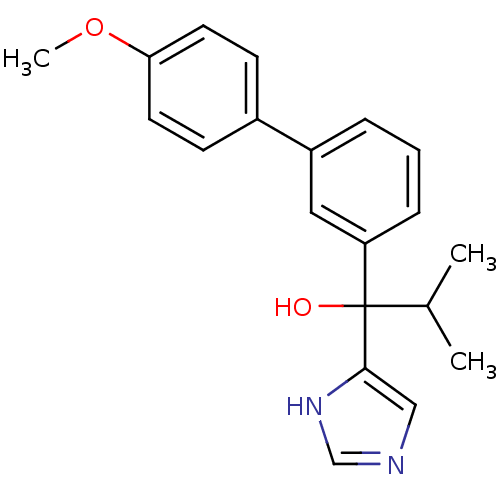
(CHEMBL1765101 | rac-1-(1H-Imidazol-4-yl)-1-(4'-met...)Show SMILES COc1ccc(cc1)-c1cccc(c1)C(O)(C(C)C)c1cnc[nH]1 Show InChI InChI=1S/C20H22N2O2/c1-14(2)20(23,19-12-21-13-22-19)17-6-4-5-16(11-17)15-7-9-18(24-3)10-8-15/h4-14,23H,1-3H3,(H,21,22) | KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company, Ltd
Curated by ChEMBL
| Assay Description
Inhibition of 17,20 lyase activity in Sprague-Dawley rat testicular microsomes |
Bioorg Med Chem 19: 2428-42 (2011)
Article DOI: 10.1016/j.bmc.2011.02.009
BindingDB Entry DOI: 10.7270/Q2FJ2H3G |
More data for this
Ligand-Target Pair | |
Steroid 17-alpha-hydroxylase/17,20 lyase
(Rattus norvegicus (Rat)) | BDBM50338355
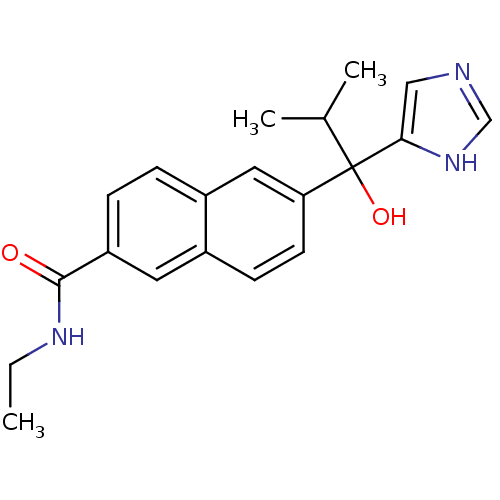
(CHEMBL1682894 | rac-N-Ethyl-6-[1-Hydroxy-1-(1H-imi...)Show SMILES CCNC(=O)c1ccc2cc(ccc2c1)C(O)(C(C)C)c1cnc[nH]1 Show InChI InChI=1S/C20H23N3O2/c1-4-22-19(24)16-6-5-15-10-17(8-7-14(15)9-16)20(25,13(2)3)18-11-21-12-23-18/h5-13,25H,4H2,1-3H3,(H,21,23)(H,22,24) | KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 11 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company, Ltd
Curated by ChEMBL
| Assay Description
Inhibition of 17,20 lyase activity in Sprague-Dawley rat testicular microsomes |
Bioorg Med Chem 19: 1751-70 (2011)
Article DOI: 10.1016/j.bmc.2011.01.017
BindingDB Entry DOI: 10.7270/Q2X92C9F |
More data for this
Ligand-Target Pair | |
Steroid 17-alpha-hydroxylase/17,20 lyase
(Rattus norvegicus (Rat)) | BDBM50338349
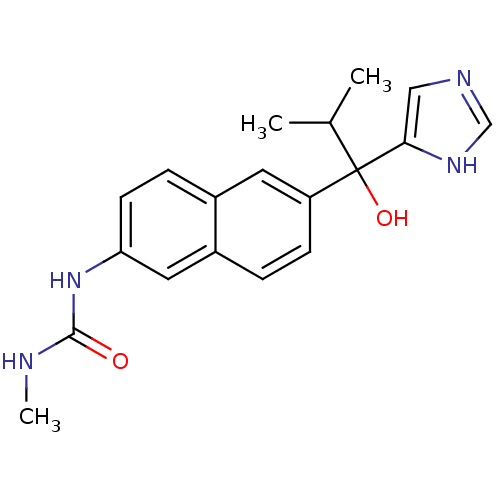
(CHEMBL1682890 | rac-N'-{6-[1-Hydroxy-1-(1H-imidazo...)Show SMILES CNC(=O)Nc1ccc2cc(ccc2c1)C(O)(C(C)C)c1cnc[nH]1 Show InChI InChI=1S/C19H22N4O2/c1-12(2)19(25,17-10-21-11-22-17)15-6-4-14-9-16(23-18(24)20-3)7-5-13(14)8-15/h4-12,25H,1-3H3,(H,21,22)(H2,20,23,24) | KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 11 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company, Ltd
Curated by ChEMBL
| Assay Description
Inhibition of 17,20 lyase activity in Sprague-Dawley rat testicular microsomes |
Bioorg Med Chem 19: 1751-70 (2011)
Article DOI: 10.1016/j.bmc.2011.01.017
BindingDB Entry DOI: 10.7270/Q2X92C9F |
More data for this
Ligand-Target Pair | |
Steroid 17-alpha-hydroxylase/17,20 lyase
(Rattus norvegicus (Rat)) | BDBM50338353
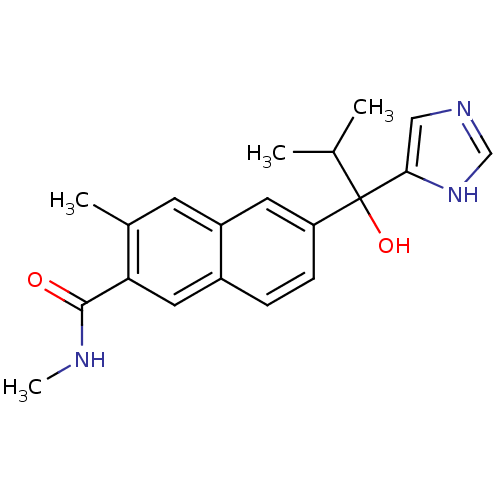
(CHEMBL1682899 | rac-6-[1-Hydroxy-1-(1H-imidazol-4-...)Show SMILES CNC(=O)c1cc2ccc(cc2cc1C)C(O)(C(C)C)c1cnc[nH]1 Show InChI InChI=1S/C20H23N3O2/c1-12(2)20(25,18-10-22-11-23-18)16-6-5-14-9-17(19(24)21-4)13(3)7-15(14)8-16/h5-12,25H,1-4H3,(H,21,24)(H,22,23) | KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 12 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company, Ltd
