

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Bifunctional dihydrofolate reductase-thymidylate synthase (Plasmodium falciparum (isolate K1 / Thailand)) | BDBM18793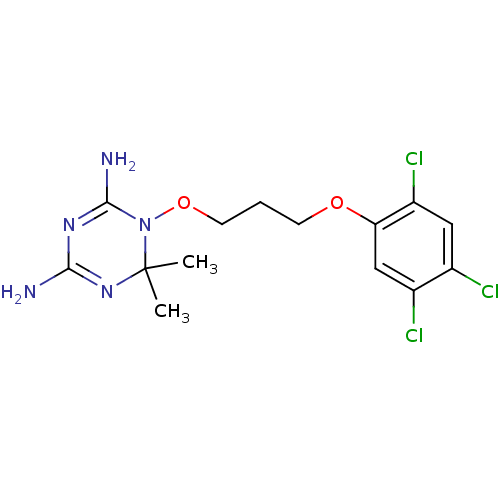 (6,6-dimethyl-1-[3-(2,4,5-trichlorophenoxy)propoxy]...) | PDB MMDB KEGG UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE MMDB PC cid PC sid PDB UniChem Patents | PDB Article PubMed | 0.0110 | -62.5 | 0.570 | n/a | n/a | n/a | n/a | 7.0 | 25 |
Mahidol University | Assay Description The concentration of inhibitor that inhibited 50% of the parasite growth (IC50) was determined from the sigmoidal curve obtained by plotting the perc... | Nat Struct Biol 10: 257-65 (2003) Article DOI: 10.1038/nsb921 BindingDB Entry DOI: 10.7270/Q2HH6HBD | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Bifunctional dihydrofolate reductase-thymidylate synthase (Plasmodium falciparum (isolate K1 / Thailand)) | BDBM18793 (6,6-dimethyl-1-[3-(2,4,5-trichlorophenoxy)propoxy]...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE MMDB PC cid PC sid PDB UniChem Patents | PDB Article PubMed | 0.0200 | -61.1 | 2.30 | n/a | n/a | n/a | n/a | 7.0 | 25 |
Mahidol University | Assay Description The concentration of inhibitor that inhibited 50% of the parasite growth (IC50) was determined from the sigmoidal curve obtained by plotting the perc... | Nat Struct Biol 10: 257-65 (2003) Article DOI: 10.1038/nsb921 BindingDB Entry DOI: 10.7270/Q2HH6HBD | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Bifunctional dihydrofolate reductase-thymidylate synthase [N51I,C59R,S108N,I164L] (Plasmodium falciparum (isolate K1 / Thailand)) | BDBM18793 (6,6-dimethyl-1-[3-(2,4,5-trichlorophenoxy)propoxy]...) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE MMDB PC cid PC sid PDB UniChem Patents | PDB Article PubMed | 0.0370 | -59.5 | 18 | n/a | n/a | n/a | n/a | 7.0 | 25 |
Mahidol University | Assay Description The concentration of inhibitor that inhibited 50% of the parasite growth (IC50) was determined from the sigmoidal curve obtained by plotting the perc... | Nat Struct Biol 10: 257-65 (2003) Article DOI: 10.1038/nsb921 BindingDB Entry DOI: 10.7270/Q2HH6HBD | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Bifunctional dihydrofolate reductase-thymidylate synthase (Plasmodium falciparum (isolate K1 / Thailand)) | BDBM18512 (5-(4-chlorophenyl)-6-ethylpyrimidine-2,4-diamine |...) | PDB MMDB KEGG UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | 0.200 | -55.4 | 80 | n/a | n/a | n/a | n/a | 7.0 | 25 |
Mahidol University | Assay Description The concentration of inhibitor that inhibited 50% of the parasite growth (IC50) was determined from the sigmoidal curve obtained by plotting the perc... | Nat Struct Biol 10: 257-65 (2003) Article DOI: 10.1038/nsb921 BindingDB Entry DOI: 10.7270/Q2HH6HBD | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Bifunctional dihydrofolate reductase-thymidylate synthase (Plasmodium falciparum (isolate K1 / Thailand)) | BDBM18792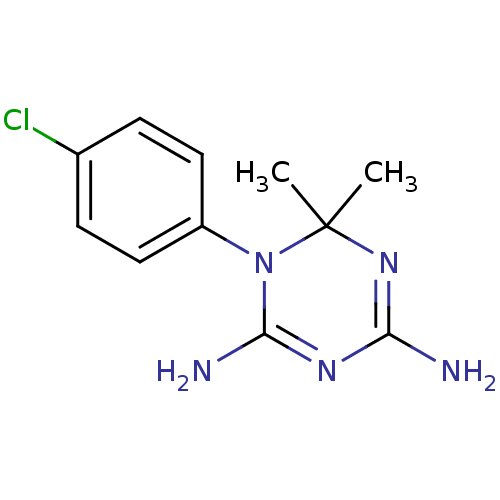 (1-(4-chlorophenyl)-6,6-dimethyl-1,6-dihydro-1,3,5-...) | PDB MMDB KEGG UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | 0.300 | -54.4 | 37 | n/a | n/a | n/a | n/a | 7.0 | 25 |
