

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 5-hydroxytryptamine receptor 1B (Homo sapiens (Human)) | BDBM50271131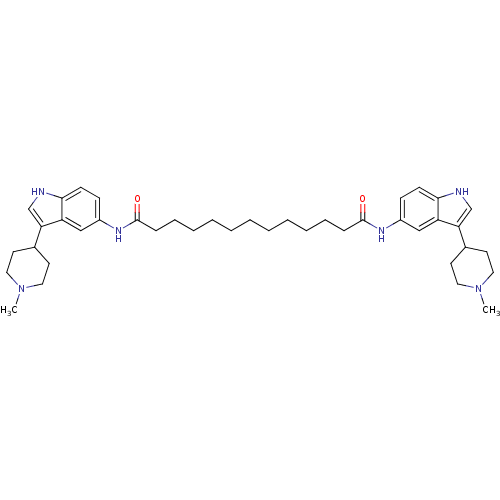 (CHEMBL374973 | N1,N13-bis(3-(1-methylpiperidin-4-y...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Theravance, Inc. Curated by ChEMBL | Assay Description Displacement of [3H]5-hydroxytryptamine from human cloned 5HT1B receptor expressed in CHOK1 cells by liquid scintillation counting | J Med Chem 51: 3609-16 (2008) Article DOI: 10.1021/jm7011722 BindingDB Entry DOI: 10.7270/Q26T0MDK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1B (Homo sapiens (Human)) | BDBM50054974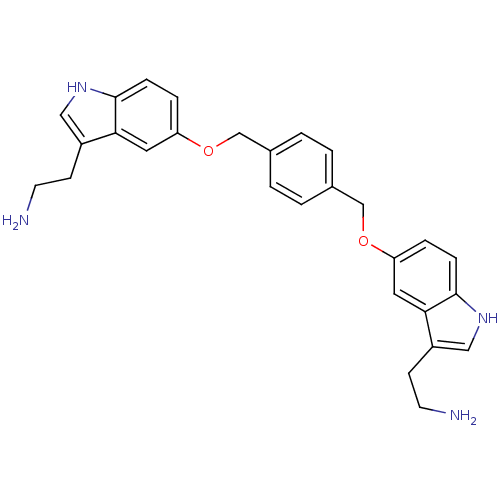 (2-(5-((4-((3-(2-aminoethyl)-1H-indol-5-yloxy)methy...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Theravance, Inc. Curated by ChEMBL | Assay Description Displacement of [3H]5-hydroxytryptamine from human cloned 5HT1B receptor expressed in CHOK1 cells by liquid scintillation counting | J Med Chem 51: 3609-16 (2008) Article DOI: 10.1021/jm7011722 BindingDB Entry DOI: 10.7270/Q26T0MDK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1D (Homo sapiens (Human)) | BDBM50054974 (2-(5-((4-((3-(2-aminoethyl)-1H-indol-5-yloxy)methy...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.110 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Theravance, Inc. Curated by ChEMBL | Assay Description Displacement of [3H]5-hydroxytryptamine from human cloned 5HT1D receptor expressed in CHOK1 cells by liquid scintillation counting | J Med Chem 51: 3609-16 (2008) Article DOI: 10.1021/jm7011722 BindingDB Entry DOI: 10.7270/Q26T0MDK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1D (Homo sapiens (Human)) | BDBM50271019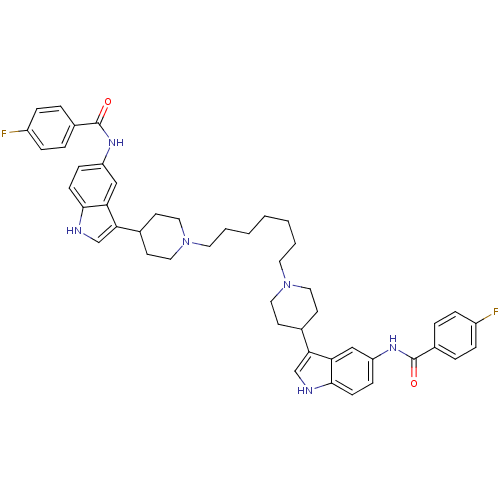 (4-fluoro-N-(3-{1-[7-(4-{5-[(4-fluorobenzene)amido]...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Theravance, Inc. Curated by ChEMBL | Assay Description Displacement of [3H]5-hydroxytryptamine from human cloned 5HT1D receptor expressed in CHOK1 cells by liquid scintillation counting | J Med Chem 51: 3609-16 (2008) Article DOI: 10.1021/jm7011722 BindingDB Entry DOI: 10.7270/Q26T0MDK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-2 adrenergic receptor (Homo sapiens (Human)) | BDBM50324835 ((R)-4-hydroxy-7-(1-hydroxy-2-(3-(methyl(phenyl)ami...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research Curated by ChEMBL | Assay Description Displacement of [3H]CGP12177 from human beta2 adrenoceptor | Bioorg Med Chem Lett 20: 5302-7 (2010) Article DOI: 10.1016/j.bmcl.2010.06.136 BindingDB Entry DOI: 10.7270/Q2HD7VVM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-2 adrenergic receptor (Homo sapiens (Human)) | BDBM50055260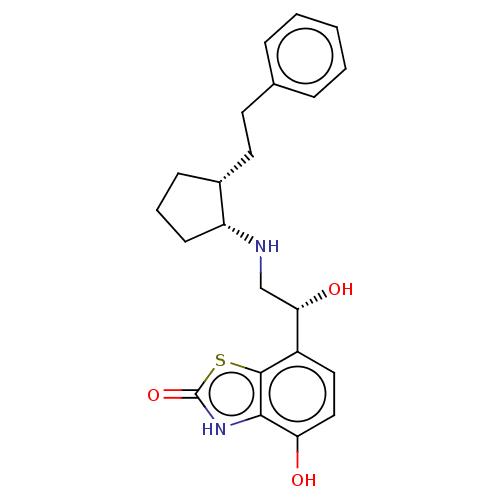 (CHEMBL3323658) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research Curated by ChEMBL | Assay Description Displacement of [125I]iodo-(+/-)-cyanopindolol from human adrenergic beta2 receptor expressed in CHO cells after 3 hrs by radio-ligand binding assay | Bioorg Med Chem Lett 24: 4341-7 (2014) Article DOI: 10.1016/j.bmcl.2014.06.014 BindingDB Entry DOI: 10.7270/Q2Z89F2R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-2 adrenergic receptor (Homo sapiens (Human)) | BDBM50324847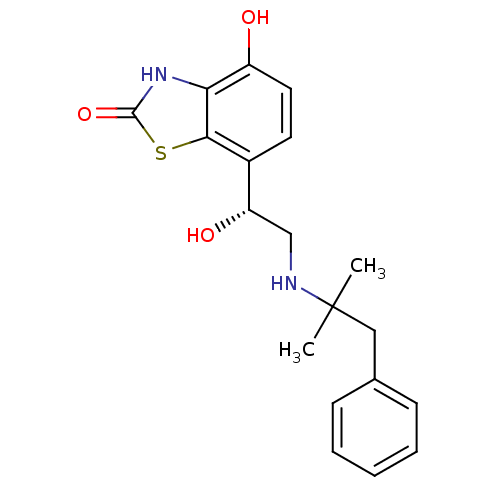 ((R)-4-hydroxy-7-(1-hydroxy-2-(2-methyl-1-phenylpro...