 Found 315 hits with Last Name = 'thalji' and Initial = 'r'
Found 315 hits with Last Name = 'thalji' and Initial = 'r' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
P2Y purinoceptor 1
(Homo sapiens (Human)) | BDBM50245983
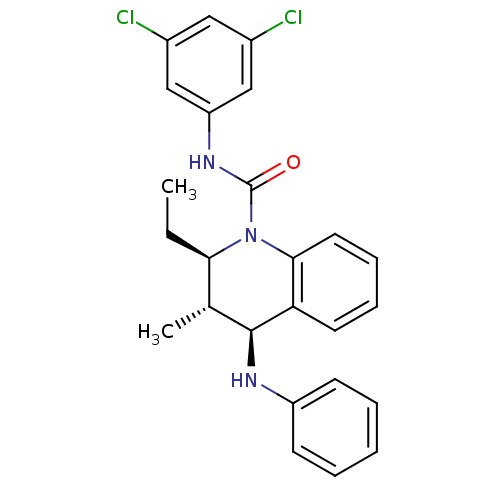
((2R,3S,4S)-2-Ethyl-3-methyl-N-[3,5-(chloro)phenyl]...)Show SMILES CC[C@@H]1[C@@H](C)[C@H](Nc2ccccc2)c2ccccc2N1C(=O)Nc1cc(Cl)cc(Cl)c1 |r| Show InChI InChI=1S/C25H25Cl2N3O/c1-3-22-16(2)24(28-19-9-5-4-6-10-19)21-11-7-8-12-23(21)30(22)25(31)29-20-14-17(26)13-18(27)15-20/h4-16,22,24,28H,3H2,1-2H3,(H,29,31)/t16-,22-,24+/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Displacement of [33P]2-Mes-ADP from human recombinant P2Y1 receptor expressed in human U20S cells |
Bioorg Med Chem Lett 18: 6222-6 (2008)
Article DOI: 10.1016/j.bmcl.2008.09.102
BindingDB Entry DOI: 10.7270/Q26Q1X42 |
More data for this
Ligand-Target Pair | |
P2Y purinoceptor 1
(Homo sapiens (Human)) | BDBM50245772
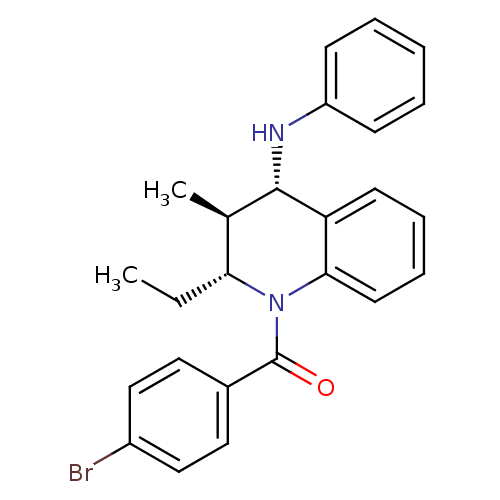
((4-bromophenyl)((2R,3S,4S)-2-ethyl-3-methyl-4-(phe...)Show SMILES CC[C@@H]1[C@@H](C)[C@H](Nc2ccccc2)c2ccccc2N1C(=O)c1ccc(Br)cc1 |r| Show InChI InChI=1S/C25H25BrN2O/c1-3-22-17(2)24(27-20-9-5-4-6-10-20)21-11-7-8-12-23(21)28(22)25(29)18-13-15-19(26)16-14-18/h4-17,22,24,27H,3H2,1-2H3/t17-,22-,24+/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Displacement of [33P]2-Mes-ADP from human recombinant P2Y1 receptor expressed in human U20S cells |
Bioorg Med Chem Lett 18: 6222-6 (2008)
Article DOI: 10.1016/j.bmcl.2008.09.102
BindingDB Entry DOI: 10.7270/Q26Q1X42 |
More data for this
Ligand-Target Pair | |
P2Y purinoceptor 1
(Homo sapiens (Human)) | BDBM50245937
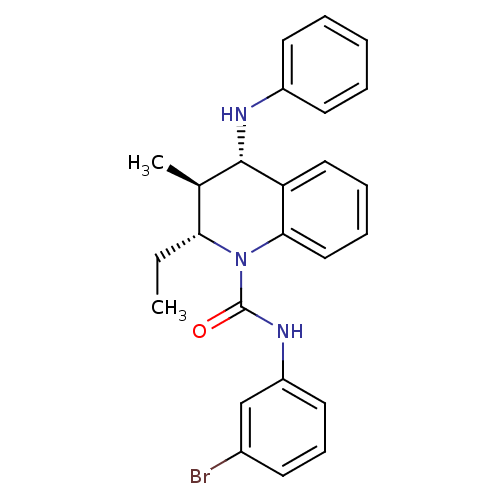
((2R,3S,4S)-N-(3-bromophenyl)-2-ethyl-3-methyl-4-(p...)Show SMILES CC[C@@H]1[C@@H](C)[C@H](Nc2ccccc2)c2ccccc2N1C(=O)Nc1cccc(Br)c1 |r| Show InChI InChI=1S/C25H26BrN3O/c1-3-22-17(2)24(27-19-11-5-4-6-12-19)21-14-7-8-15-23(21)29(22)25(30)28-20-13-9-10-18(26)16-20/h4-17,22,24,27H,3H2,1-2H3,(H,28,30)/t17-,22-,24+/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Displacement of [33P]2-Mes-ADP from human recombinant P2Y1 receptor expressed in human U20S cells |
Bioorg Med Chem Lett 18: 6222-6 (2008)
Article DOI: 10.1016/j.bmcl.2008.09.102
BindingDB Entry DOI: 10.7270/Q26Q1X42 |
More data for this
Ligand-Target Pair | |
P2Y purinoceptor 1
(Homo sapiens (Human)) | BDBM50246518
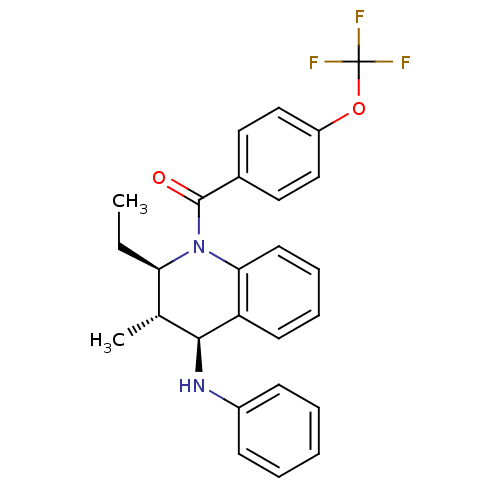
(((2R,3S,4S)-2-ethyl-3-methyl-4-(phenylamino)-3,4-d...)Show SMILES CC[C@@H]1[C@@H](C)[C@H](Nc2ccccc2)c2ccccc2N1C(=O)c1ccc(OC(F)(F)F)cc1 |r| Show InChI InChI=1S/C26H25F3N2O2/c1-3-22-17(2)24(30-19-9-5-4-6-10-19)21-11-7-8-12-23(21)31(22)25(32)18-13-15-20(16-14-18)33-26(27,28)29/h4-17,22,24,30H,3H2,1-2H3/t17-,22-,24+/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Displacement of [33P]2-Mes-ADP from human recombinant P2Y1 receptor expressed in human U20S cells |
Bioorg Med Chem Lett 18: 6222-6 (2008)
Article DOI: 10.1016/j.bmcl.2008.09.102
BindingDB Entry DOI: 10.7270/Q26Q1X42 |
More data for this
Ligand-Target Pair | |
P2Y purinoceptor 1
(Homo sapiens (Human)) | BDBM50321586
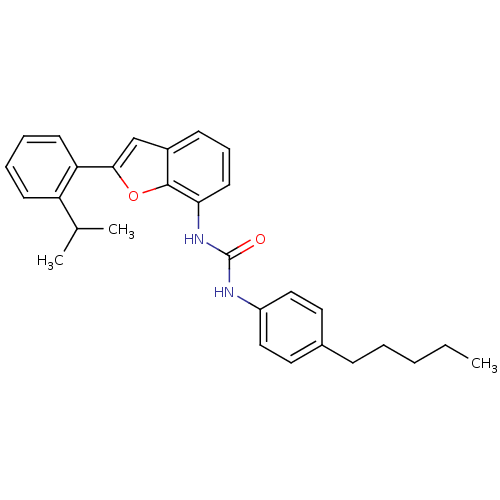
(1-(2-(2-isopropylphenyl)benzofuran-7-yl)-3-(4-pent...)Show SMILES CCCCCc1ccc(NC(=O)Nc2cccc3cc(oc23)-c2ccccc2C(C)C)cc1 Show InChI InChI=1S/C29H32N2O2/c1-4-5-6-10-21-15-17-23(18-16-21)30-29(32)31-26-14-9-11-22-19-27(33-28(22)26)25-13-8-7-12-24(25)20(2)3/h7-9,11-20H,4-6,10H2,1-3H3,(H2,30,31,32) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 140 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Displacement of [33P]2-MeS-ADP from human P2Y1 receptor expressed in human U2OS cells by scintillation counting |
Bioorg Med Chem Lett 20: 4104-7 (2010)
Article DOI: 10.1016/j.bmcl.2010.05.072
BindingDB Entry DOI: 10.7270/Q2HX1DNF |
More data for this
Ligand-Target Pair | |
P2Y purinoceptor 1
(Homo sapiens (Human)) | BDBM50245982
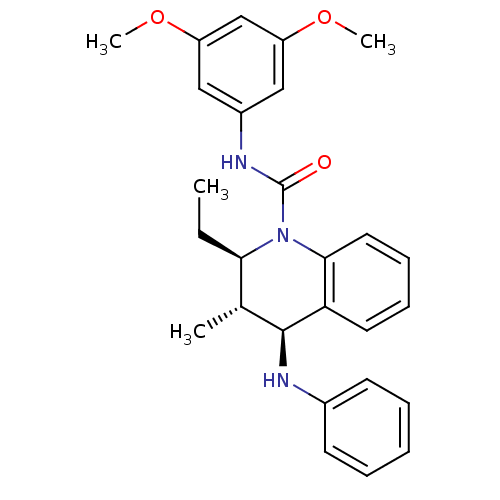
((2R,3S,4S)-N-(3,5-dimethoxyphenyl)-2-ethyl-3-methy...)Show SMILES CC[C@@H]1[C@@H](C)[C@H](Nc2ccccc2)c2ccccc2N1C(=O)Nc1cc(OC)cc(OC)c1 |r| Show InChI InChI=1S/C27H31N3O3/c1-5-24-18(2)26(28-19-11-7-6-8-12-19)23-13-9-10-14-25(23)30(24)27(31)29-20-15-21(32-3)17-22(16-20)33-4/h6-18,24,26,28H,5H2,1-4H3,(H,29,31)/t18-,24-,26+/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Displacement of [33P]2-Mes-ADP from human recombinant P2Y1 receptor expressed in human U20S cells |
Bioorg Med Chem Lett 18: 6222-6 (2008)
Article DOI: 10.1016/j.bmcl.2008.09.102
BindingDB Entry DOI: 10.7270/Q26Q1X42 |
More data for this
Ligand-Target Pair | |
P2Y purinoceptor 1
(Homo sapiens (Human)) | BDBM50245934
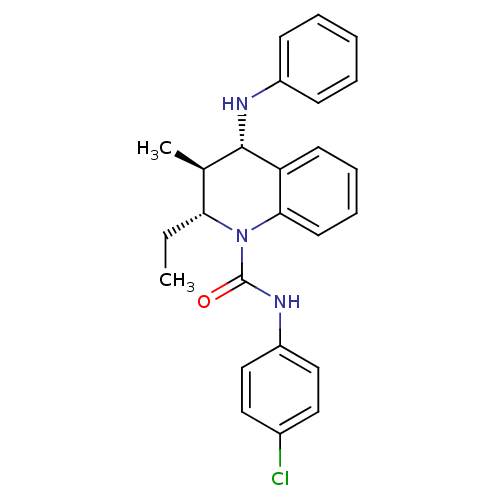
((2R,3S,4S)-N-(4-chlorophenyl)-2-ethyl-3-methyl-4-(...)Show SMILES CC[C@@H]1[C@@H](C)[C@H](Nc2ccccc2)c2ccccc2N1C(=O)Nc1ccc(Cl)cc1 |r| Show InChI InChI=1S/C25H26ClN3O/c1-3-22-17(2)24(27-19-9-5-4-6-10-19)21-11-7-8-12-23(21)29(22)25(30)28-20-15-13-18(26)14-16-20/h4-17,22,24,27H,3H2,1-2H3,(H,28,30)/t17-,22-,24+/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Displacement of [33P]2-Mes-ADP from human recombinant P2Y1 receptor expressed in human U20S cells |
Bioorg Med Chem Lett 18: 6222-6 (2008)
Article DOI: 10.1016/j.bmcl.2008.09.102
BindingDB Entry DOI: 10.7270/Q26Q1X42 |
More data for this
Ligand-Target Pair | |
P2Y purinoceptor 1
(Homo sapiens (Human)) | BDBM50245835
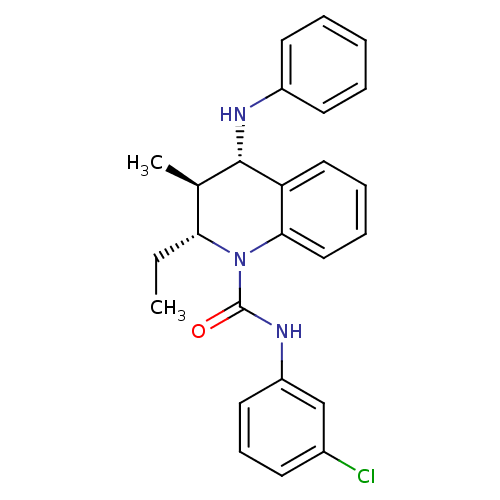
