

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 5-hydroxytryptamine receptor 1A (Homo sapiens (Human)) | BDBM50166903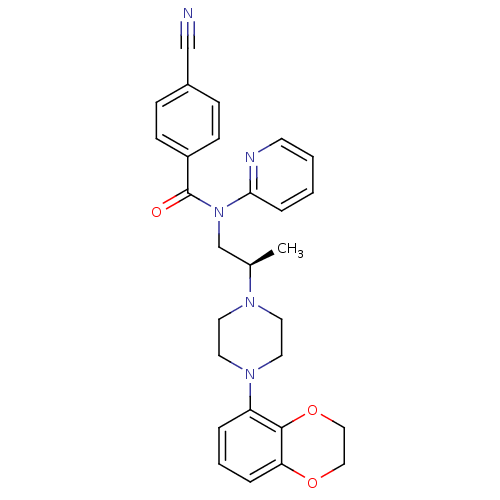 (4-Cyano-N-{(R)-2-[4-(2,3-dihydro-benzo[1,4]dioxin-...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 4.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Curated by PDSP Ki Database | J Pharmacol Exp Ther 314: 1274-89 (2005) Article DOI: 10.1124/jpet.105.086363 BindingDB Entry DOI: 10.7270/Q2057DJ4 | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(4) dopamine receptor (Homo sapiens (Human)) | BDBM50166903 (4-Cyano-N-{(R)-2-[4-(2,3-dihydro-benzo[1,4]dioxin-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 98 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Curated by PDSP Ki Database | J Pharmacol Exp Ther 314: 1274-89 (2005) Article DOI: 10.1124/jpet.105.086363 BindingDB Entry DOI: 10.7270/Q2057DJ4 | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Alpha-1A adrenergic receptor (Rattus norvegicus (Rat)) | BDBM50166903 (4-Cyano-N-{(R)-2-[4-(2,3-dihydro-benzo[1,4]dioxin-...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 248 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Curated by PDSP Ki Database | J Pharmacol Exp Ther 314: 1274-89 (2005) Article DOI: 10.1124/jpet.105.086363 BindingDB Entry DOI: 10.7270/Q2057DJ4 | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(3) dopamine receptor (Homo sapiens (Human)) | BDBM50166903 (4-Cyano-N-{(R)-2-[4-(2,3-dihydro-benzo[1,4]dioxin-...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 320 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Curated by PDSP Ki Database | J Pharmacol Exp Ther 314: 1274-89 (2005) Article DOI: 10.1124/jpet.105.086363 BindingDB Entry DOI: 10.7270/Q2057DJ4 | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(2) dopamine receptor (Homo sapiens (Human)) | BDBM50166903 (4-Cyano-N-{(R)-2-[4-(2,3-dihydro-benzo[1,4]dioxin-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 1.55E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Curated by PDSP Ki Database | J Pharmacol Exp Ther 314: 1274-89 (2005) Article DOI: 10.1124/jpet.105.086363 BindingDB Entry DOI: 10.7270/Q2057DJ4 | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM8960 ((+/-)-2-[(1-benzylpiperidin-4-yl)methyl]-5,6-dimet...) | UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 50 | n/a | n/a | n/a | n/a | n/a | n/a |
University of South Carolina Curated by ChEMBL | Assay Description Inhibition of electric eel AChE using acetylthiocholine as substrate preincubated for 8 mins followed by substrate addition by Ellman's method | Bioorg Med Chem Lett 26: 5573-5579 (2016) Article DOI: 10.1016/j.bmcl.2016.09.072 BindingDB Entry DOI: 10.7270/Q22F7QDF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50199147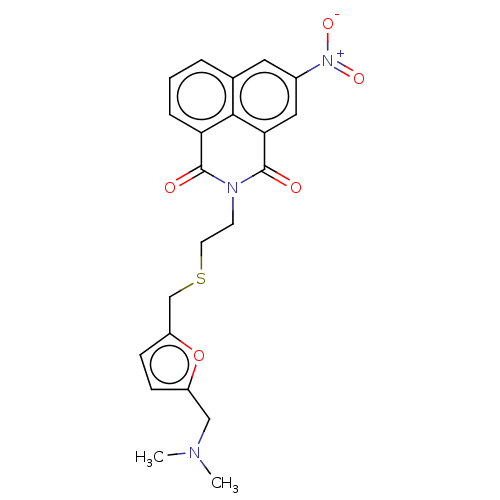 (CHEMBL3963904) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 150 | n/a | n/a | n/a | n/a | n/a | n/a |
University of South Carolina Curated by ChEMBL | Assay Description Inhibition of electric eel AChE using acetylthiocholine as substrate preincubated for 8 mins followed by substrate addition by Ellman's method | Bioorg Med Chem Lett 26: 5573-5579 (2016) Article DOI: 10.1016/j.bmcl.2016.09.072 BindingDB Entry DOI: 10.7270/Q22F7QDF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50199161 (CHEMBL3961411) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 160 | n/a | n/a | n/a | n/a | n/a | n/a |