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research Curated by ChEMBL | Assay Description Displacement of [3H]CGP12177 from human beta2 adrenoceptor | Bioorg Med Chem Lett 20: 5302-7 (2010) Article DOI: 10.1016/j.bmcl.2010.06.136 BindingDB Entry DOI: 10.7270/Q2HD7VVM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1D (Homo sapiens (Human)) | BDBM50070409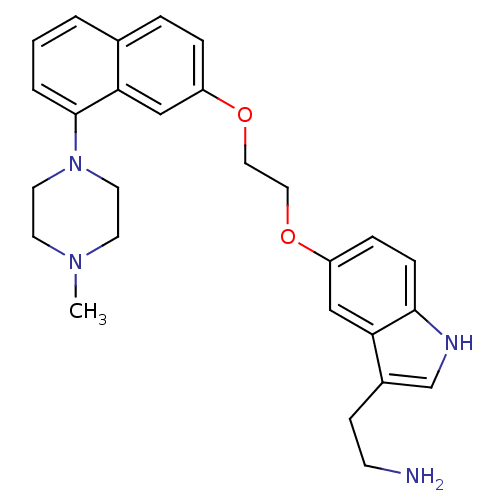 (2-(5-(2-(8-(4-methylpiperazin-1-yl)naphthalen-2-yl...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Theravance, Inc. Curated by ChEMBL | Assay Description Displacement of [3H]5-hydroxytryptamine from human cloned 5HT1D receptor expressed in CHOK1 cells by liquid scintillation counting | J Med Chem 51: 3609-16 (2008) Article DOI: 10.1021/jm7011722 BindingDB Entry DOI: 10.7270/Q26T0MDK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1B (Homo sapiens (Human)) | BDBM50271132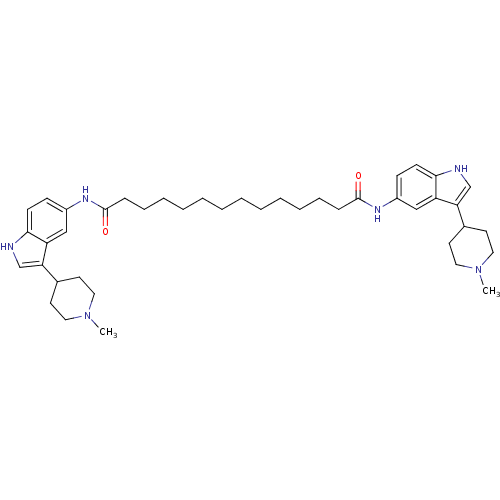 (CHEMBL502138 | N1,N14-bis(3-(1-methylpiperidin-4-y...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Theravance, Inc. Curated by ChEMBL | Assay Description Displacement of [3H]5-hydroxytryptamine from human cloned 5HT1B receptor expressed in CHOK1 cells by liquid scintillation counting | J Med Chem 51: 3609-16 (2008) Article DOI: 10.1021/jm7011722 BindingDB Entry DOI: 10.7270/Q26T0MDK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-2 adrenergic receptor (Homo sapiens (Human)) | BDBM50324854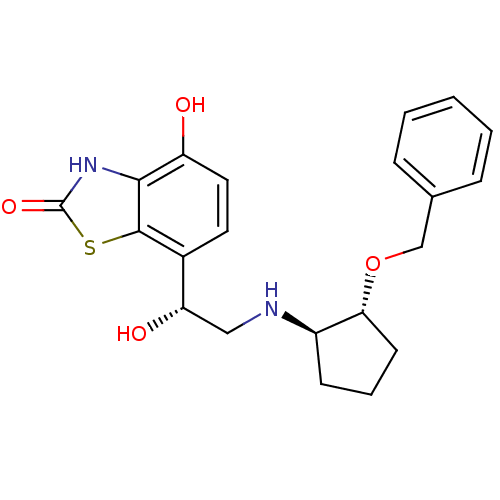 (7-((R)-2-((1R,2R)-2-(benzyloxy)cyclopentylamino)-1...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research Curated by ChEMBL | Assay Description Displacement of [3H]CGP12177 from human beta2 adrenoceptor | Bioorg Med Chem Lett 20: 5302-7 (2010) Article DOI: 10.1016/j.bmcl.2010.06.136 BindingDB Entry DOI: 10.7270/Q2HD7VVM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1D (Homo sapiens (Human)) | BDBM10755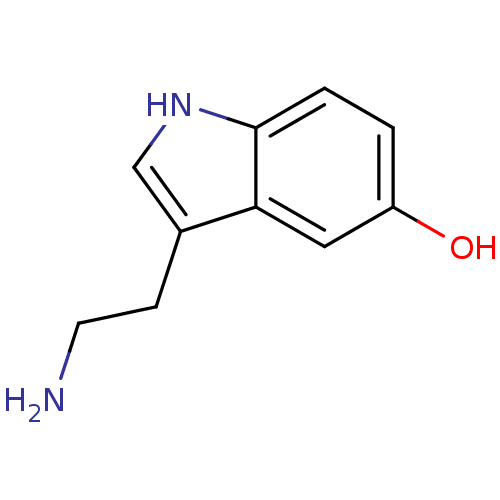 (14C-5-hydroxy tryptamine creatinine disulfate | 2-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG PC cid PC sid PDB UniChem Similars | PDB Article PubMed | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Theravance, Inc. Curated by ChEMBL | Assay Description Displacement of [3H]5-hydroxytryptamine from human cloned 5HT1D receptor expressed in CHOK1 cells by liquid scintillation counting | J Med Chem 51: 3609-16 (2008) Article DOI: 10.1021/jm7011722 BindingDB Entry DOI: 10.7270/Q26T0MDK | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| 5-hydroxytryptamine receptor 1B (Homo sapiens (Human)) | BDBM50271133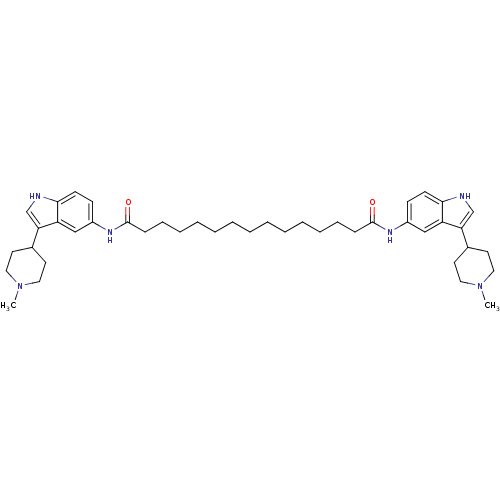 (CHEMBL500284 | N1,N15-bis(3-(1-methylpiperidin-4-y...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.580 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Theravance, Inc. Curated by ChEMBL | Assay Description Displacement of [3H]5-hydroxytryptamine from human cloned 5HT1B receptor expressed in CHOK1 cells by liquid scintillation counting | J Med Chem 51: 3609-16 (2008) Article DOI: 10.1021/jm7011722 BindingDB Entry DOI: 10.7270/Q26T0MDK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1D (Homo sapiens (Human)) | BDBM50271018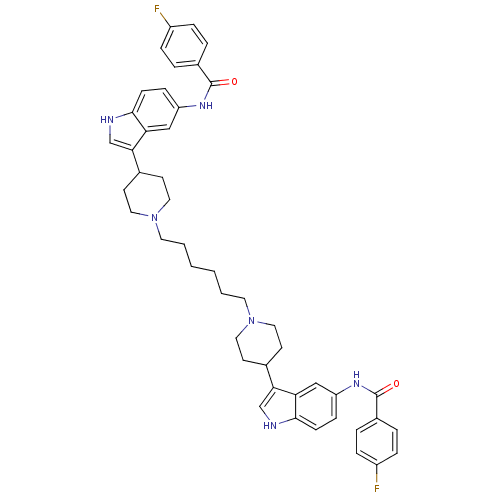 (4-fluoro-N-(3-{1-[6-(4-{5-[(4-fluorobenzene)amido]...