((2R,3S,4S)-N-(3-chlorophenyl)-2-ethyl-3-methyl-4-(...)Show SMILES CC[C@@H]1[C@@H](C)[C@H](Nc2ccccc2)c2ccccc2N1C(=O)Nc1cccc(Cl)c1 |r| Show InChI InChI=1S/C25H26ClN3O/c1-3-22-17(2)24(27-19-11-5-4-6-12-19)21-14-7-8-15-23(21)29(22)25(30)28-20-13-9-10-18(26)16-20/h4-17,22,24,27H,3H2,1-2H3,(H,28,30)/t17-,22-,24+/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Displacement of [33P]2-Mes-ADP from human recombinant P2Y1 receptor expressed in human U20S cells |
Bioorg Med Chem Lett 18: 6222-6 (2008)
Article DOI: 10.1016/j.bmcl.2008.09.102
BindingDB Entry DOI: 10.7270/Q26Q1X42 |
More data for this
Ligand-Target Pair | |
P2Y purinoceptor 1
(Homo sapiens (Human)) | BDBM50245981
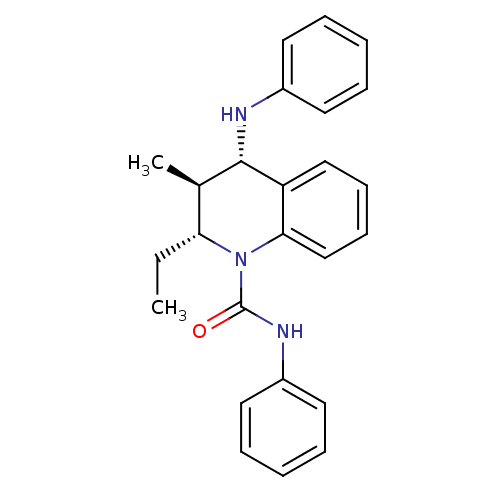
((2R,3S,4S)-2-ethyl-3-methyl-N-phenyl-4-(phenylamin...)Show SMILES CC[C@@H]1[C@@H](C)[C@H](Nc2ccccc2)c2ccccc2N1C(=O)Nc1ccccc1 |r| Show InChI InChI=1S/C25H27N3O/c1-3-22-18(2)24(26-19-12-6-4-7-13-19)21-16-10-11-17-23(21)28(22)25(29)27-20-14-8-5-9-15-20/h4-18,22,24,26H,3H2,1-2H3,(H,27,29)/t18-,22-,24+/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Displacement of [33P]2-Mes-ADP from human recombinant P2Y1 receptor expressed in human U20S cells |
Bioorg Med Chem Lett 18: 6222-6 (2008)
Article DOI: 10.1016/j.bmcl.2008.09.102
BindingDB Entry DOI: 10.7270/Q26Q1X42 |
More data for this
Ligand-Target Pair | |
P2Y purinoceptor 1
(Homo sapiens (Human)) | BDBM50246516
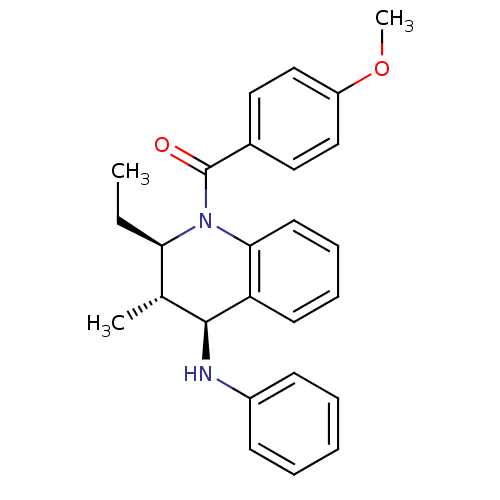
(((2R,3S,4S)-2-ethyl-3-methyl-4-(phenylamino)-3,4-d...)Show SMILES CC[C@@H]1[C@@H](C)[C@H](Nc2ccccc2)c2ccccc2N1C(=O)c1ccc(OC)cc1 |r| Show InChI InChI=1S/C26H28N2O2/c1-4-23-18(2)25(27-20-10-6-5-7-11-20)22-12-8-9-13-24(22)28(23)26(29)19-14-16-21(30-3)17-15-19/h5-18,23,25,27H,4H2,1-3H3/t18-,23-,25+/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Displacement of [33P]2-Mes-ADP from human recombinant P2Y1 receptor expressed in human U20S cells |
Bioorg Med Chem Lett 18: 6222-6 (2008)
Article DOI: 10.1016/j.bmcl.2008.09.102
BindingDB Entry DOI: 10.7270/Q26Q1X42 |
More data for this
Ligand-Target Pair | |
P2Y purinoceptor 1
(Homo sapiens (Human)) | BDBM50246517
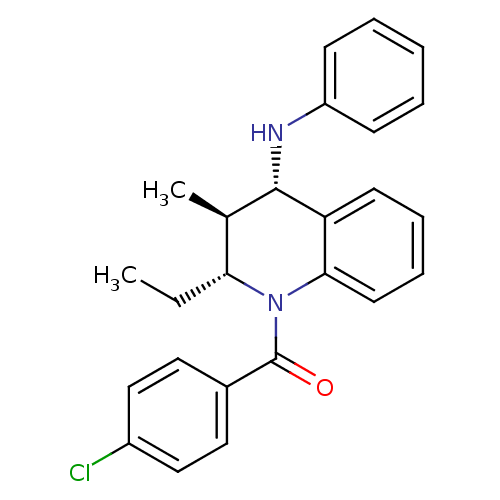
((4-chlorophenyl)((2R,3S,4S)-2-ethyl-3-methyl-4-(ph...)Show SMILES CC[C@@H]1[C@@H](C)[C@H](Nc2ccccc2)c2ccccc2N1C(=O)c1ccc(Cl)cc1 |r| Show InChI InChI=1S/C25H25ClN2O/c1-3-22-17(2)24(27-20-9-5-4-6-10-20)21-11-7-8-12-23(21)28(22)25(29)18-13-15-19(26)16-14-18/h4-17,22,24,27H,3H2,1-2H3/t17-,22-,24+/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Displacement of [33P]2-Mes-ADP from human recombinant P2Y1 receptor expressed in human U20S cells |
Bioorg Med Chem Lett 18: 6222-6 (2008)
Article DOI: 10.1016/j.bmcl.2008.09.102
BindingDB Entry DOI: 10.7270/Q26Q1X42 |
More data for this
Ligand-Target Pair | |
P2Y purinoceptor 1
(Homo sapiens (Human)) | BDBM50321585
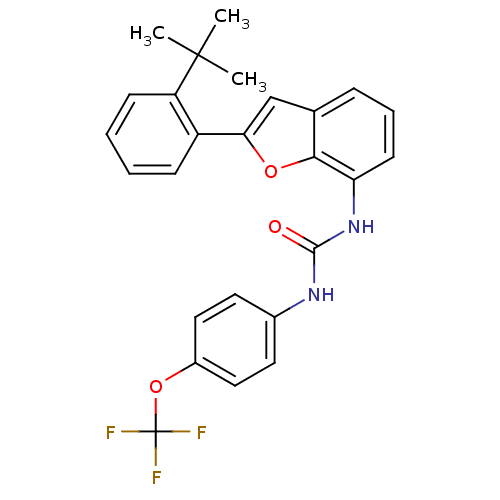
(1-(2-(2-tert-butylphenyl)benzofuran-7-yl)-3-(4-(tr...)Show SMILES CC(C)(C)c1ccccc1-c1cc2cccc(NC(=O)Nc3ccc(OC(F)(F)F)cc3)c2o1 Show InChI InChI=1S/C26H23F3N2O3/c1-25(2,3)20-9-5-4-8-19(20)22-15-16-7-6-10-21(23(16)33-22)31-24(32)30-17-11-13-18(14-12-17)34-26(27,28)29/h4-15H,1-3H3,(H2,30,31,32) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Displacement of [33P]2-MeS-ADP from human P2Y1 receptor expressed in human U2OS cells by scintillation counting |
Bioorg Med Chem Lett 20: 4104-7 (2010)
Article DOI: 10.1016/j.bmcl.2010.05.072
BindingDB Entry DOI: 10.7270/Q2HX1DNF |
More data for this
Ligand-Target Pair | |
P2Y purinoceptor 1
(Homo sapiens (Human)) | BDBM50246468
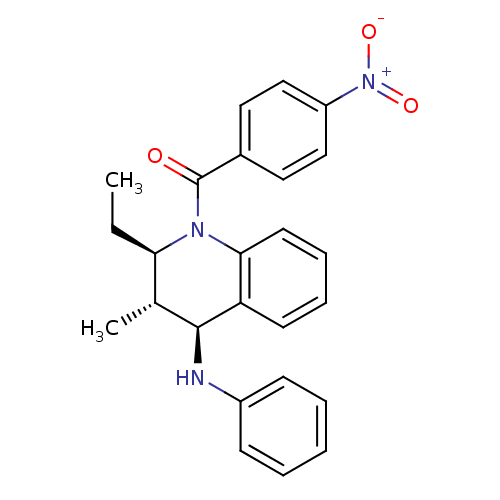
(((2R,3S,4S)-2-ethyl-3-methyl-4-(phenylamino)-3,4-d...)Show SMILES CC[C@@H]1[C@@H](C)[C@H](Nc2ccccc2)c2ccccc2N1C(=O)c1ccc(cc1)[N+]([O-])=O |r| Show InChI InChI=1S/C25H25N3O3/c1-3-22-17(2)24(26-19-9-5-4-6-10-19)21-11-7-8-12-23(21)27(22)25(29)18-13-15-20(16-14-18)28(30)31/h4-17,22,24,26H,3H2,1-2H3/t17-,22-,24+/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 500 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Displacement of [33P]2-Mes-ADP from human recombinant P2Y1 receptor expressed in human U20S cells |
Bioorg Med Chem Lett 18: 6222-6 (2008)
Article DOI: 10.1016/j.bmcl.2008.09.102
BindingDB Entry DOI: 10.7270/Q26Q1X42 |
More data for this
Ligand-Target Pair | |
P2Y purinoceptor 1
(Homo sapiens (Human)) | BDBM50245935
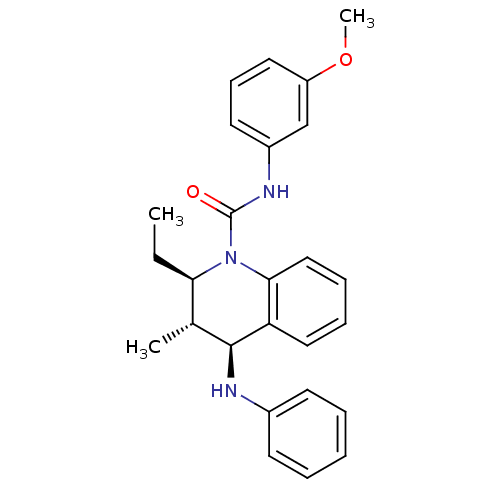
((2R,3S,4S)-2-ethyl-N-(3-methoxyphenyl)-3-methyl-4-...)Show SMILES CC[C@@H]1[C@@H](C)[C@H](Nc2ccccc2)c2ccccc2N1C(=O)Nc1cccc(OC)c1 |r| Show InChI InChI=1S/C26H29N3O2/c1-4-23-18(2)25(27-19-11-6-5-7-12-19)22-15-8-9-16-24(22)29(23)26(30)28-20-13-10-14-21(17-20)31-3/h5-18,23,25,27H,4H2,1-3H3,(H,28,30)/t18-,23-,25+/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 500 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Displacement of [33P]2-Mes-ADP from human recombinant P2Y1 receptor expressed in human U20S cells |
Bioorg Med Chem Lett 18: 6222-6 (2008)
Article DOI: 10.1016/j.bmcl.2008.09.102
BindingDB Entry DOI: 10.7270/Q26Q1X42 |
More data for this
Ligand-Target Pair | |
P2Y purinoceptor 1
(Homo sapiens (Human)) | BDBM50321584
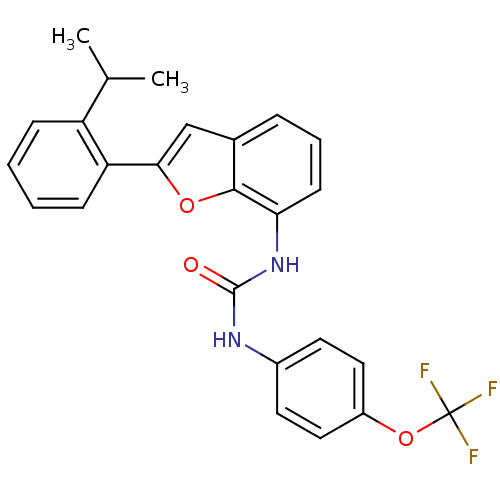
(1-(2-(2-isopropylphenyl)benzofuran-7-yl)-3-(4-(tri...)Show SMILES CC(C)c1ccccc1-c1cc2cccc(NC(=O)Nc3ccc(OC(F)(F)F)cc3)c2o1 Show InChI InChI=1S/C25H21F3N2O3/c1-15(2)19-7-3-4-8-20(19)22-14-16-6-5-9-21(23(16)32-22)30-24(31)29-17-10-12-18(13-11-17)33-25(26,27)28/h3-15H,1-2H3,(H2,29,30,31) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Displacement of [33P]2-MeS-ADP from human P2Y1 receptor expressed in human U2OS cells by scintillation counting |
Bioorg Med Chem Lett 20: 4104-7 (2010)
Article DOI: 10.1016/j.bmcl.2010.05.072
BindingDB Entry DOI: 10.7270/Q2HX1DNF |
More data for this
Ligand-Target Pair | |
P2Y purinoceptor 1
(Homo sapiens (Human)) | BDBM50321587

