

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Proprotein convertase subtilisin/kexin type 6 (Homo sapiens (Human)) | BDBM50303774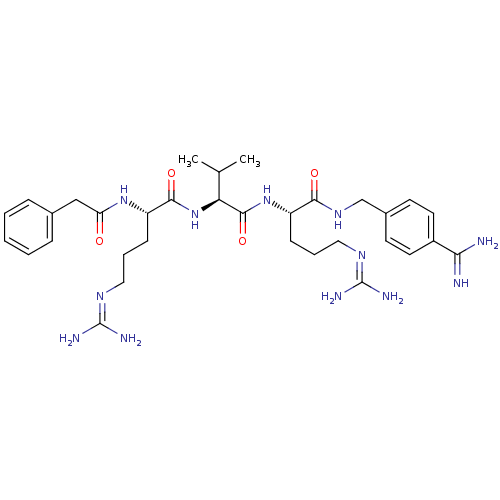 (CHEMBL566340 | phenylacetyl-Arg-Val-Arg-4-amidinob...) | Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Philipps University Marburg Curated by ChEMBL | Assay Description Inhibition of human PACE4 expressed in Drosophila schneider 2 cells by fluorescence assay | J Med Chem 53: 1067-75 (2010) Article DOI: 10.1021/jm9012455 BindingDB Entry DOI: 10.7270/Q2DN455Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuroendocrine convertase 1 (Homo sapiens (Human)) | BDBM50303774 (CHEMBL566340 | phenylacetyl-Arg-Val-Arg-4-amidinob...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.75 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Philipps University Marburg Curated by ChEMBL | Assay Description Inhibition of human PC1/3 expressed in Drosophila schneider 2 cells by fluorescence assay | J Med Chem 53: 1067-75 (2010) Article DOI: 10.1021/jm9012455 BindingDB Entry DOI: 10.7270/Q2DN455Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Furin (Homo sapiens (Human)) | BDBM50303774 (CHEMBL566340 | phenylacetyl-Arg-Val-Arg-4-amidinob...) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.810 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Philipps University Marburg Curated by ChEMBL | Assay Description Inhibition of human furin by fluorescence assay | J Med Chem 53: 1067-75 (2010) Article DOI: 10.1021/jm9012455 BindingDB Entry DOI: 10.7270/Q2DN455Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Furin (Homo sapiens (Human)) | BDBM50303775 ((S)-2-acetamido-N-((S)-1-((S)-1-(4-carbamimidoylbe...) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Philipps University Marburg Curated by ChEMBL | Assay Description Inhibition of human furin by fluorescence assay | J Med Chem 53: 1067-75 (2010) Article DOI: 10.1021/jm9012455 BindingDB Entry DOI: 10.7270/Q2DN455Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Suppressor of tumorigenicity 14 protein (Homo sapiens (Human)) | BDBM23921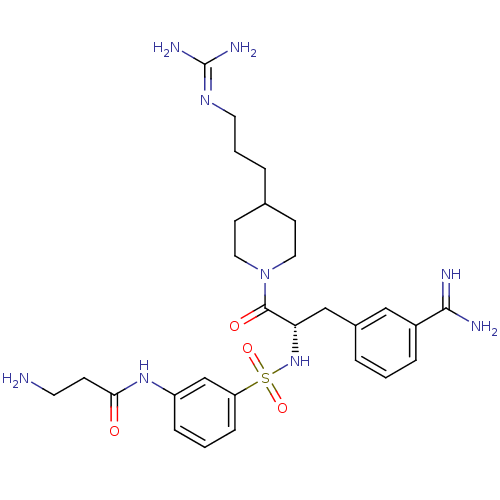 (3-amidinophenylalanine deriv., 63 | 3-amino-N-(3-{...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 1.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Curacyte Chemistry GmbH | Assay Description The measurements were carried out on a microplate reader. Two concentrations of the substrate and five concentrations of the inhibitor were used. Af... | J Med Chem 49: 4116-26 (2006) Article DOI: 10.1021/jm051272l BindingDB Entry DOI: 10.7270/Q21C1V64 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Proprotein convertase subtilisin/kexin type 5 (Homo sapiens (Human)) | BDBM50303774 (CHEMBL566340 | phenylacetyl-Arg-Val-Arg-4-amidinob...) | Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Philipps University Marburg Curated by ChEMBL | Assay Description Inhibition of human PC5/6 expressed in Drosophila schneider 2 cells by fluorescence assay | J Med Chem 53: 1067-75 (2010) Article DOI: 10.1021/jm9012455 BindingDB Entry DOI: 10.7270/Q2DN455Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Furin (Homo sapiens (Human)) | BDBM50303776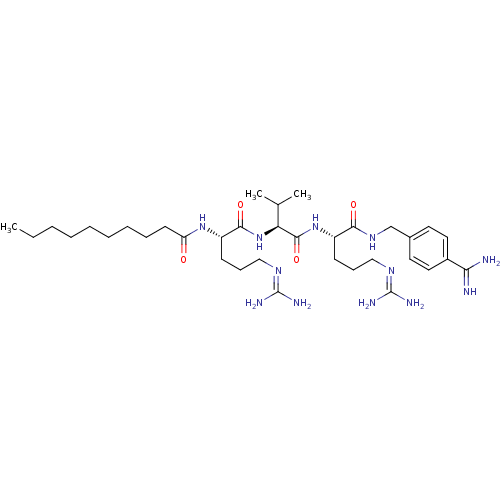 (CHEMBL568525 | N-((6S,9S,12S)-1,17-diamino-6-(4-ca...) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Philipps University Marburg Curated by ChEMBL | Assay Description Inhibition of human furin by fluorescence assay | J Med Chem 53: 1067-75 (2010) Article DOI: 10.1021/jm9012455 BindingDB Entry DOI: 10.7270/Q2DN455Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuroendocrine convertase 1 (Homo sapiens (Human)) | BDBM50303775 ((S)-2-acetamido-N-((S)-1-((S)-1-(4-carbamimidoylbe...