

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Methionine aminopeptidase 2 (Homo sapiens (Human)) | BDBM17428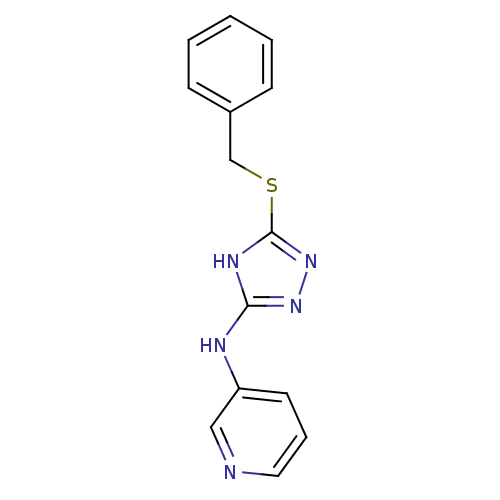 (1,2,4-Triazole Compound, 86 | N-[5-(benzylsulfanyl...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 0.0400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GSK | Assay Description MetAP2 activity was monitored by measuring the initial velocity of turnover of the artificial substrate Met-AMC. Assays were performed in 96-well mi... | J Med Chem 50: 3777-85 (2007) Article DOI: 10.1021/jm061182w BindingDB Entry DOI: 10.7270/Q2B856D7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Methionine aminopeptidase 2 (Homo sapiens (Human)) | BDBM17355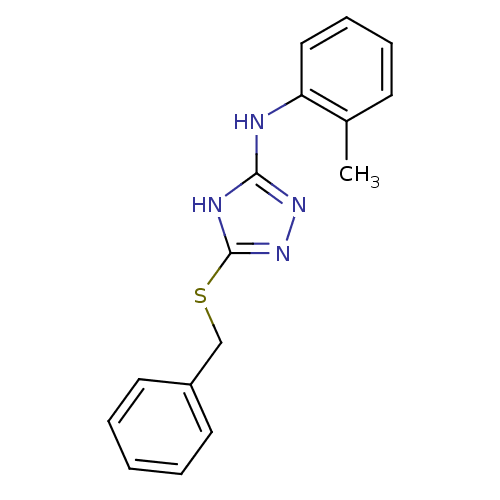 (1,2,4-Triazole Compound, 13 | 5-(benzylsulfanyl)-N...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 0.0400 | -58.8 | n/a | n/a | n/a | n/a | n/a | 7.5 | 22 |
GSK | Assay Description MetAP2 activity was monitored by measuring the initial velocity of turnover of the artificial substrate Met-AMC. Assays were performed in 96-well mi... | J Med Chem 50: 3777-85 (2007) Article DOI: 10.1021/jm061182w BindingDB Entry DOI: 10.7270/Q2B856D7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Methionine aminopeptidase 2 (Homo sapiens (Human)) | BDBM17388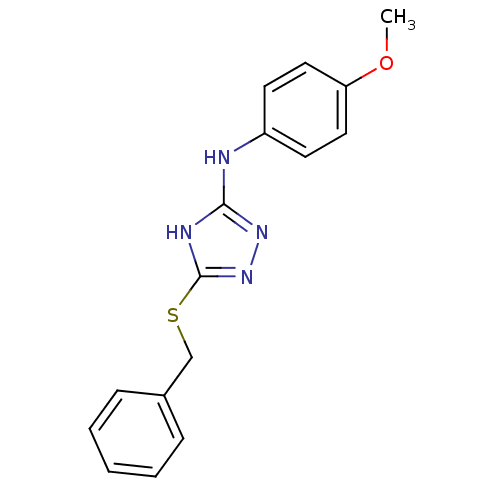 (1,2,4-Triazole Compound, 46 | 5-(benzylsulfanyl)-N...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 0.0500 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GSK | Assay Description MetAP2 activity was monitored by measuring the initial velocity of turnover of the artificial substrate Met-AMC. Assays were performed in 96-well mi... | J Med Chem 50: 3777-85 (2007) Article DOI: 10.1021/jm061182w BindingDB Entry DOI: 10.7270/Q2B856D7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Methionine aminopeptidase 2 (Homo sapiens (Human)) | BDBM17365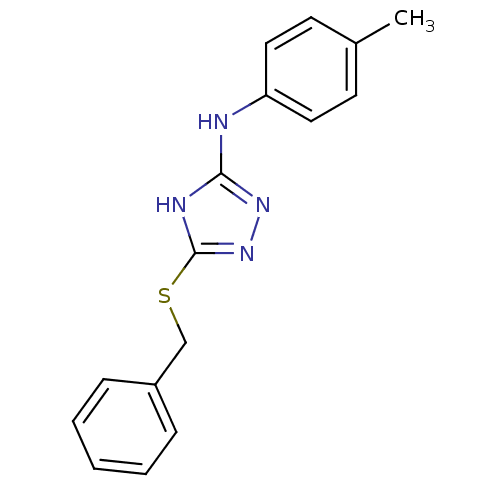 (1,2,4-Triazole Compound, 23 | 5-(benzylsulfanyl)-N...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 0.0700 | -57.4 | n/a | n/a | n/a | n/a | n/a | 7.5 | 22 |
GSK | Assay Description MetAP2 activity was monitored by measuring the initial velocity of turnover of the artificial substrate Met-AMC. Assays were performed in 96-well mi... | J Med Chem 50: 3777-85 (2007) Article DOI: 10.1021/jm061182w BindingDB Entry DOI: 10.7270/Q2B856D7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Methionine aminopeptidase 2 (Homo sapiens (Human)) | BDBM17390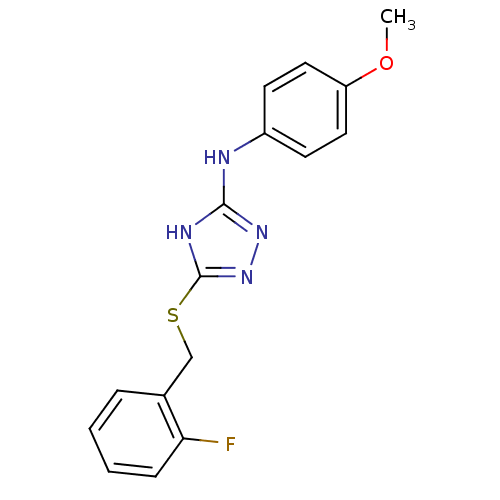 (1,2,4-Triazole Compound, 48 | 5-{[(2-fluorophenyl)...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 0.130 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GSK | Assay Description MetAP2 activity was monitored by measuring the initial velocity of turnover of the artificial substrate Met-AMC. Assays were performed in 96-well mi... | J Med Chem 50: 3777-85 (2007) Article DOI: 10.1021/jm061182w BindingDB Entry DOI: 10.7270/Q2B856D7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Methionine aminopeptidase 2 (Homo sapiens (Human)) | BDBM17362 (1,2,4-Triazole Compound, 20 | 5-[(3-methylbut-2-en...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 0.150 | -55.5 | n/a | n/a | n/a | n/a | n/a | 7.5 | 22 |
