

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Cannabinoid receptor 2 (Homo sapiens (Human)) | BDBM50257851 ((S)-4-((2',3'-dichlorobiphenyl-4-yl)methyl)-2-phen...) | PDB Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 104 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim Pharmaceuticals Curated by ChEMBL | Assay Description Displacement of [3H]Win55212 form human CB2 receptor transfected in HEK cells | Bioorg Med Chem Lett 19: 1604-9 (2009) Article DOI: 10.1016/j.bmcl.2009.02.033 BindingDB Entry DOI: 10.7270/Q2K937DD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 2 (Homo sapiens (Human)) | BDBM50257851 ((S)-4-((2',3'-dichlorobiphenyl-4-yl)methyl)-2-phen...) | PDB Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 312 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim Pharmaceuticals Curated by ChEMBL | Assay Description Displacement of [3H]CP55940 form human CB2 receptor transfected in HEK cells | Bioorg Med Chem Lett 19: 1604-9 (2009) Article DOI: 10.1016/j.bmcl.2009.02.033 BindingDB Entry DOI: 10.7270/Q2K937DD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 2 (Homo sapiens (Human)) | BDBM50258007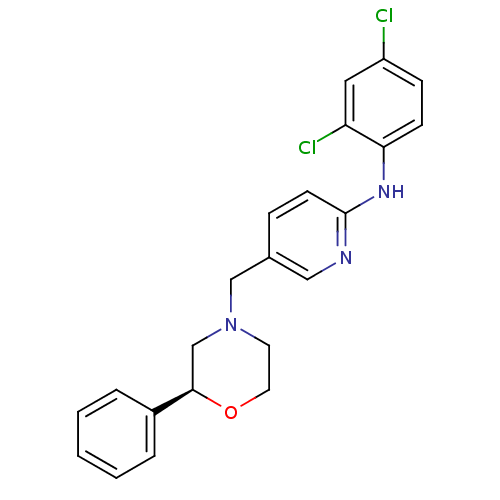 ((S)-N-(2,4-dichlorophenyl)-5-((2-phenylmorpholino)...) | PDB Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 2.65E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim Pharmaceuticals Curated by ChEMBL | Assay Description Displacement of [3H]Win55212 form human CB2 receptor transfected in HEK cells | Bioorg Med Chem Lett 19: 1604-9 (2009) Article DOI: 10.1016/j.bmcl.2009.02.033 BindingDB Entry DOI: 10.7270/Q2K937DD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 2 (Homo sapiens (Human)) | BDBM50211843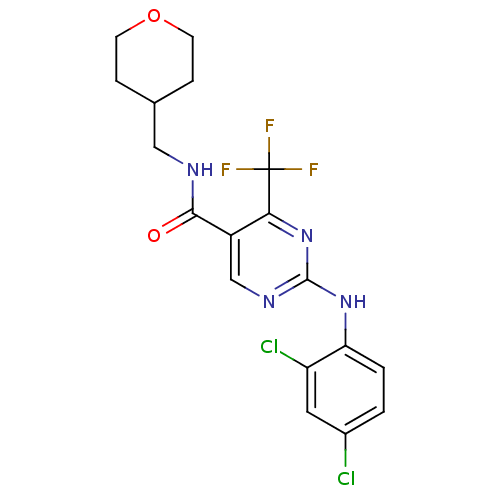 (2-(2,4-dichlorophenylamino)-4-trifluoromethyl-pyri...) | PDB Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | >5.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim Pharmaceuticals Curated by ChEMBL | Assay Description Displacement of [3H]CP55940 form human CB2 receptor transfected in HEK cells | Bioorg Med Chem Lett 19: 1604-9 (2009) Article DOI: 10.1016/j.bmcl.2009.02.033 BindingDB Entry DOI: 10.7270/Q2K937DD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 2 (Homo sapiens (Human)) | BDBM50258007 ((S)-N-(2,4-dichlorophenyl)-5-((2-phenylmorpholino)...) | PDB Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim Pharmaceuticals Curated by ChEMBL | Assay Description Displacement of [3H]CP55940 form human CB2 receptor transfected in HEK cells | Bioorg Med Chem Lett 19: 1604-9 (2009) Article DOI: 10.1016/j.bmcl.2009.02.033 BindingDB Entry DOI: 10.7270/Q2K937DD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 2 (Homo sapiens (Human)) | BDBM50211843 (2-(2,4-dichlorophenylamino)-4-trifluoromethyl-pyri...) | PDB Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim Pharmaceuticals Curated by ChEMBL | Assay Description Displacement of [3H]Win55212 form human CB2 receptor transfected in HEK cells | Bioorg Med Chem Lett 19: 1604-9 (2009) Article DOI: 10.1016/j.bmcl.2009.02.033 BindingDB Entry DOI: 10.7270/Q2K937DD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin G/H synthase 2 (Homo sapiens (Human)) | BDBM50070289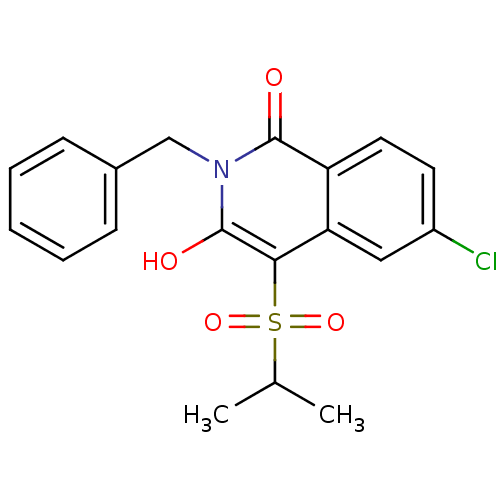 (2-Benzyl-6-chloro-3-hydroxy-4-(propane-2-sulfonyl)...