

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Cannabinoid receptor 2 (Homo sapiens (Human)) | BDBM50330773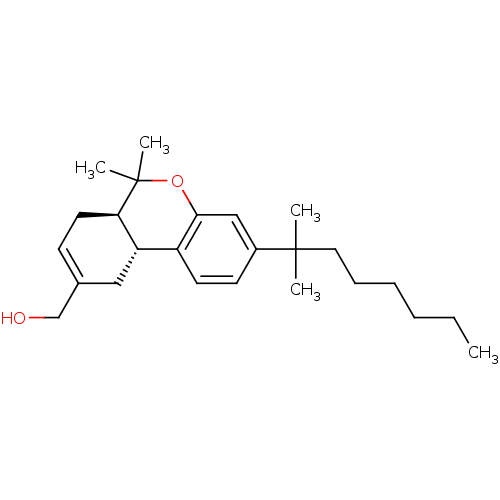 (((6aR,10aR)-6,6-dimethyl-3-(2-methyloctan-2-yl)-6a...) | PDB Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 0.0320 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Clemson University Curated by ChEMBL | Assay Description Binding affinity to CB2 receptor | Bioorg Med Chem 18: 7809-15 (2010) Article DOI: 10.1016/j.bmc.2010.09.061 BindingDB Entry DOI: 10.7270/Q2ZK5HP7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium-dependent serotonin transporter (Homo sapiens (Human)) | BDBM50135249 ((S)-1-((2S,4R)-4-(5-fluorobenzo[b]thiophen-2-yl)-2...) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 0.240 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories Curated by ChEMBL | Assay Description Displacement of [3H]paroxetine from 5HT reuptake site | Bioorg Med Chem Lett 16: 2347-51 (2006) Article DOI: 10.1016/j.bmcl.2005.11.007 BindingDB Entry DOI: 10.7270/Q2W958RV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium-dependent serotonin transporter (Homo sapiens (Human)) | BDBM50130163 ((S)-1-(1H-Indol-4-yloxy)-3-[(4S,6R)-4-(4-methoxy-b...) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 0.270 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories Curated by ChEMBL | Assay Description Displacement of [3H]paroxetine from 5HT reuptake site | Bioorg Med Chem Lett 16: 2347-51 (2006) Article DOI: 10.1016/j.bmcl.2005.11.007 BindingDB Entry DOI: 10.7270/Q2W958RV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium-dependent serotonin transporter (Homo sapiens (Human)) | BDBM50130152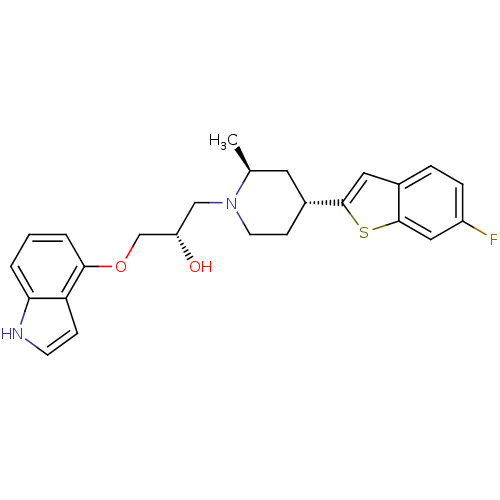 ((S)-1-(1H-indol-4-yloxy)-3-((2S,4R)-4-(6-fluoroben...) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 0.310 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories Curated by ChEMBL | Assay Description Displacement of [3H]paroxetine from 5HT reuptake site | Bioorg Med Chem Lett 16: 2347-51 (2006) Article DOI: 10.1016/j.bmcl.2005.11.007 BindingDB Entry DOI: 10.7270/Q2W958RV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium-dependent serotonin transporter (Homo sapiens (Human)) | BDBM50130168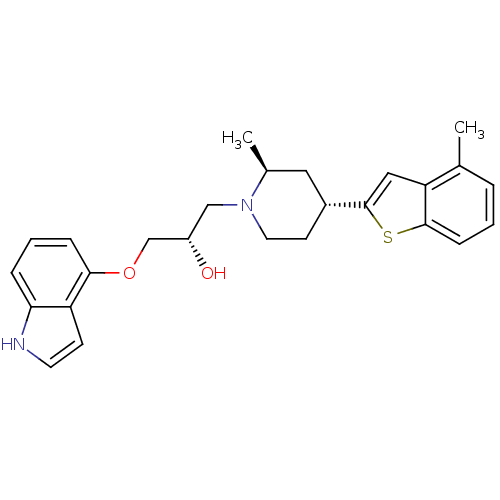 ((S)-1-(1H-Indol-4-yloxy)-3-[(4S,6R)-2-methyl-4-(4-...) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 0.440 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories Curated by ChEMBL | Assay Description Displacement of [3H]paroxetine from 5HT reuptake site | Bioorg Med Chem Lett 16: 2347-51 (2006) Article DOI: 10.1016/j.bmcl.2005.11.007 BindingDB Entry DOI: 10.7270/Q2W958RV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium-dependent serotonin transporter (Rattus norvegicus (rat)) | BDBM84745 (CAS_136434-34-9 | DULOXETINE | LY-248686 | LY24868...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase DrugBank MCE PC cid PC sid PDB UniChem Patents Similars | Article PubMed | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories Curated by PDSP Ki Database | Neuropsychopharmacology 25: 871-80 (2001) Article DOI: 10.1016/S0893-133X(01)00298-6 BindingDB Entry DOI: 10.7270/Q25M648W | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium-dependent serotonin transporter (Homo sapiens (Human)) | BDBM50130157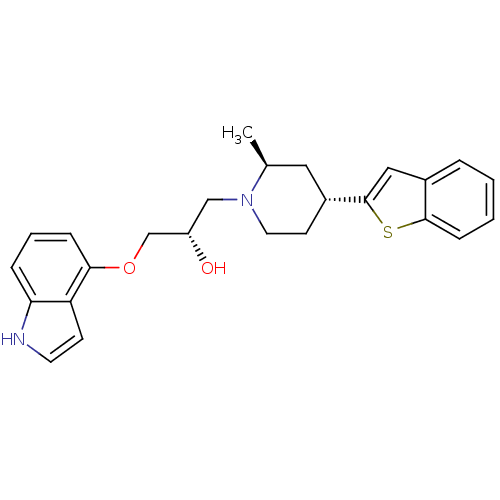 ((S)-1-((4S,6R)-4-Benzo[b]thiophen-2-yl-2-methyl-pi...) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.510 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories Curated by ChEMBL | Assay Description Displacement of [3H]paroxetine from 5HT reuptake site | Bioorg Med Chem Lett 16: 2347-51 (2006) Article DOI: 10.1016/j.bmcl.2005.11.007 BindingDB Entry DOI: 10.7270/Q2W958RV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium-dependent serotonin transporter (Homo sapiens (Human)) | BDBM50135246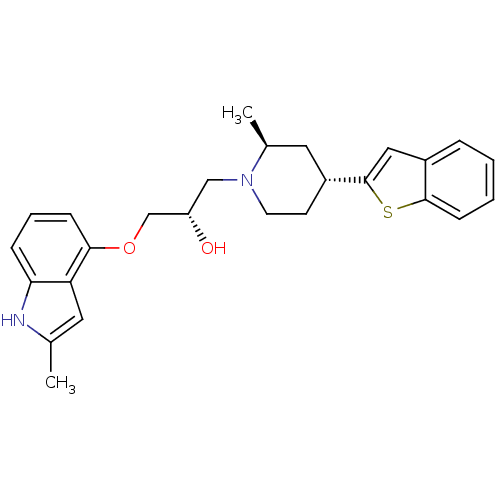 ((S)-1-((2S,4R)-4-(benzo[b]thiophen-2-yl)-2-methylp...) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.530 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories Curated by ChEMBL | Assay Description Displacement of [3H]paroxetine from 5HT reuptake site | Bioorg Med Chem Lett 16: 2347-51 (2006) Article DOI: 10.1016/j.bmcl.2005.11.007 BindingDB Entry DOI: 10.7270/Q2W958RV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Rattus norvegicus (rat)) | BDBM50072775 (2-((1R,2R,5R)-5-hydroxy-2-(3-hydroxypropyl)cyclohe...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | 0.580 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Clemson University Curated by ChEMBL | Assay Description Displacement of [3H]CP-55940 from CB1 receptor in Sprague-Dawley rat brain membrane | Bioorg Med Chem 16: 322-35 (2008) Article DOI: 10.1016/j.bmc.2007.09.033 BindingDB Entry DOI: 10.7270/Q22V2H0T | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Sodium-dependent serotonin transporter (Homo sapiens (Human)) | BDBM50130169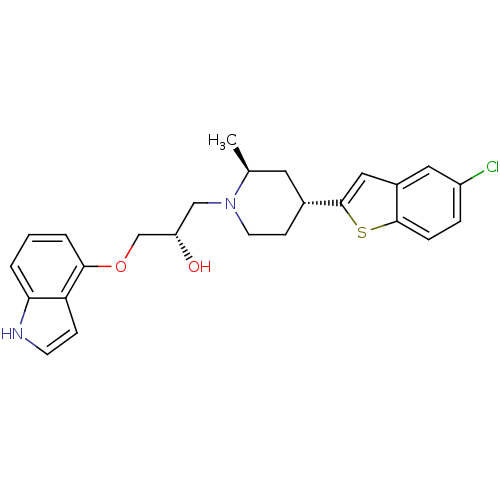 ((S)-1-(1H-indol-4-yloxy)-3-((2S,4R)-4-(5-chloroben...) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 0.580 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories Curated by ChEMBL | Assay Description Displacement of [3H]paroxetine from 5HT reuptake site | Bioorg Med Chem Lett 16: 2347-51 (2006) Article DOI: 10.1016/j.bmcl.2005.11.007 BindingDB Entry DOI: 10.7270/Q2W958RV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Imidazoleglycerol-phosphate dehydratase (Cryptococcus neoformans) | BDBM50079743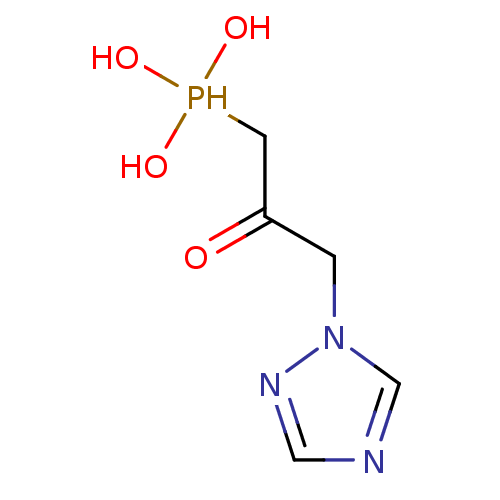 ((2-Hydroxy-3-[1,2,4]triazol-1-yl-propyl)-phosphoni...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | PubMed | 0.600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Monsanto Company Curated by ChEMBL | Assay Description Binding affinity imidazole glycerol phosphate dehydratase (IGPD) obtained from Cryptococcus neoformans | Bioorg Med Chem Lett 9: 2053-8 (1999) BindingDB Entry DOI: 10.7270/Q29P30T6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 2 (Homo sapiens (Human)) | BDBM50072775 (2-((1R,2R,5R)-5-hydroxy-2-(3-hydroxypropyl)cyclohe...) | PDB Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | 0.690 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Clemson University Curated by ChEMBL | Assay Description Displacement of [3H]CP-55940 from human cloned CB2 receptor | Bioorg Med Chem 16: 322-35 (2008) Article DOI: 10.1016/j.bmc.2007.09.033 BindingDB Entry DOI: 10.7270/Q22V2H0T | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Sodium-dependent serotonin transporter (Homo sapiens (Human)) | BDBM50136680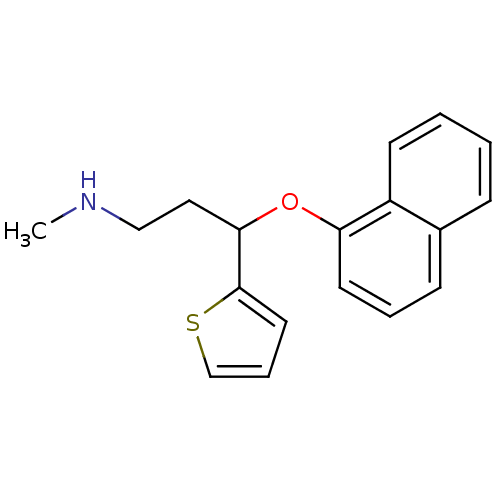 (CHEMBL424660 | N-methyl-3-(1-naphthyloxy)-3-(2-thi...) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid PDB UniChem Patents Similars | Article PubMed | 0.800 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories Curated by PDSP Ki Database | Neuropharmacology 45: 935-44 (2003) Article DOI: 10.1016/s0028-3908(03)00268-5 BindingDB Entry DOI: 10.7270/Q2VQ318R | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium-dependent serotonin transporter (Homo sapiens (Human)) | BDBM50135256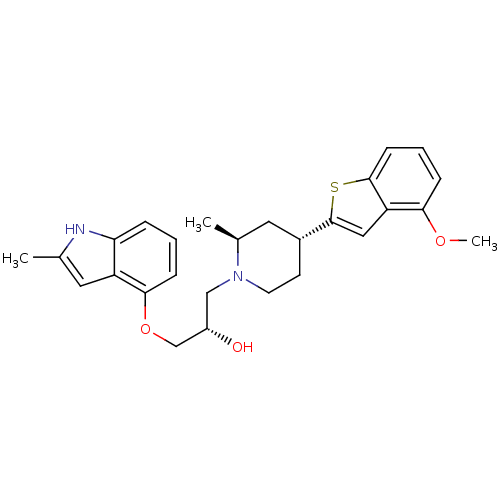 ((S)-1-((2S,4R)-4-(4-methoxybenzo[b]thiophen-2-yl)-...) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 0.860 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories Curated by ChEMBL | Assay Description Displacement of [3H]paroxetine from 5HT reuptake site | Bioorg Med Chem Lett 16: 2347-51 (2006) Article DOI: 10.1016/j.bmcl.2005.11.007 BindingDB Entry DOI: 10.7270/Q2W958RV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Presenilin-1 (Homo sapiens (Human)) | BDBM50476859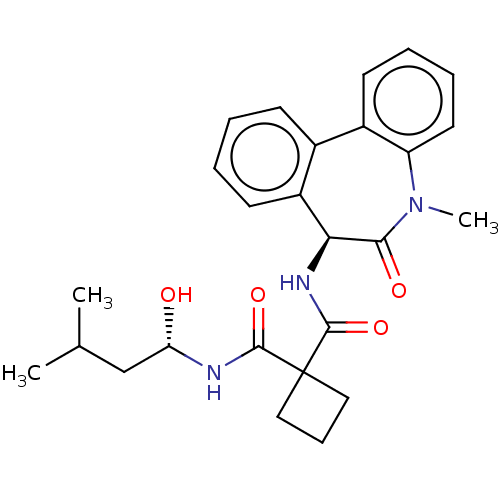 (CHEMBL232938) | PDB MMDB UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.900 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Co. Curated by ChEMBL | Assay Description Displacement of [3H]succinamide from gamma secretase in THP1 cell membranes | Bioorg Med Chem Lett 17: 3910-5 (2007) Article DOI: 10.1016/j.bmcl.2007.04.102 BindingDB Entry DOI: 10.7270/Q2M90CFP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium-dependent serotonin transporter (Homo sapiens (Human)) | BDBM86421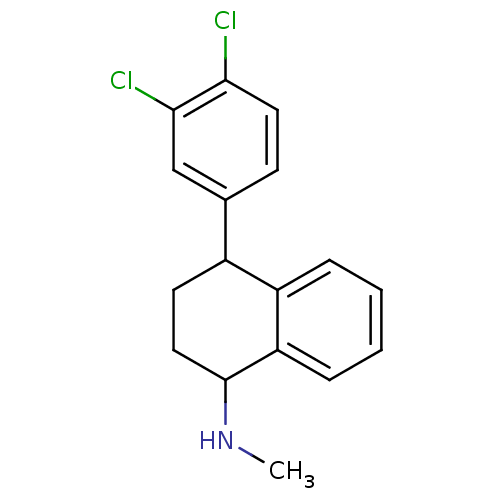 (CAS_79617-96-2 | NSC_68617 | SERTRALINE) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | 0.900 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories Curated by PDSP Ki Database | Neuropharmacology 45: 935-44 (2003) Article DOI: 10.1016/s0028-3908(03)00268-5 BindingDB Entry DOI: 10.7270/Q2VQ318R | ||||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Adenosine receptor A2b (Homo sapiens (Human)) | BDBM50150074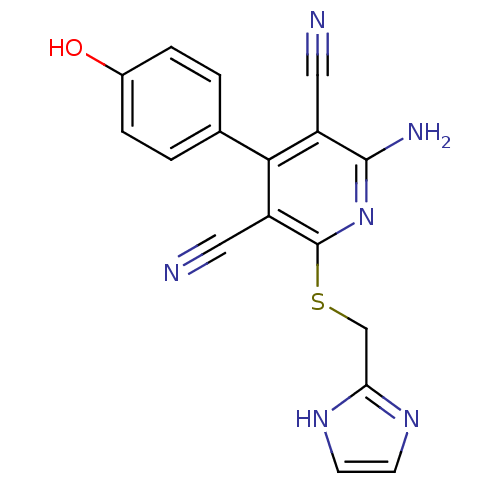 (2-Amino-4-(4-hydroxy-phenyl)-6-(1H-imidazol-2-ylme...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Citation and Details Article DOI: 10.1021/acsmedchemlett.2c00099 BindingDB Entry DOI: 10.7270/Q23200WW | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium-dependent serotonin transporter (Homo sapiens (Human)) | BDBM50130167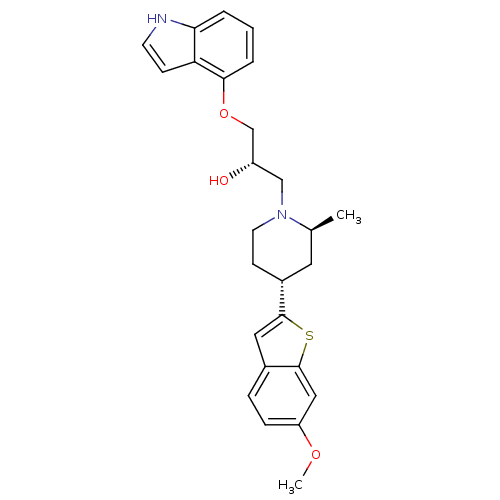 ((S)-1-(1H-Indol-4-yloxy)-3-[(4S,6R)-4-(6-methoxy-b...) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 1.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories Curated by ChEMBL | Assay Description Displacement of [3H]paroxetine from 5HT reuptake site | Bioorg Med Chem Lett 16: 2347-51 (2006) Article DOI: 10.1016/j.bmcl.2005.11.007 BindingDB Entry DOI: 10.7270/Q2W958RV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Presenilin-1 (Homo sapiens (Human)) | BDBM50476878 (CHEMBL399204) | PDB MMDB UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 1.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Co. Curated by ChEMBL | Assay Description Displacement of [3H]succinamide from gamma secretase in THP1 cell membranes | Bioorg Med Chem Lett 17: 3910-5 (2007) Article DOI: 10.1016/j.bmcl.2007.04.102 BindingDB Entry DOI: 10.7270/Q2M90CFP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium-dependent serotonin transporter (Homo sapiens (Human)) | BDBM50130165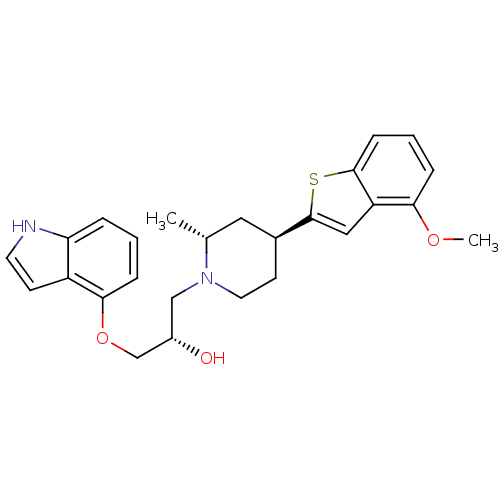 ((S)-1-(1H-Indol-4-yloxy)-3-[(4R,6S)-4-(4-methoxy-b...) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 1.15 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories Curated by ChEMBL | Assay Description Displacement of [3H]paroxetine from 5HT reuptake site | Bioorg Med Chem Lett 16: 2347-51 (2006) Article DOI: 10.1016/j.bmcl.2005.11.007 BindingDB Entry DOI: 10.7270/Q2W958RV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Homo sapiens (Human)) | BDBM50330773 (((6aR,10aR)-6,6-dimethyl-3-(2-methyloctan-2-yl)-6a...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 1.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Clemson University Curated by ChEMBL | Assay Description Binding affinity to CB1 receptor | Bioorg Med Chem 18: 7809-15 (2010) Article DOI: 10.1016/j.bmc.2010.09.061 BindingDB Entry DOI: 10.7270/Q2ZK5HP7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Presenilin-1 (Homo sapiens (Human)) | BDBM50476861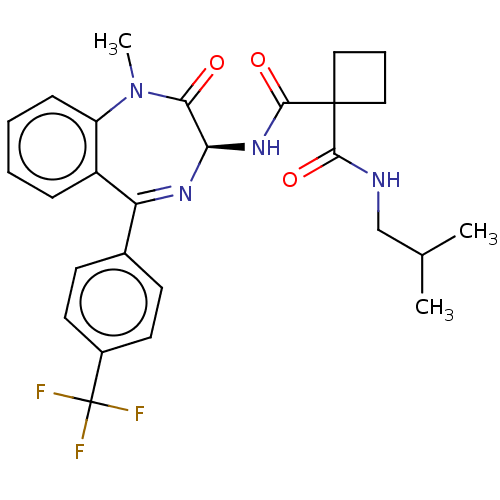 (CHEMBL401044) | PDB MMDB UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 1.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Co. Curated by ChEMBL | Assay Description Displacement of [3H]succinamide from gamma secretase in THP1 cell membranes | Bioorg Med Chem Lett 17: 3910-5 (2007) Article DOI: 10.1016/j.bmcl.2007.04.102 BindingDB Entry DOI: 10.7270/Q2M90CFP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Presenilin-1 (Homo sapiens (Human)) | BDBM50476880 (CHEMBL450279) | PDB MMDB UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 1.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Co. Curated by ChEMBL | Assay Description Displacement of [3H]succinamide from gamma secretase in THP1 cell membranes | Bioorg Med Chem Lett 17: 3910-5 (2007) Article DOI: 10.1016/j.bmcl.2007.04.102 BindingDB Entry DOI: 10.7270/Q2M90CFP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Presenilin-1 (Homo sapiens (Human)) | BDBM50476871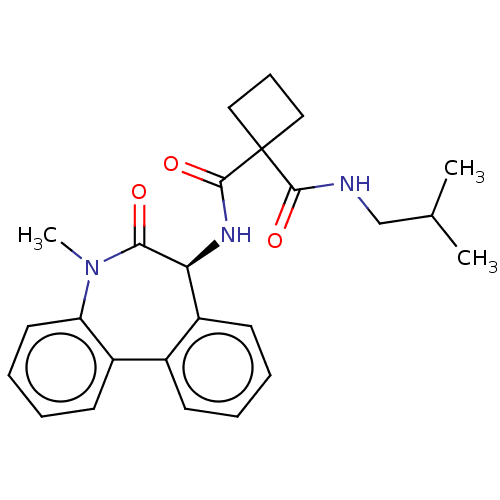 (CHEMBL232182) | PDB MMDB UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 1.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Co. Curated by ChEMBL | Assay Description Displacement of [3H]succinamide from gamma secretase in THP1 cell membranes | Bioorg Med Chem Lett 17: 3910-5 (2007) Article DOI: 10.1016/j.bmcl.2007.04.102 BindingDB Entry DOI: 10.7270/Q2M90CFP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glutamate receptor ionotropic, NMDA 2B (Rattus norvegicus (Rat)) | BDBM198694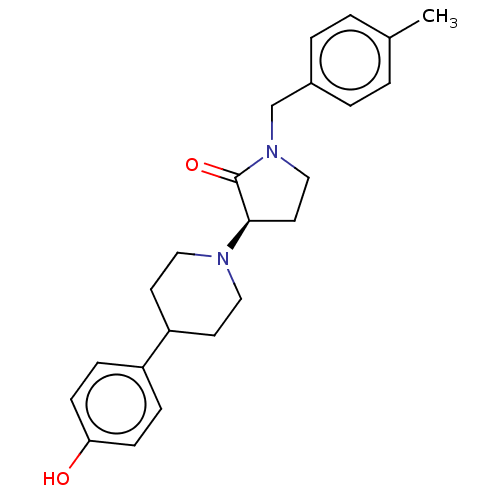 (US9221796, 23b) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 1.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Research and Development Curated by ChEMBL | Assay Description Displacement of [3H]Ro 25-6981 from GluN2B receptor in Sprague-Dawley rat forebrain membranes incubated for 1 hr by topcount micro scintillation coun... | ACS Med Chem Lett 9: 472-477 (2018) Article DOI: 10.1021/acsmedchemlett.8b00080 BindingDB Entry DOI: 10.7270/Q2PR7ZJT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glutamate receptor ionotropic, NMDA 2B (Rattus norvegicus (Rat)) | BDBM198694 (US9221796, 23b) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 1.40 | -54.8 | n/a | n/a | n/a | n/a | n/a | n/a | 50 |