Mahidol University | Assay Description The concentration of inhibitor that inhibited 50% of the parasite growth (IC50) was determined from the sigmoidal curve obtained by plotting the perc... | Nat Struct Biol 10: 257-65 (2003) Article DOI: 10.1038/nsb921 BindingDB Entry DOI: 10.7270/Q2HH6HBD | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Nociceptin receptor (RAT) | BDBM21842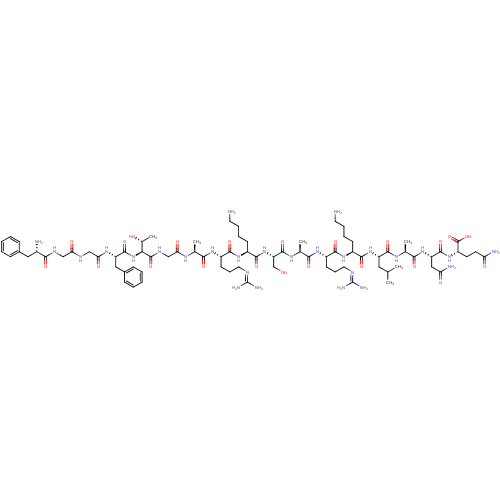 ((2S)-2-[(2S)-2-[(2S)-2-[(2S)-2-[(2S)-6-amino-2-[(2...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | KEGG PC cid PC sid UniChem Similars | Article PubMed | 0.380 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Michigan Curated by PDSP Ki Database | J Chem Neuroanat 25: 233-47 (2003) Article DOI: 10.1016/s0891-0618(03)00032-2 BindingDB Entry DOI: 10.7270/Q2XP73G2 | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nicotinic acetylcholine receptor (RAT) | BDBM50107863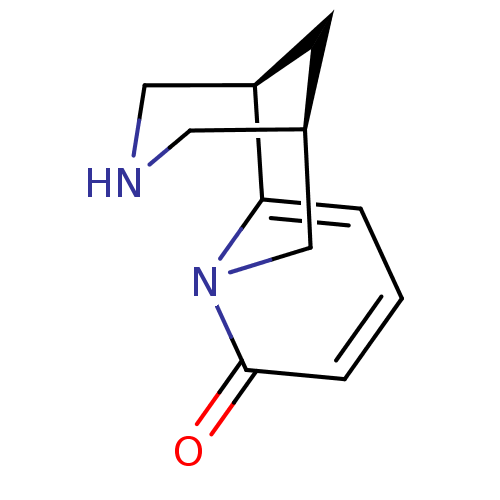 ((-)-cytisine | (1R,9R)-7,11-diazatricyclo[7.3.1.0~...) | UniProtKB/TrEMBL GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | PubMed | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of California Curated by PDSP Ki Database | J Pharmacol Exp Ther 270: 159-66 (1994) BindingDB Entry DOI: 10.7270/Q22Z141C | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nicotinic acetylcholine receptor (RAT) | BDBM50107863 ((-)-cytisine | (1R,9R)-7,11-diazatricyclo[7.3.1.0~...) | UniProtKB/TrEMBL GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | PubMed | 0.710 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of California Curated by PDSP Ki Database | J Pharmacol Exp Ther 270: 159-66 (1994) BindingDB Entry DOI: 10.7270/Q22Z141C | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholine-binding protein (Lymnaea stagnalis) | BDBM91624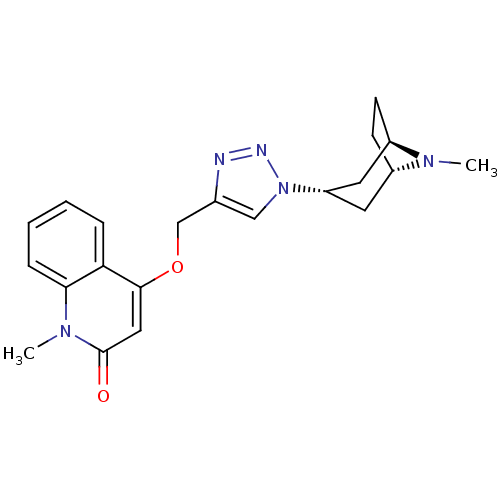 (alpha7 agonists 4t) | PDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 1.30 | -49.9 | n/a | n/a | n/a | n/a | n/a | 7.4 | 20 |
University of California, San Diego | Assay Description Assay Description 2 Assays used to generate Ki or EC50 values. 1) SPA Assay - Quick screen binding assays were performed using 100 ?l of 0.2 mg/ml an... | Taylor Research Group (2014) Article DOI: 10.1124/mol.112.080291 BindingDB Entry DOI: 10.7270/Q2805163 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nicotinic acetylcholine receptor (RAT) | BDBM82070 (CAS_29790-52-1 | NICOTINE-L (BASE) | Nicotine-D sa...) | UniProtKB/TrEMBL GoogleScholar AffyNet | Purchase KEGG PC cid PC sid PDB UniChem Similars | PubMed | 1.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of California Curated by PDSP Ki Database | J Pharmacol Exp Ther 270: 159-66 (1994) BindingDB Entry DOI: 10.7270/Q22Z141C | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Rattus norvegicus (rat)) | BDBM50045114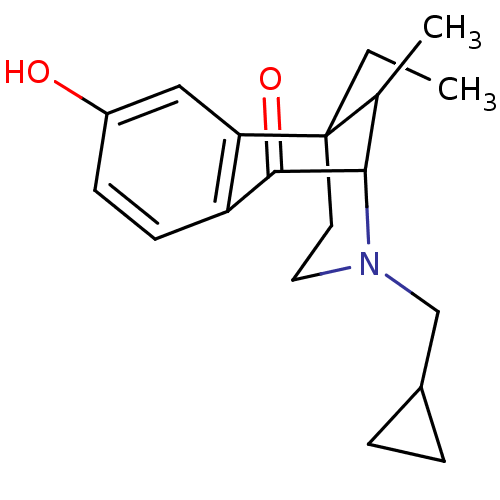 (3-Cyclopropylmethyl-6-ethyl-8-hydroxy-11-methyl-3,...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.93 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Michigan Curated by PDSP Ki Database | J Chem Neuroanat 25: 233-47 (2003) Article DOI: 10.1016/s0891-0618(03)00032-2 BindingDB Entry DOI: 10.7270/Q2XP73G2 | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholine-binding protein (Lymnaea stagnalis) | BDBM91603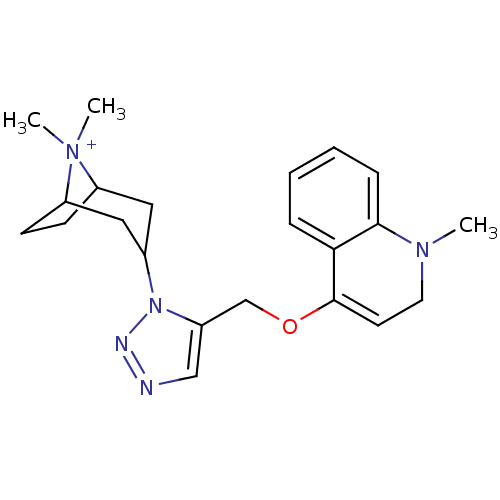 (4' | alpha7 agonists 4') | PDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 2 | -48.8 | n/a | n/a | n/a | n/a | n/a | 7.4 | 20 |
