 Found 2262 hits with Last Name = 'thoma' and Initial = 'g'
Found 2262 hits with Last Name = 'thoma' and Initial = 'g' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Glutamate carboxypeptidase 2
(Homo sapiens (Human)) | BDBM17659

((R,S)-2-phosphonomethylpentanedioic acid | 2-(phos...)Show InChI InChI=1S/C6H11O7P/c7-5(8)2-1-4(6(9)10)3-14(11,12)13/h4H,1-3H2,(H,7,8)(H,9,10)(H2,11,12,13) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
PubMed
| 0.200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Guilford Pharmaceuticals Inc.
Curated by ChEMBL
| Assay Description
Concentration of the compound required for the neuroprotective effect determined by inhibition of GCP II |
Bioorg Med Chem Lett 13: 2097-100 (2003)
BindingDB Entry DOI: 10.7270/Q2Z60NF2 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Beta-2 adrenergic receptor
(Homo sapiens (Human)) | BDBM50021888
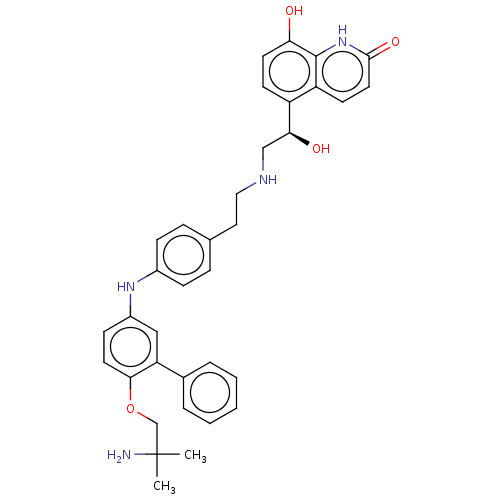
(CHEMBL3298987)Show SMILES CC(C)(N)COc1ccc(Nc2ccc(CCNC[C@H](O)c3ccc(O)c4[nH]c(=O)ccc34)cc2)cc1-c1ccccc1 |r| Show InChI InChI=1S/C35H38N4O4/c1-35(2,36)22-43-32-16-12-26(20-29(32)24-6-4-3-5-7-24)38-25-10-8-23(9-11-25)18-19-37-21-31(41)27-13-15-30(40)34-28(27)14-17-33(42)39-34/h3-17,20,31,37-38,40-41H,18-19,21-22,36H2,1-2H3,(H,39,42)/t31-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Theravance, Inc.
Curated by ChEMBL
| Assay Description
Displacement of [3H]dihydroalprenolol from human beta2 adrenergic receptor expressing cell membrane by competition binding assay |
Bioorg Med Chem Lett 24: 2871-6 (2014)
Article DOI: 10.1016/j.bmcl.2014.04.095
BindingDB Entry DOI: 10.7270/Q22J6DFT |
More data for this
Ligand-Target Pair | |
Beta-2 adrenergic receptor
(Homo sapiens (Human)) | BDBM50021866
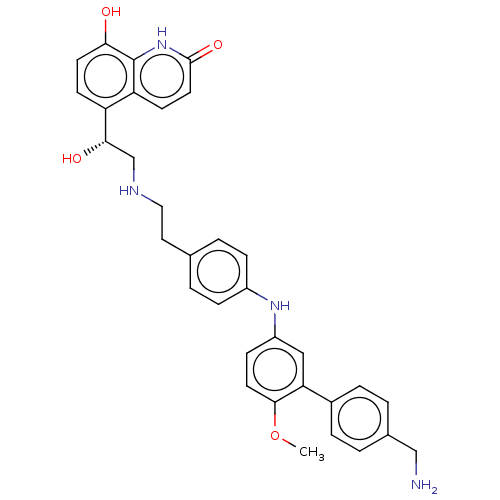
(CHEMBL3298326)Show SMILES COc1ccc(Nc2ccc(CCNC[C@H](O)c3ccc(O)c4[nH]c(=O)ccc34)cc2)cc1-c1ccc(CN)cc1 |r| Show InChI InChI=1S/C33H34N4O4/c1-41-31-14-10-25(18-28(31)23-6-2-22(19-34)3-7-23)36-24-8-4-21(5-9-24)16-17-35-20-30(39)26-11-13-29(38)33-27(26)12-15-32(40)37-33/h2-15,18,30,35-36,38-39H,16-17,19-20,34H2,1H3,(H,37,40)/t30-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Theravance, Inc.
Curated by ChEMBL
| Assay Description
Displacement of [3H]dihydroalprenolol from human beta2 adrenergic receptor expressing cell membrane by competition binding assay |
Bioorg Med Chem Lett 24: 2871-6 (2014)
Article DOI: 10.1016/j.bmcl.2014.04.095
BindingDB Entry DOI: 10.7270/Q22J6DFT |
More data for this
Ligand-Target Pair | |
Beta-2 adrenergic receptor
(Homo sapiens (Human)) | BDBM50021865

(CHEMBL3298325)Show SMILES COc1ccc(Nc2ccc(CCNC[C@H](O)c3ccc(O)c4[nH]c(=O)ccc34)cc2)cc1-c1cccc(CN)c1 |r| Show InChI InChI=1S/C33H34N4O4/c1-41-31-13-9-25(18-28(31)23-4-2-3-22(17-23)19-34)36-24-7-5-21(6-8-24)15-16-35-20-30(39)26-10-12-29(38)33-27(26)11-14-32(40)37-33/h2-14,17-18,30,35-36,38-39H,15-16,19-20,34H2,1H3,(H,37,40)/t30-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 1.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Theravance, Inc.
Curated by ChEMBL
| Assay Description
Displacement of [3H]dihydroalprenolol from human beta2 adrenergic receptor expressing cell membrane by competition binding assay |
Bioorg Med Chem Lett 24: 2871-6 (2014)
Article DOI: 10.1016/j.bmcl.2014.04.095
BindingDB Entry DOI: 10.7270/Q22J6DFT |
More data for this
Ligand-Target Pair | |
Bacterial leucyl aminopeptidase
(Vibrio proteolyticus) | BDBM50129200
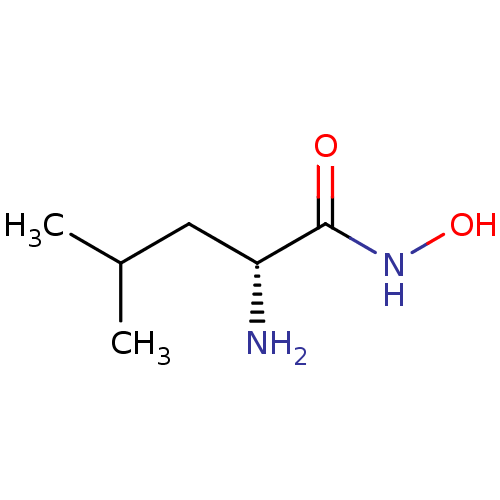
((R)-2-Amino-4-methyl-pentanoic acid hydroxyamide |...)Show InChI InChI=1S/C6H14N2O2/c1-4(2)3-5(7)6(9)8-10/h4-5,10H,3,7H2,1-2H3,(H,8,9)/t5-/m1/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Guilford Pharmaceuticals Inc.
Curated by ChEMBL
| Assay Description
Inhibition of metalloprotease from family M28, Aeromonas proteolytica aminopeptidase |
Bioorg Med Chem Lett 13: 2097-100 (2003)
BindingDB Entry DOI: 10.7270/Q2Z60NF2 |
More data for this
Ligand-Target Pair | |
Beta-2 adrenergic receptor
(Homo sapiens (Human)) | BDBM25771
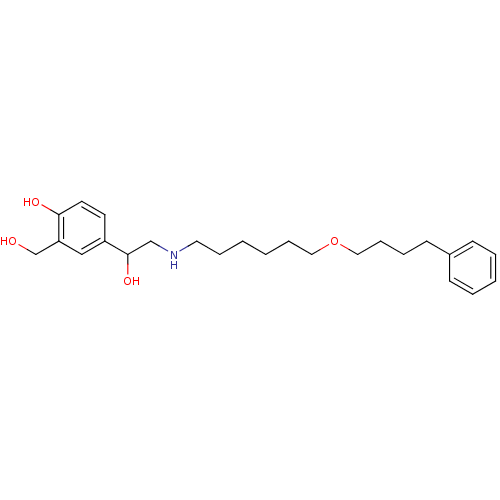
(1-hydroxy-2-naphthoic acid;4-[1-hydroxy-2-[6-(4-ph...)Show InChI InChI=1S/C25H37NO4/c27-20-23-18-22(13-14-24(23)28)25(29)19-26-15-7-1-2-8-16-30-17-9-6-12-21-10-4-3-5-11-21/h3-5,10-11,13-14,18,25-29H,1-2,6-9,12,15-17,19-20H2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
DrugBank
MCE
KEGG
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
PDB
Article
PubMed
| 2.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Theravance, Inc.
Curated by ChEMBL
| Assay Description
Displacement of [3H]dihydroalprenolol from beta2 receptor (unknown origin) by liquid scintillation counting and cell based assay |
Bioorg Med Chem Lett 24: 2625-30 (2014)
Article DOI: 10.1016/j.bmcl.2014.04.069
BindingDB Entry DOI: 10.7270/Q2M32X96 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Beta-2 adrenergic receptor
(Homo sapiens (Human)) | BDBM25771