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Theravance, Inc. Curated by ChEMBL | Assay Description Displacement of [3H]5-hydroxytryptamine from human cloned 5HT1D receptor expressed in CHOK1 cells by liquid scintillation counting | J Med Chem 51: 3609-16 (2008) Article DOI: 10.1021/jm7011722 BindingDB Entry DOI: 10.7270/Q26T0MDK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-2 adrenergic receptor (Homo sapiens (Human)) | BDBM50055263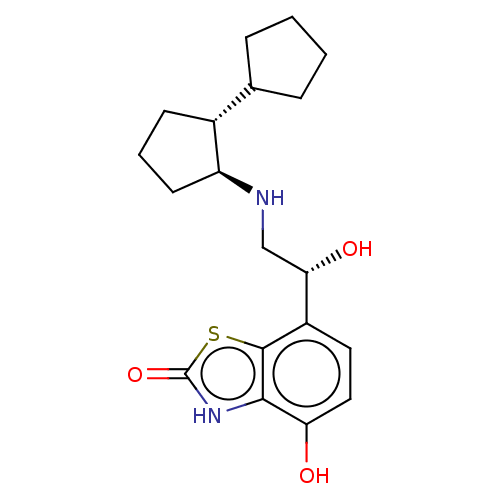 (CHEMBL3323654) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research Curated by ChEMBL | Assay Description Displacement of [125I]iodo-(+/-)-cyanopindolol from human adrenergic beta2 receptor expressed in CHO cells after 3 hrs by radio-ligand binding assay | Bioorg Med Chem Lett 24: 4341-7 (2014) Article DOI: 10.1016/j.bmcl.2014.06.014 BindingDB Entry DOI: 10.7270/Q2Z89F2R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-2 adrenergic receptor (Homo sapiens (Human)) | BDBM50324856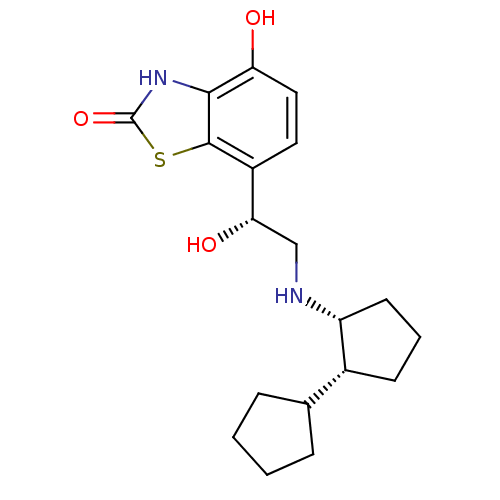 (7-((R)-2-((cis)-bi(cyclopentan)-2-ylamino)-1-hydro...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research Curated by ChEMBL | Assay Description Displacement of [3H]CGP12177 from human beta2 adrenoceptor | Bioorg Med Chem Lett 20: 5302-7 (2010) Article DOI: 10.1016/j.bmcl.2010.06.136 BindingDB Entry DOI: 10.7270/Q2HD7VVM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1B (Homo sapiens (Human)) | BDBM50069315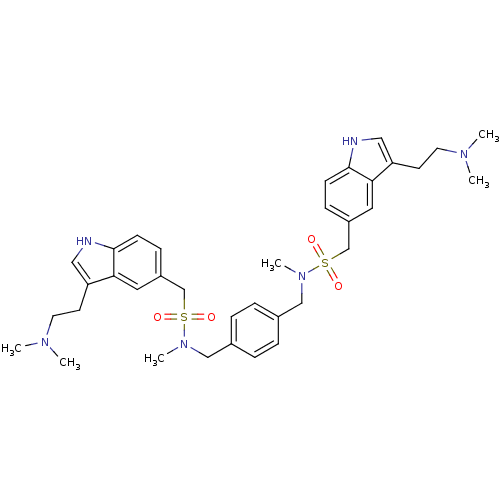 (C-[3-(2-Dimethylamino-ethyl)-1H-indol-5-yl]-N-[4-(...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | 0.640 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Theravance, Inc. Curated by ChEMBL | Assay Description Displacement of [3H]5-hydroxytryptamine from human cloned 5HT1B receptor expressed in CHOK1 cells by liquid scintillation counting | J Med Chem 51: 3609-16 (2008) Article DOI: 10.1021/jm7011722 BindingDB Entry DOI: 10.7270/Q26T0MDK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1B (Homo sapiens (Human)) | BDBM50271127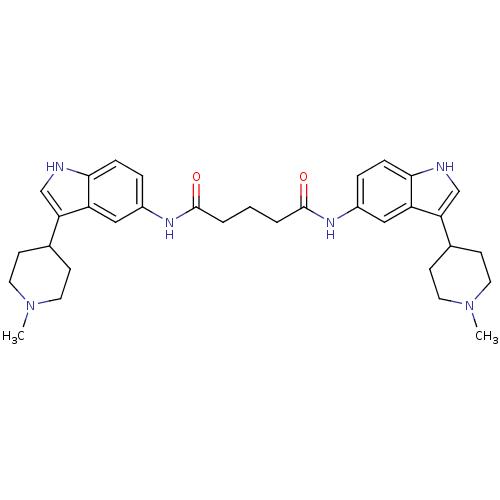 (CHEMBL444404 | N1,N5-bis(3-(1-methylpiperidin-4-yl...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.760 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Theravance, Inc. Curated by ChEMBL | Assay Description Displacement of [3H]5-hydroxytryptamine from human cloned 5HT1B receptor expressed in CHOK1 cells by liquid scintillation counting | J Med Chem 51: 3609-16 (2008) Article DOI: 10.1021/jm7011722 BindingDB Entry DOI: 10.7270/Q26T0MDK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1B (Homo sapiens (Human)) | BDBM50070409 (2-(5-(2-(8-(4-methylpiperazin-1-yl)naphthalen-2-yl...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.760 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Theravance, Inc. Curated by ChEMBL | Assay Description Displacement of [3H]5-hydroxytryptamine from human cloned 5HT1B receptor expressed in CHOK1 cells by liquid scintillation counting | J Med Chem 51: 3609-16 (2008) Article DOI: 10.1021/jm7011722 BindingDB Entry DOI: 10.7270/Q26T0MDK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1B (Homo sapiens (Human)) | BDBM50271129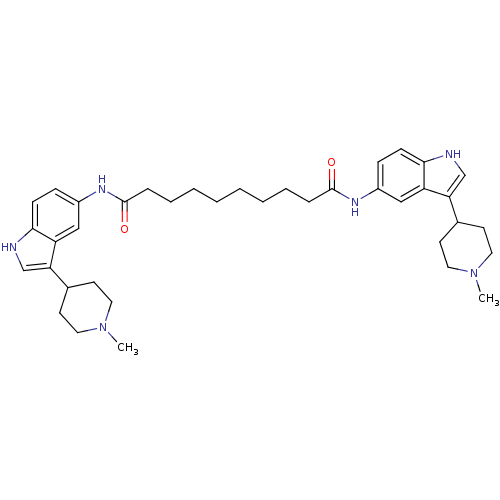 (CHEMBL452387 | N1,N10-bis(3-(1-methylpiperidin-4-y...