Curated by ChEMBL
| Assay Description
Inhibition of 17,20 lyase activity in Sprague-Dawley rat testicular microsomes |
Bioorg Med Chem 19: 1751-70 (2011)
Article DOI: 10.1016/j.bmc.2011.01.017
BindingDB Entry DOI: 10.7270/Q2X92C9F |
More data for this
Ligand-Target Pair | |
Steroid 17-alpha-hydroxylase/17,20 lyase
(Rattus norvegicus (Rat)) | BDBM50338358
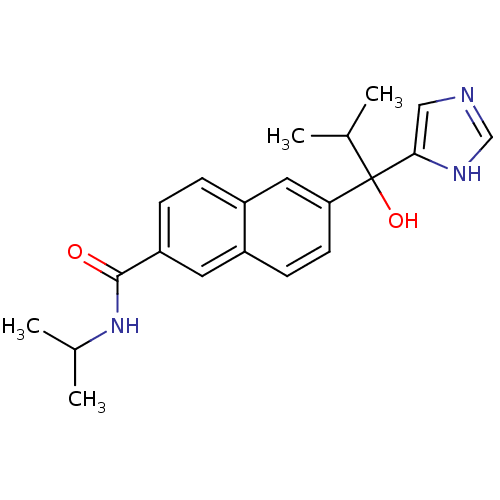
(CHEMBL1682896 | rac-6-[1-Hydroxy-1-(1H-imidazol-4-...)Show SMILES CC(C)NC(=O)c1ccc2cc(ccc2c1)C(O)(C(C)C)c1cnc[nH]1 Show InChI InChI=1S/C21H25N3O2/c1-13(2)21(26,19-11-22-12-23-19)18-8-7-15-9-17(6-5-16(15)10-18)20(25)24-14(3)4/h5-14,26H,1-4H3,(H,22,23)(H,24,25) | KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 12 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company, Ltd
Curated by ChEMBL
| Assay Description
Inhibition of 17,20 lyase activity in Sprague-Dawley rat testicular microsomes |
Bioorg Med Chem 19: 1751-70 (2011)
Article DOI: 10.1016/j.bmc.2011.01.017
BindingDB Entry DOI: 10.7270/Q2X92C9F |
More data for this
Ligand-Target Pair | |
Steroid 17-alpha-hydroxylase/17,20 lyase
(Homo sapiens (Human)) | BDBM50358195
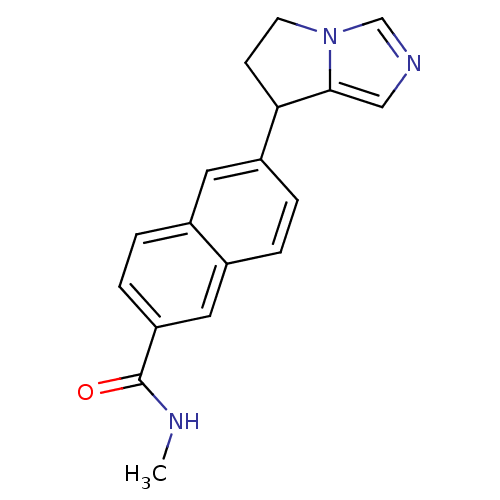
(CHEMBL1921979)Show InChI InChI=1S/C18H17N3O/c1-19-18(22)15-5-3-12-8-14(4-2-13(12)9-15)16-6-7-21-11-20-10-17(16)21/h2-5,8-11,16H,6-7H2,1H3,(H,19,22) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
| Article
PubMed
| n/a | n/a | 13 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company, Ltd.
Curated by ChEMBL
| Assay Description
Inhibition of 17,20-lyase activity of human CYP17A1 |
Bioorg Med Chem 19: 6383-99 (2011)
Article DOI: 10.1016/j.bmc.2011.08.066
BindingDB Entry DOI: 10.7270/Q2X63NDF |
More data for this
Ligand-Target Pair | |
Steroid 17-alpha-hydroxylase/17,20 lyase
(Rattus norvegicus (Rat)) | BDBM50338351
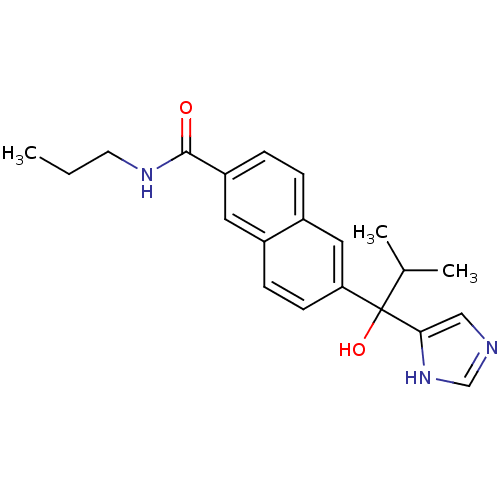
(CHEMBL1682895 | rac-6-[1-Hydroxy-1-(1H-imidazol-4-...)Show SMILES CCCNC(=O)c1ccc2cc(ccc2c1)C(O)(C(C)C)c1cnc[nH]1 Show InChI InChI=1S/C21H25N3O2/c1-4-9-23-20(25)17-6-5-16-11-18(8-7-15(16)10-17)21(26,14(2)3)19-12-22-13-24-19/h5-8,10-14,26H,4,9H2,1-3H3,(H,22,24)(H,23,25) | KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 13 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company, Ltd
Curated by ChEMBL
| Assay Description
Inhibition of 17,20 lyase activity in Sprague-Dawley rat testicular microsomes |
Bioorg Med Chem 19: 1751-70 (2011)
Article DOI: 10.1016/j.bmc.2011.01.017
BindingDB Entry DOI: 10.7270/Q2X92C9F |
More data for this
Ligand-Target Pair | |
Steroid 11-beta-monooxygenase
(Rattus norvegicus) | BDBM50338356