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Philipps University Marburg Curated by ChEMBL | Assay Description Inhibition of human PC1/3 expressed in Drosophila schneider 2 cells by fluorescence assay | J Med Chem 53: 1067-75 (2010) Article DOI: 10.1021/jm9012455 BindingDB Entry DOI: 10.7270/Q2DN455Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Suppressor of tumorigenicity 14 protein (Homo sapiens (Human)) | BDBM23916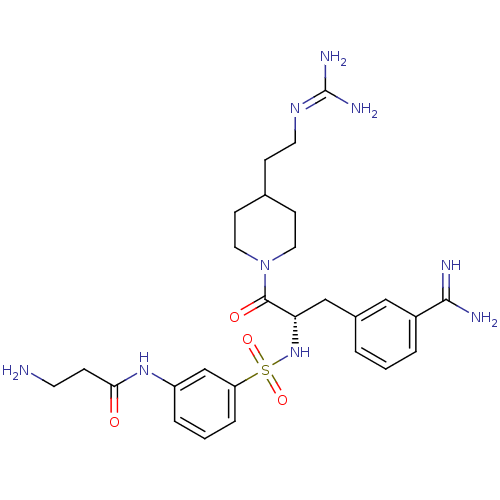 (3-amidinophenylalanine deriv., 12 | 3-amino-N-(3-{...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Curacyte Chemistry GmbH | Assay Description The measurements were carried out on a microplate reader. Two concentrations of the substrate and five concentrations of the inhibitor were used. Af... | J Med Chem 49: 4116-26 (2006) Article DOI: 10.1021/jm051272l BindingDB Entry DOI: 10.7270/Q21C1V64 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Proprotein convertase subtilisin/kexin type 6 (Homo sapiens (Human)) | BDBM50303775 ((S)-2-acetamido-N-((S)-1-((S)-1-(4-carbamimidoylbe...) | Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 2.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Philipps University Marburg Curated by ChEMBL | Assay Description Inhibition of human PACE4 expressed in Drosophila schneider 2 cells by fluorescence assay | J Med Chem 53: 1067-75 (2010) Article DOI: 10.1021/jm9012455 BindingDB Entry DOI: 10.7270/Q2DN455Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Proprotein convertase subtilisin/kexin type 6 (Homo sapiens (Human)) | BDBM50303776 (CHEMBL568525 | N-((6S,9S,12S)-1,17-diamino-6-(4-ca...) | Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Philipps University Marburg Curated by ChEMBL | Assay Description Inhibition of human PACE4 expressed in Drosophila schneider 2 cells by fluorescence assay | J Med Chem 53: 1067-75 (2010) Article DOI: 10.1021/jm9012455 BindingDB Entry DOI: 10.7270/Q2DN455Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Furin (Homo sapiens (Human)) | BDBM50303781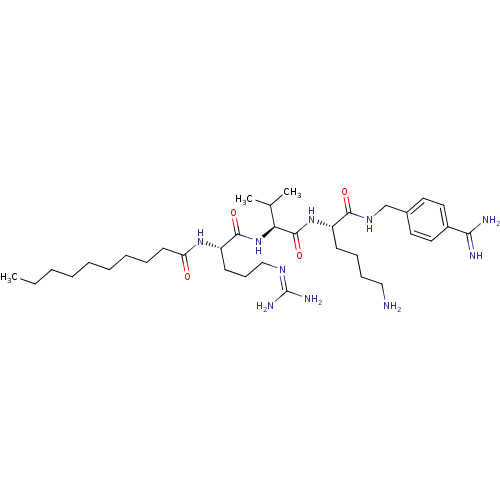 (CHEMBL565931 | N-((4S,7S,10S)-15-amino-4-(4-aminob...) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 3.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Philipps University Marburg Curated by ChEMBL | Assay Description Inhibition of human furin by fluorescence assay | J Med Chem 53: 1067-75 (2010) Article DOI: 10.1021/jm9012455 BindingDB Entry DOI: 10.7270/Q2DN455Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Proprotein convertase subtilisin/kexin type 5 (Homo sapiens (Human)) | BDBM50303775 ((S)-2-acetamido-N-((S)-1-((S)-1-(4-carbamimidoylbe...) | Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 3.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Philipps University Marburg Curated by ChEMBL | Assay Description Inhibition of human PC5/6 expressed in Drosophila schneider 2 cells by fluorescence assay | J Med Chem 53: 1067-75 (2010) Article DOI: 10.1021/jm9012455 BindingDB Entry DOI: 10.7270/Q2DN455Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuroendocrine convertase 1 (Homo sapiens (Human)) | BDBM50303776 (CHEMBL568525 | N-((6S,9S,12S)-1,17-diamino-6-(4-ca...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 3.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Philipps University Marburg Curated by ChEMBL | Assay Description Inhibition of human PC1/3 expressed in Drosophila schneider 2 cells by fluorescence assay | J Med Chem 53: 1067-75 (2010) Article DOI: 10.1021/jm9012455 BindingDB Entry DOI: 10.7270/Q2DN455Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Suppressor of tumorigenicity 14 protein (Homo sapiens (Human)) | BDBM23918 (3-amidinophenylalanine deriv., 60 | 4-amino-N-(3-{...