University of California, San Diego | Assay Description Assay Description 2 Assays used to generate Ki or EC50 values. 1) SPA Assay - Quick screen binding assays were performed using 100 ?l of 0.2 mg/ml an... | Taylor Research Group (2014) Article DOI: 10.1124/mol.112.080291 BindingDB Entry DOI: 10.7270/Q2805163 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Rattus norvegicus (rat)) | BDBM50045114 (3-Cyclopropylmethyl-6-ethyl-8-hydroxy-11-methyl-3,...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 2.12 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Michigan Curated by PDSP Ki Database | J Chem Neuroanat 25: 233-47 (2003) Article DOI: 10.1016/s0891-0618(03)00032-2 BindingDB Entry DOI: 10.7270/Q2XP73G2 | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nicotinic acetylcholine receptor (RAT) | BDBM82070 (CAS_29790-52-1 | NICOTINE-L (BASE) | Nicotine-D sa...) | UniProtKB/TrEMBL GoogleScholar AffyNet | Purchase KEGG PC cid PC sid PDB UniChem Similars | PubMed | 2.31 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of California Curated by PDSP Ki Database | J Pharmacol Exp Ther 270: 159-66 (1994) BindingDB Entry DOI: 10.7270/Q22Z141C | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Delta-type opioid receptor (Rattus norvegicus (rat)) | BDBM50045114 (3-Cyclopropylmethyl-6-ethyl-8-hydroxy-11-methyl-3,...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 2.46 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Michigan Curated by PDSP Ki Database | J Chem Neuroanat 25: 233-47 (2003) Article DOI: 10.1016/s0891-0618(03)00032-2 BindingDB Entry DOI: 10.7270/Q2XP73G2 | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholine-binding protein (Lymnaea stagnalis) | BDBM91615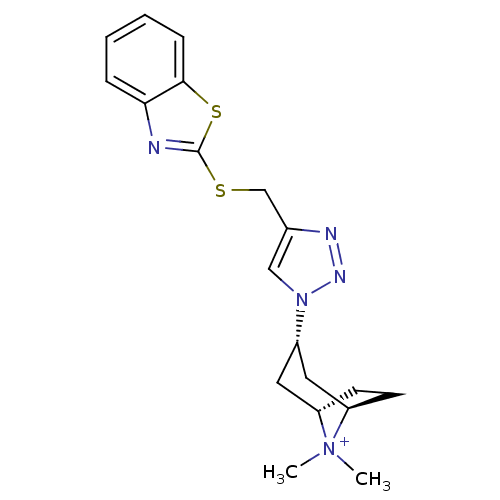 (15q | alpha7 agonists 15q) | PDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 4 | -47.1 | n/a | n/a | n/a | n/a | n/a | 7.4 | 20 |
University of California, San Diego | Assay Description Assay Description 2 Assays used to generate Ki or EC50 values. 1) SPA Assay - Quick screen binding assays were performed using 100 ?l of 0.2 mg/ml an... | Taylor Research Group (2014) Article DOI: 10.1124/mol.112.080291 BindingDB Entry DOI: 10.7270/Q2805163 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nicotinic acetylcholine receptor (RAT) | BDBM82546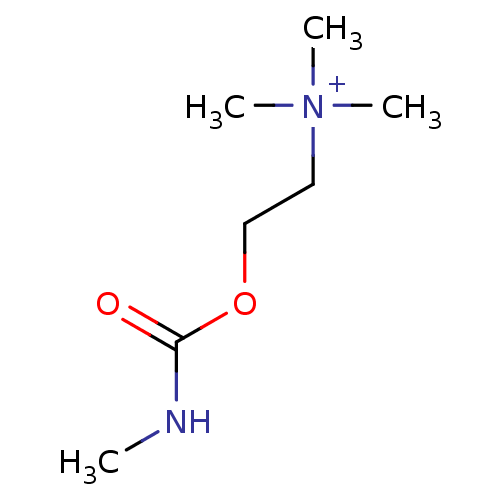 (CAS_14721-69-8 | MCC | N-methylcarbamylcholine | N...) | UniProtKB/TrEMBL GoogleScholar AffyNet | KEGG PC cid PC sid UniChem Patents Similars | PubMed | 5.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of California Curated by PDSP Ki Database | J Pharmacol Exp Ther 270: 159-66 (1994) BindingDB Entry DOI: 10.7270/Q22Z141C | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bifunctional dihydrofolate reductase-thymidylate synthase (Plasmodium falciparum (isolate K1 / Thailand)) | BDBM18792 (1-(4-chlorophenyl)-6,6-dimethyl-1,6-dihydro-1,3,5-...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | 6.20 | -46.8 | 2.40E+3 | n/a | n/a | n/a | n/a | 7.0 | 25 |
Mahidol University | Assay Description The concentration of inhibitor that inhibited 50% of the parasite growth (IC50) was determined from the sigmoidal curve obtained by plotting the perc... | Nat Struct Biol 10: 257-65 (2003) Article DOI: 10.1038/nsb921 BindingDB Entry DOI: 10.7270/Q2HH6HBD | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Nicotinic acetylcholine receptor (RAT) | BDBM50061562 ((12R,13aR)-12-Methoxy-1,4,5,6,9,11,12,13-octahydro...) | UniProtKB/TrEMBL GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | PubMed | 6.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of California Curated by PDSP Ki Database | J Pharmacol Exp Ther 270: 159-66 (1994) BindingDB Entry DOI: 10.7270/Q22Z141C | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuronal acetylcholine receptor subunit alpha-7 (Rattus norvegicus (Rat)) | BDBM50356471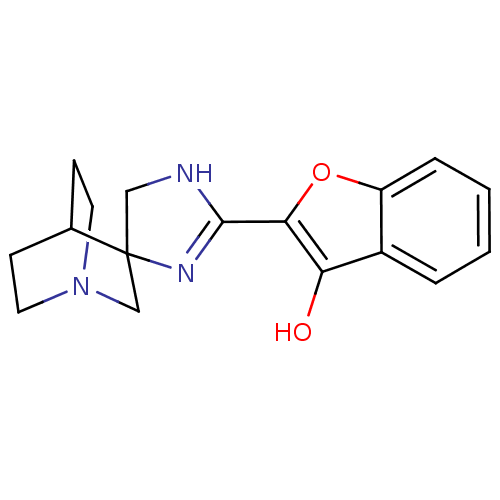 (CHEMBL1911861) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | 7.