((+)-7-[1-Hydroxy-1-(1H-imidazol-4-yl)-2-methylprop...)Show SMILES CC(C)C(O)(c1cnc[nH]1)c1ccc2c3CNC(=O)c3ccc2c1 Show InChI InChI=1S/C19H19N3O2/c1-11(2)19(24,17-9-20-10-22-17)13-4-6-14-12(7-13)3-5-15-16(14)8-21-18(15)23/h3-7,9-11,24H,8H2,1-2H3,(H,20,22)(H,21,23) | UniProtKB/TrEMBL
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 13 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company, Ltd
Curated by ChEMBL
| Assay Description
Inhibition of 11-hydroxylase activity in Sprague-Dawley rat adrenal gland |
Bioorg Med Chem 19: 1751-70 (2011)
Article DOI: 10.1016/j.bmc.2011.01.017
BindingDB Entry DOI: 10.7270/Q2X92C9F |
More data for this
Ligand-Target Pair | |
Steroid 11-beta-monooxygenase
(Rattus norvegicus) | BDBM50338356

((+)-7-[1-Hydroxy-1-(1H-imidazol-4-yl)-2-methylprop...)Show SMILES CC(C)C(O)(c1cnc[nH]1)c1ccc2c3CNC(=O)c3ccc2c1 Show InChI InChI=1S/C19H19N3O2/c1-11(2)19(24,17-9-20-10-22-17)13-4-6-14-12(7-13)3-5-15-16(14)8-21-18(15)23/h3-7,9-11,24H,8H2,1-2H3,(H,20,22)(H,21,23) | UniProtKB/TrEMBL
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 13 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company, Ltd
Curated by ChEMBL
| Assay Description
Inhibition of 11-hydroxylase activity in Sprague-Dawley rat adrenal gland |
Bioorg Med Chem 19: 1751-70 (2011)
Article DOI: 10.1016/j.bmc.2011.01.017
BindingDB Entry DOI: 10.7270/Q2X92C9F |
More data for this
Ligand-Target Pair | |
Androgen receptor
(Homo sapiens (Human)) | BDBM50360445