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 3.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Curacyte Chemistry GmbH | Assay Description The measurements were carried out on a microplate reader. Two concentrations of the substrate and five concentrations of the inhibitor were used. Af... | J Med Chem 49: 4116-26 (2006) Article DOI: 10.1021/jm051272l BindingDB Entry DOI: 10.7270/Q21C1V64 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Suppressor of tumorigenicity 14 protein (Homo sapiens (Human)) | BDBM23915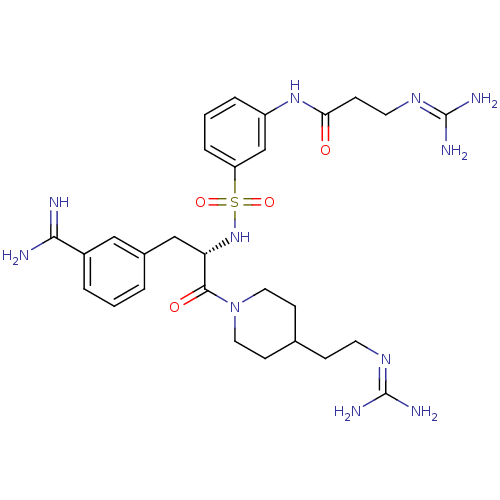 (3-amidinophenylalanine deriv., 58 | 3-carbamimidam...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 4.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Curacyte Chemistry GmbH | Assay Description The measurements were carried out on a microplate reader. Two concentrations of the substrate and five concentrations of the inhibitor were used. Af... | J Med Chem 49: 4116-26 (2006) Article DOI: 10.1021/jm051272l BindingDB Entry DOI: 10.7270/Q21C1V64 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Suppressor of tumorigenicity 14 protein (Homo sapiens (Human)) | BDBM23920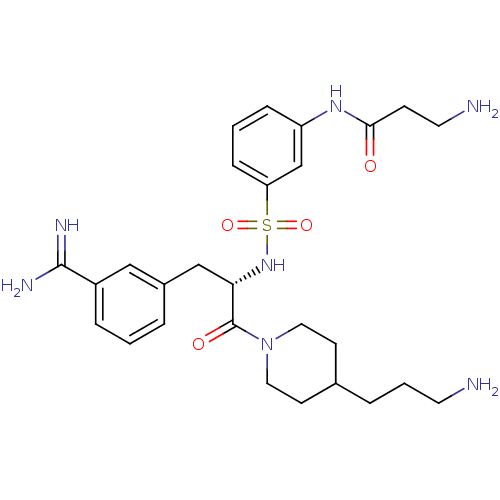 (3-amidinophenylalanine deriv., 62 | 3-amino-N-(3-{...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Curacyte Chemistry GmbH | Assay Description The measurements were carried out on a microplate reader. Two concentrations of the substrate and five concentrations of the inhibitor were used. Af... | J Med Chem 49: 4116-26 (2006) Article DOI: 10.1021/jm051272l BindingDB Entry DOI: 10.7270/Q21C1V64 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Proprotein convertase subtilisin/kexin type 5 (Homo sapiens (Human)) | BDBM50303776 (CHEMBL568525 | N-((6S,9S,12S)-1,17-diamino-6-(4-ca...) | Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 6.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Philipps University Marburg Curated by ChEMBL | Assay Description Inhibition of human PC5/6 expressed in Drosophila schneider 2 cells by fluorescence assay | J Med Chem 53: 1067-75 (2010) Article DOI: 10.1021/jm9012455 BindingDB Entry DOI: 10.7270/Q2DN455Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Suppressor of tumorigenicity 14 protein (Homo sapiens (Human)) | BDBM23917 (3-amidinophenylalanine deriv., 59 | 3-amino-N-(3-{...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 6.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Curacyte Chemistry GmbH | Assay Description The measurements were carried out on a microplate reader. Two concentrations of the substrate and five concentrations of the inhibitor were used. Af... | J Med Chem 49: 4116-26 (2006) Article DOI: 10.1021/jm051272l BindingDB Entry DOI: 10.7270/Q21C1V64 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Suppressor of tumorigenicity 14 protein (Homo sapiens (Human)) | BDBM23913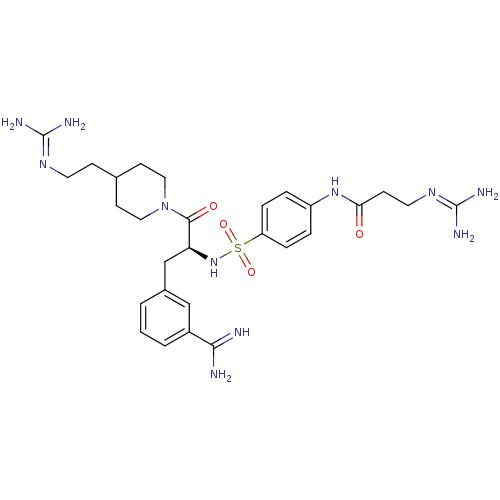 (3-amidinophenylalanine deriv., 56 | 3-carbamimidam...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 8.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Curacyte Chemistry GmbH | Assay Description The measurements were carried out on a microplate reader. Two concentrations of the substrate and five concentrations of the inhibitor were used. Af... | J Med Chem 49: 4116-26 (2006) Article DOI: 10.1021/jm051272l BindingDB Entry DOI: 10.7270/Q21C1V64 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (docked) | ||||||||||||