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Targacept Inc Curated by ChEMBL | Assay Description Displacement of [3H]-methyllycaconitine from alpha7 nAChR in rat hippocampal membranes after 2 hrs by liquid scintillation counting | Eur J Med Chem 46: 5625-35 (2011) Article DOI: 10.1016/j.ejmech.2011.09.033 BindingDB Entry DOI: 10.7270/Q2639Q4D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Soluble acetylcholine receptor (Aplysia Californica) | BDBM50143314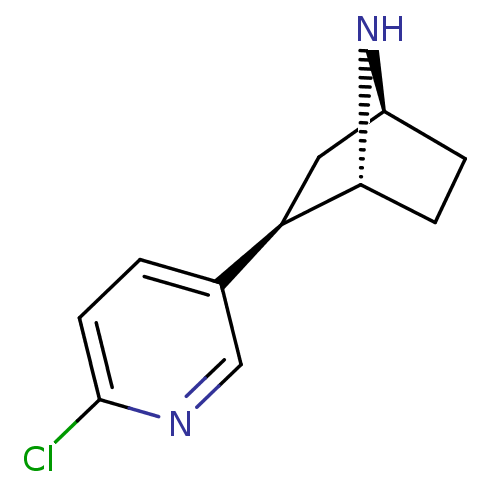 ((+)-Epibatidine | (-)-epibatidine | (1R,2R,4S)-2-(...) | PDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | Purchase CHEMBL DrugBank MMDB PC cid PC sid PDB UniChem Patents Similars | PDB | 7.40 | -45.6 | n/a | n/a | n/a | n/a | n/a | 7.4 | 20 |
UC San Diego | Assay Description Assay Description 2 Assays used to generate Ki or EC50 values. 1) SPA Assay - Quick screen binding assays were performed using 100 ul of 0.2 mg/ml an... | Taylor Research Group (2014) BindingDB Entry DOI: 10.7270/Q2DR2T3Z | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Soluble acetylcholine receptor (Aplysia Californica) | BDBM91615 (15q | alpha7 agonists 15q) | PDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 8 | -45.4 | n/a | n/a | n/a | n/a | n/a | 7.4 | 20 |
University of California, San Diego | Assay Description Assay Description 2 Assays used to generate Ki or EC50 values. 1) SPA Assay - Quick screen binding assays were performed using 100 ?l of 0.2 mg/ml an... | Taylor Research Group (2014) Article DOI: 10.1124/mol.112.080291 BindingDB Entry DOI: 10.7270/Q2805163 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nicotinic acetylcholine receptor (RAT) | BDBM50054820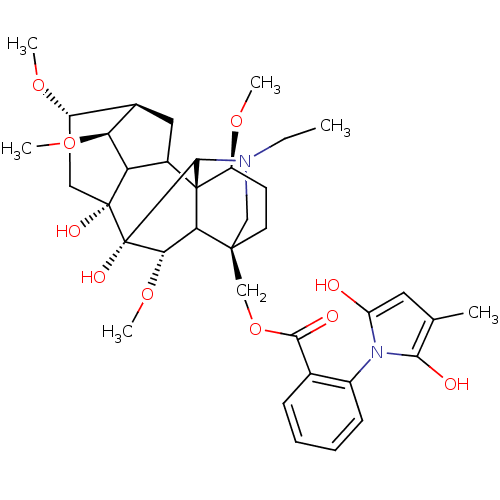 (Methyllycaconitine | [(1S,4S,5R,6S,8R,9R,13S,16S,1...) | UniProtKB/TrEMBL GoogleScholar AffyNet | PC cid PC sid UniChem Similars | PubMed | 8.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of California Curated by PDSP Ki Database | J Pharmacol Exp Ther 270: 159-66 (1994) BindingDB Entry DOI: 10.7270/Q22Z141C | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bifunctional dihydrofolate reductase-thymidylate synthase (Plasmodium falciparum (isolate K1 / Thailand)) | BDBM18512 (5-(4-chlorophenyl)-6-ethylpyrimidine-2,4-diamine |...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | DrugBank PDB Article PubMed | 9.80 | -45.7 | 3.09E+4 | n/a | n/a | n/a | n/a | 7.0 | 25 |
Mahidol University | Assay Description The concentration of inhibitor that inhibited 50% of the parasite growth (IC50) was determined from the sigmoidal curve obtained by plotting the perc... | Nat Struct Biol 10: 257-65 (2003) Article DOI: 10.1038/nsb921 BindingDB Entry DOI: 10.7270/Q2HH6HBD | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Soluble acetylcholine receptor (Aplysia Californica) | BDBM91615 (15q | alpha7 agonists 15q) | PDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 13 | -44.3 | n/a | n/a | n/a | n/a | n/a | 7.4 | 20 |
University of California, San Diego | Assay Description Assay Description 2 Assays used to generate Ki or EC50 values. 1) SPA Assay - Quick screen binding assays were performed using 100 ?l of 0.2 mg/ml an... | Taylor Research Group (2014) Article DOI: 10.1124/mol.112.080291 BindingDB Entry DOI: 10.7270/Q2805163 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Soluble acetylcholine receptor (Aplysia Californica) | BDBM91615 (15q | alpha7 agonists 15q) | PDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 13 | -44.3 | n/a | n/a | n/a | n/a | n/a | 7.4 | 20 |