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.790 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Theravance, Inc. Curated by ChEMBL | Assay Description Displacement of [3H]5-hydroxytryptamine from human cloned 5HT1B receptor expressed in CHOK1 cells by liquid scintillation counting | J Med Chem 51: 3609-16 (2008) Article DOI: 10.1021/jm7011722 BindingDB Entry DOI: 10.7270/Q26T0MDK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-1 adrenergic receptor (Homo sapiens (Human)) | BDBM50055260 (CHEMBL3323658) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.800 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research Curated by ChEMBL | Assay Description Displacement of [125I]iodo-(+/-)-cyanopindolol from human adrenergic beta1 receptor expressed in CHO cells after 3 hrs by radio-ligand binding assay | Bioorg Med Chem Lett 24: 4341-7 (2014) Article DOI: 10.1016/j.bmcl.2014.06.014 BindingDB Entry DOI: 10.7270/Q2Z89F2R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-2 adrenergic receptor (Homo sapiens (Human)) | BDBM50324856 (7-((R)-2-((cis)-bi(cyclopentan)-2-ylamino)-1-hydro...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.800 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research Curated by ChEMBL | Assay Description Displacement of [125I]iodo-(+/-)-cyanopindolol from human adrenergic beta2 receptor expressed in CHO cells after 3 hrs by radio-ligand binding assay | Bioorg Med Chem Lett 24: 4341-7 (2014) Article DOI: 10.1016/j.bmcl.2014.06.014 BindingDB Entry DOI: 10.7270/Q2Z89F2R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-2 adrenergic receptor (Homo sapiens (Human)) | BDBM50324857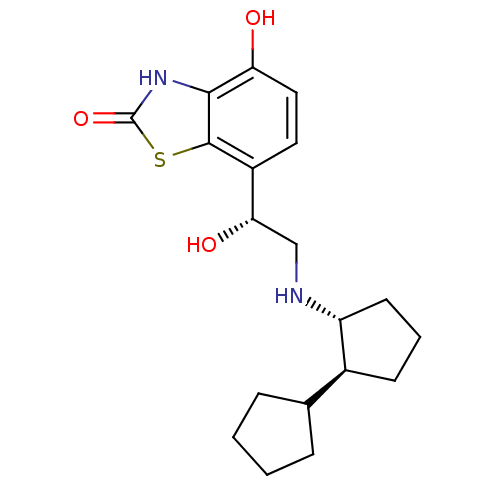 (7-((R)-2-((trans)-bi(cyclopentan)-2-ylamino)-1-hyd...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.800 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research Curated by ChEMBL | Assay Description Displacement of [3H]CGP12177 from human beta2 adrenoceptor | Bioorg Med Chem Lett 20: 5302-7 (2010) Article DOI: 10.1016/j.bmcl.2010.06.136 BindingDB Entry DOI: 10.7270/Q2HD7VVM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1B (Homo sapiens (Human)) | BDBM50271134 (CHEMBL500488 | N1,N16-bis(3-(1-methylpiperidin-4-y...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.810 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Theravance, Inc. Curated by ChEMBL | Assay Description Displacement of [3H]5-hydroxytryptamine from human cloned 5HT1B receptor expressed in CHOK1 cells by liquid scintillation counting | J Med Chem 51: 3609-16 (2008) Article DOI: 10.1021/jm7011722 BindingDB Entry DOI: 10.7270/Q26T0MDK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1D (Homo sapiens (Human)) | BDBM50069315 (C-[3-(2-Dimethylamino-ethyl)-1H-indol-5-yl]-N-[4-(...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | 0.890 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Theravance, Inc. Curated by ChEMBL | Assay Description Displacement of [3H]5-hydroxytryptamine from human cloned 5HT1D receptor expressed in CHOK1 cells by liquid scintillation counting | J Med Chem 51: 3609-16 (2008) Article DOI: 10.1021/jm7011722 BindingDB Entry DOI: 10.7270/Q26T0MDK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1D (Homo sapiens (Human)) | BDBM50271128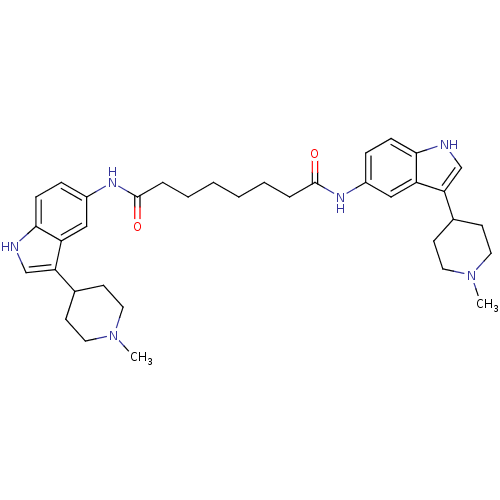 (CHEMBL448902 | N1,N8-bis(3-(1-methylpiperidin-4-yl...