| Suppressor of tumorigenicity 14 protein (Homo sapiens (Human)) | BDBM23911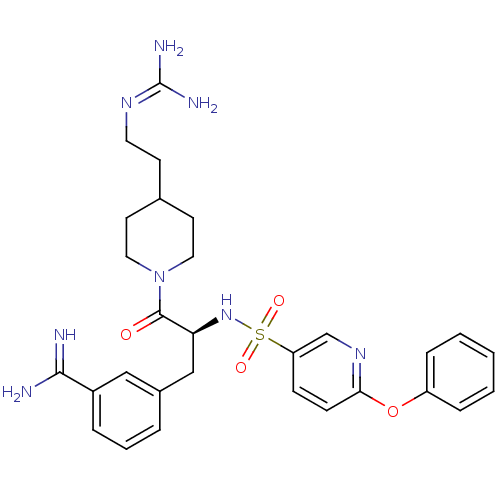 (3-[(2S)-3-[4-(2-carbamimidamidoethyl)piperidin-1-y...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 13 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Curacyte Chemistry GmbH | Assay Description The measurements were carried out on a microplate reader. Two concentrations of the substrate and five concentrations of the inhibitor were used. Af... | J Med Chem 49: 4116-26 (2006) Article DOI: 10.1021/jm051272l BindingDB Entry DOI: 10.7270/Q21C1V64 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Suppressor of tumorigenicity 14 protein (Homo sapiens (Human)) | BDBM23896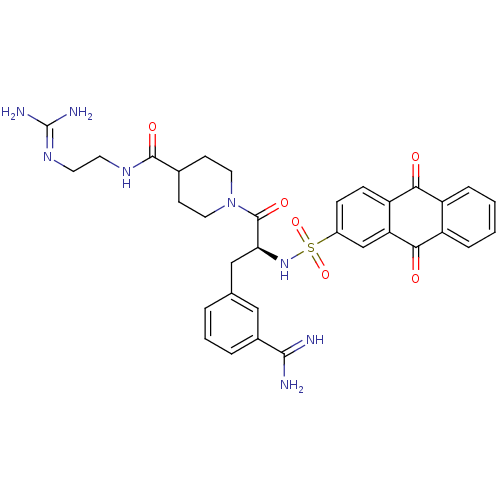 (3-amidinophenylalanine deriv., 40 | N-(2-carbamimi...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 13 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Curacyte Chemistry GmbH | Assay Description The measurements were carried out on a microplate reader. Two concentrations of the substrate and five concentrations of the inhibitor were used. Af... | J Med Chem 49: 4116-26 (2006) Article DOI: 10.1021/jm051272l BindingDB Entry DOI: 10.7270/Q21C1V64 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Suppressor of tumorigenicity 14 protein (Homo sapiens (Human)) | BDBM23887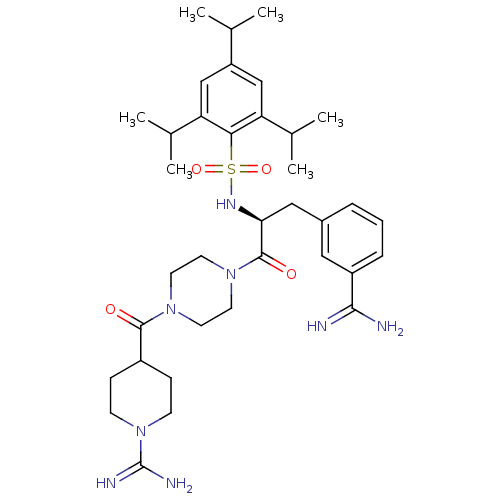 (3-amidinophenylalanine deriv., 31 | 4-({4-[(2S)-3-...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | MMDB PC cid PC sid PDB UniChem Similars | MMDB PDB Article PubMed | 14 | -44.8 | n/a | n/a | n/a | n/a | n/a | 8.0 | 25 |
Curacyte Chemistry GmbH | Assay Description The measurements were carried out on a microplate reader. Two concentrations of the substrate and five concentrations of the inhibitor were used. Af... | J Med Chem 49: 4116-26 (2006) Article DOI: 10.1021/jm051272l BindingDB Entry DOI: 10.7270/Q21C1V64 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Suppressor of tumorigenicity 14 protein (Homo sapiens (Human)) | BDBM23895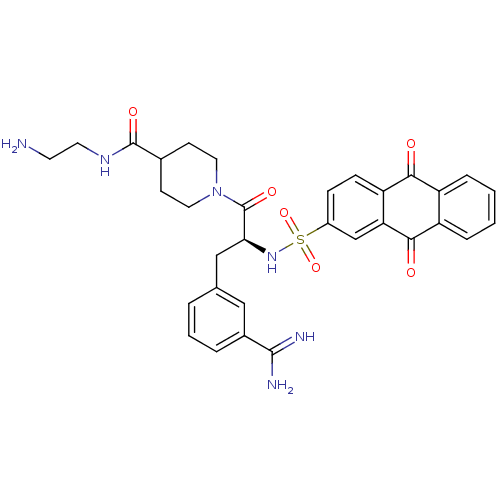 (3-amidinophenylalanine deriv., 39 | N-(2-aminoethy...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 21 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Curacyte Chemistry GmbH | Assay Description The measurements were carried out on a microplate reader. Two concentrations of the substrate and five concentrations of the inhibitor were used. Af... | J Med Chem 49: 4116-26 (2006) Article DOI: 10.1021/jm051272l BindingDB Entry DOI: 10.7270/Q21C1V64 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Suppressor of tumorigenicity 14 protein (Homo sapiens (Human)) | BDBM23900 (3-amidinophenylalanine deriv., 44 | 4-({1-[(2S)-3-...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 26 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Curacyte Chemistry GmbH | Assay Description The measurements were carried out on a microplate reader. Two concentrations of the substrate and five concentrations of the inhibitor were used. Af... | J Med Chem 49: 4116-26 (2006) Article DOI: 10.1021/jm051272l BindingDB Entry DOI: 10.7270/Q21C1V64 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Suppressor of tumorigenicity 14 protein (Homo sapiens (Human)) | BDBM23914 (3-amidinophenylalanine deriv., 57 | 3-amino-N-(4-{...