(CHEMBL1934445)Show SMILES Cc1c(C#N)c(c(C)n1Cc1cncc(CO)c1)-c1ccc(cc1)C#N Show InChI InChI=1S/C21H18N4O/c1-14-20(9-23)21(19-5-3-16(8-22)4-6-19)15(2)25(14)12-17-7-18(13-26)11-24-10-17/h3-7,10-11,26H,12-13H2,1-2H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 14 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company Ltd
Curated by ChEMBL
| Assay Description
Displacement of [17-alpha-methyl-3H]-mibolerone from human androgen receptor expressed in HEK293 derived FreeStyle293F cells after 3 hrs |
Bioorg Med Chem 20: 422-34 (2011)
Article DOI: 10.1016/j.bmc.2011.10.067
BindingDB Entry DOI: 10.7270/Q2251JM1 |
More data for this
Ligand-Target Pair | |
Steroid 17-alpha-hydroxylase/17,20 lyase
(Rattus norvegicus (Rat)) | BDBM50342186
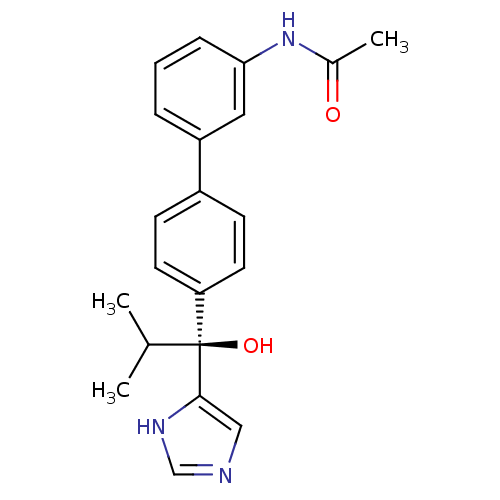
((S)-N-(4'-(1-hydroxy-1-(1H-imidazol-4-yl)-2-methyl...)Show SMILES CC(C)[C@@](O)(c1cnc[nH]1)c1ccc(cc1)-c1cccc(NC(C)=O)c1 |r| Show InChI InChI=1S/C21H23N3O2/c1-14(2)21(26,20-12-22-13-23-20)18-9-7-16(8-10-18)17-5-4-6-19(11-17)24-15(3)25/h4-14,26H,1-3H3,(H,22,23)(H,24,25)/t21-/m0/s1 | KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 14 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company, Ltd
Curated by ChEMBL
| Assay Description
Inhibition of 17,20 lyase activity in Sprague-Dawley rat testicular microsomes |
Bioorg Med Chem 19: 2428-42 (2011)
Article DOI: 10.1016/j.bmc.2011.02.009
BindingDB Entry DOI: 10.7270/Q2FJ2H3G |
More data for this
Ligand-Target Pair | |
Steroid 17-alpha-hydroxylase/17,20 lyase
(Rattus norvegicus (Rat)) | BDBM50338362
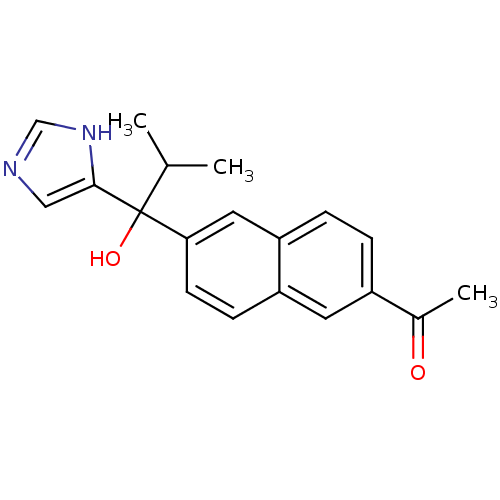
(CHEMBL1682888 | rac-1-{6-[1-Hydroxy-1-(1H-imidazol...)Show SMILES CC(C)C(O)(c1cnc[nH]1)c1ccc2cc(ccc2c1)C(C)=O Show InChI InChI=1S/C19H20N2O2/c1-12(2)19(23,18-10-20-11-21-18)17-7-6-15-8-14(13(3)22)4-5-16(15)9-17/h4-12,23H,1-3H3,(H,20,21) | KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 14 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company, Ltd
Curated by ChEMBL
| Assay Description
Inhibition of 17,20 lyase activity in Sprague-Dawley rat testicular microsomes |
Bioorg Med Chem 19: 1751-70 (2011)
Article DOI: 10.1016/j.bmc.2011.01.017
BindingDB Entry DOI: 10.7270/Q2X92C9F |
More data for this
Ligand-Target Pair | |
Steroid 17-alpha-hydroxylase/17,20 lyase
(Rattus norvegicus (Rat)) | BDBM50358195