Bristol-Myers Squibb Company US Patent | Assay Description To perform the competition binding assay, thawed membrane homogenate was added to each well of a 96-well plate (20 ug/well). The experimental compo... | US Patent US9221796 (2015) BindingDB Entry DOI: 10.7270/Q20V8BK1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glutamate receptor ionotropic, NMDA 2B (Rattus norvegicus (Rat)) | BDBM198665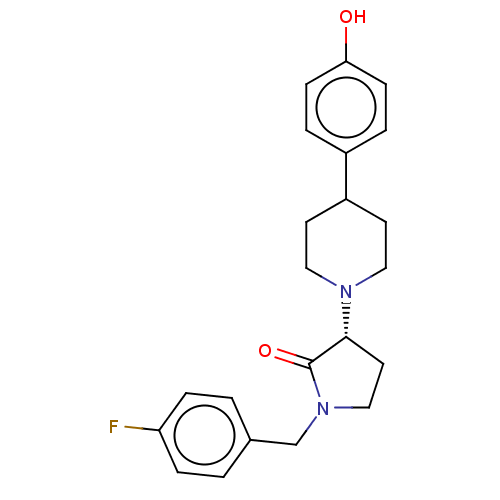 (US9221796, 2b) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 1.40 | -54.8 | n/a | n/a | n/a | n/a | n/a | n/a | 50 |
Bristol-Myers Squibb Company US Patent | Assay Description To perform the competition binding assay, thawed membrane homogenate was added to each well of a 96-well plate (20 ug/well). The experimental compo... | US Patent US9221796 (2015) BindingDB Entry DOI: 10.7270/Q20V8BK1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glutamate receptor ionotropic, NMDA 2B (Rattus norvegicus (Rat)) | BDBM198665 (US9221796, 2b) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 1.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Research and Development Curated by ChEMBL | Assay Description Displacement of [3H]Ro 25-6981 from GluN2B receptor in Sprague-Dawley rat forebrain membranes incubated for 1 hr by topcount micro scintillation coun... | ACS Med Chem Lett 9: 472-477 (2018) Article DOI: 10.1021/acsmedchemlett.8b00080 BindingDB Entry DOI: 10.7270/Q2PR7ZJT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glutamate receptor ionotropic, NMDA 2B (Rattus norvegicus (Rat)) | BDBM198687 (US9221796, 18b) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 1.5 | -54.6 | n/a | n/a | n/a | n/a | n/a | n/a | 50 |
Bristol-Myers Squibb Company US Patent | Assay Description To perform the competition binding assay, thawed membrane homogenate was added to each well of a 96-well plate (20 ug/well). The experimental compo... | US Patent US9221796 (2015) BindingDB Entry DOI: 10.7270/Q20V8BK1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glutamate receptor ionotropic, NMDA 2B (Rattus norvegicus (Rat)) | BDBM198685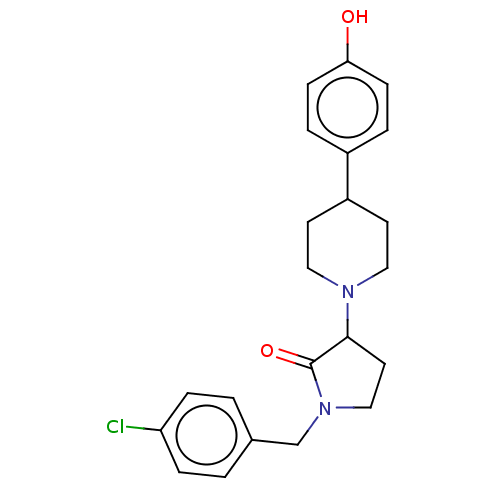 (US9221796, 17) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 1.60 | -54.4 | n/a | n/a | n/a | n/a | n/a | n/a | 50 |
Bristol-Myers Squibb Company US Patent | Assay Description To perform the competition binding assay, thawed membrane homogenate was added to each well of a 96-well plate (20 ug/well). The experimental compo... | US Patent US9221796 (2015) BindingDB Entry DOI: 10.7270/Q20V8BK1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glutamate receptor ionotropic, NMDA 2B (Rattus norvegicus (Rat)) | BDBM198681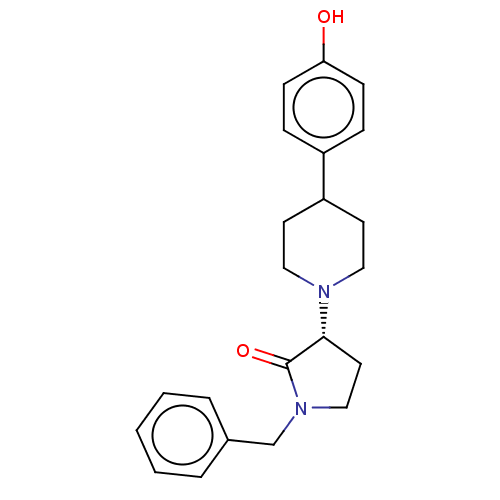 (US9221796, 13b) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 1.60 | -54.4 | n/a | n/a | n/a | n/a | n/a | n/a | 50 |