University of South Carolina Curated by ChEMBL | Assay Description Inhibition of electric eel AChE using acetylthiocholine as substrate preincubated for 8 mins followed by substrate addition by Ellman's method | Bioorg Med Chem Lett 26: 5573-5579 (2016) Article DOI: 10.1016/j.bmcl.2016.09.072 BindingDB Entry DOI: 10.7270/Q22F7QDF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50199163 (CHEMBL3952735) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 190 | n/a | n/a | n/a | n/a | n/a | n/a |
University of South Carolina Curated by ChEMBL | Assay Description Inhibition of electric eel AChE using acetylthiocholine as substrate preincubated for 8 mins followed by substrate addition by Ellman's method | Bioorg Med Chem Lett 26: 5573-5579 (2016) Article DOI: 10.1016/j.bmcl.2016.09.072 BindingDB Entry DOI: 10.7270/Q22F7QDF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50199148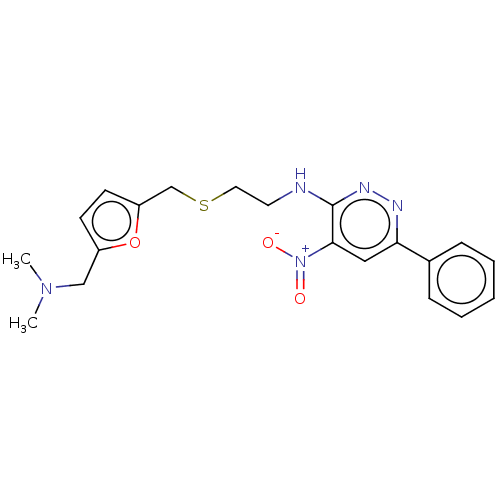 (CHEMBL3917462) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 260 | n/a | n/a | n/a | n/a | n/a | n/a |
University of South Carolina Curated by ChEMBL | Assay Description Inhibition of electric eel AChE using acetylthiocholine as substrate preincubated for 8 mins followed by substrate addition by Ellman's method | Bioorg Med Chem Lett 26: 5573-5579 (2016) Article DOI: 10.1016/j.bmcl.2016.09.072 BindingDB Entry DOI: 10.7270/Q22F7QDF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50199194 (CHEMBL3925028) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 350 | n/a | n/a | n/a | n/a | n/a | n/a |
University of South Carolina Curated by ChEMBL | Assay Description Inhibition of electric eel AChE using acetylthiocholine as substrate preincubated for 8 mins followed by substrate addition by Ellman's method | Bioorg Med Chem Lett 26: 5573-5579 (2016) Article DOI: 10.1016/j.bmcl.2016.09.072 BindingDB Entry DOI: 10.7270/Q22F7QDF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50199171 (CHEMBL3944527) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 460 | n/a | n/a | n/a | n/a | n/a | n/a |
University of South Carolina Curated by ChEMBL | Assay Description Inhibition of electric eel AChE using acetylthiocholine as substrate preincubated for 8 mins followed by substrate addition by Ellman's method | Bioorg Med Chem Lett 26: 5573-5579 (2016) Article DOI: 10.1016/j.bmcl.2016.09.072 BindingDB Entry DOI: 10.7270/Q22F7QDF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50004659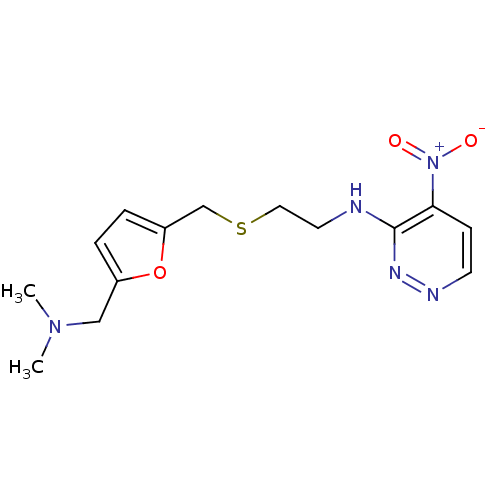 (CHEMBL321808 | [2-(5-Dimethylaminomethyl-furan-2-y...) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 470 | n/a | n/a | n/a | n/a | n/a | n/a |
University of South Carolina Curated by ChEMBL | Assay Description Inhibition of electric eel AChE using acetylthiocholine as substrate preincubated for 8 mins followed by substrate addition by Ellman's method | Bioorg Med Chem Lett 26: 5573-5579 (2016) Article DOI: 10.1016/j.bmcl.2016.09.072 BindingDB Entry DOI: 10.7270/Q22F7QDF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50199157 (CHEMBL3921095) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 680 | n/a | n/a | n/a | n/a | n/a | n/a |