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 60 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim Pharmaceuticals Curated by ChEMBL | Assay Description Concentration that caused a 50% decrease in the maximal inhibition of Prostaglandin G/H synthase 2 activity as measured by PGE-2 production. | Bioorg Med Chem Lett 8: 1181-6 (1999) BindingDB Entry DOI: 10.7270/Q2TQ60PJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin G/H synthase 2 (Homo sapiens (Human)) | BDBM50070287 (2-(4-Fluoro-benzyl)-3-hydroxy-4-(propane-2-sulfony...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 90 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim Pharmaceuticals Curated by ChEMBL | Assay Description Concentration that caused a 50% decrease in the maximal inhibition of Prostaglandin G/H synthase 2 activity as measured by PGE-2 production; Imax=84% | Bioorg Med Chem Lett 8: 1181-6 (1999) BindingDB Entry DOI: 10.7270/Q2TQ60PJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin G/H synthase 2 (Homo sapiens (Human)) | BDBM50070291 (6-Chloro-2-(3,4-difluoro-benzyl)-3-hydroxy-4-(prop...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 100 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim Pharmaceuticals Curated by ChEMBL | Assay Description Concentration that caused a 50% decrease in the maximal inhibition of Prostaglandin G/H synthase 2 activity as measured by PGE-2 production. | Bioorg Med Chem Lett 8: 1181-6 (1999) BindingDB Entry DOI: 10.7270/Q2TQ60PJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin G/H synthase 2 (Homo sapiens (Human)) | BDBM50070286 (4-Benzenesulfonyl-2-benzyl-3-hydroxy-2H-isoquinoli...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 140 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim Pharmaceuticals Curated by ChEMBL | Assay Description Concentration that caused a 50% decrease in the maximal inhibition of Prostaglandin G/H synthase 2 activity as measured by PGE-2 production. | Bioorg Med Chem Lett 8: 1181-6 (1999) BindingDB Entry DOI: 10.7270/Q2TQ60PJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin G/H synthase 2 (Homo sapiens (Human)) | BDBM50056998 (4-Hydroxy-1,1-dioxo-1,2-dihydro-1lambda*6*-benzo[e...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Similars | DrugBank PDB PubMed | n/a | n/a | 160 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim Pharmaceuticals Curated by ChEMBL | Assay Description Concentration that caused a 50% decrease in the maximal inhibition of Prostaglandin G/H synthase 2 activity as measured by PGE-2 production. | Bioorg Med Chem Lett 8: 1181-6 (1999) BindingDB Entry DOI: 10.7270/Q2TQ60PJ | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Prostaglandin G/H synthase 2 (Homo sapiens (Human)) | BDBM50070288 (2-(3,4-Difluoro-benzyl)-3-hydroxy-4-(propane-2-sul...