Bristol-Myers Squibb Company US Patent | Assay Description To perform the competition binding assay, thawed membrane homogenate was added to each well of a 96-well plate (20 ug/well). The experimental compo... | US Patent US9221796 (2015) BindingDB Entry DOI: 10.7270/Q20V8BK1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1A (Homo sapiens (Human)) | BDBM50128368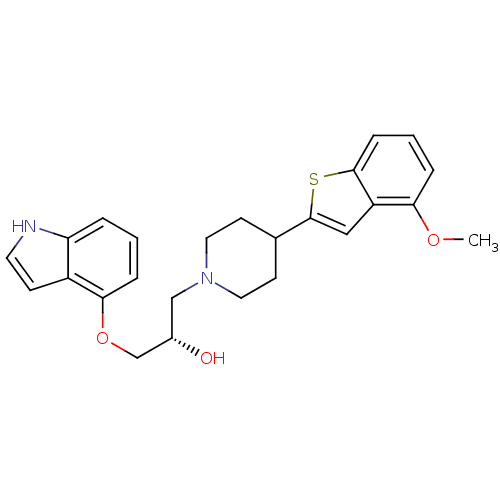 ((S)-1-(1H-Indol-4-yloxy)-3-[4-(4-methoxy-benzo[b]t...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.89 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories Curated by ChEMBL | Assay Description Displacement of [3H]8-OH-DPAT from 5HT1A receptor | Bioorg Med Chem Lett 16: 2347-51 (2006) Article DOI: 10.1016/j.bmcl.2005.11.007 BindingDB Entry DOI: 10.7270/Q2W958RV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium-dependent serotonin transporter (Homo sapiens (Human)) | BDBM50128367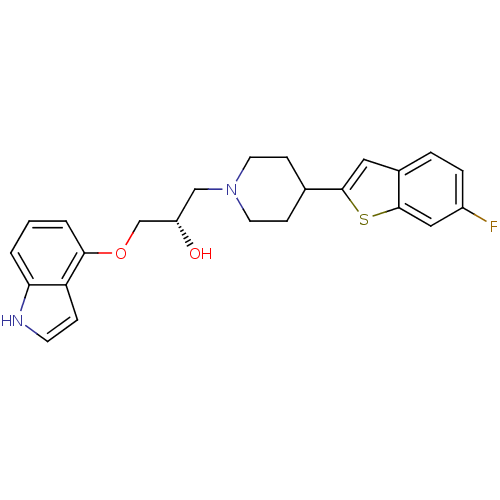 ((S)-1-(1H-indol-4-yloxy)-3-(4-(6-fluorobenzo[b]thi...) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.99 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories Curated by ChEMBL | Assay Description Displacement of [3H]paroxetine from 5HT reuptake site | Bioorg Med Chem Lett 16: 2347-51 (2006) Article DOI: 10.1016/j.bmcl.2005.11.007 BindingDB Entry DOI: 10.7270/Q2W958RV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glutamate receptor ionotropic, NMDA 2B (Rattus norvegicus (Rat)) | BDBM198835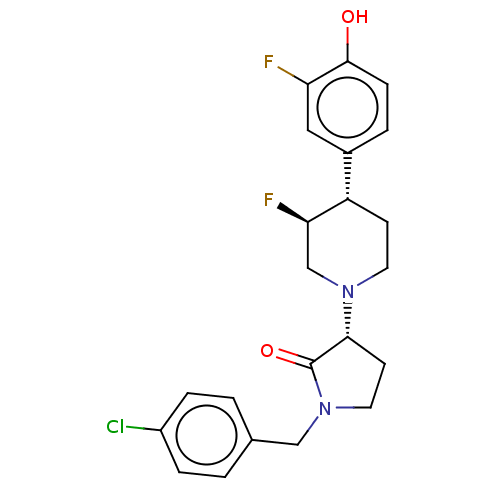 (US9221796, 116) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 2 | -53.8 | n/a | n/a | n/a | n/a | n/a | n/a | 50 |
Bristol-Myers Squibb Company US Patent | Assay Description To perform the competition binding assay, thawed membrane homogenate was added to each well of a 96-well plate (20 ug/well). The experimental compo... | US Patent US9221796 (2015) BindingDB Entry DOI: 10.7270/Q20V8BK1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Presenilin-1 (Homo sapiens (Human)) | BDBM50476864 (CHEMBL429438) | PDB MMDB UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Co. Curated by ChEMBL | Assay Description Displacement of [3H]succinamide from gamma secretase in THP1 cell membranes | Bioorg Med Chem Lett 17: 3910-5 (2007) Article DOI: 10.1016/j.bmcl.2007.04.102 BindingDB Entry DOI: 10.7270/Q2M90CFP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Presenilin-1 (Homo sapiens (Human)) | BDBM50476860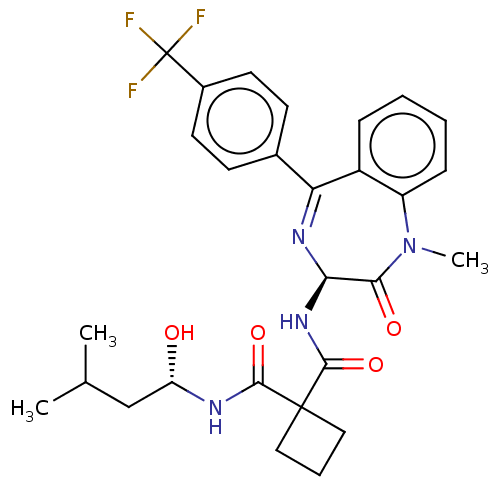 (CHEMBL232936) | PDB MMDB UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 2.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Co. Curated by ChEMBL | Assay Description Displacement of [3H]succinamide from gamma secretase in THP1 cell membranes | Bioorg Med Chem Lett 17: 3910-5 (2007) Article DOI: 10.1016/j.bmcl.2007.04.102 BindingDB Entry DOI: 10.7270/Q2M90CFP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Rattus norvegicus (rat)) | BDBM50323910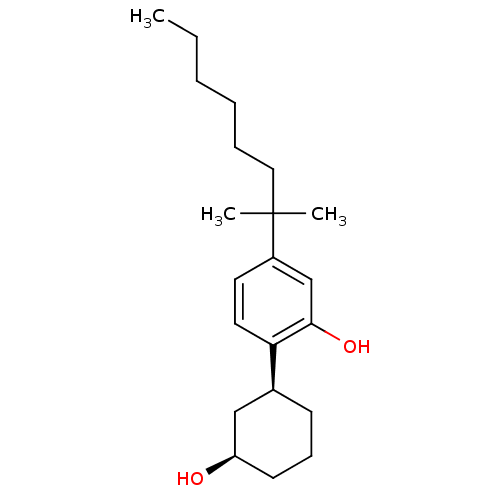 (2-((1S,3R)-3-hydroxycyclohexyl)-5-(2-methyloctan-2...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 2.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Clemson University Curated by ChEMBL | Assay Description Displacement of [3H]CP-55940 from CB1 receptor in Sprague-Dawley rat brain membrane | Bioorg Med Chem 16: 322-35 (2008) Article DOI: 10.1016/j.bmc.2007.09.033 BindingDB Entry DOI: 10.7270/Q22V2H0T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Presenilin-1 (Homo sapiens (Human)) | BDBM50476870 (CHEMBL232346) | PDB MMDB UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 2.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Co. Curated by ChEMBL | Assay Description Displacement of [3H]succinamide from gamma secretase in THP1 cell membranes | Bioorg Med Chem Lett 17: 3910-5 (2007) Article DOI: 10.1016/j.bmcl.2007.04.102 BindingDB Entry DOI: 10.7270/Q2M90CFP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glutamate receptor ionotropic, NMDA 2B (Rattus norvegicus (Rat)) | BDBM198724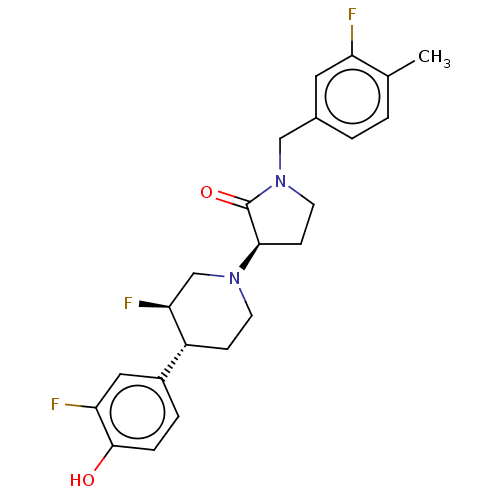 (US9221796, 45, P-4) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 2.5 | -53.2 | n/a | n/a | n/a | n/a | n/a | n/a | 50 |