(1-hydroxy-2-naphthoic acid;4-[1-hydroxy-2-[6-(4-ph...)Show InChI InChI=1S/C25H37NO4/c27-20-23-18-22(13-14-24(23)28)25(29)19-26-15-7-1-2-8-16-30-17-9-6-12-21-10-4-3-5-11-21/h3-5,10-11,13-14,18,25-29H,1-2,6-9,12,15-17,19-20H2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
DrugBank
MCE
KEGG
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
PDB
Article
PubMed
| 2.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Theravance, Inc.
Curated by ChEMBL
| Assay Description
Displacement of [3H]dihydroalprenolol from human beta2 adrenergic receptor expressing cell membrane by competition binding assay |
Bioorg Med Chem Lett 24: 2871-6 (2014)
Article DOI: 10.1016/j.bmcl.2014.04.095
BindingDB Entry DOI: 10.7270/Q22J6DFT |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Beta-2 adrenergic receptor
(Homo sapiens (Human)) | BDBM50021864

(CHEMBL3298324)Show SMILES COc1ccc(Nc2ccc(CCNC[C@H](O)c3ccc(O)c4[nH]c(=O)ccc34)cc2)cc1-c1ccccc1CN |r| Show InChI InChI=1S/C33H34N4O4/c1-41-31-14-10-24(18-28(31)25-5-3-2-4-22(25)19-34)36-23-8-6-21(7-9-23)16-17-35-20-30(39)26-11-13-29(38)33-27(26)12-15-32(40)37-33/h2-15,18,30,35-36,38-39H,16-17,19-20,34H2,1H3,(H,37,40)/t30-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 3.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Theravance, Inc.
Curated by ChEMBL
| Assay Description
Displacement of [3H]dihydroalprenolol from human beta2 adrenergic receptor expressing cell membrane by competition binding assay |
Bioorg Med Chem Lett 24: 2871-6 (2014)
Article DOI: 10.1016/j.bmcl.2014.04.095
BindingDB Entry DOI: 10.7270/Q22J6DFT |
More data for this
Ligand-Target Pair | |
Beta-2 adrenergic receptor
(Homo sapiens (Human)) | BDBM50419652

(MILVETEROL)Show SMILES O[C@@H](CNCCc1ccc(NC[C@H](O)c2ccccc2)cc1)c1ccc(O)c(NC=O)c1 |r| Show InChI InChI=1S/C25H29N3O4/c29-17-28-22-14-20(8-11-23(22)30)24(31)15-26-13-12-18-6-9-21(10-7-18)27-16-25(32)19-4-2-1-3-5-19/h1-11,14,17,24-27,30-32H,12-13,15-16H2,(H,28,29)/t24-,25-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
KEGG
PC cid
PC sid
UniChem
| Article
PubMed
| 3.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Theravance, Inc.
Curated by ChEMBL
| Assay Description
Displacement of [3H]dihydroalprenolol from beta2 receptor (unknown origin) by liquid scintillation counting and cell based assay |
Bioorg Med Chem Lett 24: 2625-30 (2014)
Article DOI: 10.1016/j.bmcl.2014.04.069
BindingDB Entry DOI: 10.7270/Q2M32X96 |
More data for this
Ligand-Target Pair | |
Beta-2 adrenergic receptor
(Homo sapiens (Human)) | BDBM50419652

(MILVETEROL)Show SMILES O[C@@H](CNCCc1ccc(NC[C@H](O)c2ccccc2)cc1)c1ccc(O)c(NC=O)c1 |r| Show InChI InChI=1S/C25H29N3O4/c29-17-28-22-14-20(8-11-23(22)30)24(31)15-26-13-12-18-6-9-21(10-7-18)27-16-25(32)19-4-2-1-3-5-19/h1-11,14,17,24-27,30-32H,12-13,15-16H2,(H,28,29)/t24-,25-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
KEGG
PC cid
PC sid
UniChem
| Article
PubMed
| 3.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Theravance, Inc.
Curated by ChEMBL
| Assay Description
Displacement of [3H]dihydroalprenolol from human beta2 adrenergic receptor expressing cell membrane by competition binding assay |
Bioorg Med Chem Lett 24: 2871-6 (2014)
Article DOI: 10.1016/j.bmcl.2014.04.095
BindingDB Entry DOI: 10.7270/Q22J6DFT |
More data for this
Ligand-Target Pair | |
Beta-2 adrenergic receptor
(Homo sapiens (Human)) | BDBM50021869
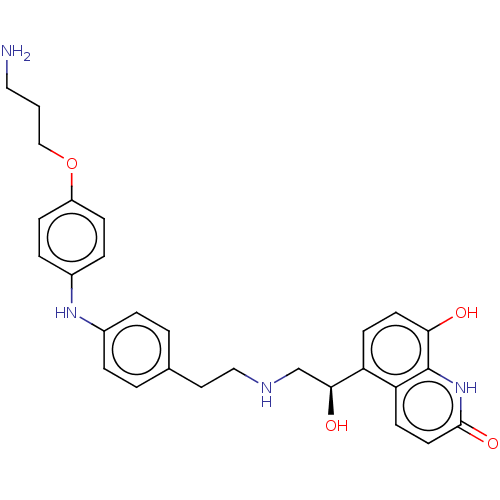
(CHEMBL3298329)Show SMILES NCCCOc1ccc(Nc2ccc(CCNC[C@H](O)c3ccc(O)c4[nH]c(=O)ccc34)cc2)cc1 |r| Show InChI InChI=1S/C28H32N4O4/c29-15-1-17-36-22-8-6-21(7-9-22)31-20-4-2-19(3-5-20)14-16-30-18-26(34)23-10-12-25(33)28-24(23)11-13-27(35)32-28/h2-13,26,30-31,33-34H,1,14-18,29H2,(H,32,35)/t26-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Theravance, Inc.
Curated by ChEMBL
| Assay Description
Displacement of [3H]dihydroalprenolol from human beta2 adrenergic receptor expressing cell membrane by competition binding assay |
Bioorg Med Chem Lett 24: 2871-6 (2014)
Article DOI: 10.1016/j.bmcl.2014.04.095
BindingDB Entry DOI: 10.7270/Q22J6DFT |
More data for this
Ligand-Target Pair | |
Beta-2 adrenergic receptor
(Homo sapiens (Human)) | BDBM50021873