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.900 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Theravance, Inc. Curated by ChEMBL | Assay Description Displacement of [3H]5-hydroxytryptamine from human cloned 5HT1D receptor expressed in CHOK1 cells by liquid scintillation counting | J Med Chem 51: 3609-16 (2008) Article DOI: 10.1021/jm7011722 BindingDB Entry DOI: 10.7270/Q26T0MDK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 11-beta-hydroxysteroid dehydrogenase 1 (Homo sapiens (Human)) | BDBM50507371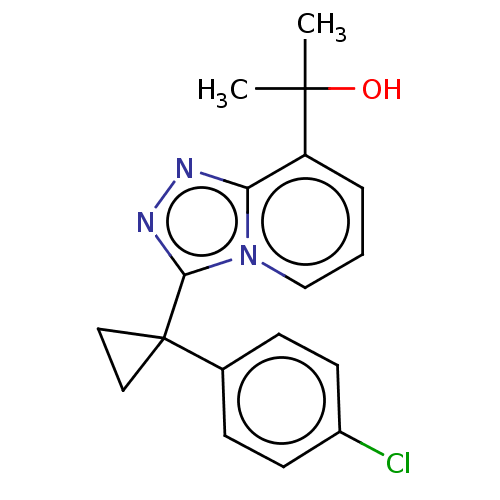 (BMS-823778) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid PDB UniChem | PDB Article PubMed | 0.900 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Curated by ChEMBL | Assay Description Inhibition of recombinant human 11beta-HSD1 expressed in HEK293 cell microsomes using [3H]cortisone as substrate after 4 hrs by homogeneous immuno-ra... | ACS Med Chem Lett 9: 1170-1174 (2018) Article DOI: 10.1021/acsmedchemlett.8b00307 BindingDB Entry DOI: 10.7270/Q20R9SP3 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| 5-hydroxytryptamine receptor 1D (Homo sapiens (Human)) | BDBM50271130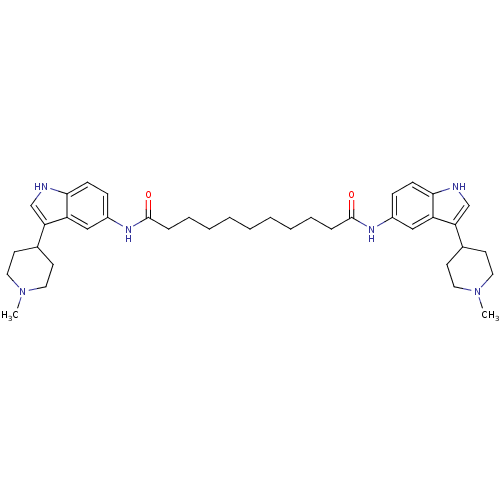 (CHEMBL446745 | N1,N11-bis(3-(1-methylpiperidin-4-y...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.900 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Theravance, Inc. Curated by ChEMBL | Assay Description Displacement of [3H]5-hydroxytryptamine from human cloned 5HT1D receptor expressed in CHOK1 cells by liquid scintillation counting | J Med Chem 51: 3609-16 (2008) Article DOI: 10.1021/jm7011722 BindingDB Entry DOI: 10.7270/Q26T0MDK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-1 adrenergic receptor (Homo sapiens (Human)) | BDBM50324854 (7-((R)-2-((1R,2R)-2-(benzyloxy)cyclopentylamino)-1...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.900 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research Curated by ChEMBL | Assay Description Displacement of [3H]CGP12177 from human beta-1 adrenoceptor | Bioorg Med Chem Lett 20: 5302-7 (2010) Article DOI: 10.1016/j.bmcl.2010.06.136 BindingDB Entry DOI: 10.7270/Q2HD7VVM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1D (Homo sapiens (Human)) | BDBM50064786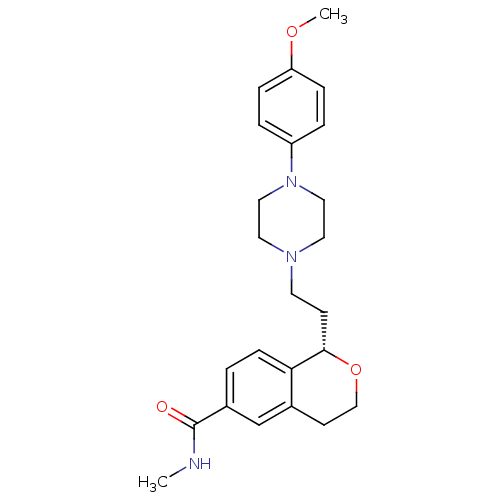 ((S)-1-(2-(4-(4-methoxyphenyl)piperazin-1-yl)ethyl)...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | 0.900 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Theravance, Inc. Curated by ChEMBL | Assay Description Displacement of [3H]5-hydroxytryptamine from human cloned 5HT1D receptor expressed in CHOK1 cells by liquid scintillation counting | J Med Chem 51: 3609-16 (2008) Article DOI: 10.1021/jm7011722 BindingDB Entry DOI: 10.7270/Q26T0MDK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1D (Homo sapiens (Human)) | BDBM50271127 (CHEMBL444404 | N1,N5-bis(3-(1-methylpiperidin-4-yl...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.950 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Theravance, Inc. Curated by ChEMBL | Assay Description Displacement of [3H]5-hydroxytryptamine from human cloned 5HT1D receptor expressed in CHOK1 cells by liquid scintillation counting | J Med Chem 51: 3609-16 (2008) Article DOI: 10.1021/jm7011722 BindingDB Entry DOI: 10.7270/Q26T0MDK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1D (Homo sapiens (Human)) | BDBM50271129 (CHEMBL452387 | N1,N10-bis(3-(1-methylpiperidin-4-y...