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 200 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim Pharmaceuticals Curated by ChEMBL | Assay Description Concentration that caused a 50% decrease in the maximal inhibition of Prostaglandin G/H synthase 2 activity as measured by PGE-2 production; Imax=84% | Bioorg Med Chem Lett 8: 1181-6 (1999) BindingDB Entry DOI: 10.7270/Q2TQ60PJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin G/H synthase 1 (Homo sapiens (Human)) | BDBM50070287 (2-(4-Fluoro-benzyl)-3-hydroxy-4-(propane-2-sulfony...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 250 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim Pharmaceuticals Curated by ChEMBL | Assay Description Concentration that caused a 50% decrease in the maximal inhibition of Prostaglandin G/H synthase 1 activity as measured by PGE-2 production. | Bioorg Med Chem Lett 8: 1181-6 (1999) BindingDB Entry DOI: 10.7270/Q2TQ60PJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin G/H synthase 2 (Homo sapiens (Human)) | BDBM50070290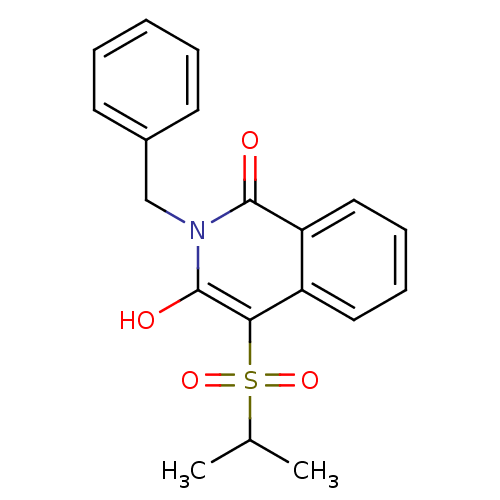 (2-Benzyl-3-hydroxy-4-(propane-2-sulfonyl)-2H-isoqu...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 290 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim Pharmaceuticals Curated by ChEMBL | Assay Description Concentration that caused a 50% decrease in the maximal inhibition of Prostaglandin G/H synthase 2 activity as measured by PGE-2 production. | Bioorg Med Chem Lett 8: 1181-6 (1999) BindingDB Entry DOI: 10.7270/Q2TQ60PJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin G/H synthase 1 (Homo sapiens (Human)) | BDBM50070290 (2-Benzyl-3-hydroxy-4-(propane-2-sulfonyl)-2H-isoqu...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 340 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim Pharmaceuticals Curated by ChEMBL | Assay Description Concentration that caused a 50% decrease in the maximal inhibition of Prostaglandin G/H synthase 1 activity as measured by PGE-2 production. | Bioorg Med Chem Lett 8: 1181-6 (1999) BindingDB Entry DOI: 10.7270/Q2TQ60PJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin G/H synthase 1 (Homo sapiens (Human)) | BDBM50070288 (2-(3,4-Difluoro-benzyl)-3-hydroxy-4-(propane-2-sul...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 570 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim Pharmaceuticals Curated by ChEMBL | Assay Description Concentration that caused a 50% decrease in the maximal inhibition of Prostaglandin G/H synthase 1 activity as measured by PGE-2 production. | Bioorg Med Chem Lett 8: 1181-6 (1999) BindingDB Entry DOI: 10.7270/Q2TQ60PJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin G/H synthase 1 (Homo sapiens (Human)) | BDBM50070286 (4-Benzenesulfonyl-2-benzyl-3-hydroxy-2H-isoquinoli...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 730 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim Pharmaceuticals Curated by ChEMBL | Assay Description Concentration that caused a 50% decrease in the maximal inhibition of Prostaglandin G/H synthase 1 activity as measured by PGE-2 production. | Bioorg Med Chem Lett 8: 1181-6 (1999) BindingDB Entry DOI: 10.7270/Q2TQ60PJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin G/H synthase 1 (Homo sapiens (Human)) | BDBM50070291 (6-Chloro-2-(3,4-difluoro-benzyl)-3-hydroxy-4-(prop...