(CHEMBL3298330)Show SMILES NCCCCOc1ccc(Nc2ccc(CCNC[C@H](O)c3ccc(O)c4[nH]c(=O)ccc34)cc2)cc1 |r| Show InChI InChI=1S/C29H34N4O4/c30-16-1-2-18-37-23-9-7-22(8-10-23)32-21-5-3-20(4-6-21)15-17-31-19-27(35)24-11-13-26(34)29-25(24)12-14-28(36)33-29/h3-14,27,31-32,34-35H,1-2,15-19,30H2,(H,33,36)/t27-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Theravance, Inc.
Curated by ChEMBL
| Assay Description
Displacement of [3H]dihydroalprenolol from human beta2 adrenergic receptor expressing cell membrane by competition binding assay |
Bioorg Med Chem Lett 24: 2871-6 (2014)
Article DOI: 10.1016/j.bmcl.2014.04.095
BindingDB Entry DOI: 10.7270/Q22J6DFT |
More data for this
Ligand-Target Pair | |
Beta-2 adrenergic receptor
(Homo sapiens (Human)) | BDBM50018589
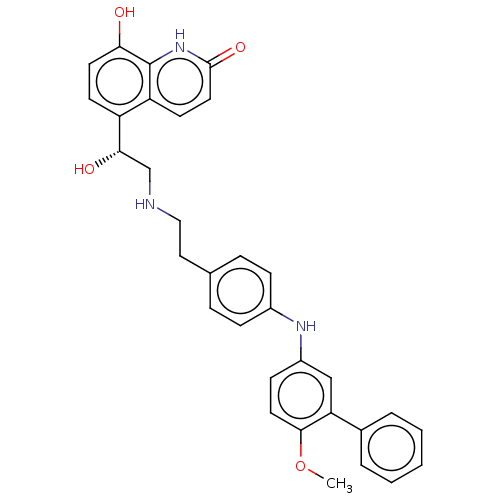
(CHEMBL3290999)Show SMILES COc1ccc(Nc2ccc(CCNC[C@H](O)c3ccc(O)c4[nH]c(=O)ccc34)cc2)cc1-c1ccccc1 |r| Show InChI InChI=1S/C32H31N3O4/c1-39-30-15-11-24(19-27(30)22-5-3-2-4-6-22)34-23-9-7-21(8-10-23)17-18-33-20-29(37)25-12-14-28(36)32-26(25)13-16-31(38)35-32/h2-16,19,29,33-34,36-37H,17-18,20H2,1H3,(H,35,38)/t29-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 6.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Theravance, Inc.
Curated by ChEMBL
| Assay Description
Displacement of [3H]dihydroalprenolol from human beta2 adrenergic receptor expressing cell membrane by competition binding assay |
Bioorg Med Chem Lett 24: 2871-6 (2014)
Article DOI: 10.1016/j.bmcl.2014.04.095
BindingDB Entry DOI: 10.7270/Q22J6DFT |
More data for this
Ligand-Target Pair | |
Beta-2 adrenergic receptor
(Homo sapiens (Human)) | BDBM50021868
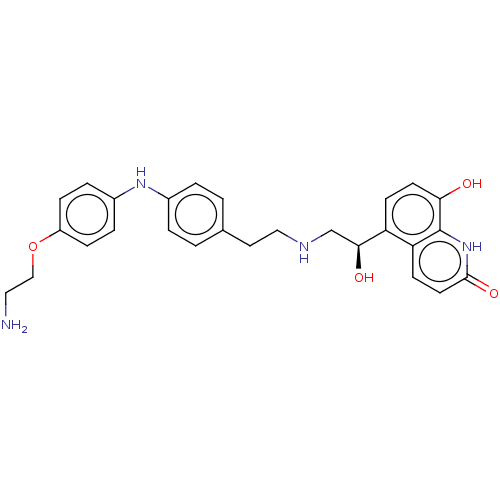
(CHEMBL3298328)Show SMILES NCCOc1ccc(Nc2ccc(CCNC[C@H](O)c3ccc(O)c4[nH]c(=O)ccc34)cc2)cc1 |r| Show InChI InChI=1S/C27H30N4O4/c28-14-16-35-21-7-5-20(6-8-21)30-19-3-1-18(2-4-19)13-15-29-17-25(33)22-9-11-24(32)27-23(22)10-12-26(34)31-27/h1-12,25,29-30,32-33H,13-17,28H2,(H,31,34)/t25-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 6.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Theravance, Inc.
Curated by ChEMBL
| Assay Description
Displacement of [3H]dihydroalprenolol from human beta2 adrenergic receptor expressing cell membrane by competition binding assay |
Bioorg Med Chem Lett 24: 2871-6 (2014)
Article DOI: 10.1016/j.bmcl.2014.04.095
BindingDB Entry DOI: 10.7270/Q22J6DFT |
More data for this
Ligand-Target Pair | |
Beta-2 adrenergic receptor
(Homo sapiens (Human)) | BDBM50021885
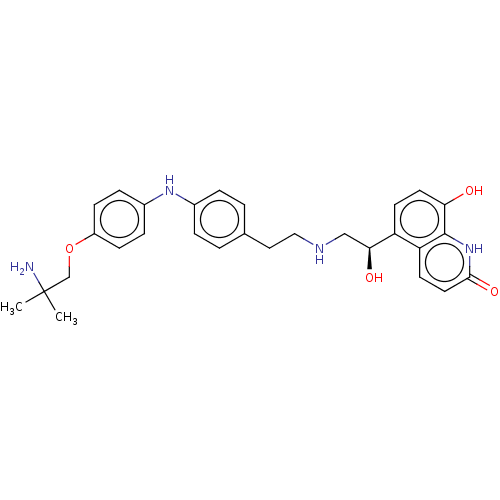
(CHEMBL3298986)Show SMILES CC(C)(N)COc1ccc(Nc2ccc(CCNC[C@H](O)c3ccc(O)c4[nH]c(=O)ccc34)cc2)cc1 |r| Show InChI InChI=1S/C29H34N4O4/c1-29(2,30)18-37-22-9-7-21(8-10-22)32-20-5-3-19(4-6-20)15-16-31-17-26(35)23-11-13-25(34)28-24(23)12-14-27(36)33-28/h3-14,26,31-32,34-35H,15-18,30H2,1-2H3,(H,33,36)/t26-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 6.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Theravance, Inc.
Curated by ChEMBL
| Assay Description
Displacement of [3H]dihydroalprenolol from human beta2 adrenergic receptor expressing cell membrane by competition binding assay |
Bioorg Med Chem Lett 24: 2871-6 (2014)
Article DOI: 10.1016/j.bmcl.2014.04.095
BindingDB Entry DOI: 10.7270/Q22J6DFT |
More data for this
Ligand-Target Pair | |
Beta-2 adrenergic receptor
(Homo sapiens (Human)) | BDBM50021875
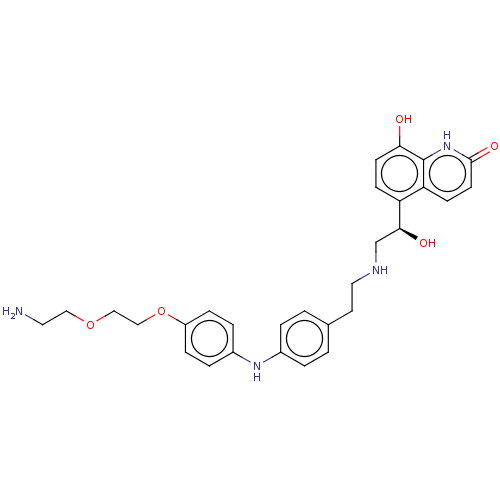
(CHEMBL3298691)Show SMILES NCCOCCOc1ccc(Nc2ccc(CCNC[C@H](O)c3ccc(O)c4[nH]c(=O)ccc34)cc2)cc1 |r| Show InChI InChI=1S/C29H34N4O5/c30-14-16-37-17-18-38-23-7-5-22(6-8-23)32-21-3-1-20(2-4-21)13-15-31-19-27(35)24-9-11-26(34)29-25(24)10-12-28(36)33-29/h1-12,27,31-32,34-35H,13-19,30H2,(H,33,36)/t27-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 6.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Theravance, Inc.
Curated by ChEMBL
| Assay Description
Displacement of [3H]dihydroalprenolol from human beta2 adrenergic receptor expressing cell membrane by competition binding assay |
Bioorg Med Chem Lett 24: 2871-6 (2014)
Article DOI: 10.1016/j.bmcl.2014.04.095
BindingDB Entry DOI: 10.7270/Q22J6DFT |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase PLK1
(Homo sapiens (Human)) | BDBM181016

(US9133199, I-253)Show SMILES COc1cc(ccc1Nc1ncc2N(C)C(=O)C3(CC3)CN(C3CCCC3)c2n1)C(=O)NN1CCCC1 Show InChI InChI=1S/C27H35N7O3/c1-32-21-16-28-26(30-23(21)34(19-7-3-4-8-19)17-27(11-12-27)25(32)36)29-20-10-9-18(15-22(20)37-2)24(35)31-33-13-5-6-14-33/h9-10,15-16,19H,3-8,11-14,17H2,1-2H3,(H,31,35)(H,28,29,30) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| <10 | n/a | n/a | n/a | n/a | n/a | n/a | 7.5 | n/a |
Cyclacel Limited
US Patent
| Assay Description
CDC25C (2 ug/well) with PLKl (1 ug/well) in 20 mM Tris/HCl buffer pH 7.5, supplemented with 25 mM beta-glycerophosphate, 5 mM EGTA, 1 mM DTT and 1 mM... |
US Patent US9133199 (2015)
BindingDB Entry DOI: 10.7270/Q21C1VNZ |
More data for this
Ligand-Target Pair | |
Beta-2 adrenergic receptor
(Homo sapiens (Human)) | BDBM50021881
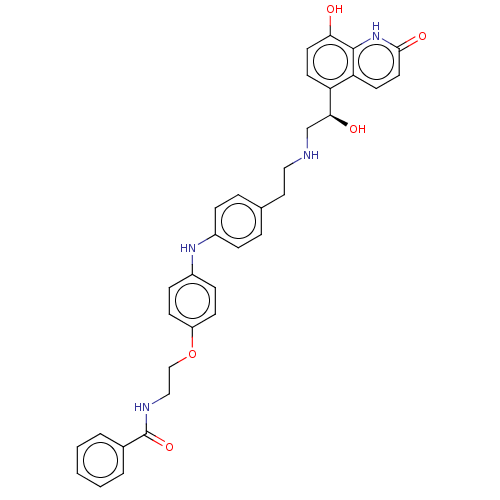
(CHEMBL3298762)Show SMILES O[C@@H](CNCCc1ccc(Nc2ccc(OCCNC(=O)c3ccccc3)cc2)cc1)c1ccc(O)c2[nH]c(=O)ccc12 |r| Show InChI InChI=1S/C34H34N4O5/c39-30-16-14-28(29-15-17-32(41)38-33(29)30)31(40)22-35-19-18-23-6-8-25(9-7-23)37-26-10-12-27(13-11-26)43-21-20-36-34(42)24-4-2-1-3-5-24/h1-17,31,35,37,39-40H,18-22H2,(H,36,42)(H,38,41)/t31-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Theravance, Inc.
Curated by ChEMBL
| Assay Description
Displacement of [3H]dihydroalprenolol from human beta2 adrenergic receptor expressing cell membrane by competition binding assay |
Bioorg Med Chem Lett 24: 2871-6 (2014)
Article DOI: 10.1016/j.bmcl.2014.04.095
BindingDB Entry DOI: 10.7270/Q22J6DFT |
More data for this
Ligand-Target Pair | |
Beta-2 adrenergic receptor
(Homo sapiens (Human)) | BDBM50021884
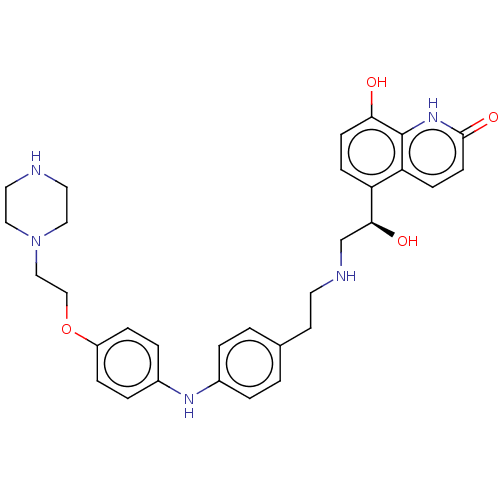
(CHEMBL3298897)Show SMILES O[C@@H](CNCCc1ccc(Nc2ccc(OCCN3CCNCC3)cc2)cc1)c1ccc(O)c2[nH]c(=O)ccc12 |r| Show InChI InChI=1S/C31H37N5O4/c37-28-11-9-26(27-10-12-30(39)35-31(27)28)29(38)21-33-14-13-22-1-3-23(4-2-22)34-24-5-7-25(8-6-24)40-20-19-36-17-15-32-16-18-36/h1-12,29,32-34,37-38H,13-21H2,(H,35,39)/t29-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Theravance, Inc.
Curated by ChEMBL
| Assay Description
Displacement of [3H]dihydroalprenolol from human beta2 adrenergic receptor expressing cell membrane by competition binding assay |
Bioorg Med Chem Lett 24: 2871-6 (2014)
Article DOI: 10.1016/j.bmcl.2014.04.095
BindingDB Entry DOI: 10.7270/Q22J6DFT |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase PLK1
(Homo sapiens (Human)) | BDBM181015