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.950 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Theravance, Inc. Curated by ChEMBL | Assay Description Displacement of [3H]5-hydroxytryptamine from human cloned 5HT1D receptor expressed in CHOK1 cells by liquid scintillation counting | J Med Chem 51: 3609-16 (2008) Article DOI: 10.1021/jm7011722 BindingDB Entry DOI: 10.7270/Q26T0MDK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1A (Homo sapiens (Human)) | BDBM50070409 (2-(5-(2-(8-(4-methylpiperazin-1-yl)naphthalen-2-yl...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.960 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Theravance, Inc. Curated by ChEMBL | Assay Description Binding affinity to 5HT1A receptor | J Med Chem 51: 3609-16 (2008) Article DOI: 10.1021/jm7011722 BindingDB Entry DOI: 10.7270/Q26T0MDK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Substance-K receptor (Rattus norvegicus (Rat)) | BDBM50470598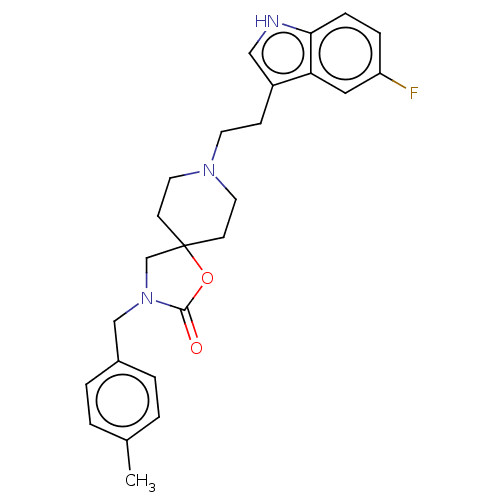 (CHEMBL126050) | PDB Reactome pathway UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Glaxo Wellcome Medicines Research Centre Curated by ChEMBL | Assay Description Binding affinity against tachykinin receptor 2 from rat colon. | J Med Chem 38: 3772-9 (1995) Article DOI: 10.1021/jm00019a006 BindingDB Entry DOI: 10.7270/Q2JM2DCF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-2 adrenergic receptor (Homo sapiens (Human)) | BDBM50324840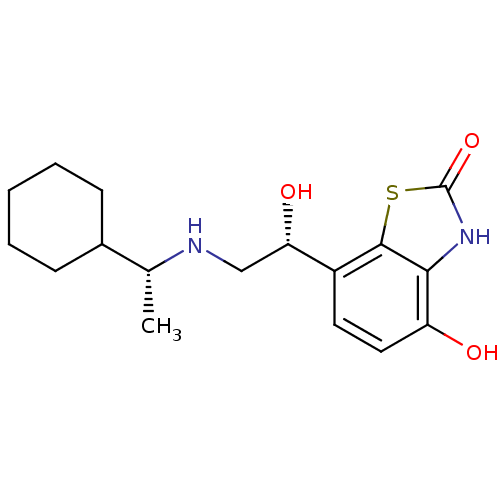 (7-((R)-2-((R)-1-cyclohexylethylamino)-1-hydroxyeth...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research Curated by ChEMBL | Assay Description Displacement of [3H]CGP12177 from human beta2 adrenoceptor | Bioorg Med Chem Lett 20: 5302-7 (2010) Article DOI: 10.1016/j.bmcl.2010.06.136 BindingDB Entry DOI: 10.7270/Q2HD7VVM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1D (Homo sapiens (Human)) | BDBM50271020 (4-fluoro-N-(3-{1-[9-(4-{5-[(4-fluorobenzene)amido]...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Theravance, Inc. Curated by ChEMBL | Assay Description Displacement of [3H]5-hydroxytryptamine from human cloned 5HT1D receptor expressed in CHOK1 cells by liquid scintillation counting | J Med Chem 51: 3609-16 (2008) Article DOI: 10.1021/jm7011722 BindingDB Entry DOI: 10.7270/Q26T0MDK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuraminidase (Influenza A virus (A/Puerto Rico/8/34/Mount Sinai(...) | BDBM50040516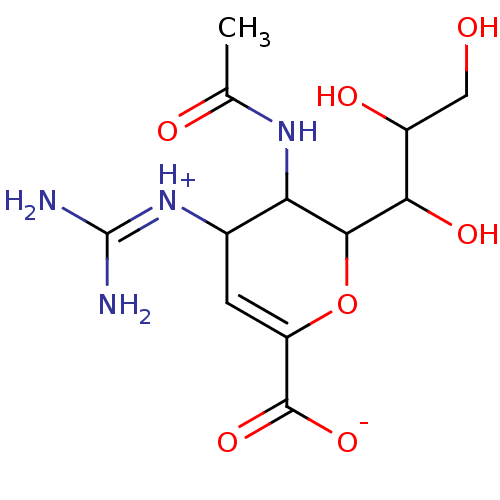 (3-(acetylamino)-4-{[amino(iminio)methyl]amino}-2-[...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | PubMed | 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Monash University Curated by ChEMBL | Assay Description inhibition of Influenza A Sialidase | J Med Chem 37: 616-24 (1994) Checked by Author BindingDB Entry DOI: 10.7270/Q27P901J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1B (Homo sapiens (Human)) | BDBM10755 (14C-5-hydroxy tryptamine creatinine disulfate | 2-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG PC cid PC sid PDB UniChem Similars | Article PubMed | 1.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Theravance, Inc. Curated by ChEMBL | Assay Description Displacement of [3H]5-hydroxytryptamine from human cloned 5HT1B receptor expressed in CHOK1 cells by liquid scintillation counting | J Med Chem 51: 3609-16 (2008) Article DOI: 10.1021/jm7011722 BindingDB Entry DOI: 10.7270/Q26T0MDK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1D (Homo sapiens (Human)) | BDBM50005835 ((3-[2-(dimethylamino)ethyl]-1H-indol-5-yl)-N-methy...