University of California, San Diego | Assay Description Assay Description 2 Assays used to generate Ki or EC50 values. 1) SPA Assay - Quick screen binding assays were performed using 100 ?l of 0.2 mg/ml an... | Taylor Research Group (2014) Article DOI: 10.1124/mol.112.080291 BindingDB Entry DOI: 10.7270/Q2805163 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nicotinic acetylcholine receptor (RAT) | BDBM50047021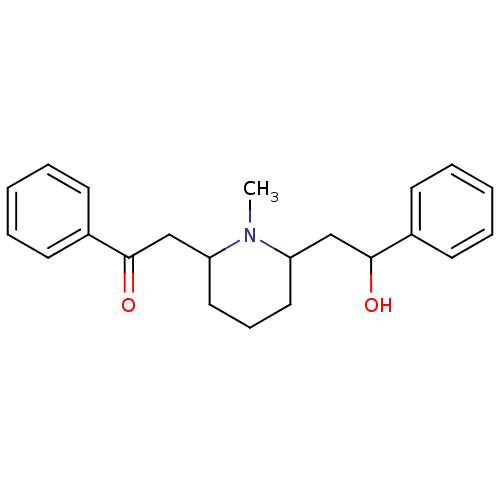 (2-(6-(2-hydroxy-2-phenylethyl)-1-methylpiperidin-2...) | UniProtKB/TrEMBL GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid PDB UniChem Patents Similars | PubMed | 15.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of California Curated by PDSP Ki Database | J Pharmacol Exp Ther 270: 159-66 (1994) BindingDB Entry DOI: 10.7270/Q22Z141C | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Soluble acetylcholine receptor (Aplysia Californica) | BDBM91624 (alpha7 agonists 4t) | PDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 16 | -43.8 | n/a | n/a | n/a | n/a | n/a | 7.4 | 20 |
University of California, San Diego | Assay Description Assay Description 2 Assays used to generate Ki or EC50 values. 1) SPA Assay - Quick screen binding assays were performed using 100 ?l of 0.2 mg/ml an... | Taylor Research Group (2014) Article DOI: 10.1124/mol.112.080291 BindingDB Entry DOI: 10.7270/Q2805163 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Soluble acetylcholine receptor (Aplysia Californica) | BDBM91621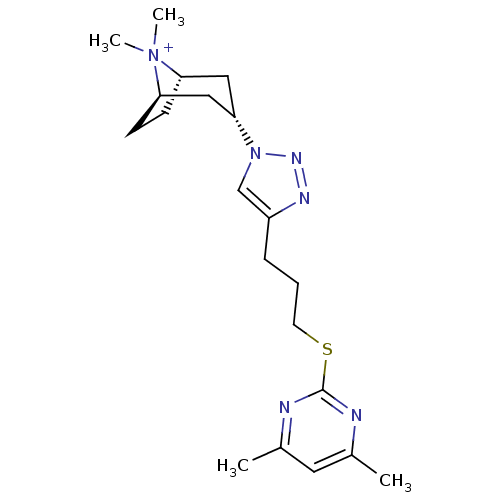 (21q | alpha7 agonists 21q) | PDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 18 | -43.5 | n/a | n/a | n/a | n/a | n/a | 7.4 | 20 |
University of California, San Diego | Assay Description Assay Description 2 Assays used to generate Ki or EC50 values. 1) SPA Assay - Quick screen binding assays were performed using 100 ?l of 0.2 mg/ml an... | Taylor Research Group (2014) Article DOI: 10.1124/mol.112.080291 BindingDB Entry DOI: 10.7270/Q2805163 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholine-binding protein (Lymnaea stagnalis) | BDBM91613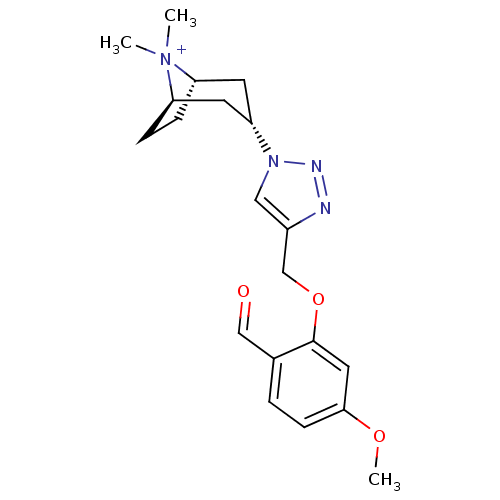 (13q | alpha7 agonists 13q) | PDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 20 | -43.2 | n/a | n/a | n/a | n/a | n/a | 7.4 | 20 |
University of California, San Diego | Assay Description Assay Description 2 Assays used to generate Ki or EC50 values. 1) SPA Assay - Quick screen binding assays were performed using 100 ?l of 0.2 mg/ml an... | Taylor Research Group (2014) Article DOI: 10.1124/mol.112.080291 BindingDB Entry DOI: 10.7270/Q2805163 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nuclear receptor ROR-gamma (Homo sapiens (Human)) | BDBM50466897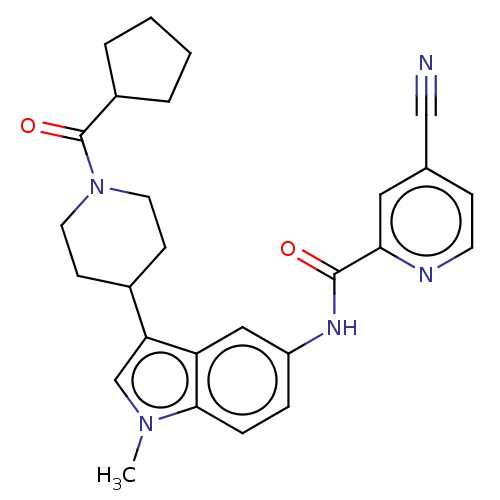 (CHEMBL4287715) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Karo Bio AB (now Karo Pharma AB) Curated by ChEMBL | Assay Description Displacement of [3H]-25-hydroxycholesterol from human N-terminal 6His-tagged-RORC2 LBD (259 to 483 residues) expressed in Escherichia coli (DE3) cell... | J Med Chem 61: 10415-10439 (2018) Article DOI: 10.1021/acs.jmedchem.8b00392 BindingDB Entry DOI: 10.7270/Q27P922T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nuclear receptor ROR-gamma (Homo sapiens (Human)) | BDBM50466891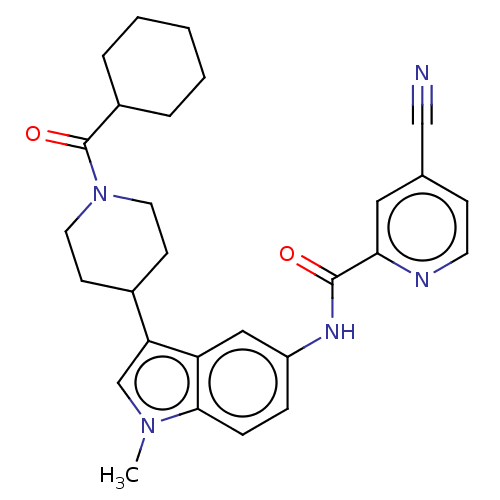 (CHEMBL4281109) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid PDB UniChem | PDB Article PubMed | 20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Karo