(CHEMBL1921979)Show InChI InChI=1S/C18H17N3O/c1-19-18(22)15-5-3-12-8-14(4-2-13(12)9-15)16-6-7-21-11-20-10-17(16)21/h2-5,8-11,16H,6-7H2,1H3,(H,19,22) | KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
| Article
PubMed
| n/a | n/a | 15 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company, Ltd.
Curated by ChEMBL
| Assay Description
Inhibition of 17,20-lyase activity of Sprague-Dawley rat testicular microsomal CYP17A1 using [1,2-3H]-17a-hydroxyprogesterone as substrate after 15 m... |
Bioorg Med Chem 19: 6383-99 (2011)
Article DOI: 10.1016/j.bmc.2011.08.066
BindingDB Entry DOI: 10.7270/Q2X63NDF |
More data for this
Ligand-Target Pair | |
Steroid 17-alpha-hydroxylase/17,20 lyase
(Rattus norvegicus (Rat)) | BDBM50338360

((+)-(R)-7-(1-hydroxy-1-(1H-imidazol-4-yl)-2-methyl...)Show SMILES CC(C)[C@](O)(c1cnc[nH]1)c1ccc2c3CN(C)C(=O)c3ccc2c1 |r| Show InChI InChI=1S/C20H21N3O2/c1-12(2)20(25,18-9-21-11-22-18)14-5-7-15-13(8-14)4-6-16-17(15)10-23(3)19(16)24/h4-9,11-12,25H,10H2,1-3H3,(H,21,22)/t20-/m1/s1 | KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 15 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company, Ltd
Curated by ChEMBL
| Assay Description
Inhibition of 17,20 lyase activity in Sprague-Dawley rat testicular microsomes |
Bioorg Med Chem 19: 1751-70 (2011)
Article DOI: 10.1016/j.bmc.2011.01.017
BindingDB Entry DOI: 10.7270/Q2X92C9F |
More data for this
Ligand-Target Pair | |
Steroid 17-alpha-hydroxylase/17,20 lyase
(Rattus norvegicus (Rat)) | BDBM50342167