Bristol-Myers Squibb Company US Patent | Assay Description To perform the competition binding assay, thawed membrane homogenate was added to each well of a 96-well plate (20 ug/well). The experimental compo... | US Patent US9221796 (2015) BindingDB Entry DOI: 10.7270/Q20V8BK1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Presenilin-1 (Homo sapiens (Human)) | BDBM209909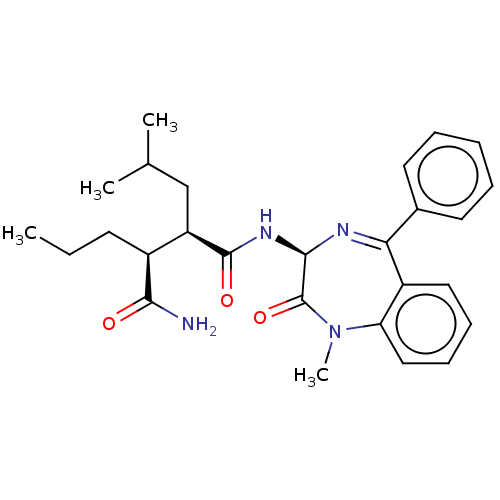 (US9273014, Comparative Compound 45 | US9427442, Co...) | PDB MMDB UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 2.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Co. Curated by ChEMBL | Assay Description Displacement of [3H]succinamide from gamma secretase in THP1 cell membranes | Bioorg Med Chem Lett 17: 3910-5 (2007) Article DOI: 10.1016/j.bmcl.2007.04.102 BindingDB Entry DOI: 10.7270/Q2M90CFP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glutamate receptor ionotropic, NMDA 2B (Rattus norvegicus (Rat)) | BDBM198834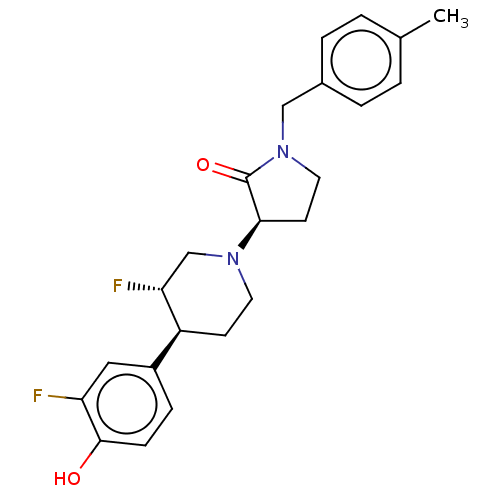 (US9221796, 115) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 2.70 | -53.0 | n/a | n/a | n/a | n/a | n/a | n/a | 50 |
Bristol-Myers Squibb Company US Patent | Assay Description To perform the competition binding assay, thawed membrane homogenate was added to each well of a 96-well plate (20 ug/well). The experimental compo... | US Patent US9221796 (2015) BindingDB Entry DOI: 10.7270/Q20V8BK1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1A (Homo sapiens (Human)) | BDBM50130161 ((S)-1-(1H-Indol-4-yloxy)-3-[(4R,6R)-4-(4-methoxy-b...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 2.76 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories Curated by ChEMBL | Assay Description Displacement of [3H]8-OH-DPAT from 5HT1A receptor | Bioorg Med Chem Lett 16: 2347-51 (2006) Article DOI: 10.1016/j.bmcl.2005.11.007 BindingDB Entry DOI: 10.7270/Q2W958RV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glutamate receptor ionotropic, NMDA 2B (Rattus norvegicus (Rat)) | BDBM198837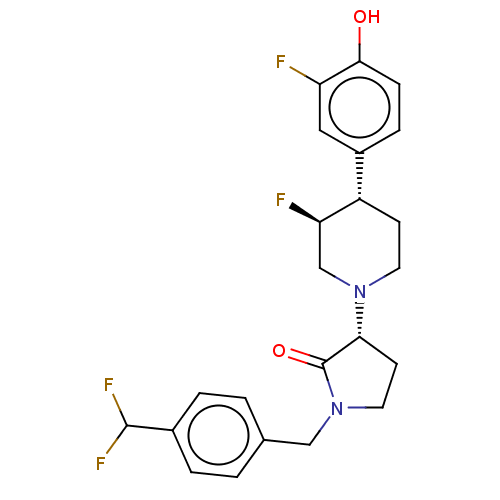 (US9221796, 117, P-2) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 2.90 | -52.8 | n/a | n/a | n/a | n/a | n/a | n/a | 50 |
Bristol-Myers Squibb Company US Patent | Assay Description To perform the competition binding assay, thawed membrane homogenate was added to each well of a 96-well plate (20 ug/well). The experimental compo... | US Patent US9221796 (2015) BindingDB Entry DOI: 10.7270/Q20V8BK1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glutamate receptor ionotropic, NMDA 2B (Rattus norvegicus (Rat)) | BDBM198692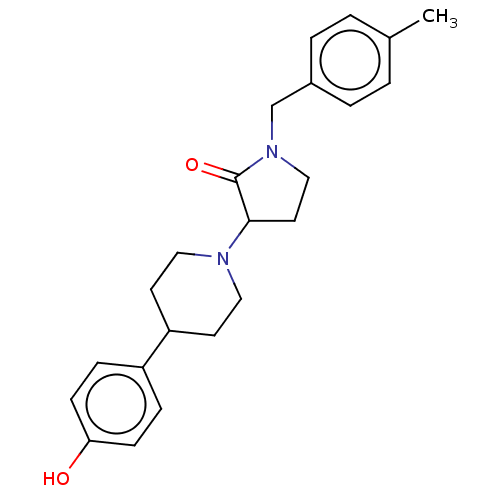 (US9221796, 22) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 2.90 | -52.8 | n/a | n/a | n/a | n/a | n/a | n/a | 50 |