(US9133199, 254)Show SMILES COc1cc(ccc1Nc1ncc2N(C)C(=O)C3(CC3)CN(C3CCCC3)c2n1)C(=O)NN1CCN(C)CC1 Show InChI InChI=1S/C28H38N8O3/c1-33-12-14-35(15-13-33)32-25(37)19-8-9-21(23(16-19)39-3)30-27-29-17-22-24(31-27)36(20-6-4-5-7-20)18-28(10-11-28)26(38)34(22)2/h8-9,16-17,20H,4-7,10-15,18H2,1-3H3,(H,32,37)(H,29,30,31) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| <10 | n/a | n/a | n/a | n/a | n/a | n/a | 7.5 | n/a |
Cyclacel Limited
US Patent
| Assay Description
CDC25C (2 ug/well) with PLKl (1 ug/well) in 20 mM Tris/HCl buffer pH 7.5, supplemented with 25 mM beta-glycerophosphate, 5 mM EGTA, 1 mM DTT and 1 mM... |
US Patent US9133199 (2015)
BindingDB Entry DOI: 10.7270/Q21C1VNZ |
More data for this
Ligand-Target Pair | |
Beta-2 adrenergic receptor
(Homo sapiens (Human)) | BDBM50021867
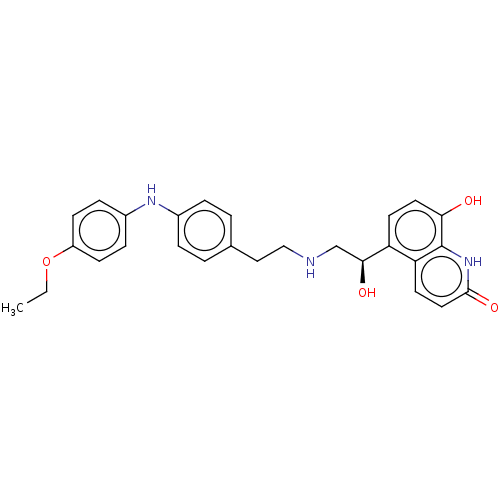
(CHEMBL3298327)Show SMILES CCOc1ccc(Nc2ccc(CCNC[C@H](O)c3ccc(O)c4[nH]c(=O)ccc34)cc2)cc1 |r| Show InChI InChI=1S/C27H29N3O4/c1-2-34-21-9-7-20(8-10-21)29-19-5-3-18(4-6-19)15-16-28-17-25(32)22-11-13-24(31)27-23(22)12-14-26(33)30-27/h3-14,25,28-29,31-32H,2,15-17H2,1H3,(H,30,33)/t25-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 13 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Theravance, Inc.
Curated by ChEMBL
| Assay Description
Displacement of [3H]dihydroalprenolol from human beta2 adrenergic receptor expressing cell membrane by competition binding assay |
Bioorg Med Chem Lett 24: 2871-6 (2014)
Article DOI: 10.1016/j.bmcl.2014.04.095
BindingDB Entry DOI: 10.7270/Q22J6DFT |
More data for this
Ligand-Target Pair | |
Beta-2 adrenergic receptor
(Homo sapiens (Human)) | BDBM50021877
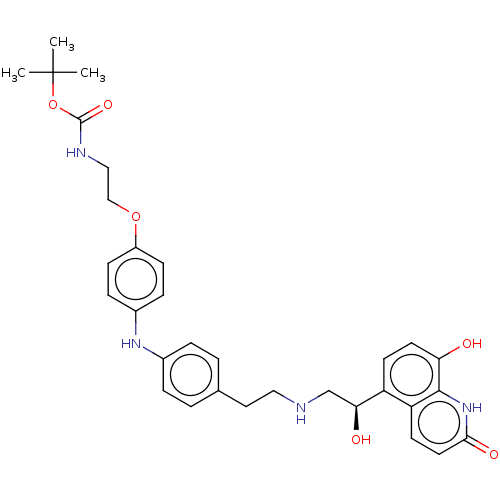
(CHEMBL3298692)Show SMILES CC(C)(C)OC(=O)NCCOc1ccc(Nc2ccc(CCNC[C@H](O)c3ccc(O)c4[nH]c(=O)ccc34)cc2)cc1 |r| Show InChI InChI=1S/C32H38N4O6/c1-32(2,3)42-31(40)34-18-19-41-24-10-8-23(9-11-24)35-22-6-4-21(5-7-22)16-17-33-20-28(38)25-12-14-27(37)30-26(25)13-15-29(39)36-30/h4-15,28,33,35,37-38H,16-20H2,1-3H3,(H,34,40)(H,36,39)/t28-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Theravance, Inc.
Curated by ChEMBL
| Assay Description
Displacement of [3H]dihydroalprenolol from human beta2 adrenergic receptor expressing cell membrane by competition binding assay |
Bioorg Med Chem Lett 24: 2871-6 (2014)
Article DOI: 10.1016/j.bmcl.2014.04.095
BindingDB Entry DOI: 10.7270/Q22J6DFT |
More data for this
Ligand-Target Pair | |
Beta-2 adrenergic receptor
(Homo sapiens (Human)) | BDBM50021882
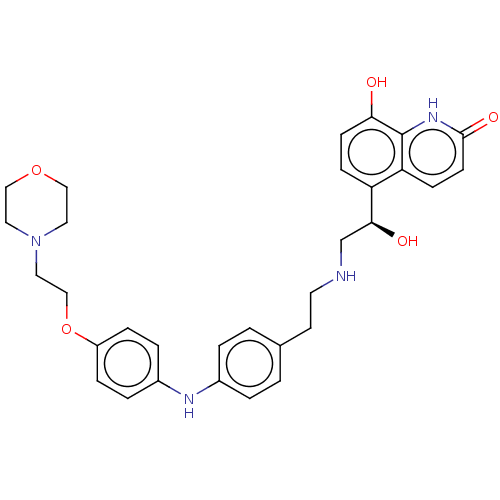
(CHEMBL3298831)Show SMILES O[C@@H](CNCCc1ccc(Nc2ccc(OCCN3CCOCC3)cc2)cc1)c1ccc(O)c2[nH]c(=O)ccc12 |r| Show InChI InChI=1S/C31H36N4O5/c36-28-11-9-26(27-10-12-30(38)34-31(27)28)29(37)21-32-14-13-22-1-3-23(4-2-22)33-24-5-7-25(8-6-24)40-20-17-35-15-18-39-19-16-35/h1-12,29,32-33,36-37H,13-21H2,(H,34,38)/t29-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Theravance, Inc.
Curated by ChEMBL
| Assay Description
Displacement of [3H]dihydroalprenolol from human beta2 adrenergic receptor expressing cell membrane by competition binding assay |
Bioorg Med Chem Lett 24: 2871-6 (2014)
Article DOI: 10.1016/j.bmcl.2014.04.095
BindingDB Entry DOI: 10.7270/Q22J6DFT |
More data for this
Ligand-Target Pair | |
Beta-2 adrenergic receptor
(Homo sapiens (Human)) | BDBM50021889
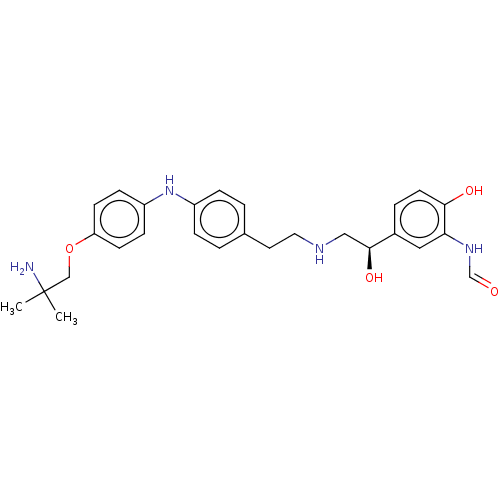
(CHEMBL3298213)Show SMILES CC(C)(N)COc1ccc(Nc2ccc(CCNC[C@H](O)c3ccc(O)c(NC=O)c3)cc2)cc1 |r| Show InChI InChI=1S/C27H34N4O4/c1-27(2,28)17-35-23-10-8-22(9-11-23)31-21-6-3-19(4-7-21)13-14-29-16-26(34)20-5-12-25(33)24(15-20)30-18-32/h3-12,15,18,26,29,31,33-34H,13-14,16-17,28H2,1-2H3,(H,30,32)/t26-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 25 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Theravance, Inc.
Curated by ChEMBL
| Assay Description
Displacement of [3H]dihydroalprenolol from human beta2 adrenergic receptor expressing cell membrane by competition binding assay |
Bioorg Med Chem Lett 24: 2871-6 (2014)
Article DOI: 10.1016/j.bmcl.2014.04.095
BindingDB Entry DOI: 10.7270/Q22J6DFT |
More data for this
Ligand-Target Pair | |
Beta-2 adrenergic receptor
(Homo sapiens (Human)) | BDBM50021883
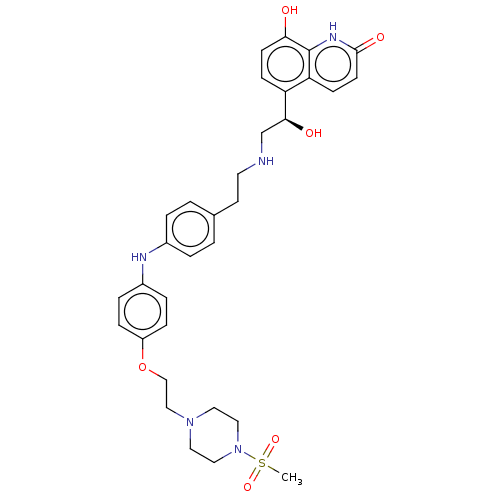
(CHEMBL3298832)Show SMILES CS(=O)(=O)N1CCN(CCOc2ccc(Nc3ccc(CCNC[C@H](O)c4ccc(O)c5[nH]c(=O)ccc45)cc3)cc2)CC1 |r| Show InChI InChI=1S/C32H39N5O6S/c1-44(41,42)37-18-16-36(17-19-37)20-21-43-26-8-6-25(7-9-26)34-24-4-2-23(3-5-24)14-15-33-22-30(39)27-10-12-29(38)32-28(27)11-13-31(40)35-32/h2-13,30,33-34,38-39H,14-22H2,1H3,(H,35,40)/t30-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 32 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Theravance, Inc.
Curated by ChEMBL
| Assay Description
Displacement of [3H]dihydroalprenolol from human beta2 adrenergic receptor expressing cell membrane by competition binding assay |
Bioorg Med Chem Lett 24: 2871-6 (2014)
Article DOI: 10.1016/j.bmcl.2014.04.095
BindingDB Entry DOI: 10.7270/Q22J6DFT |
More data for this
Ligand-Target Pair | |
Beta-2 adrenergic receptor
(Homo sapiens (Human)) | BDBM86453