(1-(4-butoxyphenyl)-3-(2-(2-isopropylphenyl)benzofu...)Show SMILES CCCCOc1ccc(NC(=O)Nc2cccc3cc(oc23)-c2ccccc2C(C)C)cc1 Show InChI InChI=1S/C28H30N2O3/c1-4-5-17-32-22-15-13-21(14-16-22)29-28(31)30-25-12-8-9-20-18-26(33-27(20)25)24-11-7-6-10-23(24)19(2)3/h6-16,18-19H,4-5,17H2,1-3H3,(H2,29,30,31) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 640 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Displacement of [33P]2-MeS-ADP from human P2Y1 receptor expressed in human U2OS cells by scintillation counting |
Bioorg Med Chem Lett 20: 4104-7 (2010)
Article DOI: 10.1016/j.bmcl.2010.05.072
BindingDB Entry DOI: 10.7270/Q2HX1DNF |
More data for this
Ligand-Target Pair | |
P2Y purinoceptor 1
(Homo sapiens (Human)) | BDBM50321588
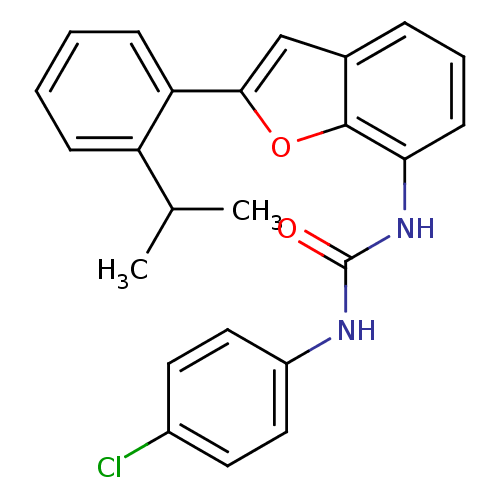
(1-(4-chlorophenyl)-3-(2-(2-isopropylphenyl)benzofu...)Show SMILES CC(C)c1ccccc1-c1cc2cccc(NC(=O)Nc3ccc(Cl)cc3)c2o1 Show InChI InChI=1S/C24H21ClN2O2/c1-15(2)19-7-3-4-8-20(19)22-14-16-6-5-9-21(23(16)29-22)27-24(28)26-18-12-10-17(25)11-13-18/h3-15H,1-2H3,(H2,26,27,28) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 760 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Displacement of [33P]2-MeS-ADP from human P2Y1 receptor expressed in human U2OS cells by scintillation counting |
Bioorg Med Chem Lett 20: 4104-7 (2010)
Article DOI: 10.1016/j.bmcl.2010.05.072
BindingDB Entry DOI: 10.7270/Q2HX1DNF |
More data for this
Ligand-Target Pair | |
P2Y purinoceptor 1
(Homo sapiens (Human)) | BDBM50245984
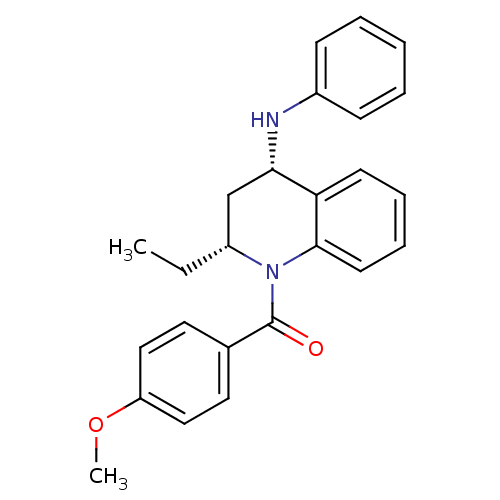
(CHEMBL516508 | cis-(2(R)-ethyl-4-(phenylamino)-3,4...)Show SMILES CC[C@@H]1C[C@H](Nc2ccccc2)c2ccccc2N1C(=O)c1ccc(OC)cc1 |r| Show InChI InChI=1S/C25H26N2O2/c1-3-20-17-23(26-19-9-5-4-6-10-19)22-11-7-8-12-24(22)27(20)25(28)18-13-15-21(29-2)16-14-18/h4-16,20,23,26H,3,17H2,1-2H3/t20-,23+/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 800 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Displacement of [33P]2-Mes-ADP from human recombinant P2Y1 receptor expressed in human U20S cells |
Bioorg Med Chem Lett 18: 6222-6 (2008)
Article DOI: 10.1016/j.bmcl.2008.09.102
BindingDB Entry DOI: 10.7270/Q26Q1X42 |
More data for this
Ligand-Target Pair | |
P2Y purinoceptor 1
(Homo sapiens (Human)) | BDBM50321583