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 28 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Curacyte Chemistry GmbH | Assay Description The measurements were carried out on a microplate reader. Two concentrations of the substrate and five concentrations of the inhibitor were used. Af... | J Med Chem 49: 4116-26 (2006) Article DOI: 10.1021/jm051272l BindingDB Entry DOI: 10.7270/Q21C1V64 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Suppressor of tumorigenicity 14 protein (Homo sapiens (Human)) | BDBM23910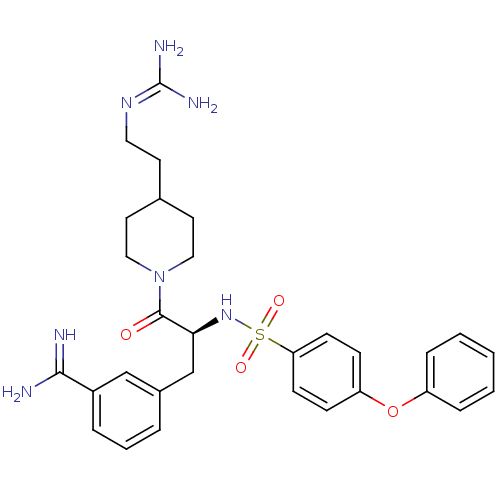 (3-[(2S)-3-[4-(2-carbamimidamidoethyl)piperidin-1-y...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 38 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Curacyte Chemistry GmbH | Assay Description The measurements were carried out on a microplate reader. Two concentrations of the substrate and five concentrations of the inhibitor were used. Af... | J Med Chem 49: 4116-26 (2006) Article DOI: 10.1021/jm051272l BindingDB Entry DOI: 10.7270/Q21C1V64 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (docked) | ||||||||||||
| Proprotein convertase subtilisin/kexin type 6 (Homo sapiens (Human)) | BDBM50303777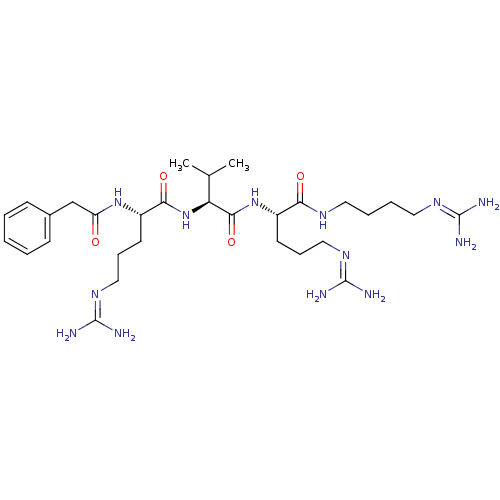 ((S)-5-guanidino-N-((S)-1-((S)-5-guanidino-1-(3-gua...) | Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 42 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Philipps University Marburg Curated by ChEMBL | Assay Description Inhibition of human PACE4 expressed in Drosophila schneider 2 cells by fluorescence assay | J Med Chem 53: 1067-75 (2010) Article DOI: 10.1021/jm9012455 BindingDB Entry DOI: 10.7270/Q2DN455Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Suppressor of tumorigenicity 14 protein (Homo sapiens (Human)) | BDBM23907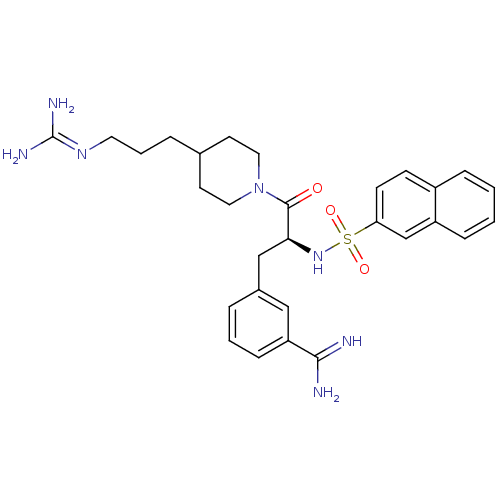 (3-[(2S)-3-[4-(3-carbamimidamidopropyl)piperidin-1-...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 42 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Curacyte Chemistry GmbH | Assay Description The measurements were carried out on a microplate reader. Two concentrations of the substrate and five concentrations of the inhibitor were used. Af... | J Med Chem 49: 4116-26 (2006) Article DOI: 10.1021/jm051272l BindingDB Entry DOI: 10.7270/Q21C1V64 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (docked) | ||||||||||||
| Suppressor of tumorigenicity 14 protein (Homo sapiens (Human)) | BDBM23877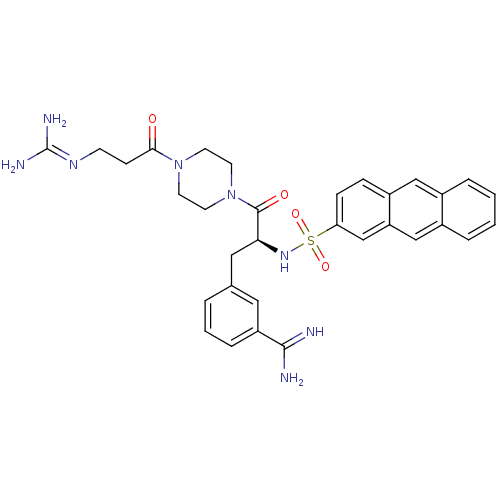 (3-[(2S)-2-(anthracene-2-sulfonamido)-3-[4-(3-carba...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | 44 | -42.0 | n/a | n/a | n/a | n/a | n/a | 8.0 | 25 |
Curacyte Chemistry GmbH | Assay Description The measurements were carried out on a microplate reader. Two concentrations of the substrate and five concentrations of the inhibitor were used. Af... | J Med Chem 49: 4116-26 (2006) Article DOI: 10.1021/jm051272l BindingDB Entry DOI: 10.7270/Q21C1V64 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (docked) | ||||||||||||
| Suppressor of tumorigenicity 14 protein (Homo sapiens (Human)) | BDBM23909 (3-amidinophenylalanine deriv., 52 | 4-{1-[(2S)-3-(...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 45 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Curacyte Chemistry GmbH | Assay Description The measurements were carried out on a microplate reader. Two concentrations of the substrate and five concentrations of the inhibitor were used. Af... | J Med Chem 49: 4116-26 (2006) Article DOI: 10.1021/jm051272l BindingDB Entry DOI: 10.7270/Q21C1V64 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (docked) | ||||||||||||