(CHEMBL1766169 | rac-1-[1,1'-biphenyl]-3-yl-1-(1H-i...)Show InChI InChI=1S/C19H20N2O/c1-14(2)19(22,18-12-20-13-21-18)17-10-6-9-16(11-17)15-7-4-3-5-8-15/h3-14,22H,1-2H3,(H,20,21) | KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 15 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company, Ltd
Curated by ChEMBL
| Assay Description
Inhibition of 17,20 lyase activity in Sprague-Dawley rat testicular microsomes |
Bioorg Med Chem 19: 2428-42 (2011)
Article DOI: 10.1016/j.bmc.2011.02.009
BindingDB Entry DOI: 10.7270/Q2FJ2H3G |
More data for this
Ligand-Target Pair | |
Steroid 17-alpha-hydroxylase/17,20 lyase
(Homo sapiens (Human)) | BDBM50338356

((+)-7-[1-Hydroxy-1-(1H-imidazol-4-yl)-2-methylprop...)Show SMILES CC(C)C(O)(c1cnc[nH]1)c1ccc2c3CNC(=O)c3ccc2c1 Show InChI InChI=1S/C19H19N3O2/c1-11(2)19(24,17-9-20-10-22-17)13-4-6-14-12(7-13)3-5-15-16(14)8-21-18(15)23/h3-7,9-11,24H,8H2,1-2H3,(H,20,22)(H,21,23) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 15 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company, Ltd
Curated by ChEMBL
| Assay Description
Inhibition of 17,20 lyase activity in human |
Bioorg Med Chem 19: 1751-70 (2011)
Article DOI: 10.1016/j.bmc.2011.01.017
BindingDB Entry DOI: 10.7270/Q2X92C9F |
More data for this
Ligand-Target Pair | |
Steroid 17-alpha-hydroxylase/17,20 lyase
(Homo sapiens (Human)) | BDBM50338356

((+)-7-[1-Hydroxy-1-(1H-imidazol-4-yl)-2-methylprop...)Show SMILES CC(C)C(O)(c1cnc[nH]1)c1ccc2c3CNC(=O)c3ccc2c1 Show InChI InChI=1S/C19H19N3O2/c1-11(2)19(24,17-9-20-10-22-17)13-4-6-14-12(7-13)3-5-15-16(14)8-21-18(15)23/h3-7,9-11,24H,8H2,1-2H3,(H,20,22)(H,21,23) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 15 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company, Ltd
Curated by ChEMBL
| Assay Description
Inhibition of 17,20 lyase activity in human |
Bioorg Med Chem 19: 1751-70 (2011)
Article DOI: 10.1016/j.bmc.2011.01.017
BindingDB Entry DOI: 10.7270/Q2X92C9F |
More data for this
Ligand-Target Pair | |
Steroid 17-alpha-hydroxylase/17,20 lyase
(Homo sapiens (Human)) | BDBM50338363

(CHEMBL1682893 | rac-6-[1-Hydroxy-1-(1H-imidazol-4-...)Show SMILES CNC(=O)c1ccc2cc(ccc2c1)C(O)(C(C)C)c1cnc[nH]1 Show InChI InChI=1S/C19H21N3O2/c1-12(2)19(24,17-10-21-11-22-17)16-7-6-13-8-15(18(23)20-3)5-4-14(13)9-16/h4-12,24H,1-3H3,(H,20,23)(H,21,22) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 16 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company, Ltd
Curated by ChEMBL
| Assay Description
Inhibition of 17,20 lyase activity in human |
Bioorg Med Chem 19: 1751-70 (2011)
Article DOI: 10.1016/j.bmc.2011.01.017
BindingDB Entry DOI: 10.7270/Q2X92C9F |
More data for this
Ligand-Target Pair | |
Steroid 17-alpha-hydroxylase/17,20 lyase
(Homo sapiens (Human)) | BDBM50338363