(1-(4-(trifluoromethoxy)phenyl)-3-(2-(2-(trifluorom...)Show SMILES FC(F)(F)Oc1ccc(NC(=O)Nc2cccc3cc(oc23)-c2ccccc2C(F)(F)F)cc1 Show InChI InChI=1S/C23H14F6N2O3/c24-22(25,26)17-6-2-1-5-16(17)19-12-13-4-3-7-18(20(13)33-19)31-21(32)30-14-8-10-15(11-9-14)34-23(27,28)29/h1-12H,(H2,30,31,32) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 2.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Displacement of [33P]2-MeS-ADP from human P2Y1 receptor expressed in human U2OS cells by scintillation counting |
Bioorg Med Chem Lett 20: 4104-7 (2010)
Article DOI: 10.1016/j.bmcl.2010.05.072
BindingDB Entry DOI: 10.7270/Q2HX1DNF |
More data for this
Ligand-Target Pair | |
Histone acetyltransferase p300
(Homo sapiens (Human)) | BDBM50607602

(CHEMBL5220994)Show SMILES F[C@@H]1C[C@@H](N(C1)C(=O)C1(CC(F)(F)C1)c1ccc(Cl)cc1)C(=O)Nc1ccc2[nH]ncc2n1 |r| | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.0794 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00670
BindingDB Entry DOI: 10.7270/Q27P93H2 |
More data for this
Ligand-Target Pair | |
Histone acetyltransferase p300
(Homo sapiens (Human)) | BDBM50607596