| Suppressor of tumorigenicity 14 protein (Homo sapiens (Human)) | BDBM23902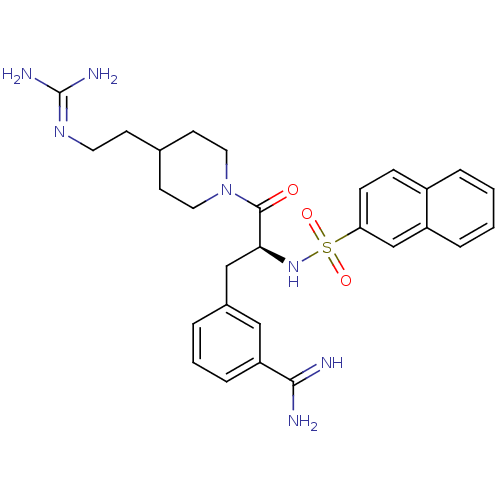 (3-[(2S)-3-[4-(2-carbamimidamidoethyl)piperidin-1-y...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | MMDB PC cid PC sid PDB UniChem Patents Similars | MMDB PDB Article PubMed | 46 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Curacyte Chemistry GmbH | Assay Description The measurements were carried out on a microplate reader. Two concentrations of the substrate and five concentrations of the inhibitor were used. Af... | J Med Chem 49: 4116-26 (2006) Article DOI: 10.1021/jm051272l BindingDB Entry DOI: 10.7270/Q21C1V64 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Suppressor of tumorigenicity 14 protein (Homo sapiens (Human)) | BDBM23890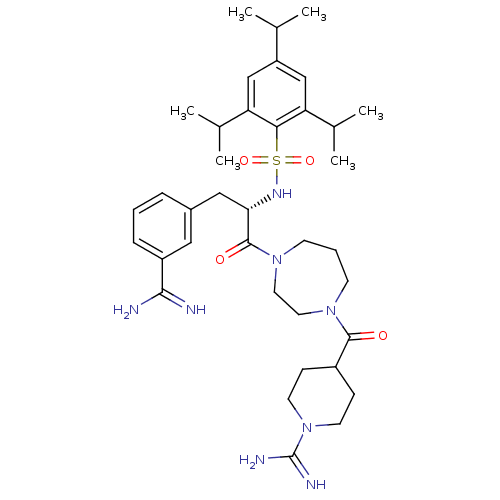 (3-amidinophenylalanine deriv., 34 | 4-({4-[(2S)-3-...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 50 | -41.7 | n/a | n/a | n/a | n/a | n/a | 8.0 | 25 |
Curacyte Chemistry GmbH | Assay Description The measurements were carried out on a microplate reader. Two concentrations of the substrate and five concentrations of the inhibitor were used. Af... | J Med Chem 49: 4116-26 (2006) Article DOI: 10.1021/jm051272l BindingDB Entry DOI: 10.7270/Q21C1V64 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Bos taurus (Bovine)) | BDBM23904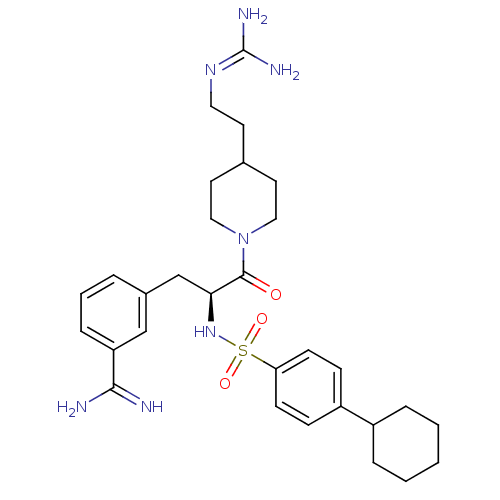 (3-[(2S)-3-[4-(2-carbamimidamidoethyl)piperidin-1-y...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 51 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Curacyte Chemistry GmbH | Assay Description The measurements were carried out on a microplate reader. Two concentrations of the substrate and five concentrations of the inhibitor were used. Af... | J Med Chem 49: 4116-26 (2006) Article DOI: 10.1021/jm051272l BindingDB Entry DOI: 10.7270/Q21C1V64 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuroendocrine convertase 1 (Homo sapiens (Human)) | BDBM50303777 ((S)-5-guanidino-N-((S)-1-((S)-5-guanidino-1-(3-gua...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 53 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Philipps University Marburg Curated by ChEMBL | Assay Description Inhibition of human PC1/3 expressed in Drosophila schneider 2 cells by fluorescence assay | J Med Chem 53: 1067-75 (2010) Article DOI: 10.1021/jm9012455 BindingDB Entry DOI: 10.7270/Q2DN455Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Suppressor of tumorigenicity 14 protein (Homo sapiens (Human)) | BDBM23876 (3-[(2S)-3-[4-(3-carbamimidamidopropanoyl)piperazin...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 53 | -41.5 | n/a | n/a | n/a | n/a | n/a | 8.0 | 25 |
Curacyte Chemistry GmbH | Assay Description The measurements were carried out on a microplate reader. Two concentrations of the substrate and five concentrations of the inhibitor were used. Af... | J Med Chem 49: 4116-26 (2006) Article DOI: 10.1021/jm051272l BindingDB Entry DOI: 10.7270/Q21C1V64 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Suppressor of tumorigenicity 14 protein (Homo sapiens (Human)) | BDBM23912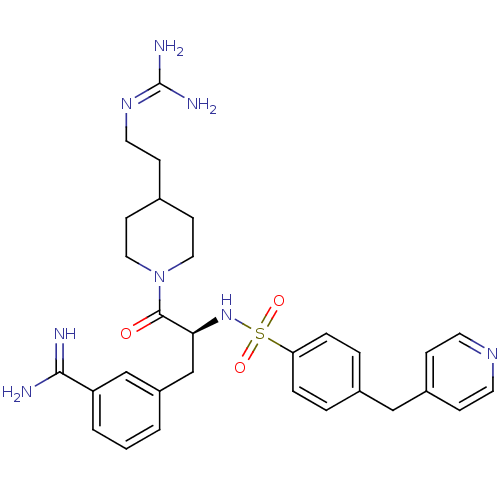 (3-[(2S)-3-[4-(2-carbamimidamidoethyl)piperidin-1-y...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 53 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Curacyte Chemistry GmbH | Assay Description The measurements were carried out on a microplate reader. Two concentrations of the substrate and five concentrations of the inhibitor were used. Af... | J Med Chem 49: 4116-26 (2006) Article DOI: 10.1021/jm051272l BindingDB Entry DOI: 10.7270/Q21C1V64 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (docked) | ||||||||||||