(CHEMBL1682893 | rac-6-[1-Hydroxy-1-(1H-imidazol-4-...)Show SMILES CNC(=O)c1ccc2cc(ccc2c1)C(O)(C(C)C)c1cnc[nH]1 Show InChI InChI=1S/C19H21N3O2/c1-12(2)19(24,17-10-21-11-22-17)16-7-6-13-8-15(18(23)20-3)5-4-14(13)9-16/h4-12,24H,1-3H3,(H,20,23)(H,21,22) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 16 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company, Ltd.
Curated by ChEMBL
| Assay Description
Inhibition of 17,20-lyase activity of human CYP17A1 |
Bioorg Med Chem 19: 6383-99 (2011)
Article DOI: 10.1016/j.bmc.2011.08.066
BindingDB Entry DOI: 10.7270/Q2X63NDF |
More data for this
Ligand-Target Pair | |
Steroid 17-alpha-hydroxylase/17,20 lyase
(Rattus norvegicus (Rat)) | BDBM50342175

(CHEMBL1766176 | rac-N-{4-[1-Hydroxy-1-(1H-imidazol...)Show SMILES CC(C)C(O)(c1cnc[nH]1)c1ccc(cc1)-c1cccc(NC(C)=O)c1 Show InChI InChI=1S/C21H23N3O2/c1-14(2)21(26,20-12-22-13-23-20)18-9-7-16(8-10-18)17-5-4-6-19(11-17)24-15(3)25/h4-14,26H,1-3H3,(H,22,23)(H,24,25) | KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 17 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company, Ltd
Curated by ChEMBL
| Assay Description
Inhibition of 17,20 lyase activity in Sprague-Dawley rat testicular microsomes |
Bioorg Med Chem 19: 2428-42 (2011)
Article DOI: 10.1016/j.bmc.2011.02.009
BindingDB Entry DOI: 10.7270/Q2FJ2H3G |
More data for this
Ligand-Target Pair | |
Steroid 17-alpha-hydroxylase/17,20 lyase
(Homo sapiens (Human)) | BDBM50342167

(CHEMBL1766169 | rac-1-[1,1'-biphenyl]-3-yl-1-(1H-i...)Show InChI InChI=1S/C19H20N2O/c1-14(2)19(22,18-12-20-13-21-18)17-10-6-9-16(11-17)15-7-4-3-5-8-15/h3-14,22H,1-2H3,(H,20,21) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 18 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company, Ltd
Curated by ChEMBL
| Assay Description
Inhibition of human 17,20 lyase activity |
Bioorg Med Chem 19: 2428-42 (2011)
Article DOI: 10.1016/j.bmc.2011.02.009
BindingDB Entry DOI: 10.7270/Q2FJ2H3G |
More data for this
Ligand-Target Pair | |
Steroid 17-alpha-hydroxylase/17,20 lyase
(Homo sapiens (Human)) | BDBM50338356