(CHEMBL5221053)Show SMILES Clc1ccc(cc1)C1(CCC1)C(=O)N1CCC[C@@H]1C(=O)Nc1ccc2[nH]ncc2n1 |r| | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.100 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00670
BindingDB Entry DOI: 10.7270/Q27P93H2 |
More data for this
Ligand-Target Pair | |
Histone acetyltransferase p300
(Homo sapiens (Human)) | BDBM50607597
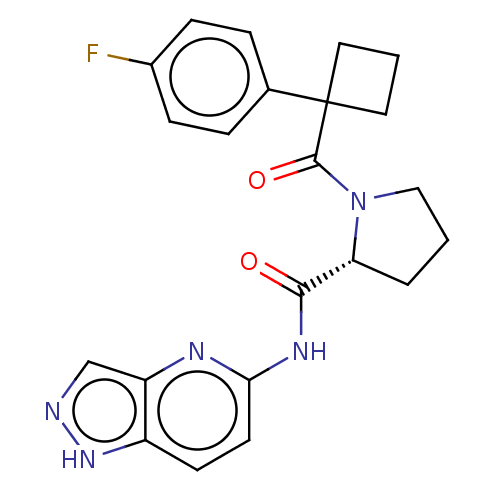
(CHEMBL5219157)Show SMILES Fc1ccc(cc1)C1(CCC1)C(=O)N1CCC[C@@H]1C(=O)Nc1ccc2[nH]ncc2n1 |r| | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.126 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00670
BindingDB Entry DOI: 10.7270/Q27P93H2 |
More data for this
Ligand-Target Pair | |
Histone acetyltransferase p300
(Homo sapiens (Human)) | BDBM50607593
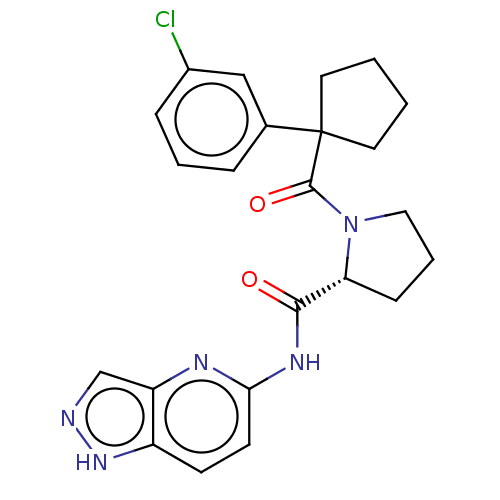
(CHEMBL5219693)Show SMILES Clc1cccc(c1)C1(CCCC1)C(=O)N1CCC[C@@H]1C(=O)Nc1ccc2[nH]ncc2n1 |r| | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.158 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00670
BindingDB Entry DOI: 10.7270/Q27P93H2 |
More data for this
Ligand-Target Pair | |
Histone acetyltransferase p300
(Homo sapiens (Human)) | BDBM50607595

(CHEMBL5219030)Show SMILES Clc1cccc(c1)C1(CCCC1)C(=O)N1CCC[C@@H]1C(=O)Nc1ccc2[nH]ccc2n1 |r| | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.158 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00670
BindingDB Entry DOI: 10.7270/Q27P93H2 |
More data for this
Ligand-Target Pair | |
Histone acetyltransferase p300
(Homo sapiens (Human)) | BDBM50607601
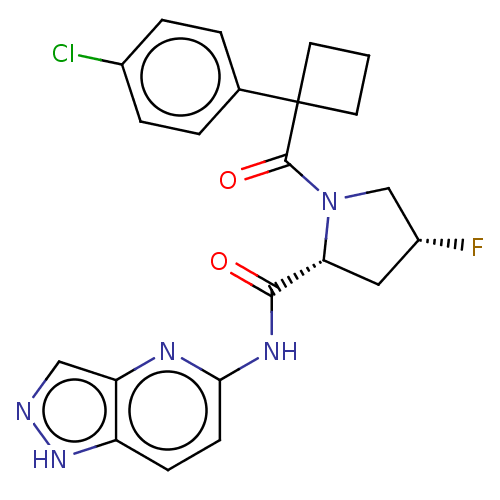
(CHEMBL5219667)Show SMILES F[C@@H]1C[C@@H](N(C1)C(=O)C1(CCC1)c1ccc(Cl)cc1)C(=O)Nc1ccc2[nH]ncc2n1 |r| | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00670
BindingDB Entry DOI: 10.7270/Q27P93H2 |
More data for this
Ligand-Target Pair | |
Histone acetyltransferase p300
(Homo sapiens (Human)) | BDBM50607599
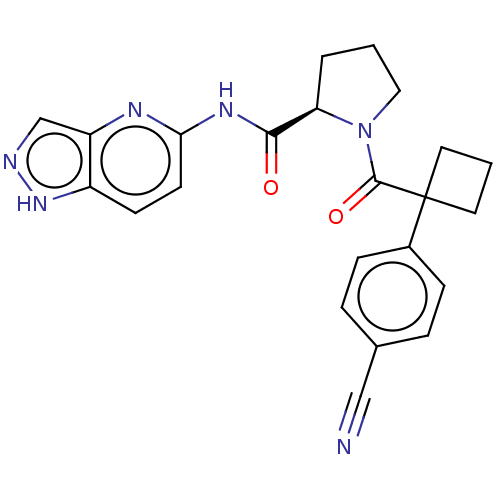
(CHEMBL5220732)Show SMILES O=C(Nc1ccc2[nH]ncc2n1)[C@H]1CCCN1C(=O)C1(CCC1)c1ccc(cc1)C#N |r| | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.251 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00670
BindingDB Entry DOI: 10.7270/Q27P93H2 |
More data for this
Ligand-Target Pair | |
Histone acetyltransferase p300
(Homo sapiens (Human)) | BDBM50607598
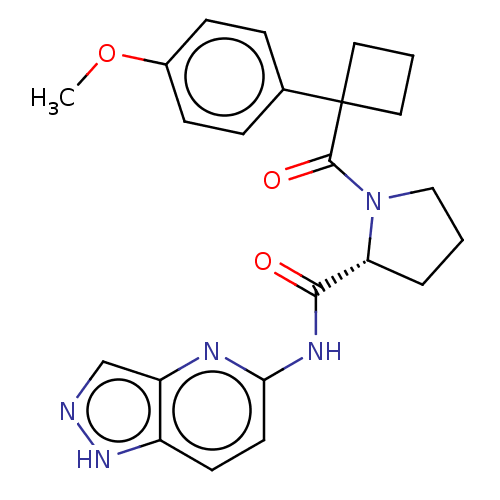
(CHEMBL5220447)Show SMILES COc1ccc(cc1)C1(CCC1)C(=O)N1CCC[C@@H]1C(=O)Nc1ccc2[nH]ncc2n1 |r| | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.251 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00670
BindingDB Entry DOI: 10.7270/Q27P93H2 |
More data for this
Ligand-Target Pair | |
Histone acetyltransferase p300
(Homo sapiens (Human)) | BDBM50607600

(CHEMBL5220956)Show SMILES FC1(F)CC(C1)(C(=O)N1CCC[C@@H]1C(=O)Nc1ccc2[nH]ncc2n1)c1ccc(Cl)cc1 |r| | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.316 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00670
BindingDB Entry DOI: 10.7270/Q27P93H2 |
More data for this
Ligand-Target Pair | |
Histone acetyltransferase p300
(Homo sapiens (Human)) | BDBM50607589
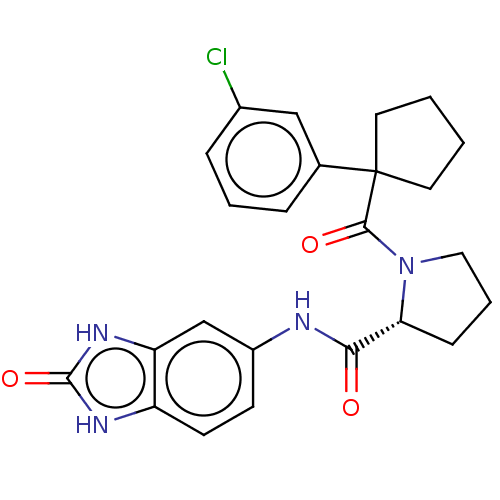
(CHEMBL5219678)Show SMILES Clc1cccc(c1)C1(CCCC1)C(=O)N1CCC[C@@H]1C(=O)Nc1ccc2[nH]c(=O)[nH]c2c1 |r| | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.398 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00670
BindingDB Entry DOI: 10.7270/Q27P93H2 |
More data for this
Ligand-Target Pair | |
Bifunctional epoxide hydrolase 2
(Homo sapiens (Human)) | BDBM50435764