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG PC cid PC sid UniChem Patents | DrugBank Article PubMed | 1.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Theravance, Inc. Curated by ChEMBL | Assay Description Displacement of [3H]5-hydroxytryptamine from human cloned 5HT1D receptor expressed in CHOK1 cells by liquid scintillation counting | J Med Chem 51: 3609-16 (2008) Article DOI: 10.1021/jm7011722 BindingDB Entry DOI: 10.7270/Q26T0MDK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-2 adrenergic receptor (Homo sapiens (Human)) | BDBM50324846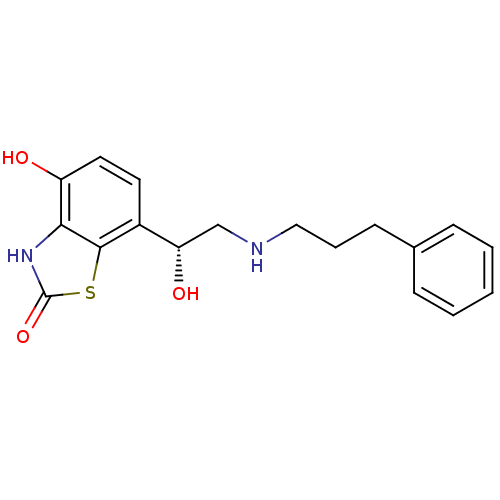 ((R)-4-hydroxy-7-(1-hydroxy-2-(3-phenylpropylamino)...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research Curated by ChEMBL | Assay Description Displacement of [3H]CGP12177 from human beta2 adrenoceptor | Bioorg Med Chem Lett 20: 5302-7 (2010) Article DOI: 10.1016/j.bmcl.2010.06.136 BindingDB Entry DOI: 10.7270/Q2HD7VVM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1B (Homo sapiens (Human)) | BDBM50271130 (CHEMBL446745 | N1,N11-bis(3-(1-methylpiperidin-4-y...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Theravance, Inc. Curated by ChEMBL | Assay Description Displacement of [3H]5-hydroxytryptamine from human cloned 5HT1B receptor expressed in CHOK1 cells by liquid scintillation counting | J Med Chem 51: 3609-16 (2008) Article DOI: 10.1021/jm7011722 BindingDB Entry DOI: 10.7270/Q26T0MDK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Substance-K receptor (Rattus norvegicus (Rat)) | BDBM50470604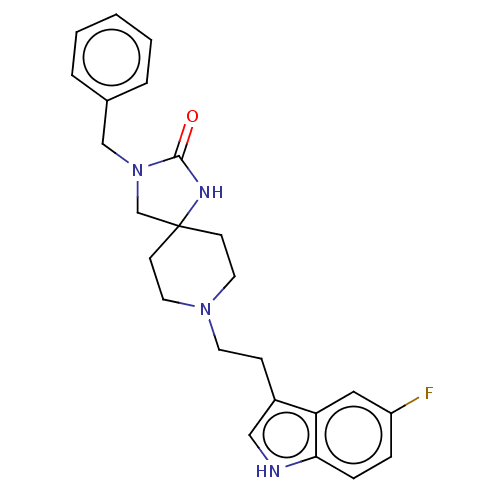 (CHEMBL338825) | PDB Reactome pathway UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 1.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Glaxo Wellcome Medicines Research Centre Curated by ChEMBL | Assay Description Binding affinity against tachykinin receptor 2 from rat colon. | J Med Chem 38: 3772-9 (1995) Article DOI: 10.1021/jm00019a006 BindingDB Entry DOI: 10.7270/Q2JM2DCF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1B (Homo sapiens (Human)) | BDBM50271128 (CHEMBL448902 | N1,N8-bis(3-(1-methylpiperidin-4-yl...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Theravance, Inc. Curated by ChEMBL | Assay Description Displacement of [3H]5-hydroxytryptamine from human cloned 5HT1B receptor expressed in CHOK1 cells by liquid scintillation counting | J Med Chem 51: 3609-16 (2008) Article DOI: 10.1021/jm7011722 BindingDB Entry DOI: 10.7270/Q26T0MDK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Substance-K receptor (Rattus norvegicus (Rat)) | BDBM50470590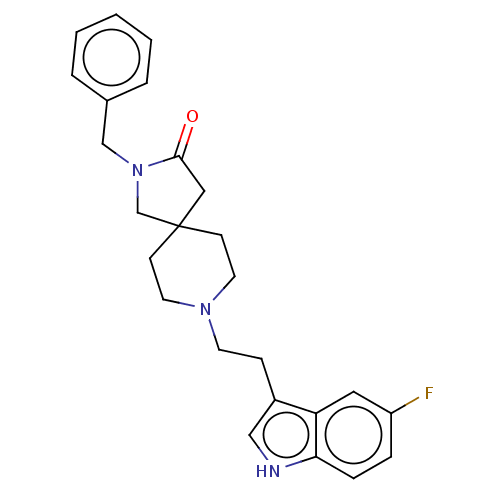 (CHEMBL341357) | PDB Reactome pathway UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 1.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Glaxo Wellcome Medicines Research Centre Curated by ChEMBL | Assay Description Binding affinity against tachykinin receptor 2 from rat colon. | J Med Chem 38: 3772-9 (1995) Article DOI: 10.1021/jm00019a006 BindingDB Entry DOI: 10.7270/Q2JM2DCF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Substance-K receptor (Rattus norvegicus (Rat)) | BDBM50470591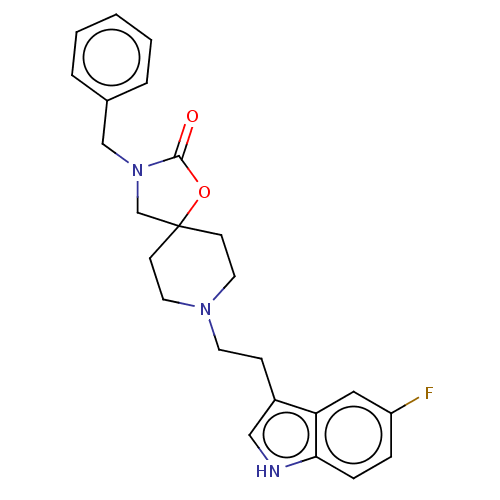 (CHEMBL124208) | PDB Reactome pathway UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 1.