| Furin (Homo sapiens (Human)) | BDBM50303778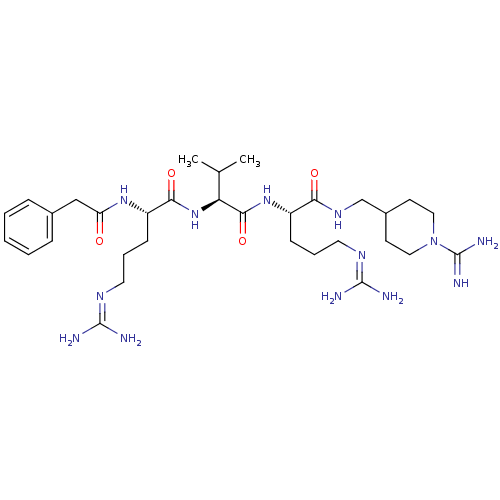 ((S)-N-((1-carbamimidoylpiperidin-4-yl)methyl)-5-gu...) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 53 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Philipps University Marburg Curated by ChEMBL | Assay Description Inhibition of human furin by fluorescence assay | J Med Chem 53: 1067-75 (2010) Article DOI: 10.1021/jm9012455 BindingDB Entry DOI: 10.7270/Q2DN455Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Bos taurus (Bovine)) | BDBM23892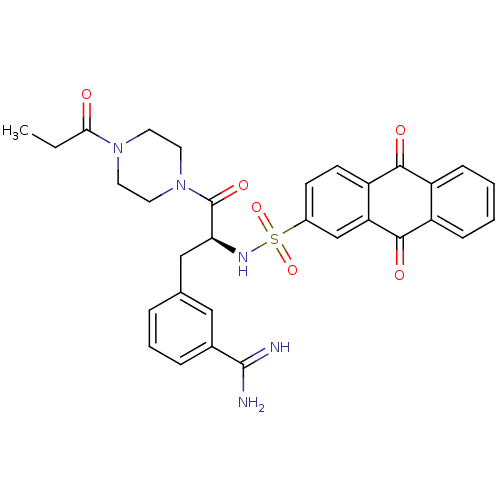 (3-[(2S)-2-[(9,10-dioxo-9,10-dihydroanthracene-2-)s...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 54 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Curacyte Chemistry GmbH | Assay Description The measurements were carried out on a microplate reader. Two concentrations of the substrate and five concentrations of the inhibitor were used. Af... | J Med Chem 49: 4116-26 (2006) Article DOI: 10.1021/jm051272l BindingDB Entry DOI: 10.7270/Q21C1V64 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuroendocrine convertase 2 (Homo sapiens (Human)) | BDBM50303776 (CHEMBL568525 | N-((6S,9S,12S)-1,17-diamino-6-(4-ca...) | Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 55 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Philipps University Marburg Curated by ChEMBL | Assay Description Inhibition of human PC2 expressed in Drosophila schneider 2 cells by fluorescence assay | J Med Chem 53: 1067-75 (2010) Article DOI: 10.1021/jm9012455 BindingDB Entry DOI: 10.7270/Q2DN455Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Suppressor of tumorigenicity 14 protein (Homo sapiens (Human)) | BDBM23867 (3-[(2S)-3-[4-(3-carbamimidamidopropanoyl)piperazin...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 57 | -41.3 | n/a | n/a | n/a | n/a | n/a | 8.0 | 25 |
Curacyte Chemistry GmbH | Assay Description The measurements were carried out on a microplate reader. Two concentrations of the substrate and five concentrations of the inhibitor were used. Af... | J Med Chem 49: 4116-26 (2006) Article DOI: 10.1021/jm051272l BindingDB Entry DOI: 10.7270/Q21C1V64 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Suppressor of tumorigenicity 14 protein (Homo sapiens (Human)) | BDBM23870 (3-[(2S)-3-[4-(4-carbamimidamidobutanoyl)piperazin-...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 61 | -41.2 | n/a | n/a | n/a | n/a | n/a | 8.0 | 25 |
Curacyte Chemistry GmbH | Assay Description The measurements were carried out on a microplate reader. Two concentrations of the substrate and five concentrations of the inhibitor were used. Af... | J Med Chem 49: 4116-26 (2006) Article DOI: 10.1021/jm051272l BindingDB Entry DOI: 10.7270/Q21C1V64 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Suppressor of tumorigenicity 14 protein (Homo sapiens (Human)) | BDBM23888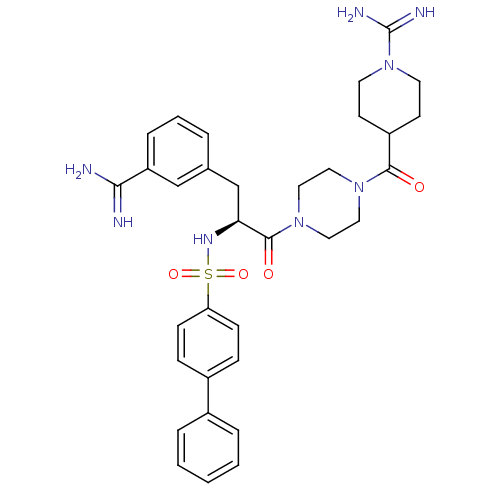 (3-amidinophenylalanine deriv., 32 | 4-({4-[(2S)-3-...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 61 | -41.2 | n/a | n/a | n/a | n/a | n/a | 8.0 | 25 |
Curacyte Chemistry GmbH | Assay Description The measurements were carried out on a microplate reader. Two concentrations of the substrate and five concentrations of the inhibitor were used. Af... | J Med Chem 49: 4116-26 (2006) Article DOI: 10.1021/jm051272l BindingDB Entry DOI: 10.7270/Q21C1V64 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Furin (Homo sapiens (Human)) | BDBM50303783 ((S)-5-guanidino-N-((S)-1-((S)-5-guanidino-1-(4-gua...) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 63 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Philipps University Marburg Curated by ChEMBL | Assay Description Inhibition of human furin by fluorescence assay | J Med Chem 53: 1067-75 (2010) Article DOI: 10.1021/jm9012455 BindingDB Entry DOI: 10.7270/Q2DN455Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Suppressor of tumorigenicity 14 protein (Homo sapiens (Human)) | BDBM23874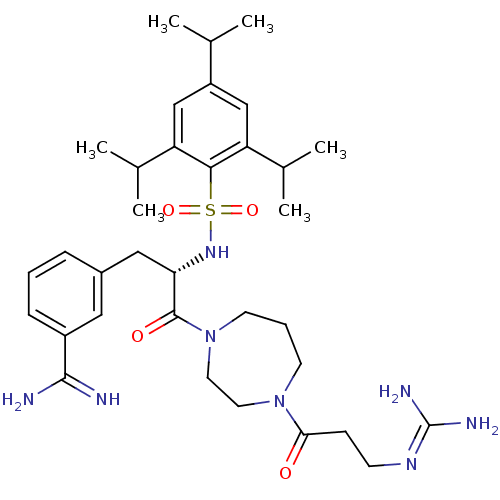 (3-[(2S)-3-[4-(3-carbamimidamidopropanoyl)-1,4-diaz...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 65 | -41.0 | n/a | n/a | n/a | n/a | n/a | 8.0 | 25 |