University of South Carolina Curated by ChEMBL | Assay Description Inhibition of electric eel AChE using acetylthiocholine as substrate preincubated for 8 mins followed by substrate addition by Ellman's method | Bioorg Med Chem Lett 26: 5573-5579 (2016) Article DOI: 10.1016/j.bmcl.2016.09.072 BindingDB Entry DOI: 10.7270/Q22F7QDF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50199162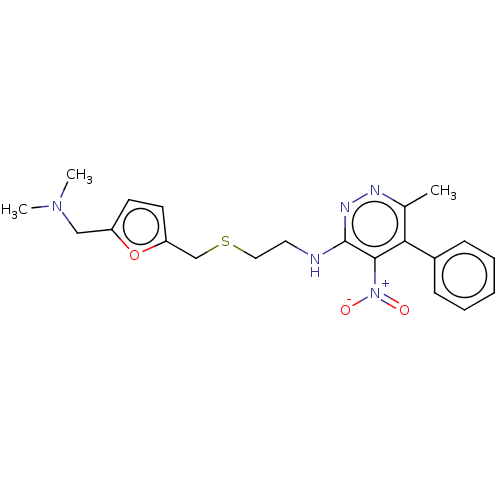 (CHEMBL3933927) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 720 | n/a | n/a | n/a | n/a | n/a | n/a |
University of South Carolina Curated by ChEMBL | Assay Description Inhibition of electric eel AChE using acetylthiocholine as substrate preincubated for 8 mins followed by substrate addition by Ellman's method | Bioorg Med Chem Lett 26: 5573-5579 (2016) Article DOI: 10.1016/j.bmcl.2016.09.072 BindingDB Entry DOI: 10.7270/Q22F7QDF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50199145 (CHEMBL3923597) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 1.18E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of South Carolina Curated by ChEMBL | Assay Description Inhibition of electric eel AChE using acetylthiocholine as substrate preincubated for 8 mins followed by substrate addition by Ellman's method | Bioorg Med Chem Lett 26: 5573-5579 (2016) Article DOI: 10.1016/j.bmcl.2016.09.072 BindingDB Entry DOI: 10.7270/Q22F7QDF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50199170 (CHEMBL3979679) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.27E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of South Carolina Curated by ChEMBL | Assay Description Inhibition of electric eel AChE using acetylthiocholine as substrate preincubated for 8 mins followed by substrate addition by Ellman's method | Bioorg Med Chem Lett 26: 5573-5579 (2016) Article DOI: 10.1016/j.bmcl.2016.09.072 BindingDB Entry DOI: 10.7270/Q22F7QDF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50199169 (CHEMBL3928312) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.65E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of South Carolina Curated by ChEMBL | Assay Description Inhibition of electric eel AChE using acetylthiocholine as substrate preincubated for 8 mins followed by substrate addition by Ellman's method | Bioorg Med Chem Lett 26: 5573-5579 (2016) Article DOI: 10.1016/j.bmcl.2016.09.072 BindingDB Entry DOI: 10.7270/Q22F7QDF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM50199194 (CHEMBL3925028) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.28E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of South Carolina Curated by ChEMBL | Assay Description Inhibition of equine serum BuChE using butyrylthiocholine as substrate preincubated for 8 mins followed by substrate addition by Ellman's method | Bioorg Med Chem Lett 26: 5573-5579 (2016) Article DOI: 10.1016/j.bmcl.2016.09.072 BindingDB Entry DOI: 10.7270/Q22F7QDF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50199153 (CHEMBL3893982) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 2.54E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of South Carolina Curated by ChEMBL | Assay Description Inhibition of electric eel AChE using acetylthiocholine as substrate preincubated for 8 mins followed by substrate addition by Ellman's method | Bioorg Med Chem Lett 26: 5573-5579 (2016) Article DOI: 10.1016/j.bmcl.2016.09.072 BindingDB Entry DOI: 10.7270/Q22F7QDF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50199160 (CHEMBL3964368) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.72E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of South Carolina Curated by ChEMBL | Assay Description Inhibition of electric eel AChE using acetylthiocholine as substrate preincubated for 8 mins followed by substrate addition by Ellman's method | Bioorg Med Chem Lett 26: 5573-5579 (2016) Article DOI: 10.1016/j.bmcl.2016.09.072 BindingDB Entry DOI: 10.7270/Q22F7QDF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM50199145 (CHEMBL3923597) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 2.96E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of South Carolina Curated by ChEMBL | Assay Description Inhibition of equine serum BuChE using butyrylthiocholine as substrate preincubated for 8 mins followed by substrate addition by Ellman's method | Bioorg Med Chem Lett 26: 5573-5579 (2016) Article DOI: 10.1016/j.bmcl.2016.09.072 BindingDB Entry DOI: 10.7270/Q22F7QDF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50199158 (CHEMBL3971565) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 3.34E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of South Carolina Curated by ChEMBL | Assay Description Inhibition of electric eel AChE using acetylthiocholine as substrate preincubated for 8 mins followed by substrate addition by Ellman's method | Bioorg Med Chem Lett 26: 5573-5579 (2016) Article DOI: 10.1016/j.bmcl.2016.09.072 BindingDB Entry DOI: 10.7270/Q22F7QDF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50199155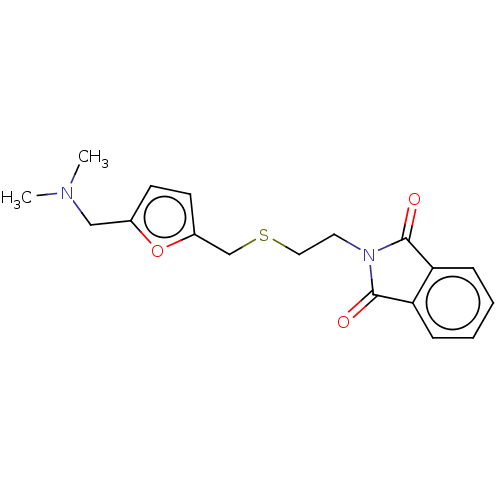 (CHEMBL3966045) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3.57E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of South Carolina Curated by ChEMBL | Assay Description Inhibition of electric eel AChE using acetylthiocholine as substrate preincubated for 8 mins followed by substrate addition by Ellman's method | Bioorg Med Chem Lett 26: 5573-5579 (2016) Article DOI: 10.1016/j.bmcl.2016.09.072 BindingDB Entry DOI: 10.7270/Q22F7QDF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM50199161 (CHEMBL3961411) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 3.71E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of South Carolina Curated by ChEMBL | Assay Description Inhibition of equine serum BuChE using butyrylthiocholine as substrate preincubated for 8 mins followed by substrate addition by Ellman's method | Bioorg Med Chem Lett 26: 5573-5579 (2016) Article DOI: 10.1016/j.bmcl.2016.09.072 BindingDB Entry DOI: 10.7270/Q22F7QDF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50199164 (CHEMBL3941167) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 4.04E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of