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 1.36E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim Pharmaceuticals Curated by ChEMBL | Assay Description Concentration that caused a 50% decrease in the maximal inhibition of Prostaglandin G/H synthase 1 activity as measured by PGE-2 production. | Bioorg Med Chem Lett 8: 1181-6 (1999) BindingDB Entry DOI: 10.7270/Q2TQ60PJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin G/H synthase 1 (Homo sapiens (Human)) | BDBM50070289 (2-Benzyl-6-chloro-3-hydroxy-4-(propane-2-sulfonyl)...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 1.42E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim Pharmaceuticals Curated by ChEMBL | Assay Description Concentration that caused a 50% decrease in the maximal inhibition of Prostaglandin G/H synthase 1 activity as measured by PGE-2 production. | Bioorg Med Chem Lett 8: 1181-6 (1999) BindingDB Entry DOI: 10.7270/Q2TQ60PJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin G/H synthase 1 (Homo sapiens (Human)) | BDBM50056998 (4-Hydroxy-1,1-dioxo-1,2-dihydro-1lambda*6*-benzo[e...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Similars | PDB PubMed | n/a | n/a | 2.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim Pharmaceuticals Curated by ChEMBL | Assay Description Concentration that caused a 50% decrease in the maximal inhibition of Prostaglandin G/H synthase 1 activity as measured by PGE-2 production. | Bioorg Med Chem Lett 8: 1181-6 (1999) BindingDB Entry DOI: 10.7270/Q2TQ60PJ | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Cannabinoid receptor 2 (Homo sapiens (Human)) | BDBM50257851 ((S)-4-((2',3'-dichlorobiphenyl-4-yl)methyl)-2-phen...) | PDB Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | n/a | n/a | 6 | n/a | n/a | n/a | n/a |
Boehringer Ingelheim Pharmaceuticals Curated by ChEMBL | Assay Description Agonist activity at human CB2 receptor expressed in CHO cells assessed as inhibition of forskolin-induced cAMP release | Bioorg Med Chem Lett 19: 1604-9 (2009) Article DOI: 10.1016/j.bmcl.2009.02.033 BindingDB Entry DOI: 10.7270/Q2K937DD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 2 (Homo sapiens (Human)) | BDBM50257852 ((S)-4-((2'-chloro-5'-methylbiphenyl-4-yl)methyl)-2...) | PDB Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | n/a | n/a | 3 | n/a | n/a | n/a | n/a |
Boehringer Ingelheim Pharmaceuticals Curated by ChEMBL | Assay Description Agonist activity at human CB2 receptor expressed in CHO cells assessed as inhibition of forskolin-induced cAMP release | Bioorg Med Chem Lett 19: 1604-9 (2009) Article DOI: 10.1016/j.bmcl.2009.02.033 BindingDB Entry DOI: 10.7270/Q2K937DD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 2 (Homo sapiens (Human)) | BDBM50257853 ((S)-4'-((2-phenylmorpholino)methyl)biphenyl-2-carb...) | PDB Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents | Article PubMed | n/a | n/a | n/a | n/a | 3 | n/a | n/a | n/a | n/a |