GSK | Assay Description MetAP2 activity was monitored by measuring the initial velocity of turnover of the artificial substrate Met-AMC. Assays were performed in 96-well mi... | J Med Chem 50: 3777-85 (2007) Article DOI: 10.1021/jm061182w BindingDB Entry DOI: 10.7270/Q2B856D7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Methionine aminopeptidase 2 (Homo sapiens (Human)) | BDBM17395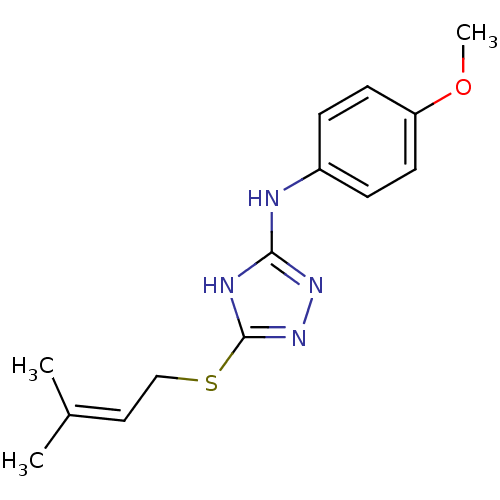 (1,2,4-Triazole Compound, 53 | N-(4-methoxyphenyl)-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 0.160 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GSK | Assay Description MetAP2 activity was monitored by measuring the initial velocity of turnover of the artificial substrate Met-AMC. Assays were performed in 96-well mi... | J Med Chem 50: 3777-85 (2007) Article DOI: 10.1021/jm061182w BindingDB Entry DOI: 10.7270/Q2B856D7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Methionine aminopeptidase 2 (Homo sapiens (Human)) | BDBM17358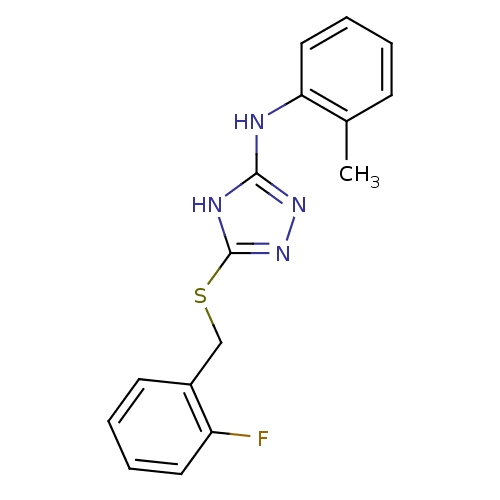 (1,2,4-Triazole Compound, 16 | 5-{[(2-fluorophenyl)...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 0.230 | -54.5 | n/a | n/a | n/a | n/a | n/a | 7.5 | 22 |
GSK | Assay Description MetAP2 activity was monitored by measuring the initial velocity of turnover of the artificial substrate Met-AMC. Assays were performed in 96-well mi... | J Med Chem 50: 3777-85 (2007) Article DOI: 10.1021/jm061182w BindingDB Entry DOI: 10.7270/Q2B856D7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium-dependent serotonin transporter (Homo sapiens (Human)) | BDBM50135249 ((S)-1-((2S,4R)-4-(5-fluorobenzo[b]thiophen-2-yl)-2...) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 0.240 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories Curated by ChEMBL | Assay Description Displacement of [3H]paroxetine from 5HT reuptake site | Bioorg Med Chem Lett 16: 2347-51 (2006) Article DOI: 10.1016/j.bmcl.2005.11.007 BindingDB Entry DOI: 10.7270/Q2W958RV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Progesterone receptor (Homo sapiens (Human)) | BDBM18627 ((10S,11S,14S,15S,17R)-17-[4-(dimethylamino)phenyl]...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | DrugBank PDB Article PubMed | 0.251 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Inhibition of 4 nM progesterone-stimulated transactivation of MMTV-Luc reporter in CV-1 cells expressing PR-B | Bioorg Med Chem Lett 15: 3203-6 (2005) Article DOI: 10.1016/j.bmcl.2005.05.001 BindingDB Entry DOI: 10.7270/Q2J67KP9 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Sodium-dependent serotonin transporter (Homo sapiens (Human)) | BDBM50130163 ((S)-1-(1H-Indol-4-yloxy)-3-[(4S,6R)-4-(4-methoxy-b...) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 0.270 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories Curated by ChEMBL | Assay Description Displacement of [3H]paroxetine from 5HT reuptake site | Bioorg Med Chem Lett 16: 2347-51 (2006) Article DOI: 10.1016/j.bmcl.2005.11.007 BindingDB Entry DOI: 10.7270/Q2W958RV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Methionine aminopeptidase 2 (Homo sapiens (Human)) | BDBM17430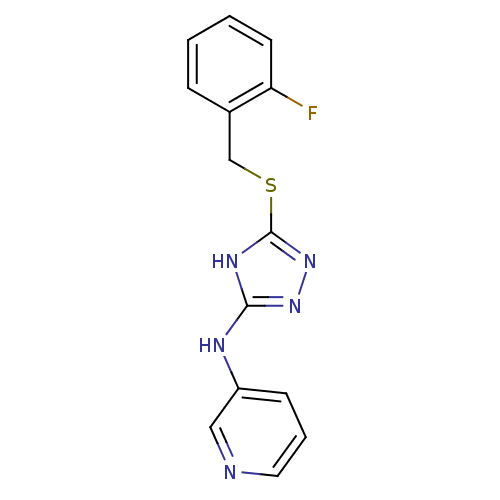 (1,2,4-Triazole Compound, 88 | N-(5-{[(2-fluorophen...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GSK | Assay Description MetAP2 activity was monitored by measuring the initial velocity of turnover of the artificial substrate Met-AMC. Assays were performed in 96-well mi... | J Med Chem 50: 3777-85 (2007) Article DOI: 10.1021/jm061182w BindingDB Entry DOI: 10.7270/Q2B856D7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium-dependent serotonin transporter (Homo sapiens (Human)) | BDBM50130152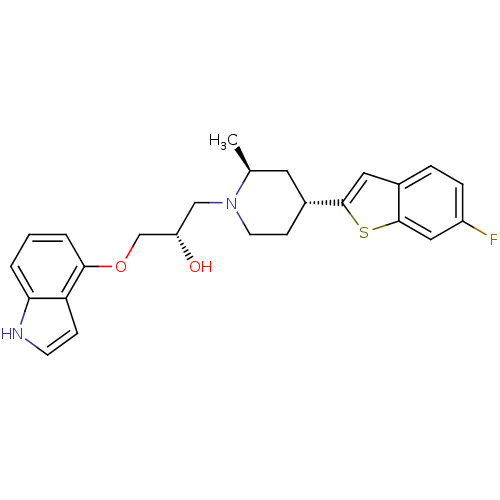 ((S)-1-(1H-indol-4-yloxy)-3-((2S,4R)-4-(6-fluoroben...) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 0.310 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories Curated by ChEMBL | Assay Description Displacement of [3H]paroxetine from 5HT reuptake site | Bioorg Med Chem Lett 16: 2347-51 (2006) Article DOI: 10.1016/j.bmcl.2005.11.007 BindingDB Entry DOI: 10.7270/Q2W958RV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Methionine aminopeptidase 2 (Homo sapiens (Human)) | BDBM17411 (1,2,4-Triazole Compound, 69 | methyl 4-[(5-{[(2-fl...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 0.410 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GSK | Assay Description MetAP2 activity was monitored by measuring the initial velocity of turnover of the artificial substrate Met-AMC. Assays were performed in 96-well mi... | J Med Chem 50: 3777-85 (2007) Article DOI: 10.1021/jm061182w BindingDB Entry DOI: 10.7270/Q2B856D7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Methionine aminopeptidase 2 (Homo sapiens (Human)) | BDBM17377 (1,2,4-Triazole Compound, 35 | 5-(benzylsulfanyl)-N...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 0.430 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GSK | Assay Description MetAP2 activity was monitored by measuring the initial velocity of turnover of the artificial substrate Met-AMC. Assays were performed in 96-well mi... | J Med Chem 50: 3777-85 (2007) Article DOI: 10.1021/jm061182w BindingDB Entry DOI: 10.7270/Q2B856D7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium-dependent serotonin transporter (Homo sapiens (Human)) | BDBM50130168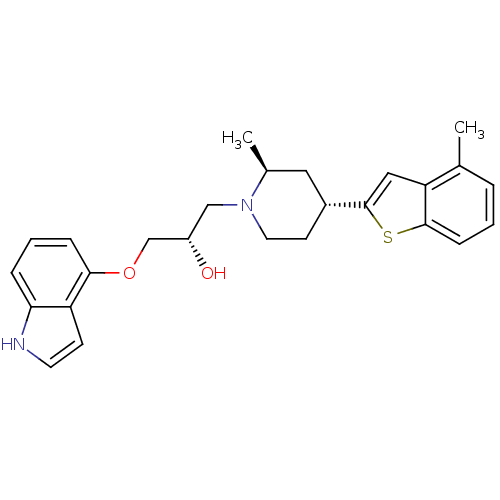 ((S)-1-(1H-Indol-4-yloxy)-3-[(4S,6R)-2-methyl-4-(4-...) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 0.440 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories Curated by ChEMBL | Assay Description Displacement of [3H]paroxetine from 5HT reuptake site | Bioorg Med Chem Lett 16: 2347-51 (2006) Article DOI: 10.1016/j.bmcl.2005.11.007 BindingDB Entry DOI: 10.7270/Q2W958RV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Methionine aminopeptidase 2 (Homo sapiens (Human)) | BDBM17416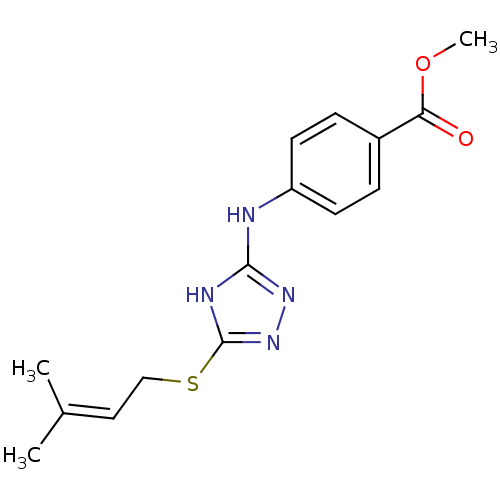 (1,2,4-Triazole Compound, 74 | methyl 4-({5-[(3-met...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GSK | Assay Description MetAP2 activity was monitored by measuring the initial velocity of turnover of the artificial substrate Met-AMC. Assays were performed in 96-well mi... | J Med Chem 50: 3777-85 (2007) Article DOI: 10.1021/jm061182w BindingDB Entry DOI: 10.7270/Q2B856D7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Methionine aminopeptidase 2 (Homo sapiens (Human)) | BDBM17431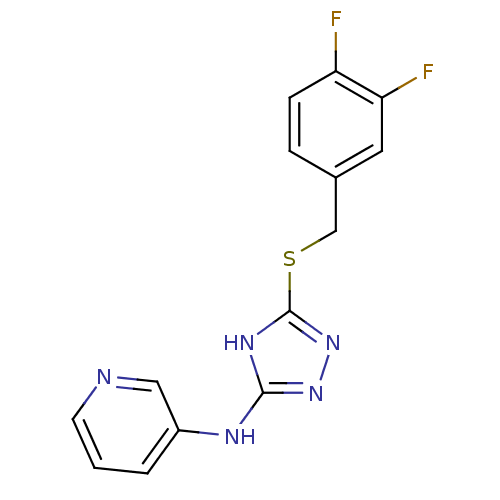 (1,2,4-Triazole Compound, 89 | N-(5-{[(3,4-difluoro...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GSK | Assay Description MetAP2 activity was monitored by measuring the initial velocity of turnover of the artificial substrate Met-AMC. Assays were performed in 96-well mi... | J Med Chem 50: 3777-85 (2007) Article DOI: 10.1021/jm061182w BindingDB Entry DOI: 10.7270/Q2B856D7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Methionine aminopeptidase 2 (Homo sapiens (Human)) | BDBM17352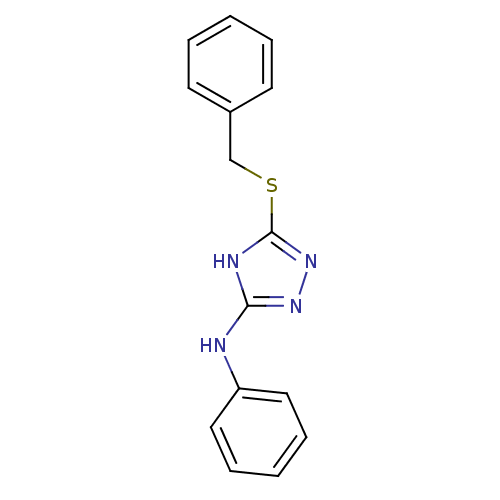 (1,2,4-Triazole Compound, 6 | 5-(benzylsulfanyl)-N-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 0.5 | -52.6 | n/a | n/a | n/a | n/a | n/a | 7.5 | 22 |