South Carolina Curated by ChEMBL | Assay Description Inhibition of electric eel AChE using acetylthiocholine as substrate preincubated for 8 mins followed by substrate addition by Ellman's method | Bioorg Med Chem Lett 26: 5573-5579 (2016) Article DOI: 10.1016/j.bmcl.2016.09.072 BindingDB Entry DOI: 10.7270/Q22F7QDF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM50199148 (CHEMBL3917462) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 4.46E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of South Carolina Curated by ChEMBL | Assay Description Inhibition of equine serum BuChE using butyrylthiocholine as substrate preincubated for 8 mins followed by substrate addition by Ellman's method | Bioorg Med Chem Lett 26: 5573-5579 (2016) Article DOI: 10.1016/j.bmcl.2016.09.072 BindingDB Entry DOI: 10.7270/Q22F7QDF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50199159 (CHEMBL3927243) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 5.01E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of South Carolina Curated by ChEMBL | Assay Description Inhibition of electric eel AChE using acetylthiocholine as substrate preincubated for 8 mins followed by substrate addition by Ellman's method | Bioorg Med Chem Lett 26: 5573-5579 (2016) Article DOI: 10.1016/j.bmcl.2016.09.072 BindingDB Entry DOI: 10.7270/Q22F7QDF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50199150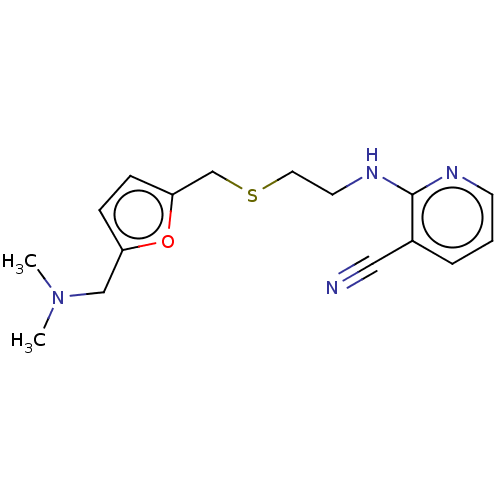 (CHEMBL3916017) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 5.34E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of South Carolina Curated by ChEMBL | Assay Description Inhibition of electric eel AChE using acetylthiocholine as substrate preincubated for 8 mins followed by substrate addition by Ellman's method | Bioorg Med Chem Lett 26: 5573-5579 (2016) Article DOI: 10.1016/j.bmcl.2016.09.072 BindingDB Entry DOI: 10.7270/Q22F7QDF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM50199163 (CHEMBL3952735) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 7.05E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of South Carolina Curated by ChEMBL | Assay Description Inhibition of equine serum BuChE using butyrylthiocholine as substrate preincubated for 8 mins followed by substrate addition by Ellman's method | Bioorg Med Chem Lett 26: 5573-5579 (2016) Article DOI: 10.1016/j.bmcl.2016.09.072 BindingDB Entry DOI: 10.7270/Q22F7QDF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM50199169 (CHEMBL3928312) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 7.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of South Carolina Curated by ChEMBL | Assay Description Inhibition of equine serum BuChE using butyrylthiocholine as substrate preincubated for 8 mins followed by substrate addition by Ellman's method | Bioorg Med Chem Lett 26: 5573-5579 (2016) Article DOI: 10.1016/j.bmcl.2016.09.072 BindingDB Entry DOI: 10.7270/Q22F7QDF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM50199170 (CHEMBL3979679) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 7.21E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of South Carolina Curated by ChEMBL | Assay Description Inhibition of equine serum BuChE using