((+)-7-[1-Hydroxy-1-(1H-imidazol-4-yl)-2-methylprop...)Show SMILES CC(C)C(O)(c1cnc[nH]1)c1ccc2c3CNC(=O)c3ccc2c1 Show InChI InChI=1S/C19H19N3O2/c1-11(2)19(24,17-9-20-10-22-17)13-4-6-14-12(7-13)3-5-15-16(14)8-21-18(15)23/h3-7,9-11,24H,8H2,1-2H3,(H,20,22)(H,21,23) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 18 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company, Ltd
Curated by ChEMBL
| Assay Description
Inhibition of 17,20 lyase activity in human |
Bioorg Med Chem 19: 1751-70 (2011)
Article DOI: 10.1016/j.bmc.2011.01.017
BindingDB Entry DOI: 10.7270/Q2X92C9F |
More data for this
Ligand-Target Pair | |
Steroid 17-alpha-hydroxylase/17,20 lyase
(Homo sapiens (Human)) | BDBM50358201
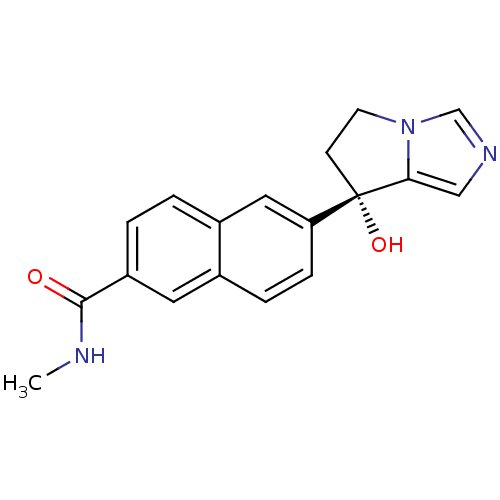
(CHEMBL1921976 | US9611270, orteronel)Show SMILES CNC(=O)c1ccc2cc(ccc2c1)[C@@]1(O)CCn2cncc12 |r| Show InChI InChI=1S/C18H17N3O2/c1-19-17(22)14-3-2-13-9-15(5-4-12(13)8-14)18(23)6-7-21-11-20-10-16(18)21/h2-5,8-11,23H,6-7H2,1H3,(H,19,22)/t18-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| n/a | n/a | 19 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company, Ltd.
Curated by ChEMBL
| Assay Description
Inhibition of 17,20-lyase activity of human CYP17A1 |
Bioorg Med Chem 19: 6383-99 (2011)
Article DOI: 10.1016/j.bmc.2011.08.066
BindingDB Entry DOI: 10.7270/Q2X63NDF |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Steroid 17-alpha-hydroxylase/17,20 lyase
(Homo sapiens (Human)) | BDBM50338357

(CHEMBL1682903 | rac-6-[1-Hydroxy-1-(1H-imidazol-4-...)Show SMILES CC(C)C(O)(c1cnc[nH]1)c1ccc2cc3C(=O)N(C)Cc3cc2c1 Show InChI InChI=1S/C20H21N3O2/c1-12(2)20(25,18-9-21-11-22-18)16-5-4-13-8-17-15(6-14(13)7-16)10-23(3)19(17)24/h4-9,11-12,25H,10H2,1-3H3,(H,21,22) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 19 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company, Ltd
Curated by ChEMBL
| Assay Description
Inhibition of 17,20 lyase activity in human |
Bioorg Med Chem 19: 1751-70 (2011)
Article DOI: 10.1016/j.bmc.2011.01.017
BindingDB Entry DOI: 10.7270/Q2X92C9F |
More data for this
Ligand-Target Pair | |
Steroid 17-alpha-hydroxylase/17,20 lyase
(Homo sapiens (Human)) | BDBM50342169

(CHEMBL1766170 | rac-1-(4'-Fluoro[1,1'-biphenyl]-3-...)Show SMILES CC(C)C(O)(c1cnc[nH]1)c1cccc(c1)-c1ccc(F)cc1 Show InChI InChI=1S/C19H19FN2O/c1-13(2)19(23,18-11-21-12-22-18)16-5-3-4-15(10-16)14-6-8-17(20)9-7-14/h3-13,23H,1-2H3,(H,21,22) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 19 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company, Ltd
Curated by ChEMBL
| Assay Description
Inhibition of human 17,20 lyase activity |
Bioorg Med Chem 19: 2428-42 (2011)
Article DOI: 10.1016/j.bmc.2011.02.009
BindingDB Entry DOI: 10.7270/Q2FJ2H3G |
More data for this
Ligand-Target Pair | |
Steroid 17-alpha-hydroxylase/17,20 lyase
(Rattus norvegicus (Rat)) | BDBM50342170

(CHEMBL1766171 | rac-1-(4'-Chloro[1,1'-biphenyl]-3-...)Show SMILES CC(C)C(O)(c1cnc[nH]1)c1cccc(c1)-c1ccc(Cl)cc1 Show InChI InChI=1S/C19H19ClN2O/c1-13(2)19(23,18-11-21-12-22-18)16-5-3-4-15(10-16)14-6-8-17(20)9-7-14/h3-13,23H,1-2H3,(H,21,22) | KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 19 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company, Ltd
Curated by ChEMBL
| Assay Description
Inhibition of 17,20 lyase activity in Sprague-Dawley rat testicular microsomes |
Bioorg Med Chem 19: 2428-42 (2011)
Article DOI: 10.1016/j.bmc.2011.02.009
BindingDB Entry DOI: 10.7270/Q2FJ2H3G |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data