GSK | Assay Description MetAP2 activity was monitored by measuring the initial velocity of turnover of the artificial substrate Met-AMC. Assays were performed in 96-well mi... | J Med Chem 50: 3777-85 (2007) Article DOI: 10.1021/jm061182w BindingDB Entry DOI: 10.7270/Q2B856D7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium-dependent serotonin transporter (Homo sapiens (Human)) | BDBM50130157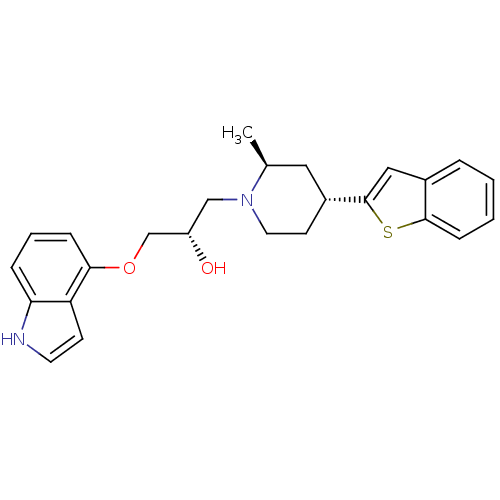 ((S)-1-((4S,6R)-4-Benzo[b]thiophen-2-yl-2-methyl-pi...) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.510 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories Curated by ChEMBL | Assay Description Displacement of [3H]paroxetine from 5HT reuptake site | Bioorg Med Chem Lett 16: 2347-51 (2006) Article DOI: 10.1016/j.bmcl.2005.11.007 BindingDB Entry DOI: 10.7270/Q2W958RV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Methionine aminopeptidase 2 (Homo sapiens (Human)) | BDBM17385 (1,2,4-Triazole Compound, 43 | N-(4-chlorophenyl)-5...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 0.520 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GSK | Assay Description MetAP2 activity was monitored by measuring the initial velocity of turnover of the artificial substrate Met-AMC. Assays were performed in 96-well mi... | J Med Chem 50: 3777-85 (2007) Article DOI: 10.1021/jm061182w BindingDB Entry DOI: 10.7270/Q2B856D7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium-dependent serotonin transporter (Homo sapiens (Human)) | BDBM50135246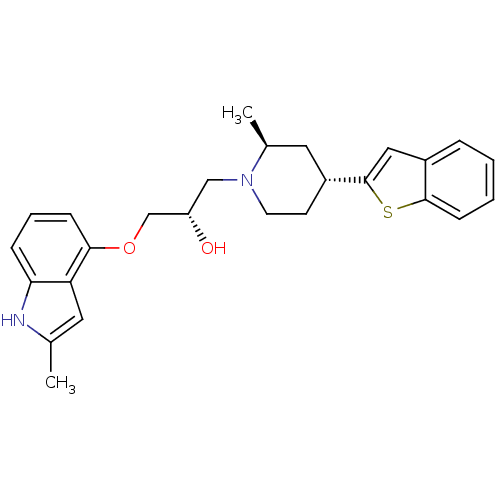 ((S)-1-((2S,4R)-4-(benzo[b]thiophen-2-yl)-2-methylp...) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.530 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories Curated by ChEMBL | Assay Description Displacement of [3H]paroxetine from 5HT reuptake site | Bioorg Med Chem Lett 16: 2347-51 (2006) Article DOI: 10.1016/j.bmcl.2005.11.007 BindingDB Entry DOI: 10.7270/Q2W958RV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium-dependent serotonin transporter (Homo sapiens (Human)) | BDBM50130169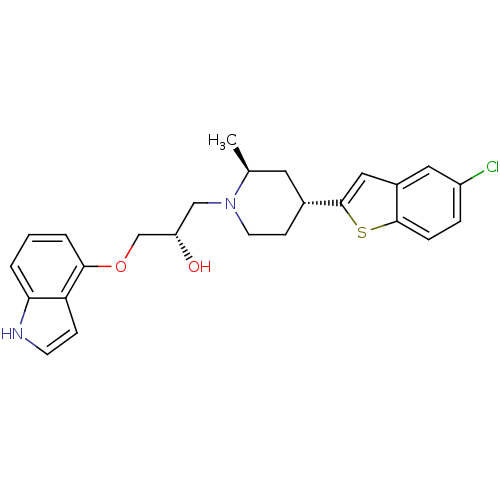 ((S)-1-(1H-indol-4-yloxy)-3-((2S,4R)-4-(5-chloroben...) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 0.580 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories Curated by ChEMBL | Assay Description Displacement of [3H]paroxetine from 5HT reuptake site | Bioorg Med Chem Lett 16: 2347-51 (2006) Article DOI: 10.1016/j.bmcl.2005.11.007 BindingDB Entry DOI: 10.7270/Q2W958RV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aurora kinase A (Homo sapiens (Human)) | BDBM13534 (CHEMBL572878 | N-[4-({4-[(3-methyl-1H-pyrazol-5-yl...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MMDB PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | 0.600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Centre de Recherches Curated by ChEMBL | Assay Description In vitro inhibition constant for Aurora-A | J Med Chem 49: 955-70 (2006) Article DOI: 10.1021/jm050786h BindingDB Entry DOI: 10.7270/Q24J0FXV | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM50403349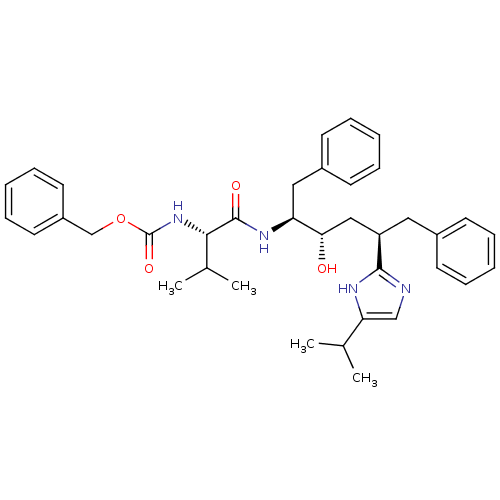 (CHEMBL407551) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | 0.600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibitory activity against HIV-1 protease | Bioorg Med Chem Lett 4: 2441-2446 (1994) Article DOI: 10.1016/S0960-894X(01)80406-6 BindingDB Entry DOI: 10.7270/Q2X63P48 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM50037121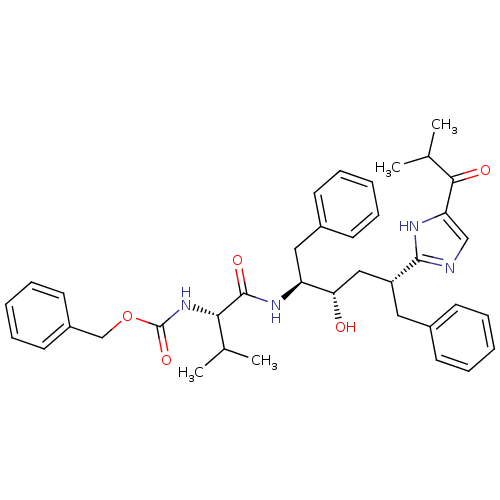 (2-[(1R,3S,4S)-1-BENZYL-4-[N-(BENZYLOXYCARBONYL)-L-...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL MMDB PC cid PC sid PDB UniChem Patents Similars | MMDB PDB PubMed | 0.600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