Boehringer Ingelheim Pharmaceuticals Curated by ChEMBL | Assay Description Agonist activity at human CB2 receptor expressed in CHO cells assessed as inhibition of forskolin-induced cAMP release | Bioorg Med Chem Lett 19: 1604-9 (2009) Article DOI: 10.1016/j.bmcl.2009.02.033 BindingDB Entry DOI: 10.7270/Q2K937DD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 2 (Homo sapiens (Human)) | BDBM50257854 ((S)-N-phenyl-4-((2-phenylmorpholino)methyl)aniline...) | PDB Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | n/a | n/a | 615 | n/a | n/a | n/a | n/a |
Boehringer Ingelheim Pharmaceuticals Curated by ChEMBL | Assay Description Agonist activity at human CB2 receptor expressed in CHO cells assessed as inhibition of forskolin-induced cAMP release | Bioorg Med Chem Lett 19: 1604-9 (2009) Article DOI: 10.1016/j.bmcl.2009.02.033 BindingDB Entry DOI: 10.7270/Q2K937DD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 2 (Homo sapiens (Human)) | BDBM50257910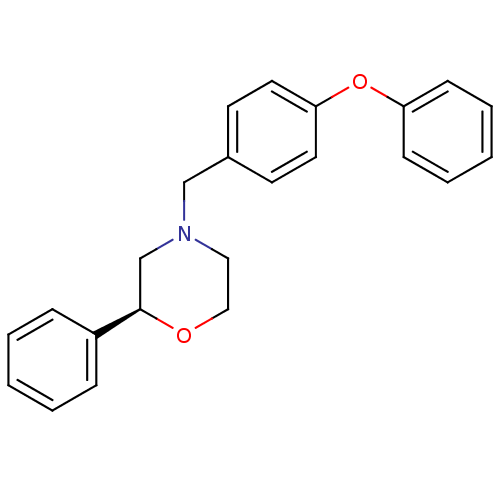 ((S)-4-(4-phenoxybenzyl)-2-phenylmorpholine | CHEMB...) | PDB Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents | Article PubMed | n/a | n/a | n/a | n/a | 1.50E+4 | n/a | n/a | n/a | n/a |
Boehringer Ingelheim Pharmaceuticals Curated by ChEMBL | Assay Description Agonist activity at human CB2 receptor expressed in CHO cells assessed as inhibition of forskolin-induced cAMP release | Bioorg Med Chem Lett 19: 1604-9 (2009) Article DOI: 10.1016/j.bmcl.2009.02.033 BindingDB Entry DOI: 10.7270/Q2K937DD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 2 (Homo sapiens (Human)) | BDBM50257911 ((S)-N-phenyl-5-((2-phenylmorpholino)methyl)pyridin...) | PDB Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | n/a | n/a | 140 | n/a | n/a | n/a | n/a |
Boehringer Ingelheim Pharmaceuticals Curated by ChEMBL | Assay Description Agonist activity at human CB2 receptor expressed in CHO cells assessed as inhibition of forskolin-induced cAMP release | Bioorg Med Chem Lett 19: 1604-9 (2009) Article DOI: 10.1016/j.bmcl.2009.02.033 BindingDB Entry DOI: 10.7270/Q2K937DD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 2 (Homo sapiens (Human)) | BDBM50257912 ((S)-N-(2,3-dichlorophenyl)-5-((2-phenylmorpholino)...) | PDB Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | n/a | n/a | 1.60E+3 | n/a | n/a | n/a | n/a |
Boehringer Ingelheim Pharmaceuticals Curated by ChEMBL | Assay Description Agonist activity at human CB2 receptor expressed in CHO cells assessed as inhibition of forskolin-induced cAMP release | Bioorg Med Chem Lett 19: 1604-9 (2009) Article DOI: 10.1016/j.bmcl.2009.02.033 BindingDB Entry DOI: 10.7270/Q2K937DD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 2 (Homo sapiens (Human)) | BDBM50257913 ((S)-N-(2-chlorophenyl)-5-((2-phenylmorpholino)meth...) | PDB Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | n/a | n/a | 30 | n/a | n/a | n/a | n/a |