Curacyte Chemistry GmbH | Assay Description The measurements were carried out on a microplate reader. Two concentrations of the substrate and five concentrations of the inhibitor were used. Af... | J Med Chem 49: 4116-26 (2006) Article DOI: 10.1021/jm051272l BindingDB Entry DOI: 10.7270/Q21C1V64 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Proprotein convertase subtilisin/kexin type 6 (Homo sapiens (Human)) | BDBM50303778 ((S)-N-((1-carbamimidoylpiperidin-4-yl)methyl)-5-gu...) | Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 67 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Philipps University Marburg Curated by ChEMBL | Assay Description Inhibition of human PACE4 expressed in Drosophila schneider 2 cells by fluorescence assay | J Med Chem 53: 1067-75 (2010) Article DOI: 10.1021/jm9012455 BindingDB Entry DOI: 10.7270/Q2DN455Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuroendocrine convertase 1 (Homo sapiens (Human)) | BDBM50303778 ((S)-N-((1-carbamimidoylpiperidin-4-yl)methyl)-5-gu...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Philipps University Marburg Curated by ChEMBL | Assay Description Inhibition of human PC1/3 expressed in Drosophila schneider 2 cells by fluorescence assay | J Med Chem 53: 1067-75 (2010) Article DOI: 10.1021/jm9012455 BindingDB Entry DOI: 10.7270/Q2DN455Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Suppressor of tumorigenicity 14 protein (Homo sapiens (Human)) | BDBM23903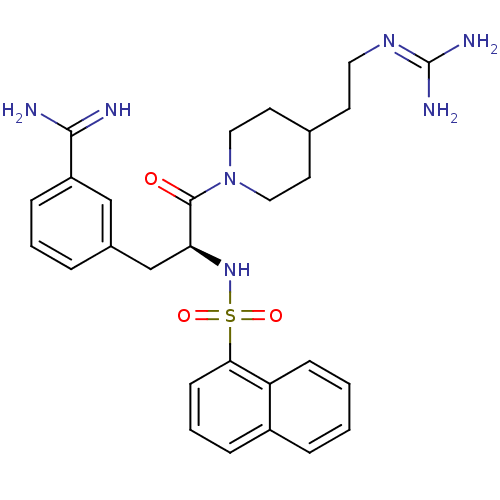 (3-[(2S)-3-[4-(2-carbamimidamidoethyl)piperidin-1-y...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 72 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Curacyte Chemistry GmbH | Assay Description The measurements were carried out on a microplate reader. Two concentrations of the substrate and five concentrations of the inhibitor were used. Af... | J Med Chem 49: 4116-26 (2006) Article DOI: 10.1021/jm051272l BindingDB Entry DOI: 10.7270/Q21C1V64 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (docked) | ||||||||||||
| Suppressor of tumorigenicity 14 protein (Homo sapiens (Human)) | BDBM23919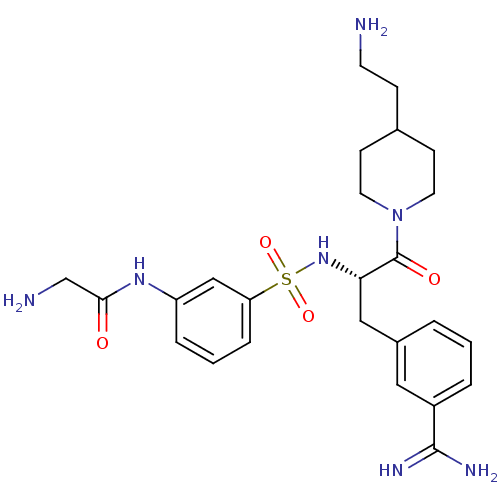 (2-amino-N-(3-{[(2S)-1-[4-(2-aminoethyl)piperidin-1...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 75 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Curacyte Chemistry GmbH | Assay Description The measurements were carried out on a microplate reader. Two concentrations of the substrate and five concentrations of the inhibitor were used. Af... | J Med Chem 49: 4116-26 (2006) Article DOI: 10.1021/jm051272l BindingDB Entry DOI: 10.7270/Q21C1V64 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Furin (Homo sapiens (Human)) | BDBM50303777 ((S)-5-guanidino-N-((S)-1-((S)-5-guanidino-1-(3-gua...) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 78 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Philipps University Marburg Curated by ChEMBL | Assay Description Inhibition of human furin by fluorescence assay | J Med Chem 53: 1067-75 (2010) Article DOI: 10.1021/jm9012455 BindingDB Entry DOI: 10.7270/Q2DN455Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 372 total ) | Next | Last >> |