Bio AB (now Karo Pharma AB) Curated by ChEMBL | Assay Description Displacement of [3H]-25-hydroxycholesterol from human N-terminal 6His-tagged-RORC2 LBD (259 to 483 residues) expressed in Escherichia coli (DE3) cell... | J Med Chem 61: 10415-10439 (2018) Article DOI: 10.1021/acs.jmedchem.8b00392 BindingDB Entry DOI: 10.7270/Q27P922T | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Acetylcholine-binding protein (Lymnaea stagnalis) | BDBM91601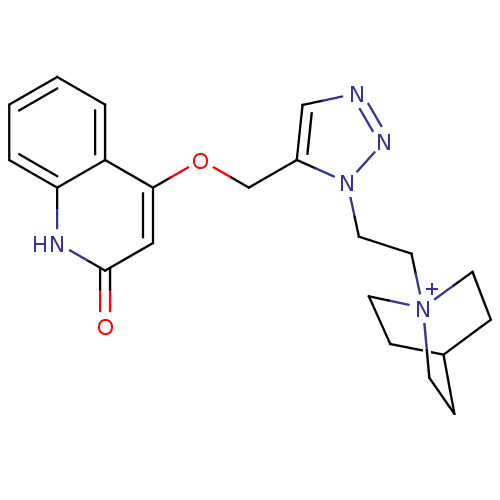 (3' | alpha7 agonists 3') | PDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 24 | -42.8 | n/a | n/a | n/a | n/a | n/a | 7.4 | 20 |
University of California, San Diego | Assay Description Assay Description 2 Assays used to generate Ki or EC50 values. 1) SPA Assay - Quick screen binding assays were performed using 100 ?l of 0.2 mg/ml an... | Taylor Research Group (2014) Article DOI: 10.1124/mol.112.080291 BindingDB Entry DOI: 10.7270/Q2805163 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholine-binding protein (Lymnaea stagnalis) | BDBM91629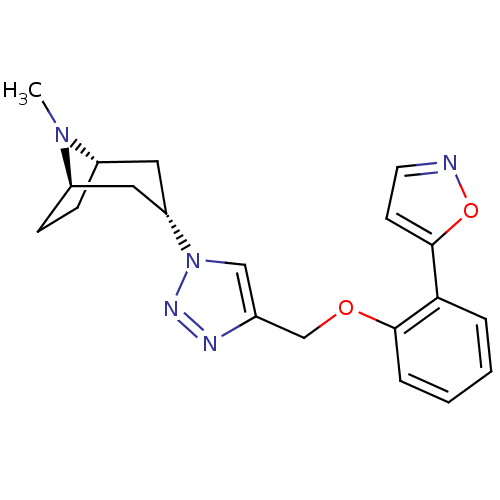 (14t | alpha7 agonists 14t) | PDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 25 | -42.7 | n/a | n/a | n/a | n/a | n/a | 7.4 | 20 |
University of California, San Diego | Assay Description Assay Description 2 Assays used to generate Ki or EC50 values. 1) SPA Assay - Quick screen binding assays were performed using 100 ?l of 0.2 mg/ml an... | Taylor Research Group (2014) Article DOI: 10.1124/mol.112.080291 BindingDB Entry DOI: 10.7270/Q2805163 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholine-binding protein (Lymnaea stagnalis) | BDBM91628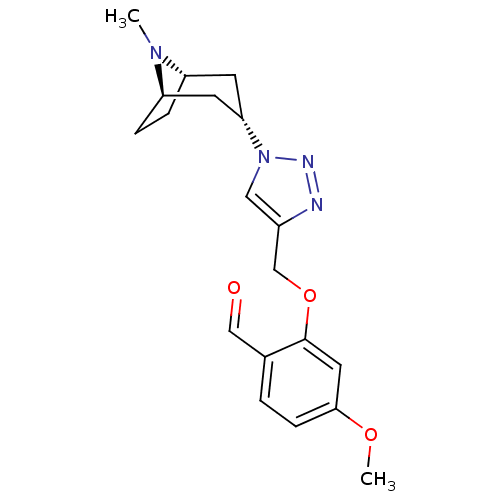 (13t | alpha7 agonists 13t) | PDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 26 | -42.6 | n/a | n/a | n/a | n/a | n/a | 7.4 | 20 |
University of California, San Diego | Assay Description Assay Description 2 Assays used to generate Ki or EC50 values. 1) SPA Assay - Quick screen binding assays were performed using 100 ?l of 0.2 mg/ml an... | Taylor Research Group (2014) Article DOI: 10.1124/mol.112.080291 BindingDB Entry DOI: 10.7270/Q2805163 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nicotinic acetylcholine receptor (RAT) | BDBM50061562 ((12R,13aR)-12-Methoxy-1,4,5,6,9,11,12,13-octahydro...) | UniProtKB/TrEMBL GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | PubMed | 28 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of California Curated by PDSP Ki Database | J Pharmacol Exp Ther 270: 159-66 (1994) BindingDB Entry DOI: 10.7270/Q22Z141C | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nicotinic acetylcholine receptor (RAT) | BDBM50061567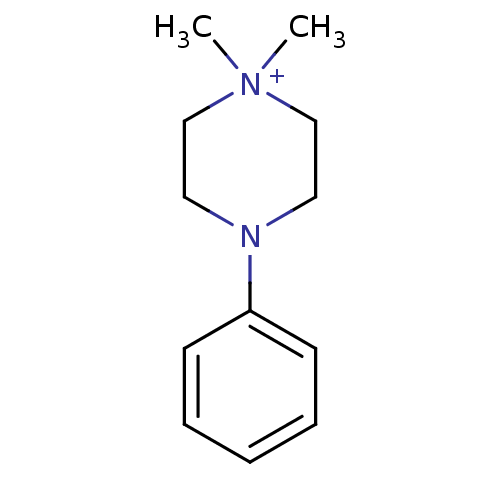 (1,1-Dimethyl-4-phenyl-piperazin-1-ium | CHEMBL1347...) | UniProtKB/TrEMBL GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG PC cid PC sid UniChem Patents Similars | PubMed | 28.8 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of California Curated by PDSP Ki Database | J Pharmacol Exp Ther 270: 159-66 (1994) BindingDB Entry DOI: 10.7270/Q22Z141C | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Soluble acetylcholine receptor (Aplysia Californica) | BDBM91621 (21q | alpha7 agonists 21q) | PDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 30 | -42.2 | n/a | n/a | n/a | n/a | n/a | 7.4 | 20 |