(CHEMBL2392692)Show SMILES CNc1nc(C)nc(n1)N1CCC(CC1)C(=O)NCc1ccc(Br)cc1OC(F)(F)F Show InChI InChI=1S/C19H22BrF3N6O2/c1-11-26-17(24-2)28-18(27-11)29-7-5-12(6-8-29)16(30)25-10-13-3-4-14(20)9-15(13)31-19(21,22)23/h3-4,9,12H,5-8,10H2,1-2H3,(H,25,30)(H,24,26,27,28) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibition of human soluble epoxide hydrolase using PHOME as substrate assessed as formation of 6-methoxy-2-naphthaldehyde incubated for 10 mins prio... |
Bioorg Med Chem Lett 23: 3584-8 (2013)
Article DOI: 10.1016/j.bmcl.2013.04.019
BindingDB Entry DOI: 10.7270/Q2J967S5 |
More data for this
Ligand-Target Pair | |
Histone acetyltransferase p300
(Homo sapiens (Human)) | BDBM50607585
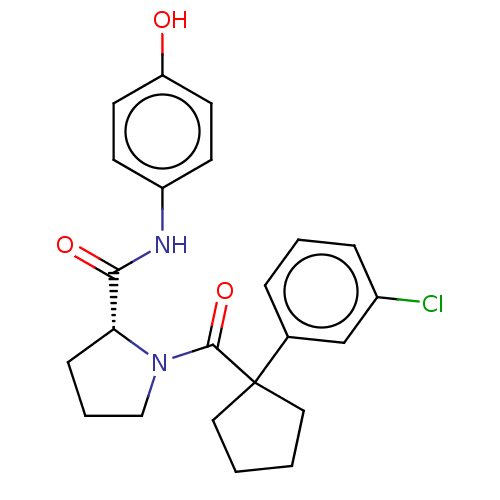
(CHEMBL5219512)Show SMILES Oc1ccc(NC(=O)[C@H]2CCCN2C(=O)C2(CCCC2)c2cccc(Cl)c2)cc1 |r| | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.631 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00670
BindingDB Entry DOI: 10.7270/Q27P93H2 |
More data for this
Ligand-Target Pair | |
Histone acetyltransferase p300
(Homo sapiens (Human)) | BDBM50607600

(CHEMBL5220956)Show SMILES FC1(F)CC(C1)(C(=O)N1CCC[C@@H]1C(=O)Nc1ccc2[nH]ncc2n1)c1ccc(Cl)cc1 |r| | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.631 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00670
BindingDB Entry DOI: 10.7270/Q27P93H2 |
More data for this
Ligand-Target Pair | |
Histone acetyltransferase p300
(Homo sapiens (Human)) | BDBM50607586
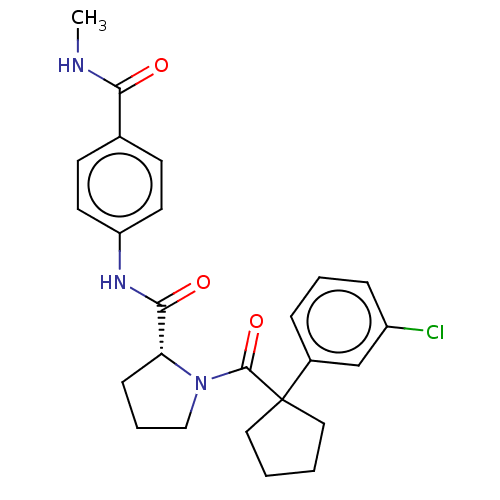
(CHEMBL5221030)Show SMILES CNC(=O)c1ccc(NC(=O)[C@H]2CCCN2C(=O)C2(CCCC2)c2cccc(Cl)c2)cc1 |r| | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.794 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00670
BindingDB Entry DOI: 10.7270/Q27P93H2 |
More data for this
Ligand-Target Pair | |
Histone acetyltransferase p300
(Homo sapiens (Human)) | BDBM50607588
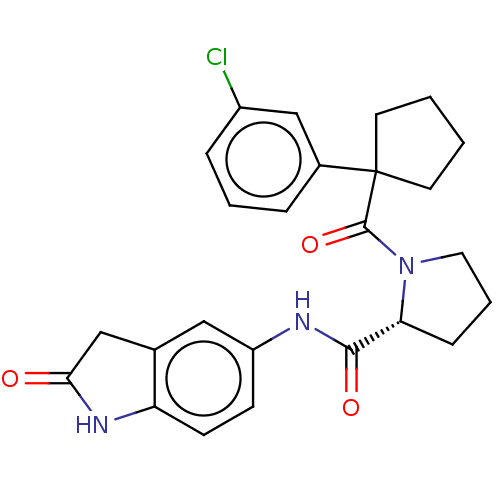
(CHEMBL5220546)Show SMILES Clc1cccc(c1)C1(CCCC1)C(=O)N1CCC[C@@H]1C(=O)Nc1ccc2NC(=O)Cc2c1 |r| | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.794 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00670
BindingDB Entry DOI: 10.7270/Q27P93H2 |
More data for this
Ligand-Target Pair | |
Bifunctional epoxide hydrolase 2
(Homo sapiens (Human)) | BDBM50435745
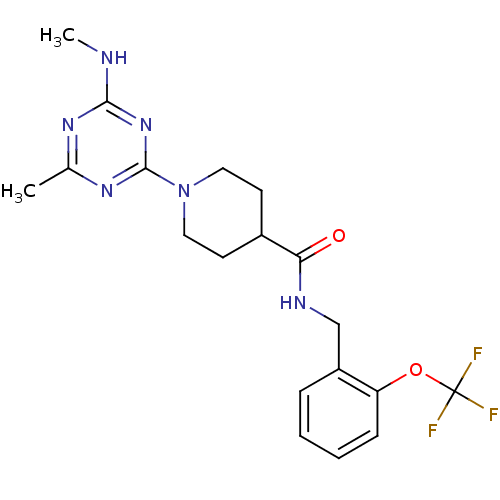
(CHEMBL2392706)Show SMILES CNc1nc(C)nc(n1)N1CCC(CC1)C(=O)NCc1ccccc1OC(F)(F)F Show InChI InChI=1S/C19H23F3N6O2/c1-12-25-17(23-2)27-18(26-12)28-9-7-13(8-10-28)16(29)24-11-14-5-3-4-6-15(14)30-19(20,21)22/h3-6,13H,7-11H2,1-2H3,(H,24,29)(H,23,25,26,27) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibition of human soluble epoxide hydrolase using PHOME as substrate assessed as formation of 6-methoxy-2-naphthaldehyde incubated for 10 mins prio... |
Bioorg Med Chem Lett 23: 3584-8 (2013)
Article DOI: 10.1016/j.bmcl.2013.04.019
BindingDB Entry DOI: 10.7270/Q2J967S5 |
More data for this
Ligand-Target Pair | |
Bifunctional epoxide hydrolase 2
(Homo sapiens (Human)) | BDBM50435753

(CHEMBL2392698)Show SMILES COCCNc1nc(C)nc(n1)N1CCC(CC1)C(=O)NCc1ccccc1C(F)(F)F Show InChI InChI=1S/C21H27F3N6O2/c1-14-27-19(25-9-12-32-2)29-20(28-14)30-10-7-15(8-11-30)18(31)26-13-16-5-3-4-6-17(16)21(22,23)24/h3-6,15H,7-13H2,1-2H3,(H,26,31)(H,25,27,28,29) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibition of human soluble epoxide hydrolase using PHOME as substrate assessed as formation of 6-methoxy-2-naphthaldehyde incubated for 10 mins prio... |
Bioorg Med Chem Lett 23: 3584-8 (2013)
Article DOI: 10.1016/j.bmcl.2013.04.019
BindingDB Entry DOI: 10.7270/Q2J967S5 |
More data for this
Ligand-Target Pair | |
Bifunctional epoxide hydrolase 2
(Homo sapiens (Human)) | BDBM50435754
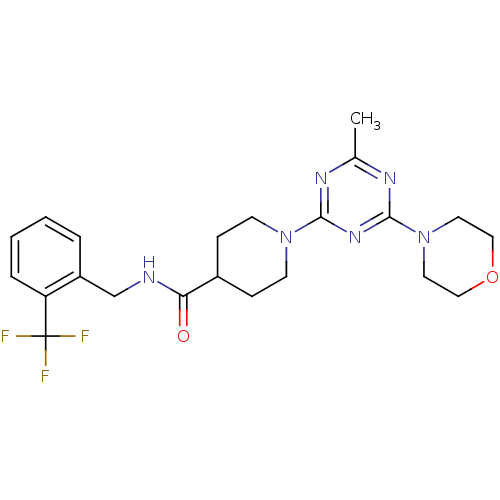
(CHEMBL2392697)Show SMILES Cc1nc(nc(n1)N1CCC(CC1)C(=O)NCc1ccccc1C(F)(F)F)N1CCOCC1 Show InChI InChI=1S/C22H27F3N6O2/c1-15-27-20(29-21(28-15)31-10-12-33-13-11-31)30-8-6-16(7-9-30)19(32)26-14-17-4-2-3-5-18(17)22(23,24)25/h2-5,16H,6-14H2,1H3,(H,26,32) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibition of human soluble epoxide hydrolase using PHOME as substrate assessed as formation of 6-methoxy-2-naphthaldehyde incubated for 10 mins prio... |
Bioorg Med Chem Lett 23: 3584-8 (2013)
Article DOI: 10.1016/j.bmcl.2013.04.019
BindingDB Entry DOI: 10.7270/Q2J967S5 |
More data for this
Ligand-Target Pair | |
Bifunctional epoxide hydrolase 2
(Homo sapiens (Human)) | BDBM50435755