(CAS_73573-87-2 | Formoterol | NSC_3083544)Show InChI InChI=1S/C19H24N2O4/c1-13(9-14-3-6-16(25-2)7-4-14)20-11-19(24)15-5-8-18(23)17(10-15)21-12-22/h3-8,10,12-13,19-20,23-24H,9,11H2,1-2H3,(H,21,22) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
DrugBank
KEGG
PC cid
PC sid
UniChem
Patents
Similars
| DrugBank
Article
PubMed
| 32 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Theravance, Inc.
Curated by ChEMBL
| Assay Description
Displacement of [3H]dihydroalprenolol from human beta2 adrenergic receptor expressing cell membrane by competition binding assay |
Bioorg Med Chem Lett 24: 2871-6 (2014)
Article DOI: 10.1016/j.bmcl.2014.04.095
BindingDB Entry DOI: 10.7270/Q22J6DFT |
More data for this
Ligand-Target Pair | |
Beta-2 adrenergic receptor
(Homo sapiens (Human)) | BDBM86453

(CAS_73573-87-2 | Formoterol | NSC_3083544)Show InChI InChI=1S/C19H24N2O4/c1-13(9-14-3-6-16(25-2)7-4-14)20-11-19(24)15-5-8-18(23)17(10-15)21-12-22/h3-8,10,12-13,19-20,23-24H,9,11H2,1-2H3,(H,21,22) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
DrugBank
KEGG
PC cid
PC sid
UniChem
Patents
Similars
| DrugBank
Article
PubMed
| 32 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Theravance, Inc.
Curated by ChEMBL
| Assay Description
Displacement of [3H]dihydroalprenolol from beta2 receptor (unknown origin) by liquid scintillation counting and cell based assay |
Bioorg Med Chem Lett 24: 2625-30 (2014)
Article DOI: 10.1016/j.bmcl.2014.04.069
BindingDB Entry DOI: 10.7270/Q2M32X96 |
More data for this
Ligand-Target Pair | |
D-amino-acid oxidase
(Homo sapiens (Human)) | BDBM50117763
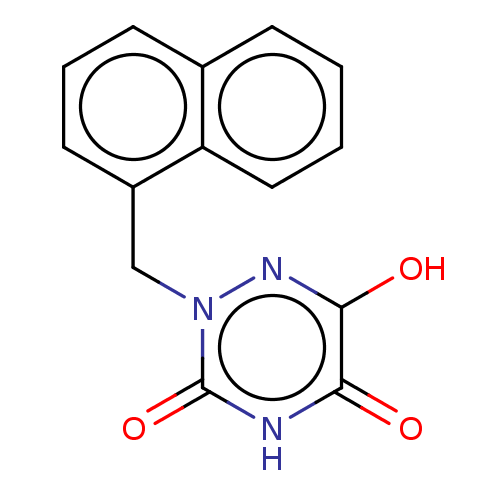
(CHEMBL3613921 | US9505753, 5u)Show InChI InChI=1S/C14H11N3O3/c18-12-13(19)16-17(14(20)15-12)8-10-6-3-5-9-4-1-2-7-11(9)10/h1-7H,8H2,(H,16,19)(H,15,18,20) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johns Hopkins University
Curated by ChEMBL
| Assay Description
Competitive inhibition of recombinant human DAAO expressed in HEK cells by double reciprocal plot analysis in presence of D-serine |
J Med Chem 58: 7258-72 (2015)
Article DOI: 10.1021/acs.jmedchem.5b00482
BindingDB Entry DOI: 10.7270/Q2SF2XZQ |
More data for this
Ligand-Target Pair | |
Beta-2 adrenergic receptor
(Homo sapiens (Human)) | BDBM50018608
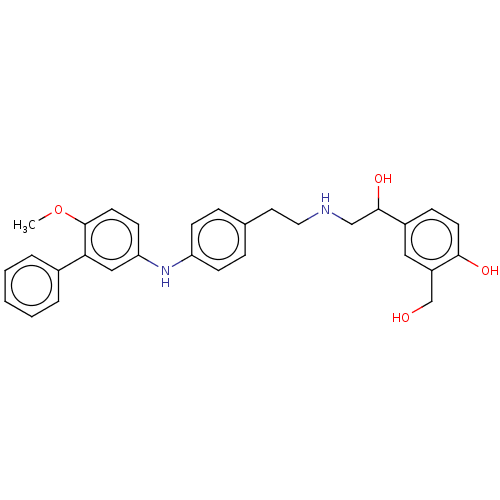
(CHEMBL3290989)Show SMILES COc1ccc(Nc2ccc(CCNCC(O)c3ccc(O)c(CO)c3)cc2)cc1-c1ccccc1 Show InChI InChI=1S/C30H32N2O4/c1-36-30-14-12-26(18-27(30)22-5-3-2-4-6-22)32-25-10-7-21(8-11-25)15-16-31-19-29(35)23-9-13-28(34)24(17-23)20-33/h2-14,17-18,29,31-35H,15-16,19-20H2,1H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 63 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Theravance, Inc.
Curated by ChEMBL
| Assay Description
Displacement of [3H]dihydroalprenolol from beta2 receptor (unknown origin) by liquid scintillation counting and cell based assay |
Bioorg Med Chem Lett 24: 2625-30 (2014)
Article DOI: 10.1016/j.bmcl.2014.04.069
BindingDB Entry DOI: 10.7270/Q2M32X96 |
More data for this
Ligand-Target Pair | |
Beta-2 adrenergic receptor
(Homo sapiens (Human)) | BDBM50018557
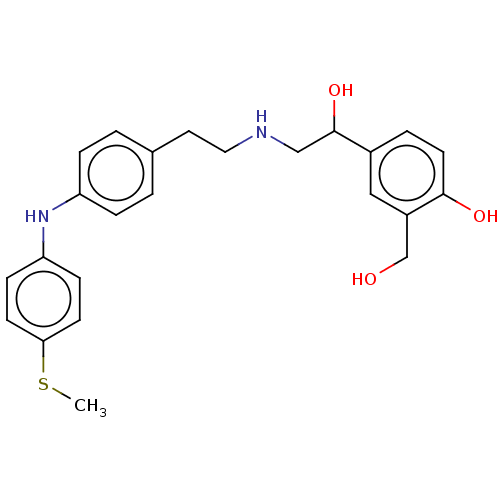
(CHEMBL3290992)Show SMILES CSc1ccc(Nc2ccc(CCNCC(O)c3ccc(O)c(CO)c3)cc2)cc1 Show InChI InChI=1S/C24H28N2O3S/c1-30-22-9-7-21(8-10-22)26-20-5-2-17(3-6-20)12-13-25-15-24(29)18-4-11-23(28)19(14-18)16-27/h2-11,14,24-29H,12-13,15-16H2,1H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 79 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Theravance, Inc.
Curated by ChEMBL
| Assay Description
Displacement of [3H]dihydroalprenolol from beta2 receptor (unknown origin) by liquid scintillation counting and cell based assay |
Bioorg Med Chem Lett 24: 2625-30 (2014)
Article DOI: 10.1016/j.bmcl.2014.04.069
BindingDB Entry DOI: 10.7270/Q2M32X96 |
More data for this
Ligand-Target Pair | |
3-phosphoshikimate 1-carboxyvinyltransferase
(Escherichia coli (strain K12)) | BDBM50123088
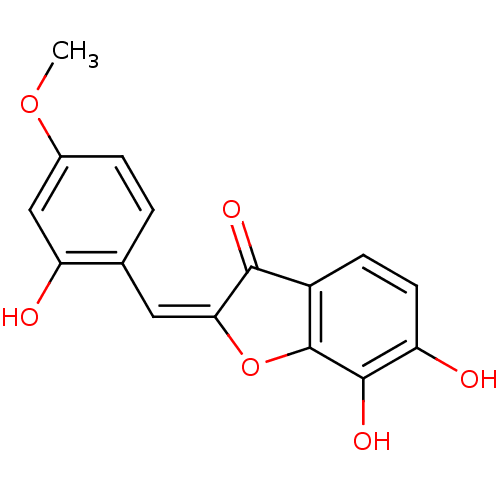
(6,7-Dihydroxy-2-[1-(2-hydroxy-4-methoxy-phenyl)-me...)Show InChI InChI=1S/C16H12O6/c1-21-9-3-2-8(12(18)7-9)6-13-14(19)10-4-5-11(17)15(20)16(10)22-13/h2-7,17-18,20H,1H3/b13-6+ | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
PanTherix Ltd.
Curated by ChEMBL
| Assay Description
Competitive inhibitory activity of the compound was determined with respect to EPSP (5-enolpyruvylshikimate- 3-phosphate) synthase |
Bioorg Med Chem Lett 13: 423-6 (2003)
BindingDB Entry DOI: 10.7270/Q2TH8M2M |
More data for this
Ligand-Target Pair | |
Beta-2 adrenergic receptor
(Homo sapiens (Human)) | BDBM50018558
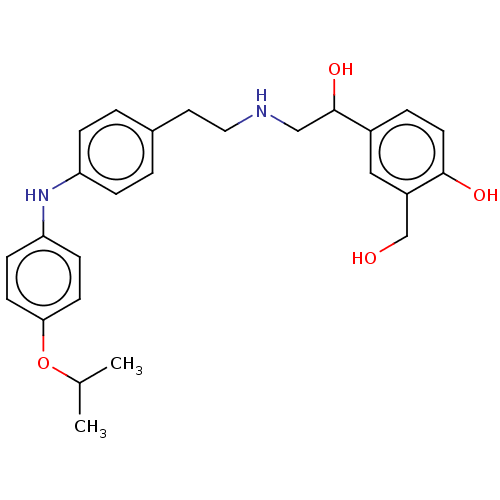
(CHEMBL3290993)Show SMILES CC(C)Oc1ccc(Nc2ccc(CCNCC(O)c3ccc(O)c(CO)c3)cc2)cc1 Show InChI InChI=1S/C26H32N2O4/c1-18(2)32-24-10-8-23(9-11-24)28-22-6-3-19(4-7-22)13-14-27-16-26(31)20-5-12-25(30)21(15-20)17-29/h3-12,15,18,26-31H,13-14,16-17H2,1-2H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Theravance, Inc.
Curated by ChEMBL
| Assay Description
Displacement of [3H]dihydroalprenolol from beta2 receptor (unknown origin) by liquid scintillation counting and cell based assay |
Bioorg Med Chem Lett 24: 2625-30 (2014)
Article DOI: 10.1016/j.bmcl.2014.04.069
BindingDB Entry DOI: 10.7270/Q2M32X96 |
More data for this
Ligand-Target Pair | |
Beta-2 adrenergic receptor
(Homo sapiens (Human)) | BDBM50018563
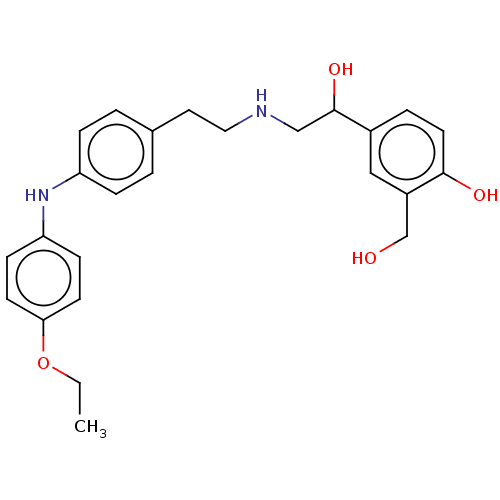
(CHEMBL3290991)Show SMILES CCOc1ccc(Nc2ccc(CCNCC(O)c3ccc(O)c(CO)c3)cc2)cc1 Show InChI InChI=1S/C25H30N2O4/c1-2-31-23-10-8-22(9-11-23)27-21-6-3-18(4-7-21)13-14-26-16-25(30)19-5-12-24(29)20(15-19)17-28/h3-12,15,25-30H,2,13-14,16-17H2,1H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 158 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Theravance, Inc.
Curated by ChEMBL
| Assay Description
Displacement of [3H]dihydroalprenolol from beta2 receptor (unknown origin) by liquid scintillation counting and cell based assay |
Bioorg Med Chem Lett 24: 2625-30 (2014)
Article DOI: 10.1016/j.bmcl.2014.04.069
BindingDB Entry DOI: 10.7270/Q2M32X96 |
More data for this
Ligand-Target Pair | |
Beta-2 adrenergic receptor
(Homo sapiens (Human)) | BDBM50018562
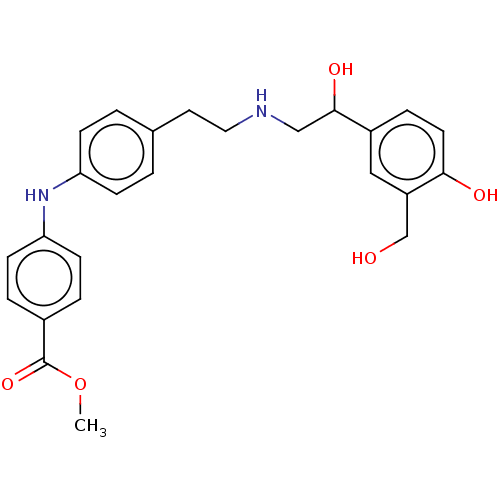
(CHEMBL3290996)Show SMILES COC(=O)c1ccc(Nc2ccc(CCNCC(O)c3ccc(O)c(CO)c3)cc2)cc1 Show InChI InChI=1S/C25H28N2O5/c1-32-25(31)18-4-9-22(10-5-18)27-21-7-2-17(3-8-21)12-13-26-15-24(30)19-6-11-23(29)20(14-19)16-28/h2-11,14,24,26-30H,12-13,15-16H2,1H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 251 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Theravance, Inc.
Curated by ChEMBL
| Assay Description
Displacement of [3H]dihydroalprenolol from beta2 receptor (unknown origin) by liquid scintillation counting and cell based assay |
Bioorg Med Chem Lett 24: 2625-30 (2014)
Article DOI: 10.1016/j.bmcl.2014.04.069
BindingDB Entry DOI: 10.7270/Q2M32X96 |
More data for this
Ligand-Target Pair | |
Beta-2 adrenergic receptor
(Homo sapiens (Human)) | BDBM50018606
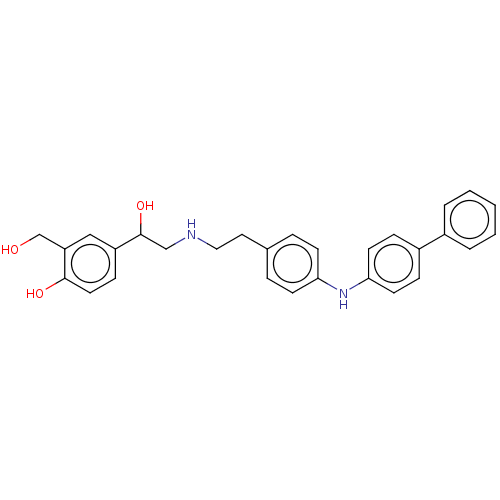
(CHEMBL3290987)Show SMILES OCc1cc(ccc1O)C(O)CNCCc1ccc(Nc2ccc(cc2)-c2ccccc2)cc1 Show InChI InChI=1S/C29H30N2O3/c32-20-25-18-24(10-15-28(25)33)29(34)19-30-17-16-21-6-11-26(12-7-21)31-27-13-8-23(9-14-27)22-4-2-1-3-5-22/h1-15,18,29-34H,16-17,19-20H2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 316 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Theravance, Inc.
Curated by ChEMBL
| Assay Description
Displacement of [3H]dihydroalprenolol from beta2 receptor (unknown origin) by liquid scintillation counting and cell based assay |
Bioorg Med Chem Lett 24: 2625-30 (2014)
Article DOI: 10.1016/j.bmcl.2014.04.069
BindingDB Entry DOI: 10.7270/Q2M32X96 |
More data for this
Ligand-Target Pair | |
Beta-2 adrenergic receptor
(Homo sapiens (Human)) | BDBM50018607