University of California, San Diego | Assay Description Assay Description 2 Assays used to generate Ki or EC50 values. 1) SPA Assay - Quick screen binding assays were performed using 100 ?l of 0.2 mg/ml an... | Taylor Research Group (2014) Article DOI: 10.1124/mol.112.080291 BindingDB Entry DOI: 10.7270/Q2805163 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholine-binding protein (Lymnaea stagnalis) | BDBM91633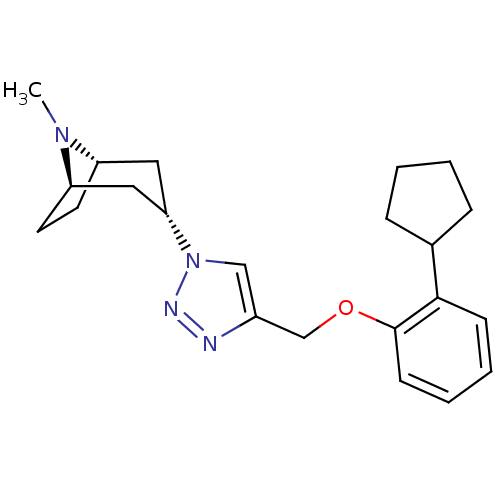 (18t | alpha7 agonists 18t) | PDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 30 | -42.2 | n/a | n/a | n/a | n/a | n/a | 7.4 | 20 |
University of California, San Diego | Assay Description Assay Description 2 Assays used to generate Ki or EC50 values. 1) SPA Assay - Quick screen binding assays were performed using 100 ?l of 0.2 mg/ml an... | Taylor Research Group (2014) Article DOI: 10.1124/mol.112.080291 BindingDB Entry DOI: 10.7270/Q2805163 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Rattus norvegicus (rat)) | BDBM86253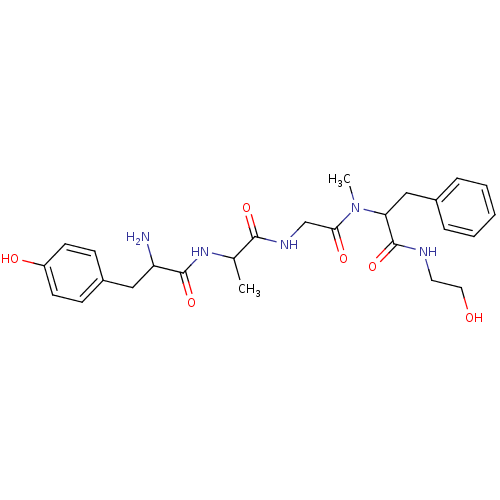 (CAS_100929-53-1 | DAMGO | NSC_104742 | US10836728,...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | KEGG PC cid PC sid UniChem Patents Similars | Article PubMed | 32 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Michigan Curated by PDSP Ki Database | J Chem Neuroanat 25: 233-47 (2003) Article DOI: 10.1016/s0891-0618(03)00032-2 BindingDB Entry DOI: 10.7270/Q2XP73G2 | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nicotinic acetylcholine receptor (RAT) | BDBM82068 (CHEMBL9732 | Nicotine-D salicylate | Nicotine-L sa...) | UniProtKB/TrEMBL GoogleScholar AffyNet | Purchase CHEMBL KEGG PC cid PC sid UniChem Similars | PubMed | 35 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of California Curated by PDSP Ki Database | J Pharmacol Exp Ther 270: 159-66 (1994) BindingDB Entry DOI: 10.7270/Q22Z141C | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholine-binding protein (Lymnaea stagnalis) | BDBM91621 (21q | alpha7 agonists 21q) | PDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 40 | -41.5 | n/a | n/a | n/a | n/a | n/a | 7.4 | 20 |
University of California, San Diego | Assay Description Assay Description 2 Assays used to generate Ki or EC50 values. 1) SPA Assay - Quick screen binding assays were performed using 100 ?l of 0.2 mg/ml an... | Taylor Research Group (2014) Article DOI: 10.1124/mol.112.080291 BindingDB Entry DOI: 10.7270/Q2805163 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholine-binding protein (Lymnaea stagnalis) | BDBM91599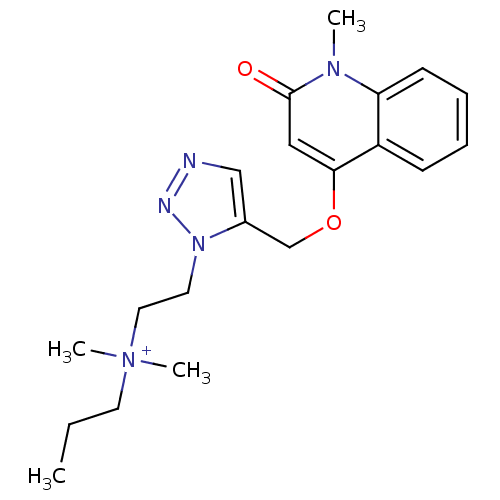 (2' | alpha7 agonists 2') | PDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 40 | -41.5 | n/a | n/a | n/a | n/a | n/a | 7.4 | 20 |
University of California, San Diego | Assay Description Assay Description 2 Assays used to generate Ki or EC50 values. 1) SPA Assay - Quick screen binding assays were performed using 100 ?l of 0.2 mg/ml an... | Taylor Research Group (2014) Article DOI: 10.1124/mol.112.080291 BindingDB Entry DOI: 10.7270/Q2805163 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholine-binding protein (Lymnaea stagnalis) | BDBM91630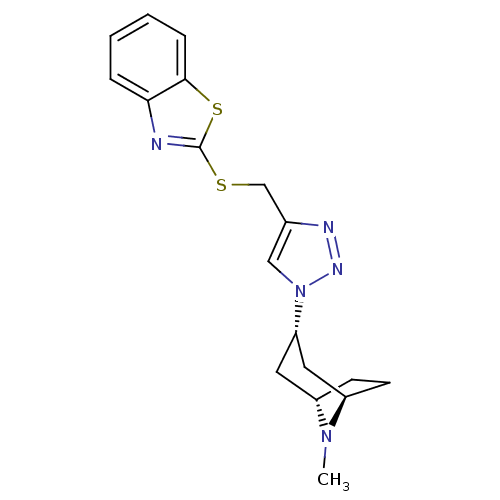 (15t | alpha7 agonists 15t) | PDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 40 | -41.5 | n/a | n/a | n/a | n/a | n/a | 7.4 | 20 |