butyrylthiocholine as substrate preincubated for 8 mins followed by substrate addition by Ellman's method | Bioorg Med Chem Lett 26: 5573-5579 (2016) Article DOI: 10.1016/j.bmcl.2016.09.072 BindingDB Entry DOI: 10.7270/Q22F7QDF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM50199144 (CHEMBL3963436) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 7.65E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of South Carolina Curated by ChEMBL | Assay Description Inhibition of equine serum BuChE using butyrylthiocholine as substrate preincubated for 8 mins followed by substrate addition by Ellman's method | Bioorg Med Chem Lett 26: 5573-5579 (2016) Article DOI: 10.1016/j.bmcl.2016.09.072 BindingDB Entry DOI: 10.7270/Q22F7QDF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM50199159 (CHEMBL3927243) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 7.65E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of South Carolina Curated by ChEMBL | Assay Description Inhibition of equine serum BuChE using butyrylthiocholine as substrate preincubated for 8 mins followed by substrate addition by Ellman's method | Bioorg Med Chem Lett 26: 5573-5579 (2016) Article DOI: 10.1016/j.bmcl.2016.09.072 BindingDB Entry DOI: 10.7270/Q22F7QDF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM50199147 (CHEMBL3963904) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 8.01E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of South Carolina Curated by ChEMBL | Assay Description Inhibition of equine serum BuChE using butyrylthiocholine as substrate preincubated for 8 mins followed by substrate addition by Ellman's method | Bioorg Med Chem Lett 26: 5573-5579 (2016) Article DOI: 10.1016/j.bmcl.2016.09.072 BindingDB Entry DOI: 10.7270/Q22F7QDF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM50199150 (CHEMBL3916017) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 8.36E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of South Carolina Curated by ChEMBL | Assay Description Inhibition of equine serum BuChE using butyrylthiocholine as substrate preincubated for 8 mins followed by substrate addition by Ellman's method | Bioorg Med Chem Lett 26: 5573-5579 (2016) Article DOI: 10.1016/j.bmcl.2016.09.072 BindingDB Entry DOI: 10.7270/Q22F7QDF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM50004659 (CHEMBL321808 | [2-(5-Dimethylaminomethyl-furan-2-y...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of South Carolina Curated by ChEMBL | Assay Description Inhibition of equine serum BuChE using butyrylthiocholine as substrate preincubated for 8 mins followed by substrate addition by Ellman's method | Bioorg Med Chem Lett 26: 5573-5579 (2016) Article DOI: 10.1016/j.bmcl.2016.09.072 BindingDB Entry DOI: 10.7270/Q22F7QDF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50199152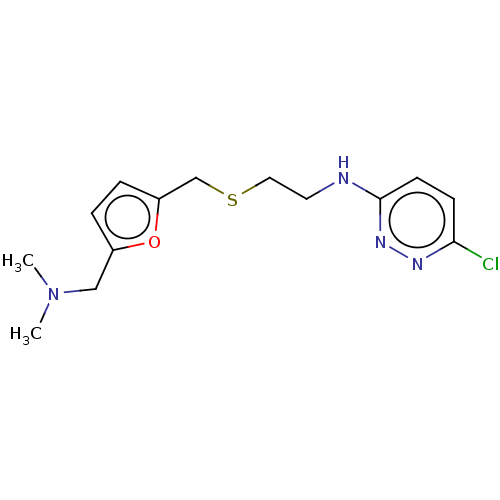 (CHEMBL3935020) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.04E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of South Carolina Curated by ChEMBL | Assay Description Inhibition of electric eel AChE using acetylthiocholine as substrate preincubated for 8 mins followed by substrate addition by Ellman's method | Bioorg