(CHEMBL3290988)Show SMILES OCc1cc(ccc1O)C(O)CNCCc1ccc(Nc2cccc(c2)-c2ccccc2)cc1 Show InChI InChI=1S/C29H30N2O3/c32-20-25-17-24(11-14-28(25)33)29(34)19-30-16-15-21-9-12-26(13-10-21)31-27-8-4-7-23(18-27)22-5-2-1-3-6-22/h1-14,17-18,29-34H,15-16,19-20H2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 316 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Theravance, Inc.
Curated by ChEMBL
| Assay Description
Displacement of [3H]dihydroalprenolol from beta2 receptor (unknown origin) by liquid scintillation counting and cell based assay |
Bioorg Med Chem Lett 24: 2625-30 (2014)
Article DOI: 10.1016/j.bmcl.2014.04.069
BindingDB Entry DOI: 10.7270/Q2M32X96 |
More data for this
Ligand-Target Pair | |
Beta-2 adrenergic receptor
(Homo sapiens (Human)) | BDBM50018602
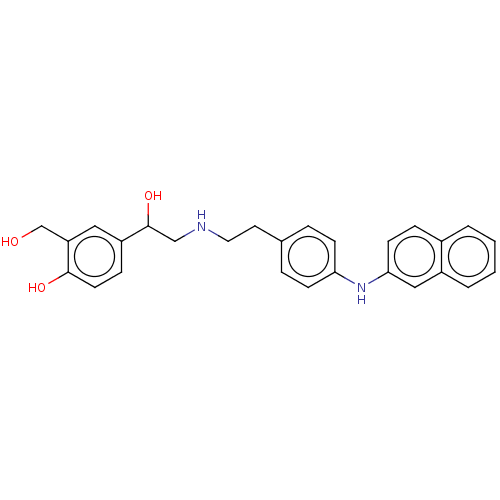
(CHEMBL3290983)Show SMILES OCc1cc(ccc1O)C(O)CNCCc1ccc(Nc2ccc3ccccc3c2)cc1 Show InChI InChI=1S/C27H28N2O3/c30-18-23-15-22(8-12-26(23)31)27(32)17-28-14-13-19-5-9-24(10-6-19)29-25-11-7-20-3-1-2-4-21(20)16-25/h1-12,15-16,27-32H,13-14,17-18H2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 316 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Theravance, Inc.
Curated by ChEMBL
| Assay Description
Displacement of [3H]dihydroalprenolol from beta2 receptor (unknown origin) by liquid scintillation counting and cell based assay |
Bioorg Med Chem Lett 24: 2625-30 (2014)
Article DOI: 10.1016/j.bmcl.2014.04.069
BindingDB Entry DOI: 10.7270/Q2M32X96 |
More data for this
Ligand-Target Pair | |
Beta-2 adrenergic receptor
(Homo sapiens (Human)) | BDBM50018601
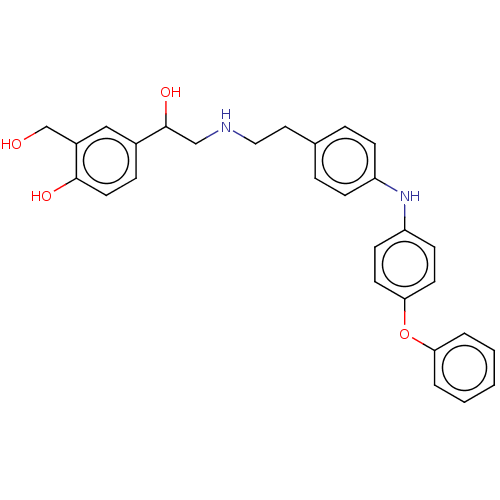
(CHEMBL3290982)Show SMILES OCc1cc(ccc1O)C(O)CNCCc1ccc(Nc2ccc(Oc3ccccc3)cc2)cc1 Show InChI InChI=1S/C29H30N2O4/c32-20-23-18-22(8-15-28(23)33)29(34)19-30-17-16-21-6-9-24(10-7-21)31-25-11-13-27(14-12-25)35-26-4-2-1-3-5-26/h1-15,18,29-34H,16-17,19-20H2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 316 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Theravance, Inc.
Curated by ChEMBL
| Assay Description
Displacement of [3H]dihydroalprenolol from beta2 receptor (unknown origin) by liquid scintillation counting and cell based assay |
Bioorg Med Chem Lett 24: 2625-30 (2014)
Article DOI: 10.1016/j.bmcl.2014.04.069
BindingDB Entry DOI: 10.7270/Q2M32X96 |
More data for this
Ligand-Target Pair | |
Bacterial leucyl aminopeptidase
(Vibrio proteolyticus) | BDBM50129202
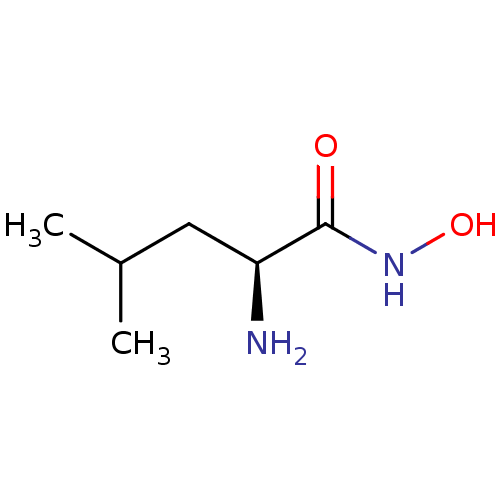
((S)-2-Amino-4-methyl-pentanoic acid hydroxyamide |...)Show InChI InChI=1S/C6H14N2O2/c1-4(2)3-5(7)6(9)8-10/h4-5,10H,3,7H2,1-2H3,(H,8,9)/t5-/m0/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PubMed
| 350 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Guilford Pharmaceuticals Inc.
Curated by ChEMBL
| Assay Description
Inhibition of metalloprotease from family M28, Aeromonas proteolytica aminopeptidase |
Bioorg Med Chem Lett 13: 2097-100 (2003)
BindingDB Entry DOI: 10.7270/Q2Z60NF2 |
More data for this
Ligand-Target Pair | |
Beta-2 adrenergic receptor
(Homo sapiens (Human)) | BDBM50018609
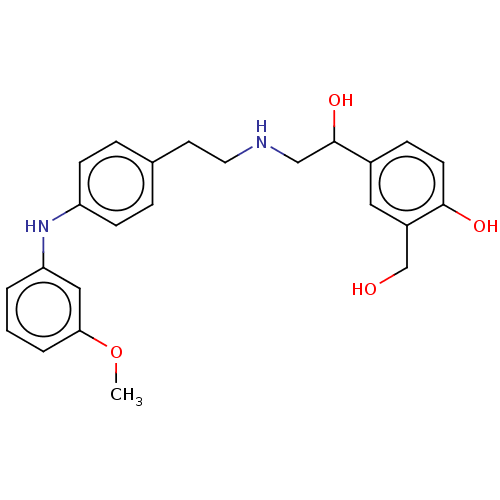
(CHEMBL3290990)Show SMILES COc1cccc(Nc2ccc(CCNCC(O)c3ccc(O)c(CO)c3)cc2)c1 Show InChI InChI=1S/C24H28N2O4/c1-30-22-4-2-3-21(14-22)26-20-8-5-17(6-9-20)11-12-25-15-24(29)18-7-10-23(28)19(13-18)16-27/h2-10,13-14,24-29H,11-12,15-16H2,1H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 631 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Theravance, Inc.
Curated by ChEMBL
| Assay Description
Displacement of [3H]dihydroalprenolol from beta2 receptor (unknown origin) by liquid scintillation counting and cell based assay |
Bioorg Med Chem Lett 24: 2625-30 (2014)
Article DOI: 10.1016/j.bmcl.2014.04.069
BindingDB Entry DOI: 10.7270/Q2M32X96 |
More data for this
Ligand-Target Pair | |
3-phosphoshikimate 1-carboxyvinyltransferase
(Escherichia coli (strain K12)) | BDBM50123114