Boehringer Ingelheim Pharmaceuticals Curated by ChEMBL | Assay Description Agonist activity at human CB2 receptor expressed in CHO cells assessed as inhibition of forskolin-induced cAMP release | Bioorg Med Chem Lett 19: 1604-9 (2009) Article DOI: 10.1016/j.bmcl.2009.02.033 BindingDB Entry DOI: 10.7270/Q2K937DD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 2 (Homo sapiens (Human)) | BDBM50257914 ((S)-N-(3-chlorophenyl)-5-((2-phenylmorpholino)meth...) | PDB Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | n/a | n/a | 200 | n/a | n/a | n/a | n/a |
Boehringer Ingelheim Pharmaceuticals Curated by ChEMBL | Assay Description Agonist activity at human CB2 receptor expressed in CHO cells assessed as inhibition of forskolin-induced cAMP release | Bioorg Med Chem Lett 19: 1604-9 (2009) Article DOI: 10.1016/j.bmcl.2009.02.033 BindingDB Entry DOI: 10.7270/Q2K937DD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 2 (Homo sapiens (Human)) | BDBM50257957 ((S)-N-(4-chlorophenyl)-5-((2-phenylmorpholino)meth...) | PDB Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | n/a | n/a | 30 | n/a | n/a | n/a | n/a |
Boehringer Ingelheim Pharmaceuticals Curated by ChEMBL | Assay Description Agonist activity at human CB2 receptor expressed in CHO cells assessed as inhibition of forskolin-induced cAMP release | Bioorg Med Chem Lett 19: 1604-9 (2009) Article DOI: 10.1016/j.bmcl.2009.02.033 BindingDB Entry DOI: 10.7270/Q2K937DD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 2 (Homo sapiens (Human)) | BDBM50257958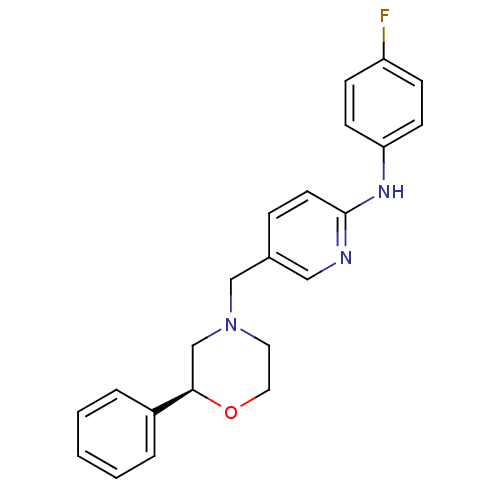 ((S)-N-(4-fluorophenyl)-5-((2-phenylmorpholino)meth...) | PDB Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | n/a | n/a | 35 | n/a | n/a | n/a | n/a |
Boehringer Ingelheim Pharmaceuticals Curated by ChEMBL | Assay Description Agonist activity at human CB2 receptor expressed in CHO cells assessed as inhibition of forskolin-induced cAMP release | Bioorg Med Chem Lett 19: 1604-9 (2009) Article DOI: 10.1016/j.bmcl.2009.02.033 BindingDB Entry DOI: 10.7270/Q2K937DD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 2 (Homo sapiens (Human)) | BDBM50257959 ((S)-5-((2-phenylmorpholino)methyl)-N-p-tolylpyridi...) | PDB Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | n/a | n/a | 100 | n/a | n/a | n/a | n/a |
Boehringer Ingelheim Pharmaceuticals Curated by ChEMBL | Assay Description Agonist activity at human CB2 receptor expressed in CHO cells assessed as inhibition of forskolin-induced cAMP release | Bioorg Med Chem Lett 19: 1604-9 (2009) Article DOI: 10.1016/j.bmcl.2009.02.033 BindingDB Entry DOI: 10.7270/Q2K937DD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 2 (Homo sapiens (Human)) | BDBM50257960 ((S)-N-(4-isopropylphenyl)-5-((2-phenylmorpholino)m...) | PDB Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | >2.00E+4 | n/a | n/a | n/a | n/a |
Boehringer Ingelheim Pharmaceuticals Curated by ChEMBL | Assay Description Agonist activity at human CB2 receptor expressed in CHO cells assessed as inhibition of forskolin-induced cAMP release | Bioorg Med Chem Lett 19: 1604-9 (2009) Article DOI: 10.1016/j.bmcl.2009.02.033 BindingDB Entry DOI: 10.7270/Q2K937DD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 2 (Homo sapiens (Human)) | BDBM50257961 ((S)-N-(4-methoxyphenyl)-5-((2-phenylmorpholino)met...) | PDB Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | n/a | n/a | 65 | n/a | n/a | n/a | n/a |