Med Chem Lett 26: 5573-5579 (2016) Article DOI: 10.1016/j.bmcl.2016.09.072 BindingDB Entry DOI: 10.7270/Q22F7QDF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50199149 (CHEMBL3954887) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 1.18E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of South Carolina Curated by ChEMBL | Assay Description Inhibition of electric eel AChE using acetylthiocholine as substrate preincubated for 8 mins followed by substrate addition by Ellman's method | Bioorg Med Chem Lett 26: 5573-5579 (2016) Article DOI: 10.1016/j.bmcl.2016.09.072 BindingDB Entry DOI: 10.7270/Q22F7QDF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM50199155 (CHEMBL3966045) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.45E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of South Carolina Curated by ChEMBL | Assay Description Inhibition of equine serum BuChE using butyrylthiocholine as substrate preincubated for 8 mins followed by substrate addition by Ellman's method | Bioorg Med Chem Lett 26: 5573-5579 (2016) Article DOI: 10.1016/j.bmcl.2016.09.072 BindingDB Entry DOI: 10.7270/Q22F7QDF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50199144 (CHEMBL3963436) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 1.62E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of South Carolina Curated by ChEMBL | Assay Description Inhibition of electric eel AChE using acetylthiocholine as substrate preincubated for 8 mins followed by substrate addition by Ellman's method | Bioorg Med Chem Lett 26: 5573-5579 (2016) Article DOI: 10.1016/j.bmcl.2016.09.072 BindingDB Entry DOI: 10.7270/Q22F7QDF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM50199153 (CHEMBL3893982) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 1.88E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of South Carolina Curated by ChEMBL | Assay Description Inhibition of equine serum BuChE using butyrylthiocholine as substrate preincubated for 8 mins followed by substrate addition by Ellman's method | Bioorg Med Chem Lett 26: 5573-5579 (2016) Article DOI: 10.1016/j.bmcl.2016.09.072 BindingDB Entry DOI: 10.7270/Q22F7QDF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM50199164 (CHEMBL3941167) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.12E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of South Carolina Curated by ChEMBL | Assay Description Inhibition of equine serum BuChE using butyrylthiocholine as substrate preincubated for 8 mins followed by substrate addition by Ellman's method | Bioorg Med Chem Lett 26: 5573-5579 (2016) Article DOI: 10.1016/j.bmcl.2016.09.072 BindingDB Entry DOI: 10.7270/Q22F7QDF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM50199171 (CHEMBL3944527) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 2.27E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of South Carolina Curated by ChEMBL | Assay Description Inhibition of equine serum BuChE using butyrylthiocholine as substrate preincubated for 8 mins followed by substrate addition by Ellman's method | Bioorg Med Chem Lett 26: 5573-5579 (2016) Article DOI: 10.1016/j.bmcl.2016.09.072 BindingDB Entry DOI: 