Bristol-Myers Squibb Company US Patent | Assay Description To perform the competition binding assay, thawed membrane homogenate was added to each well of a 96-well plate (20 ug/well). The experimental compo... | US Patent US9221796 (2015) BindingDB Entry DOI: 10.7270/Q20V8BK1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 2 (Homo sapiens (Human)) | BDBM50287933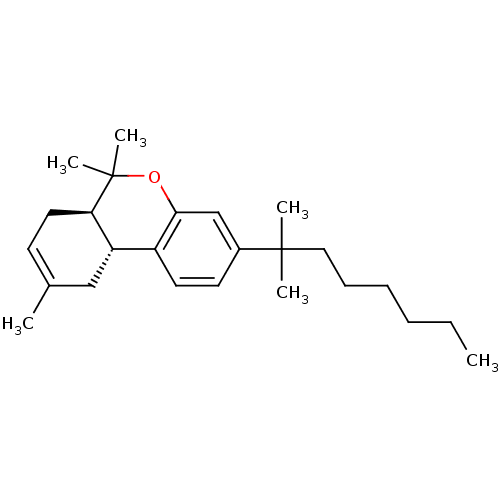 ((6aR,10aR)-3-(1,1-Dimethyl-heptyl)-6,6,9-trimethyl...) | PDB Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 2.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Clemson University Curated by ChEMBL | Assay Description Binding affinity to CB2 receptor | Bioorg Med Chem 18: 7809-15 (2010) Article DOI: 10.1016/j.bmc.2010.09.061 BindingDB Entry DOI: 10.7270/Q2ZK5HP7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glutamate receptor ionotropic, NMDA 2B (Rattus norvegicus (Rat)) | BDBM198663 (US9221796, 1) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 3 | -52.7 | n/a | n/a | n/a | n/a | n/a | n/a | 50 |
Bristol-Myers Squibb Company US Patent | Assay Description To perform the competition binding assay, thawed membrane homogenate was added to each well of a 96-well plate (20 ug/well). The experimental compo... | US Patent US9221796 (2015) BindingDB Entry DOI: 10.7270/Q20V8BK1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Presenilin-1 (Homo sapiens (Human)) | BDBM50476872 (CHEMBL401045) | PDB MMDB UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Co. Curated by ChEMBL | Assay Description Displacement of [3H]succinamide from gamma secretase in THP1 cell membranes | Bioorg Med Chem Lett 17: 3910-5 (2007) Article DOI: 10.1016/j.bmcl.2007.04.102 BindingDB Entry DOI: 10.7270/Q2M90CFP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1A (Homo sapiens (Human)) | BDBM50130157 ((S)-1-((4S,6R)-4-Benzo[b]thiophen-2-yl-2-methyl-pi...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 3.09 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories Curated by ChEMBL | Assay Description Displacement of [3H]8-OH-DPAT from 5HT1A receptor | Bioorg Med Chem Lett 16: 2347-51 (2006) Article DOI: 10.1016/j.bmcl.2005.11.007 BindingDB Entry DOI: 10.7270/Q2W958RV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glutamate receptor ionotropic, NMDA 2B (Rattus norvegicus (Rat)) | BDBM198722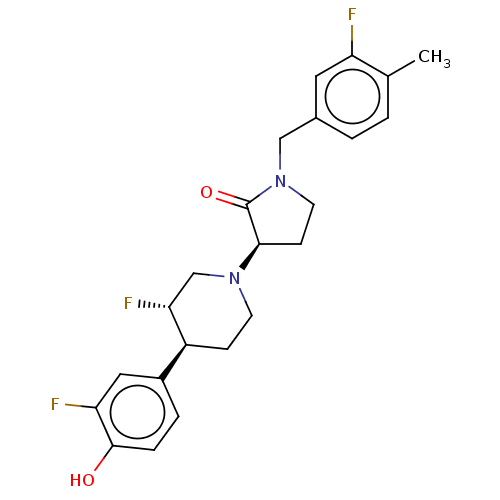 (US9221796, 45, P-2) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 3.10 | -52.6 | n/a | n/a | n/a | n/a | n/a | n/a | 50 |
Bristol-Myers Squibb Company US Patent | Assay Description To perform the competition binding assay, thawed membrane homogenate was added to each well of a 96-well plate (20 ug/well). The experimental compo... | US Patent US9221796 (2015) BindingDB Entry DOI: 10.7270/Q20V8BK1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Presenilin-1 (Homo sapiens (Human)) | BDBM50476865 (CHEMBL392265) | PDB MMDB UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | KEGG PC cid PC sid UniChem | Article PubMed | 3.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Co. Curated by ChEMBL | Assay Description Displacement of [3H]succinamide from gamma secretase in THP1 cell membranes | Bioorg Med Chem Lett 17: 3910-5 (2007) Article DOI: 10.1016/j.bmcl.2007.04.102 BindingDB Entry DOI: 10.7270/Q2M90CFP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 1938 total ) | Next | Last >> |