(CHEMBL2392696)Show SMILES CC(C)Nc1nc(C)nc(n1)N1CCC(CC1)C(=O)NCc1ccccc1C(F)(F)F Show InChI InChI=1S/C21H27F3N6O/c1-13(2)26-19-27-14(3)28-20(29-19)30-10-8-15(9-11-30)18(31)25-12-16-6-4-5-7-17(16)21(22,23)24/h4-7,13,15H,8-12H2,1-3H3,(H,25,31)(H,26,27,28,29) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibition of human soluble epoxide hydrolase using PHOME as substrate assessed as formation of 6-methoxy-2-naphthaldehyde incubated for 10 mins prio... |
Bioorg Med Chem Lett 23: 3584-8 (2013)
Article DOI: 10.1016/j.bmcl.2013.04.019
BindingDB Entry DOI: 10.7270/Q2J967S5 |
More data for this
Ligand-Target Pair | |
Bifunctional epoxide hydrolase 2
(Homo sapiens (Human)) | BDBM50435756

(CHEMBL2392695)Show SMILES CN(C)c1nc(C)nc(n1)N1CCC(CC1)C(=O)NCc1ccccc1C(F)(F)F Show InChI InChI=1S/C20H25F3N6O/c1-13-25-18(28(2)3)27-19(26-13)29-10-8-14(9-11-29)17(30)24-12-15-6-4-5-7-16(15)20(21,22)23/h4-7,14H,8-12H2,1-3H3,(H,24,30) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibition of human soluble epoxide hydrolase using PHOME as substrate assessed as formation of 6-methoxy-2-naphthaldehyde incubated for 10 mins prio... |
Bioorg Med Chem Lett 23: 3584-8 (2013)
Article DOI: 10.1016/j.bmcl.2013.04.019
BindingDB Entry DOI: 10.7270/Q2J967S5 |
More data for this
Ligand-Target Pair | |
Bifunctional epoxide hydrolase 2
(Homo sapiens (Human)) | BDBM50435757
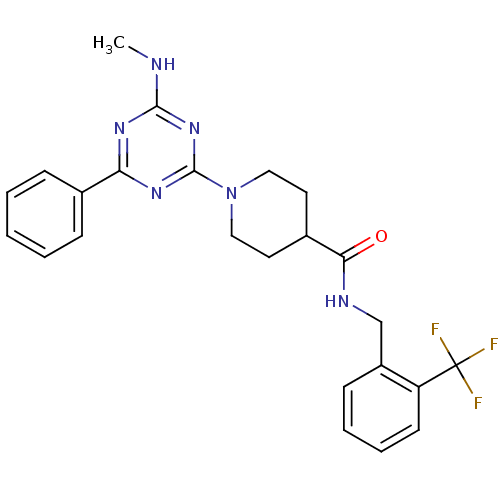
(CHEMBL2392694)Show SMILES CNc1nc(nc(n1)-c1ccccc1)N1CCC(CC1)C(=O)NCc1ccccc1C(F)(F)F Show InChI InChI=1S/C24H25F3N6O/c1-28-22-30-20(16-7-3-2-4-8-16)31-23(32-22)33-13-11-17(12-14-33)21(34)29-15-18-9-5-6-10-19(18)24(25,26)27/h2-10,17H,11-15H2,1H3,(H,29,34)(H,28,30,31,32) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibition of human soluble epoxide hydrolase using PHOME as substrate assessed as formation of 6-methoxy-2-naphthaldehyde incubated for 10 mins prio... |
Bioorg Med Chem Lett 23: 3584-8 (2013)
Article DOI: 10.1016/j.bmcl.2013.04.019
BindingDB Entry DOI: 10.7270/Q2J967S5 |
More data for this
Ligand-Target Pair | |
Histone acetyltransferase p300
(Homo sapiens (Human)) | BDBM50607590
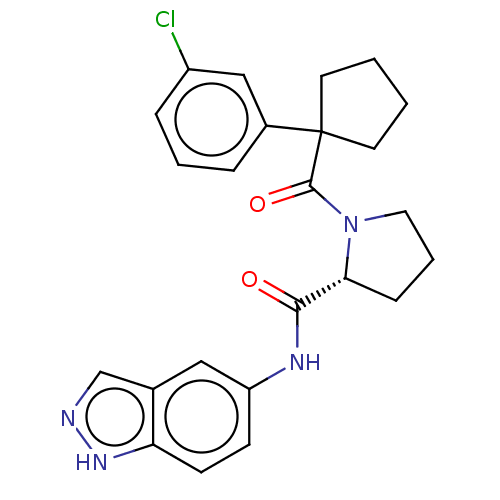
(CHEMBL5220332)Show SMILES Clc1cccc(c1)C1(CCCC1)C(=O)N1CCC[C@@H]1C(=O)Nc1ccc2[nH]ncc2c1 |r| | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00670
BindingDB Entry DOI: 10.7270/Q27P93H2 |
More data for this
Ligand-Target Pair | |
Bifunctional epoxide hydrolase 2
(Homo sapiens (Human)) | BDBM50435753

(CHEMBL2392698)Show SMILES COCCNc1nc(C)nc(n1)N1CCC(CC1)C(=O)NCc1ccccc1C(F)(F)F Show InChI InChI=1S/C21H27F3N6O2/c1-14-27-19(25-9-12-32-2)29-20(28-14)30-10-7-15(8-11-30)18(31)26-13-16-5-3-4-6-17(16)21(22,23)24/h3-6,15H,7-13H2,1-2H3,(H,26,31)(H,25,27,28,29) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibition of human soluble epoxide hydrolase overexpressed in HEK293F cells using EET as substrate assessed as formation of DHET incubated for 30 mi... |
Bioorg Med Chem Lett 23: 3584-8 (2013)
Article DOI: 10.1016/j.bmcl.2013.04.019
BindingDB Entry DOI: 10.7270/Q2J967S5 |
More data for this
Ligand-Target Pair | |
Bifunctional epoxide hydrolase 2
(Homo sapiens (Human)) | BDBM50435758