SmithKline Beecham Pharmaceuticals Curated by ChEMBL | Assay Description Apparent inhibition constant against recombinant HIV-1 protease | J Med Chem 37: 3100-7 (1994) BindingDB Entry DOI: 10.7270/Q2T72GG9 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Methionine aminopeptidase 2 (Homo sapiens (Human)) | BDBM17380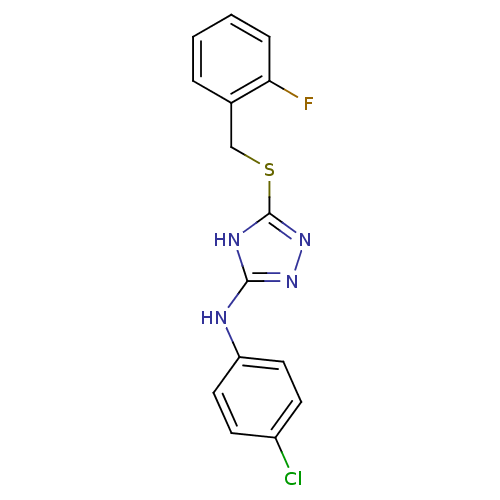 (1,2,4-Triazole Compound, 38 | N-(4-chlorophenyl)-5...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 0.610 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GSK | Assay Description MetAP2 activity was monitored by measuring the initial velocity of turnover of the artificial substrate Met-AMC. Assays were performed in 96-well mi... | J Med Chem 50: 3777-85 (2007) Article DOI: 10.1021/jm061182w BindingDB Entry DOI: 10.7270/Q2B856D7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Methionine aminopeptidase 2 (Homo sapiens (Human)) | BDBM17368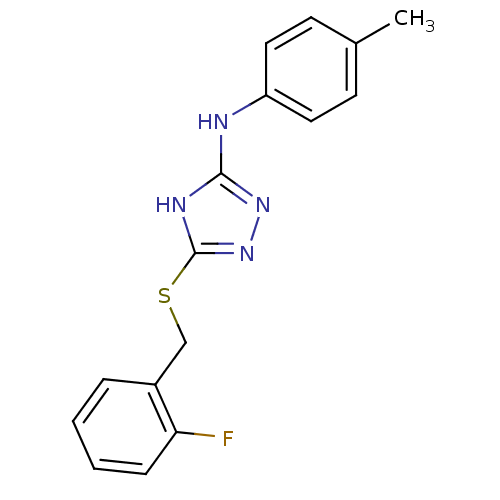 (1,2,4-Triazole Compound, 26 | 5-{[(2-fluorophenyl)...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 0.650 | -51.9 | n/a | n/a | n/a | n/a | n/a | 7.5 | 22 |
GSK | Assay Description MetAP2 activity was monitored by measuring the initial velocity of turnover of the artificial substrate Met-AMC. Assays were performed in 96-well mi... | J Med Chem 50: 3777-85 (2007) Article DOI: 10.1021/jm061182w BindingDB Entry DOI: 10.7270/Q2B856D7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Methionine aminopeptidase 2 (Homo sapiens (Human)) | BDBM17408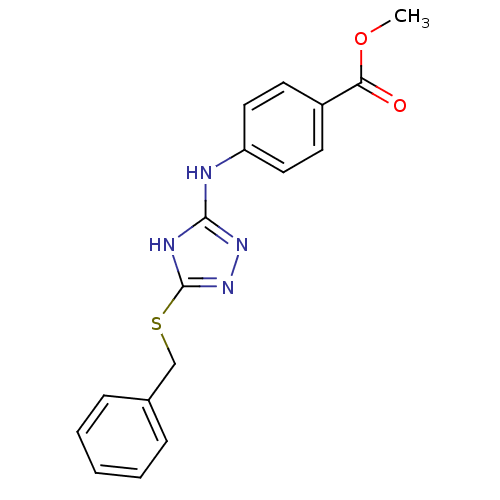 (1,2,4-Triazole Compound, 66 | methyl 4-{[5-(benzyl...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 0.700 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GSK | Assay Description MetAP2 activity was monitored by measuring the initial velocity of turnover of the artificial substrate Met-AMC. Assays were performed in 96-well mi... | J Med Chem 50: 3777-85 (2007) Article DOI: 10.1021/jm061182w BindingDB Entry DOI: 10.7270/Q2B856D7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cathepsin K (Homo sapiens (Human)) | BDBM50408519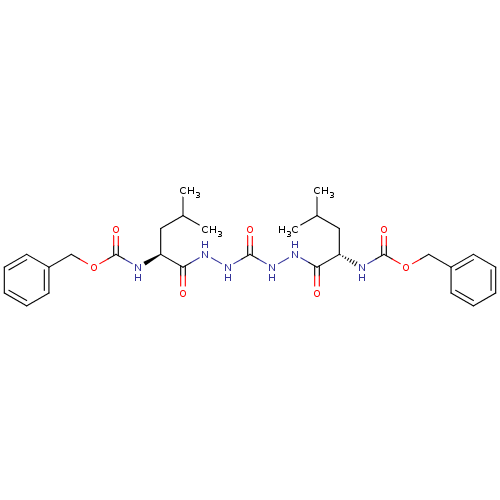 (CHEMBL115357) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid PDB UniChem Similars | Article PubMed | 0.700 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
SmithKline Beecham Pharmaceuticals Curated by ChEMBL | Assay Description Relative binding affinity was measured for Cathepsin K | J Med Chem 41: 3923-7 (1998) Article DOI: 10.1021/jm980474x BindingDB Entry DOI: 10.7270/Q2Q81F87 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM50037124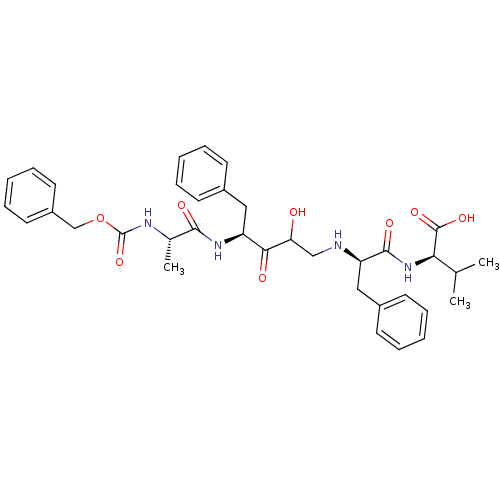 ((R)-2-{(R)-2-[(S)-4-((S)-2-Benzyloxycarbonylamino-...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.700 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
SmithKline Beecham Pharmaceuticals Curated by ChEMBL | Assay Description Apparent inhibition constant against recombinant HIV-1 protease | J Med Chem 37: 3100-7 (1994) BindingDB Entry DOI: 10.7270/Q2T72GG9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Methionine aminopeptidase 2 (Homo sapiens (Human)) | BDBM17354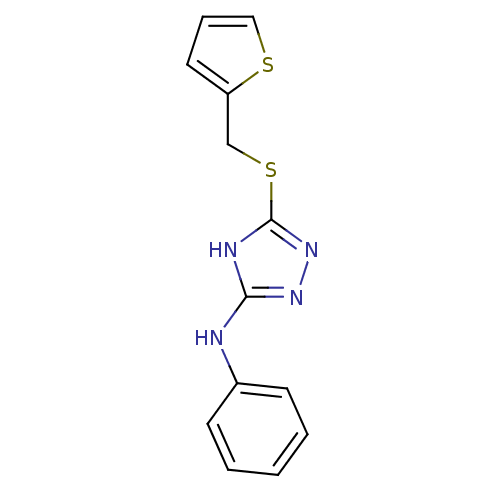 (1,2,4-Triazole Compound, 12 | N-phenyl-5-[(thiophe...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 0.75 | -51.6 | n/a | n/a | n/a | n/a | n/a | 7.5 | 22 |