University of California, San Diego | Assay Description Assay Description 2 Assays used to generate Ki or EC50 values. 1) SPA Assay - Quick screen binding assays were performed using 100 ?l of 0.2 mg/ml an... | Taylor Research Group (2014) Article DOI: 10.1124/mol.112.080291 BindingDB Entry DOI: 10.7270/Q2805163 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuronal acetylcholine receptor subunit alpha-7 (Rattus norvegicus (Rat)) | BDBM50356467 (CHEMBL1911855) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 42 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Targacept Inc Curated by ChEMBL | Assay Description Displacement of [3H]-methyllycaconitine from alpha7 nAChR in rat hippocampal membranes after 2 hrs by liquid scintillation counting | Eur J Med Chem 46: 5625-35 (2011) Article DOI: 10.1016/j.ejmech.2011.09.033 BindingDB Entry DOI: 10.7270/Q2639Q4D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholine-binding protein (Lymnaea stagnalis) | BDBM91614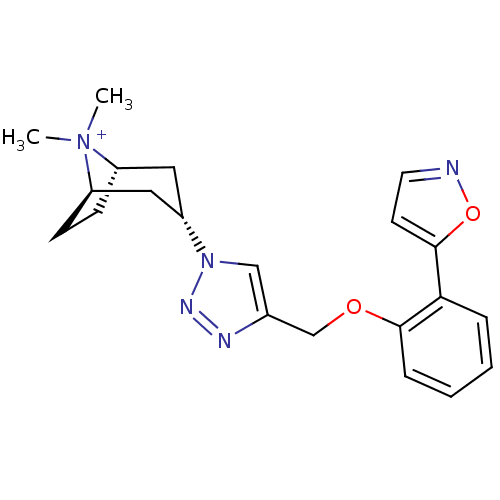 (14q | alpha7 agonists 14q) | PDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 50 | -41.0 | n/a | n/a | n/a | n/a | n/a | 7.4 | 20 |
University of California, San Diego | Assay Description Assay Description 2 Assays used to generate Ki or EC50 values. 1) SPA Assay - Quick screen binding assays were performed using 100 ?l of 0.2 mg/ml an... | Taylor Research Group (2014) Article DOI: 10.1124/mol.112.080291 BindingDB Entry DOI: 10.7270/Q2805163 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholine-binding protein (Lymnaea stagnalis) | BDBM91620 (20q | alpha7 agonists 20q) | PDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 50 | -41.0 | n/a | n/a | n/a | n/a | n/a | 7.4 | 20 |
University of California, San Diego | Assay Description Assay Description 2 Assays used to generate Ki or EC50 values. 1) SPA Assay - Quick screen binding assays were performed using 100 ?l of 0.2 mg/ml an... | Taylor Research Group (2014) Article DOI: 10.1124/mol.112.080291 BindingDB Entry DOI: 10.7270/Q2805163 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Soluble acetylcholine receptor (Aplysia Californica) | BDBM91611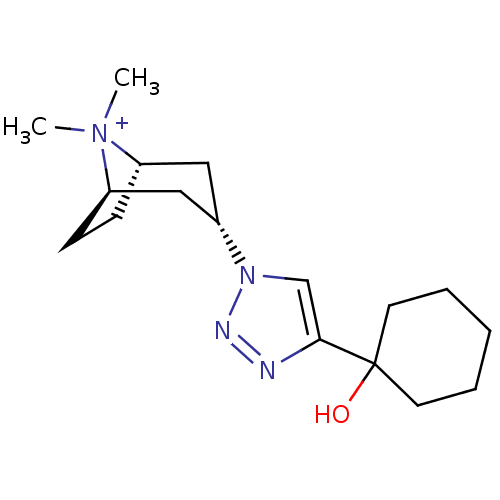 (11q | alpha7 agonists 11q) | PDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 50 | -41.0 | n/a | n/a | n/a | n/a | n/a | 7.4 | 20 |
University of California, San Diego | Assay Description Assay Description 2 Assays used to generate Ki or EC50 values. 1) SPA Assay - Quick screen binding assays were performed using 100 ?l of 0.2 mg/ml an... | Taylor Research Group (2014) Article DOI: 10.1124/mol.112.080291 BindingDB Entry DOI: 10.7270/Q2805163 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nuclear receptor ROR-gamma (Homo sapiens (Human)) | BDBM50466893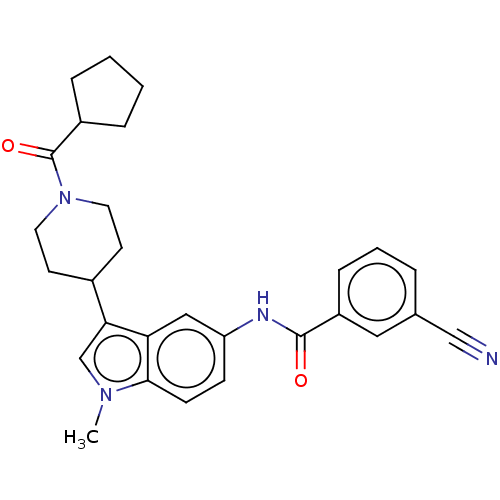 (CHEMBL4291727) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 50 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Karo Bio AB (now Karo Pharma AB) Curated by ChEMBL | Assay Description Displacement of [3H]-25-hydroxycholesterol from human N-terminal 6His-tagged-RORC2 LBD (259 to 483 residues) expressed in Escherichia coli (DE3) cell... | J Med Chem 61: 10415-10439 (2018) Article DOI: 10.1021/acs.jmedchem.8b00392 BindingDB Entry DOI: 10.7270/Q27P922T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuronal acetylcholine receptor subunit alpha-7 (Rattus norvegicus (Rat)) | BDBM50356469 (CHEMBL1911857) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 58 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Targacept Inc Curated by ChEMBL | Assay Description Displacement of [3H]-methyllycaconitine from alpha7 nAChR in rat hippocampal membranes after 2 hrs by liquid scintillation counting | Eur J Med Chem 46: 5625-35 (2011) Article DOI: 10.1016/j.ejmech.2011.09.033 BindingDB Entry DOI: 10.7270/Q2639Q4D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 1094 total ) | Next | Last >> |