(CHEMBL2392693)Show SMILES CNc1nc(nc(n1)N1CCC(CC1)C(=O)NCc1ccccc1C(F)(F)F)C1CCCCC1 Show InChI InChI=1S/C24H31F3N6O/c1-28-22-30-20(16-7-3-2-4-8-16)31-23(32-22)33-13-11-17(12-14-33)21(34)29-15-18-9-5-6-10-19(18)24(25,26)27/h5-6,9-10,16-17H,2-4,7-8,11-15H2,1H3,(H,29,34)(H,28,30,31,32) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibition of human soluble epoxide hydrolase using PHOME as substrate assessed as formation of 6-methoxy-2-naphthaldehyde incubated for 10 mins prio... |
Bioorg Med Chem Lett 23: 3584-8 (2013)
Article DOI: 10.1016/j.bmcl.2013.04.019
BindingDB Entry DOI: 10.7270/Q2J967S5 |
More data for this
Ligand-Target Pair | |
Bifunctional epoxide hydrolase 2
(Homo sapiens (Human)) | BDBM50435765
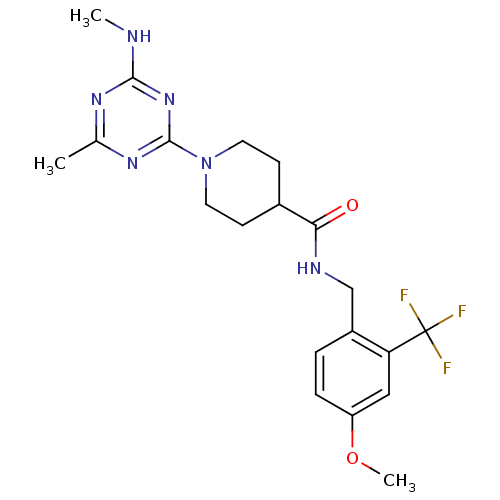
(CHEMBL2392691)Show SMILES CNc1nc(C)nc(n1)N1CCC(CC1)C(=O)NCc1ccc(OC)cc1C(F)(F)F Show InChI InChI=1S/C20H25F3N6O2/c1-12-26-18(24-2)28-19(27-12)29-8-6-13(7-9-29)17(30)25-11-14-4-5-15(31-3)10-16(14)20(21,22)23/h4-5,10,13H,6-9,11H2,1-3H3,(H,25,30)(H,24,26,27,28) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibition of human soluble epoxide hydrolase using PHOME as substrate assessed as formation of 6-methoxy-2-naphthaldehyde incubated for 10 mins prio... |
Bioorg Med Chem Lett 23: 3584-8 (2013)
Article DOI: 10.1016/j.bmcl.2013.04.019
BindingDB Entry DOI: 10.7270/Q2J967S5 |
More data for this
Ligand-Target Pair | |
Histone acetyltransferase p300
(Homo sapiens (Human)) | BDBM50607605
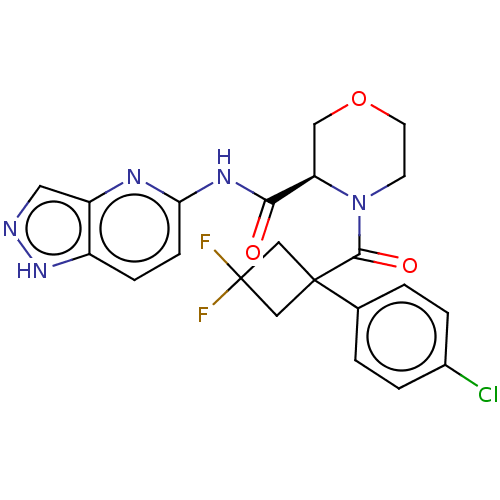
(CHEMBL5219177)Show SMILES FC1(F)CC(C1)(C(=O)N1CCOC[C@@H]1C(=O)Nc1ccc2[nH]ncc2n1)c1ccc(Cl)cc1 |r| | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00670
BindingDB Entry DOI: 10.7270/Q27P93H2 |
More data for this
Ligand-Target Pair | |
Bifunctional epoxide hydrolase 2
(Homo sapiens (Human)) | BDBM50435765

(CHEMBL2392691)Show SMILES CNc1nc(C)nc(n1)N1CCC(CC1)C(=O)NCc1ccc(OC)cc1C(F)(F)F Show InChI InChI=1S/C20H25F3N6O2/c1-12-26-18(24-2)28-19(27-12)29-8-6-13(7-9-29)17(30)25-11-14-4-5-15(31-3)10-16(14)20(21,22)23/h4-5,10,13H,6-9,11H2,1-3H3,(H,25,30)(H,24,26,27,28) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibition of human soluble epoxide hydrolase overexpressed in HEK293F cells using EET as substrate assessed as formation of DHET incubated for 30 mi... |
Bioorg Med Chem Lett 23: 3584-8 (2013)
Article DOI: 10.1016/j.bmcl.2013.04.019
BindingDB Entry DOI: 10.7270/Q2J967S5 |
More data for this
Ligand-Target Pair | |
Histone acetyltransferase p300
(Homo sapiens (Human)) | BDBM50607592
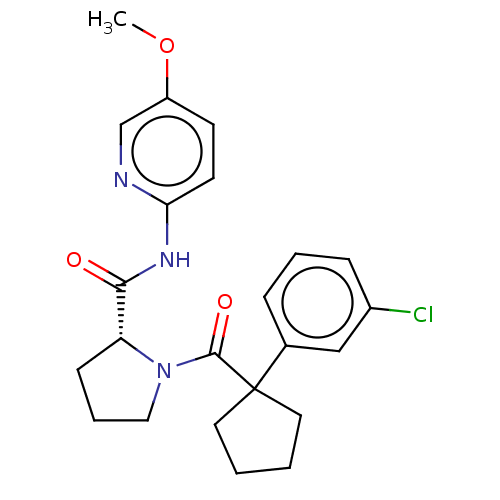
(CHEMBL5219466)Show SMILES COc1ccc(NC(=O)[C@H]2CCCN2C(=O)C2(CCCC2)c2cccc(Cl)c2)nc1 |r| | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00670
BindingDB Entry DOI: 10.7270/Q27P93H2 |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase Chk1
(Homo sapiens (Human)) | BDBM50218733
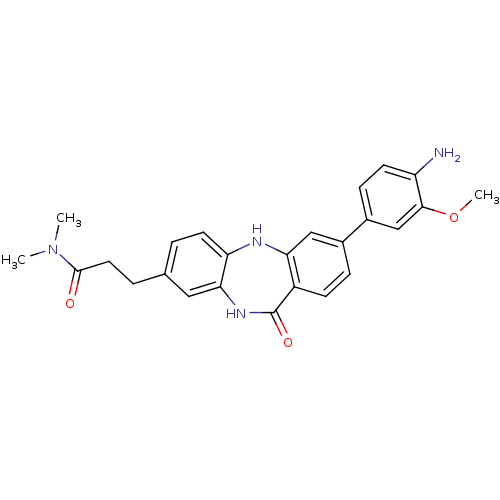
(3-[3-(3-methoxy-4-nitrophenyl)-11-oxo-10,11-dihydr...)Show SMILES COc1cc(ccc1N)-c1ccc2c(Nc3ccc(CCC(=O)N(C)C)cc3NC2=O)c1 Show InChI InChI=1S/C25H26N4O3/c1-29(2)24(30)11-5-15-4-10-20-22(12-15)28-25(31)18-8-6-16(13-21(18)27-20)17-7-9-19(26)23(14-17)32-3/h4,6-10,12-14,27H,5,11,26H2,1-3H3,(H,28,31) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant Chk1 |
J Med Chem 50: 4162-76 (2007)
Article DOI: 10.1021/jm070105d
BindingDB Entry DOI: 10.7270/Q22J6BKS |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase Chk1
(Homo sapiens (Human)) | BDBM50218701

(8-(1,1-dioxo-1l6-isothiazolidin-2-yl)-3-(3-methoxy...)Show SMILES COc1cc(ccc1N)-c1ccc2c(Nc3ccc(cc3NC2=O)N2CCCS2(=O)=O)c1 Show InChI InChI=1S/C23H22N4O4S/c1-31-22-12-15(4-7-18(22)24)14-3-6-17-20(11-14)25-19-8-5-16(13-21(19)26-23(17)28)27-9-2-10-32(27,29)30/h3-8,11-13,25H,2,9-10,24H2,1H3,(H,26,28) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant Chk1 |
J Med Chem 50: 4162-76 (2007)
Article DOI: 10.1021/jm070105d
BindingDB Entry DOI: 10.7270/Q22J6BKS |
More data for this
Ligand-Target Pair | |
Bifunctional epoxide hydrolase 2
(Rattus norvegicus) | BDBM50435764

(CHEMBL2392692)Show SMILES CNc1nc(C)nc(n1)N1CCC(CC1)C(=O)NCc1ccc(Br)cc1OC(F)(F)F Show InChI InChI=1S/C19H22BrF3N6O2/c1-11-26-17(24-2)28-18(27-11)29-7-5-12(6-8-29)16(30)25-10-13-3-4-14(20)9-15(13)31-19(21,22)23/h3-4,9,12H,5-8,10H2,1-2H3,(H,25,30)(H,24,26,27,28) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibition of rat soluble epoxide hydrolase using PHOME as substrate assessed as formation of 6-methoxy-2-naphthaldehyde incubated for 10 mins prior ... |
Bioorg Med Chem Lett 23: 3584-8 (2013)
Article DOI: 10.1016/j.bmcl.2013.04.019
BindingDB Entry DOI: 10.7270/Q2J967S5 |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data