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Glaxo Wellcome Medicines Research Centre Curated by ChEMBL | Assay Description Binding affinity against tachykinin receptor 2 from rat colon. | J Med Chem 38: 3772-9 (1995) Article DOI: 10.1021/jm00019a006 BindingDB Entry DOI: 10.7270/Q2JM2DCF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1D (Homo sapiens (Human)) | BDBM50271131 (CHEMBL374973 | N1,N13-bis(3-(1-methylpiperidin-4-y...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Theravance, Inc. Curated by ChEMBL | Assay Description Displacement of [3H]5-hydroxytryptamine from human cloned 5HT1D receptor expressed in CHOK1 cells by liquid scintillation counting | J Med Chem 51: 3609-16 (2008) Article DOI: 10.1021/jm7011722 BindingDB Entry DOI: 10.7270/Q26T0MDK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-1 adrenergic receptor (Homo sapiens (Human)) | BDBM50324851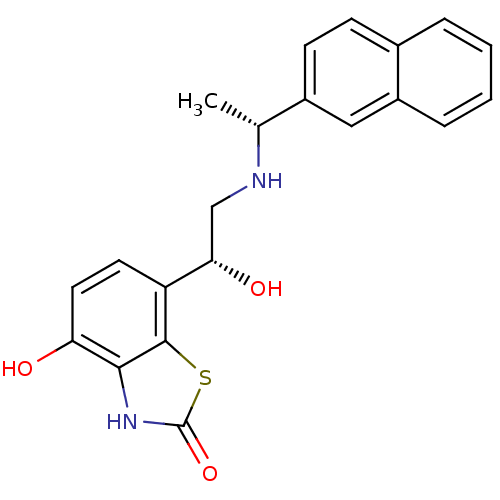 (4-hydroxy-7-((R)-1-hydroxy-2-((R)-1-(naphthalen-2-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research Curated by ChEMBL | Assay Description Displacement of [3H]CGP12177 from human beta-1 adrenoceptor | Bioorg Med Chem Lett 20: 5302-7 (2010) Article DOI: 10.1016/j.bmcl.2010.06.136 BindingDB Entry DOI: 10.7270/Q2HD7VVM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-2 adrenergic receptor (Homo sapiens (Human)) | BDBM50055245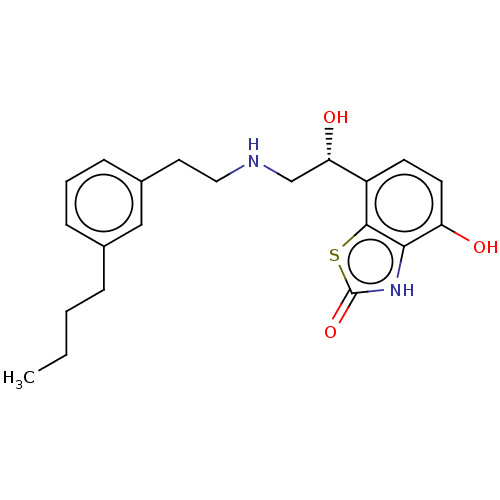 (CHEMBL3323668) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research Curated by ChEMBL | Assay Description Displacement of [125I]iodo-(+/-)-cyanopindolol from human adrenergic beta2 receptor expressed in CHO cells after 3 hrs by radio-ligand binding assay | Bioorg Med Chem Lett 24: 4341-7 (2014) Article DOI: 10.1016/j.bmcl.2014.06.014 BindingDB Entry DOI: 10.7270/Q2Z89F2R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-1 adrenergic receptor (Homo sapiens (Human)) | BDBM50324840 (7-((R)-2-((R)-1-cyclohexylethylamino)-1-hydroxyeth...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research Curated by ChEMBL | Assay Description Displacement of [3H]CGP12177 from human beta-1 adrenoceptor | Bioorg Med Chem Lett 20: 5302-7 (2010) Article DOI: 10.1016/j.bmcl.2010.06.136 BindingDB Entry DOI: 10.7270/Q2HD7VVM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1D (Homo sapiens (Human)) | BDBM50271024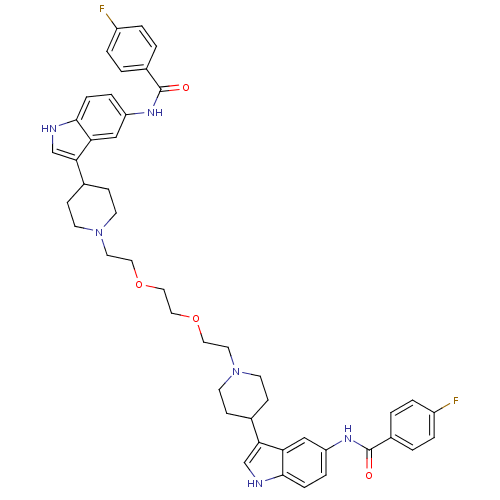 (4-fluoro-N-{3-[1-(2-{2-[2-(4-{5-[(4-fluorobenzene)...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Theravance, Inc. Curated by ChEMBL | Assay Description Displacement of [3H]5-hydroxytryptamine from human cloned 5HT1D receptor expressed in CHOK1 cells by liquid scintillation counting | J Med Chem 51: 3609-16 (2008) Article DOI: 10.1021/jm7011722 BindingDB Entry DOI: 10.7270/Q26T0MDK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1A (Homo sapiens (Human)) | BDBM50054974 (2-(5-((4-((3-(2-aminoethyl)-1H-indol-5-yloxy)methy...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Theravance, Inc. Curated by ChEMBL | Assay Description Binding affinity to 5HT1A receptor | J Med Chem 51: 3609-16 (2008) Article DOI: 10.1021/jm7011722 BindingDB Entry DOI: 10.7270/Q26T0MDK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 1799 total ) | Next | Last >> |