Boehringer Ingelheim Pharmaceuticals Curated by ChEMBL | Assay Description Agonist activity at human CB2 receptor expressed in CHO cells assessed as inhibition of forskolin-induced cAMP release | Bioorg Med Chem Lett 19: 1604-9 (2009) Article DOI: 10.1016/j.bmcl.2009.02.033 BindingDB Entry DOI: 10.7270/Q2K937DD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 2 (Homo sapiens (Human)) | BDBM50258004 ((S)-N1,N1-dimethyl-N4-(5-((2-phenylmorpholino)meth...) | PDB Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | >2.00E+4 | n/a | n/a | n/a | n/a |
Boehringer Ingelheim Pharmaceuticals Curated by ChEMBL | Assay Description Agonist activity at human CB2 receptor expressed in CHO cells assessed as inhibition of forskolin-induced cAMP release | Bioorg Med Chem Lett 19: 1604-9 (2009) Article DOI: 10.1016/j.bmcl.2009.02.033 BindingDB Entry DOI: 10.7270/Q2K937DD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 2 (Homo sapiens (Human)) | BDBM50258005 ((S)-N-(biphenyl-4-yl)-5-((2-phenylmorpholino)methy...) | PDB Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | >2.00E+4 | n/a | n/a | n/a | n/a |
Boehringer Ingelheim Pharmaceuticals Curated by ChEMBL | Assay Description Agonist activity at human CB2 receptor expressed in CHO cells assessed as inhibition of forskolin-induced cAMP release | Bioorg Med Chem Lett 19: 1604-9 (2009) Article DOI: 10.1016/j.bmcl.2009.02.033 BindingDB Entry DOI: 10.7270/Q2K937DD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 2 (Homo sapiens (Human)) | BDBM50258006 ((S)-N-(3,4-dichlorophenyl)-5-((2-phenylmorpholino)...) | PDB Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | n/a | n/a | 50 | n/a | n/a | n/a | n/a |
Boehringer Ingelheim Pharmaceuticals Curated by ChEMBL | Assay Description Agonist activity at human CB2 receptor expressed in CHO cells assessed as inhibition of forskolin-induced cAMP release | Bioorg Med Chem Lett 19: 1604-9 (2009) Article DOI: 10.1016/j.bmcl.2009.02.033 BindingDB Entry DOI: 10.7270/Q2K937DD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 2 (Homo sapiens (Human)) | BDBM50258007 ((S)-N-(2,4-dichlorophenyl)-5-((2-phenylmorpholino)...) | PDB Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | n/a | n/a | 10 | n/a | n/a | n/a | n/a |
Boehringer Ingelheim Pharmaceuticals Curated by ChEMBL | Assay Description Agonist activity at human CB2 receptor expressed in CHO cells assessed as inhibition of forskolin-induced cAMP release | Bioorg Med Chem Lett 19: 1604-9 (2009) Article DOI: 10.1016/j.bmcl.2009.02.033 BindingDB Entry DOI: 10.7270/Q2K937DD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 2 (Homo sapiens (Human)) | BDBM50257800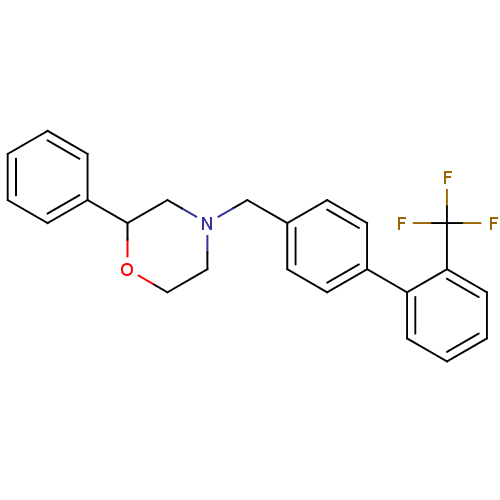 (CHEMBL494589 | rac-2-phenyl-4-((2'-(trifluoromethy...) | PDB Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | n/a | n/a | 330 | n/a | n/a | n/a | n/a |