10.7270/Q22F7QDF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM50199157 (CHEMBL3921095) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 2.84E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of South Carolina Curated by ChEMBL | Assay Description Inhibition of equine serum BuChE using butyrylthiocholine as substrate preincubated for 8 mins followed by substrate addition by Ellman's method | Bioorg Med Chem Lett 26: 5573-5579 (2016) Article DOI: 10.1016/j.bmcl.2016.09.072 BindingDB Entry DOI: 10.7270/Q22F7QDF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50199146 (CHEMBL3936203) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 3.34E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of South Carolina Curated by ChEMBL | Assay Description Inhibition of electric eel AChE using acetylthiocholine as substrate preincubated for 8 mins followed by substrate addition by Ellman's method | Bioorg Med Chem Lett 26: 5573-5579 (2016) Article DOI: 10.1016/j.bmcl.2016.09.072 BindingDB Entry DOI: 10.7270/Q22F7QDF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50199167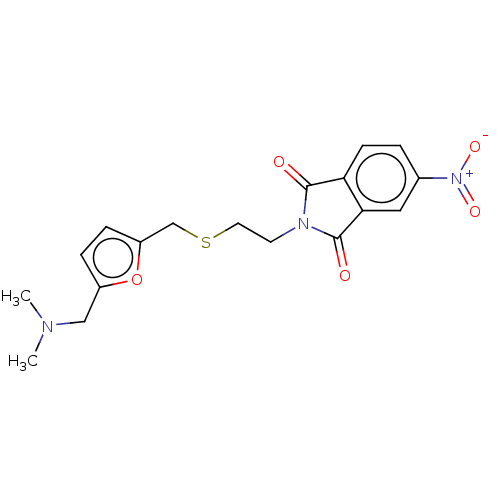 (CHEMBL3912004) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 4.60E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of South Carolina Curated by ChEMBL | Assay Description Inhibition of electric eel AChE using acetylthiocholine as substrate preincubated for 8 mins followed by substrate addition by Ellman's method | Bioorg Med Chem Lett 26: 5573-5579 (2016) Article DOI: 10.1016/j.bmcl.2016.09.072 BindingDB Entry DOI: 10.7270/Q22F7QDF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM50199165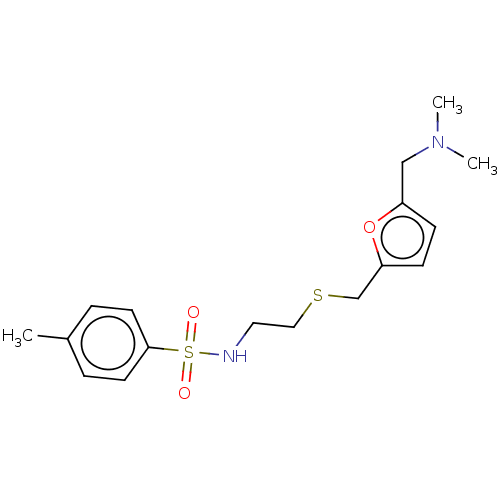 (CHEMBL3937312) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 4.72E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of South Carolina Curated by ChEMBL | Assay Description Inhibition of equine serum BuChE using butyrylthiocholine as substrate preincubated for 8 mins followed by substrate addition by Ellman's method | Bioorg Med Chem Lett 26: 5573-5579 (2016) Article DOI: 10.1016/j.bmcl.2016.09.072 BindingDB Entry DOI: 10.7270/Q22F7QDF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50199151 (CHEMBL3926121) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | >5.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of South Carolina Curated by ChEMBL | Assay Description Inhibition of electric eel AChE using acetylthiocholine as substrate preincubated for 8 mins followed by substrate addition by Ellman's method | Bioorg Med Chem Lett 26: 5573-5579 (2016) Article DOI: 10.1016/j.bmcl.2016.09.072 BindingDB Entry DOI: 10.7270/Q22F7QDF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50199156 (CHEMBL3937756) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >5.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of South Carolina Curated by ChEMBL | Assay Description Inhibition of electric eel AChE using acetylthiocholine as substrate preincubated for 8 mins followed by substrate addition by Ellman's method | Bioorg Med Chem Lett 26: 5573-5579 (2016) Article DOI: 10.1016/j.bmcl.2016.09.072 BindingDB Entry DOI: 10.7270/Q22F7QDF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 64 total ) | Next | Last >> |