GSK | Assay Description MetAP2 activity was monitored by measuring the initial velocity of turnover of the artificial substrate Met-AMC. Assays were performed in 96-well mi... | J Med Chem 50: 3777-85 (2007) Article DOI: 10.1021/jm061182w BindingDB Entry DOI: 10.7270/Q2B856D7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Methionine aminopeptidase 2 (Homo sapiens (Human)) | BDBM17418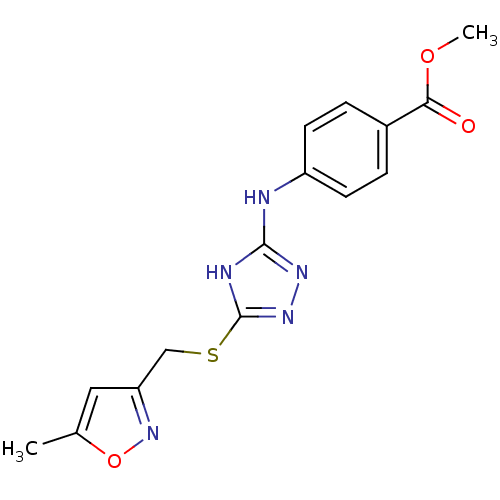 (1,2,4-Triazole Compound, 76 | methyl 4-[(5-{[(5-me...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 0.800 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GSK | Assay Description MetAP2 activity was monitored by measuring the initial velocity of turnover of the artificial substrate Met-AMC. Assays were performed in 96-well mi... | J Med Chem 50: 3777-85 (2007) Article DOI: 10.1021/jm061182w BindingDB Entry DOI: 10.7270/Q2B856D7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium-dependent serotonin transporter (Homo sapiens (Human)) | BDBM50136680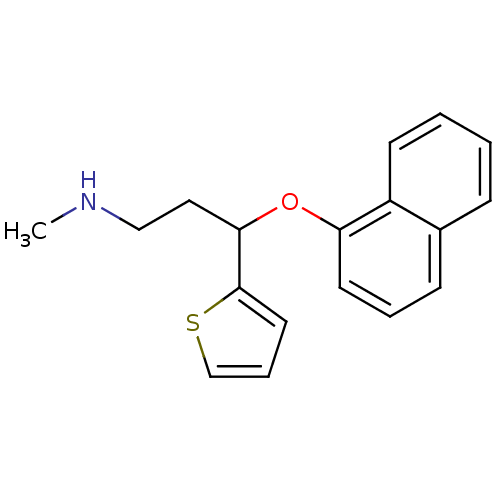 (CHEMBL424660 | N-methyl-3-(1-naphthyloxy)-3-(2-thi...) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid PDB UniChem Patents Similars | Article PubMed | 0.800 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories Curated by PDSP Ki Database | Neuropharmacology 45: 935-44 (2003) Article DOI: 10.1016/s0028-3908(03)00268-5 BindingDB Entry DOI: 10.7270/Q2VQ318R | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cathepsin K (Homo sapiens (Human)) | BDBM50408522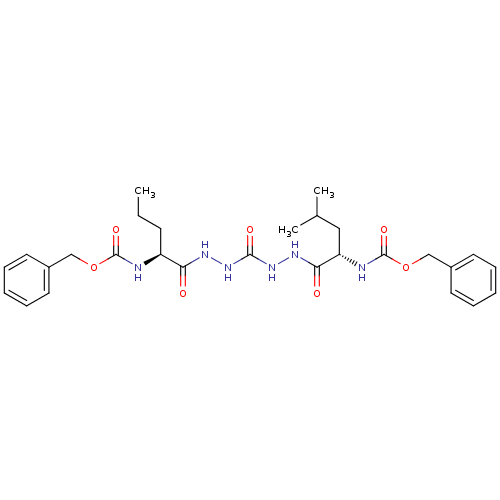 (CHEMBL126820) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.800 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
SmithKline Beecham Pharmaceuticals Curated by ChEMBL | Assay Description Relative binding affinity was measured for Cathepsin K | J Med Chem 41: 3923-7 (1998) Article DOI: 10.1021/jm980474x BindingDB Entry DOI: 10.7270/Q2Q81F87 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium-dependent serotonin transporter (Homo sapiens (Human)) | BDBM50135256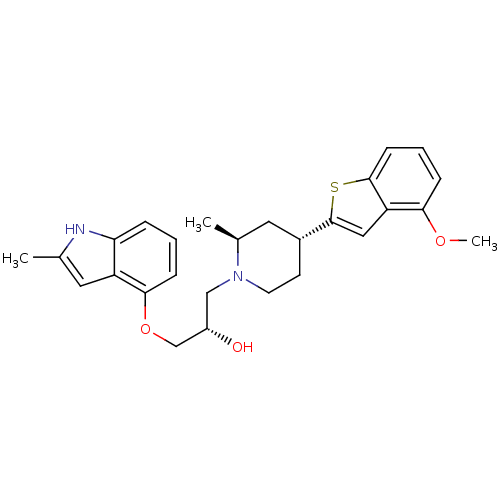 ((S)-1-((2S,4R)-4-(4-methoxybenzo[b]thiophen-2-yl)-...) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 0.860 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories Curated by ChEMBL | Assay Description Displacement of [3H]paroxetine from 5HT reuptake site | Bioorg Med Chem Lett 16: 2347-51 (2006) Article DOI: 10.1016/j.bmcl.2005.11.007 BindingDB Entry DOI: 10.7270/Q2W958RV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium-dependent serotonin transporter (Homo sapiens (Human)) | BDBM86421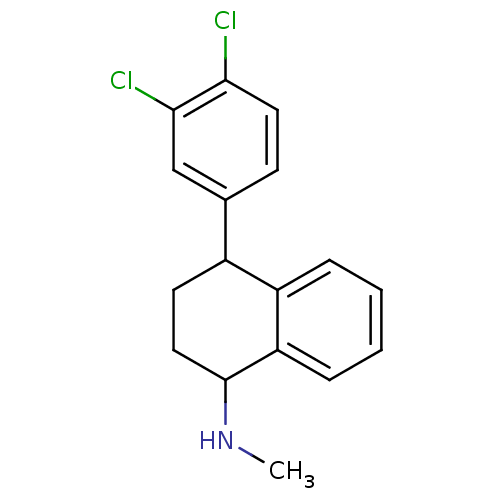 (CAS_79617-96-2 | NSC_68617 | SERTRALINE) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | 0.900 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories Curated by PDSP Ki Database | Neuropharmacology 45: 935-44 (2003) Article DOI: 10.1016/s0028-3908(03)00268-5 BindingDB Entry DOI: 10.7270/Q2VQ318R | ||||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Methionine aminopeptidase 2 (Homo sapiens (Human)) | BDBM17413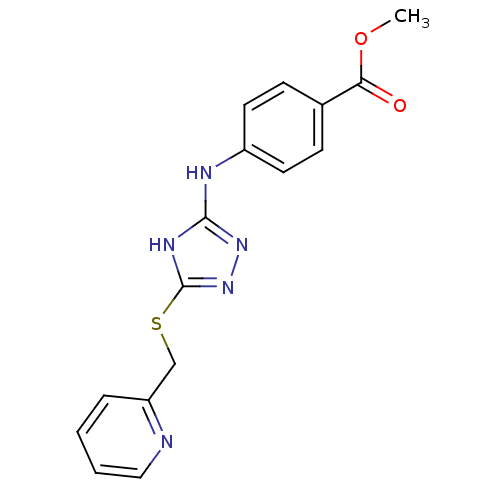 (1,2,4-Triazole Compound, 71 | methyl 4-({5-[(pyrid...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 0.900 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GSK | Assay Description MetAP2 activity was monitored by measuring the initial velocity of turnover of the artificial substrate Met-AMC. Assays were performed in 96-well mi... | J Med Chem 50: 3777-85 (2007) Article DOI: 10.1021/jm061182w BindingDB Entry DOI: 10.7270/Q2B856D7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Methionine aminopeptidase 2 (Homo sapiens (Human)) | BDBM17414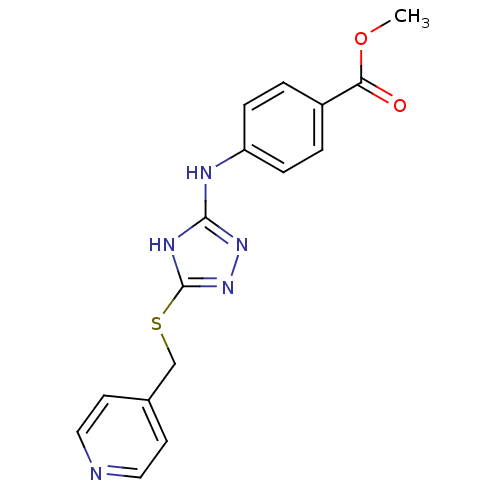 (1,2,4-Triazole Compound, 72 | methyl 4-({5-[(pyrid...