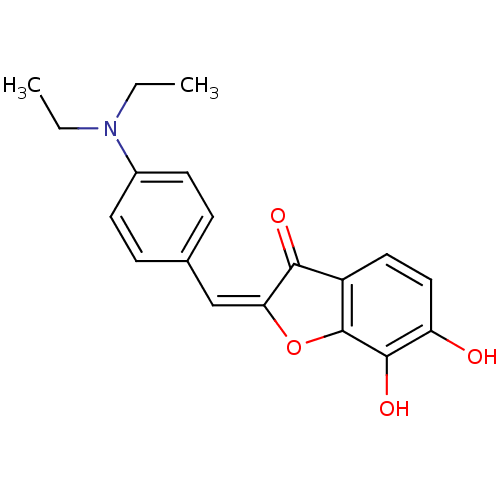
(2-[1-(4-Diethylamino-phenyl)-meth-(E)-ylidene]-6,7...)Show InChI InChI=1S/C19H19NO4/c1-3-20(4-2)13-7-5-12(6-8-13)11-16-17(22)14-9-10-15(21)18(23)19(14)24-16/h5-11,21,23H,3-4H2,1-2H3/b16-11+ | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 650 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
PanTherix Ltd.
Curated by ChEMBL
| Assay Description
Competitive inhibitory activity of the compound was determined with respect to EPSP (5-enolpyruvylshikimate- 3-phosphate) synthase |
Bioorg Med Chem Lett 13: 423-6 (2003)
BindingDB Entry DOI: 10.7270/Q2TH8M2M |
More data for this
Ligand-Target Pair | |
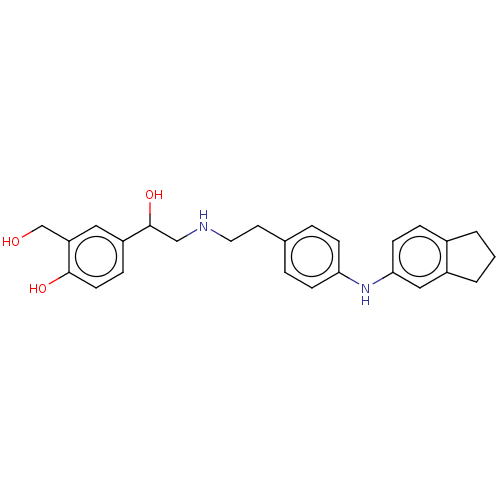
Beta-2 adrenergic receptor
(Homo sapiens (Human)) | BDBM50018605
(CHEMBL3290986)Show SMILES OCc1cc(ccc1O)C(O)CNCCc1ccc(Nc2ccc3CCCc3c2)cc1 Show InChI InChI=1S/C26H30N2O3/c29-17-22-14-21(7-11-25(22)30)26(31)16-27-13-12-18-4-8-23(9-5-18)28-24-10-6-19-2-1-3-20(19)15-24/h4-11,14-15,26-31H,1-3,12-13,16-17H2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 794 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Theravance, Inc.
Curated by ChEMBL
| Assay Description
Displacement of [3H]dihydroalprenolol from beta2 receptor (unknown origin) by liquid scintillation counting and cell based assay |
Bioorg Med Chem Lett 24: 2625-30 (2014)
Article DOI: 10.1016/j.bmcl.2014.04.069
BindingDB Entry DOI: 10.7270/Q2M32X96 |
More data for this
Ligand-Target Pair | |
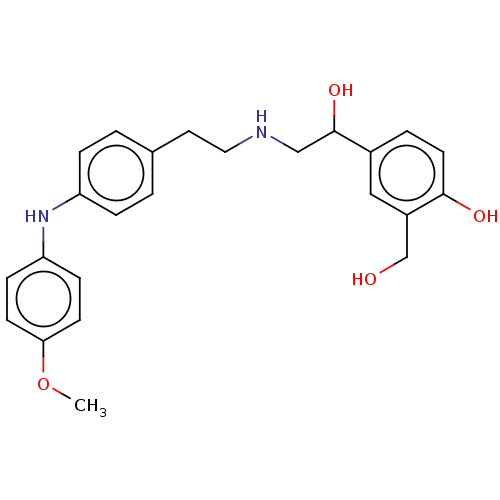
Beta-2 adrenergic receptor
(Homo sapiens (Human)) | BDBM50018603
(CHEMBL3290984)Show SMILES COc1ccc(Nc2ccc(CCNCC(O)c3ccc(O)c(CO)c3)cc2)cc1 Show InChI InChI=1S/C24H28N2O4/c1-30-22-9-7-21(8-10-22)26-20-5-2-17(3-6-20)12-13-25-15-24(29)18-4-11-23(28)19(14-18)16-27/h2-11,14,24-29H,12-13,15-16H2,1H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.26E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Theravance, Inc.
Curated by ChEMBL
| Assay Description
Displacement of [3H]dihydroalprenolol from beta2 receptor (unknown origin) by liquid scintillation counting and cell based assay |
Bioorg Med Chem Lett 24: 2625-30 (2014)
Article DOI: 10.1016/j.bmcl.2014.04.069
BindingDB Entry DOI: 10.7270/Q2M32X96 |
More data for this
Ligand-Target Pair | |
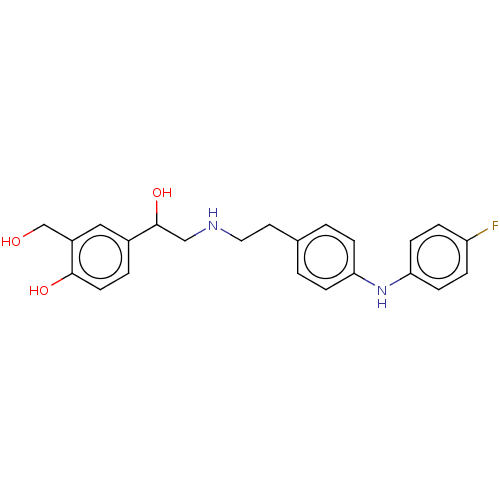
Beta-2 adrenergic receptor
(Homo sapiens (Human)) | BDBM50018561
(CHEMBL3290995)Show SMILES OCc1cc(ccc1O)C(O)CNCCc1ccc(Nc2ccc(F)cc2)cc1 Show InChI InChI=1S/C23H25FN2O3/c24-19-4-8-21(9-5-19)26-20-6-1-16(2-7-20)11-12-25-14-23(29)17-3-10-22(28)18(13-17)15-27/h1-10,13,23,25-29H,11-12,14-15H2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.26E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Theravance, Inc.
Curated by ChEMBL
| Assay Description
Displacement of [3H]dihydroalprenolol from beta2 receptor (unknown origin) by liquid scintillation counting and cell based assay |
Bioorg Med Chem Lett 24: 2625-30 (2014)
Article DOI: 10.1016/j.bmcl.2014.04.069
BindingDB Entry DOI: 10.7270/Q2M32X96 |
More data for this
Ligand-Target Pair | |
Beta-2 adrenergic receptor
(Homo sapiens (Human)) | BDBM50018559
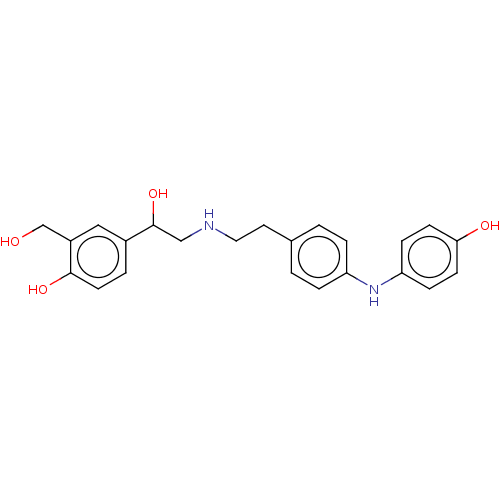
(CHEMBL3290994)Show SMILES OCc1cc(ccc1O)C(O)CNCCc1ccc(Nc2ccc(O)cc2)cc1 Show InChI InChI=1S/C23H26N2O4/c26-15-18-13-17(3-10-22(18)28)23(29)14-24-12-11-16-1-4-19(5-2-16)25-20-6-8-21(27)9-7-20/h1-10,13,23-29H,11-12,14-15H2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.59E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Theravance, Inc.
Curated by ChEMBL
| Assay Description
Displacement of [3H]dihydroalprenolol from beta2 receptor (unknown origin) by liquid scintillation counting and cell based assay |
Bioorg Med Chem Lett 24: 2625-30 (2014)
Article DOI: 10.1016/j.bmcl.2014.04.069
BindingDB Entry DOI: 10.7270/Q2M32X96 |
More data for this
Ligand-Target Pair | |
Beta-1 adrenergic receptor
(Homo sapiens (Human)) | BDBM86453