Boehringer Ingelheim Pharmaceuticals Curated by ChEMBL | Assay Description Agonist activity at human CB2 receptor expressed in CHO cells assessed as inhibition of forskolin-induced cAMP release | Bioorg Med Chem Lett 19: 1604-9 (2009) Article DOI: 10.1016/j.bmcl.2009.02.033 BindingDB Entry DOI: 10.7270/Q2K937DD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 2 (Homo sapiens (Human)) | BDBM50257799 (CHEMBL494408 | rac-2-(methyl((2'-(trifluoromethyl)...) | PDB Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | n/a | n/a | 2 | n/a | n/a | n/a | n/a |
Boehringer Ingelheim Pharmaceuticals Curated by ChEMBL | Assay Description Agonist activity at human CB2 receptor expressed in CHO cells assessed as inhibition of forskolin-induced cAMP release | Bioorg Med Chem Lett 19: 1604-9 (2009) Article DOI: 10.1016/j.bmcl.2009.02.033 BindingDB Entry DOI: 10.7270/Q2K937DD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 2 (Homo sapiens (Human)) | BDBM50211843 (2-(2,4-dichlorophenylamino)-4-trifluoromethyl-pyri...) | PDB Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | n/a | n/a | 350 | n/a | n/a | n/a | n/a |
Boehringer Ingelheim Pharmaceuticals Curated by ChEMBL | Assay Description Agonist activity at human CB2 receptor expressed in CHO cells assessed as inhibition of forskolin-induced cAMP release | Bioorg Med Chem Lett 19: 1604-9 (2009) Article DOI: 10.1016/j.bmcl.2009.02.033 BindingDB Entry DOI: 10.7270/Q2K937DD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 2 (Homo sapiens (Human)) | BDBM50257801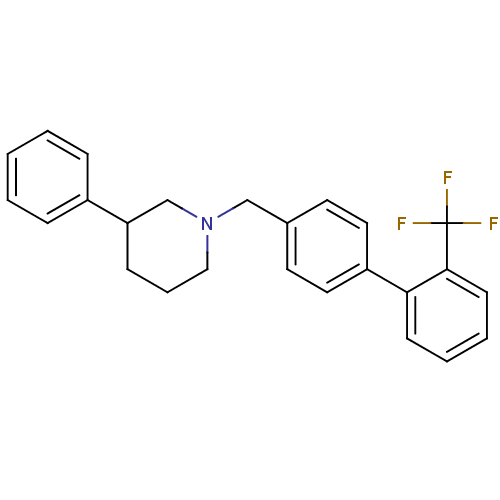 (CHEMBL494590 | rac-3-phenyl-1-((2'-(trifluoromethy...) | PDB Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents | Article PubMed | n/a | n/a | n/a | n/a | 30 | n/a | n/a | n/a | n/a |
Boehringer Ingelheim Pharmaceuticals Curated by ChEMBL | Assay Description Agonist activity at human CB2 receptor expressed in CHO cells assessed as inhibition of forskolin-induced cAMP release | Bioorg Med Chem Lett 19: 1604-9 (2009) Article DOI: 10.1016/j.bmcl.2009.02.033 BindingDB Entry DOI: 10.7270/Q2K937DD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 2 (Homo sapiens (Human)) | BDBM50257802 ((S)-2-phenyl-4-((2'-(trifluoromethyl)biphenyl-4-yl...) | PDB Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | n/a | n/a | 40 | n/a | n/a | n/a | n/a |
Boehringer Ingelheim Pharmaceuticals Curated by ChEMBL | Assay Description Agonist activity at human CB2 receptor expressed in CHO cells assessed as inhibition of forskolin-induced cAMP release | Bioorg Med Chem Lett 19: 1604-9 (2009) Article DOI: 10.1016/j.bmcl.2009.02.033 BindingDB Entry DOI: 10.7270/Q2K937DD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 2 (Homo sapiens (Human)) | BDBM50257850 ((S)-4-((2'-chlorobiphenyl-4-yl)methyl)-2-phenylmor...) | PDB Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | n/a | n/a | 25 | n/a | n/a | n/a | n/a |
Boehringer Ingelheim Pharmaceuticals Curated by ChEMBL | Assay Description Agonist activity at human CB2 receptor expressed in CHO cells assessed as inhibition of forskolin-induced cAMP release | Bioorg Med Chem Lett 19: 1604-9 (2009) Article DOI: 10.1016/j.bmcl.2009.02.033 BindingDB Entry DOI: 10.7270/Q2K937DD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||