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 0.900 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GSK | Assay Description MetAP2 activity was monitored by measuring the initial velocity of turnover of the artificial substrate Met-AMC. Assays were performed in 96-well mi... | J Med Chem 50: 3777-85 (2007) Article DOI: 10.1021/jm061182w BindingDB Entry DOI: 10.7270/Q2B856D7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Methionine aminopeptidase 2 (Homo sapiens (Human)) | BDBM17405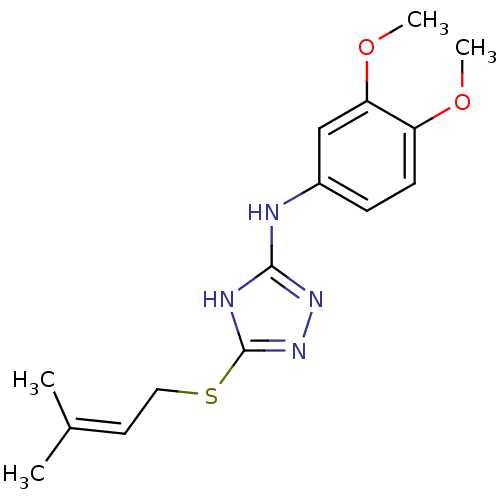 (1,2,4-Triazole Compound, 63 | N-(3,4-dimethoxyphen...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 0.900 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GSK | Assay Description MetAP2 activity was monitored by measuring the initial velocity of turnover of the artificial substrate Met-AMC. Assays were performed in 96-well mi... | J Med Chem 50: 3777-85 (2007) Article DOI: 10.1021/jm061182w BindingDB Entry DOI: 10.7270/Q2B856D7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aurora kinase B (Homo sapiens (Human)) | BDBM26292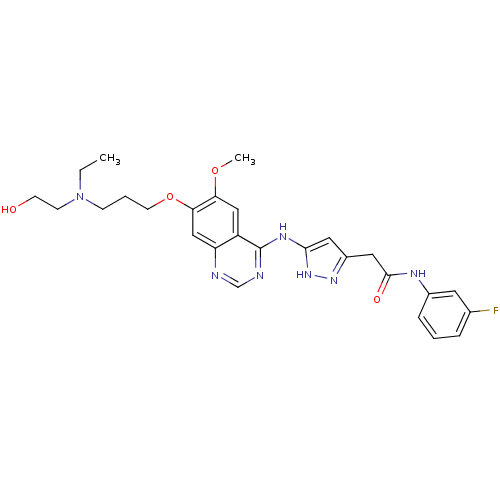 (2-{3-[(7-{3-[ethyl(2-hydroxyethyl)amino]propoxy}-6...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1 | -50.9 | n/a | n/a | n/a | n/a | n/a | 7.5 | 22 |
AstraZeneca | Assay Description In vitro kinase assay using recombinant Aurora B bound to INCENP was incubated at room temperature with substrate, and test compounds in the presence... | J Med Chem 50: 2213-24 (2007) Article DOI: 10.1021/jm061335f BindingDB Entry DOI: 10.7270/Q2GQ6W24 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aurora kinase B (Homo sapiens (Human)) | BDBM26284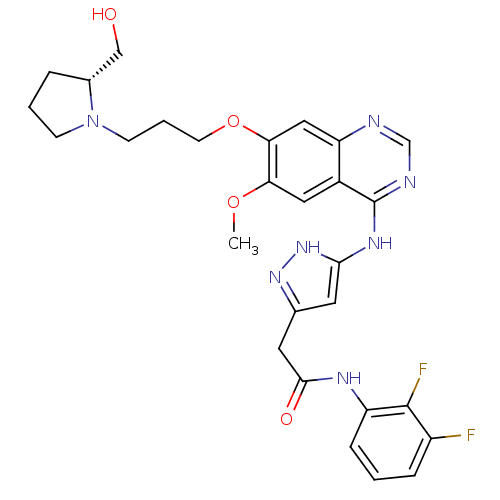 (N-(2,3-difluorophenyl)-2-{3-[(7-{3-[(2R)-2-(hydrox...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 1 | -50.9 | n/a | n/a | n/a | n/a | n/a | 7.5 | 22 |
AstraZeneca | Assay Description In vitro kinase assay using recombinant Aurora B bound to INCENP was incubated at room temperature with substrate, and test compounds in the presence... | J Med Chem 50: 2213-24 (2007) Article DOI: 10.1021/jm061335f BindingDB Entry DOI: 10.7270/Q2GQ6W24 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aurora kinase B (Homo sapiens (Human)) | BDBM26290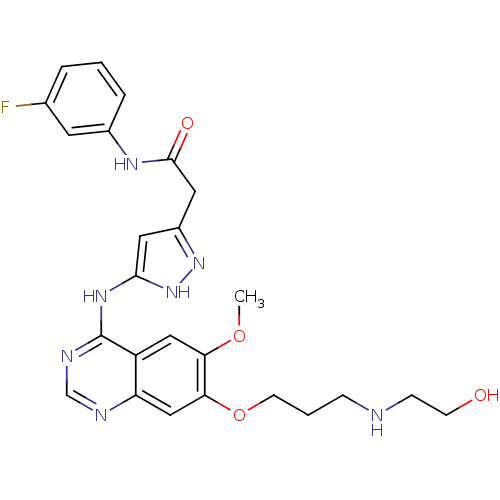 (N-(3-fluorophenyl)-2-{3-[(7-{3-[(2-hydroxyethyl)am...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 1 | -50.9 | n/a | n/a | n/a | n/a | n/a | 7.5 | 22 |
AstraZeneca | Assay Description In vitro kinase assay using recombinant Aurora B bound to INCENP was incubated at room temperature with substrate, and test compounds in the presence... | J Med Chem 50: 2213-24 (2007) Article DOI: 10.1021/jm061335f BindingDB Entry DOI: 10.7270/Q2GQ6W24 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aurora kinase B (Homo sapiens (Human)) | BDBM26295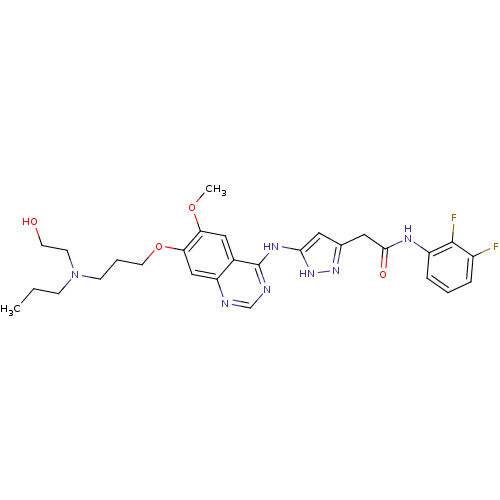 (CHEMBL214849 | N-(2,3-difluorophenyl)-2-{3-[(7-{3-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | <1 | <-50.9 | n/a | n/a | n/a | n/a | n/a | 7.5 | 22 |
AstraZeneca | Assay Description In vitro kinase assay using recombinant Aurora B bound to INCENP was incubated at room temperature with substrate, and test compounds in the presence... | J Med Chem 50: 2213-24 (2007) Article DOI: 10.1021/jm061335f BindingDB Entry DOI: 10.7270/Q2GQ6W24 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aurora kinase B (Homo sapiens (Human)) | BDBM26296 (CHEMBL216053 | N-(2,3-difluorophenyl)-2-{3-[(7-{3-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | <1 | <-50.9 | n/a | n/a | n/a | n/a | n/a | 7.5 | 22 |