(CAS_73573-87-2 | Formoterol | NSC_3083544)Show InChI InChI=1S/C19H24N2O4/c1-13(9-14-3-6-16(25-2)7-4-14)20-11-19(24)15-5-8-18(23)17(10-15)21-12-22/h3-8,10,12-13,19-20,23-24H,9,11H2,1-2H3,(H,21,22) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
DrugBank
KEGG
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 2.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Theravance, Inc.
Curated by ChEMBL
| Assay Description
Displacement of [3H]dihydroalprenolol from beta1 receptor (unknown origin) by liquid scintillation counting and cell based assay |
Bioorg Med Chem Lett 24: 2625-30 (2014)
Article DOI: 10.1016/j.bmcl.2014.04.069
BindingDB Entry DOI: 10.7270/Q2M32X96 |
More data for this
Ligand-Target Pair | |
Glutaminase kidney isoform, mitochondrial
(Homo sapiens (Human)) | BDBM108460

(CHEMBL2178393 | US11191732, Example 1 | US8604016,...)Show InChI InChI=1S/C16H18N6OS3/c17-15-21-19-13(25-15)6-8-24-9-7-14-20-22-16(26-14)18-12(23)10-11-4-2-1-3-5-11/h1-5H,6-10H2,(H2,17,21)(H,18,22,23) | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 2.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johns Hopkins University
Curated by ChEMBL
| Assay Description
Uncompetitive inhibition of human kidney glutaminase (124 to 669) assessed as reduction of glutamine hydrolysis by double-reciprocal plot analysis |
J Med Chem 55: 10551-63 (2012)
Article DOI: 10.1021/jm301191p
BindingDB Entry DOI: 10.7270/Q2VD70M7 |
More data for this
Ligand-Target Pair | |
Androgen receptor
(Homo sapiens (Human)) | BDBM50432188

(CHEMBL2346976)Show SMILES CC(=O)N1CCN(CCOc2ccc(cc2)C2CCN(CC2)C2=Nn3c(CC2)nnc3C(F)(F)F)CC1 |t:24| Show InChI InChI=1S/C25H32F3N7O2/c1-18(36)33-14-12-32(13-15-33)16-17-37-21-4-2-19(3-5-21)20-8-10-34(11-9-20)23-7-6-22-29-30-24(25(26,27)28)35(22)31-23/h2-5,20H,6-17H2,1H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| 2.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Displacement of [3H]R1881 from full length androgen receptor in human LNCAP cells |
Bioorg Med Chem Lett 23: 1945-8 (2013)
Article DOI: 10.1016/j.bmcl.2013.02.056
BindingDB Entry DOI: 10.7270/Q21J9C5N |
More data for this
Ligand-Target Pair | |
Beta-1 adrenergic receptor
(Homo sapiens (Human)) | BDBM25771

(1-hydroxy-2-naphthoic acid;4-[1-hydroxy-2-[6-(4-ph...)Show InChI InChI=1S/C25H37NO4/c27-20-23-18-22(13-14-24(23)28)25(29)19-26-15-7-1-2-8-16-30-17-9-6-12-21-10-4-3-5-11-21/h3-5,10-11,13-14,18,25-29H,1-2,6-9,12,15-17,19-20H2 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
DrugBank
MCE
KEGG
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 2.51E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Theravance, Inc.
Curated by ChEMBL
| Assay Description
Displacement of [3H]dihydroalprenolol from beta1 receptor (unknown origin) by liquid scintillation counting and cell based assay |
Bioorg Med Chem Lett 24: 2625-30 (2014)
Article DOI: 10.1016/j.bmcl.2014.04.069
BindingDB Entry DOI: 10.7270/Q2M32X96 |
More data for this
Ligand-Target Pair | |
Androgen receptor
(Rattus norvegicus (Rat)) | BDBM50432188

(CHEMBL2346976)Show SMILES CC(=O)N1CCN(CCOc2ccc(cc2)C2CCN(CC2)C2=Nn3c(CC2)nnc3C(F)(F)F)CC1 |t:24| Show InChI InChI=1S/C25H32F3N7O2/c1-18(36)33-14-12-32(13-15-33)16-17-37-21-4-2-19(3-5-21)20-8-10-34(11-9-20)23-7-6-22-29-30-24(25(26,27)28)35(22)31-23/h2-5,20H,6-17H2,1H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| 5.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Binding affinity to rat androgen receptor ligand binding domain by fluorescence polarization assay |
Bioorg Med Chem Lett 23: 1945-8 (2013)
Article DOI: 10.1016/j.bmcl.2013.02.056
BindingDB Entry DOI: 10.7270/Q21J9C5N |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data