AstraZeneca | Assay Description In vitro kinase assay using recombinant Aurora B bound to INCENP was incubated at room temperature with substrate, and test compounds in the presence... | J Med Chem 50: 2213-24 (2007) Article DOI: 10.1021/jm061335f BindingDB Entry DOI: 10.7270/Q2GQ6W24 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aurora kinase B (Homo sapiens (Human)) | BDBM26297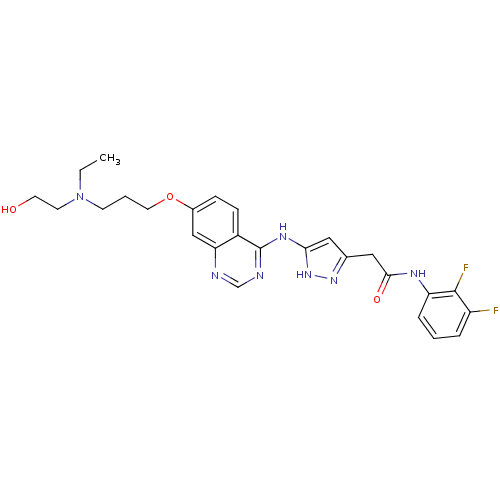 (CHEMBL215322 | N-(2,3-difluorophenyl)-2-{3-[(7-{3-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | <1 | <-51.4 | n/a | n/a | n/a | n/a | n/a | n/a | 25 |
AstraZeneca | Assay Description In vitro kinase assay using recombinant Aurora B bound to INCENP was incubated at room temperature with substrate, and test compounds in the presence... | J Med Chem 50: 2213-24 (2007) Article DOI: 10.1021/jm061335f BindingDB Entry DOI: 10.7270/Q2GQ6W24 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aurora kinase B (Homo sapiens (Human)) | BDBM26298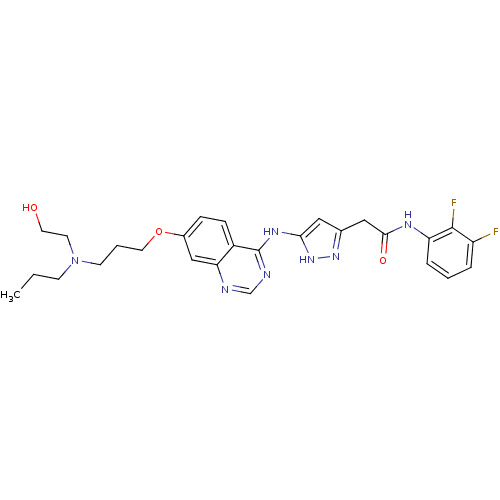 (N-(2,3-difluorophenyl)-2-{3-[(7-{3-[(2-hydroxyethy...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | <1 | <-51.4 | n/a | n/a | n/a | n/a | n/a | n/a | 25 |
AstraZeneca | Assay Description In vitro kinase assay using recombinant Aurora B bound to INCENP was incubated at room temperature with substrate, and test compounds in the presence... | J Med Chem 50: 2213-24 (2007) Article DOI: 10.1021/jm061335f BindingDB Entry DOI: 10.7270/Q2GQ6W24 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aurora kinase B (Homo sapiens (Human)) | BDBM26299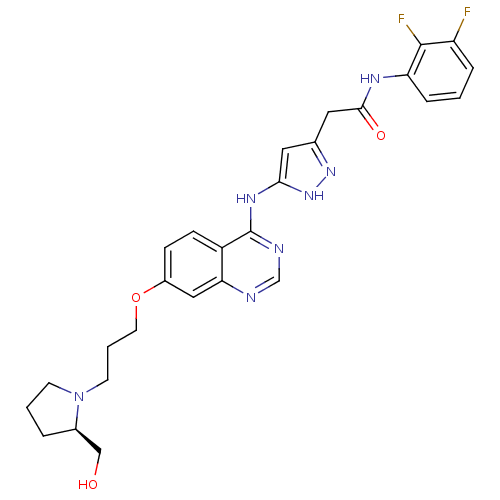 (N-(2,3-difluorophenyl)-2-{3-[(7-{3-[(2R)-2-(hydrox...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | <1 | <-51.4 | n/a | n/a | n/a | n/a | n/a | n/a | 25 |
AstraZeneca | Assay Description In vitro kinase assay using recombinant Aurora B bound to INCENP was incubated at room temperature with substrate, and test compounds in the presence... | J Med Chem 50: 2213-24 (2007) Article DOI: 10.1021/jm061335f BindingDB Entry DOI: 10.7270/Q2GQ6W24 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aurora kinase B (Homo sapiens (Human)) | BDBM26300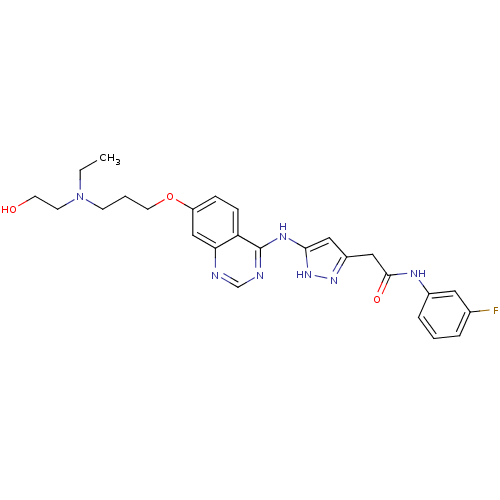 (2-{3-[(7-{3-[ethyl(2-hydroxyethyl)amino]propoxy}qu...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | <1 | <-51.4 | n/a | n/a | n/a | n/a | n/a | n/a | 25 |
AstraZeneca | Assay Description In vitro kinase assay using recombinant Aurora B bound to INCENP was incubated at room temperature with substrate, and test compounds in the presence... | J Med Chem 50: 2213-24 (2007) Article DOI: 10.1021/jm061335f BindingDB Entry DOI: 10.7270/Q2GQ6W24 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aurora kinase B (Homo sapiens (Human)) | BDBM26301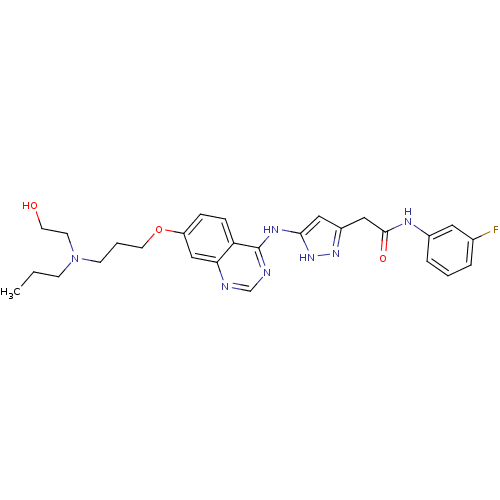 (N-(3-fluorophenyl)-2-{3-[(7-{3-[(2-hydroxyethyl)(p...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | <1 | <-51.4 | n/a | n/a | n/a | n/a | n/a | n/a | 25 |
AstraZeneca | Assay Description In vitro kinase assay using recombinant Aurora B bound to INCENP was incubated at room temperature with substrate, and test compounds in the presence... | J Med Chem 50: 2213-24 (2007) Article DOI: 10.1021/jm061335f BindingDB Entry DOI: 10.7270/Q2GQ6W24 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 1254 total ) | Next | Last >> |